Abstract
Recent efforts in our laboratory have enabled access to an unprecedented number (~90) of quantifiable metabolites in human blood by a simple NMR spectroscopy method, which includes energy coenzymes, redox coenzymes and antioxidants that are fundamental to cellular functions [J. Magn. Reson. Open, 12-13, 100082, (2022)]. The coenzymes and antioxidants, however, are notoriously labile and are extremely sensitive to specimen harvesting, extraction and measurement conditions. This problem is largely underappreciated and carries the risk of grossly inaccurate measurements and incorrect study outcomes. As a part of addressing this challenge, in this study, human blood specimens were comprehensively and quantitatively investigated using 1H NMR spectroscopy. Freshly drawn human blood specimens were treated or not treated with methanol, ethanol or a mixture of methanol and chloroform, and stored on ice or on bench, at room temperature for different time periods from 0 to 24 hrs, prior to storing at −80 °C. Interestingly, the labile metabolite levels were stable in blood treated with an organic solvent. However, their levels in blood in untreated samples increased or decreased by factors of up to 5 or more within 3 hrs. Further, surprisingly, and contrary to the current knowledge about metabolite stability, the variation of coenzyme levels was more dramatic in blood stored on ice than on bench, at room temperature. In addition, unlike the generally observed phenomenon of oxidation of redox coenzymes, reduction was observed in untreated blood. Such preanalytical dynamics of the labile metabolites potentially arises from the active cellular metabolism. From the metabolomics perspective, the massive variation of the labile metabolite levels even in blood stored on ice is alarming and stresses the critical need to immediately quench the cellular metabolism for reliable analyses. Overall, the results provide compelling evidence that warrants a paradigm shift in sample collection protocol for blood metabolomics involving labile metabolites.
Keywords: Blood, 1H NMR spectroscopy, labile metabolites, energy coenzymes, redox coenzymes, preanalytical stability, quenching metabolism
Graphical Abstract
INTRODUCTION
Coenzymes of energy and redox reactions mediate biochemical reactions that are fundamental to the functioning of all living cells. Due to their essential role in fueling a vast number of energy dependent biochemical processes, the energy coenzyme, ATP (adenosine triphosphate) and its precursors, ADP (adenosine diphosphate) and AMP (adenosine monophosphate), constitute the energy currency of living cells. Hence, their levels in biological specimens represent a measure of the cellular energetics.1 The redox coenzymes, NAD+ (nicotinamide adenine dinucleotide, oxidized), NADH (nicotinamide adenine dinucleotide, reduced), NADP+ (nicotinamide adenine dinucleotide phosphate, oxidized), and NADPH (nicotinamide adenine dinucleotide phosphate, reduced), mediate fundamental cellular functions; they are important indicators of health, and pathological conditions for major diseases including heart disease, diabetes and cancer.2-4 The need to measure these energy and redox coenzymes reliably in biological specimens is therefore of immense interest for uncovering fundamental cellular functions as well as prevention and treatment of diseases.
Human blood is the most widely used biospecimen in the clinic and the metabolomics field.5-9 NMR spectroscopy and mass spectrometry (MS) are the two major analytical platforms widely used for the analysis of blood metabolites. MS is a highly sensitive method and it detects several hundreds to thousands of metabolites in a single step. NMR spectroscopy, on the hand, is far less sensitive, but is highly reproducible and quantitative, which are unparalleled characteristics for metabolite analysis. Further, importantly, NMR enables the establishment of the identity for unknown metabolites, which is a persisting challenge in the metabolomics field. Efforts over the years, in our laboratory,10-13 have led to the development of NMR methods that enable the quantitative analysis of nearly 90 aqueous metabolites in human blood, which includes the energy coenzymes, redox coenzymes and antioxidants;14-16 to date, this is the highest number of aqueous blood metabolites that can be analyzed using a simple 1D 1H NMR spectrum.16
Unconnected with any analytical method, however, the energy coenzymes and redox coenzymes are notoriously labile and are extremely sensitive to enzyme activity and oxidation. Hence, their levels are sensitive to specimen harvesting, extraction and measurement conditions. This challenge is largely underappreciated and carries the risk of grossly inaccurate measurements, which can lead to incorrect biological inferences. In the last several years, major efforts in our laboratory have been focused on understanding specific factors that affect the stability of these compounds, and developing methods and protocols for their analysis. Since NMR enables the global visualization of metabolites using a simple 1D NMR spectrum, reproducibly and quantitatively, it represents a valuable tool for the evaluation of the stability of the labile metabolites and for establishing a reliable method for their analysis. To date, we have addressed some of the factors that affect their stability in a variety of biological specimens including human blood.12-15
In the current study, with the goal of investigating the stability of the labile metabolites in blood with respect to sample harvesting conditions, human blood specimens were comprehensively investigated using 1H NMR spectroscopy under a variety of preanalytical conditions. Freshly drawn blood specimens were either treated with methanol, ethanol or a mixture of methanol and chloroform, or left untreated, and stored on ice or at room temperature for different time periods from 0 to 24 hrs, prior to freezing at −80 °C. 1H NMR investigation of these specimens showed that the labile metabolite levels were stable in blood treated with an organic solvent, however, their levels in untreated blood samples increased or decreased enormously. Further, a number of findings were contrary to the current knowledge about the metabolite stability. Importantly, the massive variation of labile metabolite levels even in blood that was stored on ice for a short time is concerning, which stresses the critical need for quenching the cellular metabolism for reliable analyses of blood metabolites.
