Abstract
Pain is strongly modulated by expectations and beliefs. Across species, subregions of ventromedial prefrontal cortex (VMPFC) are implicated in a variety of functions germane to pain, predictions, and learning. Human fMRI studies show that VMPFC activity tracks expectations about pain and mediates expectancy effects on pain-related activity in other brain regions. Prior lesion studies suggest that VMPFC may instead play a more general role in generating affective responses to painful stimuli. To test whether VMPFC is required to generate affective responses to pain or is more specifically involved in expectancy-based pain modulation, we studied responses to heat stimuli in five adults with bilateral surgical lesions of VMPFC and twenty healthy adults without brain damage. All participants underwent a quantitative sensory testing procedure followed by a pain expectancy task in which cues predicting either low or high pain were followed by intermittent medium intensity heat stimuli. Compared to adults without brain damage, individuals with VMPFC lesions reported larger differences in expected pain based on predictive cues and failed to update expectations following the covert introduction of unexpected medium temperature stimuli. Consistent with observed expectancy differences, subjective pain unpleasantness ratings in the VMPFC lesion group were more strongly modulated by cue during thermal stimulation. We found no group differences in overall pain sensitivity, nor in relationships between pain and autonomic arousal, suggesting that VMPFC damage specifically enhances the effect of expectations on pain processing, likely driven by impaired integration of new sensory feedback to update expectations about pain. These results provide essential new data regarding the specific functional contribution of VMPFC to pain modulation.
Keywords: Pain, Lesion, Learning, Expectancy, Emotion, VMPFC
1. Introduction
Acute pain is highly susceptible to modification by expectations and by predictive cues signaling pain or relief (Atlas & Wager, 2014; Forsberg et al., 2017; Zunhammer et al., 2021). In prior work, the magnitude of expectation-related activation in human ventromedial prefrontal cortex (VMPFC) is associated with the strength of expectancy effects on subjective pain (Tinnermann et al., 2017; Wager et al., 2007). Further, VMPFC responses to pain-predictive cues mediate cue effects on responses to noxious stimuli in a distributed pain processing network, which in turn gives rise to subjective pain (Atlas et al., 2010). Inactivation of homologous regions of infralimbic cortex in rodents leads to specific impairments in the modulation of pain-related behaviors by reward-associated cues (Schwartz et al., 2017).
Although there are no prior studies of pain processing in patients with focal VMPFC lesions, case reports of humans with frontal lobe damage indicate that medial prefrontal cortex lesions can elicit dramatic reductions in the “strong aversive drive and negative affect characteristic of pain.” (Foltz & White, 1962; Freeman & Watts, 1947; Melzack & Wall, 1988) Human neuroimaging studies offer further support for a specific contribution of medial prefrontal structures to pain affect (Kulkarni et al., 2005; Rainville et al., 1997; Winston et al., 2014) and preclinical studies find analogous impairments in pain escape/avoidance behaviors after targeted medial frontal lesions in rodents (Fuchs et al., 2014; Gu et al., 2015; Johansen et al., 2001). In contrast to the specific blunting of pain affect seen in prior literature, more recent reports of humans with mixed frontal lesions find widespread effects on ratings of both pain intensity and pain unpleasantness, challenging the specificity of VMPFC function in pain to affective processing (Daum et al., 1995; Davis et al., 1994; Talbot et al., 1995).
