Abstract
Background:
Numerous studies summarized in a recent meta-analysis have shown sleep deprivation rapidly improves depressive symptoms in approximately 50% of individuals with major depressive disorder (MDD), however those studies were typically conducted in clinical settings. Here we investigated the effects of sleep deprivation utilizing a highly controlled experimental approach.
Methods:
36 antidepressant-free individuals with MDD and 10 healthy controls (HC) completed a 5 day/4-night protocol consisting of adaptation, baseline, total sleep deprivation (TSD), and recovery phases. Light was kept consistently dim (lux<=50), meals were regulated, and activity was restricted. In-the-moment mood was assessed using a modified Hamilton Rating Scale for Depression (HRSD) at screening and each morning following the experimental nights.
Results:
Day of study had a significant effect on mood in both groups. Post-hoc analyses revealed that significant effects were attributed to mood improvement in the MDD group following study initiation prior to beginning TSD, and in the HC group following recovery sleep, but were not due to mood improvement in the MDD group during TSD. No further improvement in mood occurred during 36 hours of TSD.
Limitations:
Strict eligibility requirements may limit generalizability. The requirement to be medication free may have biased toward a less severely depressed sample.
Conclusions:
Results revealed that individuals with moderate MDD can experience a significant reduction in depressive symptoms upon entering a highly controlled laboratory environment. Environmental effects on mood can be substantial and need to be considered.
Keywords: Sleep deprivation, Mood, Major Depressive Disorder
Introduction
Acute sleep deprivation has been shown to result in rapid symptom improvement in approximately 50% of individuals with major depressive disorder or bipolar disorder in a depressed episode (Boland et al., 2017; Giedke & Schwarzler, 2002; Gillin, Buchsbaum, Wu, Clark, & Bunney, 2001; Wu, J. C. & Bunney, 1990). The improvements caused by sleep deprivation occur within hours, producing much more rapid effects compared to most other treatments (Gillin et al., 2001; Hemmeter, Hemmeter-Spernal, & Krieg, 2010). Unfortunately, this antidepressant response is quickly followed by a relapse in symptoms following a state of rest, even as brief as a nap (Borbely & Wirz-Justice, 1982), rendering sleep deprivation a poor candidate as a stand-alone intervention. Although the antidepressant effects of sleep deprivation have been empirically investigated since the late 1970s, the majority of these studies have been conducted in clinical settings, limiting our understanding of the specific elements necessary for the antidepressant effect.
Prior studies in this area have sought to identify individual characteristics that can predict more a robust response. It has been shown that chronotype may influence the therapeutic response to sleep deprivation, such that individuals with evening type show greater response rates (Dallaspezia, Suzuki, Clara, Colombo, & Benedetti, 2018). Additionally, one study showed that individuals who exhibit shortened REM latencies, tend to respond more favorably to sleep deprivation (Riemann, Wiegand, & Berger, 1991). Furthermore, utilization of depression subtype classifications may be important in predicting therapeutic response to sleep deprivation. For example, previous research has shown that individuals with the melancholic subtype of depression, characterized by anhedonia or the lack of mood reactivity, diurnal mood variation with worse evening mood, and psychomotor changes, may have a more positive response to sleep deprivation (Hemmeter et al., 2010; Riemann et al., 1991). Identifying the characteristics of those who may benefit from sleep deprivation may allow us to develop personalized medicine approaches for more rapid and effective treatment of MDD.
Because experimental sleep deprivation protocols require continuous behavioral supervision, most prior studies were conducted in inpatient psychiatric clinics as part of clinical care (Holsboer-Trachsler et al., 1994). This is in contrast to the extensive history of research on the impact of sleep deprivation, which has been conducted in highly controlled settings to reduce potential confounds. While clinical settings provide the opportunity for optimal medical oversight, many of the conditions within inpatient units are uncontrolled and it is possible that factors other than sleep loss are producing clinical gains. For example, ambient light levels have been shown to be especially high in ICU and inpatient hospital settings (Pisani et al., 2015; Macfarlane et al., 2019). This increase in light can have dramatic effects on mood as light is an essential zeitgeber for the regulation of circadian rhythms (Roenneberg, Kantermann, Juda, Vetter, & Allebrandt, 2013), known to be impacted in depression (Germain & Kupfer, 2008). It is therefore essential that the antidepressant effect of sleep deprivation be investigated with a more comprehensive and methodologically robust approach that limits environmental confounds.
Here we report the trajectory of mood before, during and after a 36-hour sleep deprivation period in the context of a highly controlled laboratory study. Based on prior literature documenting the antidepressant effects of sleep deprivation in MDD (Boland et al., 2017), we hypothesized that approximately 50% of those with MDD would exhibit significant mood improvement on both clinician-rated and self-report measures during sleep deprivation, similar to what is seen in clinical settings. Further, we assessed whether those with shorter REM latency and the melancholic subtype of MDD would still be more likely to exhibit a clinical response in the context of a laboratory environment that was tightly controlled (e.g., constant dim light, isocaloric meals, consistent social zeitgebers). Additionally, because our own group recently found that no demographic variables across numerous studies were able to predict the mood response to sleep deprivation (Boland et al., 2017), we hypothesized that demographic variables would not be able to distinguish responders from non-responders.
Methods
Participants
All study participants were recruited from the Philadelphia metro area and were between the ages of 21 and 50. Eligibility criteria included body mass index within 15% of normal and a stable sleep-wake schedule with sleep duration between 6 and 9 hours and habitual wake times between 6:00am and 8:00am as determined via interview, one week of daily sleep logs, and one week of actigraphy. Females were not pregnant or lactating. Participants were not taking any psychotropics or other medications with known effects on sleep for at least 2 weeks. Exclusion criteria included trans-meridian travel within past two months, shift work, current sleep disorder other than insomnia, history of bipolar disorder, delirium, dementia, schizophrenia, or other psychotic-spectrum disorders, or history of serious medical illness, significant head injury, seizure or unconsciousness for more than 5 minutes. All participants provided written informed consent, and the protocol was approved by the Institutional Review Board.
