Abstract
The role of inflammation in nervous system injury and disease is attracting increased attention. Much of that research has focused on microglia in the central nervous system (CNS) and macrophages in the peripheral nervous system (PNS). Much less attention has been paid to the roles played by neutrophils. Neutrophils are part of the granulocyte subtype of myeloid cells. These cells, like macrophages, originate and differentiate in the bone marrow from which they enter the circulation. After tissue damage or infection, neutrophils are the first immune cells to infiltrate into tissues and are directed there by specific chemokines, which act on chemokine receptors on neutrophils. We have reviewed here the basic biology of these cells, including their differentiation, the types of granules they contain, the chemokines that act on them, the subpopulations of neutrophils that exist, and their functions. We also discuss tools available for identification and further study of neutrophils. We then turn to a review of what is known about the role of neutrophils in CNS and PNS diseases and injury, including stroke, Alzheimer’s disease, multiple sclerosis, amyotrophic lateral sclerosis, spinal cord and traumatic brain injuries, CNS and PNS axon regeneration, and neuropathic pain. While in the past studies have focused on neutrophils deleterious effects, we will highlight new findings about their benefits. Studies on their actions should lead to identification of ways to modify neutrophil effects to improve health.
Keywords: Neutrophil, polymorphonuclear leukocyte, Wallerian degeneration, phagocytosis, neutrophil extracellular trap, chemokine, nerve injury
1.0. Introduction
Neutrophils, previously known as polymorphonuclear leukocytes or PMNs, are the primary immune cell found in the blood (approximately 70% of the cells in the blood in humans and 30% in rodents; Burn et al., 2021). The father of innate immunity, Elie Metchnikoff, did extensive research into phagocytosis and investigated specific phagocytic cells, which he called macrophages and microphages. The latter became known as neutrophils, which are granulocytes, a subtype of myeloid cells. Neutrophils are the smallest granulocytes (6–15 μm in diameter) and have a polylobed nucleus (Amulic et al., 2012). They originate and differentiate in the bone marrow and can be found in blood and in tissues, e.g., spleen, lungs, lymph nodes, and injured nervous tissue. They are involved in the innate immune response, helping to eliminate bacteria and fungi from a site of infection. As first responders, they follow chemokines, pathogen-associated molecular patterns (PAMPs), and damage-associated molecular patterns (DAMPs) to areas of infection or injury (Herwald and Egesten, 2016). They remove foreign organisms and facilitate healthy repair of host tissue via five modes of action: degranulation, phagocytosis, neutrophil extracellular traps (NETs), release of reactive oxygen and nitrogen species (ROS and RNS), and immunomodulation/paracrine signaling (Burn et al., 2021). Normally, the inflammatory state resolves following recovery, but if the inflammation becomes chronic, neutrophils can damage healthy tissues (Pierson et al., 2018). While neutrophils have been identified since the beginning of the field of immunology, they can be difficult to investigate. They differentiate in the bone marrow, and any manipulation that would affect their progenitor cells would affect other cell types as well, e.g., eosinophils and monocytes. It is important to note that the idea that neutrophils have a short lifespan has been questioned recently (Koenderman et al., 2022). They are now believed to live 4–5 days instead of 12–18 hours in humans (Koenderman et al., 2022; Manz and Boettcher, 2014). New findings and ideas about neutrophil life span, tissue migration, and sub-populations have changed our knowledge of them.
Less is known about the roles of neutrophils in the nervous system than is known for macrophages/microglia. As macrophages and neutrophils are similar, with some similar cellular processes and similar roles, they can easily be confused. Research in the last twenty years has shown that neutrophils have a more significant role in neurological injury and disease than previously thought. In the beginning, neutrophils were thought to be primarily detrimental; however, recent experiments have shown some of their beneficial effects. Most of this work has been done in the CNS, and determining their effects in the PNS is a newer area of research. One of these effects that could have vast implications for several neurodegenerative diseases is the role that neutrophils play in axon regeneration after neural injury. In addition, neutrophils are thought to be responsible for the recruitment or suppression of other immune cells and to secrete certain neurotrophic growth factors and proteins e.g., oncomodulin (Kurimoto et al., 2013; Singh and Plemel, 2014). New neutrophils subtypes are being identified in both the CNS and PNS, which have pro-regenerative benefits, as we will discuss. Distinguishing between some of these cell types is difficult. These findings have opened new avenues of research in neuroimmunology.
Neuroimmunology is the intersection of neuroscience and immunology. We will begin by providing a concise review of neutrophil biology, since many neuroscientists do not have training in immunology. This review is written for general neuroscientists and investigators new to the field. We begin with a review of the basic biology of neutrophils, including their differentiation, granules formation, chemokines and chemokine receptors, functions, subpopulations, and tools used to investigate them. In some of these sections, we will highlight similarities to macrophages, which all future research should consider when designing experiments. We then review the literature on their roles of neutrophils in neurological diseases and injury, highlighting their beneficial roles and suggesting interesting lines of research that could advance the field.
2. Neutrophil biology
There are four types of granulocytes: neutrophils, eosinophils, basophils, and mast cells, which are involved in the innate and adaptive immune response (Rosales, 2020). Each is characterized by having a multilobed nucleus and granules, but their nuclear shapes differ from one another (Rosales, 2020). This means all of them could be described as PMNs, and the use of PMN to refer specifically to neutrophils can cause confusion. Basophils and eosinophils help the host fight off parasites, and basophils and mast cells are involved in the allergic response (Gibbs and Falcone, 2014; Klion et al., 2020). The latter release histamine to induce an allergic response and inflammation. Neutrophils and eosinophils clear bacteria and fungi (Ravin and Loy, 2015). The best way to identify and separate these cells is based on their cellular markers: basophils are CD117− and FcEpsilonR1+; mast cells are CD23+, CD117+, and FcEpsilonR1+; and eosinophils are CD193+, Siglec-F+, and F4/80 medium. Neutrophils are F4/80medium and Ly6G+ and can be separated from monocytes which are Ly6C+, F4/80− and Ly6G− and macrophages that are F4/80high, CD68+, and Iba1+, as shown in Fig. 1(Bochner, 2009; Horny et al., 2003; Park et al., 2014). All these immune cells develop in the bone marrow.
Fig. 1. Diagram of Identification Markers to Separate Neutrophils from Basophils, Eosinophils, Mast cells, Macrophages, and Monocytes.
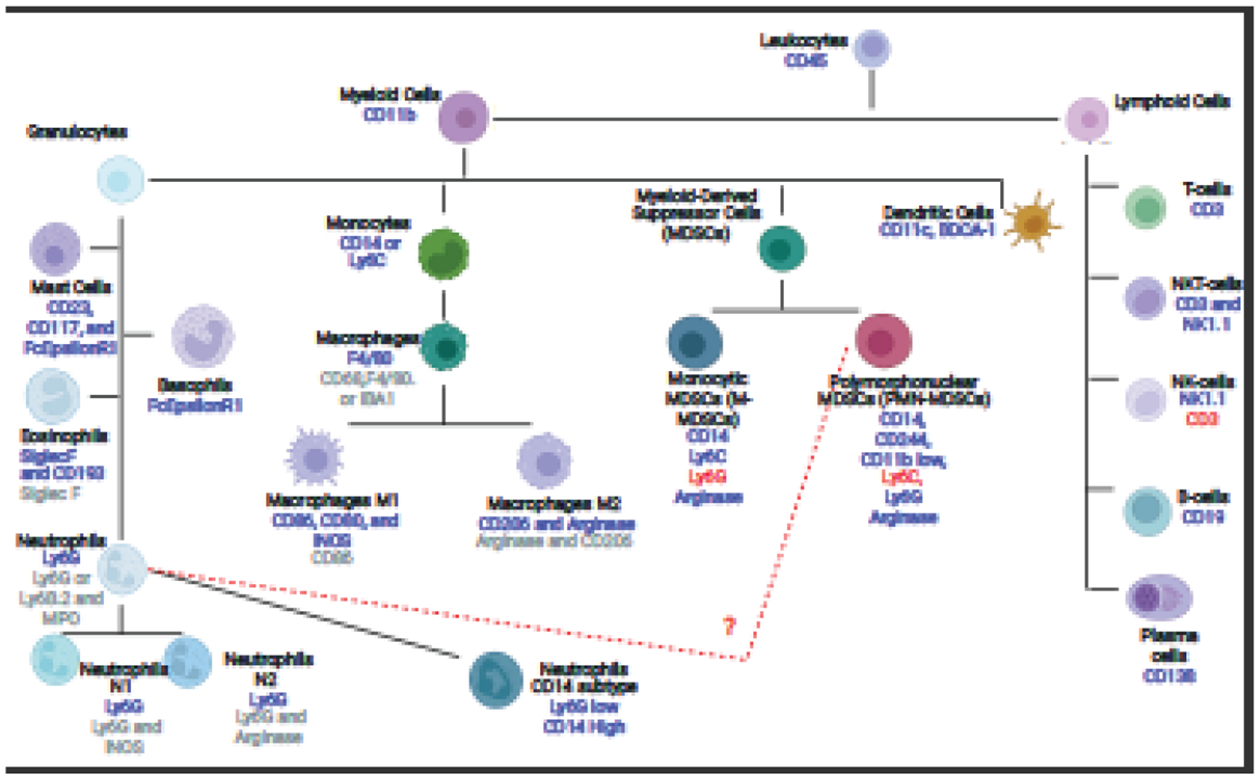
The diagram shows the different cell types within the myeloid and lymphoid lineages. The blue text under each cell type indicates a positive flow marker for that cell type, while the red text indicates a cell marker that is not present in the cell type. The grey text for macrophages, neutrophils, and eosinophils are markers that can be used in immunohistofluorescence to detect these cell types. This list is not an extensive list of cell types as some can be further divided, nor an exhaustive list of cellular markers.
2.1. Neutrophil differentiation
Over 100 billion neutrophils enter and exit the bloodstream every day in humans. To do this, the bone marrow is constantly producing new neutrophils (Pruchniak et al., 2013). Complete development of neutrophils from progenitors takes 14 days. This is such a vital process that interruption or malfunction in neutrophil production causes neutropenia, which is a reduced number of neutrophils and leads to an increased infection rate and an increase in the severity of infection (Leiding, 2017). One protein involved in their production is granulocyte colony stimulating factor (G-CSF). In mice lacking G-CSF, there is a 25% reduction in the number of neutrophils present compared to that in wild type animals (Lieschke et al., 1994). Like all leukocytes, neutrophils develop from hematopoietic stem cells. These stem cells become neutrophils as the result of lineage-specific growth factors (Borregaard, 2010). Myeloblast and promyelocytes develop into granulocytes and monocytes. Those cell that have a higher expression of GRF interactive factor 1 (Gif-1) become granulocytes, and those that express higher levels of PU.1 become monocytes (Fig. 2) (Hock et al., 2003; Karsunky et al., 2002; Radomska et al., 1998; Zhang et al., 1997). Cells that will develop into neutrophils continue to develop into myelocytes and metamyelocytes and decrease Gif-1 expression (Bjerregaard et al., 2003; Hock et al., 2003; Karsunky et al., 2002; Morosetti et al., 1997; Radomska et al., 1998; Yamanaka et al., 1997b, 1997a; Zhang et al., 1997). These cells develop near osteoblasts and bone marrow stromal cells, which produce CXCL12, while the developing neutrophils express CXCR4, the corresponding chemokine receptor (Eash et al., 2010, 2009; Hernandez et al., 2003). Band cells (i.e., immature neutrophils) and mature neutrophils are identified as having increased expression of C/EBPβ, ζ, and ξ in the bone marrow, and in circulation mature neutrophils can be identified as they have higher expression of CD101 (Bjerregaard et al., 2003). While band cells are released from the bone marrow during emergency granulopoiesis, they are typically found in the bone marrow (Amulic et al., 2012). During emergency granulopoiesis, band cells finish maturing in the injured tissue (Manz and Boettcher, 2014). Emergency granulopoiesis occurs during major injuries, when a large number of immune cells are needed at once. When serum levels of G-CSF are 100-fold greater than the normal state, the process of emergency granulopoiesis can be activated, resulting in an increased number of neutrophils. While emergency granulopoiesis may not play a role in all neurological conditions, it would be interesting to determine if it plays a role in spinal cord injury (SCI) or traumatic brain injury (TBI).
Fig. 2. Development of Neutrophils.
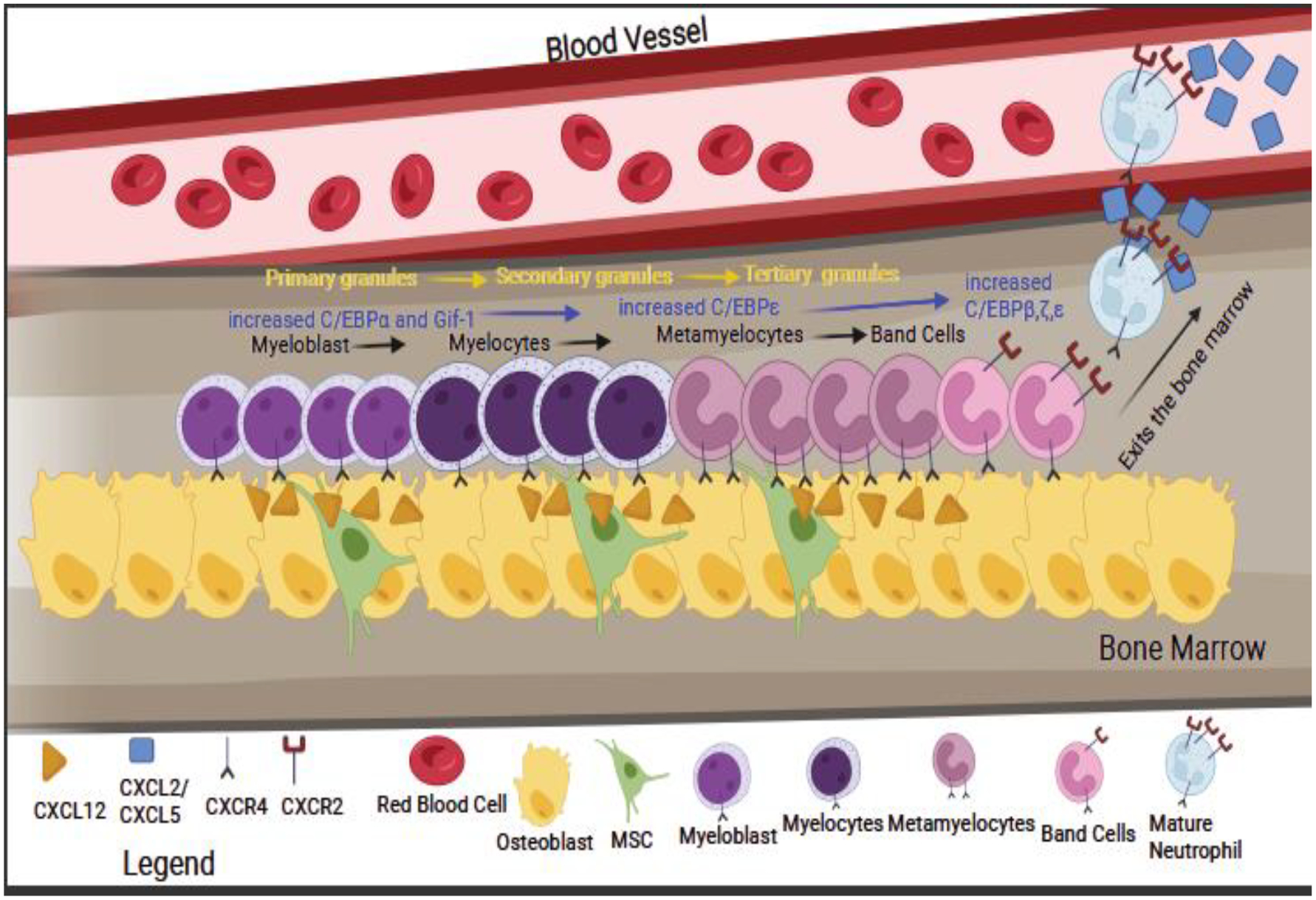
The figure shows the development of neutrophils in the bone marrow from myeloblast to myelocytes, where C/EBPε expression is increased, and primary granules start to form. These cells develop in close contact with osteoblasts and mesenchymal stem cells (MSC), as they produce CXCL12 that binds to the receptor CXCR4 present on the neutrophil precursors. As the cells further develop, they will become metamyelocytes and then band cells with an increase in expression of Gif-1 and C/EBPβ,ζ, and ζ, with secondary and tertiary granules forming. As band cells, the receptor CXCR2 is upregulated, resulting in the mature neutrophils leaving the bone marrow. The yellow text indicates the order of granule formation, the blue text indicates gene expression, and the black text indicates the order of cell differentiation during development.
2.2. Neutrophil granules
It is important to note that granule formation occurs during the development of the neutrophil, with different granules forming at different points in development, as shown in Fig. 2. Neutrophil granules are classically divided into three categories: primary (azurophilic), secondary (specific), and tertiary (gelatinase), based on staining and the presence of specific proteins important to neutrophil function (Borregaard, 2010). Azurophilic granules contain myeloperoxidase (MPO), neutrophil elastase, proteinase 3, and defensins, and they are the first granules to form during development. They can be labeled by anti-CD63 (Faurschou and Borregaard, 2003; Lacy, 2005; Nusse and Lindau, 1988). Secondary granules have lactoferrin but not MPO, form after the primary granules, and are labeled by CD66b (Faurschou and Borregaard, 2003; Lacy, 2005). Tertiary granules are also MPO negative but contain gelatinase and other metalloproteinases (MMP) e.g., MMP9.
2.3. Neutrophil chemokines
After maturation, neutrophil release from the bone marrow is dependent on the expression of CXCR2 and CXCR4. CXCR2 stimulates their release, while CXCR4 stimulates retention. G-CSF interferes with the CXCL12-CXCR4 interaction and stimulates CXCR2 expression (Christopher et al., 2009; Eash et al., 2010). Injured tissues (e.g., endothelial cells, muscle cells, nerve cells, fibroblasts, and Schwann cells), will produce the ligands for CXCR1 and CXCR2, (e.g., CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8) to summon neutrophils (Ahuja et al., 1996; Ahuja and Murphy, 1996; Borregaard, 2010; Clark-Lewis et al., 1995; Green et al., 1996; Jones et al., 1996; Katancik et al., 2000; Kobayashi, 2008; Lowman et al., 1997; Nasser et al., 2009; Rajarathnam et al., 2006, 1997, 1994; Ravindran et al., 2013; Stillie et al., 2009; Wolf et al., 1998). A list of all neutrophil chemokines and their receptors is presented in Table 1. Additional neutrophil chemoattractive and activating agents produced by injured tissues are C5a, leukotriene B4, G-CSF, interleukin 6 (IL-6), IL-18, and GM-CSF (Brennan et al., 2019; Kobayashi, 2008). The roles of CXCL3, 5, 6, and 7 in neurological disorders is not known.
Table 1.
Neutrophil Chemokines
| Standard Names | Aliases | Species known to express the protein | Receptor Affinity |
|---|---|---|---|
| CXCL1 | growth regulator oncogene α (Gro-α) neutrophil activating protein 3 melanoma growth stimulatory activity (MGSA) cytokine-induced neutrophil chemoattractant 1 (Cinc-1) platelet-derived growth factor inducible protein (KC) |
human (h), rodent (r) | CXCR2>CXCR1 |
| CXCL2 | macrophage inflammatory protein 2 (MIP-2) cytokine-induced neutrophil chemoattractant 3 (Cinc-3) growth-regulated oncogene beta (Gro-γ) |
h, r | CXCR2>CXCR1 |
| CXCL3 | growth regulator oncogene γ (Gro-γ) macrophage inflammatory protein 2 β (MIP-2β) cytokine-induced neutrophil chemoattractant 2 (Cinc-2) |
h, r | CXCR2>CXCR1 |
| CXCL5 | epithelial cell-derived neutrophil-activating protein 78 (EAN 78) small-Inducible cytokine B5 lipopolysaccharide-induced CXC chemokine (LIX) |
h, r | CXCR2>CXCR1 |
| CXCL6 | granulocyte chemotactic protein 2 (GCP-2) small-Inducible cytokine B6 chemokine alpha 3 (CKA-3) |
h, r | CXCR2>CXCR1 |
| CXCL7 | platelet basic protein leukocytic-derived growth factor (LDGF) macrophage-derived growth factor (MDGF) thymus chemokine 1 protein |
h, r | CXCR2>CXCR1 |
| CXCL8 | tumor necrosis factor (TNF) stimulated gene 6 (TSG-6) tumor necrosis factor α-induced (TNFα-induced) |
h | CXCR2=CXCR1 |
| C5a | CD88 | h, r | C5aR |
| Leukotriene B4 (LTB4) | ----------------- | h, r | LTB4 receptor 1 |
| CXCL12 | stromal cell-derived factor 1 (SDF1) | h, r | CXCR4 |
It is important to note that other signaling molecules that attract neutrophils are PAMPs, and DAMPs, although they are not neutrophil-specific (Gong et al., 2020; Herwald and Egesten, 2016). There are many receptors for DAMPs including high mobility group box 1 (HMGBI), receptor for advanced glycation end products, and triggering receptors expressed on myeloid cells (Amulic et al., 2012; Gong et al., 2020; Parker et al., 2005). Since DAMPs activate the inflammatory response without infection from a pathogen, (i.e., in chronic inflammation or autoimmune disease), they are often called sterile inflammatory markers. An in-depth review of DAMPs is outside the scope of this article (see Gong et al. 2020 for an excellent review). Distinguishing the initial signal that causes neutrophil accumulation is important. If DAMPs were the initial signal that would indicate, that they are homing to the area because of tissue damage. The use of Cxcr2 KO mice would be useful in investigating if the chemokines are attracting the cells during neurological conditions.
Neutrophils follow a chemoattractive gradient via the receptors on their membrane, which prime them to eventually exit the bloodstream via the leukocyte adhesion cascade as shown in Fig. 3 (Ley, 2002; Mueller et al., 2010; Yago et al., 2010). Endothelial cells near the area of interest will express E- and P-selectins, which will then bind to glycoproteins on the neutrophil (i.e., P-selectin glycoprotein ligand-1 and L-selectin), and the neutrophil will “roll” along the endothelium(Finger et al., 1996; Lawrence et al., 1997). This will cause the βII integrins on the neutrophils to enter a high-affinity state, causing them to bind their ligands (i.e., leukocyte function associated-antigen 1), resulting in “firm adhesion”. This is the same process that monocytes use to enter tissue. As they both enter the tissue via the leukocyte adhesion cascade inhibiting this mechanism would inhibit macrophages as well, making it difficult to separate neutrophils’ role from macrophages’ on the bases of this alone. To enter the tissue, the neutrophil must travel between the endothelial cells, which is mediated by βII integrin and intracellular adhesion molecules (Kansas, 1996; McEver and Cummings, 1997). Once through the endothelial layer, neutrophils contact the basement membrane, and it is speculated that granule proteases (tertiary granules) assist in the crossing of this layer (Phillipson et al., 2006; Schenkel et al., 2004; Wong et al., 2010). The tissue is a complex environment containing chemoattractive and inflammatory signals from the host. DAMPs, C5a, and chemokines activate many of the cellular processes of neutrophils: degranulation, phagocytosis, the release of ROS and RNS, NET formation, and immunomodulation (Ley, 2002; Ley et al., 2007; Parker et al., 2005).
Fig. 3. Neutrophil Chemotaxis and Mechanism of Action.
