Abstract
Background:
The risk of solid cancers from low-level, protracted ionising radiation is not well characterized. Nuclear workers provide valuable information on the effects of ionising radiation in contemporary exposure scenarios relevant to workers and the public.
Methods:
We evaluated the association between penetrating ionising radiation exposure and solid cancer mortality among a pooled cohort of nuclear workers in the United States, with extended follow-up to examine cancers with long latencies.
This analysis includes 101,363 workers from five nuclear facilities, with 12,069 solid cancer deaths between 1944 and 2016. The association between cumulative equivalent dose measured in Sieverts (Sv) and solid cancer subtypes were modelled as the excess relative rate per Sv (ERR Sv−1) using Cox regression.
Results:
For the association between ionising radiation exposure and all solid cancer mortality we observed an elevated rate (ERR Sv−1=0.19; 95%CI: −0.10, 0.52), which was higher among a contemporary subcohort of workers first hired 1960 or later (ERR Sv−1= 2.23; 95% CI: 1.13, 3.49). Similarly, we observed an elevated rate for lung cancer mortality (ERR Sv−1= 0.65; 0.09, 1.30) which was higher among contemporary hires (ERR Sv−1= 2.90; 95% CI: 1.00, 5.26).
Conclusions:
Although concerns remain about confounding, measurement error, and precision, this analysis strengthens the evidence base indicating there are radiogenic risks for several solid cancer types.
Keywords: Ionising radiation, cancer, nuclear workers, radiation protection, pooled study
Introduction
Ionising radiation is a carcinogen,1 but quantifying the risks of solid cancer from low dose and low dose-rate ionising radiation exposure requires additional study.2 Uncertainties in the effects of ionizing radiation on solid cancers have direct implications for radiation protection standards and worker compensation.3 Current radiation protection standards are mainly based on studies of the Japanese atomic bomb survivors who were acutely exposed to ionising radiation.4 Over 10 million United States (US) workers receive low dose (<100 mGy) and low dose rate (<6 mGy/hour) radiation from their jobs in medicine, radiography, commercial and government nuclear work, mining, milling, and air travel.3 5 Over 100 million US citizens receive low dose radiation from diagnostic medical exposures.5 Studies of nuclear workers provide more information on the risks from low dose and low dose-rate ionising radiation exposure, which better reflect the contemporary radiation scenarios to which hundreds of millions of Americans are exposed.
Epidemiologists have studied nuclear workers and reported on their solid cancer risks for several decades.6–8 In the 2005 mortality update of five US nuclear worker cohorts, which we expand upon in the present analysis, investigators reported slightly elevated rates of all cancer excluding leukaemia, all non-smoking related cancer, and lung cancer.9 In a recent study of cancer incidence among of nuclear workers in the United Kingdom (UK), investigators reported elevated rates of all solid cancer, and cancers of the lung, colon, bladder, and pleura.10 In France, investigators reported elevated rates of all solid cancers, lung cancer, and suggestive associations for several subtypes.11 These cohorts of nuclear workers from the US, UK, and France were pooled in the International Nuclear Workers Study (INWORKS) 2 in which investigators found elevated rates for all solid cancer, and several site-specific cancers, including but not limited to stomach, colon, lung, bone, skin, ovary, and thyroid cancer. 12 13
The findings from these studies are informative, but several types of solid cancer have long latency and induction periods, requiring additional follow-up time. The Life Span Study of Japanese atomic bomb survivors indicates that the that latency period from ionising radiation exposure is longer for solid cancers than for hematopoietic cancers.14 15 To address questions of low-dose and low-dose rate exposure from ionising radiation and solid cancer risks over a longer latent period, we report solid cancer mortality associations in the pooled cohort of US nuclear workers, extending follow-up for an additional decade, with almost 4 million person-years at risk.
First, we characterize the occupational experience of the cohorts compared to the general population using standardized mortality ratios (SMR). We then report associations between exposure to external penetrating ionising radiation and solid cancer subtypes of interest as excess relative rates per Sievert (ERR Sv−1). We evaluate temporal modification and we conduct several sensitivity analyses to address concerns about potential confounding by environmental and occupational co-exposures, and to evaluate exposure measurement error.
Methods
The cohorts.
The cohort data in this study was enumerated from records of US nuclear workers employed at four Department of Energy (DOE) sites: the Hanford site,16 17 Idaho National Laboratory,18 Oak Ridge National Laboratory,19 20 and Savannah River Site,21 22; and one Department of Defense site, Portsmouth Naval Shipyard (PNS).23 24 Workers are eligible for inclusion in the cohort if they were ever monitored for external ionising radiation and were employed for at least one year at any of the five facilities. The prior update of this cohort had a 30-day employment criterion, but in the current study a one year criterion was chosen to be comparable with INWORKS; therefore, there are 17,832 fewer workers in this update.
Employment records were available at the start of all facility operations except for PNS, where records were available starting in 1952. Facility employment records for the cohort members could not be updated for this analysis, but work history dates were extended based on a combination of employment records and updated dosimetry badge records. Date of first hire is based on the earliest record of employment. Date of last employment was based on either the latest employment record at any of the five facilities, or the last dose record, whichever was later.
Additional details of cohort assembly can be found in a prior publication.9 This study was approved by Institutional Review Boards of the National Institute for Occupational Safety and Health and the US DOE. Informed consent was waived for this records-based study.
Vital status and mortality outcomes.
Vital status and cause of death were extended from December 31, 2005, through December 31, 2016 by linkage to the US National Death Index (NDI). Deaths were coded to the International Classification of Disease (ICD) code in effect at the time of death. Linkages to the Social Security Administration Death Masterfile and the Internal Revenue Service were used to confirm vital status and to ensure NDI matches, when needed. Deaths which occurred prior to the establishment of NDI were obtained from prior facility-specific studies, and from state death certificates when necessary.
