Abstract
Growing evidence suggests that psychopathy is related to altered connectivity within and between three large-scale brain networks that support core cognitive functions, including allocation of attention. In healthy individuals, default mode network (DMN) is involved in internally-focused attention and cognition such as self-reference. Frontoparietal network (FPN) is anticorrelated with DMN and is involved in externally-focused attention to cognitively demanding tasks. A third network, salience network (SN), is involved in detecting salient cues and, crucially, appears to play a role in switching between the two anticorrelated networks, DMN and FPN, to efficiently allocate attentional resources. Psychopathy has been related to reduced anticorrelation between DMN and FPN, suggesting SN’s role in switching between these two networks may be diminished in the disorder. To test this hypothesis, we used independent component analysis to derive DMN, FPN, and SN activity in resting-state fMRI data in a sample of incarcerated men (N = 148). We entered the activity of the three networks into dynamic causal modeling to test SN’s switching role. The previously established switching effect of SN among young, healthy adults was replicated in a group of low psychopathy participants (posterior model probability = 0.38). As predicted, SN’s switching role was significantly diminished in high psychopathy participants (t(145) = 26.39, p < .001). These findings corroborate a novel theory of brain function in psychopathy. Future studies may use this model to test whether disrupted SN switching is related to high psychopathy individuals’ abnormal allocation of attention.
Keywords: psychopathy, MRI, neuroimaging, salience network, dynamic causal modeling
1. INTRODUCTION
People with psychopathy are notorious for their deceitful interpersonal style, callousness, impulsivity, and irresponsible lifestyle. Psychopathy is a significant risk factor for violent and non-violent criminal behavior [1–3], is overrepresented in prisons [4], and has been estimated to cost the United States $460 billion per year [5], making psychopathy one of the most costly mental health disorders. Clarifying the neurobiology of psychopathy could lead to applications for diagnosis, treatment, and criminal justice [6,7]. Yet, the neurobiology of the disorder remains poorly understood. In particular, the search for a reliable biomarker has fallen short1, with neuroimaging studies producing evidence for altered activity in widespread brain regions [8] and inconsistent evidence for altered activity within focal brain regions or in specific contexts [9]. Another branch of research has taken a different approach to identifying core neural mechanisms associated with psychopathy, analyzing interactions among large-scale brain networks, with promising results [10–19].
Three large-scale brain networks in particular appear to serve core cognitive functions [20,21]: the default mode network (DMN), frontoparietal network2 (FPN), and salience network (SN). Neuroimaging studies have consistently identified these networks both in the presence and absence of experimental tasks (i.e., during tasks and “resting state,” respectively). In healthy populations, DMN and FPN are anticorrelated networks that respond in opposing patterns to externally-focused, cognitively demanding tasks. FPN increases activity during such tasks and plays a critical role in the allocation of selective attention [22,23]. In contrast, DMN tends to decrease activity during such tasks and appears to support internally-focused cognition such as self-reference or recollecting prior experiences [24]. SN plays a critical role in detecting novel and emotionally relevant cues and recruiting attentional networks (e.g., FPN) to engage with the salient task/stimuli [25,26]. Crucially, SN appears responsible for switching between the two anticorrelated networks [22,23,27,28]. Two key SN regions, the anterior cingulate cortex and anterior insula, have faster temporal dynamics than regions in DMN and FPN, flexibly change connectivity with other networks over time, and maintain high network centrality over time [29,30]. SN thus appears to be a critical hub for facilitating interactions between other networks.
The switching effect of SN on the two anticorrelated networks may be diminished in psychopathy, according to the Impaired Integration theory [31]. While this hypothesis remains to be tested, extant data corroborate the broader hypothesis that interactions among these three large-scale networks are altered. Psychopathy has been related to more positive functional connectivity (i.e., reduced anticorrelation) between DMN and FPN nodes in resting state [14,15,19], increased connectivity between SN and FPN nodes [13,15], and decreased connectivity between SN and DMN nodes [12,32]. Intriguingly, one study has extended these findings of altered static connectivity by observing altered dynamic changes in whole-brain connectivity states (i.e., including other large-scale brain networks). Espinoza and colleagues found that individuals with higher interpersonal/affective features of psychopathy (e.g., grandiosity, callousness) made less frequent switches between whole-brain connectivity states and spent more time in a state characterized by weaker functional connectivity overall [16]. In addition to altered functional connectivity, reduced white matter integrity has been observed in pathways between DMN nodes [33] as well as between the three networks [34]. Together, these findings suggest that psychopathy is associated with altered functional and structural connectivity between the three large-scale brain networks, notably increased competition between DMN and FPN, and reduced dynamic switching between connectivity states.
Diminished SN switching could be a neural correlate of cognitive deficits associated with psychopathy. In particular, people with psychopathy display impaired attention [35]. During cognitively demanding tasks, people with psychopathy display deficits in orienting to salient, goal-irrelevant information and recruiting attentional resources [31,36–39]. One prominent theory has posited that people with psychopathy often fail to attend, for example, to another person’s emotional state or to the threat of punishment, and thus commit acts that cause others distress or result in punishment [35]. In addition, people with psychopathy display associative learning deficits and have difficulty using past experience to guide future behavior [31]. The Impaired Integration theory hypothesizes [31] that these cognitive deficits result from reduced coordination and flexible switching between a number of large-scale brain networks, including reduced SN switching.
The current study aimed to test the hypothesis that SN’s role in switching between states predominated by DMN activity and states predominated by FPN activity is impaired in psychopathy. This is the first study to investigate this hypothesis directly. We analyzed resting-state fMRI data from a sample of incarcerated men and examined SN’s switching role using methods that have previously been validated in a study of healthy adults. We hypothesized that 1) low psychopathy participants would show the expected switching effect of SN, and 2) this switching effect of SN would be diminished in high psychopathy participants. Importantly, our analyses of resting-state fMRI data will not allow for conclusions about SN’s switching role in attention, or about SN’s switching role during cognitively demanding tasks. We conducted additional preliminary analyses testing our hypotheses within the context of a cognitively demanding task in a smaller sample of incarcerated men (see Supplemental Methods and Results, Tables S2–3, Figures S4–8).
2. MATERIALS AND METHODS
2.1. Participants
Incarcerated men in medium-security correctional facilities in the Midwest were recruited. A total of 351 participants completed resting-state fMRI scans. All participants were included in previous reports [15,16]. Included participants had no history of psychosis, bipolar disorder, PTSD, epilepsy, stroke, or head injury with loss of consciousness >30 min; were not currently using psychotropic medications; demonstrated >4th grade English reading level; had intact audition and vision; and had no MRI contraindications. Initially, analyses included participants 18-55 years old. However, because initial models that included low psychopathy participants older than 30 failed to replicate the SN switching effect that has previously been observed in participants 18-30 years old [22,23], primary analyses for this study were limited to N = 148 participants age 18-30 (see sample characteristics in Table 1). All participants provided written informed consent.
Table 1.
Sample characteristics (N = 148)
| Psychopathy Group |
||||
|---|---|---|---|---|
| Measure | Low (n = 48) | Intermediate (n = 53) | High (n = 47) | |
| PCL-R Total | M (SD) | 15.6 (3.3) | 24.7 (2.3) | 32.2 (2.2) |
| Range | 6.7-20.0 | 21.0-29.0 | 30.0-40.0 | |
| Age | M (SD) | 25.0 (2.7) | 25.9 (2.6) | 25.9 (3.0) |
| Range | 19.4-30.0 | 20.9-30.0 | 19.4-29.9 | |
| IQ | M (SD) | 96.8 (12.7) | 96.5 (14.9) | 98.1 (13.3) |
| Range | 74.0-123.0 | 70.9-126.0 | 72.0-134.0 | |
| Race/Ethnicity (White) | % | 68.8% | 54.7% | 51.1% |
| Substance Use Disorder | % | 81.2% | 100.0% | 93.6% |
| Head Injury | % | 16.2% | 32.7% | 41.5% |
Note: Psychopathy groups were formed according to recommended cut-offs (Hare, 2003): low (PCL-R ≤ 20), intermediate (PCL-R > 20 and < 30), and high (PCL-R ≥ 30).