Several authors argue that a core function of VMPFC is to generate and update predictions related to expected outcome value in the context of decision-making (Schneider & Koenigs, 2017; Spalding et al., 2018; Wikenheiser & Schoenbaum, 2016; Zeithamova et al., 2012). VMPFC lesions are associated with reliable reversal learning deficits, particularly in the face of evolving valued (i.e., rewarding or punishing) outcomes and contingency reversals (Fellows & Farah, 2005; Ghazizadeh et al., 2012; Murray et al., 2007; Quirk et al., 2000; Schoenbaum et al., 2007). Humans with VMPFC lesions exhibit significantly attenuated physiological arousal during aversive learning (Battaglia et al., 2020; Bechara et al., 1996, 2000, 2005; Damasio & Carvalho, 2013a) and this failure to generate the physiologic components of an emotional response during learning is thought to explain observed decision-making deficits (Damasio, 1996; Damasio & Carvalho, 2013b; Roy et al., 2012). In the context of pain processing, VMPFC damage may therefore contribute to overall impairments of pain processing due to a failure to generate appropriate physiological and affective responses to painful stimuli, or may instead specifically disrupt the integrative processes required for dynamic computation of relative value during the subjective appraisal of a painful stimulus (Atlas, 2021; Büchel et al., 2014; Colloca, Sigaudo, & Benedetti, 2008; Colloca, Tinazzi, et al., 2008; Wiech, 2016).
Despite the substantial body of clinical and experimental evidence implicating VMPFC as a critical link between cognitive context and pain affect, there are as-yet no systematic studies in humans investigating the effects of VMPFC lesions on pain perception and modulation. Here, we use a validated experimental procedure (Atlas et al., 2010, 2013; Johnston et al., 2012; Michalska et al., 2018) in five neurosurgical patients with focal, bilateral VMPFC lesions and twenty healthy adult comparison subjects (HC) to test three central hypotheses about VMPFC function in the context of pain. We hypothesized that VMPFC lesions would (1) alter pain sensitivity, pain thresholds, and pain tolerance; (2) alter the acquisition and evolution of cue-based expectations about pain and thus alter the impact of predictive cues on subjective pain ratings; and (3) alter associations between subjective pain ratings and autonomic responses to painful stimuli. To account for the small sample size of patients with lesions, we evaluated the practical significance of our findings with Bayesian analyses that maintain low type-1 error rates in small samples (Ukyo et al., 2019; van de Schoot et al., 2015). In support of a specific modulatory function of VMPFC in the context of pain, we show that lesions do not lead to overall reductions in pain affect nor alter the modulation of autonomic responses, but instead bias subjective pain ratings toward explicit expectations and impair the updating of expectations after unexpected sensory feedback.
2. Materials and methods
2.1. Participants
The study was approved by the University of Wisconsin – Madison Institutional Review Board (IRB). No part of the study procedures or analysis plans was preregistered prior to the research being conducted. In the following sections, we report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria (which were established prior to data analysis), all manipulations, and all measures in the study.
The target lesion group consisted of five adult neurosurgical patients with acquired brain lesions from meningioma growth and ultimate resection via anterior skull base craniotomy. To be included, lesions had to involve substantial portions of the VMPFC, could not extend significantly outside the VMPFC, and could not involve other brain regions implicated in pain. All lesions reflect a combination of direct parenchymal damage from long standing meningiomas, vasogenic edema, and definitive surgical resection. Initial clinical presentations included subtle or obvious personality changes over at least several months preceding surgery. On postsurgical MRI, there were areas of encephalomalacia and persistent T2-weighted signal changes consistent with gliosis in VMPFC (Fig. 1A). At the time of testing, all patients had focal, stable resection cavities on MRI and were free of dementia and substance use disorder. All patients had no history of pain disorder, myocardial infarction, or presence of implanted cardiac device, and denied current use of analgesic medication. Retrospective chart review revealed additional pain-relevant medication prescriptions in four of five patients (levetiracetam for seizure prophylaxis in 3/5 patients, SSRI in 2/5 patients, and 2/5 with active opioid prescriptions). All patients denied current opioid use at the time of testing and both opioid prescriptions originated from the initial surgical encounter. Twenty healthy adults with no history of brain injury, myocardial infarction, presence of implanted cardiac device, neurological or psychiatric illness, pain disorder, or current use of psychoactive or analgesic medication were recruited as a healthy comparison (HC) group. Demographic and neuropsychological data for both groups are summarized in Table 1.