Outcomes of interest in this analysis are site-specific solid cancer groups of a priori interest based on prior studies of nuclear workers, and broader informative groupings of solid cancers of interest for radiation protection purposes or to assess for confounding. National Institute for Occupational Safety and Health cause of death categories (NIOSH-92) or ICD-10 cause of death categories for each outcome are listed in Supplementary Table S1. Broad groups include: (1) all solid cancers, which is of interest for radiation protection models; (2) solid cancer excluding lung; (3) all non-smoking related solid cancers, to evaluate the potential confounding impacts of cigarette smoking; and (4) solid cancer excluding lung, liver, and bone cancer, to evaluate the impact of organ sites affected by plutonium deposition.12 16 Estimates for chronic obstructive pulmonary disease (COPD) and for mesothelioma and pleural cancer are reported to indirectly assess the magnitude of smoking and asbestos, respectively, as potential unmeasured confounders of the association between ionising radiation and lung cancer mortality.25
Ionising radiation exposure.
Methods for exposure assessment are detailed in a previous update of this pooled cohort9 and in previous studies of individual facilities. Briefly, the main exposure in this analysis stems from occupational sources of whole-body penetrating low linear energy transfer radiations external to the body. Estimates of annual equivalent penetrating dose were obtained primarily from individual dosimetry records of personal monitoring conducted at study facilities. Additional dosimetry records were obtained from available records from other DOE facilities and by linking the cohort data to the US DOE Radiation Exposure Monitoring System and the US Nuclear Regulatory Commission Radiation Exposure Information Reporting System databases. Dose estimates were available through 2016 with the exception of PNS which was last updated in 1996.26 However, most PNS employees in the cohort had stopped working since the last time dosimetry records were updated.9
In addition to external penetrating ionising radiation, some workers received doses from internally deposited radionuclides. For comparability to INWORKS, doses from tritium assimilation were not included. We also did not account for internal deposition of radionuclides in cumulative radiation dose estimates because only a small proportion of workers (<2%) were known to be exposed, and internal deposition is responsible for a very small proportion of the collective dose in this cohort. 9 To evaluate the impact of neutron exposure, workers were flagged if they were ever monitored for neutrons; among monitored workers, neutron exposure was categorized as either 10% less or more of their total cumulative external dose.
The quality of dosimetry records and film badge technology improved over time. Earlier dosimetry records were subject to missing values 17, but starting in the late 1950s and early 1960s, improvements in dosimeter technology (such as the introduction of the multi-element dosimeter and improvements in dosimeter badge film processing) improved the quality of dosimetry records. Around the same time, workplace safety and health improvements led to improved working conditions and lower occupational exposures. To evaluate the impact of improved dosimetry in a workforce that more closely resembles contemporary working conditions, we examined a subcohort of workers first hired in 1960 or later.
Statistical methods.
To characterize the occupational experience of the cohort, we calculated standardized mortality ratios (SMRs) using US mortality reference rates and calculated corresponding 95% confidence intervals using the Byar approximation of the Poisson distribution.27 US population mortality rates are available starting January 1, 1940. SMRs were standardized by sex, race, 5-year age group, and 5-year calendar period. Person-time began one year after the start of employment or the date of first radiation monitoring, whichever was later. Since PNS employment records do not start until 1952, person-time for PNS workers began one year after the start of employment, the date of first radiation monitoring, or 1952. Person-time ended at the date of death or the end of follow-up (December 31st, 2016), whichever was earlier. For workers last observed alive prior to the establishment of the NDI in 1979, person-time ended at the last date of employment.
SMRs were calculated for all solid cancers as defined in the NIOSH-92 major rate file categories.28 Excess mortality caused by asbestos exposure is documented among US nuclear workers, and is an ongoing public health concern. Therefore, we conducted additional SMR analyses on mesothelioma mortality, stratifying by duration of employment, facility of longest employment duration, occupational status, calendar period, and period of hire.
We estimated the association between external penetrating ionising radiation and excess relative rates of cancer per Sievert (ERR Sv−1) using Cox regression 29 with attained age as the time scale. Risk sets in the main analyses were created by matching cases to all cohort members alive and at risk at the age of death of the case, as well as matching on date of birth (+/− 5 years of index case), sex, facility at first hire, neutron monitoring flag, occupational status (based on job title at hire: professional and technical workers, administrative staff, skilled manual workers, unskilled manual workers, and uncertain), and duration of employment or radiation monitoring (in 10 year intervals). We matched on occupational status because it is associated with exposure and unmeasured lifestyle factors such as cigarette smoking, and we matched on duration of employment or radiation monitoring to partially account for healthy worker survivor bias.
The relationship between cumulative ionising radiation exposure and solid cancer deaths was modelled as a linear excess relative rates per unit dose (ERR Sv−1) and 95% profile likelihood-based confidence intervals using the general model form where represents the ERR Sv−1 of cumulative radiation exposure d, which includes an a priori 10-year lag. 30 represents the baseline rate by the matching variables. To test the impact of a shorter lag, we fit a model with a 5-year lag and evaluated goodness of fit using likelihood ratio tests. In addition to a linear model, we plotted the relative rate (RR) in nine categories of cumulative exposure, and fit a spline model with knots at 50, 100, and 150 mSv.
We evaluated potential temporal modifiers of the associations between external penetrating ionising radiation and solid cancer types. We separately evaluated age at exposure in three windows (<30 years, 30-<50 years, and 50+ years of age), and time since exposure in three windows (10-<25 years, 25-<40 years, and 40+ years since exposure) by treating cumulative exposure as a time-dependent variable defined by categories of these temporal modifiers. We used the model form where is the parameter representing the ERR/Sv in age at exposure or time since exposure in windows, . Temporal modifier analyses were restricted to groups or sites with 300 deaths or more. All analyses were conducted in SAS 9.4.
Sensitivity analyses.
We conducted several sensitivity analyses: (1) we repeated the main analysis restricted to workers first hired in 1960 or to evaluate rates per unit dose among contemporary workers; (2) we restricted analyses to workers with a cumulative exposure of less than 200 mSv; and (3) we repeated the main analysis restricted to workers without neutron monitoring.