Individuals with MDD
Individuals with MDD were recruited from the general community and were symptomatic and unmedicated at the time of the study. All individuals were experiencing a current depressive episode with diagnoses based on the Structured Clinical Interview for DSM5. The 17-item Hamilton Depression Rating Scale (Hamilton, 1960)) was used to assess symptom severity. Eligible individuals who were taking psychotropic medications and who were willing to discontinue use for the duration of the study met with a study psychiatrist (MT) to assess safety and create a tapering plan. All individuals were unmedicated for at least 2 weeks (4 weeks for fluoxetine) prior to the laboratory portion of the study,
Healthy Controls
Healthy controls (HC) were recruited from the general community through flyers, advertisements, and online recruitment sources. SCID confirmed the absence of current or past personal or family history of psychopathology.
Procedures
Eligible participants were asked to keep their habitual sleep schedule, as verified by sleep diary and actigraphy for 7 days prior to study, and to refrain from napping, and using alcohol and drugs. Participants spent four consecutive nights in a private room at the University of Pennsylvania Center for Human Phenomic Science. Ambient light was kept at 50 lux during scheduled wakefulness, and off during sleep, for the entirety of the study. Subjects received three standardized meals per day. Intake of caffeine, alcohol, or tobacco was prohibited. The first experimental night served as an adaptation to the laboratory environment and polysomnographic screening for independent sleep disorders, while the second night served as a baseline. Total available time in bed was held constant at 9 hours (10:00pm – 7:00am). Following awakening on Day 3, participants began a period of 36 hours of total sleep deprivation followed by a 12-hour recovery sleep opportunity which began at 7:00pm on night 4. During the sleep deprivation period, participants were instructed to stay awake and were monitored by two study staff members at all times to ensure compliance, and to engage with the subject to help maintain wakefulness. Participants completed cognitive and mood assessments approximately every 2 and 4 hours, respectively. The Hamilton Depression Rating Scale (HRSD) was used to assess changes in mood at approximately 10:00am each morning following baseline sleep, during sleep deprivation, and following recovery sleep. The morningness-eveningness questionnaire (MEQ) was administered at screening to assess for trait morningness-eveningness.
Hamilton Rating Scale for Depression (HRSD)
The HRSD (Hamilton, 1960) is a 17‐item, semi-structured clinician-rating scale that assesses depression severity in the past week. Items include questions about depressed mood, suicidal ideation, interest and motivation, irritability, and libido. Items are scored from 0–4, with not all items utilizing the entire score range. Total scores can range from 0 to 52, with higher scores indicating greater severity of depressive symptomatology. The HRSD has been shown to have adequate reliability and validity (Bagby, Ryder, Schuller, & Marshall, 2004). Participants in the present study completed a modified version of the HRSS, the HRSD-NOW (Leibenluft, Moul, Schwartz, Madden, & Wehr, 1993), which prompts for responses in the context of the present moment and excludes items 4, 5, and 6 (insomnia symptoms), and item 16 (weight loss) which are less appropriate for multi-day assessment. Following previous accounts (Benedetti et al., 2008), depressed participants were designated as treatment responders if they experienced a 30% reduction in HRSD scores during sleep deprivation as compared to following baseline. To assess for depression with melancholic features, a composite of HRSD symptoms was computed following Rasmussen and colleagues (Rasmussen, Stevens, Kung, & Mohan, 2010) that included guilt, early morning awakening, loss of interest, psychomotor retardation and agitation, loss of appetite, loss of libido, weight loss, and AM diurnal mood variation. Total score on these items was computed and a median split was conducted, such that individuals in the upper half of scores were identified as having depression with melancholic features.
Visual Analogue Scale of Positive and Negative Mood (VAS)
The VAS (McCormack, David, & Sheather, 1988) is a self-report measure of mood where participants are asked to indicate where their mood falls along a continuous line. For positive mood, participants are asked to indicate their agreement with the statement “Right now, I am feeling positive” with 0 being “not at all” and 100 being “very much.” VAS has been shown to have high levels of sensitivity and be appropriate for repeated measurements of mood (Wu, J. C. & Bunney, 1990). Participants completed a measure of both positive and negative mood during 12 assessments during the course of the study, with 8 of these assessments occurring during the 36-hour sleep-deprivation period.
Beck Depression Inventory, 2nd edition (BDI-11)
The BDI-II (Beck, Steer, & Brown, 1996) is a 21-item, self-report rating scale designed to assess the severity of depressive symptoms. Scores range from 0 to 63, with higher scores indicating greater severity of depressive symptomatology. BDI scores were used to assess depression severity upon arrival at the sleep laboratory.
Morningness Eveningness Questionnaire (MEQ)
Chronotype, or an individual’s base circadian tendency, was assessed with the Morningness/Eveningness Questionnaire (MEQ) (Horne & Ostberg, 1976). The MEQ is a 13-item scale with total scores ranging from 13 to 52. Score <23 indicate a later chronotype (i.e. evening type) and high scores indicate greater morningness tendencies.
Sleep EEG
The EEG montage included frontal (Fz), central (C3, C4), and occipital (Oz) EEG (referenced against A1/A2), C3, C4, F3, F4, P3, P4, O1, and O2 EEG, left and right EOG, and a bipolar EMG. All signals were collected using digital ambulatory physiological recorders (Compumedics Profusion PSG3 recording system; Compumedics Limited, Abbotsford, Victoria, Australia), with a sampling rate of 256 Hz, displayed digitally during acquisition. For sleep scoring, high-pass filters were set at 0.3 Hz for EEGs and EOGs and 10 Hz for EMG. Low-pass filters were set at 30 Hz for EEG and EOG and 100 Hz for EMG. Trained research personnel visually scored sleep records in continuous 30-second epochs following standard criteria (Rechtschaffen & Kales, 1968). Thereafter, any epochs that contained movement, breathing muscle artifact, or recording difficulties were omitted from further analysis.
