Abstract
Development of next-generation vaccines against Plasmodium falciparum (Pf) is a priority. Many malaria vaccines target the pre-erythrocytic sporozoite (SPZ) and liver stages. These include subunit vaccines based on the Pf circumsporozoite protein (CSP) and attenuated PfSPZ vaccines. However, these strategies require 3-4 doses and have not achieved optimal efficacy against field-transmitted malaria. Prime-and-trap is a recently developed two-step heterologous vaccine strategy that combines priming with DNA encoding CSP followed by a single dose of attenuated SPZ. This strategy aims to induce CD8+ T cells that can eliminate parasites in the liver. Prior data has demonstrated that prime-and-trap with P. yoelii CSP and PySPZ was immunogenic and protective in mice. Here we report preliminary data on the immunogenicity of PfCSP prime and PfSPZ trap vaccine in rhesus macaques. This vaccine induced PfCSP-specific antibodies and T cell responses in all animals. However, response magnitude differed between individuals, suggesting further study is required.
Keywords: Malaria, vaccine, macaque
Introduction
Malaria is caused by Plasmodium parasites, of which P. falciparum (Pf) is the most lethal. In 2021, there were an estimated 247 million infections and 619,000 deaths, most of which were in infants and children1. Malaria parasites are transmitted from mosquitoes to humans as sporozoites, which migrate out of the skin and travel via the circulation to the liver. These sporozoites invade hepatocytes and replicate in a clinically-silent liver stage. Thereafter, parasites enter the circulation, where they begin cycles of replication in red blood cells, giving rise to malaria symptoms. To prevent symptoms, many vaccine strategies target the sporozoite and liver stages. This includes the first WHO recommended Pf malaria vaccine, RTS,S/AS01E, an adjuvanted subunit vaccine based on the circumsporozoite protein (CSP)2. RTS,S induces antibodies that are intended to block sporozoite invasion of the liver2. However, four-doses of RTS,S confers only modest protection from severe disease3. Development of next-generation Pf malaria vaccines is thus a priority.
Live-attenuated sporozoites are a promising class of vaccines in clinical development. These consist of Pf sporozoites that have been attenuated by irradiation, gene deletion, or concurrent delivery of drugs, such that they can invade the liver and express thousands of antigens, but arrest or are eliminated before causing disease4. The most advanced of these are Pf sporozoite (PfSPZ) based vaccines, which are administered intravenously (IV) as multi-dose regimens5. PfSPZ vaccines induce both antibody and T cell responses4, with studies in nonhuman primates (NHP) implicating liver CD8+ T cells in protection6,7. PfSPZ-based vaccines have achieved up to 100% sterile protection against challenge with homologous or heterologous parasites in controlled human malaria infection trials8-10 and have shown some field efficacy11,12. However, they are yet to achieve >90% protection against blood stage infection in the field, a strategic goal in the WHO preferred product characteristics for malaria vaccines13. Thus, it is anticipated that late-arresting PfSPZ vaccines and other improvements will likely be required to achieve this goal in all populations.
We previously reported a two-step heterologous malaria vaccine strategy called prime-and-trap14,15. This strategy involves priming with DNA encoding CSP followed by a single IV dose of irradiated sporozoites, thus combining elements of subunit and live-attenuated sporozoite vaccines. The intent is to induce CSP-specific CD8+ T cells that can eliminate parasites in the liver. Our prior studies demonstrated that prime-and-trap is immunogenic and confers sterile protection in the P. yoelii (Py) mouse malaria model14,15. However, this involved priming with DNA encoding the PyCSP CD8+ epitope, which is defined in BALB/c mice, but not suitable for translation. Here, we report preliminary data on the immunogenicity of a near full-length PfCSP-based prime-and-trap vaccine in rhesus macaques. We find that prime-and-trap induces PfCSP-specific T cell responses in all animals, but the response magnitude differs between individuals. The data from this small pilot study suggest that further investigation is necessary in order to achieve optimal performance of prime-and-trap vaccines in NHPs.
Methods
Animals
Malaria-naïve Indian origin rhesus macaques were housed at the Washington National Primate Research Center as described16. Procedures were conducted in accordance with an approved University of Washington Institutional Animal Care and Use Committee Protocol, and animals were cared for in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Animals were sedated with ketamine by intramuscular injection for all procedures. Additional anesthesia and analgesia were given for surgery. Euthanasia was performed by overdose of pentobarbital in compliance with American Veterinary Medical Association guidelines.