Results
A total of 101,363 workers met cohort inclusion criteria (Table 1). Workers contributed about 4 million person-years of follow-up time between 1944 and 2016. Median follow-up time was 39 years, and 51% of the cohort was deceased by the end of follow-up. Cause of death was unknown among 1.8% of decedents, and 0.9% of the cohort was lost to follow-up. Cumulative gamma dose ranged from 0 to 1109 mSv and was skewed towards lower exposures, with a median of 4 mSv. Facility-specific characteristics are reported in Supplementary Table S2.
Table 1.
Characteristics of the pooled United States nuclear worker cohort
| Characteristic | |
|---|---|
| Number of workers in cohort | 101 363 |
| Calendar years of follow-up | 1944–2016 |
| Person-years of follow-up | 3 980 319 |
| Facility, n (%) | |
| Portsmouth Naval Shipyard | 9 488 (9%) |
| Oak Ridge National Laboratory | 14 213 (14%) |
| Hanford site | 28 412 (28%) |
| Savannah River site | 20 005 (20%) |
| Idaho National Laboratory | 29 245 (29%) |
| Years of follow-up, median (IQR) | 39 (30–49) |
| Year of birth, median (IQR) | 1934 (1922–1948) |
| Age (years) at hire, median (IQR) | 28 (23–35) |
| Years of employment, median (IQR) | 20 (7–30) |
| Age (years) at death among deceased workers, median (IQR) | 75 (65–83) |
| Male, n (%) | 81 799 (81%) |
| Occupational status, n (%) | |
| Professional and technical | 38 070 (38%) |
| Skilled non-manual | 14 932 (15%) |
| Skilled manual | 34 363 (34%) |
| Partly skilled and unskilled | 10 515 (10%) |
| Missing | 3 483 (3%) |
| Deceased, n (%) | 51 350 (51%) |
| All cancer | 13 568 (13%) |
| All solid cancer | 12 069 (12%) |
| Unknown cause of death | 1 789 (2%) |
| Lost to follow-up | 941 (1%) |
| Cumulative exposure (mSv), mean, mediana (range) | |
| Gamma | 26.5, 4.6 (0–1109) |
| Neutron | 5.1, 0.8 (0–251) |
| Tritium | 4.4, 0.6 (0–583) |
IQR, interquartile range.
Among workers with exposures >0.
Standardized mortality ratio results.
Summary SMR estimates were all below or near expectation with the exception of pleural cancer (SMR = 2.71; 95% CI: 1.83, 3.86) and mesothelioma (SMR = 2.75; 95% CI 2.33, 3.23) (Supplementary Table S3). We examined mesothelioma mortality by facility (Table 2). While mortality rates were higher than expected for all facilities, PNS had a higher excess mortality rate of mesothelioma than the other facilities (SMR = 8.62; 95%CI: 6.36, 11.44). Skilled manual workers had a higher excess mortality rate than other types of workers (SMR = 5.87; 95%CI 4.78, 7.14), and there was an increase in the excess mortality rate by duration of employment, with the highest excess mortality rate among workers employed 25 years or longer (SMR = 3.33; 9%CI: 2.60, 4.21). Mesothelioma excess mortality remained elevated across all calendar periods. We did not observe substantial variation of SMRs for all solid cancer or all solid cancer other than lung by job type or other factors.
Table 2.
Standardized mortality ratiosa and 95% confidence intervals for mesothelioma, stratified by duration of employment, job type, period of hire, calendar period, and facility of longest employment duration in pooled cohort of workers from five United States nuclear facilities, follow-up through 2016
| Characteristic | Observed | SMR (95% CI) |
|---|---|---|
| Duration of employment | ||
| <5 years | 31 | 2.30 (1.57, 3.27) |
| 5 to <15 years | 26 | 2.37 (1.55, 3.48) |
| 15 to <25 years | 21 | 2.51 (1.55, 3.84) |
| ≥25 years | 70 | 3.33 (2.60, 4.21) |
| Job type | ||
| Professional and technical | 31 | 1.19 (0.81, 1.69) |
| Skilled non-manual | 6 | 1.55 (0.57, 3.38) |
| Skilled manual | 100 | 5.87 (4.78, 7.14) |
| Partly skilled and unskilled | 7 | 1.29 (0.52, 2.65) |
| Missing | <5 | 2.91 (0.78, 7.45) |
| Period of hire | ||
| <1955 | 61 | 3.03 (2.31, 3.89) |
| 1955 to <1965 | 57 | 3.43 (2.60, 4.44) |
| 1965 to <1975 | 19 | 2.15 (1.29, 3.35) |
| 1975 to <1985 | 9 | 1.38 (0.63, 2.61) |
| 1985+ | <5 | 1.24 (0.14, 4.49) |
| Calendar periodb | ||
| <2000 | 8 | 2.91 (1.25, 5.74) |
| 2000 to <2010 | 85 | 2.80 (2.24, 3.47) |
| 2010+ | 55 | 2.66 (2.00, 3.46) |
| Facility | ||
| Portsmouth Naval Shipyard | 48 | 8.62 (6.36, 11.44) |
| Hanford site | 10 | 1.15 (0.55, 2.12) |
| Oak Ridge National Laboratory | 35 | 2.38 (1.66, 3.31) |
| Savannah River site | 12 | 1.19 (0.61, 2.07) |
| Idaho National Laboratory | 43 | 2.92 (2.11, 3.94) |
SMR, standardized mortality ratio.
Standardized by sex, 5-year age group, and 5-year calendar period.
Deaths are limited to mesotheliomas coded into the ICD-10 revision mesothelioma category, which began in 1999.
Exposure-response results.
