Abstract
Background:
Sleep disturbances, as manifested in insomnia symptoms of difficulties falling asleep or frequent nighttime awakenings, are a strong risk factor for a diverse range of diseases involving immunopathology. Low-grade systemic inflammation has been frequently found associated with sleep disturbances and may mechanistically contribute to increased disease risk. Effects of sleep disturbances on inflammation have been observed to be long lasting and remain after recovery sleep has been obtained, suggesting that sleep disturbances may not only affect inflammatory mediators, but also the so-called specialized pro-resolving mediators (SPMs) that actively resolve inflammation. The goal of this investigation was to test for the first time whether the omega-3 fatty acid-derived D- (RvD) and E-series (RvE) resolvins are impacted by prolonged experimental sleep disturbance (ESD).
Methods:
Twenty-four healthy participants (12F, age 20–42 years) underwent two 19-day in-hospital protocols (ESD/control), separated by >2 months. The ESD protocol consisted of repeated nights of short and disrupted sleep with intermittent nights of undisturbed sleep, followed by three nights of recovery sleep at the end of the protocol. Under the control sleep condition, participants had an undisturbed sleep opportunity of 8 hours/night throughout the protocol. The D- and E-series resolvins were measured in plasma using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Results:
The precursor of the D-series resolvins, 17-HDHA, was downregulated in the ESD compared to the control sleep condition (p<.001 for condition), and this effect remained after the third night of recovery sleep has been obtained. This effect was also observed for the resolvins RvD3, RvD4, and RvD5 (p<.001 for condition), while RvD1 was higher in the ESD compared to the control sleep condition (p<.01 for condition) and RvD2 showed a mixed effect of a decrease during disturbed sleep followed by an increase during recovery sleep in the ESD condition (p<.001 for condition*day interaction). The precursor of E-series resolvins, 18-HEPE, was downregulated in the ESD compared to the control sleep condition (p<.01 for condition) and remained low after recovery sleep has been obtained. This effect of downregulation was also observed for RvE2 (p<.01 for condition), while there was no effect for RvE1 (p>.05 for condition or condition*day interaction). Sex-differential effects were found for two of the D-series resolvins, i.e., RvD2 and RvD4.
Conclusion:
This first investigation on the effects of experimental sleep disturbance on inflammatory resolution processes shows that SPMs, particularly resolvins of the D-series, are profoundly downregulated by sleep disturbances and remain downregulated after recovery sleep has been obtained, suggesting a longer lasting impact of sleep disturbances on these mediators. These findings also suggest that sleep disturbances contribute to the development and progression of a wide range of diseases characterized by immunopathology by interfering with processes that actively resolve inflammation. Pharmacological interventions aimed at promoting inflammatory resolution physiology may help to prevent future disease risk as a common consequence of sleep disturbances.
Trial Registration:
Keywords: Sleep disturbance, Recovery sleep, Resolution of inflammation, Specialized pro-resolving mediators (SPMs), D-series resolvins (RvD), E-series resolvins (RvE), 17-HDHA, 18-HEPE
1. Introduction
Sleep disturbances are prospectively associated with an increased risk of a wide range of common diseases involving immunopathology, such as cancer, chronic pain, cardiovascular, neurodegenerative, neuropsychiatric, and metabolic diseases, including diabetes and obesity (Besedovsky et al., 2019; Irwin, 2015). Poor sleep also increases risk and deteriorates progression and outcome of infection, including upper airway infections (Cohen et al., 2009; Prather et al., 2015), pneumonia (Patel et al., 2012), as well as infections with SARS-CoV-2 (Li et al., 2021). Chronic low-grade systemic inflammation is assumed to be one of the main reasons for the negative health consequences of sleep disturbance (Besedovsky et al., 2019; Irwin, 2019; Irwin and Opp, 2017). Most of the approximately 150 studies conducted in the last decades found that disturbed or insufficient sleep resulted in an upregulation of mainly pro-inflammatory acting mediators, including interleukin-6 (IL-6), C-reactive protein (CRP), and lipid-derived prostaglandins (Besedovsky et al., 2019; Irwin et al., 2016). Recent findings in humans and rodents revealed that insufficient sleep affects hematopoiesis of neutrophils and monocytes (McAlpine et al., 2022; McAlpine et al., 2019). Such inflammatory upregulation has been shown to be longer lasting as indicated by incomplete resolution after recovery sleep has been obtained (Lasselin et al., 2015; McAlpine et al., 2022; Simpson et al., 2016; van Leeuwen et al., 2009), suggesting that mediators that actively resolve inflammation may also be affected by sleep disturbances.
The resolution of inflammation is an active process orchestrated by so-called specialized pro-resolving lipid mediators (SPMs) (Panigrahy et al., 2021; Serhan, 2011; Serhan and Levy, 2018). The mediators of inflammatory resolution mainly derive from omega-3 polyunsaturated fatty acids (PUFAs) and are converted to specific SPMs, such as D-series and E-series resolvins that derive from the omega-3 PUFAs docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), respectively (Serhan, 2014). Resolvins promote the return to inflammatory homeostasis through counter-regulation of pro-inflammatory signals, involving a class-switch from pro-inflammatory lipid mediators to SPMs including the resolvins, which coincides with the transformation of inflammatory neutrophil infiltration into non-phlogistic monocyte/macrophage recruitment (Serhan, 2014; Serhan and Levy, 2018). Thus, failure to mount inflammatory resolution processes can result in chronic unresolved inflammation and the development of inflammatory diseases (Panigrahy et al., 2021).
Recent studies in humans revealed dysregulation of resolvins and their precursors in various health conditions, such as autoimmune disorders (Kooij et al., 2020; Prüss et al., 2013; Sorokin et al., 2018), asthma (Johnson et al., 2022), metabolic disorders (Barden et al., 2019; López-Vicario et al., 2019; Schulte et al., 2020), cardiovascular diseases (Bazan et al., 2017; Keeley et al., 2022), neurodegenerative disorders (Do et al., 2023; Zhu et al., 2016), chronic pain (Valdes et al., 2017), and severe COVID-19 (Palmas et al., 2021). Moreover, the functional beneficial effect of resolvins on health is suggested from studies showing that consumption of resolvin precursors, i.e., the omega-3 PUFAs DHA and EPA available in concentrated form as dietary supplements, can mitigate severity and progression of various diseases involving inflammation, including cardiovascular diseases, arthritis, and certain cancers (Calder, 2018; Djuricic and Calder, 2021).
Whether sleep disturbances disrupt the inflammatory resolution mediator pathways, e.g., resolvins, has not previously been investigated to our knowledge. We here investigated the effect of prolonged experimental sleep disturbances and subsequent recovery sleep on resolvins of the D-series and E-series and their precursors in healthy humans. We hypothesized that experimental sleep disturbances compromise the production of resolvins, and that effects are long lasting as indicated by incomplete normalization following recovery sleep. While sex differences in inflammatory responses to disturbed or insufficient sleep have been shown previously (Besedovsky et al., 2022; Irwin et al., 2010), we explored in the present study whether females and males differ in their inflammatory resolution responses to sleep disturbances.
2. Methods
2.1. Study protocol
This investigation is an exploratory analysis of a study, for which the primary outcomes of inflammation and pain were published recently (Besedovsky et al., 2022; Haack et al., 2023). The study employed a randomized crossover design with two 19-day (18 nights) in-hospital protocols. Each participant was assigned to an experimental sleep disturbance (ESD) stay and a control stay in randomized order with an interval of at least 2 months between stays in order to prevent potential carry-over effects of the prolonged experimental sleep disturbance protocol. Each in-hospital stay started with 3 nights with a sleep opportunity of 8 hours per night (23:00–07:00) for adaptation and baseline measurements. Then, under the ESD condition, participants were exposed to 3 cycles of ESD, each consisting of 3 nights of disturbed sleep (later sleep onset, hourly awakenings, earlier sleep offset, resulting in a total sleep opportunity of 4 hours) followed by 1 night of recovery sleep with an undisturbed sleep opportunity of 8 hours. Such sleep patterns are common in individuals experiencing pain or stress, for instance, in relation to economic, societal or health challenges (Haack et al., 2020; Hirotsu et al., 2015). The model was designed to mimic sleep patterns that are common in chronic pain populations, in which consecutive nights of shortened and disrupted sleep are followed by intermittent nights of undisturbed sleep as a result of build-up of homeostatic sleep pressure following several nights of disrupted sleep (Perlis et al., 2010; Vallières et al., 2005). The ESD protocol ended with 3 additional nights of recovery sleep with a sleep opportunity of 8 hours per night (Fig. 1). Under the control condition, participants had a sleep opportunity of 8 hours per night throughout the entire protocol.
Fig. 1. Study protocol.
