Abstract
Background.
Mortality related to hepatitis C virus (HCV) infection is a key indicator for elimination. We assessed the impact of HCV infection and treatment on mortality in the country of Georgia during 2015–2020.
Methods.
We conducted a population-based cohort study using data from Georgia’s national HCV Elimination Program and death registry. We calculated all-cause mortality rates in 6 cohorts: (1) Negative for anti-HCV; (2) anti-HCV positive, unknown viremia status; (3) current HCV infection and untreated; (4) discontinued treatment; (5) completed treatment, no sustained virologic response (SVR) assessment; (6) completed treatment and achieved SVR. Cox proportional hazards models were used to calculate adjusted hazards ratios and confidence intervals. We calculated the cause-specific mortality rates attributable to liver-related causes.
Results.
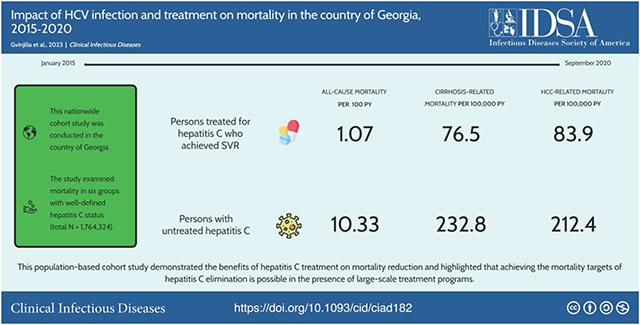
After a median follow-up of 743 days, 100 371 (5.7%) of 1 764 324 study participants died. The highest mortality rate was observed among HCV infected patients who discontinued treatment (10.62 deaths per 100 PY, 95% confidence interval [CI]: 9.65, 11.68), and untreated group (10.33 deaths per 100 PY, 95% CI: 9.96, 10.71). In adjusted Cox proportional hazards model, the untreated group had almost 6-times higher hazard of death compared to treated groups with or without documented SVR (adjusted hazard ratio [aHR] = 5.56, 95% CI: 4.89, 6.31). Those who achieved SVR had consistently lower liver-related mortality compared to cohorts with current or past exposure to HCV.
Conclusions.
This large population-based cohort study demonstrated the marked beneficial association between hepatitis C treatment and mortality. The high mortality rates observed among HCV infected and untreated persons highlights the need to prioritize linkage to care and treatment to achieve elimination goals.
Graphical Abstract
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/impact-of-hcv-infection-and-treatment-on-mortality-in-the-country-of-georgia-2015-2020.
Globally, an estimated 58 million people were living with chronic hepatitis C virus (HCV) infection in 2019 [1]. That same year, 290 000 deaths were attributable to HCV, mainly from cirrhosis, hepatocellular carcinoma (HCC), and extrahepatic complications, making HCV infection one of the leading causes of death worldwide [2]. In 2016, at the 69th World Health Assembly, the World Health Organization (WHO) adopted the Global Health Sector Strategy on Viral Hepatitis 2016–2021, which aims to reduce hepatitis-related mortality by 65% [3]. More recent guidance from the WHO shifted the focus to an absolute measure of mortality and set the target of ≤2 annual HCV-related deaths per 100 000 population [4]. As multiple countries aim to eliminate hepatitis C as a public health problem by 2030, demonstrating the impact of treatment programs on mortality is a priority.
Since 2011, with the development of highly effective and well-tolerated direct-acting antivirals (DAAs), vast improvements have been made in hepatitis C control that have reduced the public health burden related to this infection [5–7]. Growing evidence shows that treatment with DAAs in patients with chronic HCV infection substantially reduces long-term HCV-related morbidity and mortality [8–10]. The comparison of the clinical outcomes between DAA-treated adult patients and those with untreated HCV infection demonstrated a reduced all-cause mortality rate among those treated [10]. However, this benefit has not been demonstrated at the population level among different subgroups with an accurately defined level of exposure, treatment and sustained virologic response (SVR) status. DAA-induced SVR, that is, cure, is associated with fibrosis regression, reduced risk of death and incidence of HCC when compared to patients who did not achieve cure [9, 11]. However, patients achieving SVR still have higher mortality rates relative to the general population [8].
Georgia, a small middle-income country with a population of 3.7 million, had a high prevalence of HCV infection with 5.4% of the adult population infected with HCV in 2015 (an estimated 150 000 individuals). Most infections were associated with receipt of a blood transfusion and injection drug use [12, 13]. In 2015, in collaboration with the US Centers for Disease Control and Prevention (CDC) and other partners, the country embarked on a national Hepatitis C Elimination Program, with the goal of achieving a 90% reduction in prevalence by 2020 [14, 15]. Since June 2016, all persons in Georgia with chronic HCV infection are eligible for free treatment with DAAs [16, 17]. As of December 2021, over 2.2 million Georgians have been screened for hepatitis C, and 76 644 HCV-infected persons initiated treatment within the program [18].
Georgia’s Hepatitis C Elimination Program and the availability of nationwide electronic registries for hepatitis C and vital statistics provide an opportunity to explore mortality rates in a large national cohort of people with known HCV infection status. The objective of this study was to evaluate the impact of HCV infection and DAA treatment on all-cause mortality and assess liver-related mortality across 6 comparison groups with different HCV infection and treatment statuses. The findings of this study can inform the impact of DAA treatment at the population level, demonstrating the effect of nationwide elimination programs on the reduction in mortality.
METHODS
Study Design and Population
We conducted a nationwide population-based cohort study among all adult (age ≥18 years) residents of Georgia tested for HCV antibodies (anti-HCV) during 1 January 2015 through 30 September 2020. The study population was categorized into six cohorts based on hepatitis C status: (1) Negative for anti-HCV (never exposed); (2) anti-HCV positive, unknown viremia status (not tested for the presence of HCV virus by RNA or core antigen); (3) current HCV infection (ie, positive HCV RNA or core antigen result) and untreated; (4) HCV infection, discontinued treatment; (5) completed treatment, no SVR assessment; (6) completed treatment and achieved SVR. We excluded entries with a missing national ID number or missing/erroneous dates necessary for calculating mortality rates and hazards ratios (n = 2183), persons who died while undergoing HCV treatment (n = 486), and persons who completed the treatment but did not achieve SVR (n = 582).
