Abstract
Chronic hypoxia (CH) from birth attenuates the acute hypoxic ventilatory response (HVR) in rats and other mammals, but CH is often reported to augment the HVR in adult mammals. To test the hypothesis that this transition – from blunting to augmenting the HVR – occurs in the third or fourth postnatal week in rats, juvenile and adult rats were exposed to normobaric CH (12% O2) for 7 days and the HVR was assessed by whole-body plethysmography. No transition was observed, however, and the acute HVR was reduced by 61 – 85% across all ages studied. The failure to observe an augmented HVR in adult rats could not be explained by the substrain of Sprague Dawley rats used, the duration of the CH exposure, the order in which test gases were presented, the level of hypoxia used for CH and to assess the HVR, or the effects of CH on the metabolic response to hypoxia and the hypercapnic ventilatory response. A literature survey revealed several distinct patterns of ventilatory acclimatization to hypoxia (VAH) in adult rats, with most studies (77%) revealing a decrease or no change in the acute HVR after CH. In conclusion, the effects of CH on respiratory control are qualitatively similar across age groups, at least within the populations of Sprague Dawley rats used in the present study, and there does not appear to be one “typical” pattern for VAH in adult rats.
Keywords: hypoxia, control of breathing, respiratory plasticity, acclimation, ventilatory acclimatization to hypoxia, rat substrain
1. Introduction
Mammals often respond to a decrease in inspired O2 through a combination of increased ventilation and, particularly in small mammals, a decrease in metabolic rate. However, the acute hypoxic ventilatory response (HVR) can be modified during chronic exposures to sustained hypoxia. When newborn mammals are exposed to chronic hypoxia (CH), this respiratory plasticity typically manifests as a blunting of the HVR in rat pups (Eden and Hanson, 1987; Wyatt et al., 1995; Bavis, 2005; Bavis et al., 2020), lambs (Sladek et al., 1993), and kittens (Hanson et al., 1989a; Hanson et al., 1989b). The smaller increase in ventilation has been attributed to a decreased O2 sensitivity of carotid body chemoreceptors (Hanson et al., 1989a; Hanson et al., 1989b; Wyatt et al., 1995; Sterni et al., 1999; Bavis et al., 2020).
In contrast to neonatal rats, it is widely accepted that adult mammals increase their HVR in response to CH through the process of ventilatory acclimatization to hypoxia (VAH) (Powell et al., 1998; Teppema and Dahan, 2010; Pamenter and Powell, 2016). VAH is broadly described as a progressive increase in ventilation during sustained (hours to days) exposure to normobaric or hypobaric hypoxia that exceeds the increase in ventilation (or hyperventilation) achieved during acute exposure to the same level of hypoxia. VAH in adult rats is typically observed as an increase in ventilation (or decrease in arterial partial pressure of CO2, PCO2) across all levels of inspired partial pressures of O2PO2 compared to chronically normoxic rats (Olson and Dempsey, 1978; Powell et al., 1998; Pamenter and Powell, 2016). This means that the absolute level of ventilation is greater in hypoxia, but also that ventilation is greater when acutely returned to normoxia.1 VAH may also be observed as an increase in the acute HVR (i.e., a steeper slope of the ventilation – inspired PO2 relationship), particularly when measured under isocapnic conditions; the poikilocapnic HVR may increase less after CH, or appear unchanged, since acute hyperventilation lowers arterial PCO2 and constrains further increases in breathing (Aaron and Powell, 1993). Although the precise molecular mechanisms underlying VAH are still being investigated (Pamenter and Powell, 2016), the changes in breathing have been attributed to increased carotid body O2 sensitivity and an increased sensitivity of the CNS to carotid chemoafferent input (Dwinell and Powell, 1999; Powell et al., 2000b; Wilkinson et al., 2010; Dempsey et al., 2014). CH appears to have little or no effect on the metabolic response to hypoxia in rats (e.g., Olson and Dempsey, 1978; Reeves et al., 2003; Popa et al., 2011; Pamenter et al., 2015).
Given the apparent switch from CH-induced blunting of the HVR to CH-induced augmentation of the HVR between the neonatal period and adulthood, the present study initially sought to determine the age at which this transition occurs in rats. Since the carotid body is required to initiate VAH in adult mammals (Dempsey et al., 2014), we hypothesized that this transition would occur during the third or fourth postnatal week when the rat carotid body becomes functionally and morphologically mature (e.g., Bavis et al., 2013; Carroll and Kim, 2013). Although we expected to see the HVR become less blunted, if not augmented, when CH commenced later in life, the acute HVR appeared blunted in both juvenile and adult rats after one week of CH. Subsequent experiments explored potential methodological explanations for the blunted HVR in adult rats in the present study versus prior studies. Factors that were considered included the substrain of Sprague Dawley rats used, the duration of the CH exposure, the order in which test gases were presented, the level of hypoxia used for CH and to assess the HVR, and the effects of CH on the metabolic response to hypoxia and the hypercapnic ventilatory response. Finally, we systematically reviewed the literature to determine how often other studies have reported blunted HVR in adult rats after CH.
2. Methods
2.1. Experimental animals
Most experiments were completed using SAS Sprague Dawley rats (Crl:SD) obtained from Charles River Laboratories (Wilmington, MA USA). For the juvenile rats (14 – 22 days of age and 21–29 days of age) used in these experiments, individuals were born in-house to mothers obtained as late-gestation dams. To determine whether the specific genetic population of rat influenced the response to CH, adult Sprague Dawley rats from a second substrain (Hsd:SD) were obtained from Envigo (Frederick, MD, USA).
Throughout the study, rats were housed with food and water ad libitum and maintained on a 12:12-h light-dark cycle. All procedures were approved by the Institutional Animal Care and Use Committees at Bates College.
2.2. Surgical preparation
In rats studied as adults, body temperature was recorded during the ventilation measurements using temperature-sensitive transponders. Accordingly, transponders (E-mitter G2; Mini-Mitter, Bend, OR USA) were surgically implanted into the abdominal cavity at least one week prior to initiating chronic hypoxia. Rats were anesthetized with isoflurane (~2.5% isoflurane, balance O2) and transponders were placed into the abdominal cavity through a ventral, midline incision. Carprofen (5 mg kg−1, s.c.; Pfizer Animal Health, Exton, PA, USA) was administered as an analgesic.
2.3. Chronic hypoxia (CH) exposures
Environmental chambers were flushed with room air or with a mixture of room air and N2 at sufficient flow rates to maintain the desired levels of O2 (i.e., 21% O2 for Control and 10% O2 or 12% O2 for CH) and to minimize accumulation of CO2 (< 0.4% CO2).
In the youngest group of juvenile rats, the entire litter was placed with their mothers into either the Control chamber (3 litters) or 12% O2 CH chamber (4 litters) at 14 days of age (P14) and maintained there for 7 – 8 days until breathing was measured (i.e., at P21–22). For the older group of juvenile rats, pups from six litters were weaned at P21 and housed with subsets of their siblings (2–3 per cage). These cages were then placed into either the Control chamber or 12% O2 CH chamber at P21 or P22 and maintained there for 7 days until breathing was measured (i.e., at P28–29); approximately half of each litter was assigned to each treatment group.
Adult rats were typically placed into the Control chamber or CH chamber (10% O2 or 12% O2) for 7 days, but some Control and CH rats were returned to the chambers after completing breathing measurements (~ 2 h) and restudied after an additional 7 days in 12% O2 (i.e., 14 days total exposure). In some experiments, rats were removed from the Control and CH chambers after only 3 days to complete breathing measurements (~ 2 h) and promptly returned to the environmental chambers for the remainder of the 7-day exposure. Adult rats were approximately 2 – 5 months of age when they were placed into the environmental chambers; age was estimated from growth charts provided by the vendors. However, to investigate whether the order in which test gases were presented might impact the calculated HVR, a subset of rats from the Control group in one of the earlier experiments was subsequently used again 2 – 3 months later (i.e., at 6 – 7 months of age); nine of these rats were placed the Control chamber again and ten were placed into the 12% O2 CH chamber for 7 days.