Data analysis
All data were first coded for group (HC, MDD). Independent samples and paired t-tests were computed for all demographic and each macroarchitectural variables for between and within-group analyses. Chi square was used to detect differences in categorical variables. Repeated measures ANOVA was used to test for mood differences across the study with group (HC, MDD) as the between-subjects factor and day (screening, baseline, sleep deprivation, recovery) as within-subjects factor. Univariate statistics for each variable are only reported if a main effect or interaction was obtained. Post-hoc analyses were computed using one-way ANOVA or paired t-tests, where appropriate. Correlational analyses were used to assess the relationship between baseline variables and change in HRSD scores from screening to baseline.
Results
Participant Characteristics
The present sample included 36 individuals with MDD (age M = 32.1) and 11 HC (M = 35.4) (Table 1). Across both groups, participants were predominantly female (67.3%), non-Hispanic (91.9%). The total sample self-identified as 59.2% Caucasian, 38.8% Black or African American, and 2.6% Asian. Individuals with MDD were moderately depressed as measured by the BDI-II, (M = 26.41), and as expected, the had higher levels of depression t(23) =−3.26, p<0.01, than HC but did not differ in any other respect including age or percentage of number of females.
Table 1.
Participant Characteristics
| Full Sample | MDD | Healthy Controls | |
|---|---|---|---|
| N = 47 | N = 36 | N = 11 | |
| Age | 32.9 (10.5) | 32.1 ( 10.8) | 35.4 (9.5) |
| Gender | |||
| Male | 15 (31.9%) | 9 (25.0%) | 6 (54.5%) |
| Female | 30 (63.8%) | 25 (69.4%) | 5 (45.5%) |
| Other | 2 (4.3%) | 2 (5.6%) | 0 |
| Race | |||
| Black | 18 (38.3%) | 13 (36.1%) | 5 (45.5%) |
| Caucasian | 24 (51.1%) | 20 (55.6%) | 4 (36.4%) |
| Asian | 1 (2.1%) | 1 (2.8%) | 0 |
| Other/Unknown | 4 (8.5%) | 2 (5.6%) | 2 (18.2%) |
| Ethnicity | |||
| Hispanic | 4 (8.5%) | 3 (8.33%) | 1 (9.1%) |
| Non-Hispanic | 36 (76.6%) | 28 (77.78%) | 8 (72.7%) |
| Other/Unknown | 7 (14.9%) | 5 (13.89%) | 2 (18.2%) |
| BDI-II Score (St. Dev) | 25.19 (9.73) | 2.45 (5.79) |
Macroarchitectural Sleep Variables
Polysomnographic variables (PSG) were examined at baseline and recovery. Complete PSG data was not available for 9 individuals with MDD and for 4 HC. Summary data for the remaining participants are presented in Table 2. No significant differences were found between individuals with MDD and healthy controls at baseline. During recovery sleep following sleep deprivation, those with MDD exhibited significantly longer sleep latency t(29) =−2.79, p<0.01, than those in the healthy control group. Within-group comparisons revealed that both HC and individuals with MDD exhibited increases in total sleep time during recovery sleep. Additionally, individuals with MDD demonstrated an increase in sleep efficiency, t(26) =−2.73, p<0.05, decrease in percentage of stage 1, t(26) =3.70, p=0.001, and stage 2 sleep, t(26) =2.08, p<0.05, and an increase in slow-wave sleep, t(26) =−4.18, p<0.001 on the recovery sleep night compared to the baseline night.
Table 2.
Means and standard deviations of polysomnographic variables, by group and condition.
| HC (n=7) | MDD (n=27) | |||
|---|---|---|---|---|
| Baseline | Recovery | Baseline | Recovery | |
| Total Sleep Time (min) | 427.84 (56.83)* | 606.14 (77.82)* | 461.76 (54.82)* | 638.32 (79.21)* |
| Sleep Efficiency (%) | 79.3 (10.50) | 84.19 (10.83) | 85.52 (10.16)* | 89.47 (9.84)* |
| Sleep Latency (min) | 20.43 (22.48) | 4.86 (3.08) † | 21.15 (28.24) | 18.02 (23.76) † |
| % Stage 1 | 4.66 (1.17) | 4.37 (3.48) | 3.96 (3.04)* | 2.31 (1.14)* |
| % Stage 2 | 48.97 (9.10) | 48.29 (8.33) | 48.25 (6.73)* | 45.90 (7.82)* |
| % Slow-wave Sleep | 23.7 (9.68) | 26.07 (6.95) | 21.47 (7.71)* | 24.82 (8.02)* |
| REM (%) | 22.64 (3.18) | 21.29 (5.16) | 26.33 (5.49) | 26.98 (7.25) |
| REM Latency (min) | 75.79 (17.44) | 101.21 (52.12) | 59.43 (31.66) | 58.11 (28.33) |
denotes significant group difference
denotes significant, within group condition difference
Mood Response to the Sleep Deprivation
Clinician-Rated
Figure 1a shows HRSD scores at the initial screening visit, following baseline sleep, during sleep deprivation, and following recovery sleep. Data from all four timepoints was not available for 1 individual with MDD. Results revealed a significant main effect of group, F(1,44) = 25.60, p<0.001, with those with MDD demonstrating higher score on the HRSD than HC, as expected. Results also revealed a main effect of condition, F(1,44) = 18.46, p<0.001, and a significant interaction, F(1,44) = 22.08, p<0.001 (see Figure 1a). A priori post-hoc analyses revealed that in those with MDD, mood significantly improved from the initial screening assessment to baseline, F(1,34) = 66.86, p<0.001, whereas mood did not change in response to sleep deprivation. As discussed, studies of the antidepressant effects of sleep deprivation usually find that whereas 50% of individuals respond to sleep deprivation (Boland et al., 2017), 50% show no response. As in a number of previous studies, response to sleep deprivation was defined as a 30% reduction in HRSD score during sleep deprivation as compared to baseline (Boland et al., 2017). According to this definition, only two individuals with MDD in the sample qualified as responders.
