Abstract
The current study examined transactional associations between maternal internalizing symptoms, infant negative emotionality, and infant resting respiratory sinus arrhythmia (RSA). We used data from the Longitudinal Attention and Temperament Study (N=217) to examine the associations between maternal internalizing symptoms, infant negative emotionality, and infant resting RSA from 4-months to 18-months using a random-intercepts cross-lagged panel model. We found that mothers with higher average internalizing symptoms have infants with higher levels of resting RSA. However, there were no stable, between-individual differences in infant negative emotionality across time. Additionally, we found significant negative within-dyad cross-lagged associations from maternal internalizing symptoms to subsequent measures of infant negative emotionality, as well as a significant negative cross-lagged association from maternal internalizing symptoms to child resting RSA after 12-months of age. Lastly, we find evidence for infant-directed effects of negative emotionality and resting RSA to maternal internalizing symptoms. Results highlight the complex, bidirectional associations in maternal-infant dyads during the first two years of life, and the importance of considering the co-development of infant reactivity and regulatory processes in the context of maternal internalizing symptoms.
Keywords: negative emotionality, respiratory sinus arrhythmia, temperament, infancy, maternal internalizing symptoms
Infancy is characterized by rapid development in both biological and behavioral domains. During the first two years of life, changes in infants’ emotional reactivity and regulation mark important and meaningful developmental transitions and lead to the development of individual differences associated with later socioemotional outcomes, including risk for psychopathology (Bornstein et al., 2014; Klein et al., 2012). During infancy, caregivers play a large role in providing the environmental context and social experiences that may shape socioemotional development. It is important, then, to consider how parental factors, such as maternal distress, may play a role in the development of individual differences in reactivity and regulation.
One method of assessing maternal distress is through maternal internalizing symptoms, which include both depression and anxiety symptoms. Maternal internalizing symptoms are often associated with child socioemotional outcomes, and numerous studies have demonstrated intergenerational associations between maternal and child internalizing symptoms (Eley et al., 2015; Goodman, 2020; McAdams et al., 2015). Despite evidence for links between maternal and child internalizing symptoms, the mechanisms are still complex and open to study. One possibility is that maternal internalizing symptoms may impact the development of children’s emotional reactivity and regulation, which in turn lead to increased risk for children’s development of internalizing behaviors (Goodman & Gotlib, 1999). Specifically, the interaction between parasympathetic nervous system (PNS) activity and early childhood temperament may be one pathway for the development of associations between maternal and child internalizing symptoms. For example, maternal internalizing symptoms are associated with greater autonomic arousal in their infants, which is associated with higher levels of temperamental fear in toddlerhood (de Vente et al., 2020). Drawing from biopsychosocial models of development (e.g., Gottlieb, 2007; Sameroff, 2010) and prior research, the child’s daily context is likely impacting the development of both emotional reactivity and regulation. However, less is known about how within-individual changes in maternal internalizing symptoms, PNS activity, and negative emotionality development may be associated with one another, and how negative emotionality and PNS activity may co-develop across infancy.
Additionally, there are bidirectional associations between the infant and parents that occur across this developmental period, which may have implications for both infant development as well as parental mental health and behaviors. Infant and child negative emotionality (i.e., fear, sadness, and anger) elicits negative affect in mothers (Murray, 1979; Soltis, 2004), increases in maternal stress (Pesonen et al., 2008), and increases in anxiety symptoms (Behrendt et al., 2020). Taken together, there may be bidirectional associations within dyads between maternal internalizing symptoms and infant negative emotionality, and developmental changes in infant physiology may modulate these relations over time. The present study leverages a longitudinal design with repeated measures of maternal internalizing symptoms, infant negative emotionality, and infant PNS activity to examine these inter-relations across infancy as a potential mechanism by which internalizing symptoms develop and are maintained in families.
Associations between the Development of Infant Resting Respiratory Sinus Arrhythmia and Maternal Internalizing Symptoms
Maturation in physiological systems underlying reactivity and regulation may act as a marker of risk for the development of internalizing symptoms. The stress response system has three main biological functions: to coordinate physiological and behavioral responses to threats and opportunities, to encode and filter information from the environment, and to translate that information into broad-band individual differences in behavior and physiology (Del Giudice et al., 2011). The Adaptive Calibration Model (ACM) of stress responsivity provides a framework for considering the ways in which individual differences in stress regulation may arise due developmental changes in response to early experiences (Del Giudice et al., 2011). The ACM posits that individual differences in stress regulation develop as the organism modifies its functioning—and in turn, its developmental trajectory—to match the conditions of the social and physical environment. This would suggest that the activation of the child’s stress response system during the first few years of life plays a key role in recalibration of physiological systems underlying stress regulation, as early activation signals important information about the environment in which development is occurring. For example, early activation may lead to signaling that there are environmental dangers and threats that the body needs to cope with, that may lead to up- or down-regulation of the stress-response system (Del Giudice et al., 2011). This highlights the importance of considering the early environment in the development of physiological systems.
One branch of the autonomic nervous system (ANS) is the parasympathetic nervous system (PNS), which plays an important role in how the body copes with stress. The PNS responds very quickly to environmental cues as a first-response coping system by allowing individuals to mobilize resources and engage with objects and/or other individuals to achieve homeostasis (Porges, 2007). PNS activity is commonly assessed through respiratory sinus arrhythmia (RSA), which captures variability in the inter-beat interval of the heart and the impact of the vagus nerve (Porges, 2007). Resting RSA is thought to reflect the general capacity for behavioral and self regulation (including emotion regulation), with higher levels of resting RSA reflecting capacity for better emotion regulation in the face of contextual stressors. While task-based measures of RSA capture regulatory processes during specific contexts, resting RSA is putatively less context-specific and can capture individual differences in emotion regulation more broadly, and, perhaps ironically, remove ‘noise’ created by individual differences in emotional and behavioral responses to task-based triggers. Resting RSA has been associated with risk for internalizing symptom development as a transdiagnostic marker of emotion regulation associated with various forms of psychopathology, with lower levels of resting RSA associated with higher levels of internalizing symptoms (Beauchaine, 2015).
Recent work provides evidence that the ANS matures throughout the fetal period and in infancy (Mulkey & du Plessis, 2019; Porges & Furman, 2011), and may be susceptible to environmental influences during early development. There is some emerging evidence that postnatal maternal factors may shape the development of infant RSA. Factors such as maternal psychopathology (history of psychiatric disorder) and marital conflict that are associated with maternal distress are also associated with higher infant heart rate and lower infant resting RSA (Dierckx et al., 2009; Moore, 2010; Propper & Holochwost, 2013). This body of work provides evidence that postnatal maternal distress, such as maternal internalizing symptoms, are associated with infant ANS activity. It is important to note that most prior research examining associations between maternal internalizing symptoms and infant RSA does not use repeated measures of maternal internalizing symptoms or infant RSA, which limits our understanding of timing of associations between maternal internalizing symptoms and infant RSA.
Thus, one gap that remains in the literature and aligns with the goals of this study is how postnatal maternal distress may be associated with developmental change in RSA across infancy. Overall, researchers have found that there is rank-order stability in resting RSA across infancy and toddlerhood, with mean increases across time (Bornstein & Suess, 2000; Wagner et al., 2021). However, Wagner and colleagues’s systematic review (2021) concluded that while there is moderate stability in resting RSA across the first three years, there are considerable individual differences in initial levels of resting RSA and variability in trajectories that follow. Additionally, one limitation in previous work is the lack of examination of between-individual versus within-individual changes in resting RSA during development. While there is some evidence for rank-order stability (Bornstein & Suess, 2000), prior analyses examining growth trajectories of RSA reflect a combination of between-individual and within-individual change. Taken together, this suggests that while there may be trait-like components to resting RSA, resting RSA may also be open to environmental influences during this infancy period that may contribute to within-individual changes across time.
Associations between the Development of Negative Emotionality and Maternal Internalizing Symptoms
Temperament traits are biologically-based individual differences that include the domains of activity, reactivity, emotionality, and sociability (Shiner et al., 2012). One dimension of temperamental reactivity is negative emotionality, which typically includes the expression of fear, sadness, and anger. Individual differences in emotional reactivity (i.e., the intensity and duration of negative emotionality in response to contextual demands) may be modulated by emotion regulation processes, and are distinct but related constructs (Davidson, 1998; Sheppes et al., 2015). Anger, sadness, and fear individually, as well as negative emotionality more broadly, have been associated with the development of children’s internalizing symptoms (e.g., Buss et al., 2018; Klein et al., 2012; Crawford et al., 2011; Liu et al., 2018; Eisenberg et al., 2009).
While higher levels of negative emotionality are associated with greater risk for developing internalizing behaviors, not all children high in negative emotionality go on to develop high levels of internalizing symptoms (Buss & McDoniel, 2016; Klein et al., 2012). One possible reason for this inconsistency is that in addition to normative (mean-level) developmental changes in negative emotionality, patterns of change in negative emotionality may also affect risk for developing internalizing symptoms. Longitudinal work on temperamental traits has mainly focused on rank order stability across early childhood, and demonstrates that there is stability across infancy through childhood across multiple temperamental traits (Durbin et al., 2007; Roberts & DelVecchio, 2000). Negative emotionality increases across infancy and toddlerhood, with increases in parent-reported negative emotionality from 9-months to 27-months (Cioffi et al., 2021a; Lipscomb et al., 2011). Studies examining individual domains of parent-reported negative emotionality also find increases across the first year of life in fear (Braungart-Rieker et al., 2010; Gartstein et al., 2010, 2018) and anger (Braungart-Rieker et al., 2010; Dollar & Calkins, 2019). However, studies examining change in rank-order stability of temperament has shown that there is relatively less stability in temperamental traits during infancy (Lemery et al., 1999).