2.2. Assessments
Psychopathy was assessed using the Psychopathy Checklist-Revised (PCL-R) [4]. Twenty psychopathic traits were rated on a 0-2 scale based on information obtained during a 90-minute interview and file review. Groups of low (PCL-R ≤ 20; n = 48), intermediate (PCL-R > 20 and < 30; n = 53), and high psychopathy (PCL-R ≥ 30; n = 47) participants were formed according to recommended cut-offs of PCL-R Total scores [4]. IQ was estimated from the Wechsler Adult Intelligence Scale 3rd Ed. [40]. Lifetime substance use disorder diagnoses (for any substance) were determined using the Structured Clinical Interview for the DSM-IV [41]. Participants self-reported their number of lifetime head injuries that resulted in loss of consciousness, loss of memory, or symptoms such as headaches, dizziness, or nausea.
2.3. fMRI Acquisition and Preprocessing
All fMRI images were acquired on prison grounds in the Mind Research Network’s Siemens 1.5T Avanto mobile scanner. Resting-state scans were acquired by a 12-element head coil, with the following parameters applied to an EPI gradient-echo pulse sequence: TR = 2000ms, TE = 39ms, flip angle = 90°, FOV = 24 x 24cm, 64 x 64 matrix, 3.4 x 3.4mm in-plane resolution, 4mm slice thickness, 1mm gap, 30 slices. During resting-state scans, participants were asked to lay still, eyes open, focusing on a fixation cross during the five-minute scan period.
All EPI volumes were despiked (ArtDespike in SPM), aligned to the first volume in the time series (Inrialign), registered to MNI EPI template space, and smoothed with 6mm FWHM kernel. The first 8 TRs (16000ms) were removed from each resting-state scan.
2.4. Independent Component Analysis
Independent component analysis (ICA) and non-linear dynamic causal modeling (DCM) methods replicated those implemented in a prior study of SN’s modulatory effect on DMN-FPN connectivity [23]. ICA was carried out in the Group ICA of fMRI Toolbox (GIFT, http://www.trendscenter.org/software/gift/) in Matlab. The networks of interest were identified via constrained ICA, a semiblind ICA method that derives components that most closely match a specified anatomical template. For hypotheses about specific networks, this method holds advantages over blind ICA, resolving ambiguity regarding the number of components the model should define. Templates from a network atlas derived from resting-state whole-brain connectivity were used to specify DMN, FPN, and SN (Figure S1) [42]. Time courses were extracted from the ICA components and entered into DCM analyses.
2.5. Dynamic Causal Modeling
Effective connectivity of the three networks was modeled by stochastic, non-linear DCM in Statistical Parametric Mapping 12 (SPM12; http://www.fil.ion.ucl.ac.uk/spm/). Three fully connected models with no extrinsic input were compared to test the robustness of the switching effect of SN on DMN-FPN connectivity (Figure 1). DCM models tested 1) SN’s modulatory effect on the bidirectional connections between DMN and FPN (the “SN modulation model”), 2) DMN’s modulatory effect on the bidirectional connections between SN and FPN (the “DMN modulation model”), and 3) FPN’s modulatory effect on the bidirectional connections between SN and DMN (the “FPN modulation model”). Random-effects Bayesian model selection (RFX-BMS) was performed to identify the model that best fit the data [43], with greater values of expected posterior model probability (i.e., the probability of a model generating the data of a randomly selected subject) and exceedance probability (i.e., the probability, given the group data, that a model is more likely than any other) indicating better model fit. RFX-BMS was performed separately for the low, intermediate, and high psychopathy groups, in line with a prior study comparing DCM model probabilities between two groups, cognitively impaired individuals and healthy individuals [28]. Modulation parameters (e.g., the modulatory effect of SN on DMN-to-FPN connectivity) were derived via Bayesian Model Averaging (BMA), which weights the estimated parameter by the posterior probability of each model [43,44].
Figure 1.
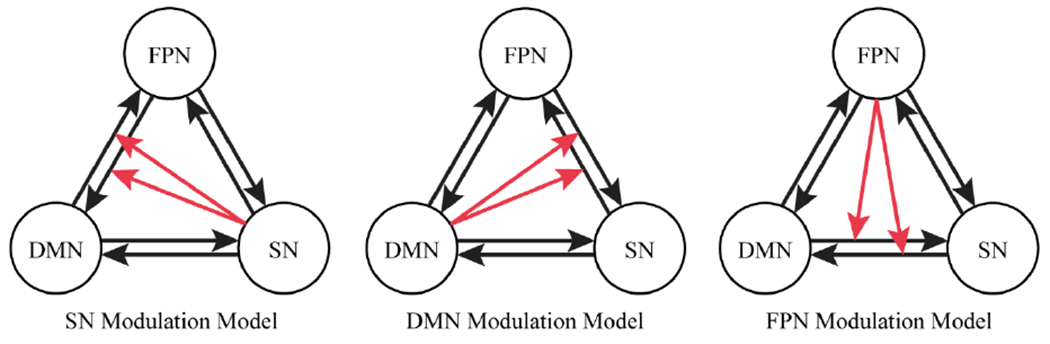
Three fully connected DCM models specified for each participant and compared using random-effects Bayesian model selection. Sets of black arrows represent bidirectional connectivity between each network. Red arrows represent one network’s modulatory effect on the connectivity between the other two networks. DMN = default mode network, FPN = frontoparietal network, SN = salience network
2.6. SN Switching and Group Comparisons
To examine the replicability of a switching role of SN, we performed RFX-BMS in the low psychopathy group. We first performed RFX-BMS in the sample of n = 118 low psychopathy participants age 18-55 (sample characteristics in Table S1). However, upon failing to replicate the switching role of SN in this sample, we attempted to more closely replicate the methods of prior studies that had reported the SN switching effect in participants 18-30 years old [22,23]. We restricted RFX-BMS analyses to n = 48 low psychopathy participants age 18-30. All subsequent analyses included participants age 18-30.
Next, we made group comparisons to test for a reduced switching role of SN in high psychopathy participants. We treated psychopathy as a categorical rather than continuous variable, as this is an established method for associating psychopathology with effective connectivity as measured by DCM [28,45]. One model with reference-coded variables compared the high psychopathy group (coded 0 in each reference variable) separately to each of the low and intermediate groups (coded 1 or 0) on posterior probabilities for the SN modulation model. A post-hoc t-test compared posterior probabilities between the low and intermediate psychopathy groups. Thus, we compared each psychopathy group separately to the other two. Finally, the reference-coded model and post-hoc t-test were repeated to examine the relationship between membership in the high psychopathy group and the SN modulation parameters. Tests were initially conducted without additional covariates, then repeated including the covariates of race, substance use disorder, and head injury (dichotomous variables coded white/non-white, present/absent, and present/absent, respectively), as well as age and IQ. One participant missing IQ scores and 18 participants missing head injury data were excluded from the covariate models.