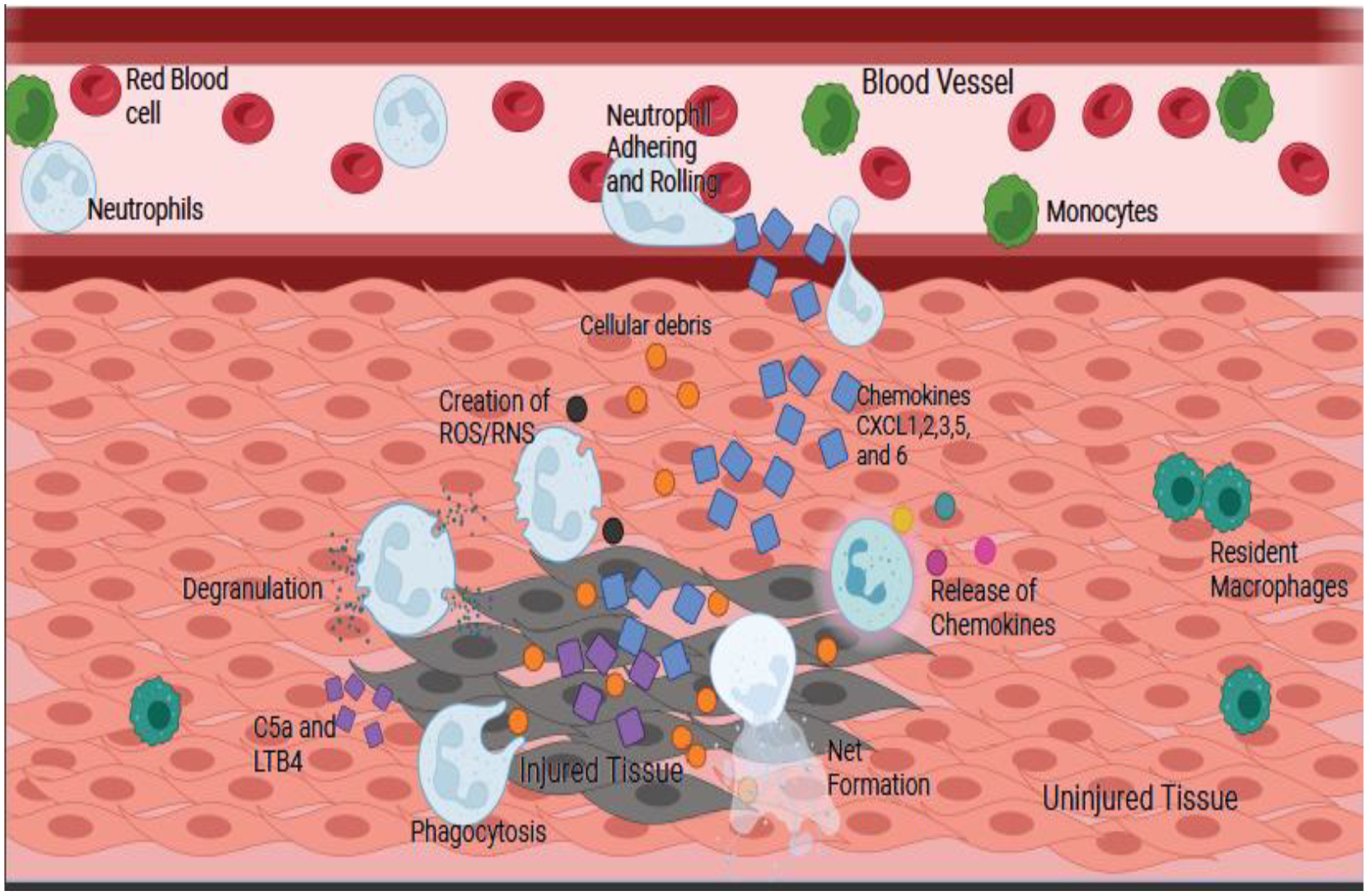
Neutrophils will follow the gradient of chemokines to the injury site, then adhere and roll along the vessel before firmly adhering and entering the tissue. Entering the tissue is accomplished by degranulation of the tertiary granules. Once in the tissue, the cytokines CXCL1, 2, 3, 5, 6, LTB4 and C5a, which are released by the injured tissue, will trigger different cellular functions leading to degranulation, phagocytosis, creation of reactive oxygen and nitrogen species (ROS/RNS), formation of NETs, and release of chemokines. The orange circles are cellular debris, the blue squares are chemokines, and the purple squares are C5a and LTB4. The cells are labeled in the figure.
Neutrophil clearance was once thought to be relatively straightforward, as neutrophils in tissue will undergo apoptosis at the end of their life and are cleared by macrophages, an example of efferocytosis (Martin et al., 2003). This clearance of neutrophil debris is important to control their numbers as dead neutrophils triggers an anti-inflammatory response (Stark et al., 2005). Circulating neutrophils at the end of their life cycle upregulate CXCR4 and return to the bone marrow. New evidence suggests that neutrophils can reverse transmigrate out of the injured tissue, so how these cells do this is an exciting new area of research (de Oliveira et al., 2016; Mathias et al., 2006; Wang et al., 2017). What is known so far is that endothelial cells expression of JAM-C prevents reverse transmigration by neutrophils (Nourshargh et al., 2016). This process is similar to macrophage reverse migration. One interesting idea to come from this is what do these cells do after leaving the tissue. If they still have granules, they could still be used in the defense of the host, but as granules are made during development, more cannot be produced. Future studies could investigate this reverse transmigration as a method to investigate neutrophils’ roles in disease as well as potential treatments to decrease unwanted neutrophil migration and accumulation.
2.4. Neutrophil functions
Granules can either fuse with a phagosome or with the cell membrane, the latter resulting in the release of their contents into the surrounding tissue (Burn et al., 2021; Peiseler and Kubes, 2019; Swamydas et al., 2016). This degranulation is essential to neutrophils’ antimicrobial properties, as the contents of the granules will kill microbes, though they can also cause damage to host tissues. Most of the work done on understanding neutrophil degranulation is from cell culture experiments due to the difficulties of imaging degranulation in vivo. Results of degranulation in vivo are indicated via increased extracellular levels of granular proteins, e.g., MPO (Rajarathnam et al., 2019). Its mechanism is highly controlled by different intracellular signaling pathways, as the contents are very destructive.
The process of degranulation requires four key steps: cytoskeletal remodeling, moving the granules to the membrane, docking and release (Lacy, 2006). One important protein for degranulation is CXCR1, as a Cxcr1 KO model showed decreased granule release (Swamydas et al., 2016). CXCR1 and N-formyl peptide receptor 2 are proteins that increase intracellular Ca+, which is known to be important for degranulation, as you can artificially elevate Ca+ level and induce degranulation (Sengelov. et al., 1993). The granules are moved to the cell membrane after the actin cytoskeleton is remodeled, which is the result of Rac2 (Rac family small GTPase 2) activation, as Rac2 KO fails to move granules to the cell membrane (Abdel-Latif et al., 2004).
Interestingly, from investigations into bacterial methods of altering degranulation has come a method to inhibit it. Studying Chlamydia trachomatis has shown they produce chlamydial protease-like activity factor, which cleaves the surface portion of N-formyl peptide receptor 2 on neutrophils, which decreases degranulation by decreasing Ca+ influx (Rajeeve et al., 2018). Inhibitors of N-formyl peptide receptor 2 are available and could become a valuable tool to investigate degranulation in neurological conditions. KOs of Mpo, neutrophil elastase, and cathepsin G can be useful in investigating their roles (Stackowicz et al., 2020). It is important to remember that degranulation has a second function, which is to present proteins to the membrane for the creation of ROS and RNS.
These reactive species can modify and damage other molecules. While they are crucial in pathogen clearance, their specific role in neurological injury and disease has not been shown; however, the ROS and RNS are not only harmful to pathogens, they can degrade host proteins as well. CXCL8 binding to CXCR1 and CXCL1 or CXCL5 binding to CXCR2 has been shown to amplify superoxide release, indicating their importance in ROS/RNS production (Planagumà et al., 2015; Rajarathnam et al., 2019). Down-stream mediators are Rac1 & 2, which are upregulated in human neutrophils and have been shown to regulate NADPH oxidase (Bokoch, 1995; Bokoch and Diebold, 2002; El-Benna et al., 2016). NADPH oxidase converts molecular oxygen to superoxide, which then creates hydrogen peroxide, which when combined with nitric oxide produces peroxynitrite (Winterbourn et al., 2016).
One of the more interesting and unique functions of neutrophils is the formation of NETs, which involves the release of granular proteins into the cytosol, release of decondensed chromatin together with modification of histones and release of cytoplasmic proteins into the extracellular space, which can be a unique form of cell death (Amulic et al., 2012). NETs trap pathogens and aid in their killing, in part by macrophages (Monteith et al., 2021). The mechanism for NET formation is not well understood; however, it is known that neutrophil elastase, NADPH oxidase, MPO, and citrullination of histones are required for NET formation (Burn et al., 2021; Fuchs et al., 2007; Metzler et al., 2011; Patel et al., 2010). In NET formation, neutrophil elastase, cathepsin G, and MPO are released in the cell and degrade the lamina of the nucleus (Papayannopoulos et al., 2010; N. V. Vorobjeva and Chernyak, 2020). This allows peptidyl-arginine deaminase 4 (PAD4) to enter the nucleus and citrullinate histone 3 (CitH3), which leads to chromatin decondensation (Li et al., 2010). Pores then form on the nucleus and the membrane to allow the chromatin with granular proteins attached to enter the extracellular space. NETs can have detrimental effects on the host as they can lead to autoimmune disorders. For example, NET formation has been associated with systemic lupus erythematosus, an autoimmune disease characterized by autoantibodies to chromatin and neutrophil components (Leiding, 2017). NET formation can lead to neutrophil cell death, but cell death does not always result from NET formation. The latter case is referred to as “vital NETosis”(Yipp and Kubes, 2013). Some believe the word “NETosis” should not be used when referring to this process as the cell survives. What is thought to separate these two processes is that during NETosis nuclear DNA is released, while mitochondrial DNA is released instead in the other process (N. V Vorobjeva and Chernyak, 2020). Pharmacologic methods of inhibiting NETs include PAD4 inhibitors and DNase 1 administration. Recently, a protein called neonatal NETs inhibitory factor has been demonstrated to significantly decrease NETs without inhibiting other function of neutrophils (Denorme et al., 2022; Yost et al., 2016). Additionally, a S1008a KO mouse model (S1008a is a calcium-binding protein) has been helpful in looking at the role of NET formation, as these mice have decreased NET formation. It would be interesting to see if KO mice or NET inhibitors have any effects in the context of the neurological injuries and pathologies that are discussed in later sections. While western blots for NETosis proteins (i.e., CitH3 and neutrophil elastase) and flow cytometry for CitH3 have been used, images of the NETs will be key for demonstrating NETosis. For visualization of NETs via immunohistochemistry CitH3+, DNA+ (DAPI), and a neutrophil marker, e.g., Ly6G+, are best. This is important as macrophages are said to produce extracellular traps also (Doster et al., 2018).
Phagocytosis is a well-known function of neutrophils (Amulic et al., 2012; DeFrancesco-Lisowitz et al., 2015); however, in the field of neuroscience, this has been largely ignored as the phagocytosis of myelin and cellular debris after injury has been attributed to macrophages in the PNS and microglia in the CNS (Gaudet et al., 2011; Rotshenker, 2011). Nevertheless, in the early 1900s, Cajal described leukocytes with a polylobed nucleus in the distal nerve within 2 days after a nerve injury, which had phagocytosed lipid debris (DeFelipe et al., 1991). Phagocytosis in neutrophils is similar to that in macrophage/microglia (Lee et al., 2003). It can be initiated by recognition of IgG-opsonized particles or by a complement receptor-mediated process (Swamydas et al., 2015). The phagosome fuses with the granules, similar to the phagosome fusing with lysosomes in macrophages via the LC3-associated phagocytosis pathway, creating an acidic environment, and the enzymes degrade the contents (Borregaard, 2010).
It has been shown that neutrophils are required for myelin clearance from the distal nerve after a sciatic nerve transection injury. This possibility was first indicated in Ccr2 KO mice, which are deficient in infiltrating macrophages but show normal myelin clearance at 7 days after nerve transection (Niemi et al., 2013). It was later shown that clearance of myelin was inhibited when neutrophils were depleted with an anti-Ly6G antibody in wild type mice after sciatic nerve transection, as seen in Fig. 4 (Lindborg et al., 2017). Additionally, by using fluorescent polystyrene beads, Lindborg et al. were able to show that neutrophils were phagocytic at 3 days after an injury, suggesting they could be phagocytosing cellular debris and perhaps myelin.
Fig. 4. Neutrophils are Involved in Myelin Clearance.
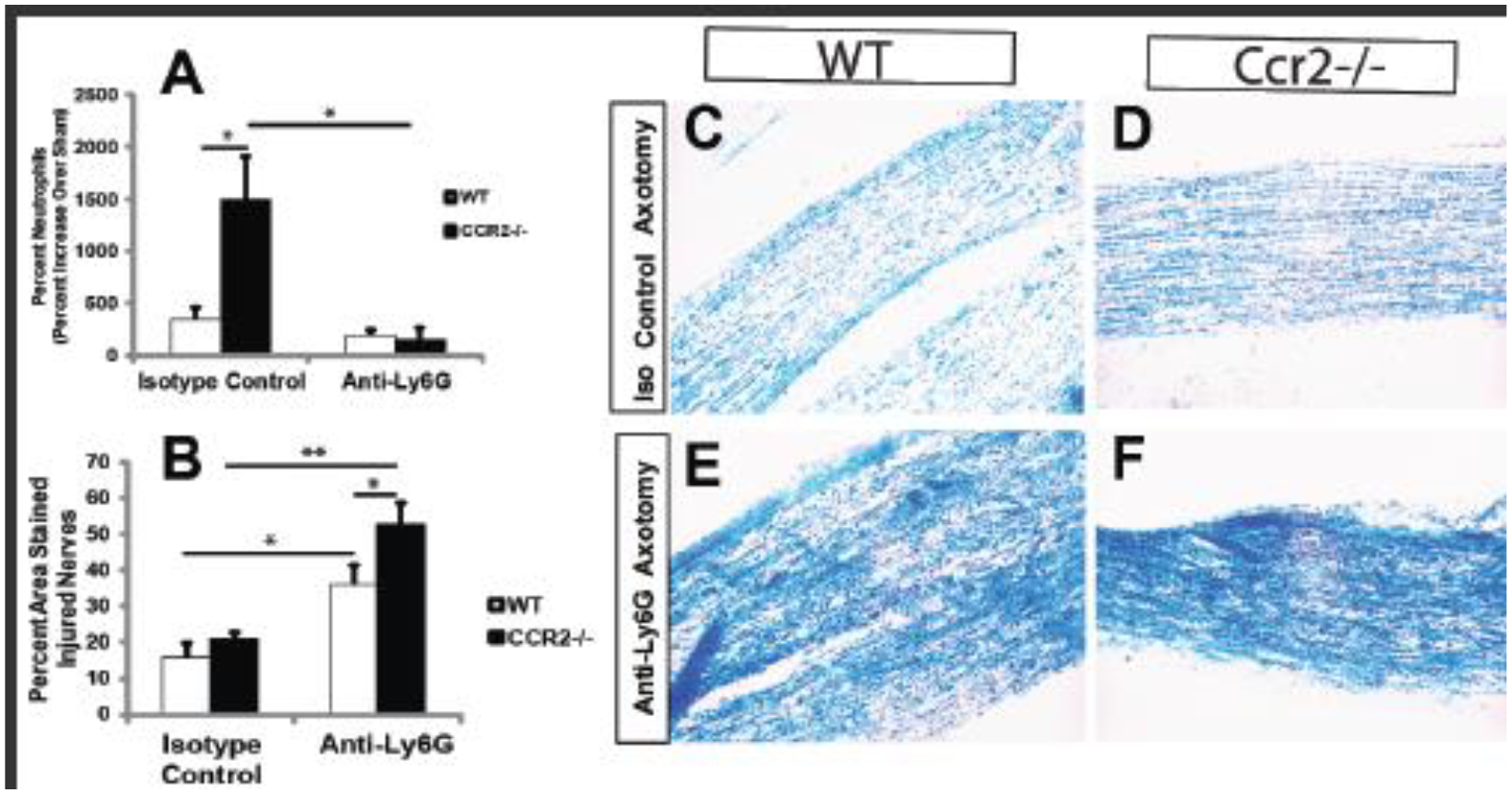
Wild type and Ccr2 KO mice were pretreated with antibodies against Ly6G to deplete neutrophils or were given an isotope control antibody. The sciatic nerve was then transected unilaterally, and seven days later, the nerve was stained with luxol fast blue (LFB) as an index of intact myelin. The top graph shows the effectiveness of the deletion strategy (A). Graph B shows the percent area stained. The LFB images show residual staining after neutrophil depletion (C-F). (Adapted from Lindborg et al., 2017).
In the last 20 years, researchers have demonstrated neutrophils’ roles as immunomodulators, which release cytokines and chemokines that help launch the adaptive immune response. These factors can be released directly into the extracellular space or via exosomes. Neutrophils are known to produce interleukin (IL-1)-β and tumor necrosis factor (TNF)-α, which promote other cells to produce cytokines that can attract immune cells (Kasama et al., 2005; Scapini et al., 2000; Sica et al., 1990). As neutrophils enter damaged tissue, they recruit monocytes by expressing CCL2, CCL3, CCL19, and CCL20, known monocyte/macrophage chemokines (Scapini et al., 2001). Neutrophils also have been shown to release TNF ligand superfamily member 13B (BAFF), which is known to play a role in B cell proliferation and maturation (Scapini et al., 2008). Decreased expression of any of these proteins could decrease immune cell activation thereby changing the immune response in a neurological condition.
However, this cellular communication is not unidirectional. T cells can secrete interferon γ, which prolongs neutrophils’ lifespan (Nagase et al., 2002). Helper T cell 17 (Th17) can secrete IL-17, upregulating CXCL8, G-CSF, and TNF-α in endothelial and stromal cells thereby recruiting more neutrophils (Ouyang et al., 2008; Yang et al., 2010). This shows a cross talk between neutrophils and immune and non-immune cells by which neutrophils enhance or inhibit the immune response, and, in turn, the other cells continue to recruit neutrophils. In later sections, we will highlight interactions between neutrophils and other cells, but this is an exciting avenue of research as modulation of immune response or neutrophil response could benefits patients greatly.
2.5. Neutrophil subpopulations
Once thought to be a homogeneous cell population, neutrophils are now known to be heterogeneous, with different ways to classify these subpopulations. Neutrophils can undergo changes during their life cycle while circulating in the blood. There are multiple different ways to separate neutrophils. One way is by centrifugation with a Ficoll density gradient, where high density PMNs are found in the bottom of the tube and low density PMNs and PMN myeloid-derived suppressor cells (PMN-MDSC) are found in the layer between soluble and insoluble solution (García-García et al., 2013; Rosales, 2018). Neutrophils have been classified similarly to macrophages, with N1 neutrophils being pro-inflammatory and N2 being anti-inflammatory. These groups are distinguished using the classic M1 and M2 markers: iNOS+ and CD86+ for N1 cells and arginase-1+ cells for N2 (Burn et al., 2021); however, this binary division is likely an oversimplification as is now recognized in the macrophage field (Martinez and Gordon, 2014).
One cell type that could easily be mistaken for neutrophils are PMN-MDSCs; however, there is some debate on whether PMN-MDSC are truly a separate cell type or just a subtype of neutrophils. These cells are Ly6G+, Ly6Clow, CD15+, and CD16+ positive, and their nuclei are similar to immature neutrophils (Rosales, 2018; Solito et al., 2017). There is a debate as to whether they are CD14 positive. It has been suggested that PMN-MDSC are CD244+, Lox1 and CD11blow, making these possible markers to separate them from neutrophils, but these markers are not agreed on (Bronte et al., 2016; Knier et al., 2018). Since neutrophils and PMN-MDSC have such similar phenotypes, it is impossible to distinguish them based on markers alone. Test of function is required to demonstrate whether the cells suppress the immune response (Bronte et al., 2016; Pillay et al., 2013). Recently a Ly6Glow,CD14hi subpopulation of neutrophils has been found in CNS and PNS injuries, which will be discuss in later sections.
2.6. Tools used to investigate neutrophils
There are many tools used to investigate neutrophil biology and roles in neurological disorder. Freshly harvested cells are the gold standard for studying neutrophil biology. Harvested neutrophils have a short lifespan after isolation (~8 h) prior to undergoing apoptosis (Borregaard, 2010). This is important because if an experiment requires isolating molecules released from the neutrophils, one only has 8 hours to collect the molecules/exosomes, before the pool is contaminated with molecules from dying cells.
As there are many markers used to label neutrophils, it can be difficult to compare results between different studies. A previous standard marker in the field Gr-1 is no longer considered appropriate, as Gr-1 antibodies recognizes both Ly6G (a neutrophil marker) and Ly6C (a monocyte marker). In vivo MPO, Ly6G, and Ly6B.2 have all been used to identify neutrophils immunohistochemically. CD11b+Ly6G+ expression is an appropriate combination of markers in flow cytometry experiments, but the addition of the identifier Ly6C intermediate is also correct (Lindborg et al., 2017; Stackowicz et al., 2020). It is important to note that many of the markers used to identify macrophages are also expressed in neutrophils though not necessarily as strongly (e.g., F4/80, CD68, and CD11b), and MPO, often used to stain neutrophils, also stains macrophages (Sasmono et al., 2007). It is best to identify neutrophils by using a Ly6G antibody either alone or in combination with other markers.
Many animal models have been used in investigation of neutrophils’ roles in injury and diseases (Stackowicz et al., 2020). Zebrafish are useful for in vivo imaging of heterophils (the zebrafish version of neutrophils). These cells are morphologically, biochemically, and functionally similar to mammalian neutrophils (Henry et al., 2013). Zebrafish models allow for clear in vivo imaging due to their transparency and are useful in investigating reverse transmigration.
Several models have been created to deplete neutrophils (Stackowicz et al., 2020); however, there are some limitations in using deletion strategies as emergency granulopoiesis can result in a rapid production of neutrophils, requiring continuous administration of the depleting agent (Stackowicz et al., 2020). The MRP8-Cre MCL1 fl/fl mice show severe neutropenia with the deletion of MCL1, with a 99% reduction in circulating neutrophils compared to wild type, with no effect on the number of monocytes, B, or T cells (Csepregi et al., 2018). One line that shows great promise is the Catchup mouse line, where the Ly6G gene has a knock-in Cre recombinase and fluorescent protein tdTomato (Hasenberg et al., 2015). While the Cre expression is primarily found in neutrophils, there were some eosinophils and basophils with transgene activity. Myeloid-specific KOs can be created using the LysM and CD11b promoters (Clausen et al., 1999; Gowing et al., 2006); however, it must be taken into account the possibility of a shared function between neutrophils and other myeloid cells, e.g., monocytes/macrophages (Barrette et al., 2008). Similarly, models created using the CSF1R promoter would have some effect on other granulocytes and macrophages, as they also express the CSF1R (Sasmono et al., 2007). No matter what mouse model is used careful analysis is needed to determine which cell types are being affected. As neutrophils develop in the bone marrow alongside monocytes, basophils, and eosinophils and descend from common progenitors, it is not surprising that some of these models are not specific to neutrophils, but, with appropriate controls, they can be used to investigate the role of neutrophils in neurological injuries and diseases.
