Abstract
Hering’s Opponent-Colors Theory has been central to understanding color appearance for 150 years. It aims to explain the phenomenology of colors with two linked propositions. First, a psychological hypothesis stipulates that any color is necessarily and sufficiently described by the extent to which it appears reddish-versus-greenish, bluish-versus-yellowish, and blackish-versus-whitish. Second, a physiological hypothesis stipulates that these perceptual mechanisms are encoded by three innate brain mechanisms. We review the evidence and conclude that neither side of the linking proposition is accurate: the theory is wrong. We sketch out an alternative, Utility-Based Coding, by which the known retinal cone-opponent mechanisms represent optimal encoding of spectral information given competing selective pressure to extract high-acuity spatial information; and phenomenological color categories represent an adaptive, efficient, output of the brain governed by behavioral demands.
Keywords: linking hypothesis, perceptual mechanisms, neural networks, color perception, cones
The brain basis for color appearance
The color of the year is Viva Magenta, “a pulsating color whose exuberance promotes a joyous and optimistic celebration”.i Or it is Spanish Moss, “a midnight green that has a strong connection with the richness of nature”.ii Or Raspberry Blush, “a vibrant orange-red shade that instantly brings joy to your home”.iii The kaleidoscope of options illustrates the importance of color, while the baroque names underscore a peculiar paradox. Each color can be specified by three numbers — the activity of the three cone types — but this trichromatic code doesn’t capture color appearance. The same code can appear to be very different colors, depending on context and expectation [1].
In the 19th century, Ewald Hering proposed an alternative to trichromacy as the explanation for color appearance [2]. His Opponent-Colors Theory (Figure 1) seeks to explain what colors look like with two propositions. First, a perceptual hypothesis invoking a specific instantiation of color opponency (see Glossary) which stipulates that any color can necessarily and sufficiently be described by the extent to which it appears reddish-versus-greenish, bluish-versus-yellowish, and blackish-versus-whitish; the six colors defining Hering’s theory came to be called unique hues. Second, a physiological hypothesis which stipulates that the perceptual mechanisms are coded by three innate and discrete neurobiological processes. The theory is a showcase linking proposition [3].
Figure 1. Opponent-Colors Theory.
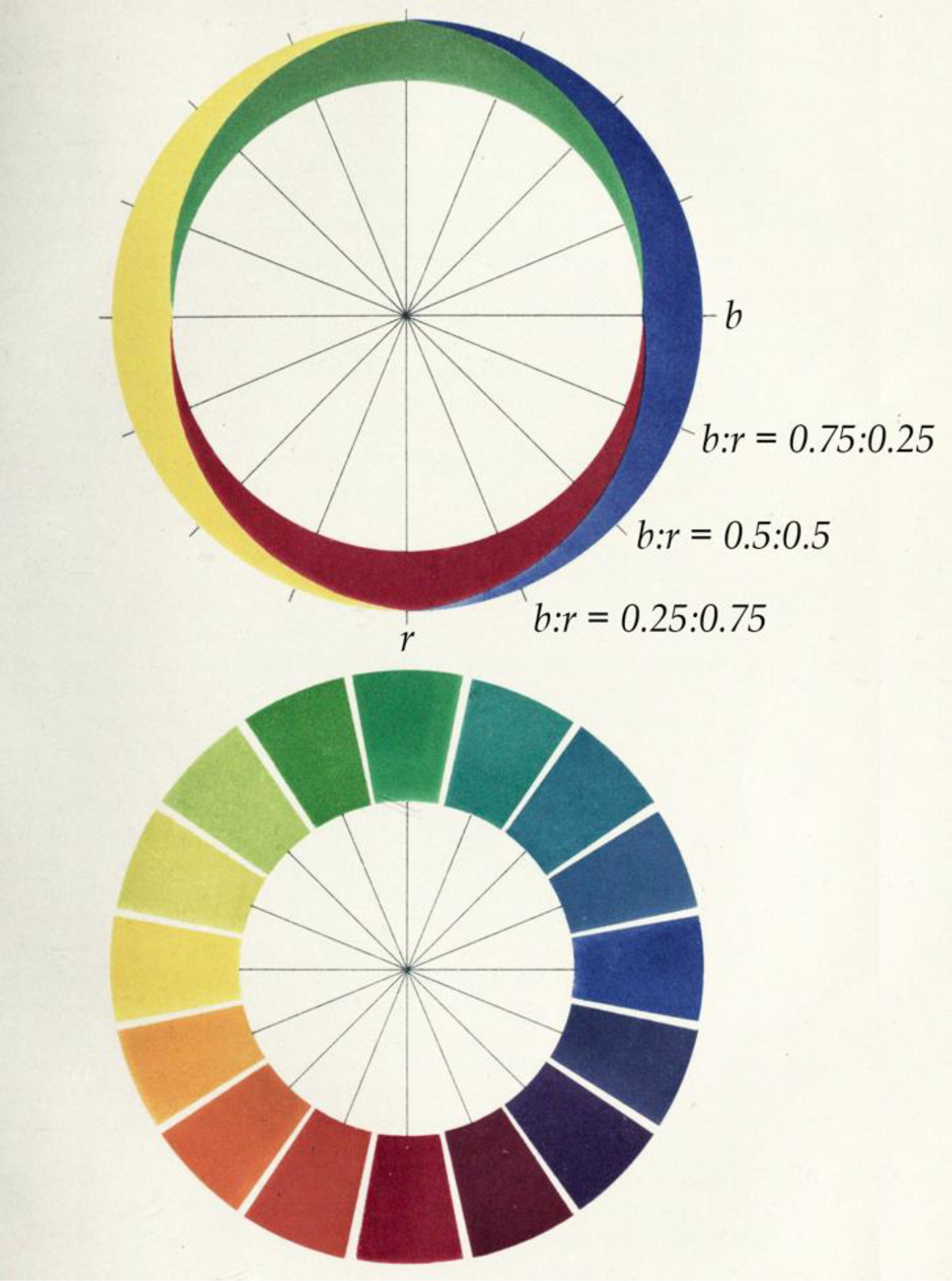
Diagram by Ewald Hering illustrating Opponent-Colors Theory. According to Hering, “the six basic sensations of the visual substance are arranged in three pairs: Black and white, blue and yellow, green and red. Each of these three pairs corresponds to a distinct process of dissimilation and assimilation, such that the visual substance can undergo chemical or metabolic change in three different ways” ([2], §. 42.). In the top panel, “r” is red, “b” is blue, and the ratios indicate the combinations of these components in each color mixture of the bottom panel. So, purple has a ratio of blue to red (b:r) of 0.5:0.5. Hering’s theory boils down to two ideas (1) that the appearance of any color is necessarily and sufficiently described by the extent to which it is reddish-versus-greenish, bluish-versus-yellowish, and blackish-versus-whitish; and (2) that these appearance mechanisms are hardwired in the nervous system.
Shortfalls of the theory notwithstanding [4–11], it remains an accepted view. The expert’s handbook defines hue as that “attribute of visual perception according to which an area appears to be similar to one of the colors, red, yellow, green, and blue” [12]. Commercial color systems are defined by Hering’s opponent-color pairs [13,14]. And textbooks state that color appearance is arranged around “four basic [unique] colors in two opponent pairs: red versus green, and blue versus yellow” [15]. On the other side of the linking proposition, contemporary neurophysiological studies are often guided by Hering’s theory [16–19]. Here we review the evidence on each side of the linking proposition and argue that the theory does not need modification, it needs to be discarded. We then sketch out an alternative.
Hering’s Opponent-Colors Theory
Two intuitions dating to pre-Socratic philosophers are that existence depends on the unity of opposites (love/strife; hot/cold; light/dark, etc.); and that some colors are elementary. The number of elementary colors has never been settled. Empedocles and Aristotle identified two (white and black). Leonardo DaVinci declared that Nature produces eight colors, but he said that “Blue and green are not simple colours … for blue is composed of light and darkness [and]…green is composed of a simple and a mixed colour” [20], pg. 98). Peter Paul Reubens argued for three. Isaac Newton thought there were five and then revised to seven. Johann Wolfgang Goethe recognized six. Hering entered the fray in the 1870s, penning his theory in the same year that his countryman Wilhelm Kühne coined the term “enzyme”. Hering’s theory married the two ancient intuitions and consecrated the union with a mechanistic hypothesis borrowed from the new field of physiological chemistry: there are four chromatic colors, and each derives its special status as the unopposed product of a biochemical process. Hering had no data. Rather, he used introspection to formulate, mathematically, his experience (Figure 1), surmising that each color-opponent experience “corresponds to a distinct process of dissimilation and assimilation” of a “visual substance” ([2], pg. 74). Hering’s hypothesis was compelling, for it not only invoked Johannes Müller’s idea of “specific nerve energies” but also gave scientific muscle to Goethe’s poetic descriptions of color afterimages, which imply that opponency of some form must underwrite color appearance.
In the 20th century, Hering’s theory came to mean “the ‘coding’ of color experience at the neural level” by “the simplest linkage between receptor events and neural events” ([21] pg. 128–136). In the 1950s, proof seemed in hand. Hue-cancelation experiments were taken as support for the first proposition [21]. And neurophysiological recordings in macaque monkeys, obtained at the same time and institution [22], were considered evidence of the second proposition. In Horace Barlow’s words, “a startling confirmation of Hering’s long-standing hypothesis about the reciprocal organization of colour systems” [23] (neural excitation/inhibition substituted for biochemical assimilation/dissimilation). The combination of behavioral and neurophysiological data “catapulted opponent-colors theory from a special-purpose model, known only to color specialists, to a central idea in vision science” ([24], pg. 319).
