Abstract
Purpose
The purpose of this study was to isolate and culture human conjunctival mesenchymal stromal cells (Conj-MSCs) from cadaveric donor tissue, and to obtain and characterize their extracellular vesicles (EVs) and their effect on conjunctival epithelium.
Methods
Stromal cells isolated from cadaveric donor conjunctival tissues were cultured and analyzed to determine whether they could be defined as MSCs. Expression of MSC markers was analyzed by flow cytometry. Cells were cultured in adipogenic, osteogenic, and chondrocyte differentiation media, and stained with Oil Red, Von Kossa, and Toluidine Blue, respectively, to determine multipotent capacity. EVs were isolated from cultured Conj-MSCs by differential ultracentrifugation. EV morphology was evaluated by atomic force microscopy, size distribution analyzed by dynamic light scattering, and EVs were individually characterized by nanoflow cytometry. The effect of EVs on oxidative stress and viability was analyzed in in vitro models using the conjunctival epithelial cell line IM-HConEpiC.
Results
Cultured stromal cells fulfilled the criteria of MSCs: adherence to plastic; expression of CD90 (99.95 ± 0.03% positive cells), CD105 (99.04 ± 1.43%), CD73 (99.99 ± 0.19%), CD44 (99.93 ± 0.05%), and absence of CD34, CD11b, CD19, CD45 and HLA-DR (0.82 ± 0.91%); and in vitro differentiation into different lineages. Main Conj-MSC EV subpopulations were round, small EVs that expressed CD9, CD63, CD81, and CD147. Conj-MSC EVs significantly decreased the production of reactive oxygen species in IM-HConEpiCs exposed to H2O2 in similar levels than adipose tissue-MSC-derived EVs and ascorbic acid, used as controls.
Conclusions
It is possible to isolate human Conj-MSCs from cadaveric tissue, and to use these cells as a source of small EVs with antioxidant activity on conjunctival epithelial cells.
Keywords: extracellular vesicles (EVs), conjunctiva, mesenchymal stromal cells (MSCs), exosomes, oxidative stress
In recent years, significant progress has been made in understanding the role of extracellular vesicles (EVs) in the eye and their involvement in various ocular diseases.1–3 In addition, the potential therapeutic role of EVs in the treatment of ocular diseases has been explored.4–6 However, much of the latter research has been conducted using EVs from non-ocular sources. Most EVs are derived from mesenchymal stromal cells (MSCs), adult multipotent cells with special immunomodulatory, secretory, and homing potential that have been used in cell therapy because of these properties. However, despite their low immunogenicity, MSCs are not completely free of some undesirable effects that can occur with any living cell therapy, such as uncontrolled differentiation of engrafted cells or malignant transformation. There is increasing evidence that much of the therapeutic effect displayed by MSCs is due to their paracrine action,7 which is why their secretome is also being studied. More recently, interest has focused on MSC-derived EVs, which are emerging as a new cell-free therapy.8,9
Although all MSCs share common properties, it is well known that they have some differences depending on the tissue of origin, and different MSC-derived EVs also have specific functions and effects.8,10 Therefore, it is relevant to investigate the presence, characteristics, and effects of ocular MSC and their EVs. The eye is an extremely complex organ in which many tissues and cells work together interconnectedly to form different units, such as the lacrimal functional unit (LFU). Therefore, the study of key players in intercellular communication, such as EVs, is paramount to understand ocular pathophysiology. The LFU mainly includes the lacrimal glands, the ocular surface, and the interconnecting innervation.11 The conjunctiva, whose epithelium forms part of the ocular surface, is the tissue responsible for many of the protective functions that maintain the health of the LFU, and for this reason conjunctival EVs may be particularly relevant both as biomarkers of disease and as therapeutic tools. The conjunctiva is composed of a stratified epithelium containing squamous epithelial cells and goblet cells and by a loose stroma containing, among other cells, conjunctival MSCs (Conj-MSCs).12 There are several studies investigating ocular surface EVs, most of them focused on EVs derived from corneal epithelium,13–15 with just one study analyzing conjunctival epithelial EVs.16 Regarding ocular MSC-derived EVs, Shojaati et al. showed that EVs isolated from MSCs from corneal stromal stem cells (CSSCs) had an essential role in the regeneration of the cornea after wounding.17 However, to the best of our knowledge, there are no reports on Conj-MSC-derived EVs. Due to the interesting effect exerted by other MSC-EVs, in this study we characterize Conj-MSC EVs obtained from Conj-MSCs isolated from human cadaveric conjunctival tissue.
Materials and Methods
Human Tissue
This study was approved by the IOBA Research Committee and the Ethics Committee of the University of Valladolid. This research followed the Tenets of the Declaration of Helsinki and all Spanish Regulations concerning the use of human tissue for biomedical research.
Corneoscleral rings were obtained from cadaveric donors (n = 6) with informed research consent from Barraquer Eye Bank of Barcelona (Spain). Donors (4 men and 2 women, 76.7 ± 10.1 years old) did not have any ocular disease. Bulbar conjunctival tissue was carefully isolated from the rest of the tissues and used for research purposes.
Human adipose tissue MSCs that form part of IOBA registered culture collection were cultured to isolate EVs used as controls. These cells were originally obtained from lipoaspirates after informed consent was signed.
Isolation of Fibroblast-Like Cells From Human Conjunctival Tissue
Fibroblast-like cells were isolated from human bulbar conjunctiva, as previously described.18 Briefly, conjunctival stroma was minced in small pieces that were plated in 12-well plastic plates (Nunc, Roskilde, Denmark). Cells were cultured in Dulbecco's modified Eagle's Medium (DMEM)/F12 (Invitrogen-Gibco, Inchinnan, UK) supplemented with 10% fetal bovine serum (FBS; Gibco, Waltham, MA, USA), 2.5 µg/mL fungizone (Invitrogen), and 50 units/mL penicillin/streptomycin (Invitrogen). Primary cells were expanded, passaged up to 10 times, and stored in liquid nitrogen until further use.
