Although extensive investigation has clarified multiple aspects of pulmonary aspergillosis in immunocompromised patients, cutaneous aspergillosis occurs relatively less frequently and therefore remains poorly characterized. Previous reports have described cutaneous aspergillosis as either primary (2, 17, 25, 38) or secondary (15, 19) infection. Primary cutaneous aspergillosis usually involves sites of skin injury, namely, at or near intravenous access catheter sites, at sites of traumatic inoculation, and at sites associated with occlusive dressings, burns, or surgery. Secondary cutaneous lesions result either from contiguous extension to the skin from infected underlying structures or from widespread blood-borne seeding of the skin. Herein, we present a review of cutaneous aspergillosis among immunocompromised patient populations. With this review, we have attempted to better define risk factors and common clinical presentations, as well as to formulate a reasonable approach to the diagnosis and management of cutaneous aspergillosis.
HIV-RELATED CUTANEOUS ASPERGILLOSIS
Human immunodeficiency virus (HIV)-infected individuals infrequently develop cutaneous aspergillosis, with previous reports describing a total of 10 patients with primary cutaneous aspergillosis (21, 29, 30, 54, 57, 58, 63). In Table 1, we summarize the clinical features and outcomes of the 10 previously reported patients with primary cutaneous aspergillosis. Interestingly, to the best of our knowledge, previous reports have not documented secondary cutaneous aspergillosis among HIV-infected patients.
TABLE 1.
Primary cutaneous aspergillosis in HIV-infected patientsa
| Reference | Neutropenia | CD4 count (cells/mm3) | Lesion morphology | Species | Classification | Treatment | Treatment response | Patient outcome |
|---|---|---|---|---|---|---|---|---|
| 29 | ND | ND | ND | ND | ND | None | Not applicable | Not applicable (autopsy specimen) |
| 30 | No | ND | Umbilicated papule | A. fumigatus | Catheter tape associated | Local, no antifungal | Some lesions involuted; one cutaneous biopsy site recrudesced in the week prior to death | Patient died 4 months later of CNS CMV, with 2-cm focus of pulmonary aspergillosis |
| 30 | ND | ND | Umbilicated papule | ND | Catheter tape associated | None (fluconazole) | Biopsy sites healed, no new lesions | Patient died several months later of disseminated MAC |
| 54 | No | 67 | Verrucous plaque with micropustules | A. fumigatus | Catheter associated | Amphotericin B at 0.6 mg/kg/day i.v. for 14 days and local wound care | Modest decrease in lesion size | Patient died 3 months later after gradual decline |
| 21 | No | 24 | Indurated erythema | A. fumigatus | Catheter associated | Itraconazole at 200 mg/day and catheter removal for 25 days, then replaced by amphotericin B at 1.0 mg/kg/day i.v. | Cutaneous infection progressively improved | Amphotericin B added 4 weeks later when pulmonary lesions cavitated; patient died 2 weeks later |
| 57 | No | 143 | Ulcer | A. glaucus | Trauma wound associated | Amphotericin B at 1.0 mg/kg/day i.v. for 4 days and surgical debridement, then itraconazole at 200 mg p.o. b.i.d. for 6 months | Lesion resolution | No subsequent aspergillosis |
| 58 | No | ND | Pruritic, exophytic lesion | A. fumigatus | Catheter, transparent tape associated | ND | ND | ND |
| 57 | Yes | 2 | Nodule | A. fumigatus | Catheter associated | Amphotericin B at 1.0 mg/kg/day i.v. for 19 days and surgical debridement, then skin graft | Lesion resolution | No subsequent aspergillosis |
| 63 | Yes | 15 | Nodule | A. fumigatus | Catheter tape associated | Itraconazole at 200 mg p.o. b.i.d. for 9 weeks | Lesion resolution | No subsequent aspergillosis |
| 63 | Yes | 0 | Nodule | A. fumigatus | Catheter tape associated | Itraconazole at 200 mg p.o. b.i.d. for 4 weeks | Lesion excised by biopsy, then possibly recrudesced off therapy | Patient died of wasting within 1 month of recrudescence of skin lesion |
Abbreviations: ND, no data; CNS CMV, central nervous system cytomegalovirus; MAC, M. avium complex; i.v., intravenously; p.o., orally; b.i.d., twice daily.
In a 1984 report of necropsy findings in AIDS patients, Hui and colleagues (29) described cutaneous aspergillosis in a 30-year-old Hispanic homosexual man who died from pulmonary failure caused by Pneumocystis carinii, cytomegalovirus, and acid-fast bacilli. The investigators did not provide details regarding the numbers or locations of lesions, the proximity of lesions to an intravenous catheter site, or neutrophil count. We presume that this patient had primary cutaneous aspergillosis because the investigators did not describe any evidence of disseminated aspergillosis.
In 1992, Hunt and colleagues (30) described two men who developed foci of cutaneous aspergillosis beneath an adhesive dressing near a central venous catheter site. The first patient developed several pruritic umbilicated papules that resembled molluscum contagiosum. Prior to the onset of the lesions, the patient had received doxorubicin, bleomycin, vincristine, ganciclovir, and fluconazole. Neutrophil counts ranged between 1.8 and 6.7 cells/mm3 with the use of granulocyte colony-stimulating factor during the 10 weeks of chemotherapy. Biopsy specimens showed fungal hyphae typical of Aspergillus species, and culture later confirmed Aspergillus fumigatus, but the patient did not receive antifungal therapy. Nonocclusive dressings and local wound care with hydrogen peroxide and bacitracin-polymyxin ointment resulted in the involution of some lesions. The patient died 4 months later from central nervous system cytomegalovirus infection; one of the cutaneous lesions had recrudesced in the week prior to death. An incidental 2-cm focus of pulmonary aspergillosis found at autopsy did not appear on premortem radiographs, and the investigators did not believe that it contributed to the patient’s death. The second patient described by Hunt and colleagues (30) developed two papular lesions that also resembled molluscum contagiosum. Biopsy revealed hyphae consistent with Aspergillus species. No evidence of disseminated aspergillosis was found, and no new lesions developed, even though the patient received treatment with fluconazole (at 200 mg/day), an agent without significant activity against Aspergillus species. This patient died several months later from disseminated Mycobacterium avium complex infection.