The historical importance of Hering’s theory is hard to overstate. It signaled a shift in the search for causal explanations of color from the physical (spectrum) to the physiological (eye/brain), [25,26]. One long-recognized problem, though, is that the physiology does not clearly line up with Hering’s opponent colors. Experts, therefore, think the theory is inaccurate, but they nonetheless think it accounts for something essential. To sort through the muddle, we need to maintain the distinction between trichromacy, Hering’s Opponent Colors (the unique hues), Hering’s theory (the linking proposition), and general concepts of color opponency and cone opponency. Opponent-Colors Theory neither contests nor establishes trichromacy, proven by the color-matching experiments of James Clerk Maxwell [27] and implemented by the three classes of cones [28]. Hering’s theory is also independent of the idea that color depends on some form of opponency. As Maxwell pointed out, the brain must compare photoreceptor signals, by subtraction or ratio, to achieve color appearance. Proof of color opponency was established decades before Hering, with the discovery of complementary-color pairs and color afterimages [29,30]; color opponency is implemented by retinal cone-opponent neurons [22]. Trichromacy and complementarity organize colorimetric space [31], and Hering’s theory can be discarded without threatening these well-established principles.
Lack of Neurophysiological Evidence for Opponent-Colors Theory
Neurons in the lateral geniculate nucleus (LGN) of macaque monkeys, a model of the human, show overt spectral opponency: their firing rates increase or decrease depending on stimulus wavelength [22]. These cells were christened “red excitatory and green inhibitory” or “blue excitatory and yellow inhibitory” to endorse the view that they are the substrate for Hering’s theory [32]. But despite the nomenclature, the colors that maximally modulate LGN cells are not Hering’s opponent colors (Figure 2b) [33]. For example, “blue-yellow” cells respond best to lavender and lime [34], colors that strongly modulate S cones. Neurophysiologists continue to discover varieties of cone-opponent neurons, often describing them with Hering’s opponent-color terms [35,36], a “quirk of nomenclature” [9] that evidences Hering’s grip.
Figure 2. Color encoding and neural representation.
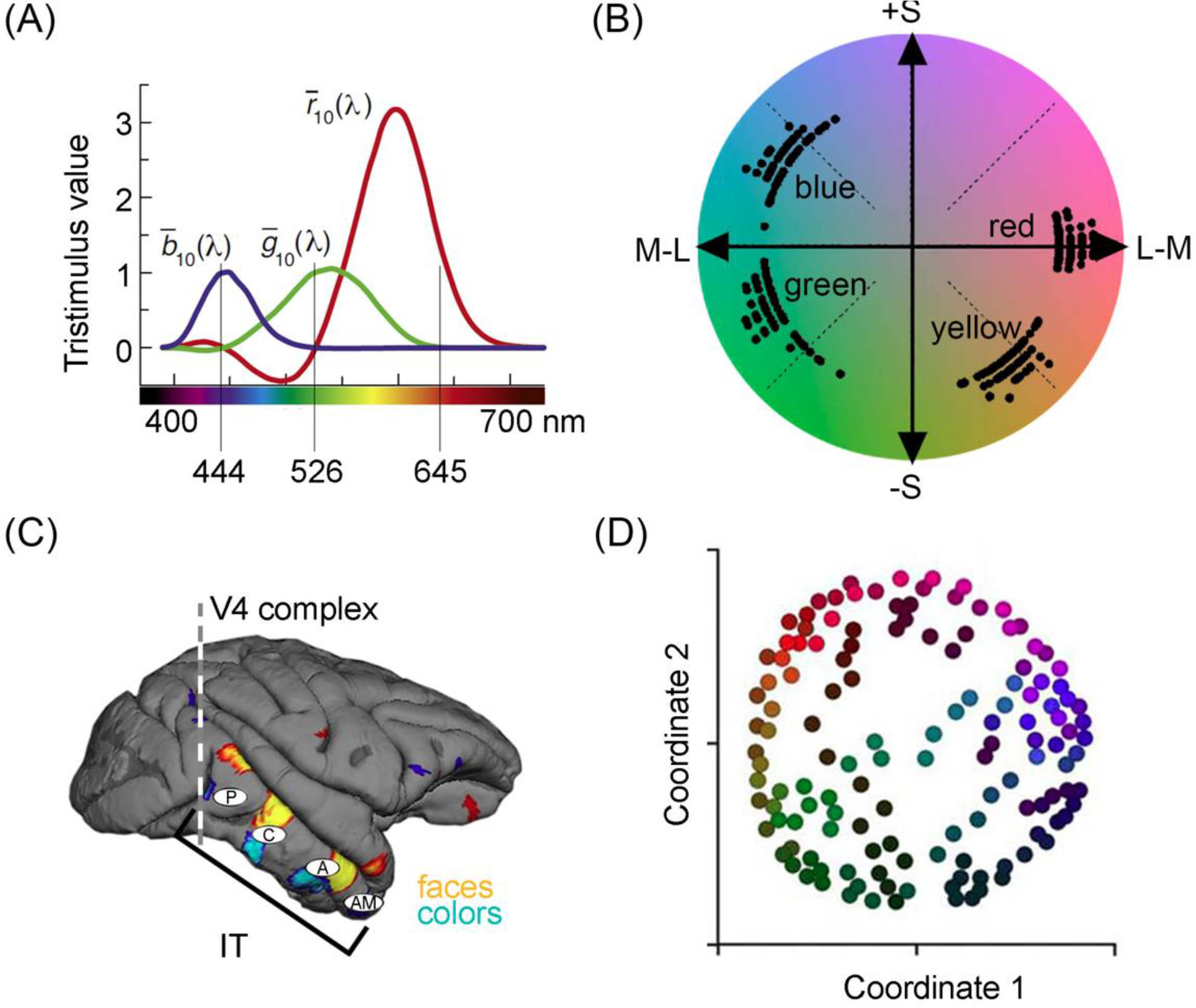
(A). Color matching functions [28]. The y-axis shows the value of each of three primary lights (444nm, 526nm, 645nm) required to match each monochromatic test light on the x-axis. To match some test lights, the primary must be added to the test not to the other primaries, indicated by negative numbers. Color matching data provide evidence of the essential trichromacy of human color vision. (B) Distribution of unique hue settings for 51 observers projected onto a cone-opponent color space [33]. The axes isolate the two cone-opponent mechanisms of the retina; colors along the x-axis vary only in their L and M modulation; colors along the y-axis vary only in modulation of the S cone. (C). Lateral view of the macaque brain showing functional domains biased for colors and faces, identified with fMRI. The vertical line shows the plane of section of the V4 complex. The white ovals indicate four stages in inferior temporal cortex defined by functional and anatomical data (P, posterior; C, central; A, anterior; AM anterior-medial) [55,59]. The existence of color-biased domains in inferior temporal cortex implies that color depends on high-level perceptual and cognitive operations. (D). Geometry of the neural representation of color for neurons within the color-responsive subcompartments of the V4 Complex, calculated by multidimensional scaling [57]. Stimuli are plotted by the two-dimensional embedding determined by the responses of 300 cells.
Can we keep Hering’s theory afloat by dismissing as trivial the mismatch in color tuning of cone-opponent cells and Hering’s opponent colors? No, we cannot. Hering’s theory was important precisely because it directly linked color appearance with physiological mechanisms, so the mismatch is fatal to the theory. In fact, contrary to Hering’s theory, the visual system seems set up to overwrite any privilege in the perception of colors associated with cone-opponent mechanisms, for there are few supra-threshold color-appearance phenomena distinguished by the colors that best modulate the mechanisms.
Perhaps Hering’s theory finds its implementation in V1, the target of the LGN? Many studies have measured V1 responses, and many mysteries remain. But one thing is clear: V1 responses do not line up with Hering’s opponent colors [37–43], not even for the cells with overt chromatic opponency [44]. Yet V1 responses, like retinal cone-opponent responses, are still often characterized using Hering’s theory [19].
Is Hering’s theory implemented downstream of V1? Neurons that respond to color are found in many brain regions, including V2 [45–48], the V4 Complex [49–52], Inferior Temporal cortex (IT) [53–55], and frontal cortex [56] (Figure 2c). Color-responsive cells throughout these regions typically do not show overt color opponency, but perhaps they serve Hering’s theory through a bias for unique hues? Here, again, the answer is “no”. We initially thought that color-responsive cells in V4 represent unique hues [16], but this conclusion reflected a confound of saturation and hue [57]. When unconfounded, the population shows a relatively uniform representation of colors (Figure 2d). Meanwhile, Inferior Temporal cortex, the culmination of object vision, reflects the color statistics of objects, which are also not aligned with unique hues [58]. Only one study has found neural evidence that unique hues are privileged [17]. That evidence reflects activity far beyond perceptual encoding and is therefore irrelevant to Hering’s theory.
Taken together, the neurophysiology shows that the biological side of Hering’s theory is wrong. Color engages surprisingly vast cortical resources [59], which implies that color appearance is not accomplished with so simple a mechanism as Hering imagined. So, what about the other side of Hering’s linking proposition?
Hering’s Original Argument
Hering put forward two arguments. First, that mixtures of his opponent colors (“reddish green”) are inconceivable. This may be true, but it is not compelling. There are an infinite number of inconceivable mixtures, including mixtures of all complementary pairs, e.g., “greenish magenta”, “cyanish pink”, “orangish cobalt”, “indigoish yellow”. Second, Hering argued that unique hues are unique insofar as they describe all colors and cannot themselves be described. Yet many colors are arguably not adequately described as mixtures of unique hues. Is the saturated peel of an orange really “reddish yellow”? Or is orange “unique”? What about purple?