Conjunctival Epithelial Cell Culture
The commercially available human conjunctival epithelial cell line IM-HConEpiC (Innoprot, Derio, Spain) was cultured in DMEM/F12 medium supplemented with 10% FBS, 10 ng/mL epidermal growth factor (Invitrogen), 1 µg/mL insulin (Sigma-Aldrich, St. Louis, MO, USA), and 50 units/mL penicillin and 50 µg/mL streptomycin (Gibco), as previously described.19 Cells were cultured at 37°C and 5% CO2, cell growth was monitored daily, and culture medium was changed every second day.
Characterization of Conjunctival Mesenchymal Stromal Cells by Flow Cytometry
Fibroblast-like cells in passages 2 to 4 were thawed and cultured in DMEM (1x) + GlutaMAX medium (Gibco) supplemented with 10% FBS and 2% antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin; Gibco).
To determine whether these fibroblast-like cells were MSCs, we followed the criteria described in the International Society for Cell Therapy (ISCT) position statement.20 Expression of MSC markers (positive expression of CD105, CD90, CD73, and CD44 and negative expression of hematopoietic markers CD34, CD11b, CD19, CD45, and HLA-DR) was analyzed by flow cytometry in a Beckman Coulter Gallios cytometer (Beckman Coulter Inc., Brea, CA, USA) using the Human MSC Analysis kit (BD Biosciences, Franklin Lakes, NJ, USA). Five independent experiments were performed.
Differentiation of Conjunctival Mesenchymal Stromal Cells Into Adipocytes, Osteocytes, and Chondrocytes
To determine in vitro multipotent capacity, cells in passages 4 to 5 were cultured in adipogenic, osteogenic, and chondrogenic differentiation media (n = 3 for each lineage).
Cells were seeded at 10,000 cells/cm2 density and grown in the StemPro Adipogenesis Differentiation Kit (Invitrogen-Gibco) for 15 days. Then, the cells were fixed, and stained with Oil Red to detect lipids. Cells (10,000 cells/cm2) cultured with StemPro Osteogenesis Differentiation Kit (Invitrogen-Gibco) were cultured for 21 days, fixed, and stained with Von Kossa staining to determine the presence of calcium deposits. For chondroblast differentiation, a pellet of 5·105 cells was cultured for 26 days in StemXVivo Chondrogenic Base Media with StemXVivo Chondrogenic Supplement (RD Systems, Minneapolis, MN, USA) for 27 days. Pellets were then fixed with 4% paraformaldehyde, embedded in Optimal Cutting Temperature Compound (OCT), sectioned, and stained with Toluidine Blue to detect the proteoglycans produced by chondrocytes.
EV Isolation by Differential Ultracentrifugation
Conjunctival MSCs and AT-MSCs (used as control) in passages 4 to 10 were grown until cells reached 70% confluence and then, medium was changed to EVs-depleted FBS-supplemented medium. Secretomes were collected after 48 hours. EVs were isolated from these secretomes by differential centrifugation and ultracentrifugation at 4°C (300 g for 10 minutes; 2000 g for 20 minutes; 10,000 g for 30 minutes; and 2 steps of 75 minutes at 100,000 g) using a Beckman Coulter L8-70M ultracentrifuge and SW28 rotor (Beckman Coulter Inc.). The pellet containing the EVs was recovered in PBS + 25 mM D-(+)-Trehalose dehydrate (from Saccharomyces cerevisiae, powder, ≥99%; Sigma-Aldrich) and kept at −80°C until further use.
Atomic Force Microscopy
EV topography was evaluated by atomic force microscopy (AFM) using the tapping mode in air in an Asylum Research MFP3D Bio AFM microscope (Oxford Instruments, Abingdon, UK). Samples were deposited in 15 mm Highest Grade V1 AFM Mica Discs (Ted Pella, Inc., Redding, CA, USA) and left overnight at 4°C. Then, the mica discs with the samples were rinsed with ultrapure water to remove salts and dried with N2. A 240AC probe (MikroMasch, Tallinn, Estonia) was used. We followed the procedure published by Skilar and Chemysev.21 Image analysis was performed with Asylum AR 16.23.224 and Gwyddion 2.56 software.
Dynamic Light Scattering
The size distribution and the zeta potential of the extracted EVs (n = 3) were analyzed by dynamic light scattering (DLS) using a Malvern Zetasizer Advance Pro Red Label (Malvern Panalytical, Malvern, UK). EVs were diluted in PBS/trehalose (25 mM) to a final volume of 1 mL (refractive index = 1.33 and viscosity = 1 mPa.s) and filtered through a 0.45 µm polytetrafluoroethylene polymer (PTFE) filter. Measurements were performed at 25°C using ZEN0040 and DTS1070 cuvettes for size distribution and zeta potential measurements, respectively. Distribution of vesicle size was determined with intensity spectra data. Three different samples were analyzed, and each sample was measured three times.
EV Detection and Counting
Flow cytometry analysis was performed on the Beckman Coulter CytoFLEX LX Flow Cytometer using a modified protocol from Brennan et al.22 with a VSSC gain = 300 and VSSC-H threshold = 5500. Events were gated on the VSSC-width log × VSSC-H log cytogram to remove EV aggregates (singlet gate). A rectangular gate was set on the VSSC-H log × RSSC-H log cytogram containing the 80 nm and 500 nm bead populations and defined as “PS beads 80 nm to 500 nm gate” followed by a “stable time gate” set on the time histogram in order to identify the microparticle region (Supplementary Figure S1). Data were re-analyzed using the Beckman Coulter CytExpert version 2.5.0.77 software and the DeNovo Software FCSExpress Plus version 7.
