Abstract
Affective disorders such as depression and anxiety are among the most prevalent psychiatric illnesses and causes of disability worldwide. The recent FDA-approval of a novel antidepressant treatment, ZULRESSO® (Brexanolone), a synthetic neurosteroid has fueled interest into the role of neurosteroids in the pathophysiology of depression as well as the mechanisms mediating the antidepressant effects of these compounds. The majority of studies examining the impact of neurosteroids on affective states have relied on the administration of exogenous neurosteroids; however, neurosteroids can also be synthesized endogenously from cholesterol or steroid hormone precursors. Despite the well-established influence of exogenous neurosteroids on affective states, we still lack an understanding of the role of endogenous neurosteroids in modulating affective tone. This review aims to summarize the current literature supporting the influence of neurosteroids on affective states in clinical and preclinical studies, as well as recent evidence suggesting that endogenous neurosteroids may set a baseline affective tone.
Keywords: neurosteroids, neurosteroidogenesis, allopregnanolone, depression, anxiety, PTSD
Introduction
Psychiatric disorders are among the most prevalent forms of disability worldwide, with affective disorders such as depression and anxiety being the most common (Center for Behavioral Health Statistics, 2017; Evaluation, 2019; James et al., 2018). Upwards of 50% of individuals diagnosed with an affective disorder fail to achieve full symptom remission, and almost a third of those treated exhibit treatment resistance with currently available antidepressant and antipsychotic treatments (Fournier et al., 2010; Howes et al., 2021; John Rush et al., 2006; Zhdanava et al., 2021). Of particular concern is the length of time it takes these treatments to become effective, a delay which poses a significant risk for treatment noncompliance as well as increased suicide risk (Fergusson et al., 2005; Hammad et al., 2006; Healy & Aldred, 2009; Jick et al., 2004; Simon & Savarino, 2007; Stone et al., 2009). Therapeutic advances for affective disorders have been delayed due to the complex neurobiological heterogeneity of these disorders. Numerous mechanisms have been implicated in the pathophysiology of mood disorders, such as the predominant monoamine hypothesis of depression (Hirschfeld, 2000) as well as newer theories including the GABAergic hypothesis of depression (Lüscher et al., 2011).The GABAergic hypothesis of depression has gained recent interest and validation (Lüscher et al., 2011) given the FDA-approval of, ZULRESSO® (Brexanolone), a synthetic neurosteroids as an antidepressant with a novel mechanism of action as a positive allosteric modulator of GABAA receptors (GABAARs) (Althaus et al., 2020; Epperson et al., 2023; Lüscher & Möhler, 2019a).
Neurosteroids are a class of steroids that are endogenously synthesized in the brain de novo from cholesterol or from steroid hormone precursors; whereas neurosteroids are steroids which, independent of their origin, can exert actions in the brain. Neurosteroids have been shown to exert anxiolytic and antidepressant effects (Zorumski et al., 2012, 2019; Zorumski & Mennerick, 2013), demonstrating their ability to modulate affective tone. In fact, treatment of affective disorders with exogenous neurosteroids has demonstrated effectiveness in clinical studies (Arnaud et al., 2021; Epperson et al., 2023; Meltzer-Brody et al., 2018; Suthoff et al., 2022). Despite the well-established influence of exogenous neurosteroids on affective states, we still lack an understanding of the role of endogenous neurosteroids in modulating affective tone. This review aims to summarize the current literature supporting the influence of neurosteroids on affective states in clinical and preclinical studies as well as recent evidence suggesting that endogenous neurosteroids may set a baseline affective tone, one which is impacted by risk factors for psychiatric illnesses and may be a useful target for the treatment of mood disorders. The neurosteroids that are discussed in this review are listed in table 1.
Table 1.
List of neurosteroids and analogs described in this review
| Steroids/Neurosteroids |
|---|
| Pregnenolone |
| Progesterone |
| Dihydroprogesterone (5a-DHP) |
| 5a-Dihydroprogesterone (5a-DHPROG) |
| Allopregnanolone (ALLO) |
| Dehydroepiandrosterone (DHEA) |
| Androstenedione |
| Testosterone (T) |
| Dihydrotestosterone (DHT) |
| Neurosteroid Analogs |
| Zuranolone (SAGE-217) |
| ZULRESSO® (Brexanolone) |
| Ganaxolone |
1. Endogenous Neurosteroid Synthesis
Etienne Baulieu coined the term, “neurosteroid” in 1981, describing steroid hormones synthesized endogenously in the brain that are derived from cholesterol. Endogenous neurosteroid synthesis requires the sequential action of enzymes 5α-reductase and 3α-hydroxysteroid dehydrogenase (3α-HSD) (Figure 1). Both 5α-reductase types I and II convert either a. progesterone into 5α-dihydroprogesterone or b. deoxycorticosterone into 5α-deoxycorticosterone. 3α-HSD bidirectionally reduces 5α-dihydroprogesterone into allopregnanolone (Figure 1). The same steroidogenic enzymes utilized in the endogenous production of neurosteroids, 5α-reductase and 3α-HSD, are present outside of the brain to synthesize neurosteroids in the adrenal glands, reproductive organs, placenta, and skin (Liang & Rasmusson, 2018; Mellon & Griffin, 2002). Within the brain however, transcriptional expression of these enzymes is temporally- and regionally- specific, implicating their role in development and maintenance of neuronal circuits.
Figure 1. 5α-reduced neurosteroid synthesis.
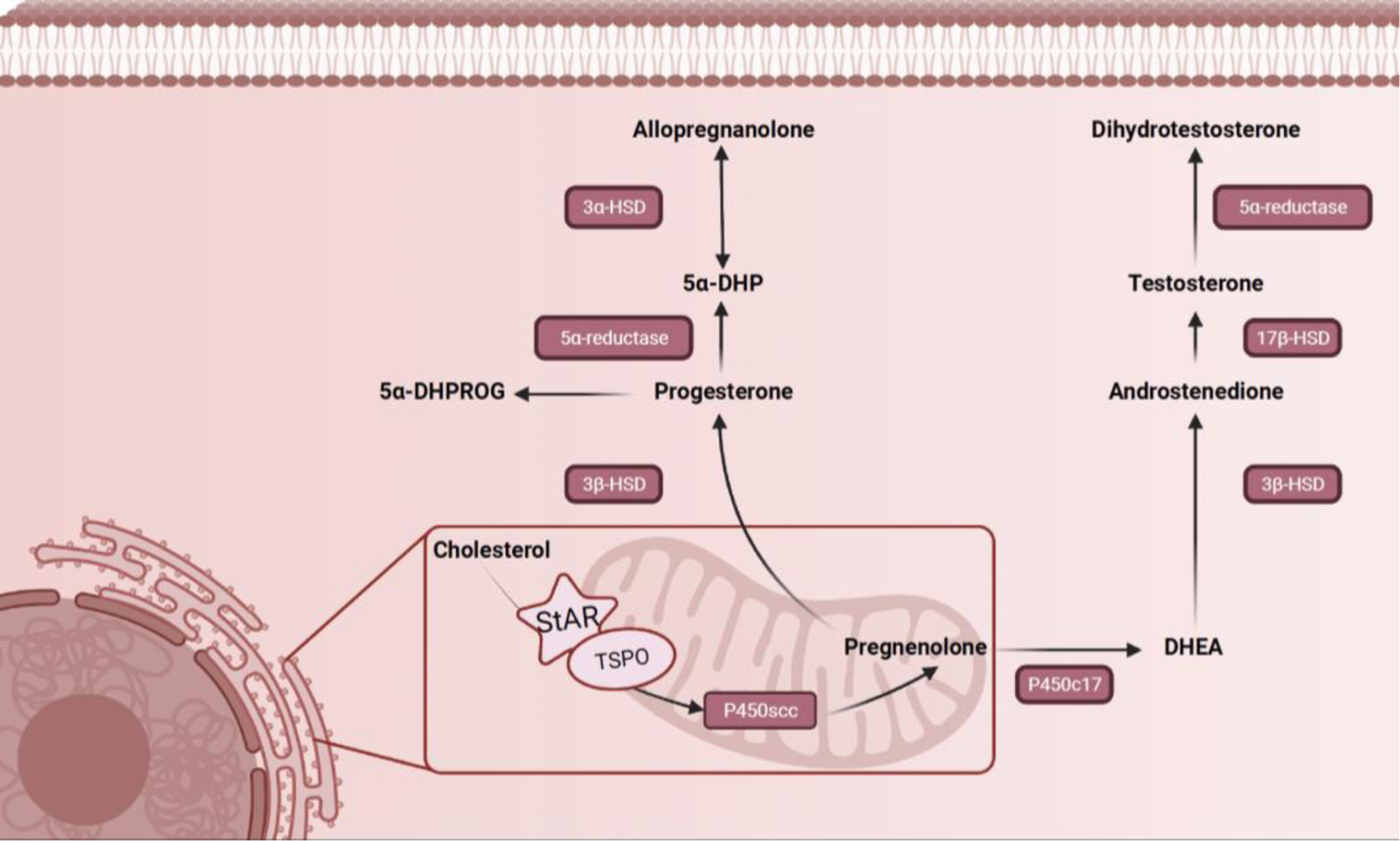
Illustration of the synthesis pathway for 5α-reduced metabolites of progesterone described in this review.
5α-reduced metabolites are synthesized in both neurons and glial cells. Key enzymes necessary for the synthesis of endogenous neurosteroids have been discovered in the brain of several mammals, submammalian vertebrates, and invertebrates. While technical limitations have hindered the precise localization of protein levels of some steroidogenic enzymes and neurosteroids, mRNA of 5α-reductase type I and 3α-HSD have both been found to colocalize in glutamatergic pyramidal neurons and glial cells in brain regions rich in white matter such as the cortex, hippocampus(CA1–3), olfactory bulb, striatum, thalamus, amygdala, corpus callosum and cerebellum (Agís-Balboa et al., 2006). 3α-HSD has been discovered to be expressed in the hypothalamus, thalamus, caudate nucleus, frontal cortex, olfactory bulb, and olfactory tubercle (For review see (Mellon, 2007)). Genes for enzymes in the Cytochrome P450 family that catalyze the cleavage of cholesterol into pregnenolone, are present in white matter throughout the brain as well as in cerebellum, hypothalamus, cortex, hippocampus, and amygdala(Compagnone et al., 1995). These regions are considered the main hubs for neurosteroidogenesis in the brain, as genes for both 5α-reductase and 3α-HSD enzymes have also been discovered in these regions (For review see (Mellon, 2007)). These hubs for neurosteroidogenesis are also key nodes for emotional processing, consistent with the impact of neurosteroids on affective states.
2. Effect of 5α-reduced neurosteroids on affective tone
Steroid hormones are known to exert their biological effects by binding to their intracellular receptors, translocating to the nucleus, and regulating gene transcription. However, steroid hormones have also been shown to exert rapid effects on neuronal excitability by interacting with specific neurotransmitter receptors, including GABAARs and NMDA receptors (for review see (Carver & Reddy, 2013; Park-Chung et al., 1997; Rasmusson et al., 2022; Rupprecht & Holsboer, 1999; Ziolkowski et al., 2021)). While there are three main classes of neurosteroids that exist based on their structural characteristics (pregnane, sulfated, and androstane), this review will focus on 5α-reduced neurosteroids that have been indicated in a variety of affective states (for review see (Reddy & Bakshi, 2020)). 5α-reduced metabolites of both progesterone and testosterone (3α-diol) have been demonstrated to impact affective states (see review (Melcangi et al., 2021)), although there is more evidence supporting a role for 5α-reduced progesterone metabolites and will therefore be the focus of this review.
2.1. Clinical evidence
While some of the first clinical observations of neurosteroids demonstrated their potent anesthetic and sedative properties, accumulating evidence has highlighted their efficacy as antidepressants, anxiolytics, and antipsychotic treatments.
2.1.1. Anxiety
Fluctuations in neurosteroid levels have been observed throughout the human lifespan, with more drastic changes across the estrous cycle, pregnancy, and a decline in late adulthood (Figure 2). These changes have been observed to occur in concert with changing GABAAR expression during periods with increased risk of developing an anxiety disorder (Figure 2). Researchers have identified significantly lower levels of pregnenolone in patients with generalized anxiety disorder (GAD) (Semeniuk et al., 2001). Dysregulation of GABAA/benzodiazepine receptor complexes have also been noted in individuals with GAD (Semeniuk et al., 2001), suggesting impaired neurosteroid signaling and inhibitory tone that may contribute to anxiety pathology. In line with this notion, disequilibrium of neurosteroids in individuals with premenstrual syndrome have also been noted where lower levels of progesterone during the luteal phase were correlated with increase symptom severity with PMS (Wang et al., 1996). Conversely, in individuals with panic disorders it was demonstrated that plasma concentrations of allopregnanolone were elevated while progesterone and 5α-DHP were comparable to levels of healthy controls (Ströhle et al., 2002). Elevated plasma levels of allopregnanolone were also observed in women during the follicular and premenstrual phase of the estrous cycle, periods where anxiety and other symptoms of premenstrual dysphoric disorder (PMDD) arise (Brambilla et al., 2003). These findings could be in part due to the increase in HPA-axis activation during panic attacks that would result in the hypersecretion of neurosteroids as a counter-regulatory mechanism against this anxiogenic condition. In follow up studies where panic attacks were induced using sodium lactate or cholecystokinin-tetrapeptide in individuals with a history of panic attacks, both allopregnanolone and pregnanolone were significantly reduced compared to levels in healthy controls without a history of panic attacks (Ströhle et al., 2002, 2003).
Figure 2. Neurosteroid and GABAAR expression across the lifespan.
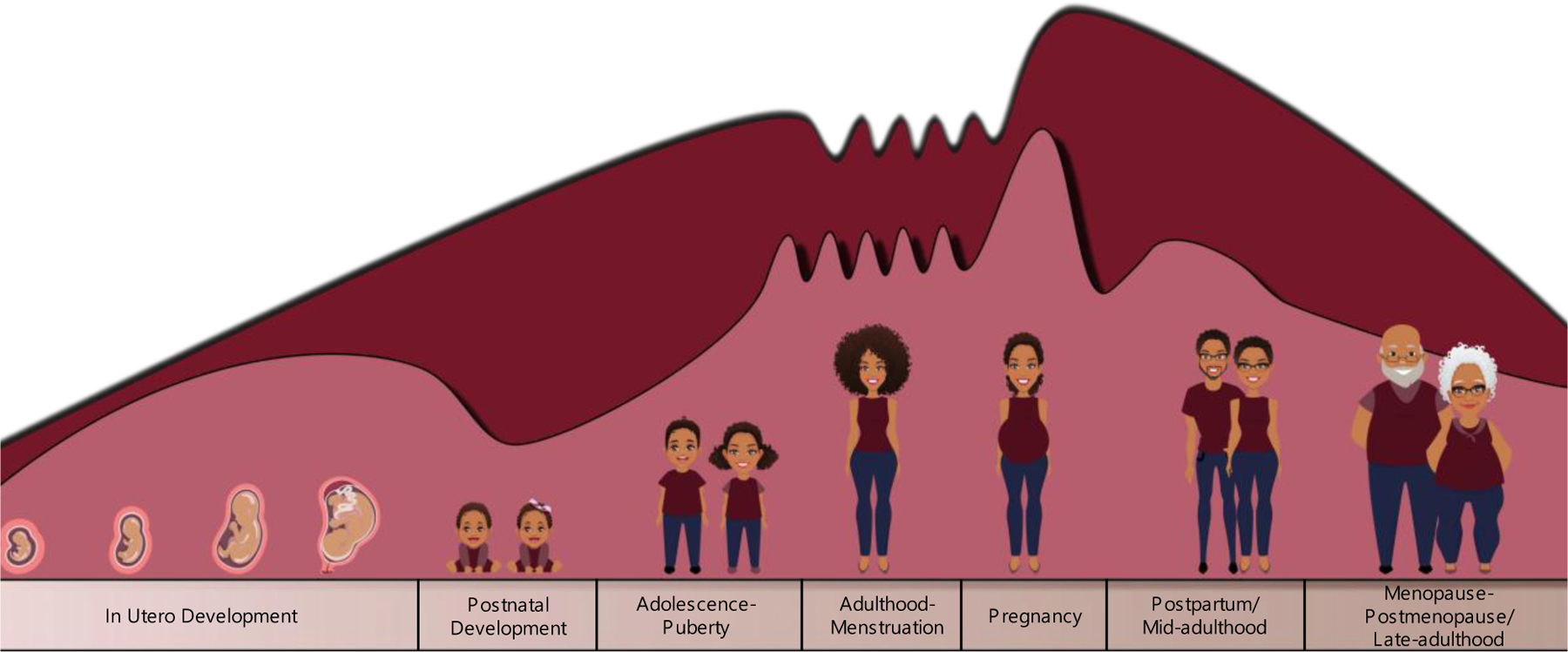
Illustration of the relative fluctuation of neurosteroid and GABAAR expression across the lifespan. These fluctuations also occur in men with a downward shift in neurosteroid expression occurring in mid-adulthood. (for review see Muller et al. 2003; Tannenbeaum et al. 2004; Mellon & Griffin, 2002; Gilfarb and Leuner, 2022; Maguire and Mody, 2008).