Early debates went in circles struggling to reconcile Opponent-Colors Theory with Maxwell’s trichromacy. A way forward was provided by “zone theories” [60,61], which implement Hering’s opponency immediately downstream of the cones. The approach required a bit of math to relate the cones’ wavelength sensitivity with Hering’s “valence” curves (Box 1, Figure I). Erwin Schrödinger (the physicist with the imaginary cat) conjured the math [62], relying on Hering’s logic that all colors can be described by linear combinations of unique hues (Figure 1). The synthesis yielded many testable predictions. Hering’s theory has failed almost all of them (Box 1). So, is there any behavioral evidence for the theory?
Box 1. Opponent-Colors Theory fails many psychophysical tests.
Objective tests of Opponent-Colors Theory became possible when the theory was formulated as a hypothesis about how cone signals are transmitted to perception. The formal exposition spells out the mathematical transformation of cone responses to opponent-color pairs [132] (Figure I); baked into the math is the linearity of Opponent-Colors Theory implied by the description of color appearances as simple (mathematical) combinations of red-vs-green, yellow-vs-blue, and black-vs-white (see Figure 1). Many psychophysical experiments have tested whether judgments of the redness-versus-greenness, blueness-versus-yellowness, and blackness-versus-whiteness of colors is predicted by the linear readout of cone (or cone-opponent) responses. One prediction is that in any color space, the line connecting unique red and unique green, and the line connecting unique blue and unique yellow, should intersect at the achromatic point in the space. This prediction fails: neither of these lines pass through the achromatic point; their intersection is yellowish and the line connecting blue and yellow passes through pink, not white [27]. Considerable work has confirmed the nonlinear relationship of cone responses to color appearance [133–136]. Moreover, the extent to which a light partakes of a unique hue sensation varies with the energy level of the light (the Bezold-Brücke phenomenon; [137]) and with its saturation (the Abney effect; [134]), which further confirms that the Hering opponent mechanisms do not correspond to linear combinations of the cones. In addition, the color appearance associated with an increase in S-cone activity can be blue or yellow depending on the activity of the L an M cones, which would imply that blue-yellow judgements are not only nonlinear but also nonmonotonic [136,138]. In other psychophysical tests of the theory, Mollon and Cavonius measured wavelength discriminations around unique yellow following low-intensity adaptation to monochromatic lights. They found that discriminations of wavelengths around unique yellow were different following adaptation short wavelength (blue) light compared to middle wavelength (yellow) light, which violates Opponent-Colors Theory since discriminations of unique yellow should only be impacted when adapted to lights that appear reddish or greenish [139]. Finally, psychophysical studies have tested the predicted number of color-opponent mechanisms and the independence of the color opponent and luminance opponent mechanisms; these studies have found more color channels than predicted by Hering’s theory [140–142] and interactions among the channels that violate Hering’s theory [143,144].
Box 1 Figure. Hering’s valence curves (left) and Hurvich and Jameson’s hue-cancelation curves (right).
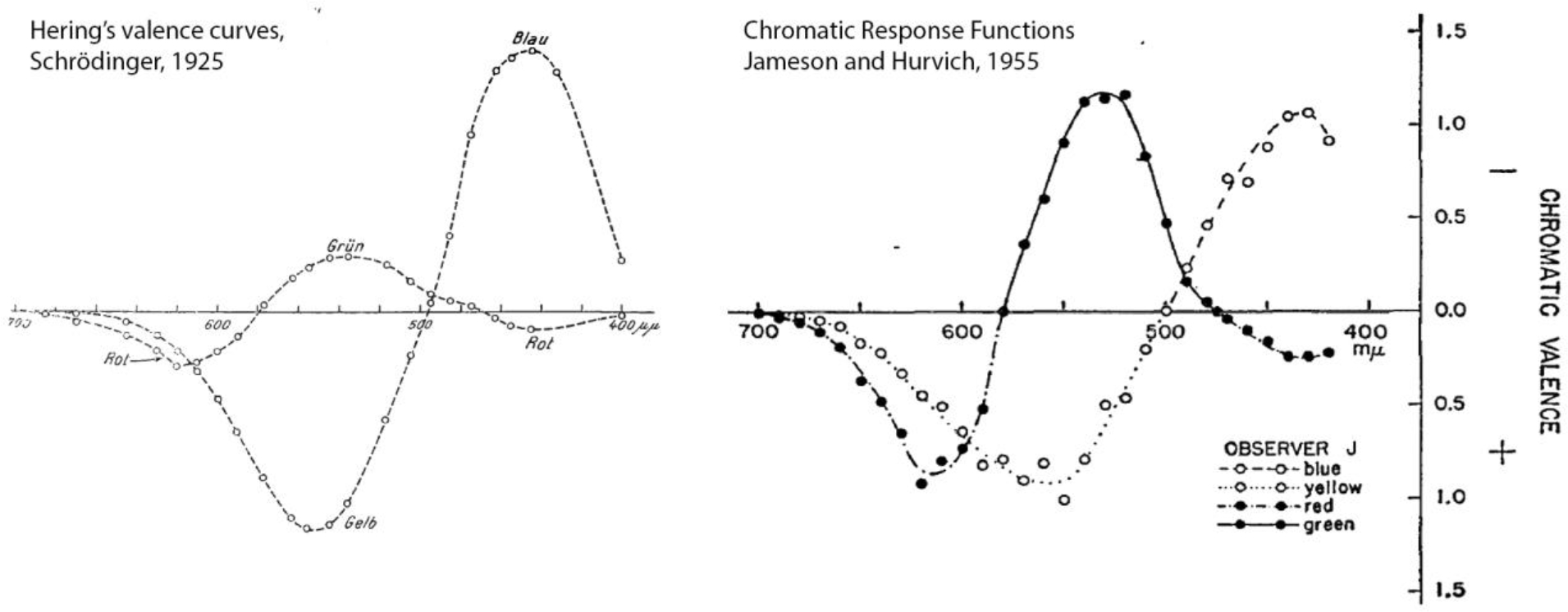
The y-axis scaling is arbitrary. Both curves plot the amount of redness, greenness, blueness, and yellowness associated with each wavelength, and show the same pattern of results (note the location of the curve crossings; grün, green; rot, red; blau, blue; gelb, yellow). The valence curves were derived on introspection; the hue-cancelation curves were based on hue-cancelation experiments.
Hue Cancelation
Classic hue-cancelation experiments remain the primary evidence. The data are quantitative, but the evidence they provide turns out to be no stronger than that offered by Hering’s intuition [5]. Indeed, hue-cancelation curves are identical to Hering’s valence curves, which were obtained without running any experiments at all (Box 1, Figure I). The difficulty is that both sets of curves beg the question they are ostensibly trying to answer [63]. They are what you get if you privilege red, green, blue, and yellow, not a test of the privilege of these colors. They test sufficiency not necessity.
If unique hues are truly privileged, then it should be unimaginable for participants to rate the proportion of teal, purple, orange, and lime in test colors that are uniquely “red”, “yellow”, “green”, and “blue”. Philosophers assumed this was unimaginable [64], but surprisingly participants are perfectly capable of seeing mixtures of intermediate colors within unique hues (Figure 3a) [63]. Other work confirms that the best criteria for demarcating unique hues also demarcate secondary colors [65]. The formal distinction between unique and secondary colors has fallen away.
Figure 3. Lime, purple, orange and teal can be taken as unique hues.