3. RESULTS
The constrained ICA yielded three components as specified (Figure 2). To check that these components represented the hypothesized networks, the percentage of overlap between voxels in the template and significant voxels (uncorrected p < .001, pFWE < .05, cluster extent threshold = 27 voxels) in the corresponding component was computed. The first component overlapped with the DMN template (100.0% of voxels in the DMN template were in the first component, 12.0% of significant voxels in the first component were in the DMN template), the second component overlapped with the FPN template (85.5% of voxels in the FPN template were in the second component, 13.7% of significant voxels in the second component were in the FPN template), and the third component overlapped with the SN template (100.0% of voxels in the SN template were in the third component, 8.3% of significant voxels in the third component were in the SN template). A similar method was used to ensure that the mean components did not differ between the low, intermediate, and high psychopathy groups (Supplemental Methods and Results, Figure S2).
Figure 2.
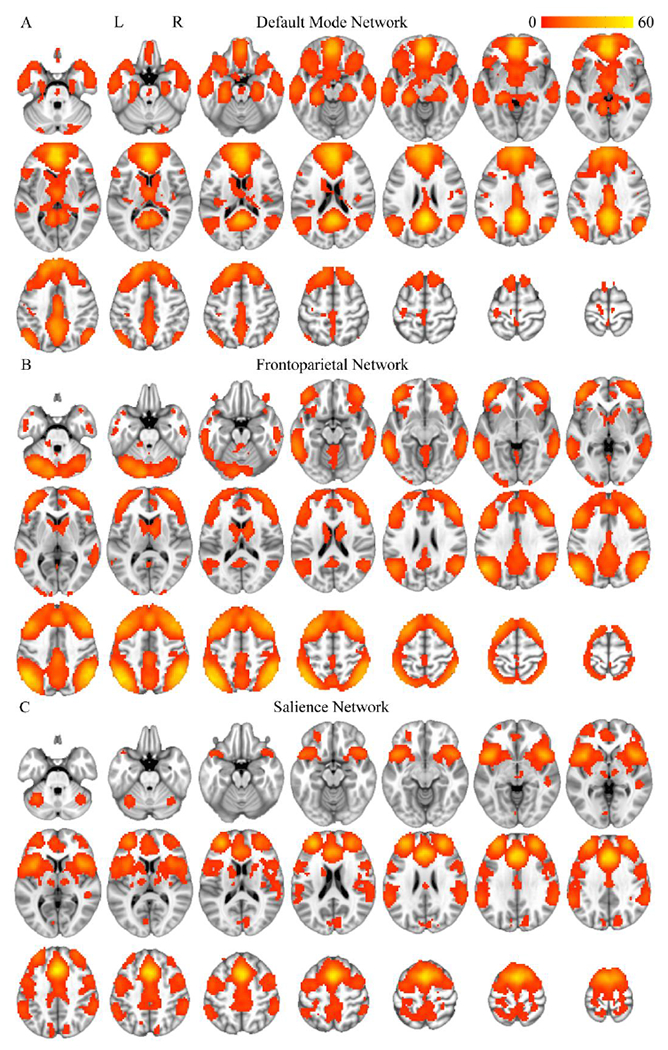
Components derived from the constrained ICA, averaged across subjects. The first component corresponded to default mode network (DMN; A), the second component corresponded to frontoparietal network (FPN; B), and the third component corresponded to salience network (SN; C). The most inferior slice in each panel is z = −30, the most superior slice is z = 70, and each slice is separated by five mm. Voxels with significant (uncorrected p < .001, pFWE < .05, cluster extent threshold = 27 voxels) t-values (displayed in the color bar) are shown.
When we performed RFX-BMS among low psychopathy participants age 18-55, we failed to replicate the SN switching effect (Figure S3). The SN modulation model did not show higher expected posterior model probability (0.262, 0.563, 0.175, respectively) or exceedance probability (0.202, 0.717, 0.081, respectively) than the DMN or FPN modulation model.
In the sample of participants younger than 30, we replicated the predicted SN switching effect in the low psychopathy group (Figure 3). The SN modulation model showed higher expected posterior model probability (0.383, 0.337, 0.280, respectively) and exceedance probability (0.415, 0.344, 0.241, respectively) than the DMN and FPN modulation models. The SN modulation model probabilities were similar to those reported in a prior study of healthy young adults (posterior model probability: sample 1 ≈ 0.440, sample 2 ≈ 0.520; exceedance probability: sample 1 = 0.514, sample 2 = 0.939) [23].
Figure 3.
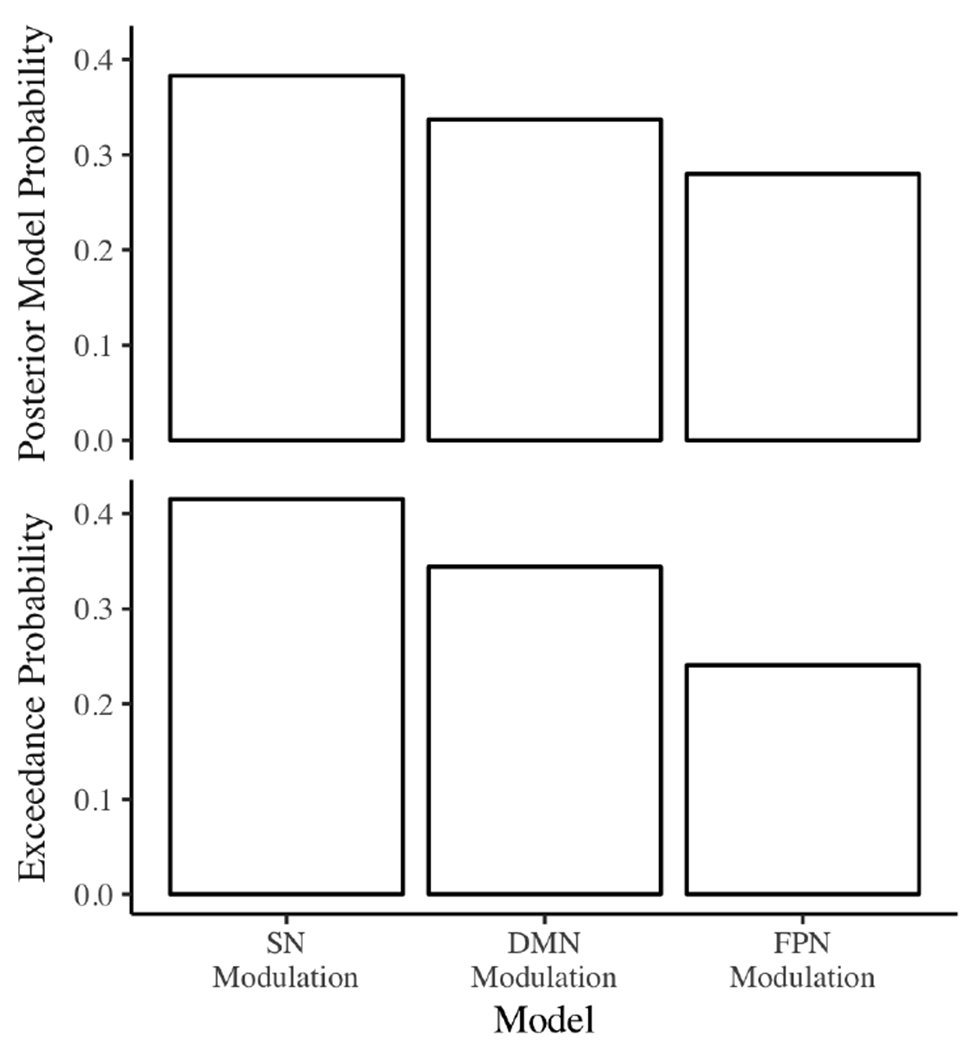
Posterior model probabilities and exceedance probabilities for the low psychopathy group (PCL-R ≤ 20). DMN = default mode network, FPN = frontoparietal network, SN = salience network
Next, and as predicted, posterior probabilities for the SN modulation model were significantly lower in the high psychopathy group compared to the low psychopathy group, t(145) = 26.39, p < .001 (Figure 4A). These posterior probabilities did not differ between the intermediate and high psychopathy groups, t(145) = 0.79, p = .43. Posterior probabilities for the SN modulation model were also significantly lower in the intermediate compared to low psychopathy group, t(99) = 27.98, p < .001. Results were the same when covariates were included in the model.
