Abstract
Although depression is a risk and prognostic factor for cardiovascular disease (CVD), clinical trials treating depression in patients with CVD have not demonstrated cardiovascular benefits. We proposed a novel explanation for the null results for CVD-related outcomes: the late timing of depression treatment in the natural history of CVD. Our objective was to determine whether successful depression treatment before, versus after, clinical CVD onset reduces CVD risk in depression. We conducted a single-center, parallel-group, assessor-blinded randomized controlled trial. Primary care patients with depression and elevated CVD risk from a safety net healthcare system (N = 216, Mage = 59 years, 78% female, 50% Black, 46% with income <$10,000/year) were randomized to 12 months of the eIMPACT intervention (modernized collaborative care involving internet cognitive-behavioral therapy [CBT], telephonic CBT, and/or select antidepressants) or usual primary care for depression (primary care providers supported by embedded behavioral health clinicians and psychiatrists). Outcomes were depressive symptoms and CVD risk biomarkers at 12 months. Intervention participants, versus usual care participants, exhibited moderate-to-large (Hedges’ g = −0.65, p < 0.01) improvements in depressive symptoms. Clinical response data yielded similar results – 43% of intervention participants, versus 17% of usual care participants, had a ≥50% reduction in depressive symptoms (OR = 3.73, 95% CI: 1.93–7.21, p < 0.01). However, no treatment group differences were observed for the CVD risk biomarkers – i.e., brachial flow-mediated dilation, high-frequency heart rate variability, interleukin-6, high-sensitivity C-reactive protein, β-thromboglobulin, and platelet factor 4 (Hedges’ gs = −0.23 to 0.02, ps ≥ 0.09). Our modernized collaborative care intervention – which harnessed technology to maximize access and minimize resources – produced clinically meaningful improvements in depressive symptoms. However, successful depression treatment did not lower CVD risk biomarkers. Our findings indicate that depression treatment alone may not be sufficient to reduce the excess CVD risk of people with depression and that alternative approaches are needed. In addition, our effective intervention highlights the utility of eHealth interventions and centralized, remote treatment delivery in safety net clinical settings and could inform contemporary integrated care approaches.
Keywords: depression, cardiovascular disease, endothelial dysfunction, autonomic dysfunction, systemic inflammation, platelet activation, collaborative care, internet interventions, clinical trial
1. Introduction
Over 30 years of rigorous research indicates that depression is an independent risk and prognostic factor for atherosclerotic cardiovascular disease (CVD).1 Despite this strong evidence base, few clinical trials have evaluated whether depression interventions improve CVD-related outcomes. In those trials, the expected cardiovascular benefits were generally not observed, even though improvements in other important endpoints were detected.1 While other explanations for the null results for CVD-related outcomes have been suggested,2 we proposed that the depression interventions may have been delivered too late in the natural history of CVD,3 as nearly all prior trials involved patients with clinical CVD. Further, we hypothesized that successful depression treatment before clinical CVD onset could yield cardiovascular benefits3 because: (1) evidence suggests that depression begins to exert a cardiotoxic influence early in CVD pathogenesis;4, 5 (2) depression interventions may have greater antidepressive efficacy before clinical CVD onset;3, 6 (3) there is a growing consensus that earlier treatment of other risk factors produces greater CVD risk reductions;7 and (4) conventional prognostic factors may override depression effects in later CVD stages.8, 9
This hypothesis was supported by our preliminary study – an 8-year follow-up3 of the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) trial.10 Among those without clinical CVD, primary care patients with depression randomized to collaborative care had a 48% lower risk of incident CVD events than patients randomized to usual care for depression. Among those with clinical CVD, there was no cardioprotective effect of the intervention. However, because our analysis was post hoc, it remains unknown if the depression-CVD risk relationship is causal and if depression treatment reduces CVD risk.11
Accordingly, we conducted the eIMPACT randomized controlled trial (RCT), which compared modernized collaborative care to usual care for depression in primary care patients with depression and elevated CVD risk from a safety net healthcare system. We modernized the IMPACT trial’s effective intervention10 by incorporating an established internet cognitive-behavioral therapy (CBT),12 delivering an established manualized CBT13 by phone, and optimizing the antidepressant algorithm for CVD risk reduction. The primary outcome was endothelial dysfunction assessed by brachial flow-mediated dilation (FMD),14, 15 as FMD is impaired in depression,16 FMD is considered an index of CVD risk,17 and intervention-related FMD improvements are associated with reduced CVD incidence.18–20 The secondary outcomes were depressive symptoms and other CVD risk biomarkers – i.e., measures of leading candidate mechanisms (autonomic dysfunction, systemic inflammation, and platelet activation) underlying the depression-incident CVD association.1, 21
2. Methods
2.1. Trial Design
The eIMPACT trial was a 24-month, phase II, single-center RCT conducted at eight primary care clinics of a safety net healthcare system (Eskenazi Health) in Indianapolis, IN from 2015–2020. Participants attended the pre-treatment visit at a clinical research center (CRC) of the Indiana Clinical and Translational Science Institute (CTSI). Staff instructed participants to fast and avoid tobacco and exercise for ≥8 hours before the visit. Participants provided written informed consent; completed self-report scales; had their height, weight, and blood pressure (BP) measured; and underwent a blood draw and heart rate variability (HRV) and FMD assessments. After baseline assessments, participants were randomized 1:1, stratified by age group (50–59 years, ≥60 years) and sex (male, female), to the eIMPACT intervention or usual primary care for depression. For each stratum, a randomization sequence was computer generated using random block sizes of 2 and 4,22 and sequentially numbered opaque envelopes containing treatment assignment were prepared. Staff assigned participants to treatment groups by opening the next envelope from the appropriate stratum. Participants completed three additional assessment contacts – 6-month mid-treatment call, 12-month post-treatment visit, and 24-month follow-up call. For the mid-treatment and follow-up calls, participants were administered self-report scales. For the post-treatment visit, participants returned to the CRC to undergo the same assessments as the pre-treatment visit. Outcomes assessors were blinded to treatment assignment. Due to the nature of our intervention, participants, the intervention team, and staff running the assessment contacts (but not assessing outcomes) were not blinded. This trial was approved by Indiana University Institutional Review Board and the Eskenazi Health Research Committee. The full study protocol and statistical analysis plan are available at ClinicalTrials.gov (NCT02458690), and the data and statistical code that support the main findings of this study are available at NHLBI BioData Catalyst (insert dbGaP link when available).
2.2. Participants
All 216 participants were recruited using a 3-stage process, which consisted of electronic health record (EHR) searches to generate lists of potentially eligible patients, an opportunity for primary care providers (PCPs) to opt all their patients out of this study (a very rare occurrence), and phone or in-clinic screening interviews conducted by staff of Indiana University’s primary care practice-based research network. Participants were screened and enrolled from August 13, 2015 to July 31, 2018. Data collection ended on July 31, 2020.
Inclusion criteria were: age ≥50 years, current depression, and elevated CVD risk. Current depression was defined as a Patient Health Questionnaire-9 (PHQ-9) score ≥1023 and a PHQ-9 major or other depressive disorder diagnosis (which require depressed mood and/or anhedonia).24 The PHQ-9 cut point of ≥10 has 88% sensitivity and 88% specificity for major depressive disorder diagnosed by clinical interview.23 To help equate risk across participants, elevated CVD risk was defined as ≥1 (if ≥60 years) or ≥2 (if 50–59 years) of the following risk factors in the EHR in the past 5 years (diagnostic code, lab value, or medication indicating presence): hypertension, hypercholesterolemia, diabetes, or current smoking.
Exclusion criteria were: clinical CVD (diagnostic/procedural code in the EHR or self-report of myocardial infarction, coronary artery disease, cerebrovascular disease, heart failure, percutaneous coronary intervention, or coronary artery bypass graft); HIV/AIDS, chronic kidney disease, or active cancer/current cancer treatment (self-report); current pregnancy (self-report or positive test); continuous treatment for a systemic inflammatory condition in the past 3 months (nonsteroidal anti-inflammatory drugs were allowed; self-report); current use of anticoagulant medications (aspirin, lipid-lowering agents, and antihypertensive agents were allowed; self-report); severe cognitive impairment (≥3 errors on a 6-item screener25); bipolar or psychotic disorder (diagnostic code in the EHR or self-report); acute risk of suicide (determined by clinical staff); and ongoing depression treatment with a psychiatrist outside of Eskenazi Health (self-report). Patients unable to understand English were also not eligible, as our intervention was available in English only.
2.3. Interventions
2.3.1. eIMPACT Intervention
The eIMPACT intervention is a collaborative care intervention in which a multidisciplinary team delivers established depression treatments consistent with patient preference. It uses a stepped, flexible, treat-to-target approach that modernized the IMPACT intervention10 by: incorporating an internet CBT (Beating the Blues US [BtB]; Workpartners UPMC), delivering another CBT (Problem Solving Treatment in Primary Care [PST-PC]) and other psychological components (psychoeducation, behavioral activation, and antidepressant adherence support) by phone or FaceTime, and optimizing the antidepressant algorithm for CVD risk reduction. The remaining IMPACT intervention components were not altered. The intervention team consisted of a Master’s-level behavioral health clinician (K.L.M.) as the depression clinical specialist (DCS), a supervising psychiatrist (J.I.N.), a primary care liaison (C.M.C.), and the participants’ usual PCPs.