Data Sources and Data Collection
Hepatitis C-related information was obtained from 2 nationwide electronic databases: the national hepatitis C screening registry and the Hepatitis C Elimination Program clinical treatment database “Elimination C” (ElimC) [19]. Vital statistics were obtained from the national death registry; for deceased individuals, the date and causes of death were ascertained. Data were linked using patients’ national ID number—a unique identifier utilized in all the data sources, which were encrypted prior to analysis.
Variables and Definitions
Basic demographic variables, such as age and gender, were obtained from the screening registry. For the three cohorts that initiated HCV treatment (discontinued treatment; no SVR assessment; achieved SVR), additional clinical variables were available and obtained from ElimC, all of which were measured upon enrollment in the program. These included body mass index (BMI), advanced liver fibrosis stage defined as fibroscan score ≥F3 or FIB-4 > 3.25 (priority given to fibroscan results), liver enzyme (transaminase) tests, HCV genotype, treatment regimens and co-infections—chronic hepatitis B virus (HBV) infection measured using hepatitis B surface antigen (HBsAg), and human immunodeficiency virus (HIV) infection measured using antibodies against HIV (anti-HIV).
For mortality rate calculations and Cox proportional hazards models, the baseline date for start of follow-up varied by cohort and corresponded to the date of: first screening for never exposed cohort, first positive screening for the cohort with unknown viremia status, viremia testing for the cohort with untreated HCV infection, treatment initiation for the cohort with discontinued treatment, and treatment completion for the cohorts with no SVR assessment and achieved SVR. The end of follow-up was the date of death for deceased individuals, or end of the study period (30 September 2020) for surviving individuals. Person-time was calculated as the number of days from the baseline date to the end of follow-up.
Liver-related causes of death were defined using International Classification of Diseases—10—Clinical Modification (ICD-10-CM) codes recorded in the death registry. Both primary and underlying causes were considered. The following ICD-10 codes were used for each of the group of liver-related causes: (1) viral hepatitis: B15.0-B19.9; (2) Cirrhosis: K70.3, K74.5, and K74.6; (3) liver cancer: C22.0-C22.9; (4) HCC: C22.0.
Statistical Analysis
We calculated crude mortality rates by different socio-demographic characteristics and HCV-related clinical factors for each of the 6 cohorts. To visually examine the difference between cohorts with regard to mortality rate, we created Kaplan-Meier curves adjusted for age, sex and hospitalization, the latter of which was determined by the venue in which a patient was last screened for anti-HCV. The Cox proportional hazards model was used to quantify the difference between cohorts in terms of time to death, and unadjusted and adjusted hazards ratios (HR and aHR) and confidence intervals (CIs) were calculated. Age-standardized liver-related mortality rates were calculated using the age distribution of Georgian adults taken from the most recent, 2014 census as a reference. All analysis was performed in SAS version 9.4 (Cary, North Carolina, USA).
Ethical Considerations
Data for this analysis derive from Georgia’s Hepatitis C Elimination Program, which was deemed by the Institutional Review Board of National Center for Disease Control to be a public health program activity. CDC determined this activity was not research involving human subjects.
RESULTS
Description of the Study Population
A total of 1 764 324 people were included in the analysis. The majority were female (n = 983 249; 55.7%) and the median age was 46 years (interquartile range [IQR]: 31–62 years). In terms of the HCV infection and treatment status, most of the study participants were anti-HCV negative (n = 1 660 573; 94.1%), followed by those with HCV infection who were treated and achieved SVR (n = 50,953, 2.9%), anti-HCV positive with unknown viremia status (n = 18,994, 1.1%), with treated HCV infection and no SVR assessment (n = 16,164, 0.9%), with untreated HCV infection (n = 15,747, 0.9%), and those with HCV infection who discontinued treatment (n = 1,893, 0.1%). Anti-HCV negative individuals were majority female (57.6%) and had the highest representation of the youngest (18–29 years) and oldest (≥60) age groups (23.9% and 28.8%, respectively), whereas all other cohorts were predominantly male with the smallest proportion made up of persons in the youngest age group (Table 1).
Table 1.
