Abstract
Background
Specialized valve endothelial cell (VEC) populations are localized oriented to blood flow in developing aortic and mitral valves, but their roles in valve development and disease are unknown. In the aortic valve (AoV), a population of VECs on the fibrosa side expresses the transcription factor Prox1 together with genes found in lymphatic ECs. In this study, we examine Prox1’s role in regulating a lymphatic-like gene network and promoting VEC diversity required for the development of the stratified trilaminar extracellular matrix (ECM) of murine AoV leaflets.
Methods
To determine if disruption of Prox1 localization affects heart valve development, we generated mice (NFATc1enCre Prox1 GoF) in which Prox1 is overexpressed on the ventricularis side of the AoV beginning in embryonic development. To identify potential targets of Prox1, we performed CUT&RUN on WT and NFATc1enCre Prox1 GoF AoVs with validation by colocalization in vivo using RNA in situ hybridization in NFATc1enCre Prox1 GoF AoVs. Natural induction of Prox1 and target gene expression was evaluated in myxomatous AoVs in a mouse model of Marfan syndrome (Fbn1C1039G/+).
Results
The overexpression of Prox1 is sufficient to cause enlargement of AoVs by postnatal day (P)0, as well as a decrease in ventricularis-specific gene expression and disorganized interstitial ECM layers at P7. We identified potential targets of Prox1 known to play roles in lymphatic ECs including Flt1, Efnb2, Egfl7, and Cx37. Ectopic Prox1 colocalized with induced Flt1, Efnb2, and Cx37 expression in NFATc1enCre Prox1 GoF AoVs. Moreover, in Marfan syndrome myxomatous AoVs, endogenous Prox1, and its identified targets, were ectopically induced in ventricularis side VECs.
Conclusions
Our results support a role for Prox1 in localized lymphatic-like gene expression on the fibrosa side of the AoV. Furthermore, localized VEC specialization is required for development of the stratified trilaminar ECM critical for AoV function and is dysregulated in congenitally malformed valves.
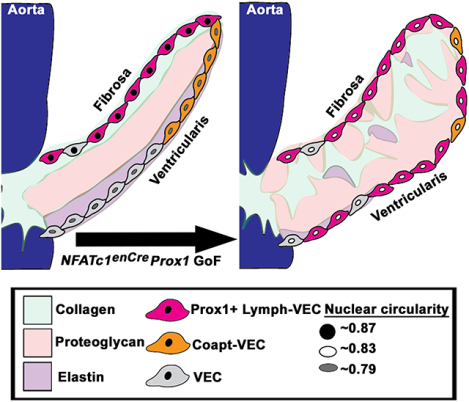
Graphical Abstract
Introduction
Representing a significant public health burden, approximately 2.5% of individuals in the USA are diagnosed with valvular heart disease, with frequency increasing with age1. Heart valves have the critical function of ensuring blood moves unidirectionally through the heart. Mature heart valves consist of internal layers of stratified extracellular matrix (ECM), composed primarily of collagens, proteoglycans, and elastin, interspersed with valve interstitial cells (VICs) and surrounded by a monolayer of valve endothelial cells (VECs). Congenital myxomatous valve disease, such as occurs in Marfan syndrome (MFS), is typically characterized by malformed leaflets and loss of ECM organization including increased deposition of collagens and proteoglycans2. Little is known about the contributions of VECs to valve morphogenesis and disease, as most studies focus on VICs which are largely responsible for producing ECM proteins3. Interestingly, recent single-cell RNA sequencing of remodeling heart valves in mice demonstrated regional specialization of VECs4. One of these populations shares features with lymphatic ECs, including expression of the transcription factor Prox1. However, the specific functions of specialized VECs, as defined by localized transcriptional regulators such as Prox1, have not been previously reported.
Specialized VECs on the ventricularis and fibrosa sides of the aortic valve (AoV) leaflet demonstrate differential gene expression, potentially in response to localized fluid forces, soon after endocardial cushion formation2,4. Beginning approximately at embryonic day (E)12.5, the ventricularis side VECs express markers such as Klf2, Wnt9B, and Notch15,6. Meanwhile VECs on the fibrosa side express markers such as Prox1, Cx37, and Efnb22,4,7. Postnatally, genetically distinct VEC populations continue to be localized to opposite sides of the AoV leaflet4. However, the potential contributions of localized VEC populations in driving the development of the stratified trilaminar ECM structure, as well as the disorganization of ECM found in valve disease, are not known. One of these specialized VEC populations (Lymphatic-VEC (Lymph-VEC)) expresses multiple genes characteristic of the lymphatic ECs, including Prox1 (Figure S1)4,7. In both the developing and adult AoVs, Prox1 is localized to the fibrosa side of the leaflet adjacent to the collagen ECM layer where VECs are subject to low bidirectional blood-flow8,9. Prox1 is not expressed in the Coaptation-VEC (Coapt-VEC) population on the opposite side of the AoV leaflet where the leaflets come together (Figure S1)4. The Coapt-VECs are subject to a combination of high unidirectional blood-flow when the valve is open and pressure when the valve is closed4,8. The importance of localized Prox1 and its influence on specialized VEC populations and overall AoV organization has not been demonstrated previously.
Prox1 is homeobox transcription factor with key roles in the development of multiple organ systems. One of Prox1’s roles is to regulate progenitor cell differentiation by interacting with multiple regulatory pathways including NFATc1, Klf2, and Notch110–12. During development, Prox1 has critical functions in muscle, central nervous system, pancreas, and lymphatic system10,12. The role of Prox1 in the lymphatic system is particularly interesting as Prox1 required for the differentiation of vein ECs into lymphatic ECs. Furthermore, Prox1 is upregulated in lymphatic ECs in sites of future valve development in response to low bidirectional flow, similar to the blood-flow pattern the Prox1+ Lymph-VECs experience in the heart9. Interestingly, many of the genes that are differentially expressed in the Prox1+ Lymph-VEC population have known roles in lymphatic valve development, suggesting that Prox1 may be regulating these same genes in cardiac valve development4,7.
In this study, we aimed to evaluate the role of Prox1 and specialized VEC populations in heart valve development and disease. In parallel, mice were generated with exogenous ectopic Prox1 expression in the AoV, and increased endogenous ectopic Prox1 expression was observed in the myxomatous AoV of a Fbn1C1039G/+ MFS mouse model. In addition, Prox1 transcriptional targets were identified using CUT&RUN and validated in remodeling and diseased AoVs using RNA in situ hybridization. Altogether, these studies demonstrate the importance of localized Prox1 in VEC specialization during normal and abnormal development of AoV, as well as identify AoV-specific Prox1 targets.
Materials and Methods
Data availability
The authors declare that all supporting data are available within the article and its supplementary files. The corresponding author may provide additional data supporting the findings of this study upon reasonable request. Cleavage under targets and release using nuclease (CUT&RUN) files have been deposited in GEO under accession number GSE231336.
Mice
All animals were maintained in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. The Cincinnati Children’s Medical Center Animal Care and Use Committee (IACUC2020–0060) approved all animal procedures which were also performed according to institutional guidelines. Both sexes were used for all analyses, with no differences observed between males and females.
Fbn1C1039G/+ mice were purchased from the Jackson Laboratory (stock no. 012885). NFATc1enCre Prox1 gain-of-function (GoF) mice were generated by crossing NFATc1-enhancer Cre transgenic male mice (NFATc1enCre) with a conditional Prox1-overexpressing knock-in transgenic mouse line (Prox1 KI) to produce NFATc1enCre Prox1KI/+ GoF offspring. The NFATc1enCre transgenic mouse line was previously described and was obtained from Dr. Bin Zhou (Albert Einstein College of Medicine)13. The Prox1 KI transgenic mouse line has also been previously described and was obtained from Dr. Zhiyong Liu (Chinese Academy of Sciences)14. All mice were maintained on a C57BL/6 background.
Histology, immunofluorescence, and RNA in situ hybridization
Murine hearts at embryonic, postnatal, and adult timepoints were harvested and processed for paraffin embedding. Female mice were checked for plugs prior to 12pm and positive plugs were considered at embryonic day (E) 0.5. Hearts were fixed in 4% paraformaldehyde (PFA) overnight at 4°C, dehydrated in a graded ethanol series and cleared in xylenes before being embedded in paraffin wax. Hearts were sectioned at either 5 or 7μm using a Leica RM2235 microtome. For histology and immunofluorescence studies, slides were baked for 1hour at 60°C, cleared in xylenes, and rehydrated in a graded ethanol series.
For all histology studies, murine AoVs were stained with Russell-Movat pentachrome according to the manufacturer’s protocol (StatLab Medical Products, Ref# KTRMPPT). Slides were submerged in Verhoeff’s Elastic Stain for 20minutes, Alcian Blue for 30minutes, Crocein Scarlet for 2minutes, and Alcoholic Saffron Solution for 15minutes. To calculate the average leaflet area per section, a minimum of two slides for E12.5 and E14.5 timepoints and a minimum of 3 slides for all later timepoints were blindly chosen from experimental and littermate controls. A minimum of 4 sections were randomly chosen from each slide and stained with Russell-Movat pentachrome and imaged at 20x. Images were captured using Olympus BX51 microscope either retrofitted with the Nikon DS-Ri1 camera and DS-U3 controller or with the DS-F13 camera. Both cameras were operated with NIS-Elements BR 3.2 software. Each AoV leaflet was manually traced in ImageJ FIJI (Version: 2.9.0/1.53t) and the average area per section was calculated.
For all immunofluorescence studies, slides were submerged in 3% H2O2 for 20minutes before being boiled for 5 minutes in a citrate-based Antigen Unmasking Solution (Vector Laboratories H-3300–250), washed in 1% TritonX-100, and incubated with the antibodies outlined in Table S1 overnight at 4°C. For the fluorescent detection of antibody staining, Alexa Fluor-488 or −568 conjugated secondary antibodies (Abcam, Life Technologies) were used at a 1:400 dilution for 2hours at room temperature (Table S1). Nuclei were counterstained with DAPI (4’, 6- Diamidino- 2-Phenylindole) (Life Technologies, 1:10,000) for 20minutes at room temperature and mounted in VECTASHIELD® HardSet™ Antifade Mounting Medium (Vector Laboratories, H-1400). Images were captured using a Nikon A1-R confocal system with NIS-Elements D 3.2 software. To quantify ectopic Prox1+ cells in NFATc1encre Prox1 GoF or Fbn1C1039G/+ MFS valves, a minimum of 2 slides equally spaced in the middle of the valve were blindly chosen from experimental and littermate controls. A minimum of 2 sections were blindly chosen from each slide and co-stained for CD31 (an endothelial marker) and Prox1 (Table S1). The number of Prox1+ CD31+ cells on the ventricularis side of the AoV leaflets was then manually counted in ImageJ FIJI (Version: 2.9.0/1.53t) and the average number of ectopic Prox1 per section was calculated. To quantify the average number of AoV nuclei per section, a minimum of 3 sections on a minimum of 3 equally spaced slides were blindly selected and manually counted in ImageJ FIJI (Version: 2.9.0/1.53t). Similarly, the average number of pHH3+ or TUNEL+ nuclei was quantified. Briefly, 3 sections from 1 slide from a similar location for each P0 NFATc1enCre Prox1 GoF and littermate control AoV were stained for pHH3 or TUNEL (TUNEL Label Mix, Roche, 11767291910 according to the manufacturer’s protocol), co-stained with DAPI, and manually counted in ImageJ FIJI (Version: 2.9.0/1.53t). The amount of CHP or HABP immunostaining in NFATc1enCre Prox1 GoF or Control AoVs was quantified as the total number of CHP+ or HABP+ pixels per leaflet area per section in a minimum of 4 equally spaced sections using the NIS-Elements AR (version 5.20.00) software. To calculate endothelial nuclear circularity, the perimeter and area of 12 DAPI-stained CD31+ nuclei from 1 slide from a minimum of 2 sections were measured in ImageJ FIJI (Version: 2.9.0/1.53t). Nuclear circularity was calculated using the following equation as previously described15:
A circularity closer to 0 indicates a more elongated nuclei while a circularity closer to 1 indicates rounder nuclei.
