SUMMARY
Saccadic eye movements are known to cause saccadic suppression, a temporary reduction in visual sensitivity and visual cortical firing rates. While saccadic suppression has been well characterized at the level of perception and single neurons, relatively little is known about the visual cortical networks governing this phenomenon. Here we examine the effects of saccadic suppression on distinct neural subpopulations within visual area V4. We find subpopulation-specific differences in the magnitude and timing of peri-saccadic modulation. Input-layer neurons show changes in firing rate and inter-neuronal correlations prior to saccade onset, and putative inhibitory interneurons in the input layer elevate their firing rate during saccades. A computational model of this circuit recapitulates our empirical observations and demonstrates that an input-layer-targeting pathway can initiate saccadic suppression by enhancing local inhibitory activity. Collectively, our results provide a mechanistic understanding of how eye movement signaling interacts with cortical circuitry to enforce visual stability.
Graphical Abstract
In brief
Denagamage et al. investigate perisaccadic neural dynamics in the laminar cortical microcircuit of visual area V4 and uncover an input-layer-targeting pathway that mediates saccadic suppression via local inhibitory neurons. An E-I network model at the cusp of inhibition-stabilized network regime explains saccadic suppression.
INTRODUCTION
As we observe the world around us, our eyes dart from point to point. Each of these shifts in gaze is a saccade, a ballistic movement of both eyes. Saccades significantly improve the efficiency of the primate visual system by allowing us to flexibly deploy our fovea, the dedicated high-acuity zone at the center of the retina, toward objects of interest in our environment. However, saccades also pose a substantial challenge for ongoing visual processing, as each saccade produces abrupt and rapid motion across the retina. Therefore, saccades are also accompanied by a temporary reduction in visual sensitivity, termed saccadic suppression, which serves to blunt our perception of this motion.1,2 Perhaps reflecting the diminished sensory processing during saccades, the firing rates of neurons in many regions of the visual cortex are also transiently suppressed.3–6 However, despite extensive psychophysical characterization and singleneuron electrophysiological analysis, relatively little is known about the circuit-level mechanisms underlying saccadic suppression in the visual cortex. One prevailing hypothesis suggests that saccadic suppression is mediated by a corollary discharge signal originating in the brain regions responsible for initiating eye movements.7–9 Given the synaptic and motor delays accompanying the execution of the saccadic motor command, a coincident corollary discharge to the visual cortex could trigger compensatory mechanisms prior to the start of the eye movement. Consistent with this idea, changes in neural activity before saccade onset have been observed in several visual cortical areas.5 However, despite clear neural evidence that the visual cortex receives information about upcoming saccades, the pathway by which a saccadic signal arrives in the visual cortex is unknown, and how that signal interacts with local circuitry to mediate saccadic suppression remains unclear.
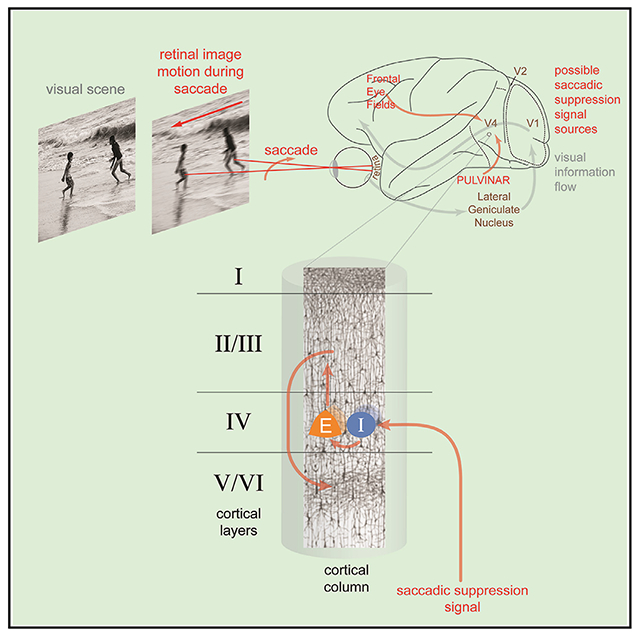
We examined these questions in visual area V4, a critical hub in the visual processing stream responsible for object recognition.10 V4 is known to exhibit peri-saccadic suppression in both humans4 and macaques,6 and undergoes dynamic shifts in stimulus sensitivity during the preparation of saccades.11 To better interrogate the underlying neural mechanisms, we studied peri-saccadic suppression of neural activity in the context of the laminar cortical circuit, which is composed of six layers with highly stereotyped patterns of intra- and inter-laminar connectivity.12,13 In higher-order visual areas, such as V4, layer IV (the input layer) is the primary target of projections carrying visual information from lower-order areas, such as V1, V2, and V3.14,15 After arriving in the input layer, visual information is then processed by local neural subpopulations as it is sent to layers II/ III (the superficial layer) and layers V/VI (the deep layer), which serve as output nodes in the laminar circuit.12,16 In V4, the superficial and deep layers feed information forward to downstream visual areas, such as ST,17 TEO,18 and TE.19 Alongside feedforward input from lower-order visual areas, V4 also receives projections from other cortical regions, such as the prefrontal cortex,15 as well as from subcortical structures.20 Accordingly, several pathways targeting V4 could be responsible for relaying information about upcoming saccades: (1) projections from lower visual areas (V1/V2/V3), which predominantly target the input layer14,15; (2) a projection from the frontal eye fields (FEFs), which predominantly targets the superficial and deep layers21,22; and (3) a projection from the pulvinar, which predominantly targets the superficial and input layers.23–25 Neurons in V1,26,27 FEF,28–30 and pulvinar31–33 are known to respond to saccades, and the corresponding pathways are therefore strong candidates for initiating suppression in V4.
Given the organization of cortical circuitry, we reasoned that a saccadic signaling pathway should target the site of entry for visual information, the input layer, to suppress visual processing most efficiently. Furthermore, considering the similarities between peri-saccadic stimulus processing and general reductions in visual gain,6,26 we considered whether saccadic suppression might be mediated in a manner comparable to visual gain modulation. Specifically, we hypothesized that an elevation in local inhibitory activity, a common mechanism for gain control,34–36 functionally suppresses neural processing in V4 during saccades. We sought to confirm our predictions by utilizing a cortical-layer-specific recording approach in combination with electrophysiological cell-type classification to simultaneously record from six well-defined neural subpopulations in V4.
RESULTS
Dissociating the effects of vision and eye movements
Past approaches for studying peri-saccadic modulation of neural activity have typically involved cued saccade tasks,4,6,26,37 which risk conflating the neural effects of visual stimulus processing with the neural effects of saccades. To dissociate saccadic signaling from visually induced activity in area V4, we performed a series of spontaneous recordings while two rhesus macaques were seated in the dark. We found that, despite their inability to observe specific objects in the environment, both animals continued to make saccades freely under these conditions. Spontaneous neural activity also persisted (Figure S1A). We identified individual saccades by estimating the onset and offset times of ballistic eye movements (see STAR Methods). To remove fixational eye movements (microsaccades), we discarded saccades with amplitudes less than 0.7° of visual angle. Additionally, to prevent the neural effects of one eye movement from overlapping with those of the next, we only included saccades that were separated from one another by at least 500 ms. This approach provided us with a set of 4,420 saccades that were used for all subsequent analyses. We evaluated the quality of our eye movement dataset by examining amplitude and velocity traces from individual saccades, as well as by characterizing the main sequence. The main sequence is the approximately linear amplitude-velocity relationship observed across all saccades,38 which we found held true for our data (Figure 1A).
Figure 1. Laminar recordings and saccade identification.

(A) The amplitude-velocity relationship (“main sequence”) for all saccades (n = 4420) in the dataset. An approximately linear trend is expected for saccades, as is found here (R2 = 0.555). dva, degrees of visual angle.
(B) Stacked contour plot showing spatial receptive fields (RFs) along the laminar probe from an example session. The RFs are well aligned, indicating perpendicular penetration down a cortical column. Zero depth represents the center of the input layer as estimated with current source density (CSD) analysis.
(C) CSD displayed as a colored map. The x axis represents time from stimulus onset; the y axis represents cortical depth. The CSD map has been spatially smoothed for visualization.
(D) Example of continuous eye-tracker and electrophysiological recordings. Eye traces are plotted at the top in gray. LFP traces are plotted by depth below, and spikes are overlaid on the corresponding channel. LFP traces have been color coded by layer identity; spikes have been color coded by cell type.
While the monkeys were making spontaneous saccades, we simultaneously recorded neural activity from well-isolated single-units (n = 211), multi-units (n = 110), and local field potentials (LFPs) using linear array probes in area V4. The use of recording chambers containing an optically transparent artificial dura allowed us to make electrode penetrations perpendicular to the cortical surface and therefore in good alignment with individual cortical columns (see STAR Methods). The alignment of each penetration was confirmed by mapping receptive fields along the depth of cortex, with overlapping receptive fields indicating proper alignment (Figure 1B). Laminar boundaries were identified with current source density (CSD) analysis.39,40 The CSD, defined as the second spatial derivative of the LFP, maps the location of current sources and sinks along the depth of the probe. Each layer of the visual cortex produces a characteristic current source-sink pattern in response to visual stimulation, with the input layer producing a current sink followed by a current source, and the superficial and deep layers producing the opposite pattern of activity (Figure 1C). By identifying transitions between current sources and sinks, we assigned laminar identities to each of our recording sites (Figure 1D, LFP traces colored by layer). We also separated well-isolated single-units into two populations based on their waveform duration: narrow-spiking units, corresponding to putative inhibitory interneurons, and broadspiking units, corresponding to putative excitatory neurons (Figure 1D, spikes colored by unit type).41–43 The three cortical layers and two unit types thus gave us six distinct neural subpopulations to consider in subsequent analyses.