Figure 4.
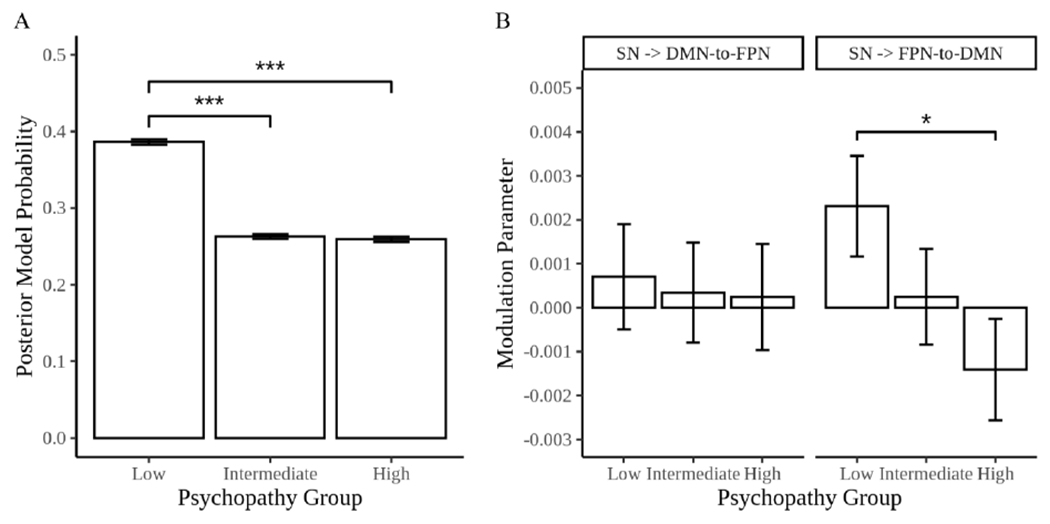
A) Posterior model probabilities for the SN modulation model in the low (PCL-R ≤ 20), intermediate (PCL-R > 20 and < 30), and high (PCL-R ≥ 30) psychopathy groups. B) Estimates from the SN modulation model of the modulatory effect of SN on DMN-to-FPN connectivity (left) and FPN-to-DMN connectivity (right) in the low, intermediate, and high psychopathy groups. In both panels, error bars represent 1 standard error above and below the point estimate of the reference-coded model comparing the high psychopathy group to the low and intermediate psychopathy groups. DMN = default mode network, FPN = frontoparietal network, SN = salience network, * p < .05, *** p < .001
Finally, analysis of modulation parameters revealed significantly reduced SN modulation of FPN-to-DMN connectivity in the high psychopathy group compared to the low psychopathy group, t(145) = 2.28, p < .03, but not the intermediate psychopathy group, t(145) = 1.04, p = .30 (Figure 4B). Post-hoc analysis revealed no significant difference between low and intermediate psychopathy groups for this modulation parameter, t(99) = 1.27, p = .21. There were no group differences for SN modulation of DMN-to-FPN connectivity: high compared to low psychopathy group, t(145) = 0.27, p = .79, high compared to intermediate psychopathy group, t(145) = 0.06, p = .95, and low compared to intermediate psychopathy group, t(99) = 0.21, p = .83. Results were the same with covariates included in the model.
For additional analyses of constituent clusters of psychopathic traits see Supplemental Methods and Results.
4. DISCUSSION
A growing body of research suggests that psychopathy may be related to altered connectivity between large-scale brain networks that support core functions. Following this line of research, we conducted novel analyses to test the hypothesis (first put forth by the Impaired Integration theory [31]) that the salience network’s role in switching between states predominated by DMN activity and states predominated by FPN activity is impaired among individuals with psychopathy. First, we replicated the switching effect of SN during resting state in a subset of low psychopathy participants [22,23]. Second, as predicted, we observed that SN’s switching role was significantly reduced in high psychopathy participants (as well as, unexpectedly, participants in the intermediate psychopathy group). As predicted, further analysis of modulation parameters revealed that SN modulation of FPN-to-DMN connectivity was uniquely impaired in the high psychopathy group. These findings may have implications for cognitive and affective deficits associated with psychopathy.
Among people with psychopathy, SN’s role in switching between two networks (DMN and FPN) that are typically anticorrelated was impaired. This finding is best understood in light of ample research on healthy populations, which has identified a canonical pattern of activity in DMN and FPN during cognitively demanding tasks. FPN increases activity during such tasks and plays a critical role in the allocation of selective attention [22,23], while DMN tends to decrease activity [24]. Importantly, deviations from this canonical pattern are associated with impaired task performance. Healthy individuals make more errors (e.g., response inhibition errors in a stop signal task) when DMN remains active during a cognitively demanding task [46]. Similarly, healthy individuals display slower and more variable processing speed when DMN-FPN anticorrelation is reduced [47,48]. This canonical pattern of activity and connectivity appears to be altered in psychopathy. People with psychopathy have shown reduced deactivation of (medial) DMN regions during cognitively demanding tasks [12,49,50] and reduced DMN-FPN anticorrelation [14,15]. Thus, competition between DMN and FPN could be related to cognitive abnormalities associated with psychopathy. We elaborate on this hypothesis in our discussion of the Impaired Integration theory below. The current study further suggests that SN switching may be a disrupted mechanism that contributes to competition between the two typically anticorrelated networks.
SN is a critical network for coordinating activity among other networks, possibly functioning at the top of a hierarchy of large-scale networks [51,52]. Among healthy individuals, two key SN nodes, the anterior insula and anterior cingulate cortex, appear to jointly coordinate the allocation of attentional resources through bottom-up attention switching and top-down biasing of sensory information [53]. Anterior insula receives input from multiple sensory modalities, suggesting the region likely plays the role of detecting salient sensory information [53]. Anterior insula also appears to send inhibitory signals to DMN and excitatory signals to FPN [22,51,54]. In contrast, anterior cingulate sends output to motor regions, suggesting the region likely plays the role of guiding action and maintaining control signals to other attention networks [53]. SN dysfunction in psychopathy has been documented in many studies. During cognitively demanding tasks, people with psychopathy have shown reduced activity when presented with salient sensory information in anterior insula [55–57] and anterior cingulate (based on meta-analyses [45]). Moreover, prior studies have found increased functional connectivity between SN and FPN nodes [13,15] and decreased connectivity between SN and DMN nodes [12]. However, connectivity within SN may be unaltered in psychopathy [17]. The extant evidence thus points to the following intriguing possibility: in psychopathy, SN’s capacity to detect salient information may be diminished; consequently, SN might fail to exert inhibitory control of DMN and excitatory control of FPN, resulting in hyperactivity of medial DMN regions [12,49,50] and competition between DMN and FPN [14,15]. This hypothesis could have important implications for cognition and behavior, but requires further testing (for preliminary analyses, see Supplemental Methods and Results, Tables S2–3, Figures S4–8).