EV-Bead Conjugated Flow Cytometry
The levels of CD9, CD63, CD81, and CD147 on the EVs were assessed by EV-bead conjugated flow cytometry analysis, which was performed on the Beckman Coulter CytoFLEX LX Flow Cytometer using a modified protocol from Brennan et al.22 The 1.25 × 107 EVs/test was mixed with 0.2 µL/test aldehyde/sulfate latex beads (4 µm; Thermo Fisher Scientific, Waltham, MA, USA) in 200 µL PBS rotating overnight at 4°C, with beads without EVs being used as a negative control. To block nonspecific protein binding to beads 200 µL 2% BSA (2% BSA, 2 mM EDTA, and 0.1% sodium azide in PBS) was then added to the samples to a final volume of 400 µL for 1 hour at room temperature (RT), followed by 45 µL of 1 M glycine for 30 minutes at RT. The samples were then centrifuged at 5500 g for 5 minutes, the supernatant was removed, the beads were resuspended in 100 µL PBS, and 2 µL Fc block was added for 10 minutes at RT. The samples were then centrifuged at 5500 g for 5 minutes and washed with 500 µL PBS 3 times. The beads were resuspended in 1% BSA 100 µL/test and aliquoted into fresh tubes. The beads were stained with antibodies for 30 minutes on ice and then centrifuged at 5500 × g for 5 minutes and washed with 500 µL PBS 3 times. The samples were then resuspended in 200 µL PBS and flow cytometry analysis was performed on the Beckman Coulter CytoFLEX LX Flow Cytometer. Gating of EV-decorated 4 µm diameter beads was performed based on FSC/SSC parameters, so that unbound EVs or possible antibody aggregates were excluded from the analysis.
Measurement of Antioxidant Effect
To analyze the effect of EVs on oxidative stress, IM-HConEpiCs (20,000 cells/cm2) in passages 15 to 18 were seeded in 96-well plates. When the cells reached confluency (after 24 hours), they were exposed for 3 hours to 50 µg/mL MSC-derived EVs or to 250 µM ascorbic acid (AnalaR NORMADUR, VWR International bvba, Levven, Belgium) in serum-free medium. Ascorbic acid (also known as vitamin C) was used as a positive control for antioxidant activity. Cells were then loaded with 2′,7′-dichlorofluorescin diacetate (H2DCF-DA; Sigma-Aldrich) in serum-free medium for 30 minutes at 37°C. H2DCF-DA passively diffuses into the cells and indicates reactive oxygen species (ROS) upon conversion to the fluorescent metabolite dichlorofluorescein (DCF). Finally, cells were exposed to 200 µM H2O2 to induce oxidative stress. To quantify ROS, fluorescence was measured at 488 nm excitation wavelength and 522 nm emission wavelength using the SpectraMax M5 fluorescence plate reader (Molecular Devices, Sunnyvale, CA, USA). Three independent experiments were performed in duplicates.
Cell Viability Assay
After exposure to the EVs, the viability of conjunctival epithelial IM-HconEpiC cells was measured by AlamarBlue assay (n = 3). Cells were treated with 50 µg/mL MSCs-derived EVs in serum-free medium for 3 hours. Later, cells were incubated with 10% AlamarBlue reagent (BioRad Laboratories) for 2 hours, and the fluorescence was measured in the SpectraMax M5 fluorescence plate reader at 560 nm excitation and 590 nm emission wavelengths. Three independent experiments were performed in duplicates.
Statistical Analysis
Data are shown as mean ± standard deviation (SD). After assuring homoscedasticity with the Brown-Forsythe test, the analysis of differences between groups was conducted by 1-way analysis of variance (ANOVA) followed by post hoc multiple comparison tests (Sidak test), using GraphPad Prism 8.2.0 software. The P values ≤ 0.05 were considered statistically significant.
Results
Cultured Conjunctival Stromal Cells Isolated From Cadaveric Tissue Fulfilled the Criteria of MSCs
Human conjunctival stromal cells were successfully isolated from cadaveric donor tissue and expanded in vitro. Cultured stromal cells fulfilled the minimal criteria of MSCs established in the ISCT position statement20: (i) adherence to plastic; (ii) expression/absence of specific markers; and (iii) in vitro differentiation into different cell lineages.
Conjunctival stromal cells easily adhered to the plastic surface of culture flasks and plates and acquired a spindle-shape morphology when cultured in standard conditions.
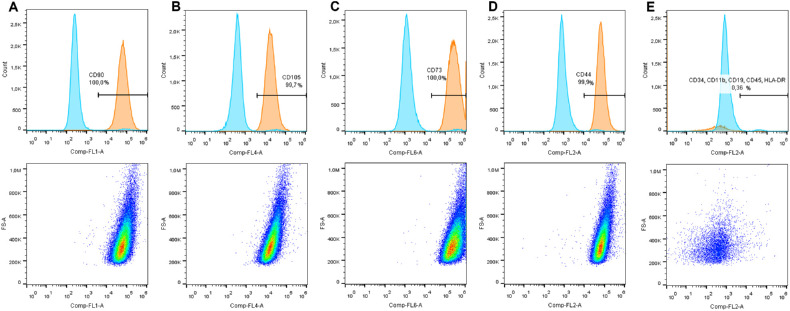
As recommended by the ISCT, the expression of specific markers20 was assessed by flow cytometry (Fig. 1). Cultured conjunctival stromal cells expressed CD90 (99.95 ± 0.03% positive cells), CD105 (99.04 ± 1.43% positive cells), CD73 (99.99 ± 0.19% positive cells), CD44 (99.93 ± 0.05% positive cells), and lacked CD34, CD11b, CD19, CD45, and HLA-DR markers (0.82 ± 0.91% positive cells).