In 1995, Romero and Hunt (54) described a nonneutropenic patient with AIDS who presented with a slowly growing verrucous plaque and small pustules 4 cm inferior to a previous Hickman catheter insertion site. The investigators did not provide any details on the use of occlusive dressings. A skin biopsy specimen showed branching hyphae, and culture of the specimen grew A. fumigatus. Amphotericin B, given intravenously (0.6 mg/kg of body weight/day) for 2 weeks and continued as 30-mg doses (approximately 0.5 mg/kg/day) once weekly thereafter, resulted in undetailed diminution in the sizes of the lesions. The patient died of unclear causes after 3 months of general decline.
Also in 1995, Girmenia and colleagues (21) described catheter-associated cutaneous aspergillosis in an HIV-infected patient with acute lymphocytic leukemia. Following recovery from neutropenia, the patient developed an erythematous and indurated Hickman catheter exit site infection (use of occlusive dressings was not detailed). Blood drawn through the catheter, purulent catheter exit site secretions, and the catheter tip all grew A. fumigatus, whereas peripheral blood cultures repeatedly did not grow any fungal organisms. Despite removal of the Hickman catheter, prompt treatment with oral itraconazole (200 mg/day), improvement of the cutaneous infection, subsequent negative blood cultures, and improvement of the underlying malignancy, the infection in this patient progressed 1 month later to pulmonary aspergillosis with a rapidly fatal outcome. Thus, this case most likely represents primary cutaneous aspergillosis that disseminated to the lungs.
In 1997, Smith and Wallace (58) reported on a patient with AIDS who developed a cutaneous lesion under the transparent dressing of a venous catheter. Histologic examination of the lesion revealed suppurative granulomatous inflammation in the dermis with numerous branching hyphae within the follicular infundibulum. The patient was receiving radiation therapy for non-Hodgkin’s lymphoma of the ethmoid sinus, and his neutrophil count had ranged from 600 to 2,500 cells/mm3 over the 6 months prior to aspergillosis.
In a 1997 report of HIV-infected children with aspergillosis, Shetty and colleagues (57) described two patients who developed cutaneous aspergillosis in the absence of deep tissue invasion or dissemination. The first patient, a 14-year-old boy, developed a chronic, nonhealing ulcer on the right calf. The investigators did not describe the type of trauma or any wound dressing. A skin biopsy specimen revealed hyphal elements, and culture of the specimen grew Aspergillus glaucus. Treatment with surgical debridement and intravenous amphotericin B (1.0 mg/kg/day) for 4 days, followed by itraconazole at 200 mg orally twice daily for 6 months, clinically cured the ulcer. The second patient, a 10-year-old girl, presented with neutropenia and a tender nodule below an intravenous catheter exit site on her chest. The investigators did not provide any detail about the use of adhesive dressings. A skin biopsy specimen showed branching hyphae, and culture of the specimen grew A. fumigatus. The lesion responded to surgical debridement and 19 days of intravenous amphotericin B (1.0 mg/kg/day), although the wound subsequently required skin grafting.
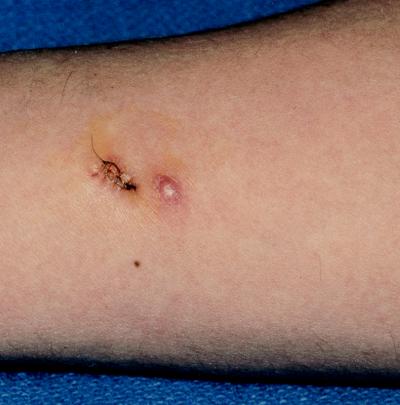
In our 1998 report, we have described two HIV-infected patients treated with itraconazole for primary cutaneous aspergillosis. Both of the patients had advanced AIDS and intermittent episodes of neutropenia that preceded the diagnosis of primary cutaneous aspergillosis. In addition, both patients developed nodular cutaneous Aspergillus lesions under an adhesive dressing near the exit site for an intravenous catheter, and neither had evidence of disseminated aspergillosis. One patient received itraconazole for 9 weeks and had complete resolution of the lesions by 4 weeks. The other patient received amphotericin B for 4 days as initial treatment after a skin biopsy had excised the nodule, followed by 4 weeks of itraconazole therapy (Fig. 1); a new lesion (probably aspergillosis) appeared less than 1 week after the discontinuation of itraconazole.
FIG. 1.
Nodular cutaneous aspergillosis in a patient with AIDS. The patient had two nodules on the right forearm that arose under an occlusive dressing distal to a previous peripherally inserted central catheter. One of the lesions had been excised by biopsy, and residual sutures are present. The biopsy specimen grew A. fumigatus.
NON-HIV-INFECTED POPULATIONS WITH CUTANEOUS ASPERGILLOSIS
Numerous reports have described primary or secondary cutaneous aspergillosis in an array of non-HIV-infected immunocompromised patients, including burn victims, neonates, individuals with cancer, and bone marrow and solid-organ transplant recipients (1, 2, 4, 6–9, 11, 20, 22–24, 26, 32–38, 43–46, 48–51, 53, 55, 56, 59, 60, 62, 66, 68). In addition, otherwise healthy hosts can develop cutaneous aspergillosis in surgical wounds (3, 8, 40), by traumatic inoculation (5, 12, 13, 27, 41, 42), or by exposure to high spore counts in occupations such as farming (10, 19, 61, 64), although such infections are rare. In general, burn victims, neonates, and solid-organ transplant recipients develop cutaneous inoculation after prolonged local skin injury, whereas bone marrow transplant recipients tend to develop secondary cutaneous aspergillosis lesions either from contiguous extension from infected structures underlying the skin, such as the paranasal sinuses, nasal cavity, or orbit, or from hematogenously disseminated embolic lesions. Cancer patients, particularly leukemia patients, develop both primary and secondary infections. The different classifications of both primary and secondary cutaneous aspergillosis infections are as follows. Primary infections include those at intravenous catheter sites (cutaneous exit sites and subcutaneous tunnels), infections associated with adhesives such as occlusive dressings and tape, and infections associated with burn wounds, surgical wounds, and trauma wounds. Secondary infections include those caused by direct extension and embolic lesions.
