Abstract
Introduction:
Studies from more than 10 years ago showed epinephrine treatment of food-induced anaphylaxis in the emergency department (ED) was unacceptably low. We investigated whether epinephrine treatment of food-induced and other cause anaphylaxis in United States and Canadian EDs has changed over time.
Methods:
Guided by a health sciences librarian, we performed a systematic search in Medline, Embase and Web of Science on January 11, 2023. We included observational studies that reported epinephrine use to treat anaphylaxis in the ED. We stratified by anaphylaxis etiology (food-, venom-, medication-induced, any cause). Associations between year and epinephrine use were tested using Spearman correlation, and proportional meta-analysis.
Results:
Of 2,458 records identified in our initial search, 40 met inclusion criteria. Of these, 14 examined food-induced, 4 venom-induced, 0 medication-induced, and 24 any cause anaphylaxis. For epinephrine treatment of food-induced anaphylaxis in the ED, among studies using similar definition of anaphylaxis, meta-analysis showed a pooled value of 20.7% (95% CI 17.8, 23.8) for studies performed >10 years ago, and 45.1% (95% CI 38.4, 52.0) from those in the last 10 years. For anaphylaxis of any cause, there was no change over time, with a pooled value of 45.0% (95% CI 39.8, 50.3) over the last 10 years.
Discussion:
Epinephrine treatment of food-induced anaphylaxis in the ED has increased over time. There was no clear change for anaphylaxis of any cause. Over the last 10 years, approximately 45% of ED patients with anaphylaxis received epinephrine. A limitation of the evidence is heterogeneity in anaphylaxis definitions.
Keywords: anaphylaxis, epinephrine, food-induced anaphylaxis, meta-analysis, proportion, systematic review, treatment
1.0. Background
Anaphylaxis is a serious allergic reaction that is rapid in onset and can be life-threatening.1 It is relatively common, with lifetime prevalence of anaphylaxis from all triggers estimated to be 0.05% to 5%.2,3 The incidence of anaphylaxis appears to be rising and food-induced anaphylaxis is the leading cause of anaphylactic reactions treated in the emergency department (ED).4,5 Although use of adjunctive medications (e.g., antihistamines, glucocorticoids) to treat anaphylaxis is common, epinephrine administration is most clearly associated with decreased morbidity and mortality and is the single first-line management strategy.6–8 Early studies showed that real world use of epinephrine to treat food-induced and other cause anaphylaxis in the ED was lower than expected.9,10 Over the last two decades there seems to be increasing awareness about the primacy of epinephrine in anaphylaxis management by ED healthcare providers primarily due to increased emphasis of this principal in anaphylaxis management guidelines.1,11–16 However, there have been no systematic reviews that have investigated this topic.
Our objective was to examine whether epinephrine use to treat food-induced and other cause anaphylaxis in United States and Canadian EDs has changed over time. Secondarily, we examined pre-ED epinephrine use, any epinephrine use (pre-ED or ED), prescription for epinephrine, and referral to allergy clinic on discharge from the ED.
2.0. Methods
We prospectively registered this study in the international prospective register of systematic reviews (PROSPERO) under the registration number: CRD42023389616. This can be accessed at crd.york.ac.uk/prospero. Amendments to the protocol are listed in Supplement Table 1. We adhered to Preferred Reporting Items of Systematic Review and Meta-Analyses (PRISMA) for reporting of this systematic review and meta-analysis.17 This systematic review did not involve human subjects and therefore Institutional Review Board approval was waived.
2.1. Search strategy
We identified studies reporting the use of epinephrine to treat anaphylaxis in the ED by searching Medline/PubMed (National Library of Medicine, NCBI); Embase (Elsevier, embase.com), and Web of Science Core Collection (Clarivate). Controlled vocabulary terms (i.e., MeSH, Emtree) were included when available and appropriate. The search strategies were designed and carried out by a health sciences librarian (CM). A language limit was applied to include studies published in English and French due to the geographic region of interest. No publication date restrictions were applied. The search was conducted on January 11, 2023. The exact search terms used for each of the databases are provided in Supplement Table 2. To reduce the risk of missing any related citation, we also manually searched the reference list of included original articles.
2.2. Study selection
We included studies that reported the frequency of epinephrine treatment in the ED for anaphylaxis in a population of people who presented to the ED for anaphylaxis (food-induced, venom-induced, medication-induced, any cause) in the United States or Canada. Other inclusion criteria were observational study design, English or French language and report was a published manuscript. We excluded studies that did not report the sample size of the anaphylaxis population or the timeframe of data collection, surveys of patients and case reports/case series. Studies of interventions, reviews, systematic review and meta-analyses were also excluded.
Four reviewers performed title/abstract screening using Covidence, a web-based collaboration software platform that streamlines production of systematic and other literature reviews (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org). As a calibration exercise, all reviewers screened the same 50 titles and abstracts and discussed questions/discrepancies prior to moving on to the formal reviewing phase. Each title/abstract was screened to meet the inclusion/exclusion criteria by two reviewers, independently. For titles/abstracts where there was disagreement, a third senior reviewer adjudicated. Each full text was screened to meet the inclusion/exclusion criteria by two reviewers, independently. For full texts where there was disagreement, a third senior reviewer adjudicated.
2.3. Data extraction and quality assessment
Three reviewers performed data extraction from included full texts. Two reviewers independently extracted data from each full text and consensus was reached on any discrepancies. We contacted corresponding authors for data clarification as needed. For each selected full text manuscript, we extracted the following primary outcome data: number of participants with anaphylaxis, number of participants treated with epinephrine in the ED, and etiology of anaphylaxis. We also extracted the following variables: lead author name, year of publication, study design, country of data collection, study period, age of participants, definition of anaphylaxis (Supplement Table 3), number of participants treated with epinephrine pre-ED (defined as self/parent/school/other administration or emergency medical services [EMS] administration), number of participants treated with epinephrine pre-ED or in the ED, number of participants prescribed epinephrine on discharge, and number of participants who received allergy clinic referral on discharge. Studies of anaphylaxis of any cause were examined for sub-analysis reporting of epinephrine use in the ED for food-, venom- and medication-induced anaphylaxis. Food-induced anaphylaxis was our primary diagnosis of interest.
To assess risk of bias among the full texts included, we used the Joanna Briggs Institute Prevalence Critical Appraisal Tool.18–20 This tool uses nine questions with four standard answer options yes/no/unclear/not applicable. Two reviewers independently evaluated each article and consensus was reached on any discrepancy.
2.4. Data analysis
Interrater reliability was assessed using Cohen’s kappa statistic. For studies that collected data over multiple years, the median year of the study period was used for data analysis. To assess for change in epinephrine treatment of anaphylaxis over time, we first examined the data qualitatively using scatter plots (x-axis = median year, y-axis = percent of patients with anaphylaxis treated with epinephrine in the ED). If there were sufficient number of studies, we used Spearman correlation and meta-analysis stratified by time period to quantitatively assess the relationship. We performed meta-analysis using a user-written Stata command called metaprop to calculate pooled proportions and 95% CI overall and for two time periods, 2013–2022 (last 10 years) and prior to 2013.21,22 Pooled proportions are presented as percentages for clarity. Heterogeneity was determined by I2 values. Given concern for bias, we excluded from the Spearman correlation and meta-analysis studies with overlapping cohorts or where the number treated with epinephrine was not stated. For overlapping cohorts, we selected those with shorter time frames, that included multiple timeframes within the study, or that increased total number of studies in the meta-analysis, and excluded the others. Secondary outcomes were not meta-analyzed due to concern for incomplete capture of relevant literature as our systematic search was optimized for capture of our primary outcome. Analyses were performed using Stata 15.1 (Stata Corp, College Station, TX, USA).