Impaired attention and associative learning have been frequently observed among people with psychopathy. Impaired SN switching may be a critical neural correlate of these cognitive deficits, as posited by the Impaired Integration theory [31]. Specifically, the theory predicts that impaired SN switching, along with other disruptions to communication between large-scale brain networks, results in inattention to perceptual features that are irrelevant to a current goal [31]. Indeed, ample research has associated psychopathy with deficits in orienting to salient, goal-irrelevant information (e.g., negative outcomes) and recruiting attentional resources during the performance of cognitively demanding tasks [31,35–39], two functions supported by SN [26]. It is possible that SN fails to orient to goal-irrelevant information and recruit attentional resources (e.g., FPN) during cognitively demanding tasks. Future studies are needed to test this hypothesis, as the current study examined SN switching during resting state, and our preliminary analysis of SN switching during a cognitively demanding task was likely underpowered (see Supplemental Methods and Results). Interestingly, prior studies have shown that people with psychopathy are capable of attending to goal-irrelevant information when it is presented prior to goal-relevant information [39,58]. This suggests SN may be capable of recruiting attentional resources when psychopathic people are not already processing goal-relevant information. Additionally, the Impaired Integration theory posits that altered brain network interactions result in impaired binding of perceptual features into multimodal mental representations [31]. This hypothesis is supported by studies of perceptual processing [59,60] and associative learning [61–63]. However, another study failed to corroborate this hypothesis in an illusory visual paradigm [64]. In sum, diminished SN switching might contribute to impaired attention and associative learning, and further research is needed to test this hypothesis.
Impaired SN switching may also be related to psychopathic individuals’ affective deficits. Psychopathic people are notoriously callous towards others and report shallow affective experience. In the broader emotion science literature, increasing evidence suggests that affect and emotion are not represented in specialized brain regions or circuits. Instead, large-scale networks including SN and DMN may interact to construct emotional experiences [65–67]. In addition to facilitating attention, SN plays a primary role processing signals from the body, which are thought to be essential components of affective experience [53,65,68,69]. Furthermore, DMN has been argued to map affect (i.e., pleasantness and arousal) onto discrete emotion categories such as fear, anger, and happiness [67]. Examining interactions among these large-scale networks while psychopathic people respond to emotionally evocative stimuli is a promising avenue for future research. In fact, one prior study has linked dysfunctional interactions between DMN and SN to psychopathic people’s affective deficits. Decety and colleagues found reduced connectivity between anterior insula (of SN) and posterior cingulate cortex (of DMN) when psychopathic participants were taking the perspective of another person in pain [32].
To further characterize the nature of SN switching impairments in psychopathy, we analyzed the modulation parameters of the SN modulation model. Psychopathy was related specifically to SN modulation of FPN-to-DMN connectivity (but not DMN-to-FPN connectivity). However, the nature of the data make this finding difficult to interpret. In a typical DCM study, sensory input and task demands, along with knowledge of feedforward and feedback connections between regions, afford interpretations about the direction of information flow. A classic DCM study analyzed how attention and features of a visual stimulus affected connectivity between primary visual cortex (V1) and extrastriate visual cortex [70]. Given a wealth of literature on the flow of visual information through visual cortex, the study was able to conclude that attention modulates feedforward (V1-to-V5) but not feedback (V5-to-V1) connectivity. The current study analyzed communication between large-scale networks with varied anatomical connections (precluding interpretations about the direction of information flow between networks) during resting state (precluding interpretations about the information flowing between networks). Thus, SN modulation of FPN-to-DMN connectivity may be further examined in studies that employ a psychological task or that model connectivity between specific network nodes with known anatomical connections.
Dysfunction in these large-scale brain networks has been proposed to underlie a range of psychiatric disorders, not just psychopathy [71,72]. In fact, a diminished switching effect of SN on DMN-FPN connectivity has been observed in patients with schizophrenia [73] and seniors with mild cognitive impairment [28]. Notably, diminished influence of SN on DMN activity has been observed in patients with behavioral variant frontotemporal dementia [27], whose behavioral profile resembles that of psychopathic individuals. Patients with this form of dementia undergo a “personality change” that often involves a lack of drive to engage in work and other personal obligations, irresponsiveness to the feelings of loved ones, and a disinclination for embarrassment (similar to irresponsibility, lack of empathy, and lack of remorse in psychopathy) [26]. However, psychiatric disorders may be distinguished by unique dysfunction within these core networks. For example, while mild cognitive impairment in advanced age has been uniquely negatively related to SN modulation of DMN-to-FPN connectivity [28], psychopathy was uniquely negatively related to SN modulation of FPN-to-DMN connectivity in the current study. Further inquiry is necessary to establish whether the observed abnormalities in large-scale brain network interactions are unique to psychopathy or shared among other disorders.
The current findings, though requiring replication, could potentially influence the development of treatments. Empirically validated treatments for psychopathy are lacking (although see [67]), perhaps in part because few treatments have targeted psychological or neurobiological mechanisms that are theoretically relevant to the disorder’s etiology [75]. Aberrant connectivity among large-scale brain networks could serve as a mechanism of change for treatments that use existing techniques. For example, real-time neurofeedback is a non-invasive, though resource-intensive, technique that can change momentary functional connectivity [76] and has yielded lasting treatment effects for other disorders such as ADHD [77]. Two studies of psychopathy have provided initial evidence that neurofeedback can alter SN function and change behavior [78,79]. Transcranial magnetic stimulation is another non-invasive technique for modulating cortical activity that has shown efficacy in treating other psychiatric disorders [80], but remains to be tested as an intervention for psychopathy. Alternatively, cognitive remediation targets cognitive processes such as attention, rather than neural activity, and has shown promising treatment efficacy for people with psychopathy [81]. Further study is needed to establish the efficacy of these techniques for treating psychopathy and to identify normalized interactions among large-scale brain networks as a key mechanism of change.
Several limitations of this study require consideration. First, the sample was limited to participants between 18 and 30 years old. Thus, the current findings may not generalize to older adults. We decided to limit the sample at a preliminary analysis stage in order to replicate prior studies that observed SN’s switching effect in a sample of participants age 18-30 [22], including a study by Goulden et al. [23]. However, Goulden et al. also observed evidence for SN’s switching effect in a second sample of participants older than 30. Another study also observed the effect in a sample of adults older than 30 [28]. To date, no other study has examined SN’s switching effect in incarcerated people of any age. More work is needed to understand large-scale network dynamics in incarcerated people older than 30. Furthermore, the current analyses of resting-state data did not address whether SN switching is diminished in response to cognitively demanding tasks. Our preliminary analysis of SN switching during such a task was likely underpowered (see Supplemental Methods and Results). The current study also fails to account for interactions between other networks (outside DMN, FPN, and SN), including those involved in executive function and attention [82]. Dysfunctional interactions among these other networks, such as the dorsal attention network and visual network, could also be related to psychopathic individuals’ attention deficits [31,83]. Lastly, the current study sought to replicate the switching effect of the salience network as a whole, but did not provide specificity about which SN regions drove the switching effect or which DMN or FPN regions were most causally affected. Analyzing the time series of specific nodes of interest rather than time series collapsed across each network, using Granger causality analyses or DCM, would help to address this issue.
5. CONCLUSIONS
In sum, mounting evidence has linked psychopathy to altered interactions between large-scale brain networks. This study provides novel evidence for a mechanistic explanation of these altered interactions, namely dysfunction of the salience network’s switching role. Further characterizing these dynamic network interactions promises to advance neurobiological models of the disorder and influence the development of treatment.
Supplementary Material
Highlights.