Figure 1a.
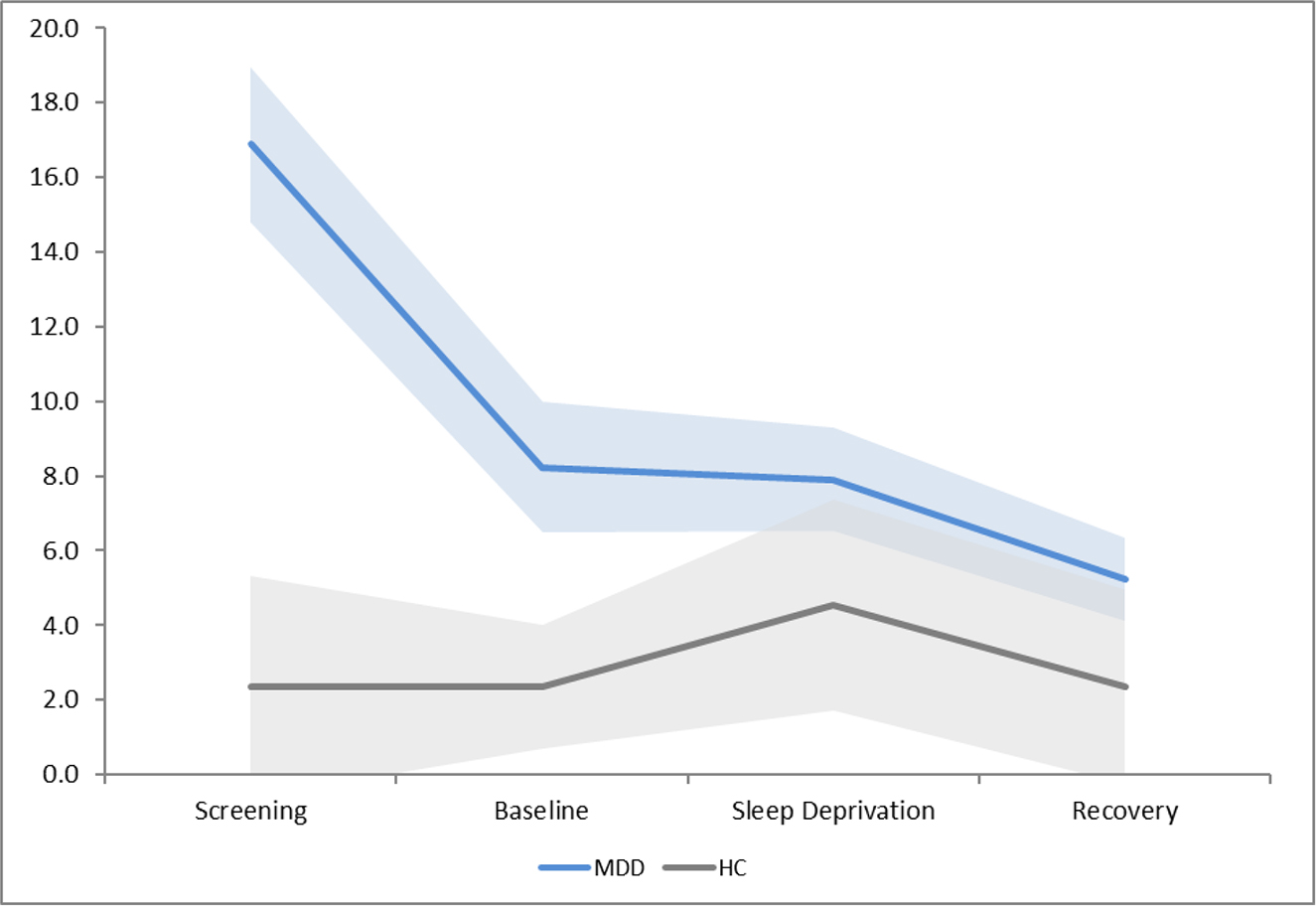
Hamilton Rating Scale of Depression scores at each condition, with 95% confidence intervals
Self-Report
Data from all four timepoints was not available for 6 individuals with MDD and 2 HC. Utilizing the VAS positive self-report measure of mood, results showed a slightly different pattern (See Figure 1b). Repeated measures ANOVA revealed a significant main effect of group, F(1,37) = 4.25, p<0.05 and a significant main effect of condition, F(3,111) = 10.87, p<0.001, but no interaction. Post-hoc t-tests demonstrated a significant increase in positive mood for those with MDD following recovery sleep as compared to during sleep deprivation, t(32) = −2.45, p=0.02, but not for HC.
Figure 1b.