All RNA in situ hybridization analyses were performed using an RNA in situ hybridization (ISH) assay (ACD- a Bio-Techne brand, RNAscope® Multiplex Fluorescent Reagent Kit v2, Cat. # 323100) according to manufacturer’s instructions (RNAscope® Multiplex Fluorescent Reagent Kit v2 User Manual). Slides were baked for 1hour at 60°C, cleared in xylenes and submerged in 100% ethanol. Sections were then outlined with a hydrophobic barrier pen and treated with RNAscope Hydrogen Peroxide for 10minutes before being submerged in heated RNAscope Antigen Retrieval buffer for 15minutes. Sections were then treated with RNAscope Protease Plus for 30minutes at 40°C. Each probe solution was prepared by adding 1 volume of C2 probe and/or C3 probe to 50 volumes of C1 probe (RNAscope in situ Probe, Table S1). The probe solution was then warmed at 40°C for 10minutes and cooled to room temperature. After slides were submerged in water, the probe solution was added to each section for 2hours at 40°C. After being washed in RNAscope Wash Buffer, slides were stored in 5x Sodium Chloride-Sodium Citrate Buffer (SCC) overnight at room temperature. Each probe signal was increased using a series of amplification solutions after which each channel was assigned either TSA Plus Fluorescein (Perkin Elmer, NEL741001KT), TSA Plus Cyanine 3 (Perkin Elmer, NEL744001KT), or TSA Plus Cyanine 5 (Perkin Elmer, NEL745001KT) at a 1:1500 dilution. After washing, nuclei were counterstained with DAPI (4’, 6- Diamidino- 2-Phenylindole) (Life Technologies, 1:10,000) for 20minutes at room temperature and mounted in VECTASHIELD® HardSet™ Antifade Mounting Medium (Vector Laboratories, H-1400). Images were captured using a Nikon A1-R confocal system with NIS-Elements D 3.2 software.
3D Valve reconstruction
NFATc1enCre; Prox1KI/+ GoF and littermate control (Prox1KI/+ or NFATc1enCre) hearts were harvested at P0 and processed for paraffin embedding. Hearts were fixed in 4% paraformaldehyde (PFA) overnight at 4°C, dehydrated in a graded ethanol series, and cleared in xylenes before being embedded in paraffin wax. Hearts were then sectioned at 5μm using a Leica RM2235 microtome. All sections containing AoV leaflets were collected in order and stained with Russell-Movat pentachrome according to the manufacturer’s protocol (StatLab Medical Products, Ref# KTRMPPT). Serial sections containing the murine AoV were then imaged using the Olympus BX51 microscope retrofitted with the Nikon DS-Ri1 camera and DS-U3 controller or with the DS-F13 camera along with NIS-Elements BR 3.2 software at 20x. The serial section images were aligned using ImageJ FIJI (Version: 2.9.0/1.53t) and imported into Imaris 10.0. The AoV was reconstructed by manually tracing each section of AoV leaflet in the Imaris 10.0 software to create a unique surface. These traces were then combined to reveal the 3D structure and corresponding volume.
Cleavage under targets and release using nuclease (CUT&RUN)
Mice were euthanized with isoflurane and sacrificed using cervical dislocation. Murine hearts were harvested quickly and washed in ice-cold PBS to remove blood. Each AoV was carefully and quickly isolated under a microscope. The tissue was than disassociated to optimize VEC isolation with a light digestion buffer. For V5-tagged NFATc1enCre Prox1 GoF CUT&RUN, 4 AoVs (female=1, male=3) were placed in 400μl digestion buffer (composed of 2mg/mL collagenase type IV (Worthington Biochemical Corporation), 1.2 U/mL dispase II (Sigma-Aldrich) in Hanks’ Balanced Salt Solution (HBSS)) for 20minutes at 37°C with rocking. Supernatant was collected and placed in a separate tube. 400μl of fresh digestion buffer was added to AoVs and rocked for 20minutes at 37°C and the supernatant was collected again. This process was repeated for a total of 3 times. The 1600μl of collected supernatant was than filtered using a 40μL cell strain and centrifuged at 400g for 10minutes. The supernatant was removed and discarded. The cell pellet was resuspended in 500μL ice-cold PBS. The cells were counted using a Bio-Rad TC20 Automated Counter. The H3K27ac CUT&RUN was completed using a similar process, in which 17 control mice (female=7, male=10) were used and 1% trypsin was added to the digestion buffer for the last two 20minute incubations.
CUT&RUN experiments were performed according to Meers et. al. 2019 with minor modifications16. Briefly, nuclei were isolated from ~250,000 VECs by resuspending cells in ice-cold NE buffer [20mM HEPES, pH 7.9, 10mM KCl, 0.1% Triton X-100, 20% Glycerol, 1mM MnCl2, supplemented with protease inhibitors and 0.5mM Spermidine]. Cells were incubated on ice for 10minutes, and then centrifuged at 600xg for 3 min at 4°C. The supernatant was discarded and the nuclear pellet resuspended in 100μl NE buffer per reaction. Simultaneously, Concanavalin A beads [PolySciences, 86057] were activated by washing in ice cold Bead Activation Buffer [20mM HEPES pH 7.9, 10mM KCl, 1mM CaC2, 1mM MnCl2] twice. After two washes, activated ConA beads were resuspended in 10μl/reaction Bead Activation Buffer and kept on ice until needed. After nuclei isolation, 100μl of nuclei were added to 10μl activated ConA beads and the bead-nuclei slurry was incubated at RT for 10 minutes for cells to adsorb. The beads were then pelleted on a magnet, and the supernatant was discarded. 50μl/reaction Antibody Buffer [20mM HEPES pH 7.5, 150mM NaCl, 0.5mM Spermidine, 0.01% digitonin, 2mM EDTA supplemented with fresh protease inhibitors] was added to each reaction and the beads were gently vortexes to thoroughly resuspend. The following antibodies were added: V5 (Invitrogen, 46–0705), mouse IGG (Milipore Sigma, 12–371), H3K27ac (Active Motif, 39133), or Rabbit IGG (Cell Signaling Technology, 2729). The bead-antibody reactions were incubated on a rotator overnight at 4°C. The next day, the beads were washed twice with Wash Buffer [20mM HEPES pH 7.5, 150mM NaCl, 0.5mM Spermidine, 0.01% digitonin]. The beads were then pelleted and 2.5μl pAG-MNase [Epicypher, Cat#15–1016] was added to each IP in 50μl of Wash buffer and incubated for 10 minutes at RT to allow pAG-MNase to bind to the bead slurry. After 10 minutes, the bead slurry was washed twice with Wash Buffer and targeted chromatin digestion was performed by adding 1μl 100mM CaCl2 per reaction in 50μl Wash Buffer. The beads were then rotated for 2 hours at 4°C for chromatin release to occur. After 2 hours, 33μl of STOP buffer [340mM NaCl, 20mM EDTA, 4mM EGTA, 50μg/ml RNase A, 50μg/ml Glycogen] was added per reaction, and the beads were incubated for 10 min at 37°C. The beads were pelleted to collect supernatant, and DNA was purified using the Minelute PCR Purification Kit [Qiagen, 28004]. DNA concentration was measured using the Qubit fluorometer and libraries were constructed using the CUTANA CUT&RUN Library Prep kit [Epicypher, 14–1001] with the following modifications: 1) Enzyme activation during end repair PCR was performed at 50°C for 1 hour. 2) PCR cleanup was performed using 1.8x reaction volume of SPRI beads. DNA was eluted in a final volume of 10μl 0.1xTE buffer and libraries were pooled in equimolar concentration. Purified libraries were sequenced using a NextSeq 500 instrument with a 2×75bp read length. CUT&RUN data are publicly available with GEO accession number GSE231336.
CUT&RUN data analyses
Raw reads were quality-checked using FASTQC17, and adapters were trimmed using cutadapt. Sequences were aligned to the mm10 genome using bowtie2 v2.2.318 with the following parameters: --local, --very-sensitive, --no-mixed, --no-discordant. –q –phred33, --minIns 10 –maxIns 1000. For V5 CUT&RUN, the resultant bam files were then split to retain <120bp fragments indicative of TF binding signature. BAM files were then sorted, indexed and converted to BigWig files for data visualizing using deeptools19. Tracks were visualized using IGV20. BAM files were then converted to bedgraph files for peak calling using bedtools genomecov21. Peaks were called using SEACR22 and the following parameters: signal threshold ‘stringent’ and normalization control ‘norm’. Heatmaps were generated using deeptools computeMatrix and plotHeatmap. The MEME suite23 of tools were used for motif enrichment analysis. Briefly, 100bp across the peak summits were extracted using awk and converted to the fasta format using bedtools getfasta. De-novo motif analysis across peak sets was performed using DREME24 and default parameters, and motifs were identified using TOMTOM25. Top enriched motifs were identified based on their E-values. To determine the presence of Prox1 motifs, the Prox1 position weight matrices were downloaded from JASPAR, and sequences were scanned for the presence of the motif using FIMO. For cofactor analysis, differential motif enrichment analysis was performed using AME26. Briefly, sequences corresponding to 20 bp across the peak submit of Prox1 peaks were extracted for AME analysis. 20bp across the peak summits of IgG peaks was used as background for calculating motif enrichment.
Genes enriched for V5-tagged Prox1 binding sites (Table S2) and/or H3K27ac binding sites (Table S3) were compared to differentially expressed genes of the P7 Lymph-VEC population previously reported (GEO accession number: GSE117011, Table S4)4. P7 Lymph-VEC genes were listed in order of average log fold change (Table S4)4. Downstream functional annotation GO Enrichment Analysis for biological processes (Table S6) was performed for the 135 genes enriched for P30 V5-tagged Prox1 binding sites, P30 H3K27ac binding sites, and P7 Lymph-VEC differentially expressed genes together with the 3,974 genes enriched for P30 V5-tagged Prox1 binding sites and P30 H3K27ac binding sites (Table S5) using The Gene Ontology Resource27. A heatmap was generated depicting aggregated normalized expression counts of genes differentially enriched in Lymph-VECs compared to previously reported VEC and Coapt-VEc aggregated normalized gene counts using the pheatmap package in R (GEO accession number: GSE117011, Table S7)4,28,29.
Statistical analyses
Nonparametric Mann-Whitney U-tests were used to determine significance using PRISM9 software package (GraphPad Prism (Version 9.3.1)) for sample sizes noted in the figure legend. Significance of differences in nuclear circularity, perimeter, and area was determined using a two-way ANOVA. Individual Mann-Whitney U-tests depicted on the same graphs were separated with a dashed line. Data are reported as mean ± SEM. A p-value < 0.05 was considered statistically significant.
Results
Prox1 is localized to the fibrosa side of the developing aortic valve by embryonic day 12.5
To determine when and where Prox1 is expressed in the developing AoV, we performed immunostaining on wildtype (WT) AoVs at key embryonic timepoints. Confocal immunofluorescence microscopy confirmed that Prox1 is found only on the fibrosa side of the AoV endocardial cushions by embryonic day (E) 12.5, after endothelial-to-mesenchymal transition and before the AoV primordia begin to elongate and mature (Figure S2A–B)3. Furthermore, Prox1 localization is restricted to the fibrosa side throughout fetal and postnatal development, as well as in the adult valve (Figure S2C–F). This localization of Prox1 is conserved in the pulmonary valve (Figure S2G), the mitral valve (Figure S2H), and in the AoVs of large animals, including pigs (Figure S2I). Thus, in mice, Prox1 is localized in fibrosa side VECs just prior to when the valve elongates and is maintained throughout postnatal stratification and adult homeostasis of the trilaminar ECM.