Individual patterns of continuity and discontinuity in negative emotionality across development could be indicative of risk status for internalizing symptom development (Aksan & Lemery, 2000; Goldsmith & Lemery, 2000). Goldsmith and Lemery (2000) suggest that there may be more stability in negative emotionality across time for children who have higher levels of negative emotionality compared to other children their age, which could be indicative of higher risk of developing internalizing disorders. Consistent with this perspective, past research suggests that temperament profiles characterized by more reactivity show more stability across time (Beekman et al., 2015). Additionally, empirical studies in children have consistently demonstrated that stable, high levels of fear are associated with greater anxiety and depressive symptoms at a later assessment (Brooker et al., 2013; Van Hulle et al., 2017). Thus, it is important to consider between-individual differences within normative changes in negative emotionality during infancy, and as well as what factors may contribute to the development of negative emotionality.
Developmental changes in temperament may stem from maturation in systems underlying regulation. These systems have a protracted developmental trajectory, and maturation of these systems (such as the stress response system) may lead to changes in the expression of temperament (Rothbart & Bates, 2006; Shiner et al., 2012). In particular, self-regulation increases across development, and this increase in regulation may begin to influence domains of temperamental reactivity, leading to increases in stability (Shiner et al., 2012). Children’s development of negative emotionality may be more sensitive to social and contextual influences earlier in development as biological mechanisms underlying reactivity and regulation are maturing.
Parents are one source of social influence on the development of reactivity. For example, there is some evidence that parental internalizing symptoms are associated with changes in negative emotionality, including fear and anger (e.g., Potapova et al., 2014; Gartstein et al., 2010). Gartstein and colleagues (2010) demonstrated that fear increased in a linear trend over the first year of life, and that infants with mothers who reported more depressive symptoms exhibited greater increases in fear across the first year and had higher levels of toddler anxiety. Both mothers’ and fathers’ internalizing symptoms have been demonstrated to influence infants’ negative emotionality during the first year of life, although parental internalizing symptoms are often assessed at a single time point. It is less clear how stability (or lack thereof) in parental internalizing symptoms across the infancy period may be associated with developmental changes in infant negative emotionality.
Taken together, it appears there may be both between-individual differences in negative emotionality and within-individual processes that may have important implications for the development of internalizing psychopathology risk. Additionally, examining developmental changes in negative emotionality in the context of infants’ social environment is particularly important as parents play a large role in infant development. Given that individual differences in negative emotionality may play a role in the intergenerational transmission of internalizing symptoms, it is important to consider these between-infant differences and within-infant changes in negative emotionality in the context of maternal internalizing symptoms.
Infant Directed Effects on Maternal Internalizing Symptoms
Lastly, it is important to consider the role that the infant may have on parents. Mothers’ own internalizing symptoms change across time, especially during the early years of parenthood (Ashman et al., 2008; Gump et al., 2009; Skipstein et al., 2012; van der Waerden et al., 2015). Transactional models of development (e.g., Sameroff, 2010) highlight the importance of considering bidirectional influences. Prior work finds evidence for transactional processes within families that maintain anxiety and depressive symptoms of both parents and children across time (e.g., Ahmadzadeh et al., 2019). While there are some studies that have measured maternal internalizing symptoms at multiple time points, most of the work examining associations between maternal internalizing symptoms and either infant negative emotionality or infant resting RSA have only examined maternal internalizing symptoms at a single time point. As a result, less is known about how changes in maternal internalizing symptoms may be associated with the development of infants’ reactivity and regulation.
Additionally, children may play a role in regulating their parent’s affective states and may elicit both greater parent negative affect and stress that, in turn, lead to changes in maternal internalizing symptoms. Infants with more difficult temperaments may exacerbate depressed mothers’ moods (Whiffen & Gotlib, 1989). More specifically, infant and child negative emotionality elicits stronger negative affect in mothers (Murray, 1979; Soltis, 2004), increases in maternal stress (Pesonen et al., 2008), parents’ depressive symptoms (Cioffi et al., 2021b), and anxiety symptoms (Behrendt et al., 2020). Mothers who have internalizing symptoms may struggle to regulate their emotional responses when faced with infant distress, which could negatively impact both early dyadic interactions as well as increase subsequent maternal internalizing symptoms.
Less work has been conducted on how children’s physiological regulation may be associated with changes in maternal internalizing symptoms, making this a critical gap this study will address. One study has demonstrated that trajectories of stable, higher levels of maternal depressive symptoms across time are associated with greater child RSA during a stressor (Gump et al., 2009). Although the work conducted by Gump and colleagues (2009) does not directly test child-directed effects on maternal internalizing symptoms, it suggests that stability/instability in parent internalizing symptoms over time may be associated with children’s physiological regulation. However, there is a lack of evidence for transactional influences with repeated measures of both RSA and maternal internalizing symptoms. In addition, it is unknown if child-directed effects are driven by behavioral responses to stress that are coupled with physiological responses, or if there may be specific biological processes that are influencing the parent.
The Present Study
Understanding the inter-related associations between maternal internalizing symptoms, infant negative emotionality, and infant PNS activity is important because it may help researchers better understand how internalizing symptoms develop and are maintained in families through (1) the development of child negative emotionality and physiological regulation as precursors to child internalizing symptoms, as well as (2) infant-directed effects on maternal postnatal distress. Prior studies have often assessed infant negative emotionality, resting RSA, and maternal internalizing symptoms at a single time point, limiting our understanding of how these constructs may co-develop across time. The current study aims to examine whether the associations between infant markers of risk (i.e., negative emotionality and resting RSA) and maternal internalizing symptoms suggest between-dyad trait-like levels across time, or if there may be time-varying, within-dyad processes. Specifically, the present study examined associations between maternal internalizing symptoms, infant negative emotionality, and infant PNS activity using a random-intercepts cross-lagged panel model (RI-CLPM; Hamaker et al., 2015; Mulder & Hamaker, 2021).
The RI-CLPM is an analytic strategy that allows for a more nuanced understanding of bidirectional associations within dyads across multiple measures, and addresses limitations in other analytic strategies that conflate between- and within-person associations (Hamaker et al., 2015). Using an RI-CLPM, we can examine both within- and between-dyad associations for maternal internalizing symptoms and negative emotionality, as well as maternal internalizing symptoms and resting RSA. Additionally, we will be able to examine within-person and between-person (trait level) associations of negative emotionality and resting RSA.
We hypothesized that we would find associations between trait-levels of maternal internalizing symptoms and negative emotionality, such that higher levels of maternal internalizing symptoms are associated with higher levels of negative emotionality. We also hypothesized that there would be negative associations between trait levels of maternal internalizing symptoms and resting RSA, as well as negative associations between trait levels of negative emotionality and resting RSA. Additionally, we predicted cross-lagged associations between maternal internalizing symptoms and negative emotionality, as well as maternal internalizing symptoms and resting RSA. Lastly, we hypothesized that the only direct child-to-parent effects we would see in the cross-lagged associations would be from negative emotionality to maternal internalizing symptoms, as prior studies suggest that child-directed effects on maternal internalizing symptoms may be behaviorally mediated, as they are the risk factors most directly visible to parents. Thus, we expected to find that RSA may be indirectly associated with maternal internalizing symptoms via children’s negative emotionality.
Method
Participants.
357 infants (50.7% female, 49.2% male) and their parents were recruited from areas surrounding State College, PA, Harrisburg, PA, and Newark, NJ (Perez-Edgar et al., 2021). 298 infants were recruited when infants were 4-months of age (Mage = 4.80 months; SDage = 0.80), with an additional 46 participants enrolled at 8-months (Mage = 8.83 months, SDage = 0.73) and 13 participants enrolled at 12 months (Mage = 12.73, SDage = 1.12). Caregivers identified 16% of infants as African American/Black, 3% as Asian/Pacific Islander, 22% as Latinx, 50% as white, and 8% as mixed race. Across all participants, 14% families reported a household income of $15,000 or less, 6% reported $16,000–20,000, 6% reported $21,000–30,000, 5% reported $31,000–40,000, 6% reported $41,000–50,000, 8% reported $51,000–60,000, and 39% reported an income above $60,000. 17% caregivers declined to provide their annual household income.
For mother’s education, 3% completed grade school only, 5% had some high school, 10% graduated from high school, 16% had some college or trade/technical degree, 20% were college graduates, 16% had graduate training, and 19% had a graduate degree. 11% did not provide their education level. For fathers, 3% completed grade school only, 4% had some high school, 14% graduated from high school, 17% had some college or trade/technical degree, 20% were college graduates, 12% had graduate training and 16% had a graduate degree. 15% did not provide this information.
For the present study, a subset of 217 infants (51.6% female) were included, as they provided at least one time point of usable data for our variables of interest, and mothers were the caregiver that completed the questionnaires.
Procedures and Measures.
As part of the larger study, data were collected when infants were 4-, 12-, 18-, and 24-months. Due to the COVID-19 pandemic prohibiting in lab data collection and the amount of missing physiology data at 24-months (79% of full sample), only data at 4-, 12-, and 18-months were used in the present study.
Questionnaires.
Most parents completed questionnaires via Qualtrics before each laboratory visit at 4-, 8-, 12-, and 18-months. For parents who were unable to complete the questionnaires ahead of time, they were instead completed using a laptop during a laboratory visit. Versions of each questionnaire were available in both English and Spanish and were administered based on the caregiver’s first language.
Maternal internalizing symptoms.
Maternal internalizing symptoms were assessed using the Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI), which were collected at 4-, 8-, 12-, and 18-months. We created a mean score of the BDI and BAI scores at each time point as a composite of maternal internalizing symptoms given the high positive correlations between both measures across timepoints (rs= .50 to .71).
The BDI is a 21-item self-report questionnaire for evaluating the severity of depression in healthy and psychiatric populations. Each item consists of a group of related statements and respondents self-report how they have been feeling for the past week on a four-point scale (0 - symptoms absent, 1 - mildly, 2 - moderately, 3 - severe symptoms). The BDI is scored by adding the ratings for all 21 items, with a score range from 0 to 63. The BDI has good to excellent scale reliability (4M α = .89; 8M α =.86; 12M α =.90; 18M α =.91).