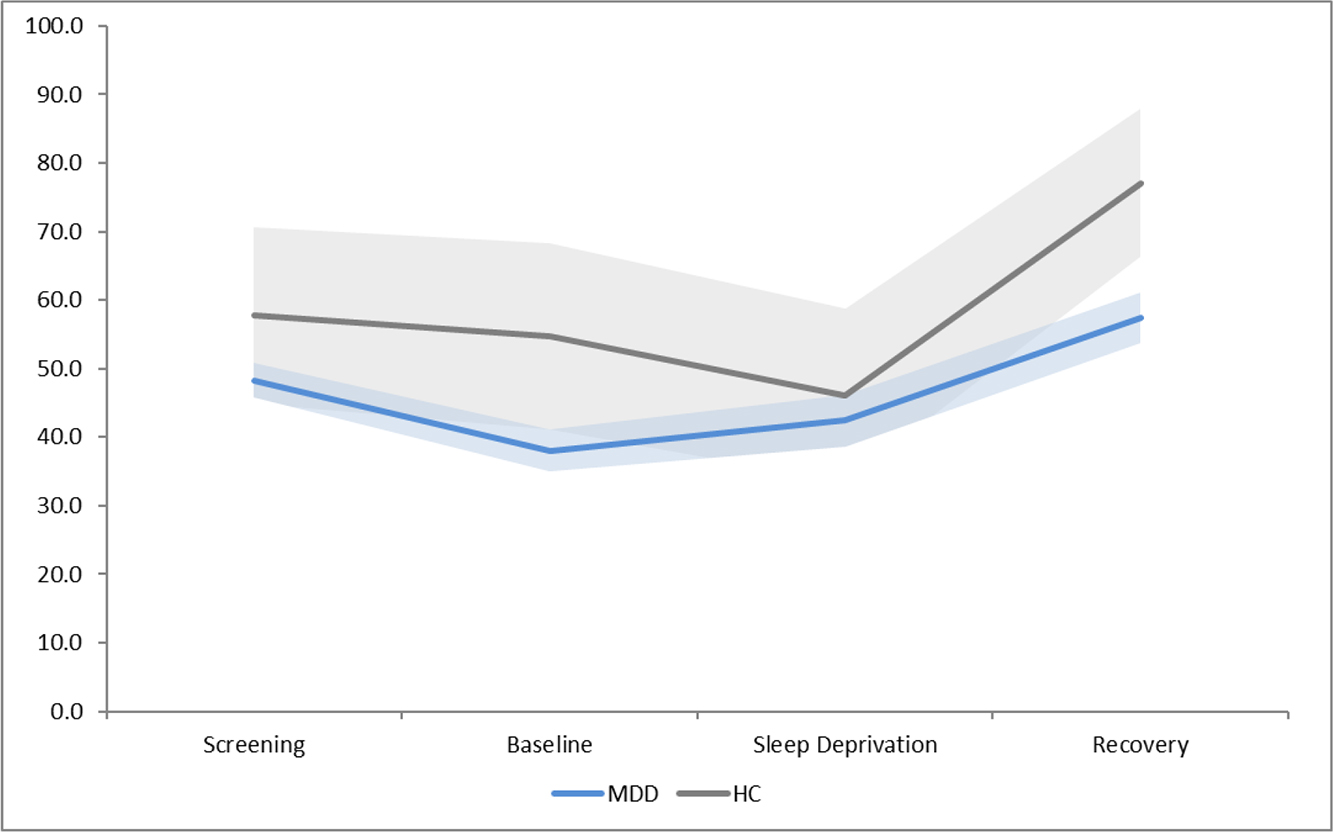
Self-report positive mood scores at each condition, with 95% confidence intervals
Exploratory Analysis
Due to the unexpected lack of antidepressant effects during the sleep deprivation paradigm, we were interested in examining if characteristics previously shown to be associated with mood improvements during sleep deprivation would differentiate the mood response in our participants.
Short REM latency
REM latency at baseline may be a predictor of mood response to sleep deprivation, with individuals with shorter REM latency showing more improved mood (Riemann et al., 1991). Individuals with MDD were categorized based on median split. Results showed a main effect of day, F(3,72) = 42.19, p < .001, but no effect of group or interaction, indicating that those with shorter REM latency showed the same pattern of response in mood ratings during sleep deprivation as those with longer REM latency.
Melancholic Features
Studies have consistently shown that individuals with melancholic features of major depression respond particularly well to therapeutic sleep deprivation (Hemmeter et al., 2010; Riemann et al., 1991). Therefore, we examined if individuals with melancholic features would show a differential response to our sleep deprivation paradigm. Repeated measures ANOVA revealed main effects of day F(1,26) = 51.28, p < .001, and group, F(1,26) = 19.88, p < .001 and a significant interaction, F (1,26) = 3.93, p =0.01, where individuals with melancholic features showed continued improvement in mood during sleep deprivation, individuals without melancholic features showed a worsening of mood during sleep deprivation, t(14) =2.02, p=0.03.
Post-Hoc Analyses: Mood Response to the Lab Environment
Although we did not observe a marked improvement in mood in response to sleep deprivation, we did observe a significant mood improvement from screening to baseline, t(34) =8.17, p<0.001 and from sleep deprivation to recovery, t(34) =4.45, p<0.001, in individuals with MDD (Figure 1a). Those in the healthy control group also showed a significant mood improvement following recovery, t(10) =3.25, p<0.01. We conducted post-hoc exploratory analyses to examine the relationship between change in mood and baseline sleep variables. Results revealed that for those individuals who showed less total minutes spent in SWS at baseline showed more improvement in mood, r=−.42, p=0.03 (Figure 2a). For percent of time spent in SWS, a trend in the same direction was found, r=−.36, p=0.06 We also found that higher MEQ scores, indicating more morning type, were associated with greater improvements in mood, r=.35, p=0.04 (Figure 2b).
Figure 2a.