Generation of NFATc1enCre Prox1 gain-of-function mice with induction of Prox1 on the ventricularis side of the aortic valve during embryonic development
To determine the importance of Prox1 localization in AoV development, we generated mice with increased Prox1 expression on both sides of the developing valve primordia. Cardiac VEC-specific NFATc1 enhancer Cre (NFATc1enCre) mice were bred with Prox1 gain-of-function (GoF) (Rosa26-LSL-Prox1 (Prox1KI/+)) mice for Cre-dependent expression of V5-tagged Prox113,14. Prox1-mediated effects on valve leaflet morphogenesis and ECM stratification in NFATc1enCre Prox1 GoF (NFATc1enCre Prox1KI/+) and Control (NFATc1encre/+, Prox1KI/+, or WT) AoVs at embryonic, postnatal, juvenile, and adult timepoints were examined (Figure 1A). Prox1 is expressed on the ventricularis side of the AoV leaflets of NFATc1enCre Prox1 GoF mice by E12.5 and is maintained in the later stages of AoV development (Figure 1B, 1D–1F’). Additionally, V5-tagged Prox1 is expressed in VECs on both the fibrosa and ventricularis side of the AoV at E14.5 and is maintained on both sides of postnatal and juvenile AoVs (Figure S3). While NFATc1enCre Prox1 GoF AoVs are not enlarged by E14.5, there is a trend towards enlargement by E18.5 (n=5–6, p=0.1255) (Figure 1C). Together, these analyses demonstrate ectopic expression of Prox1 in NFATc1enCre Prox1 GoF ventricularis and fibrosa side VECs by E12.5 in contrast to the normal fibrosa side-specific expression of endogenous Prox1 found in AoV leaflets during embryonic development.
Figure 1. Prenatal NFATc1enCre Prox1 GoF aortic valve endothelial cells express ectopic Prox1.
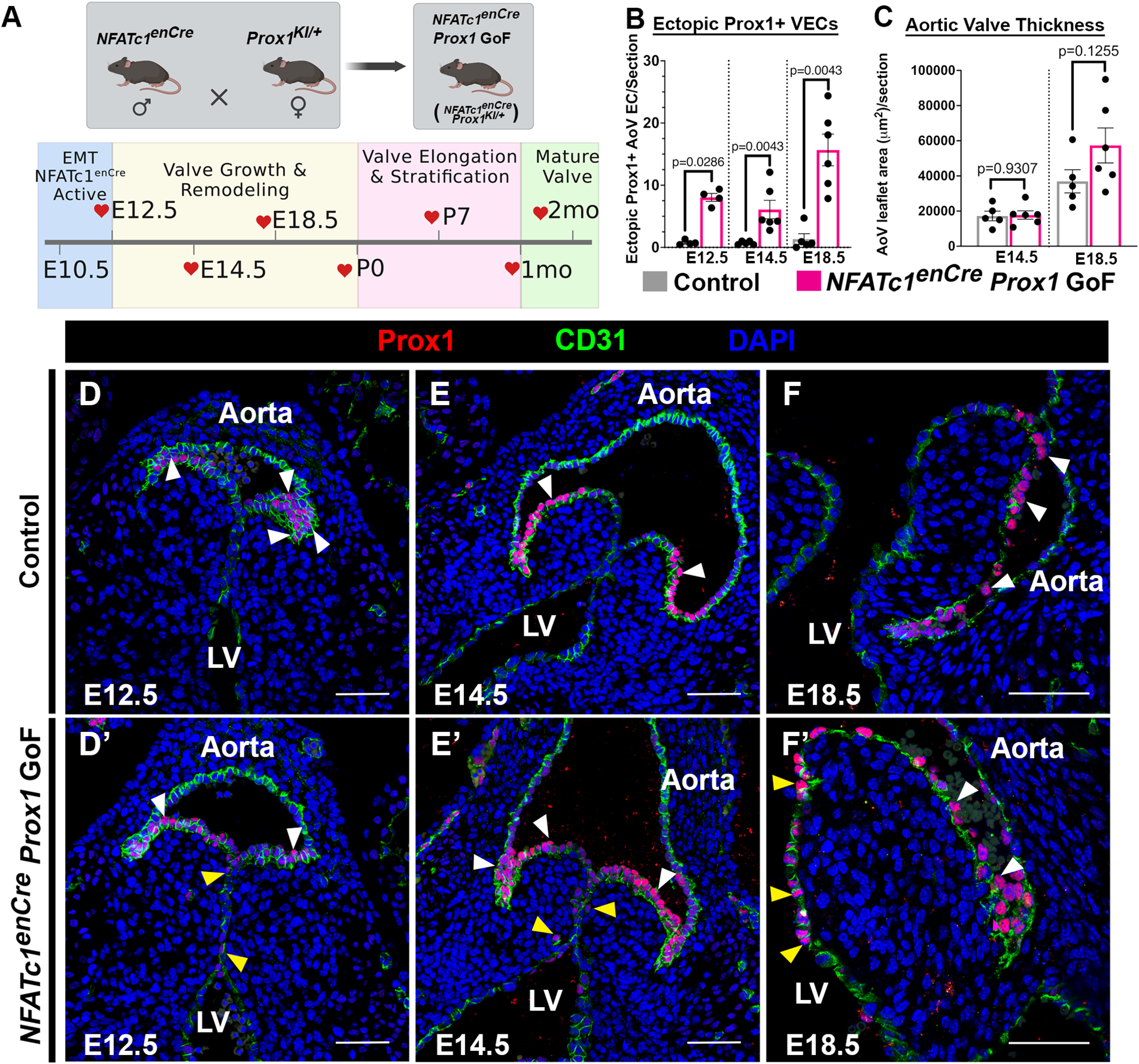
A, The NFATc1enCre mice were bred to the Rosa26-LSL-Prox1 (Prox1KI/+) mice to generate NFATc1enCre; Prox1KI/+ (NFATc1enCre Prox1 GoF) mice where Prox1 is ectopically induced in ventricularis side VECs during embryonic development. Heart valves were then collected at key embryonic, postnatal, and adult timepoints. B, The average number of ectopic Prox1+ nuclei present on the ventricularis side were quantified at embryonic (E12.5, E14.5 E18.5) timepoints. C, The average areas of NFATc1enCre Prox1 GoF and littermate control AoV leaflets were quantified at embryonic (E12.5, E14.5, E18.5) timepoints. D-F’, Representative immunofluorescence of AoV sections of Prox1+ (red) CD31+ (green) VECs in Control (D-F) and NFATc1enCre Prox1 GoF (D’-F’) AoV at key embryonic (E12.5, E14.5, E18.5) timepoints. White arrowheads indicate Prox1+ fibrosa VEC. Yellow arrowheads indicate ectopic Prox1+ ventricularis VEC adjacent to the left ventricle (LV). Black circles indicate mixed population of unknown sex (B, C). Scale bar =50μm. p-values were determined with a Mann-Whitney test with p-values<0.05 considered significant (n=4–6).
NFATc1enCre Prox1 gain-of-function aortic valves have abnormal postnatal leaflet morphogenesis
NFATc1enCre Prox1 GoF mice were born in Mendelian ratios and are viable at birth. However, NFATc1enCre Prox1 GoF mice are significantly smaller than littermate controls by 1 month-of-age (Figure S4A, S4E). Additionally, NFATc1enCre Prox1 GoF mice have significantly increased heart weight per body weight by 1 month-of-age (Figure S4B, S4F). A subset of the NFATc1enCre Prox1 GoF mice were obviously smaller than NFATc1enCre Prox1 GoF littermates with failure to thrive and increased lethality soon after weaning (Figure S4A, S4C). Pathology of deceased animals showed loss of VEC barrier and increased deposition of mucin by Movat’s pentachrome stain (Figure S4D). Mucin accumulation in the heart together with increased CD45+ immune cells detected by immunofluorescent microscopy (Figure S4G), is consistent with lethal endocarditis30. Remaining NFATc1enCre Prox1 GoF animals survived to adulthood alongside their littermate controls (Figure S4A, S4C).
NFATc1enCre Prox1 GoF AoVs maintain ectopic Prox1 on the ventricularis side of the leaflet and exhibit valve abnormalities soon after birth. The number of ectopic Prox1+ VECs are significantly increased in NFATc1enCre Prox1 GoF AoVs at postnatal and adult timepoints compared to littermate controls (Figure 2A–C’, 2F). Valve leaflet morphogenesis was evaluated at P0 in three-dimensional (3D) reconstructions of NFATc1enCre Prox1 GoF and littermate control AoVs using Movat’s pentachrome stained serial sections. The 3D reconstructions of serial sections revealed that NFATc1enCre Prox1 GoF AoVs have a significantly higher volume then littermate control AoVs by postnatal day (P)0 (Figure 2D–D’, 2E). As the AoV matures into its adult structure, NFATc1enCre Prox1 GoF leaflets continue to demonstrate a significantly larger area per section than littermate controls (Figure 2A–C’, 2G). To determine if NFATc1enCre Prox1 GoF AoVs are enlarged due to an increase in cell number, we quantified the average number of nuclei per leaflet section. NFATc1enCre Prox1 GoF AoVs exhibit a trend towards increased number of nuclei at E18.5 that becomes significant by P7 (Figure S5A). In order to determine where the additional cells come from, proliferation marker Phospho-Histone H3 (pHH3) and cell death marker TUNEL immunostaining was performed on P0 NFATc1enCre Prox1 GoF and littermate control AoVs (Figure S5B–E). No changes in proliferation or cell death were observed at this stage. Morphologically, NFATc1enCre Prox1 GoF AoVs are short and wide, in contrast to the long and thin AoVs of control littermates. Thus, NFATc1enCre Prox1 GOF AoVs are enlarged by P0 when leaflets are normally elongating and undergoing ECM stratification.
Figure 2. NFATc1enCre Prox1 GoF aortic valves are enlarged at P0.