The ERR Sv−1 for all solid cancer was 0.19 (95% CI: −0.10, 0.52), which decreased when restricted to all solid cancer except lung, liver, and bone (ERR Sv−1 = 0.07; 95% CI: −0.27, 0.47) and was close to the null when restricted to all solid cancer except lung (ERR Sv−1 = −0.01; 95% CI: −0.34, 0.36). The ERR Sv−1 was elevated for all non-smoking related solid cancers (ERR Sv−1 = 0.11; 95% CI: −0.37, 0.67) (Table 3). Compared to a 5-year lag, the 10-year lag was the best fit for most outcomes. Even when likelihood ratio tests indicated the 5-year lag was a better fit, the differences in ERR Sv−1 estimates were negligible. When solid cancer rates were examined categorically, the RR increased with exposure in a non-monotonic fashion, and the spline and linear models had similar slopes (Figure 1, panel a).
Table 3.
Excess relative rate per sievert for solid cancer types and COPD among a pooled cohort of workers from five United States nuclear facilities, follow-up through 2016a
| Solid cancer type | Deaths | ERR/Sv (95% CI) |
|---|---|---|
| Solid cancer (all) | 12 069 | 0.19 (−0.10, 0.52) |
| Solid cancer excluding lung | 8 198 | −0.01 (−0.34, 0.36) |
| Solid cancer excluding lung, liver and bone | 7 828 | 0.07 (−0.27, 0.47) |
| Non-smoking-related solid cancer | 3 819 | 0.11 (−0.37, 0.67) |
| Mouth and pharynx | 193 | NC |
| Oesophagus | 348 | 0.04 (−1.33, 2.21) |
| Stomach | 333 | 0.51 (−0.86, 2.90) |
| Colon | 1 141 | 0.08 (−0.81, 1.32) |
| Rectum | 224 | −1.03 (−1.99, 1.40) |
| Liver | 348 | −1.57 (−2.18, −0.23) |
| Pancreas | 784 | −0.29 (−1.09, 0.98) |
| Larynx | 84 | 2.35 (−0.79, 11.5) |
| Lung | 3 871 | 0.65 (0.09, 1.30) |
| Mesothelioma and pleural | 178 | 2.54 (−0.25, 7.10) |
| Skin (all) | 333 | 0.99 (−0.56, 3.43) |
| Skin excluding melanoma | 78 | 0.51 (−1.76, 5.19) |
| Skin (melanoma) | 255 | 1.24 (−0.67, 4.47) |
| Female breast | 385 | −1.16 (−3.26, 5.44) |
| Uterus | 30 | NC |
| Ovary | 128 | 5.74 (−5.65, 37.3) |
| Prostate | 1 324 | −0.19 (−0.75, 0.57) |
| Bladder | 405 | 0.04 (−0.96, 1.87) |
| Kidney | 371 | 0.93 (−0.98, 3.99) |
| Brain and CNS | 388 | −0.92 (−1.64, 0.64) |
| Thyroid | 30 | 0.77 (−2.82, 13.9) |
| Bone | 22 | −8.31 (−13.5, 9.93) |
| COPD | 2 527 | −0.04 (−0.61, 0.67) |
ERR/Sv, excess relative rate per sievert; NC, non-convergence; CNS, central nervous system; COPD, chronic obstructive pulmonary disease.
Matched on age, sex, date of birth, facility, duration of employment, neutron monitoring, and job type.
Figure 1.
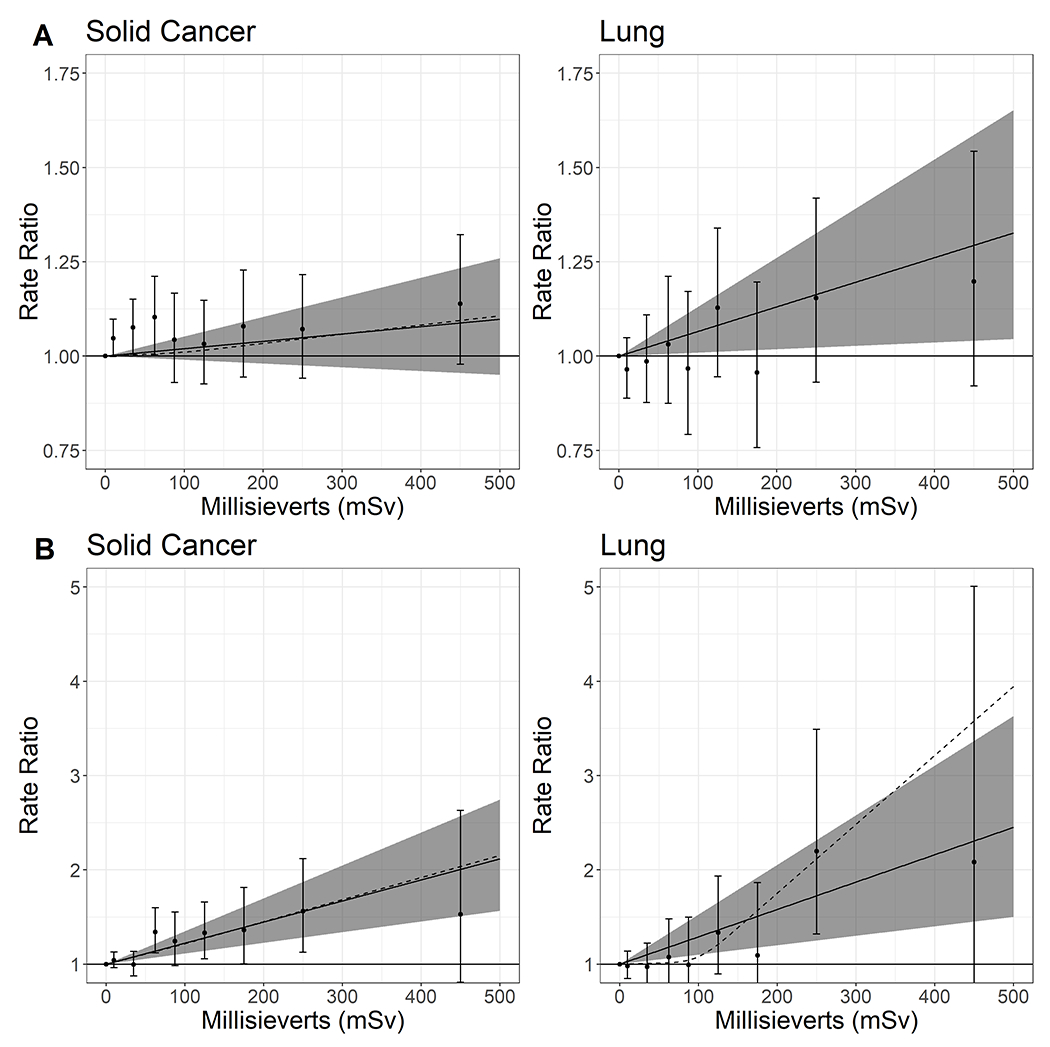
Relative rate and 95% confidence intervals for all solid cancer deaths and lung cancer deaths among a pooled cohort of workers from five United States nuclear facilities
Panel A includes the full cohort, panel B is restricted to workers first hired in 1960 or later. Solid lines represent the linear relative rates per sievert, and grey areas represent the ranges of the corresponding 95% confidence intervals.