MATERIALS AND METHODS
Chemicals and solvents:
Methanol, ethanol, chloroform, sodium phosphate (monobasic; NaH2PO4), sodium phosphate (dibasic; Na2HPO4), fumaric acid, and 3-(trimethylsilyl)propionic acid-2,2,3,3-d4 sodium salt (TSP) were obtained from Sigma-Aldrich (St. Louis, MO). Deuterium oxide (D2O) was obtained from Cambridge Isotope laboratories, Inc. (Andover, MA). Deionized (DI) water was purified using an in-house Synergy Ultrapure Water System from Millipore (Billerica, MA). All chemicals were used with no further purification.
Blood collection and pretreatment:
Human blood from healthy individuals (~ 25 mL; n = 6) were collected in heparinized BD Vacutainer tubes (BioVision, CA). The biospecimen collection protocol was approved by the IRB from the University of Washington. Figure S1 describes the preanalytical treatments of blood specimens. Briefly, each freshly drawn blood was immediately aliquoted (200 μL each) into 84 Eppendorf tubes (2 mL). The aliquots were split into three extraction groups, with each group having 28 aliquots, and were further divided into four subgroups, with each subgroup having 7 aliquots. In each group, tubes from two subgroups were stored on ice (prior to aliquoting the blood) and tubes from the remaining two subgroups were stored on the bench at room temperature. Blood specimens from one subgroup that was stored on ice and another subgroup that was stored on the bench were mixed with methanol (1:2 v/v), ethanol (1:2 v/v) or a mixture of methanol and chloroform (1:2:2 v/v/v). Subsequently, one sample from each of the 12 subgroups (3 groups x 4 subgroups =12) was stored at −80 °C at seven different time points varying from 0 to 24 hrs (0, 0.5, 1, 3, 4, 8, and 24 hrs) until used for further analysis.
Preparation of phosphate buffer:
Buffer solution (100 mM) was prepared by dissolving 1124 mg anhydrous Na2HPO4 and 250 mg anhydrous NaH2PO4 in 100 g D2O. TSP (19.2 or 17.9 μM) and fumaric acid (121.9 or 98.61 μM) were added as internal references for the chemical shift scaling and quantitation, respectively.17 The concentrations of the internal standards, TSP and fumaric acid, were calibrated using acetanilide. Acetanilide is a primary standard for calibration of internal standards using NMR.18 The calculated pH of the buffer solution was 7.4 and the measured pH was 7.33. This buffer was used without further pH correction.
Metabolite extraction:
Frozen blood samples that had not been treated with an organic solvent prior to storing at −80 °C were mixed with methanol (1:2 v/v), ethanol (1:2 v/v) or a mixture of methanol and chloroform (1:2:2 v/v/v/) (see Figure S1). No further solvent was added to the remaining samples since they were already treated with solvents prior to storing at −80 °C. Next, all samples were vortexed for 2 min or until a homogenous mixture was formed, sonicated for 20 min at 4 °C, and vortexed again for 30 s. The mixtures were then centrifuged at 13,400×g for 30 min to pellet proteins/cell debris. Clear solutions were transferred to fresh vials and dried using nitrogen gas. The dried samples were mixed with 200 μL phosphate buffer in D2O containing fumaric acid and TSP and transferred to 3 mm NMR tubes. The D2O buffer was degassed using helium gas prior to mixing with samples and the NMR tubes were flushed with the gas before and after transferring solutions and sealed with parafilm to prevent the air from entering the tube.14
NMR Spectroscopy and labile metabolite analyses:
NMR experiments were performed at 298 K on a Bruker Avance III 800 MHz spectrometer equipped with a cryogenically cooled probe and Z-gradients suitable for inverse detection. The CPMG (Carr-Purcell-Meiboom-Gill) pulse sequence with residual water suppression using presaturation was used for 1H 1D NMR experiments. Spectra were obtained using a 9615 Hz spectral width, 32,768 time-domain points, 5 s recycle delay, and 256 ms CPMG pulse train length. The raw data were Fourier transformed using a spectral size of 32,768 points. Prior to Fourier transformation, the raw data were multiplied by an exponential window function with a line broadening of 0.5 Hz. Separately, a Gaussian window function with a Gaussian broadening of 0.3 Hz and exponential broadening of −0.5 Hz was used prior to Fourier transformation for separating closely spaced labile metabolite peaks. The spectra were phase and baseline corrected and the chemical shifts were referenced to the internal standard, TSP. Bruker Topspin versions 4.1.4 and 3.6.5 software packages were used for NMR data acquisition, processing or analyses. Assignment of labile metabolite peaks was based on the 1H NMR spectral assignment templates established previously.11,12,14-16 Concentrations of metabolites were obtained based on the integration of the characteristic peaks using the Bruker AMIX software with reference to the internal standard, fumaric acid. Metabolite concentrations were compared among the blood specimens with different preanalytical treatments.