Figure 1.
Immunophenotypic characterization of Conj-MSCs by flow cytometry. Cells expressed CD90 (A), CD105 (B), CD73 (C), and CD44 (D), and lacked the hematopoietic markers CD34, CD11b, CD19, CD45, and HLA-DR (E). Representative graphs of one experiment (n = 5).
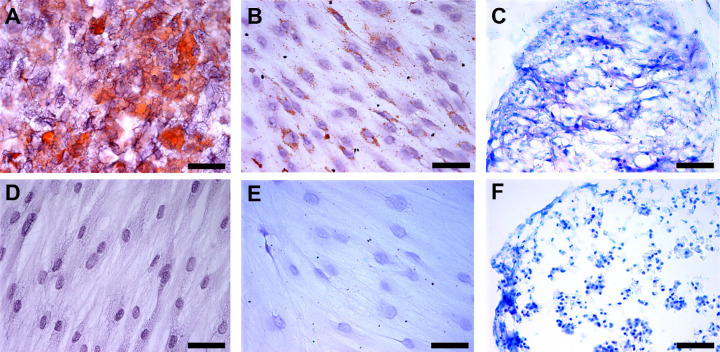
When cells were cultured under adipogenic conditions they showed intracellular lipid droplets that stained in red with Oil Red (Fig. 2A). When cultured under osteogenic conditions, cells showed calcium deposits, as observed with Von Kossa brown staining (Fig. 2B). Finally, cells grown under chondrogenic conditions formed a mass that was sectioned and stained with Toluidine Blue to detect the presence of sulfated proteoglycans in the extracellular matrix which appear stained in purple (Fig. 2C). Cells grown in regular conditions did not differentiate (Figs. 2D-F).
Figure 2.
In vitro differentiation potential of human Conj-MSCs. Cells differentiated into three different lineages. (A) Conj-MSCs differentiated into adipocytes. Lipids are marked in red by Oil Red staining. (B) Cells differentiated into osteocytes that have calcium deposits as seen in brown by Von Kossa staining. (C) Cells differentiated into chondroblasts, as observed in chondroblast pellet sections stained with Toluidine Blue dye that stain the proteoglycans in purple. Cells cultured in the same conditions but without differentiation media were also stained with Oil Red (D), Von Kossa (E), and Toluidine blue (F), but no lipids, calcium deposits, or proteoglycans were detected. Bar = 50 µm.
Conj-MSCs-Derived EV Characterization
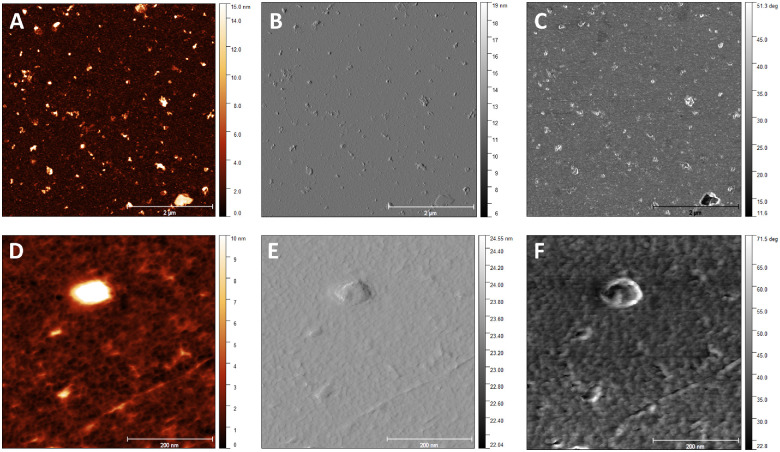
EVs were successfully isolated from the Conj-MSC secretome using differential centrifugation and ultracentrifugation procedures. Isolated Conj-MSC-derived EVs were characterized by different means. First, EVs were morphologically analyzed by AFM (Fig. 3). Wide-field images revealed the presence of EVs with different sizes (Figs. 3A-C), most of them in the range of 50 to 120 nm, with an average diameter of 70 to 80 nm, and a height of 13 to 15 nm, as seen in analyzed images in height retrace mode. Close-up images (Figs. 3D-F) confirmed that Conj-MSC-derived EVs have a globular structure.
Figure 3.
Characterization of Conj-MSC-derived EVs by AFM. Representative wide-field (A, B, C) and close-up (D, E) AFM topographic images showing Conj-MSC-derived EVs. Different sizes were observed in height (A), amplitude (B), and phases (C) images. A single EV of 80 nm is shown in height (D), amplitude (E), and phases (F) images.
Conj-MSC-derived EVs were also evaluated by DLS (see the Table). Intensity-distribution size showed heterogenicity, analyzed with the polydispersity index (see the Table). The samples showed a bimodal distribution, with two main peaks, being the most abundant (peak 1 = 91.5 ± 5.07 %) in the range of small EVs. The width of peak 1 suggests the presence of 2 subpopulations with sizes between 100 nm and 400 nm. The surface charge of Conj-MSC-derived EVs was −21,17 ± 3,741 mV.
Table.