3.0. Results
3.1. Literature search and study selection
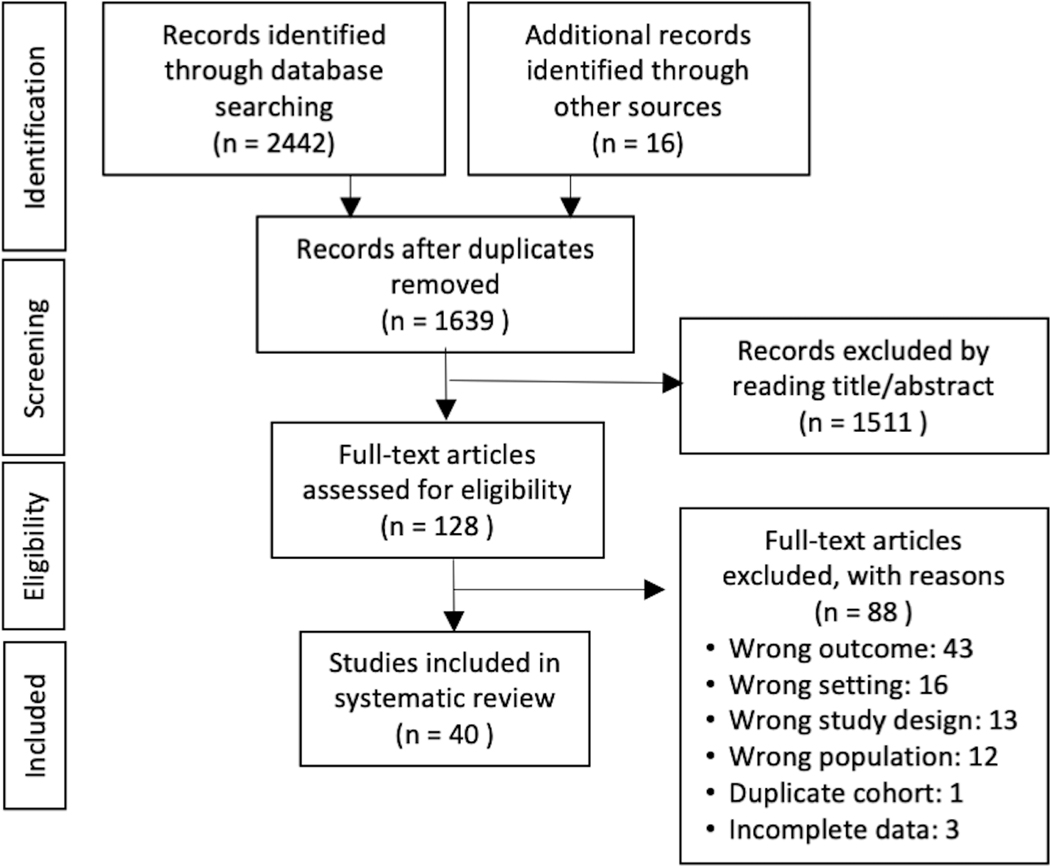
We identified 2,442 records though our systematic database search and then an additional 16 records by manual search of references from included articles (Figure 1). There were 1,639 records after removal of duplicates. Title/abstract review excluded 1,511 records, the remaining 128 were assessed for eligibility. Of these, 88 were excluded and a total of 40 were included in the systematic review. A sample of studies that may have appeared to meet the inclusion criteria, but which were excluded are available in Supplement Table 4.
Figure 1.
PRISMA flowchart: selection process of the included articles
Of 1639 titles/abstracts, there was disagreement on 61 (3.7%). Of 128 full texts, there was disagreement on 15 (12%). The interrater reliability of the title/abstract screening ranged from 0.61–0.80 among reviewers who reviewed >10 of the same titles/abstracts.
3.2. Characteristics of eligible studies
Of the 40 eligible studies reporting epinephrine treatment of anaphylaxis in the ED, 14 reported on food-induced anaphylaxis, four venom-induced anaphylaxis, and 25 anaphylaxis of any cause (Table 1).
Table 1.
Summary of emergency department studies included in systematic review.
| Study (Author, Year Published) | Reference Number | Study Period | Age Group | Study Setting | Study Design | Anaphylaxis Definition | Number of participants |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Food-induced anaphylaxis | |||||||
|
| |||||||
| Banerji 2010* | 23 | 2001–2006 | Adult | Multi-center | Observational, retrospective | NIAID/FAAN | 295 |
| Clark 2004 | 9 | 1999–2001 | Both | Multi-center | Observational, retrospective | NIAID/FAAN | 290 |
| Clark 2019 | 24 | 2013–2015 | Both | Multi-center | Observational, retrospective | NIAID/FAAN | 459 |
| Ducharme 2022 | 25 | 2011–2020 | Pediatric | Multi-center | Observational, retro-/prospective | NIAID/FAAN | 540 |
| Fleming 2015 | 26 | 2004–2009 | Pediatric | Single center | Observational, retrospective | NIAID/FAAN | 384 |
| Gabrielli 2021 | 27 | 2011–2020 | Both | Multi-center | Observational, retro-/prospective | NIAID/FAAN | 250 |
| Kim 2022 | 28 | 2007–2015 | Pediatric | Multi-center | Observational, retrospective | Anaphylaxis ICD-9/−10 codes | 15318 |
| Rehimini 2021† | 29 | 2012–2019 | Pediatric | Single center | Observational, prospective | NIAID/FAAN | 51 |
| Ross 2008 | 30 | 2003 | Both | Multi-center | Observational, retrospective | Other | 21 |
| Rudders 2010 A | 31 | 2001–2002 2003–2004 2005–2006 |
Pediatric | Single center | Observational, retrospective | NIAID/FAAN | 160 155 531 |
| Rudders 2010 B‡ | 32 | 2001–2006 | Pediatric | Single center | Observational, retrospective | NIAID/FAAN | 658 |
| Sehayek 2022 | 33 | 2011–2020 | Pediatric | Multi-center | Observational, retro-/prospective | NIAID/FAAN | 146 |
| Sillcox 2022 | 34 | 2011–2021 | Pediatric | Multi-center | Observational, retro-/prospective | NIAID/FAAN | 130 |
|
| |||||||
| Venom-induced anaphylaxis | |||||||
|
| |||||||
| Clark 2005 | 10 | 1999–2001 | Both | Multi-center | Observational, retrospective | NIAID/FAAN | 182 |
| Clark 2018 | 35 | 2013–2015 | Both | Multi-center | Observational, retrospective | NIAID/FAAN | 148 |
| Rudders 2013 | 36 | 2002–2008 | Both | Multi-center | Observational, retrospective | Anaphylaxis ICD-9/−10 codes | 807 |
|
| |||||||
| Medication-induced anaphylaxis | |||||||
|
| |||||||
| None | |||||||
|
| |||||||
| Anaphylaxis of any cause | |||||||
|
| |||||||
| Arroyo 2021 | 37 | 2006–2014 | Adult | Multi-center | Observational, retrospective | Anaphylaxis ICD-9/−10 codes | 459304 |
| Asai 2014 | 38 | 2011–2012 | Adult | Single center | Observational, retrospective | NIAID/FAAN | 98 |
| Baalmann 2016§ | 39 | 2010–2013 | Both | Single center | Observational, prospective | Other | 61 |
| Calderon 2013 | 40 | 2007–2009 | Adult | Single center | Observational, retrospective | NIAID/FAAN | 31 |
| Carrillo-Martin 2020 | 41 | 2007–2015 | Both | Multi-center | Observational, retrospective | Anaphylaxis ICD-9/−10 codes | 278000 |
| Castilano 2018 | 42 | 2012–2015 | Both | Single center | Observational, retrospective | NIAID/FAAN | 60 |
| Chiang 2021ǁ | 43 | 2010–2018 | Both | Single center | Observational, retro-/prospective | NIAID/FAAN | 1090 |
| Cohen 2021 | 44 | 2018–2019 | Pediatric | Single center | Observational, retrospective | NIAID/FAAN | 368 |
| Gabrielli 2019 | 45 | 2011–2017 | Both | Multi-center | Observational, retro-/ prospective | NIAID/FAAN | 3498 |