Results and Discussion
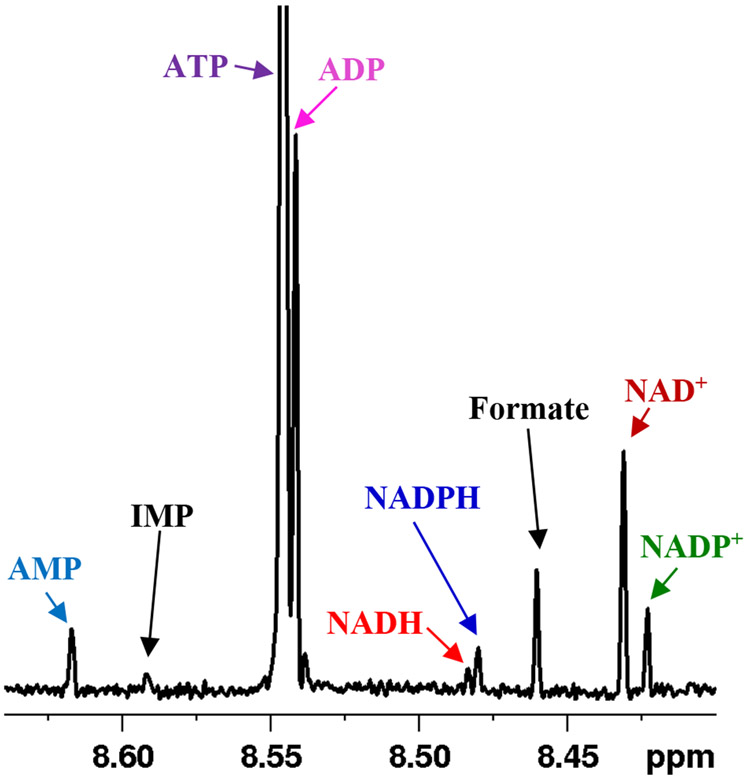
This study was focused on the investigation of the preanalytical stability and dynamics of the labile energy and redox coenzymes in human blood. Freshly drawn blood specimens were studied comprehensively using 1H NMR spectroscopy after treating or not treating with methanol, ethanol or a mixture of methanol and chloroform, and keeping the specimens on ice or at room temperature for different durations (Figure S1). Irrespective of the preanalytical condition, 1H NMR spectra of all blood specimens showed peaks for the energy and redox coenzymes along with other metabolites (Figure S2). The characteristic fingerprint region (~ 8.3 to 8.7 ppm) that exhibited distinct peaks for the major energy and redox coenzymes, as shown in Figure 1, was used to determine the concentrations of the coenzymes. The levels of coenzymes extracted using a mixture of methanol and chloroform were generally higher when compared to the extraction using methanol or ethanol (Figure S3), and the results are in agreement with those reported previously.14
Figure 1:
Portion of a typical 800 MHz 1H NMR spectrum of human whole blood obtained after extraction using a mixture of methanol and chloroform in a 1:2:2 ratio (v/v/v) with annotation of the characteristic peaks of the energy (ATP, ADP, AMP) and redox (NAD+, NADP+, NADH, NADPH) coenzymes.
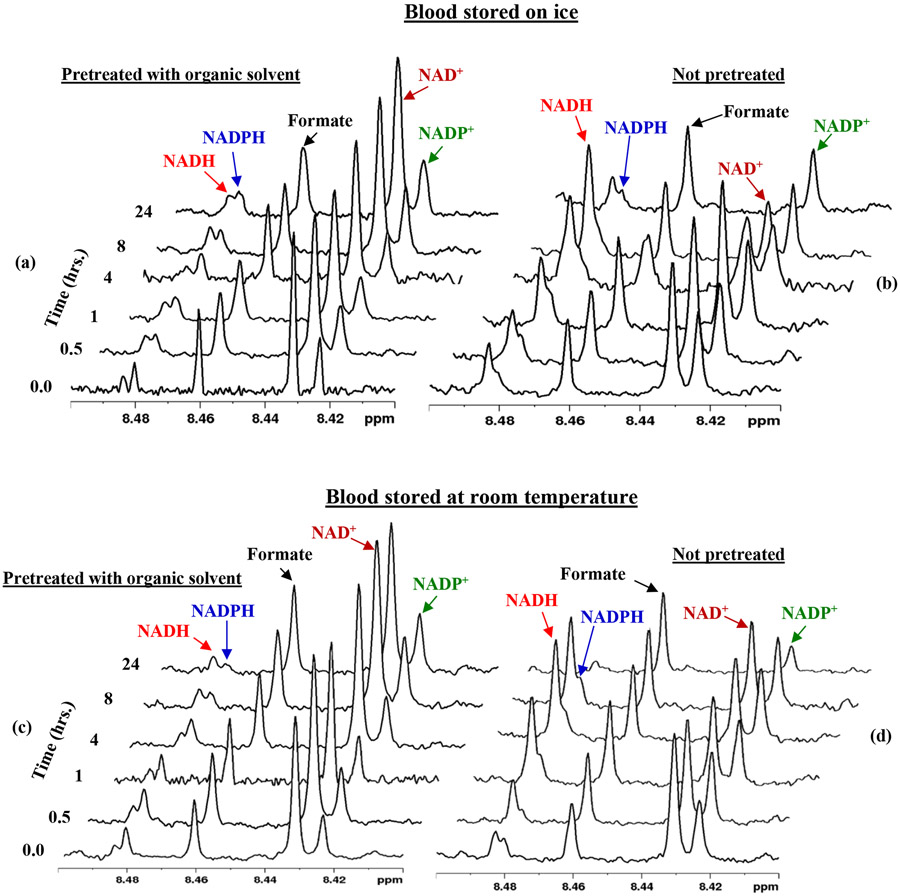
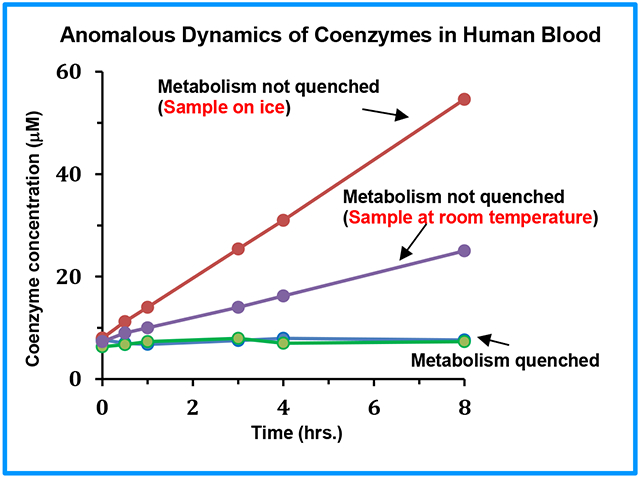
An evaluation of the coenzyme levels revealed a number of interesting phenomena: Specifically, in untreated blood the coenzyme levels increased or decreased by a factor of up to 5 or more within 3 hrs. and >20 within 24 hrs. (Figures 2 and 3). The massive variation of the metabolite levels indicates their susceptibility to enzyme activity from live blood cells, which leads to the oxidation, reduction, or hydrolysis of the labile metabolites. Red blood cells (RBCs), constitute more than 99% of blood cells; they are metabolically active and use glycolysis to meet the cellular energy demand. On the other hand, in blood treated with an organic solvent, the coenzyme levels were stable throughout the time period investigated (Figure 2-5, S4, S5). These results indicate that the organic solvents quench the cellular activity, and thus prevent the oxidation, reduction, or hydrolysis of the coenzymes, and lead to the stable levels for the coenzymes. Using a mixture of methanol and chloroform to quench enzyme activity yields a higher concentration of the coenzymes than using methanol or ethanol. This is because, the mixture of methanol and chloroform extracts the labile metabolites more optimally when compared to methanol or ethanol, and the results agree with our previous findings.14