Study design
This pilot study enrolled three groups of n=3 animals. Group 1 was mock vaccinated, Group 2 received the PfCSP prime-and-trap vaccine on day 0 and 28, and Group 3 received a comparator vaccine consisting of three doses of PfSPZ on day 0, 7 and 28, hereafter called 3x PfSPZ (Fig 1). Sample size was determined by cost. Animals were randomized to groups based on sex and weight (Table). All underwent baseline blood draws. Group 1 was mock vaccinated with DNA encoding no antigen on day 0 and with vaccine diluent only administered IV on days 0, 7 and 28. Group 2 received the prime-and-trap vaccine consisting of 15 μg DNA encoding near full-length PfCSP mixed with the Escherichia coli heat-labile toxin LT adjuvant administered by gene gun on day 0, followed by a dose of 3x106 PfSPZ administered IV on day 28. Group 3 received three doses of 1x106 PfSPZ by IV on days 0, 7 and 28. This dose was selected so that the total number of PfSPZ received by Groups 2 and 3 were equivalent. Note that these doses were higher than used in prior studies for repeated IV PfSPZ vaccination in rhesus macaques7. All animals had blood draws throughout the study and underwent liver biopsy surgery on day 56. We did not obtain liver biopsy samples prior to vaccination since this would have necessitated an additional surgery, which we deemed unnecessary because the animals were malaria naïve. All groups were then administered 1x106 non-attenuated PfSPZ IV on day 85 or 86 as a recall exposure, before necropsy to collect the liver and spleen six days later on day 90 or 91. The timing of the recall exposure and necropsy were intended to capture the peak T cell response to the non-attenuated PfSPZ, since this human-adapted parasite cannot be used for a true challenge in the rhesus macaque model. The study was staggered so that no more than two surgeries or necropsies occurred per day. Treatment order was not randomized and staff were not blinded to study groups.
Figure 1. Study diagram.
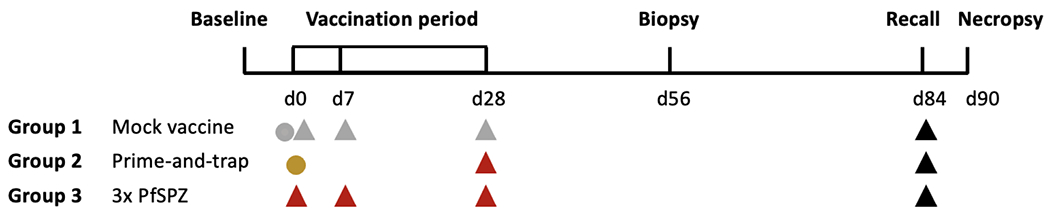
Groups of n=3 rhesus macaques were vaccinated as follows. Group 1 received mock vaccines consisting of DNA encoding no antigen delivered by gene gun on day 0 (grey circle) and vaccine diluent only administered intravenously on days 0, 7 and 28 (grey triangle). Group 2 received the prime-and-trap vaccine consisting of 15 ug DNA encoding the near full-length PfCSP and LT adjuvant administered by gene gun on day 0 (gold circle), followed by 3x106 attenuated PfSPZ administered intravenously on day 28 (red triangle). Group 3 received a comparator vaccine consisting of three doses of 1x106 attenuated PfSPZ administered intravenously on days 0, 7 and 28 (red triangle). All groups had a liver biopsy on day 56. All groups received 1x106 non-attenuated PfSPZ administered intravenously on day 84 or 85 as a recall exposure (black triangle). Necropsies for all animals occurred on day 90 or 91.
DNA vaccination
DNA vaccine production was similar to prior studies14,15. The PfCSP sequence, without the NANP repeat region, was codon optimized for human expression and inserted in the pUb.4 vector with a N-terminal ubiquitin tag (Supplementary Figure). The LT-encoding plasmid was used as an adjuvant in a 1:10 ratio with the PfCSP plasmid. Animals were vaccinated on the skin of both legs over an area of approx. 30 cm2 per leg using a PowderJect-style gene gun.
