Abstract
Aims:
There is limited research regarding insulin dosing changes following adoption of plant-based diets. We conducted a nonrandomized crossover trial utilizing two plant-based diets (Dietary Approaches to Stop Hypertension, or DASH, and Whole Food, Plant-Based, or WFPB) to assess acute changes in insulin requirements and associated markers among individuals with insulin-treated type 2 diabetes.
Methods:
Participants (n=15) enrolled in a 4-week trial with sequential, one-week phases: Baseline, DASH 1, WFPB, and DASH 2. Each diet was ad libitum and meals were provided.
Results:
Compared to baseline, daily insulin usage was 24%, 39%, and 30% lower after DASH 1, WFPB, and DASH 2 weeks respectively (all p <0.01). Insulin resistance (HOMA-IR) was 49% lower (p <0.01) and the insulin sensitivity index was 38% higher (p <0.01) at the end of the WFPB week before regressing toward baseline during DASH 2. Total, LDL, and HDL cholesterol, leptin, urinary glucose, and hsCRP decreased to a nadir at the end of the WFPB week before increasing during DASH 2.
Conclusions:
Adopting a DASH or WFPB diet can result in significant, rapid changes in insulin requirements, insulin sensitivity, and related markers among individuals with insulin-treated type 2 diabetes, with larger dietary changes producing larger benefits.
Keywords: DASH Diet, Insulin Resistance, Medical Nutrition Therapy, Plant-Based Diet, Type 2 Diabetes Mellitus, Vegan Diet
1.0. Introduction
Obesity prevalence has increased in recent decades[1] and now 14.7% of American adults have diabetes[2]. Type 2 diabetes accounts for 90-95% of total cases. Improving dietary habits is an integral part of disease self-management and is recognized by the American Diabetes Association as offering benefits for glycemic control, weight control and other risk factor management[3]. The Dietary Guidelines for Americans 2020-2025[4] recommends a healthy dietary pattern across the lifespan and describes three dietary patterns as examples: A “Healthy US-style” dietary pattern, a “Mediterranean” dietary pattern, and a “Vegetarian” dietary pattern.
These plant-enriched, or plant-based, dietary patterns are all richer in fruits, vegetables, legumes, and whole grains and lower in processed foods, saturated fat, added sugar, and salt, than typical American diets. A moderately plant-based approach is the Dietary Approaches to Stop Hypertension (DASH) diet, a “healthy US-style” diet where no food choice is eliminated but instead the emphasis is on consuming more fruits, vegetables, whole grains and low-fat dairy, and reducing saturated fats, sugar and salt. At the ‘end’ of the plant-based dietary spectrum is a whole-foods, plant-based (WFPB) diet comprised of beans, whole grains, fruits and vegetables that minimizes or fully excludes animal foods, added fats, and most added sugars.
Both the DASH and WFPB diets have been shown to lower blood pressure[5, 6], cholesterol[7, 8], weight[9], and blood sugar[10, 11]. Treatment programs integrating a WFPB diet have been shown to lead to atherosclerotic regression, improvement in angina, and reduced risk of cardiac events among people with diagnosed coronary artery disease[12, 13]. These benefits beyond glycemic control are particularly important in type 2 diabetes. According to 2015-2018 NHANES survey, only 21% of people with diabetes achieved control of their combined risk factors of blood sugar, cholesterol, and blood pressure[14].
If dietary factors change quickly, then metabolism, and specifically glycemic control, also changes quickly. Within hours, intralipid infusions increase intramyocellular lipid and reduce glucose disposal by up to 40% during hyperinsulinemic-euglycemic clamp procedure[15, 16]. Similarly, a high fat diet consumed for just three days can increase intramyocellular lipid levels and result in a 17% reduction in glucose infusion rate during clamp procedure[15]. Conversely, six days of a very low-calorie diet has been shown to significantly reduce intramyocellular lipid, increase glucose disposal rate and decrease insulin resistance[17].
Despite knowing that large dietary changes can have rapid effects, there is limited guidance regarding acute changes in insulin requirements among insulin-requiring individuals with type 2 diabetes[18] in response to dietary intervention. This is problematic because up to 24% of Americans with type 2 diabetes require insulin therapy[19] and nutrition management, as part of diabetes selfmanagement, is recommended for all patients with diabetes. Given the prevalence of patients with insulin-requiring type 2 diabetes and the hope that they might improve outcomes by adopting dietary change, this is an important gap in the literature.
Given this background, we conducted a nonrandomized crossover study of individuals with insulin-treated type 2 diabetes and a BMI of 27 or greater, using a DASH diet and a WFPB diet, to better characterize the acute effects of these plant-based interventions on total daily insulin requirements and, secondarily, to describe changes in related cardiometabolic and obesity biomarkers.
2.0. Methods
2.1. Participants and Study Design
Participants were recruited from the University of Rochester endocrinology clinic. Inclusion criteria included: being over the age of 18, having type 2 diabetes requiring daily insulin, having a body mass index (BMI) of at least 27kg/m2, and a hemoglobin A1c (HbA1c) between 6.5 - 9.5% (48 and 80 mmol/mol). English fluency was required. Exclusion criteria included diagnosis of type 1 diabetes or latent autoimmune diabetes in adulthood, use of insulin pump, or medical conditions presenting confounders or potential risks related to dietary change, including recent estimated glomerular filtration rate of 45 mL/min/1.73 m2 or lower, recent hyperkalemia, pregnancy, liver cirrhosis, or active malabsorption disorder. Individuals taking the following medications were excluded: warfarin, aspirin (if >500mg daily), vitamin C (if >1000mg daily), antipsychotics, systemic glucocorticoids within three months, phentermine, orlistat, lorcaserin, phentermine/topiramate or bupropion/naloxone in the past three months, sulfonylureas or glinides in the past three months. If used, the dose of metformin, GLP-1 agonist, or SGLT2 inhibitor had to be stable over the past three months prior to the study, and the participant’s dose of insulin had to be stable over the past three months (no changes in their prescription of > 10%). Individuals with illicit drug use (not including marijuana), high risk alcohol use, food allergies interfering with study adherence, or recent vegan or vegetarian diet were also excluded.