Fig. 1 –
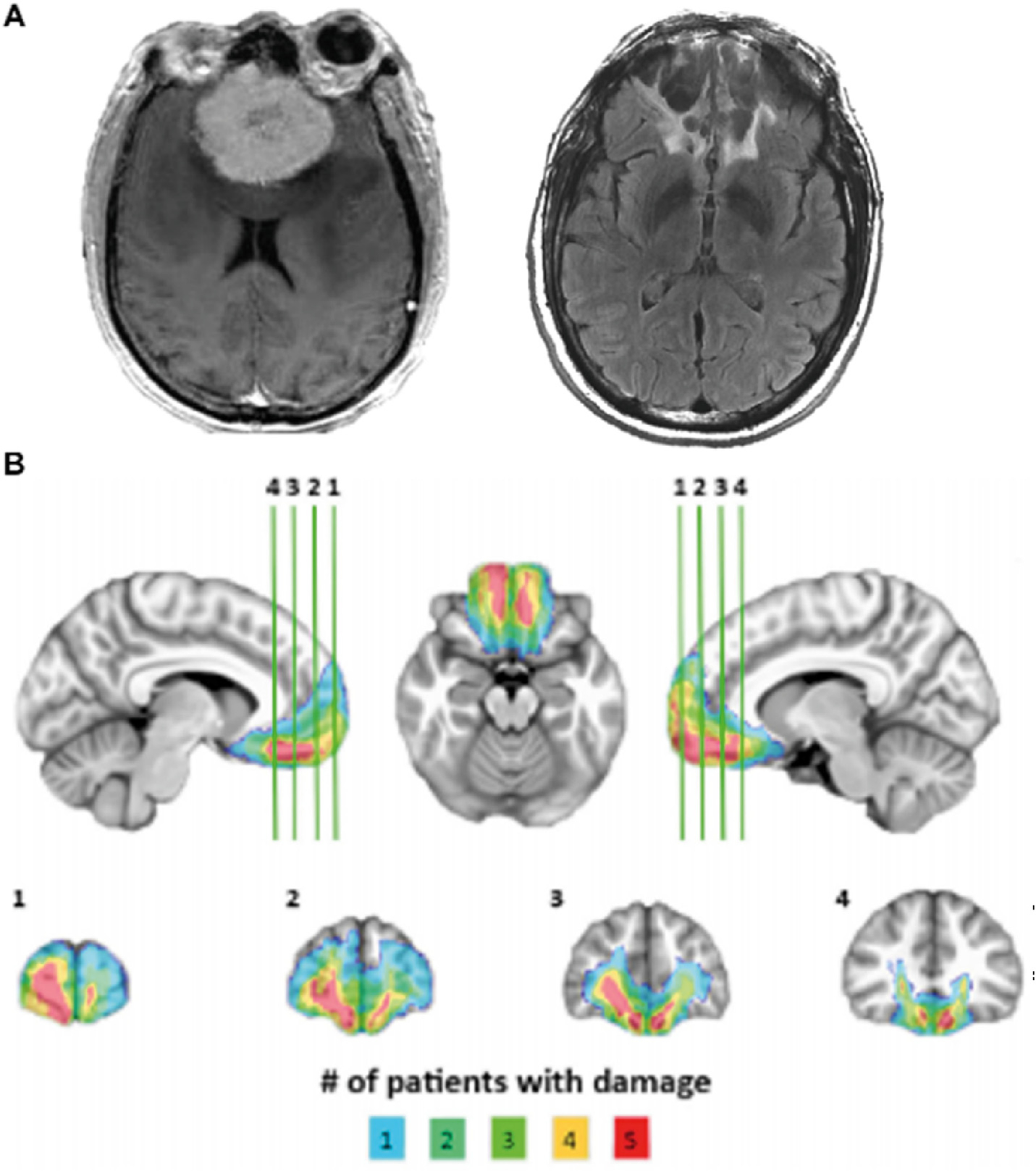
VMPFC lesions. (A) Example lesion from one patient showing appearance of skull-base meningioma on preoperative clinical T1-weighted MRI (left) and cavitary parenchymal VMPFC lesion postoperative clinical T2-weighted MRI (right). (B) Lesion overlap map depicting lesion extent across the VMPFC lesion group. All subjects have bilateral lesions involving the ventral third of the medial frontal cortex and medial third of the orbitofrontal cortex. Colors indicate number of patients with damage in a particular region.