Live-attenuated sporozoite vaccination
Irradiated, purified, cryopreserved, P. falciparum NF54 vaccine sporozoites (PfSPZ) and non-attenuated PfSPZ were produced by Sanaria, Inc. Vials were shipped and stored in liquid nitrogen. PfSPZ were thawed in a 37°C water bath for 30 sec, diluted in 1% human albumin (AlbuRx, CSL Behring) in sterile phosphate buffered saline, and administered IV using a catheter fitted with a small-bore loop into the saphenous vein. PfSPZ were administered within 30 mins of thawing. Entry of the catheter into the vein was confirmed before administration.
Blood sampling
Whole blood for plasma and peripheral blood mononuclear cell (PBMC) isolation was collected into EDTA tubes, transported at room temperature, and processed according to standard protocols. Briefly, blood was centrifuged to isolate plasma, which was frozen at −80°C. The remaining blood cells were applied to a Ficoll gradient (Ficoll Paque Plus, GE) and centrifuged to separate PBMCs. PMBCs were then washed and cryopreserved in heat inactivated FBS containing 10% DMSO (Sigma).
Liver lymphocyte isolation
Liver tissue from biopsy or necropsy was gently perfused before being transferred to media for transport on ice. Tissue was cut into pieces and incubated with DNAse I (Sigma) and collagenase (Sigma) with agitation at 37°C for 1.5-2 hrs. Softened tissue was pushed through 212 μm mesh to obtain a single cell suspension, which was centrifuged at 50xg to pellet gross hepatocytes. Liver lymphocytes were isolated by applying the remaining cells over a cushion of 20% iodixanol (Optiprep, Sigma) and centrifuging at 1500xg for 25 mins with no brake. Liver lymphocytes were collected, washed and cryopreserved as above.
Splenocyte isolation
Spleens were transferred to media for transport on ice. Tissue was cut into pieces and pushed through 212 μm mesh to obtain a single cell suspension. The cells were applied to a Ficoll gradient and centrifuged to separate splenic mononuclear cells, then washed and cryopreserved as above.
ELISPOT
ELISPOT assays were performed using the Monkey IFN-gamma ELISpot PRO (ALP) kit (Mab Technologies). Cryopreserved cells were thawed, counted, and rested before being plated in duplicate at the desired density and incubating overnight with 1 μg/mL PfCSP peptide pool, DMSO (Sigma), or Concanavalin A (Sigma). The PfCSP peptide pool was a gift of Robert Seder (NIH) and consisted of 15-mers that overlapped by 11 amino acids. The pool contained 87 individual peptides spanning the majority of the PfCSP sequence, from the first peptide QEYQCYGSSSNTRVL to the final peptide EKKICKMEKCSSVFN. Spots were counted using an ELISPOT plate reader (CTL ImmunoSpot). Normalized spot forming units (nSFU) per million cells were calculated by taking the mean SFU from the PfCSP peptide pool stimulated cells and subtracting the mean SFU from DMSO control wells. Animals were considered to have responded if nSFU per million cells was ≥10 fold greater than at baseline (for PBMCs) or ≥10 fold greater than the mock vaccinated controls (liver lymphocytes and splenocytes).
ELISA
PfCSP ELISA was performed using recombinant full length PfCSP protein as previously described7. Plasma samples were analyzed in triplicate. Data was collected using Softmax Pro GXP v5 and fit to a 4-parameter logistic curve to calculate the plasma dilution required for an optical density (OD) of 1.0. The net OD 1.0 was calculated by subtracting the baseline OD 1.0 from the sample OD 1.0. Animals were considered to have made a positive response if the net OD 1.0 was ≥50.
Statistics
Data was analyzed in Graphpad Prism v9. Descriptive statistics were used to summarize responses in each vaccine treatment group. Due to the small sample size, statistical tests were not used to make comparisons between vaccine treatment groups, nor to evaluate the impact of biological sex on vaccine response.