Dotted lines represent the relative rates per sievert using spline models with knots at 50, 100, and 150 mSv.
Points and error bars are relative rates categorized by 0, >0 −20, >20-50, >50-75, >75-100, >100-150, >150-200, >200-300, and >300 mSv.
For site-specific solid cancers, we observed an association between cumulative ionising radiation and lung cancer (ERR Sv−1 = 0.65; 95% CI: 0.09, 1.30). When lung cancer was examined by categories, the RR increased with exposure in a non-monotonic fashion, and the spline and linear models had similar slopes (Figure 1, panel a). We also observed elevated ERRs for stomach, larynx, skin, mesothelioma/pleural, ovarian, kidney, and thyroid cancer (Table 3). However, for several of these solid cancer groups, there were few deaths.
When rates were examined by windows of time since exposure, there were elevated ERRs in the earliest window of time since exposure for stomach, breast, and bladder (Supplementary Table S4) compared to other windows. When rates were examined as windows of time since exposure, there were elevated ERRs in the longest window of time since exposure for all solid cancer, all non-smoking solid cancer, all solid cancer excluding lung, all solid cancer excluding lung, liver, and bone, and for subtypes of stomach, prostate, and bladder cancer compared to other windows (Supplementary Table S4). Many cancers had few deaths in each temporal window.
Sensitivity analyses results.
Restricting analyses to workers first hired in 1960 or later had a large impact on estimates (Table 4). The estimate for all solid cancers among 1960 or later hires (ERR Sv−1 = 2.23; 95% CI: 1.13, 3.49) was an order of magnitude higher than the full cohort estimate. Large increases in estimates were also observed for lung cancer (ERR Sv−1 = 2.90; 95% CI: 1.00, 5.26), colon cancer (ERR/Sv = 7.15; 95% CI: 2.13, 14.5), mesothelioma and pleura cancer (ERR/Sv = 16.9; 95% CI: 5.34, 38.5), and all other outcomes, with the exception of rectum, skin, thyroid and bone cancers which decreased among 1960 or later hires. When lung and solid cancer mortality estimates among the 1960 or later hires were evaluated categorically, relative rates increased non-monotonically. The linear and spline models were similar for all solid cancer but diverged for lung cancer, which resembled an exponential model (Figure 1, panel b). ERR Sv−1 for COPD was substantially higher among these workers, compared with the full cohort, but the magnitude of the estimate was much lower than for lung cancer and most of the outcomes noted above.
Table 4.
Excess relative rate per sievert for solid cancer types and COPD among a pooled cohort of workers from five United States nuclear facilities: sensitivity analyses restricted to workers first hired in 1960 or later, restricted to cumulative exposures less than 200 mSv, among workers employed for 30 days or more, and restricted to workers without neutron monitoring, follow-up through 2016a
| Main analysis (n = 101 363) |
First hired 1960+ (n = 56 260) |
Cumulative exposure <200 mSv (n = 98 918) |
Not flagged for neutron monitoring (n = 87 426) |
|
|---|---|---|---|---|
|
|
||||
| Solid cancer type | ERR/Sv (95% CI) | ERR/Sv (95% CI) | ERR/Sv (95% CI) | ERR/Sv (95% CI) |
| Solid cancer (all) | 0.19 (−0.10, 0.52) | 2.23 (1.13, 3.49) | 0.15 (−0.41, 0.74) | 0.16 (−0.25, 0.62) |
| Solid cancer excluding lung | −0.01 (−0.34, 0.36) | 1.88 (0.59, 3.41) | −0.01 (−0.68, 0.72) | −0.15 (−0.61, 0.38) |
| Solid cancer excluding lung, liver and bone | 0.07 (−0.27, 0.47) | 2.12 (0.75, 3.73) | 0.22 (−0.48, 0.98) | −0.13 (−0.60, 0.43) |
| Non-smoking-related solid cancer | 0.11 (−0.37, 0.67) | 1.63 (−0.15, 3.92) | 0.09 (−0.86, 1.17) | −0.06 (−0.74, 0.76) |
| Mouth and pharynx | NC | 7.60 (−3.69, 35.30) | −0.31 (−4.05, 5.96) | −3.44 (−6.08, 1.12) |
| Oesophagus | 0.04 (−1.33, 2.21) | 2.07 (−1.89, 9.05) | −1.10 (−3.23, 2.03) | −0.78 (−1.99, 1.37) |
| Stomach | 0.51 (−0.86, 2.90) | 6.04 (−1.48, 20.13) | 1.71 (−1.59, 6.70) | −0.68 (−2.57, 2.92) |
| Colon | 0.08 (−0.81, 1.32) | 7.15 (2.13, 14.54) | 0.65 (−1.24, 3.01) | −0.03 (−1.37, 1.78) |
| Rectum | −1.03 (−1.99, 1.40) | −4.05 (−6.45, 2.63) | −2.39 (−4.57, 1.76) | −1.57 (−2.79, 1.43) |
| Liver | −1.57 (−2.18, −0.23) | −1.44 (−3.92, 4.43) | −4.06 (−5.34, −1.75) | −0.55 (−2.31, 2.37) |
| Pancreas | −0.29 (−1.09, 0.98) | 0.80 (−1.50, 4.95) | −0.20 (−2.11, 2.34) | −0.79 (−2.11, 1.27) |
| Larynx | 2.35 (−0.79, 11.5) | NC | 5.85 (−2.36, 22.05) | 3.61 (−0.56, 14.11) |
| Lung | 0.65 (0.09, 1.30) | 2.90 (1.00, 5.26) | 0.45 (−0.50, 1.52) | 0.76 (0.02, 1.61) |
| Mesothelioma and pleural | 2.54 (−0.25, 7.10) | 16.94 (5.34, 38.5) | 10.1 (3.95, 19.1) | 3.30 (−0.27, 8.79) |