Narrow-spiking input-layer units show positive perisaccadic modulation
To evaluate neural subpopulation-specific responses, we first estimated the peri-saccadic firing rate of well-isolated single-units with a kernel-based approach, in which each spike is convolved with a Gaussian kernel44 (Figure S1B). We selected the kernel bandwidth on a unit-by-unit basis at each point in time using an optimization algorithm that minimizes mean integrated squared error.45 When looking at units grouped by cell type, narrow-spiking units exhibited less peri-saccadic suppression than broad-spiking units (Figure 2A). The cause of this difference became clear when the units were further separated by layer. All laminar subpopulations of broad-spiking units displayed peri-saccadic suppression, as did narrow-spiking units in the superficial and deep layers. In contrast, narrow-spiking input-layer units elevated their firing rate in response to a saccade (Figures 2B and 2C; see Figure S2 for additional single-unit examples).
Figure 2. Narrow-spiking input-layer units display peri-saccadic enhancement of firing.

(A) Average peri-saccadic normalized firing rate for broad- (n = 102 units) and narrow-spiking (n = 95 units) populations. Thin lines indicate standard error of the mean.
(B) As in (A), but including only broad-spiking units from the superficial (n = 33), input (n = 35), and deep (n = 34) layer populations.
(C) As in (A), but including only narrow-spiking units from the superficial (n = 25 units), input (n = 24 units), and deep (n = 46 units) layer populations.
(D) Average peri-saccadic modulation index for the six neural subpopulations. Two-way ANOVA (, p = 0.3151; , p = 0.1765; , p = 0.0030) with Tukey’s multiple comparison tests (narrow-spiking superficial vs. narrow-spiking input, *p = 0.0423; narrow-spiking input vs. narrow-spiking deep, *p = 0.0495; narrow-spiking input vs. broad-spiking input, **p = 0.0070; narrow-spiking input vs. broad-spiking deep, #p = 0.0817). Error bars indicate standard error of the mean.
(E) Average peri-saccadic modulation index for positively modulated units from the six neural subpopulations. Two-way ANOVA (, p = 0.1666; , p = 0.1355; , p = 0.0077) with Tukey’s multiple comparison tests (narrow-spiking superficial vs. narrow-spiking input, #p = 0.0833; narrow-spiking input vs. narrow-spiking deep, **p = 0.0073; narrow-spiking input vs. broad-spiking input, *p = 0.0106; narrow-spiking input vs. broad-spiking deep, *p = 0.0384). Error bars indicate standard error of the mean.
(F) Proportion of units in each neural subpopulation with a positive peri-saccadic modulation index.
To quantify these observations, we calculated a modulation index that represents the degree to which the firing rate of each unit deviated from its pre-saccadic baseline activity (see STAR Methods). We found that narrow-spiking input-layer units were the only group with a positive modulation index (indicating an increase in firing rate), which was significantly different from most other subpopulations (Figure 2D). We also noted that, at the single-unit level, each of the six subpopulations had some proportion of positively modulated units. Therefore, the difference in subpopulation-level firing rates could be caused by narrowspiking input-layer neurons having a greater average magnitude of positive modulation or by having a greater proportion of positively modulated units. We determined both to be true. Narrow-spiking input-layer neurons displayed a larger magnitude of positive modulation (Figure 2E) and were also more likely to be positively modulated (Figure 2F). We found it particularly striking that, in accordance with our hypothesis, only the narrow-spiking (putative inhibitory interneurons) input-layer subpopulation showed an enhancement in firing. This led us to believe that an elevation in input-layer inhibitory activity could be responsible for the peri-saccadic suppression of firing within the visual cortex reported previously.3–6
Input-layer units respond prior to saccades
To gate visual signals received during a saccade, neurons in the cortical layer initiating suppression would need to be activated prior to the start of the eye movement. To confirm that this was true for the input layer, we performed a timing analysis to determine whether any of the recorded subpopulations show significant changes in firing rate prior to saccade onset. For each single unit, we found the time at which their activity first deviated outside a 95% confidence interval calculated from their presaccadic baseline activity, an event that was marked as the time of first significant response. The results of this approach for six example units (the same example units as in Figure S1B) are illustrated in Figure 3A. While there was response variability at the level of single units, the input-layer subpopulations (both narrow and broad spiking) were the only ones whose activity was modulated prior to saccade onset (Figure 3B). This provides strong evidence that the input layer receives information about upcoming saccades with sufficient time to enact changes in activity throughout the cortical column.
Figure 3. Input-layer units display modulation prior to saccade onset.
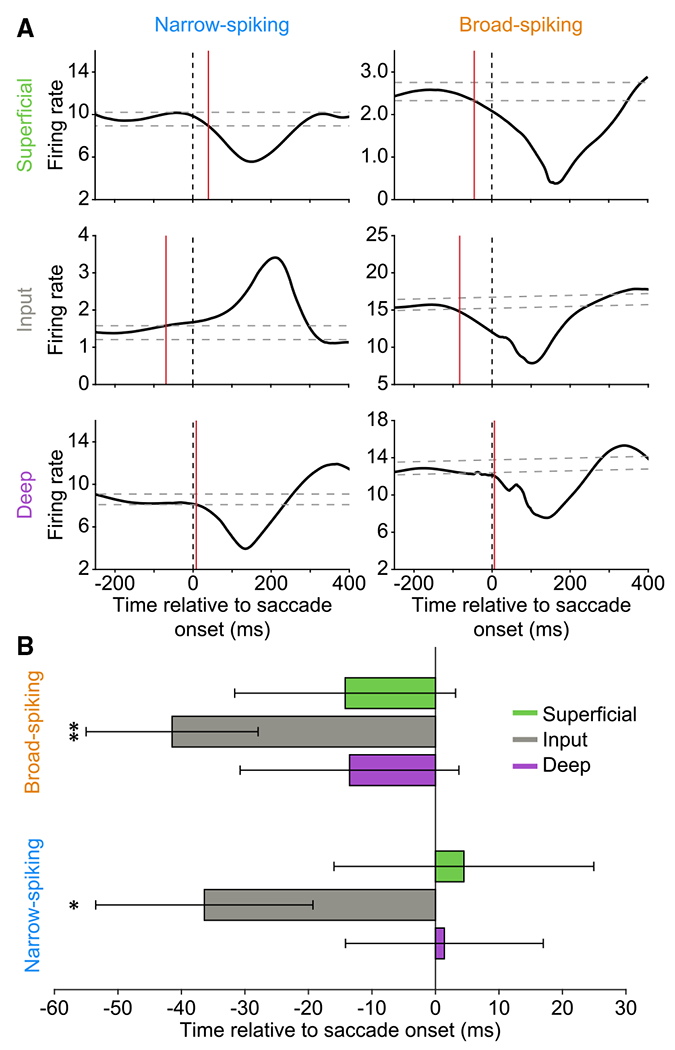
(A) Six example single units demonstrating the approach for identifying the time of significant peri-saccadic firing-rate modulation. The 95% confidence intervals were calculated for the baseline activity of each unit (dashed gray lines) and the first deviation outside of those bounds was marked as the time of first significant response (red line). The example units shown are the same as those from Figure S1B.
(B) Average timing of first significant peri-saccadic modulation for the six neural subpopulations. Input-layer units, both broad and narrow spiking, show modulation prior to saccade onset. Two-tailed one-sample t test (broad-spiking input layer, **p = 0.0049; narrow-spiking input layer, *p = 0.0492). Error bars indicate standard error of the mean.
Saccades increase correlated variability in the input layer
If input-layer neurons receive pre-saccadic excitation from a common source, that signaling should also manifest itself as an increase in correlated variability among pairs of input-layer units prior to saccade onset. To test this, we computed spike-count correlations (SCCs) between pairs of simultaneously recorded single and multi-units as a function of time and pooled these results into six groups on the basis of their pairwise laminar locations. All pair types showed a substantial increase in SCC around or after the time of saccade onset (Figure S3A). We quantified the time at which correlations first began to rise with a bootstrap analysis (see STAR Methods). SCC began increasing prior to saccade onset in input-input pairs (Figure 4A) but after saccade onset in all other pairs (Figure 4B). This pattern of activity is consistent with the pre-saccadic arrival of a signal that is shared among input-layer units. The delayed response of all other pair types suggests that they inherit their activity from the input layer. Additionally, the time at which input-input correlations began to rise (~40 ms before saccade onset) is remarkably similar to the time at which input-layer units began to show firing-rate modulation (also ~40 ms before saccade onset), further indicating that these two observations have a common cause: the activation of a saccadic signaling pathway.
Figure 4. Saccade onset increases correlated variability most prominently in the input layer.

(A) SCCs as a function of time relative to saccade onset for input-input unit pairs (n = 240 pairs). Correlations were calculated with a sliding 201-ms window. Thin lines indicate bootstrapped 95% confidence intervals.
