Abstract
Background:
Increased duration of breastfeeding improves maternal cardiovascular health and may be especially beneficial in high-risk populations, such as those with chronic hypertension. Others have shown that individuals with hypertension are less likely to breastfeed, and there has been limited research aimed at supporting breastfeeding goals in this population. The impact of perinatal blood pressure control on breastfeeding outcomes among people with chronic hypertension is unknown.
Objective:
The aim of this study was to evaluate whether breastfeeding initiation and short-term duration assessed at the postpartum clinic visit differed based on perinatal blood pressure treatment strategy (targeting blood pressure <140/90 mm Hg vs. reserving antihypertensive treatment for blood pressure ≥160/105 mm Hg).
Study Design:
We performed a secondary analysis of the Chronic Hypertension and Pregnancy (CHAP) Trial. This was an open-label, multicenter, randomized trial where pregnant participants with mild chronic hypertension were randomized to receive antihypertensive medications with goal blood pressure <140/90 mm Hg (active treatment) or deferred treatment until blood pressure ≥160/105 mm Hg (control). Primary outcome was initiation and duration of breastfeeding, assessed at the postpartum clinic visit. We performed bivariate analyses and log-binomial and cumulative logit regression models, adjusting models for variables that were unbalanced in bivariate analyses. We performed additional analyses to explore the relationship between breastfeeding duration and blood pressure measurements at the postpartum visit.
Results:
1444/2408 (60%) participants from the CHAP trial attended the postpartum study visit and provided breastfeeding information. Participants in the active treatment group had different body mass index class distribution and earlier gestational age at enrollment, and (by design) were more often discharged on antihypertensives. Breastfeeding outcomes did not differ significantly by treatment group. In the active and control treatment groups, 563 (77.5%) and 561 (78.1%) initiated breastfeeding, and mean duration of breastfeeding was 6.5 ± 2.3 and 6.3 ± 2.1 weeks, respectively. The probability of ever breastfeeding (aRR 0.99, 95% CI 0.93–1.05), current breastfeeding at postpartum visit (aRR 1.01, 95% CI 0.94–1.10), and weeks of breastfeeding (aOR 0.87, 95% CI 0.68–1.12) did not differ by treatment group. Increased duration (≥2 weeks vs. <2 weeks) of breastfeeding was associated with slightly lower blood pressure measurements at the postpartum visit, but these differences were not significant in adjusted models.
Conclusions:
In a secondary analysis of the cohort of CHAP participants who attended the postpartum study visit and provided breastfeeding information (60% of original trial participants), breastfeeding outcomes did not differ significantly by treatment group. This suggests that maintaining goal blood pressure <140/90 mm Hg throughout the perinatal period is associated with neither harm nor benefit for short-term breastfeeding goals. Further study is needed to understand long-term breastfeeding outcomes among individuals with chronic hypertension and how to support this population in achieving their breastfeeding goals.
Keywords: Blood pressure, breastfeeding, cardiovascular health, chronic hypertension, lactation, pregnancy, postpartum
Graphical Abstract
Tweetable statement
Pregnant people with mild chronic hypertension randomized to different blood pressure treatment goals (< 140/90 vs. <160/105 mm Hg) had similar short-term breastfeeding outcomes.
Paper Presentation Information
This study was presented as a poster presentation (Abstract 937) at the Society of Maternal Fetal Medicine’s 43rd Annual Pregnancy Meeting in San Francisco, California (February 6 – 11, 2023).
Introduction
There is considerable evidence demonstrating that breastfeeding reduces risk for maternal cardiovascular disease. Studies examining the association between breastfeeding and hypertension later in life have consistently shown that mothers who breastfeed longer are less likely to develop hypertension.1–4 Breastfeeding has also been associated with reduced risk for diabetes,3–5 hyperlipidemia,3,4 coronary heart disease,6 and metabolic syndrome.2 A recent meta-analysis including data from over 1 million parous women demonstrated that breastfeeding reduced maternal risk of cardiovascular disease, coronary heart disease, stroke, and fatal cardiovascular disease.7 There is less evidence regarding short-term impacts of breastfeeding on cardiovascular health; two small studies demonstrated that breastfeeding is associated with short-term improvements in blood pressure at one month postpartum8 and as soon as two days postpartum.9 Possible mechanisms linking breastfeeding to cardiovascular health include weight loss,10 a “reset” of maternal metabolism,11 and central neuroendocrine hormones including oxytocin12 and prolactin.13
The benefits of breastfeeding may be especially important in high-risk populations such as those with chronic hypertension and hypertensive disease of pregnancy, who are at increased risk for cardiovascular morbidity and mortality later in life.14–16 However, those with hypertensive disorders in pregnancy are less likely to breastfeed and report more difficulties initiating and continuing breastfeeding.17–19 There are multiple potential barriers to breastfeeding among mothers with hypertension: higher rates of cesarean delivery and preterm birth, maternal-neonate separation, effect of medications used to treat hypertension and preeclampsia (i.e. diuretics, magnesium sulfate), and underlying endocrine and metabolic changes that may interfere with lactation.20–25 Complications during pregnancy may decrease overall maternal confidence and thereby impact breastfeeding self-efficacy.26,27 Improved perinatal blood pressure control has the potential to influence many of these barriers to breastfeeding. The impact of perinatal blood pressure control on breastfeeding outcomes is unknown.
The Chronic Hypertension and Pregnancy (CHAP) Trial was a multi-center, randomized controlled trial in pregnant participants with mild chronic hypertension which demonstrated that a strategy of targeting blood pressure <140/90 mm Hg was associated with improved pregnancy outcomes, as opposed to deferring antihypertensive treatment until blood pressure ≥160/105 mm Hg.28 The aim of this secondary analysis was to evaluate whether breastfeeding outcomes assessed at the postpartum clinic visit differed between the active treatment and control groups in the CHAP trial. We hypothesized that those in the active treatment group with lower target blood pressures would have increased initiation and duration of breastfeeding.
Materials and Methods
This was a secondary analysis of the CHAP Trial, an open-label, randomized controlled trial conducted at over 70 sites in the United States. The details of the trial methodology have previously been published.28 Briefly, pregnant participants with known or new diagnosis of chronic hypertension and a viable singleton fetus prior to 23 weeks were eligible for enrollment. Known chronic hypertension was defined by documented elevation in blood pressure and prior or current antihypertensive therapy (including lifestyle changes). New chronic hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or both on at least two separate instances at least four hours apart, prior to 20 weeks’ gestation. Patients were randomized to one of two groups: antihypertensive therapy given for goal blood pressure of <140/90 mm Hg (active treatment group) or deferred antihypertensive medications until development of severe hypertension (systolic blood pressure ≥160 mm Hg or diastolic blood pressure ≥105 mm Hg) (control group). If individuals in the control group developed severe hypertension, the target blood pressure for treatment was <140/90 mm Hg. The study was approved by the institutional review board at each hospital.