Characterization of EVs by DLS
| Mean | SD | RSD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Z-average (nm) | 208.4 | 34.4 | 16.51 | 174.2 | 267.5 |
| Polydispersity index (PI) | 0.3782 | 0.0628 | 16.62 | 0.3006 | 0.4912 |
| Peak 1 mean by intensity (nm) | 226.3 | 40.3 | 17.8 | 183.3 | 308 |
| Peak 1 area by intensity (%) | 91.5 | 5.066 | 5.536 | 82.03 | 98.95 |
| Peak 2 mean by intensity (nm) | 3858 | 1612 | 41.79 | 46.21 | 5468 |
| Peak 2 area by intensity (%) | 7.54 | 4.012 | 53.2 | 1.047 | 12.18 |
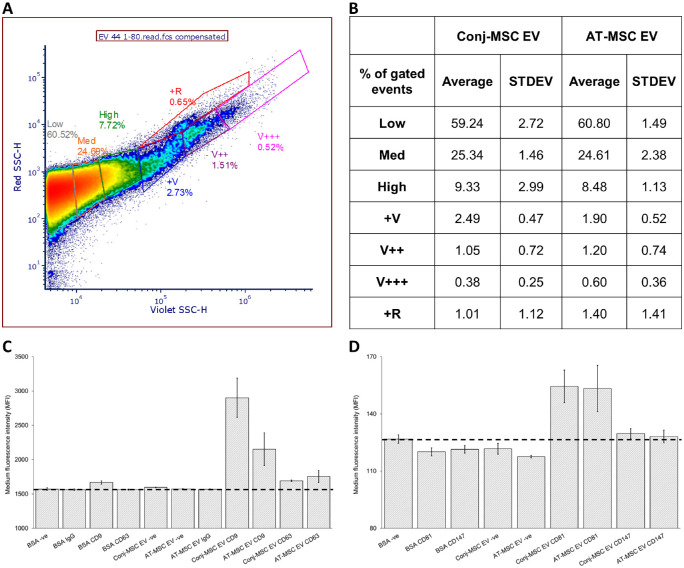
Nano-flow cytometry analysis of Conj-MSC and AT-MSC-derived EVs revealed that the majority of the EV population was in the low gate (approximately 180 nm-300 nm using Rosetta calibration; Supplementary Fig. S2). There were different amounts of the larger EV subpopulations between the two cell types. However, these differences were not significant, with Conj-MSC and AT-MSC-derived EVs appearing broadly similar (Figs. 4A, 4B). EV-bead conjugated flow cytometry analysis revealed that Conj-MSC and AT-MSC-derived EVs were positive for the EV markers: CD9, CD63, CD81, and CD147, with CD9, and CD81 being more abundant in both cell types (Figs. 4C, 4D).
Figure 4.
Characterization of Conj-MSC-derived EVs by flow cytometry. (A) A violet SSC-H/ red SSC-H dot-plot with a representative Conj-MSC-derived EV sample was gated into low, med, high, +V, V++, V+++, and +R regions, which were created based differences between biological samples and their replicates. (B) A table showing the percentage events within each gate for Conj-MSC and AT-MSC-derived EVs for three independent experiments. (C, D) The 1.25 × 107 EVs/test from 3 Conj-MSC and 3 AT-MSC-derived EV samples were bound to the surface of 4 µm aldehyde/sulfate latex beads and stained with antibodies for the EV markers CD9, CD63, CD81, and CD147 for 30 minutes on ice.
Conj-MSC-Derived EVs Show Antioxidant Effect on Conjunctival Epithelial Cells
The effect of Conj-MSC-derived EVs on conjunctival epithelium was tested on IM-HConEpiC cells (Fig. 5). An in vitro model of oxidative stress using H2O2 as the oxidative agent was used to determine antioxidant properties of EVs (Fig. 5A). H2O2 increased ROS by 1.61 ± 0.31-fold (P = 0.0061) compared to untreated cells set as 1. Conj-MSC EVs decreased the H2O2-induced ROS increase to 0.42 ± 0.46 (P < 0.0001), in similar levels than adipose tissue-MSC-derived EVs (0.65 ± 0.50, P = 0.0002) and 250 µM ascorbic acid (0.50 ± 0.59, P < 0.0001), used as control. Cell viability was not affected by either of the treatments, as shown by the AlamarBlue assay results (Fig. 5B).
Figure 5.
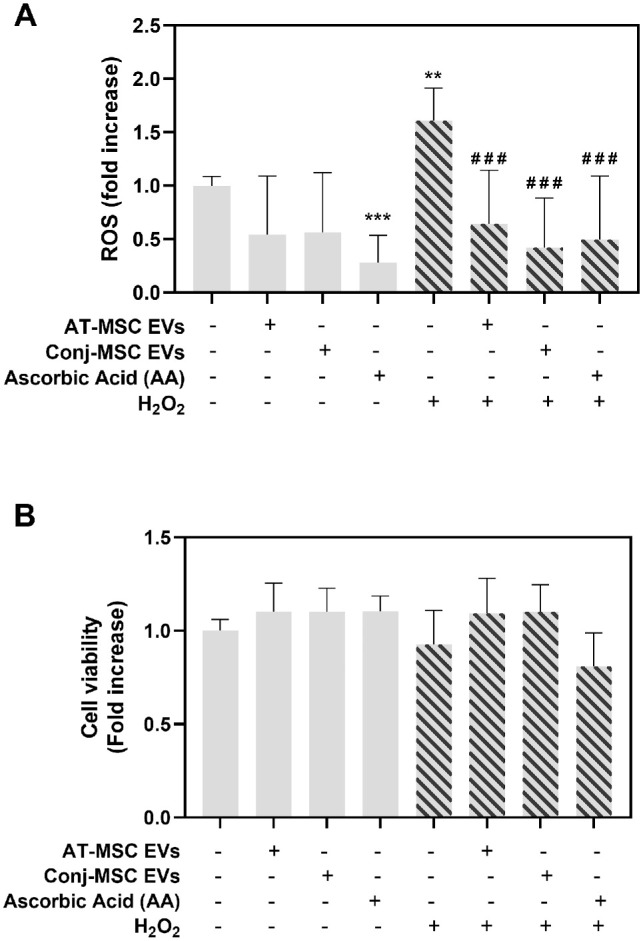
Effect of Conj-MSC-derived EVs on conjunctival epithelial cells. (A) Effect of EVs on oxidative stress produced by exposure to H2O2. EVs significantly reduced the increase in reactive oxygen species (ROS) induced by H2O2. * Indicates significance compared to untreated cells, and # compared to untreated cells exposed to H2O2. (B) Cell viability measured with AlamarBlue assay. No significant differences were observed after EV treatment (n = 3).