Demographic and Clinical Characteristics of Persons Tested or Treated for HCV Infection—Georgia, 2015–2020
| Never Exposed (N = 1 660 573) | Anti-HCV (+), Unknown Viremia Status (N = 18 994) | Current HCV Infection and Untreated (N = 15 747) | Discontinued Treatment (N = 1893) | Completed Treatment, No SVR Assessment (N = 16 164) | Completed Treatment, Achieved SVR (N = 50 953) | |
|---|---|---|---|---|---|---|
| Age, (y) median [IQR] | 45 [30, 62] | 52 [40, 68] | 52 [41, 65] | 48 [39, 59] | 45 [38, 54] | 47 [39, 55] |
| Age group, n (%) | ||||||
| 18–29 | 396 571 (23.9) | 1571 (8.3) | 691 (4.4) | 100 (5.3) | 758 (4.7) | 2466 (4.8) |
| 30–39 | 296 479 (17.9) | 3067 (16.1) | 2596 (16.5) | 385 (20.3) | 3839 (23.8) | 10 292 (20.2) |
| 40–49 | 234 814 (14.1) | 4051 (21.3) | 3643 (23.1) | 535 (28.3) | 5688 (35.2) | 17 318 (34.0) |
| 50–59 | 254 512 (15.3) | 3513 (18.5) | 3436 (21.8) | 436 (23.0) | 3877 (24.0) | 13 135 (25.8) |
| ≥60 | 478 197 (28.8) | 6792 (35.8) | 5381 (34.2) | 437 (23.1) | 2002 (12.4) | 7742 (15.2) |
| Sex, n (%) | ||||||
| Female | 956 940 (57.6) | 6850 (36.1) | 4395 (27.9) | 342 (18.1) | 2605 (16.1) | 12 117 (23.8) |
| Male | 703 633 (42.4) | 12 144 (63.9) | 11 352 (72.1) | 1551 (81.9) | 13 559 (83.9) | 38 836 (76.2) |
| BMI, n (%) | ||||||
| <18.5 | … | … | … | 32 (2.1) | 163 (1.5) | 557 (1.5) |
| 18.5–24.9 | … | … | … | 723 (47.7) | 4746 (42.8) | 14 153 (39.1) |
| 25.0–30.0 | … | … | … | 557 (36.7) | 4242 (38.3) | 14 162 (39.1) |
| >30.0 | … | … | … | 204 (13.5) | 1928 (17.4) | 7335 (20.3) |
| Missing | … | … | … | 377 | 5085 | 14 746 |
| Advanced fibrosisa | ||||||
| Yes | 710 (37.8) | 5969 (37.2) | 15 934 (31.4) | |||
| No | 1166 (62.2) | 10 098 (62.8) | 34 826 (68.6) | |||
| Missing | 17 | 97 | 193 | |||
| Liver function tests,b n (%) | ||||||
| ALT >2×ULNc | … | … | … | 494 (26.4) | 5108 (31.8) | 14 873 (29.3) |
| AST >2×ULNd | … | … | … | 512 (27.3) | 4309 (26.8) | 11 787 (23.2) |
| Billirubin >1.1 mg/dL | … | … | … | 1777 (95.0) | 14 753 (91.9) | 45 044 (89.0) |
| Albumin <35 g/L | … | … | … | 429 (22.9) | 2814 (17.5) | 9715 (19.2) |
| Coinfections,b n (%) | ||||||
| HBsAg + | … | … | … | 47 (2.5) | 375 (2.5) | 1053 (2.1) |
| HBsAg − | … | … | … | 1808 (97.5) | 14 833 (97.5) | 48 464 (97.9) |
| Missing | 38 | 956 | 1436 | |||
| Anti-HIV + | … | … | … | 29 (1.5) | 719 (4.4) | 30 (0.1) |
| Anti-HIV − | … | … | … | 1864 (98.5) | 15 445 (95.6) | 50 923 (99.9) |
| Genotype, n (%) | ||||||
| 1 | … | … | … | 790 (45.2) | 6515 (43.0) | 22 646 (46.7) |
| 2 | … | … | … | 281 (16.1) | 2712 (17.9) | 9195 (18.9) |
| 3 | … | … | … | 610 (34.9) | 5408 (35.7) | 15 266 (31.5) |
| Other | … | … | … | 67 (3.8) | 511 (3.4) | 1427 (2.9) |
| Missing | … | … | … | 145 | 1018 | 2419 |
| Treatment regimen, n (%) | ||||||
| SOF + RBV | … | … | … | 174 (9.2) | 1559 (9.6) | 4131 (8.1) |
| SOF/LED | … | … | … | 676 (35.7) | 5561 (34.4) | 20 152 (39.6) |
| SOF/LED + RBV | … | … | … | 646 (34.1) | 5698 (35.3) | 18 626 (36.6) |
| SOF/VEL | … | … | … | 360 (19.0) | 3201 (19.8) | 7674 (15.1) |
| SOF/VEL + RBV | … | … | … | 37 (2.0) | 138 (0.9) | 354 (0.7) |
| ELB/GRZ | … | … | … | … | 7 (0.0) | 16 (0.0) |
| Follow-up (d)e Median (IQR) | 736 (375, 1135) | 754 (285, 1203) | 574 (244, 864) | 694 (336, 1149) | 954 (408, 1365) | 1115 (531, 1379) |
Abbreviations: anti-HIV, antibodies against human immunodeficiency virus; ALT, alanine aminotransferase; Anti-HCV, antibodies against hepatitis C virus; AST, aspartate aminotransferase; BMI, body-mass index; FIB-4, fibrosis-4 index; ELB, elbasvir; GRZ, Grazoprevir; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; IQR, interquartile range; LED, ledipasvir; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virologic response; ULN, upper limit of normal; VEL, velpatasvir.
Advanced fibrosis defined as fibroscan score ≥F3 or FIB-4 score >3.25. Not advanced defined as fibroscan <F3 or FIB-4 score <1.45. Priority given to fibroscan results for all determinations.
Evaluated at baseline prior to initiating HCV treatment.
LT ULN = 42.
AST ULN = 37.
Follow-up began when definitive testing was performed and ended at the date of death for deceased individuals, or end of the study period (30 September 2020) for surviving individuals.
Among 3 cohorts of individuals that initiated treatment and had clinical data available, advanced liver fibrosis was present in 32.8% of people, and prevalence of chronic HBV infection was 2.1%. HCV genotype distribution did not vary meaningfully—genotype 1 was the most common in all 3 cohorts, ranging from 43.0% to 46.7%, followed by genotype 3 (31.5%–35.7%) and genotype 2 (16.1%–18.9%) (Table 1). There were no clinical or diagnostic testing data available for persons with HCV infection who did not enter the treatment program (not treated and unknown viremia groups).