Burn victims.
The predisposition for burn victims to develop cutaneous aspergillosis likely involves physical cutaneous barrier disruption and depression of several host defense mechanisms, such as impaired or decreased phagocytosis, bacterial flora disturbances from the use of systemic antimicrobial agents, and hyperglycemia from hyperalimentation. Cutaneous aspergillosis usually involves those patients with burns that average 50 to 60% of the total body surface area and occurs 50 to 60 days after the burn, usually within 10 to 35 days (7, 56, 60). Importantly, the burned skin serves as a portal of entry for Aspergillus organisms (44). In one study, approximately 0.4% of burn wounds became cutaneously infected with Aspergillus organisms (4). Reports have implicated construction in the hospital area (8) and interruptions in procedures for servicing of air-conditioning ducts and filters (60) in approximately 60% of cases. For one patient without an implicated source of contamination, the patient had underlying diabetes in addition to the burn wounds (46). In the burn patient population, primary lesions may disseminate or cause a contiguous osteomyelitis by direct extension (7). A successful outcome in the burn patient involves treatment with intravenous and topical antifungal agents, surgical excision to the level of noninvaded viable tissue, and, in some instances, amputation of the affected limb (60).
Neonates.
Preterm neonates have an increased risk of developing fungal infections, presumably because of impaired phagocytic function. Reports have described primary cutaneous aspergillosis at 5 to 30 days after birth in preterm infants whose birth weight ranged from 800 to 1,500 g (23, 26, 48, 49, 55). These cases all involved mechanical impairment of the skin’s barrier function: tape adhesive (48), tape adhesive associated with a chest tube (23), occlusion under a pulse oximetry sensor (49), and prolonged placement in the supine position (55). Cutaneous aspergillosis in neonates has a range of lesions including papules, nodules, pustules, and ulcers. Most infected neonates received only medical treatment (55), and 50% survived the infection. Deaths caused by secondary disseminated aspergillosis have also occurred in neonates at 18 days (full-term infant) (1) and 32 days (55) after birth.
Cancer patients.
Reports in the literature have described cutaneous aspergillosis in more than 50 cancer patients. Although most of these patients had leukemia as the underlying oncologic diagnosis, reports have described other diseases, including aplastic anemia (2, 51, 66), astrocytoma (38), chronic granulomatous disease (14), and agranulocytosis treated with antithymocyte globulin (45). In greater than 85% of cancer-related cases, primary cutaneous aspergillosis was associated with intravenous catheters, arm boards, or tape securing arm boards (2, 9, 11, 17, 22, 25, 37, 38, 51, 68). Other associations have included breaks in the epithelium during insertion of a vaginal clotrimazole troche (53) and phlebotomy (34). Direct extension from the sinuses accounts for most cases of secondary cutaneous aspergillosis (15, 45, 66), but reports have also described emboli (20, 68) and inoculation by a percutaneous biopsy needle (62) as the source of secondary infection. In addition, Buescher and colleagues (9) reported on a patient with a Hickman catheter tunnel Aspergillus infection leading to thrombosis of subclavian (and other central) veins that required surgical resection and reconstruction for control of the infection. In another patient, a pulmonary aspergilloma invaded the left subclavian artery with subsequent development of a lesion on the left upper extremity (65). In general, cancer patients with cutaneous aspergillosis have received treatment with intravenous amphotericin B with or without adjunctive surgical debridement. On the basis of the outcomes presented in the literature reports, approximately 50% of cancer patients had no subsequent aspergillosis after therapy.
Bone marrow transplant recipients.
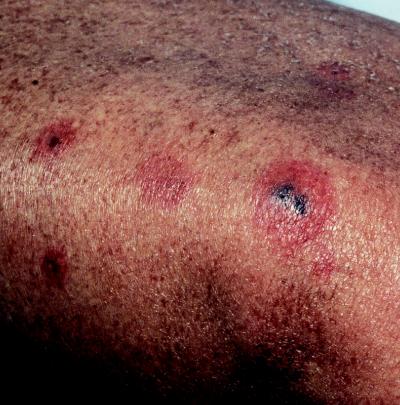
Cutaneous aspergillosis is a poorly described entity among bone marrow transplant recipients mainly because literature reports have focused on the more frequent and severe clinical scenario of pulmonary or disseminated infection. Nevertheless, existing information suggests that current or recent neutropenia is the common risk factor for cutaneous aspergillosis in bone marrow transplant recipients (31, 32, 65). Lesions are often multiple when disseminated infection presents with cutaneous lesions (Fig. 2). In one report, a myeloma patient developed primary cutaneous Aspergillus niger infection from minor trauma and subsequent repetitive pressure from a plaster cast used to stabilize a pathologic fracture (32). The investigators subsequently found fungal contamination in stockinette packages from the plaster room. In another report, an allogeneic bone marrow transplant recipient in a laminar airflow room developed grade III graft-versus-host disease and epidermolysis. Although the patient had remained in his laminar airflow room from the graft onward, he subsequently developed primary cutaneous aspergillosis that disseminated. The investigators speculate that the patient was probably infected when he left the sterile room for endoscopy (6).
FIG. 2.
Multiple cutaneous lesions on the leg of a bone marrow transplant recipient who had disseminated aspergillosis. Cultures of both a skin biopsy specimen and blood grew A. fumigatus.
Solid-organ transplant recipients.