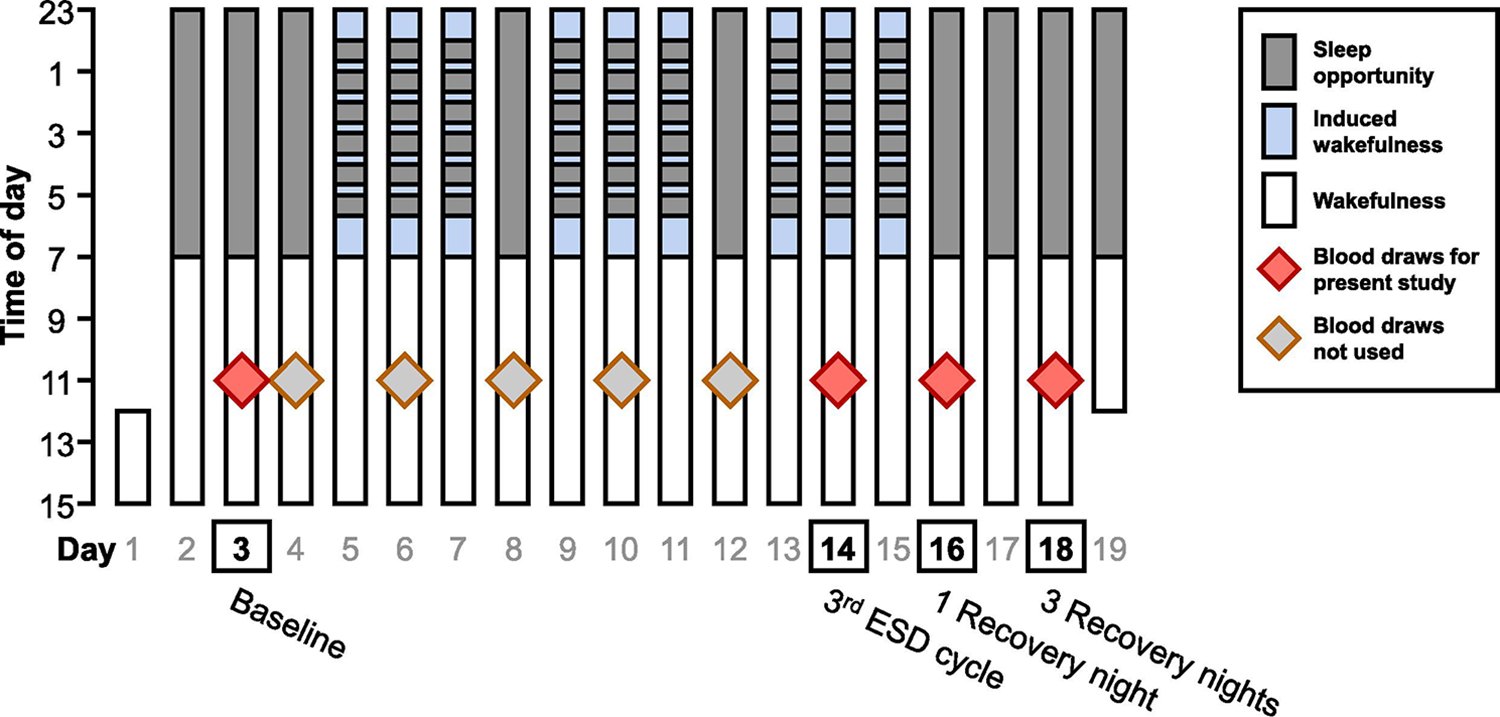
Depicted is the 19-day in-hospital protocol of the experimental sleep disturbance (ESD) model. Gray shading indicates sleep opportunity. Light blue shading indicates induced wakefulness under dim light conditions. After 3 nights of 8 h baseline sleep, participants were exposed to 3 cycles of ESD, each consisting of 3 nights of disturbed sleep (later sleep onset, hourly awakenings, earlier sleep offset, total sleep opportunity 4 hours) followed by 1 night of undisturbed sleep. The protocol ended with 3 additional nights of 8 h recovery sleep. Blood was drawn at two days during baseline and then every second day throughout the protocol. The present study includes samples collected at baseline (day 3), during the 3rd ESD cycle (day 14), after 1 night of recovery sleep (day 16), and after 3 nights of recovery sleep (day 18). During the control condition, participants had a sleep opportunity of 8 hours per night throughout the entire protocol.
During each of the ESD nights, the timing of sleep onset was delayed by one hour (from 23:00 to 00:00). This was followed by a six-hour interval (between 00:00 and 06:00), in which 40 min of sleep opportunity were followed by 20 min of induced wake time every hour, totaling 6 induced awakenings during the night (5 in-between + 1 final) and resulting in a 4-hour sleep opportunity and 2 hours of induced wake time. Lastly, sleep offset was advanced by one hour (from 07:00 to 06:00). To implement nighttime awakenings, the research nurse entered the room, turned on the light to less than 20 lx, and woke up the participant by calling their name. During the induced 20-min awakenings, participants interacted with the attending research assistant while staying in bed in a semi-recumbent position until the next scheduled sleep opportunity started.
During each of the two 19-day in-hospital stays, participants had 8 days of intensive monitoring (every second day of the study protocol starting with day 4). Measurements included polysomnographic (PSG) recordings and quantitative somatosensory testing (Besedovsky et al., 2022; Haack et al., 2023). Blood samples were collected on the first baseline day and on all 8 intensive monitoring days. The present study includes blood samples collected on the first baseline day (day 3), during the third ESD cycle (day 14), after 1 night of recovery sleep (day 16), and after 3 nights of recovery sleep (day 18) of each ESD stay and the same days of each control stay (Fig. 1).
2.2. Participants
The present study was approved by the Institutional Review Board for Research Involving Human Subjects at the Beth Israel Deaconess Medical Center (BIDMC) in Boston, MA, and registered at ClinicalTrials.gov (NCT02484742). Participants were recruited via community and website advertisements. Twenty-four healthy participants (12F/12M) were included in the analysis (Table 1). Twenty-two participants completed both 19-day in-hospital stays. Two participants did not complete the second in-hospital stay due to work/family requirements and difficulties in following study procedures, and data of one stay of one participant could not be analyzed due to missing sample material.
Table 1.
Participant characteristics.
| All (N = 24) |
Control (n = 23) |
ESD (n = 22) |
|
|---|---|---|---|
|
| |||
| Age in years, mean (SD) | 28.3 (5.9) | 28.4 (5.9) | 28.2 (6.0) |
| Sex, n (%) | |||
| Female | 12 (50.0) | 11 (47.8) | 11 (50.0) |
| Male | 12 (50.0) | 12 (52.2) | 11 (50.0) |
| BMI in kg/m2, mean (SD) | 23.9 (3.8) | 24.0 (4.0) | 23.8 (3.7) |
| Race, n (%) | |||
| Black/African American | 10 (41.7) | 10 (43.5) | 9 (40.9) |
| White | 10 (41.7) | 9 (39.1) | 10 (45.5) |
| Asian | 1 (4.2) | 1 (4.3) | 1 (4.5) |
| Multiracial a | 1 (4.2) | 1 (4.3) | 1 (4.5) |
| Otherb | 1 (4.2) | 1 (4.3) | 1 (4.5) |
| Not provided | 1 (4.2) | 1 (4.3) | 0 (0.0) |
| Ethnicity, n (%) | |||
| Hispanic | 6 (25.0) | 6 (26.1) | 4 (18.2) |
| Non-Hispanic | 14 (58.3) | 13 (56.5) | 14 (63.6) |
| Not provided | 4 (16.7) | 4 (17.4) | 4 (18.2) |
| Dietary preference, n (%) | |||
| Omnivore | 18 (75.0) | 17 (73.9) | 16 (72.7) |
| Pescetarian | 2 (8.3) | 2 (8.7) | 2 (9.1) |
| Vegetarian | 2 (8.3) | 2 (8.7) | 2 (9.1) |
| Vegan | 2 (8.3) | 2 (8.7) | 2 (9.1) |
| Menstrual cycle phase c, n (%) | |||
| Follicular | - | 4 (36.4) | 3 (27.3) |
| Luteal | - | 7 (63.6) | 8 (72.7) |
Reported as “mixed Black and Alaska Native”.
Reported as “Peruvian”.
Menstrual cycle phase at day 1 of the experimental protocol; two females did not have regular menstrual cycles, one naturally, one due to anintrauterine device.