Following randomization, participants were followed by trained research staff and outcomes were abstracted from the medical record. During clinic visits, research staff assessed patient adherence to antihypertensive medication. Other assessments were performed according to the usual practices at each site. Patients were followed until their postpartum clinic visit, which was targeted for six weeks after delivery (range four to 12 weeks postpartum). Blood pressure measurements were performed at all clinic visits, including the postpartum visit. At their postpartum visit, patients completed a questionnaire asking about initiation and duration of breastfeeding. The following questions were asked: “Was your baby ever breastfed or fed breast milk?” “If Yes, are you currently breastfeeding/feeding breast milk?” “If Yes, how long have you been breastfeeding/feeding breast milk?” “If No, how old was your baby when you stopped?” Lactation support was provided according to the usual practices at each site with no changes per the study protocol.
Analyses by treatment group were performed according to the intention-to-treat principle. Bivariate analyses summarized data for participants by their treatment group assignment. We compared continuous variables using two-sample t-tests (assuming unequal variance) or Wilcoxon rank sum test, as appropriate, and categorical variables using chi-square or Fisher’s exact tests, as appropriate. The effect of treatment group on breastfeeding was determined using log-binomial regression models for ever and current breastfeeding, and cumulative logit regression models for total weeks of breastfeeding (defined as an ordinal variable: < 2 weeks, 2 to < 6 weeks, and ≥ 6 weeks of breastfeeding). All models compared active treatment group (administer antihypertensive medications with goal blood pressure of <140/90 mm Hg) to control group (defer antihypertensive treatment until blood pressure ≥160/105 mm Hg). All models were adjusted for variables that were unbalanced between treatment groups in bivariate analyses.
As additional analyses to add to the limited literature available regarding short-term cardiovascular effects of breastfeeding, we performed bivariate analyses and linear regression models to examine associations of breastfeeding duration on blood pressure measurements at the postpartum visit. Our exposure was breastfeeding duration, and we dichotomized breastfeeding duration into less than two weeks and two or more weeks based on the timing of transition to mature breastmilk.29 Our outcome was blood pressure recorded at the postpartum visit. Linear regression models were adjusted for variables that were unbalanced between exposure groups in bivariate analyses. In all descriptive statistics and bivariate analyses, we chose to include self-reported race and ethnicity, recognizing race as a social construct that acts as a social determinant of health due to structural racism.30 Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R version 4.1.3.31
Results
Of the 2408 individuals randomized and included in the final analysis sample for the CHAP trial, 1444 (60.0%) attended the postpartum study visit and provided information on breastfeeding outcomes; this constituted the cohort for this secondary analysis. A subset (n=336) reported “Yes” to ever breastfeeding but were missing weeks in breastfeeding, so were excluded from analyses regarding breastfeeding duration. The active treatment (n=726) and control (n=718) groups were similar in baseline characteristics (Table 1) and delivery characteristics (Table 2), though participants in the active treatment group were more likely to have body mass index (BMI) class ≥40, slightly earlier gestational age at enrollment, and were (by design) more often discharged on antihypertensive medications. The median timing for the postpartum clinic visit for the overall cohort was 6.43 weeks post-delivery (interquartile range 5.86 – 7.71 weeks), and this was similar between the active treatment and control groups (data not shown).
Table 1.
Maternal baseline characteristics by treatment group.
| Characteristics | Activea (n=726) |
Controlb (n=718) |
P |
|---|---|---|---|
| Age (years) | 32.4 ± 5.5 | 32.3 ± 5.8 | 0.76 |
| Race/Ethnicity | |||
| White, non-Hispanic | 226 (31.1) | 210 (29.2) | 0.20 |
| Black, non-Hispanic | 336 (46.3) | 333 (46.4) | |
| Hispanic | 140 (19.3) | 135 (18.8) | |
| Other | 24 (3.3) | 40 (5.6) | |
| Mother’s insurance | |||
| Government assisted insurance/Medicaid | 384 (52.9) | 368 (51.3) | 0.50 |
| Private insurance | 299 (41.2) | 313 (43.6) | |
| None/self-paid | 35 (4.8) | 28 (3.9) | |
| Missing | 8 (1.1) | 9 (1.3) | |
| Chronic hypertension type | |||
| Newly diagnosed | 131 (18.0) | 139 (19.4) | 0.37 |
| Known - on medication | 416 (57.3) | 424 (59.1) | |
| Known - not on medication | 179 (24.7) | 155 (21.6) | |
| Systolic blood pressure (mm Hg) at randomization | 136.8 ± 13.9 | 136.9 ± 15.3 | 0.88 |
| Diastolic blood pressure (mm Hg) at randomization | 83.6 ± 10.5 | 83.7 ± 10.8 | 0.91 |
| Prior pregnancy | 594 (81.8) | 579 (80.6) | 0.57 |
| BMI (kg/m2) at enrollment | 38.0 ± 10.6 | 37.4 ± 9.7 | 0.27 |
| BMI (kg/m2) class at enrollment | |||
| BMI < 30 | 184 (25.3) | 161 (22.4) | 0.01 |
| 30 to < 40 | 258 (35.5) | 309 (43.0) | |
| ≥ 40 | 268 (36.9) | 232 (32.3) | |
| Missing in BMI class | 16 (2.2) | 16 (2.2) | |
| Gestational Age (weeks) at enrollment | 10.1 ± 3.3 | 10.5 ± 3.7 | 0.04 |
| Gestational Age (weeks) at delivery | 37.3 ± 2.1 | 37.0 ± 2.7 | 0.01† |
| Pre-existing diabetes at baseline | 115 (15.8) | 113 (15.7) | 0.96 |
| Current smoker at baseline | 51 (7.0) | 49 (6.8) | 0.88 |
| Aspirin use at baseline | 381 (52.5) | 368 (51.3) | 0.64 |
| Prior hypertensive disease of pregnancy | 192 (26.4) | 203 (28.3) | 0.44 |
| Maternal education | |||
| Less than high school | 72 (10.4) | 69 (9.9) | 0.90 |
| High school (diploma or GED) | 215 (31.2) | 205 (29.5) | |
| Some college | 147 (21.3) | 155 (22.3) | |
| College graduate | 184 (26.7) | 188 (27) | |
| Missing in education | 72 (10.4) | 79 (11.4) | |
| Marital status | |||
| Married | 356 (49.0) | 360 (50.1) | 0.73 |
| Not married | 359 (49.4) | 350 (48.7) | |
| Missing in marital status | 11 (1.5) | 8 (1.1) | |
BMI, body mass index; GED, General Education Development Test.