BtB is an efficacious internet CBT for depression and/or anxiety that is appropriate for adults with little computer experience and ≥5th grade reading level.26–28 BtB uses an interactive, multimedia format to deliver 8 weekly sessions, the structure and content of which mirror face-to-face CBT. Covered topics include challenging dysfunctional thoughts, activity scheduling, problem-solving, graded exposure, task breakdown, sleep management, and relapse prevention. Patients are encouraged to complete homework assignments between sessions. BtB sessions occurred at a location with internet access selected by participants (e.g., home or work). Those with no internet access or limited computer skills could complete sessions in the principal investigator’s laboratory with the DCS available for assistance. Participants were mailed a folder containing BtB worksheets, were oriented to the program, were instructed to complete 1 session/week, and worked through the sessions on their own. The DCS provided weekly support via phone by reviewing progress/content and addressing questions/issues.
PST-PC is a manualized and efficacious CBT for depression developed for primary care.13, 29 The focus of the 6–8, weekly, 30-minute sessions is teaching patients the seven problem-solving steps (i.e., defining the problem, setting a realistic goal, brainstorming potential solutions, evaluating potential solutions, selecting a solution, implementing the solution, and reviewing the outcome) and helping them apply these steps to current problems contributing to their depression. In the final session, the therapist and patient also collaboratively formulate a relapse prevention plan. The DCS (certified in PST-PC) delivered sessions by phone, which is feasible and effective.30 In-person sessions were allowed if they were the participant’s strong preference. Participants were mailed a folder containing PST-PC worksheets that they completed in collaboration with the DCS.
The IMPACT intervention manual31 guidelines for managing antidepressants were followed after our psychiatrist made necessary updates to dosing/titrating. To optimize the algorithm for CVD risk reduction, the IMPACT medication list was restricted to selective serotonin reuptake inhibitors (SSRIs), duloxetine, bupropion, and mirtazapine. These FDA-approved antidepressants are the safest from a cardiovascular perspective.32, 33 We prohibited the use of most serotonin-norepinephrine reuptake inhibitors (SNRIs) and all tricyclic antidepressants due to their potential adverse effects on cardiovascular parameters.32, 33 The intervention team made antidepressant recommendations, which the DCS communicated to participants and PCPs. PCPs wrote all prescriptions, and the intervention team and PCPs collaboratively managed pharmacotherapy.
The intervention process followed the IMPACT manual31 with modifications noted below. To end the pre-treatment visit, the DCS met with participants for 20 minutes over FaceTime to build rapport, review depression education materials, deliver a behavioral activation intervention,13 and schedule the initial visit within the next week. During the initial visit by phone, the DCS completed an assessment interview and discussed treatment options and preferences. At meetings every 2 weeks, the DCS presented cases to the intervention team, who formulated a Step 1 plan. The DCS collaborated with the PCP to implement this plan. Step 1 treatment was 2–3 months of CBT or an antidepressant, largely driven by patient preference. BtB and PST-PC were the first- and second-line CBTs, respectively; SSRIs and the other medications were the first- and second-line antidepressants, respectively. For participants on an antidepressant at entry, the dosage was adjusted, CBT was added, and/or a different antidepressant was recommended. For participants with a strong preference for CBT but with barriers to BtB delivery (e.g., no internet access), PST-PC was offered at Step 1. Our DCS followed patients for 12 months, monitoring response and staffing cases with the intervention team at least every 3 months. DCS contacts (typically 30 minutes by phone) occurred every 1–2 weeks during active treatment. In addition to delivering CBT (if prescribed), the DCS assessed depressive symptoms, maintained engagement in behavioral activation, and provided antidepressant adherence support including side effect monitoring (for those receiving antidepressants) at most contacts. For participants who achieved remission (≥50% PHQ-9 score reduction and <3 symptoms for ≥6 weeks10), the DCS developed a relapse prevention plan and followed-up every 2–4 weeks by phone. If remission was not achieved after Step 1, Step 2 treatment – i.e., augmenting Step 1 treatment with CBT or an antidepressant or switching to another CBT or antidepressant – was delivered for an additional 2–3 months. If remission was still not achieved after Step 2, Step 3 treatment was delivered, which consisted of additional CBT and/or adjustments to the antidepressant regimen and, if indicated, a phone evaluation with our psychiatrist.
2.3.2. Usual Primary Care for Depression
The comparator was modeled after that of the IMPACT trial.10 Eskenazi Health primary care clinics utilize a team care approach for behavioral health issues, with PCPs supported by embedded Master’s-level behavioral health clinicians and affiliated psychiatrists available for brief counseling and antidepressant management. To end the pre-treatment visit, participants were informed of their current depression and group assignment, were encouraged to follow-up with their usual PCP regarding their depression, and were provided a list of local mental health services. They were also reminded that all depression treatments that participants in this group receive during the study are delivered by their usual providers. Participants’ PCPs received a letter or EHR message from the trial team indicating their patient’s group assignment, encouraging them to address their patient’s depression, and providing the same list of services. This correspondence also noted that, like participants in the intervention group, there were no care restrictions. Although a PCP could have patients in both treatment groups, crossover effects were unlikely, as (a) PCPs did not have access to the intervention protocol and did not attend intervention team meetings and (b) internet and telephonic CBT was not available in the targeted clinics.
2.4. Assessments
2.4.1. Primary Outcome
The primary outcome was brachial FMD at 12 months. It was measured at the Indiana CTSI Human Vascular Imaging Core per consensus guidelines14 using a GE LOGIQe high-resolution ultrasound with a 15-MHz vascular transducer. After a 10-minute supine rest period, a BP cuff was placed on the forearm and inflated to 250 mmHg for five minutes. Brachial diameter was measured at pre-inflation and 60- and 90-seconds post-deflation using AccessPoint 2011 software (version 8.2; Freeland Systems). FMD was computed the maximum % increase in brachial diameter at 60- or 90-seconds post-deflation.
2.4.2. Secondary Outcomes
Secondary outcomes were depressive symptoms, autonomic dysfunction, systemic inflammation, and platelet activation at 12 months. As in the IMPACT trial,10 participants completed the reliable and valid Hopkins Symptom Checklist-20 (SCL-20)34 to assess depressive symptoms. Total scores (mean of items responses) and a clinical response variable (≥50% reduction in total score) were computed.
To quantify autonomic dysfunction, we measured high-frequency heart rate variability (HF HRV), an index of parasympathetic cardiac control, per established guidelines35 and as described elsewhere.36 HF HRV estimates (ln of ms2/Hz) were derived by spectral analysis (bandwidth: 0.15–0.40 Hz) from 1-minute epochs of electrocardiographic data obtained during the last 5 minutes of the 10-minute supine rest period using MindWare Technologies HRV analysis software (version 3.1.2). Mean HF HRV was computed as the average of the five estimates. To control for respiration rate, participants completed a paced-breathing computer task set to 12 breaths/minute.
To quantify systemic inflammation and platelet activation, fasting blood samples obtained by CRC nurses were collected in EDTA tubes and centrifuged within 20 minutes. Plasma aliquots were frozen at −80°C until the time of assay at the Indiana University Center for Diabetes and Metabolic Diseases Translation Core. Using enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions, we measured levels of inflammatory markers, interleukin-6 (IL-6; R&D Systems HS600C) and high-sensitivity C-reactive protein (hsCRP; R&D Systems DCRP00), and platelet-specific release products, β-thromboglobulin (BTG; Biomatik EKU02712) and platelet factor 4 (PF4; R&D Systems DPF40). All samples were measured in duplicate. The limit of detection was 0.09 pg/mL, 0.022 ng/mL, 6.7 pg/mL, and 0.032 ng/mL for IL-6, hsCRP, BTG, and PF4, respectively.
2.4.3. Other Factors
At pre-treatment, sociodemographic factors, CVD risk factors, mental health variables, and current medications were assessed by self-report (Table 1). Medical and mental health conditions were assessed by the questions: “Have you ever been told by a doctor or other health professional that you have [high cholesterol, hypertension or high blood pressure, diabetes, a depressive disorder, an anxiety disorder, an alcohol or drug problem]? Smoking status was assessed by a Behavioral Risk Factor Surveillance System measure.37 Current psychotherapy/counseling for depression was assessed by the question: “Are you currently receiving talk therapy or counseling for depression?” Participants were instructed to bring a list of current prescription medications. Medication types – CVD prevention (lipid-lowering and antihypertensive agents), diabetes (oral/injectable hypoglycemic agents and insulin products), and antidepressants – were coded by staff. Lipid fractions were measured in duplicate using a Daytona Clinical Analyzer. Three seated BP readings separated by two minutes were obtained by a CRC nurse after five minutes of rest.38 Systolic and diastolic BP (SBP and DBP) were computed as the mean of the last two readings. Body mass index (BMI) was calculated from weight and height measured by a CRC nurse using a Scale-Tronix 5002 medical scale.