Inclusion criteria were age between 18 to 45 years, a body mass index (BMI) between 18.5 and 30 kg/m2, habitual nightly sleep duration between 7 and 9 hours (verified by sleep diary data collected over seven days), sleep efficiency >85% as per screening PSG, habitual time of sleep onset within one hour of the study bedtime of 23:00 (to ensure entrainment), blood chemistry levels within the normal range (including white blood cell and differential blood cell counts, T cell subsets, thyroid hormones, glucose, insulin, creatinine, liver enzymes, erythrocyte sedimentation rate), and negative urine toxicology. Female participants were eligible if they had regular menstrual cycles and no significant discomfort during pre-menses/menses.
Exclusion criteria included presence or history of medical or psychiatric disorders (determined by diagnostic interview, physician’s medical history and physical examination), sleep disorders (based on questionnaires and in-hospital PSG), pregnant or nursing status, regular medication use other than hormonal contraceptives, non-steroidal anti-inflammatory drug (NSAID) or cough medicine use in the two weeks prior to the in-hospital stays, and donation of blood or platelets three months prior to (or in-between) in-hospital stays. Blood tests and urine toxicology screening were repeated prior to the second 19-day in-hospital stay to ensure values remained in the normal range. None of the participants reported taking omega-3 PUFA supplements.
2.3. Research environment
Throughout both in-hospital stays, participants stayed in a private room at the Clinical Research Center at BIDMC. Ambient room temperature was based on the individually tailored comfort level and kept stable throughout all study days. Participants were maintained on a balanced diet controlled for macronutrients (15% proteins, 30% fats, 55% carbohydrates) and electrolytes (3 mg sodium, 3 mg potassium adjusted for caloric intake) and regimented fluid intake (no caffeine) in order to prevent changes in body weight/composition throughout the study. Meals (breakfast 07:30, lunch 12:30, dinner 18:30, snack 20:50) and fluids (every 2 hours from 7:00 to 21:00) were served at standardized times. Food that was not consumed was weighed back to calculate actual caloric intake (ProNutra software, Viocare, Princeton, NJ). To prevent sedentary conditions and maintain constant activity levels, the attending research assistant took participants on a 10 to 15-min walk within the Clinical Research Center or outside on hospital property every 2 to 3 hours throughout the waking periods of the protocol (except during induced wake periods at night). Participants were encouraged to follow their pre-study exercise habits by visiting the hospital gym on the non-intensive monitoring days. Participants could have visitors during daytime periods and had access to internet and phone, in order to minimize disruptions to their social networks.
2.4. Sample collection and processing
Blood samples were taken through direct venipuncture at 11:00 in the morning. This time point for blood collection was selected based on previous research from our lab (Simpson et al., 2016) showing significant differences in immune parameters and glucocorticoid sensitivity between sleep restriction and control sleep at this time of day and to avoid the potential influence of the awakening cortisol peak and meal intake scheduled at 07:30. Prior to blood collection, participants refrained from food and fluid intake for 60 min and remained in a seated position for 15 min. Blood was collected in K2 EDTA tubes (BD Vacutainer, Franklin Lakes, NJ, USA) on ice. Then, plasma was separated by centrifugation for 10 min at 1500 × g at 4°C and stored at −80°C until measurement. The samples were express-shipped on dry ice to Ambiotis SAS, Toulouse, France for SPM analysis.
2.5. Lipid mediator analysis
Lipid mediators were measured in plasma using liquid chromatography-tandem mass spectrometry (LC-MS/MS) in the external contract research laboratory Ambiotis SAS, Toulouse, France as previously described (Le Faouder et al., 2013). The present study focused on the SPM omega-3 PUFA derivatives D-series resolvins (RvD1, RvD2, RvD3, RvD4, and RvD5) and E-series resolvins (RvE1 and RvE2), and their precursors 17-hydroxydocosahexaenoic acid (17-HDHA) and 18-hydroxyeicosapentaenoic acid (18-HEPE), respectively (refer to Table S1 for structural details). Values below the lower limit of quantification (LOQ) were included in the analysis as measured. Values below the limit of detection (LOD) were set to half the respective LOD (portion of values below LOD: RvD1 0.6%, RvD2 6%, RvD3 4%, RvD4 14%, RvD5 23%, 17-HDHA 0%, RvE1 26%, RvE2 0.6%, 18-HEPE 0%, refer to Table S2). No significant differences in the frequency of samples below LOD were found on the baseline day, neither for condition nor for sex. On day 14 (sleep disturbance day), there also was no difference in the frequency of samples below LOD for sex and for most of the investigated resolvins, except for RvD5, for which 0.6% of values were below LOD in the control condition and 4.4% of values were below LOD in the ESD condition. The mean intra-assay and inter-assay coefficients of variation (CV) were 6.2% and 7.5%, respectively, and the mean recovery rate was 101.4% (refer to Table S3). Original data are depicted in the supplementary material (Fig. S1, S2).
2.6. Statistical analysis
The effect of experimental sleep disturbances on plasma concentrations of resolvins and their precursors was assessed using IBM SPSS Statistics 28. Generalized linear mixed model (GLMM) analysis was performed with repeated measurements across multiple days of the two in-hospital stays (stay*day, diagonal covariance structure). Satterthwaite’s approximation was used to calculate denominator degrees of freedom. Robust estimation was used in order to protect against potential violations of model assumptions. In all models, the intercept, the three factors condition (ESD/control), day (14/16/18), and sex (female/male) as well as their interactions were included as fixed factors. The baseline day 3 was handled as a covariate in all models to account for potential baseline differences between conditions. Participant’s identification number was set as a random effect with a variance components (VC) covariance structure. To identify the best fitting model structure, all models were calculated with different combinations of target probability distributions (normal, gamma, inverse Gaussian) and link functions (identity, log, power(−1)). For each variable, the most appropriate model structure was identified by evaluation of homoscedasticity and normality by plotting residuals vs. predicted values and by histograms of residuals, respectively. In all models, a p-value of <.05 for main or interaction effects was considered significant. A significant condition effect or condition*day interaction effect was considered appropriate for pairwise comparisons at specific days using the least significant difference (LSD) tests. A significant condition*sex interaction effect was considered appropriate for pairwise comparisons at specific days for females and males separately. Data are presented as estimated marginal means (EMM) ± standard errors (SEM), which account for the between-participant variation as well as the within-participant variation across days and across conditions (intra-individual study design).
3. Results
Participant characteristics are listed in Table 1. Average daily caloric intake was 2259 kcal and daily intake of omega-3 fatty and omega-6 PUFAs was 1.4 mg and 12.9 mg, respectively, which corresponds to a typical American diet. For details on macro- and micronutrients in both conditions, see supplementary Table S4.
All of the investigated resolvins of the D-series were affected by ESD compared to the control condition (Fig. 2). The precursor of the D-series resolvins, 17-HDHA, was downregulated following disturbed sleep in the ESD condition compared to the control condition and levels remained downregulated after the first and third night of recovery sleep (p<.001 for condition). This effect was also observed for RvD3, RvD4, and RvD5, which were all lower following disturbed sleep in the ESD condition compared to the control condition (p<.001 for condition). Whereas the RvD4 level was still significantly lower after 1 night of recovery sleep only, the RvD3 level displayed a significant decrease after 3 nights of recovery sleep only, and the RvD5 levels were lower after 1 and 3 nights of recovery sleep compared to the control condition. In contrast, the plasma concentration of RvD1 following disturbed sleep in the ESD condition was higher compared to the control condition and continued to be higher following the first night of recovery sleep (p<.01 for condition). Finally, a mixed effect was found for RvD2 levels with a decrease during disturbed sleep followed by an increase after 1 night of recovery sleep in the ESD condition compared to the control condition (p<.001 for condition*day interaction).
Fig. 2. The effect of experimental sleep disturbances (ESD) on D-series resolvins and their precursor in healthy humans.