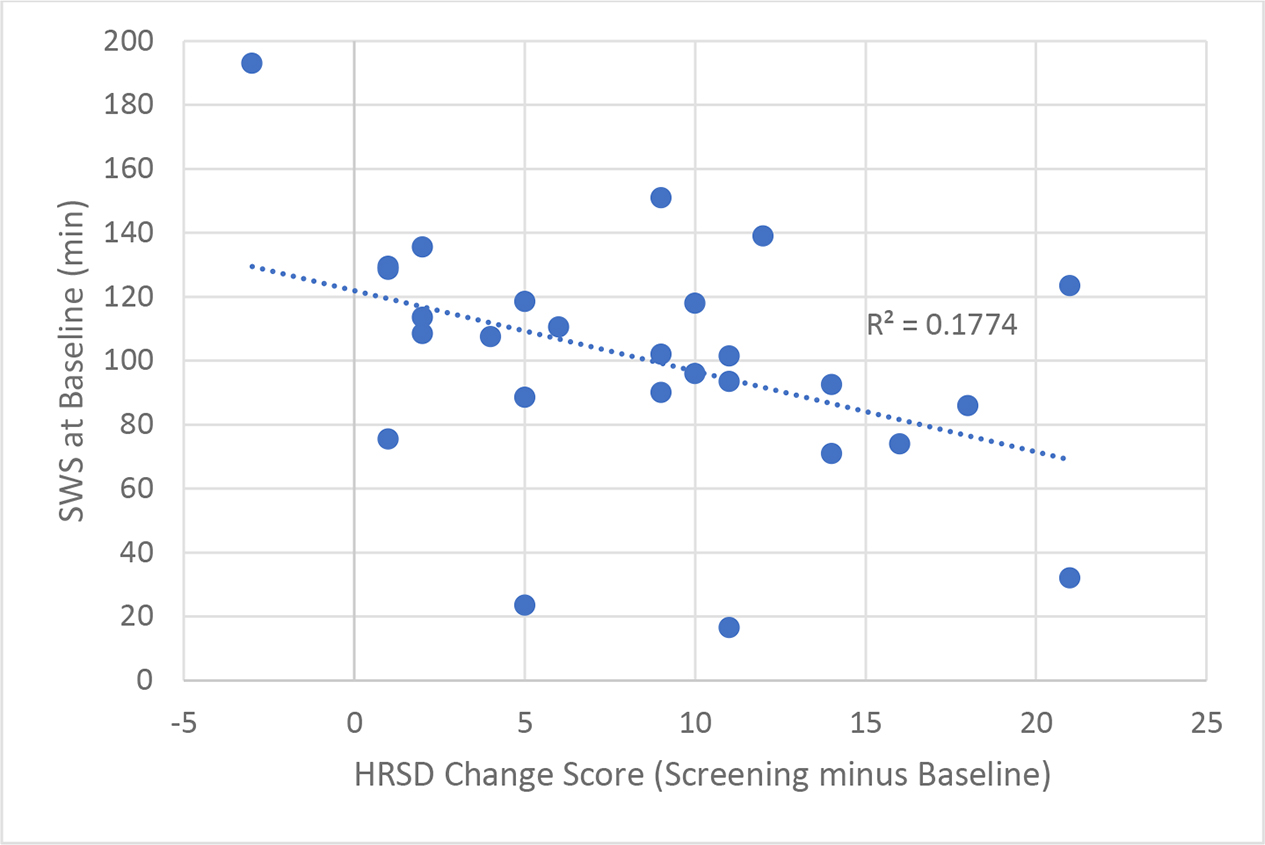
Minutes of Baseline Slow-wave Sleep and Mood Improvement from Screening to Baseline
Figure 2b.

Morningness Eveningness Questionnaire Score and Mood Improvement from Screening to Baseline
Discussion
In the present study, we examined the effects of total sleep deprivation on recovery sleep and mood in a highly controlled laboratory environment in unmedicated individuals with MDD and age and sex-matched healthy controls.
With regard to changes in mood, we did not observe an overall response to total sleep deprivation from the MDD group on either clinician-administered or self-reported measures of mood. We anticipated that our sample would be stratified into responders and non-responders as previous studies have indicated that 50% of individuals with MDD will not respond to sleep deprivation. However, according to the commonly used definition of response of a 30% reduction in HRSD scores, only two participants were classified as responders.
We were also not able to observe an antidepressant response to sleep deprivation with sleep characteristics that have previously been shown to be associated with greater response including REM latency. We did, however, observe that those individuals with melancholic depression showed continued mood improvement throughout the experimental protocol whereas those with non-melanchoic depression showed a slight worsening of mood in response to sleep deprivation. This may indicate that symptoms of melancholic depression (e.g., severe anhedonia, lack of emotional reactivity to positive events, excessive guilt feelings and feelings of hopelessness, significant sleep disturbance and weight loss/low appetite) may be connected to the observed responses to acute sleep deprivation. It has been demonstrated that individuals with a melancholic subtype of depression have greater hypothalamic-pituitary-adrenal (HPA) axis activation relative to those presenting with atypical depression symptoms (Lamers et al., 2013). Previous research has shown reductions in HPA axis activity following sleep deprivation, including one study which hypothesized that lowered HPA activity may be a mechanism through which sleep deprivation exerts its antidepressant effects (Vgontzas et al., 1999). HPA activity was not measured in this study, however these findings may suggest that the study of individuals with a melancholic subtype warrants further investigation.
The sleep deprivation protocol resulted in expected changes to the sleep architecture of the MDD group. Following sleep deprivation, individuals with MDD exhibited less stage 1 and stage 2 sleep and more slow-wave sleep. These changes were expected as the literature shows that slow-wave sleep is a marker of sleep homeostasis or sleep pressure (Borbely, 1982), as it increases proportionally to time spent awake. These increases in slow-wave sleep following sleep deprivation have been demonstrated in healthy and depressed samples, so it is unclear why in the present study, the HC group did not exhibit similar sleep architectural changes.
While we did not observe a reduction in depressive symptoms during sleep deprivation, we did find a significant reduction in symptoms from screening to baseline in the MDD group. One likely explanation for the lack of antidepressant response to sleep deprivation observed here could be a floor effect of mood symptoms which may have limited the ability for individuals to continue to show improvement during sleep deprivation.
