Abstract
Heparin-induced thrombocytopenia (HIT) is an antibody-mediated immune response against platelet factor 4 (PF4) bound to heparin anticoagulants. A priori identification of patients at-risk for HIT remains elusive and a number of risk factors have been identified, but these associations and their effect sizes have limited validation in large cohorts of suspected HIT patients. The aim of this study was to investigate existing anti-PF4/heparin antibody thresholds and model the relationship of demographic variables and anti-PF4/heparin antibody levels with functional assay positivity across multiple institutions in the absence of detailed clinical data. In a large collection of suspected HIT patients (n=8,904), we tested for associations between laboratory and demographic variables and functional assay positive status as well as anti-PF4/heparin antibody levels. We also tested for correlation between IgG-specific and polyspecific (IgG/IgA/IgM) anti-PF4/heparin antibody values and their ability to predict functional assay positive status using area under the receiver operating characteristic (AUROC). Logistic regression identified increasing anti-PF4/heparin antibody OD levels (OR=51.84 [37.27-74.34], p<2.0x10−16) and female sex (OR=1.47 [1.19-1.82], p=3.5x10−4) as risk factors for positive functional assay in the largest cohort with consistent effect sizes in two other cohorts. In a subset of 1175 patients, polyspecific and IgG-specific anti-PF4/heparin antibody values were heterogeneous (mean coefficient of variation=31.9%), but strongly correlated (rho=0.878; p<2x10−16) with similar prediction of functional assay positivity (polyspecific AUROC=0.976 and IgG-specific AUROC=0.980). Thus, we recapitulate previously identified risk factors of functional assay positivity, providing precise effect sizes in a large observational population of suspected HIT patients. Our data reinforce the necessity of functional assay confirmation and suggest that, despite heterogeneity, polyspecific and IgG-specific anti-PF4/heparin antibody assays predict functional assay positive status similarly , even in the absence of 4Ts scores and detailed clinical data.
Keywords: heparin-induced thrombocytopenia, anti-PF4/heparin antibodies, heparin, adverse drug reaction, functional assay testing, risk factors
INTRODUCTION
The anticoagulant drug heparin carries the risk for the antibody-mediated adverse drug reaction heparin-induced thrombocytopenia (HIT). HIT is characterized by low platelet count, with platelet count fall generally beginning 5-10 days after onset of heparin exposure, and a significant risk for thromboembolic events(1). Pathogenesis of HIT is initiated when heparin binds to circulating platelet factor 4 (PF4), creating a neoepitope for anti-PF4/heparin IgG antibodies to bind and leads to platelet activation, release of procoagulant microparticles, and thrombocytopenia(2, 3). Diagnosis of HIT poses multiple challenges, including a requirement of a compatible clinical picture and anti-PF4/heparin antibody thresholds for both triage of treatment and laboratory confirmation. Functional assays are time-intensive, technically challenging, and offered by a limited number of laboratories, often leading to limited role in immediate decision-making and delays in laboratory confirmation of HIT(4). Enzyme-linked immunosorbent assays (ELISAs) for the presence of anti-PF4/heparin antibodies are widely available, but only a small subset of patients with detectable levels of anti-PF4/heparin antibodies will go on to develop full-blown HIT, leading to a high number of false positives. Clinical scoring systems such as the 4Ts scoring system have been implemented to aid diagnosis, but 4Ts has a low specificity and can only be performed after the manifestation of symptoms(5, 6). Given the challenges presented with HIT diagnosis, identification of risk factors of functional assay positivity could aid clinical decision making for heparin-treated patients at risk for HIT.
While a priori identification of patients at-risk for HIT remains elusive, a number of risk factors for HIT have been identified, including type of heparin anticoagulant, surgical intervention, female sex, and higher levels of anti-PF4/heparin antibodies, judged by optical density [OD] levels(7, 8, 9, 10, 11, 12, 13, 14, 15). Recent data from the External Quality Control for Assays and Tests (ECAT) foundation have also suggested that, despite significant heterogeneity, IgG-specific and polyspecific anti-PF4/heparin ELISAs have similar concordance for HIT prediction(16). Clinical guidelines for management of HIT recommend an ELISA threshold (OD<0.4 units) to classify patients as HIT negative in the absence of a functional assay test result(11, 17). Guidelines also state that functional assay confirmation of HIT may not be necessary for individuals with a higher 4Ts score and strongly positive ELISA (OD>2.0). However, such HIT risk factors, and guideline directed anti-PF4/heparin antibody thresholds have limited validation in large, observational cohorts of suspected HIT patients. In this study, we accumulated the largest collection of suspected HIT patients to date, using three large retrospective cohorts (total n=8,904). The aim of this study was to investigate existing anti-PF4/heparin antibody thresholds and model the relationship of demographic variables and antibody levels with functional assay positivity across multiple institutions to identify/recapitulate risk factors of functional assay positive status in the absence of detailed clinical data (4Ts scores). We also aimed to assess heterogeneity between IgG-specific and polyspecific enzyme immunoassay results and the ability to predict functional assay positive status. Finally, we sought to develop predictive models for functional assay positivity based on limited demographic and laboratory data.
METHODS
Study Populations
McMaster Cohort
Patients who underwent laboratory testing for anti-PF4/heparin antibodies via ELISA and functional assay via serotonin-release assay (SRA), at the McMaster University Platelet Immunology Laboratory (Hamilton, Ontario, Canada) comprised the largest cohort. Patients 18 years of age or older were identified from the 3 hospitals in the Hamilton area between 1984-2021. Obstetrical patients were excluded from the study. Details of ELISA and SRA tests are previously described(18, 19, 20). In brief, samples were tested for platelet activation with two pharmacological (at 0.1 and/or 0.3 U/mL) and one high concentration (100 U/mL) of heparin. SRA positive patients were defined as having at least 50% serotonin release at pharmacological concentrations and 50% or more inhibition with the high heparin step. ELISA was performed for all samples prior to 2016 using a IgG-specific ELISA, as described(19). In September 2015, the McMaster Platelet Immunobiology Lab began utilizing the Immucor® PF4 Enhanced Assay for detecting PF4-PVS polyspecific antibodies (IgG/IgA/IgM class), combined with an IgG-specific ELISA for select individuals (n = 1175)(18). ELISA results were calculated as optical density [OD405] values on plate readers with a maximum OD of 4.0 units. Unless otherwise noted, when individuals possessed values from both IgG-specific and polyspecific (IgG/IgA/IgM class) ELISAs, analyses used IgG-specific ELISA OD values. Clinical data for this study population included: age, sample year, sex, surgical intervention, ELISA result, and SRA results. While patients for this cohort were accrued due to clinical suspicion of HIT, the observational study design limited data extraction from electronic health records (EHR). As such, detailed clinical data such as 4T scores are unavailable for this cohort. This study was approved by the Hamilton Integrated Research Ethics Board.
Greifswald Cohort
An observational patient cohort was accrued from a central laboratory at Greifswald University (Greifswald, Germany) as previously described(21, 22). The Greifswald University central testing facility is utilized for HIT assessment across multiple institutions. Briefly, the central laboratory performed in-house immunoglobulin class-specific ELISAs (IgG, IgA and IgM, tested separately) as previously described(23) for quantification of anti-PF4/heparin antibody levels and heparin-induced platelet aggregation (HIPA) assay for functional assay testing. Optical density values were calculated for each ELISA using a plate reader with a maximum OD of 3.5 units. Comparisons between the two functional assays, SRA and HIPA, have been extensive, showing similar metrics in HIT confirmation and the validity of HIPA in lieu of SRA testing(5, 24, 25, 26). Since samples were tested across multiple hospitals without centralized EHRs, only limited clinical data were available prior to the de-identification of samples. The available data for this cohort included anti-PF4/heparin antibody optical density (OD) levels, HIPA test results, and basic demographic data such as age and sex. Detailed clinical data for a definitive diagnosis of HIT, such as 4T scores, were also not captured for this study. This research received ethical approval from the institutional ethics committee at Greifswald University.