All-cause Mortality by HCV Status
After a median follow-up of 743 days (IQR: 377–1147days) 100 371 (5.7%) study participants died, corresponding to an overall all-cause mortality rate of 2.62 per 100 person-years (PY) (95% CI: 2.60, 2.64). Among cohorts who ever screened anti-HCV positive, the overall all-cause mortality rate was 3.65 (95% CI: 3.58, 3.73) per 100 PY. The highest mortality rate was observed among HCV infected patients who discontinued treatment (10.62 deaths per 100 PY, 95% CI: 9.65, 11.68), followed by the HCV infected untreated cohort (10.33 deaths per 100 PY, 95% CI: 9.96, 10.71) and people with anti-HCV positive result who did not receive viremia test (9.06 deaths per 100 PY, 95% CI: 8.77, 9.36) (Table 2). People who completed HCV treatment had markedly lower mortality rates, regardless of whether they had documented SVR or not; the all-cause mortality rate among those who achieved SVR was 1.07 deaths per 100 PY (95% CI: 1.02, 1.13), and among persons without SVR assessment, but presumed high cure rate, the mortality rate was 1.69 deaths per 100 PY (95% CI: 1.56, 1.82). Both cohorts had a lower mortality rate than the anti-HCV negative cohort (2.55 deaths per 100 PY, 95% CI: 2.53, 2.56), and the difference was most pronounced in the 60 + age group, in which anti-HCV negative cohort had mortality rate of 8.84 compared to 2.73 in SVR-achieved group and 4.75 in those without SVR assessment. The mortality rate increased with age in all 6 study cohorts (Table 2).
Table 2.
All-cause Mortality Rate by Different Socio-demographic and Clinical Factors Among Six Study Cohorts—Georgia, 2015–2020
| Never Exposed (N = 1 660 573) | Anti-HCV (+), Unknown Viremia Status (N = 18 994) | Current HCV Infection and Untreated (N = 15 747) | Discontinued Treatment (N = 1893) | Completed Treatment, No SVR Assessment (N = 16 164) | Completed Treatment, Achieved SVR (N = 50 953) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | Deaths/100 PY (95% CI) | Deaths | Deaths/100 PY (95% CI) | Deaths | Deaths/100 PY (95% CI) | Deaths | Deaths/100 PY (95% CI) | Deaths | Deaths/100 PY (95% CI) | Deaths | Deaths/100 PY (95% CI) | |
| Total | 91 157 | 2.55 (2.53–2.56) | 3774 | 9.06 (8.77–9.36) | 2885 | 10.33 (9.96–10.71) | 424 | 10.62 (9.65–11.68) | 673 | 1.69 (1.56–1.82) | 1458 | 1.07 (1.02–1.13) |
| Age categories | ||||||||||||
| 18–29 | 929 | 0.09 (.09–.10) | 23 | 0.47 (.31–.72) | 14 | 0.89 (.53–1.51) | 2 | 1.01 (.25–4.02) | 4 | 0.23 (.09–.62) | 12 | 0.18 (.10–.33) |
| 30–39 | 1337 | 0.20 (.19–.21) | 150 | 1.83 (1.55–2.15) | 108 | 1.87 (1.55–2.26) | 32 | 3.67 (2.60–5.19) | 36 | 0.38 (.27–.52) | 100 | 0.35 (.29–.43) |
| 40–49 | 3132 | 0.61 (.59–.64) | 368 | 3.67 (3.30–4.07) | 348 | 4.74 (4.26–5.26) | 90 | 7.24 (5.89–8.90) | 160 | 1.08 (.92–1.26) | 327 | 0.67 (.60–.75) |
| 50–59 | 9129 | 1.72 (1.68–1.76) | 671 | 8.79 (8.13–9.49) | 728 | 12.60 (11.72–13.56) | 149 | 16.63 (14.17–19.53) | 275 | 2.85 (2.53–3.21) | 542 | 1.53 (1.41–1.67) |
| ≥60 | 76 630 | 8.84 (8.77–8.90) | 2562 | 23.19 (22.30–24.12) | 1687 | 22.74 (21.67–23.85) | 151 | 19.29 (16.45–22.63) | 198 | 4.75 (4.13–5.46) | 477 | 2.73 (2.50–2.99) |
| Sex | ||||||||||||
| Female | 44 018 | 2.11 (2.09–2.13) | 1441 | 9.90 (9.40–10.43) | 891 | 12.35 (11.56–13.19) | 69 | 10.73 (8.48–13.59) | 84 | 1.47 (1.18–1.82) | 235 | 0.81 (.71–0.92) |
| Male | 47 139 | 3.16 (3.13–3.19) | 2333 | 8.60 (8.25–8.96) | 1994 | 9.62 (9.21–10.05) | 355 | 10.60 (9.55–11.76) | 589 | 1.72 (1.59–1.87) | 1223 | 1.14 (1.08–1.21) |
| BMI | ||||||||||||
| < 18.5 | 5 | 8.71 (3.63–20.93) | 2 | 0.64 (.16–2.54) | 12 | 1.06 (.60–1.86) | ||||||
| 18.5–24.9 | 147 | 11.20 (9.53–13.17) | 109 | 1.20 (.99–1.44) | 268 | 0.86 (.76–.97) | ||||||