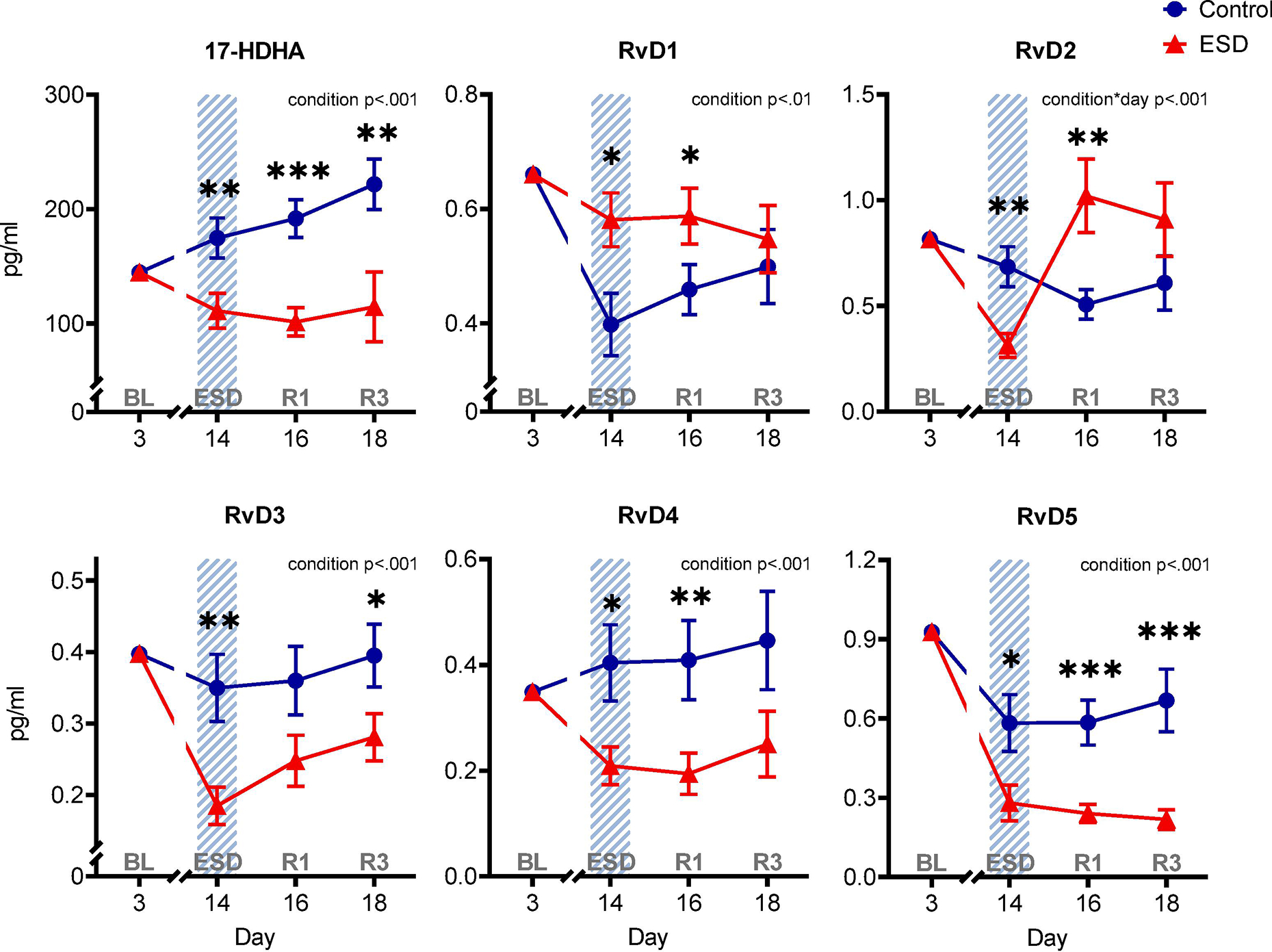
The graphs show results of GLMM analyses (EMM ± SEM) for plasma concentrations in pg/ml of the precursor 17-hydroxydocosahexaenoic acid (17-HDHA) and the D-series resolvins RvD1, RvD2, RvD3, RvD4, and RvD5. Shaded area indicates ESD. Red triangles indicate ESD condition (n = 22), blue dots indicate control condition (n = 23). Blood samples were collected at baseline (day 3 = BL), during the 3rd ESD cycle (day 14 = ESD), after 1 night of recovery sleep (day 16 = R1), and after 3 nights of recovery sleep (day 18 = R3). Detailed model results can be found in Table S6. Asterisks indicate results of pairwise comparisons between conditions: ***p<.001, **p<.01, *p<.05. N = 24 (12F/12M) participants in total, refer to Table 1 for details.
In contrast to the distinct differences between ESD and control conditions for the resolvins of the D-series, the resolvins of the E-series were less affected by ESD (Fig. 3). The concentration of the precursor of the E-series resolvins 18-HEPE was lower in the ESD condition compared to the control condition (p<.01 for condition), which manifested in lower levels following the third night of recovery sleep. There was no significant difference in the concentration of RvE1 between the ESD and the control condition (p>.05 for condition or condition*day interaction). The concentration of RvE2 was lower in the ESD condition compared to the control condition (p<.01 for condition), which manifested in lower levels following disturbed sleep.
Fig. 3. The effect of experimental sleep disturbances (ESD) on E-series resolvins and their precursor in healthy humans.
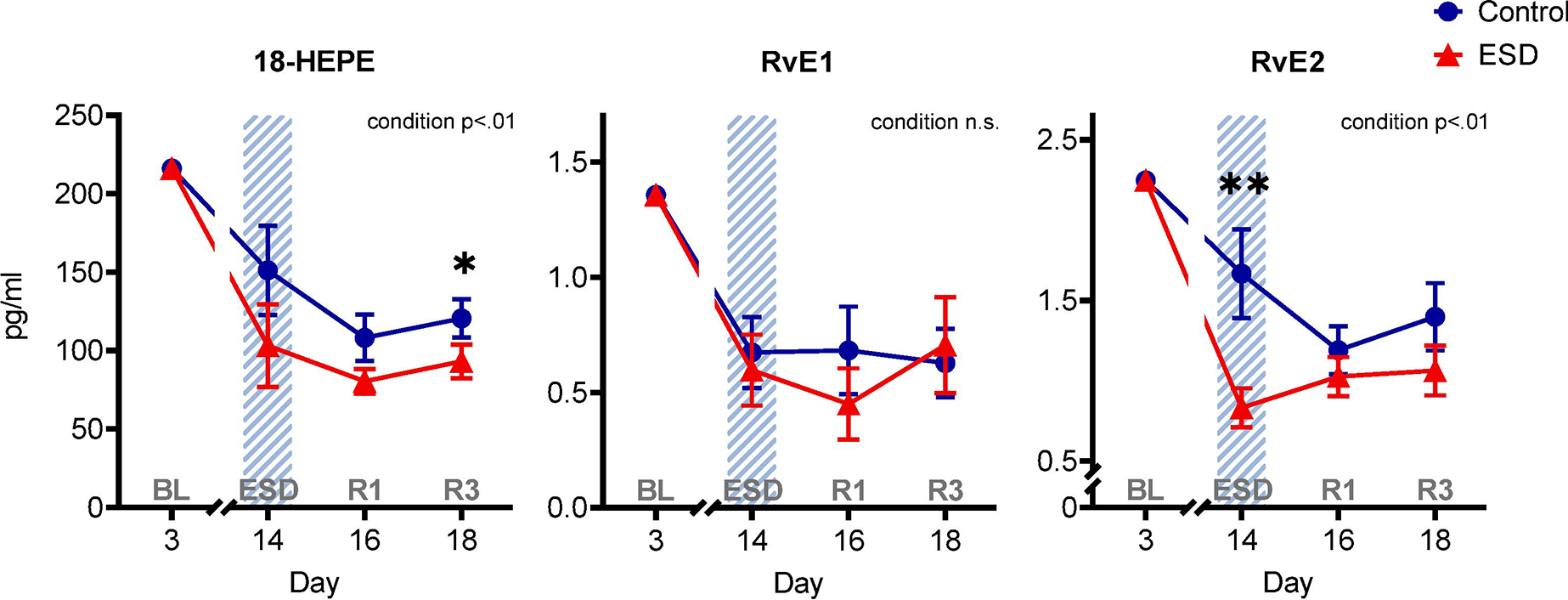
The graphs show results of GLMM analyses (EMM ± SEM) for plasma concentrations in pg/ml of the precursor 18-hydroxyeicosapentaenoic acid (18-HEPE) and the E-series resolvins RvE1 and RvE2. Shaded area indicates ESD. Red triangles indicate ESD condition (n = 22), blue dots indicate control condition (n = 23). Blood samples were collected at baseline (day 3 = BL), during the 3rd ESD cycle (day 14 = ESD), after 1 night of recovery sleep (day 16 = R1), and after 3 nights of recovery sleep (day 18 = R3). Detailed model results can be found in Table S6 (n.s. = not significant). Asterisks indicate results of pairwise comparisons between conditions: **p<.01, *p<.05. N = 24 (12F/12M) participants in total, refer to Table 1 for details.
The GLMM analyses revealed sex-differential effects for two of the D-series resolvins, RvD2 and RvD4 (Fig. 4). The condition*day*sex interaction effect (p<.05) for RvD2 indicated that females showed a distinct decrease of RvD2 concentration after disturbed sleep followed by an increase after 1 night of recovery sleep in the ESD condition compared to the control condition. RvD2 levels in males were not different between conditions. The condition*sex interaction effect (p<.05) for RvD4 was due to reduced levels in the ESD condition compared to the control condition in males, while there was no difference between conditions in females. In males, the RvD4 concentration was lower after disturbed sleep as well as after the first and third nights of recovery sleep. No sex-differential effects were found for neither the D-series resolvins RvD1, RvD3, RvD5, and their precursor 17-HDHA (Fig. 4) nor the E-series resolvins RvE1, RvE2, and their precursor 18-HEPE in the present study (Fig. 5). Pairwise comparisons at baseline (day 3) revealed that the concentration of SPMs in plasma was not different between sexes for most of the investigated variables, i.e., 17-HDHA, RvD2, RvD3, RvD5, 18-HEPE, RvE1, and RvE2. However, for RvD1 and RvD4 significant differences between sexes were found with females having lower levels than males at baseline (refer to Table S5).