| Skin (all) | 0.99 (−0.56, 3.43) | −4.01 (−6.04, 1.50) | −1.49 (−3.55, 1.75) | 0.03 (−1.40, 2.63) |
| Skin excluding melanoma | 0.51 (−1.76, 5.19) | −16.43 (−21.41, 6.90) | −3.17 (−6.24, 3.20) | NC |
| Skin (melanoma) | 1.24 (−0.67, 4.47) | −3.66 (−5.99, 2.75) | −0.89 (−3.42, 3.31) | 1.06 (−0.93, 4.92) |
| Female breast | −1.16 (−3.26, 5.44) | 49.0 (−17.3, 171.3) | −2.39 (−4.70, 4.96) | −3.40 (−5.68, 3.87) |
| Uterus | NC | NC | NC | −1.89 (−30.77, 94.8) |
| Ovary | 5.74 (−5.65, 37.3) | NC | 6.04 (−5.56, 37.81) | 7.56 (−5.33, 42.7) |
| Prostate | −0.19 (−0.75, 0.57) | 1.04 (−1.25, 4.92) | −0.61 (−1.98, 1.09) | −0.82 (−1.63, 0.37) |
| Bladder | 0.04 (−0.96, 1.87) | 1.79 (−5.75, 15.9) | −0.40 (−2.82, 3.33) | −0.09 (−1.12, 2.42) |
| Kidney | 0.93 (−0.98, 3.99) | 3.38 (−2.74, 14.26) | 2.93 (−0.62, 8.07) | 1.04 (−1.41, 4.85) |
| Brain and CNS | −0.92 (−1.64, 0.64) | 0.76 (−3.41, 8.05) | −2.57 (−4.47, 0.46) | −2.49 (−3.82, −0.09) |
| Thyroid | 0.77 (−2.82, 13.9) | −3.93 (−11.64, 48.53) | −5.44 (−7.80, 6.76) | NC |
| Bone | −8.31 (−13.5, 9.93) | −38.4 (−44.8, 14.1) | −5.92 (−13.6, 20.0) | −8.31 (−13.5, 9.93) |
| COPD | −0.04 (−0.61, 0.67) | 0.97 (−0.90, 3.52) | 0.26 (−0.92, 1.62) | 0.66 (−0.27, 1.76) |
ERR/Sv, excess relative rater per sievert; NC, non-convergence; CNS, central nervous system; COPD, chronic obstructive pulmonary disease.
Matched on age, sex, date of birth, facility, duration of employment, neutron monitoring, and job type.
Analyses restricted to workers with <200 mSv cumulative exposure showed increases in ERRs compared to the main results for all solid cancer excluding lung, liver, and bone, for cancers of the stomach, colon, larynx, ovary, and kidney, and for COPD, and decreases for cancers of oesophagus, lung, skin, bladder, and thyroid. When analyses were restricted to workers without neutron monitoring, several cause-specific estimates decreased, with an ERR Sv−1 of 0.16 (95% CI: −0.25, 0.62) for solid cancer and −0.15 (−0.61, 0.38) for solid cancer except for lung. The estimates for lung cancer and for COPD increased among workers not monitored for neutrons (ERR Sv−1 = 0.76; 95% CI: 0.02, 1.61).
Discussion
This extended mortality analysis of over 100,000 US nuclear workers contributes empirical evidence of the radiogenic risks of solid cancer and some solid cancer subtypes. We observed a substantially higher lung cancer ERR compared to the 2005 update of this cohort (ERR Sv−1 = 0.069; 95% CI: −0.43, 0.66).9 This may be due in part to differences in study design; in the updated analysis we had a longer duration of employment criterion for cohort inclusion, excluded radionuclide deposition for the cumulative exposure estimates, and chose to adjust for a different set of variables (in this analysis we did not adjust for race, however, matching on race did not impact results in our preliminary models). Another reason for the differences in results is the prolonged follow-up in our analysis, which allows for the estimation of risks for solid cancer types with longer latencies. Extended follow-up also increased the number of events; in the 2005 update only 35% of workers were deceased, whereas 50% of workers were deceased through 2016. With additional events, we were able to estimate cancer rates among a contemporary subcohort of nuclear workers. Workers hired after 1960 had improved dosimetry, and lower average exposures to gamma radiation (mean = 14.5 mSv, median = 2.5 mSv). We observed positive associations for several cancer subtypes, notably all cancer, all solid cancer excluding lung, and cancers of the colon, and lung. Although imprecise, several other subtypes also had positive associations in the contemporary subcohort.
The SMR analyses provided updated information on the occupational experiences of nuclear workers. The majority of SMR results were at or below the null. This was not unexpected since nuclear workers are subject to the healthy worker effect due to additional medical and security screenings as a requirement for employment in a nuclear facility.17 However, the elevated SMRs for mesothelioma indicate that workers experience a higher rate of mesothelioma mortality compared to the general population, particularly among skilled manual workers, which is likely attributable to occupational asbestos exposure. Some of the most common job titles among skilled manual workers were electrician, pipefitter, metal worker, and machinist. Additionally, rates of mesothelioma among nuclear workers by calendar period continue to be elevated relative to the standard US population. Clinicians and public health practitioners need to be aware of the continued asbestos burden faced by some nuclear workers.