Cutaneous aspergillosis among solid-organ transplant recipients usually occurs as primary infection directly in the surgical wound among liver or renal transplantation patients (33, 35, 43, 50) or as nodules near a site of a break in the integument that is different from the primary surgical transplantation wound, most frequently among cardiac transplant recipients (24, 28, 36, 59). Primary cutaneous aspergillosis in solid-organ transplant recipients generally occurs in the setting of a normal neutrophil count. Similar to the cases of neonatal aspergillosis, these cases of primary skin infection have diverse causes, including adhesive tape (24, 59), pressure sores from a shoe (28) or rigid brace (59), and venous access catheters (36, 59). Hospital contamination was documented in three liver transplant patients with wound (50) or pressure sore (59) infection. In general, treatment of these solid-organ transplant patients with cutaneous aspergillosis has combined surgical excision of the infected tissue with antifungal chemotherapy. When comparing the outcomes for these patients, the liver transplant recipients fared worse overall, with complications that included further wound necrosis (50), fatal pulmonary aspergillosis concurrent with wound infection (35), and fatal transplantation complications (59).
CLINICAL MANIFESTATIONS
The initial lesions of cutaneous aspergillosis may appear as macules, papules, nodules, or plaques. Pustules or lesions with purulent discharge generally have occurred among neonates (23, 25, 26, 49). For infections arising from arm board use or occlusive tape used to secure an access catheter, a hemorrhagic bulla may begin at the site of the skin trauma (25). Infections arising at the site of an intravenous catheter puncture typically begin with erythema and induration at the skin puncture site and progress to necrosis that extends radially from the initial focus (2). A patient with primary cutaneous aspergillosis arising in a wound generally presents with significant fever, a change in the character of the wound surface, swelling, induration, and tenderness (7, 8, 42, 44, 46, 60). The pace of the infection varies from indolent to fulminant, and the mortality rate is approximately 30 to 75%. Patients with primary cutaneous aspergillosis appear to present with significantly less necrosis and systemic toxicity than wound zygomycosis (18, 39, 47, 67).
Non-HIV-related secondary cutaneous aspergillosis lesions initially appear as erythematous macules or papules that evolve to hemorrhagic bullae or ulcerative nodules (65, 68). With time, they often develop eschar. Importantly, secondary lesions can resemble ecthyma gangrenosum, traditionally caused by Pseudomonas aeruginosa (27). Embolic lesions occur in approximately 11% of patients with disseminated aspergillosis (65), similar to the 10 to 13% incidence of skin lesions seen among patients with disseminated candidiasis (52). The angiotropic nature of the Aspergillus organism helps to explain the usual lesion morphology in secondary dissemination to the skin.
MICROBIOLOGY
Among patients with HIV-related cutaneous aspergillosis, seven patients had A. fumigatus infection, one had A. glaucus infection, and two had aspergillosis demonstrated by histopathology alone. The reason for this high proportion of primary A. fumigatus isolates is not known. In contrast, among cases of cutaneous aspergillosis that did not involve HIV-infected or burn patients, the following organisms accounted for the indicated proportion of cases: Aspergillus flavus, 44%; A. fumigatus, 26%; Aspergillus spp. (the species of Aspergillus was not determined), 10%; Aspergillus terreus, 6% (13, 38, 45, 61); Aspergillus niger, 6% (10, 25, 32, 38, 55); A. glaucus, 4% (15, 66, 68); Aspergillus chevalieri, 3% (42); and Aspergillus ustus, 1% (59). The proportions of species differed by at-risk populations: A. flavus accounted for approximately one-half of non-burn-related primary infection, whereas A. flavus and A. fumigatus each accounted for approximately one-third of secondary or metastatic skin lesions. Determination of the species causing aspergillosis did not guide therapy in any of the reports reviewed.
LABORATORY DIAGNOSIS
In some instances, a presumptive diagnosis of primary cutaneous aspergillosis can be made immediately by staining the roof of a bulla (25) or examining a potassium hydroxide preparation of a biopsy specimen. Generally, however, the diagnosis of most primary and secondary Aspergillus infections requires biopsy of a skin lesion taken for both culture and histopathology. A skin biopsy specimen for a suspected fungal lesion should be taken from the center of the lesion and should reach the subcutaneous fat because Aspergillus tends to invade blood vessels of the dermis and subcutis, resulting in an ischemic cone above it. If a single biopsy specimen is taken, the biopsy specimen should be divided and one half should be sent in saline (or a similar transport medium) to the microbiology laboratory and the other half should be sent in formalin to the pathology laboratory.
In the microbiology laboratory, fungal hyphal structures can be stained directly from tissue specimens with the whitening agent calcofluor, which will fluoresce when exposed to UV light. The specimen should be minced and plated on medium specific for the recovery of yeast (e.g., bromcresol green), mold (e.g., potato dextrose agar), and dermatophytes (e.g., Mycobiotic) and should be held for 6 weeks. The remaining specimen should be used for the recovery of bacteria by plating homogenized specimen on blood agar for 48 h and incubating the specimen in thioglycolate broth for 7 days. Fungal isolates from culture media are identified on the basis of colony morphology, color, and sporulation. Catheter tip cultures have confirmed diagnoses made by culture of biopsied lesions or catheter drainage but are relatively insensitive for the initial diagnosis of cutaneous aspergillosis. Unfortunately, blood cultures have low sensitivity, even for Aspergillus endocarditis (16).
In the pathology laboratory, histopathologic examination with routine stains, such as hematoxylin and eosin, variably demonstrates Aspergillus hyphae, sometimes staining the nucleus or cytoplasm of the fungus or revealing the cell wall by a negatively staining shadow. The cellular infiltrate of Aspergillus lesions is not distinct. The Gomori methenamine silver stain, however, clearly and reliably detects hyphae, since the hyphal cell wall stains black, whereas the tissue background stains green. Aspergillus hyphae should have acute-angle branching and frequent septations. The appearance of hyphae with acute-angle branching alone, however, is not specific enough to distinguish Aspergillus hyphae from other those of other medically important filamentous molds such as Pseudallescheria boydii and Fusarium spp. In addition, with certain angles of specimen sectioning, acute-angle Aspergillus branches may appear as right-angle branches, thus resembling the right-angle branching of pauciseptated hyphae of zygomycete-like species. Use of antifungal agents by the patient will alter the morphology of hyphae. The fruiting bodies of Aspergillus (ascospores with conidia) are rarely observed in tissue samples unless there is an overwhelming burden of organisms at the site (46). Thus, although a tentative diagnosis of aspergillosis can be made by histopathologic Gomori methenamine silver staining, a definitive diagnosis requires identification of Aspergillus that has grown in culture.