(B) Average start time of spike-count correlation rise relative to saccade onset. The y axis labels indicate layer identity of unit pairs (S, superficial; I, input; D, deep). Error bars represent bootstrapped 95% confidence intervals. Input-input pairs begin to rise before saccade onset and before all other pair combinations. All other pairs respond after saccade onset.
(C) Average SSC between pairs of simultaneously recorded units (single units and multi units) before (−200 to 0 ms) and after (0–200 ms) saccade onset as a function of frequency. Superficial layer, n = 299 pairs; input layer, n = 191 pairs; deep layer, n = 604 pairs. Thin lines indicate standard error of the mean. Dashed lines indicate SSC for shuffled data.
(D) Same data as in (C), but averaged across three frequency bands and plotted as a modulation index: (SSCafter − SSCbefore)/(SSCafter + SSCbefore). Two-tailed one-sample t tests (superficial 0–12 Hz, ***p = 1.64 × 10−10; superficial 15–25 Hz, **p = 0.0027; superficial 30–100 Hz, p = 0.5468; input 0–12 Hz, ***p = 2.2131 × 10−7; input 15–25 Hz, ***p = 5.5458 × 10−4; input 30–100 Hz, ***p = 1.6503 × 10−5; deep 0–12 Hz, **p = 0.0013; deep 15–25 Hz, *p = 0.0116; deep 30–100 Hz, **p = 0.0050). Error bars indicate standard error of the mean.
Alongside the expected rise in correlations, we also found a reduction in correlations that began ~250 ms prior to saccade onset among input-input pairs. Because the timing of this drop precedes saccadic initiation, which begins ~150 ms before saccade onset,46,47 it could not be the result of a corollary discharge signal. Instead, it may reflect the shift in internal state that occurs before saccadic initiation as subjects shift their attention toward a potential target location.48 In accordance with this possibility, pupil diameter, a well-established correlate of internal state,49,50 begins to ramp up several hundred milliseconds prior to saccade onset (Figure S3B).51 Similar shifts in internal state are known to be accompanied by comparable reductions in correlated variability.50,52–54
To further characterize changes in coordinated peri-saccadic activity, we examined differences in spike-spike coherence (SSC) before and after saccade onset. SSC is a measure of the degree to which the activity of two signals, in this case a pair of simultaneously recorded single and/or multi-units, fluctuates together. Unlike SCC, coherence measures covariance as a function of frequency. We found that saccade onset increases wide-band coherence most prominently in the input layer, with weaker, but still significant, effects in the superficial and deep layers (Figure 4C). When quantified as a modulation index (Figure 4D), we observed significant modulation in all layers at all frequencies, with the exception of the 30- to 100-Hz band in the superficial layer. The input layer exhibits larger increases in coherence, particularly at higher frequencies, while the deep layer experiences smaller increases, particularly at lower frequencies. Collectively, these data suggest that saccade onset leads to a temporary elevation of correlated activity, which manifests itself most prominently in the input layer. The timing and magnitude of changes in correlated variability, measured with both SCC and SSC, further suggests that an external signal arrives prior to saccade onset within the input layer before then propagating to the other layers.
Saccades increase the strength of low-frequency wideband activity
Increases in coordinated activity at the level of single neurons can result in an elevation of population-level wide-band activity. To determine whether this held true for saccades, we calculated the spectral power, a frequency-resolved measure of signal strength, of the LFP around the time of saccade onset. Since LFP signals are highly correlated across the depth of the cortex, we chose to perform this analysis by collapsing across laminar compartments. Saccade onset was associated with a large elevation in low-frequency power, and a slight reduction in high-frequency power (Figure 5A). Comparing the power spectrum before and after saccade onset, we found that these differences were significant in all three frequency bands tested, with an elevation in the 0- to 12-Hz and 15- to 25-Hz bands and a reduction in the 30- to 80-Hz band after saccade onset (Figure 5B). Next, we sought to investigate whether the increased strength of low-frequency wide-band activity entrains spiking units, as reported previously in other contexts.55–57 We found that saccade onset significantly increases low-frequency spike-LFP phase locking (Figure 5C), as measured by pairwise phase consistency (PPC). These results indicate that saccade onset increases the strength of low-frequency population-level activity, which could in turn entrain the activity of local neurons.
Figure 5. Saccade onset increases low-frequency power and spike-LFP locking.

(A) Time-frequency representation (spectrogram) of LFP power around saccade onset. Signal strength is represented as percentage change in power (i.e., the raw power at each time point is divided by the average power in the baseline period, defined here as 250–150 ms before saccade onset).
(B) Top: average power spectra before (−200 to 0 ms) and after (0–200 ms) saccade onset. Thin lines indicate standard error of the mean. Bottom: same data as in top, but averaged across three frequency bands and normalized to data before saccade onset for visualization. Two-tailed paired-sample t tests (0–12 Hz, ***p = 3.4669 × 10−92; 15–25 Hz, ***p = 9.3690 × 10−74; 30–100 Hz, ***p = 2.7322 × 10−9). Error bars indicate standard error of the mean.
(C) Top: average spike-LFP locking spectra before (−200 to 0 ms) and after (0–200 ms) saccade onset. Thin lines indicate standard error of the mean. Bottom: same data as in top, but averaged across three frequency bands. Two-tailed paired-sample t tests (0–12 Hz, ***p = 1.7147 × 10−19; 15–25 Hz, p = 0.1598; 30–100 Hz, p = 0.1091). Error bars indicate standard error of the mean.
A computational model demonstrates that activation of an input-layer-targeting projection is sufficient for inducing saccadic suppression
Given that input-layer units exhibit significant changes in firing properties prior to saccade onset, we hypothesized that a projection targeting this layer could serve as the initiator of saccadic suppression within area V4. To determine whether such a projection would be sufficient for initiating suppression across all cortical layers, as well as to gain further insight into the associated circuit-level mechanisms, we developed a computational model of peri-saccadic activity changes within a cortical column. Our model draws inspiration from previous cortical circuit models that describe population-specific firingrate changes in response to the activation of an external input.58 To simulate cortical circuit-level dynamics more faithfully, our model consists of six interconnected populations across three layers of the cortex (Figure 6A, left). Each cortical layer contains an excitatory (E) and inhibitory (I) population, which project to each other as well as to themselves. Prior studies of local E-I networks in the visual cortex suggest that such networks operate in multiple regimes. Of particular relevance to our study is the finding that E-I networks switch to an inhibition-stabilized network (ISN) regime59,60 from a non-ISN one during stimulus processing. The ISN prevents recurrent networks from becoming overexcited or unstable, and explains many observed dynamics of sensory cortical areas.58,59,61 We thus tuned the connections within a layer to allow the local E-I network to switch in and out of the ISN regime: the baseline regime of the network in the absence of stimulus processing is non-ISN, but direct external input to the E population switches it to ISN (see STAR Methods; Figures S4A–S4C). Connections between layers exist in the form of projections from E neurons in the source layer to both E and I neurons in the target layer, with E-E and E-I connections tuned to effect a net increase in E activity in the target layer in response to an increase in E activity in the source layer. In our model, the input layer projects to the superficial layer, which then projects to the deep layer; this connectivity motif highlights the primary pathway for information flow within a visual cortical column.13,62 Considering our empirical firing-rate analysis, which found that both broad- and narrow-spiking units in the input layer show responses prior to saccade onset (Figure 3B), we chose to model the input layer as the primary recipient of the saccadic signal input. We tuned the strength of the excitatory saccadic signal pathway to the local E and I populations in this layer such that its activation affected a net suppression of the E activity.
Figure 6. A computational model confirms that activation of an input-layer-targeting projection induces saccadic suppression.

(A) Connectivity between populations in our computational model. Excitatory (E) and inhibitory (I) populations within each layer project to each other as well as themselves. The input-layer excitatory population projects to the superficial layer, while the superficial excitatory population projects to the deep layer. In our model of spontaneous saccades (left), we activate an external excitatory projection that selectively targets the input layer. In our model of saccades executed in the presence of visual stimuli (right), we add an additional input that selectively targets input-layer excitatory neurons.
(B) Normalized peri-saccadic firing rate of simulated neural subpopulations during spontaneously executed saccades (n = 110 simulated saccades). The input-layer inhibitory subpopulation shows enhancement of firing, while all other subpopulations show suppression.
(C) Further dissection of input-layer activity. (Top) The saccadic signal arriving in the input layer was simulated with a ramping function. Represented here are saccadic signals of three different strengths. From weakest to strongest, these are represented by dotted, continuous, and dashed lines, both here and in the following subpanels. (Middle) The excitatory and inhibitory input-layer subpopulation firing rates in response to three saccadic signals of varying strength. (Bottom) Net excitatory drive onto the inhibitory input-layer subpopulation in response to three saccadic signals of varying strength. The excitatory drive is the sum of drive from the external saccadic input and drive from the local excitatory population.
(D) Normalized peri-saccadic firing rate of simulated neural subpopulations during saccades executed in the presence of visual stimulation (n = 102 simulated saccades). Visual stimulation is represented by the activation of a second input, which selectively targets the input-layer excitatory subpopulation. Here, all subpopulations within the cortical column show peri-saccadic suppression.