3.0. Neutrophils in CNS diseases, injuries and regeneration
3.1. Stroke
Stroke is the second leading cause of death globally at 11% in 2019, and it is a main cause for living with disabilities (Feigin et al., 2021; Li et al., 2020). Strokes cause a decreased blood flow to the brain resulting in the brain not receiving the oxygen and nutrients it needs to perform its vital functions. The result of this hypoxia is brain damage, which can lead to impaired motor function, the loss of sensation, chronic pain, emotional disturbances, aphasia, as well as memory and cognitive problems depending on where the stroke occurs (Sarikaya et al., 2015). There are two main types of strokes: 1) ischemic stroke, caused by a blockage of an artery, or 2) hemorrhagic stroke, caused by a blood vessel bursting. Neutrophils effects are negative but could also be an effective tool to aid recovery (see below). Platelets and neutrophils are among the first cells to respond to stroke injury (Cai et al., 2020; García-Culebras et al., 2018; Perez-de-Puig et al., 2015). Following the initial injury, the inflammatory response can worsen the situation by producing further damage (Perez-de-Puig et al., 2015; Wang et al., 2020). Clinically, the ratio of neutrophils-to-lymphoid cells and platelets-to-lymphoid cells are strong indicators of neurological deficiencies after a stroke and after treatment (Gong et al., 2021). Neutrophils home to the injury area, peaking in numbers between 1–3 days. During this time, microglia are necessary to inhibit the effects of neutrophils, as seen in a stroke model in which microglia engulf infiltrating neutrophils. When microglia are eliminated, this leads to a greater accumulation of neutrophils causing a greater lesion size (Otxoa-de-Amezaga et al., 2019). One way neutrophils could worsen the effects of stroke is through NETs, because inhibiting NET formation improved blood vessel formation in a stroke model (Cahilog et al., 2020; Essig et al., 2020; Kang et al., 2020; Kim et al., 2019). This indicates that modulating neutrophil function or presence after a stroke could be beneficial for recovery (Chen et al., 2021; Silvestre-Roig et al., 2020). These was demonstrated in a recent mouse stroke model study that used a NET inhibitor factor and found decreased infarct size, improved long term neurological recovery, and increased survival. Additionally, this study found increased serum levels of HMGBI associated with HMGBI expression by platelets. When HMGBI was inhibited, there was a decrease in NET formation. This indicates HMGBI from platelets could be priming or activating neutrophils to produce NETs, demonstrates the importance of understanding the many different interactions neutrophils have with other cell types.
Neutrophils have also been shown to have beneficial effects in stroke. In a mouse model, pushing neutrophils towards an N2 phenotype with TGF-β injections resulted in a decrease in the infarct size, showing that the N2 cell type is protective (García-Culebras et al., 2019). While these N2 cells are most likely PMN-MDSCs, an analysis of PMN-MDSCs presences after stroke is needed to determine that. If they are found to be PMN-MDSCs, the goal would be to determine when they normally enter the tissue, and see what molecules are being released and what cells are being affected to aid recovery.
3.2. Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by β-amyloid plaques, neurofibrillary tangles of hyperphosphorylated tau, amyloid angiopathy, reduced blood flow and neuronal loss. The disease results in a progressive loss of cognitive function and impaired memory formation and recall. Within the last fifteen years, there has been growing evidence that inflammation plays a role in AD pathophysiology; however this idea is not accepted by all (Breijyeh and Karaman, 2020; Chen et al., 2022; Ernesto et al., 2020; Heneka et al., 2015; Herrup, 2010). The pathology of AD is complex and modeled in a number of different mouse models (Yokoyama et al., 2022). Some models investigate the importance of β-amyloid plaques (i.e., 5xFAD or APP/PSI), tau neurofibrillary tangles (i.e., 3xTG), or brain insulin resistance (Bondi et al., 2017). While neutrophils role in AD is just beginning to be uncovered, there is a great deal of evidence that manipulations to their biology could be beneficial. Neutrophil staining of human brains with AD demonstrated more neutrophils in the vessels and parenchyma compared to those in control brains, with some neutrophils forming NETs (Zenaro et al., 2015). It has been suggested that β-amyloid is a DAMP that is recognized by the complement system and that activates human and mouse neutrophils’ formation of ROS. Other DAMPs (i.e., HMGBI) have been associated with AD (Paudel et al., 2020). Additionally, β-amyloid increases the affinity state of lymphocyte function-associated antigen-1 (LFA-1), which would enhance neutrophil adhesion in the brain (Zenaro et al., 2015).
Zenaro et al. (2015) showed in the 5xFAD and the 3xTG mouse the presence of neutrophils at 4, 6, and 8 months of age, demonstrating neutrophils are present when cognitive impairment can be assessed. Inhibition of neutrophil trafficking via an LFA-1 antibody reduced AD symptoms and improved blood flow in both mouse models (Zenaro et al., 2015). Depletion of neutrophils in the 3xTG mouse with the Ly6G antibody produced a return to normal response in the y-maze and contextual fear conditioning test (Silvin et al., 2022). Similar finding with Ly6G antibody in the APP/PSI mouse showed improved cognitive function and increased blood flow with compelling in vivo imaging (Bracko et al., 2020; Cruz Hernández et al., 2019). While it has been shown in a couple of different mouse models of AD that neutrophils affect blood flow, what has not been shown is the accumulation of neutrophils before cognitive decline. The neutrophils could be accumulating because of DAMPs (i.e., HGMBI), which would suggest that neutrophil accumulation leads to the clearing of debris and dying cells and causing decreased blood flow. Additionally, it will be interesting to see what role NETs play in the pathophysiology. Are the NETs an attempt to help clear the β-amyloid? The role of PMN-MDSCs is unknown, and a line of future research.
3.3. Multiple sclerosis (MS), Experimental Autoimmune Encephalomyelitis (EAE), & Neuromyelitis Optica Spectrum Disorders
Multiple sclerosis (MS) is a nontraumatic neurological debilitating disease, which affects 2.5 million people worldwide (Pierson et al., 2018). It is an autoimmune disease with onset between 20–45 years of age, affecting both sexes but affecting women at a greater rate. T cells play an important role in the inflammation through self-reaction to myelin protein that cause lesions in the brain, with some lesions occurring in the spinal cord. While there are many forms of MS, some have an initial onset followed by a stable phase then worsening of the symptoms. In humans, neutrophils are not commonly found in CSF of MS patients (Knier et al., 2018); however, there is debate on whether neutrophils-to-lymphoid ratio in the blood is useful in predicting symptom severity or relapse in patients (Hasselbalch et al., 2018; Huang et al., 2022).
There are two theories of neutrophils role in MS, which is studied in mice via the experimental autoimmune encephalomyelitis model (EAE). While EAE is not MS, it is the best model we currently have in which to test hypotheses in rodents. The first theory takes the classical view that neutrophils have a deleterious effect, while the second has strong evidence that they have a beneficial effect (Khorooshi et al., 2020; Zehntner et al., 2004). Neutrophils are also known to expand in the bone marrow and accumulate in the blood and spleen before symptom onset in EAE. These responses are induced by the release of G-CSF and CXCL1 as elimination of these molecules blocks neutrophil accumulation (Rumble et al., 2015; Simmons et al., 2014; Zhang et al., 2019). Recently, a publication showed that in the EAE model, T cells home to the bone marrow causing an increase in hematopoiesis (Shi et al., 2022). Blockade of CXCR2 in EAE decreased lesion size, suggesting that neutrophils are involved (Kerstetter et al., 2009). Studies with Cxcr2 KO mice and function-blocking antibodies in EAE models suggest the initial demyelination is the work of neutrophils, while reoccurring demyelination is associated with non-hematopoietic cells (Liu et al., 2010b, 2010a). One mechanism proposed is that neutrophil secretions activate dendritic cells, which in concert with CNS-infiltrating T cells, present the myelin antigen, resulting in an autoimmune response (Casserly et al., 2017).
The beneficial effects have been demonstrated in three recent publications. In these papers the immune suppressive and paracrine activities are demonstrated to be beneficial to recovery from EAE. One paper showed using antiserum to Ly6G to deplete Ly6G+ cells (neutrophils and PMN-MDSCs) that there was an increased accumulation of B cells in the CNS, which promoted activation of microglia and progression of EAE, indicating neutrophils beneficial effect (Knier et al., 2018). In addition to that, in the EAE model it was shown that neutrophils that enter the CNS interacted with B cells, transitioning to a PMN-MDSCs state. This demonstrates PMN-MDSCs can control the pro-inflammatory B-cells in the inflamed CNS aiding recovery. These PMN-MDSCs had a clearing effect on B-cells in the CSF with EAE improvement. This could be the result of PMN-MDSCs reducing their production of BAFF, which is needed for B-cell development. The administration of microparticle immune stimulator-416 (MIS416) to mice with EAE is known to suppress EAE symptoms (White et al., 2018). MIS416 is a micro-particle of bacteria cell wall with PAMPs (bacterial DNA and proteins) that activate the host immune system (Girvan et al., 2011; Luckey et al., 2015). Khorooshi et al, showed EAE mice that received neutrophils from mice treated with MIS416, had suppressed EAE, demonstrating MIS416 caused the neutrophils to have an immune suppressive effect. Since MIS416 can affect both neutrophils and macrophages and some evidence macrophages play a role in EAE, they did the complimentary experiment with monocytes. However, this effect was not seen when pretreated monocytes were transferred, suggesting that neutrophils were the immune suppressing cell (Khorooshi et al., 2020). These two studies show the beneficial immunomodulating effects neutrophils/PMN-MDSCs can have in EAE, but a recent paper has suggested that their effects could also be on microglia. Using nanovesicles from unmanipulated neutrophils injected into EAE mice showed enhance clearance of myelin by microglia and delayed symptom onset and decreased lesion size (Shen et al., 2022). Analysis of the effects of these nanovesicles in the tissue via bulk RNA sequencing showed changes in immune cell regulation processes. While there is no clear consensus on neutrophils in EAE and MS, it is possible that early effects are detrimental, but symptom remission is based on neutrophil/PMN-MDSCs paracrine/immunomodulator signaling.
While it is debated whether neutrophils play a role in the pathogenesis of MS, it is known they are involved in neuromyelitis optica spectrum disorders (NOS)(Carnero-Contentti and Correale, 2021; Hertwig et al., 2016; Huda et al., 2019). Until recently NOS was thought to be a form of MS, but it is now known to be a separate disorder. NOS is rare, but it results in sudden loss of vision and paralysis, with relapsing-remitting episodes. There is ample evidence that NOS is the result of autoimmune response to aquaporin-4 on microglia, which results in blood-brain-barrier (BBB) disruption allowing neutrophils to enter the CNS and damage the myelin (Huda et al., 2019). Some relapsing patients see a 60% increase in neutrophils in their CSF, and some patients show an increase in CXCL5 and CXCL8 levels in their serum. These clinical findings are supported by mouse models as depletion of neutrophils decrease tissue damage, while increasing the number of neutrophils enhanced damage, which is thought to occur by degranulation. Inhibiting their entry into the tissue with a neutrophil elastase inhibitor led to an improvement in symptoms. It would be interesting to see if these neutrophils could be manipulated into a PMN-MDSCs subtype to improve outcomes for patients.
3.4. Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS), or Lou Gehrig’s disease, is the most common motor neurodegenerative disease, which affects both upper and lower motor neurons (Mejzini et al., 2019; Therrien et al., 2016). ALS is fatal, and, typically, symptoms first appear in midlife, leading to muscle weakness and atrophy. Eventually, weakness of the respiratory muscles leads to death usually within 2–4 years from symptom onset. ALS pathophysiology is unclear and is believed to be a multifactorial disease. Clinically, a higher neutrophil-to-lymphocyte ratio is a useful predictor of the severity of symptoms in ALS patients (Leone et al., 2022; Murdock et al., 2017). ALS patients have increased levels of G-CSF and CCL3 in their cerebrospinal fluid, which could cause and increase the number of neutrophils (Chen et al., 2018). Additionally, monocytes of ALS patients show an increased expression of CXCL1 & 2, possibly enhancing neutrophil homing and activation (Zhao et al., 2017). Treatment to inhibit neutrophils and mast cell infiltration in the SOD-1G93A rat showed improved axonal pathology and decreased demyelination (Trias et al., 2018). These findings suggest targeting neutrophils or their cytokines as a possible therapeutic target for ALS patients.
As with many other fields, the gut microbiome effect is just beginning to be explored in ALS mouse studies. In the C9orf72 loss of function model of familial ALS, the mice have different survival rates depending on their environment. Recently this was found to be the result of differences in the gut microbiome (Mejzini et al., 2019; Polymenidou, 2020; Burberry et al., 2020). These changes were associated with increased expression in G-CSF, CCL3, and MIP-1β. The research indicated suppression of the gut microbiome by antibiotics decreases neutrophil neuroinflammation; however, in a SOD-1 mouse study, reducing bacterial load led to a faster onset of symptoms (Blacher et al., 2019). This faster onset was attributed to decreased nicotinamide; however, neutrophil sub-populations were not characterized in these studies, so it is unclear if that could have played a role in the different outcomes. Further investigation into the role of gut microbiome’s role in ALS could lead to new understanding of the disease. In the future, it would be interesting to see if gut microbiome influenced patient’s neutrophils-to-lymphoid ratio. Additionally, it would be interesting to see if the gut microbiome affects neutrophil and PMN-MSDC populations.
3.5. Spinal cord injury (SCI) and traumatic brain injury (TBI)
SCI.
Spinal cord and traumatic brain injuries (SCI and TBI) are life-threatening injuries, which can produce life-long disabilities. Shortly after the injuries, there is an acute phase of inflammation, which causes more damage to the surrounding tissues. In mice, within hours after SCI, astrocytes and microglia start to produce pro-inflammatory cytokines: IL-1β, CXCL1, CXCL2, and CXCL5 (Kobayashi et al., 2018; Pelisch et al., 2020). It has been shown in a mouse model of SCI that IL-1β peaks within 12 hours after the injury, which coincides with maximum neutrophil infiltration at the site of injury (Pineau and Lacroix, 2007; Saiwai et al., 2010). Deleting the IL-1 receptor or using an antagonist to IL-1 reduced neutrophil infiltration (Pineau et al., 2010; Yates et al., 2021). It would be interesting to see if lesion size was decreased under these conditions and whether the glial scar was affected, the latter being a known factor in regeneration failure after SCI (Cregg et al., 2014; Silver and Miller, 2004).
Most studies of neutrophils in spinal cord injury mainly focus on the detrimental effects that neutrophils have (McCreedy et al., 2021; Neirinckx et al., 2014). Lacroix et al. (2002) looked at the IL-6 family of cytokines, which includes leukemia inhibitory factor and ciliary neurotrophic factor (Lacroix et al., 2002). Using an experimentally created hybrid protein called hyper-IL-6, they stimulated the family’s signaling receptor subunit (gp130) to test if it would elicit regeneration after SCI. IL-6 has been shown to act as a checkpoint protein that controls the movement of neutrophils during an inflammatory response (Fielding et al., 2008). When IL-6 is blocked in rheumatoid arthritis patients, an acute neutropenia occurred, suggesting impaired neutrophil release from the bone marrow (Wright et al., 2014). Delivery of this protein was found to increase the number of neutrophils which was associated with an increase in spinal cord lesion size and reduced axonal growth (Lacroix et al., 2002). The presence of neutrophils and other leukocytes were found to increase after IL-6 administration, but unfortunately with that came a large production of ROS. This pro-inflammatory characteristic outweighed any pro-regenerative capacities the cells might have in this situation and suggests that infiltrating immune cells, including neutrophils, play a role in causing secondary damage after spinal cord injury. However, after depleting neutrophils using a neutrophil antiserum, de Castro et al. (2004) found there were no changes in ROS, suggesting neutrophils do not contribute to ROS damage after an SCI (de Castro et al., 2004). Degranulation is known to play a role in neutrophils entering the injury site, and the release of neutrophil elastase and MPO can cause damage to surrounding tissue. Neutrophil elastase has been shown to damage new vasculature and causes apoptosis of endothelial cells. After SCI, administration of sivelestat (an inhibitor of neutrophil elastase) increased angiopoietin 1, a vascular regenerative factor with anti-inflammatory properties (Kumar et al., 2018). Additionally, Mpo KO mice are known to have reduced pro-inflammatory cytokines and exhibit a better recovery of locomotor function (Hausmann, 2003; Kubota et al., 2012). These studies indicate the presences of neutrophils at some point after the SCI injury is detrimental. While neutrophils have a detrimental effect, completely removing them doesn’t improve recovery (Neirinckx et al., 2014). One study that depleted neutrophils using antibodies against Ly6G and Gr-1 in combination hindered functional recovery in SCI (Stirling et al., 2009); however, as previously discussed the Gr-1 antibody is not specific to neutrophils, as it also affects Ly6C monocytes/macrophages.
Newly described Ly6GlowCD14hi neutrophils supported axonal regeneration in an SCI model (Fig. 5) (Sas et al., 2020). These neutrophils differ from mature neutrophils in that they have ring-shaped nuclei and do not use CXCR2 to navigate to sites of injury (Sas et al., 2020). They also have an N2-like phenotype. It is important to note that these cells resemble immature neutrophils and as previously discussed, PMN-MDSC are similar to immature neutrophils. The Ly6GlowCD14hi neutrophils aided axonal regeneration through the injury site, supporting the idea that neutrophils have a beneficial effect on SCI injury. It will be interesting to determine if these neutrophils that support regeneration are a subset of neutrophils or the PMN-MDSCs subtype. Further work with these cells could lead to potential treatment options.
Fig. 5. Neutrophils in Spinal Cord Injury.
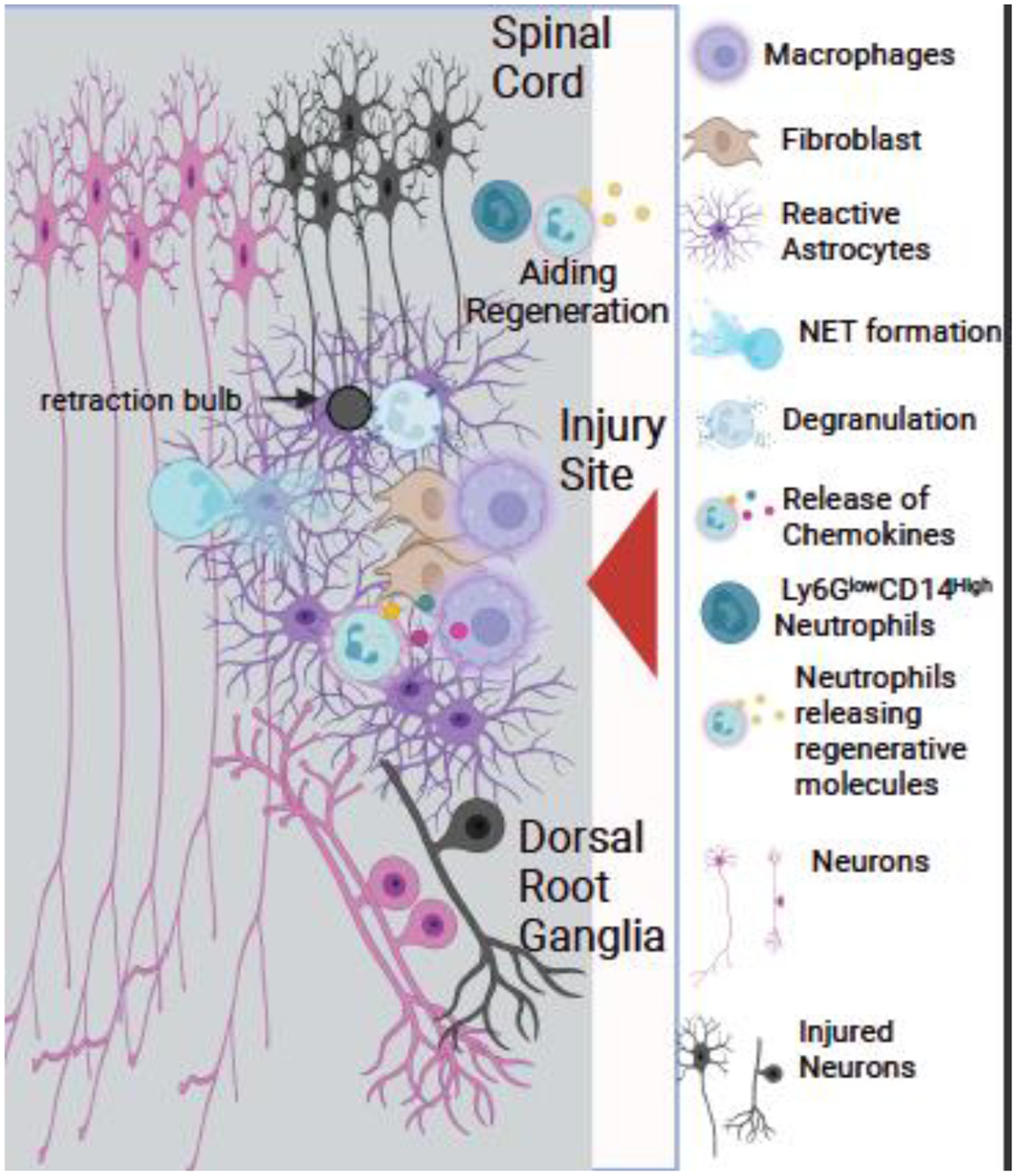
After a spinal cord injury (SCI), neutrophils enter the injury site, followed by macrophages. Activated astrocytes keep the neutrophils from spreading in the injury site. Mpo KO mice have a decreased lesion size compared to wild type demonstrating Mpo can have a detrimental effect. Several molecules released by neutrophils are beneficial to regeneration. Additionally, the neutrophil Ly6GlowCD14+ subtype has been shown to aid regeneration. Darkened neurons (motor and sensory) indicate injured neurons.
TBI.
Previous studies have shown that neutrophils play a role in hypoperfusion of the brain after TBI, which is correlated with poor neurological outcomes (Coles et al., 2004; Inoue et al., 2005; Kelly et al., 1997; Stein et al., 2011). Neutrophils are known to affect circulation in the small vessels within the brain and help maintain blood pressure (Roca-Cusachs et al., 2006). This was demonstrated in a neutropenic rat model, which showed reduced blood flow compared to normal rats after TBI (Uhl et al., 1994). Neutrophils can hinder blood flow by extending pseudopodia that attach to the vessel walls (Zhang et al., 2014). Leading to hypoperfusion of the brain during the early stages of a TBI (Palmer et al., 2004). It is well established that neutrophils weaken the integrity of the BBB (Aubé et al., 2014). Neutrophil elastase causes the BBB to become highly permeable (Allport et al., 1997; Bolton et al., 1998). Additionally, blocking MMP9 after TBI protects the BBB in the early phase of inflammation (Asahi et al., 2000; Chen et al., 2007; Gasche et al., 2001; Mori et al., 2002).
Similar to what is seen in SCI, astrocytes, microglia and oligodendrocytes after a TBI produce many chemokines to attract neutrophils (i.e., CXCL1–5 and GM-CSF) (Chen et al., 2002; Johnson et al., 2011; Lee et al., 1993; Liu et al., 2015; Lu et al., 2005; Pineau et al., 2010; Tani et al., 1996). There is emerging evidence that neutrophil-astrocyte interaction can profoundly affect the response after TBI. In a cell culture experiment, direct cell contact between astrocytes and neutrophils resulted in a decrease in degranulation, apoptosis, and ROS production by neutrophils and a decrease in pro-inflammatory cytokines (Xie et al., 2010). Additionally, another study showed neutrophils could induce astrogliogenesis (Hooshmand et al., 2017). These results suggest that these two cell types work together and could improve recovery.
The evidence suggests that early on after a TBI, neutrophils can have a detrimental effect on blood flow and the BBB, which will have a negative effect on outcomes; however, new studies demonstrate neutrophils’ protective and positive effects on regeneration. While neutrophil-astrocyte interaction in vivo in TBI and SCI are exciting, it could be similar to what was observe in the nanovesicle experiment in the EAE model, where neutrophils exosomes are changing the activities of astrocytes.