Tours Cohort
Two previously described cohorts were prospectively recruited from the University of Tours, France and comprised the third cohort(21, 22, 27, 28). HIT cases in the Tours cohort had definite HIT based on ELISA, SRA, and clinical presentation consistent with HIT. All patients in the cohort were tested for anti-PF4/heparin antibodies via polyspecific PF4-polyvinylsulfonate ELISA (IgG/A/M class). Results from ELISAs were calculated as OD values on plate readers with a maximal readout of 4.0 units. All patients not diagnosed with definite HIT were treated with unfractionated heparin (UFH) and underwent cardiopulmonary bypass (CPB). Clinical data available for analysis included age, sex, type of heparin administered (UFH vs. low molecular weight [LMWH]), platelet count prior to and after heparin treatment, and CPB surgery status. This research received ethical approval from both the University of Tours ethics committee and the Ministry of Research.
Statistical Analyses
In McMaster and Greifswald cohorts, the limited clinical data precluded our ability to definitively diagnose HIT as recommended in clinical guidelines. Instead, positive functional assay status is defined as the outcome variable throughout the manuscript. Basic demographics information was compared between cohorts and by functional assay positive status using chi-squared tests for categorical variables and one-way ANOVA for continuous variables. Beyond the exclusion criteria included for each cohort, any individual less than 18 years old and any individual with missing ELISA optical density values were excluded from all analyses. All p values less than 2x10−16 are presented as p<2x10−16. All analyses were performed in R (4.1.2).
Spearman test was utilized to identify correlation between ELISA results where multiple results were available for the same sample. These included the subset of the McMaster cohort where both IgG-specific and polyspecific (IgG/IgA/IgM class) ELISAs were available and the subset of the Greifswald cohort where immunoglobulin class-specific IgG, IgA and IgM ELISAs were available. In the McMaster subset, coefficients of variation (CoVs) were also calculated within each individual with both IgG-specific and polyspecific results as previously described(16).
Logistic regression was performed to model the relationship between independent predictors and functional assay positivity. Similarly, linear regression was performed to model the relationship between independent predictors and anti-PF4/heparin antibody levels. P-values, odds ratios (OR) and 95% confidence intervals, and/or betas and standard errors (SE) were calculated for each regression. Interactions between independent predictor variables were tested using the cross-product terms in regression models. High collinearity was assessed in the multivariable model using the R package Car for variance inflation factor (VIF) scoring and variables with VIF>2 were removed prior to regression analysis.
Modeling of functional assay positivity risk was performed in a two-fold approach; first anti-PF4/heparin antibody OD levels were used to model likelihood of a positive functional assay; then backward stepwise regression was performed on all available clinical and demographic variables included in each cohort. For stepwise regression modeling, we utilized the R package MASS with Akaike information criterion (AIC) as estimator of best model fit. The final model in each cohort was then compared to the model that included only anti-PF4/heparin antibody OD values using a likelihood ratio test to determine if the full model outperformed the antibody only model in prediction of functional assay positivity. A likelihood ratio test was used to establish significance of a better fitting model using the R package lmtest. Heterogeneity of effect sizes for statistically significant associations was assessed post-hoc using Cochran’s Q statistic under the assumption of a random effects model using the R package metafor. The Youden’s Index (J) test statistic(29) and area under the receiver operating characteristic (AUROC) curve were used to quantify the predictive performance of the final model for functional assay positivity.
Several sensitivity analyses were also performed. In the McMaster cohort, polyspecific ELISA results were used instead of IgG-specific ELISAs in the subset of individuals where both assays were performed to investigate effects on both associations from logistic regressions and AUROC values. In the Greifswald cohort, IgM and IgA showed collinearity with IgG (VIF>2) and were initially removed prior to regression in primary models. A sensitivity analysis was performed to test the influence of incorporating IgM and IgA ELISA values on predictive models for functional assay positive status.
As antibodies are necessary but not sufficient to cause HIT, we sought to identify anti-PF4/heparin antibody level thresholds which best distinguished between positive and negative functional assay results. Thresholds were manually assigned at an OD of 0.4 units, then sequentially every 0.5 OD starting from 1.0 to 3.0 units. Specificity and sensitivity were calculated for each threshold value to compare performance of each OD value cut point. We then utilized the Youden’s Index (J) test statistic(29) and AUROC for identifying the optimal antibody OD value for functional assay dichotomization within our three cohorts using the R package cutpointr. The phenotypic data for Tours & Greifswald cohorts is available in the database of Genotypes and Phenotypes (dbGaP: phs accession number phs002863.v1.p1). Code used in this study is available on Github (https://github.com/karneslab) and data from the McMaster cohort are available from the corresponding author upon reasonable request.
RESULTS
After removal of patients with missing anti-PF4/heparin antibody OD values, the McMaster cohort consisted of 3451 individuals with 1503 females (43.6%) and 400 functional assay positive cases (Table 1). The mean age was 67.60 (standard deviation [SD] 14.50) and mean anti-PF4/heparin antibody OD level was 0.52 (0.66). The Greifswald cohort consisted of 4622 patients with 1810 female participants (39.3%) and 1203 with positive functional assay. The cohort had a mean age of 66.6 (13.71) and mean anti-PF4/heparin antibody OD level of 0.78 (0.77). The Tours cohort had 831 individuals with 194 having positive functional assay and 294 female patients (35.5%). The average age was 68.37 (12.55) and mean anti-PF4/heparin antibody OD level was 1.05 (1.00). Differences between cohorts were highly significant in terms of functional assay positivity rate and all variables assessed, likely reflecting the different approaches to selection of patients for inclusion in these cohorts. For example, the McMaster and Greifswald cohorts are population-based and observational, in which the laboratory collects data on individuals who had ELISA and/or SRA testing ordered due to clinical suspicion of HIT. The Tours cohort accrued patients with definitive HIT and non-HIT patients undergoing CPB(28).
Table 1:
Demographic and laboratory characteristics of the cohorts
| Trait | McMaster | Greifswald | Tours | P-value |
|---|---|---|---|---|
| Total, n | 3451 | 4622 | 831 | |
| Age | 67.60 (14.5) | 66.60 (13.71) | 68.37 (12.55) | <0.001 |
| Sex (female), n (%) | 1503 (43.6) | 1810 (39.3) | 294 (35.5) | <0.001 |
| Anti-PF4/heparin OD1 | 0.52 (0.66) | 0.78 (0.77) | 1.05 (1.00) | <0.001 |
| Antibody positive (OD>0.5), n (%) | 799 (23.2) | 2491 (54.0) | 470 (56.6) | <0.001 |
| Functional Assay Positive, n (%)2 | 400 (14.3) | 1203 (26.6) | 194 (23.3) | <0.001 |
| Surgery, n (%) | 800 (39.5) | - | 686 (82.8)3 | <0.001 |
| Unfractionated Heparin, n (%) | - | - | 763 (95.3) | - |
| Platelet Count Before Heparin Therapy (1x109 platelets/L) | - | - | 216.13 (66.09) | - |
| Anti-PF4/heparin IgA OD1 | - | 0.45 (0.49) | - | - |
| Anti-PF4/heparin IgM OD 1 | - | 0.55 (0.40) | - | - |
(–) indicates that data were unavailable for analysis. Values represent mean (standard deviation) unless otherwise specified; n [%]: Total count in each category and the percentage of the overall sample size; OD indicates optical density.
PF4/heparin antibody levels were determined using enzyme-linked immunosorbent assay (ELISA). IgG-specific ELISAs were utilized to obtain OD values in the McMaster cohort. IgG, IgA, and IgM-specific ELISAs were performed separately in the Greifswald cohort. Poly-specific IgG/A/M ELISA kit was used to determine OD values in the Tours cohort.
Functional Assay (Positive %): functional assay positive status was determined using the heparin-induced platelet activation (HIPA) test in the Greifswald cohort and the serotonin release assay (SRA) in the McMaster and Tours cohorts.
All functional assay negative patients underwent CPB surgery in the Tours cohort.