In response to the activation of a saccadic signal of sufficient strength slightly before the time of saccade onset, the E inputlayer population rate was suppressed. In addition, our model simultaneously recapitulated our experimental findings in the input-layer I population (enhancement) as well as the E/I populations in other layers (suppression) by virtue of the inter- and intra-layer connectivity (Figures 6B, S4B). Since local E activity is a major source of excitation to the I population within a layer, we next explored the role of the saccadic signal in sustaining input-layer I activity despite a concurrent decline in local E activity. We explored three scenarios with saccadic signals of varying magnitudes ramping up and down over a fixed period (Figure 6C, top). We found that the increase in I activity in the input layer indeed depended on the strength of the saccadic signal and was sustained at sufficiently high magnitudes. Examination of the contribution of different excitatory pathways to the I population showed that sufficiently high saccadic signal magnitude led to an increase in net excitation onto the I population that exceeded the loss in excitation resulting from the reduction of local E firing rates (Figure 6C, middle and bottom). Our model thus demonstrates that an input-layer-specific saccadic signal of adequate strength can increase local I activity, which is sufficient for inducing suppression across the depth of the cortex. These findings correspond closely to our empirical observations. The model further predicted that the time course of the increase in input-layer I activity was dependent on the baseline operating regime of the E-I network. When the network was robustly within the non-ISN regime, I activity increased immediately following saccadic signal onset. However, when the network was still in the non-ISN regime but close to the switching point between the two regimes, the I activity showed little change below a saccadic signal of sufficient magnitude, even when the local E activity showed significant suppression (Figure S4E). This latter scenario corresponds closely to our empirical observations in the input layer (Figures 2B and 2C), suggesting that the baseline state of the cortical network lies in the non-ISN regime but close to the boundary between regimes.
Our experimental paradigm examined peri-saccadic neural activity in the absence of visual input. While this allowed us to investigate the cortical dynamics that are purely a result of the saccadic signal and avoid potential stimulus-induced artifacts in our recordings, it leaves open the question of how activity patterns may change in the presence of visual stimulation. To explore this further, we added a second external excitatory input (visual signal) to the input-layer E population (Figure 6A, right), which is the major target of feedforward visual input into V4.14 The addition of this second input shifted the local E-I network into an ISN regime,59 in which E and I activity increase or decrease concurrently. As a result, simultaneous activation of the visual signal led to a net reduction in the firing rate of both E and I input-layer populations (Figures 6D and S4C). This produced a similar reduction in excitatory drive to the superficial and deep layers, where firing was again suppressed. We confirmed this prediction experimentally by performing an additional set of spontaneous recordings in a third monkey, as described previously, but now in a non-scotopic environment such that the subject was able to visualize its surroundings. Under these conditions, we found that all recorded subpopulations, including the input-layer narrow-spiking subpopulation, showed suppression (Figure S5). These results are also consistent with prior reports of suppression from uncategorized neurons in the visual cortex in the presence of visual stimulation.3,5,6
DISCUSSION
To contextualize our findings regarding neurophysiological saccadic suppression in area V4, we must also consider how this phenomenon may relate to perceptual saccadic suppression, which has been extensively studied. Perceptual suppression has disputed origins: some groups have argued for extra-retinal origins63 such as corollary discharge, while others have argued for visual masking mechanisms.64,65 There is also significant uncertainty about the prevalence of suppression in the dorsal (motion detection) versus ventral (object recognition) visual streams, with cortical suppression having been studied predominantly in the dorsal stream3,5 despite conflicting psychophysical evidence on the impact of saccades on motion perception.64,66 Recent studies have demonstrated that saccadic suppression effects are prevalent in the ventral stream as well.6,26 Given this body of work, it is important to note that the mechanism described here is not likely to be solely responsible for neural suppression across all visual areas, nor is it likely to be the singular initiator of perceptual suppression during saccades. Instead, perceptual suppression almost certainly arises from a coordinated wave of effects across visual and non-visual areas around the time of saccades.
Our data suggest that neural suppression in V4 peaks around 125 ms after saccade onset. This is consistent with the hypothesis that saccadic suppression acts to minimize the effects of visual motion caused by an eye movement. Saccades can last 20–100 ms, depending on their magnitude, and synaptic delays cause visual information to arrive in V4 65–75 ms after it is first received by the retina.67 These response delays can be even longer (up to 160 ms) depending on stimulus conditions and the behavioral task.67,68 Therefore, the time of maximal suppression in V4 overlaps the time at which V4 is processing visual input collected while the eyes are in motion. We also find that firing rates are increased after this transient suppression, which may reflect increased excitability after fixation onset, allowing the visual system to collect new inputs after the saccade.69
The results described here provide evidence that the input layer of area V4 receives the earliest information about upcoming saccades. Units in the input layer showed changes in firing rate prior to saccade onset, while units in other layers did not. In addition, elevated SCCs, a common byproduct of activating a shared input to a neural population,70–73 were observed earliest among pairs of input-layer neurons. Consistent with a single input source (i.e., projection) producing both phenomena, changes in the firing rate and correlational structure of input-layer units were initiated with similar timing, approximately 40 ms before saccade onset. These convergent results indicate that the saccadic signaling pathway in question predominantly targets the input layer, which thereby allows us to infer its source. Given the lack of pre-saccadic modulation in the superficial and deep layers, it is unlikely that a projection from the FEF is responsible for the observed suppression. Likewise, it is doubtful that suppression is fed forward by projections from lower visual areas (V1/V2/V3), as such a mechanism would not explain the enhancement of narrow-spiking input-layer firing rates in V4. Furthermore, to our knowledge, there have been no clear reports of saccadic suppression in V2 or V3. On the other hand, V126,74,75 and its afferent inputs in the lateral geniculate nucleus (LGN)76,77 show suppression and may indeed be modulated presaccadically. However, a quantitative analysis of the suppression timing in these regions to confirm this hypothesis remains to be done. Thus, we propose that saccadic suppression in V4 is likely initiated via the pulvinar, which sends projections to the input layer of V4.23–25
Several lines of evidence are consistent with this view. First, the pulvinar is known to be the target of highly organized projections from the superior colliculus,78 which is a critical component of the saccadic initiation network.79,80 Second, peri-saccadic modulation has been observed in the pulvinar of macaques, where neurons respond to saccades made in the dark without visual stimulation.32,33 Neurons in the pulvinar of cats are able to distinguish between internally generated saccades and simulated saccades produced with image motion, demonstrating that they encode the underlying motor command.31 Lastly, the pulvinar is known to play a crucial role in spatial attention,81–83 which is necessary for the proper execution of saccades toward target stimuli; indeed, temporary inactivation of the pulvinar produces significant deficits in saccadic target selection and execution.84,85 Thus, pulvinar neurons encode the necessary information about saccadic motor planning to be able to provide a meaningful pre-saccadic signal to their efferent targets in the visual cortex.
We also found that narrow-spiking units in the input layer showed a peri-saccadic enhancement in firing. Narrow-spiking units are thought to correspond to parvalbumin-expressing (PV) inhibitory interneurons,86,87 and input-layer PV interneurons are known to receive strong and selective thalamocortical excitation.88–90 This led us to speculate that an elevated level of inhibition within the input layer could suppress local excitatory neurons, as well as neurons in other cortical layers (Figure 6A). To explore this hypothesis, we developed a six-population firingrate model of the visual cortex and its modulation during saccades. The model demonstrated that pre-saccadic activation of an input-layer-targeting projection is sufficient for suppressing signaling within a cortical column. A compelling aspect of our model is its simplicity: with straightforward inter-population connectivity rules, we were able to both replicate our empirical findings as well as reconcile the opposing patterns of activity observed in the input-layer subpopulations. The model also reproduces trends in our experimental observations on the relative time course of broad and narrow spiking activity under a set of conditions wherein the input layer operates on the cusp of ISN and non-ISN operating regimes. Given these results, we propose that inhibitory input-layer neurons initiate saccadic suppression and gate the processing of visual information entering the local cortical network.
We also observed increases in correlated activity across all cortical layers during saccades, in the form of elevated SCCs, SSC, low-frequency power, and low-frequency spike-phase locking. Comparable increases in neural correlation have been associated with diminished stimulus sensitivity in multiple model organisms across a variety of sensory modalities.50,91–94 This may be a result of heightened correlations functionally limiting the ability of a neural population to encode information, as suggested by previous experimental and theoretical work.95–98 Similarly, saccadic suppression at the level of neurons and circuits is also accompanied by a behaviorally significant reduction in stimulus detection capabilities,1,2 indicating that the increases in correlated variability reported here may reflect or contribute to that deficit.
To isolate the effects of the corollary discharge signal in initiating saccadic suppression in V4, we chose to examine neurophysiological responses to saccades executed in the dark, a paradigm that has been employed extensively by previous studies.6,31,99 Under these conditions, the time course of suppression that we observed was similar to reports of V4 activity during saccades made with visual stimulation,6 suggesting that the saccadic signaling network remains intact in dark conditions. Our model demonstrates that a network regime change, which has been shown to occur when the cortical network is actively engaged in sensory processing,59,60 can flip the modulation of the inhibitory input-layer subpopulation in the presence of visual stimulation. This result is consistent with the lack of evidence for subpopulation-specific differences in modulation from previous studies employing cued saccade tasks.4,6,26,37 In summary, our study provides a mechanistic understanding of perisaccadic neural dynamics in a defined cortical circuit.