MANAGEMENT
If aspergillosis is diagnosed, subsequent efforts should be directed at determining whether the patient has a primary infection or whether there is secondary dissemination from a primary focus such as the lung. The workup should begin with an assessment of risk factors (neutropenia, recent or concurrent presence of a central venous access catheter, the presence of adhesive or occlusive dressings, or other local skin injury). Special attention to pulmonary symptoms and/or signs may determine whether an evaluation for pulmonary aspergillosis is needed. If there are indications of pulmonary infection, a computed tomographic scan of the chest would be the best first diagnostic test. If that test is abnormal, evaluation by bronchoscopy should follow. The detection of antigen or antibody in serum has not been studied for cutaneous aspergillosis, and the sensitivities of these tests are anticipated to be sufficiently low that there will be no immediate role for their clinical application.
The treatment approach to cutaneous aspergillosis generally depends on the underlying status of the patient. For example, cutaneous aspergillosis in burn victims occurs as primary disease, treated principally with a surgical approach that may involve amputation (7, 60). Conversely, the approach in premature neonates with cutaneous aspergillosis, who do not tolerate skin surgery well, is antifungal chemotherapy without surgery (55). Cancer and bone marrow transplant recipients have received a variety of medical and surgical treatments that have included immunomodulating granulocyte transfusions in one case (17) and skin grafting in other cases (2). With a combination of organism-directed medical therapy and surgical excision, most HIV-related cases of primary cutaneous aspergillosis lesions did not recur. The risk of dissemination with either tape-associated or catheter-related primary cutaneous aspergillosis cases among HIV-infected patients appeared low but did occur in two of nine patients.
Itraconazole has been used for the treatment of cutaneous aspergillosis in four patients with HIV-related cases of aspergillosis (Table 1) (21, 57, 63) and two immunocompromised patients not infected with HIV (36, 62). Successful first-line itraconazole treatment courses occurred for three patients with nodular primary cutaneous aspergillosis: a heart transplant recipient who had nodules located near a catheter site (although amphotericin B was used for several days prior to the use of itraconazole) (36) and two HIV-infected patients with tape- and catheter-related cases of infection (63). Itraconazole was also successfully used after surgical debridement of a chronic ulcer for a pediatric HIV-infected patient (57). Itraconazole, however, failed when it was used as first-line therapy for an HIV-related catheter infection that started as a primary infection but that had already embolized to the pulmonary tree by the time that itraconazole was started (21). Furthermore, itraconazole used as maintenance therapy after several days of amphotericin B therapy for secondary cutaneous aspergillosis failed for a leukemia patient (62).
CONCLUSIONS
In conclusion, we have reviewed the cases of cutaneous aspergillosis reported in the literature. The use of adhesive tape dressings was the most consistent risk factor associated with the 10 cases in HIV-infected patients. Intermittent tape stripping of the stratum corneum of the skin with dressing changes likely caused sufficient mechanical trauma that predisposed the patients to this infection, although trapping of Aspergillus spores under the adhesive dressing could have played a role. Because most of the 10 patients did not have neutropenia, we conclude that neutropenia is not the most important risk factor in the development of cutaneous aspergillosis in HIV-infected patients. In this patient population, the range of clinical findings of primary cutaneous aspergillosis included nodules, molluscum-like papules, plaques, and ulcers.
Our review of non-HIV-infected immunocompromised patients with cutaneous aspergillosis revealed five major groups at risk for this infection: burn victims, neonates, individuals with cancer, bone marrow transplant recipients, and solid-organ transplant recipients. In these non-HIV-infected patients with cutaneous aspergillosis, the clinical manifestations, approach to therapy, and outcomes varied significantly depending on the underlying risk.
In order to diagnose cutaneous aspergillosis accurately, a skin biopsy of the lesion with histologic evaluation and silver staining should be performed. Culture should also be carried out to confirm the microscopic findings. If Aspergillus infection is diagnosed, subsequent efforts should determine whether the infection has spread to or arisen from an extracutaneous site, such as the lung. On the basis of our review, we recommend using itraconazole as first-line therapy if the patient has localized primary aspergillosis at least several centimeters from a vascular catheter exit site and no evidence of extracutaneous aspergillosis. Patients receiving itraconazole should be monitored closely. Therapy should be changed to intravenous amphotericin B if the lesions worsen or if there is other evidence of clinical failure. We do not recommend the use of itraconazole as first-line therapy for the treatment of cutaneous aspergillosis infections involving vascular catheter exit sites or tunnel infections, secondary cutaneous aspergillosis, or extensive primary cutaneous disease such as in a burn victim. These infections require treatment with intravenous amphotericin B, along with surgical therapy if it is clinically indicated.
ACKNOWLEDGMENT
J.-A. van Burik is a recipient of a Clinician Scientist Award (K08 AI-01411) from the National Institutes of Health.