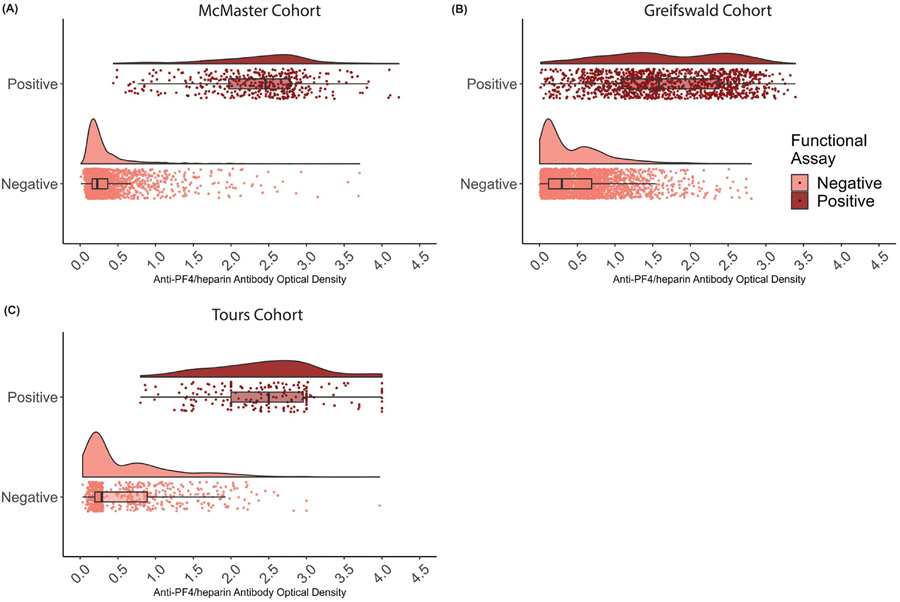
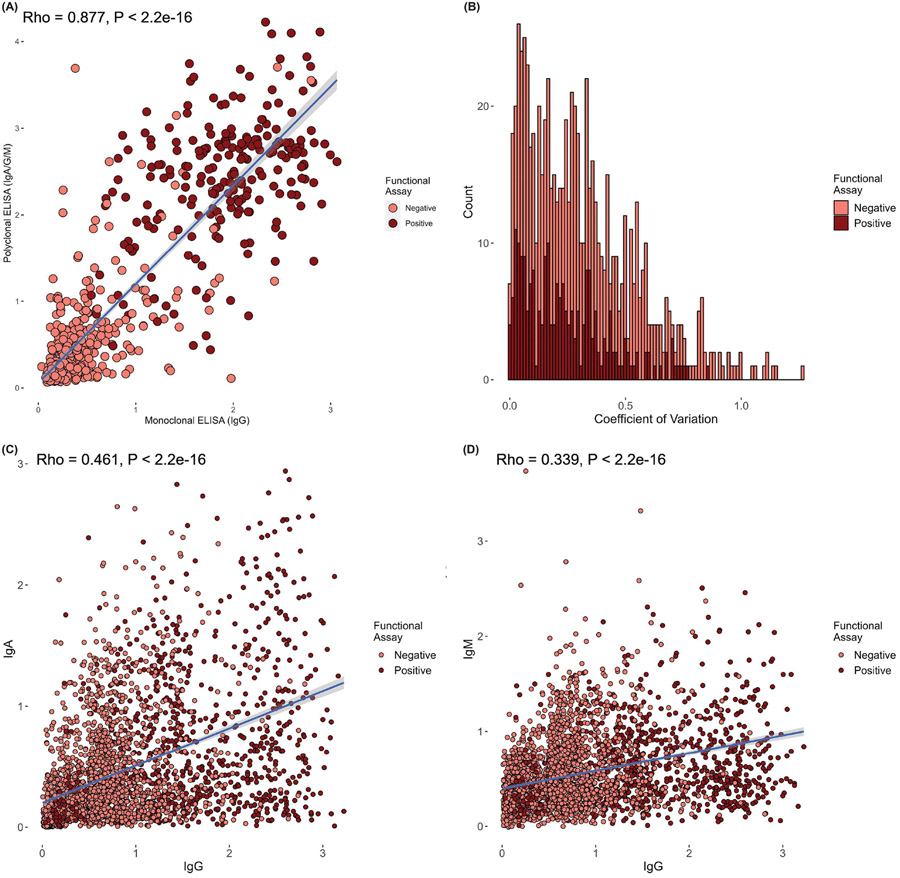
Significant differences were observed between individuals with positive and negative functional assay for PF4/heparin antibody levels in all cohorts, and for sex in McMaster and Tours (Table 2). Figure 1 shows the distribution of anti-PF4/heparin antibody levels between positive and negative functional assay across all three cohorts. A large overlap of OD values was observed between functional assay positive and negative individuals in all three cohorts, with higher variability in OD values observed in patients with a positive functional assay. In the McMaster cohort tested for both polyspecific and IgG-specific ELISAs, polyspecific ELISA OD values showed a strongly significant correlation with IgG-specific ELISA values (rho=0.878; p<2x10−16) (Figure 2A). Mean CoV in this subset of individuals was 31.9% and CoVs ranged from 0% to 126% (Figure 2B). The majority of functional assay positive individuals (70.6%) exhibited CoV values below accepted thresholds of 30%(30, 31). In the Greifswald cohort, strong correlations were observed between IgG OD values and IgA (rho=0.466; p<2x10−16) as well as IgM (rho=0.343; p<2x10−16) (Figure 2C and 2D).
Table 2:
Demographic and laboratory characteristics for individuals with positive and negative functional assay status
| Trait | Functional assay negative1 |
Functional assay positive1 |
P-value2 |
|---|---|---|---|
| McMaster (n) | 2405 | 400 | |
| Age (mean (SD)) | 67.69 (14.70) | 68.29 (12.92) | 0.442 |
| Sex, female n (%) | 1037 (43.1) | 211 (52.8) | <0.001 |
| OD (mean (SD)) 3 | 0.33 (0.32) | 2.06 (0.61) | <0.001 |
| Surgery | 575 (38.3) | 78 (44.6) | 0.125 |
| Greifswald (n) | 3313 | 1203 | |
| Age (mean (SD)) | 66.67 (13.79) | 66.51 (13.37) | 0.719 |
| Sex, female n (%) | 1272 (38.6) | 495 (41.2) | 0.12 |
| OD (mean (SD)) 3 | 0.46 (0.45) | 1.67 (0.78) | <0.001 |
| Immunoglobulin A (IgA) 3 | 0.34 (0.38) | 0.72 (0.60) | <0.001 |
| Immunoglobulin M (IgM)3 | 0.50 (0.37) | 0.68 (0.43) | <0.001 |
| Tours (n) | 637 | 194 | |
| Age (mean (SD)) | 68.73 (12.12) | 67.08 (13.94) | 0.122 |
| Sex, female n (%) | 189 (29.7) | 105 (55.0) | <0.001 |
| OD (mean (SD)) 3 | 0.62 (0.60) | 2.46 (0.71) | <0.001 |
| Cardiopulmonary Bypass (CPB) Surgery4 | 637 (100.0) | 49 (25.5) | <0.001 |
| Platelet Count Before Heparin Therapy (1x109 platelets/L) | 213.27 (56.44) | 228.07 (95.87) | 0.014 |
Values represent mean (standard deviation) unless otherwise specified; n [%]: Total count in each category and the percentage of the overall sample size. OD indicates optical density.
ns indicate number of individuals with all relevant, non-missing variables, therefore totals in table may not sum to the total cohort as some patients contained specific variables with missingness.
P-values listed are from comparison of the trait between positive and negative functional assay status in each cohort
PF4/heparin antibody levels were determined using enzyme-linked immunosorbent assay (ELISA).
All functional assay negative patients underwent CPB surgery in the Tours cohort.
Figure 1:
Raincloud plot of distribution of anti-PF4/heparin antibody OD values from primary analyses by cohort by functional assay test result. Box and whisker plots indicate the mean, interquartile range, and 95 percentiles of OD values in the A) McMaster cohort, B) Greifswald cohort, and C) Tours cohort.