After consent, participants attended a baseline visit where a 14-day continuous glucose monitor (iCGM), Freestyle Libre Pro, was placed on their upper arm, baseline questionnaires were completed, and they were provided with food and insulin diaries as well as glucose tablets and instructions on how to use them in case of non-emergent hypoglycemia. To avoid unanticipated behavioral adjustments, participants were unable to access results from the CGM and were asked to continue their baseline routine of checking blood glucose. After seven days on their normal baseline diet, they came to the University of Rochester Medical Center research clinic between 7AM and 9AM, in a fasted state, where they underwent a 2-hour oral glucose tolerance test (OGTT) with blood drawn at time 0, 60 and 120 minutes. They were instructed to withhold all of their morning diabetes medication, including insulin, until after the testing was completed. Immediately following the baseline OGTT they started a DASH diet for seven days (DASH 1). At the end of this DASH 1 week, they completed the same OGTT testing, a new continuous glucose monitor was placed, and then they started a WFPB diet for 7 days. They completed the same OGTT testing at the end of the WFPB diet week, and then completed a second week of the DASH diet with repeat testing at the end (DASH 2) (Figure 1).
Figure 1.
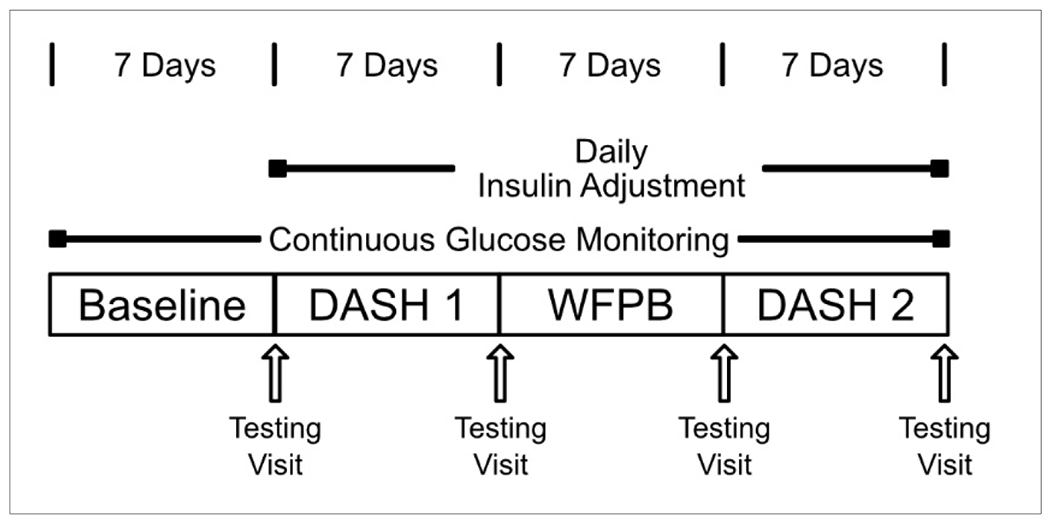
Study Schema
As a safety measure, participants were contacted daily by a study physician after they started study food in order to monitor blood sugar and adjust insulin as needed to prevent hypoglycemia. In anticipation of lower blood glucose levels, the night before the first DASH week and again the night before starting the WFPB week, basal insulin doses were reduced by approximately 20%. Prandial dose regimens were also adjusted downward by 10-20% at the start of DASH 1 and WFPB weeks, however the adjustment was individualized. After the initial adjustments, insulin doses were adjusted to a target of normal or near normal blood glucose levels, with allowance for individual variation.
Participants were instructed to eat as often and as much as they wanted to be comfortably full. Snacks and three prepared meals a day were provided in order to enhance adherence to the study diet, but participants were encouraged to consume their own food in place of, or in addition to, the provided food, as long as their food was ‘on plan’. To ensure a consistent provision of food throughout the study, the provided food consisted of approximately 1800 calories per day. The DASH diet was based on the dietary pattern described by the National Heart, Lung, and Blood Institute[20], emphasizing increased fruits, vegetables, whole grains, and low-fat dairy while including fish, poultry, and some vegetable oils. Foods higher in saturated fat, including red meats, full fat dairy, and solid fats were limited, as were foods high in added sugar and sodium. The ad libitum WFPB diet consisted of fruits, vegetables, whole grains, legumes, potatoes, nuts and seeds. The WFPB diet fully excluded all animal products and all added oils/solid fats. The study was reviewed and approved by the University of Rochester Research Subjects Review Board and registered on clinicaltrials.gov (NCT04048642).
2.2. Study Outcomes
Participants recorded and reported daily insulin usage. Blood glucose was tracked by a Freestyle Libre Pro continuous glucose monitor throughout the study, but both study participants and physicians were blinded to the CGM readings during the intervention. Study physicians used participants’ regular blood glucose tracking results to adjust insulin. Freestyle Libre Pro data are reported here. At each weekly clinic visit participants were assessed for weight, blood pressure, and resting heart rate. Weight was measured in a fasting state in the morning, in light clothing, without shoes, and blood pressure was assessed by an automated blood pressure cuff taken after 5 minutes of rest. Resting heart rate was assessed by the blood pressure cuff during the blood pressure assessment. In addition, a urine sample was collected to assess urinary glucose. After weight and blood pressure, the participant had a blood draw, considered time 0, and then consumed 75g of glucose in a standard glucose tolerance beverage within 5 minutes.
The baseline blood draw included a lipid panel, high sensitivity C-reactive protein, glucose, insulin, and C-peptide, which were sent to the University of Rochester Medical Center’s CLIA-certified clinical laboratory and assessed using standard methods. Separately, blood samples to measure leptin and adiponectin were collected in serum separator tubes and centrifuged at 1300 RCF for 10 minutes in a swinging bucket centrifuge (Thermo Scientific) to separate plasma. The plasma was transferred to new tubes and stored at −80°C in an ultra-low temperature freezer until their analysis. Leptin and total adiponectin levels were measured in these samples using ELISA kits (11-LEPHU-E01 and 80-ADPHU-E01, ALPCO) per the manufacturer’s instructions. Absorbance was measured using a microplate spectrophotometer (BioTek).
At 60 minutes and 120 minutes following the first blood draw, another blood sample was collected to measure insulin, glucose, and C-peptide to complete the OGTT. This testing process was repeated 4 times, at the end of each study phase. Insulin sensitivity was assessed by HOMA-IR ((Fasting insulin, uIU/mL) x (Fasting glucose,mg/dL)/405) and the insulin sensitivity index (ISI) was calculated from the OGTT results by using a modified Matsuda index (ISI = k/sqrt(G0 x I0 x G120 x I120) where k = 10,000, G0 and G120 are plasma glucose concentrations at 0 and 120 min, I0 and I120 are plasma insulin concentrations at 0 and 120 min)[21, 22]. Participants completed a 3-day food diary every week, starting with the baseline diet, and these were reviewed and analyzed by a dietitian using Nutrition Data System for Research, version 2017.
2.3. Statistical Methods
The study was 80% powered to detect a 20% difference in the insulin requirements between DASH and the plant-based diet using a 2-sided 0.05 level test. Distributions of baseline characteristics were summarized via means and standard deviations (SD) for continuous variables and counts and proportions for categorical variables. Since most readers are more familiar with the usual arithmetic mean as a summary of total daily insulin dose, while it is arguably better linearly modeled on the log scale and thus summarized via its geometric mean (which is generally smaller), both arithmetic and geometric mean total daily insulin dose appear in Table 1. All p-values are two-sided.