REFERENCES
- 1.Allan G W, Andersen D H. Generalized aspergillosis in an infant 18 days of age. Pediatrics. 1960;26:432–440. [PubMed] [Google Scholar]
- 2.Allo M D, Miller J, Townsend T, Tan C. Primary cutaneous aspergillosis associated with Hickman intravenous catheters. N Engl J Med. 1987;317:1105–1108. doi: 10.1056/NEJM198710293171802. [DOI] [PubMed] [Google Scholar]
- 3.Anderson L L, Giandoni M B, Keller R A, Grabski W J. Surgical wound healing complicated by Aspergillus infection in a nonimmunocompromised host. Dermatol Surg. 1995;21:799–801. doi: 10.1111/j.1524-4725.1995.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 4.Becker W K, Cioffi W G, Jr, McManus A T, Kim S H, McManus W F, Mason A D, Pruitt B A., Jr Fungal burn wound infection. A 10-year experience. Arch Surg. 1991;126:44–48. doi: 10.1001/archsurg.1991.01410250048008. [DOI] [PubMed] [Google Scholar]
- 5.Bohler K, Metze D, Poitschek C, Jurecka W. Cutaneous aspergillosis. Clin Exp Dermatol. 1990;15:446–450. doi: 10.1111/j.1365-2230.1990.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 6.Bretagne S, Bart-Delabesse E, Wechsler J, Kuentz M, Dhedin N, Cordonnier C. Fatal primary cutaneous aspergillosis in a bone marrow transplant recipient: nosocomial acquisition in a laminar-air flow room. J Hosp Infect. 1997;36:235–239. doi: 10.1016/s0195-6701(97)90199-7. [DOI] [PubMed] [Google Scholar]
- 7.Bruck H M, Nash G, Foley D, Pruitt B A., Jr Opportunistic fungal infection of the burn wound with phycomycetes and Aspergillus. A clinical-pathologic review. Arch Surg. 1971;102:476–482. doi: 10.1001/archsurg.1971.01350050042014. [DOI] [PubMed] [Google Scholar]
- 8.Bryce E A, Walker M, Scharf S, Lim A T, Walsh A, Sharp N, Smith J A. An outbreak of cutaneous aspergillosis in a tertiary-care hospital. Infect Control Hosp Epidemiol. 1996;17:170–172. doi: 10.1086/647266. [DOI] [PubMed] [Google Scholar]
- 9.Buescher T M, Moritz D M, Killyon G W. Resection of chest wall and central veins for invasive cutaneous Aspergillus infection in an immunocompromised patient. Chest. 1994;105:1283–1285. doi: 10.1378/chest.105.4.1283. [DOI] [PubMed] [Google Scholar]
- 10.Cahill K M, Mofty A M, Kawaguchi T P. Primary cutaneous aspergillosis. Arch Dermatol. 1967;96:545–547. [PubMed] [Google Scholar]
- 11.Carlile J R, Millet R E, Cho C T, Vats T S. Primary cutaneous aspergillosis in a leukemic child. Arch Dermatol. 1978;114:78–80. [PubMed] [Google Scholar]
- 12.Cawley E P. Aspergillosis and the aspergilli: report of a unique case of the disease. Arch Intern Med. 1947;80:423–434. doi: 10.1001/archinte.1947.00220160002001. [DOI] [PubMed] [Google Scholar]
- 13.Cheetham H D. Subcutaneous infection due to Aspergillus terreus. J Clin Pathol. 1964;17:251–253. doi: 10.1136/jcp.17.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dohil M, Prendiville J S, Crawford R I, Speert D P. Cutaneous manifestations of chronic granulomatous disease. A report of four cases and review of the literature. J Am Acad Dermatol. 1997;36:899–907. doi: 10.1016/s0190-9622(97)80269-1. [DOI] [PubMed] [Google Scholar]
- 15.Dreizen S, Bodey G P, McCredie K B, Keating M J. Orofacial aspergillosis in acute leukemia. Oral Surg Oral Med Oral Pathol. 1985;59:499–504. doi: 10.1016/0030-4220(85)90091-x. [DOI] [PubMed] [Google Scholar]
- 16.Duthie R, Denning D W. Aspergillus fungemia: report of two cases and review. Clin Infect Dis. 1995;20:598–605. doi: 10.1093/clinids/20.3.598. [DOI] [PubMed] [Google Scholar]
- 17.Estes S A, Hendricks A A, Merz W G, Prystowsky S D. Primary cutaneous aspergillosis. J Am Acad Dermatol. 1980;3:397–400. doi: 10.1016/s0190-9622(80)80334-3. [DOI] [PubMed] [Google Scholar]
- 18.Everett E D, Pearson S, Rogers W. Rhizopus surgical wound infection with elasticized adhesive tape dressings. Arch Surg. 1979;114:738–739. doi: 10.1001/archsurg.1979.01370300092019. [DOI] [PubMed] [Google Scholar]
- 19.Findlay G H, Roux H F, Simson I W. Skin manifestations of disseminated aspergillosis. Br J Dermatol. 1971;85:94–97. [Google Scholar]
- 20.Gerstl B, Weidman W H, Newmann A V. Pulmonary aspergillosis: report of two cases. Ann Intern Med. 1948;28:662–671. doi: 10.7326/0003-4819-28-3-662. [DOI] [PubMed] [Google Scholar]
- 21.Girmenia C, Gastaldi R, Martino P. Catheter-related cutaneous aspergillosis complicated by fungemia and fatal pulmonary infection in an HIV-positive patient with acute lymphocytic leukemia. Eur J Clin Microbiol Infect Dis. 1995;14:524–526. doi: 10.1007/BF02113431. [DOI] [PubMed] [Google Scholar]
- 22.Googe P B, DeCoste S D, Herold W H, Mihm M C., Jr Primary cutaneous aspergillosis mimicking dermatophytosis. Arch Pathol Lab Med. 1989;113:1284–1286. [PubMed] [Google Scholar]
- 23.Granstein R D, First L R, Sober A J. Primary cutaneous aspergillosis in a premature neonate. Br J Dermatol. 1980;103:681–684. doi: 10.1111/j.1365-2133.1980.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 24.Greenbaum R S, Roth J S, Grossman M E. Subcutaneous nodule in a cardiac transplant. Cutaneous aspergillosis. Arch Dermatol. 1993;129:1191. doi: 10.1001/archderm.129.9.1191. , 1194. [DOI] [PubMed] [Google Scholar]