At mid- and post-treatment, the DCS conducted EHR chart reviews to quantify depression treatment received as part of usual primary care for both groups. We recorded participant withdrawals as they occurred. We identified adverse events by conducting EHR searches every 6 months and administering a brief self-report questionnaire at mid-treatment, post-treatment, and follow-up to capture events occurring outside of Eskenazi Health. We identified deaths via EHR searches every 6 months, secondary contacts provided by participants, and internet searches for obituaries.
2.5. Statistical Analysis
Based on results of our pilot trial (see NCT01605552), the eIMPACT trial was powered to detect d ≥ 0.43 for the effect of the eIMPACT intervention on the primary and secondary outcomes. For an independent t test with two-tailed α = 0.05, 172 participants are needed to achieve 80% power to detect a d = 0.43. Using an attrition rate of 20%, we randomized 216 participants to ensure at least 172 completers.
Analyses were conducted using SAS 9.4. P values are two-tailed (p < 0.05 considered significant). Outcome variables exhibiting considerable skew were log transformed (HF HRV: ln; IL-6 and hsCRP: log10[Xi+1]). Untransformed values for IL-6 and hsCRP are provided in Supplemental Table 2 to facilitate comparisons with other reported data. Change scores for each outcome were computed as post-treatment minus pre-treatment level. To compare treatment groups on baseline characteristics, independent t tests and χ2 tests were performed. To compare treatment groups on outcome variables at baseline, ANCOVA models adjusting for age group and sex were conducted with baseline levels of the outcomes as dependent variables. To characterize intervention delivery, descriptive statistics were computed for intervention process variables. To compare treatment groups on depression treatment received as part of usual care, withdrawals, adverse events, and deaths, χ2 tests were performed.
For hypothesis-testing analyses, an intention-to-treat approach employing multiple imputation was used, in accordance with recent recommendations for RCTs when the missing completely at random (MCAR) assumption may be implausible.39, 40 First, to properly handle missing data (0.9–5.6% for pre-treatment outcomes and 7.4–12.0% for post-treatment outcomes; see Table 4 Note), 10 multiple imputation datasets were generated from the observed data using the SAS MI procedure and the fully conditional specification method, where missing pre-treatment outcomes were imputed from regression models using age, sex, race, and treatment group, and missing post-treatment outcomes were imputed from models using pre-treatment level for the same outcome variable, age, sex, race, and treatment group. Second, to compare treatment groups on the outcomes, primary ANCOVA models adjusting for the stratification variables of age group and sex and with post-treatment outcomes as dependent variables were conducted on each imputed dataset, and results were combined using the SAS MIANALYZE procedure. A parallel set of supplemental ANCOVA models was run with outcome change scores as dependent variables. Third, secondary ANCOVA models further adjusting for baseline characteristics (education, income, and SBP) exhibiting imbalance between treatment groups (p < 0.10) and pre-treatment level for the outcome were conducted on each imputed dataset, and results were combined. Fourth, between-group effect sizes (Hedges’ g) for each outcome were computed using the group means and standard deviations derived from multiple imputation. In addition, a χ2 test was performed to assess for treatment group differences in the depression clinical response variable. Finally, as a sensitivity analysis, hypothesis-testing models were rerun using only the observed data (complete case analysis40).
3. Results
3.1. Participant Flow and Characteristics
As indicated in Figure 1, 4,474 patients were contacted for screening. Of the 304 patients eligible and interested after screening, 216 (71%) were randomized. Retention was high and balanced across the treatment groups (intervention: 93%; usual care: 92%).
Figure 1.
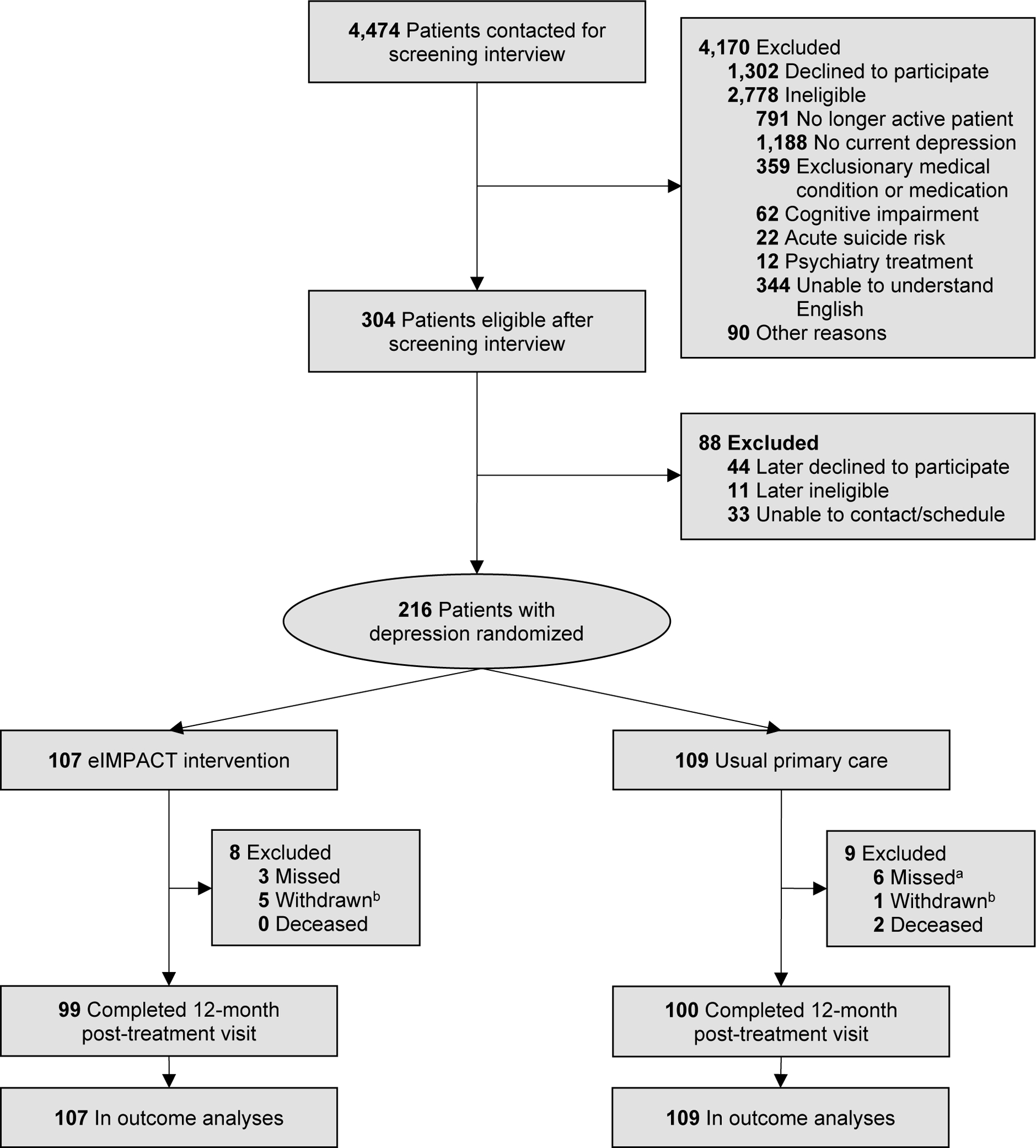
Flowchart of Screening, Enrollment, Randomization, and Follow-Up for the eIMPACT Trial
Note. Participants were screened and enrolled from August 13, 2015 to July 31, 2018. Data collection ended on July 31, 2020.
aFor two of these participants, 12-month survey data were obtained.
bFor all withdrawn by investigator (intervention: 3; usual care: 1), it was determined after randomization that the participant met an exclusion criterion.
As shown in Table 1, our randomized sample had good representation of women and Black/African American adults, people with low incomes, and individuals with high CVD risk factor burden. The mean pre-treatment PHQ-9 score was 15.1, falling in the moderately severe depression range. There was a high prevalence of depressive disorder history and a high frequency of current antidepressant use. Treatment groups were balanced on baseline characteristics, although the intervention group had somewhat higher education and incomes and lower SBP (ps < 0.10).
Table 1.