Figure 2:
Portions of 1H NMR spectra of human blood stored on ice (a, b) or at room temperature (c, d) prior to freezing at −80 °C at different duration until used for metabolite extraction and analyses. The blood in (a, c) was pretreated with a mixture of methanol and chloroform (1:2:2 v/v/v).
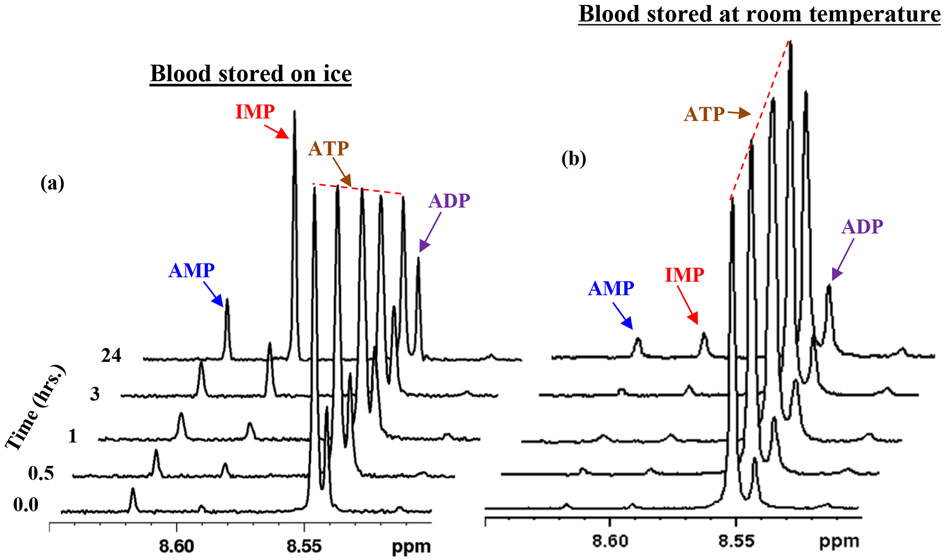
Figure 3:
Portions of 1H NMR spectra of human blood kept on ice (a) or at room temperature (b), for different duration prior to freezing at −80 °C. The spectra for blood kept on ice show that the levels of IMP increase with time with a concomitant decrease of ATP; AMP and ADP also increase with time. AMP, IMP and ATP were more stable in blood kept at room temperature than on ice.
Figure 5:
Concentrations of IMP and AMP in human blood kept on ice or bench, at room temperature (RT), with or without treating with a solvent (a mixture of methanol and chloroform 1/2/2 v/v/v) prior to freezing at −80 °C at different duration. In untreated blood, IMP and AMP increase with time and the rate of increase is more in blood kept on ice than at room temperature.
The preanalytical variabilities of blood metabolites continues to be a topic of intense investigations in the metabolomics field owing to the critical need to establish an appropriate sample protocol for the reliable analysis of metabolites.19-24 A consistent finding of the studies to date is that metabolite levels change more rapidly when a freshly drawn blood is stored on the bench at room temperature when compared to samples stored on ice.21,22, 25-30 Surprisingly, contrary to this, in the current study, we show that the coenzyme levels change more rapidly in blood that was stored on ice than on bench (Figures 2-5, S4, S5). The trend was identical irrespective of the organic solvent used for metabolite extraction (Figures S6). Such an anomalous behavior of the labile metabolites was hitherto unknown. Although the mechanism for such a behavior is not understood, the results suggest an increased hypothermal response of the blood cells, which potentially results in an increased enzyme activity causing a rapid change in the coenzyme levels at lower temperature when compared to room temperature.