- 25.Grossman M E, Fithian E C, Behrens C, Bissinger J, Fracaro M, Neu H C. Primary cutaneous aspergillosis in six leukemic children. J Am Acad Dermatol. 1985;12:313–318. doi: 10.1016/s0190-9622(85)80042-6. [DOI] [PubMed] [Google Scholar]
- 26.Gupta M, Weinberger B, Whitley-Williams P N. Cutaneous aspergillosis in a neonate. Pediatr Infect Dis J. 1996;15:464–465. doi: 10.1097/00006454-199605000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Harmon C B, Su W P, Peters M S. Cutaneous aspergillosis complicating pyoderma gangrenosum. J Am Acad Dermatol. 1993;29:656–658. doi: 10.1016/s0190-9622(08)81879-8. [DOI] [PubMed] [Google Scholar]
- 28.Hartman P D, Kaplan R P. Transepithelial elimination in cutaneous aspergillosis. J Am Acad Dermatol. 1986;15:1305–1307. doi: 10.1016/s0190-9622(86)80055-x. [DOI] [PubMed] [Google Scholar]
- 29.Hui A N, Koss M N, Meyer P R. Necropsy findings in acquired immunodeficiency syndrome: a comparison of premortem diagnoses with postmortem findings. Hum Pathol. 1984;15:670–676. doi: 10.1016/s0046-8177(84)80293-2. [DOI] [PubMed] [Google Scholar]
- 30.Hunt S J, Nagi C, Gross K G, Wong D S, Mathews W C. Primary cutaneous aspergillosis near central venous catheters in patients with the acquired immunodeficiency syndrome. Arch Dermatol. 1992;128:1229–1232. [PubMed] [Google Scholar]
- 31.Iwen P C, Reed E C, Armitage J O, Bierman P J, Kessinger A, Vose J M, Arneson M A, Winfield B A, Woods G L. Nosocomial invasive aspergillosis in lymphoma patients treated with bone marrow or peripheral stem cell transplants. Infect Control Hosp Epidemiol. 1993;14:131–139. doi: 10.1086/646698. [DOI] [PubMed] [Google Scholar]
- 32.Johnson A S, Ranson M, Scarffe J H, Morgenstern G R, Shaw A J, Oppenheim B A. Cutaneous infection with Rhizopus oryzae and Aspergillus niger following bone marrow transplantation. J Hosp Infect. 1993;25:293–296. doi: 10.1016/0195-6701(93)90116-h. [DOI] [PubMed] [Google Scholar]
- 33.Langlois R P, Flegel K M, Meakins J L, Morehouse D D, Robson H G, Guttmann R D. Cutaneous aspergillosis with fatal dissemination in a renal transplant recipient. Can Med Assoc J. 1980;122:673–676. [PMC free article] [PubMed] [Google Scholar]
- 34.Larkin J A, Greene J N, Sandin R L, Houston S H. Primary cutaneous aspergillosis: case report and review of the literature. Infect Control Hosp Epidemiol. 1996;17:365–366. doi: 10.1086/647318. [DOI] [PubMed] [Google Scholar]
- 35.Le Conte P, Blanloeil Y, Michel P, Francois T, Paineau J. Cutaneous aspergillosis in a patient with orthotopic hepatic transplantation. Transplantation. 1992;53:1153–1154. [PubMed] [Google Scholar]
- 36.Loria K M, Salinger M H, Frohlich T G, Gendelman M D, Cook F V, Arentzen C E. Primary cutaneous aspergillosis in a heart transplant recipient treated with surgical excision and oral itraconazole. J Heart Lung Transplant. 1992;11:156–159. [PubMed] [Google Scholar]
- 37.Magid M L, Prendiville J S, Esterly N B. Violaceous nodules on the arm of a child with acute lymphocytic leukemia. Primary cutaneous aspergillosis (Aspergillus flavus) Arch Dermatol. 1988;124:125–126. doi: 10.1001/archderm.124.1.122. [DOI] [PubMed] [Google Scholar]
- 38.McCarty J M, Flam M S, Pullen G, Jones R, Kassel S H. Outbreak of primary cutaneous aspergillosis related to intravenous arm boards. J Pediatr. 1986;108:721–724. doi: 10.1016/s0022-3476(86)81051-4. [DOI] [PubMed] [Google Scholar]
- 39.Mead J H, Lupton G P, Dillavou C L, Odom R B. Cutaneous Rhizopus infection. Occurrence as a postoperative complication associated with an elasticized adhesive dressing. JAMA. 1979;242:272–274. doi: 10.1001/jama.242.3.272. [DOI] [PubMed] [Google Scholar]
- 40.Mowad C M, Nguyen T V, Jaworsky C, Honig P J. Primary cutaneous aspergillosis in an immunocompetent child. J Am Acad Dermatol. 1995;33:136–137. doi: 10.1016/0190-9622(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 41.Myers J T, Dunn A D. Aspergillus infection of the hand. JAMA. 1930;95:794–796. [Google Scholar]
- 42.Naidu J, Singh S M. Aspergillus chevalieri (Mangin) Thom and Church: a new opportunistic pathogen of human cutaneous aspergillosis. Mycoses. 1994;37:271–274. doi: 10.1111/j.1439-0507.1994.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 43.Nampoory M R, Khan Z U, Johny K V, Constandi J N, Gupta R K, Al-Muzairi I, Samhan M, Mozavi M, Chugh T D. Invasive fungal infections in renal transplant recipients. J Infect. 1996;33:95–101. doi: 10.1016/s0163-4453(96)92986-2. [DOI] [PubMed] [Google Scholar]
- 44.Nash G, Foley F D, Goodwin M N, Jr, Bruck H M, Greenwald K A, Pruitt B A., Jr Fungal burn wound infection. JAMA. 1971;215:1664–1666. [PubMed] [Google Scholar]