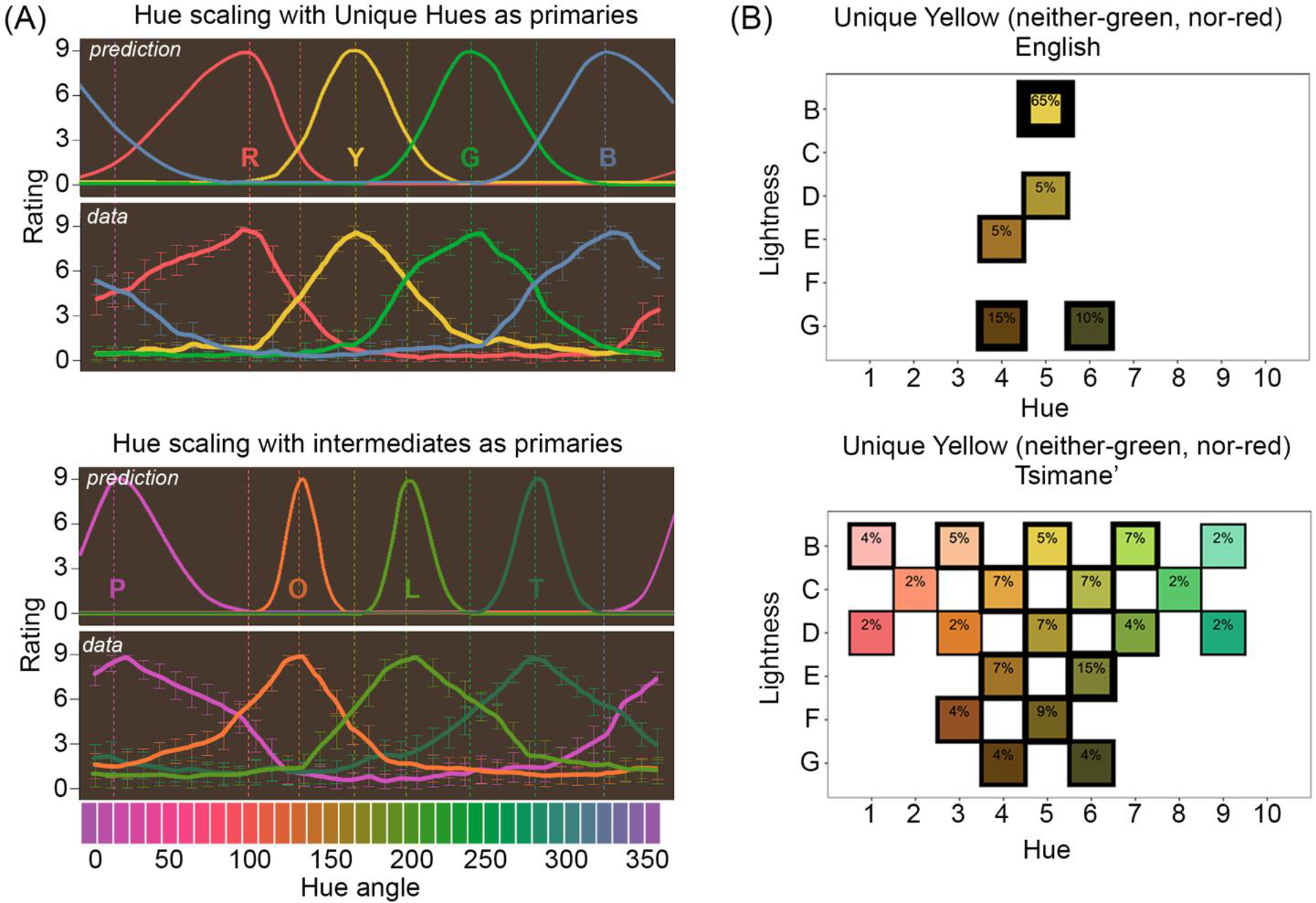
(A). Hue scaling in which participants rated the proportion of unique hues (top) or intermediate colors (bottom) for a complete set of colors; each panel shows predictions from Opponent-Colors Theory and the data, adapted from [63]. The data in the top panel are consistent with the theory and the classic hue-cancellation experiments; the data in the bottom panel violates the predictions because participants observed unique hues as composed of proportions of intermediate colors. (B) Distribution of color chips selected by English speakers (top) or mono-lingual Tsimane’ speakers (bottom) tasked with picking the color chip that is neither reddish nor greenish, adapted from [131]. The experiment focused on yellow, the most consistent unique hue in classic studies, and it used a paradigm that is thought to be effortless. Participants in both language groups first identified the best exemplars of their terms for red and blue/green. Answers are comparable across groups, showing that the Tsimane’ understand the task instructions. The results support the conclusion that the Tsimane’ do not have an innate sense of unique yellow.
To reject Hering’s theory one might ask for studies showing that participants can adjust the proportion of lime to neutralize the appearance of purple in a test. If so, is the result unique orange? This would be analogous to the case where the green needed to neutralize the red in an orange test is a probe of unique yellow. But such measurements are unnecessary to reject Hering’s theory, given that people’s “unique” settings change depending on the prompt. Settings for unique yellow are different if asked to pick a color that is “neither greenish nor reddish”, “neither tealish nor reddish”, or “neither greenish nor purplish” [63]. Such task dependence is incompatible with Hering’s hardwired encoding.
Hue Cancelation in Non-Western Cultures
The generalizability of Hering’s theory has been tested in other cultures. The Tsimane’ of the Amazon jungle have a word corresponding to “red” but no consistent term for “yellow”. “Green” and “blue” are represented together. One might think that the lack of words for unique hues would speak against Opponent-Colors Theory, but the theory pertains to color encoding and appearance, not how colors are discussed. Color-encoding mechanisms are likely the same in all people with normal color-vision genetics, are functional without industrialization [66], and are similar for trichromatic non-human primates (who have no language) [67,68]. When asked to pick the color that is neither reddish nor greenish in an array spanning reds to greens, the Tsimane’ do not pick focal yellow (or brown) as predicted by Hering’s theory. Instead, they show tremendous variability (Figure 3b).
In related work, Somali speakers were asked to identify yellow samples that contain no red or green [11]. The Somali behaved similarly to English speakers, seemingly consistent with Hering’s theory. But in another experiment, participants were asked to name the unique hues apparent in colors across the array. The results were very different for English versus Somali speakers, violating Hering’s theory and supporting the idea that hue decomposition is lexical [69].
Perceptual properties of colors
Opponent-Colors Theory states that the unique hues are innate, which might suggest that they are consistent across observers, as Hering assumed. But there is tremendous variability in unique hue selections [33] (Figure 2b). Moreover, the variations across hues are uncorrelated within observers, suggesting the hues are not encoded jointly as stipulated by Hering’s theory [70].
Many other perceptual results violate Hering’s theory. The unique hues can be influenced by changes in environmental color statistics [71] and transient use of colored glasses [72]. Unique hues are not selected with lower variability than intermediate hues [73,74]; they do not appear more colorful [75]; they are insufficient to describe all colors [76]; they are not especially salient [77,78]; they are not necessarily exclusive [79]; they are not readily explained by cone ratios [80], or post-receptoral encoding [74], or looking behavior in infants [81]; and at least one unique hue (green) changes throughout life [82]. Moreover, the unique hues vary depending on methodology [83], they are not predicted by afterimages [84], and they are not complementary (although Hering thought they were)[9]. The afterimage (and complement) of red is bluish-green (not green).
Another way to test Hering’s theory is by asking how trichromatic non-human primates categorize colors. Three studies have attempted this. The first showed that macaques bin colors into “red”, “green”, “blue”, and “yellow” as humans do [85]. But the categories were predefined, so the experiment suffers the same flaw as classic hue-cancellation studies. The second study tested for a category boundary between green and blue. One was found in humans but not monkeys [86]. The third study used a data-driven approach and found that macaques have two color categories, neither of which align with unique hues (they align with “warm” and “cool”) [87].
Patterns of Color Naming
Hering sought to quell opposition to his theory by invoking language, asserting that “language has simple names for [the unique hues] that are not borrowed from colored natural bodies” [2](pg. 109). But it is now recognized that language is not a read-out of perceptual processes, and even unique-hue names likely derive from diagnostically colored stuff (“red” is related to words for blood). In addition, one cannot always map color terms across languages, probably because color concepts vary across cultures [88]. Failure to recognize this fact led William Gladstone to declare that the ancient Greeks were blue-blind because he found no Greek term mapping onto English “blue”. In fact, the ancient Greeks had several words to describe blues in various contexts [89]. The idea of “primitive” cultures, and that they are color deficient, is now recognized as racist [90]. Yet the notion that color-naming patterns provide evidence regarding innate mechanisms took root, popularized by Berlin and Kay who proposed that all languages “evolve” on a predictable trajectory dictated by Hering’s theory [91].
As with classic hue-cancelation studies, Berlin and Kay’s experiment may have begged the conclusion it was trying to test, for the task required that participants use specified “basic color terms” [92]. Two studies of non-industrialized cultures obtained data without these task constraints. One study discovered that the Hazda people in Africa label orange chips more consistently than blue chips, which violates Hering’s theory since orange is not unique [93]. The other study asked the Tsimane’ of South America to name 80 color chips; some participants were required to use basic-color terms, while others were simply asked to “describe the color chip to someone in your language” [94]. The data, analyzed in an information-theoretic framework, show that communication efficiency of color is surprisingly similar regardless of the instructions, so the analysis can be applied to the 110 languages in the World Color Survey [95]. Across languages, communication is less efficient for blue and green than for many colors including pink, orange, and beige, again violating Hering’s theory. The color-naming studies imply either that color naming does not tell us much about how color is encoded by the brain, or that Opponent-Colors Theory is not how color is encoded, or both.
Taken together, the psychological research shows that colors can be described without appeal to Opponent-Colors Theory, and there is nothing perceptually special about the unique hues. If we follow the data, Hering’s theory must be rejected.
Utility-Based Coding
It’s one thing to show a theory is invalid. It’s another thing to toss it out, for doing so requires a replacement [96]. We think that a replacement is emerging, which we call Utility-Based Coding. Germs of this idea can be found in the literature [10,59,94,97–100].
The first part of Utility-Based Coding interprets retinal physiology not as evidence of appearance mechanisms but as a solution to constraints imposed by a camera-type eye, a solution that provides an optimal representation of the retinal image [101]. Because the retina is a fixed distance from the lens, only some wavelengths can be in focus at a time (short wavelengths bend more than long). This chromatic aberration poses a problem for a system under selective pressure to extract both chromatic and spatial information [102]. The spectral sensitivity of L and M cones vastly overlap. This means that light will be focused to the same degree for both classes of cone, affording good spatial resolution (S cones are sparse). Post-receptoral neurons, meanwhile, use cone opponency to compare cone responses. This comparison, together with normalization mechanisms, allows the system to amplify slight differences in cone responses to capture chromatic information in the retinal image. By this argument, cone opponency has little to do with appearance. Instead, it is a way of achieving both color and spatial vision from the same cone mosaic.