The numbers of individual rats studied in each experiment are presented in the appropriate figure legends and supplementary tables (Appendix A).
2.4. Breathing measurements
Breathing was measured using a commercially available whole-body barometric plethysmograph system for rats (Data Sciences International (DSI), St. Paul, MN, USA). Gas flow through the plethysmograph chamber (P/N 601-1427-001; DSI) was set at 3 L min−1 (STPD) using a gas-mixing mass flow controller (MFC-4; Sable Systems, Las Vegas, NV USA) and valves (series 840; Sierra Instruments, Monterey, CA USA). This flow was monitored with an integrated pneumotach and a differential pressure transducer (Buxco TRD5700; DSI) that was calibrated prior to each animal with known gas flow rates. Respiratory-related oscillations in air flow, temperature and humidity inside the chamber (temp/humidity probe, P/N 601-2249-001; DSI), and body temperature (see below) were then used to calculate tidal volume (VT), respiratory frequency (fR), and ventilation using Ponemah software (version 5.20; DSI) in one-minute bins during the final two minutes (juvenile rats) or every fifth minute (adult rats) of each test gas; movement artifacts and irregular breaths (e.g., sniffing, sighs) were manually removed if not already excluded by the software filters.
Individual rats were weighed and sealed into the plethysmograph chamber during the light portion of the light cycle and exposed to 21% O2 (balance N2); approximately 10 min elapsed between removing rats from the environmental chamber (Control or CH) and sealing them into the plethysmograph chamber. All measurements were made at room temperature. For juvenile rats, body temperature (Tb) was determined using a rectal probe (RET-3 probe and BAT-12 microprobe thermometer; Physitemp, Clifton, NJ, USA) during baseline conditions (21% O2) and at the end of hypoxia (12% O2). Accordingly, the plethysmograph chamber was opened briefly about 30 min into the trial (when the rat appeared calm) and Tb was recorded. The chamber was then re-sealed and baseline breathing was monitored for approximately 30 min before switching to hypoxia (12% O2, balance N2) for 20 min. The plethysmograph chamber was then promptly opened and the hypoxic Tb was recorded.
For adult rats, Tb was recorded continuously via telemetry (VitalView 4.1, Starr Life Sciences Corp., Oakmont, PA USA) so it was not necessary to open the plethysmograph chamber during the trial. The typical protocol consisted of a 60 min baseline exposure to 21% O2 (balance N2) followed by a 20 min exposure to either 12% O2 (balance N2) or 7% CO2 (21% O2, balance N2). In one set of experiments, after recording the baseline breathing, rats were exposed to both 12% and 10% O2, in that order, separated by a recovery period in 21% O2; hypoxic exposures and the recovery period were each 20 min. Finally, to investigate whether the order that test gases were presented might impact breathing measurements, the baseline period for one group of CH rats was set at 12% O2 (i.e., the same inspired O2 as their CH exposure). After 60 min of this 12% O2 baseline, the inspired gas was switched to 21% O2 for 20 min.
2.5. Metabolism measurements
Gaseous metabolism was measured in adult rats using a custom-built, open-system respirometer chamber. Individual rats were weighed and sealed into the clear, acrylic chamber (3.2 L) during the light portion of the light cycle and exposed to 21% O2 (balance N2). Air flow through the chamber was set at 1.5 L min−1 (STPD) using a gas-mixing mass flow controller (MFC-4; Sable Systems) and valves (series 840; Sierra Instruments). The experimental protocol consisted of 60 min in 21% O2 (balance N2) followed by 20 min in 12% O2 (balance N2). All measurements were made at room temperature.
Fractional concentrations of O2 and CO2 in the air exiting the respirometer chamber (i.e., FEO2 and FECO2) were measured continuously (ML206 gas analyzer; ADInstruments, Colorado Springs, CO, USA) over the final five minutes of each test gas exposure and recorded to a computer (PowerLab 8/30 and LabChart 8; ADInstruments); the air was dried (Drierite; W.A. Hammond Drierite Co., Xenia, OH, USA) before entering the gas analyzer. Gas flow to the chamber was interrupted for approximately one minute at the end of the baseline period and again at the end of the hypoxic exposure to measure the fractional concentrations of O2 and CO2 in the air entering the chamber (i.e., FIO2 and FICO2). These values were then used to calculate O2 consumption and CO2 production using the equations of Frappell et al. (1992). The respiratory exchange ratio (RER) was calculated as .
2.6. Statistical analysis
Ventilation and metabolism data were compared among groups using 3-way repeated measures ANOVA when both male and female rats were studied (factors: treatment group, sex, and FIO2). Sex did not influence the response to CH, so some experiments were conducted with only male rats. In these cases, ventilation data were compared among groups using either a 2-way repeated measures ANOVA (factors: treatment group and FIO2) or 3-way repeated measures ANOVA (factors: treatment group, exposure duration, and FIO2). Tukey’s post hoc test was used when significant interactions were detected among factors with 3-way ANOVA while Šídák’s multiple comparison test was used following 2-way ANOVA. Although the HVR in adult rats was initially characterized every fifth minute of the 20-min hypoxic exposure, there was no evidence for ventilatory roll-off (consistent with other studies on Sprague Dawley rats (Hodges et al., 2002)). Therefore, the final two bins (minutes 15 and 20) were averaged for each individual and used for subsequent statistical comparisons. Due to the large number of experiments described, only data are presented in the Results section to enhance clarity and concision; corresponding values for VT and fR, along with data for body mass and Tb, are available in Appendix A (Supplementary Tables 1 – 9). All statistical tests were run using Prism 9.5.1 (GraphPad Software, Boston, MA, USA) and significance was determined at P≤0.05. Values in the text and figures are reported as mean±SEM; individual data points are presented in supplementary figures (Appendix A).
2.7. Literature survey of ventilatory responses to CH in adult rats
Studies have reported a variety of ventilatory responses to CH in rats. In an attempt to quantify the frequency of different patterns of responses, a literature search was conducted on Scopus (https://www.scopus.com/) by searching in the title, abstract and keywords for the following combination of terms: (acclimation OR acclimatization OR chronic OR sustained) AND (hypoxia OR altitude) AND rat AND (breathing OR ventilation OR ventilatory) for all years through April 2023. Each of the 811 search results (ranging in publication year from 1961 to 2023) was examined to determine whether it contained ventilation data for rats exposed to CH; a few additional references were identified through the references in key review articles (Teppema and Dahan, 2010; Pamenter and Powell, 2016). Studies were excluded if the CH exposure was less than 6 hours, if the protocol consisted of chronic intermittent hypoxia rather than chronic sustained hypoxia, if the rats were returned to room air after CH for more than 3 hours prior to commencing breathing measurements, or if rats were less than 4 weeks of age at the start of the CH exposure. Studies were also excluded if rats were anesthetized during the breathing measurements. Finally, since the goal was to assess the effects of CH on the HVR, studies were excluded if they reported normoxic ventilation after CH but did not also include ventilation measurements made in hypoxia.
Ventilation data were retrieved from those studies meeting the inclusion criteria (Appendix B). Extracted data included normoxic for CH and Control rats (NXCH and NXCon, respectively), hypoxic for CH and Control rats (HXCH and HXCon), and the acute HVR [calculated as hypoxic minus normoxic during an acute (minutes to hours) challenge] for CH and Control rats (HVRCH and HVRCon). If the study did not report mass-specific ventilation, it was estimated by dividing the mean value for ventilation by the mean value for body mass for each treatment group. Where exact values were not reported in the text or in tables, the values were extracted from graphs using WebPlotDigitizer 4.2 (https://automeris.io/WebPlotDigitizer). If ventilation was measured at multiple levels of hypoxia, the test gas most similar to the CH exposure was selected. For studies in which drugs were administered, only data for the vehicle-injected groups were used.