This pattern may be attributed to environmental aspects of the sleep laboratory (e.g., regularized routines, near constant staff interaction during waking hours, perceived feelings of safety in the hospital environment) or demand characteristics of study participation (e.g., expectations and/or hope for improvement). In some cases, merely checking into a hospital setting for a prolonged period may have served as a respite from environmental or social issues that are proximately related to mood symptoms, resulting in pre-sleep deprivation improvements. We also found a significant improvement in mood following recovery sleep in both individuals with MDD and those in the HC group. This may have been attributed for some, by the financial compensation for the multi-day protocol which may have provided some more immediate sense of relief if affected by economic stressors. Thus, while the study attempted to control for routine and environmental variability inherent in most trials of sleep deprivation in clinical settings, the current protocol may have over-corrected to the point where aspects of the study environment were in and of themselves antidepressant. Future studies employing such tightly controlled conditions can examine their impact by evaluating depression post-discharge. There is also the possibility that the constant dim light conditions, regardless of routine regularity, influenced response. The degree to which the absence of strong, light-driven zeitgebers may blunt antidepressant response is a worthwhile focus of future research and may help us better understand whether sleep deprivation operates through circadian mechanisms as has been hypothesized (Bunney & Bunney, 2013).
Given that we did observe an improvement in symptoms from screening to post-baseline, we were interested in investigating if this improvement could be predicted using demographic variables or those collected from baseline sleep. We found that compared to those who spent more time in SWS, individuals with MDD who spent less time in SWS showed greater mood improvements. This is in line with our previous work demonstrating a relationship between impairments in the homeostatic regulation of SWS and mood disturbance in MDD (Goldschmied et al., 2018), and other studies showing that the appropriate dissipation of SWA from the first to the second NREM period is a predictor of the response to sleep deprivation (Nissen et al., 2001). Additionally, we also demonstrated that the morning chronotype was associated with greater mood improvements, in line with previous research indicating that MDD is associated with a more evening chronotype, and that sleep phase advance can result in antidepressant effects (Wirz-Justice, 2008). These results add to prior studies that have identified a range of factors associated with the response to sleep deprivation including peripheral inflammation (Benedetti et al., 2017), patterns of activation on neuroimaging (Wu, Joseph et al., 1999), and genetic burden of depression (Trautmann et al., 2018).
Our observed rate of symptom reduction was substantially lower than the 50% response rate observed in previous studies (Boland et al., 2017). As we have stated, although we removed a number of environmental confounds we may have inadvertently introduced other environmental/psychological supports that may have improved subjects’ depression at baseline, thus making it more difficult to observe sleep deprivation effects from already reduced depression severity scores. As such, we cannot make claims about the degree to which factors relevant to the clinical setting may influence antidepressant effects of sleep deprivation. That said, to further evaluate sleep deprivation mechanisms, greater attention to homogeneity in clinical setting across studies is warranted. Isolating the biological mechanisms from the psychosocial effects of changes in environment, interpersonal interaction, and constant observation is an important goal.
Our findings should be interpreted in light of several study limitations. As previously stated, laboratory conditions may have promoted improved depression severity prior to SD, limiting our observation of antidepressant effects specific to sleep deprivation. Additionally, strict eligibility requirements (e.g., BMI restrictions, early morning habitual wake times, regular sleep schedule, free of antidepressant medications) may have limited generalizability to the greater population of individuals with MDD and may have been related to the lower-than-expected rate of antidepressant response. In particular, the requirement of participants to be medication free may have biased the sample towards less severe depression since it could have been clinically contraindicated for more severely depressed individuals on antidepressants to taper off for participation in the study. An advantage of prior studies being conducted in clinical inpatient settings is that they can provide an environment in which it is safer to include unmedicated, severely depressed individuals. Our lack of post-discharge follow-up did not allow us to statistically test whether laboratory conditions contributed to lack of antidepressant response to SD. It is possible that individuals with mild to moderate MDD respond more to changes in their environment than individuals with more severe cases of MDD, resulting in the pattern of findings noted here. Furthermore, it is important to note that milder samples of MDD may respond differently in general to certain interventions compared to individuals with more severe MDD. Therefore, the results of any study that includes a predominantly mild sample may not directly translate to those with more severe cases of the disorder. Future studies should aim to recruit a sample with a broader range of impairment. Lastly, this study included a relatively small sample of participants with MDD, and an even smaller sample of HC. The sample size may have contributed to the high variability with regard to sleep parameters and other outcome measures. Future studies should seek to confirm these results in larger samples.
In summary, this study aimed to examine the effects of total sleep deprivation on recovery sleep and mood in individuals with MDD and age and sex-matched HC and found that individuals with MDD demonstrated significant mood improvements upon entering the highly controlled laboratory environment, but not during the experimental sleep deprivation period. As the extant literature has consistently indicated that sleep deprivation results in rapid antidepressant effects, better understanding of this effect may allow us uncover more information regarding the mechanisms of depression and inform better treatment targets and prevention efforts (Boland et al., 2017; Wu, J. C. & Bunney, 1990).
Supplementary Material
Highlights.
A lower than expected antidepressant response rate was observed in a highly controlled experimental environment.
Participants showed a robust antidepressant response following baseline sleep in the sleep lab.
Those with melancholic features showed continued mood improvement during sleep deprivation.
Role of the Funding Source
National Institute of Mental Health
Award Number: 5R01MH107571
Recipient: Philip Gehrman
National Institute of Mental Health
Award Number: 5K23MH118580
Recipient: Jennifer Goldschmied
U.S. Department of Veterans Affairs
Award Number: IK2-CX001501
Recipient: Elaine Boland
Footnotes
Declarations of interest:
Dr. Philip Gehrman has received research support from Merck, Inc. and has been a paid consultant for Eight Sleep, Eisai Inc., Fisher Wallace Laboratories, and Idorsia.