| Gaeta 2007 | 46 | 1993–2004 | Both | Multi-center | Observational, prospective | Anaphylaxis ICD-9/−10 codes | 143000 |
| Goetz 2019 | 47 | 2009–2010 | Pediatric | Single center | Observational, retrospective | Anaphylaxis ICD-9/−10 codes, then NIAID/FAAN | 211 |
| Hemler 2017 | 48 | 2013 | Pediatric | Single center | Observational, retrospective | Anaphylaxis ICD-9/−10 codes, then NIAID/FAAN | 64 |
| Hochstadter 2016 | 49 | 2011–2015 | Pediatric | Single center | Observational, retro-/prospective | NIAID/FAAN | 965 |
| Huang 2012 | 50 | 2004–2008 | Pediatric | Single center | Observational, retrospective | NIAID/FAAN | 213 |
| Lee 2017 | 51 | 2014–2016 | Pediatric | Multi-center | Observational, retro-/prospective | NIAID/FAAN | 977 |
| Liu 2020 | 52 | 2010–2018 | Both | Multi-center | Observational, prospective | NIAID/FAAN | 430 |
| Manivannan 2009 | 53 | 1990–2000 | Both | Multi-center | Observational, retrospective | Other | 208 |
| Meir 2022 | 54 | 2016–2017 | Adult | Multi-center | Observational, retrospective | Anaphylaxis ICD-9/−10 codes, then NIAID/FAAN | 82 |
| Owusu-Ansah 2019 | 55 | 2012–2014 | Pediatric | Multi-center | Observational, retrospective | NIAID/FAAN | 86 |
| Russell 2010 | 56 | 2002–2006 | Pediatric | Single center | Observational, retrospective | NIAID/FAAN | 124 |
| Sidhu 2016 | 57 | 2004–2006 2007–2011 |
Pediatric | Single center | Observational, retrospective | Anaphylaxis ICD-9/−10 codes, then NIAID/FAAN | 61 126 |
| Tiyyagura 2014 | 58 | 2008–2010 | Pediatric | Single center | Observational, retrospective | NIAID/FAAN | 218 |
| Trainor 2022 | 59 | 2015–2017 | Pediatric | Single center | Observational, retrospective | Other | 414 |
| Wright 2017 | 60 | 2014 | Pediatric | Single center | Observational, retrospective | NIAID/FAAN | 40 |
NIAID/FAAN National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network 2006 definition of anaphylaxis; ICD-9/ICD-10 International Classification of Disease, Ninth Revision/Tenth Revision.
Study reports unweighted frequencies for epinephrine use and weighted frequencies for epinephrine prescription and allergy clinical referral.
Overlapping cohort with Ducharme 2022, Gabrielli 2021, Sehayek 2022, Sillcox 2022.
Overlapping cohort with Rudders 2010 A.
Study stratifies by food- and venom-induced anaphylaxis.
Overlapping study cohort with Baalmann 2016 and Liu 2022.
No included studies examined medication-induced anaphylaxis. Most studies were retrospective (n = 28), though some were prospective (n = 4), or a combination of the two (n = 8). Most studies used the National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network (NIAID/FAAN) definition of anaphylaxis (n = 27). Fewer studies used International Classification of Disease, Ninth Revision or Tenth Revision (ICD-9 or ICD-10) codes for anaphylaxis (n = 5), ICD-9 or ICD-10 codes for anaphylaxis followed by application of the NIAID/FAAN definition (n = 4), or other definitions of anaphylaxis (n = 4). There was a mix of studies that examined pediatric (n = 20), adult (n = 5) or both age groups (n = 15). There were 9 publications that reported on overlapping cohorts/timeframes: excluding three of these studies removed all potentially overlapping cases.29,32,43 Gaeta 2007 only reported a percentage for epinephrine use in the ED, but not the number treated with epinephrine and therefore was also excluded from further analyses.46
3.3. Bias assessment
Overall, the quality of the studies included was good. Potential bias mainly came from differences in sample size, differences in the definition of anaphylaxis, and lack of reporting of important clinical factors (e.g., number of participants who received epinephrine before arriving to the ED [pre-ED]). Supplement Table 5 summarizes the potential for bias of each study.
3.4. Food-induced anaphylaxis
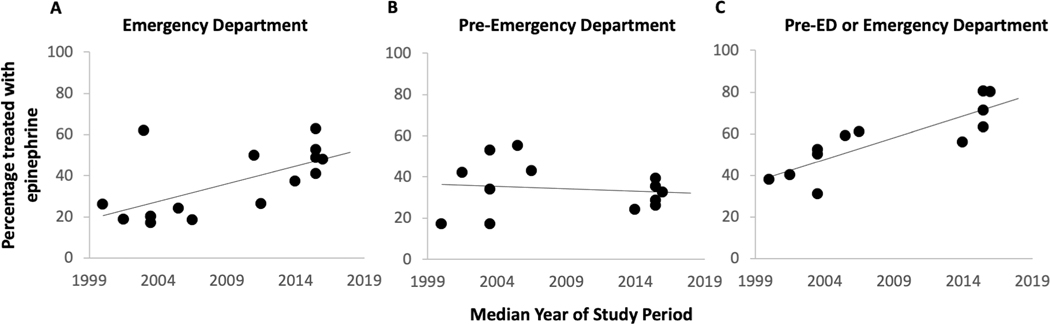
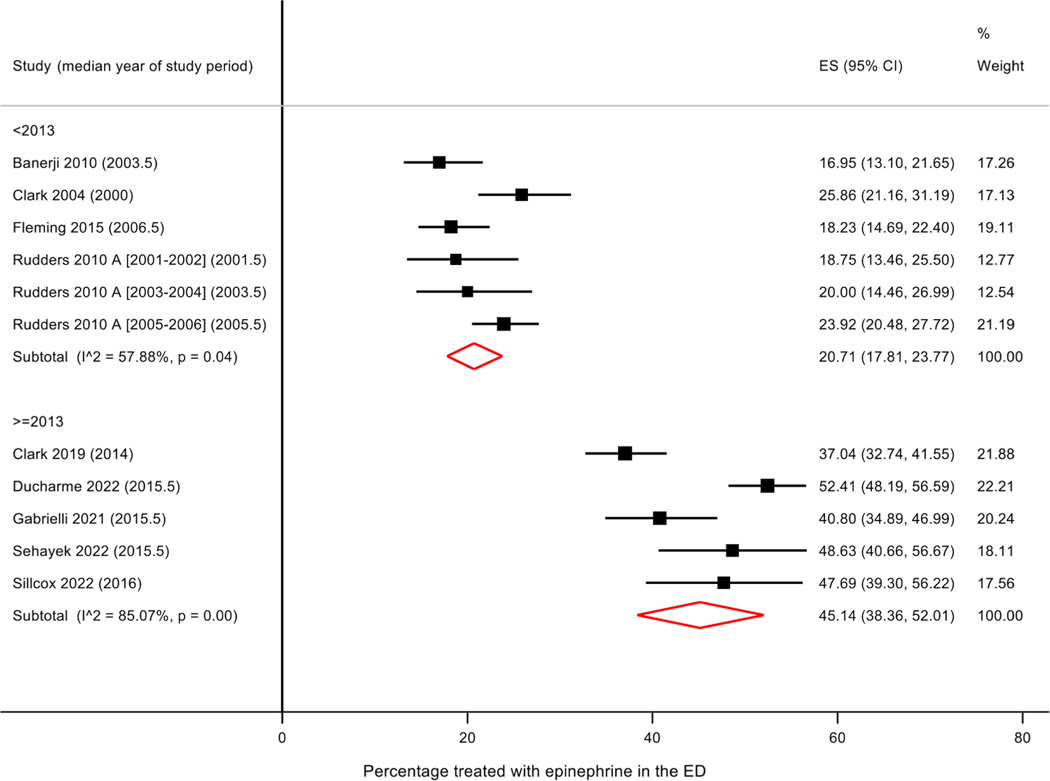
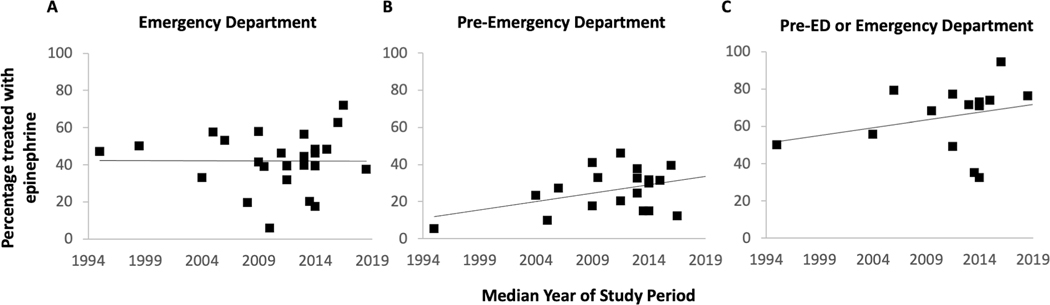
Of the 14 studies examining food-induced anaphylaxis, the median year of study period ranged from 2000 to 2016. The percentage of ED patients who received epinephrine treatment ranged from 17 to 63% (Table 2). There appeared to be a trend toward increase in epinephrine use in the ED over time (r = 0.49, p=0.07) (Figure 2A); excluding the three studies that used a non-NIAID/FAAN definition of anaphylaxis led to a stronger association (r = 0.72, p = 0.009) (Figure 2A). Meta-analysis of studies stratified by time period (prior to 2013 vs 2013–2022) showed trend toward an improvement in epinephrine use over time (prior to 2013: pooled 27.6% [95% CI 15.8, 41.1], I2 = 98.7%, p < 0.001; 2013–2022: pooled 45.1% [95% CI 38.4, 52.0], I2 = 85.1%, p < 0.001); however, heterogeneity was high (Supplement Figure 1). Sensitivity analysis examining only studies using the NIAIAD/FAAN definition of anaphylaxis, stratified by time period, showed an improvement over time with a pooled value of 20.7% (95% CI 17.8, 23.7; I2 = 57.9; p = 0.04) for studies performed prior to 2013, and a pooled value of 45.1% (95% CI 38.4, 52.0; I2 = 85.1; p < 0.001) from those performed from 2013–2022 (Figure 3).