Patterns of VAH were identified based on whether normoxic ventilation, hypoxic ventilation, or the change in ventilation (HVR) differed between CH and Control groups. These comparisons were made by calculating the relevant ratios (NXCH / NXCon, HXCH / HXCon, and HVRCH / HVRCon); CH was considered to have had an effect if the difference between groups exceeded 10% (i.e., ratios greater than or equal to 1.1, or ratios less than or equal to 0.9). The relative frequency of each pattern was then calculated using individual published articles as the unit of replication. Since articles often contained multiple relevant data sets (e.g., several independent experiments or several durations of CH in for the same group of animals), each data set was counted as a fraction of the full study. For example, if an article contained data sets corresponding to different patterns of VAH, each would count as 0.50 if there were two data sets, 0.33 if there were three data sets, etc.
3. Results
3.1. CH in juvenile rats after 7 – 8 d in 12% O2
Chronic exposure to 12% O2 during either the third or fourth postnatal week altered the HVR in juvenile rats (Fig. 1). At P21–22, CH rats (i.e., exposed for 7 – 8 d from P14) exhibited a much smaller HVR (treatment × FIO2, P<0.001; Fig. 1A). This effect was similar between males and females (main effect and all interaction terms containing sex, P>0.05) was greater in CH rats than in Control rats at 21% O2 (P=0.001 for females, <0.001 for males) but not at 12% O2. Accordingly, the increase in between baseline and hypoxia was reduced by approximately 67% in CH rats (Δ = 12.0 ± 4.7 vs. 29.7 ± 4.9 mL min−1 100g−1 for females; 7.9 ± 4.2 vs. 30.3 ± 4.9 mL min−1 100g−1 for males).
Fig. 1.

Hypoxic ventilatory response of juvenile rats (Crl:SD) exposed to 21% O2 (Control) or 12% O2 (CH) for approximately one week. (A) P21–22 rats were exposed for 7–8 days beginning at P14 (Control: n= 10 females, 15 males; CH: n= 17 females, 19 males). (B) P28–29 rats were exposed for 7 days beginning at P21 or P22 (Control: n= 10 females, 16 males; CH: 9 females, 18 males). Ventilation (mean±SEM) was measured in 21% O2 and again after 20 min in 12% O2. * P≤0.05 vs. Control at the same inspired O2 level.
Similar results were obtained at P28–29 for rats exposed to CH for 7 d beginning at P21 or P22. Specifically, CH rats exhibited a much smaller HVR (treatment × FIO2, P=0.01; Fig. 1B). Overall, females had a lower mass-specific (main effect for sex, P=0.02), but sex did not affect the magnitude of the HVR or the effects of CH (all interaction terms containing sex, P>0.05). was greater in CH rats than in Control rats at 21% O2 (P=0.01 for females, 0.008 for males) but not at 12% O2. The increase in between baseline and hypoxia was reduced by approximately 62% in CH rats (Δ = 7.4 ± 3.1 vs. 19.4 ± 3.9 mL min−1 100g−1 for females; 8.6 ± 4.1 vs. 23.0 ± 5.1 mL min−1 100g−1 for males).
Values for body mass, Tb, VT, and fR are presented in Supplementary Tables 1 and 2 (Appendix A).
3.2. CH in adult rats
3.2.1. HVR after 3 d and 7 d of 12% O2
The HVR was assessed in adult, male rats after 3 d and again after 7 d in 12% O2 (Fig. 2). The CH rats exhibited a smaller increase in when switched from 21% O2 to 12% O2 after 3 d (treatment × time in hypoxia, P=0.001; Fig. 2A) and after 7 d (treatment × time in hypoxia, P<0.001; Fig. 2B). Neither group showed significant ventilatory roll-off between the early and late phases of the HVR, so the final two time points were averaged for subsequent analyses (Fig. 2C). CH attenuated the HVR (treatment × FIO2, P<0.001), but the duration of the CH exposure (3 d vs. 7 d) did not matter (main effect for duration and all interaction terms containing duration, P>0.05). Although tended to be greater in CH rats than in Control rats while breathing 21% O2 (e.g., Fig. 2B), this was not statistically significant in the pooled analysis (Fig. 2C). However, tended to be lower in CH rats than in Control rats while breathing 12% O2 (Fig. 2C), meaning that the increase in between baseline and hypoxia was reduced by approximately 85% in CH rats (Δ = 3.0 ± 2.4 vs. 13.7 ± 1.7 mL min−1 100g−1 after 3 d; 1.4 ± 2.1 vs. 14.9 ± 1.8 mL min−1 100g−1 after 7 d).
Fig. 2.

Hypoxic ventilatory response of adult, male rats (Crl:SD) after 3 days and 7 days in 21% O2 (Control; n= 11) or 12% O2 (CH; n= 13). Ventilation (mean±SEM) is reported under baseline (BL; 21% O2) conditions and throughout a 20-min exposure to 12% O2 after 3 days and 7 days (panels A and B, respectively). In panel C, the final two time points during hypoxia were averaged. * P≤0.05 vs. Control at the same time point.
To determine whether the response to CH differed among substrains of Sprague Dawley rats, the experiment was repeated with male Hsd:SD rats rather than Crl:SD rats. The results were similar: CH attenuated the HVR (treatment × FIO2, P<0.001) and the duration of the CH exposure (3 d vs. 7 d) did not modify this effect (main effect for duration and all interaction terms containing duration, all P>0.05) (Fig. 3). Whereas hypoxic tended to be lower in Crl:SD rats exposed to CH than in Controls (Fig. 2), this difference was more pronounced and statistically significant in the Hsd:SD rats breathing 12% O2 (P<0.001 after 3 d, 0.004 after 7 d; Fig. 3). Accordingly, the increase in between baseline and hypoxia was reduced by approximately 83% in CH rats (Δ = 1.2 ± 2.6 vs. 21.6 ± 2.0 mL min−1 100g−1 after 3 d; 5.4 ± 1.5 vs. 18.7 ± 1.9 mL min−1 100g−1 after 7 d).
Fig. 3.