Baseline Characteristics of Participants in the eIMPACT Trial
| All (N = 216) | Intervention (n = 107) | Usual Care (n =109) | p value | |
|---|---|---|---|---|
|
| ||||
| Sociodemographic Factors | ||||
|
| ||||
| Age, years, M (SD) | 58.7 (5.7) | 58.5 (6.0) | 58.9 (5.4) | 0.62 |
| Sex, n (%) | 0.81 | |||
| Female | 169 (78.2) | 83 (77.6) | 86 (78.9) | |
| Male | 47 (21.8) | 24 (22.4) | 23 (21.1) | |
| Race, n (%) | 0.70a | |||
| American Indian/Alaskan Native | 1 (0.5) | 0 (0.0) | 1 (0.9) | |
| Asian | 1(0.5) | 1 (0.9) | 0 (0.0) | |
| Black/African American | 107 (49.5) | 56 (52.3) | 51 (46.8) | |
| Native Hawaiian/Other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| White | 97 (44.9) | 45 (42.1) | 52 (47.7) | |
| Multi-Racial | 8 (3.7) | 4 (3.7) | 4 (3.7) | |
| Other Race | 2 (0.9) | 1 (0.9) | 1 (0.9) | |
| Ethnicity, n (%) | 0.98 | |||
| Hispanic or Latino/a/x | 10 (4.6) | 5 (4.7) | 5 (4.6) | |
| Not Hispanic or Latino/a/x | 206 (95.4) | 102 (95.3) | 104 (95.4) | |
| Education, years, M (SD) | 12.8 (2.3) | 13.1 (2.5) | 12.6 (2.0) | 0.09 |
| Annual Household Income, n (%) | 0.04 | |||
| <$10,000 | 98 (45.6) | 44 (41.1) | 54 (50.0) | |
| $10,000-$14,999 | 37 (17.2) | 16 (15.0) | 21 (19.4) | |
| $15,000-$24,999 | 45 (20.9) | 21 (19.6) | 24 (22.2) | |
| $25,000-$39,999 | 25 (11.6) | 18 (16.8) | 7 (6.5) | |
| $40,000+ | 10 (4.7) | 8(7.5) | 2 (1.9) | |
|
| ||||
| Cardiovascular Disease Risk Factors | ||||
|
| ||||
| Hypercholesterolemia, n (% yes) | 114 (53.0) | 56 (52.3) | 58 (53.7) | 0.84 |
| LDL Cholesterol, mg/dL, M (SD) | 108.8 (40.9) | 109.8(41.4) | 107.8 (40.5) | 0.72 |
| HDL Cholesterol, mg/dL, M (SD) | 48.9 (15.8) | 50.5 (18.2) | 47.3 (12.8) | 0.14 |
| Triglycerides, mg/dL, M (SD)b | 141.2 (90.8) | 140.0 (94.5) | 142.3 (87.5) | 0.72 |
| Hypertension, n (% yes) | 164 (76.3) | 82 (76.6) | 82 (75.9) | 0.90 |
| SBP, mmHg, M (SD) | 135.7 (19.7) | 133.0 (18.9) | 138.4 (20.2) | 0.04 |
| DBP, mmHg, M (SD) | 80.9 (11.8) | 79.7 (11.0) | 82.1 (12.5) | 0.14 |
| Diabetes, n (% yes) | 76 (35.3) | 34 (31.8) | 42 (38.9) | 0.28 |
| Fasting Glucose, mg/dL, M (SD)b | 130.9 (60.1) | 130.4 (59.2) | 131.5 (61.1) | 0.80 |
| Fasting Insulin, μU/mL, M (SD)b | 17.6 (17.3) | 17.6 (20.6) | 17.7 (13.3) | 0.45 |
| Body Mass Index, kg/m2, M (SD) | 33.6 (9.5) | 32.7 (9.3) | 34.6 (9.6) | 0.14 |
| Smoking Status, n (%) | 0.21 | |||
| Never Smoker | 66 (30.6) | 37 (34.6) | 29 (26.6) | |
| Former Smoker | 37 (17.1) | 14 (13.1) | 23 (21.1) | |
| Current Smoker | 113 (52.3) | 56 (52.3) | 57 (52.3) | |
|
| ||||
| Mental Health Variables | ||||
|
| ||||
| Pre-Treatment PHQ-9 Score, M (SD) | 15.1 (5.0) | 14.7 (5.2) | 15.4 (4.8) | 0.33 |
| Depressive Disorder History, n (% yes) | 125 (58.1) | 64 (59.8) | 61 (56.5) | 0.62 |
| Anxiety Disorder History, n (% yes) | 101 (47.0) | 48 (44.9) | 53 (49.1) | 0.54 |
| Alcohol or Drug Problem History, n (% yes) | 33 (15.4) | 18 (16.8) | 15 (14.0) | 0.57 |
| Current Psychotherapy/Counseling for Depression, n (% yes) | 35 (16.3) | 20 (18.9) | 15 (13.8) | 0.31 |
|
| ||||
| Current Medication Use | ||||
|
| ||||
| CVD Prevention Medication, n (% yes) | 155(73.1) | 74 (71.8) | 81 (74.3) | 0.69 |
| Diabetes Medication, n (% yes) | 65(30.7) | 27 (26.2) | 38 (34.9) | 0.17 |
| Antidepressant Medication, n (% yes) | 124 (58.5) | 63 (61.2) | 61 (56.0) | 0.44 |
Note. Values are from the observed dataset. All variables had complete data except (n): education (215), income (215), hypercholesterolemia (215), LDL cholesterol (214), HDL cholesterol (214), triglycerides (214), hypertension(215), diabetes (215), fasting glucose (214), fasting insulin (214), depressive disorder (215), anxiety disorder (215), alcohol or drug problem (214), current psychotherapy/counseling for depression (215), CVD prevention medication (212), diabetes medication (212), and antidepressant medication (212). P values are from independent samples t tests for continuous variables and χ2tests for categorical variables comparing the intervention and usual care groups. LDL = low-density lipoprotein; HDL = high-density lipoprotein; SBP = systolic blood pressure. DBP = diastolic blood pressure. PHQ-9 = Patient Health Questionnaire-9.
Due to some low cell counts, race was recoded into a 3-level variable (Black/African American, White, other race) prior to conducting the χ2 test.
For triglycerides, fasting glucose, and fasting insulin, untransformed values are shown. To normalize their distributions, these variables were log10-tranformed prior to conducting t tests.
3.2. Depression Treatment
As can be seen in Table 2, our intervention team delivered substantial depression treatment. On average, intervention participants had 21 DCS contacts, most of which were by phone. A total of 85% were prescribed CBT, with 70% prescribed internet CBT and 29% prescribed telephonic CBT. For 31%, our intervention team made an antidepressant recommendation, three quarters of which were for an SSRI. Phone evaluations with our psychiatrist were rare, and few outside referrals were made (none directly related to depression or CVD). EHR chart reviews revealed that the treatment groups received similar depression treatment as part of usual care (Table 3).
Table 2.
Depression Treatment Delivered as part of the eIMPACT Intervention During the 12-Month Treatment Period
| Intervention Process Variable | Value |
|---|---|
|
| |
| Number of Depression Clinical Specialist Contacts per Participant, M (SD) | 20.8 (9.1) |
| Cognitive-Behavioral Therapy (CBT) Prescribed, n (%) | 91(85.0) |
| Internet CBT (BtB) Prescribed, n (%) | 75 (70.1) |
| Number of BtB Sessions Completed (possible range: 0–8)a, M (SD) | 2.6 (3.0) |
| Telephonic CBT (PST-PC) Prescribed, n (%) | 31 (29.0) |
| Number of PST-PC Sessions Completed (possible range: 0–6/8), M (SD) | 4.5 (3.1) |
| Antidepressant Medication Recommended, n (%) | 33 (30.8) |
| Selective Serotonin Reuptake Inhibitor (SSRI), n (%) | 25 (23.4) |
| Duloxetine, n (%) | 3 (2.8) |
| Bupropion, n (%) | 2(1.9) |
| Mirtazapine, n (%) | 3 (2.8) |
| Phone Evaluation with Intervention Team Psychiatrist Completed, n (%) | 3 (2.8) |
| Outside Referral Madeb, n (%) | 15 (14.0) |
Note. n = 107. 95% of participants received a first-line intervention (68% BtB, 13% PST-PC, 14% antidepressant medication), 32% a second-line intervention (2% BtB, 14% PST-PC, 16% antidepressant medication), and 3% a third-line intervention (0% BtB, 2% PST-PC, 1% antidepressant medication). BtB = Beating the Blues; PST-PC = Problem Solving Treatment in Primary Care.
Thirty-one (41%) of intervention participants prescribed internet CBT completed zero BtB sessions. When there was not early BtB engagement or when there were BtB access issues (49% did not have a computer/tablet with internet access at home), our intervention team moved to another treatment option (e.g., telephonic CBT). For the 44 (59%) intervention participants who completed at least one BtB session, the mean number of BtB sessions was markedly higher at 4.4 (SD = 2.8).
12 participants had 1 referral, 2 participants had 2 referrals, and 1 participant had 3 referrals for a total of 19 referrals (8 to a medical social worker for basics needs/resources; 8 to the patien’s primary care provider or embedded behavioral health clinician for health issues unrelated to depression or cardiovascular disease; 3 to other programs [palliative care, substance use treatment, health and wellness groups]).
Table 3.