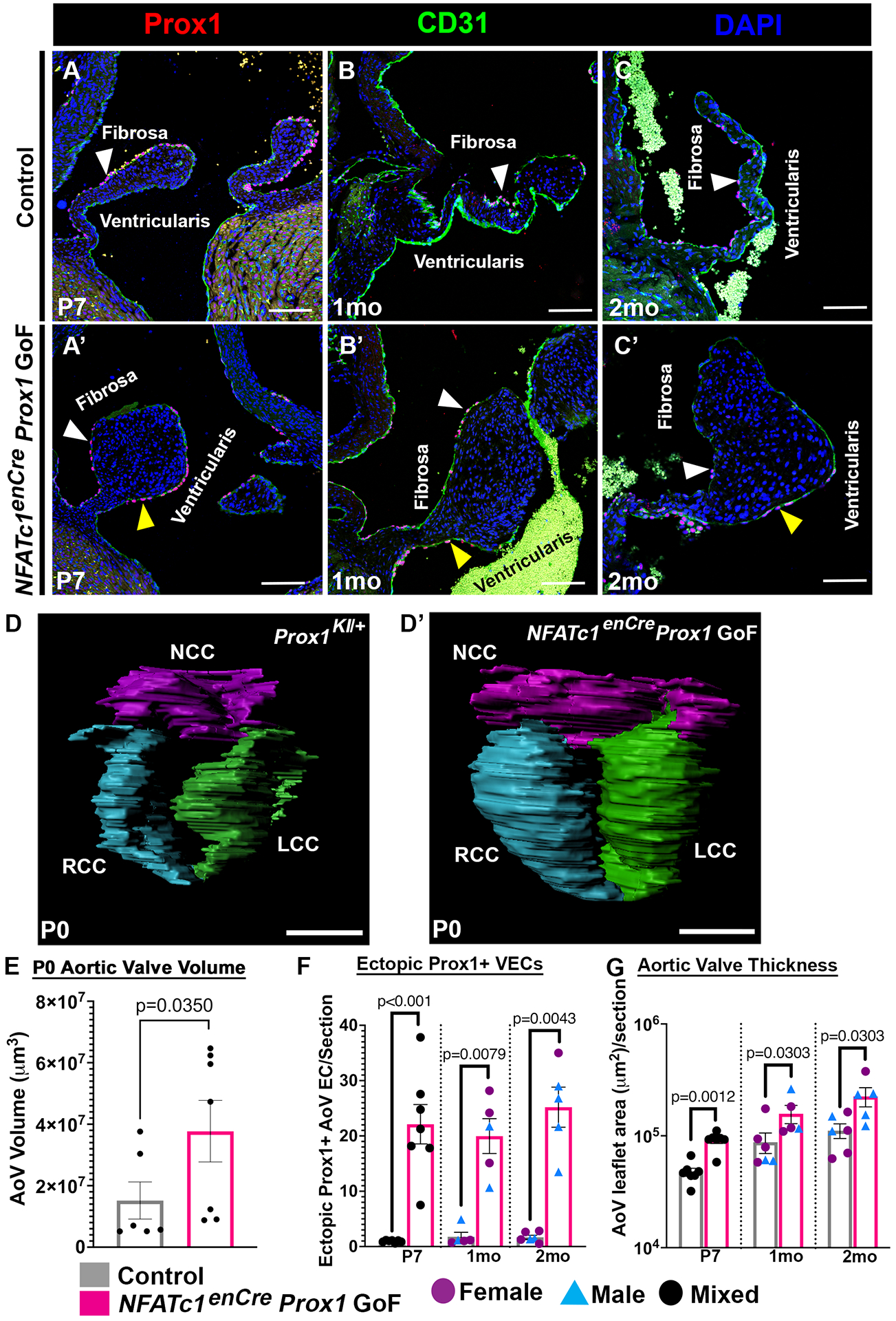
A-C’, Representative AoV sections of Prox1+ (red) CD31+ (green) VECs in Control (A-C) and NFATc1enCre Prox1 GoF (A’-C’) AoV’s at postnatal (P7), juvenile (1mo), and adult (2mo) timepoints. D-D’, Representative 3D reconstructions of serial sections of Control (D) and NFATc1enCre Prox1 GoF (D’) AoVs at P0. Non-coronary cusp (NCC, purple), Right coronary cusp (RCC, blue), and Left coronary cusp (LCC, green) are shown. E, The total volumes of NFATc1enCre Prox1 GoF and Control AoV 3D reconstructions (as shown in A-A’) for 3 leaflets combined were quantified at P0. F, The average numbers of ectopic Prox1+ nuclei in NFATc1enCre Prox1 GoF and Control AoVs were quantified at postnatal (P7), 1mo, and 2mo timepoints. G, The average areas of NFATc1enCre Prox1 GoF and Control AoV leaflets were quantified at postnatal (P7), 1mo and 2mo. White arrowheads indicate Prox1+ fibrosa side VEC. Yellow arrowheads indicate ectopic Prox1+ ventricularis side VEC. Purple circles indicate females (F, G). Blue triangles indicate males (F, G). Black circles indicate mixed population of unknown sex (E, F ,G). Scale bar =100μm. p-values were determined with a Mann-Whitney test with p-values<0.05 considered significant (n=5–7).
In addition to differential gene expression in specialized VEC populations after birth, the cell shapes of VECs on the fibrosa and ventricularis sides of the AoV leaflets in large animals have been found to have distinct morphologies31. To determine if mouse VECs have a similar difference in morphology, we analyzed nuclear circularity in NFATc1enCre Prox1 GoF and littermate control AoVs32. Control valves exhibit a significant differential circularity between the nuclei of the Prox1+ fibrosa side VECs (~0.87) and the Prox1- ventricularis side VECs (~0.79) at P7 (Figure S6A, S6B). In contrast, differential nuclear circularity is not observed on the fibrosa and ventricularis side VECs of NFATc1enCre Prox1 GoF AoVs (Figure S6A’, S6B). No significant differences were observed in nuclear area or nuclear perimeter between NFATc1enCre Prox1 GoF and littermate controls VECs (Figure S6C–D). Thus, the loss of side-specific VEC nuclear shape in NFATc1enCre Prox1 GoF AoV VECs accompanies AoV leaflet enlargement and morphological abnormalities postnatally. Together, these data suggest that the increase of Prox1 on the ventricularis side of the AoV leads to abnormalities in endothelial specialization of elongating valve leaflets after birth.
NFATc1enCre Prox1 gain-of-function aortic valves exhibit defective extracellular matrix patterning
After birth, heart valve leaflets, including AoVs, develop a distinct stratified trilaminar ECM structure, composed of layers of elastin, proteoglycans, and collagens, required for normal valve function3. In order to determine the effects of NFATc1enCre Prox1 GoF in VECs on ECM remodeling and organization, RNA in situ hybridization was performed on P7 AoV leaflets when ECM is being actively produced2. Versican (VCAN), Collagen Type 1 Alpha 1 Chain (Col1a1), and elastin (ELN) were used to visualize the proteoglycan-rich spongiosa, the collagen-rich fibrosa, and the elastin-rich ventricularis layers, respectively (Figure 3A–B’). In a normal P7 control AoV, gene expression is localized to the three distinct ECM layers (Figure 3A–A’). However, the layer-specific ECM gene expression of the P7 NFATc1enCre Prox1 GoF AoV is dysregulated. In enlarged NFATc1enCre Prox1 GoF AoVs, Col1a1 and VCAN colocalize and appear increased in the distal tip of the leaflet (Figure 3B–B’). Moreover, ELN localization is lost on the ventricularis side of the leaflet (Figure 3A–B’, pink arrow), but is misexpressed in the center of the leaflet surrounded by Col1a1-expressing interstitial cells (Figure 3A–B’, yellow arrow). At the protein level, Collagen Hybridizing Peptide (CHP) and Hyaluronan Binding Protein (HABP) were examined by immunofluorescent microscopy (Figure 3C–F’). Similar to the RNA in situ hybridization, the collagen marker CHP is normally localized the fibrosa side while the proteoglycan marker HABP is localized to the ventricularis side near the tip of the postnatal control AoV (Figure 3C–F). In contrast, the collagen marker CHP (Figure 3G) and the proteoglycan marker HABP (Figure 3H) are increased and mislocalized by P7 in the NFATc1enCre Prox1 GoF AoV leaflet (Figure 3C’–F’). Furthermore, CHP is aberrantly expressed down the center of the AoV while HABP is found at the tip of the NFATc1enCre Prox1 GoF AoV on both the fibrosa and ventricularis sides of the postnatal AoVs (Figure 3C’–F’). ECM disorganization and leaflet thickening is further shown by Movat’s pentachrome stain (Figure 3I–K’). Altogether these changes in ECM mRNA expression and protein demonstrate that Prox1 on the ventricularis side of the leaflet results in the down regulation of elastin on the ventricularis side and the misregulation of fibrillar collagen and proteoglycan compartmentalization in the abnormally remodeled and thickened AoV leaflets.
Figure 3. ECM stratification is disrupted in NFATc1enCre Prox1 GoF AoVs.
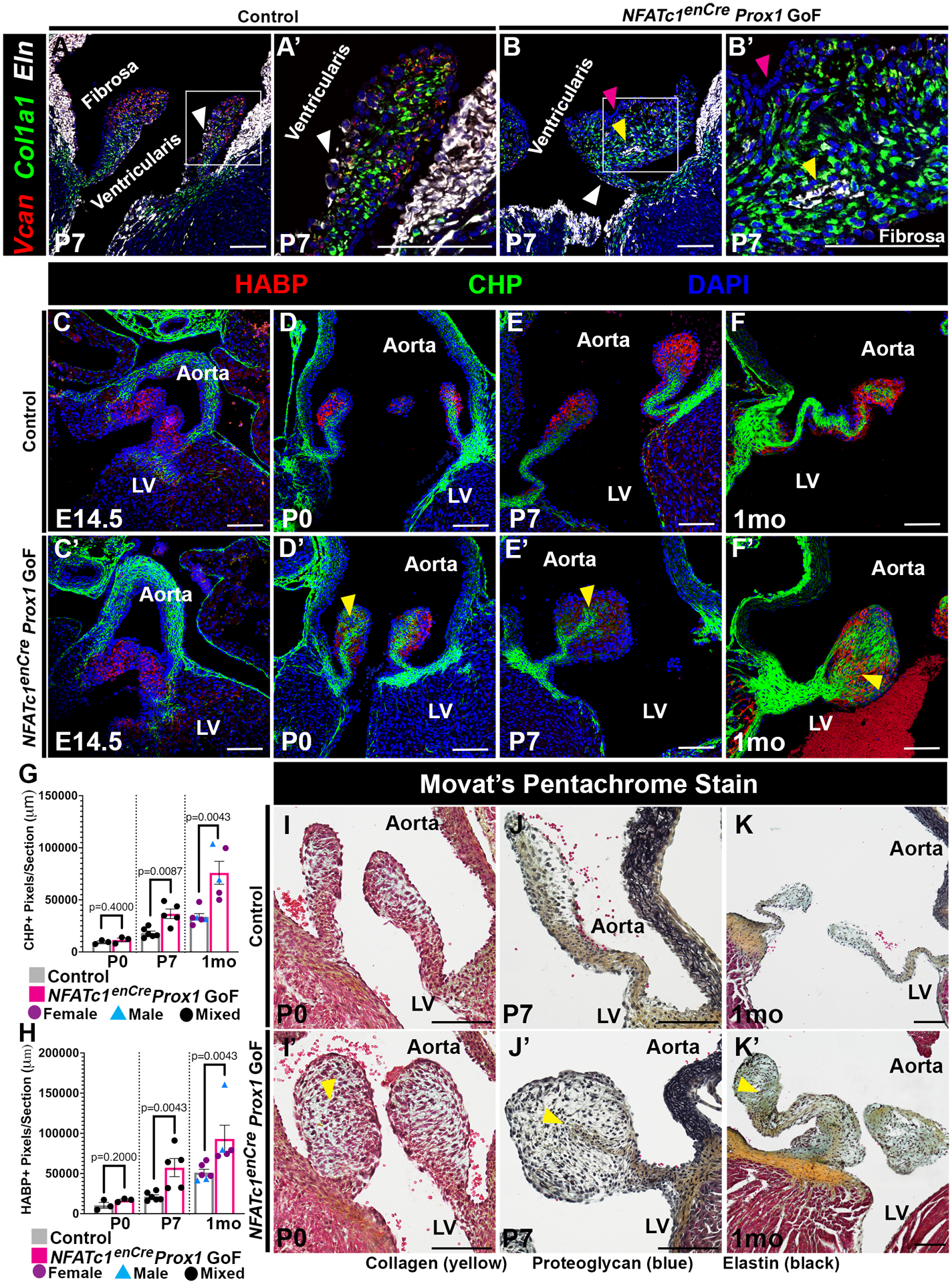
A-B’, Representative P7 AoV sections showing Versican (VCAN, proteoglycan, red), Collagen Type I Alpha 1 Chain (Col1a1, collagen, green), and Elastin (Eln, elastin, white) expression by RNA in situ hybridization. White arrowheads indicate normally localized ELN on the ventricularis side. Yellow arrowheads indicate abnormally localized ELN within the valve interstitium. Pink arrowheads indicate where ELN expression is decreased on the ventricularis side. C-F’, Representative AoV sections of Hyaluronan Binding Protein (HABP, proteoglycan, red) and collagen hybridizing peptide (CHP, collagen, green) at embryonic (E14.5), postnatal (P0, P7), and juvenile (1mo) timepoints. Yellow arrowheads indicate increased abnormally localized interstitial CHP and HABP. G-H, P0, P7, and 1mo (C-F) CHP (G) and HABP (H) positive pixels/section were quantified. I-K, Representative AoV sections of Movat’s Pentachrome Stain at postnatal (P0, P7) and juvenile (1mo) timepoints with Collagen (yellow), Proteoglycan (blue), and Elastin (black). Yellow arrowheads indicate valve leaflet thickening with abnormal ECM distribution. Purple circles indicate females (G, H). Blue triangles indicate males (G, H). Black circles indicate mixed population of unknown sex (G, H). Scale bar =100μm. p-values were determined with a Mann-Whitney test with p-values<0.05 considered significant (n=3–7).