Data presented as n (percentage) or mean ± standard deviation.
Active treatment group was randomized to receive antihypertensive medications with goal blood pressure of < 140/90 mm Hg.
Control group was randomized to defer antihypertensive treatment until blood pressure ≥ 160/105 mm Hg.
Table 2.
Delivery characteristics by treatment group.
| Characteristics | Activea (n=726) |
Controlb (n=718) |
P |
|---|---|---|---|
| Systolic blood pressure at delivery | 132.8 ± 10.3 | 133.1 ± 10.3 | 0.62 |
| Diastolic blood pressure at delivery | 79.0 ± 7.8 | 79.5 ± 8.3 | 0.19 |
| Mode of Delivery | |||
| Spontaneous vaginal | 343 (47.2) | 331 (46.1) | 0.88 |
| Operative vaginal | 11 (1.5) | 10 (1.4) | |
| Cesarean delivery | 372 (51.2) | 377 (52.5) | |
| Discharged on Medication | 638 (87.9) | 367 (51.1) | <0.0001 |
| Labetalol | 350 (48.2) | 185 (25.8) | <0.0001 |
| Nifedipine | 274 (37.7) | 183 (25.5) | <0.0001 |
Data presented as n (percentage) or mean ± standard deviation.
Active treatment group was randomized to receive antihypertensive medications with goal blood pressure of < 140/90 mm Hg.
Control group was randomized to defer antihypertensive treatment until blood pressure ≥ 160/105 mm Hg.
Overall, 1124 (77.8%) individuals initiated breastfeeding, and 797 (55.2%) were breastfeeding at the time of their postpartum visit; these rates did not differ significantly between the active treatment and control groups. Of the 1108 who initiated breastfeeding and provided information on breastfeeding duration, mean duration of breastfeeding and infant age when breastfeeding stopped did not differ between groups (Table 3). Results from all adjusted models showed no significant treatment group effect on breastfeeding outcomes (Table 4). Given that the BMI distribution among treatment groups differed slightly, we performed an additional sensitivity analysis where we tested for interaction between treatment group and BMI, and no p-values approached significance (results not shown).
Table 3.
Breastfeeding outcomes by treatment group.
| Activea (n=726) |
Controlb (n=718) |
P | |
|---|---|---|---|
| Was your baby ever breastfed or fed breast milk? | 563 (77.5) | 561 (78.1) | 0.79 |
| Currently breastfeeding | 402 (55.4) | 395 (55) | 0.89 |
| Breastfeeding duration (weeks) c | 6.5 ± 2.3 | 6.3 ± 2.1 | 0.15 |
| Infant age (weeks) when stopped breastfeeding c | 3.1 ± 2.0 | 3.4 ± 2.5 | 0.17 |
Data presented as n (percentage) or mean ± standard deviation.
Active treatment group was randomized to receive antihypertensive medications with goal blood pressure of < 140/90 mm Hg.
Control group was randomized to defer antihypertensive treatment until blood pressure ≥ 160/105 mm Hg.
336 individuals were missing data for weeks in breastfeeding (n = 1108/1444 provided information on duration of breastfeeding).
Table 4.
Results from adjusted regression models quantifying the association between treatment group and breastfeeding outcomes at the postpartum clinic visit.
| Model | Estimate | 95% CI | P | |
|---|---|---|---|---|
| Ever Breastfed (n = 1444) | Log-binomial | 0.99 (aRR) | 0.93 – 1.05 | 0.78 |
| Current Breastfeeding at postpartum visit (n = 1124) | Log-binomial | 1.01 (aRR) | 0.94 – 1.10 | 0.75 |
| Total Weeks Breastfeeding (n = 1108) | Cumulative Logit | 0.87 (aOR) | 0.68 – 1.12 | 0.30 |
CI, confidence interval; aRR, adjusted risk ratio; aOR, adjusted odds ratio.
All models compare active treatment group (receive antihypertensive medications with goal blood pressure of < 140/90 mm Hg) to control group (defer antihypertensive treatment until blood pressure ≥ 160/105 mm Hg) as a predictor for breastfeeding outcomes. All models adjusted for gestational age (at enrollment and delivery), body mass index, and discharged on antihypertensive medication.
In additional analyses examining the association between breastfeeding and short-term blood pressure control, 306 (154 active treatment and 152 control) of the 1108 individuals who reported information on breastfeeding duration were missing postpartum blood pressure measurements. While the majority (603/611) of those attending the postpartum visit had blood pressure measurements, most (222/306) of the missing postpartum blood pressures came from individuals who were unable to attend in-person visits and instead were contacted by phone for follow up. Thus our sample for this additional analysis included a total of n=802 individuals (408 in the active treatment group and 394 in the control group). Baseline characteristics differed as follows comparing those who breastfed for two or more weeks vs. less than two weeks: those who breastfed longer were more likely to be White, privately insured, married, college graduates, non-smokers, without pre-existing diabetes, and with lower BMI at enrollment (Table 5). In the active treatment group, individuals breastfeeding for two or more weeks had slightly lower postpartum diastolic blood pressure (84.9 vs. 88.4 mm Hg, p=0.003) but not systolic blood pressure (133.5 vs. 136.5 mm Hg, p=0.07), as compared to those breastfeeding for less than two weeks. In the control group, those breastfeeding for two or more weeks had slightly lower systolic blood pressure (133.9 vs. 139.0 mm Hg, p=0.004) (Figure 1). These decreases in postpartum blood pressure were no longer significant in the fully adjusted regression model (Table 6).
Table 5.