Psychopathy is a significant risk factor for criminal behavior
Prior evidence for altered interactions between large-scale brain networks
Examined salience network’s role in switching between two anticorrelated networks
Switching role estimated via dynamic causal modeling
Salience network’s switching role was reduced for incarcerated men with psychopathy
ACKNOWLEDGMENTS
We thank Keith Harenski for managing fMRI data collection and Rasmus Birn for consulting on fMRI analyses. Authors P.D. and M.K. had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by grants from the National Institutes of Health (R01DA026964, R01MH090169, R01MH087525, R01DA026505, R01DA020870, R01NS126742, R01AA026290, R01HD092331).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors declare no competing financial interests.
For a discussion of the methodological factors that may contribute to inconsistencies in the neuroimaging literature on psychopathy, see [8].
Also referred to as “central executive network.”
CRediT authorship contribution statement
Philip Deming: Conceptualization, Methodology, Formal Analysis, Writing – Original Draft, Writing – Review & Editing, Visualization
Cole J. Cook: Methodology, Writing- Review & Editing
Mary E. Meyerand: Methodology
Kent A. Kiehl: Funding Acquisition, Resources, Supervision, Writing – Review & Editing
David S. Kosson: Funding Acquisition, Writing – Review & Editing
Michael Koenigs: Conceptualization, Resources, Supervision, Writing – Review & Editing
REFERENCES
- [1].Harris GT, Rice ME, Cormier CA, Psychopathy and violent recidivism, Law Hum. Behav 15 (1991) 625–637. 10.1007/BF01065856. [DOI] [PubMed] [Google Scholar]
- [2].Anderson JR, Walsh Z, Kosson DS, Psychopathy, self-identified race/ethnicity, and nonviolent recidivism: A longitudinal study., Law Hum. Behav 42 (2018) 531–544. 10.1037/lhb0000302. [DOI] [PubMed] [Google Scholar]
- [3].Reidy DE, Kearns MC, DeGue S, Lilienfeld SO, Massetti G, Kiehl KA, Why psychopathy matters: Implications for public health and violence prevention, Aggress. Violent Behav 24 (2015) 214–225. 10.1016/j.avb.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hare RD, The Hare psychopathy checklist-revised, Multi-Health Systems, Toronto, 2003. [Google Scholar]
- [5].Kiehl KA, Hoffman MB, The Criminal Psychopath: history, neuroscience, treatment, and economics., Jurimetrics. 51 (2011) 355–397. 10.1108/17506200710779521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nadelhoffer T, Bibas S, Grafton S, Kiehl KA, Mansfield A, Sinnott-Armstrong W, Gazzaniga M, Neuroprediction, violence, and the law: Setting the stage, Neuroethics. 5 (2012) 67–99. 10.1007/s12152-010-9095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Aspinwall LG, Brown TR, Tabery J, The Double-Edged Sword: Does Biomechanism Increase or Decrease Judges’ Sentencing of Psychopaths?, Science. 337 (2012) 846–849. 10.1126/science.1224352. [DOI] [PubMed] [Google Scholar]
- [8].Koenigs MR, Baskin-Sommers AR, Zeier J, Newman JP, Investigating the neural correlates of psychopathy: a critical review, Mol. Psychiatry 16124 (2011) 792–799. 10.1038/mp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Deming P, Heilicher M, Koenigs M, How reliable are amygdala findings in psychopathy? A systematic review of MRI studies, Neurosci. Biobehav. Rev (2022) 104875. 10.1016/j.neubiorev.2022.104875. [DOI] [PubMed] [Google Scholar]
- [10].Dotterer HL, Neural Network-Level Examinations of Psychopathy: Preliminary Evidence and Future Directions, Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 (2018) 981–982. 10.1016/j.bpsc.2018.09.011. [DOI] [PubMed] [Google Scholar]
- [11].Philippi CL, Pujara MS, Motzkin JC, Newman JP, Kiehl KA, Koenigs MR, Altered Resting-State Functional Connectivity in Cortical Networks in Psychopathy, J. Neurosci 35 (2015) 6068–6078. 10.1523/JNEUROSCI.5010-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pujol J, Batalla I, Contreras-Rodríguez O, Harrison BJ, Pera V, Hernández-Ribas R, Real E, Bosa L, Soriano-Mas C, Deus J, Ló Pez-Solà M, Pifarré J, Menchó JM, Cardoner N, Hernandez-Ribas R, Real E, Bosa L, Soriano-Mas C, Deus J, Lopez-Sola M, Pifarre J, Menchón JM, Cardoner N, Hernández-Ribas R, Real E, Bosa L, Soriano-Mas C, Deus J, Ló Pez-Solà M, Pifarré J, Menchó JM, Cardoner N, Breakdown in the brain network subserving moral judgment in criminal psychopathy, Soc. Cogn. Affect. Neurosci 7 (2012) 917–923. 10.1093/scan/nsr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Contreras-Rodríguez O, Pujol J, Batalla I, Harrison BJ, Soriano-Mas C, Deus J, López-Solà M, Macià D, Pera V, Hernández-Ribas R, Pifarré J, Menchón JM, Cardoner N, Functional connectivity bias in the prefrontal cortex of psychopaths, Biol. Psychiatry 78 (2015) 647–655. 10.1016/j.biopsych.2014.03.007. [DOI] [PubMed] [Google Scholar]
- [14].Dotterer HL, Hyde LW, Shaw DS, Rodgers EL, Forbes EE, Beltz AM, Connections that characterize callousness: Affective features of psychopathy are associated with personalized patterns of resting-state network connectivity, NeuroImage Clin. 28 (2020) 102402. 10.1016/j.nicl.2020.102402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Espinoza FA, Vergara VM, Reyes D, Anderson NE, Harenski CL, Decety J, Rachakonda S, Damaraju E, Rashid B, Miller RL, Koenigs MR, Kosson DS, Harenski KA, Kiehl KA, Calhoun VD, Aberrant functional network connectivity in psychopathy from a large (N = 985) forensic sample, Hum. Brain Mapp 39 (2018) 2624–2634. 10.1002/hbm.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Espinoza FA, Anderson NE, Vergara VM, Harenski CL, Decety J, Rachakonda S, Damaraju E, Koenigs MR, Kosson DS, Harenski KA, Calhoun VD, Kiehl KA, Resting-state fMRI dynamic functional network connectivity and associations with psychopathy traits, NeuroImage Clin. 24 (2019) 101970. 10.1016/j.nicl.2019.101970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tillem S, Harenski KA, Harenski CL, Decety J, Kosson DS, Kiehl KA, Baskin-Sommers AR, Psychopathy is associated with shifts in the organization of neural networks in a large incarcerated male sample, NeuroImage Clin. 24 (2019) 102083. 10.1016/j.nicl.2019.102083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lindner P, Flodin P, Budhiraja M, Savic I, Jokinen J, Tiihonen J, Hodgins S, Associations of psychopathic traits with local and global brain network topology in young adult women, Biol. Psychiatry Cogn. Neurosci. Neuroimaging (2018) 1–10. 10.1016/j.bpsc.2018.04.010. [DOI] [PubMed] [Google Scholar]
- [19].Pu W, Luo Q, Jiang Y, Gao Y, Ming Q, Yao S, Alterations of Brain Functional Architecture Associated with Psychopathic Traits in Male Adolescents with Conduct Disorder., Sci. Rep 7 (2017) 11349. 10.1038/s41598-017-11775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rosazza C, Minati L, Resting-state brain networks: Literature review and clinical applications, Neurol. Sci 32 (2011) 773–785. 10.1007/sl0072-011-0636-y. [DOI] [PubMed] [Google Scholar]
- [21].van den Heuvel MP, Hulshoff Pol HE, Exploring the brain network: A review on resting-state fMRI functional connectivity, Eur. Neuropsychopharmacol 20 (2010) 519–534. 10.1016/jeuroneuro2010.03.008. [DOI] [PubMed] [Google Scholar]