Table 2.
Frequency of epinephrine use, epinephrine prescription, and allergy clinical referral among patients who present to the emergency department with anaphylaxis.
| Study (Author, Year Published) | Median year of study periods | Total # with anaphylaxis | Treated with epinephrine in the ED | Treated with epinephrine pre-ED | Treated with epinephrine pre-ED or ED | Epinephrine prescription on discharge | Allergy referral on discharge | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Food-induced anaphylaxis | N | N | % | N | % | N | % | N | % | N | % | |
|
| ||||||||||||
| Baalmann 2016 (food)* | 2011.5 | 19 | 5 | 26 | ||||||||
| Banerji 2010 | 2003.5 | 295 | 50 | 17 | 50 | 17 | 91 | 31 | ||||
| Banerji 2010 (weighted)† | 2003.5 | 802 | 388 | 48 | 189 | 24 | ||||||
| Clark 2004 | 2000 | 290 | 75 | 26 | 49 | 17 | 110 | 38 | 70 | 24 | 41 | 14 |
| Clark 2019 | 2014 | 459 | 170 | 37 | 110 | 24 | 257 | 56 | 248 | 54 | 110 | 24 |
| Ducharme 2022 | 2015.5 | 540 | 283 | 52 | 190 | 35 | 433 | 80 | ||||
| Fleming 2015 | 2006.5 | 384 | 70 | 18 | 164 | 43 | 234 | 61 | ||||
| Gabrielli 2021 | 2015.5 | 250 | 102 | 41 | 71 | 28 | 158 | 63 | 179 | 72 | ||
| Kim 2022 | 2011 | 15318 | 7600 | 50 | ||||||||
| Rehimini 2021 ‡ | 2015.5 | 51 | 32 | 63 | 20 | 39 | ||||||
| Ross 2008 | 2003 | 21 | 13 | 62 | ||||||||
| Rudders 2010 A‡ | 2003.5 | 658 | 132 | 20 | 222 | 34 | 329 | 50 | 434 | 66 | 249 | 38 |
| Rudders 2010 B [2001–2002] | 2001.5 | 160 | 30 | 19 | 67 | 42 | 64 | 40 | 105 | 66 | 62 | 39 |
| Rudders 2010 B [2003–2004] | 2003.5 | 155 | 31 | 20 | 82 | 53 | 81 | 52 | 101 | 65 | 81 | 52 |
| Rudders 2010 B [2005–2006] | 2005.5 | 531 | 127 | 24 | 292 | 55 | 313 | 59 | 361 | 68 | 313 | 59 |
| Sehayek 2022 | 2015.5 | 146 | 71 | 49 | 38 | 26 | 104 | 71 | ||||
| Sillcox 2022 | 2016 | 130 | 62 | 48 | 42 | 32 | 104 | 80 | 88 | 68 | 66 | 51 |
|
| ||||||||||||
| Venom-Induced anaphylaxis | ||||||||||||
|
| ||||||||||||
| Baalman 2016 (venom)* | 2011.5 | 10 | 5 | 50 | ||||||||
| Clark 2005 | 2000 | 182 | 25 | 14 | 31 | 17 | 55 | 30 | 62 | 34 | 51 | |
| Clark 2018 | 2014 | 148 | 37 | 25 | 41 | 28 | 73 | 49 | 84 | 57 | 18 | 12 |
| Rudders 2013 | 2005 | 807 | 50 | 6 | ||||||||
|
| ||||||||||||
| Anaphylaxis of any cause | ||||||||||||
|
| ||||||||||||
| Arroyo 2021 | 2010 | 459304 | 26033 | 6 | ||||||||
| Asai 2014 | 2011.5 | 98 | 31 | 32 | 20 | 20 | 48 | 49 | ||||
| Baalmann 2016 | 2011.5 | 61 | 24 | 39 | 28 | 46 | 47 | 77 | ||||
| Calderon 2013 | 2008 | 31 | 6 | 19 | 7 | 23 | ||||||
| Carrillo-Martin 2020 | 2011 | 278000 | 127880 | 46 | 41700 | 15 | ||||||
| Castilano 2018 | 2013.5 | 60 | 12 | 20 | 9 | 15 | 21 | 35 | 28 | 47 | 9 | 15 |
| Chiang 2021 ‡ | 2014 | 1090 | 503 | 46 | 324 | 30 | 780 | 72 | ||||
| Cohen 2021 | 2018.5 | 368 | 138 | 38 | 281 | 76 | 262 | 71 | ||||
| Gabrielli 2019 | 2014 | 3498 | 1686 | 48 | 1070 | 31 | 2549 | 73 | ||||
| Gaeta 2007 § | 1998.5 | 143000 | 50 | |||||||||
| Goetz 2019 | 2009.5 | 211 | 82 | 39 | 69 | 33 | 144 | 68 | 180 | 85 | 119 | 56 |
| Hemler 2017 | 2013 | 64 | 36 | 56 | 24 | 38 | 53 | 83 | 31 | 48 | ||
| Hochstadter 2016 | 2013 | 965 | 427 | 44 | 313 | 32 | 690 | 72 | ||||
| Huang 2012 | 2006 | 213 | 113 | 53 | 58 | 27 | 169 | 79 | 116 | 54 | ||
| Lee 2017 | 2015 | 977 | 471 | 48 | 305 | 31 | 722 | 74 | ||||
| Liu 2020 | 2014 | 430 | 169 | 39 | 136 | 32 | 305 | 71 | ||||
| Manivannan 2009 | 1995 | 208 | 98 | 47 | 11 | 5 | 104 | 50 | 79 | 38 | 87 | 42 |
| Meir 2022 | 2016.5 | 82 | 59 | 72 | 10 | 12 | 28 | 34 | 14 | 17 | ||
| Owusu-Ansah 2019 | 2013 | 86 | 34 | 40 | 21 | 24 | 55 | 64 | 15 | 17 | ||
| Russell 2010 | 2004 | 124 | 41 | 33 | 29 | 23 | 69 | 56 | ||||
| Sidhu 2016 [2004–2006] | 2005 | 61 | 35 | 57 | 6 | 10 | 34 | 56 | 25 | 41 | ||
| Sidhu 2016 [2007–2011] | 2009 | 126 | 52 | 41 | 22 | 17 | 81 | 64 | 60 | 48 | ||
| Tiyyagura 2014 | 2009 | 218 | 126 | 58 | 89 | 41 | ||||||
| Trainor 2022 | 2016 | 414 | 259 | 63 | 163 | 39 | 391 | 94 | ||||
| Wright 2017 | 2014 | 40 | 7 | 18 | 6 | 15 | 13 | 33 | 26 | 65 | 12 | 30 |
ED: Emergency Department.