The BAI is a 21-item self-report questionnaire for evaluating the severity of anxiety, designed to distinguish cognitive and somatic symptoms of anxiety from symptoms of depression. Parents rated individual symptoms of anxiety (e.g., fear of losing control) in the past month using a four-point Likert scale (0 - not at all, 1 - mildly, 2 - moderately, 3 - severely). The BAI is also scored by adding the ratings for all 21 items, for a score range from 0 to 63. For both measures, higher scores indicate higher levels of symptom severity. The BAI has good to excellent scale reliability (4M α = .91; 8M α =.88; 12M α =.93; 18M α =.93).
Infant Temperament.
Dimensions of infant temperament were assessed using mothers’ ratings on the Infant Behavior Questionnaire-Revised (IBQ; Putnam et al., 2014) and the Toddler Behavior Assessment Questionnaire (TBAQ; Goldsmith, 1996). Prior studies have demonstrated measurement equivalence between the subscales of the IBQ and the TBAQ (e.g., Goldsmith, 1996). For the present study, a composite of negative emotionality consisting of subscales assessing fear, sadness, and anger (distress to limitations) was used.
The IBQ-R is a 191-item survey designed to assess general patterns of behavior associated with temperament in infancy (3–12 months), Parents rated how often they observed a behavior in the past week at the 4-, 8-, and 12-month time points. Each item describes an infant’s behavior (e.g., During feeding, how often did the baby lie or sit quietly?) using a 7-point scale (1 = never, 7 = always). Parents are also given a “not applicable” response option for use when the infant has not been observed in the situation described. Each item loads onto one of 14 subscales: Activity Level, Distress to Limitations, Fear, Duration of Orienting, Smile/Laughter, High-intensity Pleasure, Low-intensity Pleasure, Soothability, Falling Reactivity, Cuddliness, Perceptual Sensitivity, Sadness, Approach, and Vocal Reactivity. The Distress to Limitations consists of 16 items and has acceptable scale reliability (4M α = .77; 8M α =.84; 12M α =.80). The Fear subscale consisted of 16 items and had excellent scale reliability (4M α =.90, 8M α =.92, 12M α =.91). The Sadness scale consists of 14 items and has acceptable scale reliability (4M α =.82, 8M α =.83, 12M α =.78). The IBQ-R has demonstrated good internal consistency, reliability, and validity, including correlations with laboratory observations (Gartstein & Marmion, 2008; Goldsmith & Campos, 1990; Parade & Leerkes, 2008).
The TBAQ is a 120-item survey designed to assess general patterns of behavior associated with temperament in young children (2–3 years). It was collected at the 12-, and 18-month time points. Parents rated how often their toddler displayed a specific behavior in the past month using a 7-point Likert scale (1 = never, 7 = always). Each item loads onto one of 11 subscales: Activity Level, Anger, Appropriate Attention Allocation, Inhibitory Control, Interest, Object Fear, Perceptual Sensitivity, Pleasure, Sadness, Social Fear, Soothability. Items from each subscale are averaged to obtain scale scores. Goldsmith (1996) reported high levels of convergence with various subscales of the IBQ. There was acceptable scale reliability at 18-months for the anger subscale (α =.77), the sadness subscale (α =.64), the object fear subscale (α =.82), and the social fear subscale (α =.73).
Respiratory Sinus Arrhythmia (RSA).
Infants and their parents participated in a lab visit when infants were 8-, 12-, and 18-months of age. Electrocardiograph (ECG) data from the infant was continuously recorded during a resting baseline. Gelled sensors were placed by the experimenter on the child’s right collarbone, and lower left and right rib prior to resting baseline. Resting RSA was 4 minutes in duration, and the infant was sitting on a parent’s lap and given non-stimulating toys to keep them occupied. Parents were instructed to avoid interacting with their infants and to keep as neutral as possible. Ambulatory data collection equipment (PDA) was attached to the back of the parent’s chair. At the 18-month visit, the infant wore the PDA inside a small backpack to allow for locomotor activity, although infants were seated on their mothers’ laps during the resting task.
ECG was sampled at a rate of 500ms using Mindware MW1000A PDA devices and BioLab system (Mindware Technologies Ltd., Westerville, OH). Data were analyzed offline using the Mindware editing program Mindware HRV, Versions 3.1.4 and 3.1.5, which identified interbeat intervals, and detected physiologically improbable intervals using a validated algorithm. Trained personnel visually inspected ECG data for R-peak and artifact identification. Artifacts in the data from infant crying were excluded from RSA calculations. RSA was calculated in 30-second epochs using the 0.240–1.040 Hz power band, consistent with prior work establishing this range as appropriate for infants and toddlers (Bar-Haim et al., 2000). For participants with less than 4 minutes of data, a minimum of 10 seconds in an epoch at the end of the episode were required to be included in the calculation in order to reduce data loss. Epoch length did not significantly predict RSA after accounting for nesting by participant within our data. On average, participants had 7.91 epochs across all ages. The mean RSA value of all epochs across the resting task was used for analyses. 96.0% of participants’ data were usable at 8-months, 94.7% of participants’ data were usable at 12-months, and 99.2% of participants’ data were usable at 18-months.
Analytic Plan.
Missing Data.
Of note, the full sample (N=357) contained data that was not missing not at random, as participants missing RSA data differed significantly on negative emotionality, with infants who did not provide any RSA data at subsequent timepoints having significantly higher negative emotionality scores at 4M than infants who provided at least 1 time point of usable data. Thus, the decision was made to use a subsample of infants who provided at least 1 time point of usable data for our variables of interest. While the subsample of data (N=217) was not missing completely at random (MCAR) based on Little’s MCAR test (Little, 1988), we tested if missing data differed based on our variables of interest as well as demographic data (e.g., if mothers with and without internalizing symptom scores at 4-months significantly differed based on infant negative emotionality scores). There were no significant differences between data that was and was not missing within the subsample, thus data was established to be missing at random. Thus, full information maximum likelihood (FIML) was used to account for missing data. FIML estimator in multiple regression models with missing data have shown to produce less biased parameter estimates, especially compared to listwise deletion, pairwise deletion and mean imputation (Enders, 2001).
Random-Intercept Cross-Lagged Panel Model.
A random-intercept cross-lagged panel model (Hamaker et al., 2015) is an extension of the traditional cross-lagged panel model that helps account for stable, trait-like differences between units. A model including random intercepts for maternal internalizing symptoms, infant negative emotionality, and infant RSA, autoregressive paths for maternal internalizing symptoms, infant negative emotionality and infant RSA, and cross-lagged paths between maternal internalizing symptoms, infant negative emotionality and infant RSA was fit to the data using the lavaan package (Rosseel, 2012) in the R Environment for Statistical Computing. Random intercepts are latent variables with the repeated measures as its indicators, and all factor loadings are fixed to 1. Auxiliary variables in the model included biological sex of the child, and prenatal medication use. While we do not hypothesize sex (as assigned at birth) differences in our analyses as prior studies on gender differences in temperament only found a very small difference between boys and girls in fear, and no differences in anger (Else-Quest et al., 2006). However, there are findings that suggest parents’ responses to children’s negative emotionality may differ based on child sex (e.g., Park et al., 1997). Thus, child sex was entered as an auxiliary variable. Significant prenatal medication use (including selective serotonin reuptake inhibitor use, anxiolytics, and psychotics) was included as an auxiliary variable as there is some evidence for associations between prenatal medications and infant neurobiology (e.g., Gemmel et al., 2018; Gentile, 2010). The lavaan package uses FIML to account for unbiased estimates of data when data is missing at random. Model fit was evaluated using χ2, the comparative fit index (CFI), standardized root mean square residual (SRMR), and the root mean square error of approximation (RMSEA). Good model fit is indicated by p < .05, CFI >= .95, TLI >= .95, SRMR <= .05, RMSEA <= .05 (Hu & Bentler, 1999).
Results
Descriptive Statistics
Table 3.1 contains the descriptive statistics and correlations among all key variables. Infant negative emotionality was significantly correlated across all timepoints (rs = .46 to .70). Maternal internalizing symptoms were significantly correlated across all timepoints (rs = .48 to .72). RSA was also significantly correlated across timepoints (rs = .39 to .54).
Random-Intercept Cross-Lagged Panel Model.
The RI-CLPM fit the data well (Figure 1), χ2 = 20.40, p = .01, CFI = 0.99, TLI = 0.97, RMSEA = .03, SRMR = .03.
Figure 1.