2.1.2. Depression
Following parturition there is a rapid decline in neurosteroid levels, particularly in levels of allopregnanolone that might trigger symptoms of postpartum depression in a subset of individuals. This precipitous decline in endogenous allopregnanolone levels is thought to create a window of vulnerability to mood disorders during this period and spurred interest in developing an exogenous neurosteroid analog to target the underlying pathology of postpartum depression. Clinical trials have demonstrated the efficacy of this treatment modality where administration of a synthetic allopregnanolone analog ZULRESSO® (Brexanolone), to individuals with severe postpartum depression resulted in a significant reduction in depressive symptoms, anxiety, and insomnia (Epperson et al., 2023; Kanes, Colquhoun, Doherty, et al., 2017; Kanes, Colquhoun, Gunduz-Bruce, et al., 2017; Meltzer-Brody et al., 2018). Treatment responses with ZULRESSO® (Brexanolone) were rapid and durable, persisting up to 90 days post-treatment (Epperson et al., 2023; Meltzer-Brody et al., 2018; Patterson et al., 2022). Follow-up studies utilizing a similar allopregnanolone analog for treatment of major depressive disorder demonstrated a significant reduction in symptoms of depression in response to 14 days of oral treatment (Gunduz-Bruce et al., 2019). In postmenopausal women with major depression that were treatment-resistant to classical antidepressants selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs), the use of a synthetic allopregnanolone analog, Ganaxalone, improved mood symptoms and insomnia associated with depression following an 8-week treatment period (Dichtel et al., 2020). Allopregnanolone concentrations have been demonstrated to be ~60% lower in patients with unipolar depression. Interestingly, treatment with SSRIs such as fluoxetine or paroxetine are able to normalize CSF allopregnanolone concentrations without altering levels of progesterone or pregnanolone, the timing of which correlates with the antidepressant treatment response (Uzunova et al., 1998). Postmortem analysis of tissue samples from patients with depression demonstrated that mRNA expression of 5α-reductase type I is downregulated in Brodmann’s area 9 (BA9) of the prefrontal cortex (Agís-Balboa et al., 2014). In patients that received antidepressant treatment, allopregnanolone levels in BA9 were significantly increased when compared to those with depression that went untreated (Agís-Balboa et al., 2014). To further probe this mechanism, in vitro studies utilizing rat and human purified 3α-HSD isoforms to evaluate the impact of SSRIs on neurosteroid synthesis revealed that fluoxetine, sertraline, and paroxetine directly decrease the affinity for 3α-HSD to the substrate DHP. This occurrence indicates that SSRIs influence neurosteroidogenesis by shifting the preference of 3α-HSD to favor its reductive reaction to convert DHP to allopregnanolone (Griffin & Mellon, 1999). These findings thus indicate that neurosteroid synthesis after SSRI treatment may contribute to the mechanism underlying the effectiveness of these therapies to aid in alleviating mood symptoms in individuals with affective disorders. To further support this hypothesis, low dose treatment with SSRIs like fluoxetine that are not sufficient to fully block serotonin reuptake, are sufficient to recover brain levels of neurosteroids and improve mood symptoms such as aggression (Pinna et al., 2003). Such findings emphasize an important mechanism of antidepressant action outside of the monoamine hypothesis of depression, to alleviate mood symptoms through normalizing neurosteroid synthesis.
In men and women, midlife (40–70 years of age) marks a downward shift in levels of neurosteroids (Figure 2). For one of the most abundant neurosteroids, dehydroepiandrosterone (DHEA), this decrease has been reported at a rate of 1–4% per year until ~80 years of age when levels begin to remain steady (Muller et al., 2003; Tannenbaum et al., 2004). Midlife also marks a period of vulnerability for many agerelated psychiatric illnesses that may be driven in part by this shift in neurosteroid production (Muller et al., 2003; Tannenbaum et al., 2004). Additionally, it has been demonstrated that DHEA levels are lower in individuals with a history of major depressive disorder and may be utilized as a predictive biomarker in individuals experiencing their first episode of depression (Agorastos et al., 2023). Previous clinical trials utilizing a sixweek treatment protocol with DHEA indicated a reduction in depressive-symptoms in individuals with midlife-onset depression (Schmidt et al., 1996, 2005). Replacement therapy with DHEA has been shown to not only be capable of restoring serum levels of neurosteroids but also improved overall mood and depression symptoms (Binder et al., 2009; Bloch et al., 2012; Morales et al., 1994; Wolkowitz et al., 1997, 1999). These findings indicate that exogenous neurosteroid treatment may be effective for both reproductive and non-reproductive mood disorders.
2.1.3. PTSD
In individuals with posttraumatic stress disorder (PTSD), deficits in emotional-processing and emotional-regulation can be attributed in part to hyperactivation of brain regions that govern emotional regulation, including the prefrontal cortex, amygdala, insula, and anterior cingulate (Sripada, King, et al., 2012). Exogenous neurosteroid treatment in individuals with PTSD decreased neuronal activity in the amygdala and insula, while increasing activity in the prefrontal cortex and rostral anterior cingulate cortex (Sripada, Marx, et al., 2012). Such findings demonstrate the ability of exogenous neurosteroid treatments to impact neural circuits that govern emotional regulation. While one clinical trial utilizing Ganaxalone, a neuroactive allopregnanolone analog, failed to show efficacy in individuals with PTSD, the dosing of Ganaxalone may have been below the necessary therapeutic level (Rasmusson et al., 2017).
There are known sex differences in the alterations of neurosteroids in male and female individuals with PTSD (Rasmusson et al., 2006, 2019). In women with PTSD, deficits in CSF levels of allopregnanolone are apparent without changes in progesterone or 5α-DHP levels, indicating an impairment in synthesis through the 3α-HSD enzyme (Rasmusson et al., 2006). Conversely, in men with PTSD, whose 3α-HSD function was not impacted, deficits in CSF levels of allopregnanolone as well as pregnanolone levels were nonetheless observed, indicating an impairment in synthesis through the 5α-reductase enzyme (Rasmusson et al., 2019). In a separate study, men with PTSD from two independent cohorts were found to have decreased allopregnanolone levels (Marx, 2018). Pregnenolone treatment in these individuals improved the primary behavioral endpoints in this study and correlated with a potential enhancement of white matter integrity (Marx, 2018). These results are supported by the findings of a functional gene variant of 5α-reductase type II that has been shown to exert influence on PTSD risk and symptom severity only in men (Gillespie et al., 2013). Further, these studies highlight the ability of exogenous neurosteroid treatment to reverse the circuit level neuropathology observed in psychiatric diseases (for review see (Almeida et al., 2021; Rasmusson et al., 2022)).
2.1.4. Bipolar Disorder
Emotional lability is a hallmark symptom of bipolar disorders (i.e., cyclical shifts of manic or hypomanic episodes and depressive states). During active depressive states in individuals with bipolar disorder, it has been demonstrated that CSF levels of pregnenolone are reduced compared to individuals without an affective illness (George et al., 1994). An association has been made between a single nucleotide polymorphism in the gene encoding TSPO in individuals with bipolar disorder that may lead to impaired neurosteroidogenesis (Colasanti et al., 2013). Low serum progesterone levels were also found to be associated with a single nucleotide polymorphism in the gene AKR1C4 that encodes for the steroidogenic enzyme 3α-HSD in men with a history of manic or hypomanic irritability; the effect was not found in females with similar diagnoses (Johansson et al., 2011). This resulting functional missense mutation may increase the risk for developing manic or hypomanic irritability, as it impairs the conversion of progesterone to allopregnanolone, resulting in less anxiolytic capacity of neurosteroid signaling during states of intense mood shifts. Conversely, in women with the same AKR1C4 missense mutation, elevated serum progesterone levels have been reported that were shown to be protective against paranoia in women with bipolar disorder during manic or hypomanic episodes. These studies also identified a mutation in the HSD3B2 gene, which encodes for the steroidogenic enzyme 3β-HSD, that was shown to be associated with an increased risk of paranoid ideation (Johansson et al., 2012).
Individuals with bipolar disorder have been demonstrated to have the highest rate of comorbid substance use disorders, with cannabis being the most abused drug (Leweke & Koethe, 2008). In bipolar depressed individuals, it has been demonstrated that previous cannabis use disorders were associated with increased basal CSF levels of pregnenolone while allopregnanolone levels were attenuated (Mason et al., 2017). These findings suggest that prior cannabis use may increase risk for bipolar disorder through preferencing neurosteroidogenesis to pregnanolone synthesis over allopregnanolone. In a randomized, double-blind, placebo-controlled trial in which individuals with bipolar disorder received 500mg of pregnenolone per day, those with bipolar disorder reported significantly reduced depressive symptoms as determined by the Hamilton Rating Scale for Depression (Brown et al., 2014; Hamilton, 1960). Furthermore, administration of pregnenolone increased serum levels of allopregnanolone, pregnenolone, and pregnanolone from baseline measurements, which may have contributed to the higher rate of symptom remission post-treatment (Brown et al., 2014). Consistent with the proposed efficacy of exogenous neurosteroids in the treatment of symptoms present in bipolar disorder, treatment with atypical antipsychotics (i.e. olanzapine, clozapine, and lithium), increase levels of pregnenolone and allopregnanolone (Marx et al., 2008; Marx, Shampine, et al., 2006). The resulting increase in neurosteroid levels may contribute to the efficacy of these therapies in stabilizing mood (for review see ((Carta et al., 2012)).
2.1.5. Schizophrenia
Neurosteroids have been linked to the pathology of schizophrenia (for review see (Cai et al., 2018; Michele et al., 2008; Ritsner, 2010)). Two brain regions that have been indicated for hypofunction and grey matter deficits in first-episode and progressive schizophrenia are the posterior cingulate cortex and the parietal cortex (Liang et al., 2020; Mitelman et al., 2005; Northoff et al., 2005). Post-mortem analysis of neurosteroid levels in individuals with schizophrenia demonstrated that pregnenolone and DHEA levels were higher in the posterior cingulate and parietal cortex, compared to individuals without a psychiatric diagnosis (Marx, Stevens, et al., 2006). Allopregnanolone levels in the parietal cortex were found to be decreased in individuals with schizophrenia (Marx, Stevens, et al., 2006). Treatment with neurosteroids have been shown to alleviate negative, depressive, and anxiety symptoms in individuals with schizophrenia. In several studies, individuals diagnosed with schizophrenia that received DHEA or pregnenolone treatment in addition to their regular antipsychotic medication demonstrated significant improvements in negative, anxiety, and depressive symptoms without varying impact on cognitive and positive symptoms associated with schizophrenia (Marx et al., 2017; Marx et al., 2009; Ritsner et al., 2014; Strous et al., 2003). These individuals had an increase in plasma neurosteroid levels that correlated with their improvement in negative symptoms (Marx et al., 2017; Marx et al., 2009; Strous et al., 2003). A separate study evaluating the impact of add-on treatment with pregnenolone to improve neurocognitive dysfunction in individuals with schizophrenia found that pregnenolone significantly reduced deficits in visual attention, sustained attention, and executive functioning (Kreinin et al., 2017). These improvements were demonstrated to occur independent of the type of antipsychotic therapy individuals were also taking or the duration of their illness (Kreinin et al., 2017). Interestingly, similar to treatment in bipolar disorder, it has been noted that second-generation antipsychotics such as clozapine, increase neurosteroid levels, an effect which may contribute to their efficacy for both positive and negative symptoms of schizophrenia (Barbaccia et al., 2001; Marx et al., 2003; Marx, Shampine, et al., 2006).
2.1.6. 5α-reductase Deficiency and Post-Finasteride Syndrome
In 1974, an article was published detailing the impact of a genetic mutation found in a group of children leading to deficiencies in 5α-reductase and dihydrotestosterone. Further studies identified that 5α-reductase type 2 is the primary isozyme absent in these individuals with no significant impairment in the expression or function of 5α-reductase type 1. Males with this mutation present with ambiguous sexual features until maturity when they present with prostate hypoplasia. Additional studies identified that these individuals do not develop androgenic alopecia, also known as male pattern hair loss. This discovery prompted interest in the development of 5α-reductase inhibitors for the treatment of both androgenic alopecia and prostate hyperplasia.
Currently there are two 5α-reductase inhibitors that are FDA-approved for the treatment of benign prostate hyperplasia and androgenic alopecia. The first in this class, Finasteride (Propecia™ or Proscar™), is a competitive 5α-reductase inhibitor with preferential inhibition of the type 2 isozyme. The second, Dutasteride (Avodart™), is a selective inhibitor for both type 1 and type 2 isozymes. Several side effects have been noted in individuals that received these therapies, even after discontinuation of the drug, including anxiety, depression, and suicidal ideation (Ganzer et al., 2014; Irwig, 2012; Melcangi et al., 2017; Rahimi-Ardabili et al., 2006; Traish et al., 2015), now termed post-finasteride syndrome (for review see (Diviccaro et al., 2019)). CSF measurement in individuals that discontinued treatment with finasteride conclude that 5α-reduced neurosteroids remained altered in these individuals which is considered to contribute to the pathology of their mood symptoms (Caruso et al., 2015; Melcangi et al., 2013, 2017) (Figure 3).
Figure 3. Clinical and Preclinical Consequences of Impaired Neurosteroid Signaling.