Table 1 –
Demographics and pain calibration data for HC and VMPFC groups.
| HC(n = 20) | VMPFC(n = 5) | HC versus | VMPFC | |||
|---|---|---|---|---|---|---|
|
|
|
|
|
|
||
| Mean | ±SD | Mean | ±SD | ChiSq/U | P-value | |
|
| ||||||
| Sex* | 10 M/10 F | 3 M/2 F | 0 | 1 | ||
| Race* | 19C/1H/0 AA | 4C/0H/1 AA | .03 | .854 | ||
| Age | 63.5 | 4.62 | 60 | 5.15 | 67 | .259 |
| Education | 17.95 | 3.27 | 15.6 | 3.58 | 66.5 | .267 |
| WRATa | 112.05 | 5.84 | 105.2 | 11.18 | 67 | .174 |
| Low Temperature | 41.65 | 3.87 | 44 | 2.37 | 30 | .184 |
| Medium Temperature | 44.92 | 2.8 | 46.3 | 1.99 | 35 | .322 |
| High Temperature | 47.83 | 1.49 | 48.4 | .89 | 38.5 | .432 |
| Calibration r2 | 0.6 | 0.2 | .61 | 0.1 | 59 | .564 |
| Temp*Intensity (Pearson) | .76 | .16 | .78 | .07 | 59 | .564 |
| Temp*Unpleasantness (Pearson) | 0.7 | 0.2 | .75 | .06 | 57 | .659 |
| Intensity*Unpleasantness (Pearson) | .86 | .19 | .92 | .04 | 42 | .61 |
Abbreviations: C=Caucasian; H=Hispanic; AA = African American; M = male; F = female; WRAT=Wide Range Achievement Test.
Low, Medium, and High Temperatures from calibration are in °C. Pearson values represent the mean of within-subject correlations for the listed values for each group. Chi–Square tests were used to compare categorical variables (*) and Mann–Whitney U-tests used to compare continuous variables between groups. There were no significant group differences in any variable.
Calculated from the WRAT3 verbal subscore.
2.2. Lesion segmentation and image normalization
Structural MRIs for VMPFC patients were obtained at least 30 months after surgery (mean: 48.2, SD: 20.25). Individual VMPFC lesions were visually identified and manually segmented on T1-weighted images. Lesion boundaries were drawn to include areas with gross tissue damage or abnormal signal characteristics on T1 or T2 FLAIR images. T1-weighted images were skullstripped and diffeomorphically aligned to the Montreal Neurological Institute (MNI) coordinate system using a symmetric normalization algorithm with constrained cost-function masking to prevent warping of tissue within the lesion mask (Avants & Gee, 2004; Brett et al., 2001). The lesion overlap map (Fig. 1B) was created by computing the sum of aligned binary lesion masks for all five VMPFC patients.