Table 1.
Baseline Characteristics (n=15)
| Age | Mean, years (SD) | 56.7 (±14.3) |
| Gender | Female, n (%) | 9 (60.0) |
| Male, n (%) | 6 (40.0) | |
| Race | White, n (%) | 10 (66.7) |
| African American, n (%) | 3 (20.0) | |
| American Indian, n (%) | 1 (6.7) | |
| No Response, n (%) | 1 (6.7) | |
| Ethnicity | Hispanic/Latino, n (%) | 2 (13.3) |
| Not Hispanic/Latino, n (%) | 13 (86.7) | |
| Weight | Mean, kg (SD) | 97.5 (±19.1) |
| BMI | Mean, kg/m2 (SD) | 34.3 (±5.3) |
| BMI category | Overweight (BMI 25-29.9), n (%) | 5 (33.3) |
| Class 1 obesity (BMI 30-34.9), n (%) | 4 (26.7) | |
| Class 2 obesity (BMI 35-39.9), n (%) | 3 (20.0) | |
| Class 3 obesity (BMI >40), n (%) | 3 (20.0) | |
| Age at diabetes diagnosis | Mean, years (SD) | 44.8 (±14.7) |
| Years elapsed since diagnosis | Mean, years (SD) | 12.0 (±6.6) |
| Most recent HgbA1C | Mean, % (SD) | 8.4 (±0.7) |
| Mean, mmol/mol (SD) | 68 (±2.2) | |
| Medicines Used | Basal Insulin, n (%) | 15 (100.0) |
| Prandial Insulin, n (%) | 12 (80.0) | |
| Metformin, n (%) | 6 (40.0) | |
| GLP-1, n (%) | 9 (60.0) | |
| SGLT2-inhibitor, n (%) | 0 (0.0) | |
| Antihypertensive, n (%) | 9 (60) | |
| Cholesterol-lowering, n (%) | 11 (73.3) | |
| Heartburn Control, n (%) | 10 (66.7) | |
| Depression/Anxiety Control, n (%) | 7 (46.7) | |
| Total Daily Insulin Dose | Mean, units/day (SD) | 90.9 (59.9) |
| Geometric mean, units/day (SD Factor) | 74 (2.0) |
Linear mixed effects models were used to model each dietary intake outcome as a function of a fixed effect for diet (Baseline, DASH 1, WFPB, DASH 2) with a random effect for subject to account for longitudinal dependence, given that each subject was sequentially placed on each diet. Results were summarized via diet-specific (arithmetic) means and their differences, along with 95% confidence intervals (CI) and p-values for mean changes. Missing data was handled automatically by the linear mixed effects models, under the common Missing At Random (MAR) assumption.
Insulin, blood glucose, and cardiometabolic outcomes were all log-transformed to symmetrize distributions prior to fitting similar linear mixed effects models with a fixed effect of diet and a random subject effect. Diet-specific means and their pairwise differences on the log scale were exponentiated and reported as geometric means and ratios thereof, along with 95% CI and p-values.
Individual trajectories of total insulin usage by day, relative to the insulin dose at the end of the baseline week were used to visualize subject-specific relative changes in total insulin, with the geometric mean overlaid to highlight trends. The first day of each diet was omitted due to non-dietary influence of the OGTT.
3.0. Results
From October 2020 to January 2022, 225 individuals were screened, out of which 117 were eligible (Figure 2). 15 participants were enrolled, and 12 participants completed all visits. Three participants withdrew after their baseline visit, including one who was scheduled unexpectedly for surgery for an unrelated, pre-existing orthopedic problem, one who was in a motor vehicle accident prior to starting the intervention, and one who started new employment and could not continue the weekly assessments.
Figure 2.
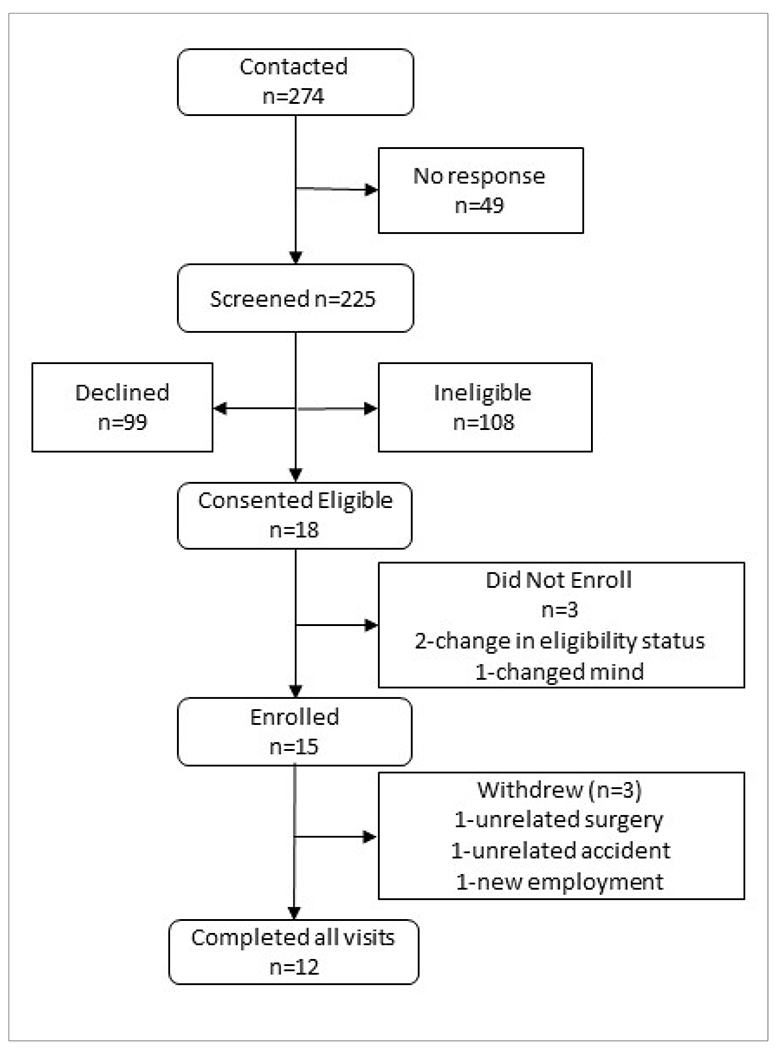
Consort Diagram
Baseline characteristics of the 15 participants who enrolled are shown in Table 1. Participants had diagnosed diabetes for an average of 12 years and their most recent HbA1c was 8.4% (68 mmol/mol). On average, participants took 90.1 units of insulin a day, with a range of 34 units/day to 205 units/day.