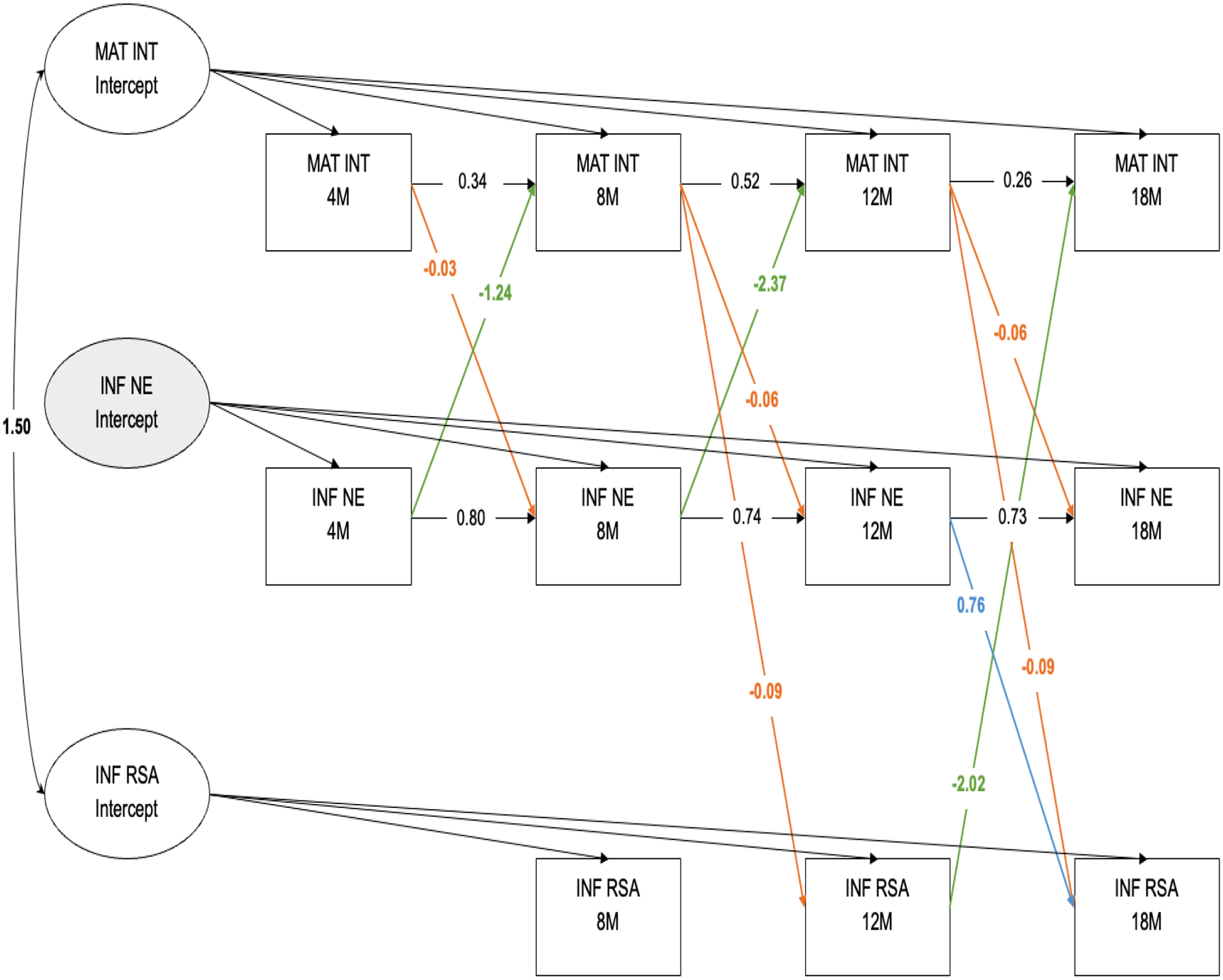
Random-Intercepts Cross-Lagged Panel Model of Maternal Internalizing Symptoms, Infant Negative Emotionality and Infant Resting RSA from 4-months to 18-months. Significant cross-lagged paths (p < .05) are indicated by estimates and solid lines.
MAT INT = Maternal Internalizing Symptoms composite. INF NE = Infant Negative Emotionality. INF RSA = Infant Resting RSA
Intercept factors in a RI-CLPM account for trait-like levels, and our model showed that there was significant variance in maternal internalizing symptoms (10.77, p < .01) and infant RSA (0.473, p < .01), indicating stable, between-persons differences across time. The intercepts for maternal internalizing symptoms and infant RSA were also significantly associated (B=1.50, p < .01). There was no significant variance in negative emotionality in this sample, suggesting that there are no stable between-infant differences, and that each infant fluctuates around the same individual level means across time. The non-significant variance for infant negative emotionality was subsequently fixed to 0 given that including a non-significant intercept in the model is redundant (Mulder & Hamaker, 2021). Thus, negative emotionality scores reflect both trait-like levels as well as within-person fluctuations as there was no meaningful random intercept.
Concurrent Covariances
Concurrent covariances were included in the model, but not included in Figure 1. At 4-months, there was a significant covariance between maternal internalizing symptoms and child negative emotionality, B=−0.64, p=.04. At 8-months, there was a significant covariance between maternal internalizing symptoms and RSA, B=−1.06, p < .01. At 12-months, there was a significant covariance between maternal internalizing symptoms and child RSA, B=−1.08, p=.02. Finally, at 18-months, there was a significant covariance between maternal internalizing symptoms and child negative emotionality, B=−1.25, p=.03.
Autoregressive Paths
Autoregressive paths for maternal internalizing symptoms were significant from 4-months to 8-months (B=0.34, p<.01), from 8-months to 12-months (B=0.52, p<.01), and from 12-months to 18-months (B=0.26, p=.03). For child negative emotionality, there was a significant autoregressive path from 4-months to 8-months (B=0.80, p<.01), from 8-months to 12-months (B=0.74, p<.01), and from 12-months to 18-months (B=0.73, p < .01). There were no significant autoregressive paths for infant RSA.
Cross-Lagged Paths
For interpretation of cross-lagged paths after accounting for the random intercepts, levels of maternal internalizing symptoms and infant RSA are relative to an individual’s mean across time. Across all time points, higher maternal internalizing symptoms relative to the individual’s mean across time were associated with infant negative emotionality at the subsequent time point. Higher maternal internalizing symptoms at 4-months was associated with lower negative emotionality at 8-months (B=−0.03, p<.01). Higher maternal internalizing symptoms at 8-months was associated with lower negative emotionality at 12-months, B=−0.06, p<.01. Similarly, higher maternal internalizing symptoms at 12-months was also associated with lower negative emotionality at 18-months, B=−0.06, p<.01. Additionally, 8-month maternal internalizing symptoms were negatively associated with 12-month infant resting RSA (B=−0.09, p < .01). Similarly, 12-month maternal internalizing symptoms were negatively associated with 18-month infant resting RSA (B=−0.09, p<.01).
Negative emotionality at 12-months was associated with resting RSA at 18-months (B=0.76, p=.03). There were no cross-lagged paths from RSA to infant negative emotionality.
Lastly, the results supported the hypothesis that there would be infant-directed effects on maternal internalizing symptoms. Negative emotionality was negatively associated with maternal internalizing symptoms at the subsequent time point prior to 18-months: 4-months to 8-months (B=−1.24, p=.04), from 8-months to 12-months (B=−2.37, p=.02). Additionally, infant RSA at 12-months was associated with maternal internalizing symptoms at 18-months (B=−2.07, p=.01) but not at any earlier timepoints.
Discussion
The present study aimed to better understand the inter-relations between maternal internalizing symptoms, infant negative emotionality, and infant PNS activity across the first two years of life as a possible mechanism by which intergenerational transmission of internalizing symptoms may occur. Specifically, our model was able to examine transactional associations that furthered our understanding of (1) the co-development of child negative emotionality and physiological regulation in the context of maternal internalizing symptoms, and (2) infant-directed effects on maternal postnatal internalizing symptoms. In particular, by accounting for between-person levels, we are able to examine how within-person changes relative to one’s mean in one construct may lead to within-person changes in another, and vice versa. While associations between observed variables may be informative for understanding how constructs are generally associated with one another, parsing out the between- and within-dyad associations can provide targets for intervention based on individual differences in risk as well as optimal points during development based on within-dyad fluctuations.
While we were not able to directly test the association between infant negative emotionality, infant resting RSA, and child internalizing symptoms, the present study provides important preliminary evidence that maternal internalizing symptoms may impact the development of child risk for developing internalizing symptoms and furthers our understanding of how internalizing symptoms may develop and be maintained within families during infancy.
Individual differences in maternal internalizing symptoms and resting RSA
We found stable, between-persons differences in both maternal internalizing symptoms during the postnatal period from 4-months to 18-months. This is consistent with prior person-centered work examining maternal internalizing symptom trajectories across infancy to childhood. For example, Ashman and colleagues (2008) show that there are three trajectories of maternal internalizing symptoms from infancy to childhood: stable, low levels of internalizing symptoms, stable/persistent high internalizing symptoms, and decreasing internalizing symptoms. Similarly, we found evidence for stable, between-persons differences in infant resting RSA, consistent with findings from prior work that demonstrates that there are individual differences in levels of resting RSA during infancy (Wagner et al., 2021). It is important to note there was no evidence for a “trait” component of resting RSA during the first two years of life in more diverse samples (e.g., low-income Mexican American infants) (Jewell et al., 2018). This work suggests that continuity in levels of resting RSA may not be universal, and the broader environment (beyond the parent-child dyad) may also play a role.
Contrary to our hypotheses, we did not find a significant intercept for infant negative emotionality, which suggests that there were no stable, between-infant differences across time, and that each infant fluctuates around the same individual level mean. One possibility is that temperamental traits such as negative emotionality are not stable during infancy, and thus we do not find stable, between-infant differences from 4- to 18-months. This is consistent with work by Lemery and colleagues (1999) that show that different dimensions of temperament, including distress-anger and fear, are less stable from 3-months to 18-months. It may be that individual differences are not emerging during this early developmental period as there is a heavy reliance on caregivers for regulation. Future research should examine these associations into early childhood and school entry as a possible transitional period where individual differences in negative emotionality may emerge.
An alternative explanation for the lack of stable, between-person differences in infant negative emotionality is rooted in our specific analytic sample, as infants who provided usable resting RSA data had significantly lower levels of negative emotionality relative to the full sample. Our RSA data were not missing at random, as infants without any usable RSA data at any time point had significantly higher scores for 4-month negative emotionality than infants who provided usable RSA data for at least one time point. It is possible that stability in negative emotionality is only present for infants who are at the higher end of the distribution compared with their same-aged peers, and thus at higher risk for internalizing symptom development (Goldsmith & Lemery, 2000). Brooker and colleagues (2013) found four different trajectories of stranger fear from 6-months to 36-months, but only 11.8% of infants remained high and stable across time while the rest of the infants exhibited change across time. In particular, the infants in the high, stable group were associated with later behavioral inhibition. Behavioral inhibition, in turn, is a temperament trait associated with sensitivity to social novelty in early childhood, and the later emergence of internalizing difficulties (Pèrez-Edgar & Fox, 2005). Indeed, the specific association between behavioral inhibition and social anxiety emerges for children with high, and stable, levels of temperamental risk. It is possible that infants in this study who may be at the higher end of the distribution may not have provided usable physiological data, and thus we did not find significant variance in the random intercept of negative emotionality.
Associations between Maternal Internalizing Symptoms and Infant Negative Emotionality
Results from the present study also provided evidence for within-dyad associations between maternal internalizing symptoms and infant negative emotionality and infant resting RSA. Higher levels of maternal internalizing symptoms (relative to the mean) were associated with lower levels of negative emotionality at the next time point from 4-months to 12-months. This is contrary to our hypotheses, as we expected to find that higher levels of maternal internalizing symptoms would be associated with higher levels of infant negative emotionality given prior research reporting positive associations between maternal internalizing symptoms and higher levels of negative emotionality (e.g., Gartstein et al., 2010).