Depression Treatment Received as part of Usual Primary Care in the Targeted Clinics During the 12-Month Treatment Period
| Interventiona (n = 107) | Usual Care (n = 109) | p value | |
|---|---|---|---|
|
| |||
| Depression Treatment | |||
|
| |||
| Started New Psychotherapy/Counseling for Depression, n (%) | 7 (6.5) | 14(12.8) | 0.12 |
| Started New Antidepressant Medication or | 33 (30.8) | 40 (36.7) | 0.36 |
| Had Dosage Adjusted, n (%) | |||
| Seen by a Provider with Psychiatry Specialty, n (%)b | 14 (13.1) | 21 (19.3) | 0.22 |
Note. Values are from the observed dataset. All variables had complete data. P values are from χ2tests comparing the intervention and usual care groups.
Includes only treatments not initiated by our intervention team
Includes both psychiatrists and psychiatric and mental health clinical nurse specialists
3.3. Primary Outcome: Endothelial Dysfunction
There was no intervention effect on endothelial dysfunction. The intention-to-treat analysis revealed that the treatment groups did not differ on post-treatment FMD (Table 4 and Figure 2: Panel A). Of note, FMD slightly worsened in both groups during the treatment period. Supplemental analyses examining FMD change scores (Table 4) or further adjusting for baseline education, income, SBP, and FMD (Supplemental Table 1) yielded similar results, as did sensitivity analyses examining the observed data (Supplemental Tables 2 and 3).
Table 4.
Results for Primary and Secondary Outcomes of the eIMPACT Trial: Intention-to-Treat Analyses using Multiple Imputation Adjusted for Age Group and Sex
| All (N = 216) M (SD) | Intervention (n = 107) M (SD) | Usual Care (n = 109) M (SD) | p value | |
|---|---|---|---|---|
|
| ||||
| Brachial Flow-Mediated Dilation (% dilation) | ||||
|
| ||||
| Pre-Treatment FMD | 2.72 (2.31) | 2.59 (2.16) | 2.85(2.45) | 0.42 |
| Post-Treatment FMDa | --- | 2.28 (2.04) | 2.44 (2.29) | 0.60 |
| Pre-to-Post Change in FMDb | --- | −0.30 (2.25) | −0.41 (2.90) | 0.78 |
|
| ||||
| Depressive Symptoms (possible range of SCL-20: 0.00–4.00) | ||||
|
| ||||
| Pre-Treatment SCL-20 | 1.91 (0.72) | 1.86 (0.76) | 1.96 (0.68) | 0.30 |
| Post-Treatment SCL-20c | --- | 1.12 (0.80) | 1.65 (0.83) | < 0.01 |
| Pre-to-Post Change in SCL-20b | --- | −0.75 (0.78) | −0.31 (0.66) | < 0.01 |
|
| ||||
| High-Frequency Heart Rate Variability (ln of ms2/Hz) | ||||
|
| ||||
| Pre-TreatmentHF HRV | 5.22 (1.71) | 5.29 (1.72) | 5.15 (1.66) | 0.49 |
| Post-Treatment HF HRVc | --- | 5.39 (1.85) | 5.35 (1.78) | 0.81 |
| Pre-to-Post Change in HF HRVb | --- | 0.10 (1.73) | 0.20 (1.66) | 0.68 |
|
| ||||
| Interleukin-6 (log10 of pg/mL) | ||||
|
| ||||
| Pre-Treatment IL-6 | 0.73 (0.27) | 0.71 (0.28) | 0.75 (0.27) | 0.22 |
| Post-Treatment IL-6c | --- | 0.73 (0.26) | 0.77 (0.27) | 0.20 |
| Pre-to-Post Change in IL-6b | --- | 0.02 (0.16) | 0.02 (0.21) | 1.00 |
|
| ||||
| High-Sensitivity C-Reactive Protein (log10 of mg/L) | ||||
|
| ||||
| Pre-Treatment hsCRP | 0.69 (0.40) | 0.67 (0.40) | 0.71 (0.40) | 0.38 |
| Post-Treatment hsCRPc | --- | 0.65 (0.40) | 0.74 (0.41) | 0.09 |
| Pre-to-Post Change in hsCRPb | --- | −0.02 (0.29) | 0.03 (0.30) | 0.27 |
|
| ||||
| β-thromboglobulin (ng/mL) | ||||
|
| ||||
| Pre-Treatment BTG | 204(113) | 194 (118) | 213 (108) | 0.22 |
| Post-Treatment BTGc | --- | 185 (125) | 205 (118) | 0.23 |
| Pre-to-Post Change in BTGb | --- | −9 (135) | −8 (123) | 0.94 |
|
| ||||
| Platelet Factor 4 (ng/mL) | ||||
|
| ||||
| Pre-Treatment PF4 | 4165 (2094) | 4038 (2047) | 4290 (2133) | 0.37 |
| Post-Treatment PF4c | --- | 3842 (2011) | 4351 (2341) | 0.09 |
| Pre-to-Post Change in PF4b | --- | −196 (2230) | 61 (2373) | 0.43 |
Note. Values are from imputed datasets. Post-treatment and pre-to-post change values are not shown for all participants (both groups combined), as it is differential change between groups that is of interest. Pre-treatment values for all participants are shown to further characterize the sample. Observed ns for each outcome were (pre-treatment; post-treatment; pre-to-post change): FMD (212, 195, 194), SCL-20 (214, 200, 198), HF HRV (204, 190, 179), IL-6 (214, 195, 194), hsCRP (214, 196, 195), BTG (214, 195, 194), and PF4 (214, 195, 194). P values are from analysis of covariance (ANCOVA) models using multiple imputation and adjusting for the stratification variables of age group and sex. FMD = flow-mediated dilation; SCL-20 = Hopkins Symptom Checklist-20; HF HRV = high-frequency heart rate variability; IL-6 = interleukin-6; hsCRP = high-sensitivity C-reactive protein; BTG = β-thromboglobulin; PF4 = platelet factor 4.
Primary outcome
Pre-to-post change values were computed as: post-treatment value - pre-treatment value.
Secondary outcomes
Figure 2.
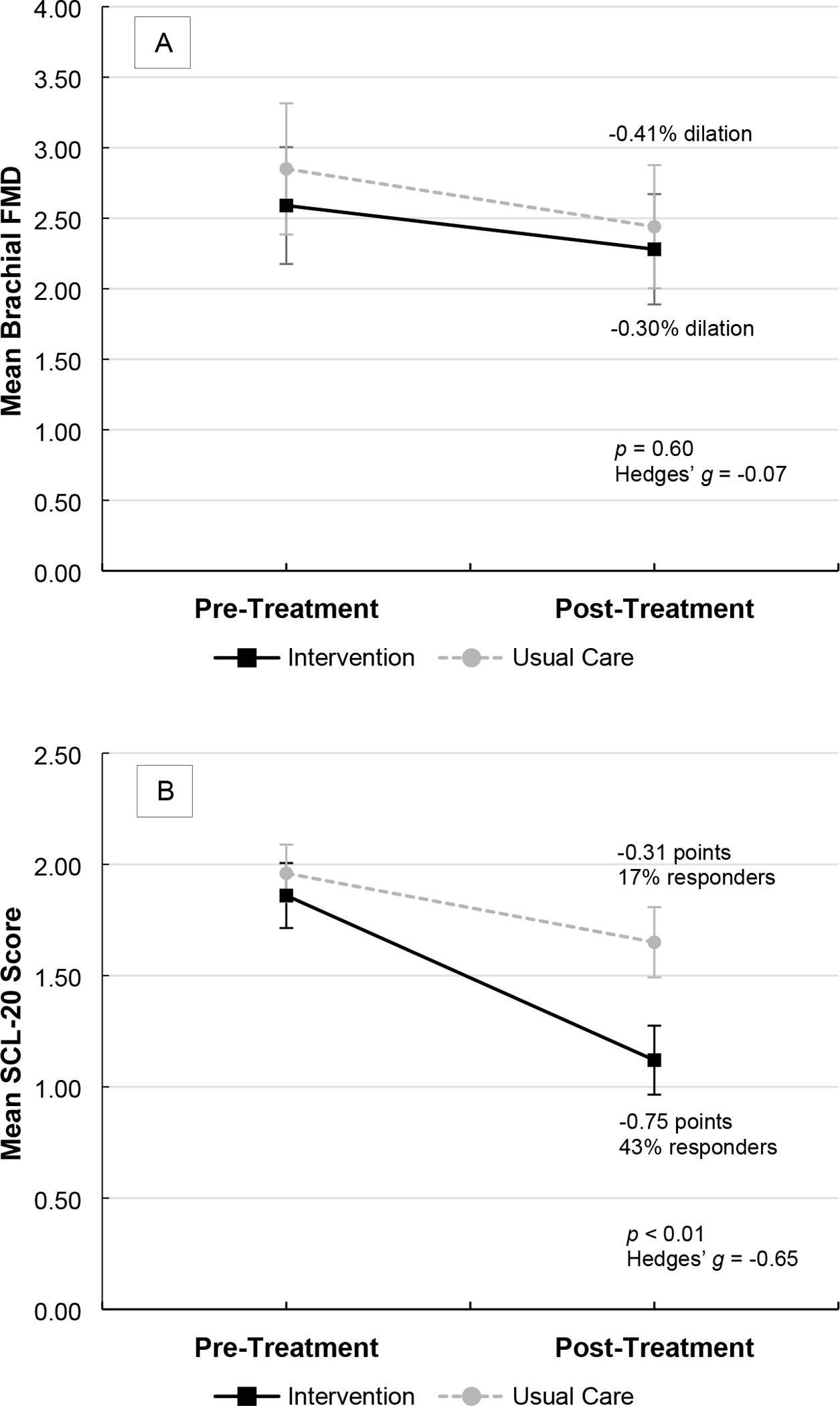
Mean Brachial Flow-Mediated Dilation (Endothelial Dysfunction; Panel A) and Hopkins Symptom Checklist-20 Scores (Depressive Symptoms; Panel B) at Pre-Treatment and Post-Treatment (12 Months) by Treatment Group
Note. Values are from imputed datasets. Error bars represent 95% confidence intervals. P values are from analysis of covariance (ANCOVA) models using multiple imputation and adjusting for the stratification variables of age group and sex. Effect sizes (Hedges’ g) were computed from imputed Ms and SDs. Responder defined as a ≥50% reduction in SCL-20 score. FMD = flow-mediated dilation; SCL-20 = Hopkins Symptom Checklist-20.