The above results demonstrate that while keeping the freshly drawn blood on ice is more detrimental to maintaining coenzyme levels than keeping it at room temperature, both are unsuitable for the reliable analysis of the labile metabolites. This is a remarkable finding considering the generally followed norm in the metabolomics field that the integrity of metabolite levels does not alter appreciably when the freshly drawn blood is stored on ice and processed within a few hours (< 4 hrs).23 However, in agreement with the current knowledge, the levels of the major blood metabolites, lactate and glucose, increased or decreased more rapidly in blood stored on bench than on ice (Figures 6 and S7, S8). Lactate is a major glycolysis end product and is sensitive to preanalytical conditions; in fact, the lactate level, which can be visualized directly from 1H NMR spectrum, is often considered a measure of blood sample quality. The sensitivity of lactate is in accordance with the decreased concentration of glucose in whole blood, which is known to occur at a rate of 5 to 7 % per hour due to the glycolysis induced by blood cells.31
Figure 6:
Concentrations of lactate and glucose in human blood stored on ice or at room temperature (RT), with or without treating with a solvent (a mixture of methanol and chloroform 1:2:2 v/v/v) prior to freezing at −80 °C at different duration. In untreated blood, lactate increases and glucose decreases with time and the rate of increase or decrease is higher in blood kept at room temperature (RT) than on ice.
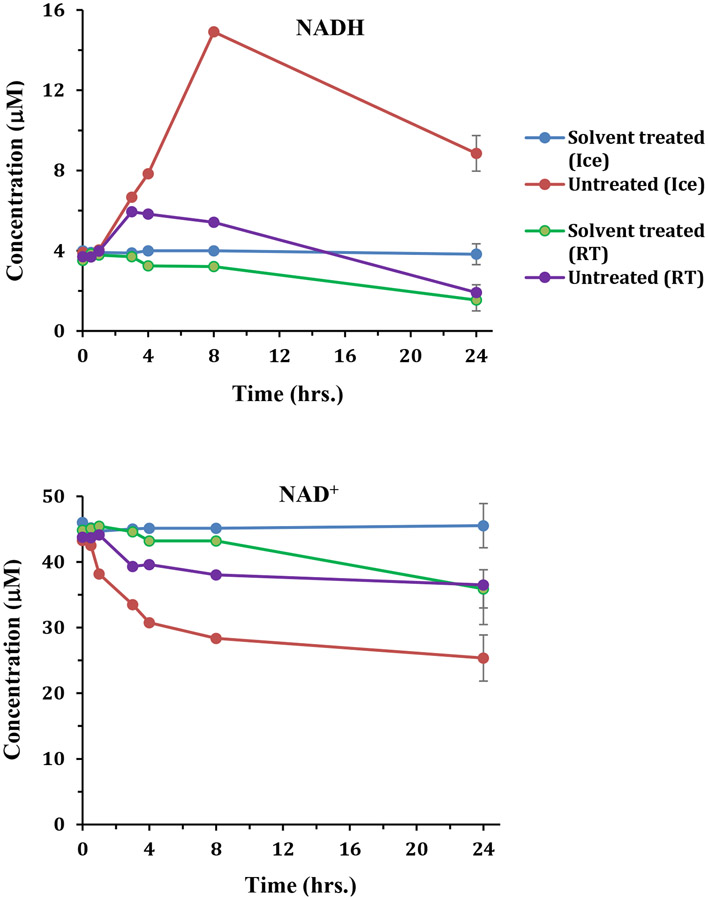
A well-known challenge to date for the analysis of redox coenzymes ex vivo is their sensitivity to oxidation. For example, the reduced forms, NADH and NADPH, oxidize to NAD+ and NADP+, respectively.12,14 By contrast, interestingly, in the current study, we find the opposite phenomenon, reduction, occurs especially for the NAD+/NADH pair. Untreated whole blood specimens exhibited the conversion of NAD+ to NADH, and the rate of conversion was higher in blood stored on ice than at room temperature (Figures 2, 4, and S4). The formation of NADH at the cost of NAD+ was similar irrespective of the solvent used for metabolite extraction. This phenomenon was less pronounced for methanol and ethanol extraction, however, when compared to the extraction using a mixture of methanol and chloroform. The ratio between the reduced and oxidized forms is an indicator of cellular function, including the overall redox status, and regulation of ion channels, cell signaling, cell survival and death.32-35 The massive increase in the levels of NADH at the cost of NAD+ induced by preanalytical conditions, as discovered in the current study, leads to grossly inaccurate NAD+ to NADH ratios and could cause incorrect study outcomes, if not accounted for.
Figure 4:
NADH and NAD+ levels in human blood stored on ice or at room temperature (RT), after treating or not treating with a solvent (mixture of methanol and chloroform (1:2:2 v/v/v)) prior to freezing at −80 °C. In untreated blood, NADH increases and NAD+ decreases with time and the rate of increase or decrease is higher in blood stored on ice than at room temperature.