Identification of candidate direct downstream targets of Prox1 using CUT&RUN
To identify the genomic binding targets of Prox1 in specialized VECs, we performed cleavage under targets and release using nuclease (CUT&RUN) followed by high-throughput sequencing using V5-tagged Prox1 on NFATc1enCre Prox1 GoF AoVs (Figure S7A). In parallel, we performed CUT&RUN for H3K27ac on wildtype AoVs to identify regions of open chromatin (Figure S7A). V5 CUT&RUN identified 5,146 binding events (Table S2) for ectopic Prox1 in NFATc1enCre Prox1 GoF AoVs that were enriched for the Prox1 DNA binding domain (Figure 4A, 4F). In addition, we identified 68,889 H3K27ac binding events (Table S3) in wildtype AoVs (Figure 4A). As expected, the majority of Prox1 binding events were localized to the promoter or intronic regions of target genes (Figure S7B). Next, we annotated and compared genes with H3K27ac and Prox1 binding sites with those previously differentially expressed in the P7 Lymph-VEC population dataset (Table S4–5)4, and found an overlap of almost 29% (135/469) (Figure 4B–C). The aggregated normalized expression counts of the 135 genes in P7 Lymph-VEC enriched for Prox1 and H3K27ac binding sites (Figure 4B, pink) were then compared to the P7 VEC and Coapt-VEC aggregated normalized expression counts previously determined by single cell RNA sequencing of P7 and P30 mouse valves (Figure S7C, Table S7)4. Several of the top candidates are known to play a role in lymphatic VECs, confirming the robustness of our analysis (Figure 4C, 4E)7. Gene ontology (GO) analyses of these 135 genes, as well as the 3,974 genes with V5-tagged Prox1 binding sites and H3K27ac binding events, identified important signaling pathways active in heart valve development, including the VEGF and Notch signaling pathways (Figure 4B, 4D, Table S6). Since Prox1 is known to regulate target genes in complexes with other transcription factors, we performed differential motif enrichment analysis to identify putative Prox1 partners (Figure 4G)26,33. Interestingly, this revealed an enrichment of KLF and NFAT motifs, proteins previously shown to physically interact with Prox1 (Figure 4G)34,35. Next, we sought to validate whether exemplar Prox1 bound genes were also induced by Prox1 in valve ECs. We focused on Connexin 37 (Cx37/Gja4), a known direct target of Prox1 in lymphatic valve ECs, which also has active enhancer signatures in WT VECs (Figure 4E)36. RNA in situ hybridization revealed that at P7, Cx37 colocalizes with ectopic Prox1 on the ventricularis side of the NFATc1enCre Prox1 GoF AoV leaflet (Figure 4H). These data indicate that the CUT&RUN of V5-tagged Prox1 is effective in identifying direct targets of Prox1, including known lymphatic VEC Prox1 target genes, expressed in AoV VECs.
Figure 4. Proximal aortic valve (AoV) Prox1 targets, including lymphatic EC genes, were identified using CUT&RUN technology.
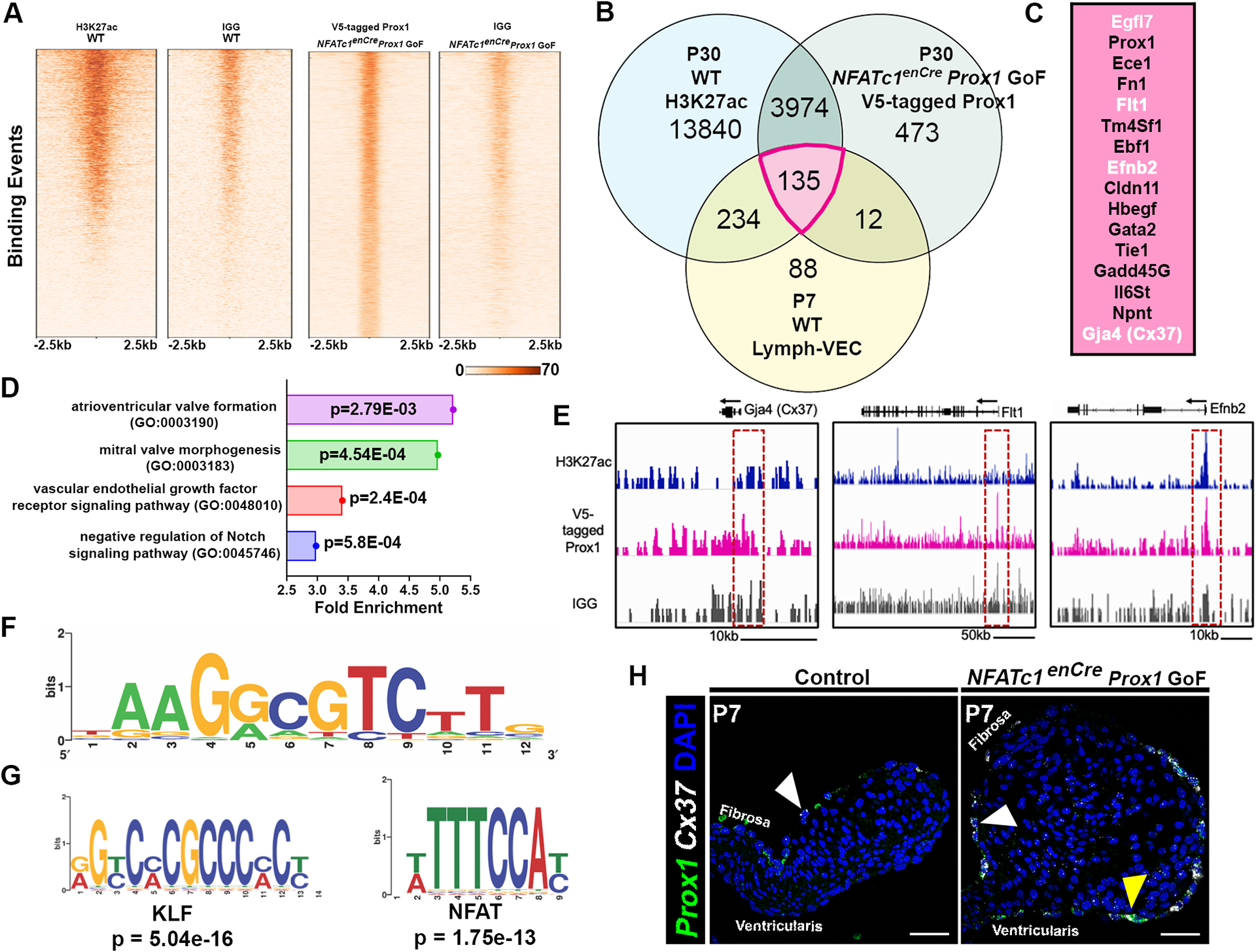
A, Peak distribution of Prox1-binding sites in the mouse genome (mm10) in one control group (mouse IgG) and one experimental (V5 antibody) group as determined by CUT&RUN. B, Venn diagram showing overlap in the number of genes with open chromatin marker H3Kac27 (blue), enriched binding sites for V5-tagged Prox1, and enrichment in the Lymph-VEC VEC population at P7. C, The top P7 Lymph-VEC differentially expressed genes with open chromatin marker H3K27ac and enriched with V5-tagged Prox1 binding sites (B, Pink (135 genes)). Genes in white font were subsequently validated in vivo. D, A biological GO term analysis of genes adjacent to V5-tagged Prox1 binding sequences includes key signaling pathways found in heart valve development including the VEGF signaling pathway. E, The visualized exogenous V5-tagged Prox1 binding peaks associated with a known Prox1 target Gja4 (Cx37), and potential VEC Prox1 targets Flt1 and Efnb2. F, A V5-tagged Prox1 binding motif similar to reported Prox1 binding motifs was identified in candidate target gene sequences. G, A differential motif enrichment analysis was performed to identify potential Prox1 partners based on consensus site localization in proximity to putative Prox1 binding sites. H, Expression of the known Prox1 target gene Cx37 (white) is colocalized with ectopic Prox1+ (green) VECs at P7 in NFATc1enCre Prox1 GoF AoV as determined by RNA in situ hybridization (G’). White arrowheads indicate Prox1+ Cx37+ fibrosa side VEC. Yellow arrowheads indicate ectopic Prox1+ Cx37+ ventricularis side VEC. Scale bar =50μm.
Prox1 promotes the Lymph-VEC gene regulatory network and leads to the loss of the Coapt-VEC gene regulatory network
In lymphatic ECs, direct Prox1 targets beyond Cx37 have not been previously identified. From our CUT&RUN analyses, we identified the VEGF receptor Flt1 and membrane-bound ligand Ephrin-B2 (Efnb2) as potential Prox1 targets. Both Flt1 and Efnb2 have been implicated in endothelial specialization and maturation in aortic and lymphatic valves2,7,37. RNA in situ hybridization revealed that Flt1 and Efbn2 colocalize with ectopic Prox1+ VECs on the ventricularis side of the NFATc1enCre Prox1 GoF AoV leaflets at P0 (Figure 5A–B’, yellow arrowhead). Thus, Flt1 and Efnb2 are expressed in response to NFATc1enCre Prox1 GoF, contain Prox1 bound DNA sequences, and are proximal targets of Prox1 in AoV VECs. By contrast, Hapln1, Wnt9b, and Prnp are all genes that are differentially expressed in the P7 Coapt-VEC population, located near the tip of the ventricularis side of the AoV at P7, that does not normally express Prox1 (Figure S1)4. RNA in situ hybridization of P7 NFATc1enCre Prox1 GoF AoVs demonstrated that the presence of Prox1 in VECs in the ventricularis side of the AoV leads to the loss of Hapln1 (Figure 5C–C’, green), Wnt9b (Figure 5C–C’, white), and Prnp (Figure 5D–D’, green), as well as the gain of Cx37 (Figure 5D–D’, white), in the same cells. Thus, the gain of Prox1 on ventricularis side VECs promotes a Lymph-VEC gene expression profile while leading to the loss of the specialized Coapt-VEC gene expression.
Figure 5. Expanded Prox1+ Lymph-VEC gene regulatory network coincides with loss of Coapt-VEC gene expression in NFATc1enCre Prox1 GoF AoVs.