Baseline characteristics comparing those who breastfed for less than two weeks and those breastfeeding for two weeks or more.
| Characteristics | Breastfed < 2 weeks (n = 214) | Breastfed ≥ 2 weeks (n = 588) | P |
|---|---|---|---|
| Age (years) | 32.45± 5.9 | 33.0 ± 5.4 | 0.25 |
| Race/Ethnicity | |||
| White, non-Hispanic | 49 (22.9) | 184 (31.3) | <0.0001 |
| Black, non-Hispanic | 128 (59.8) | 240 (40.8) | |
| Hispanic | 32 (15.0) | 132 (22.4) | |
| Other | 5 (2.3) | 32 (5.4) | |
| Mother’s insurance | |||
| Government assisted insurance/Medicaid | 140 (65.4) | 250 (42.5) | <0.0001 |
| Private insurance | 61 (28.5) | 300 (51.0) | |
| None/self-paid | 11 (5.1) | 34 (5.8) | |
| Missing | 2 (0.9) | 4 (0.7) | |
| Chronic hypertension type | |||
| Newly diagnosed | 38 (17.8) | 114 (19.4) | 0.09 |
| Known - on medication | 115 (53.7) | 350 (59.5) | |
| Known - not on medication | 61 (28.5) | 124 (21.1) | |
| Systolic blood pressure (mm Hg) at randomization | 137.3 ± 15.6 | 136.6 ± 14.4 | 0.60 |
| Diastolic blood pressure (mm Hg) at randomization | 84.9 ± 11.6 | 83.5 ± 10.0 | 0.10 |
| Prior pregnancy | 176 (82.2) | 473 (80.4) | 0.57 |
| BMI (kg/m 2 ) at Enrollment | 38.6 ± 10.1 | 36.3 ± 9.8 | 0.00 |
| BMI (kg/m 2 ) class at enrollment | |||
| BMI < 30 | 44 (20.6) | 168 (28.6) | 0.04 |
| 30 to < 40 | 88 (41.1) | 229 (39.0) | |
| ≥ 40 | 80 (37.4) | 177 (30.1) | |
| Missing in BMI class | 2 (0.9) | 14 (2.4) | |
| Gestational age (weeks) at enrollment | 10.6 ± 3.8 | 10.2 ± 3.5 | 0.14 |
| Gestational Age (weeks) at delivery | 37.2 ± 1.9 | 37.4 ± 2.4 | 0.33 |
| Pre-existing diabetes at baseline | 41 (19.2) | 70 (11.9) | 0.008 |
| Current smoker at baseline | 18 (8.4) | 27 (4.6) | 0.04 |
| Aspirin use at baseline | 104 (48.6) | 314 (53.4) | 0.23 |
| Prior hypertensive disease of pregnancy | 61 (28.5) | 150 (25.5) | 0.39 |
| Maternal education | |||
| Less than high school | 18 (8.4) | 54 (9.2) | <0.0001 |
| High school (diploma or GED) | 82 (38.3) | 108 (18.4) | |
| Some college | 45 (21.0) | 114 (19.4) | |
| College graduate | 46 (21.5) | 249 (42.4) | |
| Missing in education | 23 (10.8) | 63 (10.7) | |
| Marital status | |||
| Married | 74 (34.6) | 340 (57.8) | <0.0001 |
| Not married | 134 (62.6) | 243 (41.3) | |
| Missing in marital status | 6 (2.8) | 5 (0.8) | |
BMI, body mass index; GED, General Education Development Test.
Data presented as n (percentage) or mean ± standard deviation.
Figure 1.
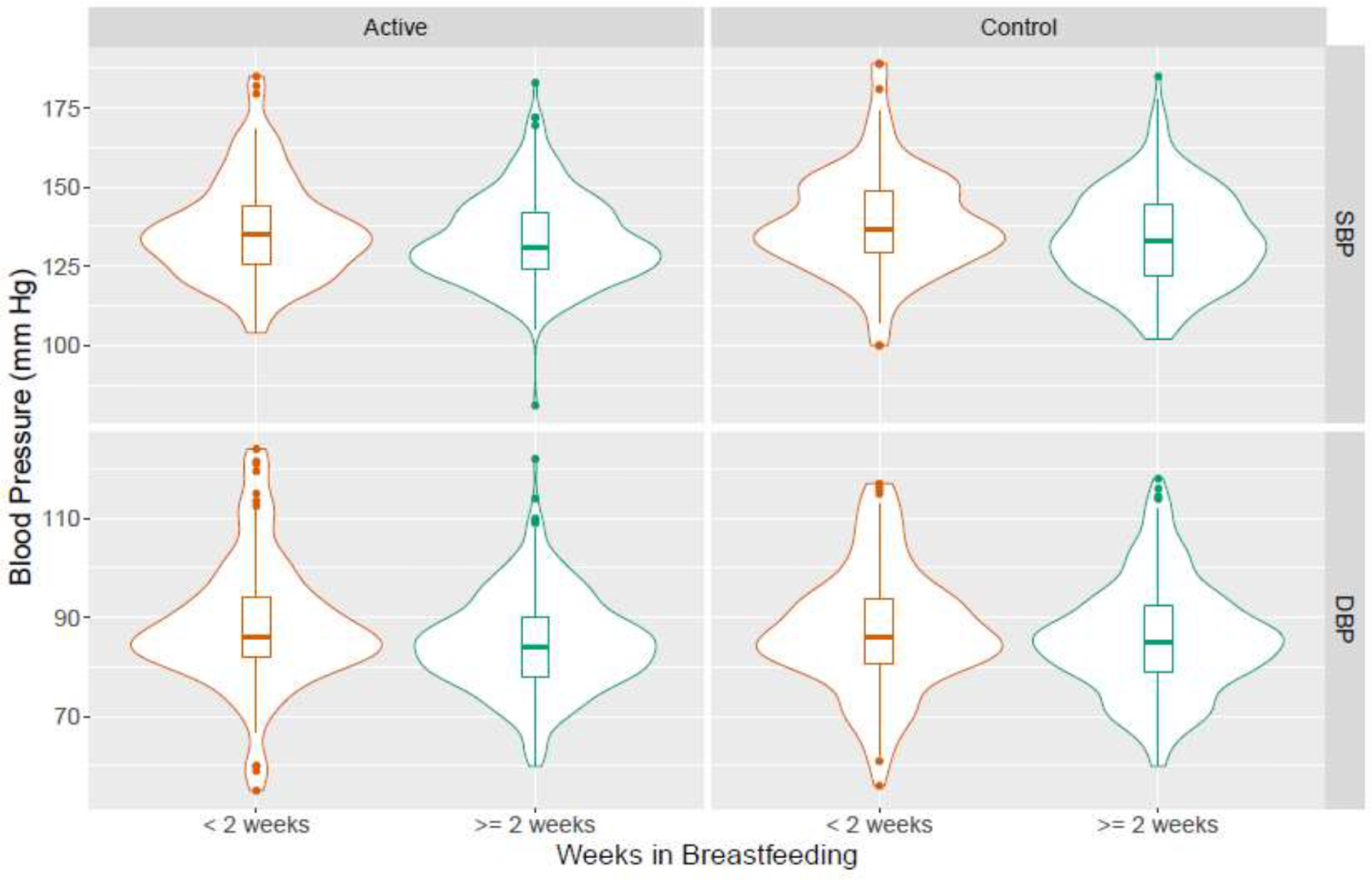
Violin plot of systolic and diastolic blood pressure by breastfeeding duration and treatment groupa.