Discussion
In this study, we demonstrate that it is possible to culture Conj-MSCs isolated from cadaveric tissue. To date, research on Conj-MSC has been sparse. In 2008, Nadri et al. reported the isolation of MSC from conjunctival biopsies.12 Since then, only a few studies using cultured Conj-MSC have been published and, to the best of our knowledge, they all follow that same protocol.23–28 Although the isolation of Conj-MSC is undoubtedly of scientific interest, obtaining Conj-MSC from conjunctival biopsies has two major drawbacks: first, conjunctival biopsies are difficult to get for ethical reasons, and, second, they are usually obtained from patients undergoing an ocular surgery. Thus, they are obtained from patients with ocular pathology and, in most cases, conjunctival pathology. For instance, in the protocol described by Nadri et al., biopsies were procured from patients with pterygium. There is evidence of alterations throughout the conjunctival tissue of patients with pterygium, suggesting that it may be a diffuse disease,29 with alterations in pterygium-free areas that are not present in healthy conjunctivas.30 Therefore, conjunctival biopsies collected from areas close to a pterygium should not be considered healthy tissue. In this study, we describe a method to isolate Conj-MSC from cadaveric conjunctival tissue. We believe that this protocol overcomes the two major drawbacks mentioned above: the tissue is obtained from deceased donors who do not have ocular diseases, and there are fewer ethical concerns in using cadaveric tissue than in taking a biopsy for research purposes. In addition, cadaveric conjunctival tissue is more accessible to researchers than ocular biopsies.
Despite the critical role of the conjunctiva in maintaining overall LFU homeostasis and the upsurge in the study of EVs in all tissues, there is almost no information on conjunctival EVs, with only one study analyzing the role of EVs derived from a conjunctival epithelial cell line.16 In that study, Ramos et al. evaluated the effect of corneal and conjunctival-derived EVs on the transdifferentiation of these two cell populations, and found that both EVs had the ability to influence in each other’s cell type. That means that communication between different ocular surface tissues, such as the cornea and the conjunctiva, may be mediated by EVs. But apart from this crosstalk between different tissues, it is also meaningful to analyze the communication between different parts of the same tissue, for instance, the epithelium and the stroma. There are some interesting studies analyzing this in the cornea.13,14,31,32 Those studies also highlight that the basement membrane appears to limit EV diffusion, what could mean that the impact of EVs released by stromal cells may depend on the basement membrane integrity. However, in different diseases, epithelial function and structure is damaged, and basement membrane integrity can be affected. In these situations, EVs may have an important role in the development and/or the onset of the disease. These studies analyzing the crosstalk between corneal epithelial and stromal cells through EVs paved the road to do similar research in other ocular tissues. To the best of our knowledge, there are no reports studying the effect of conjunctival stromal EVs on epithelial cells. Here, we have shown that it is possible to isolate EVs from cultured human Conj-MSC, and that these EVs have an antioxidant effect on the conjunctival epithelium.
We isolated EVs by classical differential ultracentrifugation, which has been widely described in the literature.33 One of the major advantages of ultracentrifugation is that it requires minimal sample pretreatment and no addition of reagents. We used 25 mM trehalose to preserve the integrity of EVs during freezing and thawing cycles,34 although EVs can be recovered in PBS without additives or in other buffers depending on the further applications of the EVs. However, ultracentrifugation also has some limitations, such as the need for large amounts of culture supernatant to obtain low yields, and that the procedure is very time-consuming. Therefore, further studies analyzing other optimized isolation methods are warranted.35
According to the MISEV 2018 guidelines,36 EVs should be characterized using techniques that provide high-resolution images of single EVs. One of these techniques is AFM, whose main advantage over transmission electron microscopy is that it provides a true 3D image of the surface topography.21,37,38 Characterization of EVs by AFM revealed that Conj-MSC-derived EVs have globular structure. The diameter distribution ranges from 50 to 120 nm, whereas the height ranges from 10 to 20 nm, which is consistent with the literature for this technique.38 DLS analysis revealed larger EV sizes than that reported by AFM, possibly due to aggregation of EVs. The z-potential values we obtained confirmed that the isolated Conj-MSC-derived EVs are stable. Characterization of EVs by flow cytometry analysis revealed that the majority Conj-MSC and AT-MSC-derived EVs were smaller than 300 nm and were positive for 4 EV markers. There were no significant differences between the EV size and marker expression of the Conj-MSC and AT-MSC-derived EVs, suggesting they share an MSC-derived EV phenotype.
Finally, we have shown that Conj-MSC-derived EVs are biologically active because they were able to significantly reduce ROS production in conjunctival epithelial cells. High ROS levels and the subsequent oxidative stress are associated with many ocular pathologies, such as dry eye disease39 or age-related macular degeneration.40 The relationship between oxidative stress and EVs is bidirectional, as EVs possess both pro-oxidant and antioxidant machinery.41 The effect of EVs in reducing oxidative stress has been demonstrated in the colon,42 kidneys,43 in specific cell types such as macrophages,44 or in the ocular drainage system.45 However, this is the first study analyzing Conj-MSC EVs and their bioactivity alleviating oxidative stress in the ocular surface and, specifically, in the conjunctiva. We did not observe any effect of EVs on conjunctival epithelial cell viability, confirming that our isolated EVs did not have any toxicity due to the EVs themselves or to the isolation procedure.