One possible explanation is that our sample is relatively low-risk with respect to overall low levels of maternal internalizing symptoms. Prior research has often recruited mothers who meet clinical criteria for an anxiety or depression diagnosis. In our sample, our measure of maternal internalizing symptoms may not reflect psychopathology, and instead reflect normative levels of maternal distress. Additionally, there is some emerging evidence that a midrange of anxiety symptoms may be associated with better infant outcomes as a function of higher maternal sensitivity to infant cues (e.g., Lemus et al., 2022). Our sample was not recruited for elevated levels of maternal internalizing symptoms, and thus our findings of negative associations between maternal internalizing symptoms and subsequent infant negative emotionality may reflect this idea that some maternal internalizing symptoms may be optimal for infant socioemotional development, as mild to moderate levels of maternal internalizing symptoms may be mediated by positive parenting behaviors.
It is also possible that when we examine maternal postpartum distress as a combination of maternal depressive and anxiety symptoms, we are not accounting for differential effects of maternal depressive and anxiety symptoms on infant socioemotional development that may be mediated by different parenting behaviors. Of note, there were low rates of maternal depressive symptoms in our sample, and so our findings may be explained by relatively higher levels of anxiety. For example, there is research that demonstrates that clinically anxious mothers do not show deficits in sensitivity; rather, higher levels of maternal anxiety symptoms were associated with increased attunement to 12-month-old infants’ emotional arousal (Kaitz et al., 2010; Smith et al., 2021). Recent work also demonstrates that maternal sensitivity is associated with lower toddler negative emotionality, but that these associations were moderated by maternal emotion dysregulation (Bailes & Leerkes, 2023). Future research should aim to examine if there are dimensions of parenting behaviors, such as parental sensitivity, that may help potentially explain the negative associations between within-person change in maternal internalizing symptoms on infant negative emotionality.
While we did not find significant variance in the intercept factor for negative emotionality, we found continuity in negative emotionality across time. All autoregressive paths for negative emotionality were significantly associated, representing within-person carry-over effects. That is, an infant higher on negative emotionality at one time point is likely to experience higher negative emotionality at the next occasion as well. This is consistent with work that demonstrates increases in negative emotionality across infancy and toddlerhood (Aktar & Pérez-Edgar, 2020; Brooker et al., 2013; Gartstein et al., 2010).
Associations between Maternal Internalizing Symptoms and Infant Resting RSA
We also found a significant, positive association between the trait-like levels of maternal internalizing symptoms and infant RSA. Mothers who have higher overall maternal internalizing symptoms across time have infants with higher levels of resting RSA. Higher levels of resting RSA are often thought to reflect a greater capacity for physiological regulation, and are linked with better socioemotional outcomes (Beauchaine, 2001; Porges, 2007). Infants with mothers higher in internalizing symptoms may be exposed to more situations that require self-regulation than infants with mothers with lower internalizing symptoms, thus developing higher levels of resting RSA in order to meet contextual demands. However, another interpretation is that higher levels of resting RSA also reflect greater sensitivity to the environment. For instance, studies have shown that resting RSA often moderates associations between environmental factors and child socioemotional outcomes (e.g., Blandon et al., 2008). Thus, it is possible that infants with higher trait levels of RSA are more sensitive to their environments, which in turn supports the emergence of positive associations between maternal traits and infant outcomes.
Additionally, there were within-dyad associations between maternal internalizing symptoms and subsequent levels of infant resting RSA even after accounting for between-dyad associations. Within each mother-infant dyad, mothers exhibiting higher levels of internalizing symptoms relative to their mean had infants with subsequent lower levels of resting RSA relative to their mean from 8- to 18-months. Importantly, the between-person associations were opposite of the within-person’s associations between maternal internalizing symptoms and resting RSA. While higher levels of overall maternal internalizing symptoms are associated with higher levels of infant resting RSA, there are negative cross-lagged associations indicating that maternal internalizing symptoms at the prior time point are associated with later lower resting RSA relative to trait levels. Mothers who exhibit higher levels of internalizing symptoms relative to their mean may have children whose resting RSA are becoming lower relative to their mean, indicating the development of poorer physiological regulation.
Co-Development of Infant Negative Emotionality and Infant Resting RSA in the context of Maternal Internalizing Symptoms
After accounting cross-lagged paths from maternal internalizing, we did not find reciprocal associations between infant negative emotionality and infant resting RSA from 4- to 12-months. Our results suggest that negative emotionality and RSA are not associated with one another during the first year of life. At 12-months, higher levels of negative emotionality were positively associated with subsequent 18-month RSA. Prior work has demonstrated negative associations between resting RSA and emotional reactivity during infancy, such that higher levels of resting RSA were associated with shorter latency to negative affect during an affective task (Calkins, 1997). However, these associations were found during concurrent measures of negative emotionality and resting RSA during early childhood (Calkins, 1997).
Turning to literature on behavioral measures of reactivity and regulation during infancy, studies have found that 5-month reactivity was negatively associated with 10-month regulation (Braungart-Rieker & Stifter, 1996). Our findings of positive associations between negative emotionality and resting RSA are opposite to findings from prior work. It is important to note that the co-development of negative emotionality and resting RSA is examined in the present study within the context of the mother-infant dyad. The direction of effects may be, in part, due to accounting for associations with maternal internalizing symptoms, as well as accounting for trait-like levels of resting RSA. The discrepancy in findings highlight the importance of considering how infants are developing within a social context, as parental characteristics may have influences on infants’ emotional reactivity and regulation. After accounting for the effects of maternal internalizing symptoms on 18-month RSA, higher levels of negative emotionality are associated with higher than expected levels of RSA after accounting for an infant’s mean resting RSA across time. Additionally, given that we did not find between-individual differences in negative emotionality and could not examine associations with RSA on a between-individuals level, it may be that we see a positive association emerge in the cross-lagged associations instead. Similarly, one way to interpret the positive cross-lagged association may be that resting RSA reflects greater sensitivity to the environment such that higher levels of negative emotionality (as a marker of greater reactivity to contextual demands) is positively associated with greater physiological sensitivity to context.
Additionally, maternal internalizing symptoms have been associated with infant negative emotionality via the development of the ANS (de Vente et al., 2020). Prior work on developmental changes in temperament also suggests that maturational changes in biological systems in the individual underlies within-person change in temperament (e.g., Gartstein et al., 2010). However, our findings did not support this hypothesis, as we did not find cross-lagged associations from resting RSA to negative emotionality. It may be that there are different biological processes underlying negative emotionality that resting RSA does not influence at this point in development, or relations are found at more extreme levels of negative emotionality. Future work should examine other biological indices that may be associated with the development of negative emotionality or other behavioral indices of reactivity.
Infant-Directed Effects on Maternal Internalizing Symptoms
Consistent with our hypotheses and extant literature, we also found evidence for infant-directed effects on maternal internalizing symptoms. Both infant negative emotionality and infant resting RSA may elicit changes in maternal internalizing symptoms, although cross-lagged associations between infant resting RSA and subsequent maternal internalizing symptoms only emerge after the first year of life.
We found negative associations between infant negative emotionality and subsequent change in maternal internalizing symptoms during the first year of life, such that higher negative emotionality was associated with lower maternal internalizing symptoms relative to the mean. This is contrary to our hypotheses as we expected to find that higher levels of negative emotionality would be associated with higher levels of maternal internalizing symptoms at the next time point. Infants expressing higher levels of negative affect and negative emotionality often elicit stronger maternal negative affect and increased internalizing symptoms (Whiffen & Gotlib, 1989; Murray, 1979; Behrendt et al., 2020). However, it is important to note that prior studies did not account for trait levels of maternal internalizing symptoms, and did not examine repeated measures of infant or maternal constructs. Effects that were not evident in prior studies, as well as bivariate correlations within the same dataset, highlight the importance of considering both individual differences and temporal precedence to understand how maternal internalizing symptoms and child negative emotionality may co-develop.
One interpretation of this finding is that not all negative emotionality may be indicative of risk for the development of socioemotional problems. It is likely that negative emotionality plays an adaptive role during infancy when infants are reliant on caregivers for regulation. From a functionalist perspective on emotion, fear, sadness, and anger have adaptive functions: for example, anger is associated with restoration of progress towards a goal and effecting a change in the behavior of a social other, while fear is associated with avoidance of pain, and alerting others to avoid the situation or help (Campos et al., 1983; Bornstein et al., 2014). Negative emotionality may be adaptive and elicit parenting behaviors such as maternal sensitivity as it alerts parents to the needs of the infant. Prior research has demonstrated that child distress is also associated with children seeking out comfort from parents (Buss et al., 2004). It may be that the adaptive nature of negative emotionality during this developmental period may be associated with fluctuations in maternal internalizing symptoms and lower parental distress. It is important to note that our measure of infant negative emotionality captures broad dimensions of negative emotionality in multiple contexts, and future research should consider examining negative emotionality in different contexts as a potential risk factor that may be positively associated with maternal internalizing symptoms. For example, work by Buss and colleagues (2021) demonstrate that behavioral fear in low-threat contexts during toddlerhood was associated with both maternal overprotection, as well as maternal general anxiety symptoms. Researchers may find different associations between negative emotionality and maternal internalizing symptoms when considering infant negative emotionality in different contexts.
Next, the finding that higher levels of resting RSA at 12-months relative to an infant’s average RSA was associated with lower maternal internalizing symptoms relative to trait levels of maternal internalizing symptoms suggests that better regulatory capability is associated with subsequent decrease in mothers’ internalizing symptoms. We found a direct effect from resting RSA to maternal internalizing symptoms from 12-months to 18-months, and did not find evidence that this association may be behaviorally mediated by negative emotionality. Infants higher in resting RSA relative to their own mean may be exhibiting more regulatory behaviors contributing to the decrease in maternal distress at the subsequent time point. As negative emotionality is capturing dimensions of behavioral reactivity, there may be behavioral regulation that may be mediating associations between resting RSA and maternal internalizing symptoms. Taken together, there is evidence for joint contributions of reactivity and regulation that may play a role in changes in maternal internalizing symptoms across infancy.