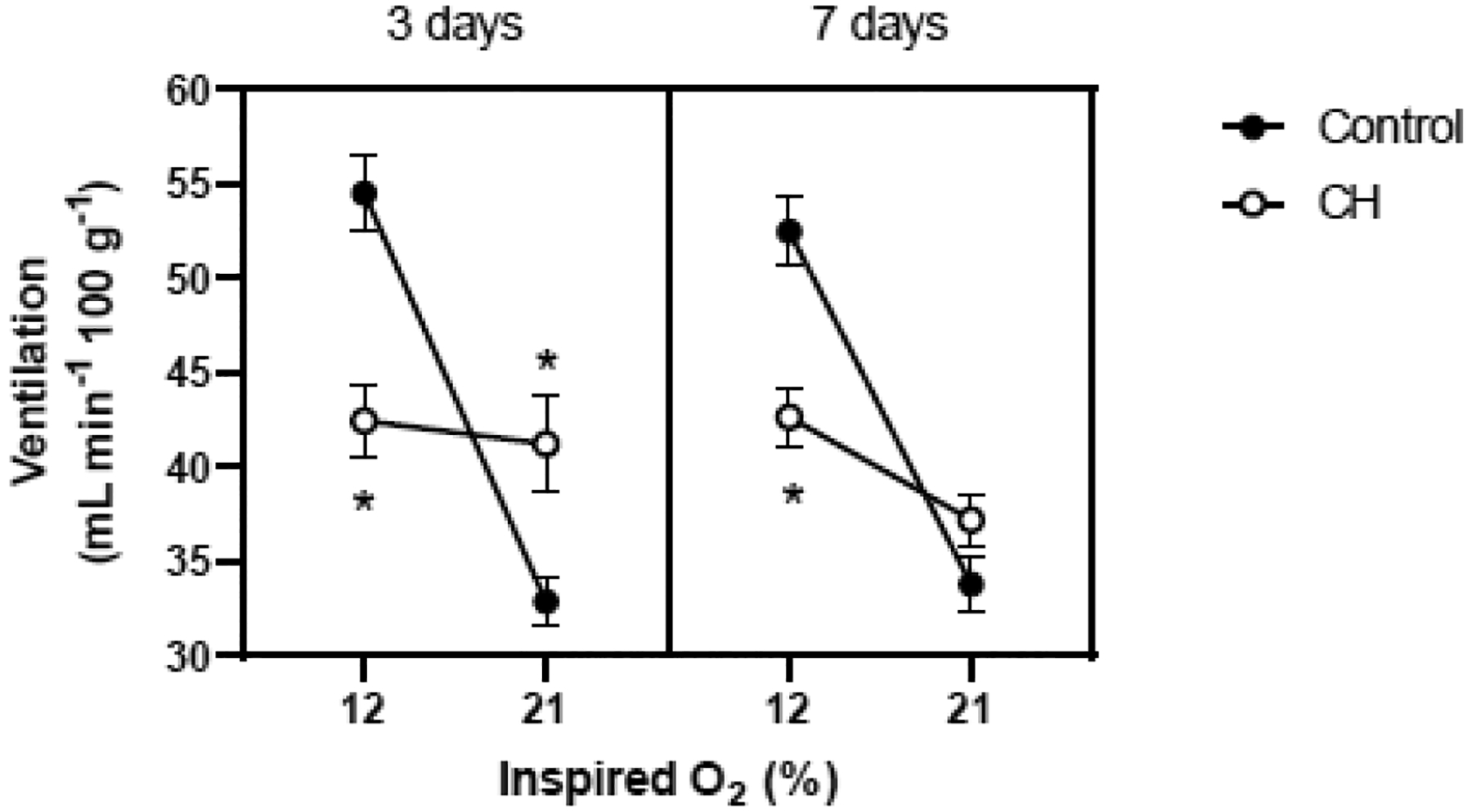
Hypoxic ventilatory response of adult, male rats (Hsd:SD) after 3 days and 7 days in 21% O2 (Control; n= 12) or 12% O2 (CH; n= 11). Ventilation (mean±SEM) was measured in 21% O2 and then in 12% O2. * P≤0.05 vs. Control at the same inspired O2 level.
Values for body mass, Tb, VT, and fR are presented in Supplementary Tables 3 and 4 (Appendix A).
3.2.2. HVR after 14 d of 12% O2
To determine whether a longer CH exposure is needed to elicit an augmented HVR, an additional cohort of male and female rats was studied after 14 d in 12% O2; note that these rats were removed from the CH chamber after 7 d for approximately 2 h to assess their hypercapnic ventilatory responses (see section 3.2.5, below) before completing the final 7 d in 12% O2. The HVR was reduced in CH rats (treatment × FIO2, P=0.006; Fig. 4). Females had a greater mass-specific overall (sex, P<0.001). Although the relationships shown in Figure 4 suggested that CH might have reduced hypoxic more in male than female rats, this was not supported by the ANOVA results (treatment × sex, P=0.06); no other interaction terms containing sex approached statistical significance. The increase in between baseline and hypoxia was reduced by approximately 69% in CH rats (Δ = 5.1 ± 4.9 vs. 14.4 ± 3.8 mL min−1 100g−1 for females; 4.2 ± 2.1 vs. 15.3 ± 2.3 mL min−1 100g−1 for males).
Fig. 4.

Hypoxic ventilatory response of adult rats (Crl:SD) after 14 days in 21% O2 (Control) or 12% O2 (CH). Ventilation (mean±SEM) was measured in 21% O2 and then in 12% O2. Sample sizes (n) were Control: 10 females, 7 males and CH: 7 females, 10 males.
Values for body mass, Tb, VT, and fR are presented in Supplmentary Table 5 (Appendix A).
3.2.3. HVR after 7 d of 12% O2 – effect of the order of test gas exposure
In the experiments presented above, both Control and CH rats were exposed to 21% O2 for 60 min (50–55 min adjustment period + 5–10 min “normoxic” ventilation recordings) before switching the test gas to 12% O2 to measure the hypoxic response. In contrast, some researchers start the CH group at the same inspired PO2 that is used during the chronic exposure (e.g., Wilkinson et al., 2010; Popa et al., 2011; Pamenter et al., 2015). To determine whether the order of the test gas exposure influences the measured HVR in CH rats, a separate group of male CH rats was maintained at 12% O2 during the adjustment period while the corresponding Control rats were maintained at 21% O2. Breathing was measured at the end of the adjustment period and then the inspired gas was switched to 21% O2 for the CH rats or to 12% O2 for the Control rats.
Despite this change in protocol, CH rats still had a diminished HVR compared to Control rats (treatment × FIO2, P=0.05; Fig. 5). was greater in CH rats than in Control rats while breathing 21% O2 (P=0.05), but not while breathing 12% O2; therefore, the increase in between normoxia and hypoxia was reduced by approximately 61% in CH rats (Δ = 4.8 ± 1.8 vs. 12.4 ± 3.4 mL min−1). Although not shown in Figure 5, CH rats were returned to 12% O2 and Control rats were returned to 21% O2 for an additional 20 min after these ventilation measurements. The HVR calculated from this second transition confirmed that CH rats had a reduced response relative to their age-matched Controls (Δ = −0.2 ± 1.5 vs. 13.2 ± 2.1 mL min−1).
Fig. 5.

Hypoxic ventilatory response of adult, male rats (Crl:SD) after 7 days in 21% O2 (Control; n= 9) or 12% O2 (CH; n= 10). To determine whether the order of the test gases influences the magnitude of the ventilatory response, ventilation (mean±SEM) was measured in 12% O2 before 21% O2 for CH rats; Control rats were presented with 21% O2 before 12% O2. * P≤0.05 vs. Control at the same inspired O2 level.
Values for body mass, Tb, VT, and fR are presented in Supplementary Table 6 (Appendix A).
3.2.4. Metabolic response to hypoxia after 7 d of 12% O2
A more pronounced metabolic suppression during hypoxia could lead to a smaller increase in pulmonary ventilation and thus, a smaller apparent HVR. Since metabolic rate was not assessed during ventilation measurements, and were measured in a separate group of male and female rats after CH. When acutely exposed to 12% O2, both treatment groups exhibited a modest decrease in (−13%; main effect for FIO2, P=0.003) and (−9%; FIO2, P=0.03) which was accompanied by an increase in the respiratory exchange ratio (FIO2, P<0.001) (Fig. 6). CH rats had higher metabolic rates overall (main effect for treatment, P=0.004 for and 0.01 for ), but the metabolic responses to hypoxia did not differ between treatment groups (treatment × FIO2, P=0.82 for , 0.94 for , and 0.68 for RER). Although females had higher mass-specific (main effect for sex, P=0.01) and tended to have higher mass-specific (sex, P=0.06) than their male counterparts, sex did not affect the magnitude of the metabolic suppression during hypoxia or the effects of CH on this response (all interaction terms containing sex, P>0.05).
Fig. 6.

Metabolic responses to hypoxia of adult rats (Crl:SD) after 7 days in in 21% O2 (Control) or 12% O2 (CH). (A) O2 consumption, (B) CO2 production, and (C) the respiratory exchange ratio (RER) were measured in 21% O2 and again after 20 min in 12% O2; values are mean±SEM. * P≤0.05 vs. Control across both inspired O2 levels (i.e., main effect for treatment group).
Values for body mass are presented in Supplementary Table 7 (Appendix A).