All other authors declare no financial and personal relationships with other people or organizations that could inappropriately influence or bias this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagby RM, Ryder AG, Schuller DR, & Marshall MB (2004). The hamilton depression rating scale: Has the gold standard become a lead weight? American Journal of Psychiatry, 161(12), 2163–2177. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Benedetti F, Barbini B, Bernasconi A, Fulgosi MC, Colombo C, Dallaspezia S, et al. (2008). Serotonin 5-HT2A receptor gene variants influence antidepressant response to repeated total sleep deprivation in bipolar depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 32(8), 1863–1866. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Poletti S, Hoogenboezem TA, Locatelli C, de Wit H, Wijkhuijs AJ, et al. (2017). Higher baseline proinflammatory cytokines mark poor antidepressant response in bipolar disorder. The Journal of Clinical Psychiatry, 78(8), 4015. [DOI] [PubMed] [Google Scholar]
- Boland EM, Rao H, Dinges DF, Smith RV, Goel N, Detre JA, et al. (2017). Meta-analysis of the antidepressant effects of acute sleep deprivation. The Journal of Clinical Psychiatry, 78(8), e1020–e1034. [DOI] [PubMed] [Google Scholar]
- Borbely AA (1982). A two process model of sleep regulation. Human Neurobiology, 1(3), 195–204. [PubMed] [Google Scholar]
- Borbely AA, & Wirz-Justice A (1982). Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation. Human Neurobiology, 1(3), 205–210. [PubMed] [Google Scholar]
- Bunney BG, & Bunney WE (2013). Mechanisms of rapid antidepressant effects of sleep deprivation therapy: Clock genes and circadian rhythms. Biological Psychiatry, 73(12), 1164–1171. [DOI] [PubMed] [Google Scholar]
- Dallaspezia S, Suzuki M, Clara L, Colombo C, & Benedetti F (2018). Chronotype influences response to antidepressant chronotherapeutics in bipolar patients. Chronobiology International, 35(9), 1319–1325. [DOI] [PubMed] [Google Scholar]
- Germain A, & Kupfer DJ (2008). Circadian rhythm disturbances in depression. Human Psychopharmacology: Clinical and Experimental, 23(7), 571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedke H, & Schwarzler F (2002). Therapeutic use of sleep deprivation in depression. Sleep Medicine Reviews, 6(5), 361–377. [PubMed] [Google Scholar]
- Gillin JC, Buchsbaum M, Wu J, Clark C, & Bunney W (2001). Sleep deprivation as a model experimental antidepressant treatment: Findings from functional brain imaging. Depression and Anxiety, 14(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Goldschmied JR, Cheng P, Hoffmann R, Boland EM, Deldin PJ, & Armitage R (2018). Effects of slow-wave activity on mood disturbance in major depressive disorder. Psychological Medicine, 49(4), 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmeter U, Hemmeter-Spernal J, & Krieg J (2010). Sleep deprivation in depression. Expert Review of Neurotherapeutics, 10(7), 1101–1115. [DOI] [PubMed] [Google Scholar]
- Holsboer-Trachsler E, Hemmeter U, Hatzinger M, Seifritz E, Gerhard U, & Hobi V (1994). Sleep deprivation and bright light as potential augmenters of antidepressant drug treatment—neurobiological and psychometric assessment of course. Journal of Psychiatric Research, 28(4), 381–399. [DOI] [PubMed] [Google Scholar]
- Horne JA, & Ostberg O (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology, 4(2) [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, De Jonge P, Beekman A, & Penninx B (2013). Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Molecular Psychiatry, 18(6), 692–699. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Moul DE, Schwartz PJ, Madden PA, & Wehr TA (1993). A clinical trial of sleep deprivation in combination with antidepressant medication. Psychiatry Research, 46(3), 213–227. [DOI] [PubMed] [Google Scholar]
- McCormack HM, David J. d. L., & Sheather S. (1988). Clinical applications of visual analogue scales: A critical review. Psychological Medicine, 18(04), 1007–1019. [DOI] [PubMed] [Google Scholar]
- Nissen C, Feige B, König A, Voderholzer U, Berger M, & Riemann D (2001). Delta sleep ratio as a predictor of sleep deprivation response in major depression. Journal of Psychiatric Research, 35(3), 155–163. [DOI] [PubMed] [Google Scholar]
- Rasmussen KG, Stevens SR, Kung S, & Mohan A (2010). Melancholic symptoms as assessed by the hamilton depression rating scale and outcomes with and without electroconvulsive therapy on an in-patient mood disorders unit. Acta Neuropsychiatrica, 22(1), 21–25. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, & Kales A (1968). A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Public Health Service, US Government Printing, , 1–12. [Google Scholar]
- Riemann D, Wiegand M, & Berger M (1991). Are there predictors for sleep deprivation response in depressed patients? Biological Psychiatry, [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kantermann T, Juda M, Vetter C, & Allebrandt KV (2013). Light and the human circadian clock. Circadian Clocks, , 311–331. [DOI] [PubMed] [Google Scholar]
- Trautmann N, Foo JC, Frank J, Witt SH, Streit F, Treutlein J, et al. (2018). Response to therapeutic sleep deprivation: A naturalistic study of clinical and genetic factors and post-treatment depressive symptom trajectory. Neuropsychopharmacology, 43(13), 2572–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, & Chrousos GP (1999). Sleep deprivation effects on the activity of the hypothalamic–pituitary–adrenal and growth axes: Potential clinical implications. Clinical Endocrinology, 51(2), 205–215. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A (2008). Diurnal variation of depressive symptoms. Dialogues in Clinical Neuroscience, 10(3), 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, & Bunney WE (1990). The biological basis of an antidepressant response to sleep deprivation and relapse: Review and hypothesis. The American Journal of Psychiatry, 147(1), 14–21. [DOI] [PubMed] [Google Scholar]
- Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, et al. (1999). Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. American Journal of Psychiatry, 156(8), 1149–1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