Fig. 4. The effect of experimental sleep disturbances (ESD) on D-series resolvins and their precursor in healthy humans by sex.
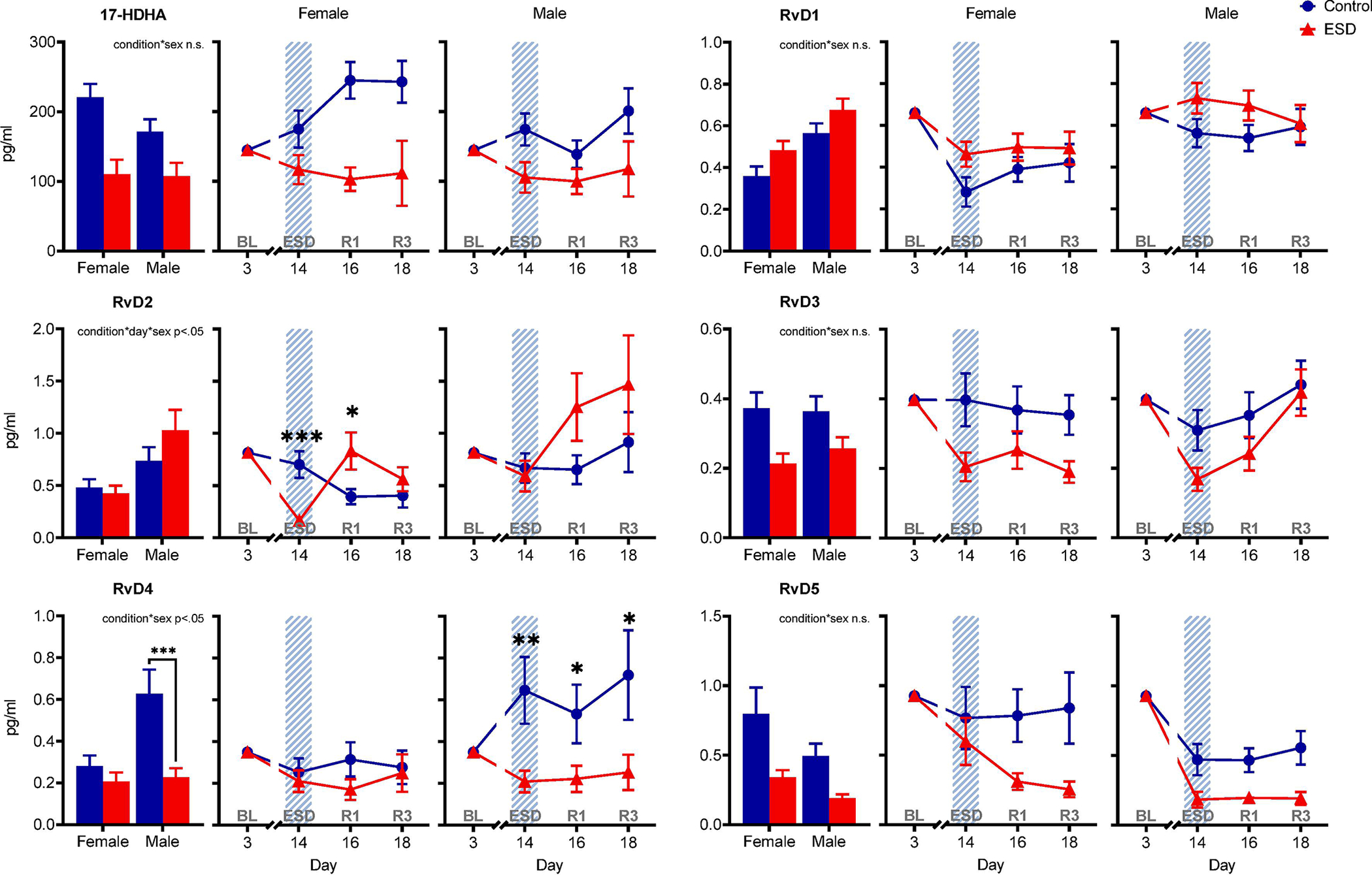
The graphs show results of GLMM analyses (EMM ± SEM) for plasma concentrations in pg/ml of the precursor 17-hydroxydocosahexaenoic acid (17-HDHA) and the D-series resolvins RvD1, RvD2, RvD3, RvD4, and RvD5 by sex. Bar graphs show main effect of condition by sex. Shaded area indicates ESD. Red triangles indicate ESD condition (n = 11F/11M), blue dots indicate control condition (n = 11F/12M). Blood samples were collected at baseline (day 3 = BL), during the 3rd ESD cycle (day 14 = ESD), after 1 night of recovery sleep (day 16 = R1), and after 3 nights of recovery sleep (day 18 = R3). Detailed model results can be found in Table S6 (n.s. = not significant). Asterisks indicate results of pairwise comparisons between conditions: ***p<.001, **p<.01, *p<.05. N = 24 (12F/12M) participants in total, refer to Table 1 for details.
Fig. 5. The effect of experimental sleep disturbances (ESD) on E-series resolvins and their precursor in healthy humans by sex.
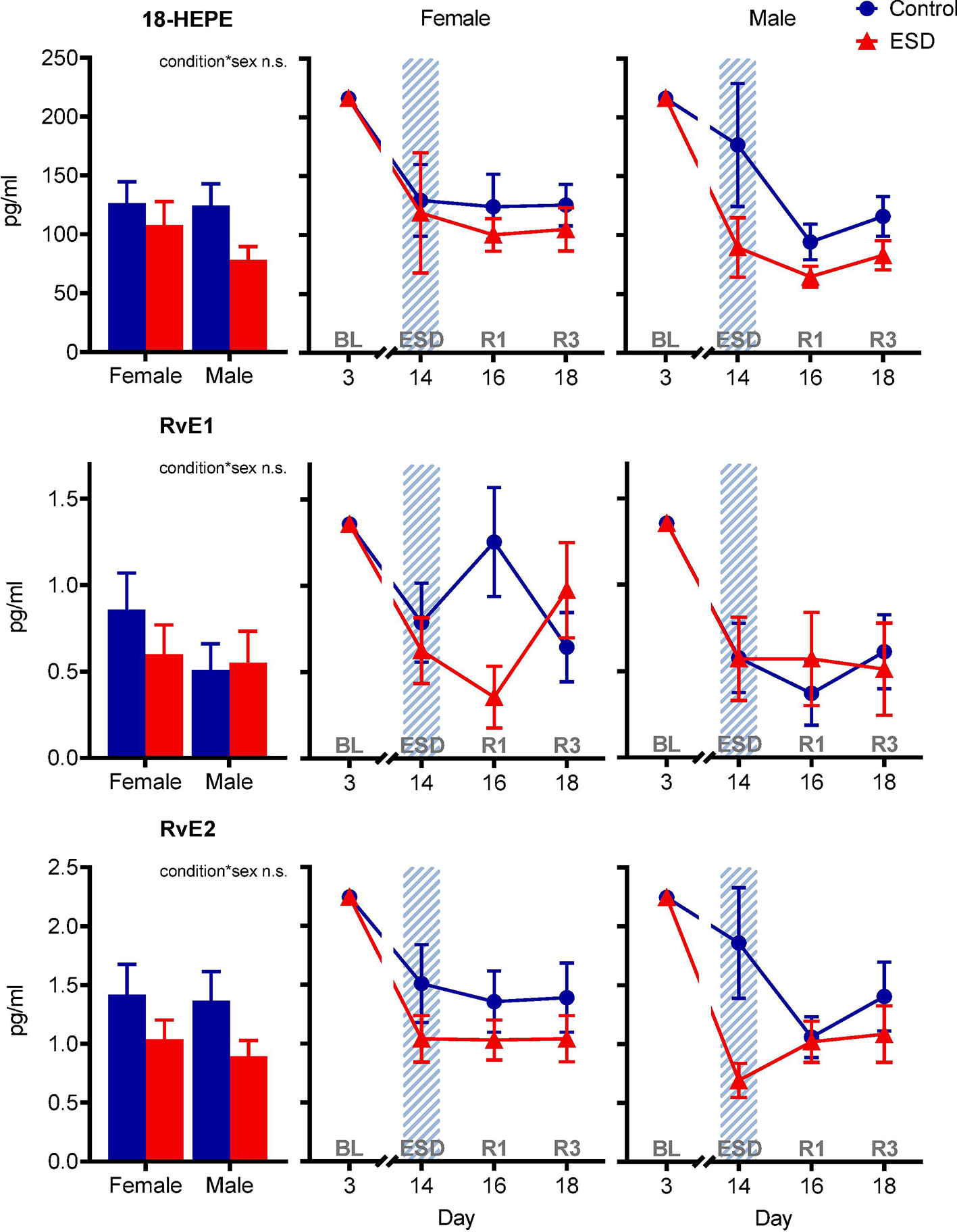
The graphs show results of GLMM analyses (EMM ± SEM) for plasma concentrations in pg/ml of the precursor 18-hydroxyeicosapentaenoic acid (18-HEPE) and the E-series resolvins RvE1 and RvE2 by sex. Bar graphs show main effect of condition by sex. Shaded area indicates ESD. Red triangles indicate ESD condition (n = 11F/11M), blue dots indicate control condition (n = 11F/12M). Blood samples were collected at baseline (day 3 = BL), during the 3rd ESD cycle (day 14 = ESD), after 1 night of recovery sleep (day 16 = R1), and after 3 nights of recovery sleep (day 18 = R3). Detailed model results can be found in Table S6 (n.s. = not significant). No significant effects of condition by sex were found for E-series resolvins and their precursor. N = 24 (12F/12M) participants in total, refer to Table 1 for details.