Limitations of the study
Our study does not establish a causal role for inhibitory interneurons in mediating peri-saccadic perception. Nor does it establish that the peri-saccadic dynamics we observe in area V4 are the same across the visual hierarchy. Our hypothesis that the pulvinar is the most likely candidate for providing excitatory drive to the input layer is largely based on anatomical work, with no direct experimental confirmation.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Anirvan Nandy (anirvan.nandy@yale.edu).
Materials availability
The study did not generate new unique reagents or materials.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request. All original code has been deposited at Zenodo (https://doi.org/10.5281/zenodo.7946057) and are publicly available as of the date of publication. DOIs are listed in the key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Organisms/strains | ||
| Rhesus Macaques (Macaca mulatta) | UC Davis, Worldwide Primates | N/A |
| Software and algorithms | ||
| MATLAB | Mathworks | R2019a |
| Custom Code and Analyses | This paper | Zenodo: https://doi.org/10.5281/zenodo.7946057 |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Experiments were conducted in three male rhesus (Macaca mulatta) monkeys (Monkey A, 11 years old; Monkey C, 19 years old; Monkey D, 8 years old). All procedures were approved by the Institutional Animal Care and Use Committee at the Salk Institute and at Yale University, and conformed to NIH guidelines.
METHOD DETAILS
Experimental design
The study did not involve randomization or blinding, and we did not estimate sample-size before carrying out the study. No subjects or data were excluded from the study.
Surgical procedures
Surgical procedures have been described in detail previously.100,101 In brief, an MRI-compatible, low-profile titanium chamber was placed over the pre-lunate gyrus on the basis of preoperative MRI imaging in three rhesus macaques (right hemisphere in monkey A, left hemisphere in monkey C, right hemisphere in monkey D). The native dura mater was then removed, and a silicone-based, optically clear artificial dura (AD) was inserted, resulting in an optical window over dorsal V4.
Electrophysiology
At the beginning of each recording session, a plastic insert with an opening for targeting electrodes was lowered into the chamber and secured. This served to stabilize the recording site against cardiac pulsations. Neurons were recorded from cortical columns in dorsal V4 using 16-channel linear array electrodes (“laminar probes”; Plexon, Plexon V-probe; monkeys A and C). The laminar probes were mounted on adjustable x-y stages attached to the recording chamber and positioned over the center of the pre-lunate gyrus under visual guidance through a microscope (Zeiss). This ensured that the probes were maximally perpendicular to the surface of the cortex and thus had the best possible trajectory to make a normal penetration down a cortical column. Across recording sessions, the probes were positioned over different sites along the center of the gyrus in the parafoveal region of V4 with receptive field eccentricities between 2 and 7 degrees of visual angle. Care was taken to target cortical sites with no surface micro-vasculature and, in fact, the surface micro-vasculature was used as reference so that the same cortical site was not targeted across recording sessions. The probes were advanced using a hydraulic microdrive (Narishige) to first penetrate the AD and then through the cortex under microscopic visual guidance. Probes were advanced until the point that the top-most electrode (toward the pial surface) registered LFP signals. At this point, the probe was retracted by about 100–200 μm to ease the dimpling of the cortex due to the penetration. This procedure greatly increased the stability of the recordings and also increased the neuronal yield in the superficial electrodes.
The distance from the tip of the probes to the first electrode contact was either 300 μm or 700 μm. The inter-electrode distance was 150 μm, thus negating the possibility of recording the same neural spikes in adjacent recording channels. Neuronal signals were recorded extra-cellularly, filtered, and stored using the Multi-channel Acquisition Processor system (Plexon). Neuronal signals were classified as either multi-unit clusters or isolated single-units using the Plexon Offline Sorter program. Single-units were identified based on two criteria: (1) if they formed an identifiable cluster, separate from noise and other units, when projected into the principal components of waveforms recorded on that electrode and (2) if the inter-spike interval (ISI) distribution had a well-defined refractory period. Single-units were classified as either narrow-spiking (putative interneurons) or broad-spiking (putative pyramidal cells) based on methods described in detail previously.41 Specifically, only units with waveforms having a clearly defined peak preceded by a trough were potential candidates. Units with trough-to-peak duration less than 225 μs were classified as narrow-spiking units; units with trough-to-peak duration greater than 225 μs were classified as broad-spiking units.
Data were collected over 17 sessions (8 sessions in monkey A and 9 in monkey C), yielding a total of 211 single-units (99 narrowspiking, 112 broad-spiking) and 110 multi-unit clusters.
Recording
Stimuli were presented on a computer monitor placed 57 cm from the eye. Eye position was continuously monitored with an infrared eye tracking system (ISCAN ETL-200). Trials were aborted if eye position deviated more than 1 degree of visual angle from fixation. Experimental control was handled by NIMH Cortex software (http://www.cortex.salk.edu/).
Receptive field mapping
At the beginning of each recording session, neuronal RFs were mapped using subspace reverse correlation in which Gabor (eight orientations, 80% luminance contrast, spatial frequency 1.2 cycles/degree, Gaussian half-width 2°) or ring (80% luminance contrast) stimuli appeared at 60 Hz while monkeys fixated. Each stimulus appeared at a random location selected from an 11 x 11 grid with 1° spacing in the appropriate visual quadrant. Spatial receptive maps were obtained by applying reverse correlation to the evoked LFP signal at each recording site. For each spatial location in the 11 x 11 grid, we calculated the time-averaged power in the stimulusevoked LFP (0–200 ms after each stimulus fash) at each recording site. The resulting spatial map of LFP power was taken as the spatial RF at the recording site. For the purpose of visualization, the spatial RF maps were smoothed using spline interpolation and displayed as stacked contours plots of the smoothed maps (Figure 1B). All RFs were in the lower visual quadrant (lower left in monkey A and lower right in monkey C) and with eccentricities between 2 and 7 degrees of visual angle.
Current source density mapping
In order to estimate the laminar identity of each recording channel, we used a CSD mapping procedure.39 Monkeys maintained fixation while 100% luminance contrast ring stimuli were flashed (30 ms), centered at the estimated RF overlap region across all channels. The size of the ring was scaled to about three-quarters of the estimated diameter of the RF. CSD was calculated as the second spatial derivative of the flash-triggered LFPs. The resulting time-varying traces of current across the cortical layers can be visualized as CSD maps (Figure 1C). Red regions depict current sinks in the corresponding region of the cortical laminae; blue regions depict current sources. The input layer (Layer 4) was identified as the first current sink followed by a reversal to current source. The superficial (Layers 1–3) and deep (Layers 5 and 6) layers had opposite sink-source patterns. LFPs and spikes from the corresponding recording channels were then assigned to one of three layers: superficial, input, or deep.
Spontaneous recordings in scotopic conditions
In the main experiment, all monitors and lights were turned off in the recording room and adjacent control room to ensure that the environment was as dark as experimentally feasible. Luminance inside the recording room was less than 9x10−4 cd/m2 (SpectraScan PR 701S, Photo Research). Eye tracker and electrophysiological data were recorded while the animals (monkeys A and C) executed eye-movements freely in the dark.
Spontaneous recordings in non-scotopic conditions
Additional spontaneous data was collected from area V4 of a third animal (monkey D) in a recording room with non-scotopic conditions (lights on in an adjacent control room). Luminance inside the recording room was 0.054 cd/m2 (Sekonic L-478D-U). 64-channel laminar probes (NeuroNexus Technologies, Inc.; 2 shanks, 32 channels/shank, 70 μm site spacing, 200 μm shank spacing) were used for these recordings. Electrical signals from the probe were collected at 30 kHz, digitized on a 64-channel headstage, and send to the recording controller (RHD Recording System, Intan Technologies). Action potential waveforms were extracted offline with Kilosort2 and manually sorted into single- and multi-unit clusters. Unit classification, RF mapping and CSD mapping procedures were identical to the above. Data were collected over 11 sessions yielding a total of 236 single-units (40 narrow-spiking, 196 broad-spiking).
QUANTIFICATION AND STATISTICAL ANALYSIS
Saccade identification
To identify saccade onset and offset times from the raw eye-tracker data, we used the ClusterFix algorithm.102 ClusterFix performs k-means clustering on four parameters (distance, velocity, acceleration, and angular velocity) to find natural partitions in the eyetracker data and identify saccades. To ensure that our set of identified eye movements was ‘clean,’ we imposed several additional criteria: 1) to avoid adjacent saccades affecting our analysis, we only considered saccades separated from neighboring saccades by at least 500 ms, 2) to exclude microsaccades, we only considered saccades that were greater in amplitude than 0.7 degrees of visual angle, 3) to exclude slower eye movements that ClusterFix had mislabeled as saccades, we only considered eye movements less than 200 ms in duration, and 4) to exclude periods of data erroneously labeled as saccades due to blinking or drowsiness, we identified those periods using pupil diameter measurements and excluded nearby saccades. To do so, pupil diameter was range normalized to the maximum and minimum values for each session. Data segments with normalized pupil diameter less than 0.1 were marked as blinks if they lasted less than 400ms, and drowsiness if they lasted longer. Saccades within 100 ms of a blink or drowsiness were excluded.