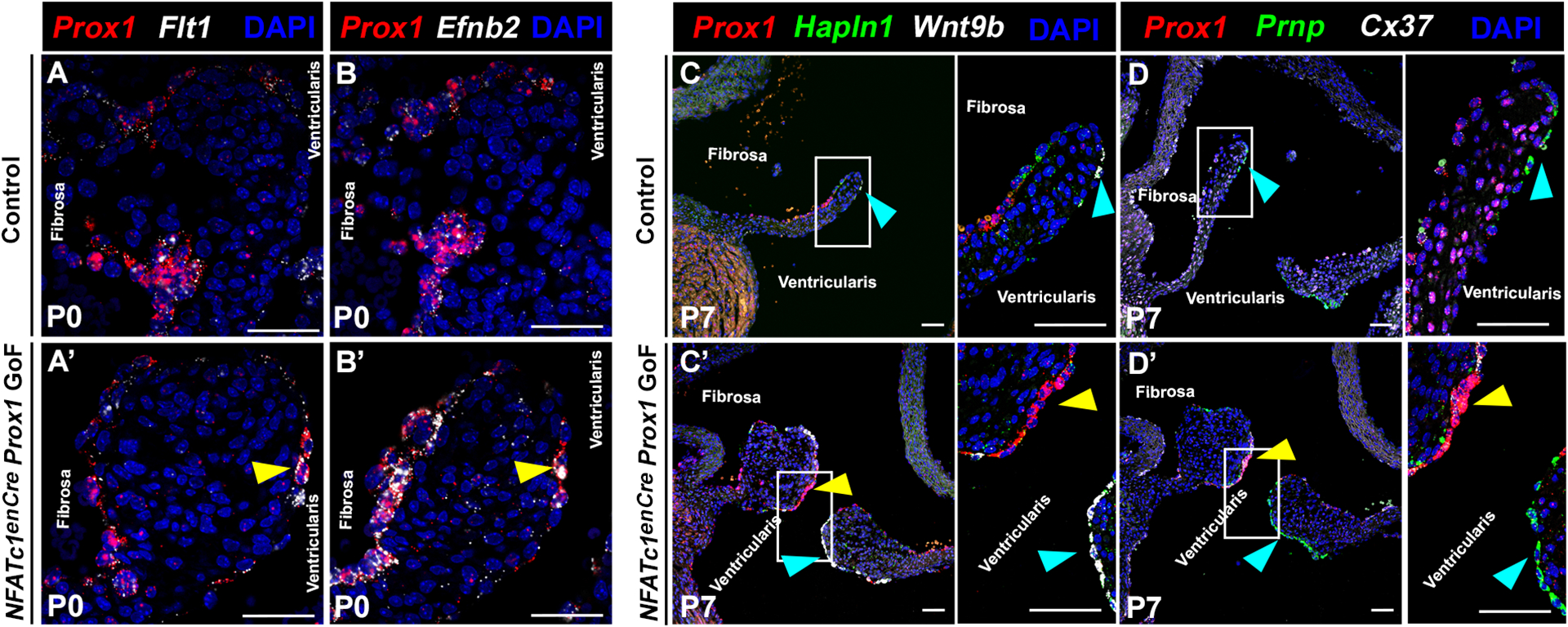
A-B, Representative AoV section of vascular endothelial growth factor receptor Flt1 (A, A’ white) and Efnb2 (B, B’ white) colocalizing with Prox1 (red) at P0 in control (A,B) and NFATc1enCre Prox1 GoF (A’, B’) AoV as determined by RNA in situ hybridization (representative of n=3). Yellow arrowheads indicate ectopic Prox1+ Flt1+ (A’) or Prox1+ Efnb2+ (B’) ventricularis VEC. C-D, Representative AoV section showing complementary staining of Prox1 (C-D, red) with Coapt-VEC markers Hapln1 (C, green), Wnt9b (C, white), and Prnp (D, green) compared with Prox1 known target Cx37 (D, white) at P7, as determined by RNA in situ hybridization. The blue arrow indicates normal localization of Hapln1 (C-C’, green), Wnt9b (C-C’, white), and Prnp (D-D’, green). The yellow arrow indicates loss of Hapln1 (green) and Wnt9b (white) in regions of ectopic Prox1 (red) (n=3–4). Scale bar =50μm.
Endogenous Prox1 is ectopically expressed in VECs on the ventricularis side of Marfan Syndrome myxomatous aortic valves
Congenital heart valve disease, particularly myxomatous valve disease, is often characterized by enlarged valve leaflets with disorganized ECM, together with valve dysfunction, notably regurgitation2. In a mouse model of Marfan syndrome (Fbn1C1039G/+ MFS), heart valves exhibit progressive ECM disorganization and AoV regurgitation38. The association of endogenous Prox1 mislocalization with enlarged dysfunctional aortic valves was examined in Fbn1C1039/+ MFS mice at 2.5 months-of-age when myxomatous remodeling is present39. Immunofluorescent images of Fbn1C1039/+ MFS AoVs revealed expansion of endogenous ectopic Prox1 to VECs on the ventricularis side of the myxomatous AoV leaflet (Figure 6A–A’, yellow arrowheads). Quantification of the number of ectopic Prox1+ VECs demonstrated that Fbn1C1039/+ MFS AoVs have significantly more Prox1+ VECs on the ventricularis side compared to littermate controls (Figure 6B). RNA in situ hybridization of the proteoglycan marker Vcan, collagen marker Col1a1, and elastin (Eln) confirmed disorganized ECM gene expression (Figure S8A–A’). Moreover, the known Prox1 target Cx37 also colocalizes with endogenous ectopic Prox1 on the ventricularis side of the myxomatous Fbn1C1039/+ MFS AoV leaflet (Figure 6C–C’). Additional Prox1 proximal targets Flt1 (Figure S8B–B’, yellow arrow), Egfl7, and Efnb2 (Figure 6D–D’, yellow arrowheads) also colocalize with endogenous ectopic Prox1, as determined by RNA in situ hybridization. Furthermore, Prox1+ induction in the ventricularis side VECs also corresponds with reduced expression of Coapt-VEC markers Hapln1, Wnt9b, and Prnp (Figure S8C–D’, yellow arrowheads). Thus, endogenous Prox1 and its proximal downstream targets are ectopically induced, alongside decreased expression of Coapt-VEC markers, in VECs on the ventricularis side of the congenitally malformed AoV in Fbn1C1039/+ MFS mice.
Figure 6. Endogenous Prox1 is ectopically induced in VECs on the ventricularis side of myxomatous AoVs in a MFS mouse model.
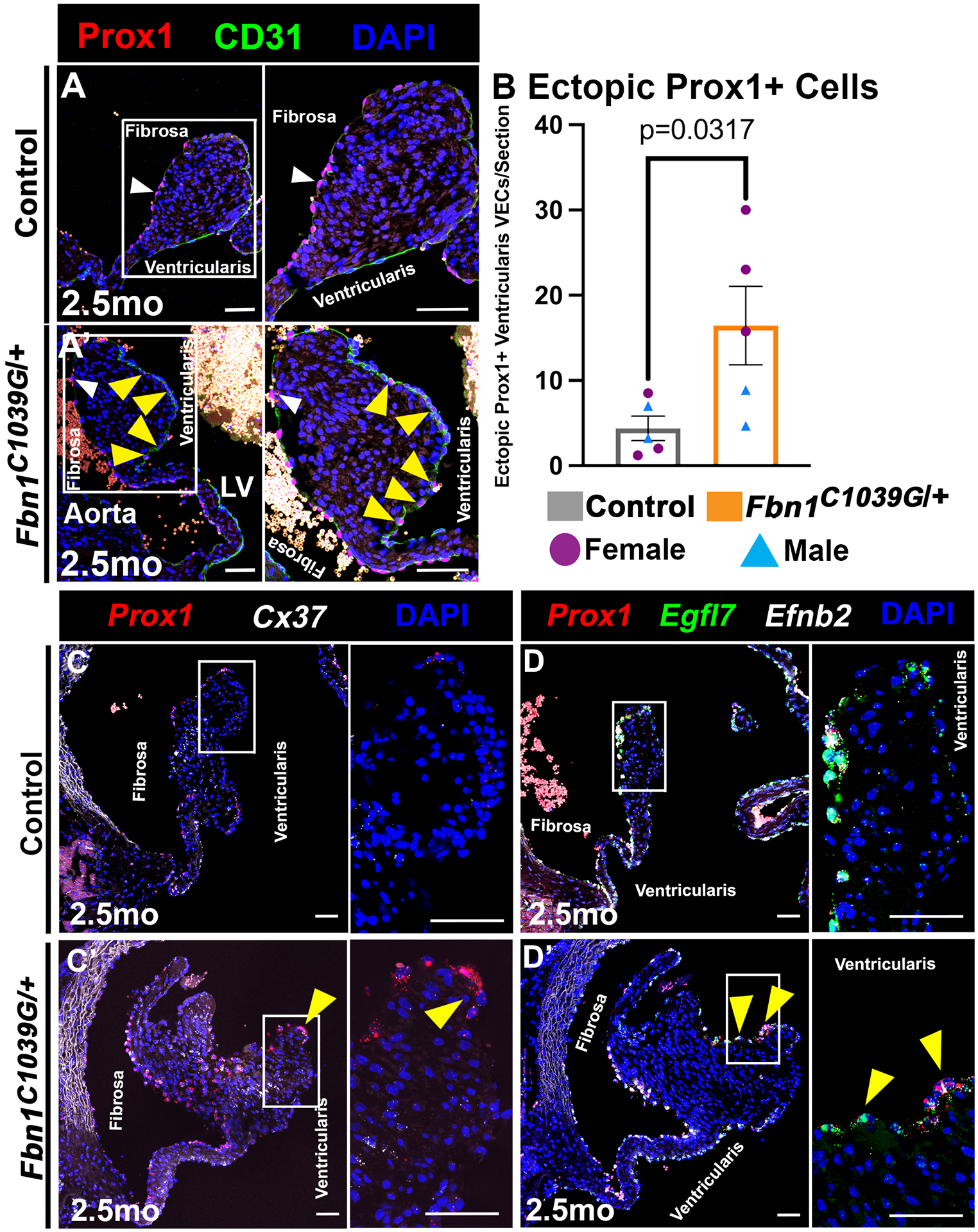
A, Representative AoV section of Prox1 (red) localized with CD31+ (green) VECs on the fibrosa side of a normal AoV (A) and on the ventricularis side of MFS (Fbn1C1039G/+) myxomatous AoVs (A’) at 2.5 months of age. White arrowheads indicate Prox1+ fibrosa side VEC. Yellow arrowheads indicate ectopic Prox1+ ventricularis side VEC. B, The average numbers of Prox1+ ventricularis VECs (termed Ectopic Prox1+ VECs) per section in Fbn1C1039G/+ MFS and Control AoVs were quantified. Purple circles indicate females. Blue triangles indicate males. p-values were determined with a Mann-Whitney test with p-values<0.05 considered significant (n=5). C-D, Representative AoV section of known Prox1 target Cx37 (C, C’white), and proximal Prox1 targets Egfl7 (D, D’green) and Efnb2 (D, D’white) colocalizing with Prox1+ (red nuclei) in ventricularis VEC (yellow arrow) in control (C,D) and myxomatous Fbn1C1039/+ MFS AoV (C’D’) as determined by RNA in situ hybridization (images are representative of n=3). Scale bar =50μm.