aActive treatment group was randomized to receive antihypertensive medications with goal blood pressure of <140/90 mm Hg, while antihypertensive treatment was deferred in the control group until blood pressure ≥160/105 mm Hg.
In the active treatment group, individuals breastfeeding for two or more weeks had slightly lower postpartum diastolic blood pressure (84.9 vs. 88.4, p=0.003), as compared to those breastfeeding for less than two weeks. In the control group, those breastfeeding for two or more weeks had slightly lower systolic blood pressure (133.9 vs. 139.0, p=0.004).
Table 6.
Results from adjusted linear regression models quantifying the association between breastfeeding duration (< 2 weeks vs. ≥ 2 weeks) and blood pressure measured at the postpartum clinic visit.
| Active (N = 408) | Control (N = 394) | All (N = 802) | ||||
|---|---|---|---|---|---|---|
| Regression coefficient (95% CI) | P | Regression coefficient (95% CI) | P | Regression coefficient (95% CI) | P | |
| Model 1 a | ||||||
| Postpartum SBP | −2.72 (−5.92, 0.48) | 0.09 | −4.24 (−7.66, −0.82) | 0.02 | −3.52 (−5.85, −1.19) | 0.003 |
| Postpartum DBP | −3.10 (−5.43, −0.77) | 0.009 | −−0.99 (−3.50, 1.51) | 0.43 | −2.05 (−3.76, −0.35) | 0.02 |
| Model 2 b | ||||||
| Postpartum SBP | −1.59 (−5.11, 1.92) | 0.37 | −3.81 (−7.65, 0.03) | 0.05 | −2.71 (−5.29, −0.14) | 0.04 |
| Postpartum DBP | −2.03 (−4.59, 0.53) | 0.12 | −0.66 (−3.48, 2.16) | 0.64 | −1.39 (−3.27, 0.50) | 0.15 |
| Model 3 c | ||||||
| Postpartum SBP | −1.52 (−5.02, 1.98) | 0.40 | −3.00 (−6.83, 0.83) | 0.12 | −2.36 (−4.92, 0.19) | 0.07 |
| Postpartum DBP | −2.12 (−4.66, 0.41) | 0.10 | −0.03 (−2.85, 2.79) | 0.98 | −1.10 (−2.97, 0.76) | 0.24 |
CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure.
All models compare breastfeeding duration (dichotomized as < 2 weeks breastfeeding vs. ≥ 2 weeks) as a predictor for blood pressure measurements at the postpartum visit.
Adjusted for delivery blood pressure.
Adjusted for delivery blood pressure, mother’s insurance, body mass index, pre-existing diabetes, maternal education, marital status.
Adjusted for delivery blood pressure, mother’s insurance, body mass index, pre-existing diabetes, maternal education, marital status, race/ethnicity, and smoking.
Comment
Principal Findings
In this secondary analysis of the CHAP trial, we found that, among a cohort of trial participants with mild chronic hypertension, an initial strategy of treating mild chronic hypertension (as opposed to deferring treatment until development of severe hypertension) was not associated with significant differences in short term breastfeeding outcomes. Additionally, we found that increased breastfeeding duration, specifically beyond two weeks, was not associated with significant changes in blood pressure readings at the postpartum visit when controlling for potential confounders.
Results in the Context of What is Known
The cardiovascular benefits of breastfeeding have been well-documented,1–7 as has the increased long term cardiovascular morbidity for individuals with hypertensive disease in pregnancy.14–16 The mechanisms underlying the association between breastfeeding and cardiovascular health are not fully understood; possible mechanisms include weight loss,10 a “reset” of maternal metabolism,11 and central neuroendocrine hormones including oxytocin12 and prolactin.13 Individuals with hypertensive disease in pregnancy are less likely to breastfeed17–19 and thus are less likely to realize the associated cardiovascular benefits.
With respect to intervention studies, a large (n =17,046 mother-infant pairs at 31 sites) randomized controlled trial conducted in Belarus from 1996–1997 randomly assigned clinical sites to an experimental intervention based on the Baby-Friendly Hospital Initiative of the World Health Organization and United Nations Children’s Fund, which aimed to increase the duration and degree of breastfeeding.32 This trial recruited a low-risk cohort, though individuals with hypertension were not explicitly excluded from this trial. We found no published information describing incidence of hypertension in this cohort. A follow up analysis failed to show lowering of maternal blood pressure 11.5 years after delivery.33 A Canadian pilot study that randomized individuals (n=45) with recent hypertensive complications of pregnancy to a breastfeeding self-efficacy intervention demonstrated increased breastfeeding rates, and no significant change in blood pressure measurements at 3, 6, and 12 months postpartum.34 There is an ongoing clinical trial studying this intervention in a larger cohort (n=323) with hypertensive disorders of pregnancy (ClinicalTrials.gov Identifier NCT04580927).25 We are not aware of other trials examining interventions with the potential to promote breastfeeding among individuals with chronic hypertension or hypertensive disorders of pregnancy, though there are many studies demonstrating that breastfeeding support interventions are associated with increased breastfeeding rates in other perinatal populations.35
In the CHAP trial, participants with mild chronic hypertension randomized to the active treatment group with blood pressure target <140/90 mm Hg had decreased risk of adverse pregnancy outcomes. Given that adverse pregnancy outcomes can impair breastfeeding,20,36,37 and that hypertension in pregnancy is generally associated with decreased rates of breastfeeding,17–19 we hypothesized that those in the active treatment group with improved perinatal blood pressure control would have improved breastfeeding outcomes. However, we found that breastfeeding outcomes did not differ significantly between the treatment groups. While decreased blood pressure and the associated decreased risk of adverse outcomes would be expected to remove some barriers to breastfeeding, lactation is a complex process with other neuroendocrine and psychosocial factors playing an important role in this population. For example, prolactin, the hormone released from the anterior pituitary that regulates breastmilk synthesis,38 has also been associated with increased risk of hypertension when elevated over general physiologic levels.13 Pre-pregnancy depression and anxiety symptoms have been linked with increased risk of hypertensive disorders in pregnancy,39 and perinatal depression is associated with decreased breastfeeding rates. Further studies are needed to better understand the mechanisms linking hypertension and breastfeeding, and to develop interventions that support breastfeeding goals in this high-risk population with the potential for long-term maternal and offspring benefit.