In conclusion, we have shown that it is possible to culture human Conj-MSC isolated from cadaveric tissue, and that these cells may be a valuable source of EVs with promising antioxidant activity on conjunctival epithelial cells, warranting further research to determine whether these EVs may have a therapeutic effect.
Supplementary Material
Acknowledgments
The authors thank Javier Gutiérrez Reguera for his technical assistance with DLS and AFM, José Miguel Ferreras for allowing us to use the ultracentrifuge and aiding with the equipment, Antonio López-García for his help with the histological staining of differentiated Conj-MSCs, and VISION R&D for scientific advice. Part of this research was carried out in the Laboratory of Instrumental Techniques (LTI) Research Facilities, University of Valladolid.
Supported by Ministerio de Ciencia, Innovación y Universidades (MCIU), Agencia Estatal de Investigación (AEI) and Fondo Europeo de Desarrollo Regional (FEDER), Grant number RTI2018–094071-B-C21. LG-P was funded by the Postdoctoral contracts 2017 call (Universidad de Valladolid). IRC was supported by PRE2019-089985 scholarship (Ministry of Science and Innovation, Spain).
Part of the research reported in this publication was supported by UCD Equip Funding Programme (ref 2021129) and Science Foundation Ireland Research Infrastructure Programme (ref 21/RI/9718); UCD Conway-CEPHR Advanced Cytometry Unit.
Disclosure: L. García-Posadas, None; I. Romero-Castillo, None; K. Brennan, None; M.M. Mc Gee, None; A. Blanco-Fernández, None; Y. Diebold, None
References
- 1. Rudraprasad D, Rawat A, Joseph J. Exosomes, extracellular vesicles and the eye. Exp Eye Res. 2022; 214: 108892. [DOI] [PubMed] [Google Scholar]
- 2. Li N, Zhao L, Wei Y, Ea VL, Nian H, Wei R.. Recent advances of exosomes in immune-mediated eye diseases. Stem Cell Res Ther. 2019; 10(1): 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu J, Jiang F, Jiang Y, et al.. Roles of exosomes in ocular diseases. Int J Nanomedicine. 2020; 15: 10519–10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu B, Li XR, Zhang XM.. Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World J Stem Cells. 2020; 12(3): 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang Y, Lin S, Gao Y.. Mesenchymal stromal cell-based therapy for dry eye: current status and future perspectives. Cell Transplant. 2022; 31: 09636897221133818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mathew B, Ravindran S, Liu X, et al.. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. 2019; 197: 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang D, Wang W, Li L, et al.. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. Qin G, ed. PLoS One. 2013; 8(3): e59020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phinney DG, Pittenger MF.. Concise Review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017; 35(4): 851–858. [DOI] [PubMed] [Google Scholar]
- 9. Moghadasi S, Elveny M, Rahman HS, et al.. A paradigm shift in cell-free approach: the emerging role of MSCs-derived exosomes in regenerative medicine. J Transl Med. 2021; 19(1): 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai J, Wu J, Wang J, et al.. Extracellular vesicles derived from different sources of mesenchymal stem cells: therapeutic effects and translational potential. Cell Biosci. 2020; 10(1): 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC.. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004; 78(3): 409–416. [DOI] [PubMed] [Google Scholar]
- 12. Nadri S, Soleimani M, Kiani J, Atashi A, Izadpanah R.. Multipotent mesenchymal stem cells from adult human eye conjunctiva stromal cells. Differentiation. 2008; 76(3): 223–231. [DOI] [PubMed] [Google Scholar]
- 13. Desjardins P, Berthiaume R, Couture C, et al.. Impact of exosomes released by different corneal cell types on the wound healing properties of human corneal epithelial cells. Int J Mol Sci. 2022; 23(20): 12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKay TB, Hutcheon AEK, Zieske JD, Ciolino JB.. Extracellular vesicles secreted by corneal epithelial cells promote myofibroblast differentiation. Cells. 2020; 9(5): 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee C, Edman MC, Laurie GW, Hamm-Alvarez SF, Mackay JA.. Biosynthesized multivalent lacritin peptides stimulate exosome production in human corneal epithelium. Int J Mol Sci. 2020; 21(17): 6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramos T, Parekh M, Kaye SB, Ahmad S.. Epithelial cell-derived extracellular vesicles trigger the differentiation of two epithelial cell lines. Int J Mol Sci. 2022; 23(3): 1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shojaati G, Khandaker I, Funderburgh ML, et al.. Mesenchymal stem cells reduce corneal fibrosis and inflammation via extracellular vesicle-mediated delivery of miRNA. Stem Cells Transl Med. 2019; 8(11): 1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. García-Posadas L, Arranz-Valsero I, López-García A, Soriano-Romaní L, Diebold Y.. A new human primary epithelial cell culture model to study conjunctival inflammation. Invest Ophthalmol Vis Sci Vis Sci. 2013; 54(10): 7143–7152. [DOI] [PubMed] [Google Scholar]
- 19. García-Posadas L, Romero-Castillo I, Katsinas N, Krstić L, López-García A, Diebold Y.. Characterization and functional performance of a commercial human conjunctival epithelial cell line. Exp Eye Res. 2022; 223: 109220. [DOI] [PubMed] [Google Scholar]
- 20. Dominici M, Le Blanc K, Mueller I, et al.. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy Position Statement. Cytotherapy. 2006; 8(4): 315–317. [DOI] [PubMed] [Google Scholar]