3.6. Regeneration after axotomy of retinal ganglion cells
Normally regeneration does not occur after a CNS injury, because of inhibitory molecules and impaired regenerative ability of neurons, but neutrophils have beneficial effects on regeneration. It is well established that when an inflammatory response occurs in the eye and the optic nerve is crushed, regeneration is enhanced (Leon et al., 2000). Several studies found that oncomodulin, a small calcium-binding protein, promotes axonal regeneration of retinal ganglion cells (Kurimoto et al., 2010; Yin et al., 2006). Originally, it was thought that oncomodulin was produced only by macrophages, but Kurimoto et al. found that there were high levels of oncomodulin at the inflammation site very early after injury, at a time when there is a minimal macrophage presence. They found evidence that neutrophils also secrete oncomodulin, suggesting that they might be crucial players in regeneration of the optic nerve. In the early stages of injury, neutrophils are the main infiltrating immune cell in the retina, and in situ hybridization showed that these neutrophils contain oncomodulin RNA (Kurimoto et al., 2013; Singh and Plemel, 2014). Both blocking oncomodulin and blocking neutrophils significantly decreased optic nerve regeneration after a nerve crush (Kurimoto et al., 2013). Several interesting questions remain with regard to this research as discussed by Singh and Plemel (2014). Given that both macrophages and neutrophils produce oncomodulin, are both cell types involved in promoting regeneration? Are their effects redundant or complementary? Is oncomodulin produced by a specific subset of neutrophils? In addition, since neutrophils are present in the retina only for the first few days after an injury, are they somehow able to produce a sustained effect on optic nerve regeneration? Equally interesting is what accounts for the fact that only a small percentage of optic nerve axons show enhanced regeneration in response to oncomodulin?
The Benowitz laboratory found that, when the eye is injected with a yeast cell wall inflammation-producing compound (i.e., zymosan) and the optic nerve is crushed, regeneration is enhanced (Leon et al., 2000). Sas et al. (2020) reported that when such mice are also pretreated with neutralizing antibodies to CXCR2, regeneration is further enhanced raising the possibility that a different population of neutrophils may inhibit regeneration. They identified a neutrophil Ly6GlowCD14hiCD101low-neg subtype, suggesting they were immature neutrophils. This Ly6GlowCD14hi neutrophil subset secreted both NGF and IGF-1, and antibodies to these growth factors reduced regeneration; however, these antibodies did not stop regeneration completely, indicating the presence of additional factors that have yet to be identified.
This picture is a long way from the idea that all neutrophils have cytotoxic effects on the CNS tissues and suggests that different subsets of neutrophils could produce different effects on regeneration. What this makes clear is that there is a lot of research that still needs to be done regarding the role of neutrophils in CNS regeneration. Are there any other markers, proteins, or cytokines involved in regeneration through neutrophil action? The differing data found in regards to the benefits and drawbacks of neutrophils and their recruitment to the site of injury is very important to consider. By shifting the long-held thought that neutrophils only have negative roles in regeneration, there is hope for new treatments to be discovered by targeting neutrophil secretion and signaling.
4.0. Neutrophils in PNS regeneration, neuropathies, and pain
4.1. Responses to PNS injury
The inflammatory response that occurs shortly after peripheral nerve injury has been shown to affect axonal regeneration. This response includes an influx of neutrophils into the distal nerve after it is crushed, followed a few days later by infiltration of macrophages e.g., (Nadeau et al., 2011; Neirinckx et al., 2014). Depletion of both macrophages and neutrophils had a negative effect on functional recovery after a sciatic nerve lesion (Barrette et al., 2008). This was followed up by Nadeau et al., which specifically examined the role of neutrophils by depleting circulating neutrophils using systemic administration of antibodies to Ly6G. They found that neutrophil depletion had no effect on axonal regeneration or on recovery of nerve function as measured by the sciatic functional index (Nadeau et al., 2011). More recently, Pan et al. (2020) reported that neutrophil depletion did not inhibit or accelerate regeneration of the sciatic nerve into acellular nerve allografts (Pan et al., 2020a, 2020b). In apparent contrast, the Ly6GlowCD14hi neutrophil subtype previously discussed showed upregulation of genes for tissue development and wound healing, which suggest they could aid regeneration in the PNS (Jerome et al., 2022; Sas et al., 2020). A recent publication of the cellular and molecule processes of the PNS after injury showed myeloid cells and neutrophils in the injured nerve undergo rapid metabolic changes (Zhao et al., 2022).
There are other avenues for neutrophils to alter PNS regeneration. For example, studies have been done on the effects of two proteins that are released by neutrophils. One such factor is neutrophil peptide-1 (NP-1), a member of a class of polypeptides known as defensins. These molecules are responsible for some of the signaling that occurs in the body associated with wound and tissue healing (Murphy et al., 1993; Nozdrachev et al., 2006). Rats injected with NP-1 have a significant increase in sciatic nerve regeneration compared to animals given physiological saline (Nozdrachev et al., 2006). NP-1 can recruit monocytes and macrophages (Territo et al., 1989), which are involved in axonal debris clearance via phagocytosis (Fig. 6); (Brück, 1997; Zigmond and Echevarria, 2019). Yuan et al. (2020) found that NP-1 increased nerve conduction velocity and functional recovery after sciatic nerve crush and hypothesized that this resulted from the clearance of tissue debris during Wallerian degeneration, thereby removing inhibitors to regeneration (Yuan et al., 2020).
Fig. 6. Neutrophils in Peripheral Nerve Injuries.
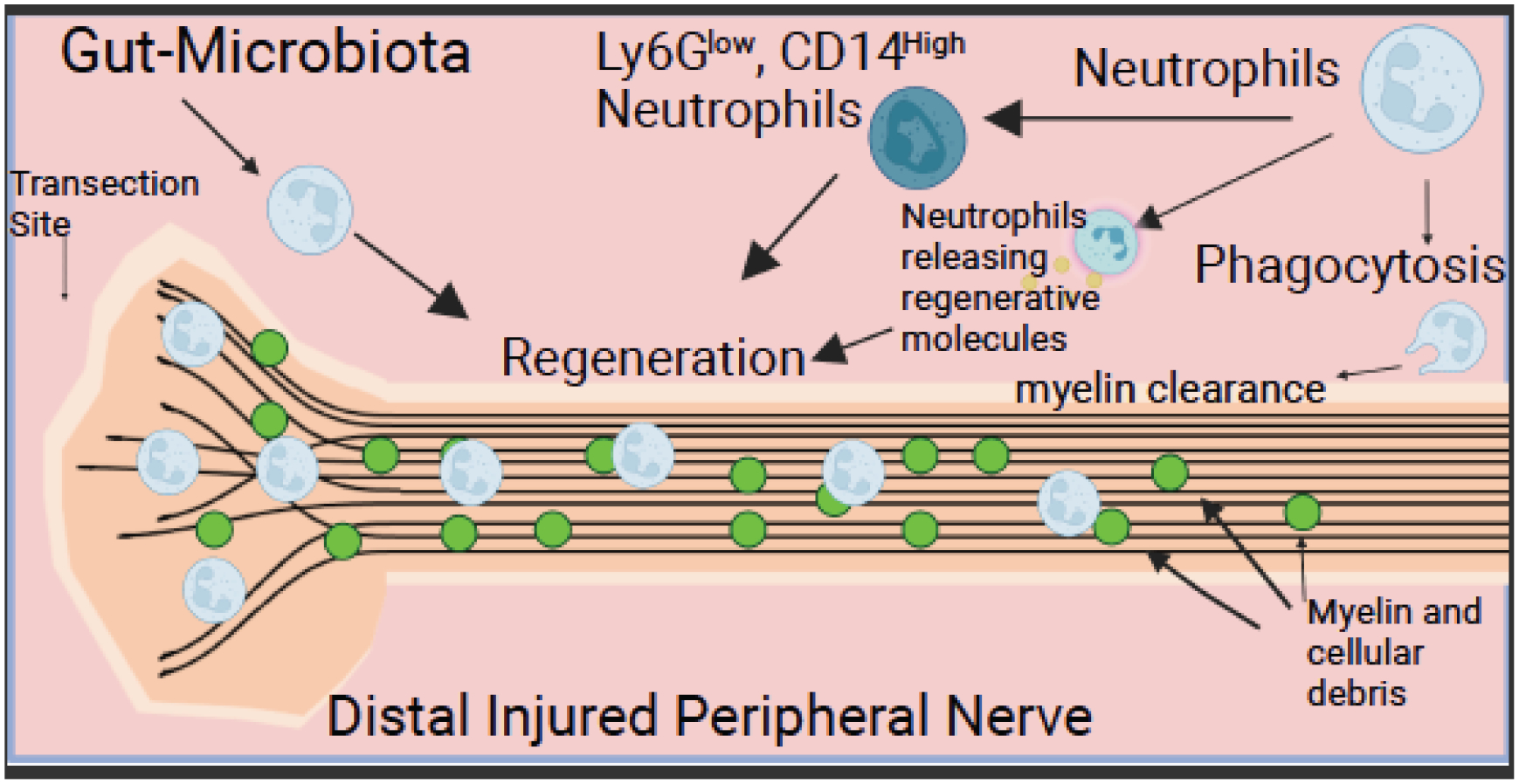
After peripheral nerve injury, the distal nerve undergoes Wallerian degeneration (WD). Neutrophils play a role in WD, as depleting neutrophils delays myelin clearance. Many molecules (i.e., oncomodulin, nerve growth factor, & neutrophil peptide-1) aid regeneration that are released from neutrophils. Changes in the gut microbiota have been shown to accelerate regeneration, which is dependent on neutrophils. Additionally, the Ly6GlowCD14+ subtype is involved in regeneration after a peripheral nerve injury.
Another substance which can be secreted by neutrophils, and which has been studied extensively with regard to nerve regeneration, though with variable results, is nerve growth factor (NGF). Intraplantar injection of NGF increases neutrophil accumulation in the hind paw; however, the ensuing effects were only studied in terms of changes in pain sensitivity (Bennett et al., 1998). The studies on NP-1 all involve exogenous administration of the proteins, and whether secretion of endogenous NP-1 or NGF by neutrophils plays a role in regeneration remains to be shown.
A recent publication demonstrates that changes in a metabolite from bacteria in the gut apparently caused a change in neutrophils, which prime sensory neurons to regenerate. Enhanced regeneration after a sciatic nerve crush was found to occur after intermittent fasting and to be dependent on an increase in blood levels of the gut bacteria-derived metabolite indole-3 propionate (IPA) (Serger et al., 2022). When an antibiotic was used to inhibit IPA production, this increased regeneration was blocked. Furthermore, IPA alone enhanced regeneration. When animals that received IPA were treated with a function-blocking antibody to the neutrophil chemokine receptor CXCR2, regeneration was not increased. That effect was associated with a decrease in the number of neutrophils present in the dorsal root ganglia (DRG). (As will be discussed later, there is controversy as to whether neutrophils infiltrate into the parenchyma of the ganglia.) These results suggest a mechanism of enhancing peripheral nerve regeneration via neutrophils, because of changes in the gut microbiome. What remains to be determined are the mechanisms of how intermittent fasting or IPA affects neutrophil migration and subsequently how neutrophils affect nerve regeneration. It is possible that intermittent fasting or IPA switches neutrophils to a regenerative phenotype or causes changes in the amount or make up of secreted molecules that neutrophils release.
A recent publication showed in a nerve crush model in mice and rats that neutrophils were detrimental to myelin clearance (Yamamoto et al., 2022). The paper reported that NETs inhibited macrophage infiltration into the nerve, slowing myelin clearance. Administration of an anti-PMN antibody significantly improved myelin clearance indicated by myelin basic protein staining. It is unclear from the Cedarlane catalogue what protein is targeted by the anti-PMN antibody used. The group then inhibited NET formation and showed a similar increase in myelin clearance. These results are dramatically different from the findings of Lindborg et al. 2017, in which a Ly6G antibody significantly decreased neutrophil numbers and inhibited or delayed myelin clearance. One factor that that is different between the two studies is that Lindborg et al. used a transection model, while Yamamoto et al. used a nerve crush. The difference in these findings demonstrate further research is needed.
These studies show neutrophils could accelerate PNS regeneration via their secreted proteins or via the Ly6GlowCD14high subpopulation. The role and presence of PMN-MDSCs in the PNS is not known. Gut-microbiome changes present new questions for research to determine how these affect neutrophils and how they enhance regeneration.
4.2. Localization of neutrophils in dorsal root ganglia (DRGs) and sciatic nerve
DRGs are covered by meninges, which connect to the epineurium of the spinal nerves. The sensory neurons are surrounded by satellite glial cells, which help to facilitate chemical regulation and neuronal responses. Several studies have looked into whether injury to the sciatic nerve prompts the invasion of neutrophils into both the distal nerve and into the corresponding DRGs. Infiltration of neutrophils into the distal sciatic nerve after injury has been shown repeatedly (e.g., Kim and Moalem-Taylor, 2011; Lindborg et al., 2017; Nadeau et al., 2011; Nukada et al., 2000; Perkins and Tracey, 2000. Lindborg et al. (2017) showed that neutrophils in the nerve are increased in KO mice for the macrophage chemokine receptor Ccr2, in which macrophages in the distal sciatic nerve are decreased.
With regard to neutrophil infiltration into the DRG, however, there is disagreement. Morin et al. (2007) reported finding neutrophils in the DRG after chronic constriction injury, a common model for studies on neuropathic pain. In a response to that paper, McLachlan et al. (2007) reported that they only found neutrophils in the DRG in the meninges covering the ganglion and not in the parenchyma (McLachlan et al., 2007). In fact, if one looks at the neutrophils in some published micrographs of the DRG, the cells seem to be few in number and localized at the perimeter of the ganglion (Kim and Moalem-Taylor, 2011b; Serger et al., 2022).
Lindborg et al. (2018), using flow cytometry, did not detect neutrophils in the DRG, while Pan et al. using a somewhat different gating scheme found some (Lindborg et al., 2018; Pan et al., 2022). Neutrophils were found to be present in the sympathetic superior cervical ganglia of wild type mice 3 days after transection of the postganglionic external and internal carotid nerves. The accumulation of neutrophils in these ganglia was significantly higher in Ccr2 KOs, and this difference correlated with increased expression of the neutrophil chemokine CXCL5.
4.3. Peripheral Neuropathies Including Those Induced by Diabetes and Chemotherapy
Peripheral neuropathies are common in the clinical setting and can result from an injury or be inherited. These can result in loss of motor function and sensation or cause neuropathic pain. Among these neuropathies are Charcot-Marie-Tooth disease, Guillain Barre syndrome, diabetic neuropathy, and chemotherapy-induced neuropathies (Pareyson et al., 2017; Rg et al., 2019; Sarejloo et al., 2022a; Wang et al., 2023a). There is growing evidence that neutrophils play a role in these pathologies. In diabetic neuropathy, patients have decreased strength and sensation in their limbs starting with their hands and feet leading to limited joint mobility (Guthrie and Guthrie, 2004). Recently, a study has shown patients with limited joint mobility have an increased neutrophil-to-lymphoid ratio compared to patients without mobility problems (Mineoka et al., 2018). Diabetic patients can also have an increase in pain sensation. Inhibiting neutrophils in an experimental model of diabetic neuropathy prevented the pain, with decreased expression of CXCL1 and 8, and decreased oxidative stress on neurons in the ventral and dorsal horn of the L4 and L5 spinal cord (Newton et al., 2017). Additionally, studies have found diabetes primes neutrophils to form NETs and impairs wound healing, which could affect nerve regeneration (Berbudi et al., 2019; Joshi et al., 2013). Further investigation is needed to see what role NETosis has on diabetic neuropathy. NETs have been found in a model of chemotherapy-induced neuropathy. A study showed increased number of neutrophils in the blood after injection of oxaliplatin, a chemotherapeutic agent, in a mouse model, with an increase in NETs measured by CitH3 western blot (Wang et al., 2023b). An increased number of cells were found in the DRG that were positive for neutrophil elastase and MPO but did not stain for Ly6G or for CitH3. Treatment with DNase to inhibit NET formation produced a decrease in pain and in CitH3 in western blots, though no immunohistochemistry was performed. These results were confirmed with the Pad4 KO mouse. Thus, NETs may play a role in peripheral neuropathies, though further investigation is needed. Like NOS and other CNS disorders, Guillain Barre syndrome has been linked with neutrophils, as a high neutrophils-to-lymphoid ratio is associated with symptoms, while treatment resulted in a lower neutrophils-to-lymphoid ratio (Sarejloo et al., 2022b). The role of neutrophil function in peripheral neuropathies needs to be further investigated.
4.4. Neuropathic pain
Chronic pain is a debilitating disorder characterized by a continuation beyond the standard healing time (Pizzo and Clark, 2012). Research demonstrates that 13–50% of adults in the United Kingdom live with chronic pain, and 10–14% have moderate-to-severe disabilities because of it (Fayaz et al., 2016). These numbers are similar to those reported in Japan and the United States, indicating the prevalence of chronic pain worldwide (Pizzo and Clark, 2012). Chronic pain is estimated to cost $600 billion annually in the United States (Gereau et al., 2014). These facts indicate the importance of understanding chronic pain’s pathophysiology and the involvement of neutrophils in order to help improve patients’ lives.
4.4.1. Neutrophils can produce a hyperalgesic effect.
Dorsal root ganglia and trigeminal ganglia contain two populations of small diameter neurons: calcitonin gene-related peptide positive and isolectin B4 positive. These cells are known as nociceptors, and they detect painful stimuli and convey the information to the CNS. An area of interaction between the immune system and the PNS that has received considerable attention is the role neutrophils play in pain; however, the picture that emerges from these studies is far from simple. Whereas many studies concluded that neutrophils increase pain (i.e., they produce a hyperalgesic effect), a number of other studies report that neutrophils either produce the opposite effect (i.e., an antinociceptive or analgesic effect) or have no effect on pain at all.
The sixteen studies listed in Table 2 all concluded that neutrophils increase pain. A group of rats or mice was treated in one of a number of ways to produce inflammatory or neuropathic pain. A second group of animals was treated identically but only after these animals were pretreated (pharmacologically or immunologically) to decrease substantially circulating neutrophils. These studies vary in terms of whether they in fact documented neutrophil depletion, whether the effect of that manipulation was specific to neutrophils, and whether they measured neutrophils directly by immunohistochemistry or indirectly by measuring MPO activity biochemically.
Table 2.
Examples of reported neutrophil-dependent hyperalgesia.
| Manipulation | Species | Effect on Neutrophils | Type of Hyperalgesia Tested | Method of Neutrophils Reduction | Effect of Neutrophils Reduction | Reference |
|---|---|---|---|---|---|---|
| Leukotriene B4 injected into the dorsum of the paw | Rat | A known potent PMN chemotactic agent | Mechanical hyperalgesia (paw pressure threshold) | Hydroxyurea or methotrexate | Decreased hyperalgesia | (Levine et al., 1984) |
| N-formyl- methionyl-leucyl-phenylalanine or C5a into the dorsum of the paw | Rat | A known potent PMN chemotactic agents | Mechanical hyperalgesia | Hydroxyurea | Decreased hyperalgesia | (Levine et al., 1985) |
| Intraplantar nerve growth factor | Rat | Increased MPO activity in plantar skin | Thermal hyperalgesia | Anti-rat PMN | Decreased hyperalgesia | (Bennett et al., 1998) |
| Partial sciatic nerve ligation | Rat | PMN accumulation in sciatic nerve | Thermal hyperalgesia | Anti-rat PMN | Decreased hyperalgesia | (Perkins and Tracey, 2000) |
| Allergen (ovalbumin) into the paw | Rat | PMN accumulation in plantar skin | Thermal hyperalgesia | Fucoidin or anti-rat PMN | Decreased hyperalgesia | (Lavich et al., 2006) |
| Serotonin into the dorsum of the paw | Rat | ND | Mechanical hyperalgesia | Fucoidin | Decreased hyperalgesia | (Oliveira et al., 2007) |
| Carrageenan into the paw | Rat | Increased MPO activity in plantar skin | Mechanical hyperalgesia | Fucoidin | Decreased hyperalgesia | (Cunha et al., 2008) |
| Antigen (methylated bovine serum albumin) into knee joint | Mouse | Increased MPO activity in knee joint | Mechanical hyperalgesia | Reparixin or DF2162 | Decreased hyperalgesia | (Coelho et al., 2008) |
| C5a, zymosan, or carrageenan into paw | Rat | Increased MPO activity in paw | Mechanical hyperalgesia | Vinblastine | Decreased hyperalgesia | (Ting et al., 2008) |
| Partial sciatic nerve ligation | Mouse | PMN accumulation in sciatic nerve | Mechanical hyperalgesia | Anti-Ly6G | Decreased hyperalgesia | (Nadeau et al., 2011) |
| Intraplantar IL-17 | Mouse | PMN accumulation in plantar skin | Thermal hyperalgesia | Fucoidin | Decreased hyperalgesia | (McNamee et al., 2011) |
| Monosodium urate into the tibio-femoral joint | Mouse | Increased MPO activity in periarticular tissue | Mechanical hyperalgesia | DF2162 or Fucoidin or 5-LOX inhibitor | Decreased hyperalgesia | (Amaral et al., 2012) |
| Paw incision | Mouse | Increased MPO activity in plantar tissue | Mechanical hyperalgesia | Anti-Ly6G or vinblastine or Ladarixin | Decreased hyperalgesia | (Carreira et al., 2013) |
| MOG35–55 subcutaneously (causing EAE) | Mouse | PMN accumulation in DRG | Mechanical hyperalgesia | Anti-Ly6G | Decreased hyperalgesia | (Harada et al., 2019) |
| MOG35–55 subcutaneously | Mouse | PMN accumulation in DRG | Mechanical hyperalgesia | Anti-Ly6G | Decreased hyperalgesia | (Zhang et al., 2019) |
| Monosodium urate or IL-33/ST2 | Mouse | Increased MPO activity | Mechanical hyperalgesia | Fucoidin | Decreased hyperalgesia | (Yin et al., 2020) |
Carrageenan, an extract from a red seaweed; EAE, experimental autoimmune encephalomyelitis; Fucoidin, a nonspecific selectin inhibitor; Ladarixin, a CXCR1/2 antagonist; 5-LOX, 5-lipoxygenase, which produces leukotriene B4; MOG, myelin oligodendrocyte glycoprotein; MPO, myeloperoxidase; ND, not determined; PMN, although PMN refers to all granulocytes, it is used here to refer specifically to neutrophils; Reparixin and DF2162, both CXCR1/2 inhibitors; ST2, suppression of tumorigenicity/IL-33 receptor.
The first reports of a hyperalgesic effect of neutrophils came from Levine et al. (1985, 1984; Table 2). These researchers produced local inflammation by injection into the dorsum of the paw either LTB4, N-formyl-methionyl-leucyl-phenylalanine, or complement factor C5a. These injections decreased the threshold for mechanical pain. A second group of animals was pretreated with hydroxyurea or methotrexate to deplete neutrophils, which produced a decrease in hyperalgesia. While hydroxyurea and methotrexate were shown to deplete circulating neutrophils, their effect was not specific as they also depleted other granulocytes and mononuclear leukocytes (Levine et al., 1984).