3.1. Dietary changes
3-day food diaries showed large changes in nutrient intakes during each dietary phase (Table 2, Figure 3). Total calories were lower during all three dietary phases compared to baseline (p<0.01 for all), though calorie intake during each dietary phase was not significantly different from other dietary phases (p>0.25 for all). The DASH diet had less total fat (p<0.01) and saturated fat (p<0.01), and more fiber (p<0.01) than the baseline diet. Protein intake was significantly higher only during DASH 1 (p<0.01). The WFPB diet was significantly lower in total and saturated fat and protein than baseline and both DASH weeks (p<0.01 for all). The WFPB diet was higher in fiber and carbohydrate (p<0.01 for both). There were no significant differences in nutrient intake between DASH 1 and DASH 2 (p>0.18 for all comparisons).
Table 2.
Mean Dietary Intake at Baseline and Changes During Each Study Phase
| Unit | Baseline Diet | DASH 1 | WFPB | DASH 2 | |
|---|---|---|---|---|---|
| Total energy | Kcal (95% CI) | 1846 (1660, 2031) | −264 (−436, −93) | −358 (−530, −187) | −350 (−526, −174) |
| Carbohydrates | grams (95% CI) | 186 (161, 212) | −5 (−33, 24) | 83 (55, 112) | −16 (−45, 13) |
| % of total kcal (95% CI) | 39.7 (36.8, 42.6) | 4.9 (0.9, 8.9) | 29.9 (25.8, 33.9) | 4.0, (0, 8.1) | |
| Total Protein | grams (95% CI) | 81 (71, 92) | 15 (4, 25) | −33 (−43, −22) | 7 (−4, 18) |
| % of total kcal (95% CI) | 18.0 (16.5, 19.6) | 5.8 (3.7, 8.0) | −6.8 (−9.0, −4.7) | 5.6 (3.4, 7.8) | |
| Vegetable Protein | grams (95% CI) | 26 (21, 31) | 5 (0, 10) | 22 (17, 28) | 4 (−1, 10) |
| Total Fat | grams (95% CI) | 88 (80, 97) | −31 (−40, −21) | −56 (−65, −46) | −32 (−41, −23) |
| % of total kcal (95% CI) | 41.6 (39.3, 44.0) | −10.1 (−13, −7.0) | −22.5 (−25.6, −19.4) | −8.9, (−12.1, −5.8) | |
| Saturated Fat | grams (95% CI) | 29 (26, 32) | −13 (−17, −10) | −23 (−27, −20) | −14 (−18, −11) |
| % of total kcal (95% CI) | 13.6 (12.5, 14.6) | −5.0 (−6.3, −3.7) | −10.3 (−11.7, −9.0) | −4.8 (−6.2, −3.5) | |
| Fiber | g/1000kcal (95% CI) | 11 (9, 13) | 9 (7, 10) | 21 (19, 23) | 9, (7, 11) |
| Sodium | mg (95% CI) | 3699 (3181, 4218) | −1644 (−2297, −992) | −1413 (−2066, −761) | −1784 (−2451, −1117) |
| Dietary Cholesterol | mg (95% CI) | 380 (325, 435) | −84 (−148, −21) | −379, (−442, −315) | −96 (−161, −31) |
Bolded values have p value of <0.05 when compared to baseline
Figure 3.
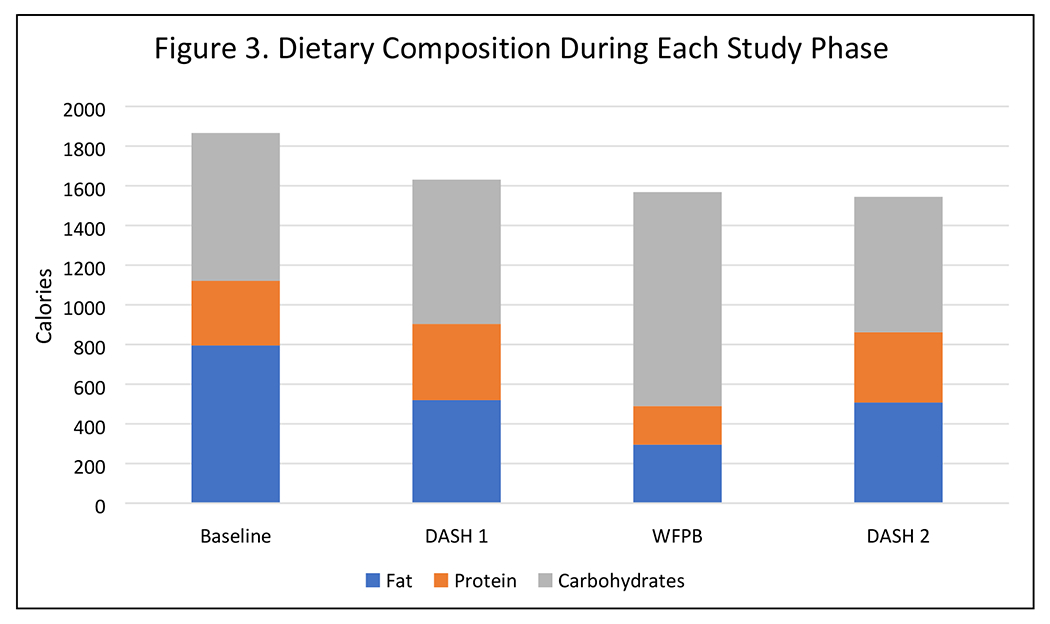
Dietary Composition During Each Study Phase
3.2. Insulin Changes
By the end of the DASH 1 phase, total daily insulin requirements had dropped 24% (p<0.01) compared to baseline (Table 3 and Figure 4). By the end of the WFPB phase, total daily insulin requirements had dropped 39% (p<0.01) compared to baseline. Upon resumption of the DASH diet, insulin dosing increased 15% from the end of the WFPB week, though the difference between WFPB and DASH 2 was not statistically significant (p=0.07).
Table 3.