Saccadic eye movements are known to have an approximately linear relationship between amplitude and peak velocity that is referred to as the main sequence.38 To ensure that our approach identified a set of eye movements that quantitatively resembled the properties of saccades, we calculated the amplitude and peak velocity for each of the identified eye movements and found an approximately linear relationship (Figure 1A).
Firing rate estimation and modulation index
To estimate the peri-saccadic firing rate, spikes were extracted for each saccade beginning 500 ms prior to saccade onset and ending 500 ms after saccade onset. To obtain a continuous estimate of the firing rate, each spike was convolved with a Gaussian kernel and the average firing rate across saccades was calculated for each unit (Figures S1B and S2). The kernel bandwidth was selected on a unit-by-unit basis at each point in time using a bandwidth optimization algorithm that minimized the mean integrated squared error.45 In order to obtain reliable firing rate estimates, only units that fired at least 75 spikes within 500 ms of saccade onset (before or after) across all saccades were included. Firing rates were normalized to a baseline period, which was defined as 200 to 250 ms before saccade onset.
A modulation index was calculated for each unit to estimate the degree to which their activity deviated from baseline following saccade onset (Figures 2D and 2E). The modulation index was defined as , where is the maximum firing rate in the 200 ms after saccade onset and is the baseline firing rate of the unit, calculated by averaging the firing rate for each unit in a time window 350 to 250 ms before saccade onset.
Spike count correlations
To obtain a continuous estimate of spike count correlations, we calculated the Pearson correlation of spike counts across saccades at each time bin for every pair of simultaneously recorded single- and multi-units (Figures 4A and S3A). The window was 201 ms centered in time and was shifted by 1 ms steps. Spike counts were z-scored to control for firing rate differences across time. Traces were smoothed with a 101 ms Gaussian weighted moving average. To perform the timing analysis, we took the local minimum in correlation as the time at which the rise begins. Bootstrapping was performed for each pair type to obtain confidence intervals on the estimate (Figure 4B).
Spike-spike coherence
We computed the coherence between simultaneously recorded, multi-unit pairs within a cortical layer using multi-taper methods103 for two different windows: the 200 ms before and after saccade onset (Figure 4C). Spike trains were tapered with a Slepian taper (TW = 3, K = 5). Magnitude of coherence estimates depend on the number of spikes used to create the estimates.104 To control for differences in firing rate across the two windows, we adopted a rate-matching procedure.53 In order to obtain a baseline for the coherence expected solely due to trends in firing time-locked to saccade onset, we also computed coherence in which saccade identities were randomly shuffled. Only units that elicited at least 50 spikes cumulatively across all saccades in both time windows were considered. A modulation index, defined as , was calculated for each pair of units at each frequency band (Figure 4D).
Power
Local field potential power was calculated with multi-tapered methods.103 Signals were tapered with a Slepian taper (TW = 3, K = 5). Spectrograms (Figure 5A) were generated by sliding a 201 ms window by increments of 1 ms. Power spectra before (−200 to 0 ms) and after (0–200 ms) saccade onset were calculated with static variants of the same methods (Figure 5B). No layer-specific differences were found, so results shown here are averaged across all recording channels.
Pairwise phase consistency
The pairwise phase consistency (PPC) is an estimate of spike-field locking that is not biased by spike count or firing rate.105 Two segments of data were analyzed: the 200 ms before and after saccade onset. In order to obtain reliable estimates, only units that fired at least 50 spikes cumulatively across all saccades during both time windows were considered. The spectro-temporal representation of the local field potential signal was generated through a continuous wavelet transform with a family of complex Morlet wavelets (spanning frequencies from 2 to 100 Hz, with 6 cycles at each frequency). Phase information was extracted at the time of each spike, and the PPC was then calculated for each unit with each LFP channel in the laminar probes (Figure 5C). We observed minimal change in the PPC as a function of LFP layer, so the results presented here were averaged across all recording channels.
Computational model
The firing rate or mean field model consists of a network of six populations representing excitatory (E) and inhibitory (I) populations in the superficial (L1), input (L2), and deep (L3) layers of the cortex. The firing rate model describes temporal changes in the firing rates (r) of each population as a function of the activity of two external inputs (, the saccadic signal, and , the visual signal) and the activity of other populations in the network. The connectivity between populations is depicted in Figure 6A. The network consists of a coupled E-I network in each layer and captures the core aspect of connectivity between cortical layers: information flow from input to superficial to deep layer. The saccadic signal is sent to both E and I populations in the input layer, while the visual signal is sent only to the E population in the input layer. The saccadic signal is described by a ramping function (Figure 6C, top). We introduced Gaussian noise in the simulations by varying the strength (), onset () and offset () of the saccadic signal, in addition to the excitability () of the E () and I () populations. The visual signal is only activated in the second set of simulations, where it is held constant to replicate sustained visual input. The six-population rate model is described by the following equations:
| Equation 1 |
| Equation 2 |
| Equation 3 |
| Equation 4 |
| Equation 5 |
| Equation 6 |
| Equation 7 |
and are the rates at which the excitatory and inhibitory populations approach their steady states. and are the population response functions, described by Equation 7, for the excitatory and inhibitory populations that transform the given inputs into a firing response. is the threshold input and m is the rate of the response function. Parameters tuned in the model to recapitulate the population level observations in experimental data: , , , , , , , , , , , , , , , , , , , , , (Figure 6B, 6D), (Figure 6C), . To better capture the laminar timing differences observed in our data, we set interlaminar delays to 20ms between the input and superficial layers and to 10ms between the superficial and deep layers. Note that these delays are arbitrary and do not influence the underlying connection-strength based dynamical systems model in this parameter regime. We implemented the above as a system of delay-differential equations in MATLAB.
A further tuning was applied to the model to switch each E-I population between an inhibitory stabilized network (ISN) regime and a non-ISN one.60 Experimental data from visual cortex suggest that a cortical network switches to an ISN regime during stimulus processing,59 hence we included this feature to generate model predictions for saccadic suppression-related laminar activity during stimulus presentation. Previous modeling work has shown that a firing-rate E-I network model can switch between these regimes either by modification of connection weights (W), change in slope or rate of response functions (m) or a combination of both.60 We implemented the simplest mechanism for the network to switch between ISN and non-ISN regime: nonlinear response functions. When visual stimulation (modeled as an excitatory input to the input layer E population) is applied to the model cortical column, the network moves to a higher slope region of its response functions, and switches to the ISN operating regime. A phase-plane analysis of the E-I network model illustrates how this results in a distinct response of the network to exactly the same saccadic signal input (Figure S4).
Supplementary Material
Highlights.
Saccadic suppression is mediated by an input-layer-targeting pathway in area V4
Input-layer putative inhibitory neurons are facilitated during spontaneous saccades in dark
Input-layer-targeting pathway can initiate saccadic suppression via local inhibitory neurons
E-I network model operating at the cusp of ISN regime explains saccadic suppression
ACKNOWLEDGMENTS
This research was supported by NIH/NEI R01 EY021827 to J.H.R. and A.S.N.; NIH/NEI R01 EY032555, NARSAD Young Investigator Grant, Ziegler Foundation Grant, and Yale Orthwein Scholar Funds to A.S.N.; NIH/NEI R00 EY025026 to M.P.J.; NIH/NINDS training grants T32-NS007224 and T32-NS041228 to S.D.; and by NIH/NEI core grants for vision research P30 EY019005 to the Salk Institute and P30 EY026878 to Yale University. We would like to thank the veterinary and husbandry staff at the Salk Institute and at Yale for excellent animal care.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.112720.