The second part of Utility-Based Coding invokes cone-opponent mechanisms as a substrate for subsequent stages of processing that compute behaviorally relevant information, including, ultimately, appearance. Thus Utility-Based Coding makes fundamentally different predictions from Hering’s theory about the relationship between encoding mechanisms and appearance: encoding mechanisms should not leave an obvious trace in perception, for color appearances are an output of the brain that tell us about the world not constraints of the input. Contrary to Hering’s theory, the colors corresponding to the spectral tuning functions of the cones or the poles of the cone-opponent mechanisms are not expected to be salient—and they are not [103–105]. By this logic, the “quirk of nomenclature” is not innocuous: the use of color names for descriptions of cones, cone-opponent mechanisms, and related color spaces steers us into a dead end about the mechanisms of color appearance.
Utility-Based Coding is supported by neurophysiological data that implicates extensive cortical resources in color processing. Neural populations from V1 to V4 are optimized to extract spectral information using a multitude of narrowly tuned mechanisms [47,52,57]. The observation that red, green, blue, and yellow can be achieved from cone spectral tuning [106] is not inconsistent with Utility Based Coding; indeed, all colors should be extractable from the cone functions. And beyond V4, there is as much “high-level” visual cortex engaged in color perception as in face recognition [55]. The function of these color-biased regions remains to be determined, but they provide a substrate for the sophisticated computations that use color in the service of behaviors such as categorization [107–109], foraging [110,111], and ultimately appearance.
Utility-Based Coding liberates neurophysiologists from a preoccupation of trying to find a substrate for Opponent-Colors Theory. Studies of neural mechanisms can focus on understanding how maximally useful representations of color are wired up [112], how the color-encoding mechanisms adapt to changing contexts and shifting behavioral demands [100], how they arise in development [81], and how other surface-appearance properties such as gloss, luster, and transparency relate to color and are represented [113,114] (see Outstanding questions). As the field advances, neural network technology may be instructive regarding how color categories emerge without hardwiring. For example, networks trained on natural scenes—photographs taken by humans—form color categories that are not so different from those generated by people [115].
Outstanding Questions.
What limits the brain’s capacity to deploy new cognitive mapping strategies for color?
What universal behavioral needs underwrite the common patterns in color naming seen across the world’s languages?
At what point in human development do color concepts and cognitive maps that organize them develop?
How similar is color categorization behavior in trichromatic non-human primates and humans?
Hues can be described with two independent chromatic dimensions, but to what extent does the brain or behavior constrain which dimensions can be used?
What linking proposition governs the relationship between neurophysiology and color perception?
How does the brain integrate across the vast network engaged in color perception to bring about the experience of color?
Which neurons and neural circuits are required to compute color categories and how do these mechanisms enable adaptation to changing contexts and demands?
What computations on the cone responses are performed by the visual circuitry to achieve narrow hue tuning, and to what extent to neurophysiological responses parallel perception under changing viewing conditions?
How can color properties that are not captured by hue, such as glitter and luster, be incorporated into a framework of color appearance, and what are the underlying neural mechanisms?
Behavioral Data as Evidence of Utility-Based Coding
According to Utility-Based Coding, color words (and preferences [116]) reflect the things we want to label, not how we see [93,94,97,99,117–119]. Anthropological work validates this idea. In Papua New Guinea, one Yélî Dnye term for “black” derives from the name of a tree whose nuts are not black until they are roasted [117]. Indeed, all color terms probably reflect behavioral relevance, not limits of discrimination. The term “orange” comes from the fruit, not the other way around. Per Utility-Based Coding, it becomes unsurprising that universal patterns of color naming correspond to the color statistics of objects [58,94], since what we label is what we care about. The lack of consistently demarcated blue and green is then explained by a paucity of cool-colored objects [58]. Within a Utility-Based Coding framework, focal colors are optimal representatives of behaviorally important categories [120]. And color naming is the efficient tradeoff between the complexity and accuracy of the lexicon [99], since color naming is governed by the ideas the speakers need to communicate not how colors are encoded [121]. Variability in color-naming patterns across cultures is then explained by differences in the ideas that different cultures need to communicate [98,119], while similarities in color naming among languages likely represent universal behavioral significance, e.g. daylight, foraging, and social signaling [122,123].
Utility-Based Coding distinguishes between Hering’s Opponent-Colors theory, a linking proposition that is wrong, from Hering’s opponent colors, a cognitive mapping strategy that is evidentially useful. But according to Utility-Based Coding, the navigating strategy is not pre-determined by color-encoding mechanisms—the strategy is sufficient to describe colors but not necessary. Accordingly, Hering’s Opponent Colors provide one of many possible ways of communicating color, just as English is not innate but one of many languages. But like a language, it derives its utility when widely used and experienced as reflexive, which can lead to a category error about its cause. Utility-Based Coding therefore seeks answers from behavior and cognitive theory (including lexical contributions), rather than neurophysiology, about why cultures settle on the color-naming and color-mapping strategies they use [69]. One prediction is that opponent colors become useful when called to mind, either explicitly [124] or, we would argue, implicitly through task structure and Western education [125]. Utility-Based Coding also makes imaginable mapping strategies besides Hering’s opponent-colors [63].
Utility-Based Coding does not deny that some colors might take on “elementary” or special status. But it stipulates that if some colors are special, their specialness should be attributed to meaningful structure in the world, structure that has utility [126]. For example, unique yellow and blue might be understood not in terms of constraints imposed by encoding mechanisms but in terms of sun and sky—environmental features of universal relevance [127]. Capturing meaningful structure must engage neural processes far beyond encoding because the relevant features might vary depending on context and behavioral goals. Utility-Based Coding, therefore, places cultural developments such as industrialization in a new light: industrialization might promote color utility [94], but industrialization might itself be driven by the potential utility of color [128]. From the perspective of Utility-Based Coding, the surprising diversity of color-adaptation mechanisms [8,100] are evidence of flexible sophistication, not limitations of the visual system.
Utility-Based Coding is a long way from formally connecting physiology and perception. But recent studies suggest a way forward. One study developed a compression theory of color naming [99]. This theory is the basis for a quantitative model integrating the shared psychophysics of color perception with language-specific communicative needs for colors [119]. The model is applied to an object-color statistics database [58,94], and experimental tests that could invalidate the model are described. Another study systematically examined contributions to color naming and suggests that capacity constraints and linguistic usage play a more substantial role than the visual environment [121]. These studies can be complemented by statistical analyses [129,130]. In these studies, Opponent-Colors Theory is, usefully, not a premise. Instead, the studies provide a quantitative data-driven framework for relating behavior, theory, and physiology.
Concluding Remarks
Color has long been useful as a tool to understand mechanisms that bring about perception and cognition. In modern times, Hering’s theory has been instrumental, for it focused attention on physiological processes as causes of color appearance. But after 150 years of testing, we can conclude that the theory is wrong. Introspection might have provided an entry point for understanding color appearance, but it can be deeply misleading about causes. To take a familiar example: Aristotle thought the earth was the center of the universe because experience tells us the sun rises and sets. The experience of color is similarly a poor guide, for it can lead to a gross underestimate not only of the sophistication of color behavior but also of the neural resources required. It’s time to let go of Opponent-Colors Theory as an idea for understanding how the brain turns light into Spanish moss and raspberry blush. A new framework, Utility-Based Coding, has emerged as an alternative for understanding both perceptual and biological mechanisms of color, and it may prove useful in uncovering the way these two domains of understanding are linked.
Highlights.
The essay reviews the psychological and physiological evidence for Opponent-Colors Theory and concludes the theory is wrong.
Behavioral work shows that the theory’s three appearance mechanisms (red-versus-green; blue-versus-yellow; black-versus-white) are not necessary to describe color.
Physiological work shows that neural color-encoding mechanisms are not characterized by tuning to the opponent colors of the theory. Contrary to Opponent-Colors Theory, the color-encoding mechanisms of the brain are not evident in perception.
A new Utility-Based Coding framework is described, by which color depends on many interacting brain areas. Encoding mechanisms efficiently capture and transmit to the cortex as much chromatic information as possible given photoreceptor tuning, while appearance reflects adaptable neural operations that optimally support behavior under changing contexts and objectives.
Acknowledgements.
For engaging discussions, we thank David Brainard, Angela Brown, Rhea Eskew, Karl Gegenfurtner, Anya Hurlbert, Delwin Lindsey, Steven Shevell, Michael Webster, Christoph Witzel, Qasim Zaidi, and Noga Zaslavsky; and for suggesting the term “Utility-Based Coding”, we thank Noga Zaslavsky. We also are indebted to five reviewers and a patient editor for many rounds of review that we hope have sharpened the arguments presented here. This work was supported by the Intramural Research Program of the National Eye Institute (to BRC). The essay was written by BRC to reflect ideas of all the authors. William Rushton wrote that “The general emotional attitude in colour vision is similar to that often seen in politics and religion—a strong partisanship towards particular views, and crusading tendencies”. But like him, we assert that our “own views, of course, are both moderate and reasonable—but so say all extremists. You must be the judge”.
Glossary
- Color afterimage
The color seen after adapting to a colored display. The afterimage of red is bluish green.
- Color-matching experiment
a test projected in one half of an aperture is matched by adjusting the intensities of three primary lights projecting to the other half of the aperture. There is a unique solution for all tests. The three primaries can have any spectral composition, so long as each cannot be matched by a combination of the other two. Results with one set of primaries can be linearly transformed to predict results obtained with other primaries. The intensity values of the three primaries needed to match each wavelength across the visible spectrum comprise color matching functions.
- Color opponency
A perceptual phenomenon whereby two colors are mutually exclusive. Hering’s Opponent Colors Theory specifies three pairs of opponent colors as necessary and sufficient for describing the appearance of all colors: red-vs-green; blue-vs-yellow; and black-vs-white.