Many results from our analysis are comparable to the findings from the French and UK nuclear worker cohorts, as well as INWORKS and the Life Span Study of Japanese atomic bomb survivors. Solid cancer risk estimates from these studies range from 0.20 to 0.53 ERR Sv−1. Our summary ERR for solid cancer is in the lower range at 0.19, but results from our sensitivity analyses ranged from 0.12 to 2.23. For lung cancer, results from the aforementioned studies range from 0.34 to 1.33. Our main analysis results fall within these estimates at 0.65 and our sensitivity analyses ranged from 0.30 to 2.90.
We conducted several types of analyses to indirectly evaluate the impact of unmeasured smoking on the association between ionising radiation and solid cancer. We estimated associations for a group of non-smoking related solid cancers. When compared with all solid cancer, the ERR for non-smoking related solid cancers is only slightly lower, suggesting that confounding by smoking does not fully explain the association between radiation and solid cancer. We also estimated the association between ionising radiation and COPD to assess unmeasured confounding by smoking of the association between ionising radiation and lung cancer, since whole-body penetrating ionising radiation does not cause COPD and smoking is an unmeasured confounder of the association between radiation and COPD. We found no association between radiation and COPD mortality in the overall cohort, which suggests that smoking is not an important confounder of the association between ionising radiation and lung cancer. The COPD estimate in the contemporary subcohort was higher than the full cohort, but the magnitude of this association was still much smaller than the lung cancer estimate in the contemporary subcohort, indicating that confounding by smoking still cannot entirely explain the observed excess lung cancer rate. And although there is a strong association between smoking and lung cancer, prior studies indicated that the association between smoking and ionising radiation exposure among US nuclear workers is weak among the overall cohort 31, meaning that confounding by smoking is unlikely to explain the association between ionising radiation and lung cancer.
In addition to smoking, unmeasured confounding by asbestos exposure is a concern. We observed a large positive ERR for mesothelioma and pleural cancer. The magnitude was similar to the estimate from the 2005 update of this cohort (ERR Sv−1 = 2.5; 95% CI: 1.3, 10)9 but was much higher among the workers hired since 1960. These elevated mesothelioma and pleural cancer rates suggest that asbestos may confound the association between ionising radiation and lung cancer. Since PNS workers had the highest SMR for mesothelioma and other studies have documented the potential for confounding by asbestos at PNS,9 32 we removed PNS workers in a post hoc analysis to indirectly evaluate the impact of asbestos exposure on our lung cancer estimate. When PNS is removed, the lung cancer association decreased but remained elevated (ERR Sv−1 = 0.46; 95% CI: −0.12, 1.16). Matching on occupational status also partially controls confounding by asbestos exposure. And while there is some association between asbestos exposure and lung cancer, the association between asbestos exposure and ionising radiation is likely weak, indicating that confounding by asbestos is unlikely to explain the association between ionising radiation and lung cancer.31
Despite some concerns with confounding by unmeasured environmental and occupational co-exposures, this analysis makes an important advance in understanding solid cancer risks among contemporary nuclear workers. There was a sufficient duration of mortality follow-up to evaluate contemporary worker risks, and we observed notable increases in rates for all solid cancers, lung cancer, and colon cancer. We chose to conduct sensitivity analyses restricted to workers hired 1960 or later because of improvements in dosimetry during this period and to provide risk estimates for workers exposed to ionising radiation contemporary exposure scenarios. There are differences between early and contemporary workers such as differences in occupational conditions, temporal trends, and differences in dose rate which may contribute to the elevated rates observed among contemporary workers. Non-differential exposure misclassification causing bias towards the null among early hires may also contribute to these elevated rates. Ultimately it provides new evidence that lower-exposed contemporary workers experience elevated rates of solid cancer.
This updated study provides insight into the continued asbestos burden of many nuclear workers. It adds to the understanding of solid cancer risks from exposure to ionising radiation, and contributes to the growing body of evidence that lung cancer, and likely some other subtypes of cancer, are radiosensitive at low doses and low dose-rates. As this cohort continues to be followed over time, and additional INWORKS and country-specific updates are conducted, we expect to observe additional evidence of health effects from low dose and low dose-rate radiation exposures.
Supplementary Material
Key Messages:
Large, long-term studies of nuclear workers can provide important evidence for contemporary radiological protection standards among workers and the general population.
In this study of over 100,000 United States nuclear workers exposed to an average 26.5 millisieverts of external penetrating ionising radiation, we observed excess relative rates of solid cancer, including lung cancer.
A contemporary sub cohort of workers first hired after 1960 had higher excess relative rates of solid cancer.
Acknowledgements:
We would like to acknowledge Lisa Thomas (NIOSH) for her assistance with data management and preparation.
Funding:
No specific funding was received. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control. Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
Footnotes
Ethics approval: This study was approved by the National Institute for Occupational Safety and Health Institutional Review Board.
Supplementary data: Supplementary data are available at IJE online
Conflict of interest: None declared
Data availability:
Data availability is subject to US data protections and regulations. For inquiries, please contact kkelly-reif@cdc.gov.