Sub-analysis of Baalmann 2016
Study reports both weighted and unweighted counts
Excluded from Spearman correlation and meta-analysis due to cohort overlap.
Excluded from Spearman correlation and meta-analysis due to no reported frequency of epinephrine use.
Figure 2.
Epinephrine use in the emergency department, pre-ED or either location for treatment of food-induced anaphylaxis. ED: Emergency Department
Figure 3.
Forest plot – Epinephrine use in the emergency department to treat food-induced anaphylaxis by time period, among studies using similar definition of anaphylaxis. All studies included in meta-analysis use National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network (NIAID/FAAN) definition of anaphylaxis
Many studies included participants of all ages. When restricting the analysis to studies that included only children (n = 8) the primary finding remained the same. There was an improvement in use of epinephrine in the ED for treatment of food-induced anaphylaxis (prior to 2013: 25.6% [95% CI 11.2, 43.5]; 2013–2022: 51.0% [95% CI 47.5, 54.4]). There were an insufficient number of studies that included only adults (n = 1) to examine this subgroup.
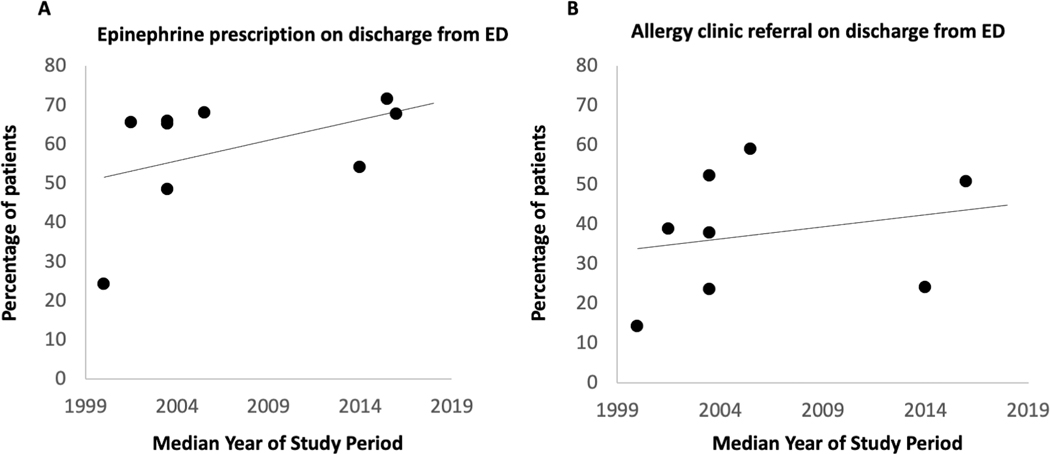
Examination of pre-ED epinephrine use over time did not show a clear improvement, with rates ranging between 17% and 55% (Figure 2B). However, when examining pre-ED or ED epinephrine use there appeared to be an improvement over time, with studies in the last 10 years ranging between 56% and 80% compared to studies prior to 2013 with range of 31% to 61% (Figure 2C). Qualitative examination of epinephrine prescription and allergy clinic referral on discharge from the ED also showed improvement (Figure 4A, B). These secondary outcomes were not meta-analyzed due to possible incomplete capture of data in the literature.
Figure 4.
Frequency of epinephrine prescription and allergy referral on emergency department discharge among patients presenting with food-induced anaphylaxis. ED: Emergency Department
3.5. Venom-induced anaphylaxis
Of the four studies examining venom-induced anaphylaxis, the median year of study period ranged from 2000 to 2014. The percentage of ED patients who received epinephrine treatment ranged from 6% to 50% (Table 2). Given the low sample size, we were not able to assess for changes in ED epinephrine use over time in this group. We also were not able to assess for changes in pre-ED or pre-ED and/or ED epinephrine use over time.
3.6. Anaphylaxis of any cause
Of the 25 studies examining anaphylaxis of any cause, the median year of study period ranged from 1995 to 2019. The percentage of ED patients who received epinephrine treatment ranged from 6% to 72% (Table 2). There did not appear to be an increase in epinephrine use in the ED over time (r = 0.08, p = 0.71) (Figure 5A). This finding did not change when we excluded studies that used a non-NIAID/FAAN definition of anaphylaxis. Meta-analysis stratified by time period also showed no significant change; a pooled value of 38.3% (95% CI 19.8, 58.6; I2 = 99.9%; p < 0.001) for studies performed prior to 2013, and a pooled value of 45.0% (95% CI 39.8, 50.3; I2 = 92.4; p < 0.001) for those performed from 2013–2022 (Supplement Figure 2). The overall pooled value was 41.0% (95% CI 27.1, 55.6, I2 = 99.9%, p < 0.001). This finding was similar when we excluded studies that used a non-NIAID/FAAN definition of anaphylaxis (Supplement Figure 3).
Figure 5.
Epinephrine use in the emergency department, pre-ED or either location for treatment of anaphylaxis of any cause. ED: Emergency Department.
When restricting the analysis to studies that include only children (n = 13) the primary findings remain the same. There was no change over time for use of epinephrine in the ED for treatment of any cause anaphylaxis (prior to 2013: 46.7% [95% CI 38.4, 55.1]; 2013–2022: 44.7% [95%CI 37.4, 52.2]). There were an insufficient number of studies that included only adults (n = 4) to examined this subgroup.
There did appear to be qualitative improvement in pre-ED use of epinephrine and pre-ED and/or ED use of epinephrine over time (Figure 5B,C). These secondary outcomes were not meta-analyzed due to possible incomplete capture of data in the literature.