- 45.Neumeister B, Hartmann W, Oethinger M, Heymer B, Marre R. A fatal infection with Alternaria alternata and Aspergillus terreus in a child with agranulocytosis of unknown origin. Mycoses. 1994;37:181–185. doi: 10.1111/j.1439-0507.1994.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 46.Panke T W, McManus A T, Jr, McLeod C G., Jr “Fruiting bodies” of Aspergillus on the skin of a burned patient. Am J Clin Pathol. 1978;69:188–189. doi: 10.1093/ajcp/69.2.188. [DOI] [PubMed] [Google Scholar]
- 47.Paparello S F, Parry R L, MacGillivray D C, Brock N, Mayers D L. Hospital-acquired wound mucormycosis. Clin Infect Dis. 1992;14:350–352. doi: 10.1093/clinids/14.1.350. [DOI] [PubMed] [Google Scholar]
- 48.Papouli M, Roilides E, Bibashi E, Andreou A. Primary cutaneous aspergillosis in neonates: case report and review. Clin Infect Dis. 1996;22:1102–1104. doi: 10.1093/clinids/22.6.1102. [DOI] [PubMed] [Google Scholar]
- 49.Perzigian R W, Faix R G. Primary cutaneous aspergillosis in a preterm infant. Am J Perinatol. 1993;10:269–271. doi: 10.1055/s-2007-994737. [DOI] [PubMed] [Google Scholar]
- 50.Pla M P, Berenguer J, Arzuaga J A, Banares R, Polo J R, Bouza E. Surgical wound infection by Aspergillus fumigatus in liver transplant recipients. Diagn Microbiol Infect Dis. 1992;15:703–706. doi: 10.1016/0732-8893(92)90074-4. [DOI] [PubMed] [Google Scholar]
- 51.Prystowsky S D, Vogelstein B, Ettinger D S, Merz W G, Kaizer H, Sulica V I, Zinkham W H. Invasive aspergillosis. N Engl J Med. 1976;295:655–658. doi: 10.1056/NEJM197609162951206. [DOI] [PubMed] [Google Scholar]
- 52.Radentz W H. Opportunistic fungal infections in immunocompromised hosts. J Am Acad Dermatol. 1989;20:989–1003. doi: 10.1016/s0190-9622(89)70124-9. [DOI] [PubMed] [Google Scholar]
- 53.Raszka W V, Jr, Shoupe B L, Edwards E G. Isolated primary cutaneous aspergillosis of the labia. Med Pediatr Oncol. 1993;21:375–378. doi: 10.1002/mpo.2950210514. [DOI] [PubMed] [Google Scholar]
- 54.Romero L S, Hunt S J. Hickman catheter-associated primary cutaneous aspergillosis in a patient with the acquired immunodeficiency syndrome. Int J Dermatol. 1995;34:551–553. doi: 10.1111/j.1365-4362.1995.tb02951.x. [DOI] [PubMed] [Google Scholar]
- 55.Rowen J L, Correa A G, Sokol D M, Hawkins H K, Levy M L, Edwards M S. Invasive aspergillosis in neonates: report of five cases and literature review. Pediatr Infect Dis J. 1992;11:576–582. [PubMed] [Google Scholar]
- 56.Salisbury R E, Silverstein P, Goodwin M N., Jr Upper extremity fungal invasions secondary to large burns. Plastic Reconstruct Surg. 1974;54:654–659. doi: 10.1097/00006534-197412000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Shetty D, Giri N, Gonzalez C E, Pizzo P A, Walsh T J. Invasive aspergillosis in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1997;16:216–221. doi: 10.1097/00006454-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Smith W F, Wallace M R. Cutaneous aspergillosis. Cutis. 1997;59:138–140. [PubMed] [Google Scholar]
- 59.Stiller M J, Teperman L, Rosenthal S A, Riordan A, Potter J, Shupack J L, Gordon M A. Primary cutaneous infection by Aspergillus ustus in a 62-year-old liver transplant recipient. J Am Acad Dermatol. 1994;31:344–347. doi: 10.1016/s0190-9622(94)70169-5. [DOI] [PubMed] [Google Scholar]
- 60.Stone H H, Cuzzell J Z, Kolb L D, Moskowitz M S, McGowan J E., Jr Aspergillus infection of the burn wound. J Trauma. 1979;19:765–767. doi: 10.1097/00005373-197910000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Suseelan A V, Gugnani H C, Ojukwu J O. Primary cutaneous aspergillosis due to Aspergillus terreus. Arch Dermatol. 1976;112:1468. [PubMed] [Google Scholar]
- 62.Tobin E H, Westenfeld F, Dietrich P A. Cutaneous infection due to Aspergillus species after transthoracic lung biopsy. Clin Infect Dis. 1993;17:955–956. doi: 10.1093/clinids/17.5.955. [DOI] [PubMed] [Google Scholar]
- 63.van Burik J-A, Colven R, Spach D H. Itraconazole treatment for primary cutaneous aspergillosis in patients with AIDS. Clin Infect Dis. 1998;27:643–644. doi: 10.1086/517138. [DOI] [PubMed] [Google Scholar]
- 64.Vedder J S, Schorr W F. Primary disseminated pulmonary aspergillosis with metastatic skin nodules. Successful treatment with inhalation nystatin therapy. JAMA. 1969;209:1191–1195. [PubMed] [Google Scholar]
- 65.Watsky K L, Eisen R N, Bolognia J L. Unilateral cutaneous emboli of Aspergillus. Arch Dermatol. 1990;126:1214–1217. [PubMed] [Google Scholar]
- 66.Weingarten J S, Crockett D M, Lusk R P. Fulminant aspergillosis: early cutaneous manifestations and the disease process in the immunocompromised host. Otolaryngol Head Neck Surg. 1987;97:495–499. doi: 10.1177/019459988709700512. [DOI] [PubMed] [Google Scholar]
- 67.White C B, Barcia P J, Bass J W. Neonatal zygomycotic necrotizing cellulitis. Pediatrics. 1986;78:100–102. [PubMed] [Google Scholar]
- 68.Young R C, Bennett J E, Vogel C L, Carbone P P, DeVita V T. Aspergillosis. The spectrum of the disease in 98 patients. Medicine. 1970;49:147–173. doi: 10.1097/00005792-197003000-00002. [DOI] [PubMed] [Google Scholar]