- [22].Sridharan D, Levitin DJ, Menon V, A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks, PNAS. 105 (2008) 12569–12574. 10.1073/pnas0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG, The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM, NeuroImage. 99 (2014) 180–190. 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- [24].Raichle ME, The Brain’s Default Mode Network, Annu. Rev. Neurosci 38 (2015) 433–447. 10.l146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- [25].Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, Dissociable intrinsic connectivity networks for salience processing and executive control, J. Neurosci 27 (2007) 2349–2356. 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Seeley WW, The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands, J. Neurosci 39 (2019) 9878–9882. 10.1523/JNEUROSCI.1138-17.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chiong W, Wilson SM, D’Esposito M, Kayser AS, Grossman SN, Poorzand P, Seeley WW, Miller BL, Rankin KP, The salience network causally influences default mode network activity during moral reasoning, Brain. 136 (2013) 1929–1941. 10.1093/brain/awt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chand GB, Wu J, Hajjar I, Qiu D, Interactions of the Salience Network and Its Subsystems with the Default-Mode and the Central-Executive Networks in Normal Aging and Mild Cognitive Impairment, Brain Connect. 7 (2017) 401–412. 10.1089/brain.2017.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Das A, Menon V, Spatiotemporal integrity and spontaneous nonlinear dynamic properties of the salience network revealed by human intracranial electrophysiology: A multicohort replication, Cereb. Cortex 30 (2020) 5309–5321. 10.1093/cercor/bhaalll. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen T, Cai W, Ryali S, Supekar K, Menon V, Distinct Global Brain Dynamics and Spatiotemporal Organization of the Salience Network, PLoS Biol. 14 (2016) 1–21. 10.1371/journal.pbio.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hamilton RKB, Racer KH, Newman JP, Impaired Integration in Psychopathy: A Unified Theory of Psychopathic Dysfunction, Psychol. Rev 122 (2015) 770–791. [DOI] [PubMed] [Google Scholar]
- [32].Decety J, Chen C, Harenski CL, Kiehl KA, Parvizi J, Uddin LQ, An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy, Front. Hum. Neurosci 7 (2013) 1–12. 10.3389/fnhum.2013.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sethi A, Gregory S, Dell’Acqua F, Periche Thomas E, Simmons A, Murphy DGMM, Hodgins S, Blackwood NJ, Craig MC, Emotional detachment in psychopathy: Involvement of dorsal default-mode connections, Cortex. 62 (2015) 11–19. 10.1016/j.cortex.2014.07.018. [DOI] [PubMed] [Google Scholar]
- [34].Dotterer HL, Waller R, Shaw DS, Plass J, Brang D, Forbes EE, Hyde LW, Antisocial behavior with callous-unemotional traits is associated with widespread disruptions to white matter structural connectivity among low-income, urban males, NeuroImage Clin. 23 (2019) 101836. 10.1016/j.nicl.2019.101836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Baskin-Sommers AR, Brazil IA, The importance of an exaggerated attention bottleneck for understanding psychopathy, Trends Cogn. Sci 26 (2022) 1–12. 10.1016/j.tics.2022.01.001. [DOI] [PubMed] [Google Scholar]
- [36].Baskin-Sommers AR, Curtin JJ, Li W, Newman JP, Psychopathy-related differences in selective attention are captured by an early event-related potential., Personal. Disord 3 (2012) 370–8. 10.1037/a0025593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wolf RC, Carpenter RW, Warren CM, Zeier JD, Baskin-Sommers AR, Newman JP, Reduced susceptibility to the attentional blink in psychopathic offenders: implications for the attention bottleneck hypothesis, Neuropsychology. 26 (2012) 102–9. 10.1037/a0026000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Newman JP, Curtin JJ, Bertsch JD, Baskin-Sommers AR, Attention moderates the fearlessness of psychopathic offenders, Biol. Psychiatry 67 (2010) 66–70. 10.1016/j.biopsych.2009.07.035.Attention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Baskin-Sommers AR, Curtin JJ, Newman JP, Specifying the attentional selection that moderates the fearlessness of psychopathic offenders, Psychol. Sci 22 (2011) 226–34. 10.1177/0956797610396227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wechsler D, Wechsler Adult Intelligence Scales, Harcourt Brace & Co, San Antonio, 1997. [Google Scholar]
- [41].First MB, Spitzer RL, Gibbon M, Williams JB, Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinical Version, Administration Booklet, American Psychiatric Pub, 2012. [Google Scholar]
- [42].Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD, Decoding Subject-Driven Cognitive States with Whole-Brain Connectivity Patterns, Cereb. Cortex 22 (2012). 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Stephan KE, Penny W.Dd., Moran RJ, den Ouden HEM, Daunizeau J, Friston KJ, Ten simple rules for dynamic causal modeling, NeuroImage. 49 (2010) 3099–3109. 10.1016/j.neuroimage.2009.ll.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Penny WD, Stephan KE, Daunizeau J, Rosa MJ, Friston KJ, Schofield TM, Leff AP, Comparing families of dynamic causal models, PLoS Comput. Biol 6 (2010). 10.1371/journal.pcbi.1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Seghier ML, Zeidman P, Neufeld NH, Leff AP, Price CJ, Identifying abnormal connectivity in patients using dynamic causal modeling of fMRI responses, Front. Syst. Neurosci 4 (2010) 1–14. 10.3389/fnsys.2010.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bednarski SR, Zhang S, Hong K-I, Sinha R, Rounsaville BJ, Li CR, Deficits in default mode network activity preceding error in cocaine dependent individuals, Drug Alcohol Depend. 119 (2012) e51–e57. 10.1016/j.drugalcdep.2011.05.026.Deficits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP, Competition between functional brain networks mediates behavioral variability, NeuroImage. 39 (2008) 527–537. 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- [48].Ng KK, Lo JC, Lim JKW, Chee MWL, Zhou J, Reduced functional segregation between the default mode network and the executive control network in healthy older adults: A longitudinal study, NeuroImage. 133 (2016) 321–330. 10.1016/j.neuroimage.2016.03.029. [DOI] [PubMed] [Google Scholar]