2.3. Stimuli and apparatus
Thermal stimuli were delivered to the volar surface of the left forearm using a 30 × 30 mm Peltier thermode (Medoc, Inc). Each stimulus lasted 11 sec, with 2-s ramp-up and ramp-down from a 32 °C baseline and 7 sec at target temperature. Target temperatures were individually calibrated for each participant (see 2.4: Pain Calibration Procedure). We did not apply any temperatures above 49 °C to avoid skin damage. Six seconds after heat offset, participants separately rated pain intensity and pain unpleasantness for each stimulus using scales adapted from Petzke et al. (Gracely et al., 1978; Petzke et al., 2005), with standardized and validated verbal descriptors displayed alongside numerical ratings that reliably distinguish between sensory and affective components of pain (see Fig. 2A). The intensity scale was a 21-box Likert scale ranging from 0 (no pain sensation) to 20 (unbearable pain). Prior to calibration, participants were informed that an intensity rating of 5 on this scale should indicate the temperature at which heat is first perceived as painful (i.e., pain threshold) and that a rating of 15 should indicate the maximum temperature they would be willing to tolerate during the experiment (i.e., pain tolerance). The unpleasantness scale was a 21-box Likert scale range from 0 (neutral) to 20 (very intolerable). Participants were instructed that intensity ratings should reflect how the pain “feels to you” whereas unpleasantness ratings should reflect how the pain “makes you feel”. Verbal descriptors were present on rating scales throughout the experiment. During the study, auditory cue presentations, thermal stimulus triggers, and collection of pain ratings were implemented using E-prime software (Psychology Software Tools, Pittsburgh, PA).
Fig. 2 –
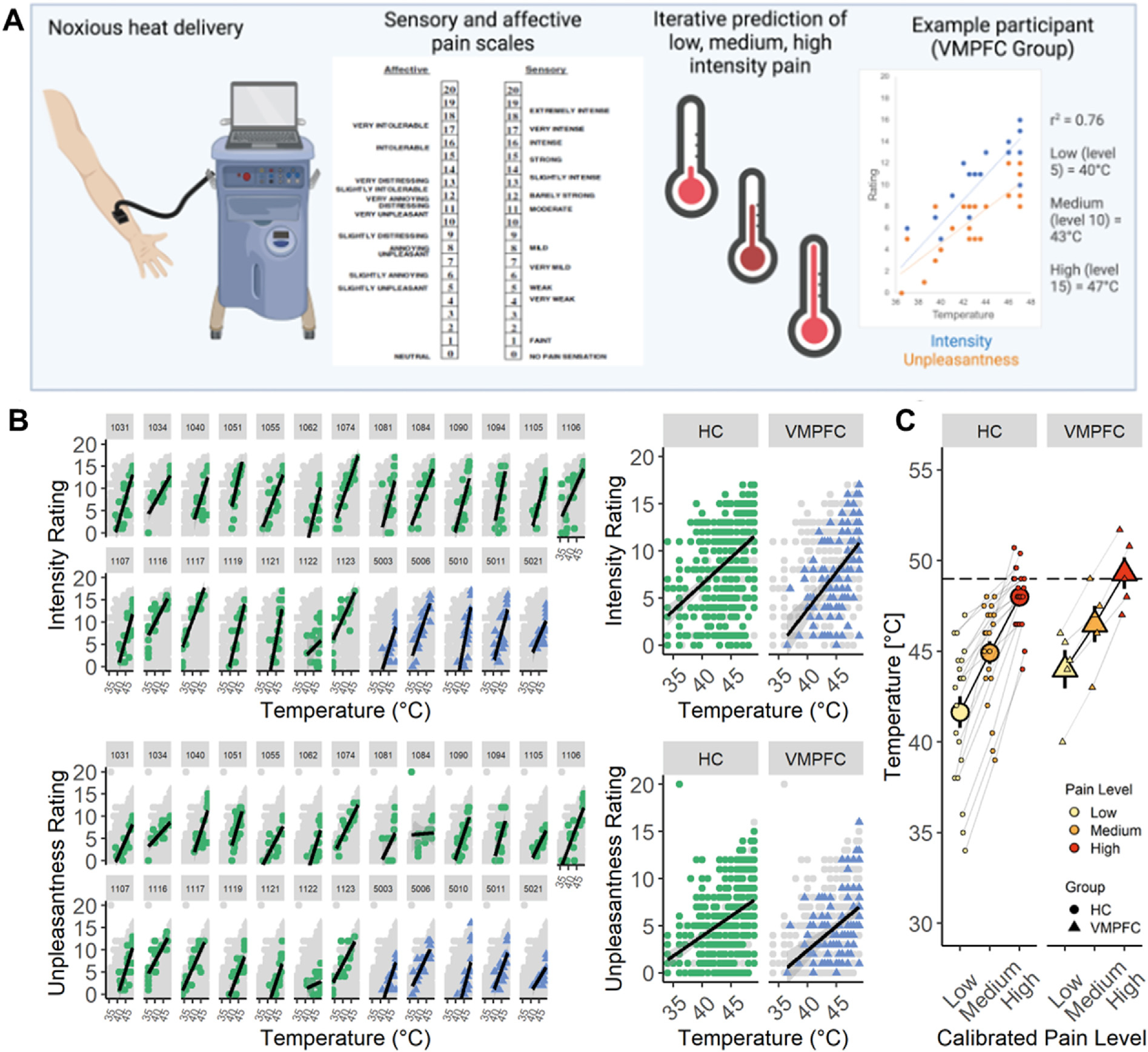
Quantitative Sensory Testing and Calibration Procedure. (A) Graphical representation of the adaptive pain calibration procedure including the pain stimulation device and thermode (left), representative scales of pain unpleasantness (affective) and intensity (sensory) with verbal groundings of numerical ratings (center), and the calibration procedure used to identify individual temperatures expected to elicit low, medium, or high levels of pain (right). Example calibration graph shows ratings of intensity (blue) and unpleasantness (orange). For this patient, calibrated low, moderate, and high pain levels are 40 °C, 43 °C, and 47 °C, respectively. (B) Scatterplots depicting correlation between applied temperatures and ratings of intensity (top row) and unpleasantness (bottom row) for individual subjects (left) and across the entire group (right). HC subjects (green circles) and VMPFC subjects (blue triangles) have similar temperature-rating