Figure 2:
Correlation and heterogeneity of various immunoassays performed on the same samples by functional assay positive status. A) Correlation of IgG-specific ELISA OD values (x-axis) and polyspecific ELISA (IgG/A/M) OD values (y-axis) for the subset of individuals with both tests (n=1175) in the McMaster cohort. B) Histogram of coefficients of variation (CoVs) for IgG-specific and polyspecific (IgG/A/M) ELISA OD values from the subset of individuals with both tests (n=1175) in the McMaster cohort. C) Correlation of IgG-specific ELISA OD values (x-axis) and IgA-specific ELISA OD values (y-axis) for the subset of individuals with both tests in the Greifswald cohort. D) Correlation of IgG-specific ELISA OD values (x-axis) and IgM-specific ELISA OD values (y-axis) for the subset of individuals with both tests in the Greifswald cohort. Spearman correlation coefficients (rho) and p-values are presented in top left of panel A, C, and D. Dark blue indicates trend line, with light blue shading showing 95% confidence intervals. Each panel displays functional assay positive patients in dark red and functional assay negative patients in light red.
Association of Clinical and Demographic Variables with Functional Assay Status
In univariate logistic regressions, unadjusted ORs for functional assay positivity increased markedly with increasing anti-PF4/heparin IgG OD levels (Table 3). A 1-unit increase in OD level resulted in significant ORs ranging between 15.48 and 51.84 for assay positivity risk in all three cohorts (all p<2x10−16). Female sex was also associated with increased risk of a positive functional assay in Tours and McMaster, with a trend towards association in the Greifswald cohort. Age was associated with positive functional assay risk only in the Tours cohort (OR=0.98 [0.96-0.99], p=0.024). Several clinical variables were collected that were not shared among all three cohorts. In the McMaster cohort, surgery was not associated with functional assay positive status (OR=1.30 [0.94-1.78], p=0.107). In the Greifswald cohort, anti-PF4/heparin immunoglobulin M (IgM) and immunoglobulin A (IgA) OD levels were both associated with functional assay positive status (Table 3). In the Tours cohort, higher platelet count prior to heparin initiation was associated with functional assay positive status (OR=1.00 [1.00-1.01] per 1x109 platelets/L, p=0.018).
Table 3:
Univariate and Stepwise logistic regression of predictor variables and functional assay positivity
| Cohort - Variable | Odds Ratio (95%CI) | P-value |
|---|---|---|
| Univariate Regression | ||
| McMaster | ||
| Anti-PF4/heparin IgG antibody1 | 51.84 (37.27-74.34) | <2*10−16 |
| Sex (female) | 1.47 (1.19,1.82) | 0.00035 |
| Age (per year) | 1.003 (0.996,1.01) | 0.442 |
| Surgery | 1.30 (0.94,1.78) | 0.107 |
| Greifswald | ||
| Anti-PF4/heparin IgG antibody1 | 15.475 (13.185,18.284) | <2*10−16 |
| Sex (female) | 1.12 (0.975,1.276) | 0.112 |
| Age (per year) | 0.999 (0.994-1.004) | 0.89 |
| Immunoglobulin A (IgA)2 | 4.578 (3.867-5.444) | <2*10−16 |
| Immunoglobulin M (IgM)2 | 3.989 (2.47-3.625) | <2*10−16 |
| Tours | ||
| Anti-PF4/heparin IgG antibody1 | 15.82 (10.21-25.87) | <2*10−16 |
| Sex (female) | 2.89 (2.08-4.04) | 3.49*10−10 |
| Age (per year) | 0.98 (0.96-0.997) | 0.024 |
| Platelet Count Before Heparin Therapy (1x109 platelets/L) | 1.003 (1.00-1.006) | 0.0184 |
| Stepwise Regression 3 | ||
| McMaster | ||
| Anti-PF4/heparin IgG antibody1 | 37.78 (24.99, 60.40) | <2*10−16 |
| Sex (female) | 1.74 (0.94, 3.26) | 0.0791 |
| Greifswald | ||
| Anti-PF4/heparin IgG antibody1 | 15.66 (13.33-18.52) | <2*10−16 |
| Sex (female) | 1.23 (1.01-1.49) | 0.0327 |
| Tours | ||
| Anti-PF4/heparin IgG antibody1 | 21.81 (13.7-37.26) | <2*10−16 |
| Sex (female) | 2.25 (1.21-4.22) | 0.011 |
Logistic regression summary statistics for association of antibodies, sex, or age and functional assay positivity across all three cohorts. Case status was determined via the results of the function assay tests. 95%CI indicates 95% confidence interval; LMWH, low molecular weight heparin; OD, optical density; PF4 platelet factor 4.
Polyspecific anti-PF4/heparin antibody levels were determined using enzyme-linked immunosorbent assay (ELISA) and ORs are calculated per 1 OD unit increase.
Anti-PF4/heparin antibody IgA and IgM levels were determined via ELISA and ORs are calculated per 1 OD unit increase.
Stepwise regression variables were retained based on AIC and model improvement, while p-values were calculated for each variable in the final model, p-values were not used for variable inclusion and as such may be greater than 0.05 for this reason.
We performed backwards stepwise regression to identify variables that may improve prediction of positive functional assay risk beyond anti-PF4/heparin antibody levels. Due to excessive missingness, the surgery variable in the McMaster cohort was removed prior to stepwise regression. UFH status was excluded from stepwise regressions in the Tours cohort since all functional assay negative patients were treated with UFH. In all three cohorts, sex and anti-PF4/heparin antibody levels were retained in the final stepwise model. The variables containing anti-PF4/heparin IgA and IgM levels also remained in the Greifswald cohort final model but were removed(32, 33, 34) due to statistical correlation between IgM and IgA with IgG antibody values. In final models for all three cohorts, a small increase in functional assay positivity prediction accuracy was observed compared to models with anti-PF4/heparin antibody OD levels alone. No multicollinearity was observed among any of the variables in multivariable regressions (all VIF<1.05). Significant heterogeneity in terms of effect size was observed across the three cohorts for anti-PF4/heparin antibody OD values (Q=38.63; p=6x10−7) and for gender (Q=28.55; p=4x10−9).