- Complementary color
the light that when added to a test renders the test colorless. Every color has a complementary color, roughly opposite in the color circle, so there are almost an infinite number of complementary colors. The existence of complementary-color pairs is evidence that the visual system encodes colors with some form of opponency.
- Cone
A type of retinal photoreceptor. There are three types of cones, called L, M, and S, for their peak wavelength sensitivity to long, middle, and short wavelengths. The spectral tuning of L, M, and S cone cells overlap extensivity; the L and the M cone cells have about 98% overlap.
- Cone opponency
A physiological operation implemented by retinal bipolar cells that recovers spectral information by pitting excitation by one cone class against inhibition by another cone class.
- Hue-Cancelation Experiment
an observer is asked to adjust a red, green, blue, or yellow light to cancel the appearance of green, red, yellow, or blue in a test. For an orange test, a participant would add enough green to cancel the reddish quality; the result defines unique yellow. These experiments were taken as evidence for Hering’s Opponent-Colors Theory, but a theory that red, green, blue, and yellow are privileged cannot be tested with an experiment where these are fixed parameters.
- Linking proposition
A hypothesis that aims to explain a psychological phenomenon by a direct link to physiological processes; also known as a linking hypothesis.
- Trichromacy
the idea that color is coded by three variables.
- Unique hues:
the six Urfarben (“source colors”) defined by Hering’s Opponent-Colors Theory, deemed psychologically pure: red, green, blue, yellow, black, white. The Theory states that each unique hue cannot be described by any other term, and all colors can be described by combinations of unique hues.
Resources
PANTONE® USA | Pantone Color of the Year 2023 / Introduction
KRYLON®|2023 Color of the Year
BenjaminMoore®|Color of the Year and Color Trends 2023
MINIWAX®|2023 Color of the Year
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brainard DH and Hurlbert AC (2015) Colour Vision: Understanding #TheDress. Curr Biol 25, R551–554. 10.1016/j.cub.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 2. Hering E (1878) Zur Lehre vom Lichtsinne Carl Gerold’s Sohn, pp141. This reference is the revised and collated selection of papers originally published between 1872–1875. A posthumous summary was published in 1920 and translated by Hurvich and Jameson as Outlines of a theory of the light sense (Harvard University Press, 1964).
- 3.Teller DY (1984) Linking propositions. Vision Research 24, 1233–1246 [DOI] [PubMed] [Google Scholar]
- 4.Abramov I and Gordon J (1994) Color appearance: on seeing red--or yellow, or green, or blue. Annu Rev Psychol 45, 451–485 [DOI] [PubMed] [Google Scholar]
- 5.Mollon JD and Jordan G (1997) On the nature of unique hues. In John Dalton’s Colour Vision Legacy (Dickenson C et al. , eds), pp. 381–392, Taylor & Francis [Google Scholar]
- 6.Jameson K and R.G., D.A. (1997) It’s not really red, green, yellow, blue: An inquiry into perceptual color space. In Color Categories in Thought and Language (HardinandMaffi, ed), pp. 295–319, Univ of Cambridge Press [Google Scholar]
- 7.Gegenfurtner KR and Kiper DC (2003) Color vision. Annu Rev Neurosci 26, 181–206 [DOI] [PubMed] [Google Scholar]
- 8.Stockman A and Brainard DH (2010) Color vision mechanisms. In the OSA Handbook of Optics (3rd edition, Bass M, ed). McGraw-Hill, New York, 11.11–11.104 [Google Scholar]
- 9.Shevell SK and Martin PR (2017) Color opponency: tutorial. J Opt Soc Am A 34, 1099–1108. 10.1364/Josaa.34.001099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witzel C and Gegenfurtner KR (2018) Color Perception: Objects, Constancy, and Categories. Annual Review of Vision Science, Vol 4 4, 475–499. 10.1146/annurev-vision-091517-034231 [DOI] [PubMed] [Google Scholar]
- 11.Lindsey DT et al. (2020) Testing the Cross-Cultural Generality of Hering’s Theory of Color Appearance. Cogn Sci 44, e12907. 10.1111/cogs.12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairchild MD (2015) Chapter 1, Color Models and systems, in Handbook of Color Psychology
- 13.Brainard DH (2003) Color appearance and color difference specification. In The Science of Color (Shevell SK, ed), pp. 191–216, Elsevier [Google Scholar]
- 14.Kuehni RG et al. (2010) Perceptual prominence of Hering’s chromatic primaries. J Opt Soc Am A Opt Image Sci Vis 27, 159–165 [DOI] [PubMed] [Google Scholar]
- 15.Wolfe JM et al. (2022) The Perception of Colour. In Sensation and Perception, International Edition (Sixth Edition), pp. 141, Oxford: Sinauer Associates [Google Scholar]
- 16.Stoughton CM and Conway BR (2008) Neural basis for unique hues. Current Biology 18, R698–R699 [DOI] [PubMed] [Google Scholar]
- 17.Forder L et al. (2017) A neural signature of the unique hues. Sci Rep 7, 42364. 10.1038/srep42364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt BP et al. (2018) Sensations from a single M-cone depend on the activity of surrounding S-cones. Scientific Reports 8, 8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li P et al. (2022) Cone opponent functional domains in primary visual cortex combine signals for color appearance mechanisms. Nature communications 13, 6344. 10.1038/s41467-022-34020-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DaVinci L.t.b.J.F.R. (1877) A treatise on painting. London: : George Bell & Sons, [Google Scholar]
- 21.Hurvich LM (1981) Color vision. Sinauer Associates Inc., [Google Scholar]
- 22.De Valois RL, Smith CJ, Kitai ST, Karoly AJ (1958) Response of single cells in monkey lateral geniculate nucleus to monochromatic light. Science 127, 238–239 [DOI] [PubMed] [Google Scholar]
- 23.Barlow HB (1972) Single Units and Sensation: A Neuron Doctrine for Perceptual Psychology? Perception 1, 371–394. 10.1068/p010371 [DOI] [PubMed] [Google Scholar]
- 24.Wandell BA (1995) Foundations of Vision. Sinauer Assoiciates Inc, Sunderland, Massachusetts, [Google Scholar]
- 25.Motokawa K (1949) Retinal processes and their role in color vision. J Neurophysiol 12, 291–303. 10.1152/jn.1949.12.5.291 [DOI] [PubMed] [Google Scholar]
- 26.Svaetichin G, MacNichol EF (1958) Retinal mechanisms for chromatic and achromatic vision. Ann. N.Y. Acad. Sci. 74, 385–404 [DOI] [PubMed] [Google Scholar]
- 27.Maxwell JC (1855) Experiments on Colour, as Perceived by the Eye, with Remarks on Colour-Blindness Transactions of the Royal Society of Edinburgh 21, 275–298 [Google Scholar]
- 28.Stockman A (2019) Cone fundamentals and CIE standards. Current Opinion in Behavioral Sciences 30, 87–93. 10.1016/j.cobeha.2019.06.005 [DOI] [Google Scholar]
- 29.Grassmann HG (1854) On the Theory of Compound Colours. The London, Edinburgh and Dublin philosophical magazine and journal of science 7, 254–264 [Google Scholar]
- 30.Helmholtz H (1855) Ueber die Zusammensetzung von Spectralfarben. Annalen Der Physick und Chemie, Ed. J. C. Poggendorff 94, 1–28 [Google Scholar]
- 31.Brainard DH and Stockman A (2010) Colorimetry. In the OSA Handbook of Optics (3rd edition, Bass M, ed). McGraw-Hill, New York, 10.11–11.56 [Google Scholar]
- 32.De Valois RL et al. (1966) Analysis of response patterns of LGN cells. J Opt Soc Am 56, 966–977. 10.1364/josa.56.000966 [DOI] [PubMed] [Google Scholar]
- 33.Webster MA et al. (2000) Variations in normal color vision. II. Unique hues. J Opt Soc Am A Opt Image Sci Vis 17, 1545–1555 [DOI] [PubMed] [Google Scholar]
- 34.Sun H et al. (2006) Specificity of cone inputs to macaque retinal ganglion cells. J Neurophysiol 95, 837–849. 95/2/837 [pii] 10.1152/jn.00714.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crook JD et al. (2009) Parallel ON and OFF cone bipolar inputs establish spatially coextensive receptive field structure of blue-yellow ganglion cells in primate retina. J Neurosci 29, 8372–8387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson SS et al. (2020) Another Blue-ON ganglion cell in the primate retina. Curr Biol 30, R1409–R1410. 10.1016/j.cub.2020.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conway BR (2001) Spatial structure of cone inputs to color cells in alert macaque primary visual cortex (V-1). Journal of Neuroscience 21, 2768–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wachtler T et al. (2003) Representation of color stimuli in awake macaque primary visual cortex. Neuron 37, 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson EN et al. (2004) Cone inputs in macaque primary visual cortex. J Neurophysiol 91, 2501–2514 [DOI] [PubMed] [Google Scholar]