We performed additional analyses to add to the limited literature describing the association between breastfeeding and maternal blood pressure. A Swedish observational study of 66 primiparae with uncomplicated deliveries found that, two days after birth, blood pressure fell significantly (within 60 minutes) after breastfeeding, with 8 mm Hg decrease in systolic blood pressure and 7.7 mm Hg decrease in diastolic blood pressure. During a 25-week follow up period, blood pressure consistently decreased after individual breastfeeding sessions, and basal systolic and diastolic blood pressures decreased.9 Another observational study of 407 low-risk mothers in rural Japan examined blood pressure one month postpartum by route of infant feeding. They found that individuals who were exclusively breastfeeding had lower systolic blood pressure, compared to those feeding via formula or mixed methods.8 Our study differs in examining a larger (n=802) high-risk population with chronic hypertension in the United States. Additionally, we were able to adjust for a number of potentially confounding factors in our models, which is of particular importance in lactation research as mothers who breastfeed are more likely to engage in other health-promoting behaviors.40 We were unable to evaluate time from breastfeeding to blood pressure evaluation given design of the parent study. We found that the modest short-term decreases in blood pressure associated with increased breastfeeding duration were no longer significant after adjustment for potentially confounding factors.
Clinical and Research Implications
Our main finding, that breastfeeding outcomes were similar regardless of blood pressure targets (<140/90 vs. <160/105 mm Hg) in a cohort of trial participants with mild chronic hypertension, suggests that a lower perinatal blood pressure goal is neither harmful nor beneficial for short-term breastfeeding goals among individuals with mild chronic hypertension. Given that most mothers express the desire to breastfeed,41 and that breastfeeding is associated with numerous maternal and child health benefits,42 it is important to collect breastfeeding outcomes when new obstetric interventions are studied. While recognizing the inherent limitations of a secondary analysis, clinicians can assure their patients that there is no evidence to suggest that lowering the target perinatal blood pressure will interfere with achievement of short-term breastfeeding goals. Further studies are needed to evaluate longer term breastfeeding outcomes and interventions to support breastfeeding goals in this high-risk population.
Findings from our additional analyses indicate that, after controlling for possible confounders, increased duration of breastfeeding is not associated with significant decreases in blood pressure at the postpartum clinic visit. However, obstetricians caring for pregnant people with chronic hypertension should continue to counsel their patients about the important long-term cardiovascular benefits of breastfeeding.1–7
Strengths and Limitations
The strengths of this study include its large sample size incorporating multiple trial centers. The study population mirrored the racial and ethnic diversity of pregnant people in the United States with chronic hypertension.43 This is one of a small number of studies examining breastfeeding outcomes in pregnant people with hypertension, a population that can especially benefit from the multiple measures of improved cardiovascular health associated with breastfeeding.
There are some limitations to note. This was a secondary analysis of the CHAP trial, and while breastfeeding outcomes were included in the data collection, the index trial was not powered for breastfeeding outcomes. Some participants in the original CHAP trial did not have information on breastfeeding outcomes due to the delayed addition of these questions to the study protocol. Furthermore, all breastfeeding outcomes were self-reported by patients. No information was available on breastfeeding intention, barriers to breastfeeding, or breastfeeding support provided to these participants, all important factors in helping patients meet their individual breastfeeding goals. Accordingly, the results of our study are intended to be hypothesis-generating, and to inform future studies designed to examine and support breastfeeding among pregnant people with hypertension.
Conclusions
In the CHAP trial, the strategy of treating mild chronic hypertension to a goal blood pressure of <140/90 mm Hg resulted in decreased risks for adverse pregnancy outcomes. This secondary analysis found that breastfeeding initiation and short-term duration, as measured at the postpartum visit, did not differ by treatment group, suggesting that perinatal blood pressure control to goal <140/90 mm Hg is neither harmful nor beneficial for breastfeeding goals. Further studies are needed to evaluate long-term breastfeeding outcomes and strategies to support breastfeeding among people with hypertension.
AJOG At A Glance.
- Why was this study conducted?
- The long-term cardiovascular health benefits of breastfeeding are known, and may be especially important in those with chronic hypertension.
- Altering blood pressure control during the perinatal period has the potential to impact breastfeeding initiation and duration.
- What are the key findings?
- Among a cohort of pregnant people with mild chronic hypertension randomized to different blood pressure treatment goals (< 140/90 vs. <160/105 mm Hg), short-term measures of breastfeeding initiation and duration did not differ significantly by treatment group.
- What does the study add to what is already known?
- These findings suggest that perinatal blood pressure control to <140/90 mm Hg is neither harmful nor beneficial for short-term breastfeeding goals.
- This study adds to the limited literature examining breastfeeding outcomes among pregnant people with chronic hypertension.
Acknowledgements
We thank Kjersti Aagaard, MD, PhD for her critical feedback in study design, results interpretation, and writing and editing of this manuscript. We thank Robin Parks for her invaluable support in managing and coordinating the CHAP trial and for her continued organizational support throughout this secondary analysis. The authors would also like to acknowledge the CHAP study participants and the many faculty, trainees, and staff at each study site involved in recruiting for this study.
Financial support
Funded by the National Heart, Lung, and Blood Institute U01HL120338; CHAP ClinicalTrials.gov number, NCT02299414. For the secondary analysis, the funder had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, nor in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no conflicts of interest.
Contributor Information
Alison N GOULDING, Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston TX.
Leah ANTONIEWICZ, Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, TX.
Justin M LEACH, Department of Biostatistics, The University of Alabama at Birmingham, Birmingham, Alabama.
Kim BOGGESS, Department of Obstetrics and Gynecology, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Lorraine DUGOFF, Department of Obstetrics and Gynecology, University of Pennsylvania, Philadelphia PA.