| 25.0–30.0 | 113 | 11.49 (9.55–13.81) | 89 | 1.11 (.91–1.37) | 297 | 0.97 (.86–1.08) | ||||||
| >30.0 | 46 | 13.04 (9.77–17.41) | 51 | 1.34 (1.02–1.76) | 165 | 1.03 (.89–1.20) | ||||||
| Advanced fibrosisa | ||||||||||||
| Yes | 260 | 15.00 (13.28–16.94) | 524 | 2.93 (2.69–3.19) | 999 | 2.10 (1.97–2.23) | ||||||
| No | 164 | 7.31 (6.27–8.52) | 143 | 0.65 (.55–0.77) | 455 | 0.51 (.47–.56) | ||||||
| Genotype | ||||||||||||
| 1 | 215 | 13.16 (11.51–15.04) | 301 | 1.81 (1.62–2.03) | 700 | 1.14 (1.06–1.23) | ||||||
| 2 | 62 | 9.03 (7.04–11.59) | 119 | 1.55 (1.30–1.86) | 276 | 1.01 (.90–1.14) | ||||||
| 3 | 141 | 9.42 (7.99–11.11) | 224 | 1.55 (1.36–1.76) | 457 | 1.04 (.95–1.14) | ||||||
| Other | 6 | 5.45 (2.45–12.12) | 19 | 2.19 (1.40–3.43) | 22 | 0.73 (.48–1.11) | ||||||
| Treatment regimen | ||||||||||||
| SOF + RBV | 51 | 6.88 (5.23–9.05) | 199 | 3.04 (2.65–3.50) | 345 | 1.91 (1.71–2.12) | ||||||
| SOF/LED | 172 | 12.85 (11.06–14.92) | 160 | 1.16 (.99–1.35) | 470 | 0.88 (.80–.96) | ||||||
| SOF/LED + RBV | 184 | 11.45 (9.91–13.23) | 254 | 1.45 (1.28–1.64) | 608 | 1.03 (.95–1.11) | ||||||
| SOF/VEL | 15 | 5.33 (3.21–8.84) | 46 | 2.32 (1.74–3.10) | 26 | 0.51 (.35–.75) | ||||||
| SOF/VEL + RBV | 2 | 8.38 (2.09–33.49) | 13 | 15.08 (8.75–25.96) | 7 | 2.50 (1.19–5.24) | ||||||
| ELB/GRZ | … | … | 1 | 5.98 (.84—42.46) | 2 | 5.46 (1.37–21.84) | ||||||
| Follow-up (d)b | ||||||||||||
| Median (IQR) | 736 (375, 1135) | 754 (286, 1203) | 574 (244, 864) | 695 (336, 1149) | 954 (408, 1365) | 1115 (531, 1379) | ||||||
Abbreviations: Anti-HCV, antibodies against hepatitis C virus; BMI, body mass index; CI, confidence interval; ELB, elbasvir; FIB-4, fibrosis-4 index; GRZ, Grazoprevir; HCV, hepatitis C virus; IQR, interquartile range; LED, ledipasvir; PY, person-years; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virologic response; VEL, velpatasvir.
Advanced fibrosis defined as fibroscan score ≥F3 or FIB-4 score >3.25. Not advanced defined as fibroscan <F3 or FIB-4 score <1.45. Priority given to fibroscan results for all determinations.
Follow-up began when definitive testing was performed and ended at the date of death for deceased individuals, or end of the study period (30 September 2020) for surviving individuals.
In survival analysis, the cohort that discontinued treatment had the worst survival, while the never exposed and SVR-achieved cohorts had the highest survival rates (Figure 1). In a Cox proportional hazards model, adjusted for age, sex, and hospitalization, persons who achieved SVR had lower hazard of death (aHR = 0.72, 95% CI: .69, .76), compared to people never exposed to HCV, whereas every other cohort had higher hazard of death (Table 3). HCV infected and untreated persons had 5.56-times (95% CI: 4.89, 6.31) higher hazard of death compared to those who completed treatment. In a separate model, we compared the three groups with clinical data available and additionally adjusted for severity of liver disease (fibrosis stage). Those who completed treatment and were not tested for SVR still had a higher hazard of death compared to those who were known to have achieved SVR (aHR = 1.47, 95% CI: 1.34, 1.61). Similarly, people who discontinued treatment had substantially higher hazard of death compared to those who completed treatment but were not tested for SVR (aHR = 4.40, 95% CI: 3.88, 5.00) and compared to those with documented SVR (aHR = 6.46, 95% CI: 5.77, 7.23).
Figure 1.
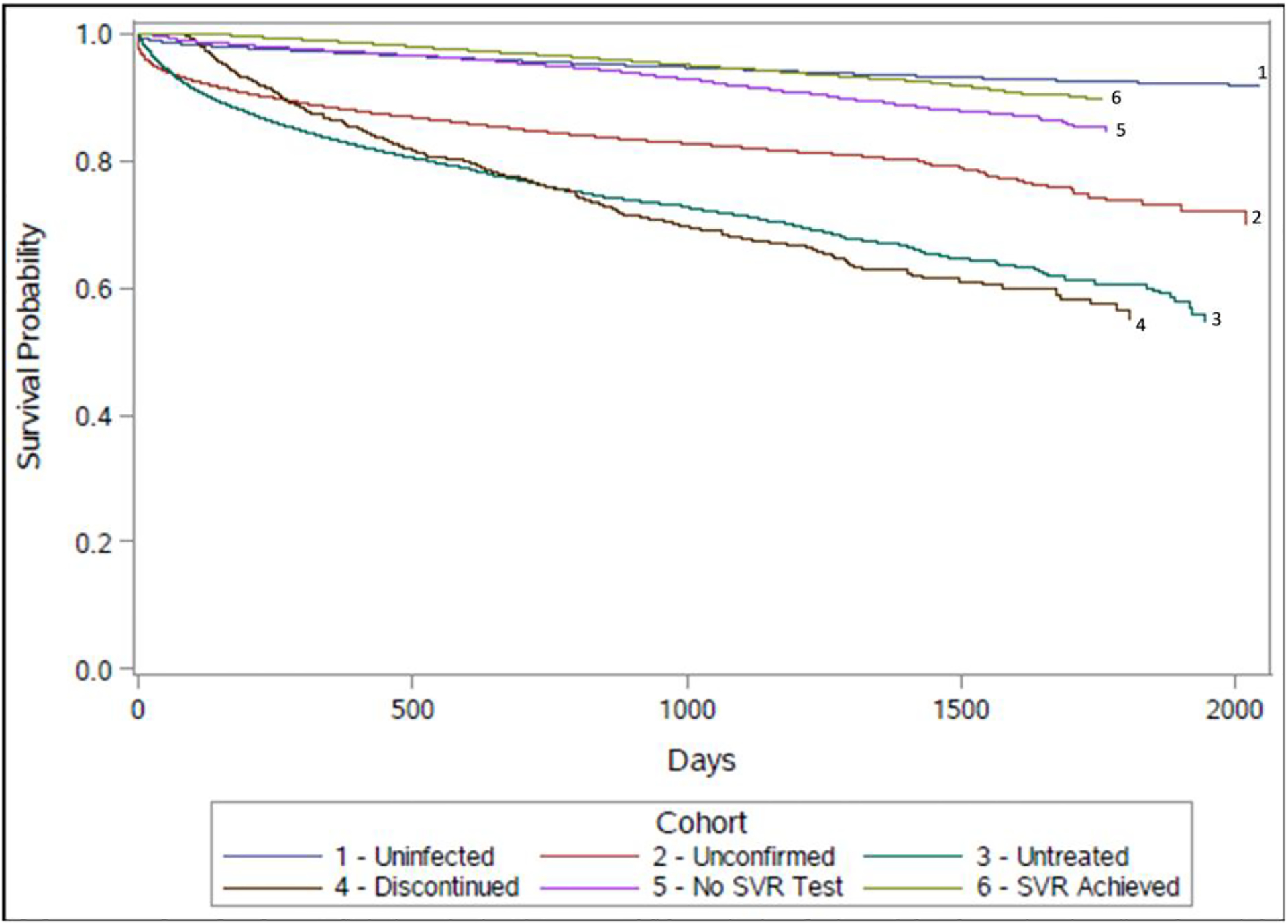
Kaplan-Meier curves of all-cause mortality rate in six cohorts, adjusted for age, sex, and hospitalization. Abbreviation: SVR, sustained virologic response.