Limitations and Future Directions
It is important to interpret findings from the current study with limitations in mind. One consideration is that mothers rated both their internalizing symptoms as well as infant temperament. There have been some mixed findings on how maternal internalizing disorders or symptoms may be associated with their perceptions of infant behavior. For example, there is some evidence that mothers higher in depressive symptoms were more likely to report more problems than their child’s self-report (van der Toorn et al., 2010). However, Olino and colleagues (2021) found little psychometric evidence for maternal psychopathology biasing reports of child psychopathology symptoms. In the current study, our measure of infant temperament may be a better reflection of mothers’ interpretations of infant negative emotionality. Future work should aim to replicate these findings using a different caregiver’s report of temperament, or external observer scores of negative emotionality to ensure that maternal symptoms are not biasing infant negative emotionality scores.
Another methodological consideration of the physiology data used in the current study is that we did not collect movement and respiration data. While movement artifacts were visually inspected by trained personnel and accounted for in data processing, respiration rate was not accounted for. Thus, measures of RSA that do not account for respiration rate may reflect a combination of both cardiac vagal control and other changes in respiration independent of the vagus nerve (Houtveen et al., 2002; Allen et al., 2007; Ritz et al., 2012). However, there are also studies that establish that RSA uncorrected for respiration is accurately reflecting PNS activity during tasks where respiration is not expected to greatly vary (Houtveen et al., 2002; Allen et al., 2007). Given that we did not expect respiration to greatly vary during our resting task, our physiology data may still primarily reflect vagal activity even without accounting for respiration rate.
Additionally, we do not have measures of prenatal maternal internalizing symptoms and distress and therefore we are unable to account for any associations between prenatal maternal internalizing symptoms, infant negative emotionality, and infant RSA. This is important as there is research suggesting that prenatal maternal distress can play a role in fetal programming, especially with stress response systems (Del Giudice, 2012). Additionally, empirical studies have found associations between prenatal maternal internalizing symptoms and infant RSA (Propper & Holochwost, 2013), as well as associations between prenatal maternal distress and behavioral fear (Nolvi et al., 2019). However, one study that accounted for both prenatal and postnatal developmental periods only found associations between maternal emotion dysregulation and infant RSA during the postnatal period, but did not find evidence for associations between prenatal maternal emotion dysregulation and postnatal infant RSA (Gao et al., 2022). These studies suggest that the associations between maternal internalizing symptoms, infant negative emotionality, and infant RSA may be largely due to genetic and environmental processes as opposed to prenatal programming—however, future studies should aim to test these associations directly to disentangle prenatal programming from environmental processes.
Paternal mental health during infancy is also associated with infant socioemotional development. For example, there is a study showing that paternal anxiety symptoms are associated with infant temperament (Potapova et al., 2014). Additionally, infant negative emotionality is also associated with fathers’ depressive symptoms (Cioffi et al., 2021b). More broadly, paternal anxiety is prospectively associated with child anxiety, but maternal anxiety is also associated with subsequent paternal anxiety (Ahmadzadeh et al., 2019), highlighting the importance of considering the family unit as a whole.
An additional consideration is the range of negative emotionality in infants and maternal internalizing symptoms in this sample. As aforementioned, infants that did not provide any RSA data were significantly higher in negative emotionality at 4-months than the infants that provided usable RSA data. Therefore, we cannot assume that these associations hold when there are infants who exhibit higher negative emotionality. Using imputation methods may help account for missing data, but one major limitation of doing so would be assuming that the associations between variables are linear even at the higher end of the distribution for negative emotionality. Similarly, our sample was not recruited for elevated, clinical levels of postpartum maternal internalizing symptoms. It is less clear if these associations would be the same in samples that include a wider range of maternal internalizing symptoms. Future research should aim to examine if these associations remain the same when samples include both infants with higher levels of negative emotionality and mothers with higher levels of internalizing symptoms.
Conclusions
The present study extends the current literature by examining both between- and within-dyad associations between maternal internalizing symptoms, infant negative emotionality and infant resting RSA during the first two years of life. Specifically, the findings from the present study provide evidence for both mother-to-infant as well as infant-to-mother associations over time. The bidirectional associations from the present study suggest that while maternal internalizing symptoms are associated with the development of risk for child internalizing behaviors, infant-directed effects may also serve to maintain the presence of internalizing behaviors and symptoms within families. A better understanding of these transactional processes provides some evidence for processes that may underlie intergenerational associations between parent and child internalizing behaviors, and may have implications for targets for prevention and intervention for both mothers and their children during infancy and toddlerhood. The use of longitudinal methods can help elucidate the nuances in development that occur on multiple levels of analysis, and further our understanding of the importance of parental internalizing symptoms in the co-development of child reactivity and regulation.
Table 1.
Means, standard deviations, and correlations with confidence intervals.
| Variable | M (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. INF NE 4 | 2.94 (0.68) | ||||||||||
| 2. INF NE 8 | 3.23 (0.73) |
.70
**
[.61,.77] |
|||||||||
| 3. INF NE 12 | 3.45 (0.71) |
.49
**
[.36,.60] |
.62
**
[.51,.71] |
||||||||
| 4. INF NE 18 | 0.64 (0.64) |
.53
**
[.39,.64] |
.47
**
[.33,.58] |
.46
**
[.33,.58] |
|||||||
| 5. MAT INT 4 | 6.01 (5.63) | .09 [−.06,.23] |
.11 [−.05,.26] |
.11 [−.06,.27] |
.12 [−.06,.28] |
||||||
| 6. MAT INT 8 | 4.86 (4.98) | .13 [−.03,.28] |
.22
**
[.08,.36] |
.16 [−.00,.32] |
.12 [−.05,.28] |
.72
**
[.63,.79] |
|||||
| 7. MAT INT 12 | 6.11 (7.06) | .07 [−.11,.24] |
.22
*
[.05,.38] |
.23
**
[.07,.38] |
−.03 [−.20,.14] |
.52
**
[.38,.64] |
.64
**
[.52,.73] |
||||
| 8. MAT INT 18 | 5.69 (6.56) | .04 [−.13,.21] |
.15 [−.02,.31] |
.17 [−.00,.33] |
−.01 [−.17,.15] |
.48
**
[.33,.61] |
.60
**
[.47,.70] |
.55
**
[.41,.66] |
|||
| 9. RSA 8 | 3.54 (0.97) | −.02 [−17,.14] |
.05 [−.09,.20] |
.08 [−.08,.23] |
.01 [−.16,.17] |
.02 [−.13,.18] |
.11 [−.04,.25] |
.10 [−.08,.27] |
.15 [−.02,.31] |
||
| 10. RSA 12 | 3.78 (0.91) | .11 [−.07,.28] |
.20
*
[.03,.36] |
.11 [−.06,.27] |
.16 [−.01,.33] |
−.08 [−.26,.10] |
.12 [−.06,.30] |
.01 [−17,.19] |
−.05 [−.23,.13] |
.54
**
[.41,.65] |
|
| 11. RSA 18 | 3.93 (0.98) | .08 [−.12,.28] |
.12 [−.09,.31] |
.07 [−.13,.26] |
.14 [−.05,.33] |
−.14 [−.33,.07] |
−.03 [−.23,.18] |
−.05 [−.26,.16] |
.07 [−.13,.27] |
.39
**
[.22,.55] |
.51
**
[.35,.65] |
Note. M and SD are used to represent mean and standard deviation, respectively. Values in square brackets indicate the 95% confidence interval for each correlation.
indicates p < .05.
indicates p < .01.