Discussion
In this study, we show that Prox1 regulates VEC specialization critical for the morphogenesis and ECM stratification of mature AoVs in mice. During development, Prox1 is expressed in the VECs localized to the fibrosa side of the valve leaflet, which is maintained in adulthood. Misexpression of endothelial Prox1 on the ventricularis side of the AoV is sufficient to cause enlarged AoVs at birth, together with dysregulation of ECM stratification and valve thickening. Notably, the compartmentalization of the valve ECM is abnormal with increased deposition of collagens and proteoglycans, as well as the mixing of the two layers. Furthermore, elastin expression is also mislocalized to the center of the AoV. Thus, the expansion of Prox1 to ventricularis side VECs is sufficient to cause defective patterning and remodeling of interstitial ECM organization. Comparative analyses of Prox1 binding sites in isolated cardiac valve cells in combination with the transcriptomic profile of the P7 Lymph-VEC population previously reported by Hulin et. al. (2019) identified potential downstream targets of Prox1, including Flt1, Efbn2, and Egfl74. Not only do Flt1 and Efnb2 colocalize with ectopic Prox1, but markers for the Coapt-VEC population, including Hapln1, Wnt9b, and Prnp, are reduced on the ventricularis side of the AoV with Prox1 GoF (Figure 7). A congenital myxomatous valve disease Fbn1C1039G/+ MFS mouse model demonstrates an abnormal ECM phenotype similar to that found in NFATc1enCre Prox1 GoF mice, and also expresses naturally induced ectopic Prox1 in VECs on the ventricularis side of the AoV. Interestingly, the proximal AoV Prox1 targets, including Flt1, Efnb2, and Egfl7 identified in NFATc1enCre Prox1 GoF mice, as well as reduced Coapt-VEC markers Hapln1, Wnt9b and Prnp, also colocalize with endogenous ectopic Prox1 in the Fbn1C1039G/+ MFS AoVs. Together, these data indicate that localized Prox1 in cardiac VECs promotes the Lymph-VEC genetic program, while inhibiting the Coapt-VEC genetic program, which is necessary for the development, maintenance, and stratification of ECM remodeling in AoV leaflets.
Figure 7. Model of Prox1 regulation of aortic valve endothelial specification compared to NFATc1enCre Prox1 GoF which disrupts endothelial diversity, leading to myxomatous extracellular matrix.
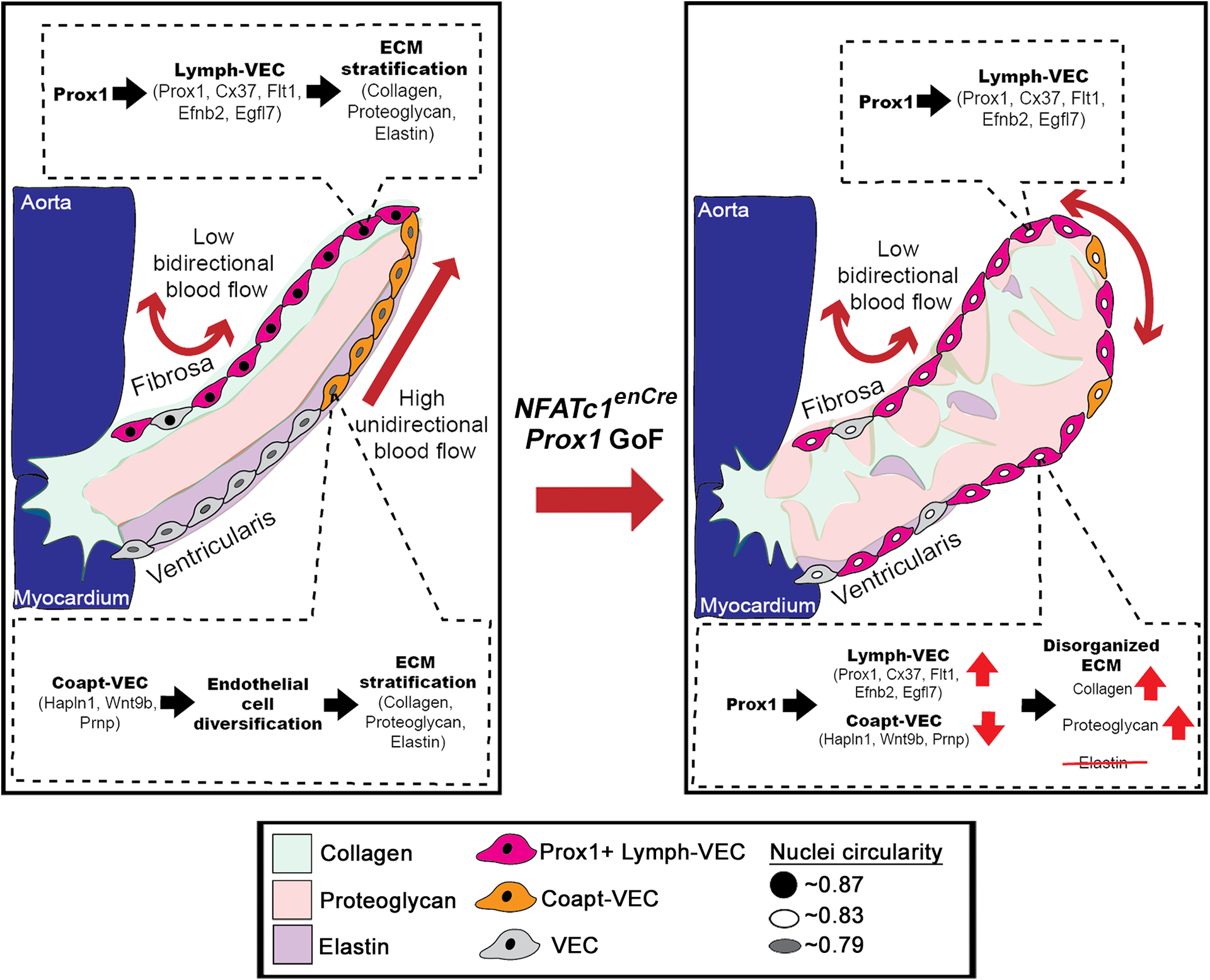
In a healthy valve, the Prox1+ Lymph-VEC population (pink cells) is localized to the fibrosa side of the AoV and is required for the development of the stratified trilaminar extracellular matrix (ECM) found in heart valves. The Coapt-VEC population (orange cells) are normally localized to tip of the leaflet on the ventricularis side of the AoV. Ectopic induction of Prox1 on the ventricularis side of the AoV increases Lymph-VEC (pink cells) and decreases Coapt-VEC (orange cells) gene expression on the ventricularis side of the AoV, disrupting VEC diversification and leading to a disorganized myxomatous ECM. Collagen (green). Proteoglycan (red), Elastin (purple).
Prox1 plays key roles in the development and maintenance of multiple organ systems including the lymphatic vasculature, central nervous system, slow muscle fibers, eye, pancreas, and heart10,11. In the heart, eye, and lymphatic VECs, Prox1 functions in cell shape regulation9,40,41. In the lymphatic ECs, central nervous system, muscle, and pancreas, Prox1 functions in the differentiation of progenitor cells11,42–44. Our study shows that Prox1 promotes the expression of differentially expressed genes in the Lymph-VEC population as well as affects nuclear cell shape, suggesting a role for Prox1 in regulating the cell fate and shape of cardiac VECs. Despite its role in many different organ systems, few direct downstream targets of Prox1 have been reported. Here we identify proximal targets, including Efnb2, Flt1, and Egfl7, that are enriched for V5-tagged Prox1 and H3K27ac binding sites while colocalizing with exogenous ectopic Prox1 in NFATc1enCre Prox1 GoF mice or with naturally induced Prox1 in Fbn1C1039G/+ MFS myxomatous AoVs. Prox1 regulates different target genes by forming complexes with other transcription factors, including NFATs and KLFs, in T cells and lymphatic ECs respectively33–35. Similarly, we identified NFAT and KLF binding sites near the V5-tagged binding sites in NFATc1enCre Prox1 GoF VECs. Cx37, Efnb2, Flt1, and Egfl7 have known roles in either the development of the lymphatic system and/or cardiac valves and are highly differentially expressed in the Lymph-VEC population4,36,45–50. Cx37, Efnb2, and Egfl7, which also contains the microRNA, miR-126, in particular are critical for the development of the lymphatic valve45–47,49,50. Gata2, known to bind a putative enhancer element upstream of Prox1 in lymphatic VECs, and Prox1 itself, are also enriched for H3K27ac and V5-tagged Prox1 binding sites, indicating a potential feed forward regulatory circuit51. Together, these data suggest that Prox1 is acting upstream of the highly differentially expressed genes in the Lymph-VEC population of developing and adult AoV.
In our study, we demonstrate that the gain of Prox1 on the ventricularis side VECs is sufficient to cause the loss of Coapt-VEC specific gene expression in remodeling AoVs. This suggests that, not only does Prox1 act to promote the Lymph-VEC gene regulatory network, it also inhibits the Coapt-VEC gene regulatory network. The Coapt-VEC population is localized to the ventricularis side, opposite of the Prox1+ Lymph-VEC population, near the tip of the AoV leaflet and highly differentially expresses Prnp, Wnt9b, and Hapln1 (Figure S1)4. Here, we show that the expansion of Prox1 to the ventricularis side of the AoV results in reduced Coapt-VEC markers Prnp, Wnt9b, and Hapln1. One of the ways in which Prox1 might be inhibiting the expression of Coapt-VEC specific genes is through the repression of Notch1 signaling which is active on the ventricularis side6. Interestingly, a biological GO term analysis of genes with H3K27ac and V5-tagged Prox1 showed enrichment for genes related to the inhibition of Notch1 signaling. Moreover, Coapt-VEC markers Hapln1 and Wnt9b have been previously shown to be reduced with Notch1 inhibition, and Notch1 and Prox1 inhibit each other’s activity in lymphatic ECs6,9,12,44,52,53. Mechanosensitive Klf2, identified as a potential Prox1 cofactor, has been implicated in the Coapt-VEC gene regulatory network. Coapt-VECs experience a combination of high unidirectional blood flow and pressure as the valve opens and closes, and Klf2 is both induced by unidirectional blood flow and promotes the expression of Coapt-VEC marker Wnt9b in AoV ECs4,5,8. Exactly how Prox1 mis-localization leads to the loss of Coapt-VEC gene expression and intersects with the existing Coapt-VEC gene regulatory network is an important unanswered question.
The gain of Prox1 expression on the ventricularis side VECs is sufficient to prevent the AoV from maturing into its stratified trilaminar ECM structure and leads to myxomatous-like ECM changes, together with altered VEC specialization. This result is in contrast to the traditional view that diversified VIC populations are primarily responsible for ECM stratification and compartmentalization2,3. Our data suggest that VEC specialization, possibly in response to blood flow, controls interstitial ECM patterning and shows that mis-localization of one VEC-specific transcription factor results in abnormal ECM deposition and defective valve morphogenesis. Normally, collagen-expressing VICs localize directly under the Prox1+ Lymph-VEC population towards the fibrosa side of the AoV, while elastin is expressed on the ventricularis side of the AoV (Figure S1)5. Overexpressing Prox1 in VECs on the ventricularis side of the AoV leaflet results in the loss of localized collagen- and proteoglycan-expressing VICs and subsequent mixing of the ECM compartments, with increased deposition of collagens and proteoglycans (Figure 7). This suggests that the VEC populations are dictating deposition and stratification of ECM proteins through a paracrine mechanism. It remains to be determined exactly how VEC populations are regulating the compartmentalization and stratification of the ECM layers.
In this study, we show that expression of Prox1, together with its proximal target genes, is expanded to the ventricularis side of diseased Fbn1C1039G/+ MFS AoV leaflets, thus implicating Prox1 and aberrant VEC specialization in morphological defects in leaflet formation and heart valve disease progression. However, it remains unclear to what extent increased Prox1 and mis-localized VEC specialization contribute to the initiation and progression of the myxomatous valve disease found in Fbn1C1039G/+ MFS mice. Interestingly, many congenital and acquired cardiac valve diseases, such as found in MFS and aging, share the common characteristics of enlarged valve leaflets and ECM disorganization2. The mis-localization of the Prox1+ Lymph- and Coapt-VEC populations in diseased valves, most likely due to abnormal mechanical forces, could underlie similarities in myxomatous valve pathology. Therefore, elucidation of the molecular pathways governing VEC specialization, including those related to Prox1, could provide novel therapeutic targets to treat valve disease resulting from multiple causes. We also found that Prox1 promotes expression of Lymph-VEC enriched genes, including genes known to be involved in lymphatic valve development, supporting a conserved role for Prox1 in cardiac and lymphatic valves.9,54 Discerning the gene regulatory networks upstream and downstream of Prox1 in cardiac VECs, as well as the gene regulatory networks involved in the specialization of other VEC populations, will provide valuable insight into understanding, diagnosing, and treating cardiac and lymphatic valve disease.