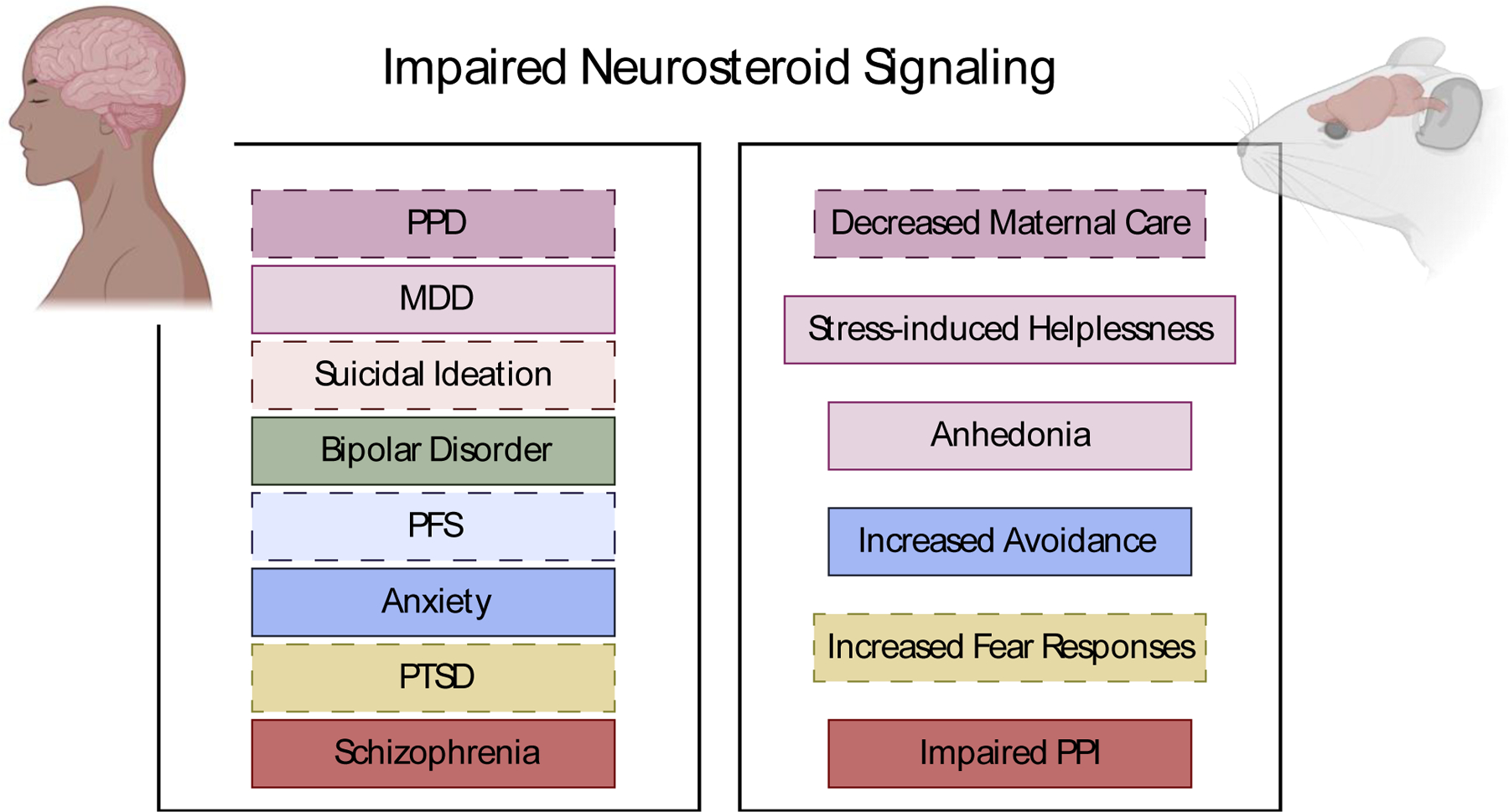
Descriptions of preclinical and clinical symptoms associated with impaired neurosteroid levels.
2.2. Preclinical evidence
2.2.1. Preclinical Models Relevant to Anxiety
Neurosteroids have also proven efficacious in improving affective deficits observed in preclinical models. To measure affective responses in rodents, several behavioral tasks are utilized such as the forced swim test, tail suspension test, open field, light/dark box, and elevated plus maze test (Cryan et al., 2002). In the forced swim test, experimenters place a rodent individually into a cylinder filled with water and measure the amount of time the rodent remains mobile as a proxy for antidepressant responsiveness (Porsolt et al., 1977). Use of exogenous neurosteroids has been shown to increase the total time rodents remain mobile in the forced swim test indicating an antidepressant response of these compounds (Frye et al., 2004; Frye & Walf, 2002; Khisti et al., 2000; Khisti & Chopde, 2000; Martínez-Mota et al., 1999). In open field testing, anxiety behavior has been inferred from the lack of avoidance behaviors measured as the number of entries and time spent in the center of the chamber. After receiving exogenous neurosteroids, rodents display less avoidance behaviors, performing more center entries and spending more time in the center of the chamber (Antonoudiou et al., 2022; Frye et al., 2004; Wieland et al., 1991). Opposingly, rodents treated with finasteride display behaviors consistent with increased anxiety and depressive responses when subjected to the forced swim and open field tests (Frye & Walf, 2002). In the light/dark box the number of transitions an animal makes between the light and dark chambers is also a measure of avoidance behaviors and used as a proxy for anxiety behaviors, whereby an increase in transitions signifies anxiolytic behavior. Following neurosteroid exposure, rodents are observed to have a decrease in anxiogenic behavior demonstrated by an increase in light/dark chamber transitions (Wieland et al., 1991). Similar results are seen in the elevated plus maze following neurosteroid treatment, where rodents display more anxiolytic behavior demonstrated by spending more time in the open arm of the maze (Frye et al., 2004). Additionally, these anxiolytic effects of exogenous neurosteroids are seen in rodents that have been devoid of peripheral neurosteroid secretion, an effect associated with dose-dependent increases in brain levels of neurosteroids (Bitran et al., 1993). Cumulatively, these studies suggest that treatment with exogenous neurosteroids have behavioral effects in part due to their ability to modulate endogenous neurosteroid levels. Another preclinical measure of rodent behavior is the Geller-Seifter conflict paradigm, in which rodents are subjected to an operant chamber that produces a reward followed by increasing amplitudes of foot shocks (Pollard & Howard, 1979). After receiving exogenous neurosteroids, rodents demonstrate an increase in anti-conflict behaviors measured as an increase in lever pressing for a reward despite the conflict of being shocked (Brot et al., 1997).
2.2.2. Preclinical Models Relevant to Depression
Chronic stress is a known precipitating factor in the emergence of depressive disorders and has been utilized in several preclinical protocols to induce behavioral deficits similar to the symptoms manifest in depression (for review see (Willner, 1984, 1990)). In work from our laboratory and others using rodent models of depression, allopregnanolone levels in the amygdala and frontal cortex have been demonstrated to decline (Uzunova et al., 2003; Walton et al., 2023). Coinciding with the decline in allopregnanolone, 5α-reductases have also been demonstrated to be reduced in the amygdala following chronic stress (Walton et al., 2023). Though acute stress has shown to briefly elevate neurosteroid levels (Purdy et al., 1991), several models of chronic stress have demonstrated deficits in neurosteroids that may contribute to the emergence of affective disorders (Dong et al., 2001; Walton et al., 2023). The use of exogenous neurosteroids has also been shown to prevent the behavioral deficits posed by chronic stress by lowering anxiety and avoidance behavioral measures (Antonoudiou et al., 2022; Evans et al., 2012; Walton et al., 2023). If treatment occurred after the period of chronic stress, the use of exogenous neurosteroids was capable of restoring behavioral measures to baseline outcomes (Antonoudiou et al., 2022; Evans et al., 2012).
2.2.3. Preclinical Models Relevant to PTSD
It has been demonstrated that neurosteroid biosynthesis is disrupted in several corticolimbic brain regions in preclinical rodent models of PTSD (for review see (Almeida et al., 2021; Aspesi & Pinna, 2019; Guidotti et al., 2001; Locci & Pinna, 2019b; Pinna, 2018)). Social isolation has been proven to induce enhanced contextual fear, impaired contextual fear extinction, as well as symptoms of anxiety and depression (Locci & Pinna, 2019a; Pibiri et al., 2008; Pinna et al., 2003; Rau et al., 2005). Following protracted social isolation, pregnenolone, 5α-DHP, 5α-reductase type I, and allopregnanolone are decreased in the frontal cortex as compared to group housed mice (Dong et al., 2001; Serra et al., 2000). Socially isolated mice also exhibit a downregulation of 5α-reductase type I in layer v-vi, CA3, dentate gyrus, and BLA, as well as a downregulation of 3α-HSD in the dentate gyrus (Agís-Balboa et al., 2007).
Treatment with neurosteroids prior to exposure of a traumatic event is protective against anxiogenic behaviors in rodent models of PTSD (Bitran et al., 2000). Restoring brain allopregnanolone levels with SSRI treatment (fluoxetine) following protracted social isolation has been demonstrated to reduce aggression that is induced by this model (Pinna et al., 2003). The aggressive phenotype displayed in this model has been attributed in part to reductions in brain levels of allopregnanolone, insofar as the intensity of aggressive behavior is dose-dependently reduced with allopregnanolone (Pinna et al., 2003). Additionally, (S)-norfluoxetine (S-NFLX), an active metabolite of fluoxetine that does not impact serotonin reuptake, was demonstrated to be more potent than fluoxetine in normalizing brain allopregnanolone levels and further, reduced the enhanced contextual fear-conditioned response that is elicited by social isolation (Pibiri et al., 2008). In preclinical models of PTSD there have also been noted reductions in the GABAAR signaling, demonstrated by a reduction in their responsiveness to GABA agonists and a decrease of α1, α2, and γ2 subunits with an increase in α4 and α5 subunits (Matsumoto et al., 2009). These changes in GABAAR subunit assembly may be attributed to the downregulation of allopregnanolone synthesis that occurs during social isolation. It has been demonstrated that GABAAR positive allosteric modulation with benzodiazepines (midazolam), which are commonly used to treat anxiety, enhances long term potentiation in the hippocampus through a mechanism dependent on neurosteroid signaling (Tokuda et al., 2010). Collectively, these findings further establish the ability of exogenous neurosteroids to impact anxiogenic behaviors in rodents and support the role of neurosteroids in maintaining affective tone despite known precipitating factors for psychiatric illnesses like PTSD.
2.2.4. Preclinical Models Relevant to Bipolar Disorder
Historically, lithium has been the gold standard in treatment for bipolar I and II to prevent manic/hypomanic and depressive episodes (Volkmann et al., 2020). In rodents, exposure 10 days of lithium treatment was demonstrated to lower brain levels of DHEA in the frontal cortex and hippocampus (Maayan et al., 2004). Serum levels of DHEA were also found to be impaired following lithium treatment (Maayan et al., 2004). Conversely, serum levels of allopregnanolone and pregnenolone were demonstrated to increase following lithium treatment. Other mood stabilizers including clozapine and olanzapine, which are also used for the treatment of bipolar disorder, have been shown to increase levels of pregnenolone in the rat hippocampus, cerebral cortex, and serum (for review see (Carta et al., 2012).
2.2.5. Preclinical Models Relevant to Schizophrenia
The stress-diathesis model provides one explanation for the increased vulnerability to the development of schizophrenia in individuals that have been chronically exposed to stressors during development (Walker & Tessner, 2008). This model has been applied in rodents utilizing isolation rearing, where rodents are subjected to chronic social deprivation from weaning until adulthood. Following isolation rearing, neurosteroids have also been found to be impacted through impairment of 5α-reductase synthesis (Paba et al., 2011). Additionally, rodents exhibit several behavioral and biochemical aberrations that are observed in individuals with schizophrenia, including: hyper-reactivity to a novel environment, impaired sensorimotor gating, anxiogenic-behaviors, anhedonia, cognitive deficits, and excess dopamine secretion (for review see (Fone & Porkess, 2008; Walker, 1994)) (Figure 3).
Dopamine hyperactivity has also been indicated in schizophrenia (for review see (Howes & Kapur, 2009), a condition in which individuals have difficulty filtering sensorimotor stimuli to effectively respond to the environment. Sensorimotor gating may be measured using prepulse inhibition (PPI) where rodent motor responses are measured during a first introduction to a weak acoustic stimulus preceding a startle-inducing acoustic stimulus. Dopaminergic agonists have been shown to produce deficits in PPI, where subsequent exposure to a startle-inducing stimulus results in a lower startle amplitude (Bortolato et al., 2008; Frau et al., 2014). Inhibition of 5α-reductase by finasteride blocks the effects of dopaminergic agonists on PPI (Bortolato et al., 2008; Frau et al., 2014). Following treatment with finasteride, reversal of PPI deficits induced by dopamine agonists was only effective after use of a D1 agonist (Frau et al., 2014). The contribution of dopamine receptors in mediating these effects of impaired 5α-reductases is further discussed in the below section titled: “Proposed mechanisms mediating effects of 5α-reduced neurosteroids on affective tone”.
3. Proposed mechanisms mediating the effects of 5α-reduced neurosteroids on affective tone
3.1. Ionotropic
In the mammalian brain, GABAA-receptors (GABAARs) mediate most of the inhibitory control over neurotransmission (Ghit et al., 2021; Michels & Moss, 2007). GABAARs are heteropentameric ligand gated chloride ion selective channels, composed of five subunit variants from 19 subunit isoforms (α1–6, β1–3, γ1–3, δ, ε, q, π, and r1–3). GABAAR subunit composition expression varies throughout neuronal circuits, with both regional and neuronal specificity (Michels & Moss, 2007; Nakamura et al., 2015). Compositional diversity of GABAARs differs between synaptically or extrasynaptically located receptors and further, determines the biophysical responses of these receptors to pharmacological manipulations and therefore their downstream impacts on behavior (Michels & Moss, 2007).
Finasteride has been demonstrated to prevent the estrous cycle-related changes in GABAergic inhibition, suggesting that these effects are mediated by endogenous neurosteroids (Maguire & Mody, 2007). Proteomic analyses indicate that treatment with finasteride results in dysregulation of pathways involved in GABA synthesis and degradation, synaptic transmission, fatty acid metabolism and steroid biosynthesis (Soggiu et al., 2016). Sub-chronic treatment with finasteride followed by withdrawal has been shown to induce downregulation of both α4- and β3-subunit containing GABAARs in the cerebral cortex in conjunction with a downregulation of estrogen receptor-α and ‒β (Giatti et al., 2016). The effects of finasteride on the downregulation of β3-subunit containing GABAARs was also observed in the gut following finasteride withdrawal (Diviccaro et al., 2022). These findings support the notion that endogenous neurosteroids impact GABAARs, which may contribute to their influence over affective tone.
Nonsulfated 5α-reduced neurosteroids act as positive allosteric modulators of GABAARs, increasing the frequency and duration of ion channel opening (Akk et al., 2005). Neurosteroid access to intracellular GABAARs is largely via lateral membrane diffusion; however, they are also capable of binding to GABAARs upon extracellular membrane sites (Akk et al., 2005). These actions by neurosteroids have been demonstrated to occur at low nanomolar (~10nM) concentrations in the presence of GABA, through a highly conserved binding site on α- subunits, however, in the absence of GABA, neurosteroids at micromolar concentrations have been shown to directly activate GABAARs through a binding site at the interphase of α/β subunits (Chen et al., 2019; Hosie et al., 2006). Neurosteroid sensitivity for GABAARs is influenced by GABAAR subunit composition (Akk et al., 2005, 2008; Belelli & Lambert, 2005; Hosie et al., 2006). Specifically, extrasynaptic δ-subunit containing GABAARs are more sensitive to neurosteroid modulation in comparison to synaptically located γ-subunit containing GABAARs (Belelli & Lambert, 2005; Carver & Reddy, 2016; Reddy, 2018). In addition to their extrasynaptic location, δ-subunit containing GABAARs also differ from γ-subunit containing GABAARs by their contribution to a tonic inhibition firing pattern, activated by low concentrations of GABA (Mody & Pearce, 2004). This contrasts with benzodiazepines, classically used for treatment of anxiety and depressive disorders, which bind to GABAARs at the interphase between α1–3, α5 and γ subunits yet α4/6 containing receptors are insensitive to benzodiazepines (Morlock & Czajkowski, 2011; Richter et al., 2012). Fluctuations in expression of δ-subunit containing GABAARs have been noted across estrous states as well as in affective disorders including postpartum depression, major depression, and anxiety disorders (Gilfarb & Leuner, 2022; Lüscher & Möhler, 2019; Maguire et al., 2005). Many of these fluctuations have been demonstrated to occur in concert with changing neurosteroid levels, suggesting that impairments in GABAergic functioning and neurosteroid signaling may underlie some of the neuropathology of affective disorders.