Highlights:
Maternal factors may play a role in infant reactivity and regulation development
Infants may also influence their mothers’ internalizing symptoms
Findings show complex, bidirectional associations between mothers and infants
Acknowledgments
Data for this study is supported by grants from National Institutes of Health awarded to Pérez-Edgar, Buss, and LoBue (R01MH109692). Drs. Buss and Pérez-Edgar’s Psychology Professorships are supported by the Social Science Research Institute of The Pennsylvania State University, and endowments through the Tracy Winfree and Ted H. McCourtney Professorship in Children, Work, and Families (Buss) and the McCourtney Professorship of Child Studies (Pérez-Edgar). The authors declare they have no conflicts of interest. We would like to thank the families involved with the study, as well as the staff and larger research team for their contributions to the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadzadeh YI, Eley TC, Leve LD, Shaw DS, Natsuaki MN, Reiss D, Neiderhiser JM, & McAdams TA (2019). Anxiety in the family: A genetically informed analysis of transactional associations between mother, father and child anxiety symptoms. Journal of Child Psychology and Psychiatry, 60(12), 1269–1277. 10.1111/jcpp.13068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksan N, & Lemery KS (2000). The Role of Emotion in the Development of Child Psychopathology. In Davidson RJ, Anxiety, Depression, and Emotion (pp. 266–280). Oxford University Press, Inc. [Google Scholar]
- Aktar E, & Pérez-Edgar K (2020). Infant emotion development and temperament. In Lockman J, Tamis-LeMonda C (Eds.) The Cambridge Handbook of Infant Development, 715–741. Cambridge University Press. [Google Scholar]
- Allen JJB, Chambers AS, & Towers DN (2007). The many metrics of cardiac chronotropy: a pragmatic primer and a brief comparison of metrics. Biological Psychology, 74(2), 243–262. 10.1016/j.biopsycho.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Ashman SB, Dawson G, & Panagiotides H (2008). Trajectories of maternal depression over 7 years: Relations with child psychophysiology and behavior and role of contextual risks. Development and Psychopathology, 20(1), 55–77. 10.1017/S0954579408000035 [DOI] [PubMed] [Google Scholar]
- Bailes LG, & Leerkes EM (2023). Transactional associations between infant negative emotionality and maternal sensitivity: Maternal emotion dysregulation as a moderator. Journal of Family Psychology. Advance online publication. 10.1037/fam0001060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ and Fox NA (2000), Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Dev. Psychobiol, 37: 44–56. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integratedmodel of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13(2), 183–214. 10.1017/S0954579401002012 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory Sinus Arrhythmia: A Transdiagnostic Biomarker of Emotion Dysregulation and Psychopathology. Current opinion in psychology, 3, 43–47. 10.1016/j.copsyc.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman C, Neiderhiser JM, Buss KA, Loken E, Moore GA, Leve LD, Ganiban JM, Shaw DS, & Reiss D (2015). The Development of Early Profiles of Temperament: Characterization, Continuity, and Etiology. Child Development, 86(6), 1794–1811. 10.1111/cdev.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt HF, Wade M, Bayet L, Nelson CA, & Bosquet Enlow M (2020). Pathways to social-emotional functioning in the preschool period: The role of child temperament and maternal anxiety in boys and girls. Development and Psychopathology, 32(3), 961–974. 10.1017/S0954579419000853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, & O’Brien M (2008). Individual differences in trajectories of emotion regulation processes: The effects of maternal depressive symptomatology and children’s physiological regulation. Developmental Psychology, 44(4), 1110–1123. 10.1037/0012-1649.44.4.1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Arterberry ME, & Lamb ME (2014). Development in infancy: A contemporary introduction (Fifth edition). Psychology Press, Taylor & Francis Group. [Google Scholar]
- Bornstein MH, & Suess PE (2000). Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Developmental Psychology, 36(1), 54–65. 10.1037/0012-1649.36.1.54 [DOI] [PubMed] [Google Scholar]
- Braungart-Rieker JM, Hill-Soderlund AL, & Karrass J (2010). Fear and anger reactivity trajectories from 4 to 16 months: The roles of temperament, regulation, and maternal sensitivity. Developmental Psychology, 46(4), 791–804. 10.1037/a0019673 [DOI] [PubMed] [Google Scholar]
- Braungart-Rieker JM, & Stifter CA (1996). Infants’ Responses to Frustrating Situations: Continuity and Change in Reactivity and Regulation. Child Development, 67(4), 1767–1779. [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, Lemery-Chalfant K, Aksan N, Davidson RJ, & Goldsmith HH (2013). The development of stranger fear in infancy and toddlerhood: Normative development, individual differences, antecedents, and outcomes. Developmental Science, 16(6), 864–878. 10.1111/desc.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Davis EL, Ram N, & Coccia M (2018). Dysregulated Fear, Social Inhibition, and Respiratory Sinus Arrhythmia: A Replication and Extension. Child Development, 89(3), e214–e228. 10.1111/cdev.12774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, & McDoniel ME (2016). Improving the Prediction of Risk for Anxiety Development in Temperamentally Fearful Children. Current Directions in Psychological Science, 25(1), 14–20. 10.1177/0963721415611601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD (1997). Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology, 31(2), 125–135. [DOI] [PubMed] [Google Scholar]
- Campos JJ, Barrett KC, Lamb ME, Goldsmith HH, & Stenberg C (1983). Socioemotional development. In Mussen P (Series Ed.) & Campos JJ & Haith MH (Vol. Eds.), Handbook of child psychology: Vol 2. Infancy and developmental psychobiology New York: Wiley. [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Pérez-Edgar K, Henderson HA, Diaz Y, Raggi VL, & Fox NA (2009). Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi CC, Griffin AM, Natsuaki MN, Shaw DS, Reiss D, Ganiban JM, Neiderhiser JM, & Leve LD (2021a). The role of negative emotionality in the development of child executive function and language abilities from toddlerhood to first grade: An adoption study. Developmental Psychology, 57(3), 347–360. 10.1037/dev0000972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi CC, Leve LD, Natsuaki MN, Shaw DS, Reiss D,C Ganiban JM, & Neiderhiser JM (2021b). Examining reciprocal associations between parent depressive symptoms and child internalizing symptoms on subsequent psychiatric disorders: An adoption study. Depression and Anxiety, 38, 1211–1224. 10.1002/da.23190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NA, Schrock M & Woodruff-Borden J (2011). Child Internalizing Symptoms: Contributions of Child Temperament, Maternal Negative Affect, and Family Functioning. Child Psychiatry Hum Dev, 42, 53–64. 10.1007/s10578-010-0202-5 [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1998). Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion, 12(3), 307–330. 10.1080/026999398379628 [DOI] [Google Scholar]
- de Vente W, Majdandžić M, & Bögels SM (2020). Intergenerational transmission of anxiety: Linking parental anxiety to infant autonomic hyperarousal and fearful temperament. Journal of Child Psychology and Psychiatry, 61(11), 1203–1212. 10.1111/jcpp.13208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M (2012). Fetal programming by maternal stress: Insights from a conflict perspective. Psychoneuroendocrinology, 37(10), 1614–1629. 10.1016/j.psyneuen.2012.05.014 [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, & Shirtcliff EA (2011). The Adaptive Calibration Model of stress responsivity. Neuroscience & Biobehavioral Reviews, 35(7), 1562–1592. 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierckx B, Tulen JHM, van den Berg MP, Tharner A, Jaddoe VW, Moll HA, Hofman A, Verhulst FC, & Tiemeier H (2009). Maternal Psychopathology Influences Infant Heart Rate Variability: Generation R Study. Psychosomatic Medicine, 71(3), 313–321. 10.1097/PSY.0b013e318198a82c [DOI] [PubMed] [Google Scholar]
- Dollar JM, & Calkins SD (2019). The Development of Anger. In LoBue V, Pérez-Edgar K, & Buss KA (Eds.), Handbook of Emotional Development (pp. 199–225). Springer International Publishing. 10.1007/978-3-030-17332-6_9 [DOI] [Google Scholar]
- Durbin CE, Hayden EP, Klein DN, & Olino TM (2007). Stability of laboratory-assessed temperamental emotionality traits from ages 3 to 7. Emotion, 7(2), 388–399. 10.1037/1528-3542.7.2.388 [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Valiente C, Spinrad TL, Cumberland A, Liew J, Reiser M, Zhou Q, & Losoya SH (2009). Longitudinal relations of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Developmental Psychology, 45(4), 988–1008. 10.1037/a0016213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC, McAdams TA, Rijsdijk FV, Lichtenstein P, Narusyte J, Reiss D, Spotts EL, Ganiban JM, & Neiderhiser JM (2015). The Intergenerational Transmission of Anxiety: A Children-of-Twins Study. American Journal of Psychiatry, 172(7), 630–637. 10.1176/appi.ajp.2015.14070818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else-Quest NM, Hyde JS, Goldsmith HH, & Van Hulle CA (2006). Gender differences in temperament: A meta-analysis. Psychological Bulletin, 132(1), 33–72. 10.1037/0033-2909.132.1.33 [DOI] [PubMed] [Google Scholar]
- Enders CK (2001). The performance of the full information maximum likelihood estimator in multiple regression models with missing data. Educational and Psychological Measurement, 61(5), 713–740. [Google Scholar]
- Gao MM, Kaliush PR, Brown MA, Shakiba N, Raby KL, Crowell SE, & Conradt E (2022). Unique Contributions of Maternal Prenatal and Postnatal Emotion Dysregulation on Infant Respiratory Sinus Arrhythmia. Research on Child and Adolescent Psychopathology. 10.1007/s10802-022-00914-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Bridgett DJ, Rothbart MK, Robertson C, Iddins E, Ramsay K, & Schlect S (2010). A latent growth examination of fear development in infancy: Contributions of maternal depression and the risk for toddler anxiety. Developmental Psychology, 46(3), 651–668. 10.1037/a0018898 [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Hancock GR, & Iverson SL (2018). Positive Affectivity and Fear Trajectories in Infancy: Contributions of Mother-Child Interaction Factors. Child Development, 89(5), 1519–1534. 10.1111/cdev.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, & Marmion J (2008). Fear and positive affectivity in infancy: Convergence/discrepancy between parent-report and laboratory-based indicators. Infant Behavior and Development, 31(2), 227–238. 10.1016/j.infbeh.2007.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmel M, Bögi E, Ragan C, Hazlett M, Dubovicky M, van den Hove DL, Oberlander TF, Charlier TD, & Pawluski JL (2018). Perinatal selective serotonin reuptake inhibitor medication (SSRI) effects on social behaviors, neurodevelopment and the epigenome. Neuroscience and biobehavioral reviews, 85, 102–116. 10.1016/j.neubiorev.2017.04.023 [DOI] [PubMed] [Google Scholar]
- Gentile S (2010), Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depression & Anxiety, 27, 675–686. 10.1002/da.20706 [DOI] [PubMed] [Google Scholar]