Table 3.
Unadjusted and Adjusted Hazards Ratios for All-cause Mortality, Comparing Study Cohorts—Country of Georgia, 2015–2020
| … | Unadjusted Hazard Ratio (95% CI) |
Adjusted Hazard Ratio (Age, Sex, Hospitalization) (95% CI) |
|---|---|---|
| Never exposed | 1 | 1 |
| Anti-HCV (+), unknown viremia status | 3.70 (3.57, 3.85) | 1.85 (1.79, 1.89) |
| Untreated HCV infection | 3.70 (3.57, 3.85) | 2.70 (2.63, 2.86) |
| Discontinued treatment | 4.17 (3.70, 4.55) | 3.70 (3.33, 4.00) |
| Completed treatment, no SVR assessment | 0.69 (.64, .75) | 1.16 (1.08, 1.27) |
| Achieved SVR | 0.45 (.43, .47) | 0.72 (.69, .76) |
Abbreviations: CI. confidence interval; HCV, hepatitis C virus; SVR, sustained virologic response.
Deaths Due to Liver-related Causes
Of the 100 371 total deaths, 1203 (1.2%) had missing ICD-10 codes for cause of death, and 11 039 (11.0%) had an unknown cause of death (ICD-10 code R99). Of the remaining 88 129 deaths, 624 were attributable to cirrhosis, 278 to HCC, 1092 to viral hepatitis and 973 to liver cancer (Table 4). Overall age-standardized cause-specific mortality rate per 100 000 person-years was 49.6 for viral hepatitis, 42.1 for liver cancer, 27.6 for cirrhosis, and 12.3 for HCC. Compared with people never exposed to HCV, cirrhosis-related mortality was approximately 4-times higher among people who achieved SVR, more than 12-times higher among people with unknown viremia status or untreated infection, and 29-times higher among cohort of people who discontinued hepatitis C treatment. Similar trend was observed for other liver-related mortality rates, with people who discontinued treatment having the highest mortality rate due to each of the examined liver-related causes, and people who achieved SVR having the second lowest liver-related mortality, after the never exposed cohort who had the lowest (Table 4).
Table 4.
Liver Related Causes of Death, Age-standardized Mortality Rates per 100 000 Person-years and Rate Ratios Among Persons Tested and Treated for Hepatitis C Georgia, 2015–2020
| Cohort | Total | Cirrhosis | HCCa | Viral Hepatitis | Liver Cancera | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | Rate | Rate ratio | Deaths | Rate | Rate ratio | Deaths | Rate | Rate Ratio | Deaths | Rate | Rate Ratio | ||
| Total | 1 764 324 | 624 | 27.6 | 278 | 12.3 | 1092 | 49.6 | 973 | 42.1 | ||||
| Never exposed | 1 660 573 | 392 | 18.2 | 1 | 114 | 5.1 | 1 | 158 | 7.3 | 1 | 563 | 25.1 | 1 |
| Anti-HCV (+), unknown viremia status | 18 994 | 67 | 229.0 | 12.6 | 27 | 98.6 | 19.2 | 238 | 799.2 | 108.9 | 81 | 282.4 | 11.3 |
| HCV infection and untreated | 15 747 | 59 | 232.8 | 12.8 | 52 | 212.4 | 41.4 | 290 | 1145.3 | 156.0 | 136 | 536.0 | 21.4 |
| Discontinued treatment | 1893 | 16 | 530.7 | 29.2 | 9 | 332.2 | 64.8 | 81 | 2746.6 | 374.1 | 25 | 957.0 | 38.2 |
| Completed treatment, no SVR assessment | 16 164 | 41 | 207.4 | 11.4 | 27 | 114.9 | 22.4 | 110 | 564.8 | 76.9 | 67 | 313.9 | 12.5 |
| Completed treatment, SVR achieved | 50 953 | 49 | 76.5 | 4.2 | 49 | 83.9 | 16.3 | 215 | 278.8 | 38.0 | 101 | 184.4 | 7.4 |
ICD10 codes: Cirrhosis: K70.3, K74.5, and K74.6; HCC: C22.0; Viral hepatitis: B15.0-B19.9; Liver cancer: C22.0-C22.9.
Abbreviations: anti-HCV: antibody to HCV; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; SVR: sustained virologic response.
HCC and Liver cancer categories are not mutually exclusive. All 278 deaths included in HCC category are also included in 973 deaths in Liver cancer category.