Results and Discussion
To assess cellular responses to vaccination, IFNγ ELISPOT assays were performed using PfCSP peptide pool stimulation. This approach was selected for this preliminary analysis because although this assay cannot distinguish if responding cells are CD4+ or CD8+ T cells, prior studies have shown that only IFNγ+ CD8+ T cells associate with protection in mice17, and IFNγ+ CD8+ T cells have previously been detected in the liver of PfSPZ-vaccinated rhesus macaques7. Since prime-and-trap mainly aims to induce liver CD8+ T cells, cellular responses in this tissue were assessed at the time of biopsy (day 56) and necropsy (day 90/91). PfCSP-specific responses were detected in the liver for two of the three prime-and-trap vaccinated animals at biopsy, with the magnitude of response differing considerably between individuals (Fig 2A). No response to PfCSP peptide stimulation were detected in animals that received 3x PfSPZ, consistent with the need for whole-parasite stimulation to detect such responses in rhesus macaques7. Similar results were observed at necropsy, although responses in the liver of prime-and-trap vaccinated animals were now more similar in magnitude (Fig 2B). We hypothesize that this was due to the PfSPZ recall exposure six days prior to necropsy, which may have induced similar levels of PfCSP-specific T cell recruitment and expansion in the two animals. Thus, prime-and-trap induced PfCSP-specific responses in the liver of two of the three animals, albeit at different magnitudes, but in each case higher than in animals receiving 3x PfSPZ.
Figure 2. Cellular response to vaccination as measured by PfCSP peptide IFNγ ELISPOT. Inset: Table of animal characteristics.
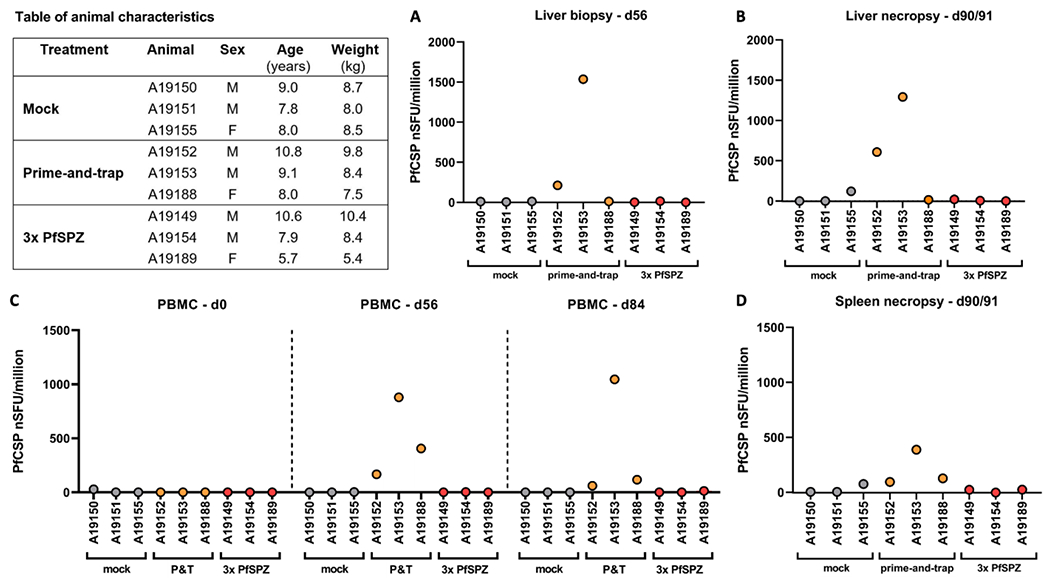
A. Responses in liver lymphocytes obtained post-vaccination by surgery on day 56. B. Responses in liver lymphocytes from six days post-PfSPZ recall exposure at necropsy. C. Responses in PBMCs on days 0, 56, and 84. D. Responses in splenocytes from six days post-PfSPZ recall exposure at necropsy. Animal ID and vaccine treatment group given below x-axis. nSFU, normalized spot forming units. Animals were considered to have responded if nSFU per million cells was ≥10 fold greater than baseline for PBMCs or ≥10 fold greater than mock vaccinated controls for liver lymphocytes and splenocytes.
Cellular responses were next assessed in PBMCs collected at baseline, and prior to both liver biopsy (day 56) and PfSPZ recall exposure (day 84/85). PfCSP-specific responses were detected in all prime-and-trap animals (Fig 2C). No responses to PfCSP peptide stimulation were detected in the PBMCs of animals that received 3x PfSPZ. Similar results were observed from splenocytes at necropsy (Fig 2D). Together with data above, this indicated that three prime-and-trap animals responded to the PfCSP prime, but only two of the three had responses boosted and directed to the liver by the PfSPZ trap. We speculate this was because PfSPZ are less fit in rhesus macaques than macaque-adapted parasites18, and thus may not effectively boost and trap T cell responses in all animals.