- Goldsmith HH (1996). Studying temperament via construction of the toddler behavior assessment questionnaire [Data set]. In Child Development (Vol. 67, Issue 1, pp. 218–235). 10.1037/t07241-000 [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, & Campos JJ (1990). The Structure of Temperamental Fear and Pleasure in Infants: A Psychometric Perspective. Child Development, 61(1), 1944–1964. [PubMed] [Google Scholar]
- Goldsmith HH, & Lemery KS (2000). Linking temperamental fearfulness and anxiety symptoms: A behavior–genetic perspective. Biological Psychiatry, 48(12), 1199–1209. 10.1016/S0006-3223(00)01003-9 [DOI] [PubMed] [Google Scholar]
- Goodman SH (2020). Intergenerational Transmission of Depression. Annual Review of Clinical Psychology, 16(1), 213–238. 10.1146/annurev-clinpsy-071519-113915 [DOI] [PubMed] [Google Scholar]
- Goodman SH, & Gotlib IH (1999). Risk for Psychopathology in the Children of Depressed Mothers: A Developmental Model for Understanding Mechanisms of Transmission. Psychological Review, 106(3), 458–490. [DOI] [PubMed] [Google Scholar]
- Gottlieb G (2007). Probabilistic epigenesis. Developmental Science, 10, 1–11. 10.1111/j.1467-7687.2007.00556.x [DOI] [PubMed] [Google Scholar]
- Gump BB, Reihman J, Stewart P, Lonky E, Darvill T, Granger DA, & Matthews KA (2009). Trajectories of maternal depressive symptoms over her child’s life span: Relation to adrenocortical, cardiovascular, and emotional functioning in children. Development and Psychopathology, 21(1), 207–225. 10.1017/S0954579409000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaker EL, Kuiper RM, & Grasman RPPP (2015). A critique of the cross-lagged panel model. Psychological Methods, 20(1), 102–116. 10.1037/a0038889 [DOI] [PubMed] [Google Scholar]
- Houtveen JH, Rietveld S, & De Geus EJ (2002). Contribution of tonic vagal modulation of heart rate, central respiratory drive, respiratory depth, and respiratory frequency to respiratory sinus arrhythmia during mental stress and physical exercise. Psychophysiology, 39(4), 427–436. [DOI] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Jewell SL, Suk HW, & Luecken LJ (2018). Respiratory sinus arrhythmia: Modeling longitudinal change from 6 weeks to 2 years of age among low-income Mexican Americans. Developmental Psychobiology, 60(2), 232–238. 10.1002/dev.21595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitz M, Maytal HR, Devor N, Bergman L, & Manjuta D (2010). Maternal anxiety, mother-infant interactions, and infants’ response to challenge. Infant Behavior and Development, 33(2), 136–148. https://doi.org/j.infbeh.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Klein DN, Dyson MW, Kujawa AJ, & Kotov R (2012). Temperament and Internalizing Disorders. In Zentner M & Shiner RL (Eds.), Handbook of Temperament (pp. 541–561). [Google Scholar]
- Lemery KS, Goldsmith HH, Klinnert MD, & Mrazek DA (1999). Developmental Models of Infant and Childhood Temperament. 35(1), 189–204. [DOI] [PubMed] [Google Scholar]
- Lemus A, Vogel SC, Greaves AN, & Brito NH (2022). Maternal anxiety symptoms associated with increased behavioral synchrony in the early postnatal period. Infancy, infa.12473. 10.1111/infa.12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb ST, Leve LD, Harold GT, Neiderhiser JM, Shaw DS, Ge X, & Reiss D (2011). Trajectories of Parenting and Child Negative Emotionality During Infancy and Toddlerhood: A Longitudinal Analysis: Parenting and Child Negative Emotionality. Child Development, 82(5), 1661–1675. 10.1111/j.1467-8624.2011.01639.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Moore GA, Beekman C, Pérez-Edgar KE, Leve LD, Shaw DS, Ganiban JM, Natsuaki MN, Reiss D, & Neiderhiser JM (2018). Developmental patterns of anger from infancy to middle childhood predict problem behaviors at age 8. Developmental Psychology, 54(11), 2090–2100. 10.1037/dev0000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams TA, Rijsdijk FV, Neiderhiser JM, Narusyte J, Shaw DS, Natsuaki MN, Spotts EL, Ganiban JM, Reiss D, Leve LD, Lichtenstein P, & Eley TC (2015). The relationship between parental depressive symptoms and offspring psychopathology: Evidence from a children-of-twins study and an adoption study. Psychological Medicine, 45(12), 2583–2594. 10.1017/S0033291715000501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA (2010). Parent conflict predicts infants’ vagal regulation in social interaction. Development and Psychopathology, 22(1), 23–33. 10.1017/S095457940999023X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder JD, & Hamaker EL (2021). Three Extensions of the Random Intercept Cross-Lagged Panel Model. Structural Equation Modeling: A Multidisciplinary Journal, 28(4), 638–648. 10.1080/10705511.2020.1784738 [DOI] [Google Scholar]
- Mulkey SB, & du Plessis AJ (2019). Autonomic nervous system development and its impact on neuropsychiatric outcome. Pediatric Research, 85(2), 120–126. 10.1038/s41390-018-0155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AD (1979). Infant crying as an elicitor of parental behavior: An examination of two models. Psychological Bulletin, 86(1), 191–215. [PubMed] [Google Scholar]
- Nolvi S, Bridgett DJ, Korja R, Kataja E-L, Junttila N, Karlsson H, & Karlsson L (2019). Trajectories of maternal pre- and postnatal anxiety and depressive symptoms and infant fear: Moderation by infant sex. Journal of Affective Disorders, 257, 589–597. 10.1016/j.jad.2019.07.055 [DOI] [PubMed] [Google Scholar]
- Olino TM, Michelini G, Mennies RJ, Kotov R, & Klein DN (2021). Does maternal psychopathology bias reports of offspring symptoms? A study using moderated non-linear factor analysis. Journal of child psychology and psychiatry, and allied disciplines, 62(10), 1195–1201. 10.1111/jcpp.13394 [DOI] [PubMed] [Google Scholar]
- Parade SH, & Leerkes EM (2008). The reliability and validity of the Infant Behavior Questionnaire-Revised. Infant Behavior and Development, 31(4), 637–646. 10.1016/j.infbeh.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Belsky J, Putnam S, & Crnic K (1997). Infant Emotionality, Parenting, and 3-Year Inhibition: Exploring Stability and Lawful Discontinuity in a Male Sample. Developmental Psychology, 33(2), 218–227. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, & Fox NA (2005). Temperament and Anxiety Disorders. Child and Adolescent Psychiatric Clinics of North America, 14(4), 681–706. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, LoBue V, Buss KA, Field AP, & the LAnTs Team. (2021). Study Protocol: Longitudinal Attention and Temperament Study. Frontiers in Psychiatry, 12, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen A-K, Räikkönen K, Heinonen K, Komsi N, Järvenpää A-L, & Strandberg T (2008). A Transactional Model of Temperamental Development: Evidence of a Relationship between Child Temperament and Maternal Stress over Five Years. Social Development, 17(2), 326–340. 10.1111/j.1467-9507.2007.00427.x [DOI] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, & Furman SA (2011). The early development of the autonomic nervous system provides a neural platform for social behaviour: A polyvagal perspective. Infant and Child Development, 20(1), 106–118. 10.1002/icd.688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova NV, Gartstein MA, & Bridgett DJ (2014). Paternal influences on infant temperament: Effects of father internalizing problems, parenting-related stress, and temperament. Infant Behavior and Development, 37(1), 105–110. [DOI] [PubMed] [Google Scholar]
- Propper CB, & Holochwost SJ (2013). The influence of proximal risk on the early development of the autonomic nervous system. Developmental Review, 33(3), 151–167. 10.1016/j.dr.2013.05.001 [DOI] [Google Scholar]
- Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, & Leerkes E (2014). Development and Assessment of Short and Very Short Forms of the Infant Behavior Questionnaire–Revised. Journal of Personality Assessment, 96(4), 445–458. 10.1080/00223891.2013.841171 [DOI] [PubMed] [Google Scholar]
- Ritz T, Enlow MB, Schulz SM, Kitts R, Staudenmayer J, & Wright RJ (2012). Respiratory Sinus Arrhythmia as an Index of Vagal Activity during Stress in Infants: Respiratory Influences and Their Control. PLOS ONE, 7(2), e52729. 10.1371/journal.pone.0052729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, & DelVecchio WF (2000). The rank-order consistency of personality traits from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin, 126(1), 3–25. 10.1037/0033-2909.126.1.3 [DOI] [PubMed] [Google Scholar]
- Rosseel Y (2012). lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48, 1–36. 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Rothbart MK, & Bates JE (2006). Temperament. In Eisenberg N (Ed.), Handbook of Child Psychology (6th ed., Vol. 3, pp. 99–166). John Wiley & Sons. [Google Scholar]
- Sameroff A (2010). A Unified Theory of Development: A Dialectic Integration of Nature and Nurture. Child Development, 81(1), 6–22. 10.1111/j.1467-8624.2009.01378.x [DOI] [PubMed] [Google Scholar]
- Sheppes G, Suri G, & Gross JJ (2015). Emotion regulation and psychopathology. Annual review of clinical psychology, 11, 379–405. 10.1146/annurev-clinpsy-032814-112739 [DOI] [PubMed] [Google Scholar]
- Shiner RL, Buss KA, McClowry SG, Putnam SP, Saudino KJ, & Zentner M (2012). What Is Temperament Now? Assessing Progress in Temperament Research on the Twenty-Fifth Anniversary of Goldsmith et al. Child Development Perspectives, 6(4), 436–444. 10.1111/j.1750-8606.2012.00254.x [DOI] [Google Scholar]
- Skipstein A, Janson H, Kjeldsen A, Nilsen W, & Mathiesen KS (2012). Trajectories of maternal symptoms of depression and anxiety over 13 years: The influence of stress, social support, and maternal temperament. BMC Public Health, 12(1), 1120. 10.1186/1471-2458-12-1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis J (2004). The signal functions of early infant crying. Behavioral and Brain Sciences, 27(4), 443–458. 10.1017/S0140525X0400010X [DOI] [PubMed] [Google Scholar]
- van der Toorn SLM, Huizink AC, Utens EMWJ, Verhulst FC, Ormel J, & Ferdinand RF (2010). Maternal depressive symptoms, and not anxiety symptoms, are associated with positive mother–child reporting discrepancies of internalizing problems in children: a report on the TRAILS Study. Eur Child Adolesc Psychiatry 19, 379–388. 10.1007/s00787-009-0062-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waerden J, Galéra C, Larroque B, Saurel-Cubizolles M-J, Sutter-Dallay A-L, & Melchior M (2015). Maternal Depression Trajectories and Children’s Behavior at Age 5 Years. The Journal of Pediatrics, 166(6), 1440–1448.e1. 10.1016/j.jpeds.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Van Hulle CA, Moore MN, Lemery-Chalfant K, Goldsmith HH, & Brooker RJ (2017). Infant stranger fear trajectories predict anxious behaviors and diurnal cortisol rhythm during childhood. Development and Psychopathology, 29(3), 1119–1130. 10.1017/S0954579417000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner NJ, Holochwost SJ, Lynch SF, Mills-Koonce R, & Propper C (2021). Characterizing change in vagal tone during the first three years of life: A systematic review and empirical examination across two longitudinal samples. Neuroscience & Biobehavioral Reviews, 129, 282–295. 10.1016/j.neubiorev.2021.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiffen VE, & Gotlib IH (1989). Infants of Postpartum Depressed Mothers: Temperament and Cognitive Status. Journal of Abnormal Psychology, 98(3), 274–279 [DOI] [PubMed] [Google Scholar]