DISCUSSION
In this large population-based cohort study that assessed the impact of HCV infection and treatment on mortality, we found a strong association between hepatitis C treatment and reduced mortality. Among persons who completed hepatitis C treatment, the all-cause mortality rate approximated the rate among persons without HCV infection and was much lower compared with those who have untreated hepatitis C or discontinued treatment. Our findings highlight the benefit of DAAs in reducing mortality and can help countries assess the impact of treatment programs on mortality among the affected population. To our knowledge, this is the largest cohort study assessing mortality among different population subgroups with an accurately defined level of exposure to hepatitis C and comparing it to mortality among those never exposed to HCV.
This study has several major implications for hepatitis C programs both locally and globally. Our findings once again highlight the importance of timely treatment to save lives. In Georgia, as of December 2021, 20 000 individuals with anti-HCV positive results have not undergone viremia testing and more than 14 000 individuals diagnosed with HCV infection have not enrolled in the treatment program [18]. Our findings suggest that these individuals are approximately 3-times more likely to die of cirrhosis and 1.5–3 times more likely to die of liver cancer than those who received hepatitis C treatment and achieved SVR. This finding is in line with previous smaller studies reporting approximately the same magnitude of the effect of treatment on liver-related mortality [20]. Hepatitis C elimination programs in Georgia and other countries should prioritize interventions targeted at these groups and develop innovative ways of linking people to care and treatment, as earlier engagement and treatment could substantially reduce mortality.
Our analyses identified several noteworthy findings about the people who achieved SVR. We found that all-cause mortality among those who achieved SVR was lower than in the general population, but the liver-related cause-specific mortality was higher than among those never exposed to HCV. Lower all-cause mortality in SVR-achieved patients could be due to engagement in medical care triggered by the hepatitis C treatment, resulting in management of other comorbidities independent of hepatitis C and improvement of general health status, especially among elderly population, indicating an indirect benefit of enrolling in hepatitis C care. A study conducted among patients who achieved SVR after interferon-based regimens found that the mortality remained higher than in the general population [8]. This discrepancy could be explained by the fact that in our cohort, we had a shorter follow-up duration than in the previous study, and patients might be more likely to engage in harmful health behavior, such as alcohol or drug use, as time after treatment advances. Another explanation could be that variety of interferon-free regimens containing DAAs often lead to higher regression of liver fibrosis and are generally safer and better tolerated than interferon-based treatment regimens [21–25].
Our cause-specific mortality analysis identified that even after SVR is achieved, the liver-related mortality rate was substantially higher than among those never exposed to HCV. This finding is consistent with the previous reports from smaller-scale studies that found higher liver-related mortality after SVR compared to the general population [26, 27]. The high post-SVR liver-related mortality rate highlights that addressing the underlying causes and consequences of liver disease is essential in reducing liver-related mortality and reminds that some damage cannot be reversed by treatment alone. Individuals, particularly those with advanced fibrosis, benefit from regular post-treatment monitoring, including imaging for early detection of HCC, and frequent check-ups to ensure any residual liver conditions are identified in time and treated adequately. Such post-treatment monitoring is not regularly conducted in Georgia but can be considered given these findings.
With cure rates of >95% globally and reaching 99% in Georgia [18, 28], it is usually assumed that people who complete treatment with DAAs achieve SVR, hence should have comparable mortality rate to those with documented SVR. Surprisingly, we observed that cohort of people who completed the treatment without SVR assessment had substantially higher all-cause and liver-related mortality rates than those with documented SVR. Some portion of this cohort could have serious health conditions that precluded them from getting SVR assessment and also caused death, which would explain higher mortality in this group than those who underwent SVR assessment. However, we cannot rule out possibility that substantial portion of the people without SVR assessment did not actually achieve SVR, which highlights the need to improve active follow-up with patients after the treatment completion and ensuring they receive SVR assessment.
Our study has several limitations. First, we could not control for some potential confounders such as socioeconomic status and behavioral factors (eg, alcohol and drug use) that may impact ability to access and adhere to full treatment course and also be associated with mortality. Second, we cannot rule out potential misclassification, especially cause of death reported to the death registry, which could cause an underestimation of our cause-specific mortality estimates. As for all-cause mortality, the sensitivity of the death registry for identifying deaths is more than >95% [29], suggesting that misclassification would not impact our all-cause mortality estimates substantially. Third, a history of hepatocellular carcinoma and other comorbidities associated with increased mortality was not assessed in our analysis. Fourth, in our data there were not enough details about the reasons for not starting or discontinuing hepatitis C treatment. Therefore, it is possible that people in this cohort had higher mortality because of untreated hepatitis C, but another plausible explanation is that some persons may have stopped DAA treatment or did not start treatment due to poor health or serious medical conditions which contributed to or caused their death.
In conclusion, this large population-based cohort study demonstrated the benefits of hepatitis C treatment on mortality reduction and highlighted that achieving the mortality targets of hepatitis C elimination is possible in the presence of large-scale treatment programs. Persons with untreated hepatitis C or discontinued treatment had mortality rates that were much greater than those of individuals who received hepatitis C treatment, making the former a priority group for efforts to enroll in treatment. Novel targeted interventions aimed at linkage to and retention in hepatitis C treatment are essential for engaging the remaining population with hepatitis C in care, reducing HCV-related mortality, and achieving the elimination goals set by the WHO.
Financial support.
D. B. reports support for this work from the Task Force for Global Health (contractor). F. A. reports support from CDC and Abbott.
Potential conflicts of interest.