3.2.5. Hypercapnic ventilatory response (HCVR) after 7 d of 12% O2
Increased CO2 sensitivity could attenuate the HVR when measured under poikilocapnic conditions, so the HCVR was compared between CH and Control rats. After 7 d in 12% O2, ventilation was greater in CH rats across all FICO2 (main effect for treatment, P<0.001; Fig. 7). However, when focusing on the HCVR itself, the change in between 0% CO2 and 7% CO2 was not different between CH and Control rats (treatment × FICO2, P=0.18). Sex did not influence the effect of CH exposure (treatment × sex and treatment × sex × FICO2, both P>0.05), but females tended to have a greater HCVR than their male counterparts independent of their treatment group (sex × FICO2, P=0.004).
Fig. 7.

Hypercapnic ventilatory response of adult rats (Crl:SD) after 7 days in 21% O2 (Control) or 12% O2 (CH). Ventilation (mean±SEM) was measured in 0% CO2 (21% O2, balance N2) and then in 7% CO2 (21% O2, balance N2). Sample sizes (n) were Control: 9 females, 7 males and CH: 6 females, 9 males. * P≤0.05 vs. Control across both inspired CO2 levels (i.e., main effect for treatment group).
Values for body mass, Tb, VT, and fR are presented in Supplementary Table 8 (Appendix A).
3.2.6. HVR after 7 d of 10% O2
Some published studies have used more severe levels of hypoxia than the 12% O2 used here for CH exposures (e.g., PO2 equivalent to 9–10.5% O2), so an additional group of male rats was exposed to 10% O2 for 7 d to determine whether more severe hypoxia is needed to augment the HVR. The HVR, measured in response to both 12% O2 and 10% O2, was once again attenuated in the CH rats (treatment × FIO2, P<0.001; Fig. 8). was significantly greater in CH rats while breathing 21% O2 (P<0.001), but was not different from Control rats at 12% O2 or 10% O2. The increase in between baseline and 12% O2 was reduced by approximately 97% in CH rats (Δ = 0.5 ± 3.1 vs. 15.9 ± 2.5 mL min−1 100g−1) while the increase in between baseline and 10% O2 was reduced by approximately 60% (13.3 ± 4.0 vs. 33.0 ± 2.3 mL min−1 100g−1).
Fig. 8.
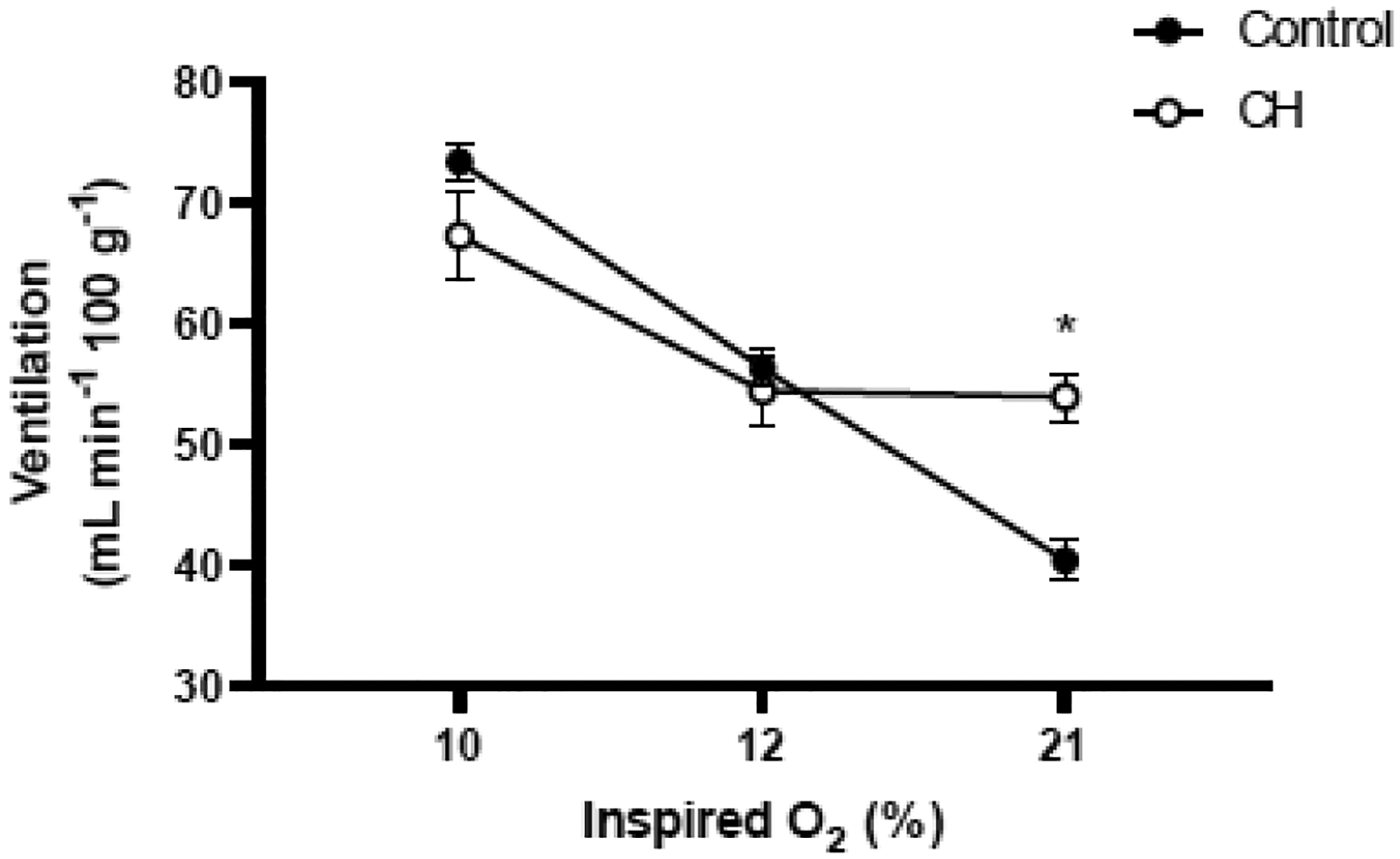
Hypoxic ventilatory response of adult, male rats (Crl:SD) after 7 days in 21% O2 (Control; n= 13) or 10% O2 (CH; n= 12). Ventilation (mean±SEM) was measured in 21% O2, 12% O2, and 10% O2. * P≤0.05 vs. Control at the same inspired O2 level.
Values for body mass, Tb, VT, and fR are presented in Supplementary Table 9 (Appendix A).
3.3. Literature survey of ventilatory responses to CH in adult rats
A literature search was performed to identify published studies on the HVR of adult rats after CH, and 32 studies (including the present study) met the inclusion criteria for further analysis (Appendix B). The most consistent evidence for VAH in these studies was an increase in normoxic (or baseline) in CH rats. Among the studies that included normoxic for both treatment groups (n=25), 94.0% found that CH increased normoxic by at least 10%; the remaining 6.0% of studies indicated that normoxic ventilation was relatively unchanged (± 10% of Control). Consistent with VAH, 73.0% of all studies (n=32) found that hypoxic was greater in CH rats than in Control rats acutely exposed to the same FIO2. Hypoxic was relatively unchanged (± 10% of Control) in 26.1% of the studies, while a lower hypoxic after CH was rarely reported (0.9% of studies, comprised exclusively the HsD:Sd rats in the present study). However, for those studies with sufficient data to calculate the acute HVR (n=26), 62.2% found that the change in was diminished after CH. The acute HVR was relatively unchanged (± 10% of Control) in 14.7% of the studies, while 23.1% found an augmented HVR.