curves. Light gray dots represent data from the entire sample, shown for reference. (C) Line graphs depicting correlation between estimated pain level (low, medium, high) and temperature for HC (left) and VMPFC (right) groups. Group means are shown in bold over individual subject values. Though temperatures appear higher in the VMPFC group, this difference did not meet criteria for statistical significance. The horizontal dotted line at 49°C represents the maximum allowable temperature for the study.
Heart rate data were acquired at 2000 Hz with a BioPac photoplethysmograph (Biopac Inc), affixed to the left index finger throughout the experiment. Skin conductance responses (SCRs) were collected at 10 Hz according to standard guidelines (Boucsein et al., 2012; Fowles et al., 1981) using 2 shielded Ag–AgCl electrodes prepared with isotonic paste and affixed to the second and third digits of the participants’ left hand. All physiologic data was collected using the BioPac MP150 system.
2.4. Pain calibration procedure
Following consent, subjects underwent quantitative sensory testing (QST) using four sites on the volar surface of the left forearm (see Fig. 2A). We used an adaptive staircase calibration, described in detail in previous work (Amir et al., 2021; Atlas et al., 2010; Dildine et al., 2020; Mischkowski et al., 2019), to estimate the dose–response relationship between applied thermal stimulation and reported pain (slope, intercept, r2) for each participant. Following heat offset, participants rated both subjective intensity and unpleasantness using the corresponding 21-item Likert scales. We used linear regression to iteratively fit intensity ratings as a function of temperature for each participant to derive temperatures predicted to elicit Low pain (i.e., pain threshold; intensity rating of 5), Medium pain (intensity rating of 10), and High pain (i.e., maximum tolerance; intensity rating of 15), which were used in the main expectancy experiment. Only participants who had an r2 > .4 at the end of the calibration procedure were included in the experimental task analyses, consistent with prior work (Atlas et al., 2010). Three healthy volunteer participants were excluded from the expectancy task on the basis of low r2 values, such that sample size in the HC group for calibration analyses was 20 and for expectancy task analyses was 17. All participants in the VMPFC group had r2 > .4 (see Table 1) and were thus included in all analyses. If a participant had an estimated high temperature >49 °C, a temperature of 49 °C was used (n = 4 HC, range 49.6 °C–50.7 °C; n = 2 VMPFC, range 50.8 °C–51.7 °C). If the estimated medium temperature was also ≥49 °C (n = 1 VMPFC, 49 °C), a temperature of 48 °C was used (i.e., 1 °C lower than the high temperature).