- [49].Freeman SM, Clewett DV, Bennett CM, Kiehl KA, Gazzaniga MS, Miller MB, Bennett DV, Kiehl CM, Miller KA, The Posteromedial Region of the Default Mode Network Shows Attenuated Task-Induced Deactivation in Psychopathic Prisoners, Neuropsychology. 29 (2015) 493–500. 10.1037/neu0000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Deming P, Koenigs MR, Functional neural correlates of psychopathy: a meta-analysis of MRI data, Transl. Psychiatry 10 (2020) 1–8. 10.1038/s41398-020-0816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhou Y, Friston KJ, Zeidman P, Chen J, Li S, Razi A, The Hierarchical Organization of the Default, Dorsal Attention and Salience Networks in Adolescents and Young Adults, Cereb. Cortex 28 (2018) 726–737. 10.1093/cercor/bhx307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chanes L, Barrett LF, Redefining the Role of Limbic Areas in Cortical Processing, Trends Cogn. Sci 20 (2016) 96–106. 10.1016/j.tics.2015.ll.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Menon V, Uddin LQ, Saliency, switching, attention and control: a network model of insula function, Brain Struct. Funct (2010) 1–13. 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jilka SR, Scott G, Ham T, Pickering A, Bonnelle V, Braga RM, Leech R, Sharp DJ, Damage to the salience network and interactions with the default mode network, J. Neurosci 34 (2014) 10798–10807. 10.1523/JNEUROSCI.0518-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Deming P, Dargis M, Haas BW, Brook M, Decety J, Harenski CL, Kiehl KA, Koenigs MR, Kosson DS, Psychopathy is associated with fear-specific reductions in neural activity during affective perspective-taking, NeuroImage. 223 (2020) 117342. 10.1016/j.neuroimage.2020.117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sethi A, McCrory E, Puetz V, Hoffmann F, Knodt AR, Radtke SR, Brigidi BD, Hariri AR, Viding E, Primary and Secondary Variants of Psychopathy in a Volunteer Sample Are Associated With Different Neurocognitive Mechanisms, Biol. Psychiatry Cogn. Neurosci. Neuroimaging (2018) 1–9. 10.1016/j.bpsc.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Meffert H, Gazzola V, Den Boer JA, Bartels AAJ, Keysers C, Reduced spontaneous but relatively normal deliberate vicarious representations in psychopathy, Brain J. Neurol 136 (2013) 2550–2562. 10.1093/brain/awtl90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Larson CL, Baskin-Sommers AR, Stout DM, Balderston NL, Curtin JJ, Schultz DH, Kiehl KA, Newman JP, The interplay of attention and emotion: top-down attention modulates amygdala activation in psychopathy, Cogn. Affect. Behav. Neurosci 13 (2013) 757–770. 10.3758/sl3415-013-0172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sadeh N, Verona E, Psychopathic personality traits associated with abnormal selective attention and impaired cognitive control, Neuropsychology. 22 (2008) 669–680. 10.1037/a0012692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Baskin-Sommers AR, Curtin JJ, Newman JP, Emotion-modulated startle in psychopathy: Clarifying familiar effects, J. Abnorm. Psychol 122 (2013) 458–468. 10.1037/a0030958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lykken DT, A study of anxiety in the sociopathic personality, J. Abnorm. Soc. Psychol 55 (1957) 6–10. [DOI] [PubMed] [Google Scholar]
- [62].Newman JP, Kosson DS, Passive avoidance learning in psychopathic and nonpsychopathic offenders., J. Abnorm. Psychol 95 (1986) 252–256. 10.1037/0021-843X.95.3.252. [DOI] [PubMed] [Google Scholar]
- [63].Patterson CM, Newman JP, Reflectivity and learning from aversive events: Toward a psychological mechanism for the syndromes of disinhibition, Psychol. Rev 100 (1993) 716–736. 10.1037/0033-295X.100.4.716. [DOI] [PubMed] [Google Scholar]
- [64].Gunschera LJ, Verschuere B, Murphy RA, Temple-McCune A, Dutton K, Fox E, No impaired integration in psychopathy: Evidence from an illusory conjunction paradigm., Personal. Disord. Theory Res. Treat (2023). 10.1037/per0000619. [DOI] [PubMed] [Google Scholar]
- [65].Barrett LF, The theory of constructed emotion: an active inference account of interoception and categorization, Soc. Cogn. Affect. Neurosci 12 (2017) 1–23. 10.1093/scan/nswl54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF, The Brain Basis of Positive and Negative Affect: Evidence from a Meta-Analysis of the Human Neuroimaging Literature, Cereb. Cortex 26 (2016) 1910–1922. 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Satpute AB, Lindquist KA, The Default Mode Network’s Role in Discrete Emotion, Trends Cogn. Sci 23 (2019) 851–864. 10.1016/j.tics.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Barrett LF, Simmons WK, Interoceptive predictions in the brain, Nat. Rev. Neurosci 16 (2015)419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Damasio A, Fundamental feelings, Nature. 413 (2001) 781. [DOI] [PubMed] [Google Scholar]
- [70].Penny WD, Stephan KE, Mechelli A, Friston KJ, Comparing dynamic causal models, NeuroImage. 22 (2004) 1157–1172. 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- [71].Menon V, Large-scale brain networks and psychopathology: A unifying triple network model, Trends Cogn. Sci 15 (2011)483–506. 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- [72].Sha Z, Wager TD, Mechelli A, He Y, Common Dysfunction of Large-Scale Neurocognitive Networks Across Psychiatric Disorders, Biol. Psychiatry 85 (2019) 379–388. 10.1016/j.biopsych.2018.l1.011. [DOI] [PubMed] [Google Scholar]
- [73].Manoliu A, Riedl V, Zherdin A, Mühlau M, Schwerthöffer D, Scherr M, Peters H, Zimmer C, Förstl H, Bäuml J, Wohlschläger AM, Sorg C, Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia, Schizophr. Bull 40 (2014) 428–437. 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Olver ME, Lewis K, Wong SCP, Risk reduction treatment of high-risk psychopathic offenders: The relationship of psychopathy and treatment change to violent recidivism., Personal. Disord. Theory Res. Treat 4 (2013) 160–167. 10.1037/a0029769. [DOI] [PubMed] [Google Scholar]
- [75].Hecht LK, Latzman RD, Lilienfeld SO, The Psychological Treatment of Psychopathy: Theory and Research, in: David D, Lynn SJ, Montgomery GH (Eds.), Evid.-Based Psychother. State Sci. Pract, Wiley-Blackwell, Hoboken, NJ, 2018: pp. 271–298. 10.1002/9781119462996chl1. [DOI] [Google Scholar]
- [76].Veit R, Singh V, Sitaram R, Caria A, Rauss K, Birbaumer N, Using real-time fmri to learn voluntary regulation of the anterior insula in the presence of threat-related stimuli, Soc. Cogn. Affect. Neurosci 7 (2012) 623–634. 10.1093/scan/nsr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Arns M, Heinrich H, Strehl U, Evaluation of neurofeedback in ADHD: The long and winding road, Biol. Psychol 95 (2014) 108–115. 10.1016/j.biopsycho.2013.ll.013. [DOI] [PubMed] [Google Scholar]
- [78].Sitaram R, Caria A, Veit R, Gaber T, Ruiz S, Birbaumer N, Volitional control of the anterior insula in criminal psychopaths using real-time fMRI neurofeedback: a pilot study, Front. Behav. Neurosci 8 (2014) 1–13. 10.3389/fnbeh.2014.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Konicar L, Veit R, Eisenbarth H, Barth B, Tonin P, Strehl U, Birbaumer N, Brain self-regulation in criminal psychopaths, Sci. Rep 5 (2015) 9426. 10.1038/srep09426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Peters SK, Dunlop K, Downar J, Cortico-striatal-thalamic loop circuits of the salience network: A central pathway in psychiatric disease and treatment, Front. Syst. Neurosci 10 (2016) 1–23. 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Baskin-Sommers AR, Curtin JJ, Newman JP, Altering the Cognitive-Affective Dysfunctions of Psychopathic and Externalizing Offender Subtypes With Cognitive Remediation, Clin. Psychol. Sci 3 (2015) 45–57. 10.1177/2167702614560744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Witt ST, van Ettinger-Veenstra H, Salo T, Riedel MC, Laird AR, What executive function network is that? An image-based meta-analysis of network labels, Brain Topogr. (2021) 1–10. [DOI] [PubMed] [Google Scholar]
- [83].Steimke R, Nomi JS, Calhoun VD, Stelzel C, Paschke LM, Gaschler R, Goschke T, Walter H, Uddin LQ, Salience network dynamics underlying successful resistance of temptation, Soc. Cogn. Affect. Neurosci 12 (2017) 1928–1939. 10.1093/scan/nsxl23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