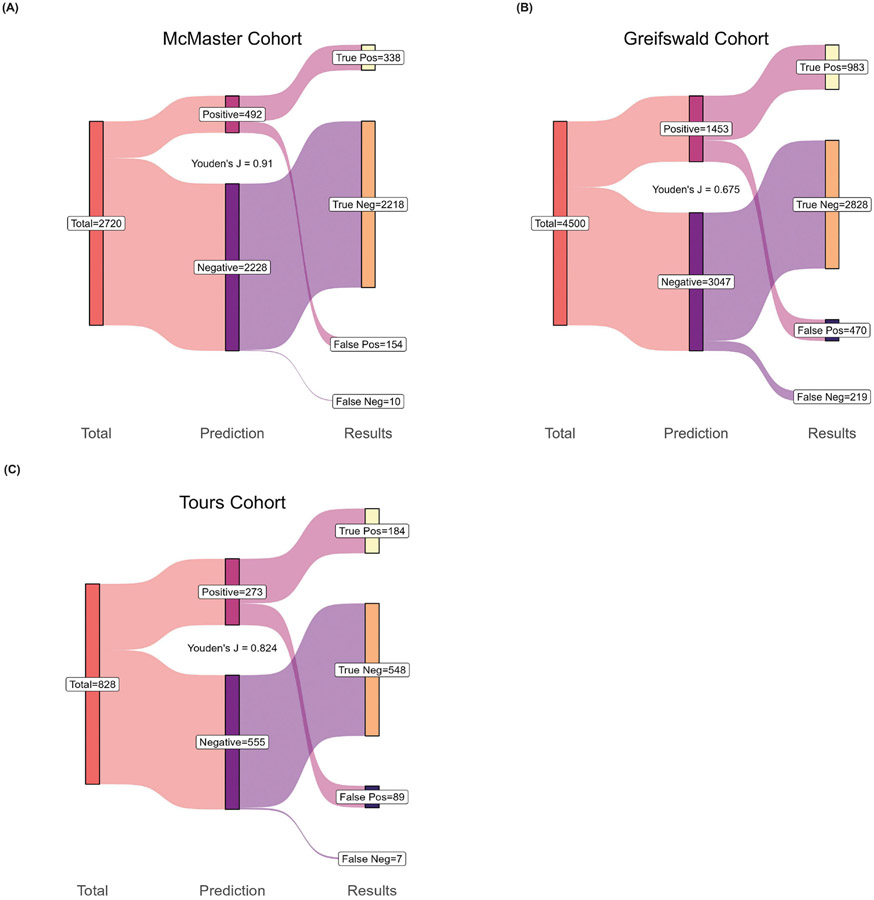
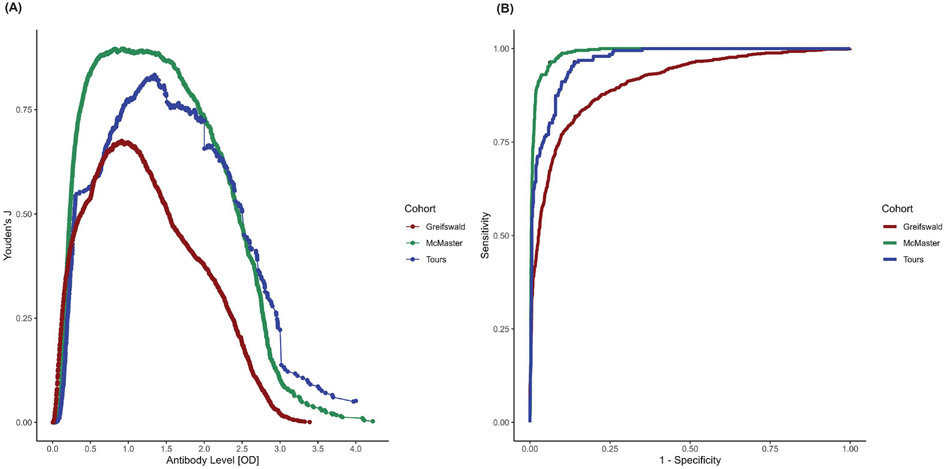
The accuracy of the final stepwise model for each cohort, including total true/false positives and true/false negatives, are summarized in Figure 3. Overall, the multivariable models had a Youden’s J of 0.906, 0.675, and 0.824 and an AUROC of 0.988, 0.909, and 0.967 in the McMaster, Greifswald, and Tours cohorts, respectively (Table 4 and Figure 4B). In the Greifswald sensitivity analysis where IgM and IgA variables were retained, AUROCs were reduced with the addition of IgA (0.893), IgM (0.892) or both variables (0.897) compared to the base model (0.909). In the McMaster sensitivity analysis that used polyspecific ELISA results instead of IgG-specific ELISA results when available, we observed a slight reduction in prediction ability (AUROC=0.9875) versus the original model (AUROC=0.9881). When analysis was restricted to the subset of individuals who had both IgG-specific and polyspecific results, the model using polyspecific ELISA values resulted in an AUROC of 0.976, while the model using IgG-specific ELISA values resulted in an AUROC of 0.980.
Figure 3:
Sankey diagram depicting the prediction for the final stepwise model in the A) McMaster cohort, B) Greifswald cohort, and C) Tours cohort. The performance of the cohorts’ model to discern true positive and negative functional assay individuals from false positive and negative patients are presented. Each diagram (starting from left) shows the number of individuals included in each cohorts’ model (individuals with missing data in any predictor variable were removed). The middle ‘Prediction’ column shows each models’ absolute count for the number of individuals predicted to be functional assay positive or negative. Listed in the right ‘Results’ column are the true functional assay positive patients, the number predicted to have a positive result but tested negative on functional assay (false positive), as well as those who are true negative and false negative (predicted to be functional assay negative but tested positive on function assay).
Table 4:
Model performance for prediction of functional assay positivity
| Antibody Model | Full Model | ||||
|---|---|---|---|---|---|
| Cohort | Youden's J | AIC | Youden's J | AIC | LR Test P value |
| McMaster | 0.8989 | 632.602 | 0.9063 | 631.807 | 0.094 |
| Greifswald | 0.6761 | 2937.655 | 0.6753 | 2934.655 | 0.03288 |
| Tours | 0.8323 | 284.53 | 0.824 | 279.94 | 0.011 |
Comparison of models for prediction of functional assay positivity. Antibody model utilizes anti-PF4/heparin optical density values. Full model includes all variable which remained after stepwise regression in each respective cohort. Youden’s J statistic (sensitivity + specificity −1) measures model performance, with higher values indicating better performance (range:0-1). AIC: Akaike information criterion - smaller values indicate better fit models. LR: likelihood ratio test - statistic tested if full prediction model is statistically significantly a better fitting model.
Figure 4:
A) Performance of anti-PF4/heparin antibody optical density units to predict functional assay positive status across three cohorts. Distribution of antibody OD units are plotted on the x-axis and on the y-axis the Youden’s index (J) value. The peak of each cohort’s plot indicates the antibody OD unit that best dichotomizes functional assay positive versus negative status. B) Area under the receiver operator characteristic (AUROC) curve showing the performance of the final stepwise model to accurately classify functional assay positive and negative individuals across all three cohorts. The legend on the right describes each cohort based on the line plot color with McMaster in green, Greifswald in red, and Tours in blue).
Performance of PF4/heparin Antibody Thresholds for Functional Assay Positivity
Prediction of functional assay positive individuals at each antibody level are presented in Table 5. Using a threshold of 0.4, sixty-five individuals were considered false negatives in the Greifswald cohort based on functional assay testing. Using a threshold of 2.0, totals of 14, 24, and 36 patients were considered false positives, indicating that these individuals would likely be considered HIT positive even though their eventual functional assay result would have been negative. However, no upper antibody threshold eliminated all false positive results in all three cohorts. We also estimated ELISA OD values that best dichotomized positive and negative functional assay status across our three cohorts (Figure 4). The McMaster cohort cut point calculated to be an antibody OD of 0.818 units with J=0.899. The Greifswald cohort exhibited a similar cut point (OD=0.911) but with a smaller Youden’s J (J=0.675). In the Tours cohort, the optimal cut point was an OD of 1.347 units (J=0.835); the higher cutoff in the Tours cohort could reflect the exclusive inclusion of postcardiac surgery individuals given the known high frequency of false-positive ELISA-positive patients in this patient population(35). A combined analysis of all cohorts calculated the overall antibody OD cutoff to be 0.91 units (J=0.744, sensitivity=0.859, and specificity=0.885).
Table 5:
Prediction of functional assay positivity across anti-PF4/heparin antibody level thresholds
| Anti- PF4/heparin IgG OD Threshold |
Sensitivity | Specificity | False Negatives |
False Positives |
|---|---|---|---|---|
| 0.4 (McMaster) | 1 | 0.785 | 0 | 516 |
| 0.4 (Greifswald) | 0.946 | 0.562 | 65 | 1451 |
| 0.4 (Tours) | 1 | 0.555 | 0 | 283 |
| 1.0 (McMaster) | 0.923 | 0.954 | 31 | 111 |
| 1.0 (Greifswald) | 0.784 | 0.887 | 260 | 375 |
| 1.0 (Tours) | 0.979 | 0.794 | 4 | 131 |
| 1.5 (McMaster) | 0.825 | 0.982 | 70 | 43 |
| 1.5 (Greifswald) | 0.549 | 0.966 | 543 | 112 |
| 1.5 (Tours) | 0.892 | 0.893 | 21 | 68 |
| 2.0 (McMaster) | 0.568 | 0.994 | 173 | 14 |
| 2.0 (Greifswald) | 0.3865 | 0.989 | 738 | 36 |
| 2.0 (Tours) | 0.763 | 0.962 | 46 | 24 |
| 2.5 (McMaster) | 0.288 | 0.998 | 285 | 4 |
| 2.5 (Greifswald) | 0.192 | 0.997 | 972 | 10 |
| 2.5 (Tours) | 0.516 | 0.992 | 94 | 5 |
| 3.0 (McMaster) | 0.025 | 1 | 390 | 0 |
| 3.0 (Greifswald) | 0.0216 | 1 | 1177 | 0 |
| 3.0 (Tours) | 0.227 | 0.997 | 150 | 2 |
Specificity and sensitivity calculated for optical density values in each cohort, including OD values currently implemented in HIT guidelines as demarcating for classifying patients as HIT negative (OD<0.4 units) or HIT positive (OD>2.0 units) in the absence of functional assay results. False negative and positive values are absolute counts for each cohort.