- 40.Tailby C et al. (2008) Habituation reveals fundamental chromatic mechanisms in striate cortex of macaque. J Neurosci 28, 1131–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garg AK et al. (2019) Color and orientation are jointly coded and spatially organized in primate primary visual cortex. Science 364, 1275–1279. 10.1126/science.aaw5868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horwitz GD (2020) Signals Related to Color in the Early Visual Cortex. Annu Rev Vis Sci 6, 287–311. 10.1146/annurev-vision-121219-081801 [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee S et al. (2021) Chromatic micromaps in primary visual cortex. Nature communications 12, 2315. 10.1038/s41467-021-22488-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lafer-Sousa R et al. (2012) Color tuning in alert macaque V1 assessed with fMRI and single-unit recording shows a bias toward daylight colors. J Opt Soc Am A Opt Image Sci Vis 29, 657–670. 231742 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Hubel DH and Livingstone MS (1987) Segregation of form, color, and stereopsis in primate area 18. J Neurosci 7, 3378–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshioka T et al. (1996) Neuronal mechanisms of color categorization in areas V1, V2 and V4 of macaque monkey visual cortex. Behavioural Brain Research. 76, 51–70 [DOI] [PubMed] [Google Scholar]
- 47.Kiper DC et al. (1997) Chromatic properties of neurons in macaque area V2. Visual Neuroscience 14, 1061–1072 [DOI] [PubMed] [Google Scholar]
- 48.Xiao Y et al. (2003) A spatially organized representation of colour in macaque cortical area V2. Nature 421, 535–539 [DOI] [PubMed] [Google Scholar]
- 49.Zeki SM (1977) Colour coding in the superior temporal sulcus of rhesus monkey visual cortex. Proceedings of the Royal Society of London - Series B: Biological Sciences. 197, 195–223 [DOI] [PubMed] [Google Scholar]
- 50.Conway BR et al. (2007) Specialized color modules in macaque extrastriate cortex. Neuron 56, 560–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conway BR and Tsao DY (2009) Color-tuned neurons are spatially clustered according to color preference within alert macaque posterior inferior temporal cortex. Proceedings of the National Academy of Science (USA) 106, 18034–18039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y et al. (2020) Hierarchical Representation for Chromatic Processing across Macaque V1, V2, and V4. Neuron. 10.1016/j.neuron.2020.07.037 [DOI] [PubMed] [Google Scholar]
- 53.Komatsu H (1998) Mechanisms of central color vision. Curr Opin Neurobiol 8, 503–508 [DOI] [PubMed] [Google Scholar]
- 54.Yasuda M et al. (2010) Color selectivity of neurons in the posterior inferior temporal cortex of the macaque monkey. Cereb Cortex 20, 1630–1646. 10.1093/cercor/bhp227bhp227 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lafer-Sousa R and Conway BR (2013) Parallel, multi-stage processing of colors, faces and shapes in macaque inferior temporal cortex. Nat Neurosci 16, 1870–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haile TM et al. (2019) Visual stimulus-driven functional organization of macaque prefrontal cortex. NeuroImage 188, 427–444. 10.1016/j.neuroimage.2018.11.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bohon KS et al. (2016) Representation of Perceptual Color Space in Macaque Posterior Inferior Temporal Cortex (the V4 Complex). Eneuro 3. 10.1523/ENEURO.0039-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenthal I et al. (2018) Color statistics of objects, and color tuning of object cortex in macaque monkey. J Vis 18, 1. 10.1167/18.11.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conway BR (2018) The Organization and Operation of Inferior Temporal Cortex. Annu Rev Vis Sci 4, 381–402. 10.1146/annurev-vision-091517-034202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ladd-Franklin C (1892) A new theory of light sensation. Proceedings of the International Congress of Experimental Psychology, London, 103–108 [Google Scholar]
- 61.von Kries J (1905) Zonentheorie. In Handbuch der Physiologie des Menschen: Dritter Band, Physiologie der Sinne (Nagel W, Ed.), pp. 269 – 274, Friedrich Vieweg und Sohn [Google Scholar]
- 62.Zaidi Q (1994) On the Relationship of 4-Color Theory to 3-Color Theory - Comment. Color Res Appl 19, 37–40 [Google Scholar]
- 63.Bosten JM and Boehm AE (2014) Empirical evidence for unique hues? J Opt Soc Am A Opt Image Sci Vis 31, A385–393. 10.1364/JOSAA.31.00A385 [DOI] [PubMed] [Google Scholar]
- 64.Broackes J (1997) Could we take lime, purple, orange, and Teal as unique hues? Behavioral and Brain Sciences 20, 183–184 [Google Scholar]
- 65.Mylonas D and Griffin LD (2020) Coherence of achromatic, primary and basic classes of colour categories. Vision Res 175, 14–22. 10.1016/j.visres.2020.06.001 [DOI] [PubMed] [Google Scholar]
- 66.Wnuk E et al. (2022) Color technology is not necessary for rich and efficient color language. Cognition 229. ARTN 105223 10.1016/j.cognition.2022.105223 [DOI] [PubMed] [Google Scholar]
- 67.Gagin G et al. (2014) Color-detection thresholds in rhesus macaque monkeys and humans. J Vis 14, 12. 10.1167/14.8.1212 [pii]14.8.12 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horwitz GD (2015) What studies of macaque monkeys have told us about human color vision. Neuroscience 296, 110–115. 10.1016/j.neuroscience.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindsey DT and Brown AM (2021) Lexical Color Categories. Annu Rev Vis Sci 7, 605–631. 10.1146/annurev-vision-093019-112420 [DOI] [PubMed] [Google Scholar]
- 70.Emery KJ and Webster MA (2019) Individual differences and their implications for color perception. Curr Opin Behav Sci 30, 28–33. 10.1016/j.cobeha.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welbourne LE et al. (2015) Human colour perception changes between seasons. Current Biology 25, R646–R647. 10.1016/j.cub.2015.06.030 [DOI] [PubMed] [Google Scholar]
- 72.Neitz J et al. (2002) Color perception is mediated by a plastic neural mechanism that is adjustable in adults. Neuron 35, 783–792 [DOI] [PubMed] [Google Scholar]
- 73.Bosten JM and Lawrance-Owen AJ (2014) No difference in variability of unique hue selections and binary hue selections. J Opt Soc Am A Opt Image Sci Vis 31, A357–364. 10.1364/JOSAA.31.00A357 [DOI] [PubMed] [Google Scholar]
- 74.Witzel C and Gegenfurtner KR (2018) Are red, yellow, green, and blue perceptual categories? Vision Research 151, 152–163. 10.1016/j.visres.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 75.Witzel C and Franklin A (2014) Do focal colors look particularly “colorful”? J Opt Soc Am A Opt Image Sci Vis 31, A365–374. 10.1364/JOSAA.31.00A365 [DOI] [PubMed] [Google Scholar]
- 76.Witzel C et al. (2019) Red, yellow, green, and blue are not particularly colorful. J Vis 19, 27. 10.1167/19.14.27 [DOI] [PubMed] [Google Scholar]
- 77.Brown AM et al. (2011) Color names, color categories, and color-cued visual search: sometimes, color perception is not categorical. J Vis 11. 10.1167/11.12.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wool LE et al. (2015) Salience of unique hues and implications for color theory. J Vis 15. 10.1167/15.2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Billock VA et al. (2001) Perception of forbidden colors in retinally stabilized equiluminant images: an indication of softwired cortical color opponency? J Opt Soc Am A Opt Image Sci Vis 18, 2398–2403 [DOI] [PubMed] [Google Scholar]
- 80.Brainard DH et al. (2000) Functional consequences of the relative numbers of L and M cones. J Opt Soc Am A Opt Image Sci Vis 17, 607–614 [DOI] [PubMed] [Google Scholar]
- 81.Skelton AE et al. (2017) Biological origins of color categorization. Proc Natl Acad Sci U S A 114, 5545–5550. 10.1073/pnas.1612881114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schefrin BE and Werner JS (1990) Loci of spectral unique hues throughout the life span. J Opt Soc Am A Opt Image Sci Vis 7, 305–311 [DOI] [PubMed] [Google Scholar]
- 83.Volbrecht V et al. (2010) Unique hue loci differ with methodology. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists) 30, 545–552. 10.1111/j.1475-1313.2010.00727.x [DOI] [PubMed] [Google Scholar]
- 84.Koenderink J et al. (2020) Hues of Color Afterimages. Iperception 11, 2041669520903553. 10.1177/2041669520903553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sandell JH et al. (1979) Color categories in macaques. Journal of Comparative & Physiological Psychology 93, 626–635 [DOI] [PubMed] [Google Scholar]
- 86.Fagot J et al. (2006) Cross-species differences in color categorization. Psychon Bull Rev 13, 275–280. 10.3758/bf03193843 [DOI] [PubMed] [Google Scholar]
- 87.Chang ALY et al. (2022) Color categorization in macaques. Journal of Vision (presented at the annual Vision Sciences Society Meeting, May 2022) in press, [Google Scholar]
- 88.Roberson D et al. (2005) Color categories: evidence for the cultural relativity hypothesis. Cogn Psychol 50, 378–411. 10.1016/j.cogpsych.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 89.Funke M (2018) Colourblind: The Use of Greek Colour Terminology in Cultural Linguistics in the Late Nineteenth and Early Twentieth Centuries. In Brill’s companion to classics and early anthropology (Varto E, ed), Brill [Google Scholar]
- 90.Deutscher G (2010) Through the language glass: why the world looks different in other languages. Macmillan, [Google Scholar]
- 91.Berlin B and Kay P (1969) Basic color terms: their universality and evolution. Berkeley, CA: University of California Press, [Google Scholar]
- 92.Saunders BAC and van Brakel J (1997) Are there nontrivial constraints on colour categorization? Behavioral and Brain Sciences 20, 167–+ [PubMed] [Google Scholar]