Plasma antibody responses were measured by ELISA. PfCSP antibodies were absent in prime-and-trap vaccinated animals before trapping (day 28) but detected in all at the time of liver biopsy (day 56) and PfSPZ recall exposure (day 84) (Fig 3). This indicated that PfCSP priming did not induce antibodies, consistent with the immunodominant repeat region19 intentionally being excluded and the N-terminal ubiquitin tag favoring class I MHC presentation20. PfCSP antibodies were detected in all animals that received 3x PfSPZ. Thus, prime-and-trap induces PfCSP antibodies, but this can be attributed to the PfSPZ trap. Since rhesus macaques cannot support blood stage infections with human-adapted Pf parasites, we were not able to perform PfSPZ challenge to assess vaccine efficacy in the present study.
Figure 3. Humoral response to vaccination as measured by PfCSP ELISA.
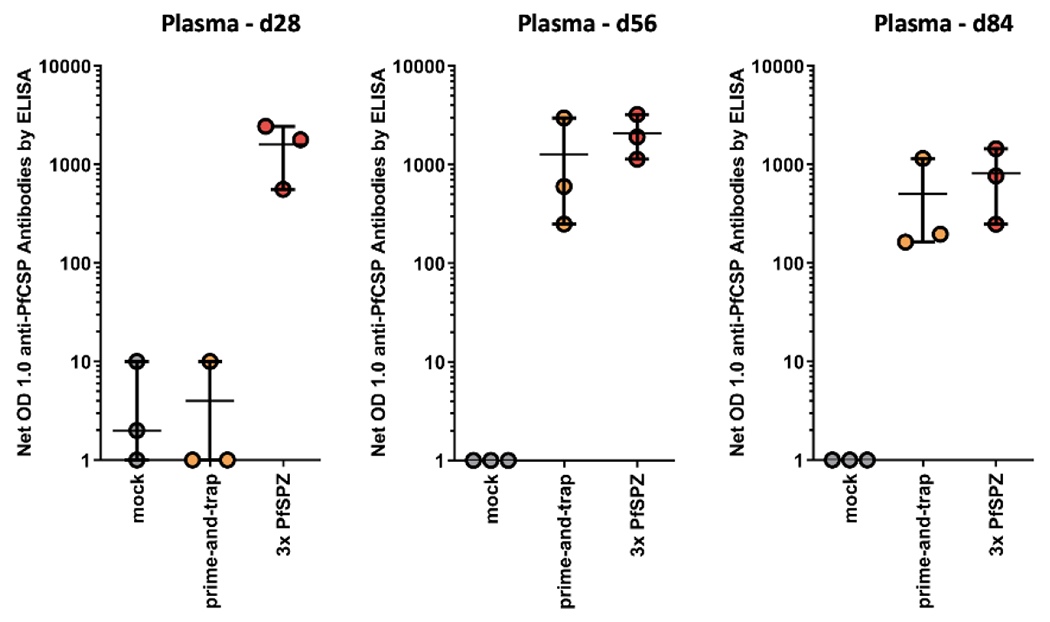
Plasma was collected at baseline, on day 28 before final vaccination, and post-vaccination on day 56 and 84. The net OD 1.0 was calculated by subtracting the baseline OD 1.0 from the sample OD 1.0. Data for individual animals is shown as circles. Error bars show mean and range for each vaccine treatment group.
In conclusion, these preliminary data indicate that prime-and-trap induced PfCSP-specific T cells in all animals, but the magnitude of response to priming and the effectiveness of the trap differed between individuals. Such a finding is not uncommon when first advancing vaccine strategies from mice to NHPs, ie. when moving from a small, inbred animal model to a larger, outbred model. Indeed, the variation in the response to priming suggests that prime-and-trap in NHPs may benefit from including additional antigens or adjuvants at the DNA priming step. Similarly, the variability in trapping response may either be attributed to differences in the response to priming, or alternatively to using the human-adapted PfSPZ for trapping, which we now know are less fit than macaque-adapted parasites in the rhesus macaque liver18. In the future, we therefore plan to re-evaluate prime-and-trap using the P. knowlesi macaque model, which permits sporozoite challenge studies to assess vaccine efficacy. The goal will be to develop a highly effective prime-and-trap malaria vaccine that is immunogenic and protective in all individuals.
Supplementary Material
Highlights.