Geometric Mean Insulin and Glucose Outcomes at Baseline and Relative Changes During Diet Phases
| Baseline | DASH1 v Baseline | WFPB v Baseline | DASH2 v Baseline | WFPB v DASH1 | WFPB v DASH2 | DASH2 v DASH1 | |
|---|---|---|---|---|---|---|---|
| Daily Insulin Dose, units | 74.0 (49.8, 110) | 0.76 (0.66, 0.87) | 0.61 (0.53, 0.71) | 0.7 (0.61, 0.82) | 0.81 (0.7, 0.94) | 0.87 (0.75, 1.01) | 0.93 (0.8, 1.08) |
| Average 24hr Blood Glucose, mg/dL | 155 (138, 173) | 0.76 (0.66, 0.87) | 0.78 (0.67, 0.9) | 0.76 (0.65, 0.87) | 1.03 (0.89, 1.18) | 1.03 (0.88, 1.19) | 1 (0.87, 1.15) |
| Fasting Blood Glucose, mg/dL | 165 (151, 180) | 0.85 (0.77, 0.94) | 0.71 (0.64, 0.78) | 0.81 (0.73, 0.9) | 0.83 (0.75, 0.92) | 0.87 (0.79, 0.97) | 0.95 (0.85, 1.05) |
| Insulin Sensitivity Index | 1.62 (0.95, 2.76) | 1.17 (0.95, 1.44) | 1.38 (1.11, 1.71) | 1.04 (0.84, 1.29) | 1.18 (0.96, 1.45) | 1.33 (1.07, 1.65) | 0.89 (0.73, 1.09) |
| HOMA-IR | 7.52 (4.34, 13.01) | 0.7 (0.54, 0.91) | 0.51 (0.39, 0.68) | 0.72 (0.54, 0.95) | 0.74 (0.56, 0.96) | 0.72 (0.54, 0.96) | 1.02 (0.77, 1.37) |
| Urinary Glucose, mg/dL | 16.5 (6.5, 41.7) | 0.11 (0.04, 0.31) | 0.08 (0.03, 0.24) | 0.19 (0.06, 0.57) | 0.76 (0.26, 2.19) | 0.43 (0.14, 1.31) | 1.75 (0.58, 5.2) |
p<0.05 shown in bold. Numbers in parentheses are 95% confidence intervals.
Figure 4. Relative Daily Insulin Usage During Four Study Phases.
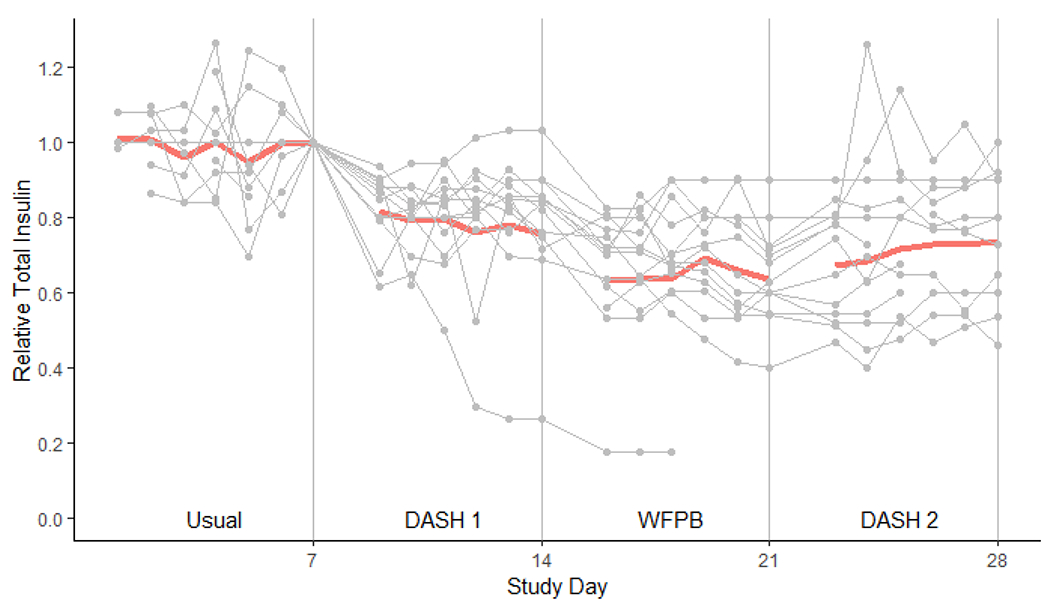
Bold line denotes the geometric mean total daily insulin usage relative to the insulin dose at the end of the baseline week. Light gray lines with circles are trajectories of each individual participant. 1st day of each diet is omitted due to non-dietary influence of the OGTT.
Average daily blood sugar was 22-24% lower across dietary phases compared to baseline (p<0.01 for all, Table 3). The WFPB resulted in the lowest fasting blood sugar, which was significant when compared to baseline (p<0.01), DASH 1 (p<0.01), and DASH 2 (p=0.01).
HOMA-IR decreased by 30.0% during DASH 1 (p<0.01, Table 3) and 49% during the WFPB diet (p<0.01). HOMA-IR subsequently increased during DASH 2 but remained 28% lower than baseline (p=0.02). The insulin sensitivity index was 17% higher at the end of the first DASH diet (p=0.14) and was 38% higher at the end of the WFPB diet (p<0.01), but decreased to near baseline by the end of DASH 2. Urinary glucose decreased across all three phases (p<0.01 for all).
3.3. Cardiometabolic Markers
Weight decreased during each dietary phase, with a 3% lower weight at the end of the third week compared to baseline (p <0.01, Table 4). Total, HDL, and LDL cholesterol all reached their nadir at the end of the WFPB week and total and LDL cholesterol significantly increased upon returning to the DASH diet (p<0.01 for both). Systolic and diastolic blood pressure did not significantly change after DASH 1 or WFPB compared to baseline, but systolic BP was lower after DASH 2 (p<0.01). hsCRP was lower during all three dietary phases compared to baseline, but only significantly so at its nadir after the WFPB week (42% reduction, p=0.01).
Table 4.