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
REFERENCES
- 1.Matin E (1974). Saccadic suppression: a review and an analysis. Psychol. Bull 81, 899–917. 10.1037/h0037368. [DOI] [PubMed] [Google Scholar]
- 2.Zuber BL, and Stark L (1966). Saccadic suppression: elevation of visual threshold associated with saccadic eye movements. Exp. Neurol 16, 65–79. 10.1016/0014-4886(66)90087-2. [DOI] [PubMed] [Google Scholar]
- 3.Thiele A, Henning P, Kubischik M, and Hoffmann KP (2002). Neural mechanisms of saccadic suppression. Science 295, 2460–2462. 10.1126/science.1068788. [DOI] [PubMed] [Google Scholar]
- 4.Kleiser R, Seitz RJ, and Krekelberg B (2004). Neural correlates of saccadic suppression in humans. Curr. Biol 14, 386–390. 10.1016/j.cub.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Bremmer F, Kubischik M, Hoffmann KP, and Krekelberg B (2009). Neural dynamics of saccadic suppression. J. Neurosci 29, 12374–12383. 10.1523/JNEUROSCI.2908-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanos TP, Mineault PJ, Guitton D, and Pack CC (2016). Mechanisms of Saccadic Suppression in Primate Cortical Area V4. J. Neurosci 36, 9227–9239. 10.1523/JNEUROSCI.1015-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wurtz RH, Joiner WM, and Berman RA (2011). Neuronal mechanisms for visual stability: progress and problems. Philos. Trans. R. Soc. Lond. B Biol. Sci 366, 492–503. 10.1098/rstb.2010.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommer MA, and Wurtz RH (2002). A pathway in primate brain for internal monitoring of movements. Science 296, 1480–1482. 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- 9.Sommer MA, and Wurtz RH (2006). Influence of the thalamus on spatial visual processing in frontal cortex. Nature 444, 374–377. 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- 10.Roe AW, Chelazzi L, Connor CE, Conway BR, Fujita I, Gallant JL, Lu H, and Vanduffel W (2012). Toward a unified theory of visual area V4. Neuron 74, 12–29. 10.1016/j.neuron.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han X, Xian SX, and Moore T (2009). Dynamic sensitivity of area V4 neurons during saccade preparation. Proc. Natl. Acad. Sci. USA 106, 13046–13051. 10.1073/pnas.0902412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch JA, and Martinez LM (2006). Laminar processing in the visual cortical column. Curr. Opin. Neurobiol 16, 377–384. 10.1016/j.conb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Douglas RJ, and Martin KAC (2004). Neuronal circuits of the neocortex. Annu. Rev. Neurosci 27, 419–451. 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 14.Felleman DJ, and Van Essen DC (1991). Distributed Hierarchical Processing in the Primate Cerebral Cortex (Citeseer). [DOI] [PubMed] [Google Scholar]
- 15.Ungerleider LG, Galkin TW, Desimone R, and Gattass R (2008). Cortical connections of area V4 in the macaque. Cerebr. Cortex 18, 477–499. 10.1093/cercor/bhm061. [DOI] [PubMed] [Google Scholar]
- 16.Rockland KS, and Pandya DN (1979). Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 179, 3–20. 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- 17.Boussaoud D, Ungerleider LG, and Desimone R (1990). Pathways for motion analysis: cortical connections of the medial superiortemporal and fundus of the superior temporal visual areas in the macaque. J. Comp. Neurol 296, 462–495. 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- 18.Distler C, Boussaoud D, Desimone R, and Ungerleider LG (1993). Cortical connections of inferior temporal area TEO in macaque monkeys. J. Comp. Neurol 334, 125–150. 10.1002/cne.903340111. [DOI] [PubMed] [Google Scholar]
- 19.Borra E, Ichinohe N, Sato T, Tanifuji M, and Rockland KS (2010). Cortical connections to area TE in monkey: hybrid modular and distributed organization. Cerebr. Cortex 20, 257–270. 10.1093/cercor/bhp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gattass R, Galkin TW, Desimone R, and Ungerleider LG (2014). Subcortical connections of area V4 in the macaque. J. Comp. Neurol 522, 1941–1965. 10.1002/cne.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson JC, Kennedy H, and Martin KAC (2011). Pathways of attention: synaptic relationships of frontal eye field to V4, lateral intraparietal cortex, and area 46 in macaque monkey. J. Neurosci 31, 10872–10881. 10.1523/JNEUROSCI.0622-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanton GB, Bruce CJ, and Goldberg ME (1995). Topography of projections to posterior cortical areas from the macaque frontal eye fields. J. Comp. Neurol 353, 291–305. 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- 23.Benevento LA, and Rezak M (1976). The cortical projections of the inferior pulvinar and adjacent lateral pulvinar in the rhesus monkey (Macaca mulatta): an autoradiographic study. Brain Res. 108, 1–24. 10.1016/0006-8993(76)90160-8. [DOI] [PubMed] [Google Scholar]
- 24.Shipp S (2003). The functional logic of cortico-pulvinar connections. Philos. Trans. R. Soc. Lond. B Biol. Sci 358, 1605–1624. 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockland KS (2019). Distinctive Spatial and Laminar Organization of Single Axons from Lateral Pulvinar in the Macaque. Vision 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFarland JM, Bondy AG, Saunders RC, Cumming BG, and Butts DA (2015). Saccadic modulation of stimulus processing in primary visual cortex. Nat. Commun 6, 8110. 10.1038/ncomms9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wurtz RH (1969). Response of striate cortex neurons to stimuli during rapid eye movements in the monkey. J. Neurophysiol 32, 975–986. 10.1152/jn.1969.32.6.975. [DOI] [PubMed] [Google Scholar]
- 28.Bizzi E (1968). Discharge of frontal eye field neurons during saccadic and following eye movements in unanesthetized monkeys. Exp. Brain Res 6, 69–80. 10.1007/BF00235447. [DOI] [PubMed] [Google Scholar]
- 29.Schall JD, and Hanes DP (1993). Neural basis of saccade target selection in frontal eye field during visual search. Nature 366, 467–469. 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- 30.Everling S, and Munoz DP (2000). Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J. Neurosci 20, 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson DL, McClurkin JW, Kertzman C, and Petersen SE (1991). Visual responses of pulvinar and collicular neurons during eye movements of awake, trained macaques. J. Neurophysiol 66, 485–496. 10.1152/jn.1991.66.2.485. [DOI] [PubMed] [Google Scholar]
- 32.Berman RA, and Wurtz RH (2011). Signals conveyed in the pulvinar pathway from superior colliculus to cortical area MT. J. Neurosci 31, 373–384. 10.1523/JNEUROSCI.4738-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudkamp S, and Schmidt M (2000). Response characteristics of neurons in the pulvinar of awake cats to saccades and to visual stimulation. Exp. Brain Res 133, 209–218. 10.1007/s002210000374. [DOI] [PubMed] [Google Scholar]
- 34.Katzner S, Busse L, and Carandini M (2011). GABAA inhibition controls response gain in visual cortex. J. Neurosci 31, 5931–5941. 10.1523/JNEUROSCI.5753-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson NR, Runyan CA, Wang FL, and Sur M (2012). Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348. 10.1038/nature11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell SJ, and Silver RA (2003). Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38, 433–445. 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- 37.Berman RA, Cavanaugh J, McAlonan K, and Wurtz RH (2017). A circuit for saccadic suppression in the primate brain. J. Neurophysiol 117, 1720–1735. 10.1152/jn.00679.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahill A, Clark MR, and Stark L (1975). The main sequence, a tool for studying human eye movements. Math. Biosci 24, 191–204. [Google Scholar]
- 39.Mitzdorf U (1985). Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol. Rev 65, 37–100. 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- 40.Nandy AS, Nassi JJ, and Reynolds JH (2017). Laminar Organization of Attentional Modulation in Macaque Visual Area V4. Neuron 93, 235–246. 10.1016/j.neuron.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell JF, Sundberg KA, and Reynolds JH (2007). Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55, 131–141. 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Tamura H, Kaneko H, Kawasaki K, and Fujita I (2004). Presumed inhibitory neurons in the macaque inferior temporal cortex: visual response properties and functional interactions with adjacent neurons. J. Neurophysiol 91, 2782–2796. 10.1152/jn.01267.2003. [DOI] [PubMed] [Google Scholar]
- 43.Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, and McCormick DA (2005). Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron 47, 423–435. 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Kass RE, Ventura V, and Cai C (2003). Statistical smoothing of neuronal data. Network 14, 5–15. 10.1088/0954-898x/14/1/301. [DOI] [PubMed] [Google Scholar]
- 45.Shimazaki H, and Shinomoto S (2010). Kernel bandwidth optimization in spike rate estimation. J. Comput. Neurosci 29, 171–182. 10.1007/s10827-009-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westheimer G (1954). Mechanism of saccadic eye movements. AMA. Arch. Ophthalmol 52, 710–724. 10.1001/archopht.1954.00920050716006. [DOI] [PubMed] [Google Scholar]
- 47.Paré M, and Munoz DP (1996). Saccadic reaction time in the monkey: Advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J. Neurophysiol 76, 3666–3681. [DOI] [PubMed] [Google Scholar]
- 48.Deubel H, and Schneider WX (1996). Saccade target selection and object recognition: evidence for a common attentional mechanism. Vis. Res 36, 1827–1837. 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- 49.Gilzenrat MS, Nieuwenhuis S, Jepma M, and Cohen JD (2010). Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cognit. Affect Behav. Neurosci 10, 252–269. 10.3758/CABN.10.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, and Tolias AS (2014). Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84, 355–362. 10.1016/j.neuron.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jainta S, Vernet M, Yang Q, and Kapoula Z (2011). The pupil reflects motor preparation for saccades-even before the eye starts to move. Front. Hum. Neurosci 5, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doiron B, Litwin-Kumar A, Rosenbaum R, Ocker GK, and Josić K (2016). The mechanics of state-dependent neural correlations. Nat. Neurosci 19, 383–393. 10.1038/nn.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell JF, Sundberg KA, and Reynolds JH (2009). Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63, 879–888. 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen MR, and Maunsell JHR (2009). Attention improves performance primarily by reducing interneuronal correlations. Nat. Neurosci 12, 1594–1600. 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anastassiou CA, Perin R, Markram H, and Koch C (2011). Ephaptic coupling of cortical neurons. Nat. Neurosci 14, 217–223. 10.1038/nn.2727. [DOI] [PubMed] [Google Scholar]