Prime-and-trap is a two-step heterologous vaccine strategy developed for malaria
Prime-and-trap aims to induce CD8+ T cells against liver stage Plasmodium parasites
Prior data show prime-and-trap is immunogenic and protective in mouse models
Here we report immunogenicity of a prime-and-trap vaccine in non-human primates
Acknowledgements
We gratefully acknowledge staff at the Washington National Primate Research Center. The Washington National Primate Research Center is supported by the NIH under awards P51OD010425 and U42OD011123. S.C.M received support from the NIH under awards R01AI141857 and R03AI144143-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
S. C. M. filed a patent application on selected aspects of the prime-and-trap concept through the University of Washington. S. C. M. has equity in a startup company (Sound Vaccines, Inc.) that is negotiating with the University of Washington for rights to this intellectual property. The relationship between the authors and Sound Vaccines, Inc., has been reviewed by the University of Washington and complies with all University and State of Washington policies on such activities. N.K.C., B. K. L. S., and S. L. H. are paid employees of Sanaria Inc.
References
- 1.WHO. World Malaria Report (2022). [Google Scholar]
- 2.Laurens MB RTS,S/AS01 vaccine (Mosquirix): an overview. Hum Vaccin Immunother 16, 480–489, doi: 10.1080/21645515.2019.1669415 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RTS, S. Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386, 31–45, doi: 10.1016/S0140-6736(15)60721-8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itsara LS et al. The Development of Whole Sporozoite Vaccines for Plasmodium falciparum Malaria. Front Immunol 9, 2748, doi: 10.3389/fimmu.2018.02748 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richie TL et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 33, 7452–7461, doi: 10.1016/j.vaccine.2015.09.096 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss WR & Jiang CG Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS One 7, e31247, doi: 10.1371/journal.pone.0031247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizuka AS et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 22, 614–623, doi: 10.1038/nm.4110 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein JE et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2, e89154, doi: 10.1172/jci.insight.89154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mordmuller B. et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542, 445–449, doi: 10.1038/nature21060 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulyok Z. et al. Heterologous protection against malaria by a simple chemoattenuated PfSPZ vaccine regimen in a randomized trial. Nat Commun 12, 2518, doi: 10.1038/s41467-021-22740-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirima SB et al. A randomized controlled trial showing safety and efficacy of a whole sporozoite vaccine against endemic malaria. Sci Transl Med 14, eabj3776, doi: 10.1126/scitranslmed.abj3776 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sissoko MS et al. Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: a randomised, controlled phase 1 trial. Lancet Infect Dis 22, 377–389, doi: 10.1016/S1473-3099(21)00332-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Malaria vaccines: preferred product characteristics and clinical development considerations. (2022). [Google Scholar]
- 14.Olsen TM, Stone BC, Chuenchob V & Murphy SC Prime-and-Trap Malaria Vaccination To Generate Protective CD8(+) Liver-Resident Memory T Cells. J Immunol 201, 1984–1993, doi: 10.4049/jimmunol.1800740 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Watson F. et al. Cryopreserved Sporozoites with and without the Glycolipid Adjuvant 7DW8-5 Protect in Prime-and-Trap Malaria Vaccination. Am J Trop Med Hyg, doi: 10.4269/ajtmh.21-1084 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakravarty S. et al. Efficient infection of non-human primates with purified, cryopreserved Plasmodium knowlesi sporozoites. Malar J 21, 247, doi: 10.1186/s12936-022-04261-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tse SW, Radtke AJ & Zavala F Induction and maintenance of protective CD8+ T cells against malaria liver stages: implications for vaccine development. Mem Inst Oswaldo Cruz 106 Suppl 1, 172–178, doi: 10.1590/s0074-02762011000900022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shears MJ, Seilie AM, Kim Lee Sim B, Hoffman SL & Murphy SC Quantification of Plasmodium knowlesi versus Plasmodium falciparum in the rhesus liver: implications for malaria vaccine studies in rhesus models. Malar J 19, 313, doi: 10.1186/s12936-020-03385-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zavala F. et al. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science 228, 1436–1440, doi: 10.1126/science.2409595 (1985). [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Marvin JM, Tatsis N & Eisenlohr LC Cutting Edge: Selective role of ubiquitin in MHC class I antigen presentation. J Immunol 186, 1904–1908, doi: 10.4049/jimmunol.1003411 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