Perkins and Tracey examined thermal hyperalgesia produced by a unilateral partial sciatic nerve ligation, which involved ⅓ to ½ of the nerve and is a classical model system for studying neuropathic pain (Perkins and Tracey, 2000; Seltzer et al., 1990). This lesion produced a large increase in endoneurial neutrophils determined immunohistochemically at and both immediately distal and proximal to the lesion site. Neutrophil levels in the nerve peaked at 24 hours but were still detectable after 7 days. Sham-operated nerves showed some neutrophils but only in the epineurium. In the lesioned animals, no increase in neutrophils was found around sensory nerve terminals in the skin. The lesion also led to thermal hyperalgesia. In one group of animals, neutrophils were depleted by an intraperitoneal injection at the time of surgery of a rabbit anti-rat neutrophil antibody, which substantially depleted circulating neutrophils for 5 days. Other leukocytes were slightly lowered compared to animals receiving normal serum, but their levels were not significantly below those found preoperatively. The depletion decreased hyperalgesia. When neutrophil depletion was delayed until 8 days after injury, at a time when thermal hyperalgesia had already been established, the anti-rat neutrophil antibody did not affect the hyperalgesia. Injection of this antibody into non-lesioned animals had no effect on the withdrawal latency to the heat stimulus, even though circulating neutrophils were shown to be reduced. The authors concluded that neutrophils “contribute to the early genesis of peripheral neuropathic pain”.
Nadeau et al. also examined the role of neutrophils after partial sciatic nerve ligation, but they measured mechanical pain using the von Frey filament assay (Nadeau et al., 2011). Neutrophils were depleted by injection of the anti-Ly6G antibody, which had no effect on the levels of M1 macrophages. The decreased threshold for paw withdrawal seen after injury in animals injected with a control serum was totally blocked at 3, 4, and 5 days after the lesion when neutrophils were depleted. As noted early in this review, this depletion had no effect on functional regeneration measured by the sciatic functional index test.
It is assumed that neutrophils induce hyperalgesia either by activation or sensitization of nociceptors (Rittner et al., 2003). Although, as already noted, neutrophils can secrete a large number of molecules, including cytokines, enzymes, and cytotoxic compounds, the specific mechanism(s) by which these immune cells produce a hyperalgesic effect is not known. Among the possible molecules that could be involved are IL-1β, TNF-α, IL-6, prostaglandin E2, ROS, neutrophil elastase, and cathepsin E (Cunha et al., 2008; Ferreira et al., 1993; Gao et al., 2022; Rittner et al., 2003). To what extent the secretion of such molecules occurs directly by neutrophils or indirectly from nearby cells has not been determined. While many of the studies are consistent with an action of neutrophils directly or indirectly on the terminals of nociceptors, that is not true for the studies of Perkins and Tracey and that of Nadeau et al., in which neutrophils were found in the lesioned nerves. It will be interesting to determine if neutrophils have a differential effect on calcitonin gene-related peptide positive and isolectin B4 positive pain neurons. In a culture study, Shaw et al. showed that neutrophils can increase the firing rate of isolectin B4 positive DRG neurons (Shaw et al., 2008).
In a further attempt to determine the mechanism between neutrophils and pain, one study examined the effect of NETs on hyperalgesia (Schneider et al., 2021). Antigen-induced arthritis was produced in mice, which led to NET formation intra-articularly and to mechanical hyperalgesia. When a PAD4 inhibitor was given to block NET formation, hyperalgesia was inhibited. Furthermore, isolation of NETs induced in vitro by a phorbol ester and their injection into articular joints in WT mice led to a rapid onset of hyperalgesia. This effect was not seen in KO animals for TNF receptor 1, IL-1 receptors, toll-like receptors 4 or 9. In addition, injection of indomethacin, a non-selective cyclooxygenase inhibitor, prevented the hyperalgesia. In studying NETs, however, it must be remembered that macrophages are also said to be able to produce extracellular traps (Doster et al., 2018).
4.4.2. Neutrophils have also been shown to produce an analgesic effect.
In other studies in which the effects of neutrophils on pain were examined, using the same depletion strategy, neutrophils were found to have the opposite effect (referred to as an antinociceptive or analgesic effect). It is known that certain immune cells, including but not restricted to neutrophils, synthesize and secrete opiate peptides, and in some of the studies listed in Table 3, these molecules have been implicated in the antinociceptive responses observed (Brack et al., 2004; Cabot, 2001; Machelska et al., 2003; Plein and Rittner, 2018; Heike L. Rittner et al., 2006a, 2006b; Rittner et al., 2003; Stein et al., 1990).
Table 3.
Situations in which neutrophils did not produce hyperalgesia.
| Manipulation | Species | Effect on Neutrophils | Type of Hyperalgesia Measured | Method of Neutrophils Reduction | Effect of Neutrophil Reduction | Reference |
|---|---|---|---|---|---|---|
| Intraplantar CFA & CRF | Rat | PMN accumulation | Mechanical hyperalgesia | Anti-rat PMN serum | CRH-induced antinociception is inhibited. | (Brack et al., 2004) |
| Intraplantar CFA & CXCL2 | Rat | PMN accumulation | Mechanical & thermal hyperalgesia | Anti-rat PMN serum | CFA-induced hyperalgesia was unaffected while CXCL2-induced antinociception is reduced. | (Rittner et al., 2006) |
| Intraplantar CFA, CXCL1, or CXCL2 | Rat | PMN accumulation | Mechanical & thermal hyperalgesia | Anti-rat PMN serum | CFA-induced hyperalgesia is independent of PMNs. Also, chemokine-induced PMN recruitment does not cause hyperalgesia. | (Rittner et al., 2006b) |
| Intraplantar CFA | Rat | PMN accumulation | Mechanical & thermal hyperalgesia | Anti-rat PMN serum | Increased hyperalgesia. | (Rittner et al., 2009) |
| Plantar hind paw incision | Mouse | PMN accumulation | Mechanical & thermal hyperalgesia | Anti-Ly6G/Gr-1 | No effect on mechanical hyperalgesia. Slightly increased thermal hyperalgesia. | (Sahbaie et al., 2012) |
| Intraplantar zymosan | Mouse | PMN accumulation | Mechanical hyperalgesia | Anti-Gr-1 | No effect on mechanical hyperalgesia. | (Suo et al., 2014) |
| Intraplantar CFA or plantar incisional wound | Mouse | PMN accumulation | Mechanical & thermal hyperalgesia | Anti-Ly6G | No effect | (Ghasemlou et al., 2015) |
CFA, Complete Freund’s adjuvant; CRH, corticotropin-releasing hormone, releases opioids from leukocytes; ND, not determined.
A number of papers beginning in the 1990s indicated that endogenous opiate peptides released by immune cells produce antinociceptive responses but had not specifically demonstrated an involvement of neutrophils (Cabot et al., 1997; Przewłocki et al., 1992). Machelska et al. demonstrated that 6 hours after complete Freund’s adjuvant (CFA)-induced inflammation, the immune cells in the paw that contained β-endorphin, met-enkephalin, and dynorphin A were primarily granulocytes (Machelska et al., 2003). At 4 days, the endogenous opiates were largely present in macrophages. Swim stress, which causes the release of opiates from leukocytes, at least in part through the action of corticotropin-releasing hormone (CRH), produced antinociception similarly at both 6 hours and 4 days, and this effect was blocked by the opiate antagonist naloxone (Schafer et al., 1996).
The first study implicating neutrophils specifically was by Brack et al. who used CFA to produce inflammation and mechanical hyperalgesia in the paw (Brack et al., 2004). CRH was injected into the inflamed paw and produced antinociception, an effect blocked by naloxone. In addition, the CRH-produced analgesia was significantly reduced by administration of an anti-rat neutrophil serum. Flow cytometry demonstrated that the antiserum produced a decrease in the inflamed paws in the number of neutrophils and opioid-containing cells, with no change in monocytes/macrophages. Nearly all of the neutrophils were positive for the chemokine receptor CXCR2, and nearly all of these cells expressed the endogenous opiates, β-endorphin and enkephalin. Opiate receptors are present on the terminals of nociceptors and are upregulated as a result of inflammation (Machelska and Stein, 2006). The two endogenous CXCR2 ligands, CXCL1 and CXCL2, reached a maximum in the paw between 12 and 24 hours after CFA injection. Combined administration of antibodies to these chemokines reduced neutrophils in the paw (without affecting monocytes/macrophages), reduced opioid-containing cells, and completely blocked CRH-induced antinociception. Interestingly, these chemokine antibodies had no effect on mechanical pain in CFA-induced inflammation in the absence of CRH, indicating that there are multiple mechanisms for inflammatory hyperalgesia.
Neutrophils are not the only leukocytes that have been implicated in pain. A number of studies have implicated macrophages in causing mechanical or thermal hyperalgesia, using clodronate or MaFIA mice to deplete macrophages (Liu et al., 2000; Shepherd et al., 2018; Yu et al., 2020). Studies on T lymphocytes have also implicated those cells, using athymic nude rats to eliminate mature T cells or antibodies to CD25 to deplete regulatory T cells. Type 1 T cell subsets cause mechanical and thermal pain hypersensitivity, while type 2 cells decrease pain hypersensitivity. Regulatory T cells also reduced mechanical hypersensitivity. Some studies have found that neutrophils play no role in inflammatory or neuropathic pain (Brack et al., 2004; Ghasemlou et al., 2015; Heike L. Rittner et al., 2006b). Ghasemlou et al. examined the role of neutrophils in hyperalgesia in two pain models: intraplantar injection of CFA and a deep plantar incision involving injury to the skin and underlying muscle (Ghasemlou et al., 2015). In both models, a rapid and prolonged hyperalgesia occurred in tests of mechanical and thermal pain. Flow cytometry revealed that the majority of immune cells were CD45+CD11b+ myeloid cells, with only a small percentage of lymphoid cells present. The levels of most myeloid cells peaked at 1 day, with Ly6Chi monocytes/macrophages peaking later at 3 days. Depletion of neutrophils was accomplished using an anti-Gr1 antibody and was confirmed using flow cytometry. The depletion procedure had no effect on the levels of monocytes/macrophage. In apparent contrast with some of the studies described above (Table 2), these researchers found that the depletion of neutrophils had no effect on mechanical or thermal hypersensitivity. Interestingly, depletion of a different population of myeloid cells, CD11b+Ly6G−, a population of monocytes/macrophages, which actually constituted the largest population of immune cells in both pain models, led to a strong alleviation of mechanical hyperalgesia with only a mild effect on thermal hyperalgesia. The authors concluded not that neutro phils are incapable of causing pain but that “they are not required, presumably because other cells are sufficient to drive the phenotype in their absence”.
While these studies present a somewhat confusing picture, much of that possibly is due to slight differences in procedure, for example the times at which pain assays are conducted. The specifics of these different studies have been discussed in some detail under the title “Neutrophils: are they hyperalgesic or anti-hyperalgesic?” (Cunha and Verri, 2006; Heike L. Rittner et al., 2006a). Two things that do seem clear is that neutrophils can produce hyperalgesia and that under other conditions favoring the release of endogenous opiates they can produce analgesia.
5.0. Conclusion
Neutrophils, the homogeneous, short-lived cells once thought to only clear pathogens from the body are now known to be a heterogeneous cell type that undergoes many changes throughout their lives. They live longer than previously thought, are involved in a lot more than just clearing pathogens, especially in the nervous system. Four functions have been implicated in neurological disease and injuries: degranulation, phagocytosis, NET formation, and paracrine/immunomodulatory factor release. As more has been learned about their biology, we have gained a greater understanding of their role in CNS and PNS injury and diseases. In some situations, their effects on neural tissue are primarily damaging; however, in others, their effects are regenerative. As reviewed in this article, increasingly neutrophils have been shown to be involved in a variety of neurological diseases and after neural injury (Fig. 7). There is still much to learn about neutrophil subtypes and their role under these conditions. They are complex cells with the ability to communicate and modulate other cells, as shown in the B cell neutrophil interactions in the EAE model, and neutrophil-astrocyte interactions. Different subtype are able to positively influence regeneration demonstrated by the Ly6Glow,CD14hi in both the CNS and PNS. Continued research into the gut-microbiome’s interactions with neutrophils is intriguing as it could provide new evidence on the susceptibility to some diseases and a possible avenue for clinical research into potential treatments for ALS. NET formation will be an interesting area to investigate in the coming decade in many neurological conditions but careful controls and specific staining will be required. Studying neutrophils will require attention to ensure their role is carefully separated from macrophages. Specific KO models will help but will need appropriate controls. With research continuing, the coming decade should offer new insights into positive roles for neutrophils in the nervous system.
Fig. 7. Summary.
In this figure, we summarize all the effects discussed in this article. Neutrophils have many vital roles in neurological disease and injuries. Degranulation affects the lesion size in multiple sclerosis (MS) and spinal cord injuries (SCI). Neutrophils possibly help with the phagocytosis of myelin debris after injury. The CD14 subtype’s role in regeneration is just starting to be investigated but holds promise. Other subtypes; (i.e., N2) involvement in other neurological conditions is being investigated. NET formation has been reported in SCI and Alzheimer’s disease (AD). Cytokine release from neutrophils can activate other cell types causing a continued immune response. Neutrophils have a significant effect on blood flow which affects clinical outcomes during traumatic brain injury (TBI) and stroke, in addition to playing a role in AD. It has been shown that neutrophils release regenerative molecules that aid regeneration in the peripheral nervous system after injury and in SCI. The role of the gut-microbiota activating neutrophils to aid in regeneration is an exciting and new avenue of research.
Highlights.
Our understanding of neutrophil’s role in the nervous system has advanced.
Subpopulations of neutrophils have been found and may have unique roles.
Neutrophil extracellular traps are found in neurological conditions.
Neutrophils play a role in neuropathic pain, although the literature are mixed.
Neutrophils are involved in Wallerian degeneration.
Acknowledgements
The research on neutrophils in our laboratory is funded by a grant from the NIH (NS114891). BMB is supported by an NIH postdoctoral fellowship (NS122918). We thank Jon Niemi, Franklin D. Echevarria, Jane Lindborg, and Thomas Disabato for helpful comments on our manuscript. Many of the figures were created with the use of BioRender.
Abbreviations:
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- ATF3
activating transcription factor 3
- BAFF
TNF ligand superfamily member 13B
- BBB
blood brain barrier
- C3a & C5a
complement component 3a & 5a
- C/EBP
CCAAT/enhancer binding proteins
- CD
cluster of differentiation
- CCL
C-C chemokine ligand
- CCR2
C-C chemokine receptor type 2
- CFA
complete Freund’s adjuvant
- CRH
corticotropin-releasing hormone
- CSF1r
colony stimulating factor 1 receptor
- CXCL1
chemokine (C-X-C motif) ligand 2
- CXCR2
C-X-C chemokine receptor type 2
- CXCR4
C-X-C chemokine receptor type 4
- CXCL8
C-X-C chemokine receptor 8
- CNS
central nervous system
- DAMPs
damage-associated molecular patterns
- DC
dendritic cells
- EAE
experimental autoimmune encephalomyelitis
- G-CSF
granulocyte colony stimulating factor
- Gif-1
GRF interactive factor 1
- Gr1
lymphocyte antigen 6 complex
- GRF1
growth related factor interactive factor
- Iba1
ionized calcium binding adaptor molecule 1
- IFN
interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IgG
immunoglobulin G
- iNOS
inducible nitric oxide synthase
- IPA
indole-3 propionate
- KO
knockout
- L
lumbar
- LFA1
lymphocyte function-associated antigen 1
- LTB4
leukotriene B4
- Ly6B.2
Lymphocyte antigen 6B
- Ly6G
lymphocyte antigen 6 complex locus G
- LysM
protein domain
- MDSC
myeloid-derived suppressor cells
- MIP
macrophage inflammatory protein
- MMP8
neutrophil collagenase
- MMP9
matrix metalloproteinase 9
- MPO
myeloperoxidase
- MS
multiple sclerosis
- NADPH
nicotinamide adenine dinucleotide phosphate
- NAT
neutrophils associated with tumors
- NET
neutrophil extracellular trap
- NGF
nerve growth factor
- NP-1
neutrophil peptide-1
- PAD4
peptidyl arginine deiminase 4
- PAMP
pathogen-associated molecular patterns
- PMN
polymorphonuclear leukocytes
- PNS
peripheral nervous system
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- SCI
spinal cord injury
- SOD1
superoxide dismutase 1
- STAT3
signal transducer and activator of transmission 3
- TBI
traumatic brain injury
- T cells
T-lymphocyte and thymocyte
- TGF-beta
transforming growth factor beta
- Th
T helper cells
- TNFα
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors have no conflict of interests to disclose.
References
- Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P, 2004. Rac2 is critical for neutrophil primary granule exocytosis. Blood 104, 832–839. 10.1182/blood-2003-07-2624 [DOI] [PubMed] [Google Scholar]
- Ahuja SK, Lee JC, Murphy PM, 1996. CXC chemokines bind to unique sets of selectivity determinants that can function independently and are broadly distributed on multiple domains of human interleukin-8 receptor B. Determinants of high affinity binding and receptor activation are distinct. J Biol Chem 271, 225–232. 10.1074/jbc.271.1.225 [DOI] [PubMed] [Google Scholar]
- Ahuja SK, Murphy PM, 1996. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem 271, 20545–20550. 10.1074/jbc.271.34.20545 [DOI] [PubMed] [Google Scholar]
- Allport JR, Ding H, Collins T, Gerritsen ME, Luscinskas FW, 1997. Endothelial-dependent mechanisms regulate leukocyte transmigration: a process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J Exp Med 186, 517–527. 10.1084/jem.186.4.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral FA, Costa VV, Tavares LD, Sachs D, Coelho FM, Fagundes CT, Soriani FM, Silveira TN, Cunha LD, Zamboni DS, Quesniaux V, Peres RS, Cunha TM, Cunha FQ, Ryffel B, Souza DG, Teixeira MM, 2012. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B4 in a murine model of gout. Arthritis Rheum 64, 474–484. 10.1002/art.33355 [DOI] [PubMed] [Google Scholar]
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A, 2012. Neutrophil function: From mechanisms to disease. Annu Rev Immunol 30, 459–489. 10.1146/annurev-immunol-020711-074942 [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH, 2000. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab 20, 1681–1689. 10.1097/00004647-200012000-00007 [DOI] [PubMed] [Google Scholar]
- Aubé B, Lévesque SA, Paré A, Chamma É, Kébir H, Gorina R, Lécuyer M-A, Alvarez JI, de Koninck Y, Engelhardt B, Prat A, Côté D, Lacroix S, 2014. Neutrophils Mediate Blood–Spinal Cord Barrier Disruption in Demyelinating Neuroinflammatory Diseases. The Journal of Immunology 193, 2438–2454. 10.4049/jimmunol.1400401 [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B, 1997. Human chemokines: an update. Annu Rev Immunol 15, 675–705. 10.1146/annurev.immunol.15.1.675 [DOI] [PubMed] [Google Scholar]
- Balasa R, Barcutean L, Balasa A, Motataianu A, Roman-Filip C, Manu D, 2020. The action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum Immunol 81, 237–243. 10.1016/j.humimm.2020.02.009 [DOI] [PubMed] [Google Scholar]
- Barrette B, Hébert M-AA, Filali M, Lafortune K, Vallières N, Gowing G, Julien J-PP, Lacroix S, 2008. Requirement of myeloid cells for axon regeneration. Journal of Neuroscience 28, 9363–9376. 10.1523/JNEUROSCI.1447-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G, Al-Rashed S, Hoult SJR, Brain DS, Hoult JRS, Brain SD, 1998. Nerve growth factor induced hyperalgesia in the rat hind paw is dependent on circulating neutrophils. Pain 77, 315–322. 10.1016/S0304-3959(98)00114-6 [DOI] [PubMed] [Google Scholar]
- Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R, 2019. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev 16, 442–449. 10.2174/1573399815666191024085838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerregaard MD, Jurlander J, Klausen P, Borregaard N, Cowland JB, 2003. The in vivo profile of transcription factors during neutrophil differentiation in human bone marrow. Blood 101, 4322–4332. 10.1182/blood-2002-03-0835 [DOI] [PubMed] [Google Scholar]
- Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, Zabari M, Brik RB-Z, Kviatcovsky D, Zmora N, Cohen Y, Bar N, Levi I, Amar N, Mehlman T, Brandis A, Biton I, Kuperman Y, Tsoory M, Alfahel L, Harmelin A, Schwartz M, Israelson A, Arike L, Johansson M.E. v, Hansson GC, Gotkine M, Segal E, Elinav E, 2019. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480. 10.1038/s41586-019-1443-5 [DOI] [PubMed] [Google Scholar]
- Bochner BS, 2009. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clinical and Experimental Allergy 39, 317–324. 10.1111/j.1365-2222.2008.03173.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM, 1995. Chemoattractant signaling and leukocyte activation. Blood 86, 1649–1660. [PubMed] [Google Scholar]
- Bokoch GM, Diebold BA, 2002. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood. 10.1182/blood-2002-04-1149 [DOI] [PubMed] [Google Scholar]
- Bolton SJ, Anthony DC, Perry VH, 1998. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience 86, 1245–1257. 10.1016/s0306-4522(98)00058-x [DOI] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Salmon DP, 2017. Alzheimer ‘ s Disease : Past, Present, and Future 818–831. 10.1017/S135561771700100X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N, 2010. Neutrophils, from Marrow to Microbes. Immunity 33, 657–670. 10.1016/j.immuni.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Borregaard N, Sørensen OE, Theilgaard-Mönch K, 2007. Neutrophil granules: a library of innate immunity proteins. Trends Immunol 28, 340–345. 10.1016/j.it.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Brack A, Rittner HL, Machelska H, Leder K, Mousa SA, Schäfer M, Stein C, 2004. Control of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cells. Pain 112, 229–238. 10.1016/j.pain.2004.08.029 [DOI] [PubMed] [Google Scholar]