- 56.Fries P (2015). Rhythms for Cognition: Communication through Coherence. Neuron 88, 220–235. 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, and Buzsáki G (2008). Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60, 683–697. 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veit J, Hakim R, Jadi MP, Sejnowski TJ, and Adesnik H (2017). Cortical gamma band synchronization through somatostatin interneurons. Nat. Neurosci 20, 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozeki H, Finn IM, Schaffer ES, Miller KD, and Ferster D (2009). Inhibitory stabilization of the cortical network underlies visual surround suppression. Neuron 62, 578–592. 10.1016/j.neuron.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsodyks MV, Skaggs WE, Sejnowski TJ, and McNaughton BL (1997). Paradoxical effects of external modulation of inhibitory interneurons. J. Neurosci 17, 4382–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jadi MP, and Sejnowski TJ (2014). Regulating cortical oscillations in an inhibition-stabilized network. Proc. IEEE 102, 830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Douglas RJ, and Martin KAC (2007). Mapping the matrix: the ways of neocortex. Neuron 56, 226–238. 10.1016/j.neuron.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 63.Diamond MR, Ross J, and Morrone MC (2000). Extraretinal control of saccadic suppression. J. Neurosci 20, 3449–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castet E, and Masson GS (2000). Motion perception during saccadic eye movements. Nat. Neurosci 3, 177–183. [DOI] [PubMed] [Google Scholar]
- 65.Campbell FW, and Wurtz RH (1978). Saccadic omission: why we do not see a grey-out during a saccadic eye movement. Vis. Res 18, 1297–1303. [DOI] [PubMed] [Google Scholar]
- 66.Burr DC, Morrone MC, and Ross J (1994). Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature 371, 511–513. [DOI] [PubMed] [Google Scholar]
- 67.Zamarashkina P, Popovkina DV, and Pasupathy A (2020). Timing of response onset and offset in macaque V4: stimulus and task dependence. J. Neurophysiol 123, 2311–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, and Leventhal AG (1998). Signal timing across the macaque visual system. J. Neurophysiol 79, 3272–3278. [DOI] [PubMed] [Google Scholar]
- 69.Rajkai C, Lakatos P, Chen C-M, Pincze Z, Karmos G, and Schroeder CE (2008). Transient cortical excitation at the onset of visual fixation. Cerebr. Cortex 18, 200–209. [DOI] [PubMed] [Google Scholar]
- 70.Bair W, Zohary E, and Newsome WT (2001). Correlated firing in macaque visual area MT: time scales and relationship to behavior. J. Neurosci 21, 1676–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kohn A, and Smith MA (2005). Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J. Neurosci 25, 3661–3673. 10.1523/JNEUR0SCI.5106-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shadlen MN, and Newsome WT (1998). The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci 18, 3870–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kriener B, Tetzlaff T, Aertsen A, Diesmann M, and Rotter S (2008). Correlations and population dynamics in cortical networks. Neural Comput. 20, 2185–2226. 10.1162/neco.2008.02-07-474. [DOI] [PubMed] [Google Scholar]
- 74.Kagan I, Gur M, and Snodderly DM (2008). Saccades and drifts differentially modulate neuronal activity in V1: effects of retinal image motion, position, and extraretinal influences. J. Vis 8, 19.1–1925. [DOI] [PubMed] [Google Scholar]
- 75.Barczak A, Haegens S, Ross DA, McGinnis T, Lakatos P, and Schroeder CE (2019). Dynamic modulation of cortical excitability during visual active sensing. Cell Rep. 27, 3447–3459.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reppas JB, Usrey WM, and Reid RC (2002). Saccadic eye movements modulate visual responses in the lateral geniculate nucleus. Neuron 35, 961–974. [DOI] [PubMed] [Google Scholar]
- 77.Royal DW, Sáry G, Schall JD, and Casagrande VA (2006). Correlates of motor planning and postsaccadic fixation in the macaque monkey lateral geniculate nucleus. Exp. Brain Res 168, 62–75. [DOI] [PubMed] [Google Scholar]
- 78.Stepniewska I, QI HX, and Kaas JH (2000). Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis. Neurosci 17, 529–549. [DOI] [PubMed] [Google Scholar]
- 79.Robinson DA (1972). Eye movements evoked by collicular stimulation in the alert monkey. Vis. Res 12, 1795–1808. [DOI] [PubMed] [Google Scholar]
- 80.Schiller PH, Sandell JH, and Maunsell JH (1987). The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J. Neurophysiol 57, 1033–1049. [DOI] [PubMed] [Google Scholar]
- 81.Saalmann YB, Pinsk MA, Wang L, Li X, and Kastner S (2012). The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337, 753–756. 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou H, Schafer RJ, and Desimone R (2016). Pulvinar-Cortex Interactions in Vision and Attention. Neuron 89, 209–220. 10.1016/j.neuron.2015.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petersen SE, Robinson DL, and Morris JD (1987). Contributions of the pulvinar to visual spatial attention. Neuropsychologia 25, 97–105. 10.1016/0028-3932(87)90046-7. [DOI] [PubMed] [Google Scholar]
- 84.Wilke M, Turchi J, Smith K, Mishkin M, and Leopold DA (2010). Pulvinar inactivation disrupts selection of movement plans. J. Neurosci 30, 8650–8659. 10.1523/JNEUROSCI.0953-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilke M, Kagan I, and Andersen RA (2013). Effects of pulvinar inactivation on spatial decision-making between equal and asymmetric reward options. J. Cognit. Neurosci 25, 1270–1283. 10.1162/jocn_a_00399. [DOI] [PubMed] [Google Scholar]
- 86.Kawaguchi Y, and Kubota Y (1993). Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J. Neurophysiol 70, 387–396. 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- 87.Galarreta M, and Hestrin S (1999). A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature 402, 72–75. 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 88.Cruikshank SJ, Urabe H, Nurmikko AV, and Connors BW (2010). Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65, 230–245. 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gibson JR, Beierlein M, and Connors BW (1999). Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402, 75–79. 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 90.Agmon A, and Connors BW (1992). Correlation between Intrinsic Firing Patterns and Thalamocortical Synaptic Responses of Neurons in Mouse Barrel Cortex. J. Neurosci 12, 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poulet JFA, and Petersen CCH (2008). Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454, 881–885. 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- 92.Vinck M, Batista-Brito R, Knoblich U, and Cardin JA (2015). Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86, 740–754. 10.1016/j.neuron.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arroyo S, Bennett C, and Hestrin S (2018). Correlation of Synaptic Inputs in the Visual Cortex of Awake, Behaving Mice. Neuron 99, 1289–1301.e2. 10.1016/j.neuron.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.John ER, Prichep LS, Kox W, Valdés-Sosa P, Bosch-Bayard J, Aubert E, Tom M, di Michele F, and Gugino LD (2001). Invariant reversible QEEG effects of anesthetics. Conscious. Cognit 10, 165–183. 10.1006/ccog.2001.0507. [DOI] [PubMed] [Google Scholar]
- 95.Zohary E, Shadlen MN, and Newsome WT (1994). Correlated neuronal discharge rate and its implications for psychophysical performance. Nature 370, 140–143. 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]
- 96.Abbott LF, and Dayan P (1999). The effect of correlated variability on the accuracy of a population code. Neural Comput. 11, 91–101. 10.1162/089976699300016827. [DOI] [PubMed] [Google Scholar]
- 97.Bartolo R, Saunders RC, Mitz AR, and Averbeck BB (2020). Information-Limiting Correlations in Large Neural Populations. J. Neurosci 40, 1668–1678. 10.1523/JNEUROSCI.2072-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Averbeck BB, Latham PE, and Pouget A (2006). Neural correlations, population coding and computation. Nat. Rev. Neurosci 7, 358–366. 10.1038/nrn1888. [DOI] [PubMed] [Google Scholar]
- 99.Lee D, and Malpeli JG (1998). Effects of saccades on the activity of neurons in the cat lateral geniculate nucleus. J. Neurophysiol 79, 922–936. [DOI] [PubMed] [Google Scholar]
- 100.Nassi JJ, Avery MC, Cetin AH, Roe AW, and Reynolds JH (2015). Optogenetic Activation of Normalization in Alert Macaque Visual Cortex. Neuron 86, 1504–1517. 10.1016/j.neuron.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruiz O, Lustig BR, Nassi JJ, Cetin A, Reynolds JH, Albright TD, Callaway EM, Stoner GR, and Roe AW (2013). Optogenetics through windows on the brain in the nonhuman primate. J. Neurophysiol 110, 1455–1467. 10.1152/jn.00153.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.König SD, and Buffalo EA (2014).A nonparametric method for detecting fixations and saccades using cluster analysis: removing the need for arbitrary thresholds. J. Neurosci. Methods 227, 121–131. 10.1016/j.jneumeth.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mitra PP, and Pesaran B (1999). Analysis of dynamic brain imaging data. Biophys. J 76, 691–708. 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeitler M, Fries P, and Gielen S (2006). Assessing neuronal coherence with single-unit, multi-unit, and local field potentials. Neural Comput. 18, 2256–2281. 10.1162/neco.2006.18.9.2256. [DOI] [PubMed] [Google Scholar]
- 105.Vinck M, Battaglia FP, Womelsdorf T, and Pennartz C (2012). Improved measures of phase-coupling between spikes and the Local Field Potential. J. Comput. Neurosci 33, 53–75. 10.1007/s10827-011-0374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request. All original code has been deposited at Zenodo (https://doi.org/10.5281/zenodo.7946057) and are publicly available as of the date of publication. DOIs are listed in the key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Organisms/strains | ||
| Rhesus Macaques (Macaca mulatta) | UC Davis, Worldwide Primates | N/A |
| Software and algorithms | ||
| MATLAB | Mathworks | R2019a |
| Custom Code and Analyses | This paper | Zenodo: https://doi.org/10.5281/zenodo.7946057 |