The energy coenzyme, ATP, is one of the most abundant metabolites in blood. ATP levels also change more rapidly in blood stored on ice than on the bench (Figure 3), similar to NADH and NAD+ (Figures 2 and 4). ATP undergoes hydrolysis to form ADP, which further hydrolyzes to form AMP.36 AMP then undergoes deamination to form IMP (inosine monophosphate).36, 37 The net result is an increased accumulation of AMP as well as IMP at the cost of ATP. We observed that the increase of AMP and IMP levels was more rapid in blood stored on ice, which is in accordance with the rate of decrease of the ATP levels (Figures 3, 5, S5, S6). Biologically, IMP is a precursor to AMP and plays an important role in intracellular purine metabolism.38 The massive increase in IMP and AMP levels arising from the red blood cellular activity induced by the preanalytical condition far outweighs their physiological concentrations and hence potentially leads to grossly incorrect inferences. These results further highlight the critical need to quench the cellular metabolism to gain insights into the physiological concentrations of these metabolites in health and diseases.
The solvent mixture consisting of methanol and chloroform not only extracts the labile metabolites optimally, but it also retains the integrity of the levels of other blood metabolite as we have shown previously.14 Further, our results indicate that quenching cellular metabolism using this solvent mixture does not affect the integrity of the levels of metabolites such as amino acids (Figure 7). In fact, the need to quench cellular metabolism for accurate analysis of intracellular metabolites was recognized back in 1960.39 Since then, numerous quenching methods including the use of hot or cold organic solvent, cold saline, liquid nitrogen, and hot air have been proposed.40-44 However, this is the first study to provide compelling evidence that points to the need to quench metabolism for accurate analysis of blood metabolites that include the notoriously labile metabolites and other important metabolites such as lactate and glucose.
Figure 7:
Concentrations of a few amino acids in a typical blood stored on ice or at room temperature (RT), with or without treating with a solvent (mixture of methanol and chloroform 1/2/2 v/v/v) prior to freezing at −80 °C at different duration.
In conclusion, this study describes a comprehensive evaluation of the major redox and energy coenzymes in human blood under a variety of preanalytical conditions with the goal of understanding the factors that impart instability to these labile metabolites. The results demonstrate several intriguing phenomena, which are contrary to the current knowledge about the blood metabolite profiling. Importantly, the coenzyme levels increased or decreased more dramatically in blood stored on ice than at room temperature, which goes against the accepted sample collection protocols currently in practice. In addition, contrary to the oxidation generally observed for redox coenzymes,12-14 reduction was observed, especially for the NAD+/NADH pair. Such anomalous preanalytical dynamics of the labile metabolites arises from the metabolically active blood cells, although the mechanism for such changes is unknown. The opposite trends in NADH and ATP observed in this study are consistent with active glycolysis in RBCs in the absence of a mitochondrial electron transport chain. The findings open new avenues for further investigation of the temperature dependent cellular metabolism in blood as well as other specimens including tissue, cells and subcellular organelles.
A key result of this work is that rapid quenching of the metabolism using an organic solvent yields stable levels for the coenzymes. Conventionally, quenching metabolism has not been a part of blood sample collection protocol in the metabolomics field, although it is an important part of cell or tissue processing. From the metabolomics perspective, the massive variation of labile metabolite levels even in whole blood preserved on ice for a short time is alarming and points to the critical need to immediately quench the cellular metabolism for reliable analyses of the labile blood metabolites. Overall, the results in this study provide compelling evidence that warrants a paradigm shift in the sample collection protocol for blood metabolomics especially that involves labile metabolites as well as other important metabolites such as lactate and glucose.
Supplementary Material
Figure S1. Study design for blood sample pretreatment, storage and extraction prior to analysis.
Figure S2. 1H NMR spectrum of blood obtained after extraction using methanol and chloroform.
Figure S3. Portions of 1H NMR spectra of blood obtained after extraction using ethanol (n=112), methanol (n=112), and a mixture of methanol and chloroform (n=112) highlighting coenzyme peaks.
Figure S4. NAD+ and NADH levels with error bars under different conditions of sample pretreatment and extraction using a mixture of methanol and chloroform.
Figure S5. AMP and IMP levels with error bars under different conditions of sample pretreatment and extraction using a mixture of methanol and chloroform.
Figure S6. AMP and IMP levels under different conditions of sample pretreatment and extraction using ethanol or methanol.
Figure S7. Lactate levels with error bars under different conditions of sample pretreatment and extraction using a mixture of methanol and chloroform.
Figure S8. Glucose and lactate levels under different conditions of sample pretreatment and extraction using ethanol or methanol.
Acknowledgements
The authors acknowledge financial support from the NIH R01GM138465 and R01GM131491.
Footnotes
Declaration of Competing Interest
The authors declare no competing interest.