Association of Clinical and Demographic Variables with Anti-PF4/heparin Antibodies
In the McMaster cohort, age, female sex, and surgery status was significantly associated with increasing anti-PF4/heparin antibody OD levels (Table 6). In the Tours cohort, female sex was also associated with increasing antibody levels, but age was associated with decreasing antibody levels. A higher platelet count prior to heparin was also associated with increasing antibody levels. As IgM and IgA variables were removed due to correlation with IgG antibody values, no significant associations were observed in the Greifswald cohort. Stepwise regression was performed for prediction of anti-PF4/heparin antibody OD levels in the same manner as was performed for HIT prediction (Table 6). No multicollinearity was observed among any of the variables in multivariable regressions (all VIF<1.1).
Table 6:
Multivariable linear regression of predictor variables and anti-PF4/heparin antibodies
| Trait | Beta [Standard Error] | P-value |
|---|---|---|
| Univariate Regression | ||
| McMaster | ||
| Sex (female) | 0.074 [0.02262] | 0.001 |
| Age (per year) | 0.0016 [0.0007742] | 0.0398 |
| Surgery | 0.082 [0.02829] | 0.00384 |
| Greifswald | ||
| Sex (female) | −0.009 [0.02327] | 0.708 |
| Age (per year) | −0.0005 [0.0008267] | 0.566 |
| Tours | ||
| Sex (female) | 0.434 [0.07069] | 1.30x10−9 |
| Age (per year) | −0.007 [0.002727] | 0.00649 |
| Unfractionated Heparin (vs. LMWH) | −1.515 [0.1516] | <2*10−16 |
| Platelet Count Before Heparin Therapy (1x109 platelets/L) | 0.002 [0.0005177] | 0.000739 |
| Stepwise Regression (Final Model) | ||
| McM aster | ||
| Sex (female) | 0.075 [0.023] | 0.000886 |
| Age (per year) | 0.0017 [0.0007742] | 0.03 |
| Greifswald | ||
| Immunoglobulin A (IgA) | 0.5887 [0.0248] | <2*10−16 |
| Immunoglobulin M (IgM) | 0.3741 [0.0304] | <2*10−16 |
| Tours | ||
| Sex (female) | 0.399 [0.067] | 3.54*10−9 |
| Age (per year) | −0.008 [0.003] | 0.0016 |
| Unfractionated Heparin (vs. LMWH) | −1.462 [0.155] | <2*10−16 |
Association of variables with anti-PF4/heparin antibody levels. Betas (β) and P-values are presented for each variable present in the respective cohorts. For example, surgery status was not available in the Greifswald cohort and as such no regression summary statistics are available for that variable. In each cohort, the model was adjusted for all variable provided in each respective row.
DISCUSSION
In this study, we utilized three large, retrospectively collected cohorts of clinically suspected HIT patients to validate prior evidence for the association of increasing anti-PF4/heparin antibody OD values and female sex as risk factors for functional assay positivity(7, 8). We also provide an evaluation of the performance of guideline directed antibody thresholds for confirmatory functional assay testing in the absence of detailed clinical data. Our results reinforce the necessity of functional assay confirmation in these patients and the importance of evaluating a clinical picture consistent with HIT. Our results also quantify the potential for incorrect diagnosis of HIT using an OD threshold of 2.0 in the absence of detailed clinical data at a rate similar to published guidelines for HIT(11, 17). Additionally, we recapitulate previous work indicating heterogeneity among IgG-specific and polyspecific ELISA OD values, but also observe similar prediction accuracy for functional assay positive status with the two assays(16). Finally, we provide new models of functional assay positivity risk based on basic clinical and laboratory data from our large cohorts of suspected HIT patients. These results suggest that female sex, which is not included in the 4Ts scoring system, might be used to improve evaluation of HIT risk prior to functional assay confirmation.
Clinical guidelines for management, treatment, and prevention of HIT indicate that functional assay confirmation may not be necessary in individuals with ELISA OD values less than 0.4 units (presumed HIT negative) and more than 2.0 (presumed HIT positive)(11, 17). Any false negatives associated with the 0.4 threshold carries a risk that a patient with HIT does not receive the recommended non-heparin, anti-thrombotic treatment to prevent HIT-related sequelae. However, any false positives associated with the 2.0 threshold might result in a thrombocytopenic patient without HIT receiving aggressive monitoring and antithrombotic treatment that is not necessary. Given the logistical challenges surrounding HIPA and SRA testing for HIT confirmation, there is a strong rationale to identify HIT patients without the need for confirmatory functional assays. ASH guidelines state that functional assay confirmation of HIT may not be necessary for individuals with a higher 4Ts score and strongly positive ELISA (OD>2.0)(11, 17). Importantly, 4Ts scoring was not available in our cohorts, which consisted of suspected HIT patients. As such, we calculated the ability to predict functional assay positive status as a proxy for HIT, based solely on ELISA OD threshold of 2.0 units. In our three cohorts we calculated high specificity, 0.994 (McMaster), 0.989 (Greifswald), and 0.962 (Tours), largely in agreement with ASH guidelines that indicate a specificity of 1.00. While the calculated specificities indicate relatively few people were misclassified as being a false positive (74 total false positives among 8,904 patients in all 3 cohorts [0.8%]), these individuals would not be treated appropriately if decision making was based solely on ELISA results. This proportion is comparable to that included in ASH guidelines, which state 10/1000 (1%) suspected HIT patients with intermediate/high 4Ts score and ELISA OD>2.0 units would be false positives. These data suggest that the performance of this anti-PF4/heparin OD threshold for prediction of functional assay positive status is consistent with published guidelines in an observational population, even in the absence of 4Ts scores and detailed clinical data.
In our cohorts, a lower anti-PF4/heparin antibody threshold of 0.4 effectively eliminated all false negatives. However, this result should be interpreted with caution since patients with OD levels less than 0.5 were excluded from receiving clinical confirmation with a functional assay in all cohorts but the Greifswald cohort. Whereas studies from the Chest guidelines indicate that a polyspecific Immucor® ELISA cutoff of 0.4 units had specificities of 0.81, 0.85 and 0.82 for HIT(17), we observed lower specificities of 0.785 (McMaster), 0.562 (Greifswald), and 0.555 (Tours) for functional assay positive status. Sensitivity was 1.00, 0.946, and 1.00 for the McMaster, Greifswald, and Tours cohorts, respectively, and 1.00, 1.00, and 0.95 for the three cohorts in Chest guidelines(17). In the ASH clinical guidelines, the OD 0.4 unit threshold had a sensitivity of 0.98 and a specificity of 0.85. A threshold of OD<0.4 units is implemented to minimize false negatives, increasing sensitivity at the cost of specificity. We recapitulated the observed high sensitivity in our study with all three cohorts but calculated lower specificities when compared to previous clinical guideline papers at an OD cutoff of 0.4 units. Our results support the continued use ASH guidelines for decision making in regard to ELISA negative individuals. This result should be interpreted with caution since our study utilized functional assay positive status rather than definitive HIT in two of our three cohorts.Beyond the guidelines’ OD unit thresholds used in clinical decision making, we were able to identify antibody OD level cut point values that divided positive and negative functional assay results. For the two larger cohorts, anti-PF4/heparin antibody OD values that best divided cases from controls were similar (0.81 and 0.91). These values are higher than the ASH and Chest guidelines for the antibody level threshold (OD<0.4) that separate suspected HIT cases from heparin-treated controls. While a single antibody cut point may simplify clinical decision making, clinical guidelines must weigh the trade-offs of false negatives and false positives, as the performance of a single value comes at a cost of a greater number of false negative and false positive diagnoses. Thus, our data do not support the use of a single antibody cut point to replace guideline-based antibody thresholds or the 4Ts system. Our results corroborate the approach of using an upper and lower OD thresholds to establish the necessity for functional assay confirmation.