Of the 25 studies for which NXCH / NXCon, HXCH / HXCon, and HVRCH / HVRCon could be calculated, 94% revealed some form of respiratory plasticity after CH (Fig. 9). Specifically, three patterns of VAH were identified for adult rats among the surveyed studies. CH increased across all FIO2 with no change in the acute HVR in 9.3% of the studies (pattern 1), while the acute HVR was augmented in 20.0% of the studies (pattern 2). Despite an increased normoxic , the acute HVR appeared to be blunted in 64.6% of the surveyed studies (pattern 3). Pattern 3 was subdivided into three subtypes (A – C) based on the hypoxic of CH and Control rats acutely exposed to the same FIO2. Hypoxic was greater than in Control rats in most of these studies (pattern 3A), and this was the most common pattern of VAH overall (43.3% of all studies). CH rats had hypoxic similar to that of Controls in 20.2% of the studies (pattern 3B) and had lower hypoxic than Controls in only 1.1% of the studies (pattern 3C).
Fig. 9.

Patterns of ventilatory acclimatization of hypoxia (VAH) observed in the literature for adult rats. (A) Patterns were characterized based on whether normoxic ventilation (NX), hypoxic ventilation (HX), or the change in ventilation (HVR) differed between CH and Control groups; CH was considered to have had an effect if the difference between groups exceeded 10% (i.e., ratios greater than or equal to 1.1, or ratios less than or equal to 0.9). The three principal patterns included no change in the acute HVR (pattern 1), an augmented HVR (pattern 2), or a blunted HVR (pattern 3); pattern 3 was subdivided based on whether hypoxic ventilation was increased compared to chronically hypoxic rats (pattern 3A), unchanged (pattern 3B), or decreased (pattern 3C). (B) Relative frequency for patterns of VAH in adult rats based on the 25 published studies (including the present study) for which sufficient data were available (see Appendix B). (C) Data from panel B separated based on whether normobaric hypoxia (Normo; n= 9.25 studies) or hypobaric hypoxia (Hypo; n= 15.75 studies) was used for the CH exposure. Individual published studies were used as the unit of replication, so fractions of studies were counted where multiple relevant data sets were reported in the same study (see Methods section for details).
Studies were further separated based on whether the CH exposure consisted of normobaric hypoxia or hypobaric hypoxia (approximately 9 and 16 studies, respectively; Appendix B). Both normobaric hypoxia and hypobaric hypoxia produced all three patterns of VAH (Fig. 9C). A blunted acute HVR (pattern 3) was the most common response to CH in both groups, but studies using hypobaric hypoxia appeared more likely to report pattern 3A than pattern 3B, while studies using normobaric hypoxia were more evenly split between these (Fig. 9C). Overall, studies using hypobaric hypoxia appeared more likely than studies using normobaric hypoxia to report an increase in hypoxic after CH (87.3 vs. 47.7% of studies, respectively, for patterns 1, 2, and 3A combined).
4. Discussion
CH elicited respiratory plasticity in both juvenile and adult rats, but this plasticity did not change qualitatively with age. Since the HVR is generally considered to increase during VAH in adult mammals (Teppema and Dahan, 2010; Dempsey et al., 2014; Pamenter and Powell, 2016), it was expected that the acute HVR would be blunted in the youngest rats after CH but that the HVR would be less blunted, or even augmented, in adult rats after CH. Instead, the acute HVR (measured as the change in between 21% O2 and 12% O2 within individual rats) was reduced by 61 – 85% after 7 d in 12% O2 across all ages studied. The absolute level of attained during the hypoxic challenge (i.e., hypoxic ) is arguably more relevant to whether pulmonary gas exchange improves during VAH than the change from normoxia, especially since normoxic is almost always increased after CH as well (including in the present study). In other words, the chronic HVR (i.e., the increase in between a chronically normoxic rat breathing a normoxic gas mixture and a chronically hypoxic rat breathing its hypoxic gas mixture) may exceed the acute HVR even if CH does not alter the acute HVR itself (e.g., patterns 1 and 3A in Fig. 9A). However, this was not the case in the present study: one week of CH did not substantially change hypoxic in juvenile rats (+4% at P21–22 and +6% at P28–29 relative to age-matched Controls) and tended to decrease hypoxic in adult rats (−3 to −19% across experiments). A systematic review of published studies revealed that these findings are not uncommon in rats: approximately two-thirds of the surveyed studies (65%, including the present study) indicated a blunted acute HVR in adult rats after CH (i.e., pattern 3; Fig. 9B) and approximately one-quarter (27%) of the studies reported no improvement in hypoxic in adult rats after CH (i.e., patterns 0, 3B, and 3C combined; Fig. 9B).
Numerous factors could influence the expression of respiratory plasticity observed across studies. For example, strain differences in the control of breathing and respiratory neuroplasticity are well established in rodent models (e.g., Tankersley et al., 1994; Strohl et al., 1997; Golder et al., 2005; Baker-Herman et al., 2010; Cramer et al., 2015). Even within a single, outbred strain such as Sprague Dawley rats, populations from different vendors or from different breeding colonies of the same vendor may diverge physiologically over time through genetic drift. This has been reported for substrains of Sprague Dawley rats in terms of the differential expression of respiratory plasticity after acute intermittent hypoxia (Fuller et al., 2001) and differential tolerance to chronic hypoxia (Ou and Smith, 1983). The present study considered whether the blunted HVR in Crl:SD rats in the initial experiments was unique to the genetic population from which these rats were sourced. On the contrary, Hsd:SD rats also exhibited pronounced blunting of the HVR after CH. These rats were sampled from only two of the many commercially available populations of Sprague Dawley rats, however, so these findings do not exclude genetic background as a source for some of the variation in patterns of rat VAH observed among studies (Fig. 9 and Appendix B).
The duration of hypoxic exposures is another potential source of variation among studies, but there is no consistent pattern for how these differences affect VAH in rats. For example, some studies have suggested that rats reach peak VAH within 1 – 3 d of CH and that VAH then either does not change (Ou et al., 1992; Ou et al., 1998; Huey et al., 2000; Wenninger et al., 2006) or diminishes (Bee and Pallot, 1995; Bonora and Vizek, 2001) with longer exposures. Other studies indicate that the magnitude of VAH increases with increasing duration length, at least over the course of weeks (Olson and Dempsey, 1978; Conde et al., 2012). However, the present study found qualitatively similar changes in the control of breathing after 3, 7, and 14 days of CH. Likewise, the level of hypoxia (10% O2 or 12% O2) used for the CH exposure or to test the acute HVR did not change any of the overall conclusions. Accordingly, the level and duration of hypoxia seem to be poor predictors for the pattern of VAH exhibited by adult rats.
There has been some debate as to whether normobaric hypoxia and hypobaric hypoxia elicit the same physiological responses (e.g., Millet et al., 2012; Mounier and Brugniaux, 2012), but it does not appear that any studies have directly compared normobaric CH and hypobaric CH for VAH in rats. However, there is no evidence in the literature that either CH protocol is more likely to augment or attenuate the acute HVR (Fig. 9C). Indeed, several studies from the same lab, all using hypobaric hypoxia and using the same protocols to measure ventilation, have reported VAH consistent with patterns 1, 2, 3A, and 3B in adult Sprague Dawley rats (e.g., Popa et al., 2011; Pamenter et al., 2014; Pamenter et al., 2015). Therefore, the use of normobaric hypoxia for the CH exposure likely cannot explain why CH did not enhance hypoxic or the acute HVR in the present study.