- Bracko O, Njiru BN, Swallow M, Ali M, Haft-Javaherian M, Schaffer CB, 2020. Increasing cerebral blood flow improves cognition into late stages in Alzheimer’s disease mice. Journal of Cerebral Blood Flow and Metabolism 40, 1441–1452. 10.1177/0271678X19873658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breijyeh Z, Karaman R, 2020. Comprehensive Review on Alzheimer ‘ s Disease : [DOI] [PMC free article] [PubMed]
- Brennan FH, Jogia T, Gillespie ER, Blomster L. v., Li XX, Nowlan B, Williams GM, Jacobson E, Osborne GW, Meunier FA, Taylor SM, Campbell KE, MacDonald KPA, Levesque JP, Woodruff TM, Ruitenberg MJ, 2019. Complement receptor C3aR1 controls neutrophil mobilization following spinal cord injury through physiological antagonism of CXCR2. JCI Insight 4, 1–18. 10.1172/jci.insight.98254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI, 2016. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7. 10.1038/ncomms12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück W, 1997. The role of macrophages in Wallerian degeneration. Brain Pathol 7, 741–752. 10.1111/j.1750-3639.1997.tb01060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burberry A, Wells MF, Limone F, Couto A, Smith KS, Keaney J, Gillet G, van Gastel N, Wang JY, Pietilainen O, Qian M, Eggan P, Cantrell C, Mok J, Kadiu I, Scadden DT, Eggan K, 2020. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 582, 89–94. 10.1038/s41586-020-2288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A, 2021. The Neutrophil. Immunity 54, 1377–1391. 10.1016/j.immuni.2021.06.006 [DOI] [PubMed] [Google Scholar]
- Cabot PJ, 2001. Immune-derived opioids and peripheral antinociception. Clin Exp Pharmacol Physiol 28, 230–232. 10.1046/j.1440-1681.2001.03425.x [DOI] [PubMed] [Google Scholar]
- Cabot PJ, Carter L, Gaiddon C, Zhang Q, Schäfer M, Loeffler JP, Stein C, 1997. Immune cell-derived beta-endorphin. Production, release, and control of inflammatory pain in rats. J Clin Invest 100, 142–148. 10.1172/JCI119506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahilog Z, Zhao H, Wu L, Alam A, Eguchi S, Weng H, Ma D, 2020. The Role of Neutrophil NETosis in Organ Injury: Novel Inflammatory Cell Death Mechanisms. Inflammation 43, 2021–2032. 10.1007/s10753-020-01294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Liu S, Hu M, Huang F, Zhu Q, Qiu W, Hu X, Colello J, Zheng SG, Lu Z, 2020. Functional Dynamics of Neutrophils After Ischemic Stroke. Transl Stroke Res 11, 108–121. 10.1007/s12975-019-00694-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero-Contentti E, Correale J, 2021. Neuromyelitis optica spectrum disorders: from pathophysiology to therapeutic strategies. J Neuroinflammation 18, 1–18. 10.1186/s12974-021-02249-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira EU, Carregaro V, Teixeira MM, Moriconi A, Aramini A, Verri WA, Ferreira SH, Cunha FQ, Cunha TM, 2013. Neutrophils recruited by CXCR1/2 signalling mediate post-incisional pain. European Journal of Pain (United Kingdom) 17, 654–663. 10.1002/j.1532-2149.2012.00240.x [DOI] [PubMed] [Google Scholar]
- Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R, A-González N, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A, Hidalgo A, 2013. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153, 1025. 10.1016/j.cell.2013.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casserly CS, Nantes JC, Whittaker Hawkins RF, Vallières L, 2017. Neutrophil perversion in demyelinating autoimmune diseases: Mechanisms to medicine. Autoimmun Rev 16, 294–307. 10.1016/j.autrev.2017.01.013 [DOI] [PubMed] [Google Scholar]
- Chen C, Huang T, Zhai X, Ma Y, Xie L, Lu B, Zhang Y, Li Y, Chen Z, Yin J, Li P, 2021. Targeting neutrophils as a novel therapeutic strategy after stroke. Journal of Cerebral Blood Flow and Metabolism 41, 2150–2161. 10.1177/0271678X211000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-C, Leach MW, Chen Y, Cai X-Y, Sullivan L, Wiekowski M, Dovey-Hartman BJ, Zlotnik A, Lira SA, 2002. Central nervous system inflammation and neurological disease in transgenic mice expressing the CC chemokine CCL21 in oligodendrocytes. J Immunol 168, 1009–1017. 10.4049/jimmunol.168.3.1009 [DOI] [PubMed] [Google Scholar]
- Chen X, Hu Y, Cao Z, Liu Q, Cheng Y, 2018. Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Front Immunol 9, 1–10. 10.3389/fimmu.2018.02122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XR, Besson VC, Palmier B, Garcia Y, Plotkine M, Marchand-Leroux C, 2007. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J Neurotrauma 24, 1119–1131. 10.1089/neu.2006.0216 [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang H, Hu X, Cai W, Ni W, Zhou K, 2022. Role of NETosis in Central Nervous System Injury. Oxid Med Cell Longev 2022. 10.1155/2022/3235524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher MJ, Liu F, Hilton MJ, Long F, Link DC, 2009. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood 114, 1331–1339. 10.1182/blood-2008-10-184754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I, Kim KS, Rajarathnam K, Gong JH, Dewald B, Moser B, Baggiolini M, Sykes BD, 1995. Structure-activity relationships of chemokines. J Leukoc Biol 57, 703–711. 10.1002/jlb.57.5.703 [DOI] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I, 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8, 265–277. 10.1023/a:1008942828960 [DOI] [PubMed] [Google Scholar]
- Coelho FM, Pinho V, Amaral FA, Sachs D, Costa V. v, Rodrigues DH, Vieira AT, Silva TA, Souza DG, Bertini R, Teixeira AL, Teixeira MM, 2008. The chemokine receptors CXCR1/CXCR2 modulate antigen-induced arthritis by regulating adhesion of neutrophils to the synovial microvasculature. Arthritis Rheum 58, 2329–2337. 10.1002/art.23622 [DOI] [PubMed] [Google Scholar]
- Coles JP, Fryer TD, Smielewski P, Rice K, Clark JC, Pickard JD, Menon DK, 2004. Defining ischemic burden after traumatic brain injury using 15O PET imaging of cerebral physiology. J Cereb Blood Flow Metab 24, 191–201. 10.1097/01.WCB.0000100045.07481.DE [DOI] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J, 2014. Functional regeneration beyond the glial scar. Exp Neurol 253, 197–207. 10.1016/j.expneurol.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csepregi JZ, Orosz A, Zajta E, Kása O, Németh T, Simon E, Fodor S, Csonka K, Barátki BL, Kövesdi D, He Y-W, Gácser A, Mócsai A, 2018. Myeloid-Specific Deletion of Mcl-1 Yields Severely Neutropenic Mice That Survive and Breed in Homozygous Form. The Journal of Immunology 201, 3793–3803. 10.4049/jimmunol.1701803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ, 2008. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol 83, 824–832. 10.1189/jlb.0907654 [DOI] [PubMed] [Google Scholar]
- Cunha TM, Verri WAJ, 2006. Neutrophils: are they hyperalgesic or anti-hyperalgesic? J Leukoc Biol 80, 727–728. 10.1189/jlb.0406244 [DOI] [PubMed] [Google Scholar]
- Daffern PJ, Pfeifer PH, Ember JA, Hugli TE, 1995. C3a Is a Chemotaxin for Human Eosinophils but Not for Neutrophils. I. C3a Stimulation of Neutrophils Is Secondary to Eosinophil Activation. Journal of Experimental Medicine 181, 2119–2127. 10.1084/jem.181.6.2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro RJ, Hughes MG, Xu G-Y, Clifton C, Calingasan NY, Gelman BB, McAdoo DJ, 2004. Evidence that infiltrating neutrophils do not release reactive oxygen species in the site of spinal cord injury. Exp Neurol 190, 414–424. 10.1016/j.expneurol.2004.05.046 [DOI] [PubMed] [Google Scholar]
- de Oliveira S, Rosowski EE, Huttenlocher A, 2016. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat Rev Immunol. 10.1038/nri.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG, May RM, 1991. Cajal’s Degeneration & Regeneration of the Nervous System. Oxford University Press, New York. [Google Scholar]
- DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE, 2015. The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience 302, 174–203. https://doi.org/S0306-4522(14)00769-6 [pii]; 10.1016/j.neuroscience.2014.09.027 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denorme F, Rustad JL, Portier I, Crandell JL, de Araujo CV, Cody MJ, Campbell RA, Yost CC, 2022. Neutrophil extracellular trap inhibition improves survival in neonatal mouse infectious peritonitis. Pediatr Res. 10.1038/s41390-022-02219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster RS, Rogers LM, Gaddy JA, Aronoff DM, 2018. Macrophage Extracellular Traps: A Scoping Review. J Innate Immun 10, 3–13. 10.1159/000480373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eash KJ, Greenbaum AM, Gopalan PK, Link DC, 2010. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. Journal of Clinical Investigation 120, 2423–2431. 10.1172/JCI41649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eash KJ, Means JM, White DW, Link DC, 2009. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 113, 4711–4719. 10.1182/blood-2008-09-177287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PMC, 2016. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev 273, 180–193. 10.1111/imr.12447 [DOI] [PubMed] [Google Scholar]
- Erdener ŞE, Tang J, Kılıç K, Postnov D, Giblin JT, Kura S, Chen I. chun A., Vayisoğlu T, Sakadžić S, Schaffer CB, Boas DA, 2021. Dynamic capillary stalls in reperfused ischemic penumbra contribute to injury: A hyperacute role for neutrophils in persistent traffic jams. Journal of Cerebral Blood Flow and Metabolism 41, 236–252. 10.1177/0271678X20914179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernesto S Jr., Hascup K, Hascup E, 2020. Alzheimer’s Disease: The Link Between Amyloid-β and Neurovascular Dysfunction. Physiol Behav 76, 1179–1198. 10.3233/JAD-200473.Alzheimer [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essig F, Kollikowski AM, Pham M, Solymosi L, Stoll G, Haeusler KG, Kraft P, Schuhmann MK, 2020. Immunohistological analysis of neutrophils and neutrophil extracellular traps in human thrombemboli causing acute ischemic stroke. Int J Mol Sci 21, 1–13. 10.3390/ijms21197387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurschou M, Borregaard N, 2003. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 5, 1317–1327. 10.1016/j.micinf.2003.09.008 [DOI] [PubMed] [Google Scholar]
- Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT, 2016. Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open 6. 10.1136/bmjopen-2015-010364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, Abbasifard M, AbbasiKangevari M, Abd-Allah F, Abedi V, Abualhasan A, Al., Et., 2021. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 20, 1–26. 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Poole S, 1993. Bradykinin initiates cytokine‐mediated inflammatory hyperalgesia. Br J Pharmacol 110, 1227–1231. 10.1111/j.1476-5381.1993.tb13946.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ, 2008. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol 181, 2189–95. 10.4049/jimmunol.181.3.2189 [DOI] [PubMed] [Google Scholar]
- Finger EB, Puri KD, Alon R, Lawrence MB, von Andrian UH, Springer TA, 1996. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature 379, 266–269. 10.1038/379266a0 [DOI] [PubMed] [Google Scholar]
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A, 2007. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176, 231–241. 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futosi K, Fodor S, Mócsai A, 2013. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 17, 638–650. 10.1016/j.intimp.2013.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Mei C, Chen P, Chen X, 2022. The contribution of neuro-immune crosstalk to pain in the peripheral nervous system and the spinal cord. Int Immunopharmacol 107, 108700. 10.1016/j.intimp.2022.108700 [DOI] [PubMed] [Google Scholar]
- García-Culebras A, Durán-Laforet V, Peña-Martínez C, Ballesteros I, Pradillo JM, Díaz-Guzmán J, Lizasoain I, Moro MA, 2018. Myeloid cells as therapeutic targets in neuroinflammation after stroke: Specific roles of neutrophils and neutrophil–platelet interactions. Journal of Cerebral Blood Flow and Metabolism 38, 2150–2164. 10.1177/0271678X18795789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Culebras A, Durán-Laforet V, Peña-Martínez C, Moraga A, Ballesteros I, Cuartero MI, de la Parra J, Palma-Tortosa S, Hidalgo A, Corbí AL, Moro MA, Lizasoain I, 2019. Role of TLR4 (Toll-like receptor 4) in N1/N2 neutrophil programming after stroke. Stroke 50, 2922–2932. 10.1161/STROKEAHA.119.025085 [DOI] [PubMed] [Google Scholar]
- García-García E, Uribe-Querol E, Rosales C, 2013. A simple and efficient method to detect nuclear factor activation in human neutrophils by flow cytometry. J Vis Exp. 10.3791/50410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH, 2001. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab 21, 1393–1400. 10.1097/00004647-200112000-00003 [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Popovich PG, Ramer MS, 2011. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammation 8, 110. https://doi.org/17422094-8-110 [pii]; 10.1186/1742-2094-8-110 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereau RW 4th, Sluka KA, Maixner W, Savage SR, Price TJ, Murinson BB, Sullivan MD, Fillingim RB, 2014. A pain research agenda for the 21st century. J Pain 15, 1203–1214. 10.1016/j.jpain.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemlou N, Chiu IM, Julien J-PP, Woolf CJ, 2015. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A 112, E6808–E6817. 10.1073/pnas.1501372112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs BF, Falcone FH, 2014. Basophils and Mast Cells Series Editor. [DOI] [PubMed]
- Girvan RC, Knight DA, O’loughlin CJ, Hayman CM, Hermans IF, Webster GA, 2011. MIS416, a non-toxic microparticle adjuvant derived from Propionibacterium acnes comprising immunostimulatory muramyl dipeptide and bacterial DNA promotes cross-priming and Th1 immunity. Vaccine 29, 545–557. 10.1016/j.vaccine.2010.10.040 [DOI] [PubMed] [Google Scholar]
- Gong P, Liu Y, Gong Y, Chen G, Zhang X, Wang S, Zhou F, Duan R, Chen W, Huang T, Wang M, Deng Q, Shi H, Zhou J, Jiang T, Zhang Y, 2021. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation 18, 1–11. 10.1186/s12974-021-02090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T, Liu L, Jiang W, Zhou R, 2020. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol 20, 95–112. 10.1038/s41577-019-0215-7 [DOI] [PubMed] [Google Scholar]
- Gorlino C. v., Ranocchia RP, Harman MF, García IA, Crespo MI, Morón G, Maletto BA, Pistoresi-Palencia MC, 2014. Neutrophils Exhibit Differential Requirements for Homing Molecules in Their Lymphatic and Blood Trafficking into Draining Lymph Nodes. The Journal of Immunology 193, 1966–1974. 10.4049/jimmunol.1301791 [DOI] [PubMed] [Google Scholar]
- Gowing G, Vallières L, Julien J-P, 2006. Mouse model for ablation of proliferating microglia in acute CNS injuries. Glia 53, 331–337. 10.1002/glia.20288 [DOI] [PubMed] [Google Scholar]
- Green SP, Chuntharapai A, Curnutte JT, 1996. Interleukin-8 (IL-8), melanoma growth-stimulatory activity, and neutrophil-activating peptide selectively mediate priming of the neutrophil NADPH oxidase through the type A or type B IL-8 receptor. J Biol Chem 271, 25400–25405. 10.1074/jbc.271.41.25400 [DOI] [PubMed] [Google Scholar]
- Guthrie RA, Guthrie DW, 2004. Pathophysiology of Diabetes Mellitus. Critical Care Neursing Quarterly 27, 113–125. [DOI] [PubMed] [Google Scholar]
- Harada Y, Zhang J, Imari K, Yamasaki R, Ni J, Wu Z, Yamamoto K, Kira JI, Nakanishi H, Hayashi Y, 2019. Cathepsin E in neutrophils contributes to the generation of neuropathic pain in experimental autoimmune encephalomyelitis. Pain 160, 2050–2062. 10.1097/j.pain.0000000000001596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenberg A, Hasenberg M, Männ L, Neumann F, Borkenstein L, Stecher M, Kraus A, Engel DR, Klingberg A, Seddigh P, Abdullah Z, Klebow S, Engelmann S, Reinhold A, Brandau S, Seeling M, Waisman A, Schraven B, Göthert JR, Nimmerjahn F, Gunzer M, 2015. Catchup: A mouse model for imaging-based tracking and modulation of neutrophil granulocytes. Nat Methods 12, 445–452. 10.1038/nmeth.3322 [DOI] [PubMed] [Google Scholar]
- Hasselbalch IC, Søndergaard HB, Koch-Henriksen N, Olsson A, Ullum H, Sellebjerg F, Oturai AB, 2018. The neutrophil-to-lymphocyte ratio is associated with multiple sclerosis. Mult Scler J Exp Transl Clin 4. 10.1177/2055217318813183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann ON, 2003. Post-traumatic inflammation following spinal cord injury. Spinal Cord 41, 369–378. 10.1038/sj.sc.3101483 [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP, 2015. Neuroinfl ammation in Alzheimer ‘ s disease 14. 10.1016/S1474-4422(15)70016-5 [DOI] [Google Scholar]
- Henry KM, Loynes CA, Whyte MKB, Renshaw SA, 2013. Zebrafish as a model for the study of neutrophil biology. J Leukoc Biol 94, 633–642. 10.1189/jlb.1112594 [DOI] [PubMed] [Google Scholar]
- Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA, 2003. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet 34, 70–74. 10.1038/ng1149 [DOI] [PubMed] [Google Scholar]
- Herrup K, 2010. Reimagining Alzheimer’s disease - An age-based hypothesis. Journal of Neuroscience. 10.1523/JNEUROSCI.4521-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertwig L, Pache F, Romero-suarez S, Stürner KH, Borisow N, Behrens J, Bellmann-strobl J, Seeger B, Asselborn N, Millward JM, Infante-duarte C, Paul F, 2016. Distinct functionality of neutrophils in multiple sclerosis and neuromyelitis optica 160–173. 10.1177/1352458515586084 [DOI] [PubMed] [Google Scholar]
- Herwald H, Egesten A, 2016. On PAMPs and DAMPs. J Innate Immun 8, 427–428. 10.1159/000448437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock H, Hamblen MJ, Rooke HM, Traver D, Bronson RT, Cameron S, Orkin SH, 2003. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity 18, 109–120. 10.1016/S1074-7613(02)00501-0 [DOI] [PubMed] [Google Scholar]
- Hooshmand MJ, Nguyen HX, Piltti KM, Benavente F, Hong S, Flanagan L, Uchida N, Cummings BJ, Anderson AJ, 2017. Neutrophils Induce Astroglial Differentiation and Migration of Human Neural Stem Cells via C1q and C3a Synthesis. J Immunol 199, 1069–1085. 10.4049/jimmunol.1600064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horny HP, Lange K, Sotlar K, Valent P, 2003. Increase of bone marrow lymphocytes in systemic mastocytosis: Reactive lymphocytosis or malignant lymphoma? Immunohistochemical and molecular findings on routinely processed bone marrow biopsy specimens. J Clin Pathol 56, 575–578. 10.1136/jcp.56.8.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Lin HC, Yang YH, Hsu CW, Chen NC, Tsai WC, Cheng BC, Tsai NW, 2022. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio are associated with a 2-year relapse in patients with multiple sclerosis. Mult Scler Relat Disord 58. 10.1016/j.msard.2022.103514 [DOI] [PubMed] [Google Scholar]
- Huda S, Whittam D, Bhojak M, Chamberlain J, Noonan C, Jacob A, 2019. Neuromyelitis optica spectrum disorders. Clinical Medicine, Journal of the Royal College of Physicians of London 19, 169–176. 10.7861/CLINMEDICINE.19-2-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Shiozaki T, Tasaki O, Hayakata T, Ikegawa H, Yoshiya K, Fujinaka T, Tanaka H, Shimazu T, Sugimoto H, 2005. Changes in cerebral blood flow from the acute to the chronic phase of severe head injury. J Neurotrauma 22, 1411–1418. 10.1089/neu.2005.22.1411 [DOI] [PubMed] [Google Scholar]
- Jerome AD, Atkinson JR, McVey Moffatt AL, Sepeda JA, Segal BM, Sas AR, 2022. Characterization of Zymosan-Modulated Neutrophils With Neuroregenerative Properties. Front Immunol 13, 1–13. 10.3389/fimmu.2022.912193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EA, Dao TL, Guignet MA, Geddes CE, Koemeter-Cox AI, Kan RK, 2011. Increased expression of the chemokines CXCL1 and MIP-1α by resident brain cells precedes neutrophil infiltration in the brain following prolonged soman-induced status epilepticus in rats. J Neuroinflammation 8, 41. 10.1186/1742-2094-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Wolf M, Qin S, Mackay CR, Baggiolini M, 1996. Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc Natl Acad Sci U S A 93, 6682–6686. 10.1073/pnas.93.13.6682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi MB, Lad A, Bharath Prasad AS, Balakrishnan A, Ramachandra L, Satyamoorthy K, 2013. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett 587, 2241–2246. 10.1016/j.febslet.2013.05.053 [DOI] [PubMed] [Google Scholar]
- Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, Cao Y, Xu H, Luo H, Lu L, Shi MJ, Tian Y, Fan W, Zhao BQ, 2020. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun 11. 10.1038/s41467-020-16191-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas GS, 1996. Selectins and their ligands: current concepts and controversies. Blood 88, 3259–3287. [PubMed] [Google Scholar]
- Karsunky H, Zeng H, Schmidt T, Zevnik B, Kluge R, Schmid KW, Dührsen U, Möröy T, 2002. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor GFi1. Nat Genet 30, 295–300. 10.1038/ng831 [DOI] [PubMed] [Google Scholar]
- Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL, 2005. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy 4, 273–279. 10.2174/1568010054022114 [DOI] [PubMed] [Google Scholar]
- Katancik JA, Sharma A, de Nardin E, 2000. Interleukin 8, neutrophil-activating peptide-2 and GROalpha bind to and elicit cell activation via specific and different amino acid residues of CXCR2. Cytokine 12, 1480–1488. 10.1006/cyto.2000.0742 [DOI] [PubMed] [Google Scholar]
- Kelly DF, Martin NA, Kordestani R, Counelis G, Hovda DA, Bergsneider M, McBride DQ, Shalmon E, Herman D, Becker DP, 1997. Cerebral blood flow as a predictor of outcome following traumatic brain injury. J Neurosurg 86, 633–641. 10.3171/jns.1997.86.4.0633 [DOI] [PubMed] [Google Scholar]
- Kerstetter AE, Padovani-Claudio DA, Bai L, Miller RH, 2009. Inhibition of CXCR2 signaling promotes recovery in models of multiple sclerosis. Exp Neurol 220, 44–56. 10.1016/j.expneurol.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorooshi R, Marczynska J, Dieu RS, Wais V, Hansen CR, Kavan S, Thomassen M, Burton M, Kruse T, Webster GA, Owens T, 2020. Innate signaling within the central nervous system recruits protective neutrophils. Acta Neuropathol Commun 8. 10.1186/s40478-019-0876-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Brumell J, Al-Alawi N, Latour S, Cheng A, Veillette A, Grinstein S, Pawson T, 1998. The Syk Protein Tyrosine Kinase Is Essential for Fc Receptor Signaling in Macrophages and Neutrophils, MOLECULAR AND CELLULAR BIOLOGY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Moalem-Taylor G, 2011a. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res 1405, 95–108. 10.1016/j.brainres.2011.06.022 [DOI] [PubMed] [Google Scholar]
- Kim CF, Moalem-Taylor G, 2011b. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. Journal of Pain 12, 370–383. 10.1016/j.jpain.2010.08.003 [DOI] [PubMed] [Google Scholar]