Baha SIBAI, Department of Maternal Fetal Medicine, University of Texas Health Center at Houston, Houston TX.
Kirsten LAWRENCE, Department of Obstetrics and Gynecology, Yale University, New Haven, CT.
Brenna L HUGHES, Department of Obstetrics and Gynecology, Duke University, Durham, NC.
Joseph BELL, Department of Obstetrics and Gynecology. St. Luke’s University Health Network, Bethlehem, PA.
Rodney K EDWARDS, Department of Obstetrics and Gynecology, University of Oklahoma Health Sciences, Oklahoma City, OK.
Kelly GIBSON, Department of Obstetrics and Gynecology, Metro Health Case Western University, Cleveland OH.
David M HAAS, Department of Obstetrics and Gynecology, Indiana University, Indianapolis, IN.
Lauren PLANTE, Department of Obstetrics and Gynecology, Penn State College of Medicine and Milton S. Hershey Medical Center, Hershey PA.
Torri D METZ, Department of Obstetrics and Gynecology, University of Utah, Salt Lake City, UT.
Brian CASEY, Center for Women’s Reproductive Health, University of Alabama, Birmingham AL; Department of Obstetrics and Gynecology, The University of Alabama, Birmingham AL.
Sean ESPLIN, Department of Obstetrics and Gynecology, Intermountain Healthcare, Salt Lake City, UT.
Sherri LONGO, Ochsner Baptist Medical Center, New Orleans, LA.
Matthew HOFFMAN, Christiana Care Health Services, Newark, DE.
George R SAADE, Department of Obstetrics and Gynecology, University of Texas Medical Branch, Galveston TX.
Kara K HOPPE, Department of Obstetrics and Gynecology, UnityPoint Health–Meriter Hospital/Marshfield Clinic, Madison, WI.
Janelle FOROUTAN, St. Peters University Hospital, New Brunswick, NJ.
Methodius TUULI, Department of Obstetrics and Gynecology, Brown University Warren Alpert Medical School, Providence RI.
Michelle Y OWENS, Department of Obstetrics and Gynecology, University of Mississippi Medical Center, Jackson, MI.
Hyagriv N SIMHAN, Department of Obstetrics and Gynecology, Magee Women’s Hospital, University of Pittsburgh, Pittsburgh, PA.
Heather FREY, Department of Obstetrics and Gynecology, The Ohio State University College of Medicine, Columbus OH.
Todd ROSEN, Department of Obstetrics and Gynecology, Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ.
Anna PALATNIK, Department of Obstetrics and Gynecology, Medical College of Wisconsin, Milwaukee, WI.
Susan BAKER, Department of Obstetrics and Gynecology, University of South Alabama, Mobile, AL.
Uma M REDDY, Department of Obstetrics and Gynecology, Columbia University, New York, NY.
Wendy KINZLER, Department of Obstetrics and Gynecology, Winthrop University Hospital, Mineola, NY.
Emily SU, Department of Obstetrics and Gynecology, University of Colorado, Boulder, CO.
Iris KRISHNA, Department of Obstetrics and Gynecology, Emory University, Atlanta, GA.
Nicki NGUYEN, Department of Obstetrics and Gynecology, Denver Health, Denver, CO.
Mary E NORTON, Department of Obstetrics and Gynecology, University of California, San Francisco, and Zuckerberg San Francisco General Hospital, San Francisco, CA.
Daniel SKUPSKI, Department of Obstetrics and Gynecology, New York Presbyterian Queens Hospital, New York, NY.
Yasser Y EL-SAYED, Department of Obstetrics and Gynecology, Stanford University, Stanford, CA.
Dotun OGUNYEMI, Department of Obstetrics and Gynecology, Arrowhead Regional Medical Center, Colton, CA; Beaumont Hospital, Southfield, MI.
Lorie M. HARPER, Department of Women’s Health, University of Texas, Austin, TX.
Namasivayam AMBALAVANAN, Division of Neonatology, Department of Pediatrics, University of Alabama, Birmingham AL; Center for Women’s Reproductive Health, University of Alabama, Birmingham AL.
Suzanne OPARIL, Division of Cardiovascular Disease, Department of Medicine, University of Alabama, Birmingham AL; Center for Women’s Reproductive Health, University of Alabama, Birmingham AL.
Jeff M SZYCHOWSKI, Department of Biostatistics, The University of Alabama, Birmingham, AL; Center for Women’s Reproductive Health, University of Alabama, Birmingham AL.
Alan T TITA, Center for Women’s Reproductive Health, University of Alabama, Birmingham AL; Department of Obstetrics and Gynecology, The University of Alabama, Birmingham AL.