4. Discussion
To our knowledge, the present study is the first investigation on the effects of experimental sleep disturbance and recovery sleep on inflammatory resolution pathways. The results demonstrate that resolvins, especially the resolvins of the D-series RvD1, RvD2, RvD3, RvD4, RvD5, and their precursor 17-HDHA are affected by sleep disturbances. In particular, 17-HDHA, RvD3, RvD4, and RvD5 were downregulated and this downregulation continued into the recovery sleep period, suggesting a lasting effect of sleep disturbances on these inflammatory resolution mediators. This might contribute to the slowed inflammatory recovery from sleep disturbances as observed in previous studies (Lasselin et al., 2015; McAlpine et al., 2022; Simpson et al., 2016; van Leeuwen et al., 2009).
A few studies have investigated single resolvins or their precursors in relation to sleep. In mice, sleep fragmentation (induced by housing the animals in a rotating drum over 14 days) has been shown to downregulate the omega-3 PUFA docosapentaenoic acid (DPA) in serum (Feng et al., 2016). In addition, some preclinical studies have investigated the effects of SPMs on sleep. An early study in rats showed that lipoxins, a class of SPMs derived from the omega-6 PUFA arachidonic acid (AA), injected to the brain increased slow wave sleep (Sri Kantha et al., 1994). More recently, a study in brain-injured mice showed that intra-peritoneal treatment with RvE1 increased sleep time during the recovery period (Harrison et al., 2015). In humans, it has been shown that serum levels of RvD1 and lipoxin A4 were reduced in patients with obstructive sleep apnea compared to healthy controls (Chen et al., 2019). However, to which degree the lower values were associated with the degree of sleep fragmentation in these patients was not reported. While there are only very few investigations on the relationship between sleep and SPMs, they suggest that there is a potential bi-directional link to be explored in future investigations.
While we found pronounced effects for the D-series resolvins and their precursor 17-HDHA, the investigated resolvins of the E-series, RvE1 and RvE2, and their precursor 18-HEPE, were less affected by the ESD condition compared to control in the present study. The reason for this apparent difference between those classes of resolvins in response to ESD is currently unknown. Different resolvins exhibit specific cell- and receptor-dependent pro-resolving actions, such as limiting neutrophil infiltration or blocking pro-inflammatory-cytokine release (Krishnamoorthy et al., 2018; Serhan et al., 2022). How these different cell-type specific actions of various resolvins translate into increased disease risk as a consequence of sleep disturbances will be an active area of research in the future.
We found sex-differential effects of ESD and recovery sleep for two of the investigated lipid mediators, namely RvD2 and RvD4. Females responded to disturbed sleep with a marked reduction in RvD2 levels, followed by a rebound effect during the recovery sleep period compared to the control sleep condition. Males, in contrast, responded with a marked reduction of RvD4 levels following disturbed sleep and subsequent recovery sleep compared to control sleep. These findings suggest that females and males may differ in their responses of certain D-series resolvins to sleep disturbances. Sex differences in the response of certain SPMs to various stimuli have been reported in a few studies in humans (Barden et al., 2018; Barden et al., 2015; Rathod et al., 2017). For instance, using an experimental blister model, females appear to have a stronger inflammatory resolution response than males (Rathod et al., 2017). On these grounds, it was suggested that differences in concentrations of individual SPMs in plasma may be small between females and males (Calder, 2020). In contrast, we found that baseline concentrations did not differ between females and males for most of the investigated variables, i.e., 17-HDHA, RvD2, RvD3, RvD5, 18-HEPE, RvE1, and RvE2, and that females had lower levels than males in two of the investigated SPMs, i.e., RvD1 and RvD4, at baseline. However, with respect to potential sex-differential effects of sleep disturbances on SPMs, future studies powered for the detection of sex differences are needed.
This investigation was an exploratory analysis of a study, for which the primary outcomes of inflammation and pain were published recently (Besedovsky et al., 2022; Haack et al., 2023). Briefly, these studies reported that PSG-derived sleep measures were affected by the ESD protocol as expected, such that the amount of slow wave sleep was reduced during all nights with disturbed sleep but increased during the subsequent nights of recovery sleep (Besedovsky et al., 2022). The pro-inflammatory response to sleep disturbance was affected in a sex-specific manner (Besedovsky et al., 2022; Haack et al., 2023), as manifested in a stronger activation of IL-6 and cyclooxygenase (COX)-2 expression in males compared to females. Sex-differential effects were also found for cortisol, with a stronger morning cortisol response in females (Besedovsky et al., 2022). To date, no associations between SPMs in plasma and glucocorticoids have been reported in studies that were stratified by sex (Barden et al., 2020; Barden et al., 2018). In the present study, two of the investigated resolvins showed sex-differential effects (i.e., RvD2 and RvD4). However, further investigations that are adequately powered on sex are needed in the future.
Given the growing clinical and preclinical evidence of the involvement of inflammatory resolution mediators in the development and progression of various health conditions, including autoimmune disorders (Kooij et al., 2020; Prüss et al., 2013; Sorokin et al., 2018), cardiovascular diseases (Bazan et al., 2017; Keeley et al., 2022), chronic pain (Valdes et al., 2017), and neurodegenerative disorders (Do et al., 2023; Zhu et al., 2016), the current findings suggest that sleep disturbances may contribute to an increased risk of these diseases through a profound downregulation of certain inflammatory resolution mediators. For some health conditions, supplementation with the precursors of resolvins, i.e. the omega-3 PUFAs EPA and DHA, has been recommended as an effective and safe option to improve health. For instance, elevated triglycerides that are commonly associated with obesity, diabetes, and cardiovascular disease can be effectively and safely reduced through supplementation with the omega-3 PUFAs EPA and DHA (Skulas-Ray et al., 2019). Furthermore, growing preclinical evidence suggests that certain resolvins have potent analgesic effects and may become effective therapeutics in the treatment of pain (Fattori et al., 2020). In humans, omega-3 PUFA supplementation standardized to the resolvin precursors 17-HDHA and 18-HEPE has been shown to reduce pain intensity in a sample of adults with chronic pain, and interestingly also reduced reported sleep disturbances (Callan et al., 2020). Pain is a very common consequence of sleep disturbances (Finan et al., 2013) and our current findings suggest that sleep disturbance may predispose to chronic pain by reducing D-series resolvins and their precursor 17-HDHA, in particular.
The present study has several strengths, in particular its intra-individual crossover design, which allows for direct assessment of changes in SPM levels within each participant by statistical modeling. A further strength of this investigation is the control group design. Some of the investigated mediators in the sleep control condition change across time during the in-hospital stay, with some of them in a sex-dependent manner. Such an effect has also been observed for other immune-related variables in the current and previous long-term in-hospital protocols (Besedovsky et al., 2022; Simpson et al., 2016). While we controlled many factors that potentially could influence SPM levels, such as diet composition and timing, we have only limited knowledge on whether other factors, for example pre-stay variables (regularity of sleep patterns, stress exposure, meal times), contributed to in-hospital and sometimes sex-differential changes in certain SPMs in the control condition. Another strength is that SPMs were assessed using a LC-MS/MS platform, which is considered the most sensitive and specific method to detect very low concentrations of lipid mediators in plasma (Liakh et al., 2020).