- 21. Skliar M, Chernyshev VS.. Imaging of extracellular vesicles by atomic force microscopy. J Vis Exp. 2019; 2019(151), doi: 10.3791/59254. [DOI] [PubMed] [Google Scholar]
- 22. Brennan K, Iversen KF, Blanco-Fernández A, Lund T, Plesner T, Mc Gee MM. Extracellular vesicles isolated from plasma of multiple myeloma patients treated with daratumumab express CD38, PD-L1, and the complement inhibitory proteins CD55 and CD59. Cells. 2022; 11(21): 3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barati G, rahmani A, Nadri S.. In vitro differentiation of conjunctiva mesenchymal stem cells into insulin producing cells on natural and synthetic electrospun scaffolds. Biologicals. 2019; 62: 33–38. [DOI] [PubMed] [Google Scholar]
- 24. Soleimanifar F, Mortazavi Y, Nadri S, Soleimani M.. Conjunctiva derived mesenchymal stem cell (CJMSCs) as a potential platform for differentiation into corneal epithelial cells on bioengineered electrospun scaffolds. J Biomed Mater Res A. 2017; 105(10): 2703–2711. [DOI] [PubMed] [Google Scholar]
- 25. Soleimanifar F, Mortazavi Y, Nadri S, Islami M, Vakilian S.. Coculture of conjunctiva derived mesenchymal stem cells (CJMSCs) and corneal epithelial cells to reconstruct the corneal epithelium. Biologicals. 2018; 54: 39–43. [DOI] [PubMed] [Google Scholar]
- 26. Forouzandeh M, Bigdeli MR, Mostafavi H, Nadri S, Eskandari M.. Therapeutic potentials of human microfluidic encapsulated conjunctival mesenchymal stem cells on the rat model of Parkinson's disease. Exp Mol Pathol. 2021; 123: 104703. [DOI] [PubMed] [Google Scholar]
- 27. Soleimannejad M, Ebrahimi-Barough S, Soleimani M, et al.. Fibrin gel as a scaffold for photoreceptor cells differentiation from conjunctiva mesenchymal stem cells in retina tissue engineering. Artif Cells Nanomed Biotechnol. 2018; 46(4): 805–814. [DOI] [PubMed] [Google Scholar]
- 28. Rahmani A, Naderi M, Barati G, Arefian E, Jedari B, Nadri S.. The potency of hsa-miR-9-1 overexpression in photoreceptor differentiation of conjunctiva mesenchymal stem cells on a 3D nanofibrous scaffold. Biochem Biophys Res Commun. 2020; 529(3): 526–532. [DOI] [PubMed] [Google Scholar]
- 29. Shahraki T, Arabi A, Feizi S.. Pterygium: an update on pathophysiology, clinical features, and management. Ther Adv Ophthalmol. 2021; 13: 25158414211020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torres J, Enríquez-de-Salamanca A, Fernández I, et al.. Activation of MAPK signaling pathway and NF-kappaB activation in pterygium and ipsilateral pterygium-free conjunctival specimens. Invest Ophthalmol Vis Sci. 2011; 52(8): 5842–5852. [DOI] [PubMed] [Google Scholar]
- 31. Yeung V, Zhang TC, Yuan L, et al.. Extracellular vesicles secreted by corneal myofibroblasts promote corneal epithelial cell migration. Int J Mol Sci. 2022; 23(6): 3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zieske JD, Hutcheon AEK, Guo X.. Extracellular vesicles and cell–cell communication in the cornea. Anatomic Rec. Published online 2019, doi: 10.1002/ar.24181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chhoy P, Brown CW, Amante JJ, Mercurio AM.. Protocol for the separation of extracellular vesicles by ultracentrifugation from in vitro cell culture models. STAR Protoc. 2021; 2(1): 100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bosch S, De Beaurepaire L, Allard M, et al.. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep. 2016; 6(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liangsupree T, Multia E, Riekkola ML.. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. 2021; 1636: 461773. [DOI] [PubMed] [Google Scholar]
- 36. Théry C, Witwer KW, Aikawa E, et al.. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018; 7(1): 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J.. The methods of choice for extracellular vesicles (EVs) characterization. Int J Molec Sci. 2017; 18(6): 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parisse P, Rago I, Ulloa Severino L, et al.. Atomic force microscopy analysis of extracellular vesicles. Eur Biophys J. 2017; 46(8): 813–820. [DOI] [PubMed] [Google Scholar]
- 39. Dogru M, Kojima T, Simsek C, Tsubotav K.. Potential role of oxidative stress in ocular surface inflammation and dry eye disease. Invest Ophthalmol Vis Sci. 2018; 59(14): DES163–DES168. [DOI] [PubMed] [Google Scholar]
- 40. Datta S, Cano M, Ebrahimi K, Wang L, Handa JT.. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017; 60: 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bodega G, Alique M, Puebla L, Carracedo J, Ramírez RM.. Microvesicles: ROS scavengers and ROS producers. J Extracell Vesicles. 2019; 8(1): 1626654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang J, Liu XX, Fan H, et al.. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. Camussi G, ed. PLoS One. 2015; 10(10): e0140551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang G, Zou X, Huang Y, et al.. Mesenchymal stromal cell-derived extracellular vesicles protect against acute kidney injury through anti-oxidation by enhancing Nrf2/ARE activation in Rats. Kidney Blood Press Res. 2016; 41(2): 119–128. [DOI] [PubMed] [Google Scholar]
- 44. Shen K, Jia Y, Wang X, et al.. Exosomes from adipose-derived stem cells alleviate the inflammation and oxidative stress via regulating Nrf2/HO-1 axis in macrophages. Free Radic Biol Med. 2021; 165: 54–66. [DOI] [PubMed] [Google Scholar]
- 45. Lerner N, Chen I, Schreiber-Avissar S, Beit-Yannai E.. Extracellular vesicles mediate anti-oxidative response—in vitro study in the ocular drainage system. Int J Molec Sci. 2020; 21(17): 6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.