3.2. Metabotropic
3.2.1. Membrane Progesterone Receptors
5α-reduced neurosteroids also act upon receptors of the progestin and adipoQ receptor (PAQR) family, which are a g-protein coupled receptor family comprised of membrane progesterone receptors (mPRs) (Pang et al., 2013; Rupprecht et al., 1993). Neurosteroid binding of mPRs activates a signaling cascade that promotes the surface expression of α4βδ-containing GABAARs (Abramian et al., 2014; Davies et al., 2017; Parakala et al., 2019). This has been shown to occur via the phosphorylation of a key regulatory residue, serine 408/409, in the β3 subunit (Modgil et al., 2017; Parakala et al., 2019). Along with the induction of cell surface expression, neurosteroid phosphorylation of the β3 subunit has been demonstrated to potentiate GABAergic inhibition (Modgil et al., 2017; Parakala et al., 2019). Evidence supports that these metabotropic actions of neurosteroids aid in facilitating anti-apoptotic actions in brain regions that coordinate emotional responses (i.e., hypothalamus, amygdala, pituitary gland, forebrain, and corpus callosum), where mPRs are abundantly expressed (Pang et al., 2013). These metabotropic actions highlight the potential for neurosteroids to impact affect tone through the preservation of cell circuits that facilitate emotional responses.
3.2.2. Mesolimbic dopamine system
Neurosteroids have been demonstrated to modulate the dopaminergic mesolimbic system (Dornellas et al., 2021; Mosher et al., 2019). In rodents treated with finasteride, increased anxiety-like behaviors in the open field where correlated with decreased dopamine synthesis in the frontal cortex, hippocampus, caudate putamen, and nucleus accumbens (Li et al., 2018). As previously mentioned, dopamine hyperactivity has been indicated in psychiatric illnesses such as schizophrenia, and results in impaired rodent responses to PPI. While treatment with finasteride was demonstrated to reverse the deficits of PPI induced by dopamine agonists, this was shown only to be effective with use of a D1 agonist (Frau et al., 2014). These effects were not seen following use of a selective D2 agonist or with the use of a non-selective D1/D2 agonist, indicating that finasteride effects sensorimotor gating primarily through D1 receptors (Frau et al., 2014). Further investigation has also identified the role of finasteride in reversing the effects of the dopaminergic agonist, pramipexole, that has high selectivity for D3 receptors and a lesser affinity for D2 receptors (Floris et al., 2022). Finasteride reversed the pramipexole-induced upregulation of D3 receptors in the nucleus accumbens (Floris et al., 2022). D1 and D3 receptors are known to form complexes that increases the affinity of dopamine. Following finasteride treatment, coimmunoprecipitation of D1- and D3-receptor complexes was dampened (Fanni et al., 2019). These actions of finasteride indicate the capacity of impaired synthesis of 5α-reductase in modulating the functional relationship of D1-and D3- receptor complexes and subsequently dopamine signaling through a key brain region involved in the dopamine mesolimbic system.
3.3. Inflammation
In addition to the ability of stress to induce alterations in neurosteroid levels, stress also induces changes in neuroimmune signaling that may contribute to the emergence of psychiatric disease states (Brenhouse et al., 2019; Bullmore, 2018; Walter et al., 2017). Recent studies have indicated that allopregnanolone and pregnenolone are capable of inhibiting neuroimmune signaling by mediating interactions with toll-like receptors (Balan et al., 2019, 2023). Toll-like receptors (TLRs) and their downstream effects on neuroimmune signaling have been previously implicated in the pathology of neuropsychiatric disorders (Liu et al., 2014). TLRs are dependent upon adaptor proteins including myeloid differentiation primary response 88 (MyD88) and TIR-domain-containing adapter-including interferon-β (TRIF) to activate cytokine and chemokine signaling pathways. Upon lipopolysaccharide (LPS)-mediated activation of neuroimmune signaling, neurosteroids including allopregnanolone and pregnenolone inhibit the levels of proinflammatory mediators (i.e. high mobility group box-1 (HMGB1), monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor alpha (TNFα)) in addition to transcription factors (i.e. the p50 subunit of nuclear factor kappa B (NF-kB p50), (phosphorylated p65 subunit of nuclear factor kappa B (pNF-kB p65), phosphorylated cyclic-AMP response element binding protein (pCREB), phosphorylated transforming growth factor-β-activated kinase-1 (pTAK1), and tumor necrosis factor receptorassociated factor 6 (TRAF6)) (Balan et al., 2019). These effects have been shown to be due in part by the ability of these neurosteroids to prevent the TLR4/MD-2 complex, which would initiate LPS-mediated TLR4 signaling (Balan et al., 2019). Finasteride has been observed to induce activation of pro-inflammatory mediators in the gut-brain axis following treatment withdrawal. In rodents, 20 days of treatment followed by one month of withdrawal, gut levels of TNFα and Interleukin-1β (IL-1β) were found to be elevated (Diviccaro et al., 2022). Brexanolone was shown to inhibit pro-inflammatory pathways in individuals with postpartum depression (Balan et al., 2023). Specifically, it was demonstrated that following the 60-hour infusion of Brexanolone, TNFα and IL-6 levels were reduced in whole blood samples and were significantly correlated with improvements in HAM-D scores (Balan et al., 2023). Further studies have demonstrated that Brexanolone blunted LPS-induced TLR activation through inhibition of TLR4 and TLR7 (Balan et al., 2023) which is in alignment with previous observations that allopregnanolone inhibits TLR activation through MyD88-dependent signaling and not TRIF signaling (Balan et al., 2021). Collectively these findings highlight a novel mechanism by which neurosteroids may mitigate neuropsychiatric disease states by dampening pro-inflammatory pathways.
3.4. Network states/Connectivity
Exogenous administration of neurosteroids has been demonstrated to alter network states in amygdalocortical brain regions critical for emotional processing across several species (Antonoudiou et al., 2022). Administration of neurosteroid analogs has been shown to alter the patterns of electroencephalographic (EEG) activity in humans and local field potential recording in rodents, potentiating the power of high theta (6–12Hz) frequency oscillations known to govern safety behavioral responses (Antonoudiou et al., 2022). Interestingly, this network effect on high theta oscillations is distinct from the effects of other positive allosteric modulators of GABAARs, suggesting a unique mechanism of action (Antonoudiou et al., 2022). Moreover, the effects on high theta power do not occur in mice lacking δ-subunit containing GABAARs, indicating that the ability of neurosteroid analogs to alter oscillations in the theta frequency are mediated through δ-subunit containing GABAARs (Antonoudiou et al., 2022).
Neurosteroid actions in limbic brain regions including the amygdala have previously been shown to mediate anxiolytic and antidepressant effects (Antonoudiou et al., 2022; Walton et al., 2023). Exogenous administration of neurosteroids either directly to the amygdala or systemically result in changes in connectivity within the amygdala as well as the regions to which it projects (Antonoudiou et al., 2022; Sripada et al., 2014; Van Wingen et al., 2008). Cortical thickness and associated disruptions in connectivity have been associated with multiple psychiatric disorders (Paus, 2021). In disorders where neurosteroid levels are impaired, there have been observed correlations in the degree of cortical thickness to serum neurosteroid levels such that a higher degree of cortical thinning and symptom severity tracks with lower levels of neurosteroids (Kinzel et al., 2020). Chronic stress has also been shown to induce brain wide alterations in network connectivity (Antonoudiou et al., 2022; Negrón-Oyarzo et al., 2016; Woo et al., 2021). These alterations are prevented with the treatment of exogenous neurosteroids during the experience of chronic stress, suggesting that neurosteroids may coordinate network activity through multiple brain regions that are necessary to maintain affective tone.
4. Therapeutic Potential of Targeting Endogenous Neurosteroidogenesis
As highlighted in this review and others, endogenous neurosteroidogenesis has been indicated in the neuropathology of several psychiatric illness (for further review see (Pinna, 2020; Porcu et al., 2016; Walton & Maguire, 2019)). Treatment with neurosteroids or pharmacological treatments (i.e., antidepressants, antipsychotics, and mood stabilizers) that increase neurosteroid levels (Table 2) have demonstrated efficacy in improving symptoms of psychiatric illnesses. This suggests that neurosteroids may contribute to the therapeutic efficacy of these treatments, in addition to potentially contributing to the underlying neuropathology of these illnesses. Known precipitating factors to the emergence of psychiatric illnesses such as social isolation and chronic stress have been shown to elicit deficits in neurosteroids. Importantly, many of these induced deficits in neurosteroidogenesis have been discovered in brain regions that mediate emotional responses, suggesting a key role for neurosteroids in maintaining affective tone.
Table 2.
Pharmacological treatments interact with neurosteroid synthesis.
| MEDICATION | CLASS | INDICATION | IMPACT ON 5a-REDUCED NEUROSTEROIDS | REFERENCE |
|---|---|---|---|---|
| ANTIDEPRESSANTS | ||||
| AMITRIPTYLINE | Tricyclic | MDD Off-label: anxiety, insomnia, treatment-resistant depression | + | (Jaworska-Feil et al., 2000) |
| DOXEPIN | Tricyclic | MDD, insomnia Off-label: anxiety | ? | |
| IMIPRAMINE | Tricyclic | MDD Off-label: insomnia | no effect | (Haduch et al., 2011; Uzunov et al., 1996; Guidotti & Costa, 1998) |
| TRAZODONE | Atypical | MDD Off-label: insomnia, anxiety | + | (Korade et al., 2017) |
| MIRTAZAPINE | Atypical | MDD Off-label: panic disorder, GAD, PTSD | + | (Schüle et al., 2006) |
| FLUOXETINE | SSRI | MDD, OCD, panic disorder, treatment-resistant depression, acute depression in Bipolar I disorder, PMDD. Off-label: social anxiety disorder and PTSD | + | (Griffin & Mellon, 1999; Guidotti & Costa, 1998; Pinna et al., 2003; Uzunova et al., 1998; Guidotti & Costa, 1998) |
| PAROXETINE | SSRI | MDD, OCD, GAD, PTSD, social anxiety disorder, PMDD | + | (Griffin & Mellon, 1999; Alessandr o Guidotti & Costa, 1998) |
| SERTRALINE | SSRI | MDD, panic disorder, OCD, PTSD, social anxiety disorder, PMDD Off-label: GAD | + | (Griffin & Mellon, 1999) |
| CITALOPRAM | SSRI | MDD Off-label: PMDD, OCD, panic disorder, GAD, PTSD, social anxiety disorder | ? | |
| ESCITALOPRAM | SSRI | MDD, GAD Off-label: panic disorder, OCD, PTSD, social anxiety disorder, PMDD | + | (Haduch et al., 2011) |
| VENLAFAXINE | SNRI | MDD, GAD, social anxiety disorder Off-label: PMDD | + | (Haduch et al., 2011) |
| DESVENLAFAXINE | SNRI | MDD | ? | |
| DULOXETINE | SNRI | MDD, GAD | ? | |
| BUPROPION | Atypical | MDD, Seasonal affective disorder | ? | |
| PHENELZINE | “Irreversible” MAOI | MDD, treatment-resistant depression | ? | |
| ISOCARBOXAZID | “Irreversible” MAOI | MDD | ? | |
| TRANYLCYPROMINE | “Reversible” MAOI | MDD Off-label: treatment-resistant depression, social anxiety disorder, panic disorder, atypical depression | ? | |
| SELEGILINE | “Reversible” MAOI | MDD | ? | |
| BREXANOLONE | Neurosteroi d Analog | PPD | + | (Balan et al., 2023) |
| KETAMINE | Dissociative Anesthetic | Treatment-resistant depression | + | (Korneyev et al., 1993; Li et al., 2016) |
| ANXIOLYTICS | ||||
| ALPRAZOLAM | Benzodiaza pine | GAD, panic disorder, anxiety associated with depression. Off-label: PMS, PMDD, insomnia, adjunct with acute mania, acute psychosis | ? | |
| CHLORDIAZEP OXIDE | Benzodiaza pine | GAD | ? | |
| CLONAZEPAM | Benzodiaza pine | Panic disorder Off-label: adjunct treatment of acute mania, acute psychosis, or insomnia | no effect | (Korneyev et al., 1993; Tokuda et al., 2010) |
| DIAZEPAM | Benzodiaza pine | anxiety disorder Off-label: insomnia | + | (Wolf et al., 2015) |
| LORAZEPAM | Benzodiaza pine | anxiety disorder Off-label: insomnia, panic disorder, adjunct with acute mania or acute psychosis | ? | |
| HYDROXYZINE | Antihistamine | Anxiety | ? | |
| MADAZOLAM | Benzodiaza pine | Anxiety | + | (Dhir & Rogawski, 2012; Tokuda et al., 2010) |
| ETIFOXINE | Benzooxazine | Anxiety | + | (Luc Do Rego et al., 2015; Wolf et al., 2015) |
| BUSPIRONE | Azapirone | GAD | ? | |
| ANTIPSYCHOTICS | ||||
| OLANZAPINE | Thio-Benzodiaze pine | Schizophrenia, Bipolar I Disorder, treatment-esistant depression | + | (Marx et al., 2003; Marx, Shampine, et al., 2006) |
| QUETIAPINE | Dibenzo-Thiazepine Derivative | Schizophrenia, depressive episodes in Bipolar I disorder, adjunctive treatment in MDD | no effect | (Marx, Shampine, et al., 2006) |
| RISPERIDONE | Benzisoxazole Derivative | Schizophrenia, acute mania/mixed episodes due to Bipolar I dDsorder, irritability associated with autism spectrum disorder | no effect | (Marx et al., 2003) |
| ZIPRASIDONE | Benzo-Thiazolyl-Piperazine Derivative | Schizophrenia, acute mania/mixed episodes due to Bipolar I Disorder | no effect | (Marx, Shampine, et al., 2006) |
| ARIPIPRAZOLE | Benzisoxazole Derivative | Schizophrenia, Bipolar I Disorder | no effect | (Marx, Shampine, et al., 2006) |
| CLOZAPINE | Dibenzo-Diazepine | Schizophrenia, suicidality in Schizophrenia | + | (Barbacci a et al., 2001; Marx et al., 2003; Marx, Shampine, et al., 2006) |
| THIOTHIXENE | Thioxanthene Derivative | Schizophrenia | ? | |
| THIORIDAZINE | Piperidine Phenothiazine | Schizophrenia. Off-label: depression with psychotic features | + | (Haduch et al., 2011) |
| CHLORPROMA ZINE | Dimethylamine Phenothiazine Derivative | Schizophrenia, psychoses, hyperexcitability, combative behavior in children Off-label: Bipolar I Disorder | ? | |
| HALOPERIDOL | Butyrophen one | Schizophrenia, manic states, drug-induced psychoses, hyperexcitability, agitation | no effect | (Barbaccia et al., 2001; Marx et al., 2003) |
| PALIPERIDONE | Benzisoxazole Derivative | schizophrenia, schizoaffective disorder | ? | |
| ASENAPINE | Dibenzo-Oxepino Pyrrroles | schizophrenia, manic/mixed episodes associated with bipolar I disorder | + | (Danek et al., 2021) |
| LURASIDONE | Benzisoxaz ole Derivative | schizophrenia, depressive episodes in Bipolar I disorder | + | (Danek & Daniel, 2022) |
| ILOPERIDONE | Benzisoxaz ole Derivative | schizophrenia | + | (Danek & Daniel, 2021) |
| CARIPRAZINE | Atypical | schizophrenia, acute mania/mixed episodes due to bipolar I disorder | ? | |
| BREXPIPRAZO LE | Serotonin-Dopamine Activity Modulators (SDAM) | schizophrenia, adjunct treatment of MDD | ? | |
| MOOD STABILIZERS | ||||
| LITHIUM | Antimanic | Bipolar I Disorder | + | (Marx et al., 2008; Maayan et al., 2004) |
| CARBAMAZEPI NE | Anticonvuls ant | Bipolar I Disorder | + | (Eagle et al., 2020; Jaworska-Feil et al., 2000; Yoshizaw a et al., 2020) |
| DIVALPROEX | Anticonvuls ant | Bipolar Disorder | ? | |
| LAMOTRIGINE | Anticonvuls ant | Bipolar Disorder | ? | |
indicates evidence of an interaction with the specified drug and neurosteroid synthesis,
indicates no known evidence of an interaction. Abbreviations: Major Depressive Disorder (MDD), Generalized Anxiety Disorder (GAD), Post Traumatic Stress Disorder (PTSD), Obsessive Convulsive Disorder (OCD), Premenstrual Dysphoric Disorder (PMDD), Postpartum Depression (PPD), Premenstrual Syndrome (PMS).