3.4. Secondary Outcomes: Depressive Symptoms, Autonomic Dysfunction, Systemic Inflammation, and Platelet Activation
The eIMPACT intervention significantly and meaningfully improved depressive symptoms. The intention-to-treat analysis demonstrated that the intervention group, versus the usual care group, exhibited moderate-to-large improvements in SCL-20 score at post-treatment (Table 4 and Figure 2: Panel B). This group difference was clinically meaningful – 43% of intervention participants, versus 17% of usual care participants, showed a clinical response (χ2 [1, N = 198] = 16.19, p < 0.01, OR = 3.73, 95% CI: 1.93–7.21). Comparable results were observed in supplemental analyses (Table 4 and Supplemental Table 1) and sensitivity analyses (Supplemental Tables 2 and 3).
No intervention effects were found for post-treatment measures reflecting autonomic dysfunction (HF HRV: Hedges’ g = 0.02), systemic inflammation (IL-6: Hedges’ g = −0.15; hsCRP: Hedges’ g = −0.22), or platelet activation (BTG: Hedges’ g = −0.16; PF4: Hedges’ g = −0.23; Table 3). Nonetheless, the small, nonsignificant improvements in hsCRP and PF4 favored the intervention group. Again, supplemental analyses (Table 4 and Supplemental Table 1) and sensitivity analyses (Supplemental Tables 2 and 3) yielded similar results.
3.5. Withdrawals, Adverse Events, and Deaths
The treatment groups (intervention vs. usual care) did not differ in the frequency of withdrawals (5 vs. 1, χ2 [1, N = 216] = 2.82, p = 0.09), adverse events possibly related to depression or study procedures (8 vs. 6, χ2 [1, N = 216] = 0.80, p = 0.37), or deaths (5 vs. 8, χ2 [1, N = 216] = 0.68, p = 0.41) through July 31, 2020. Only three adverse events were possibly related to study involvement (all mild/moderate and in the intervention group). No death was related to study involvement.
4. Discussion
We report the main results of the eIMPACT trial, the most definitive RCT to date testing whether depression treatment reduces CVD risk biomarkers among people with depression and elevated CVD risk. Our modernized collaborative care intervention, versus usual primary care for depression, produced statistically significant, moderate-to-large, and clinically meaningful improvements in depressive symptoms at 12 months. However, no group differences were observed for the primary outcome of endothelial dysfunction (FMD) and the secondary outcomes of autonomic dysfunction (HF HRV), systemic inflammation (IL-6, hsCRP), and platelet activation (BTG, PF4). Therefore, our hypothesis that successful depression treatment before clinical CVD onset yields cardiovascular benefits was not supported. Instead, our results agree with the general absence of cardiovascular benefits found in prior depression trials in patients with clinical CVD.1 Our findings indicate that depression treatment alone may not be sufficient to reduce the excess CVD risk in people with depression.
While our trial advances understanding of the depression-CVD risk link, there remains ambiguity about this relationship. Our null results for the CVD risk biomarkers fit with several possibilities. One possible explanation is that depression is not a causal risk factor for CVD and that a third factor is responsible for their association. However, recent compelling evidence suggests that depression may indeed be a causal CVD risk factor. Bidirectional Mendelian randomization analyses revealed that genetic predisposition to depression is causally associated with CVD risk but not vice versa.41 Furthermore, clinical trials in people with depression but no clinical CVD have observed some promising beneficial effects of depression treatment on indicators of CVD risk,3, 42, 43 and a recent meta-analysis found that SSRI treatment improves endothelial function.44
There are other possible explanations for our null results for the CVD risk biomarkers. First, we may have still delivered depression treatment too late. Due to the inclusion criteria, the prevalence of CVD risk factors (and perhaps advanced subclinical atherosclerosis) was high in our sample. To yield cardiovascular benefits, it might be necessary to deliver depression interventions earlier in the natural history of CVD. Second, depression may be a distal and modest11 causal risk factor for CVD. Consequently, depression treatment alone may be insufficient to meaningfully improve the putative mechanisms1, 21 and, ultimately, downstream CVD outcomes. A combined intervention approach in which depression and candidate biological mechanisms1 (e.g., autonomic dysfunction and systemic inflammation) are treated concurrently might be required. Another approach worthy of evaluation is integrated cardiologic care focused on concurrent management of depression and candidate behavioral mechanisms1 (e.g., poor diet, physical inactivity, smoking, medication nonadherence, and insomnia), as depression has been linked with poor adherence to medical recommendations to prevent CVD,45 and insomnia is common in depression46 and is associated with elevated inflammatory markers and incident CVD.47, 48 Third, only some subtypes or facets of depression may confer CVD risk, and current treatments may not adequately improve them. Accumulating evidence indicates that the atypical/immunometabolic subtype is the depression subgroup at particularly elevated CVD risk.49, 50 Other research suggests that somatic depressive symptoms (which often remain after treatment51, 52) may be stronger predictors of CVD-related outcomes than other symptoms in people initially free of CVD.53, 54 These observations imply that cardiovascular benefits of depression treatment may be present only in people with the atypical/immunometabolic subtype and only if interventions improve somatic symptoms. Again, this may call for a combined intervention approach – here, integrating depression interventions with pharmacological or psychological interventions shown to be efficacious for specific somatic symptoms (e.g., appetite and sleep disturbance).
The present findings provide direction regarding the way forward in the depression-CVD risk area. In the research domain, future work seeking to determine the causal mechanisms underlying the depression-CVD risk relationship is needed to refine conceptual frameworks and intervention targets. Additionally, future RCTs are needed in which (a) the depression subgroups at greatest CVD risk (e.g., people with the atypical/immunometabolic subtype) are enrolled and (b) optimized interventions (e.g., those concurrently targeting depression and putative mechanisms or appetite/sleep disturbance) are delivered. In addition, future RCTs should consider recruiting young and middle-aged adults with no history of clinical CVD, a group less likely to have advanced subclinical atherosclerosis. In the practice domain, it is important for clinicians engaged in CVD primary prevention to know that depression treatment alone is likely not sufficient to reduce the excess CVD risk of people with depression. Furthermore, because depression is an independent risk factor for incident CVD,1, 11 its presence should signal the need for frequent screening and intensified attention and support to improve management of traditional CVD risk factors.55
The eIMPACT trial also has important implications for the design and delivery of contemporary integrated care in CVD prevention settings and elsewhere. Our trial is among the first to integrate an eHealth intervention into a collaborative care framework for depression28 and is the first to do so in a safety net healthcare system where there are additional barriers (e.g., lower rates of internet access). Our depression results highlight the utility of our modernized approach, as we found that the eIMPACT intervention is feasible, acceptable, and effective. The substantial depression treatment delivered and the low intervention arm attrition support the feasibility and acceptability of our approach. Moreover, the eIMPACT intervention was found to be superior to usual primary care in the targeted clinics, which had implemented an integrated care model for mental health issues. Notably, intervention participants had 3.7 times greater odds of exhibiting a clinical response in depressive symptoms than usual care participants. Outperforming current integrated care underscores the potential added value of the innovative features of the eIMPACT intervention. These features also support broader dissemination and implementation. First, our intervention is practical for both patients and healthcare systems, given that nearly all our treatment was delivered remotely from a centralized location by one Master’s-level clinician. For patients, our intervention is convenient and accessible, perhaps especially for those with logistical barriers to treatment (e.g., unreliable transportation). For healthcare systems, our intervention is efficient and resource sparing, as it harnesses technology to minimize personnel, training, and space resources. Second, given our remote, centralized treatment delivery and our use of internet CBT, the eIMPACT intervention is more scalable, easier to deliver with high fidelity, and likely more cost effective than face-to-face alternatives.26
Our trial has strengths and limitations that should be considered. Concerning strengths, because this trial had pragmatic aspects (e.g., real-world setting, minimal mental health exclusions, and a flexible intervention), its results should be generalizable to routine clinical settings. Our sample also had strong representation of Black/African American adults and people with low incomes, which is uncommon in eHealth trials and enhances generalizability. Moreover, our intervention provided multiple treatment options and prioritized shared decision-making, which allowed for tailoring to participant preferences and situations. Regarding limitations, because a depressive disorder diagnosis was not required, our sample likely includes some patients with subclinical depression, which could have attenuated our depression effect sizes. However, 58% reported a depressive disorder history at entry, and our depression effect sizes compare favorably to those for collaborative care for depression.56 Furthermore, a recent meta-analysis determined that even subclinical depressive symptoms are associated with elevated CVD risk.11 There is also room for improvement in eHealth engagement, as 41% of intervention participants prescribed internet CBT completed zero sessions. Because no device with internet access at home (49% of intervention participants) and limited computer skills were common barriers, future trials should consider loaning tablets with data plans and providing basic computer training to participants in need of them. In addition, CVD prevention medication use was high in our sample at 73%, which may have masked depression intervention effects on the CVD risk biomarkers. Finally, we may have missed a depression intervention effect on other CVD-related outcomes. Future trials should consider a comprehensive assessment approach, including candidate mechanisms, CVD risk factors, clinical outcomes, and quality of life measures. Follow-up periods longer than ours may also be needed to observe depression intervention effects on CVD-related outcomes.