The present study also has some limitations. The study was not powered to detect sex differences and findings should be handled with precaution. In addition, blood samples analyzed included only those obtained at baseline and at the end of the 19-day protocol (last cycle of ESD). Thus, we could not assess whether SPM levels were changed following acute sleep disturbance, i.e., following the first cycle of ESD. Moreover, as just one blood sample per day was assessed at 11:00 in the morning, potential effects of sleep disturbances and recovery sleep on the diurnal regulation of SPMs could not be investigated. Given the report suggesting diurnal variations of DPA-derived resolvins with higher concentrations in the morning than in the evening in humans (Colas et al., 2018), future studies may investigate the diurnal regulation of resolvins and underlying mechanisms in response to sleep disturbance.
In conclusion, the present study demonstrates for the first time that sleep disturbances downregulate certain SPMs, particularly the D-series resolvins RvD2, RvD3, RvD4, RvD5, and the precursor 17-HDHA, and that their levels do not normalize after up to three nights of recovery sleep, suggesting a lasting effect of sleep disturbances on inflammatory resolution physiology. Impaired inflammatory resolution as a consequence of sleep disturbance may explain slowed inflammatory recovery from sleep disturbances. The present work suggests that sleep disturbances contribute to the development and progression of many common diseases characterized by immunopathology by interfering with processes that actively resolve inflammation. Pharmacologically targeting these pathways in the future, for instance through resolvin precursor supplementation or specific resolvins that may be soon on the market for human research, may help to limit the many negative health consequences of sleep disturbance.
Supplementary Material
Highlights:
Prolonged experimental sleep disturbances in humans downregulate inflammatory resolution mediators, in particular the D-series resolvins
Most of the D-series resolvins remain downregulated following three nights of recovery sleep, suggesting a longer lasting effect of sleep disturbances on inflammatory resolution processes
Sleep disturbances may contribute to the many diseases involving immunopathology by disruption of inflammatory resolution processes
Acknowledgements
We thank the study physicians, the nursing staff, the medical research assistants, and the dieticians at the Clinical Research Center at Beth Israel Deaconess Medical Center for their great support in carrying out the study protocols. We also thank all research assistants, the many research students, and the research associates for helping with conducting the study. Our special thank goes to Dr. Marc Dubourdeau and his team from Ambiotis SAS, Toulouse, France for SPM analysis and excellent advice. Finally, we also thank the volunteers for participating in this research study.
Funding
This work was supported by the following grants: NIH/NINDS R01NS091177 to MH, German Research Foundation (DFG) EN1291/1-1 to LCE, NIH/NCRR UL1RR02758 and M01RR01032 to the Harvard Clinical and Translational Science Center.
Abbreviations:
- 17-HDHA
17-hydroxydocosahexaenoic acid
- 18-HEPE
18-hydroxyeicosapentaenoic acid
- AA
arachidonic acid
- BMI
body mass index
- COVID-19
coronavirus disease 2019
- COX
cyclooxygenase
- CRP
C-reactive protein
- CV
coefficient of variation
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EMM
estimated marginal mean
- EPA
eicosapentaenoic acid
- ESD
experimental sleep disturbance
- F
female
- GLMM
generalized linear mixed model
- IL-6
interleukin-6
- K2 EDTA
dipotassium ethylenediaminetetraacetic acid
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LOD
limit of detection
- LOQ
lower limit of quantification
- M
male
- NSAID
non-steroidal anti-inflammatory drug
- PSG
polysomnography
- PUFA
polyunsaturated fatty acid
- RvD
D-series resolvin
- RvE
E-series resolvin
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
- SEM
standard error of the mean
- SPM
specialized-pro-resolving mediator
Footnotes
Disclosures
None of the authors declares a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barden A, Phillips M, Hill LM, Fletcher EM, Mas E, Loh PS, French MA, Ho KM, Mori TA, Corcoran TB, 2018. Antiemetic doses of dexamethasone and their effects on immune cell populations and plasma mediators of inflammation resolution in healthy volunteers. Prostaglandins Leukot. Essent. Fatty Acids 139, 31–39. [DOI] [PubMed] [Google Scholar]
- Barden A, Phillips M, Mas E, Hill LM, Mowat I, Loh PS, Corcoran T, Mori TA, 2020. Effects of antiemetic doses of dexamethasone on plasma mediators of inflammation resolution and pain after surgery in women. Prostaglandins Other Lipid Mediat. 149, 106427. [DOI] [PubMed] [Google Scholar]
- Barden A, Shinde S, Tsai I-J, Croft KD, Beilin LJ, Puddey IB, Mori TA, 2019. Effect of weight loss on neutrophil resolvins in the metabolic syndrome. Prostaglandins Leukot. Essent. Fatty Acids 148, 25–29. [DOI] [PubMed] [Google Scholar]
- Barden AE, Mas E, Croft KD, Phillips M, Mori TA, 2015. Specialized proresolving lipid mediators in humans with the metabolic syndrome after n-3 fatty acids and aspirin. Am. J. Clin. Nutr. 102, 1357–1364. [DOI] [PubMed] [Google Scholar]
- Bazan HA, Lu Y, Jun B, Fang Z, Woods TC, Hong S, 2017. Circulating inflammation-resolving lipid mediators RvD1 and DHA are decreased in patients with acutely symptomatic carotid disease. Prostaglandins Leukot. Essent. Fatty Acids 125, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky L, Dang R, Engert LC, Goldstein MR, Devine JK, Bertisch SM, Mullington JM, Simpson N, Haack M, 2022. Differential effects of an experimental model of prolonged sleep disturbance on inflammation in healthy females and males. PNAS Nexus 1, pgac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky L, Lange T, Haack M, 2019. The sleep-immune crosstalk in health and disease. Physiol. Rev. 99, 1325–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC, 2018. Very long-chain n-3 fatty acids and human health: fact, fiction and the future. Proc. Nutr. Soc. 77, 52–72. [DOI] [PubMed] [Google Scholar]
- Calder PC, 2020. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 178, 105–123. [DOI] [PubMed] [Google Scholar]
- Callan N, Hanes D, Bradley R, 2020. Early evidence of efficacy for orally administered SPM-enriched marine lipid fraction on quality of life and pain in a sample of adults with chronic pain. J. Transl. Med. 18, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-C, Su M-C, Chin C-H, Lin I-C, Hsu P-Y, Liou C-W, Huang K-T, Wang T-Y, Lin Y-Y, Zheng Y-X, Hsiao C-C, Lin M-C, 2019. Formyl peptide receptor 1 upregulation and formyl peptide receptor 2/3 down-regulation of blood immune cells along with defective lipoxin A4/resolvin D1 production in obstructive sleep apnea patients. PLoS ONE 14, e0216607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB, 2009. Sleep habits and susceptibility to the common cold. Arch. Intern. Med. 169, 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas RA, Souza PR, Walker ME, Burton M, Zasłona Z, Curtis AM, Marques RM, Dalli J, 2018. Impaired production and diurnal regulation of vascular RvDn-3 DPA increase systemic inflammation and cardiovascular disease. Circ. Res. 122, 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuricic I, Calder PC, 2021. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2021. Nutrients 13, 2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KV, Hjorth E, Wang Y, Jun B, Kautzmann M-AI, Ohshima M, Eriksdotter M, Schultzberg M, Bazan NG, 2023. Cerebrospinal fluid profile of lipid mediators in Alzheimer’s disease. Cell. Mol. Neurobiol. 43, 797–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattori V, Zaninelli TH, Rasquel-Oliveira FS, Casagrande R, Verri WA Jr., 2020. Specialized pro-resolving lipid mediators: a new class of non-immunosuppressive and non-opioid analgesic drugs. Pharmacol. Res. 151, 104549. [DOI] [PubMed] [Google Scholar]