4.0. Discussion
We performed a systematic review and meta-analysis to examine whether use of epinephrine in the ED for treatment of food-induced and other cause anaphylaxis, in the United States and Canada, has changed over time. We found that for food-induced anaphylaxis, the pooled percentage of ED patients treated with epinephrine in the ED improved from 21% for studies performed >10 years ago (prior to 2013), to 45% for those from the last 10 years (2013–2022), among studies who use the NIAID/FAAN definition of anaphylaxis. We did not find a change over time for anaphylaxis of any cause, but noted a similar frequency of treatment (45%) for studies performed over the last 10 years. There were not enough studies to analyze changes for venom- or medication-induced anaphylaxis.
4.1. Food-induced and any cause anaphylaxis
We have shown that the use of epinephrine for treatment of food-induced anaphylaxis in the ED has improved over time. Although the same cannot be said about any cause anaphylaxis, the frequency of epinephrine treatment started higher than for food-induced anaphylaxis -- and usage was similar (45%) for both food-induced and any cause anaphylaxis over the last 10 years. However, an important question remains: is 45% high enough or is there continued room for improvement in ED administration of epinephrine?
Although ED treatment is a key part of the care of a patient who experiences anaphylaxis, the pre-ED management, including self/parent/school/other and emergency medical services (EMS) administration of epinephrine will influence ED management. A recent systematic review and meta-analysis showed that only 8% of patients with anaphylaxis require more than a single dose of epinephrine.61 Therefore, if a patient receives epinephrine prior to ED arrival, on most occasions the ED would not need to administer an additional dose. Further, since early administration of epinephrine is an important predictor of morbidity and mortality associated with epinephrine use,26,62 preferably there would be increased pre-ED use of epinephrine which would necessarily and appropriately result in decreased need for ED use of epinephrine. Our secondary outcome, rate of pre-ED epinephrine use for treatment of anaphylaxis did not appear to improve over time for food-induced anaphylaxis, but it did appear to improve for any cause anaphylaxis. For any cause anaphylaxis, more pre-ED epinephrine use over the last 10 years may explain why we do not see an increase in ED epinephrine use over the same time frame.
Although we did not include studies from outside the United States or Canada in the present study, it appears that treatment of any cause anaphylaxis by a health professional in Europe may have improved over time. Using data from the European Anaphylaxis Register, Grabenhenrich and colleagues found that over the last decide, epinephrine administration from a health professional to treat anaphylaxis almost doubled to reach 30.6% in 2015–2017.63 While this study examines epinephrine use in healthcare professionals generally, rather than ED clinicians, it does suggest that epinephrine usage was very low 10 years ago and has improved over time, but may still remain suboptimal. This study does not examine epinephrine use by anaphylaxis etiology (e.g. food-induced anaphylaxis), so it is not possible to know if food-induced anaphylaxis also has an improvement over time.
We found that for both food-induced and any cause anaphylaxis, any epinephrine use (pre-ED or ED) did appear to improve over time with rates as high as 80% in two different studies. Among the patients who did not receive epinephrine for their anaphylaxis pre-ED or in the ED, it is possible that non-guideline based care was given; however, there may be alternative explanations in many cases. For example, anaphylaxis may have resolved prior to ED presentation (e.g, Pouessel and colleagues found that among 116 children seen in an ED for grade 3 or 4 anaphylaxis, 52% had rapid improvement of anaphylaxis symptoms prior to ED arrival without epinephrine administration64), the anaphylaxis may have been mild (e.g., flushing and abdominal pain without other symptoms) and the patient may have preferred not to receive an intramuscular medication, or the provider may have felt that the risk outweighed the benefit (e.g., mild anaphylaxis in a patient with severe coronary artery disease). Indeed, a study by Baalmann et al found that although more than 60% of patients with anaphylaxis did not receive epinephrine in the ED, case review by two board certified allergy immunology physicians deemed ED management appropriate in 98% of total cases.39 In sum, epinephrine should always be used as soon as anaphylaxis symptoms are recognized. However, ED clinicians may evaluate patients whose anaphylaxis has already been treated with epinephrine prior to ED arrival or whose anaphylaxis spontaneously resolved prior to evaluation, and therefore will likely not administer epinephrine to 100% of patients who are presenting for anaphylaxis.
4.2. Limitations of the evidence
Limitations of the evidence used in this systematic review include, most importantly, differences in the way anaphylaxis was defined. Some studies performed chart review of all potential allergic reaction cases applying the NIAID/FAAN criteria for anaphylaxis, which is the most widely accepted definition. Others used ICD-9 and/or ICD-10 codes for anaphylaxis to identify possible cases and then performed chart review using NIAID/FAAN criteria to verify the diagnosis, likely producing a higher severity group of patients with anaphylaxis as there may have been cases that presented to the ED who met the NIAID/FAAN criteria, but were not given an ICD-9 and/or ICD-10 code for anaphylaxis, but rather were labeled with a different code like adverse food reaction or allergic reaction not otherwise specified. Still others used only ICD-9 and/or ICD-10 codes for anaphylaxis without additional validation, which has been shown to both over and under-include true anaphylaxis cases in a sample.65 There were also other methods in identified articles, which have not been validated or present possible concern for bias. We attempted to address this by performing sensitivity analyses using anaphylaxis definition (Figure 3).
An additional limitation is that several studies used in the food allergy analysis were focused on single foods and therefore may not be representative of food allergy generally. Further, several of the more recent food allergy studies are from the Cross-Canada Anaphylaxis Registry (C-CARE). We ensured that we did not use overlapping samples by excluding the study that covered a larger time period, if there were two timeframes that overlapped, but it does give large representation to data from the C-CARE cohort, especially for food-induced anaphylaxis data over the last 10 years. Reassuringly, C-CARE is the most robust Canadian data available on anaphylaxis which includes data from multiple centers across Canada. It has both retrospective and prospective data collection and therefore is less likely to be missing substantial numbers of observations. Finally, severity of anaphylaxis was not reported in the vast majority of studies and therefore we were not able to report whether treatment of anaphylaxis with epinephrine varied between mild, moderate and severe presentations.
4.3. Limitations of the review process
Limitations of the review process include that we based our inclusion and exclusion criteria on the primary outcome, rate of use of epinephrine for treatment of anaphylaxis in the ED, and therefore studies that only reported data for our secondary outcomes (pre-ED epinephrine use, any epinephrine use [pre-ED or ED], epinephrine autoinjector prescription and allergy clinical referral on discharge) were not included in the systematic review. This limits our ability to interpret the secondary outcomes, as we likely did not capture all extant relevant literature on these different topics.
5.0. Conclusion
Epinephrine treatment of food-induced anaphylaxis in the ED has improved over time to 45% in the last 10 years. There is no clear change over time for anaphylaxis of any cause, but there was the similar usage of epinephrine (45%) over the last 10 years as seen for food-induced anaphylaxis. These data show possible improvements in pre-ED epinephrine use and any epinephrine use (pre-ED or ED) for anaphylaxis, but our systematic search was focused on ED administration and therefore may not have completely captured literature related to these secondary outcomes. It is important to emphasize that pre-ED epinephrine use will affect the rate of ED epinephrine use. We encourage future studies (including systematic reviews) on epinephrine use in the treatment of food-induced and other cause anaphylaxis to focus on pre-ED epinephrine use and any epinephrine use (pre-ED or ED) for treatment of anaphylaxis. Similar studies (and systematic reviews) could be completed for epinephrine prescriptions and allergy referral.
Supplementary Material
Funding
G Mehta was supported by grant T32 AI007306 from the National Institutes of Health. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Reviewer disclosures
One peer reviewer is a provincial lead for C-CARE. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Declaration of interest
Over the past 30 years, C Camargo has done paid consultation for several companies that make epinephrine auto-injectors (Mylan, Kaleo) or that are developing new modes of epinephrine delivery (Hikma, Bryn). He also has served on scientific advisory boards for these same companies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1. Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med 2006;47:373–80. DOI: 10.1016/j.annemergmed.2006.01.018#1. * This reference establishes the National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network (NIAID/FAAN) definition of anaphylaxis which is the most widely accepted definiton of anaphylaxis at this time. It also states that anaphylaxis is probably under recognized and undertreated in both the prehospital setting and in the ED.