- 93.Lindsey DT et al. (2015) Hunter-Gatherer Color Naming Provides New Insight into the Evolution of Color Terms. Curr Biol 25, 2441–2446. 10.1016/j.cub.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gibson E et al. (2017) Color naming across languages reflects color use. P Natl Acad Sci USA 114, 10785–10790. 10.1073/pnas.1619666114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kay P et al. (2011) The World Color Survey. Center for the Study of Language and Information; 1st edition (February 15, 2011), 620 [Google Scholar]
- 96.Kuhn T (1962) Structure of Scientific Revolutions University of Chicago Press [Google Scholar]
- 97.Wierzbicka A (1990) The meaning of color terms: semantics, culture, and cognition. Cognitive Linguistics 1, 99–150 [Google Scholar]
- 98.Conway BR et al. (2020) Communication efficiency of color naming across languages provides a new framework for the evolution of color terms. Cognition 195, 104086. 10.1016/j.cognition.2019.104086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zaslavsky N et al. (2018) Efficient compression in color naming and its evolution. Proc Natl Acad Sci U S A 115, 7937–7942. 10.1073/pnas.1800521115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Webster MA (2018) Color Vision. The Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience: Sensation, Perception and Attention Wiley; Volume 2 edition (March 13, 2018), 343–384 [Google Scholar]
- 101.Zhang LQ et al. (2022) An image reconstruction framework for characterizing initial visual encoding. Elife 11. 10.7554/eLife.71132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brainard DH (2015) Color and the Cone Mosaic. Annu Rev Vis Sci 1, 519–546. 10.1146/annurev-vision-082114-035341 [DOI] [PubMed] [Google Scholar]
- 103.Krauskopf J et al. (1986) Higher order color mechanisms. Vision Res 26, 23–32 [DOI] [PubMed] [Google Scholar]
- 104.Hansen T and Gegenfurtner KR (2006) Higher level chromatic mechanisms for image segmentation. J Vis 6, 239–259 [DOI] [PubMed] [Google Scholar]
- 105.Eskew RT Jr. (2009) Higher order color mechanisms: a critical review. Vision Res 49, 2686–2704. S0042–6989(09)00328–9 [pii] 10.1016/j.visres.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 106.Rezeanu D et al. (2023) From cones to color vision: a neurobiological model that explains the unique hues. J Opt Soc Am A 40, A4–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koida K and Komatsu H (2007) Effects of task demands on the responses of color-selective neurons in the inferior temporal cortex. Nat Neurosci 10, 108–116 [DOI] [PubMed] [Google Scholar]
- 108.Brouwer GJ and Heeger DJ (2013) Categorical clustering of the neural representation of color. J Neurosci 33, 15454–15465. 10.1523/JNEUROSCI.2472-13.2013 33/39/15454 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tajima S et al. (2017) Task-dependent recurrent dynamics in visual cortex. Elife 6. 10.7554/eLife.26868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khosla M et al. (2022) A highly selective response to food in human visual cortex revealed by hypothesis-free voxel decomposition. Curr Biol 32, 4159–4171 e4159. 10.1016/j.cub.2022.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pennock IML et al. (2023) Color-biased regions in the ventral visual pathway are food selective. Curr Biol 33, 134–146 e134. 10.1016/j.cub.2022.11.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zaidi Q and Conway BR (2019) Steps towards neural decoding of colors. Current Opinion in Behavioral Sciences 30, 169–177. 10.1016/j.cobeha.2019.10.011 [DOI] [Google Scholar]
- 113.Liao C et al. (2022) Crystal or jelly? Effect of color on the perception of translucent materials with photographs of real-world objects. J Vis 22, 6. 10.1167/jov.22.2.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nishio A et al. (2012) Neural selectivity and representation of gloss in the monkey inferior temporal cortex. J Neurosci 32, 10780–10793. 10.1523/JNEUROSCI.1095-12.2012 32/31/10780 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Vries JP et al. (2022) Emergent color categorization in a neural network trained for object recognition. Elife 11. ARTN e76472 10.7554/eLife.76472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Palmer SE and Schloss KB (2010) An ecological valence theory of human color preference. Proc Natl Acad Sci U S A 107, 8877–8882. 10.1073/pnas.0906172107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Levinson SC (2000) Yélî Dnye and the Theory of Basic Color Terms. Journal of Linguistic Antrhopology 10, 3–55. 10.1525/jlin.2000.10.1.3 [DOI] [Google Scholar]
- 118.Griber YA et al. (2018) Objects as culture-specific referents of color terms in Russian. Color Res Appl 43, 958–975. 10.1002/col.22280 [DOI] [Google Scholar]
- 119.Twomey CR et al. (2021) What we talk about when we talk about colors. Proc Natl Acad Sci U S A 118. 10.1073/pnas.2109237118 [DOI] [Google Scholar]
- 120.Abbott JT et al. (2016) Focal colors across languages are representative members of color categories. Proc Natl Acad Sci U S A 113, 11178–11183. 10.1073/pnas.1513298113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zaslavsky N et al. (2020) Communicative need in colour naming. Cogn Neuropsychol 37, 312–324. 10.1080/02643294.2019.1604502 [DOI] [PubMed] [Google Scholar]
- 122.Hasantash M et al. (2019) Paradoxical impact of memory on color appearance of faces. Nature communications 10, 3010. 10.1038/s41467-019-10073-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Henderson AJ et al. (2017) Skin colour changes during experimentally-induced sickness. Brain Behav Immun 60, 312–318. 10.1016/j.bbi.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 124.Ennis RJ and Zaidi Q (2019) Geometrical structure of perceptual color space: Mental representations and adaptation invariance. J Vis 19, 1. 10.1167/19.12.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Logvinenko AD and Beattie LL (2011) Partial hue-matching. Journal of Vision 11, 1–16 [DOI] [PubMed] [Google Scholar]
- 126.Yendrikhovskij SN (2001) Computing color categories from statistics of natural images. Journal of Imaging Science and Technology 45, 409–441 [Google Scholar]
- 127.Mollon J (2006) Monge: The Verriest lecture, Lyon, July 2005. Vis Neurosci 23, 297–309 [DOI] [PubMed] [Google Scholar]
- 128.Conway BR (2012) Color consilience: color through the lens of art practice, history, philosophy, and neuroscience. Ann N Y Acad Sci 1251, 77–94. 10.1111/j.1749-6632.2012.06470.x [DOI] [PubMed] [Google Scholar]
- 129.Josserand M et al. (2021) Environment and culture shape both the colour lexicon and the genetics of colour perception. Scientific Reports 11, 19095. 10.1038/s41598-021-98550-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hardy JL et al. (2023) Sunlight exposure cannot explain “grue” languages. Sci Rep 13, 1836. 10.1038/s41598-023-28280-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Conway BR et al. (2022) Hering’s opponent-colors theory fails a key test in a non-Western culture. Proceedings of the Cognitive Society, [Google Scholar]
- 132.Schrödinger E (1925) Über das Verhältnis der Vierfarben- zur Dreifarbentheorie. Sitzungsberichte der Akademie der Wissenschaften in Wien mathematisch-naturwissenschaftlichen Klasse. Abteilung 2a 134, 471–490 [Google Scholar]
- 133.Ikeda M and Ayama M (1980) Additivity of opponent chromatic valence. Vision Res 20, 995–999. 10.1016/0042-6989(80)90082-6 [DOI] [PubMed] [Google Scholar]
- 134.Burns SA et al. (1984) The Abney effect: chromaticity coordinates of unique and other constant hues. Vision Research 24, 479–489 [DOI] [PubMed] [Google Scholar]
- 135.Chichilnisky EJ and Wandell BA (1999) Trichromatic opponent color classification. Vision Research 39, 3444–3458. Doi 10.1016/S0042-6989(99)00033-4 [DOI] [PubMed] [Google Scholar]
- 136.Wuerger SM et al. (2005) The cone inputs to the unique-hue mechanisms. Vision Res 45, 3210–3223 [DOI] [PubMed] [Google Scholar]
- 137.Larimer J et al. (1975) Opponent process additivity. II. Yellow/blue equilibria and nonlinear models. Vision Research 15, 723–731 [DOI] [PubMed] [Google Scholar]
- 138.Knoblauch K and Shevell SK (2001) Relating cone signals to color appearance: failure of monotonicity in yellow/blue. Vis Neurosci 18, 901–906 [DOI] [PubMed] [Google Scholar]
- 139.Mollon JD and Cavonius CR (1987) The Chromatic Antagonisms of Opponent Process Theory are not the Same as Those Revealed in Studies of Detection and Discrimination. In Colour Vision Deficiencies VIII. Documenta Ophthalmologica Proceedings Series, vol 46. (Verriest G, ed), Springer [Google Scholar]
- 140.Krauskopf J et al. (1986) Higher-Order Color Mechanisms. Vision Research 26, 23–32. Doi 10.1016/0042-6989(86)90068-4 [DOI] [PubMed] [Google Scholar]
- 141.Hansen T and Gegenfurtner KR (2013) Higher order color mechanisms: evidence from noise-masking experiments in cone contrast space. J Vis 13. 10.1167/13.1.26 [DOI] [PubMed] [Google Scholar]
- 142.Shepard TG et al. (2016) A model of selective masking in chromatic detection. J Vis 16, 3. 10.1167/16.9.3 [DOI] [PubMed] [Google Scholar]
- 143.Webster MA and Mollon JD (1991) Changes in colour appearance following post-receptoral adaptation. Nature 349, 235–238 [DOI] [PubMed] [Google Scholar]
- 144.Gegenfurtner KR and Kiper DC (1992) Contrast detection in luminance and chromatic noise. J Opt Soc Am A 9, 1880–1888 [DOI] [PubMed] [Google Scholar]