2.5. Pain expectancy task instruction and training
All participants were explicitly informed of cue-pain contingencies prior to any experimental pairings between cues and heat (see Fig. 3A). Cues were 2-s auditory tones (a cymbal or a cowbell). Cue-outcome contingencies were counter-balanced across participants. Participants were instructed that one tone would predict low pain and the other tone would predict high pain. Each tone was then played along with the instruction “This is your [low/high] pain tone” three times. Following instructions, participants completed a discrimination task consisting of 10 trials in which they had to correctly identify the expected level of pain following each cue, consistent with previous work (Atlas et al., 2010). All participants successfully identified at least 90% of trials and therefore proceeded to the experimental portion of the study.
Fig. 3 –
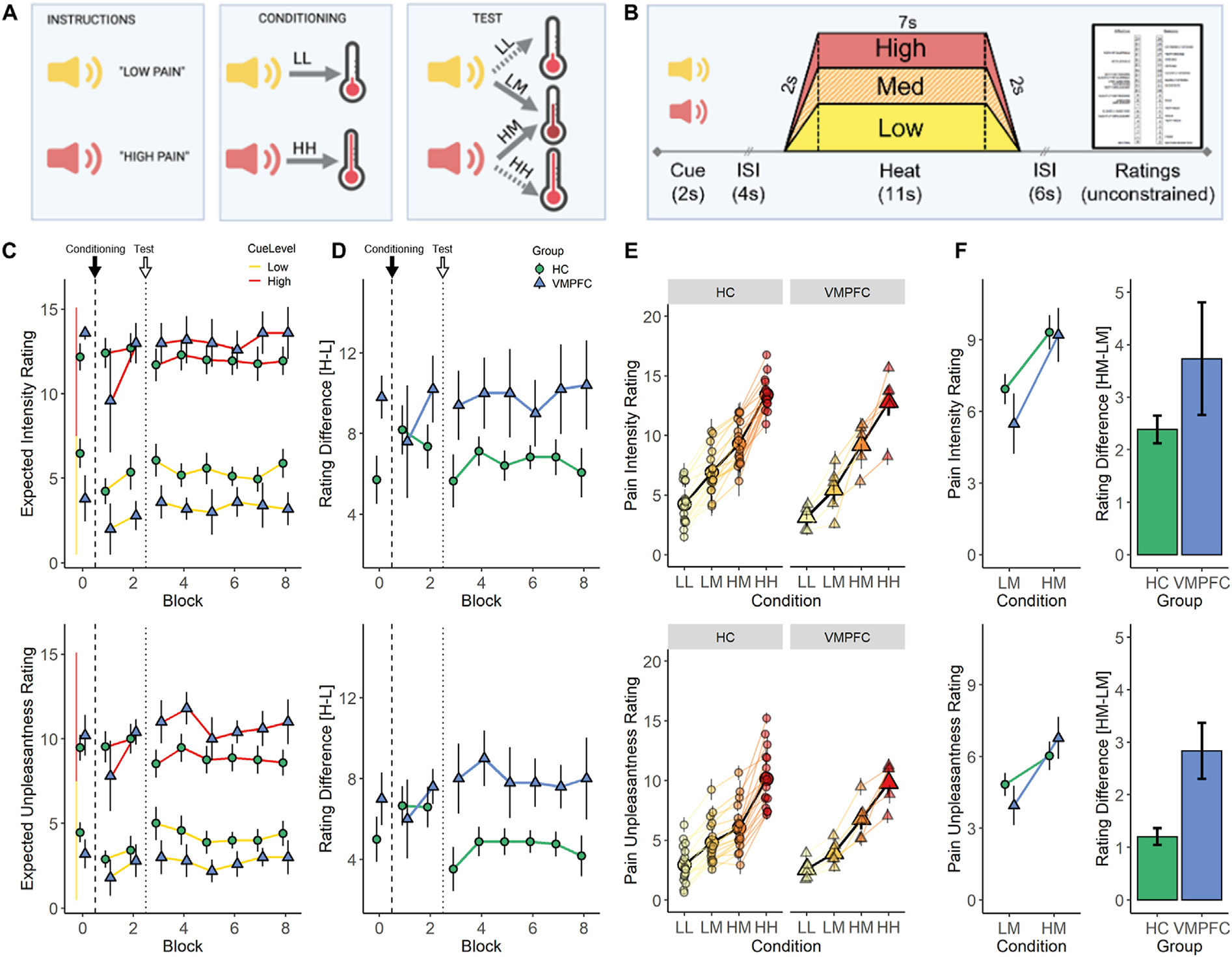
Pain Expectancy Task Results. (A) Graphical representation of the overall structure of the pain expectancy task showing initial presentation of auditory cues (left), followed by two blocks of conditioning trials in which cue is reliably followed by the predicted level of heat (center), and six blocks of test trials (right) in which low- or high-pain cues are followed either by predicted low and high temperatures (LL and HH trials) or a temperature calibrated to elicit medium pain (LM and HM trials). (B) Temporal structure of individual trials. (C) Expected pain intensity (top) and unpleasantness (bottom) after hearing High (red) or Low (yellow) pain predictive cues between blocks of experimental trials. Block 0 ratings are acquired at Baseline, before any pairings between cue and heat. Black arrows and dashed lines indicate the start of the Conditioning Phase of the task and white arrows and dotted lines indicate the introduction of medium temperature trials, signaling the beginning of the Test Phase of the task. (D) Difference in expected pain intensity (top) and unpleasantness (bottom) between High and Low pain predictive cues for HC (green circles) and VMPFC (blue triangles) groups. The VMPFC group maintained