- Kim SW, Lee HHK, Lee HHK, Kim ID, Lee JK, 2019. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol Commun 7, 94. 10.1186/s40478-019-0747-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klion AD, Ackerman SJ, Bochner BS, 2020. Contributions of Eosinophils to Human Health and Disease. Annual Review of Pathology: Mechanisms of Disease 15, 179–209. 10.1146/annurev-pathmechdis-012419-032756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knier B, Hiltensperger M, Sie C, Aly L, Lepennetier G, Engleitner T, Garg G, Muschaweckh A, Mitsdörffer M, Koedel U, Höchst B, Knolle P, Gunzer M, Hemmer B, Rad R, Merkler D, Korn T, 2018. Myeloid-derived suppressor cells control B cell accumulation in the central nervous system during autoimmunity. Nat Immunol 19, 1341–1351. 10.1038/s41590-018-0237-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi SD, Malachowa N, DeLeo FR, 2018. Neutrophils and Bacterial Immune Evasion. J Innate Immun 10, 432–441. 10.1159/000487756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, 2008. The role of chemokines in neutrophil biology. Frontiers in Bioscience 13, 2400–2407. 10.2741/2853 [DOI] [PubMed] [Google Scholar]
- Koenderman L, Tesselaar K, Vrisekoop N, 2022. Human neutrophil kinetics: a call to revisit old evidence. Trends Immunol 43, 868–876. 10.1016/j.it.2022.09.008 [DOI] [PubMed] [Google Scholar]
- Kubota K, Saiwai H, Kumamaru H, Maeda T, Ohkawa Y, Aratani Y, Nagano T, Iwamoto Y, Okada S, 2012. Myeloperoxidase exacerbates secondary injury by generating highly reactive oxygen species and mediating neutrophil recruitment in experimental spinal cord injury. Spine (Phila Pa 1976) 37, 1363–1369. 10.1097/BRS.0b013e31824b9e77 [DOI] [PubMed] [Google Scholar]
- Kumar H, Choi H, Jo M-JJ, Joshi HP, Muttigi M, Bonanomi D, Kim SB, Ban E, Kim A, Lee S-HH, Kim K-TT, Sohn S, Zeng X, Han I, 2018. Neutrophil elastase inhibition effectively rescued angiopoietin-1 decrease and inhibits glial scar after spinal cord injury. Acta Neuropathol Commun 6, 1–18. 10.1186/s40478-018-0576-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto T, Yin Y, Habboub G, Gilbert HY, Li Y, Nakao S, Hafezi-Moghadam A, Benowitz LI, 2013. Neutrophils express oncomodulin and promote optic nerve regeneration. Journal of Neuroscience 33, 14816–14824. 10.1523/JNEUROSCI.5511-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto T, Yin Y, Omura K, Gilbert H, Kim D, Cen L-P, Moko L, Kügler S, Benowitz LI, 2010. Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci 30, 15654–15663. 10.1523/JNEUROSCI.4340-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix S, Chang L, Rose-John S, Tuszynski MH, 2002. Delivery of hyper-interleukin-6 to the injured spinal cord increases neutrophil and macrophage infiltration and inhibits axonal growth. Journal of Comparative Neurology 454, 213–228. 10.1002/cne.10407 [DOI] [PubMed] [Google Scholar]
- Lacy P, 2006. Mechanisms of degranulation in neutrophils. Allergy, Asthma and Clinical Immunology. 10.2310/7480.2006.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P, 2005. The role of Rho GTPases and SNAREs in mediator release from granulocytes. Pharmacol Ther 107, 358–376. 10.1016/j.pharmthera.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Lavich TR, Siqueira R. de A., Farias-Filho FA, Cordeiro RSB, Rodrigues e Silva PM, Martins MA, Balão Cordeiro RS, Rodrigues e Silva PM, Martins MA, 2006. Neutrophil infiltration is implicated in the sustained thermal hyperalgesic response evoked by allergen provocation in actively sensitized rats. Pain 125, 180–187. 10.1016/j.pain.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Kansas GS, Kunkel EJ, Ley K, 1997. Threshold levels of fluid shear promote leukocyte adhesion through selectins (CD62L,P,E). J Cell Biol 136, 717–727. 10.1083/jcb.136.3.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW, 1993. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol 150, 2659–2667. [PubMed] [Google Scholar]
- Lee WL, Harrison RE, Grinstein S, 2003. Phagocytosis by neutrophils 5, 1299–1306. 10.1016/j.micinf.2003.09.014 [DOI] [PubMed] [Google Scholar]
- Leiding JW, 2017. Neutrophil evolution and their diseases in humans. Front Immunol 8. 10.3389/fimmu.2017.01009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI, 2000. Lens injury stimulates axon regeneration in the mature rat optic nerve. Journal of Neuroscience 20, 4615–4626. 10.1523/jneurosci.20-12-04615.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone MA, Mandrioli J, Russo S, Cucovici A, Gianferrari G, Lisnic V, Muresanu DF, Giuliani F, Copetti M, Fontana A, 2022. Neutrophils-to-Lymphocyte Ratio Is Associated with Progression and Overall Survival in Amyotrophic Lateral Sclerosis. Biomedicines 10, 1–13. 10.3390/biomedicines10020354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Gooding J, Donatoni P, Borden L, Goetzl EJ, 1985. The role of the polymorphonuclear leukocyte in hyperalgesia. Journal of Neuroscience 5, 3025–3029. 10.1523/jneurosci.05-11-03025.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Lau W, Kwiat G, Goetzl EJ, 1984. Leukotriene B4 produces hyperalgesia that is dependent on polymorphonuclear leukocytes. Science 225, 743–745. 10.1126/science.6087456 [DOI] [PubMed] [Google Scholar]
- Ley K, 2002. Integration of inflammatory signals by rolling neutrophils. Immunol Rev 186, 8–18. 10.1034/j.1600-065x.2002.18602.x [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S, 2007. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7, 678–689. 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y, 2010. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. Journal of Experimental Medicine 207, 1853–1862. 10.1084/jem.20100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Cui Y, Feng J, Guo Y, 2020. Identifying the pattern of immune related cells and genes in the peripheral blood of ischemic stroke. J Transl Med 18, 1–17. 10.1186/s12967-020-02463-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR, 1994. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 84, 1737–1746. 10.1182/blood.v84.6.1737.1737 [DOI] [PubMed] [Google Scholar]
- Lindborg JA, Mack M, Zigmond RE, 2017. Neutrophils are critical for myelin removal in a peripheral nerve injury model of Wallerian degeneration. Journal of Neuroscience 37, 10258–10277. 10.1523/JNEUROSCI.2085-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindborg JA, Niemi JP, Howarth MA, Liu KW, Moore CZ, Mahajan D, Zigmond RE, 2018. Molecular and cellular identification of the immune response in peripheral ganglia following nerve injury. J Neuroinflammation 15, 1–17. 10.1186/s12974-018-1222-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Belkadi A, Darnall L, Hu T, Drescher C, Cotleur AC, Padovani-Claudio D, He T, Choi K, Lane TE, Miller RH, Ransohoff RM, 2010a. CXCR2-positive neutrophils are essential for cuprizone-induced demyelination: Relevance to multiple sclerosis. Nat Neurosci 13, 319–326. 10.1038/nn.2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Darnall L, Hu T, Choi K, Lane TE, Ransohoff RM, 2010b. Myelin repair is accelerated by inactivating CXCR2 on nonhematopoietic cells. Journal of Neuroscience 30, 9074–9083. 10.1523/JNEUROSCI.1238-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, van Rooijen N, Tracey DJ, 2000. Depletion of macrophages reduces axonal degeneration and hyperalgesia following nerve injury. Pain 86, 25–32. 10.1016/s0304-3959(99)00306-1 [DOI] [PubMed] [Google Scholar]
- Liu Y, Holdbrooks AT, Meares GP, Buckley JA, Benveniste EN, Qin H, 2015. Preferential Recruitment of Neutrophils into the Cerebellum and Brainstem Contributes to the Atypical Experimental Autoimmune Encephalomyelitis Phenotype. The Journal of Immunology 195, 841–852. 10.4049/jimmunol.1403063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell CA, 2011. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: Signaling cross talk. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman HB, Fairbrother WJ, Slagle PH, Kabakoff R, Liu J, Shire S, Hébert CA, 1997. Monomeric variants of IL-8: effects of side chain substitutions and solution conditions upon dimer formation. Protein Sci 6, 598–608. 10.1002/pro.5560060309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Maheshwari A, Misiuta I, Fox SE, Chen N, Zigova T, Christensen RD, Calhoun DA, 2005. Neutrophil-specific chemokines are produced by astrocytic cells but not by neuronal cells. Brain Res Dev Brain Res 155, 127–134. 10.1016/j.devbrainres.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Luckey AM, Anderson T, Silverman MH, Webster G, 2015. Safety, tolerability and pharmacodynamics of a novel immunomodulator, MIS416, in patients with chronic progressive multiple sclerosis. Mult Scler J Exp Transl Clin 1. 10.1177/2055217315583385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machelska H, Schopohl JK, Mousa SA, Labuz D, Schäfer M, Stein C, 2003. Different mechanisms of intrinsic pain inhibition in early and late inflammation. J Neuroimmunol 141, 30–39. 10.1016/s0165-5728(03)00213-3 [DOI] [PubMed] [Google Scholar]
- Machelska H, Stein C, 2006. Leukocyte-derived opioid peptides and inhibition of pain. J Neuroimmune Pharmacol 1, 90–97. 10.1007/s11481-005-9002-2 [DOI] [PubMed] [Google Scholar]
- Manz MG, Boettcher S, 2014. Emergency granulopoiesis. Nat Rev Immunol. 10.1038/nri3660 [DOI] [PubMed] [Google Scholar]
- Martin C, Burdon PCE, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM, 2003. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19, 583–593. 10.1016/S1074-7613(03)00263-2 [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, 2014. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6, 13. 10.12703/P6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JR, Perrin BJ, Liu T-X, Kanki J, Look AT, Huttenlocher A, 2006. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol 80, 1281–1288. 10.1189/jlb.0506346 [DOI] [PubMed] [Google Scholar]
- McCreedy DA, Abram CL, Hu Y, Min SW, Platt ME, Kirchhoff MA, Reid SK, Jalufka FL, Lowell CA, 2021. Spleen tyrosine kinase facilitates neutrophil activation and worsens long-term neurologic deficits after spinal cord injury. J Neuroinflammation 18. 10.1186/s12974-021-02353-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver RP, Cummings RD, 1997. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest 100, S97–103. [PubMed] [Google Scholar]
- McLachlan E, Hu P, Geczy C, 2007. Neutrophils rarely invade dorsal root ganglia after peripheral nerve lesions. J Neuroimmunol 187, 212–215. 10.1016/j.jneuroim.2007.05.001.Neutrophils [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee KE, Alzabin S, Hughes JP, Anand P, Feldmann M, Williams RO, Inglis JJ, 2011. IL-17 induces hyperalgesia via TNF-dependent neutrophil infiltration. Pain 152, 1838–1845. 10.1016/j.pain.2011.03.035 [DOI] [PubMed] [Google Scholar]
- Mejzini R, Flynn LL, Pitout IL, Fletcher S, Wilton SD, Akkari PA, 2019. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front Neurosci 13, 1–27. 10.3389/fnins.2019.01310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A, 2011. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood 117, 953–959. 10.1182/blood-2010-06-290171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineoka Y, Ishii M, Hashimoto Y, Nakamura N, Katsumi Y, Fukui M, 2018. Neutrophil-lymphocyte ratio correlates with limited joint mobility of hand in patients with type 2 diabetes. Endocrine Journal 65, 1011–1017. [DOI] [PubMed] [Google Scholar]
- Mócsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA, 2006. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol 7, 1326–1333. 10.1038/ni1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith AJ, Miller JM, Maxwell CN, Chazin WJ, Skaar EP, 2021. Neutrophil extracellular traps enhance macrophage killing of bacterial pathogens. [DOI] [PMC free article] [PubMed]
- Mori T, Wang X, Aoki T, Lo EH, 2002. Downregulation of matrix metalloproteinase-9 and attenuation of edema via inhibition of ERK mitogen activated protein kinase in traumatic brain injury. J Neurotrauma 19, 1411–1419. 10.1089/089771502320914642 [DOI] [PubMed] [Google Scholar]
- Morosetti R, Park DJ, Chumakov AM, Grillier I, Shiohara M, Gombart AF, Nakamaki T, Weinberg K, Koeffler HP, 1997. A Novel, Myeloid Transcription Factor, C/EBPε, Is Upregulated During Granulocytic, But Not Monocytic, Differentiation. Blood 90, 2591–2600. 10.1182/blood.v90.7.2591 [DOI] [PubMed] [Google Scholar]
- Mueller H, Stadtmann A, van Aken H, Hirsch E, Wang D, Ley K, Zarbock A, 2010. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood 115, 3118–3127. 10.1182/blood-2009-11-254185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock BJ, Zhou T, Kashlan SR, Little RJ, Goutman SA, Feldman EL, 2017. Correlation of peripheral immunity with rapid Amyotrophic lateral sclerosis progression. JAMA Neurol 74, 1446–1454. 10.1001/jamaneurol.2017.2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CJ, Foster BA, Mannis MJ, Selsted ME, Reid TW, 1993. Defensins are mitogenic for epithelial cells and fibroblasts. J Cell Physiol 155, 408–413. 10.1002/jcp.1041550223 [DOI] [PubMed] [Google Scholar]
- Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, Iwakura Y, de Rivero Vaccari JP, Keane RW, Lacroix S, Vaccari J.P. de R., Keane RW, Lacroix S, 2011. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: Implications for neuropathic pain. Journal of Neuroscience 31, 12533–12542. 10.1523/JNEUROSCI.2840-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Miyamasu M, Yamaguchi M, Imanishi M, Tsuno NH, Matsushima K, Yamamoto K, Morita Y, Hirai K, 2002. Cytokine-mediated regulation of CXCR4 expression in human neutrophils. J Leukoc Biol 71, 711–7. 10.1189/jlb.71.4.711 [DOI] [PubMed] [Google Scholar]
- Nasser MW, Raghuwanshi SK, Grant DJ, Jala VR, Rajarathnam K, Richardson RM, 2009. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J Immunol 183, 3425–3432. 10.4049/jimmunol.0900305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neirinckx V, Coste C, Franzen R, Gothot A, Rogister B, Wislet S, 2014. Neutrophil contribution to spinal cord injury and repair. J Neuroinflammation 11, 1–9. 10.1186/s12974-014-0150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton VL, Guck JD, Cotter MA, Cameron NE, Gardiner NJ, 2017. Neutrophils Infiltrate the Spinal Cord Parenchyma of Rats with Experimental Diabetic Neuropathy. J Diabetes Res 2017. 10.1155/2017/4729284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi JP, DeFrancesco-Lisowitz A, Roldan-Hernandez L, Lindborg JA, Mandell D, Zigmond RE, 2013. A Critical Role for Macrophages Near Axotomized Neuronal Cell Bodies in Stimulating Nerve Regeneration. Journal of Neuroscience 33, 16236–16248. 10.1523/JNEUROSCI.3319-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourshargh S, Renshaw SA, Imhof BA, 2016. Reverse Migration of Neutrophils : Where, When, How, and Why ? Trends Immunol 37, 273–286. 10.1016/j.it.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Nozdrachev AD, Kolosova LI, Moiseeva AB, Ryabchikova O. v., 2006. The role of defensin NP-1 in restoring the functions of an injured nerve trunk. Neurosci Behav Physiol 36, 313–315. 10.1007/s11055-006-0018-8 [DOI] [PubMed] [Google Scholar]
- Nukada H, McMorran PD, Shimizu J, 2000. Acute inflammatory demyelination in reperfusion nerve injury. Ann Neurol 47, 71–79. [PubMed] [Google Scholar]
- Nusse O, Lindau M, 1988. The dynamics of exocytosis in human neutrophils. Journal of Cell Biology 107, 2117–2123. 10.1083/jcb.107.6.2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MCG, Pelegrini-da-Silva A, Parada CA, Tambeli CH, 2007. 5-HT acts on nociceptive primary afferents through an indirect mechanism to induce hyperalgesia in the subcutaneous tissue. Neuroscience 145, 708–714. 10.1016/j.neuroscience.2006.12.021 [DOI] [PubMed] [Google Scholar]
- Otxoa-de-Amezaga A, Miró-Mur F, Pedragosa J, Gallizioli M, Justicia C, Gaja-Capdevila N, Ruíz-Jaen F, Salas-Perdomo A, Bosch A, Calvo M, Márquez-Kisinousky L, Denes A, Gunzer M, Planas AM, 2019. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol 137, 321–341. 10.1007/s00401-018-1954-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y, 2008. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467. 10.1016/j.immuni.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C, Roberts RL, Young PI, 2004. Timing of neutrophil depletion influences long-term neuroprotection in neonatal rat hypoxic-ischemic brain injury. Pediatr Res 55, 549–556. 10.1203/01.PDR.0000113546.03897.FC [DOI] [PubMed] [Google Scholar]
- Pan D, Acevedo-Cintrón JA, Sayanagi J, Snyder-Warwick AK, Mackinnon SE, Wood MD, 2020a. The CCL2/CCR2 axis is critical to recruiting macrophages into acellular nerve allograft bridging a nerve gap to promote angiogenesis and regeneration. Exp Neurol 331, 113363. 10.1016/j.expneurol.2020.113363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Hunter DA, Schellhardt L, Fuchs A, Halevi AE, Snyder-Warwick AK, Mackinnon SE, Wood MD, 2020b. T cells modulate IL-4 expression by eosinophil recruitment within decellularized scaffolds to repair nerve defects. Acta Biomater 112, 149–163. 10.1016/j.actbio.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Schellhardt L, Acevedo-Cintron JA, Hunter D, Snyder-Warwick AK, Mackinnon SE, Wood MD, 2022. IL-4 expressing cells are recruited to nerve after injury and promote regeneration. Exp Neurol 347, 113909. 10.1016/j.expneurol.2021.113909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A, 2010. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. Journal of Cell Biology 191, 677–691. 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareyson D, Saveri P, Pisciotta C, 2017. New developments in Charcot-Marie-Tooth neuropathy and related diseases. Curr Opin Neurol. 10.1097/WCO.0000000000000474 [DOI] [PubMed] [Google Scholar]
- Park B-K, Park Y-C, Jung IC, Kim S-H, Choi J-E, Park S, Choi JJ, Jin M, 2014. Oral administration of SSC201, a medicinal herbal formula, suppresses atopic dermatitis-like skin lesions. J Med Food 17, 496–504. 10.1089/jmf.2013.2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LC, Whyte MKB, Dower SK, Sabroe I, 2005. The expression and roles of Toll-like receptors in the biology of the human neutrophil. J Leukoc Biol 77, 886–892. 10.1189/jlb.1104636 [DOI] [PubMed] [Google Scholar]
- Patel S, Kumar S, Jyoti A, Srinag BS, Keshari RS, Saluja R, Verma A, Mitra K, Barthwal MK, Krishnamurthy H, Bajpai VK, Dikshit M, 2010. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide 22, 226–234. 10.1016/j.niox.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Paudel YN, Angelopoulou E, Piperi C, Othman I, Aamir K, Shaikh MF, 2020. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s Disease (AD): From Risk Factors to Therapeutic Targeting. Cells. 10.3390/cells9020383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiseler M, Kubes P, 2019. More friend than foe: The emerging role of neutrophils in tissue repair. Journal of Clinical Investigation. 10.1172/JCI124616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelisch N, Rosas Almanza J, Stehlik KE, Aperi B. v, Kroner A, 2020. CCL3 contributes to secondary damage after spinal cord injury. J Neuroinflammation 17, 362. 10.1186/s12974-020-02037-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, Urra X, Chamorro A, Planas AM, 2015. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol 129, 239–257. 10.1007/s00401-014-1381-0 [DOI] [PubMed] [Google Scholar]
- Perkins NM, Tracey DJ, 2000. Hyperalgesia due to nerve injury: Role of neutrophils. Neuroscience 101, 745–757. 10.1016/S0306-4522(00)00396-1 [DOI] [PubMed] [Google Scholar]
- Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P, 2006. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med 203, 2569–2575. 10.1084/jem.20060925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ER, Wagner CA, Goverman JM, 2018. The contribution of neutrophils to CNS autoimmunity. Clinical Immunology 189, 23–28. 10.1016/j.clim.2016.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay J, Tak T, Kamp VM, Koenderman L, 2013. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: Similarities and differences. Cellular and Molecular Life Sciences 70, 3813–3827. 10.1007/s00018-013-1286-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau I, Lacroix S, 2007. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol 500, 267–285. 10.1002/cne.21149 [DOI] [PubMed] [Google Scholar]
- Pineau I, Sun L, Bastien D, Lacroix S, 2010. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun 24, 540–553. 10.1016/j.bbi.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Pizzo PA, Clark NM, 2012. Alleviating suffering 101--pain relief in the United States. N Engl J Med 366, 197–199. 10.1056/NEJMp1109084 [DOI] [PubMed] [Google Scholar]
- Planagumà A, Domènech T, Pont M, Calama E, García-González V, López R, Aulí M, López M, Fonquerna S, Ramos I, de Alba J, Nueda A, Prats N, Segarra V, Miralpeix M, Lehner MD, 2015. Combined anti CXC receptors 1 and 2 therapy is a promising anti-inflammatory treatment for respiratory diseases by reducing neutrophil migration and activation. Pulm Pharmacol Ther 34, 37–45. 10.1016/j.pupt.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Plein LM, Rittner HL, 2018. Opioids and the immune system – friend or foe. Br J Pharmacol 175, 2717–2725. 10.1111/bph.13750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruchniak MP, Arazna M, Demkow U, 2013. Life of neutrophil: From stem cell to neutrophil extracellular trap. Respir Physiol Neurobiol 187, 68–73. 10.1016/j.resp.2013.02.023 [DOI] [PubMed] [Google Scholar]
- Przewłocki R, Hassan AH, Lason W, Epplen C, Herz A, Stein C, 1992. Gene expression and localization of opioid peptides in immune cells of inflamed tissue: functional role in antinociception. Neuroscience 48, 491–500. 10.1016/0306-4522(92)90509-z [DOI] [PubMed] [Google Scholar]
- Radomska HS, Huettner CS, Zhang P, Cheng T, Scadden DT, Tenen DG, 1998. CCAAT/Enhancer Binding Protein α Is a Regulatory Switch Sufficient for Induction of Granulocytic Development from Bipotential Myeloid Progenitors. Mol Cell Biol 18, 4301–4314. 10.1128/mcb.18.7.4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarathnam K, Kay CM, Dewald B, Wolf M, Baggiolini M, Clark-Lewis I, Sykes BD, 1997. Neutrophil-activating peptide-2 and melanoma growth-stimulatory activity are functional as monomers for neutrophil activation. J Biol Chem 272, 1725–1729. 10.1074/jbc.272.3.1725 [DOI] [PubMed] [Google Scholar]
- Rajarathnam K, Prado GN, Fernando H, Clark-Lewis I, Navarro J, 2006. Probing receptor binding activity of interleukin-8 dimer using a disulfide trap. Biochemistry 45, 7882–7888. 10.1021/bi0605944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarathnam K, Schnoor M, Richardson RM, Rajagopal S, 2019. How do chemokines navigate neutrophils to the target site: Dissecting the structural mechanisms and signaling pathways. Cell Signal 54, 69–80. 10.1016/j.cellsig.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarathnam K, Sykes BD, Kay CM, Dewald B, Geiser T, Baggiolini M, Clark-Lewis I, 1994. Neutrophil activation by monomeric interleukin-8. Science 264, 90–92. 10.1126/science.8140420 [DOI] [PubMed] [Google Scholar]
- Rajeeve K, Das S, Prusty BK, Rudel T, 2018. Chlamydia trachomatis paralyses neutrophils to evade the host innate immune response. Nat Microbiol 3, 824–835. 10.1038/s41564-018-0182-y [DOI] [PubMed] [Google Scholar]
- Ravin KA, Loy M, 2015. The eosinophil in infection. Clin Rev Allergy Immunol 50, 214–227. 10.1007/s12016-015-8525-4 [DOI] [PubMed] [Google Scholar]