Most reports on the impact of neurosteroids have been based on the use of exogenous neurosteroids or their inhibitors. Recently, it was demonstrated that increasing neurosteroidogenesis by directly overexpressing 5α-reductases is sufficient to ameliorate behavioral deficits that are induced by chronic stress (Walton et al., 2023). These findings further support the role of endogenous neurosteroids in maintaining affective tone reinforce the potential of targeting neurosteroidogenesis for treatment of several affective illnesses. Harnessing the body’s ability to synthesize endogenous neurosteroids offers a superior therapeutic approach, ensuring synthesis and delivery at the endogenous site of action, largely in limbic regions, limiting off target and unwanted side effects. Future studies are required to explore this promising, novel therapeutic avenue.
Figure 4. Mechanisms Mediating 5α-reduced neurosteroids on affective tone.
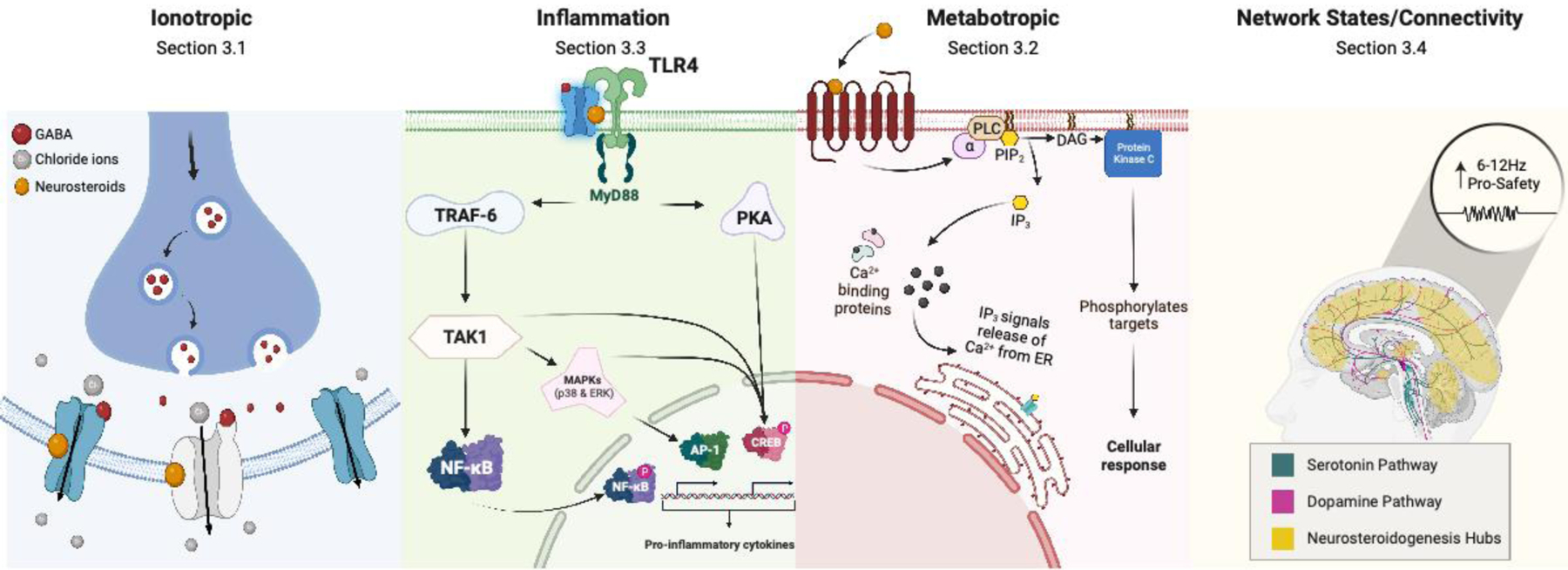
Illustration depicting the mechanisms highlighted in this review whereby 5α-reduced neurosteroids may impact affective tone. Abbreviations: Toll-like Receptor 4 (TLR4), myeloid differentiation primary response 88 (MyD88), tumor necrosis factor receptor-associated facto- 6(TRAF-6), protein kinase A(PKA), phosphorylated cyclic-AMP response element binding protein(pCREB), transforming growth factor-b-activated kinase-1(TAK1), nuclear factor kappa B(NF-kβ), phosphorylated nuclear factor kappa B(pNF-kβ), mitogen-activate protein kinases(MAPKs), activator protein-1(AP-1), phospholipase C(PLC), phosphatidylinositol 4,5-biphosphate(PIP2), diacylglycerol(DAG), inositol triphosphate(IP3), Calcium(Ca2+), Endoplasmic Reticulum(ER).
Acknowledgements
N.L.W, P.A., and J.L.M. are supported by NIH R01MH128235, and P50MH122379.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest statement
J.L.M serves as a member of the Scientific Advisory Board for SAGE Therapeutics, Inc. All other authors report no potential biomedical financial interests or conflicts of interest.
References
- Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, Maguire JL, Terunuma M, Davies PA, & Moss SJ (2014). Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America, 111(19), 7132–7137. 10.1073/pnas.1403285111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa Roberto C., Pinna G, Pibiri F, Kadriu B, Costa E, & Guidotti A (2007). Downregulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. In Proceedings of the National Academy of Sciences of the United States of America (Vol. 104, Issue 47). National Academy of Sciences. 10.1073/PNAS.0709419104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa Roberto C., Pinna G, Zhubi A, Maloku E, Veldic M, Costa, & Guidotti A (2006). Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proceedings of the National Academy of Sciences of the United States of America, 103(39), 14602. 10.1073/PNAS.0606544103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa Roberto Carlos, Guidotti A, & Pinna G (2014). 5α-reductase type I expression is downregulated in the prefrontal cortex/Brodmann’s area 9 (BA9) of depressed patients. Psychopharmacology, 231(17), 3569–3580. 10.1007/S00213-014-3567-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A, Heinig A, Sommer A, Wiedemann K, & Demiralay C (2023). Morning salivary dehydroepiandrosterone (DHEA) qualifies as the only neuroendocrine biomarker separating depressed patients with and without prior history of depression: An HPA axis challenge study. Journal of Psychiatric Research, 161, 449–454. 10.1016/j.jpsychires.2023.04.003 [DOI] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, & Steinbach JH (2008). Mutations of the GABA-A Receptor α1 Subunit M1 Domain Reveal Unexpected Complexity for Modulation by Neuroactive Steroids. Molecular Pharmacology, 74(3), 614–627. 10.1124/MOL.108.048520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu H-JJ, Wang C, Steinbach JHH, Zorumski CFF, Covey DFF, & Mennerick S (2005). Neurosteroid Access to the GABAA Receptor. Journal of Neuroscience, 25(50), 11605–11613. 10.1523/JNEUROSCI.4173-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida FB, Pinna G, & Barros HMT (2021). The Role of HPA Axis and Allopregnanolone on the Neurobiology of Major Depressive Disorders and PTSD. International Journal of Molecular Sciences, 22(11). 10.3390/IJMS22115495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althaus AL, Ackley MA, Belfort GM, Gee SM, Dai J, Nguyen DP, Kazdoba TM, Modgil A, Davies PA, Moss SJ, Salituro FG, Hoffmann E, Hammond RS, Robichaud AJ, Quirk MC, & Doherty JJ (2020). Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology, 181. 10.1016/j.neuropharm.2020.108333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonoudiou P, Colmers PLWW, Walton NL, Weiss GL, Smith AC, Nguyen DP, Lewis M, Quirk MC, Barros L, Melon LC, & Maguire JL (2022). Allopregnanolone Mediates Affective Switching Through Modulation of Oscillatory States in the Basolateral Amygdala. Biological Psychiatry, 91(3), 283–293. 10.1016/J.BIOPSYCH.2021.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud A, Suthoff E, Stenson K, Werneburg B, Hodgkins P, Bonthapally V, Jonas J, Meyer K, & O’Day K (2021). Number Needed to Treat and Number Needed to Harm analysis of the zuranolone phase 2 clinical trial results in major depressive disorder. Journal of Affective Disorders, 285, 112–119. 10.1016/J.JAD.2021.02.027 [DOI] [PubMed] [Google Scholar]
- Aspesi D, & Pinna G (2019). Animal models of post-traumatic stress disorder and novel treatment targets. Behavioural Pharmacology, 30(2and3-SpecialIssue), 130–150. 10.1097/FBP.0000000000000467 [DOI] [PubMed] [Google Scholar]
- Balan I, Beattie MC, O’buckley TK, Aurelian L, & Morrow AL (2019). endogenous Neurosteroid (3α,5α)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Activation and Pro-inflammatory Signaling in Macrophages and Brain. Scientific Reports, 9(1), 1–14. 10.1038/s41598-018-37409-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan I, Patterson R, Boero G, Krohn H, O’buckley TK, Meltzer-Brody S, & Morrow AL (2023). Brexanolone therapeutics in post-partum depression involves inhibition of systemic inflammatory pathways. EBioMedicine, 89, 104473. 10.1016/j.ebiom.2023.104473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Purdy RH, Maciocco E, Spiga F, & Biggio G (2001). Clozapine, but not haloperidol, increases brain concentrations of neuroactive steroids in the rat. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 25(4), 489–497. 10.1016/S0893-133X(01)00254-8 [DOI] [PubMed] [Google Scholar]
- Belelli D, & Lambert JJ (2005). Neurosteroids: Endogenous regulators of the GABAA receptor. In Nature Reviews Neuroscience (Vol. 6, Issue 7, pp. 565–575). Nat Rev Neurosci. 10.1038/nrn1703 [DOI] [PubMed] [Google Scholar]
- Binder G, Weber S, Ehrismann M, Zaiser N, Meisner C, Ranke MB, Maier L, Wudy SA, Hartmann MF, Heinrich U, Bettendorf M, Doerr HG, Pfaeffle RW, & Keller E (2009). Effects of dehydroepiandrosterone therapy on pubic hair growth and psychological well-being in adolescent girls and young women with central adrenal insufficiency: a double-blind, randomized, placebo-controlled phase III trial. The Journal of Clinical Endocrinology and Metabolism, 94(4), 1182–1190. 10.1210/JC.2008-1982 [DOI] [PubMed] [Google Scholar]
- Bitran D, Klibansky DA, & Martin GA (2000). The neurosteroid pregnanolone prevents the anxiogenic-like effect of inescapable shock in the rat. Psychopharmacology, 151(1), 31–37. 10.1007/S002130000472/METRICS [DOI] [PubMed] [Google Scholar]
- Bitran Daniel, Purdy RH, & Kellog CK (1993). Anxiolytic effect of progesterone is associated with increases in cortical alloprenanolone and GABAA receptor function. Pharmacology, Biochemistry and Behavior, 45(2), 423–428. 10.1016/0091-3057(93)90260-Z [DOI] [PubMed] [Google Scholar]
- Bloch M, Ish-Shalom S, Greenman Y, Klein E, & Latzer Y (2012). Dehydroepiandrosterone treatment effects on weight, bone density, bone metabolism and mood in women suffering from anorexia nervosa-a pilot study. Psychiatry Research, 200(2–3), 544–549. 10.1016/J.PSYCHRES.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orrù M, Bourov Y, Marrosu F, Mereu G, Devoto P, & Gessa GL (2008). Antipsychotic-like properties of 5-alpha-reductase inhibitors. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 33(13), 3146–3156. 10.1038/NPP.2008.39 [DOI] [PubMed] [Google Scholar]
- Brambilla F, Biggio G, Pisu MG, Bellodi L, Perna G, Bogdanovich-Djukic V, Purdy RH, & Serra M (2003). Neurosteroid secretion in panic disorder. Psychiatry Research, 118(2), 107–116. 10.1016/S0165-1781(03)00077-5 [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Danese A, & Grassi-Oliveira R (2019). Neuroimmune Impacts of Early-Life Stress on Development and Psychopathology. Current Topics in Behavioral Neurosciences, 43, 423–447. 10.1007/7854_2018_53 [DOI] [PubMed] [Google Scholar]
- Brot MD, Akwa Y, Purdy RH, Koob GF, & Britton KT (1997). The anxiolytic-like effects of the neurosteroid allopregnanolone: Interactions with GABA(A) receptors. European Journal of Pharmacology, 325(1), 1–7. 10.1016/S0014-2999(97)00096-4 [DOI] [PubMed] [Google Scholar]
- Brown ES, Park J, Marx CE, Hynan LS, Gardner C, Davila D, Nakamura A, Sunderajan P, Lo A, & Holmes T (2014). A Randomized, Double-Blind, Placebo-Controlled Trial of Pregnenolone for Bipolar Depression. Neuropsychopharmacology, 39(12), 2867. 10.1038/NPP.2014.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E (2018). Inflamed depression. The Lancet, 392(10154), 1189–1190. 10.1016/S0140-6736(18)32356-0 [DOI] [PubMed] [Google Scholar]
- Cai HL, Cao T, Zhou X, & Yao JK (2018). Neurosteroids in Schizophrenia: Pathogenic and Therapeutic Implications. Frontiers in Psychiatry, 9(73). 10.3389/FPSYT.2018.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta MG, Bhat KM, & Preti A (2012). GABAergic neuroactive steroids: A new frontier in bipolar disorders? Behavioral and Brain Functions, 8(1), 1–8. 10.1186/1744-9081-8-61/METRICS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso D, Abbiati F, Giatti S, Romano S, Fusco L, Cavaletti G, & Melcangi RC (2015). Patients treated for male pattern hair with finasteride show, after discontinuation of the drug, altered levels of neuroactive steroids in cerebrospinal fluid and plasma. The Journal of Steroid Biochemistry and Molecular Biology, 146, 74–79. 10.1016/J.JSBMB.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Carver CM, & Reddy DS (2013). Neurosteroid interactions with synaptic and extrasynaptic GABA A receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology (Berl), 230(2). 10.1007/s00213-013-3276-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, & Reddy DS (2016). Neurosteroid Structure-Activity Relationships for Functional Activation of Extrasynaptic δGABAA Receptors. The Journal of Pharmacology and Experimental Therapeutics, 357(1), 188. 10.1124/JPET.115.229302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics, S. (2017). 2017 National Survey on Drug Use and Health: Methodological Summary and Definitions.