5. Conclusions
Our modernized collaborative care intervention – which harnessed technology to maximize access and to minimize the needed resources – improved depressive symptoms in primary care patients with depression and elevated CVD risk. However, 12 months of successful depression treatment did not lower CVD risk as assessed by multiple CVD risk biomarkers. Our findings indicate that depression treatment alone may not be sufficient to reduce the excess CVD risk of people with depression and that alternative approaches are needed. In addition, our effective intervention could inform the design and delivery of contemporary integrated care approaches.
Supplementary Material
Highlights.
Our modernized depression intervention used internet and telephonic psychotherapy.
Intervention participants exhibited meaningful improvements in depressive symptoms.
Successful depression treatment did not reduce cardiovascular risk biomarkers.
Depression treatment is not sufficient to lower cardiovascular risk in depression.
Integrated care should harness technology to maximize access/minimize resources.
Acknowledgements
We thank the patients and their primary care providers for their participation in this study, as well as our clinical partner, Eskenazi Health, and our research partners, Indiana Clinical and Translational Sciences Institute, Regenstrief Institute, and Workpartners UPMC. We also thank the Data and Safety Monitoring Board members – Drs. Michael Francis (chair), Gopi Dandamudi, Abhishek Khemka, Alexia Torke, and Wanzhu Tu – for their oversight.
Funding Sources
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the NIH under Award Number R01 HL122245. In addition, the Indiana University Center for Diabetes and Metabolic Diseases Translation Core was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH under Award Number P30 DK097512. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. These funding sources had no role in the conduct of the eIMPACT trial, including results interpretation and manuscript preparation.
Footnotes
Declarations of Interest/Disclosures
The authors have no potential conflicts of interest to declare related to the subject matter of this article. Several authors (J.C.S., B.M.P., S.G., J.I.N., S.K.G., R.V.C., B.L.R., C.M.C.) received grants from the NIH. J.C.S. received advisory fees from Boston Medical Center. J.I.N. received support as an investigator from Janssen. S.K.G. received advisory fees from Gilead Sciences and ViiV Healthcare and grants from ViiV Healthcare. R.V.C. received grants from Almond Board of California, American Diabetes Association, and Eli Lilly and Company.
Trial Registration: ClinicalTrials.gov Identifier: NCT02458690
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carney RM and Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol. 2017;14:145–155. DOI: 10.1038/nrcardio.2016.181 [DOI] [PubMed] [Google Scholar]
- 2.Shapiro PA. Depression treatment and coronary artery disease outcomes: Time for reflection. Journal of Psychosomatic Research. 2013;74:4–5. DOI: 10.1016/j.jpsychores.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 3.Stewart JC, Perkins AJ and Callahan CM. Effect of collaborative care for depression on risk of cardiovascular events: data from the IMPACT randomized controlled trial. Psychosom Med. 2014;76:29–37. DOI: 10.1097/PSY.0000000000000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper DC, Tomfohr LM, Milic MS, Natarajan L, Bardwell WA, Ziegler MG and Dimsdale JE. Depressed mood and flow-mediated dilation: a systematic review and meta-analysis. Psychosomatic medicine. 2011;73:360–9. DOI: 10.1097/PSY.0b013e31821db79a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shively CA, Register TC, Adams MR, Golden DL, Willard SL and Clarkson TB. Depressive behavior and coronary artery atherogenesis in adult female cynomolgus monkeys. Psychosomatic medicine. 2008;70:637–45. DOI: 10.1097/PSY.0b013e31817eaf0b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thombs BD, de Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, Mitchell AJ, Zuidersma M, Eze-Nliam C, Lima BB, Smith CG, Soderlund K and Ziegelstein RC. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300:2161–71. DOI: 10.1001/jama.2008.667 [DOI] [PubMed] [Google Scholar]
- 7.Makover ME, Shapiro MD and Toth PP. There is urgent need to treat atherosclerotic cardiovascular disease risk earlier, more intensively, and with greater precision: A review of current practice and recommendations for improved effectiveness. Am J Prev Cardiol. 2022;12:100371. DOI: 10.1016/j.ajpc.2022.100371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheier MF and Bridges MW. Person variables and health: personality predispositions and acute psychological states as shared determinants for disease. Psychosomatic medicine. 1995;57:255–68. DOI: 10.1097/00006842-199505000-00007 [DOI] [PubMed] [Google Scholar]
- 9.Suls J and Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychological Bulletin. 2005;131:260–300. DOI: 10.1037/0033-2909.131.2.260 [DOI] [PubMed] [Google Scholar]
- 10.Unutzer J, Katon W, Callahan CM, Williams JW, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C and Treatment IIIM-PAtC. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–45. DOI: 10.1001/jama.288.22.2836 [DOI] [PubMed] [Google Scholar]
- 11.Harshfield EL, Pennells L, Schwartz JE, Willeit P, Kaptoge S, Bell S, Shaffer JA, Bolton T, Spackman S, Wassertheil-Smoller S, Kee F, Amouyel P, Shea SJ, Kuller LH, Kauhanen J, van Zutphen EM, Blazer DG, Krumholz H, Nietert PJ, Kromhout D, Laughlin G, Berkman L, Wallace RB, Simons LA, Dennison EM, Barr ELM, Meyer HE, Wood AM, Danesh J, Di Angelantonio E and Davidson KW. Association Between Depressive Symptoms and Incident Cardiovascular Diseases. Jama-J Am Med Assoc. 2020;324:2396–2405. DOI: 10.1001/jama.2020.23068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proudfoot J, Goldberg D, Mann A, Everitt B, Marks I and Gray JA. Computerized, interactive, multimedia cognitive-behavioural program for anxiety and depression in general practice. Psychological Medicine. 2003;33:217–27. DOI: 10.1017/s0033291702007225 [DOI] [PubMed] [Google Scholar]
- 13.Hegel MT, Barrett JE, Oxman TE and al e. Problem-Solving Treatment for Primary Care (PST-PC): A Treatment Manual for Depression. Hanover, NH: Dartmouth University; 1999. [Google Scholar]
- 14.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R and International Brachial Artery Reactivity Task F. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology. 2002;39:257–65. DOI: 10.1016/s0735-1097(01)01746-6 [DOI] [PubMed] [Google Scholar]
- 15.Thijssen DHJ, Bruno RM, van Mil A, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, Green DJ and Ghiadoni L. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40:2534–2547. DOI: 10.1093/eurheartj/ehz350 [DOI] [PubMed] [Google Scholar]
- 16.Waclawovsky AJ, de Brito E, Smith L, Vancampfort D, da Silva AMV and Schuch FB. Endothelial dysfunction in people with depressive disorders: A systematic review and meta-analysis. J Psychiatr Res. 2021;141:152–159. DOI: 10.1016/j.jpsychires.2021.06.045 [DOI] [PubMed] [Google Scholar]
- 17.Frick M and Weidinger F. Endothelial function: a surrogate endpoint in cardiovascular studies? Curr Pharm Des. 2007;13:1741–50. DOI: 10.2174/138161207780831211 [DOI] [PubMed] [Google Scholar]
- 18.Modena MG, Bonetti L, Coppi F, Bursi F and Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. Journal of the American College of Cardiology. 2002;40:505–10. DOI: 10.1016/s0735-1097(02)01976-9 [DOI] [PubMed] [Google Scholar]
- 19.Suessenbacher A, Frick M, Alber HF, Barbieri V, Pachinger O and Weidinger F. Association of improvement of brachial artery flow-mediated vasodilation with cardiovascular events. Vascular Medicine. 2006;11:239–44. DOI: [DOI] [PubMed] [Google Scholar]
- 20.Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K, Sano K, Kobayashi T, Yano T, Nakamura K and Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. Journal of the American College of Cardiology. 2009;53:323–30. DOI: 10.1016/j.jacc.2008.08.074 [DOI] [PubMed] [Google Scholar]