References
- 1.Nelson DL; Cox MM Lehninger Principles of Biochemistry. 6th Edition, Freeman & Company, W. H., New York. 2012. [Google Scholar]
- 2.Zhou B; Wang DD; Qiu Y; Airhart S; Liu Y; Stempien-Otero A; O'Brien. KD; Tian R Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J Clin Invest. 2020, 130(11), 6054–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiao YA; Chakraborty AD; Light CM; Tian R Sadoshima J; Shi X; Gu H; Lee CF NAD + Redox Imbalance in the Heart Exacerbates Diabetic Cardiomyopathy. Circ. Heart Fail 2021, 14(8), e008170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiarugi A; Dölle C; Felici R; Ziegler M The NAD metabolome--a key determinant of cancer cell biology. Nat. Rev. Cancer 2012, 12(11), 741–52. [DOI] [PubMed] [Google Scholar]
- 5.Bujak R; Struck-Lewicka W; Markuszewski MJ; Kaliszan R Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal 2015, 113, 108–20. [DOI] [PubMed] [Google Scholar]
- 6.Chaleckis R; Murakami I; Takada J; Yanagida M Individual variability in human blood metabolites identifies age-related differences, Proc. Natl. Academ. Sci. USA, 2016, 113 (16), 4252–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Ascenzo N; Antonecchia E; Angiolillo A; Bender V; Camerlenghi M; Xie Q; Costanzo AD Metabolomics of blood reveals age-dependent pathways in Parkinson’s Disease, Cell & Bioscience, 2022, 12, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng C; Nagana Gowda GA; Raftery D; Neuhouser ML; Tinker LF; Prentice RL; Beresford SAA; Zhang Y; Bettcher L; Pepin R; Djukovic D; Gu H; Barding GA Jr; Song X; Lampe JW Evaluation of potential metabolomic-based biomarkers of protein, carbohydrate and fat intakes using a controlled feeding study. Eur. J. Nutr 2021, 60(8), 4207–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagana Gowda GA; Raftery D (eds.) NMR based metabolomics: methods and protocols. Methods in Molecular Biology, Vol. 2037. Humana Press/Springer Science, New York. 2019. [Google Scholar]
- 10.Nagana Gowda GA; Raftery D Quantitating metabolites in protein precipitated serum using NMR spectroscopy. Anal. Chem 2014, 86(11), 5433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagana Gowda GA; Gowda YN; Raftery D Expanding the limits of human blood metabolite quantitation using NMR spectroscopy. Anal. Chem 2015, 87(1), 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagana Gowda GA; Abell L; Lee CF; Tian R; Raftery D Simultaneous Analysis of Major Coenzymes of Cellular Redox Reactions and Energy Using ex Vivo 1H NMR Spectroscopy. Anal. Chem 2016, 88(9), 4817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagana Gowda GA; Abell L; Tian R Extending the Scope of 1H NMR Spectroscopy for the Analysis of Cellular Coenzyme A and Acetyl Coenzyme A. Anal. Chem 2019, 91(3), 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagana Gowda GA; Raftery D Whole Blood Metabolomics by 1H NMR Spectroscopy Provides a New Opportunity to Evaluate Coenzymes of Redox Reactions, Coenzymes of Energy and Antioxidants. Anal. Chem 2017, 89(8), 4620–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagana Gowda GA; Pascua V; Raftery D Extending the Scope of 1H NMR-Based Blood Metabolomics for the Analysis of Labile Antioxidants: Reduced and Oxidized Glutathione. Anal. Chem 2021, 93(44) 14844–14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagana Gowda GA; Pascua V; Raftery D A new limit for blood metabolite analysis using 1H NMR spectroscopy. J. Magn. Reason. Open, 2022, 12–13, 100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagana Gowda GA; Hong NN; Raftery D Evaluation of Fumaric acid and Maleic acid as Internal Standards for NMR Analysis of Protein Precipitated Plasma, Serum, and Whole Blood. Anal. Chem 2021, 93(6), 3233–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rundlöf T; McEwen I; Johansson M; Arvidsson T Use and qualification of primary and secondary standards employed in quantitative 1H NMR spectroscopy of pharmaceuticals. J Pharm Biomed Anal. 2014, 93, 111–7. [DOI] [PubMed] [Google Scholar]
- 19.Boyanton BL Jr.; Blick KE Stability Studies of Twenty-Four Analytes in Human Plasma and Serum. Clin. Chem 2002, 48, 2242–2247. [PubMed] [Google Scholar]
- 20.Anton G; Wilson R; Yu Z-H; Prehn C; Zukunft S; Adamski J; Heier M; Meisinger C; Römisch-Margl W; Wang-Sattler R; Hveem K; Wolfenbuttel B; Peters A; Kastenmüller G; Waldenberger M Pre-analytical sample quality: metabolite ratios as an intrinsic marker for prolonged room temperature exposure of serum samples. PLoS One. 2015, 10(3), e0121495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamlage B; Maldonado SG; Bethan B; Peter E; Schmitz O; Liebenberg V; Schatz P Quality Markers Addressing Preanalytical Variations of Blood and Plasma Processing Identified by Broad and Targeted Metabolite Profiling. Clin. Chem 2014, 60(2), 399–412. [DOI] [PubMed] [Google Scholar]
- 22.Yin P; Peter A; Franken H; Zhao X; Neukamm SS; Rosenbaum L; Lucio M; Zell A; Häring HU; Xu G Lehmann R Preanalytical Aspects and Sample Quality Assessment in Metabolomics Studies of Human Blood. Clin. Chem 2013, 59 (5), 833–845. [DOI] [PubMed] [Google Scholar]