- Feng L, Wu H-W, Song G-Q, Lu C, Li Y-H, Qu L-N, Chen S-G, Liu X-M, Chang Q, 2016. Chronical sleep interruption-induced cognitive decline assessed by a metabolomics method. Behav. Brain Res. 302, 60–68. [DOI] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, Smith MT, 2013. The association of sleep and pain: an update and a path forward. J. Pain 14, 1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Engert LC, Besedovsky L, Goldstein MR, Devine JK, Dang R, Olia K, Molina V, Bertisch SM, Sethna N, Simpson N, 2023. Alterations of pain pathways by experimental sleep disturbances in humans: central pain-inhibitory, cyclooxygenase, and endocannabinoid pathways. Sleep, DOI: 10.1093/sleep/zsad061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Simpson N, Sethna N, Kaur S, Mullington J, 2020. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology 45, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JL, Rowe RK, Ellis TW, Yee NS, O’Hara BF, Adelson PD, Lifshitz J, 2015. Resolvins AT-D1 and E1 differentially impact functional outcome, post-traumatic sleep, and microglial activation following diffuse brain injury in the mouse. Brain Behav. Immun. 47, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu C, Tufik S, Andersen ML, 2015. Interactions between sleep, stress, and metabolism: from physiological to pathological conditions. Sleep Sci. 8, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, 2015. Why sleep is important for health: a psychoneuroimmunology perspective. Annu. Rev. Psychol. 66, 143–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, 2019. Sleep and inflammation: partners in sickness and in health. Nat. Rev. Immunol. 19, 702–715. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Carrillo C, Olmstead R, 2010. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav. Immun. 24, 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carroll JE, 2016. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 80, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Opp MR, 2017. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology 42, 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RK, Manke J, Campbell M, Armstrong M, Boorgula MP, Pinheiro G, Santana CVN, Mathias RA, Barnes KC, Cruz A, Reisdorph N, Figueiredo CA, 2022. Lipid mediators are detectable in the nasal epithelium and differ by asthma status in female subjects. J. Allergy Clin. Immunol. 150, 965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley EC, Li HJ, Cogle CR, Handberg EM, Merz CNB, Pepine CJ, 2022. Specialized proresolving mediators in symptomatic women with coronary microvascular dysfunction (from the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD [WARRIOR] trial). Am. J. Cardiol. 162, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij G, Troletti CD, Leuti A, Norris PC, Riley I, Albanese M, Ruggieri S, Libreros S, van der Pol SMA, van het Hof B, Schell Y, Guerrera G, Buttari F, Mercuri NB, Centonze D, Gasperini C, Battistini L, de Vries HE, Serhan CN, Chiurchiù V, 2020. Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction. Haematologica 105, 2056–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy N, Abdulnour R-EE, Walker KH, Engstrom BD, Levy BD, 2018. Specialized proresolving mediators in innate and adaptive immune responses in airway diseases. Physiol. Rev. 98, 1335–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasselin J, Rehman J-U, Åkerstedt T, Lekander M, Axelsson J, 2015. Effect of long-term sleep restriction and subsequent recovery sleep on the diurnal rhythms of white blood cell subpopulations. Brain Behav. Immun. 47, 93–99. [DOI] [PubMed] [Google Scholar]
- Le Faouder P, Baillif V, Spreadbury I, Motta J-P, Rousset P, Chêne G, Guigné C, Tercé F, Vanner S, Vergnolle N, Bertrand-Michel J, Dubourdeau M, Cenac N, 2013. LC-MS/MS method for rapid and concomitant quantification of pro-inflammatory and pro-resolving polyunsaturated fatty acid metabolites. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 932, 123–133. [DOI] [PubMed] [Google Scholar]
- Li P, Zheng X, Ulsa MC, Yang H-W, Scheer FAJL, Rutter MK, Hu K, Gao L, 2021. Poor sleep behavior burden and risk of COVID-19 mortality and hospitalization. Sleep 44, zsab138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakh I, Pakiet A, Sledzinski T, Mika A, 2020. Methods of the analysis of oxylipins in biological samples. Molecules 25, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Vicario C, Titos E, Walker ME, Alcaraz-Quiles J, Casulleras M, Durán-Güell M, Flores-Costa R, Pérez-Romero N, Forné M, Dalli J, Clària J, 2019. Leukocytes from obese individuals exhibit an impaired SPM signature. FASEB J. 33, 7072–7083. [DOI] [PubMed] [Google Scholar]
- McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, Poller WC, Frodermann V, Fenn AM, Gregory AF, Halle L, Iwamoto Y, Hoyer FF, Binder CJ, Libby P, Tafti M, Scammell TE, Nahrendorf M, Swirski FK, 2019. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine CS, Kiss MG, Zuraikat FM, Cheek D, Schiroli G, Amatullah H, Huynh P, Bhatti MZ, Wong L-P, Yates AG, Poller WC, Mindur JE, Chan CT, Janssen H, Downey J, Singh S, Sadreyev RI, Nahrendorf M, Jeffrey KL, Scadden DT, Naxerova K, St-Onge M-P, Swirski FK, 2022. Sleep exerts lasting effects on hematopoietic stem cell function and diversity. J. Exp. Med. 219, e20220081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmas F, Clarke J, Colas RA, Gomez EA, Keogh A, Boylan M, McEvoy N, McElvaney OJ, McElvaney O, Alalqam R, McElvaney NG, Curley GF, Dalli J, 2021. Dysregulated plasma lipid mediator profiles in critically ill COVID-19 patients. PLoS ONE 16, e0256226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy D, Gilligan MM, Serhan CN, Kashfi K, 2021. Resolution of inflammation: an organizing principle in biology and medicine. Pharmacol. Ther. 227, 107879. [DOI] [PubMed] [Google Scholar]
- Patel SR, Malhotra A, Gao X, Hu FB, Neuman MI, Fawzi WW, 2012. A prospective study of sleep duration and pneumonia risk in women. Sleep 35, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Swinkels CM, Gehrman PR, Pigeon WR, Matteson-Rusby SE, Jungquist CR, 2010. The incidence and temporal patterning of insomnia: a pilot study. J. Sleep Res. 19, 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Janicki-Deverts D, Hall MH, Cohen S, 2015. Behaviorally assessed sleep and susceptibility to the common cold. Sleep 38, 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss H, Rosche B, Sullivan AB, Brommer B, Wengert O, Gronert K, Schwab JM, 2013. Proresolution lipid mediators in multiple sclerosis — differential, disease severity-dependent synthesis — a clinical pilot trial. PLoS ONE 8, e55859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod KS, Kapil V, Velmurugan S, Khambata RS, Siddique U, Khan S, Van Eijl S, Gee LC, Bansal J, Pitrola K, Shaw C, D’Acquisto F, Colas RA, Marelli-Berg F, Dalli J, Ahluwalia A, 2017. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J. Clin. Invest. 127, 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte F, Asbeutah AA, Benotti PN, Wood GC, Still C, Bistrian BR, Hardt M, Welty FK, 2020. The relationship between specialized pro-resolving lipid mediators, morbid obesity and weight loss after bariatric surgery. Sci. Rep. 10, 20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, 2011. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 25, 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Levy BD, 2018. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128, 2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Libreros S, Nshimiyimana R, 2022. E-series resolvin metabolome, biosynthesis and critical role of stereochemistry of specialized pro-resolving mediators (SPMs) in inflammation-resolution: preparing SPMs for long COVID-19, human clinical trials, and targeted precision nutrition. Semin. Immunol. 59, 101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson NS, Diolombi M, Scott-Sutherland J, Yang H, Bhatt V, Gautam S, Mullington J, Haack M, 2016. Repeating patterns of sleep restriction and recovery: do we get used to it? Brain Behav. Immun. 58, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, Jacobson TA, Engler MB, Miller M, Robinson JG, Blum CB, Rodriguez-Leyva D, de Ferranti SD, Welty FK, 2019. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation 140, e673–e691. [DOI] [PubMed] [Google Scholar]
- Sorokin AV, Norris PC, English JT, Dey AK, Chaturvedi A, Baumer Y, Silverman J, Playford MP, Serhan CN, Mehta NN, 2018. Identification of proresolving and inflammatory lipid mediators in human psoriasis. J. Clin. Lipidol. 12, 1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sri Kantha S, Matsumura H, Kubo E, Kawase K, Takahata R, Serhan CN, Hayaishi O, 1994. Effects of prostaglandin D2, lipoxins and leukotrienes on sleep and brain temperature of rats. Prostaglandins Leukot. Essent. Fatty Acids 51, 87–93. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Ravipati S, Menni C, Abhishek A, Metrustry S, Harris J, Nessa A, Williams FMK, Spector TD, Doherty M, Chapman V, Barrett DA, 2017. Association of the resolvin precursor 17-HDHA, but not D- or E- series resolvins, with heat pain sensitivity and osteoarthritis pain in humans. Sci. Rep. 7, 10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallières A, Ivers H, Bastien CH, Beaulieu-Bonneau S, Morin CM, 2005. Variability and predictability in sleep patterns of chronic insomniacs. J. Sleep Res. 14, 447–453. [DOI] [PubMed] [Google Scholar]
- van Leeuwen WMA, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, Härmä M, Porkka-Heiskanen T, Alenius H, 2009. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS ONE 4, e4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Wang X, Hjorth E, Colas RA, Schroeder L, Granholm A-C, Serhan CN, Schultzberg M, 2016. Pro-resolving lipid mediators improve neuronal survival and increase Aβ42 phagocytosis. Mol. Neurobiol. 53, 2733–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