- 21.Penninx BW. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2017;74:277–286. DOI: 10.1016/j.neubiorev.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 22.SAS Institute Inc. SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc.; 2008. [Google Scholar]
- 23.Kroenke K, Spitzer RL and Williams JB. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals. 2002;32:509–515. DOI: 10.1046/j.1525-1497.2001.016009606.x [DOI] [Google Scholar]
- 25.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ and Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical Care. 2002;40:771–781. DOI: 10.1097/00005650-200209000-00007 [DOI] [PubMed] [Google Scholar]
- 26.Marks IM, Cavanagh K and Gega L. Hands-on Help: Computer-aided Psychotherapy. Hove, UK: Psychology Press; 2007. [Google Scholar]
- 27.Proudfoot J, Ryden C, Everitt B, Shapiro DA, Goldberg D, Mann A, Tylee A, Marks I and Gray JA. Clinical efficacy of computerised cognitive-behavioural therapy for anxiety and depression in primary care: randomised controlled trial. British Journal of Psychiatry. 2004;185:46–54. DOI: 10.1192/bjp.185.1.46 [DOI] [PubMed] [Google Scholar]
- 28.Rollman BL, Belnap BH, Abebe KZ, Spring MB, Rotondi AJ, Rothenberger SD and Karp JF. Effectiveness of online collaborative care for treating mood and anxiety disorders in primary care. JAMA Psychiatry. 2018;75:56–64. DOI: 10.1001/jamapsychiatry.2017.3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang A, Park S, Sullivan JE and Jing S. The Effectiveness of Problem-Solving Therapy for Primary Care Patients’ Depressive and/or Anxiety Disorders: A Systematic Review and Meta-Analysis. J Am Board Fam Med. 2018;31:139–150. DOI: 10.3122/jabfm.2018.01.170270 [DOI] [PubMed] [Google Scholar]
- 30.Davidson KW, Bigger JT, Burg MM, Carney RM, Chaplin WF, Czajkowski S, Dornelas E, Duer-Hefele J, Frasure-Smith N, Freedland KE, Haas DC, Jaffe AS, Ladapo JA, Lesperance F, Medina V, Newman JD, Osorio GA, Parsons F, Schwartz JE, Shaffer JA, Shapiro PA, Sheps DS, Vaccarino V, Whang W and Ye S. Centralized, stepped, patient preference-based treatment for patients with post-acute coronary syndrome depression: CODIACS Vanguard randomized controlled trial. JAMA Intern Med. 2013:1–8. DOI: 10.1001/jamainternmed.2013.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unutzer J and investigators tI. IMPACT Intervention Manual. 1999.
- 32.Mago R, Tripathi N and Andrade C. Cardiovascular adverse effects of newer antidepressants. Expert Rev Neurother. 2014;14:539–51. DOI: 10.1586/14737175.2014.908709 [DOI] [PubMed] [Google Scholar]
- 33.Mavrides N and Nemeroff CB. Treatment of affective disorders in cardiac disease. Dialogues Clin Neurosci. 2015;17:127–40. DOI: 10.31887/DCNS.2015.17.2/nmavrides [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH and Covi L. The Hopkins Symptom Checklist (HSCL). A measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry. 1974;7:79–110. DOI: 10.1159/000395070 [DOI] [PubMed] [Google Scholar]
- 35.Berntson GG, Bigger J, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul J, Stone PH and van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. DOI: 10.1111/j.1469-8986.1997.tb02140.x [DOI] [PubMed] [Google Scholar]
- 36.Hawkins MA, Stewart JC, Fitzgerald GJ and Kim S. Combined effect of depressive symptoms and hostility on autonomic nervous system function. Int J Psychophysiol. 2011;81:317–23. DOI: 10.1016/j.ijpsycho.2011.07.018 [DOI] [PubMed] [Google Scholar]
- 37.Nelson DE, Holtzman D, Bolen J, Stanwyck CA and Mack KA. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS). Social and Preventive Medicine. 2001;46 Suppl 1:S3–42. [PubMed] [Google Scholar]
- 38.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M and Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–70. DOI: 10.1161/01.cir.88.5.2460 [DOI] [PubMed] [Google Scholar]
- 39.Li P, Stuart EA and Allison DB. Multiple Imputation: A Flexible Tool for Handling Missing Data. Jama. 2015;314:1966–7. DOI: 10.1001/jama.2015.15281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakobsen JC, Gluud C, Wetterslev J and Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162. DOI: 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li GH, Cheung CL, Chung AK, Cheung BM, Wong IC, Fok MLY, Au PC and Sham PC. Evaluation of bi-directional causal association between depression and cardiovascular diseases: a Mendelian randomization study. Psychol Med. 2020:1–12. DOI: 10.1017/S0033291720003566 [DOI] [PubMed] [Google Scholar]
- 42.Gupta SK, Slaven JE, Liu Z, Polanka BM, Freiberg MS and Stewart JC. Effects of Internet Cognitive-Behavioral Therapy on Depressive Symptoms and Surrogates of Cardiovascular Risk in Human Immunodeficiency Virus: A Pilot, Randomized, Controlled Trial. Open Forum Infect Dis. 2020;7:ofaa280. DOI: 10.1093/ofid/ofaa280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherwood A, Blumenthal JA, Smith PJ, Watkins LL, Hoffman BM and Hinderliter AL. Effects of Exercise and Sertraline on Measures of Coronary Heart Disease Risk in Patients With Major Depression: Results From the SMILE-II Randomized Clinical Trial. Psychosom Med. 2016;78:602–9. DOI: 10.1097/PSY.0000000000000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delialis D, Mavraganis G, Dimoula A, Patras R, Dimopoulou AM, Sianis A, Ajdini E, Maneta E, Kokras N, Stamatelopoulos K and Georgiopoulos G. A systematic review and meta-analysis on the effect of selective serotonin reuptake inhibitors on endothelial function. J Affect Disord. 2022;316:71–75. DOI: 10.1016/j.jad.2022.08.007 [DOI] [PubMed] [Google Scholar]
- 45.Berntson J, Stewart KR, Vrany E, Khambaty T and Stewart JC. Depressive symptoms and self-reported adherence to medical recommendations to prevent cardiovascular disease: NHANES 2005–2010. Soc Sci Med. 2015;138:74–81. DOI: 10.1016/j.socscimed.2015.05.041 [DOI] [PubMed] [Google Scholar]
- 46.Seow LSE, Verma SK, Mok YM, Kumar S, Chang S, Satghare P, Hombali A, Vaingankar J, Chong SA and Subramaniam M. Evaluating DSM-5 Insomnia Disorder and the Treatment of Sleep Problems in a Psychiatric Population. J Clin Sleep Med. 2018;14:237–244. DOI: 10.5664/jcsm.6942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irwin MR, Olmstead R and Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry. 2016;80:40–52. DOI: 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sofi F, Cesari F, Casini A, Macchi C, Abbate R and Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21:57–64. DOI: 10.1177/2047487312460020 [DOI] [PubMed] [Google Scholar]
- 49.Case SM, Sawhney M and Stewart JC. Atypical depression and double depression predict new-onset cardiovascular disease in U.S. adults. Depress Anxiety. 2018;35:10–17. DOI: 10.1002/da.22666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milaneschi Y, Lamers F, Berk M and Penninx B. Depression Heterogeneity and Its Biological Underpinnings: Toward Immunometabolic Depression. Biol Psychiatry. 2020;88:369–380. DOI: 10.1016/j.biopsych.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 51.Conradi HJ, Ormel J and de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med. 2011;41:1165–74. DOI: 10.1017/S0033291710001911 [DOI] [PubMed] [Google Scholar]
- 52.Hieronymus F, Emilsson JF, Nilsson S and Eriksson E. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol Psychiatry. 2016;21:523–30. DOI: 10.1038/mp.2015.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawkins MA, Callahan CM, Stump TE and Stewart JC. Depressive symptom clusters as predictors of incident coronary artery disease: a 15-year prospective study. Psychosom Med. 2014;76:38–43. DOI: 10.1097/PSY.0000000000000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K and Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Archives of General Psychiatry. 2007;64:225–33. DOI: 10.1001/archpsyc.64.2.225 [DOI] [PubMed] [Google Scholar]
- 55.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B, Societies ESCNC and Group ESCSD. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–3337. DOI: 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 56.Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, Dickens C and Coventry P. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