References:
- 1.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Volume 100-D: Radiation IARC Monogr Eval Carcinog Risks Hum, Lyon, France: 2012;7–303. [PMC free article] [PubMed] [Google Scholar]
- 2.Hamra GB, Richardson DB, Cardis E et al. Cohort Profile: The International Nuclear Workers Study (INWORKS). Int J Epidemiol 2016;45:693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: The National Academies Press, 2006. [PubMed] [Google Scholar]
- 4.Leuraud K, Richardson DB, Cardis E et al. Risk of cancer associated with low-dose radiation exposure: comparison of results between the INWORKS nuclear workers study and the A-bomb survivors study. Radiat Environ Biophys 2021;60:23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Academies of Sciences E, Medicine. Leveraging Advances in Modern Science to Revitalize Low-Dose Radiation Research in the United States. Washington, DC: The National Academies Press, 2022. [PubMed] [Google Scholar]
- 6.Vrijheid M, Cardis E, Blettner M et al. The 15-Country Collaborative Study of Cancer Risk Among Radiation Workers in the Nuclear Industry: design, epidemiological methods and descriptive results. Radiat Res 2007;167:361–79. [DOI] [PubMed] [Google Scholar]
- 7.Cardis E, Vrijheid M, Blettner M et al. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ 2005;331:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frome EL, Cragle DL, Watkins JP et al. A mortality study of employees of the nuclear industry in Oak Ridge, Tennessee. Radiat Res 1997;148:64–80. [PubMed] [Google Scholar]
- 9.Schubauer-Berigan MK, Daniels RD, Bertke SJ, Tseng CY, Richardson DB. Cancer Mortality through 2005 among a Pooled Cohort of U.S. Nuclear Workers Exposed to External Ionizing Radiation. Radiat Res 2015;183:620–31. [DOI] [PubMed] [Google Scholar]
- 10.Hunter N, Haylock RGE, Gillies M, Zhang W. Extended analysis of solid cancer incidence among the Nuclear Industry Workers in the UK: 1955-2011. Radiat Res 2022;198:1–17. [DOI] [PubMed] [Google Scholar]
- 11.Metz-Flamant C, Laurent O, Samson E et al. Mortality associated with chronic external radiation exposure in the French combined cohort of nuclear workers. Occup Environ Med 2013;70:630–8. [DOI] [PubMed] [Google Scholar]
- 12.Richardson DB, Cardis E, Daniels RD et al. Site-specific Solid Cancer Mortality After Exposure to Ionizing Radiation. Epidemiology 2018;29:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson DB, Cardis E, Daniels RD et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 2015;351:h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozasa K, Shimizu Y, Suyama A et al. Studies of the Mortality of Atomic Bomb Survivors, Report 14, 1950–2003: An Overview of Cancer and Noncancer Diseases. Radiat Res 2012;177:229–243. [DOI] [PubMed] [Google Scholar]
- 15.Daniels RD, Bertke SJ, Richardson DB et al. Examining temporal effects on cancer risk in the international nuclear workers’ study. Int J Cancer 2017;140:1260–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing S, Richardson D, Wolf S, Mihlan G. Plutonium-related work and cause-specific mortality at the United States Department of Energy Hanford Site. Am J Ind Med 2004;45:153–64. [DOI] [PubMed] [Google Scholar]
- 17.Wing S, Richardson DB. Age at exposure to ionising radiation and cancer mortality among Hanford workers: follow up through 1994. Occup Environ Med 2005;62:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schubauer-Berigan MK, Macievic G, Utterback D, Tseng C. An epidemiologic study of mortality and radiation-related risk of cancer among workers at the Idaho National Engineering and Environmental Laboratory, a U.S. Department of Energy Facility. In: National Institute for Occupational Safety and Health DoS, Hazard Evaluations, and Field Studies (ed.), Cincinnati, OH: 2005. [Google Scholar]
- 19.Richardson DB, Wing S, Keil A, Wolf S. Mortality Among Workers at Oak Ridge National Laboratory. Am J Ind Med 2013;56:725–732. [DOI] [PubMed] [Google Scholar]
- 20.Richardson DB, Wing S. Radiation and mortality of workers at Oak Ridge National Laboratory: positive associations for doses received at older ages. Environ Health Perspect 1999;107:649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.-. Evidence of confounding by smoking of associations between radiation and lung cancer mortality among workers at the Savannah River Site. Am J Ind Med 2011;54:421–7. [DOI] [PubMed] [Google Scholar]
- 22.Richardson DB, Wing S, Wolf S. Mortality among workers at the Savannah River Site. Am J Ind Med 2007;50:881–91. [DOI] [PubMed] [Google Scholar]
- 23.Rinsky RA, Melius JM, Hornung RW et al. Case-control study of lung cancer in civilian employees at the Portsmouth Naval Shipyard, Kittery, Maine. Am J Epidemiol 1988;127:55–64. [DOI] [PubMed] [Google Scholar]
- 24.Rinsky RA, Zumwalde RD, Waxweiler RJ et al. Cancer mortality at a Naval Nuclear Shipyard. Lancet 1981;1:231–5. [DOI] [PubMed] [Google Scholar]
- 25.Richardson DB, Laurier D, Schubauer-Berigan MK, Tchetgen Tchetgen E, Cole SR. Assessment and indirect adjustment for confounding by smoking in cohort studies using relative hazards models. Am J Epidemiol 2014;180:933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silver SR, Daniels RD, Taulbee TD et al. Differences in mortality by radiation monitoring status in an expanded cohort of Portsmouth Naval Shipyard workers. J Occup Environ Med 2004;46:677–90. [DOI] [PubMed] [Google Scholar]
- 27.Rothman KJ, Boice JD. Epidemiologic analysis with a programmable calculator. Washington: National Institutes of Health, 1979. [Google Scholar]
- 28.Robinson CF, Schnorr TM, Cassinelli RT 2nd et al. Tenth revision U.S. mortality rates for use with the NIOSH Life Table Analysis System. J Occup Environ Med 2006;48:662–7. [DOI] [PubMed] [Google Scholar]
- 29.Richardson DB, Langholz B. Background stratified Poisson regression analysis of cohort data. Radiat Environ Biophys 2012;51:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson DB. A simple approach for fitting linear relative rate models in SAS. Am J Epidemiol 2008;168:1333–8. [DOI] [PubMed] [Google Scholar]
- 31.Schubauer-Berigan MK, Berrington De Gonzalez A, Cardis E et al. Evaluation of Confounding and Selection Bias in Epidemiological Studies of Populations Exposed to Low-Dose, High-Energy Photon Radiation. J Natl Cancer Inst Monogr 2020;2020:133–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yiin JH, Silver SR, Daniels RD, Zaebst DD, Seel EA, Kubale TL. A nested case-control study of lung cancer risk and ionizing radiation exposure at the portsmouth naval shipyard. Radiat Res 2007;168:341–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability is subject to US data protections and regulations. For inquiries, please contact kkelly-reif@cdc.gov.