- Chen ZW, Bracamontes JR, Budelier MM, Germann AL, Shin DJ, Kathiresan K, Qian MX, Manion B, Cheng WWL, Reichert DE, Akk G, Covey DF, & Evers AS (2019). Multiple functional neurosteroid binding sites on gabaa receptors. PLoS Biology, 17(3). ps:// 10.1371/JOURNAL.PBIO.3000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti A, Owen DR, Grozeva D, Rabiner EA, Matthews PM, Craddock N, & Young AH (2013). Bipolar Disorder is associated with the rs6971 polymorphism in the gene encoding 18 kDa Translocator Protein (TSPO). Psychoneuroendocrinology, 38(11), 2826–2829. 10.1016/J.PSYNEUEN.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Bulfone A, Rubenstein JLR, & Mellon SH (1995). Expression of the steroidogenic enzyme P450scc in the central and peripheral nervous systems during rodent embryogenesis. Endocrinology, 136(6), 2689–2696. 10.1210/ENDO.136.6.7750493 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, & Lucki I (2002). Assessing antidepressant activity in rodents: recent developments and future needs. Trends in Pharmacological Sciences, 23(5), 238–245. 10.1016/S0165-6147(02)02017-5 [DOI] [PubMed] [Google Scholar]
- Danek PJ, Bromek E, Haduch A, & Daniel WA (2021). Chronic treatment with asenapine affects cytochrome P450 2D (CYP2D) in rat brain and liver. Pharmacological aspects. Neurochemistry International, 151. 10.1016/J.NEUINT.2021.105209 [DOI] [PubMed] [Google Scholar]
- Danek PJ, & Daniel WA (2021). Long-Term Treatment with Atypical Antipsychotic Iloperidone Modulates Cytochrome P450 2D (CYP2D) Expression and Activity in the Liver and Brain via Different Mechanisms. Cells, 10(12). 10.3390/cells10123472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danek PJ, & Daniel WA (2022). The Atypical Antipsychotic Lurasidone Affects Brain but Not Liver Cytochrome P450 2D (CYP2D) Activity. A Comparison with Other Novel Neuroleptics and Significance for Drug Treatment of Schizophrenia. Cells, 11(21). 10.3390/cells11213513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Modgil AA, Parakala M, Ackley M, Doherty J, & Moss SJ (2017). Neurosteroids reverse tonic inhibitory deficits in Fragile X syndrome mouse model. The FASEB Journal, 31(S1), 815.12–815.12. 10.1096/FASEBJ.31.1_SUPPLEMENT.815.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir A, & Rogawski MA (2012). Role of neurosteroids in the anticonvulsant activity of midazolam. British Journal of Pharmacology, 165(8), 2684. 10.1111/J.1476-5381.2011.01733.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Michele F, Caltagirone C, & Spalletta G (2008). Neurosteroid perturbation and neuropsychiatric symptoms in schizophrenia: From the mechanisms to the treatment. Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders: Novel Strategies for Research and Treatment, 325–335. 10.1007/978-1-4020-6854-6_16/COVER [DOI] [Google Scholar]
- Dichtel LE, Nyer M, Dording C, Fisher LB, Cusin C, Shapero BG, Pedrelli P, Kimball AS, Rao EM, Mischoulon D, Fava M, & Miller KK (2020). Effects of Open-Label, Adjunctive Ganaxolone on Persistent Depression Despite Adequate Antidepressant Treatment in Postmenopausal Women: A Pilot Study. The Journal of Clinical Psychiatry, 81(4), 7602. 10.4088/JCP.19M12887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviccaro S, Giatti S, Cioffi L, Falvo E, Herian M, Caruso D, & Melcangi RC (2022). Gut Inflammation Induced by Finasteride Withdrawal: Therapeutic Effect of Allopregnanolone in Adult Male Rats. Biomolecules, 12(11). 10.3390/BIOM12111567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviccaro S, Melcangi RC, & Giatti S (2019). Post-finasteride syndrome: An emerging clinical problem. Neurobiology of Stress, 12. 10.1016/J.YNSTR.2019.100209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, & Guidotti A (2001). Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proceedings of the National Academy of Sciences of the United States of America, 98(5), 2849. 10.1073/PNAS.051628598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornellas APS, Macedo GC, McFarland MH, Gómez-A A, O’Buckley TK, Da Cunha C, Morrow AL, & Robinson DL (2021). Allopregnanolone Decreases Evoked Dopamine Release Differently in Rats by Sex and Estrous Stage. Frontiers in Pharmacology, 11. 10.3389/FPHAR.2020.608887/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle AL, Manning CE, Williams ES, Bastle RM, Gajewski PA, Garrison A, Wirtz AJ, Akguen S, Brandel-Ankrapp K, Endege W, Boyce FM, Ohnishi YN, Mazei-Robison M, Maze I, Neve RL, & Robison AJ (2020). Circuit-specific hippocampal ΔFosB underlies resilience to stress-induced social avoidance. Nature Communications, 11(1), 4484. 10.1038/s41467-020-17825-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Rubinow DR, Meltzer-Brody S, Deligiannidis KM, Riesenberg R, Krystal AD, Bankole K, Huang M-YY, Li H, Brown C, Kanes SJ, & Lasser R (2023). Effect of brexanolone on depressive symptoms, anxiety, and insomnia in women with postpartum depression: Pooled analyses from 3 double-blind, randomized, placebo-controlled clinical trials in the HUMMINGBIRD clinical program. Journal of Affective Disorders, 320, 353–359. 10.1016/J.JAD.2022.09.143 [DOI] [PubMed] [Google Scholar]
- Evaluation, I. for H. M. and. (2019). VizHub - GBD Compare. GBD Compare—Viz Hub. https://vizhub.healthdata.org/gbd-compare/ [Google Scholar]
- Evans J, Sun Y, McGregor A, & Connor B (2012). Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology, 63(8), 1315–1326. 10.1016/J.NEUROPHARM.2012.08.012 [DOI] [PubMed] [Google Scholar]
- Fanni S, Scheggi S, Rossi F, Tronci E, Traccis F, Stancampiano R, De Montis MG, Devoto P, Gambarana C, Bortolato M, Frau R, & Carta M (2019). 5alpha-reductase inhibitors dampen L-DOPA-induced dyskinesia via normalization of dopamine D1-receptor signaling pathway and D1-D3 receptor interaction. Neurobiology of Disease, 121, 120–130. 10.1016/J.NBD.2018.09.018 [DOI] [PubMed] [Google Scholar]
- Fergusson D, Doucette S, Glass KC, Shapiro S, Healy D, Hebert P, & Hutton B (2005). Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials. BMJ (Clinical Research Ed.), 330(7488), 396–399. 10.1136/BMJ.330.7488.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris G, Scheggi S, Pes R, & Bortolato M (2022). The steroidogenic inhibitor finasteride reverses pramipexole-induced alterations in probability discounting. Brain Research Bulletin, 181, 157–166. 10.1016/J.BRAINRESBULL.2022.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KCF, & Porkess MV (2008). Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neuroscience and Biobehavioral Reviews, 32(6), 1087–1102. 10.1016/J.NEUBIOREV.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, & Fawcett J (2010). Antidepressant Drug effects and Depression Severity: A Patient-Level Meta-Analysis. JAMA : The Journal of the American Medical Association, 303(1), 47. 10.1001/JAMA.2009.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, Bini V, Pes R, Pillolla G, Saba P, Devoto P, & Bortolato M (2014). Inhibition of 17α-hydroxylase/C17,20 lyase reduces gating deficits consequent to dopaminergic activation. Psychoneuroendocrinology, 39(1), 204. 10.1016/J.PSYNEUEN.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, & Walf AA (2002). Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Hormones and Behavior, 41(3), 306–315. 10.1006/hbeh.2002.1763 [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Rhodes ME, & Harney JP (2004). Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Research, 1004(1–2), 116–124. 10.1016/j.brainres.2004.01.020 [DOI] [PubMed] [Google Scholar]
- Ganzer CA, Jacobs AR, & Iqbal F (2014). Persistent Sexual, Emotional, and Cognitive Impairment Post-Finasteride. American Journal of Men’s Health, 9(3), 222–228. 10.1177/1557988314538445 [DOI] [PubMed] [Google Scholar]
- George MS, Guidotti A, Rubinow D, Pan B, Mikalauskas K, & Post RM (1994). CSF neuroactive steroids in affective disorders: Pregnenolone, progesterone, and DBI. Biological Psychiatry, 35(10), 775–780. 10.1016/0006-3223(94)91139-8 [DOI] [PubMed] [Google Scholar]
- Ghit A, Assal D, Al-Shami AS, & Hussein DEE (2021). GABAA receptors: structure, function, pharmacology, and related disorders. Journal of Genetic Engineering and Biotechnology 2021 19:1, 19(1), 1–15. 10.1186/S43141-021-00224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giatti S, Foglio B, Romano S, Pesaresi M, Panzica G, Garcia-Segura LM, Caruso D, & Melcangi RC (2016). Effects of Subchronic Finasteride Treatment and Withdrawal on Neuroactive Steroid Levels and Their Receptors in the Male Rat Brain. Neuroendocrinology, 103(6), 746–757. 10.1159/000442982 [DOI] [PubMed] [Google Scholar]
- Gilfarb RA, & Leuner B (2022). GABA System Modifications During Periods of Hormonal Flux Across the Female Lifespan. Frontiers in Behavioral Neuroscience, 16, 802530. 10.3389/FNBEH.2022.802530/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Almli LM, Smith AK, Bradley B, Kerley K, Crain DF, Mercer KB, Weiss T, Phifer J, Tang Y, Cubells JF, Binder EB, Conneely KN, & Ressler KJ (2013). Sex dependent influence of a functional polymorphism in steroid 5-α-reductase type 2 (SRD5A2) on post-traumatic stress symptoms. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 162(3), 283–292. 10.1002/AJMG.B.32147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin LD, & Mellon SH (1999). Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proceedings of the National Academy of Sciences of the United States of America, 96(23), 13512. 10.1073/PNAS.96.23.13512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, & Costa E (2001). The socially-isolated mouse: A model to study the putative role of allopregnanolone and 5α-dihydroprogesterone in psychiatric disorders. Brain Research Reviews, 37(1–3), 110–115. 10.1016/S0165-0173(01)00129-1 [DOI] [PubMed] [Google Scholar]
- Guidotti Alessandro, & Costa E (1998). Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3α, 5α- tetrahydroprogesterone (allopregnanolone) availability? Biological Psychiatry, 44(9), 865–873. 10.1016/S0006-3223(98)00070-5 [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, Li H, Lasser R, Zorumski CF, Rubinow DR, Paul SM, Jonas J, Doherty JJ, Kanes SJ, Gunduz-Bruce, et al. , Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, … Kanes SJ (2019). Trial of SAGE-217 in Patients with Major Depressive Disorder. New England Journal of Medicine, 381(10), 903–911. 10.1056/NEJMoa1815981 [DOI] [PubMed] [Google Scholar]
- Haduch A, Bromek E, & Daniel WA (2011). The effect of psychotropic drugs on cytochrome P450 2D (CYP2D) in rat brain. European Journal of Pharmacology, 651(1–3), 51–58. 10.1016/J.EJPHAR.2010.10.077 [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23(1), 56–62. 10.1136/JNNP.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad TA, Laughren T, & Racoosin J (2006). Suicidality in Pediatric Patients Treated With Antidepressant Drugs. Archives of General Psychiatry, 63(3), 332–339. 10.1001/ARCHPSYC.63.3.332 [DOI] [PubMed] [Google Scholar]
- Healy D, & Aldred G (2009). Antidepressant drug use & the risk of suicide. Http://Dx.Doi.Org/10.1080/09540260500071624, 17(3), 163–172. 10.1080/09540260500071624 [DOI] [PubMed] [Google Scholar]
- Hirschfeld RMA (2000). History and Evolution of the Monoamine Hypothesis of Depression. The Journal of Clinical Psychiatry, 61(suppl 6), 8272. https://www.psychiatrist.com/jcp/depression/history-evolution-monoamine-hypothesis-depression [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Da Silva HMA, & Smart TG (2006). Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature, 444(7118), 486–489. 10.1038/nature05324 [DOI] [PubMed] [Google Scholar]
- Howes OD, & Kapur S (2009). The Dopamine Hypothesis of Schizophrenia: Version III-The Final Common Pathway. Schizophrenia Bulletin, 35(3), 549–562. 10.1093/schbul/sbp006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Thase ME, & Pillinger T (2021). Treatment resistance in psychiatry: state of the art and new directions. Molecular Psychiatry 2021 27:1, 27(1), 58–72. 10.1038/s41380-021-01200-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwig MS (2012). Depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects. Journal of Clinical Psychiatry, 73(9), 1220–1223. 10.4088/JCP.12M07887 [DOI] [PubMed] [Google Scholar]
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, Abdollahpour I, Abdulkader RS, Abebe Z, Abera SF, Abil OZ, Abraha HN, Abu-Raddad LJ, Abu-Rmeileh NME, Accrombessi MMK, … Murray CJL (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392(10159), 1789–1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworska-Feil L, Budziszewska B, Leskiewicz M, & Lason W (2000). Effects of some centrally active drugs on the allopregnanolone synthesis in rat brain. Polish Journal of Pharmacology, 52(5), 359–365. https://pubmed.ncbi.nlm.nih.gov/11334228/ [PubMed] [Google Scholar]
- Jick H, Kaye JA, & Jick SS (2004). Antidepressants and the risk of suicidal behaviors. JAMA : The Journal of the American Medical Association, 292(23). 10.1001/jama.292.23.2833-c [DOI] [PubMed] [Google Scholar]
- Johansson AGM, Nikamo P, Schalling M, & Landén M (2011). AKR1C4 gene variant associated with low euthymic serum progesterone and a history of mood irritability in males with bipolar disorder. Journal of Affective Disorders, 133(1–2), 346–351. 10.1016/J.JAD.2011.04.009 [DOI] [PubMed] [Google Scholar]
- Johansson AGM, Nikamo P, Schalling M, & Landén M (2012). Polymorphisms in AKR1C4 and HSD3B2 and differences in serum DHEAS and progesterone are associated with paranoid ideation during mania or hypomania in bipolar disorder. European Neuropsychopharmacology, 22(9), 632–640. 10.1016/J.EURONEURO.2012.01.007 [DOI] [PubMed] [Google Scholar]
- John Rush A, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, George Niederehe M, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, & Maurizio Fava M (2006). Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. In Am J Psychiatry (Vol. 163, Issue 11). www.star-d.org [DOI] [PubMed] [Google Scholar]
- Kanes S, Colquhoun H, Doherty J, Raines S, Hoffmann E, Rubinow DRR, & Meltzer-Brody S (2017). Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Human Psychopharmacology, 32(2), e2576. 10.1002/hup.2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, Doherty J, Epperson CN, Deligiannidis KM, Riesenberg R, Hoffmann E, Rubinow D, Jonas J, Paul S, & Meltzer-Brody S (2017). Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. The Lancet, 390(10093), 480–489. 10.1016/S0140-6736(17)31264-3 [DOI] [PubMed] [Google Scholar]
- Khisti RT, & Chopde CT (2000). Serotonergic agents modulate antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice. Brain Research, 865(2), 291–300. 10.1016/S0006-8993(00)02373-8 [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT, & Jain SP (2000). Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacology, Biochemistry, and Behavior, 67(1), 137–143. 10.1016/S0091-3057(00)00300-2 [DOI] [PubMed] [Google Scholar]
- Kinzel P, Marx CE, Sollmann N, Hartl E, Guenette JP, Kaufmann D, Bouix S, Pasternak O, Rathi Y, Coleman MJ, van der Kouwe A, Helmer K, Kilts JD, Naylor JC, Morey RA, Shutter L, Andaluz N, Coimbra R, Lang AJ, … Koerte IK (2020). Serum Neurosteroid Levels Are Associated With Cortical Thickness in Individuals Diagnosed With Posttraumatic Stress Disorder and History of Mild Traumatic Brain Injury. Clinical EEG and Neuroscience, 51(4), 285–299. 10.1177/1550059420909676 [DOI] [PubMed] [Google Scholar]