- 2.Lieberman P, Camargo CA Jr., Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol 2006;97:596–602. DOI: 10.1016/S1081-1206(10)61086-1 [DOI] [PubMed] [Google Scholar]
- 3.Wood RA, Camargo CA Jr., Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol 2014;133:461–7. DOI: 10.1016/j.jaci.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 4.Sampson HA. Anaphylaxis and emergency treatment. Pediatrics 2003;111:1601–8 [PubMed] [Google Scholar]
- 5.Motosue MS, Bellolio MF, Van Houten HK, et al. Increasing Emergency Department Visits for Anaphylaxis, 2005–2014. J Allergy Clin Immunol Pract 2017;5:171–5. DOI: 10.1016/j.jaip.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 6.Brown JC, Simons E, Rudders SA. Epinephrine in the Management of Anaphylaxis. J Allergy Clin Immunol Pract 2020;8:1186–95. DOI: 10.1016/j.jaip.2019.12.015 [DOI] [PubMed] [Google Scholar]
- 7.Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy 2000;30:1144–50. DOI: 10.1046/j.1365-2222.2000.00864.x [DOI] [PubMed] [Google Scholar]
- 8.Sheikh A, Shehata YA, Brown SG et al. Adrenaline for the treatment of anaphylaxis: cochrane systematic review. Allergy 2009;64:204–12. DOI: 10.1111/j.1398-9995.2008.01926.x [DOI] [PubMed] [Google Scholar]
- 9. Clark S, Bock SA, Gaeta TJ, et al. Multicenter study of emergency department visits for food allergies. J Allergy Clin Immunol 2004;113:347–52. DOI: 10.1016/j.jaci.2003.10.053#2. * This reference is one of the earliest multi-center studies to describe low rates of epinephrine use among EDs in the United States to treat food-induced anaphylaxis.
- 10.Clark S, Long AA, Gaeta TJ, et al. Multicenter study of emergency department visits for insect sting allergies. J Allergy Clin Immunol 2005;116:643–9. DOI: 10.1016/j.jaci.2005.06.026 [DOI] [PubMed] [Google Scholar]
- 11.Campbell RL, Li JT, Nicklas RA, et al. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol 2014;113:599–608. DOI: 10.1016/j.anai.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 12.Kemp SF, Lockey RF, Simons FE, et al. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 2008;63:1061–70. DOI: 10.1111/j.1398-9995.2008.01733.x [DOI] [PubMed] [Google Scholar]
- 13.Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010;126:477–80 e1–42. DOI: 10.1016/j.jaci.2010.06.022 [DOI] [PubMed] [Google Scholar]
- 14.Joint Task Force on Practice Practice Parmeters. The diagnosis and management of anaphylaxis: an updated practice parameter. J Allergy Clin Immunol 2005;115:S483–523. DOI: 10.1016/j.jaci.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 15.Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014;69:1026–45. DOI: 10.1111/all.12437 [DOI] [PubMed] [Google Scholar]
- 16.Alrasbi M, Sheikh A. Comparison of international guidelines for the emergency medical management of anaphylaxis. Allergy 2007;62:838–41. DOI: 10.1111/j.1398-9995.2007.01434.x [DOI] [PubMed] [Google Scholar]
- 17.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372:n160. DOI: 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Migliavaca CB, Stein C, Colpani V, et al. Quality assessment of prevalence studies: a systematic review. J Clin Epidemiol 2020;127:59–68. DOI: 10.1016/j.jclinepi.2020.06.039 [DOI] [PubMed] [Google Scholar]
- 19.Munn Z, Moola S, Riitano D, et al. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag 2014;3:123–8. DOI: 10.15171/ijhpm.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015;13:147–53. DOI: 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 21.Barker TH, Migliavaca CB, Stein C, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol 2021;21:189. DOI: 10.1186/s12874-021-01381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014;72:39. DOI: 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerji A, Rudders SA, Corel B, et al. Repeat epinephrine treatments for food-related allergic reactions that present to the emergency department. Allergy Asthma Proc 2010;31:308–16. DOI: 10.2500/aap.2010.31.3375 [DOI] [PubMed] [Google Scholar]
- 24.Clark S, Boggs KM, Balekian DS, et al. Changes in Emergency Department Concordance with Guidelines for the Management of Food-Induced Anaphylaxis: 1999–2001 versus 2013–2015. J Allergy Clin Immunol Pract 2019;7:2262–9. DOI: 10.1016/j.jaip.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 25.Ducharme L, Gabrielli S, Clarke AE, et al. Tree nut-induced anaphylaxis in Canadian emergency departments: Rate, clinical characteristics, and management. Ann Allergy Asthma Immunol 2022;129:335–341. DOI: 10.1016/j.anai.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 26.Fleming JT, Clark S, Camargo CA, et al. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract 2015;3:57–62. DOI: 10.1016/j.jaip.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 27.Gabrielli S, Clarke AE, Morris J, et al. Fruit-Induced Anaphylaxis: Clinical Presentation and Management. J Allergy Clin Immunol Pract 2021;9:2825–2830.e2. DOI: 10.1016/j.jaip.2021.02.055 [DOI] [PubMed] [Google Scholar]
- 28.Kim SL, Suresh R, Mayampurath A, et al. Increase in Epinephrine Administration for Food-Induced Anaphylaxis in Pediatric Emergency Departments From 2007 to 2015. J Allergy Clin Immunol Pract 2022;10:200–5.e1. DOI: 10.1016/j.jaip.2021.09.024 [DOI] [PubMed] [Google Scholar]
- 29.Rehimini S, Gabrielli S, Langlois A, et al. Specific IgE antibody levels during and after food-induced anaphylaxis. Clin Exp Allergy 2021;51:364–368. DOI: 10.1111/cea.13796 [DOI] [PubMed] [Google Scholar]
- 30.Ross MP, Ferguson M, Street D, et al. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J Allergy Clin Immunol 2008;121:166–171. DOI: 10.1016/j.jaci.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 31.Rudders SA, Banerji A, Vassallo MF, et al. Trends in pediatric emergency department visits for food-induced anaphylaxis. J Allergy Clin Immunol 2010;126:385–388. DOI: 10.1016/j.jaci.2010.05.018 [DOI] [PubMed] [Google Scholar]
- 32.Rudders SA, Banerji A, Corel B, et al. Multicenter study of repeat epinephrine treatments for food-related anaphylaxis. Pediatrics 2010;125:e711–8. DOI: 10.1542/peds.2009-2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sehayek D, Gold MS, Gabrielli S, et al. Seafood-induced anaphylaxis in children presenting to Canadian emergency departments: Rates, clinical presentation, and management. Ann Allergy Asthma Immunol 2022;128:583–588. DOI: 10.1016/j.anai.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Sillcox C, Gabrielli S, Clarke AE, et al. Sesame-induced anaphylaxis in pediatric patients from the cross-Canada anaphylaxis registry. Ann Allergy Asthma Immunol 2022;129:342–6. DOI: 10.1016/j.anai.2022.06.005 [DOI] [PubMed] [Google Scholar]
- 35.Clark S, Boggs KM, Balekian DS, et al. Changes in emergency department concordance with guidelines for the management of stinging insect-induced anaphylaxis: 1999–2001 vs 2013–2015. Ann Allergy Asthma Immunol 2018;120:419–23. DOI: 10.1016/j.anai.2018.01.029 [DOI] [PubMed] [Google Scholar]