Geometric Mean Cardiometabolic Outcomes at Baseline and Relative Changes During Diet Phases
| Baseline | DASH1 v Baseline | WFPB v Baseline | DASH2 v Baseline | WFPB v DASH1 | WFPB v DASH2 | DASH2 v DASH1 | |
|---|---|---|---|---|---|---|---|
| Total Cholesterol, mg/dL | 156 (138, 176) | 0.96 (0.9, 1.02) | 0.82 (0.77, 0.88) | 0.9 (0.84, 0.96) | 0.86 (0.81, 0.92) | 0.91 (0.86, 0.97) | 0.94 (0.88, 1) |
| Triglycerides, mg/dL | 124 (98, 158) | 1.04 (0.94, 1.15) | 0.98 (0.88, 1.09) | 0.97 (0.87, 1.08) | 0.94 (0.85, 1.04) | 1.01 (0.91, 1.12) | 0.93 (0.84, 1.04) |
| HDL Cholesterol, mg/dL | 43 (37, 50) | 0.98 (0.92, 1.03) | 0.92 (0.87, 0.98) | 0.94 (0.88, 1) | 0.95 (0.89, 1) | 0.98 (0.93, 1.04) | 0.96 (0.91, 1.02) |
| LDL Cholesterol, mg/dL | 82 (64, 105) | 0.89 (0.79, 1.01) | 0.69 (0.61, 0.78) | 0.86 (0.75, 0.97) | 0.78 (0.69, 0.88) | 0.81 (0.71, 0.92) | 0.96 (0.85, 1.09) |
| Weight, kg | 95.3 (85.5, 106.2) | 0.98 (0.98, 0.99) | 0.97 (0.97, 0.98) | 0.97 (0.96, 0.98) | 0.99 (0.98, 1) | 1 (1, 1.01) | 0.99 (0.98, 0.99) |
| Systolic BP, mmHg | 130 (124, 137) | 0.99 (0.96, 1.02) | 0.98 (0.95, 1.01) | 0.96 (0.92, 0.99) | 0.99 (0.96, 1.03) | 1.03 (0.99, 1.06) | 0.97 (0.94, 1) |
| Diastolic BP, mmHg | 69 (64, 74) | 1.03 (0.97, 1.1) | 0.96 (0.9, 1.03) | 0.97 (0.91, 1.04) | 0.94 (0.88, 1) | 0.99 (0.93, 1.06) | 0.95 (0.89, 1.01) |
| hsCRP, mg/L | 2.3 (1.3,4.1) | 0.84 (0.56, 1.26) | 0.58 (0.38, 0.88) | 0.85 (0.55, 1.3) | 0.69 (0.45, 1.05) | 0.68 (0.44, 1.05) | 1.01 (0.66, 1.55) |
| Adiponectin, ng/mL | 3014 (2345, 3870) | 0.99 (0.8, 1.23) | 0.92 (0.74, 1.14) | 1.08 (0.86, 1.35) | 0.93 (0.75, 1.15) | 0.85 (0.68, 1.06) | 1.09 (0.87, 1.36) |
| Leptin, ng/mL | 33 (18, 62) | 0.77 (0.58, 1.01) | 0.72 (0.55, 0.96) | 0.87 (0.66, 1.17) | 0.94 (0.72, 1.24) | 0.83 (0.62, 1.1) | 1.14 (0.86, 1.51) |
p<0.05 shown in bold. Numbers in parentheses are 95% confidence intervals.
There were very few adverse events. One participant had a robust blood sugar response and had 3 episodes of hypoglycemia that resolved with snacks and/or glucose tablets at home.
3.4. Adipokines
Leptin was lower during all three dietary phases compared to baseline, but only significantly so at its nadir after the WFPB week (28% reduction, p=0.03), before rebounding at the end of DASH 2. There was insufficient evidence of any changes in Adiponectin (p > 0.15 for all).
4.0. Discussion
Both a DASH diet and WFPB diet, consumed without mandated calorie or portion restriction, result in large, rapid reductions in insulin requirements among community dwelling participants with insulin-treated type 2 diabetes. Within two weeks, participants were taking 39% less insulin while maintaining significantly lower blood glucose. Simultaneously, significant cardiometabolic improvements were observed in total and LDL cholesterol, leptin, weight, and hsCRP. While it is not possible to strictly isolate the effects of the DASH diet and WFPB diet in this study due to carryover effects from week to week, a pattern emerged. For most outcomes, initial benefits resulting from a DASH diet became significantly greater after switching to a WFPB diet for 7 days and then these benefits regressed toward baseline upon resumption of the DASH diet.
Participants in this study had more advanced diabetes than subjects had in many previous nutrition interventions. Other plant-based dietary interventions that have reported insulin adjustments include a study conducted in a metabolic ward and a study of individuals in a residential health program. Anderson et al. found that lean men in a metabolic ward who consumed a weight-maintaining high-carbohydrate, high fiber diet, reduced their insulin from 26 units/day to 11 units/day, on average, over the course of 16 days[23]. Men with higher insulin requirements were more refractory to the effects of dietary intervention. Separately, Barnard et al. published an analysis from the Pritikin program, a residential program utilizing a plant-based diet and exercise[24]. Patients more than 10% above their desirable body weight were placed on calorie restriction (900 kcal/day), while others were placed on an ad libitum plant-based diet. Of 60 individuals with type 2 diabetes, 17 were on insulin, with a range of doses from 14 to 75 units/day. After 3 weeks, 13 of the 17 individuals were off insulin entirely while fasting blood sugar improved. More recently, a randomized controlled trial using an ad libitum vegan diet found significant improvements in multiple outcomes, but excluded individuals who had been on insulin for more than 5 years[11]. Only 22% of the randomized controlled trial participants were on insulin and changes in insulin dosing were not reported. Given the more advanced nature of our participants’ diabetes, both the rapidity and scale of changes observed are notable.
Both a DASH diet and a WFPB show benefit. The design of this study does not permit a strict comparison of the two diets because there was no wash out period and there were carryover effects from one diet week to the next. Despite these limitations, the trajectory of most outcomes is similar: There were improvements during the DASH 1 week, additional improvements during the WFPB week, and then a regression toward baseline during the DASH 2 diet. This suggests that the DASH diet was less efficacious than the WFPB diet among most outcomes studied. Interestingly, most outcomes after the DASH 2 week were similar to the outcomes at the end of DASH 1. This suggests that a) many of these outcomes change very quickly and b) participants adhered to the DASH diet similarly well in both DASH weeks, despite making large changes during the WFPB diet week in between.
The benefits seen in this study are likely related to many mechanisms working simultaneously. During all diet weeks, participants reported consuming fewer calories, which is consistent with the observed weight loss of 3% of total body weight by the end of the study. Compared to baseline, there was a 13%, 18%, and 18% reduction in calorie intake during the DASH 1, WFPB, and DASH 2 weeks, respectively, which was associated with a 24%, 39%, and 30% reduction in insulin requirements. However, calorie reduction and weight loss are not the sole contributing factors resulting in the observed changes. During the final DASH 2 week, participants continued consuming fewer calories, resulting in additional weight loss, and yet most outcomes regressed back towards baseline.
Comprehensive changes in nutrient intake are likely to be contributing as well. Fiber intake has consistently been found to benefit both glycemic control and cardiovascular risk factors[25, 26]. In addition, increased fat intake can lead to increased intramyocellular lipids and decreased insulin sensitivity[15, 16, 27]. Saturated fat appears to be particularly deleterious[28]. Early observations, published case series and several controlled interventions[11, 23, 24, 29–36] have found that diets higher in carbohydrate and lower in fat can offer significant benefits for glycemic control and related comorbidities. The DASH diet results in moderate changes in the intake of these nutrients, and the WFPB results in even larger changes, which appears to mirror the difference in clinical efficacy observed in this study.