- Korade Ž, Liu W, Warren EB, Armstrong K, Porter NA, & Konradi C (2017). Effect of psychotropic drug treatment on sterol metabolism. Schizophrenia Research, 187, 74–81. 10.1016/J.SCHRES.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneyev AY, Costa E, & Guidolti A (1993). During Anesthetic-Induced Activation of Hypothalamic Pituitary Adrenal Axis, Blood-Borne Steroids Fail to Contribute to the Anesthetic Effect. Neuroendocrinology, 57(3), 559–565. 10.1159/000126405 [DOI] [PubMed] [Google Scholar]
- Kreinin A, Bawakny N, & Ritsner MS (2017). Adjunctive pregnenolone ameliorates the cognitive deficits in recent-onset schizophrenia: An 8-week, randomized, double-blind, placebo-controlled trial. Clinical Schizophrenia and Related Psychoses, 10(4), 201–210. 10.3371/CSRP.KRBA.013114 [DOI] [PubMed] [Google Scholar]
- Leweke FM, & Koethe D (2008). Cannabis and psychiatric disorders: it is not only addiction. Addiction Biology. 10.1111/j.1369-1600.2008.00106.x [DOI] [PubMed] [Google Scholar]
- Li J, Yu Y, Wang B, Wu H, Xue G, & Hou Y (2016). Selective regulation of neurosteroid biosynthesis under ketamine-induced apoptosis of cortical neurons in vitro. Molecular Medicine Reports, 13(2), 1586–1592. 10.3892/MMR.2015.4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Kang YX, Ji XM, Li YK, Li SC, Zhang XJ, Cui HX, & Shi GM (2018). Finasteride inhibited brain dopaminergic system and open-field behaviors in adolescent male rats. CNS Neuroscience & Therapeutics, 24(2), 115–125. 10.1111/CNS.12781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JJ, & Rasmusson AM (2018). Overview of the Molecular Steps in Steroidogenesis of the GABAergic Neurosteroids Allopregnanolone and Pregnanolone. Chronic Stress, 2, 247054701881855. 10.1177/2470547018818555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Deng W, Li X, Wang Q, Greenshaw AJ, Guo W, Kong X, Li M, Zhao L, Meng Y, Zhang C, Yu H, Li X. min, Ma X, & Li T (2020). Aberrant posterior cingulate connectivity classify first-episode schizophrenia from controls: A machine learning study. Schizophrenia Research, 220, 187–193. 10.1016/J.SCHRES.2020.03.022 [DOI] [PubMed] [Google Scholar]
- Liu JJ, Buisman-Pijlman F, & Hutchinson MR (2014). Toll-like receptor 4: Innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Frontiers in Neuroscience, 8(SEP), 309. 10.3389/FNINS.2014.00309/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci A, & Pinna G (2019a). Social isolation as a promising animal model of PTSD comorbid suicide: neurosteroids and cannabinoids as possible treatment options. Progress in NeuroPsychopharmacology & Biological Psychiatry, 92, 243–259. 10.1016/J.PNPBP.2018.12.014 [DOI] [PubMed] [Google Scholar]
- Locci A, & Pinna G (2019b). Stimulation of Peroxisome Proliferator-Activated Receptor-α by N-Palmitoylethanolamine Engages Allopregnanolone Biosynthesis to Modulate Emotional Behavior. Biological Psychiatry, 85(12), 1036–1045. 10.1016/J.BIOPSYCH.2019.02.006 [DOI] [PubMed] [Google Scholar]
- Luc Do Rego J, Vaudry D, & Vaudry H (2015). The Non-Benzodiazepine Anxiolytic Drug Etifoxine Causes a Rapid, Receptor-Independent Stimulation of Neurosteroid Biosynthesis. PLOS ONE, 10(3), e0120473. 10.1371/JOURNAL.PONE.0120473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B, Shen Q, & Sahir N (2011). The GABAergic Deficit Hypothesis of Major Depressive Disorder. Molecular Psychiatry, 16(4), 383. 10.1038/MP.2010.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher Bernhard, & Möhler H (2019). Brexanolone, a neurosteroid antidepressant, vindicates the GABAergic deficit hypothesis of depression and may foster resilience [version 1; peer review: 4 approved]. F1000Research, 8, 751. https://doi.org/ 10.12688/f1000research.18758.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan R, Shaltiel G, Poyurovsky M, Ramadan E, Morad O, Nechmad A, Weizman A, & Agam G (2004). Chronic lithium treatment affects rat brain and serum dehydroepiandrosterone (DHEA) and DHEA-sulphate (DHEA-S) levels. The International Journal of Neuropsychopharmacology, 7(1), 71–75. 10.1017/S1461145703003821 [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, & Mody I (2005). Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature Neuroscience, 8(6), 797–804. 10.1038/nn1469 [DOI] [PubMed] [Google Scholar]
- Maguire J, & Mody I (2007). Neurosteroid Synthesis-Mediated Regulation of GABAA Receptors: Relevance to the Ovarian Cycle and Stress. Journal of Neuroscience, 27(9), 2155–2162. 10.1523/JNEUROSCI.4945-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Mota L, Contreras CM, & Saavedra M (1999). Progesterone reduces immobility in rats forced to swim. Archives of Medical Research, 30(4), 286–289. 10.1016/S0188-0128(99)00024-X [DOI] [PubMed] [Google Scholar]
- Marx C (2018). 39. Biomarkers and New Therapeutics in PTSD and TBI: Neurosteroid Signatures to Randomized Controlled Trials. Biological Psychiatry, 83, S16. 10.1016/j.biopsych.2018.02.056 [DOI] [Google Scholar]
- Marx CE, Keefe RSE, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, Savitz AJ, Leimone LA, Dunn L, Porcu P, Morrow AL, & Shampine LJ (2009). Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology, 34(8), 1885–1903. 10.1038/npp.2009.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Shampine LJ, Duncan GE, VanDoren MJ, Grobin AC, Massing MW, Madison RD, Bradford DW, Butterfield MI, Lieberman JA, & Morrow AL (2006). Clozapine markedly elevates pregnenolone in rat hippocampus, cerebral cortex, and serum: candidate mechanism for superior efficacy? Pharmacology, Biochemistry, and Behavior, 84(4), 598–608. 10.1016/J.PBB.2006.07.026 [DOI] [PubMed] [Google Scholar]
- Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, & Lieberman JA (2006). Neuroactive Steroids are Altered in Schizophrenia and Bipolar Disorder: Relevance to Pathophysiology and Therapeutics. Neuropsychopharmacology, 31, 1249–1263. 10.1038/sj.npp.1300952 [DOI] [PubMed] [Google Scholar]
- Marx CE, Van Doren MJ, Duncan GE, Lieberman JA, & Morrow AL (2003). Olanzapine and Clozapine Increase the GABAergic Neuroactive Steroid Allopregnanolone in Rodents. Neuropsychopharmacology 2003 28:1, 28(1), 1–13. 10.1038/sj.npp.1300015 [DOI] [PubMed] [Google Scholar]
- Marx CE, Yuan P, Kilts JD, Madison RD, Shampine LJ, & Manji HK (2008). Neuroactive steroids, mood stabilizers, and neuroplasticity: alterations following lithium and changes in Bcl-2 knockout mice. The International Journal of Neuropsychopharmacology, 11(4), 547–552. 10.1017/S1461145708008444 [DOI] [PubMed] [Google Scholar]
- Marx C, Naylor J, Kilts J, Allan T, Smith K, Szabo S, Wagner R, Buchanan R, Keefe R, & Shampine L (2017). 19. Randomized Controlled Trial of a Neurosteroid Intervention in Schizophrenia. Schizophrenia Bulletin, 43(suppl_1), S14–S14. 10.1093/SCHBUL/SBX021.038 [DOI] [Google Scholar]
- Mason BL, Van Enkevort E, Filbey F, Marx CE, Park J, Nakamura A, Sunderajan P, & Brown ES (2017). Neurosteroid Levels in Patients with Bipolar Disorder and a History of Cannabis Use Disorders. Journal of Clinical Psychopharmacology, 37(6), 684–688. 10.1097/JCP.0000000000000793 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Puia G, Dong E, & Pinna G (2009). GABA A receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: Possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress the International Journal on the Biology of Stress. 10.1080/10253890701200997 [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Caruso D, Abbiati F, Giatti S, Calabrese D, Piazza F, & Cavaletti G (2013). Neuroactive Steroid Levels are Modified in Cerebrospinal Fluid and Plasma of Post-Finasteride Patients Showing Persistent Sexual Side Effects and Anxious/Depressive Symptomatology. Journal of Sexual Medicine, 10(10), 2598–2603. https://pubmed.ncbi.nlm.nih.gov/23890183/ [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Cioffi L, Diviccaro S, & Traish AM (2021). Synthesis and Actions of 5α-Reduced Metabolites of Testosterone in the Nervous System. Androgens, 2(1), 173–188. 10.1089/ANDRO.2021.0010/ASSET/IMAGES/LARGE/ANDRO.2021.0010_FIGURE4.JPEG [DOI] [Google Scholar]
- Melcangi RC, Santi D, Spezzano R, Grimoldi M, Tabacchi T, Fusco ML, Diviccaro S, Giatti S, Carrà G, Caruso D, Simoni M, & Cavaletti G (2017). Neuroactive steroid levels and psychiatric and andrological features in post-finasteride patients. The Journal of Steroid Biochemistry and Molecular Biology, 171, 229–235. 10.1016/J.JSBMB.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Mellon SH (2007). Neurosteroid regulation of CNS development. Pharmacology & Therapeutics, 116(1), 107–124. 10.1016/J.PHARMTHERA.2007.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, & Griffin LD (2002). Synthesis, regulation, and function of neurosteroids. Endocrine Research, 28(4), 463. 10.1081/ERC-120016823 [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, Li H, Sankoh AJ, Clemson C, Schacterle A, Jonas J, & Kanes S (2018). Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebocontrolled, phase 3 trials. The Lancet, 392(10152), 1058–1070. 10.1016/S01406736(18)31551-4 [DOI] [PubMed] [Google Scholar]
- Michels G, & Moss SJ (2007). GABAA receptors: Properties and trafficking. In Critical Reviews in Biochemistry and Molecular Biology (Vol. 42, Issue 1, pp. 3–14). Crit Rev Biochem Mol Biol. 10.1080/10409230601146219 [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, & Buchsbaum MS (2005). Volume of the cingulate and outcome in schizophrenia. Schizophrenia Research, 72(2–3), 91–108. 10.1016/J.SCHRES.2004.02.011 [DOI] [PubMed] [Google Scholar]
- Modgil A, Parakala ML, Ackley MA, Doherty JJ, Moss SJ, & Davies PA (2017). Endogenous and synthetic neuroactive steroids evoke sustained increases in the efficacy of GABAergic inhibition via a protein kinase C-dependent mechanism. Neuropharmacology, 113(May), 314–322. 10.1016/j.neuropharm.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, & Pearce RA (2004). Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends in Neurosciences, 27(9), 569–575. 10.1016/J.TINS.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Morales AJ, Nolan JJ, Nelson JC, & Yen SSC (1994). Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. The Journal of Clinical Endocrinology and Metabolism, 78(6), 1360–1367. 10.1210/JCEM.78.6.7515387 [DOI] [PubMed] [Google Scholar]
- Morlock EV, & Czajkowski C (2011). Different Residues in the GABAA Receptor Benzodiazepine Binding Pocket Mediate Benzodiazepine Efficacy and Binding. Molecular Pharmacology, 80(1), 14. 10.1124/MOL.110.069542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher LJ, Cadeddu R, Yen S, Staudinger JL, Traccis F, Fowler SC, Maguire JL, & Bortolato M (2019). Allopregnanolone is required for prepulse inhibition deficits induced by D1 dopamine receptor activation. Psychoneuroendocrinology, 108, 53–61. 10.1016/j.psyneuen.2019.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, den Tonkelaar I, Thijssen JHH, Grobbee DE, & van der Schouw YT (2003). Endogenous sex hormones in men aged 40–80 years. European Journal of Endocrinology, 149(6), 583–589. 10.1530/EJE.0.1490583 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Darnieder LM, Deeb TZ, & Moss SJ (2015). Regulation of GABAARs by phosphorylation. In Advances in Pharmacology (Vol. 72, pp. 97–146). Academic Press Inc. 10.1016/bs.apha.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrón-Oyarzo I, Aboitiz F, & Fuentealba P (2016). Impaired Functional Connectivity in the Prefrontal Cortex: A Mechanism for Chronic Stress-Induced Neuropsychiatric Disorders. Neural Plasticity, 2016, 1–16. 10.1155/2016/7539065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Richter A, Bermpohl F, Grimm S, Martin E, Marcar VL, Wahl C, Hell D, & Boeker H (2005). NMDA hypofunction in the posterior cingulate as a model for schizophrenia: an exploratory ketamine administration study in fMRI. Schizophrenia Research, 72(2–3), 235–248. 10.1016/J.SCHRES.2004.04.009 [DOI] [PubMed] [Google Scholar]
- Paba S, Frau R, C. Godar S, Devoto P, Marrosu F, & Bortolato M (2011). Steroid 5α-Reductase as a Novel Therapeutic Target for Schizophrenia and Other Neuropsychiatric Disorders. Current Pharmaceutical Design, 17(2), 151–167. 10.2174/138161211795049589 [DOI] [PubMed] [Google Scholar]
- Pang Y, Dong J, & Thomas P (2013). Characterization, Neurosteroid Binding and Brain Distribution of Human Membrane Progesterone Receptors and (mPR and mPR) and mPR Involvement in Neurosteroid Inhibition of Apoptosis. 10.1210/en.2012-1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parakala ML, Zhang Y, Modgil A, Chadchankar J, Vien TN, Ackley MA, Doherty JJ, Davies PA, & Moss SJ (2019). Metabotropic, but not allosteric, effects of neurosteroids on GABAergic inhibition depend on the phosphorylation of GABAA receptors. Journal of Biological Chemistry, 294(32), 12220–12230. 10.1074/jbc.RA119.008875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Chung M, Wu F Sen, Purdy RH, Malayev AA, Gibbs TT, & Farb DH (1997). Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Molecular Pharmacology, 52(6), 1113–1123. 10.1124/MOL.52.6.1113 [DOI] [PubMed] [Google Scholar]
- Patterson R, Krohn H, Richardson E, Kimmel M, & Meltzer-Brody S (2022). A Brexanolone Treatment Program at an Academic Medical Center: Patient Selection, 90-Day Posttreatment Outcomes, and Lessons Learned. Journal of the Academy of Consultation-Liaison Psychiatry, 63(1), 14–22. 10.1016/J.JACLP.2021.08.001 [DOI] [PubMed] [Google Scholar]
- Paus T (2021). Virtual Histology of Cortical Thickness and Shared Neurobiology in 6 Psychiatric Disorders Writing Committee for the Attention-Deficit/Hyperactivity Disorder; Autism Spectrum Disorder; Bipolar Disorder; Major Depressive Disorder; Obsessive-Compulsive Disorder; and Schizophrenia ENIGMA Working Groups Supplemental content. JAMA Psychiatry, 78(1), 47–63. 10.1001/jamapsychiatry.2020.2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, & Pinna G (2008). Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America, 105(14), 5567–5572. 10.1073/PNAS.0801853105/ASSET/10CE550E-234E-41C4-9191-00C7C19476D9/ASSETS/GRAPHIC/ZPQ0130800530007.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G (2018). Biomarkers for PTSD at the Interface of the Endocannabinoid and Neurosteroid Axis. Frontiers in Neuroscience, 12(AUG), 482. 10.3389/FNINS.2018.00482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G (2020). Allopregnanolone, the Neuromodulator Turned Therapeutic Agent: Thank You, Next? Frontiers in Endocrinology, 11, 236. 10.3389/FENDO.2020.00236/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, & Guidotti A (2003). In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. In Proceedings of the National Academy of Sciences of the United States of America (Vol. 18, Issue 4). https://pubmed.ncbi.nlm.nih.gov/12571361/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard GT, & Howard JL (1979). The Geller-Seifter conflict paradigm with incremental shock. Psychopharmacology, 62(2), 117–121. 10.1007/BF00427123 [DOI] [PubMed] [Google Scholar]