- 36.Rudders SA, Clark S, Wei W, et al. Longitudinal study of 954 patients with stinging insect anaphylaxis. Ann Allergy Asthma Immunol 2013;111:199–204. DOI: 10.1016/j.anai.2013.06.020 [DOI] [PubMed] [Google Scholar]
- 37.Arroyo AC, Robinson LB, Cash RE, et al. Trends in Emergency Department Visits and Hospitalizations for Acute Allergic Reactions and Anaphylaxis Among US Older Adults: 2006–2014. J Allergy Clin Immunol Pract 2021;9:2831–43. DOI: 10.1016/j.jaip.2021.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asai Y, Yanishevsky Y, Clarke A, et al. Rate, triggers, severity and management of anaphylaxis in adults treated in a Canadian emergency department. Int Arch Allergy Immunol 2014;164:246–52. DOI: 10.1159/000365631 [DOI] [PubMed] [Google Scholar]
- 39.Baalmann DV, Hagan JB, Li JT, et al. Appropriateness of epinephrine use in ED patients with anaphylaxis. Am J Emerg Med 2016;34:174–9. DOI: 10.1016/j.ajem.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 40.Calderón E, Méndez J, Nazario S. Anaphylaxis diagnosis and treatment at an emergency department in Puerto Rico. P R Health Sci J 2013;32:170–4. [PubMed] [Google Scholar]
- 41.Carrillo-Martin I, Gonzalez-Estrada A, Funni SA, et al. Increasing Allergy-Related Emergency Department Visits in the United States, 2007 to 2015. J Allergy Clin Immunol Pract 2020;8:2983–88. DOI: 10.1016/j.jaip.2020.05.056 [DOI] [PubMed] [Google Scholar]
- 42.Castilano A, Sternard B, Cummings ED, et al. Pitfalls in anaphylaxis diagnosis and management at a university emergency department. Allergy Asthma Proc 2018;39:316–321. DOI: 10.2500/aap.2018.39.4144 [DOI] [PubMed] [Google Scholar]
- 43.Chiang D, Ade JM, Liu XW, et al. Assessment of ED triage of anaphylaxis patients based on the Emergency Severity Index. Am J Emerg Med 2021;46:449–55. DOI: 10.1016/j.ajem.2020.10.057 [DOI] [PubMed] [Google Scholar]
- 44.Cohen N, Test G, Scolnik D. Predictors of epinephrine undertreatment in anaphylaxis—the experience of a busy North American tertiary care pediatric emergency department. J Allergy Clin Immunol Pract 2021;9:2090–2. DOI: 10.1016/j.jaip.2020.12.063 [DOI] [PubMed] [Google Scholar]
- 45.Gabrielli S, Clarke A, Morris J, et al. Evaluation of Prehospital Management in a Canadian Emergency Department Anaphylaxis Cohort. J Allergy Clin Immunol Pract 2019;7:2232–8. DOI: 10.1016/j.jaip.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 46.Gaeta TJ, Clark S, Pelletier AJ, et al. National study of US emergency department visits for acute allergic reactions, 1993 to 2004. Ann Allergy Asthma Immunol 2007;98:360–5. DOI: 10.1016/S1081-1206(10)60883-6 [DOI] [PubMed] [Google Scholar]
- 47.Goetz VL, Kim K, Stang AS. Pediatric Anaphylaxis in the Emergency Department: Clinical Presentation, Quality of Care, and Reliability of Consensus Criteria. Pediatr Emerg Care 2019;35:28–31. DOI: 10.1097/PEC.0000000000001136 [DOI] [PubMed] [Google Scholar]
- 48.Hemler JA, Sharma HP. Management of children with anaphylaxis in an urban emergency department. Ann Allergy Asthma Immunol 2017;118:381–3. DOI: 10.1016/j.anai.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 49.Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: A 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol 2016;137:1888–90. DOI: 10.1016/j.jaci.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 50.Huang F, Chawla K, Järvinen KM, et al. Anaphylaxis in a New York City pediatric emergency department: Triggers, treatments, and outcomes. J Allergy Clin Immunol 2012;129:162–8. DOI: 10.1016/j.jaci.2011.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee AYM, Enarson P, Clarke AE, et al. Anaphylaxis across two canadian pediatric centers: Evaluating management disparities. J Asthma and Allergy 2017;10:1–7. DOI: 10.2147/JAA.S123053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X, Lee S, Lohse CM, et al. Biphasic Reactions in Emergency Department Anaphylaxis Patients: A Prospective Cohort Study. J Allergy Clin Immunol Prac 2020;8:1230–8. DOI: 10.1016/j.jaip.2019.10.027 [DOI] [PubMed] [Google Scholar]
- 53.Manivannan V, Campbell RL, Bellolio AF, et al. Factors associated with repeated use of epinephrine for the treatment of anaphylaxis. Ann Allergy Asthma Immunol 2009;103:395–400. DOI: 10.1016/s1081-1206(10)60358-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meir LR, Habbsa S, Waqar O, et al. Anaphylaxis among elderly emergency department patients in a large health system in New York. Ann Allergy Asthma Immunol 2022;129:63–70. DOI: 10.1016/j.anai.2022.03.020 [DOI] [PubMed] [Google Scholar]
- 55.Owusu-Ansah S, Badaki O, Perin J, et al. Under Prescription of Epinephrine to Medicaid Patients in the Pediatric Emergency Department. Glob Pediatr Health 2019;6:2333794×19854960. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell S, Monroe K, Losek JD. Anaphylaxis management in the pediatric emergency department: Opportunities for improvement. Pediatr Emerg Care 2010;26:71–6. DOI: 10.1097/PEC.0b013e3181ce2e1c [DOI] [PubMed] [Google Scholar]
- 57.Sidhu N, Jones S, Perry T, et al. Evaluation of Anaphylaxis Management in a Pediatric Emergency Department. Pediatr Emerg Care 2016;32:508–513. DOI: 10.1097/PEC.0000000000000864 [DOI] [PubMed] [Google Scholar]
- 58.Tiyyagura GK, Arnold L, Cone DC, et al. Pediatric anaphylaxis management in the prehospital setting. Prehosp Emerg Care 2014;18:46–51. DOI: 10.3109/10903127.2013.825352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trainor JL, Pittsenbarger ZE, Joshi D, et al. Outcomes and Factors Associated with Prehospital Treatment of Pediatric Anaphylaxis. Pediatr Emerg Care 2022;38:E69–E74. DOI: 10.1097/PEC.0000000000002146 [DOI] [PubMed] [Google Scholar]
- 60.Wright CD, Longjohn M, Lieberman PL, et al. An analysis of anaphylaxis cases at a single pediatric emergency department during a 1-year period. Ann Allergy Asthma Immunol 2017;118:461–4. DOI: 10.1016/j.anai.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 61.Patel N, Chong KW, Yip AYG, et al. Use of multiple epinephrine doses in anaphylaxis: A systematic review and meta-analysis. J Allergy Clin Immunol 2021;148:1307–15. DOI: 10.1016/j.jaci.2021.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: A 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol 2016;137:1888–9. DOI: 10.1016/j.jaci.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 63.Grabenhenrich LB, Dolle S, Rueff F, et al. Epinephrine in Severe Allergic Reactions: The European Anaphylaxis Register. J Allergy Clin Immunol Pract 2018;6:1898–1906. DOI: 10.1016/j.jaip.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 64.Pouessel G, Antoine M, Lejeune S, et al. The time course of anaphylaxis manifestations in children is diverse and unpredictable. Clin Exp Allergy 2020;50:117–120. DOI: 10.1111/cea.13510 [DOI] [PubMed] [Google Scholar]
- 65.Castilano A, Sternard B, Cummings ED, et al. Pitfalls in anaphylaxis diagnosis and management at a university emergency department. Allergy Asthma Proc 2018;39:316–21. DOI: 10.2500/aap.2018.39.4144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.