Metabolic rate and arterial Pco2/PH are also powerful determinants of ventilation in normoxia and hypoxia. If CH rats exhibit a more profound metabolic suppression during hypoxia, for example, this could result in a smaller hypoxic and a blunted HVR. However, CH rats tend to exhibit greater and than chronically normoxic rats across all inspired Pco2 with no change in the hypoxic metabolic response (e.g., Reeves et al., 2003; Popa et al., 2011; Pamenter et al., 2015) and present study), or perhaps even a modest reduction in the hypoxic metabolic response (Olson and Dempsey, 1978). While hyperventilation-induced hypocapnia should constrain the increase in during hypoxia, effectively limiting the acute HVR after CH, it is less clear that this process would completely block any additional increase in hypoxic during CH (pattern 3B), or lead to a decrease in hypoxic as seen in Hsd:SD rats in the present study (pattern 3C). An increase in CO2 sensitivity after CH might enhance the braking effect on the poikilocapnic HVR (Powell et al., 2000a; Conde et al., 2012), but several studies, including the present one, did not detect any change in hypercapnic ventilatory response after CH in rats (e.g., Pamenter et al., 2014; Pamenter et al., 2015). Although Aaron and Powell (1993) demonstrated that the isocapnic HVR was augmented in adult rats after CH even if the poikilocapnic HVR was not, hypoxic was still increased under poikilocapnic conditions. Moreover, many studies have reported increased poikilocapnic HVR in adult rats after CH (Olson and Dempsey, 1978; Ou et al., 1984; Ou et al., 1992; Bonora and Vizek, 2001; Reid and Powell, 2005; Caceres et al., 2007; Pamenter et al., 2014; Stokes et al., 2017). Additional studies assessing the isocapnic HVR after CH are warranted, but CO2 sensitivity may not be sufficient to explain the observed variation in VAH in adult rats.
VAH is a reversible form of respiratory plasticity in adult mammals, with normoxic , blood gases, and the acute HVR gradually returning to normal within a few days, or perhaps a few weeks, after return to normoxia (Dempsey et al., 2014; Pamenter and Powell, 2016). Although the time course for this post-CH recovery (i.e., deacclimation from CH) has not been fully described for adult rats, breathing appears to fully recover to pre-CH levels in 1 – 3 days (Olson and Dempsey, 1978; Bonora and Vizek, 2001; Vizek and Bonora, 2001; Wenninger et al., 2006). All of the studies investigating VAH reviewed in Figure 9 (see also Appendix B) assessed normoxic and/or hypoxic shortly after return to room air (generally within 10 – 60 min, but up to 3 h in (Reeves et al., 2003)). Over this short period of time, there does not appear to be a correlation between the duration of time CH rats were exposed to normoxia prior to breathing measurements and the pattern of VAH exhibited. For example, normoxic was elevated by similar amounts (60–70%) in CH rats returned to normoxia for 15 min (Aaron and Powell, 1993), 1 h (Olson and Dempsey, 1978), or 3 h (Reeves et al., 2003), and VAH consistent with patterns 1, 2, 3A, and 3B have been observed in studies using similar experimental protocols (e.g., Popa et al., 2011; Pamenter et al., 2014; Pamenter et al., 2015). Likewise, in the present study, the effects of CH were similar whether adult rats were exposed to normoxia or hypoxia while adjusting to the plethysmograph chamber at the start of the breathing measurements (e.g., Fig. 2 vs. Fig. 5). Therefore, minor variation in the duration of room air exposure post-CH does not seem to explain different patterns of VAH among studies.
In conclusion, at least within the substrain of Sprague Dawley rats used in the present study, the effects of CH on respiratory control appear to be qualitatively similar across neonates (Bavis et al., 2020), juveniles, and adults. More broadly, there does not appear to be one “typical” pattern of VAH in adult rats, so caution should be used when generalizing across all studies on laboratory rats, or even across studies on the same rat strain. Persistent hyperpnea after return to normoxia is the most consistent observation after CH, but changes in hypoxic and the acute HVR are highly variable. Multiple experiments produced consistent results despite varying the level and duration of hypoxia used for CH and to assess the HVR, so there was no obvious technical reason identified for differences in VAH among rat studies. Genetic variation among strains or substrains, and perhaps even within populations over time, may contribute to these divergent results, but it will take additional studies with more genetic backgrounds to fully explore this hypothesis. Although VAH appears to be fairly reproducible in human studies (Teppema and Dahan, 2010), perhaps in part due to the widespread use of isocapnic HVR tests, the extent to which the pattern of VAH varies in other species warrants additional scrutiny. Future studies might also consider whether variation in the expression of VAH ultimately impacts O2 delivery to tissues during CH, or whether it might instead reflect the degree to which plasticity is expressed at other levels of the oxygen cascade (e.g., hematocrit, cardiac output, etc.).
Supplementary Material
Highlights.
Chronic hypoxia causes respiratory plasticity, but it reportedly varies with age.
The plasticity might transition from blunting to enhancing the HVR in older rats.
In contrast, we found that chronic hypoxia blunted the HVR across all ages studied.
A review of published studies showed blunting to be a common result for adult rats.
There is no “typical” pattern of ventilatory acclimatization to hypoxia in rats.
Acknowledgments.
The authors thank Darya Lee, KJ Gormley, and Viuro Nkemngong for their assistance while screening published articles for the literature survey presented in Section 3.3 and Appendix B. Research reported here was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM103423.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Powell et al. (1998) suggested the term ventilatory deacclimatization to hypoxia (VDH) to describe the persistent hyperpnea or hyperventilation observed immediately after return to normoxia. Deacclimation and deacclimatization are commonly used to describe the process by which plastic phenotypes “recover” after individuals are returned to their original environment or, more precisely, the process of reacclimating to their original environment. The persistent hyperpnea observed after acute return to normoxia is evidence of plasticity (not its reversal) and thus is not consistent with the latter interpretation, so we chose not to refer to this phenotype as VDH in the present paper to avoid potential confusion.
References
- Aaron EA, Powell FL, 1993. Effect of chronic hypoxia on hypoxic ventilatory response in awake rats. J. Appl. Physiol 74, 1635–1640. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Bavis RW, Dahlberg JM, Mitchell AZ, Wilkerson JE, Golder FJ, Macfarlane PM, Watters JJ, Behan M, Mitchell GS, 2010. Differential expression of respiratory long-term facilitation among inbred rat strains. Respir. Physiol. Neurobiol 170, 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, 2005. Developmental plasticity of the hypoxic ventilatory response after perinatal hyperoxia and hypoxia. Respir. Physiol. Neurobiol 149, 287–299. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Fallon SC, Dmitrieff EF, 2013. Chronic hyperoxia and the development of the carotid body. Respir. Physiol. Neurobiol 185, 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Song MJ, Smachlo JP, Hulse A, Kenison HR, Peralta JN, Place JT, Triebwasser S, Warden SE, McDonough AB, 2020. Ventilatory and carotid body responses to acute hypoxia in rats exposed to chronic hypoxia during the first and second postnatal weeks. Respir. Physiol. Neurobiol 275, 103400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee D, Pallot DJ, 1995. Acute hypoxic ventilation, carotid body cell division, and dopamine content during early hypoxia in rats. J. Appl. Physiol 79, 1504–1511. [DOI] [PubMed] [Google Scholar]
- Bonora M, Vizek M, 2001. Ventilation, EELV and diaphragmatic activity in rats during the early phase of normobaric hypoxia. Respir. Physiol 128, 131–145. [DOI] [PubMed] [Google Scholar]