References
- 1.Stuebe AM, Schwarz EB, Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174(10):1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ram KT, Bobby P, Hailpern SM, et al. Duration of lactation is associated with lower prevalence of the metabolic syndrome in midlife—SWAN, the study of women’s health across the nation. Am J Obstet Gynecol. 2008;198(3):268. e261–268. e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113(5):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Natland ST, Nilsen TI, Midthjell K, Andersen LF, Forsmo S. Lactation and cardiovascular risk factors in mothers in a population-based study: the HUNT-study. Int Breastfeed J. 2012;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294(20):2601–2610. [DOI] [PubMed] [Google Scholar]
- 6.Stuebe AM, Michels KB, Willett WC, Manson JE, Rexrode K, Rich-Edwards JW. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200(2):138.e131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tschiderer L, Seekircher L, Kunutsor SK, Peters SAE, O’Keeffe LM, Willeit P. Breastfeeding Is Associated With a Reduced Maternal Cardiovascular Risk: Systematic Review and Meta-Analysis Involving Data From 8 Studies and 1 192 700 Parous Women. J Am Heart Assoc. 2022;11(2):e022746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebina S, Kashiwakura I. Influence of breastfeeding on maternal blood pressure at one month postpartum. Int J Womens Health. 2012:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonas W, Nissen E, Ransjö-Arvidson A-B, Wiklund I, Henriksson P, Uvnäs-Moberg K. Short-and long-term decrease of blood pressure in women during breastfeeding. Breastfeed Med. 2008;3(2):103–109. [DOI] [PubMed] [Google Scholar]
- 10.Neville C, McKinley M, Holmes V, Spence D, Woodside J. The relationship between breastfeeding and postpartum weight change—a systematic review and critical evaluation. Int J Obes. 2014;38(4):577–590. [DOI] [PubMed] [Google Scholar]
- 11.Stuebe AM, Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26(01):081–088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersson M, Alster P, Lundberg T, Uvnas-Moberg K. Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiol Behav. 1996;60(5):1311–1315. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Curhan GC, Forman JP. Plasma prolactin level and risk of incident hypertension in postmenopausal women. J Hypertens. 2010;28(7):1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald EG, Dayan N, Pelletier R, Eisenberg MJ, Pilote L. Premature cardiovascular disease following a history of hypertensive disorder of pregnancy. Int J Cardiol. 2016;219:9–13. [DOI] [PubMed] [Google Scholar]
- 15.Coutinho T, Lamai O, Nerenberg K. Hypertensive Disorders of Pregnancy and Cardiovascular Diseases: Current Knowledge and Future Directions. Curr Treat Options Cardiovasc Med. 2018;20(7):56. [DOI] [PubMed] [Google Scholar]
- 16.Welters SM, de Boer M, Teunissen PW, et al. Cardiovascular mortality in women in their forties after hypertensive disorders of pregnancy in the Netherlands: a national cohort study. Lancet Healthy Longev. 2023;4(1):e34–e42. [DOI] [PubMed] [Google Scholar]
- 17.Strapasson MR, Ferreira CF, Ramos JGL. Feeding practices in the first 6 months after delivery: Effects of gestational hypertension. Pregnancy Hypertens. 2018;13:254–259. [DOI] [PubMed] [Google Scholar]
- 18.Horsley K, Chaput K, Da Costa D, et al. Hypertensive disorders of pregnancy and breastfeeding practices: A secondary analysis of data from the All Our Families Cohort. Acta Obstet Gynecol Scand. 2022;101(8):871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess A, Eichelman E, Rhodes B. Lactation patterns in women with hypertensive disorders of pregnancy: an analysis of Illinois 2012–2015 pregnancy risk assessment monitoring system (PRAMS) data. Matern Child Health J. 2021;25:666–675. [DOI] [PubMed] [Google Scholar]
- 20.Cordero L, Valentine CJ, Samuels P, Giannone PJ, Nankervis CA. Breastfeeding in women with severe preeclampsia. Breastfeed Med. 2012;7(6):457–463. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113(5):e465–471. [DOI] [PubMed] [Google Scholar]
- 22.Leeners B, Rath W, Kuse S, Neumaier-Wagner P. Breast-feeding in women with hypertensive disorders in pregnancy. J Perinat Med. 2005;33(6):553–560. [DOI] [PubMed] [Google Scholar]
- 23.Moore ER, Anderson GC, Bergman N, Dowswell T. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2012;5(5):Cd003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demirci J, Schmella M, Glasser M, Bodnar L, Himes KP. Delayed Lactogenesis II and potential utility of antenatal milk expression in women developing late-onset preeclampsia: a case series. BMC Pregnancy Childbirth. 2018;18(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dayan N, Smith G, Nedelchev A, et al. Study protocol for the sheMATTERS study (iMproving cArdiovascular healTh in new moThERS): a randomized behavioral trial assessing the effect of a self-efficacy enhancing breastfeeding intervention on postpartum blood pressure and breastfeeding continuation in women with hypertensive disorders of pregnancy. BMC Pregnancy Childbirth. 2023;23(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dennis CL. Theoretical underpinnings of breastfeeding confidence: a self-efficacy framework. J Hum Lact. 1999;15(3):195–201. [DOI] [PubMed] [Google Scholar]
- 27.Brockway M, Benzies K, Hayden KA. Interventions to Improve Breastfeeding Self-Efficacy and Resultant Breastfeeding Rates: A Systematic Review and Meta-Analysis. J Hum Lact. 2017;33(3):486–499. [DOI] [PubMed] [Google Scholar]
- 28.Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. New Engl J Med. 2022;386(19):1781–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey ZD, Feldman JM, Bassett MT. How Structural Racism Works - Racist Policies as a Root Cause of U.S. Racial Health Inequities. N Engl J Med. 2021;384(8):768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing V, Austria. URL https://www.R-project.org/. [Google Scholar]
- 32.Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–420. [DOI] [PubMed] [Google Scholar]
- 33.Oken E, Patel R, Guthrie LB, et al. Effects of an intervention to promote breastfeeding on maternal adiposity and blood pressure at 11.5 y postpartum: results from the Promotion of Breastfeeding Intervention Trial, a cluster-randomized controlled trial. American J Clin Nutr. 2013;98(4):1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dayan N, Semenic S, Fiorda A, et al. Breastfeeding and blood Pressure patterns in MOthers with recent hypertensive coMplications of pregnancy: BP-MOM Feasibility Study. Can J Cardiol. 2021;37(2):e25. [Google Scholar]
- 35.Patnode CD, Henninger ML, Senger CA, Perdue LA, Whitlock EP. Primary Care Interventions to Support Breastfeeding: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;316(16):1694–1705. [DOI] [PubMed] [Google Scholar]
- 36.Cordero L, Stenger MR, Landon MB, Nankervis CA. Breastfeeding initiation among women with preeclampsia with and without severe features. J Neonatal Perinatal Med. 2021;14(3):419–426. [DOI] [PubMed] [Google Scholar]
- 37.Chiang KV SA, Nelson JM, Olson CK, Perrine CG. Receipt of Breast Milk by Gestational Age — United States, 2017. MMWR Morb Mortal Wkly Rep 2019;68:489–493. DOI: 10.15585/mmwr.mm6822a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stuebe AM. Enabling women to achieve their breastfeeding goals. Obstet Gynecol. 2014;123(3):643–652. [DOI] [PubMed] [Google Scholar]
- 39.Thombre MK, Talge NM, Holzman C. Association between pre-pregnancy depression/anxiety symptoms and hypertensive disorders of pregnancy. J Womens Health (Larchmt). 2015;24(3):228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pesa JA, Shelton MM. Health-enhancing behaviors correlated with breastfeeding among a national sample of mothers. Public Health Nurs. 1999;16(2):120–124. [DOI] [PubMed] [Google Scholar]
- 41.Declercq ER, Sakala C, Corry MP, Applebaum S. Listening to mothers II: Report of the second national US survey of women’s childbearing experiences. Journal Perinat Educ. 2007;16(4):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. [DOI] [PubMed] [Google Scholar]
- 43.Grover S, Brandt J, Reddy U, Ananth C. Chronic hypertension, perinatal mortality and the impact of preterm delivery: a population-based study. BJOG. 2022;129(4):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]