D. B. reports consulting fees from World Health Organization (served as a consultant for piloting the tools for validation of viral hepatitis elimination). F. A. reports stock or stock options with Abbott. M. B. reports the following grants or contracts unrelated to this work: Integrated bio-behavioral surveillance survey among persons who inject drugs (PWID) in Georgia, NCDC Georgia, and missing sustained virologic response (SVR) data among patients enrolled in HCV elimination program—barriers to assessment including coronavirus disease 2019 (COVID-19) pandemic’s effects, Rustaveli Foundation. M. B. also reports payment or honoraria for Long COVID, training course for primary care physicians, Pfizer. N. C. reports the following grants or contracts unrelated to this work and paid to institution: An International Observational Study of Outpatients with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, supported by National Institutes of Health (NIH); An International Observational Study of Outpatients with SARS-CoV-2 Infection, supported by NIH; An International Observational Study of Outpatients with SARS-CoV-2 Infection, supported by NIH; and An International Observational Study of Outpatients with SARS-CoV-2 Infection, supported by NIH. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- 1.World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Geneva; 2021. [Google Scholar]
- 2.World Health Organization. Global hepatitis report 2017. Geneva, 2017. April 2017. [Google Scholar]
- 3.World Health Organization. Global Health Sector Strategy on viral hepatitis 2016–2021. Geneva, 2016. [Google Scholar]
- 4.Interim guidance for country validation of viral hepatitis elimination. Geneva: World Health Organization, 2021. [DOI] [PubMed] [Google Scholar]
- 5.Das D, Pandya M. Recent advancement of direct-acting antiviral agents (DAAs) in hepatitis C therapy. Mini Rev Med Chem 2018; 18:584–96. [DOI] [PubMed] [Google Scholar]
- 6.Scavone C, Sportiello L, Rafaniello C, et al. New era in treatment options of chronic hepatitis C: focus on safety of new direct-acting antivirals (DAAs). Expert Opin Drug Saf 2016; 15:85–100. [DOI] [PubMed] [Google Scholar]
- 7.Spengler U Direct antiviral agents (DAAs)—a new age in the treatment of hepatitis C virus infection. Pharmacol Ther 2018; 183:118–26. [DOI] [PubMed] [Google Scholar]
- 8.Innes H, McDonald S, Hayes P, et al. Mortality in hepatitis C patients who achieve a sustained viral response compared to the general population. J Hepatol 2017; 66: 19–27. [DOI] [PubMed] [Google Scholar]
- 9.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: impact on mortality in patients without advanced liver disease. Hepatology 2018; 68:827–38. [DOI] [PubMed] [Google Scholar]
- 10.Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 2019; 393:1453–64. [DOI] [PubMed] [Google Scholar]
- 11.Su F, Ioannou GN. The impact of direct-acting antiviral therapy for hepatitis C on hepatocellular carcinoma risk. Curr Hepatol Rep 2018; 17:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baliashvili D, Averhoff F, Kasradze A, et al. Risk factors and genotype distribution of hepatitis C virus in Georgia: a nationwide population-based survey. PLoS One 2022; 17:e0262935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagan LM, Kasradze A, Salyer SJ, et al. Hepatitis C prevalence and risk factors in Georgia, 2015: setting a baseline for elimination. BMC Public Health 2019; 19-(Suppl 3):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitruka K, Tsertsvadze T, Butsashvili M, et al. Launch of a nationwide hepatitis C elimination program–Georgia, April 2015. MMWR Morb Mortal Wkly Rep 2015; 64:753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gvinjilia L, Nasrullah M, Sergeenko D, et al. National progress toward hepatitis C elimination–Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep 2016; 65: 1132–5. [DOI] [PubMed] [Google Scholar]
- 16.Nasrullah M, Sergeenko D, Gvinjilia L, et al. The role of screening and treatment in national progress toward hepatitis C elimination–Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep 2017; 66:773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsertsvadze T, Gamkrelidze A, Chkhartishvili N, et al. Three years of progress towards achieving hepatitis C elimination in the country of Georgia, April 2015–March 2018. Clin Infect Dis 2020; 71:1263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgia Hepatitis Elimination Program Progress Report, 2020–2021, 2022.
- 19.Averhoff F, Shadaker S, Gamkrelidze A, et al. Progress and challenges in a pioneering hepatitis C elimination program in the country of Georgia, 2015–2018. J Hepatol 2020; 72:680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cepeda JA, Thomas DL, Astemborski J, et al. Impact of hepatitis C treatment up-take on cirrhosis and mortality in persons who inject drugs: a longitudinal, community-based cohort study. Ann Intern Med 2022; 175:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdelsameea E, Alsebaey A, Abdel-Samiee M, Abdel-Razek W, Salama M, Waked I. Direct acting antivirals are associated with more liver stiffness regression than pegylated interferon therapy in chronic hepatitis C patients. Expert Rev Anti-infect Ther 2021; 19:1053–9. [DOI] [PubMed] [Google Scholar]
- 22.Witthöft T, Möller B, Wiedmann KH, et al. Safety, tolerability and efficacy of peginterferon alpha-2a and ribavirin in chronic hepatitis C in clinical practice: the German open safety trial. J Viral Hepatitis 2007; 14:788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridruejo E, Adrover R, Cocozzella D, Fernandez N, Reggiardo MV. Efficacy, tolerability and safety in the treatment of chronic hepatitis C with combination of PEG-interferon - ribavirin in daily practice. Ann Hepatol 2010; 9:46–51. [PubMed] [Google Scholar]
- 24.Liu C-H, Liu C-J, Su T-H, et al. Real-world effectiveness and safety of sofosbuvir and ledipasvir with or without ribavirin for patients with hepatitis C virus genotype 1 infection in Taiwan. PLoS One 2018; 13:e0209299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buggisch P, Wursthorn K, Stoehr A, et al. Real-world effectiveness and safety of sofosbuvir/velpatasvir and ledipasvir/sofosbuvir hepatitis C treatment in a single centre in Germany. PLoS One 2019; 14:e0214795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Ambrosio R, Degasperi E, Anolli MP, et al. Incidence of liver- and non-liver-related outcomes in patients with HCV-cirrhosis after SVR. J Hepatol 2022; 76:302–10. [DOI] [PubMed] [Google Scholar]
- 27.Negro F Residual risk of liver disease after hepatitis C virus eradication. J Hepatol 2021; 74:952–63. [DOI] [PubMed] [Google Scholar]
- 28.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection. Ann Internal Med 2017; 166:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Center for Disease Control and Public Health of Georgia. Statistical Yearbook 2018, 2019.