Changes in cardiometabolic risk factors were large. Statistically and clinically significant decreases in total and LDL cholesterol were perhaps even more notable given that 73% of the participants were already on cholesterol-lowering medication at baseline. Similarly, inflammation, as assessed by hsCRP, was 42% lower by the end of the WFPB week. A reduction in inflammation is another mechanism by which both cardiovascular risk and glycemic control improve[37]. Given that the majority of individuals with diabetes die from cardiovascular disease, this is a welcome effect of diets that simultaneously offer large and rapid benefits for glycemic control.
Changes in leptin, which is stimulated by insulin and affects appetite and other processes[38], were consistent with other observed changes in this study. Hyperleptinemia reflects a state of leptin resistance and is implicated in obesity. Our findings are consistent with previous studies which have found that energy restriction[39], low-fat[40], and low-protein[41] dietary intakes can lower leptin levels, reflecting reduced leptin resistance. Adiponectin levels did not significantly change during this study, which may reflect the fact that adiponectin does not change as rapidly in response to acute dietary changes compared to related outcomes[42].
There are numerous strengths and limitations of this study. The study duration only allows us to characterize short-term changes in outcomes. Further, because there were carryover effects from week to week and there were no washout periods, the effects of the two dietary interventions cannot be isolated. Generalizability is reduced in our study due to the smaller sample size, particularly the relatively advanced nature of our participants’ diabetes. It is possible that individuals with more recently diagnosed diabetes, who require no or less insulin, respond differently than what is observed here. It would be reasonable to hypothesize that they may have an even more robust response.
Generalizability is also limited by the fact that we provided prepared meals. Most patients will not achieve the abrupt, large dietary changes implemented here, which were made more convenient by having prepared meals available. Nonetheless, some patients may have sufficient motivation to make large changes quickly, particularly when supported with dietary counseling. A wide array of resources is available to help motivated individuals quickly adopt a DASH diet or a WFPB diet. Our goal was to characterize the acute changes in insulin requirements from dietary patterns consumed as intended, not dietary patterns that were only partially implemented. As a result, a strength of our study was the large dietary changes achieved, which were done without mandated calorie or portion restriction and were not confounded by mandated changes in physical activity or other lifestyle changes. Our participants lived in the community and conducted their lives as normal during their participation, which may allow for greater generalizability than previous interventions that have required participants to be housed in a residential program or metabolic ward.
In conclusion, this study demonstrates that adopting a DASH diet or a WFPB diet can result in large and rapid changes in insulin requirements, insulin resistance, cholesterol, and inflammation among individuals with insulin-dependent type 2 diabetes, with greater dietary changes producing greater benefits. These findings are relevant for counseling motivated patients who may wish to adopt a plant-based diet and need guidance regarding medication deprescribing. This study also demonstrates that patients with more advanced type 2 diabetes may still realize large, comprehensive, and rapid benefits related to glycemic control, medication requirements, and cardiovascular risk factors from dietary change. These findings have important implications not only for individual health risks but also cost of care. Additional study is warranted to assess sustainability of behavioral changes and durability of outcomes over time.
Funding
Funding support was provided by the Highland Hospital Foundation, with donations made to the foundation by the Ladybug Foundation and the T. Colin Campbell Center for Nutrition Studies and multiple individual donors. Additional support was provided by NIH DK124619. The study funders were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Abbreviations:
- CGM
Continuous glucose monitor
- DASH
Dietary Approaches to Stop Hypertension
- WFPB
Whole-food, plant-based
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
TMC receives royalties from general interest books about plant-based nutrition (Benbella Books and Penguin Random House). In addition, he has received income from a medical practice focused on lifestyle medicine (Thomas Campbell, MD PLLC). EKC has no conflicts other than those of her spouse (TMC). SDW has received consulting honoraria from Medtronic Diabetes and Ascencia Diabetes. None of the other authors declare any conflicts.
Data Availability
The data underlying this article will be made available by request at www.figshare.com. Email Thomas_campbell@urmc.rochester.edu for access.
References
- [1].Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL (2018) Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. Jama 319(16): 1723–1725. 10.1001/jama.2018.3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention (2022) Prevalence of Both Diagnosed and Undiagnosed Diabetes. Available from https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html. Accessed October 13, 2022 2022
- [3].Evert AB, Dennison M, Gardner CD, et al. (2019) Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes care 42(5): 731–754. 10.2337/dci19-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].United States. Department of Health and Human Services., United States. Department of Agriculture., United States. Dietary Guidelines Advisory Committee. (2020) Dietary guidelines for Americans, 2020-2025. 9th Edition. In: HHS publication, Washington, D.C. [Google Scholar]
- [5].Appel LJ, Moore TJ, Obarzanek E, et al. (1997) A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 336(16): 1117–1124. 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- [6].Berkow SE, Barnard ND (2005) Blood pressure regulation and vegetarian diets. Nutrition reviews 63(1): 1–8 [DOI] [PubMed] [Google Scholar]
- [7].Appel LJ, Sacks FM, Carey VJ, et al. (2005) Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. Jama 294(19): 2455–2464. 10.1001/jama.294.19.2455 [DOI] [PubMed] [Google Scholar]
- [8].Macknin M, Kong T, Weier A, et al. (2015) Plant-Based, No-Added-Fat or American Heart Association Diets: Impact on Cardiovascular Risk in Obese Children with Hypercholesterolemia and Their Parents. J Pediatr. 10.1016/j.jpeds.2014.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wright N, Wilson L, Smith M, Duncan B, McHugh P (2017) The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes 7(3): e256. 10.1038/nutd.2017.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Azadbakht L, Fard NR, Karimi M, et al. (2011) Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes care 34(1): 55–57. 10.2337/dc10-0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barnard ND, Cohen J, Jenkins DJ, et al. (2006) A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes care 29(8): 1777–1783. 29/August/1777 [pii] 10.2337/dc06-0606 [DOI] [PubMed] [Google Scholar]