Stepwise regression of additional variables into a multivariable functional assay predictor model resulted in statistically better prediction of functional assay positive status compared to models using anti-PF4/heparin antibodies alone. The final models in all three cohorts included anti-PF4/heparin antibody levels and female sex as risk factors with predictive power, recapitulating prior observations from clinical trials(7, 8, 9, 10, 11, 12, 13, 14, 15). These data suggest that female sex, which is not currently a part of the 4Ts scoring system, might be used to improve HIT prediction prior to functional assay confirmation. Because our study utilized functional assay positive status in two of our three cohorts, additional studies are necessary to confirm the utility of sex in predicting definitive HIT.
The strengths of this study include the relatively large size of patients in each of our observational cohorts, whereas prior data has come primarily from small clinical trials. There are several limitations to the current study to mention. Although our study included three large cohorts, we were unable to uniformly collect clinical variables across all three cohorts beyond anti-PF4/heparin antibody levels, age, and sex. This limited our ability to build robust validated prediction models and calculate 4Ts scores. A definitive diagnosis of HIT was not possible in the McMaster and Greifswald cohorts due to the lack of clinical data, although all patients were tested due to clinical suspicion of HIT. Thus, platelet activation assay reactivity was used as the primary outcome in these two cohorts. Our data, particularly in the McMaster and Greifswald cohorts, provide evidence from real-world hospital settings, which often utilize various functional assays and ELISAs.
It should also be noted that the different functional assays used in this study, HIPA test for the Greifswald cohort and SRA in Tours and McMaster cohorts, are both ‘washed’ platelet assays but vary in their endpoints. The HIPA test measures the formation of platelet aggregation using four different random donors, while the SRA test measures radiolabeled serotonin that is released from activated platelets using two HIT positive donors(4, 26). Studies have shown however, these tests are quite similar in positivity rates across ELISA OD ranges and in positivity rates of clinically suspected HIT patients(25, 26). Both assays are considered “gold-standards” for diagnosing HIT, but we acknowledge the possibility that our results may have been affected by the different functional assays employed in this study.
Optical density readouts from multiple ELISAs enabled several sensitivity analyses, formal statistical correlation, and calculation of coefficient of variation to assess technical validity and robustness of prediction between assays. Previous studies have shown significant heterogeneity between IgG-specific and polyspecific ELISAs for anti-PF4/heparin antibodies, but still observe consistent predictive ability in terms of clinical HIT(16). We recapitulate these results in our study, where we observed an average CoV of 31.9 percent between IgG-specific and polyspecific ELISAs, but similar prediction of functional assay positive status in terms of AUROC. The AUROC in McMaster (PF4-PVS polyspecific antibodies) was 0.988, in Greifswald (IgG-specific ELISA) was 0.909, and in Tours (PF4-PVS polyspecific ELISA) was 0.967. The heterogeneity observed between IgG-specific and polyspecific ELISAs in our study and others might suggest that standardization of ELISAs, such as utilization of standard curves for IgG levels rather than OD values or exclusive utilization of IgG-specific ELISAs, is necessary. However, we observe comparable associations and AUROCs for both polyspecific and IgG-specific assays, suggesting that this heterogeneity between ELISAs is not a significant diagnostic issue.
Additional limitations of our study include the observational nature for two of the three cohorts, which precluded our ability capture detailed clinical data and to exclude false positive HIT cases since functional assay positive status was used. In the McMaster and Greifswald cohorts, we observed significant heterogeneity in our significant associations, and we cannot exclude confounding by important clinical variables such as UFH versus LMWH treatment, time between UFH exposure and assay, and thrombosis. Similarly, clinicians across hospital systems are not always familiar with HIT-specific guidelines and we cannot exclude that assays may have been ordered inappropriately in McMaster and Greifswald. The sampling design for the Tours cohort, which did utilize definitive HIT as the outcome, included exclusions for functional assay positivity based on a lower limit of OD values and enrichment for functional assay positivity. This may have biased our results for functional assay positivity prediction in our smallest cohort. CPB surgery was also an inclusion criterion for heparin-treated controls in the Tours cohort, which may have introduced additional confounding(35). The different ELISAs and photometers used for OD levels in different labs may also have resulted in inconsistencies in OD levels between cohorts, especially considering our observed heterogeneity between IgG-specific and polyspecific assays(36). Finally, the blood samples were not tested using PF4-enhanced platelet activation assays, which increase diagnostic sensitivity of functional assays for HIT. For example, functional assay-negative blood samples of OD>2.0 could reflect “SRA-negative” HIT cases that might have been identified by PF4-enhanced functional assay(37).
CONCLUSIONS
Identification of patients at-risk for HIT prior to the manifestation of symptoms remains a barrier in heparin therapy. In this study, we recapitulated previously identified risk factors of HIT, providing precise effect sizes for anti-PF4/heparin antibody OD levels and female sex. Our data suggest that utilization of an anti-PF4/heparin antibody threshold of 2.0 in the absence of clinical picture will result in a modest number of false positive functional assay results. Our data also suggest that performance of guideline based anti-PF4/heparin antibody thresholds is robust for functional assay positive status in a large observational population, even in the absence of 4Ts scores and detailed clinical data. Given further validation and clinical information, the models developed here, which included female sex in all cohorts, might be used to improve prediction models aimed at early identification of patients at-risk for HIT. Our data reinforce the necessity of functional assay confirmation and suggest that, despite heterogeneity, polyspecific and IgG-specific anti-PF4/heparin antibody assays predict functional assay positive status similarly , even in the absence of 4Ts scores and detailed clinical data.
HIGHLIGHTS.
We generate data for the largest known cohort of suspected HIT patients (n=8,904).
We validate risk factors for positive functional assay in HIT.
Our data reinforce the necessity of functional assay confirmation in HIT.
We observe heterogeneity between polyspecific and IgG-specific ELISAs.
Despite heterogeneity, different ELISAs similarly predict functional assay positivity.
Acknowledgements
We are grateful to Ulrike Strobel, Carmen Freyer, Katrin Stein, Ines Warnig, Ricarda Raschke, Jessica Fuhrmann for excellent technical support in performing all HIT assays in the Greifswald cohort. We are grateful to Kayle Lucier for support in the acquisition of the McMaster dataset.
Funding:
BG is funded by the National Institutes of Health’s (NIH’s) Environmental Health Sciences (NIEHS) T32 Training Grant – T32 ES007091. This research is funded by the NIH’s National Heart, Lung, and Blood Institute (NHLBI) under award K01HL143137 (JHK), R01HL158686 (JHK), R01 HL156993 (JHK), and U19 HL065962 (DMR) and the National Institute of General Medical Sciences (NIGMS) under award P50GM115305 (DMR). Acquisition of the replication cohort from the University of Tours with the independent replication population was supported by the IRTH (Institut pour la Recherche sur la Thrombose et l’Hémostase) and by a PHRC grant (PHRN09-YG/FRIGTIH). The study was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Project Number 374031 971–TRR240 (AG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts of Interest
Theodore (Ted) E. Warkentin, MD, has received lecture honoraria from Instrumentation Laboratory, and royalties from Informa (Taylor & Francis) and UptoDate (Wolters Kluwer); has provided consulting services to Aspen Canada, Aspen Global, CSL Behring, Ergomed, Paradigm Pharmaceuticals, Octapharma, and Veralox Therapeutics; has received research funding from Instrumentation Laboratory; and has provided expert witness testimony relating to heparin-induced thrombocytopenia (HIT) and non-HIT thrombocytopenic and coagulopathic disorders.
Andreas Greinacher reports grants and non-financial support from Aspen, Boehringer Ingelheim, MSD, Bristol Myers Squibb (BMS), Paringenix, Bayer Healthcare, Gore Inc., Rovi, Sagent, Biomarin/Prosensa, personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma, BMS, Chromatec, Instrumentation Laboratory, non-financial support from Boehringer Ingelheim, Portola, Ergomed, GTH e.V. outside the submitted work. Kathleen Selleng reports a research funding from Immucor, personal fees from Aspen and Viatris and non-financial support from SOBI outside the submitted work. There are no other conflicts.