Supplementary Material
Highlights.
Prox1 is expressed on the fibrosa side of cardiac valves by embryonic day 12.5 and its localization is conserved in postnatal and adult valves
The overexpression of Prox1 in ventricularis VECs leads to an enlarged aortic valve with disorganized extracellular matrix
Cx37, Flt1, Efbn2, and Egfl7 are enriched for Prox1 binding sites and colocalize with ectopic Prox1 on the ventricularis side of NFATc1enCre Prox1 GoF aortic valves
Prox1 in ventricularis aortic valve endothelial cells is sufficient to cause decreased expression of Coapt-VEC markers Hapln1, Wnt9b, and Prnp
Ectopic Prox1, and its identified targets, are naturally expressed in ventricularis VECs of Marfan syndrome myxomatous aortic valves
Acknowledgements
Graphic images in Figure 1A and Figure S7A were created with BioRender.com.
Sources of Funding
Support for this work was provided by a grant from the National Institutes of Health (R01HL156270) and by a National Institutes of Health Ruth L. Kirschstein Predoctoral Individual National Research Service Award (F31HL150935).
Non-standard Abbreviations and Acronyms
- VEC
Valve endothelial cell
- VIC
Valve interstitial cells
- AoV
Aortic valve
- ECM
Extracellular matrix
- E
Embryonic
- P
Postnatal
- GoF
Gain-of-function
- MFS
Marfan syndrome
- Lymph-VEC
Lymphatic valve endothelial cell
- Coapt-VEC
Coaptation valve endothelial cell
- CUT&RUN
Cleavage under targets and release using nuclease
Footnotes
Disclosures
None
- Expanded Materials & Methods
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8 [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell A, Yutzey KE. Mechanisms of heart valve development and disease. Development. 2020;147. doi: 10.1242/dev.183020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulin A, Hortells L, Gomez-Stallons MV, et al. Maturation of heart valve cell populations during postnatal remodeling. Development. 2019;146. doi: 10.1242/dev.173047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goddard LM, Duchemin AL, Ramalingan H, et al. Hemodynamic Forces Sculpt Developing Heart Valves through a KLF2-WNT9B Paracrine Signaling Axis. Dev Cell. 2017;43:274–289.e275. doi: 10.1016/j.devcel.2017.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacGrogan D, D’Amato G, Travisano S, et al. Sequential Ligand-Dependent Notch Signaling Activation Regulates Valve Primordium Formation and Morphogenesis. Circ Res. 2016;118:1480–1497. doi: 10.1161/CIRCRESAHA.115.308077 [DOI] [PubMed] [Google Scholar]
- 7.Geng X, Cha B, Mahamud MR, Srinivasan RS. Intraluminal valves: development, function and disease. Dis Model Mech. 2017;10:1273–1287. doi: 10.1242/dmm.030825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balachandran K, Sucosky P, Yoganathan AP. Hemodynamics and mechanobiology of aortic valve inflammation and calcification. Int J Inflam. 2011;2011:263870. doi: 10.4061/2011/263870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan RS, Oliver G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev. 2011;25:2187–2197. doi: 10.1101/gad.16974811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsir T, Smits A, Lindstrom MS, Nister M. Transcription factor PROX1: its role in development and cancer. Cancer Metastasis Rev. 2012;31:793–805. doi: 10.1007/s10555-012-9390-8 [DOI] [PubMed] [Google Scholar]
- 11.Kivela R, Salmela I, Nguyen YH, Petrova TV, Koistinen HA, Wiener Z, Alitalo K. The transcription factor Prox1 is essential for satellite cell differentiation and muscle fibre-type regulation. Nat Commun. 2016;7:13124. doi: 10.1038/ncomms13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murtomaki A, Uh MK, Choi YK, Kitajewski C, Borisenko V, Kitajewski J, Shawber CJ. Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development. 2013;140:2365–2376. doi: 10.1242/dev.083865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu B, Wang Y, Lui W, Langworthy M, Tompkins KL, Hatzopoulos AK, Baldwin HS, Zhou B. Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation. Circ Res. 2011;109:183–192. doi: 10.1161/CIRCRESAHA.111.245035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Z, Zhang J, Qiao L, Lu F, Liu Z. Mapping Genome-wide Binding Sites of Prox1 in Mouse Cochlea Using the CUT&RUN Approach. Neurosci Bull. 2021;37:1703–1707. doi: 10.1007/s12264-021-00757-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ankam S, Teo BKK, Pohan G, Ho SWL, Lim CK, Yim EKF. Temporal Changes in Nucleus Morphology, Lamin A/C and Histone Methylation During Nanotopography-Induced Neuronal Differentiation of Stem Cells. Front Bioeng Biotechnol. 2018;6:69. doi: 10.3389/fbioe.2018.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meers MP, Janssens DH, Henikoff S. Pioneer Factor-Nucleosome Binding Events during Differentiation Are Motif Encoded. Mol Cell. 2019;75:562–575 e565. doi: 10.1016/j.molcel.2019.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FastQC. In; 2015.
- 18.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez F, Ryan DP, Gruning B, Bhardwaj V, Kilpert F, Richter AS, Heyne S, Dundar F, Manke T. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44:W160–165. doi: 10.1093/nar/gkw257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorvaldsdottir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meers MP, Tenenbaum D, Henikoff S. Peak calling by Sparse Enrichment Analysis for CUT&RUN chromatin profiling. Epigenetics Chromatin. 2019;12:42. doi: 10.1186/s13072-019-0287-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey TL, Johnson J, Grant CE, Noble WS. The MEME Suite. Nucleic Acids Res. 2015;43:W39–49. doi: 10.1093/nar/gkv416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey TL. DREME: motif discovery in transcription factor ChIP-seq data. Bioinformatics. 2011;27:1653–1659. doi: 10.1093/bioinformatics/btr261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Stamatoyannopoulos JA, Bailey TL, Noble WS. Quantifying similarity between motifs. Genome Biol. 2007;8:R24. doi: 10.1186/gb-2007-8-2-r24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLeay RC, Bailey TL. Motif Enrichment Analysis: a unified framework and an evaluation on ChIP data. BMC Bioinformatics. 2010;11:165. doi: 10.1186/1471-2105-11-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gene Ontology C The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49:D325–D334. doi: 10.1093/nar/gkaa1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Development Core Team. R: A language and environment for statistical computing; Vienna, Austria: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 29.Kolde R pheatmap: Pretty Heatmaps; R package version 1.0.12. 2019.
- 30.Chorianopoulos E, Bea F, Katus HA, Frey N. The role of endothelial cell biology in endocarditis. Cell Tissue Res. 2009;335:153–163. doi: 10.1007/s00441-008-0687-4 [DOI] [PubMed] [Google Scholar]
- 31.Deck JD. Endothelial cell orientation on aortic valve leaflets. Cardiovasc Res. 1986;20:760–767. doi: 10.1093/cvr/20.10.760 [DOI] [PubMed] [Google Scholar]
- 32.Versaevel M, Grevesse T, Gabriele S. Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nat Commun. 2012;3:671. doi: 10.1038/ncomms1668 [DOI] [PubMed] [Google Scholar]
- 33.Bui K, Hong YK. Ras Pathways on Prox1 and Lymphangiogenesis: Insights for Therapeutics. Front Cardiovasc Med. 2020;7:597374. doi: 10.3389/fcvm.2020.597374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi D, Park E, Jung E, et al. Laminar flow downregulates Notch activity to promote lymphatic sprouting. J Clin Invest. 2017;127:1225–1240. doi: 10.1172/JCI87442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Yu N, Wang L, Liu Y, Kong Y, Liu J, Xie Y. Prox1 represses IL-2 gene expression by interacting with NFAT2. Oncotarget. 2017;8:69422–69434. doi: 10.18632/oncotarget.17278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabine A, Agalarov Y, Maby-El Hajjami H, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell. 2012;22:430–445. doi: 10.1016/j.devcel.2011.12.020 [DOI] [PubMed] [Google Scholar]
- 37.Su SA, Xie Y, Zhang Y, Xi Y, Cheng J, Xiang M. Essential roles of EphrinB2 in mammalian heart: from development to diseases. Cell Commun Signal. 2019;17:29. doi: 10.1186/s12964-019-0337-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest. 2004;114:172–181. doi: 10.1172/JCI20641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng CM, Cheng A, Myers LA, et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844 [DOI] [PubMed] [Google Scholar]
- 41.Risebro CA, Searles RG, Melville AA, et al. Prox1 maintains muscle structure and growth in the developing heart. Development. 2009;136:495–505. doi: 10.1242/dev.030007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaltezioti V, Kouroupi G, Oikonomaki M, Mantouvalou E, Stergiopoulos A, Charonis A, Rohrer H, Matsas R, Politis PK. Prox1 regulates the notch1-mediated inhibition of neurogenesis. PLoS Biol. 2010;8:e1000565. doi: 10.1371/journal.pbio.1000565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Kilic G, Aydin M, Burke Z, Oliver G, Sosa-Pineda B. Prox1 activity controls pancreas morphogenesis and participates in the production of “secondary transition” pancreatic endocrine cells. Dev Biol. 2005;286:182–194. doi: 10.1016/j.ydbio.2005.07.021 [DOI] [PubMed] [Google Scholar]
- 44.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazigou E, Lyons OT, Smith A, Venn GE, Cope C, Brown NA, Makinen T. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J Clin Invest. 2011;121:2984–2992. doi: 10.1172/JCI58050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweet DT, Jimenez JM, Chang J, Hess PR, Mericko-Ishizuka P, Fu J, Xia L, Davies PF, Kahn ML. Lymph flow regulates collecting lymphatic vessel maturation in vivo. J Clin Invest. 2015;125:2995–3007. doi: 10.1172/JCI79386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev Biol. 2004;271:263–271. doi: 10.1016/j.ydbio.2004.03.026 [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahamud MR, Geng X, Ho YC, et al. GATA2 controls lymphatic endothelial cell junctional integrity and lymphovenous valve morphogenesis through miR-126. Development. 2019;146. doi: 10.1242/dev.184218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kazenwadel J, Betterman KL, Chong CE, et al. GATA2 is required for lymphatic vessel valve development and maintenance. J Clin Invest. 2015;125:2979–2994. doi: 10.1172/JCI78888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acharya A, Hans CP, Koenig SN, Nichols HA, Galindo CL, Garner HR, Merrill WH, Hinton RB, Garg V. Inhibitory role of Notch1 in calcific aortic valve disease. PLoS One. 2011;6:e27743. doi: 10.1371/journal.pone.0027743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Wu B, Chamberlain AA, Lui W, Koirala P, Susztak K, Klein D, Taylor V, Zhou B. Endocardial to myocardial notch-wnt-bmp axis regulates early heart valve development. PLoS One. 2013;8:e60244. doi: 10.1371/journal.pone.0060244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bazigou E, Wilson JT, Moore JE Jr. Primary and secondary lymphatic valve development: molecular, functional and mechanical insights. Microvasc Res. 2014;96:38–45. doi: 10.1016/j.mvr.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all supporting data are available within the article and its supplementary files. The corresponding author may provide additional data supporting the findings of this study upon reasonable request. Cleavage under targets and release using nuclease (CUT&RUN) files have been deposited in GEO under accession number GSE231336.