- [12].Ornish D, Scherwitz LW, Billings JH, et al. (1998) Intensive lifestyle changes for reversal of coronary heart disease. JAMA : the journal of the American Medical Association 280(23): 2001–2007 [DOI] [PubMed] [Google Scholar]
- [13].Esselstyn CB Jr., Ellis SG, Medendorp SV, Crowe TD (1995) A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. Journal of Family Practice 41(6): 560–568 [PubMed] [Google Scholar]
- [14].Wang L, Li X, Wang Z, et al. (2021) Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999-2018. Jama. 10.1001/jama.2021.9883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bachmann OP, Dahl DB, Brechtel K, et al. (2001) Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes 50(11): 2579–2584 [DOI] [PubMed] [Google Scholar]
- [16].Lee S, Boesch C, Kuk JL, Arslanian S (2013) Effects of an overnight intravenous lipid infusion on intramyocellular lipid content and insulin sensitivity in African-American versus Caucasian adolescents. Metabolism 62(3): 417–423. 10.1016/j.metabol.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lara-Castro C, Newcomer BR, Rowell J, et al. (2008) Effects of short-term very low-calorie diet on intramyocellular lipid and insulin sensitivity in nondiabetic and type 2 diabetic subjects. Metabolism 57(1): 1–8. 10.1016/j.metabol.2007.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bradley Michael D., Arnold Matthew E., Biskup Bradley G., et al. (2022) Medication Deprescribing Among Patients With Type 2 Diabetes: A Qualitative Case Series of Lifestyle Medicine Practitioner Protocols. Clinical Diabetes. 10.2337/cd22-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wirtz V, Knox R, Cao C, Mehrtash H, Posner N, McClenathan J (April 2016) Insulin Market Profile. In, Health Action International [Google Scholar]
- [20].DASH Eating Plan. Available from https://www.nhlbi.nih.gov/health-topics/dash-eating-plan. Accessed February 18, 2019
- [21].Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes care 22(9): 1462–1470. 10.2337/diacare.22.9.1462 [DOI] [PubMed] [Google Scholar]
- [22].DeFronzo RA, Matsuda M (2010) Reduced time points to calculate the composite index. Diabetes care 33(7): e93. 10.2337/dc10-0646 [DOI] [PubMed] [Google Scholar]
- [23].Anderson JW, Ward K (1979) High-carbohydrate, high-fiber diets for insulin-treated men with diabetes mellitus. The American journal of clinical nutrition 32(11): 2312–2321 [DOI] [PubMed] [Google Scholar]
- [24].Barnard RJ, Lattimore L, Holly RG, Cherny S, Pritikin N (1982) Response of non-insulin-dependent diabetic patients to an intensive program of diet and exercise. Diabetes care 5(4): 370–374 [DOI] [PubMed] [Google Scholar]
- [25].Reynolds AN, Akerman AP, Mann J (2020) Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med 17(3): e1003053. 10.1371/journal.pmed.1003053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fu L, Zhang G, Qian S, Zhang Q, Tan M (2022) Associations between dietary fiber intake and cardiovascular risk factors: An umbrella review of meta-analyses of randomized controlled trials. Front Nutr 9: 972399. 10.3389/fnut.2022.972399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kitessa SM, Abeywardena MY (2016) Lipid-Induced Insulin Resistance in Skeletal Muscle: The Chase for the Culprit Goes from Total Intramuscular Fat to Lipid Intermediates, and Finally to Species of Lipid Intermediates. Nutrients 8(8). 10.3390/nu8080466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Riccardi G, Giacco R, Rivellese AA (2004) Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr 23(4): 447–456. 10.1016/j.clnu.2004.02.006 [DOI] [PubMed] [Google Scholar]
- [29].Himsworth H (1935) Diet and the incidence of diabetes mellitus. Clinical Science 2: 117–148 [Google Scholar]
- [30].Rabinowitch IM (1935) Effects of the High Carbohydrate-Low Calorie Diet Upon Carbohydrate Tolerance in Diabetes Mellitus. Canadian Medical Association journal 33(2): 136–144 [PMC free article] [PubMed] [Google Scholar]
- [31].Kempner W, Peschel RL, Schlayer C (1958) Effect of rice diet on diabetes mellitus associated with vascular disease. Postgraduate medicine 24(4): 359–371 [DOI] [PubMed] [Google Scholar]
- [32].Stone DB, Connor WE (1963) The prolonged effects of a low cholesterol, high carbohydrate diet upon the serum lipids in diabetic patients. Diabetes 12: 127–132 [DOI] [PubMed] [Google Scholar]
- [33].Simpson HC, Simpson RW, Lousley S, et al. (1981) A high carbohydrate leguminous fibre diet improves all aspects of diabetic control. Lancet 1(8210): 1–5 [DOI] [PubMed] [Google Scholar]
- [34].Barnard RJ, Massey MR, Cherny S, O’Brien LT, Pritikin N (1983) Long-term use of a high-complex-carbohydrate, high-fiber, low-fat diet and exercise in the treatment of NIDDM patients. Diabetes care 6(3): 268–273 [DOI] [PubMed] [Google Scholar]
- [35].Barnard ND, Katcher HI, Jenkins DJ, Cohen J, Turner-McGrievy G (2009) Vegetarian and vegan diets in type 2 diabetes management. Nutrition reviews 67(5): 255–263. NURE198 [pii] 10.1111/j.1753-4887.2009.00198.x [DOI] [PubMed] [Google Scholar]
- [36].Lee YM, Kim SA, Lee IK, et al. (2016) Effect of a Brown Rice Based Vegan Diet and Conventional Diabetic Diet on Glycemic Control of Patients with Type 2 Diabetes: A 12-Week Randomized Clinical Trial. PloS one 11(6): e0155918. 10.1371/journal.pone.0155918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Weber KS, Nowotny B, Strassburger K, et al. (2015) The Role of Markers of Low-Grade Inflammation for the Early Time Course of Glycemic Control, Glucose Disappearance Rate, and beta-Cell Function in Recently Diagnosed Type 1 and Type 2 Diabetes. Diabetes care 38(9): 1758–1767. 10.2337/dc15-0169 [DOI] [PubMed] [Google Scholar]
- [38].Zhao S, Kusminski CM, Elmquist JK, Scherer PE (2020) Leptin: Less Is More. Diabetes 69(5): 823–829. 10.2337/dbi19-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mendoza-Herrera K, Florio AA, Moore M, et al. (2021) The Leptin System and Diet: A Mini Review of the Current Evidence. Front Endocrinol (Lausanne) 12: 749050. 10.3389/fendo.2021.749050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Weigle DS, Cummings DE, Newby PD, et al. (2003) Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J Clin Endocrinol Metab 88(4): 1577–1586. 10.1210/jc.2002-021262 [DOI] [PubMed] [Google Scholar]
- [41].Kozlowska L, Rosolowska-Huszcz D, Rydzewski A (2004) Low protein diet causes a decrease in serum concentrations of leptin and tumour necrosis factor-alpha in patients with conservatively treated chronic renal failure. Nephrology (Carlton) 9(5): 319–324. 10.1111/j.1440-1797.2004.00327.x [DOI] [PubMed] [Google Scholar]
- [42].Merl V, Peters A, Oltmanns KM, et al. (2005) Serum adiponectin concentrations during a 72-hour fast in over- and normal-weight humans. Int J Obes (Lond) 29(8): 998–1001. 10.1038/sj.ijo.0802971 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be made available by request at www.figshare.com. Email Thomas_campbell@urmc.rochester.edu for access.