REFERENCES CITED
- 1.Prince M, Wenham T. Heparin-induced thrombocytopaenia. Postgrad Med J. 2018;94(1114):453–7. [DOI] [PubMed] [Google Scholar]
- 2.Warkentin T, Hayward C, Boshkov L, Santos A, Sheppard J, Bode A, et al. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood. 1994;84(11):3691–9. [PubMed] [Google Scholar]
- 3.Kelton J, Smith J, Warkentin T, Hayward C, Denomme G, Horsewood P. Immunoglobulin G from patients with heparin-induced thrombocytopenia binds to a complex of heparin and platelet factor 4. Blood. 1994;83(11):3232–9. [PubMed] [Google Scholar]
- 4.Bakchoul T, Zöllner H, Greinacher A. Current insights into the laboratory diagnosis of HIT. International Journal of Laboratory Hematology. 2014;36(3):296–305. [DOI] [PubMed] [Google Scholar]
- 5.Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. Journal of Thrombosis and Haemostasis. 2006;4(4):759–65. [DOI] [PubMed] [Google Scholar]
- 6.Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. Journal of Thrombosis and Haemostasis. 2008;6(8):1304–12. [DOI] [PubMed] [Google Scholar]
- 8.Bakchoul T, Giptner A, Najaoui A, Bein G, Santoso S, Sachs UJH. Prospective evaluation of PF4/heparin immunoassays for the diagnosis of heparin-induced thrombocytopenia. Journal of Thrombosis and Haemostasis. 2009;7(8):1260–5. [DOI] [PubMed] [Google Scholar]
- 9.Pouplard C, May M-A, Iochmann S, Amiral J, Vissac A-M, Marchand M, et al. Antibodies to Platelet Factor 4–Heparin After Cardiopulmonary Bypass in Patients Anticoagulated With Unfractionated Heparin or a Low-Molecular-Weight Heparin. Circulation. 1999;99(19):2530–6. [DOI] [PubMed] [Google Scholar]
- 10.Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. The American Journal of Medicine. 1996;101(5):502–7. [DOI] [PubMed] [Google Scholar]
- 11.Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Advances. 2018;2(22):3360–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhakal B, Kreuziger LB, Rein L, Kleman A, Fraser R, Aster RH, et al. Disease burden, complication rates, and health-care costs of heparin-induced thrombocytopenia in the USA: a population-based study. The Lancet Haematology. 2018;5(5):e220–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur J, Arsene C, Yadav SK, Ogundipe O, Malik A, Sule AA, et al. Risk Factors in Hospitalized Patients for Heparin-Induced Thrombocytopenia by Real World Database: A New Role for Primary Hypercoagulable States. Journal of Hematology. 2021;10(4):171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warkentin TE, Sheppard J- AI, Sigouin CS, Kohlmann T, Eichler P, Greinacher A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108(9):2937–41. [DOI] [PubMed] [Google Scholar]
- 15.Mehta BP, Sims JR, Baccin CE, Leslie-Mazwi TM, Ogilvy CS, Nogueira RG. Predictors and Outcomes of Suspected Heparin-Induced Thrombocytopenia in Subarachnoid Hemorrhage Patients. Interventional Neurology. 2013;2(4):160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liederman Z, Van Cott EM, Smock K, Meijer P, Selby R. Heparin-induced thrombocytopenia: An international assessment of the quality of laboratory testing. J Thromb Haemost. 2019;17(12):2123–30. [DOI] [PubMed] [Google Scholar]
- 17.Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e495S–e530S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nazi I, Arnold DM, Warkentin TE, Smith JW, Staibano P, Kelton JG. Distinguishing between anti-platelet factor 4/heparin antibodies that can and cannot cause heparin-induced thrombocytopenia. Journal of Thrombosis and Haemostasis. 2015;13(10):1900–7. [DOI] [PubMed] [Google Scholar]
- 19.Horsewood P, Warkentin TE, Hayward CPM, Kelton JG. The epitope specificity of heparin-induced thrombocytopenia. British Journal of Haematology. 1996;95(1):161–7. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan D, Carter C, Kelton J. A diagnostic test for heparin-induced thrombocytopenia. Blood. 1986;67(1):27–30. [PubMed] [Google Scholar]
- 21.Giles JB, Steiner HE, Rollin J, Shaffer CM, Momozawa Y, Mushiroda T, et al. Genome-wide association study of platelet factor 4/heparin antibodies in heparin-induced thrombocytopenia. Blood Adv. 2022;6(14):4137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karnes JH, Rollin J, Giles JB, Martinez KL, Steiner HE, Shaffer CM, et al. ABO O blood group as a risk factor for platelet reactivity in heparin-induced thrombocytopenia. Blood. 2022;140(3):274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juhl D, Eichler P, Lubenow N, Strobel U, Wessel A, Greinacher A. Incidence and clinical significance of anti-PF4/heparin antibodies of the IgG, IgM, and IgA class in 755 consecutive patient samples referred for diagnostic testing for heparin-induced thrombocytopenia. European Journal of Haematology. 2006;76(5):420–6. [DOI] [PubMed] [Google Scholar]
- 24.Greinacher A, Michels I, Kiefel V, Mueller-Eckhardt C. A Rapid and Sensitive Test for Diagnosing Heparin-Associated Thrombocytopenia. Thromb Haemost. 1991;66(12):734–6. [PubMed] [Google Scholar]
- 25.Eekels JJM, Althaus K, Bakchoul T, Kroll H, Kiefel V, Nazy I, et al. An international external quality assessment for laboratory diagnosis of heparin-induced thrombocytopenia. Journal of Thrombosis and Haemostasis. 2019;17(3):525–31. [DOI] [PubMed] [Google Scholar]
- 26.Greinacher A, Ittermann T, Bagemühl J, Althaus K, Fürll B, Selleng S, et al. Heparin-induced thrombocytopenia: towards standardization of platelet factor 4/heparin antigen tests. Journal of Thrombosis and Haemostasis. 2010;8(9):2025–31. [DOI] [PubMed] [Google Scholar]
- 27.Rollin J, Pouplard C, Gratacap M-P, Leroux D, May M-A, Aupart M, et al. Polymorphisms of protein tyrosine phosphatase CD148 influence FcγRIIA-dependent platelet activation and the risk of heparin-induced thrombocytopenia. Blood. 2012;120(6):1309–16. [DOI] [PubMed] [Google Scholar]
- 28.Gruel Y, Vayne C, Rollin J, Weber P, Faille D, Bauters A, et al. Comparative Analysis of a French Prospective Series of 144 Patients with Heparin-Induced Thrombocytopenia (FRIGTIH) and the Literature. Thrombosis and Haemostasis. 2020;120(07):1096–107. [DOI] [PubMed] [Google Scholar]
- 29.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5. [DOI] [PubMed] [Google Scholar]
- 30.Brown CE. Coefficient of Variation. Springer; Berlin Heidelberg; 1998. p. 155–7. [Google Scholar]
- 31.Kelley M, Desilva B. Key elements of bioanalytical method validation for macromolecules. The AAPS Journal. 2007;9(2):E156–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JH. Multicollinearity and misleading statistical results. Korean Journal of Anesthesiology. 2019;72(6):558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayman EO, Dexter F. Multicollinearity in Logistic Regression Models. Anesthesia & Analgesia. 2021;133(2):362–5. [DOI] [PubMed] [Google Scholar]
- 34.Comparison of statistical and machine learning methods in modelling of data with multicollinearity. International Journal of Modelling, Identification and Control. 2013;18(4):295–312. [Google Scholar]
- 35.Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6(8):1304–12. [DOI] [PubMed] [Google Scholar]
- 36.Greinacher A, Ittermann T, Bagemuhl J, Althaus K, Furll B, Selleng S, et al. Heparin-induced thrombocytopenia: towards standardization of platelet factor 4/heparin antigen tests. J Thromb Haemost. 2010;8(9):2025–31. [DOI] [PubMed] [Google Scholar]
- 37.Warkentin TE, Nazy I, Sheppard JI, Smith JW, Kelton JG, Arnold DM. Serotonin-release assay-negative heparin-induced thrombocytopenia. Am J Hematol. 2020;95(1):38–47. [DOI] [PubMed] [Google Scholar]