- Porcu P, Barron AM, Frye CA, Walf AA, Yang SY, He XY, Morrow AL, Panzica GC, & Melcangi RC (2016). Neurosteroidogenesis Today: Novel Targets for Neuroactive Steroid Synthesis and Action and Their Relevance for Translational Research. Journal of Neuroendocrinology, 28(2), 1–19. 10.1111/JNE.12351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, & Jalfre M (1977). Depression: a new animal model sensitive to antidepressant treatments. Nature, 266(5604), 730–732. 10.1038/266730A0 [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, & Paul SM (1991). Stress-induced elevations of γ-aminobutyric acid type a receptor-active steroids in the rat brain. Proceedings of the National Academy of Sciences of the United States of America, 88(10), 4553–4557. 10.1073/pnas.88.10.4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, & Mualeki A (2006). Finasteride induced depression: A prospective study. BMC Clinical Pharmacology, 6(1), 1–6. 10.1186/1472-6904-6-7/COMMENTS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, King MW, Valovski I, Gregor K, Scioli-Salter E, Pineles SL, Hamouda M, Nillni YI, Anderson GM, & Pinna G (2019). Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology, 102, 95. 10.1016/J.PSYNEUEN.2018.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Marx CE, Jain S, Farfel GM, Tsai J, Sun X, Geracioti TD, Hamner MB, Lohr J, Rosse R, Summerall L, Naylor JC, Cusin C, Lang AJ, Raman R, & Stein MB (2017). A randomized controlled trial of ganaxolone in posttraumatic stress disorder. 234, 2245–2257. 10.1007/s00213-017-4649-y [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Pineles SL, Brown KD, & Pinna G (2022). A role for deficits in GABAergic neurosteroids and their metabolites with NMDA receptor antagonist activity in the pathophysiology of posttraumatic stress disorder. Journal of Neuroendocrinology, 34(2), e13062. 10.1111/JNE.13062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, & Guidotti A (2006). Decreased Cerebrospinal Fluid Allopregnanolone Levels in Women with Posttraumatic Stress Disorder. Biological Psychiatry, 60(7), 704–713. 10.1016/J.BIOPSYCH.2006.03.026 [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, & Fanselow MS (2005). Stress-induced enhancement of fear learning: An animal model of posttraumatic stress disorder. Neuroscience and Biobehavioral Reviews, 29(8), 1207–1223. 10.1016/J.NEUBIOREV.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Reddy DS (2018). GABA-A Receptors Mediate Tonic Inhibition and Neurosteroid Sensitivity in the Brain. Vitamins and Hormones, 107, 177–191. 10.1016/BS.VH.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Reddy DS, & Bakshi K (2020). Neurosteroids: Biosynthesis, Molecular Mechanisms, and Neurophysiological Functions in the Human Brain. Hormonal Signaling in Biology and Medicine: Comprehensive Modern Endocrinology, 69–82. 10.1016/B978-0-12-813814-4.00004-3 [DOI] [Google Scholar]
- Richter L, De Graaf C, Sieghart W, Varagic Z, Mörzinger M, De Esch IJP, Ecker GF, & Ernst M (2012). Diazepam-bound GABAA receptor models identify new benzodiazepine binding-site ligands. Nature Chemical Biology, 8(5), 455. 10.1038/NCHEMBIO.917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsner MS (2010). Pregnenolone, Dehydroepiandrosterone, and Schizophrenia: Alterations and Clinical Trials. CNS Neuroscience & Therapeutics, 16(1), 32–44. 10.1111/J.1755-5949.2009.00118.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsner MS, Bawakny H, & Kreinin A (2014). Pregnenolone treatment reduces severity of negative symptoms in recent-onset schizophrenia: an 8-week, double-blind, randomized add-on two-center trial. Psychiatry and Clinical Neurosciences, 68(6), 432–440. 10.1111/PCN.12150 [DOI] [PubMed] [Google Scholar]
- Rupprecht R, & Holsboer F (1999). Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends in Neurosciences, 22(9), 410–416. 10.1016/S0166-2236(99)01399-5 [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JMHM, Trapp T, Steensel B van, Wetzel C, Damm K, Zieglgänsberger W, & Holsboer F (1993). Progesterone receptor-mediated effects of neuroactive steroids. Neuron, 11(3), 523–530. 10.1016/0896-6273(93)90156-L [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, Simpson St Clair L, Murphy JH, Haq N, Rubinow DR, & Simpson St Clair M (1996). Dehydroepiandrosterone Monotherapy in Midlife-Onset Major and Minor Depression. [DOI] [PubMed]
- Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, Simpson St Clair L, Murphy JH, Haq N, Rubinow DR, & Simpson St Clair M (2005). Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Archives of General Psychiatry, 62(2), 154–162. 10.1001/ARCHPSYC.62.2.154 [DOI] [PubMed] [Google Scholar]
- Schüle C, Romeo E, Uzunov DP, Eser D, Di Michele F, Baghai TC, Pasini A, Schwarz M, Kempter H, & Rupprecht R (2006). Influence of mirtazapine on plasma concentrations of neuroactive steroids in major depression and on 3alpha-hydroxysteroid dehydrogenase activity. Molecular Psychiatry, 11(3), 261–272. 10.1038/SJ.MP.4001782 [DOI] [PubMed] [Google Scholar]
- Semeniuk T, Jhangri GS, & Le Mellédo J-M (2001). CLINICAL AND RESEARCH REPORTS Neuroactive Steroid Levels in Patients With Generalized Anxiety Disorder. The Journal of Neuropsychiatry and Clinical Neurosciences, 13, 396–398. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, & Biggio G (2000). Social Isolation-Induced Decreases in Both the Abundance of Neuroactive Steroids and GABA A Receptor Function in Rat Brain. [DOI] [PubMed]
- Simon GE, & Savarino J (2007). Suicide attempts among patients starting depression treatment with medications or psychotherapy. The American Journal of Psychiatry, 164(7), 1029–1034. 10.1176/AJP.2007.164.7.1029 [DOI] [PubMed] [Google Scholar]
- Soggiu A, Piras C, Greco V, Devoto P, Urbani A, Calzetta L, Bortolato M, & Roncada P (2016). Exploring the neural mechanisms of finasteride: a proteomic analysis in the nucleus accumbens. Psychoneuroendocrinology, 74, 387–396. 10.1016/J.PSYNEUEN.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, & Liberzon I (2012). Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. Journal of Psychiatry & Neuroscience : JPN, 37(4), 241–249. 10.1503/JPN.110069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rampton JC, Ho S, & Liberzon I (2012). Allopregnanolone Elevations Following Pregnenolone Administration are Associated with Enhanced Activation of Emotion Regulation Neurocircuits. 10.1016/j.biopsych.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Welsh RC, Marx CE, & Liberzon I (2014). The neurosteroids allopregnanolone and dehydroepiandrosterone modulate resting-state amygdala connectivity. Human Brain Mapping, 35(7), 3249. 10.1002/HBM.22399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M, Laughren T, Jones ML, Levenson M, Holland PC, Hughes A, Hammad TA, Temple R, & Rochester G (2009). Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ (Clinical Research Ed.), 339(7718), 431–434. 10.1136/BMJ.B2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströhle A, Romeo E, Di Michele F, Pasini A, Hermann B, Gajewsky G, Holsboer F, & Rupprecht R (2003). Induced Panic Attacks Shift γ-Aminobutyric Acid Type A Receptor Modulatory Neuroactive Steroid Composition in Patients With Panic Disorder: Preliminary Results. Archives of General Psychiatry, 60(2), 161–168. 10.1001/ARCHPSYC.60.2.161 [DOI] [PubMed] [Google Scholar]
- Ströhle A, Romeo E, di Michele F, Pasini A, Yassouridis A, Holsboer F, & Rupprecht R (2002). GABA A Receptor-Modulating Neuroactive Steroid Composition in Patients With Panic Disorder Before and During Paroxetine Treatment. Am J Psychiatry, 159, 1105–1121. [DOI] [PubMed] [Google Scholar]
- Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, & Weizman A (2003). Dehydroepiandrosterone Augmentation in the Management of Negative, Depressive, and Anxiety Symptoms in Schizophrenia. Archives of General Psychiatry, 60, 133–141. [DOI] [PubMed] [Google Scholar]
- Suthoff E, Kosinski M, Arnaud A, Hodgkins P, Gunduz-Bruce H, Lasser R, Silber C, Sankoh AJ, Li H, Werneburg B, Jonas J, Doherty J, Kanes SJ, & Bonthapally V (2022). Patient-reported health-related quality of life from a randomized, placebo-controlled phase 2 trial of zuranolone in adults with major depressive disorder. Journal of Affective Disorders, 308, 19–26. 10.1016/J.JAD.2022.03.068 [DOI] [PubMed] [Google Scholar]
- Tannenbaum C, Barrett-Connor E, Laughlin GA, & Platt RW (2004). A longitudinal study of dehydroepiandrosterone sulphate (DHEAS) change in older men and women: the Rancho Bernardo Study. European Journal of Endocrinology, 151(6), 717–725. 10.1530/EJE.0.1510717 [DOI] [PubMed] [Google Scholar]
- Tokuda K, O’dell KA, Izumi Y, & Zorumski CF (2010). Development/Plasticity/Repair Midazolam Inhibits Hippocampal Long-Term Potentiation and Learning through Dual Central and Peripheral Benzodiazepine Receptor Activation and Neurosteroidogenesis. 10.1523/JNEUROSCI.4101-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traish AM, Melcangi RC, Bortolato M, Garcia-Segura LM, & Zitzmann M (2015). Adverse effects of 5α-reductase inhibitors: What do we know, don’t know, and need to know? Reviews in Endocrine and Metabolic Disorders 2015 16:3, 16(3), 177–198. 10.1007/S11154-015-9319-Y [DOI] [PubMed] [Google Scholar]
- Uzunov DP, Coopert TB, Costa E, Guidorri A, & Kline NS (1996). Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. 93, 12599–12604. https://www.pnas.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, & Guidotti A (1998). Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. In Neurobiology (Vol. 95). www.pnas.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova Veska, Ceci M, Kohler C, Uzunov DP, & Wrynn AS (2003). Region-specific dysregulation of allopregnanolone brain content in the olfactory bulbectomized rat model of depression. Brain Research, 976(1), 1–8. 10.1016/S0006-8993(03)02577-0 [DOI] [PubMed] [Google Scholar]
- Van Wingen GA, Van Broekhoven F, Verkes RJ, Petersson KM, Bä Ckströ M T, Buitelaar JK, & Ferná Ndez G (2008). Progesterone selectively increases amygdala reactivity in women. Molecular Psychiatry, 13, 325–333. 10.1038/sj.mp.4002030 [DOI] [PubMed] [Google Scholar]
- Volkmann C, Bschor T, & Köhler S (2020). Lithium Treatment Over the Lifespan in Bipolar Disorders. Frontiers in Psychiatry, 11. 10.3389/FPSYT.2020.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF (1994). Developmental^ Moderated Expressions of the Neuropathology Underlying Schizophrenia. Schizophrenia Bulletin, 20(3), 453–480. https://academic.oup.com/schizophreniabulletin/article/20/3/453/1864190 [DOI] [PubMed] [Google Scholar]
- Walker E, & Tessner K (2008). Schizophrenia. Perspectives on Psychological Science, 3(1), 30–37. 10.1111/J.1745-6916.2008.00059.X/ASSET/IMAGES/LARGE/10.1111_J.1745-6916.2008.00059.X-FIG1.JPEG [DOI] [PubMed] [Google Scholar]
- Walter TJ, Vetreno RP, & Crews FT (2017). Alcohol and Stress Activation of Microglia and Neurons: Brain Regional Effects. Alcoholism: Clinical and Experimental Research, 41(12), 2066–2081. 10.1111/ACER.13511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NL, Antonoudiou P, Barros L, Dargan T, DiLeo A, Evans-Strong A, Gabby J, Howard S, Paracha R, Sánchez EJ, Weiss GL, Kong D, & Maguire JL (2023). Impaired endogenous neurosteroid signaling contributes to behavioral deficits associated with chronic stress. Biological Psychiatry. 10.1016/J.BIOPSYCH.2023.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton N, & Maguire J (2019). Allopregnanolone-based treatments for postpartum depression: Why/how do they work? Neurobiology of Stress, 11. 10.1016/j.ynstr.2019.100198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, & Biickstriim T (1996). Relationship between Symptom Severity and Steroid Variation in Women with Premenstrual Syndrome: Study on Serum Pregnenolone, Pregnenoione Sulfate, k=Pregnane=3,20=Dione and 3cx-Hydroxy&u-Pregnan=20=One*. Journal of Climeal Endocrinology and Metabolism, 811076–811082. https://academic.oup.com/jcem/article/81/3/1076/2649632 [DOI] [PubMed] [Google Scholar]
- Wieland S, Lan NC, Mirasedeghi S, & Gee KW (1991). Anxiolytic activity of the progesterone metabolite 5 alpha-pregnan-3 alpha-o1–20-one. Brain Research, 565(2), 263–268. 10.1016/0006-8993(91)91658-N [DOI] [PubMed] [Google Scholar]
- Willner P (1984). The validity of animal models of depression. Psychopharmacology, 83(1), 1–16. 10.1007/BF00427414 [DOI] [PubMed] [Google Scholar]
- Willner P (1990). Animal models of depression: an overview. Pharmacology & Therapeutics, 45(3), 425–455. 10.1016/0163-7258(90)90076-E [DOI] [PubMed] [Google Scholar]
- Wolf L, Bauer A, Melchner D, Hallof-Buestrich H, Stoertebecker P, Haen E, Kreutz M, Sarubin N, Milenkovic VM, Wetzel CH, Rupprecht R, & Nothdurfter C (2015). Enhancing neurosteroid synthesis - Relationship to the pharmacology of translocator protein (18 kDa) (TSPO) ligands and benzodiazepines. Pharmacopsychiatry, 48(2), 72–77. 10.1055/S-0034-1398507 [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizendine L, & Roberts E (1999). Double-Blind Treatment of Major Depression With Dehydroepiandrosterone. Https://Doi.Org/10.1176/Ajp.156.4.646, 156(4), 646–649. 10.1176/AJP.156.4.646 [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Roberts E, Manfredi F, Chan T, Raum WJ, Ormiston S, Johnson R, Canick J, Brizendine L, & Weingartner H (1997). Dehydroepiandrosterone (DHEA) treatment of depression. Biological Psychiatry, 41(3), 311–318. 10.1016/S0006-3223(96)00043-1 [DOI] [PubMed] [Google Scholar]
- Woo E, Sansing LH, Arnsten AFT, & Datta D (2021). Chronic Stress Weakens Connectivity in the Prefrontal Cortex: Architectural and Molecular Changes. Chronic Stress, 5, 247054702110292. 10.1177/24705470211029254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa K, Nakashima K, Tabuchi M, Okumura A, Nakatake Y, Yamada M, Tsuneoka Y, & Higashi T (2020). Benzothiazepines, diltiazem and JTV-519, exert an anxiolytic-like effect via neurosteroid biosynthesis in mice. Journal of Pharmacological Sciences, 143(3), 234–237. 10.1016/J.JPHS.2020.03.003 [DOI] [PubMed] [Google Scholar]
- Zhdanava M, Pilon D, Ghelerter I, Chow W, Joshi K, Lefebvre P, & Sheehan JJ (2021). The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. The Journal of Clinical Psychiatry, 82(2), 29169. 10.4088/JCP.20M13699 [DOI] [PubMed] [Google Scholar]
- Ziolkowski L, Mordukhovich I, Chen DM, Chisari M, Shu HJ, Lambert PM, Qian M, Zorumski CF, Covey DF, & Mennerick S (2021). A neuroactive steroid with a therapeutically interesting constellation of actions at GABAA and NMDA receptors. Neuropharmacology, 183. 10.1016/J.NEUROPHARM.2020.108358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, & Mennerick S (2013). Neurosteroids as therapeutic leads in psychiatry. In JAMA Psychiatry (Vol. 70, Issue 7, pp. 659–660). American Medical Association. 10.1001/jamapsychiatry.2013.245 [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Covey DF, & Mennerick S (2019). Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiology of Stress, 11, 100196. 10.1016/j.ynstr.2019.100196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, & Mennerick S (2012). Neurosteroids, stress and depression: potential therapeutic opportunities. Neuroscience and Biobehavioral Reviews, 37(1), 109–122. 10.1016/J.NEUBIOREV.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