- Ravindran A, Sawant K. v, Sarmiento J, Navarro J, Rajarathnam K, 2013. Chemokine CXCL1 dimer is a potent agonist for the CXCR2 receptor. J Biol Chem 288, 12244–12252. 10.1074/jbc.M112.443762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rg YV, Ognnkvwu F, Qz T, Urgekgu IGP, Cfxcpegf QH, Ecvkqp IN, Rtqfwevu GPF, 2019. Diabetic neuropathy. Nat Rev Dis Primers 5, 42. 10.1038/s41572-019-0097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittner HL, Brack A, Stein C, 2003. Pro-algesic versus analgesic actions of immune cells. Curr Opin Anaesthesiol 16, 527–533. 10.1097/00001503-200310000-00014 [DOI] [PubMed] [Google Scholar]
- Rittner HL, Hackel D, Voigt P, Mousa S, Stolz A, Labuz D, Schäfer M, Schaefer M, Stein C, Brack A, 2009. Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils. PLoS Pathog 5. 10.1371/journal.ppat.1000362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittner Heike L, Labuz D, Schaefer M, Mousa SA, Schulz S, Schäfer M, Stein C, Brack A, 2006. Pain control by CXCR2 ligands through Ca2+-regulated release of opioid peptides from polymorphonuclear cells. FASEB J 20, 2627–2629. 10.1096/fj.06-6077fje [DOI] [PubMed] [Google Scholar]
- Rittner Heike L., Machelska H, Schäfer M, Stein C, Brack A, 2006a. Comment on “Neutrophils: are they hyperalgesic or anti-hyperalgesic?” J Leukoc Biol 80, 729–730. 10.1189/jlb.0506337 [DOI] [PubMed] [Google Scholar]
- Rittner Heike L., Mousa SA, Labuz D, Beschmann K, Schäfer M, Stein C, Brack A, 2006b. Selective local PMN recruitment by CXCL1 or CXCL2/3 injection does not cause inflammatory pain. J Leukoc Biol 79, 1022–1032. 10.1189/jlb.0805452 [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P, Almendros I, Sunyer R, Gavara N, Farré R, Navajas D, 2006. Rheology of passive and adhesion-activated neutrophils probed by atomic force microscopy. Biophys J 91, 3508–3518. 10.1529/biophysj.106.088831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales C, 2020. Neutrophils at the crossroads of innate and adaptive immunity. J Leukoc Biol 108, 377–396. 10.1002/JLB.4MIR0220-574RR [DOI] [PubMed] [Google Scholar]
- Rosales C, 2018. Neutrophil: A cell with many roles in inflammation or several cell types? Front Physiol 9, 1–17. 10.3389/fphys.2018.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S, 2011. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J. Neuroinflammation 8, 109. https://doi.org/1742-2094-8-109 [pii]; 10.1186/1742-2094-8-109 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, Wang L, Segal BM, 2015. Neutrophil-related factors as biomarkers in EAE and MS. Journal of Experimental Medicine 212, 23–35. 10.1084/jem.20141015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahbaie P, Li X, Shi X, David Clark J, 2012. PAIN MEDICINE Roles of Gr-1 Leukocytes in Postincisional Nociceptive Sensitization and Inflammation. [DOI] [PMC free article] [PubMed]
- Saiwai H, Ohkawa Y, Yamada H, Kumamaru H, Harada A, Okano H, Yokomizo T, Iwamoto Y, Okada S, 2010. The LTB4-BLT1 axis mediates neutrophil infiltration and secondary injury in experimental spinal cord injury. American Journal of Pathology 176, 2352–2366. 10.2353/ajpath.2010.090839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarejloo S, Khanzadeh S, Hosseini S, Gargari MK, Lucke-Wold B, Mosalamiaghili S, Azami P, Oftadehbalani S, Sadeghvand S, 2022a. Role of the Neutrophil to Lymphocyte Ratio in Guillain Barré Syndrome: A Systematic Review and Meta-Analysis. Mediators Inflamm. 10.1155/2022/3390831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarejloo S, Khanzadeh S, Hosseini S, Gargari MK, Lucke-Wold B, Mosalamiaghili S, Azami P, Oftadehbalani S, Sadeghvand S, 2022b. Role of the Neutrophil to Lymphocyte Ratio in Guillain Barré Syndrome: A Systematic Review and Meta-Analysis. Mediators Inflamm. 10.1155/2022/3390831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarikaya H, Ferro J, Arnold M, 2015. Stroke prevention - Medical and lifestyle measures. Eur Neurol 73, 150–157. 10.1159/000367652 [DOI] [PubMed] [Google Scholar]
- Sas AR, Carbajal KS, Jerome AD, Menon R, Yoon C, Kalinski AL, Giger RJ, Segal BM, 2020. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat Immunol 21, 1496–1505. 10.1038/s41590-020-00813-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasmono RT, Ehrnsperger A, Cronau SL, Ravasi T, Kandane R, Hickey MJ, Cook AD, Himes SR, Hamilton JA, Hume DA, 2007. Mouse neutrophilic granulocytes express mRNA encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J Leukoc Biol 82, 111–123. 10.1189/jlb.1206713 [DOI] [PubMed] [Google Scholar]
- Scapini P, Bazzoni F, Cassatella MA, 2008. Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol Lett 116, 1–6. 10.1016/j.imlet.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA, 2000. The neutrophil as a cellular source of chemokines. Immunol Rev 177, 195–203. 10.1034/j.1600-065x.2000.17706.x [DOI] [PubMed] [Google Scholar]
- Scapini P, Laudanna C, Pinardi C, Allavena P, Mantovani A, Sozzani S, Cassatella MA, 2001. Neutrophils produce biologically active macrophage inflammatory protein-3alpha (MIP-3alpha)/CCL20 and MIP-3beta/CCL19. Eur J Immunol 31, 1981–1988. [DOI] [PubMed] [Google Scholar]
- Schafer M, Mousa SA, Zhang Q, Carter L, Stein C, 1996. Expression of corticotropin-releasing factor in inflamed tissue is required for intrinsic peripheral opioid analgesia. Proc Natl Acad Sci U S A 93, 6096–6100. 10.1073/pnas.93.12.6096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel AR, Chew TW, Muller WA, 2004. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol 173, 6403–6408. 10.4049/jimmunol.173.10.6403 [DOI] [PubMed] [Google Scholar]
- Schneider AH, MacHado CC, Veras FP, Maganin AGDME, de Souza FFL, Barroso LC, de Oliveira RDR, Alves-Filho JC, Cunha TM, Fukada SY, Louzada-Júnior P, da Silva TA, Cunha FQ, 2021. Neutrophil extracellular traps mediate joint hyperalgesia induced by immune inflammation. Rheumatology (United Kingdom) 60, 3461–3473. 10.1093/rheumatology/keaa794 [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y, 1990. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43, 205–218. 10.1016/0304-3959(90)91074-S [DOI] [PubMed] [Google Scholar]
- Sengelov H, Kjeldsen L, Borregaard N, 1993. Control of exocytosis in early neutrophil activation. Journal of Immunology 15, 1535–1543. [PubMed] [Google Scholar]
- Serger E, Luengo-Gutierrez L, Chadwick JS, Kong G, Zhou L, Crawford G, Danzi MC, Myridakis A, Brandis A, Bello AT, Müller F, Sanchez-Vassopoulos A, de Virgiliis F, Liddell P, Dumas ME, Strid J, Mani S, Dodd D, di Giovanni S, 2022. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature 607, 585–592. 10.1038/s41586-022-04884-x [DOI] [PubMed] [Google Scholar]
- Shaw SK, Owolabi SA, Bagley J, Morin N, Cheng E, LeBlanc BW, Kim M, Harty P, Waxman SG, Saab CY, 2008. Activated Polymorphonuclear Cells promote Injury and Excitability of Dorsal Root Ganglia Neurons. Exp. Neurol 210, 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Cheng X, Zhou L, Zhao Y, Wang H, Zhang J, Sun X, Wang Y, Shu Y, Xu Y, Tao Y, Li M, Lu Z, Cai W, Nie G, Qiu W, 2022. Neutrophil Nanovesicle Protects against Experimental Autoimmune Encephalomyelitis through Enhancing Myelin Clearance by Microglia. ACS Nano 16, 18886–18897. 10.1021/acsnano.2c07798 [DOI] [PubMed] [Google Scholar]
- Shepherd AJ, Mickle AD, Golden JP, Mack MR, Halabi CM, de Kloet AD, Samineni VK, Kim BS, Krause EG, Gereau RW 4th, Mohapatra DP, 2018. Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc Natl Acad Sci U S A 115, E8057–E8066. 10.1073/pnas.1721815115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K, Li H, Chang T, He W, Kong Y, Qi C, Li R, Huang H, Zhu Z, Zheng P, Ruan Z, Zhou J, Shi FD, Liu Q, 2022. Bone marrow hematopoiesis drives multiple sclerosis progression. Cell 185, 2234–2247.e17. 10.1016/j.cell.2022.05.020 [DOI] [PubMed] [Google Scholar]
- Sica A, Matsushima K, van Damme J, Wang JM, Polentarutti N, Dejana E, Colotta F, Mantovani A, 1990. IL-1 transcriptionally activates the neutrophil chemotactic factor/IL-8 gene in endothelial cells. Immunology 69, 548–553. [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH, 2004. Regeneration beyond the glial scar. Nat Rev Neurosci 5, 146–156. 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O, 2020. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol 17, 327–340. 10.1038/s41569-019-0326-7 [DOI] [PubMed] [Google Scholar]
- Silvin A, Uderhardt S, Piot C, Amit I, Kipnis J, Ginhoux F, Silvin A, Uderhardt S, Piot C, Da Mesquita S, Yang K, Geirsdottir L, 2022. Dual ontogeny of disease-associated microglia and disease inflammatory macrophages in aging and neurodegeneration ll ll Article Dual ontogeny of disease-associated microglia and disease inflammatory macrophages in aging and neurodegeneration. Immunity 55, 1448–1465.e6. 10.1016/j.immuni.2022.07.004 [DOI] [PubMed] [Google Scholar]
- Simmons SB, Liggitt D, Goverman JM, 2014. Cytokine-regulated neutrophil recruitment is required for brain but not spinal cord inflammation during EAE. J Immunol 193, 555–563. 10.4049/jimmunol.1400807.Cytokine-regulated [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Plemel JR, 2014. Neutrophil contribution in facilitating optic nerve regeneration. Journal of Neuroscience 34, 1081–1082. 10.1523/JNEUROSCI.4827-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solito S, Pinton L, Mandruzzato S, 2017. In Brief: Myeloid-derived suppressor cells in cancer. Journal of Pathology 242, 7–9. 10.1002/path.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackowicz J, Jönsson F, Reber LL, 2020. Mouse Models and Tools for the in vivo Study of Neutrophils. Front Immunol 10. 10.3389/fimmu.2019.03130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K, 2005. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294. 10.1016/j.immuni.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Stein C, Hassan AH, Przewłocki R, Gramsch C, Peter K, Herz A, 1990. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci U S A 87, 5935–5939. 10.1073/pnas.87.15.5935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DM, Hu PF, Brenner M, Sheth KN, Liu K-H, Xiong W, Aarabi B, Scalea TM, 2011. Brief episodes of intracranial hypertension and cerebral hypoperfusion are associated with poor functional outcome after severe traumatic brain injury. J Trauma 71, 364. 10.1097/TA.0b013e31822820da [DOI] [PubMed] [Google Scholar]
- Stillie R, Farooq SM, Gordon JR, Stadnyk AW, 2009. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol 86, 529–543. 10.1189/jlb.0208125 [DOI] [PubMed] [Google Scholar]
- Stirling DP, Liu S, Kubes P, Yong VW, 2009. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci 29, 753–64. 10.1523/JNEUROSCI.4918-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo J, Linke B, Meyer dos Santos S, Pierre S, Stegner D, Zhang DD, Denis C. v., Geisslinger G, Nieswandt B, Scholich K, 2014. Neutrophils mediate edema formation but not mechanical allodynia during zymosan-induced inflammation. J Leukoc Biol 96, 133–142. 10.1189/jlb.3a1213-628r [DOI] [PubMed] [Google Scholar]
- Swamydas M, Gao JL, Break TJ, Johnson MD, Jaeger M, Rodriguez CA, Lim JK, Green NM, Collar AL, Fischer BG, Lee CCR, Perfect JR, Alexander BD, Kullberg BJ, Netea MG, Murphy PM, Lionakis MS, 2016. CXCR1-mediated neutrophil degranulation and fungal killing promote Candida clearance and host survival. Sci Transl Med 8. 10.1126/scitranslmed.aac7718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamydas M, Luo Y, Dorf ME, Lionakis MS, 2015. Isolation of mouse neutrophils. Curr Protoc Immunol 2015, 3.20.1–3.20.15. 10.1002/0471142735.im0320s110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani M, Fuentes ME, Peterson JW, Trapp BD, Durham SK, Loy JK, Bravo R, Ransohoff RM, Lira SA, 1996. Neutrophil infiltration, glial reaction, and neurological disease in transgenic mice expressing the chemokine N51/KC in oligodendrocytes. J Clin Invest 98, 529–539. 10.1172/JCI118821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Territo MC, Ganz T, Selsted ME, Lehrer R, 1989. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest 84, 2017–2020. 10.1172/JCI114394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilgaard-Mönch K, Porse BT, Borregaard N, 2006. Systems biology of neutrophil differentiation and immune response. Curr Opin Immunol 18, 54–60. 10.1016/j.coi.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Thelen M, Uguccioni M, Bösiger J, 1995. PI 3-kinase-dependent and independent chemotaxis of human neutrophil leukocytes. Biochem Biophys Res Commun 217, 1255–1262. 10.1006/bbrc.1995.2903 [DOI] [PubMed] [Google Scholar]
- Therrien M, Dion PA, Rouleau GA, 2016. ALS: Recent Developments from Genetics Studies. Curr. Neurol. Neurosci. Rep 16, 59. 10.1007/s11910-016-0658-1 [doi]; [pii] [DOI] [PubMed] [Google Scholar]
- Ting E, Guerrero ATG, Cunha TM, Verri WA, Taylor SM, Woodruff TM, Cunha FQ, Ferreira SH, 2008. Role of complement C5a in mechanical inflammatory hypernociception: Potential use of C5a receptor antagonists to control inflammatory pain. Br J Pharmacol 153, 1043–1053. 10.1038/sj.bjp.0707640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias E, King PH, Si Y, Kwon Y, Varela V, Ibarburu S, Kovacs M, Moura IC, Beckman JS, Hermine O, Barbeito L, 2018. Mast cells and neutrophils mediate peripheral motor pathway degeneration in ALS. JCI Insight 3, 1–16. 10.1172/jci.insight.123249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl MW, Biagas K. v, Grundl PD, Barmada MA, Schiding JK, Nemoto EM, Kochanek PM, 1994. Effects of neutropenia on edema, histology, and cerebral blood flow after traumatic brain injury in rats. J Neurotrauma 11, 303–315. 10.1089/neu.1994.11.303 [DOI] [PubMed] [Google Scholar]
- van Gisbergen KPJM, Sanchez-Hernandez M, Geijtenbeek TBH, van Kooyk Y, 2005. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J Exp Med 201, 1281–1292. 10.1084/jem.20041276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ziffle JA, Lowell CA, 2009. Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection. Blood 114, 4871–4882. 10.1182/blood-2009-05-220806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjeva NV, Chernyak BV, 2020. NETosis : Molecular Mechanisms,. Biochemistry (Moscow) 85, 1178–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjeva NV, Chernyak BV, 2020. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry (Moscow). 10.1134/S0006297920100065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Lin T-T, Hu L, Xu C-J, Hu F, Wan L, Yang X, Wu X-F, Zhang X-T, Li Y, Yin H-Y, Jiang C-Y, Xin H-L, Liu W-T, 2023a. Neutrophil extracellular traps as a unique target in the treatment of chemotherapy-induced peripheral neuropathy. [DOI] [PMC free article] [PubMed]
- Wang C-Y, Lin T-T, Hu L, Xu C-J, Hu F, Wan L, Yang X, Wu X-F, Zhang X-T, Li Y, Yin H-Y, Jiang C-Y, Xin H-L, Liu W-T, 2023b. Neutrophil extracellular traps as a unique target in the treatment of chemotherapy-induced peripheral neuropathy. [DOI] [PMC free article] [PubMed]
- Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes, † Paul, 2017. Visualizing the function and fate of neutrophils in sterile injury and repair. Science (1979) 358, 111–116. [DOI] [PubMed] [Google Scholar]
- Wang PL, Yim AKY, Kim KW, Avey D, Czepielewski RS, Colonna M, Milbrandt J, Randolph GJ, 2020. Peripheral nerve resident macrophages share tissue-specific programming and features of activated microglia. Nat Commun 11, 1–13. 10.1038/s41467-020-16355-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MPJ, Webster G, Leonard F, La Flamme AC, 2018. Innate IFN-γ ameliorates experimental autoimmune encephalomyelitis and promotes myeloid expansion and PDL-1 expression. Sci Rep 8, 1–11. 10.1038/s41598-017-18543-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC, Kettle AJ, Hampton MB, 2016. Reactive Oxygen Species and Neutrophil Function. Annu Rev Biochem 85, 765–792. 10.1146/annurev-biochem-060815-014442 [DOI] [PubMed] [Google Scholar]
- Wolf M, Delgado MB, Jones SA, Dewald B, Clark-Lewis I, Baggiolini M, 1998. Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. Eur J Immunol 28, 164–170. [DOI] [PubMed] [Google Scholar]
- Wong CHY, Heit B, Kubes P, 2010. Molecular regulators of leucocyte chemotaxis during inflammation. Cardiovasc Res 86, 183–191. 10.1093/cvr/cvq040 [DOI] [PubMed] [Google Scholar]
- Wright HL, Cross AL, Edwards SW, Moots RJ, 2014. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology (United Kingdom) 53, 1321–1331. 10.1093/rheumatology/keu035 [DOI] [PubMed] [Google Scholar]
- Wu D, LaRosa GJ, Simon MI, 1993. G protein-coupled signal transduction pathways for interleukin-8. Science 261, 101–103. 10.1126/science.8316840 [DOI] [PubMed] [Google Scholar]
- Xie L, Poteet EC, Li W, Scott AE, Liu R, Wen Y, Ghorpade A, Simpkins JW, Yang S-H, 2010. Modulation of polymorphonuclear neutrophil functions by astrocytes. J Neuroinflammation 7, 53. 10.1186/1742-2094-7-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Shi Q, Wu P, Zhang X, Kambara H, Su J, Yu H, Park SY, Guo R, Ren Q, Zhang S, Xu Y, Silberstein LE, Cheng T, Ma F, Li C, Luo HR, 2020. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat Immunol 21, 1119–1133. 10.1038/s41590-020-0736-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yago T, Shao B, Miner JJ, Yao L, Klopocki AG, Maeda K, Coggeshall KM, McEver RP, 2010. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood 116, 485–494. 10.1182/blood-2009-12-259556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kadoya K, Terkawi MA, Endo T, Konno K, Watanabe M, Ichihara S, Hara A, Kaneko K, Iwasaki N, Ishijima M, 2022. Neutrophils delay repair process in Wallerian degeneration by releasing NETs outside the parenchyma. Life Sci Alliance 5, 1–17. 10.26508/lsa.202201399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka R, Barlow C, Lekstrom-Himes J, Castilla LH, Liu PP, Eckhaus M, Decker T, Wynshaw-Boris A, Xanthopoulos KG, 1997a. Impaired granulopoiesis, myelodysplasia, and early lethality in CCAAT/enhancer binding protein ε-deficient mice. Proc Natl Acad Sci U S A 94, 13187–13192. 10.1073/pnas.94.24.13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka R, do Kim G, Radomska HS, Lekstrom-Himes J, Smith LT, Antonson P, Tenen DG, Xanthopoulos KG, 1997b. CCAAT/enhancer binding protein ε is preferentially up-regulated during granulocytic differentiation and its functional versatility is determined by alternative use of promoters and differential splicing. Proc Natl Acad Sci U S A 94, 6462–6467. 10.1073/pnas.94.12.6462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-W, Strong BSI, Miller MJ, Unanue ER, 2010. Neutrophils influence the level of antigen presentation during the immune response to protein antigens in adjuvants. J Immunol 185, 2927–2934. 10.4049/jimmunol.1001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates AG, Jogia T, Gillespie ER, Couch Y, Ruitenberg MJ, Anthony DC, 2021. Acute IL-1RA treatment suppresses the peripheral and central inflammatory response to spinal cord injury. J Neuroinflammation 18, 1–12. 10.1186/s12974-020-02050-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Liu Boyu, Li Y, Li X, Wang J, Chen R, Tai Y, Shou Q, Wang P, Shao X, Liang Y, Zhou H, Mi W, Fang J, Liu Boyi, 2020. IL-33/ST2 induces neutrophil-dependent reactive oxygen species production and mediates gout pain. Theranostics 10, 12189–12203. 10.7150/thno.48028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI, 2006. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci 9, 843–852. 10.1038/nn1701 [DOI] [PubMed] [Google Scholar]
- Yipp BG, Kubes P, 2013. NETosis: How vital is it? Blood 122, 2784–2794. 10.1182/blood2013-04-457671 [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Kobayashi H, Tatsumi L, Tomita T, 2022. Mouse Models of Alzheimer ‘ s Disease 15, 1–14. 10.3389/fnmol.2022.912995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost CC, Schwertz H, Cody MJ, Wallace JA, Campbell RA, Vieira-De-Abreu A, Araujo CV, Schubert S, Harris ES, Rowley JW, Rondina MT, Fulcher JM, Koening CL, Weyrich AS, Zimmerman GA, 2016. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. Journal of Clinical Investigation 126, 3783–3798. 10.1172/JCI83873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI, 2020. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 11, 264. 10.1038/s41467-019-13839-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y-SS, Niu S-PP, Yu F, Zhang Y-JJ, Han N, Lu H, Yin X-FF, Xu H-LL, Kou Y-HH, 2020. Intraoperative single administration of neutrophil peptide 1 accelerates the early functional recovery of peripheral nerves after crush injury. Neural Regen Res 15, 2108–2115. 10.4103/1673-5374.282270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Ley K, 2011. Protein tyrosine kinases in neutrophil activation and recruitment. Arch Biochem Biophys 510, 112–119. 10.1016/j.abb.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Zehntner SP, Bourbonniere L, Hassan-Zahraee M, Tran E, Owens T, 2004. Bone marrow-derived versus parenchymal sources of inducible nitric oxide synthase in experimental autoimmune encephalomyelitis. J Neuroimmunol 150, 70–79. 10.1016/j.jneuroim.2004.01.020 [DOI] [PubMed] [Google Scholar]
- Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, Turano E, Rossi B, Angiari S, Dusi S, Montresor A, Carlucci T, Nanì S, Tosadori G, Calciano L, Catalucci D, Berton G, Bonetti B, Constantin G, 2015. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 21, 880–886. 10.1038/nm.3913 [DOI] [PubMed] [Google Scholar]
- Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG, 1997. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc Natl Acad Sci U S A 94, 569–574. 10.1073/pnas.94.2.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Harada Y, Hayashi Y, 2019. A TLR–CXCL1 pathway in DRG neurons induces neutrophil accumulation in the DRG and mechanical allodynia in EAE mice. Sci Rep 9, 1–10. 10.1038/s41598-019-48558-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cheng R, Rowe D, Sethu P, Daugherty A, Yu G, Shin HY, 2014. Shear-sensitive regulation of neutrophil flow behavior and its potential impact on microvascular blood flow dysregulation in hypercholesterolemia. Arterioscler Thromb Vasc Biol 34, 587–593. 10.1161/ATVBAHA.113.302868 [DOI] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Hooten KG, Sieglaff DH, Zhang A, Kalyana-Sundaram S, Traini CM, Halsey WS, Hughes AM, Sathe GM, Livi GP, Fan GH, Appel SH, 2017. Characterization of gene expression phenotype in amyotrophic lateral sclerosis monocytes. JAMA Neurol 74, 677–685. 10.1001/jamaneurol.2017.0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X-F, Huffman LD, Hafner H, Athaiya M, Finneran MC, Kalinski AL, Kohen R, Flynn C, Passino R, Johnson CN, Kohrman D, Kawaguchi R, Yang LJS, Twiss JL, Geschwind DH, Corfas G, Arbor A, Arbor A, 2022. The injured sciatic nerve atlas (iSNAT), insights into the cellular and molecular basis of neural tissue degeneration and regeneration 1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE, Echevarria FD, 2019. Macrophage biology in the peripheral nervous system after injury. Prog Neurobiol 173, 102–121. 10.1016/j.pneurobio.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindel J, Kubes P, 2020. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annual Review of Pathology: Mechanisms of Disease 15, 493–518. 10.1146/annurevpathmechdis-012419-032847 [DOI] [PubMed] [Google Scholar]