- Caceres AI, Obeso A, Gonzalez C, Rocher A, 2007. Molecular identification and functional role of voltage-gated sodium channels in rat carotid body chemoreceptor cells. Regulation of expression by chronic hypoxia in vivo. J. Neurochem 102, 231–245. [DOI] [PubMed] [Google Scholar]
- Carroll JL, Kim I, 2013. Carotid chemoreceptor “resetting” revisited. Respir. Physiol. Neurobiol 185, 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde SV, Ribeiro MJ, Obeso A, Rigual R, Monteiro EC, Gonzalez C, 2012. Chronic caffeine intake in adult rat inhibits carotid body sensitization produced by chronic sustained hypoxia but maintains intact chemoreflex output. Mol. Pharmacol 82, 1056–1065. [DOI] [PubMed] [Google Scholar]
- Cramer NP, Xu X, Christensen C, Bierman A, Tankersley CG, Galdzicki Z, 2015. Strain variation in the adaptation of C57Bl6 and BALBc mice to chronic hypobaric hypoxia. Physiol. Behav 143, 158–165. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Powell FL, Bisgard GE, Blain GM, Poulin MJ, Smith CA, 2014. Role of chemoreception in cardiorespiratory acclimatization to, and deacclimatization from, hypoxia. J. Appl. Physiol 116, 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwinell MR, Powell FL, 1999. Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats. Journal of applied physiology (Bethesda, Md. : 1985) 87, 817–823. [DOI] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA, 1987. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J. Physiol 392, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frappell P, Lanthier C, Baudinette RV, Mortola JP, 1992. Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. Am. J. Physiol 262, R1040–1046. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS, 2001. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol. Genomics 4, 175–181. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Zabka AG, Bavis RW, Baker-Herman T, Fuller DD, Mitchell GS, 2005. Differences in time-dependent hypoxic phrenic responses among inbred rat strains. J. Appl. Physiol 98, 838–844. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Eden GJ, Nijhuis JG, Moore PJ, (1989a). Peripheral chemoreceptors and other oxygen sensors in the fetus and newborn, in: Lahiri S, Forster RE, Davies RO, Pack AI (Eds.), Chemoreceptors and Reflexes in Breathing: Cellular and Molecular Aspects. Oxford University Press, New York, pp. 113–120. [Google Scholar]
- Hanson MA, Kumar P, Williams BA, 1989b. The effect of chronic hypoxia upon the development of respiratory chemoreflexes in the newborn kitten. J. Physiol 411, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Forster HV, Papanek PE, Dwinell MR, Hogan GE, 2002. Ventilatory phenotypes among four strains of adult rats. J. Appl. Physiol 93, 974–983. [DOI] [PubMed] [Google Scholar]
- Huey KA, Brown IP, Jordan MC, Powell FL, 2000. Changes in dopamine D(2)-receptor modulation of the hypoxic ventilatory response with chronic hypoxia. Respir. Physiol 123, 177–187. [DOI] [PubMed] [Google Scholar]
- Millet GP, Faiss R, Pialoux V, 2012. Point: Hypobaric hypoxia induces different physiological responses from normobaric hypoxia. Journal of applied physiology (Bethesda, Md. : 1985) 112, 1783–1784. [DOI] [PubMed] [Google Scholar]
- Mounier R, Brugniaux JV, 2012. Counterpoint: Hypobaric hypoxia does not induce different responses from normobaric hypoxia. Journal of applied physiology (Bethesda, Md. : 1985) 112, 1784–1786. [DOI] [PubMed] [Google Scholar]
- Olson EB Jr., Dempsey JA, 1978. Rat as a model for humanlike ventilatory adaptation to chronic hypoxia. J Appl Physiol Respir Environ Exerc Physiol 44, 763–769. [DOI] [PubMed] [Google Scholar]
- Ou LC, Chen J, Fiore E, Leiter JC, Brinck-Johnsen T, Birchard GF, Clemons G, Smith RP, 1992. Ventilatory and hematopoietic responses to chronic hypoxia in two rat strains. J. Appl. Physiol 72, 2354–2363. [DOI] [PubMed] [Google Scholar]
- Ou LC, Hill NS, Tenney SM, 1984. Ventilatory responses and blood gases in susceptible and resistant rats to high altitude. Respir. Physiol 58, 161–170. [DOI] [PubMed] [Google Scholar]
- Ou LC, Salceda S, Schuster SJ, Dunnack LM, Brink-Johnsen T, Chen J, Leiter JC, 1998. Polycythemic responses to hypoxia: molecular and genetic mechanisms of chronic mountain sickness. J. Appl. Physiol 84, 1242–1251. [DOI] [PubMed] [Google Scholar]
- Ou LC, Smith RP, 1983. Probable strain differences of rats in susceptibilities and cardiopulmonary responses to chronic hypoxia. Respir. Physiol 53, 367–377. [DOI] [PubMed] [Google Scholar]
- Pamenter ME, Carr JA, Go A, Fu Z, Reid SG, Powell FL, 2014. Glutamate receptors in the nucleus tractus solitarius contribute to ventilatory acclimatization to hypoxia in rat. J. Physiol 592, 1839–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamenter ME, Go A, Fu Z, Powell FL, 2015. No evidence of a role for neuronal nitric oxide synthase in the nucleus tractus solitarius in ventilatory responses to acute or chronic hypoxia in awake rats. J. Appl. Physiol 118, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamenter ME, Powell FL, 2016. Time domains of the hypoxic ventilatory response and their molecular basis. Compr Physiol 6, 1345–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Fu Z, Go A, Powell FL, 2011. Ibuprofen blocks time-dependent increases in hypoxic ventilation in rats. Respir. Physiol. Neurobiol 178, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Dwinell MR, Aaron EA, 2000a. Measuring ventilatory acclimatization to hypoxia: comparative aspects. Respir. Physiol 122, 271–284. [DOI] [PubMed] [Google Scholar]
- Powell FL, Huey KA, Dwinell MR, 2000b. Central nervous system mechanisms of ventilatory acclimatization to hypoxia. Respir. Physiol 121, 223–236. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS, 1998. Time domains of the hypoxic ventilatory response. Respir. Physiol 112, 123–134. [DOI] [PubMed] [Google Scholar]
- Reeves SR, Gozal E, Guo SZ, Sachleben LR Jr., Brittian KR, Lipton AJ, Gozal D, 2003. Effect of long-term intermittent and sustained hypoxia on hypoxic ventilatory and metabolic responses in the adult rat. J. Appl. Physiol 95, 1767–1774. [DOI] [PubMed] [Google Scholar]
- Reid SG, Powell FL, 2005. Effects of chronic hypoxia on MK-801-induced changes in the acute hypoxic ventilatory response. J. Appl. Physiol 99, 2108–2114. [DOI] [PubMed] [Google Scholar]
- Sladek M, Parker RA, Grogaard JB, Sundell HW, 1993. Long-lasting effect of prolonged hypoxemia after birth on the immediate ventilatory response to changes in arterial partial pressure of oxygen in young lambs. Pediatr. Res 34, 821–828. [DOI] [PubMed] [Google Scholar]
- Sterni LM, Bamford OS, Wasicko MJ, Carroll JL, 1999. Chronic hypoxia abolished the postnatal increase in carotid body type I cell sensitivity to hypoxia. Am. J. Physiol 277, L645–652. [DOI] [PubMed] [Google Scholar]
- Stokes JA, Arbogast TE, Moya EA, Fu Z, Powell FL, 2017. Minocycline blocks glial cell activation and ventilatory acclimatization to hypoxia. J. Neurophysiol 117, 1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl KP, Thomas AJ, St Jean P, Schlenker EH, Koletsky RJ, Schork NJ, 1997. Ventilation and metabolism among rat strains. Journal of applied physiology (Bethesda, Md. : 1985) 82, 317–323. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Fitzgerald RS, Kleeberger SR, 1994. Differential control of ventilation among inbred strains of mice. Am. J. Physiol 267, R1371–1377. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Dahan A, 2010. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol. Rev 90, 675–754. [DOI] [PubMed] [Google Scholar]
- Vizek M, Bonora M, 2001. Ventilation, EELV and diaphragmatic activity in rats during chronic normobaric hypoxia. Respir. Physiol 128, 147–159. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Olson EB, Wang Z, Keith IM, Mitchell GS, Bisgard GE, 2006. Carotid sinus nerve responses and ventilatory acclimatization to hypoxia in adult rats following 2 weeks of postnatal hyperoxia. Respir. Physiol. Neurobiol 150, 155–164. [DOI] [PubMed] [Google Scholar]
- Wilkinson KA, Huey K, Dinger B, He L, Fidone S, Powell FL, 2010. Chronic hypoxia increases the gain of the hypoxic ventilatory response by a mechanism in the central nervous system. J. Appl. Physiol 109, 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt CN, Wright C, Bee D, Peers C, 1995. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc. Natl. Acad. Sci. U. S. A 92, 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