- 23.Yin P; Lehmann R; Xu G Effects of pre-analytical processes on blood samples used in metabolomics studies. Anal. Bioanal. Chem 2015, 407, 4879–4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens VL; Hoover E; Wang Y; Zanetti KA Pre-Analytical Factors that Affect Metabolite Stability in Human Urine, Plasma, and Serum: A Review. Metabolites. 2019, 9(8), 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teahan O; Gamble S; Holmes E; Waxman J; Nicholson JK; Bevan C; Keun HC Impact of analytical bias in metabonomic studies of human blood serum and plasma. Anal. Chem 2006, 78, 4307–4318. [DOI] [PubMed] [Google Scholar]
- 26.Nishiumi S; Suzuki M; Kobayashi T; Yoshida M Differences in metabolite profiles caused by pre-analytical blood processing procedures. J. Biosci. Bioeng 2018, 125, 613–618. [DOI] [PubMed] [Google Scholar]
- 27.Bernini P; Bertini I; Luchinat C; Nincheri P; Staderini S; Turano P Standard operating procedures for pre-analytical handling of blood and urine for metabolomic studies and biobanks. J. Biomol. NMR 2011, 49, 231–243. [DOI] [PubMed] [Google Scholar]
- 28.Brunius C; Pedersen A; Malmodin D; Karlsson BG; Andersson LI; Tybring G; Landberg R Prediction and modeling of pre-analytical sampling errors as a strategy to improve plasma NMR metabolomics data. Bioinformatics 2017, 33, 3567–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fliniaux O; Gaillard G; Lion A; Cailleu D; Mesnard F; Betsou F Influence of common preanalytical variations on the metabolic profile of serum samples in biobanks. J. Biomol. NMR 2011, 51, 457–465. [DOI] [PubMed] [Google Scholar]
- 30.Trezzi JP; Bulla A; Bellora C; Rose M; Lescuyer P; Kiehntopf M; Hiller K; Betsou F LacaScore: A novel plasma sample quality control tool based on ascorbic acid and lactic acid. Metabolomics 2016, 12, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan AY; Swaminathan R; Cockram CS Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem. 1989, 35, 315–317. [PubMed] [Google Scholar]
- 32.Kilfoil PJ; Tipparaju SM; Barski OA; Bhatnagar A Regulation of ion channels by pyridine nucleotides. Circ. Res 2013. 112(4), 721–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch-Nolte F; Haag F; Guse AH; Lund F; Ziegler M Emerging roles of NAD+ and its metabolites in cell signaling. Sci. Signal 2009, 2(57):mr1. [DOI] [PubMed] [Google Scholar]
- 34.Ying W NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal. 2008, 10(2), 179–206. [DOI] [PubMed] [Google Scholar]
- 35.Engel PC Glutamate dehydrogenases: the why and how of coenzyme specificity. Neurochem. Res 2014, 39(3), 426–32. [DOI] [PubMed] [Google Scholar]
- 36.Johnson TA; Jinnah HA; Kamatani K Shortage of Cellular ATP as a Cause of Diseases and Strategies to Enhance ATP. Front Pharmacol. 2019, 10, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogasawara N; Goto H; Yamada Y AMP Deaminase Isozymes in Human Blood Cells. In: De Bruyn CHMM, Simmonds HA, Müller MM (eds) Purine Metabolism in Man-IV. Advances in Experimental Medicine and Biology, 1984), vol 165. Springer, Boston, MA. 10.1007/978-1-4757-0390-0_12 [DOI] [PubMed] [Google Scholar]
- 38.Lovászi M; Németh ZH; Gause WC; Beesley J; Pacher P; Haskó G Inosine monophosphate and inosine differentially regulate endotoxemia and bacterial sepsis. FASEB J. 2021, 35(11), e21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wollenberger A; Ristau O; Schoffa G Eine einfache technik der extrem schnellen abkuhlung grosserer gewebestucke. Pflug. Arch. Eur. J. Physiol 1960, 270, 399–412. [PubMed] [Google Scholar]
- 40.Lu W; Su X; Klein MS; Lewis IA Fiehn O; Rabinowitz JD Metabolite Measurement: Pitfalls to Avoid and Practices to Follow. Annu Rev Biochem. 2017, 86, 277–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinu FR; Villas-Boas SG; Aggio R Analysis of Intracellular Metabolites from Microorganisms: Quenching and Extraction Protocols. Metabolites. 2017, 7(4):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahrheit J; Heinzle E Quenching methods for the analysis of intracellular metabolites. Methods Mol Biol. 2014, 1104, 211–21. [DOI] [PubMed] [Google Scholar]
- 43.Sake CL; Newman DM; Boyle NR Evaluation of quenching methods for metabolite recovery in photoautotrophic Synechococcus sp. PCC 7002. Biotechnol Prog. 2020. 36(5):e3015. [DOI] [PubMed] [Google Scholar]
- 44.Kapoore RV; Vaidyanathan S; Quenching for Microalgal Metabolomics: A Case Study on the Unicellular Eukaryotic Green Alga Chlamydomonas reinhardtii. Metabolites. 2018, 8(4):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study design for blood sample pretreatment, storage and extraction prior to analysis.
Figure S2. 1H NMR spectrum of blood obtained after extraction using methanol and chloroform.
Figure S3. Portions of 1H NMR spectra of blood obtained after extraction using ethanol (n=112), methanol (n=112), and a mixture of methanol and chloroform (n=112) highlighting coenzyme peaks.
Figure S4. NAD+ and NADH levels with error bars under different conditions of sample pretreatment and extraction using a mixture of methanol and chloroform.
Figure S5. AMP and IMP levels with error bars under different conditions of sample pretreatment and extraction using a mixture of methanol and chloroform.
Figure S6. AMP and IMP levels under different conditions of sample pretreatment and extraction using ethanol or methanol.
Figure S7. Lactate levels with error bars under different conditions of sample pretreatment and extraction using a mixture of methanol and chloroform.
Figure S8. Glucose and lactate levels under different conditions of sample pretreatment and extraction using ethanol or methanol.