significantly greater differences in expected pain unpleasantness during the test phase, driven by similar cue effects for both low and high levels of expected pain. (E) Individual subject pain rating data from the expectancy task. Line graph of pain ratings by condition, showing group mean ± s.e.m. ratings of pain intensity (top) and unpleasantness (bottom) across all 4 conditions (LL, LM, HM, and HH conditions represented from yellow to red) superimposed on individual subject values. Note the consistency of ratings across individual subjects and greater separation between LM (light orange) and HM (dark orange) columns in the VMPFC group relative to the HC group. (F) Group summary data. Line (left) and bar (right) graphs showing differences between ratings for the critical medium heat trials preceded by a low (LM) or high (HM) pain cue, showing greater expectancy effect on unpleasantness ratings in the VMPFC group (blue triangles) relative to the HC group (green circles). Similar findings were observed for intensity ratings but did not meet frequentist criteria for statistical significance. Model parameters and statistics are reported in Table 3. ISI=Interstimulus interval, HC=Healthy Comparison, VMPFC = Lesion Group. LL = Low Pain Cue + Low Heat, LM = Low Pain Cue + Moderate Heat, HM=High Pain Cue + Moderate Heat. HH=High Pain Cue + High Heat
2.6. Pain expectancy task procedure
After calibration and training, participants completed a cue-based pain expectancy task that consisted of four types of trials adapted from Atlas et al. (2010) (see Fig. 3A). On each trial, a pain-predictive cue (Low Pain Cue or High Pain Cue) was followed by a 4 sec anticipatory interval and then 11 sec of thermal stimulation, after which participants rated pain intensity and unpleasantness (see Fig. 3B for trial timing). On “Low Pain Cue + Low Heat” (LL) trials, Low Pain cues were followed by stimulation calibrated to elicit an intensity rating of 5 (pain threshold) based on pain calibration. On “High Pain Cue + High Heat” (HH) trials, High Pain cues were followed by stimulation calibrated to elicit intensity ratings of 15 (pain tolerance). On “Low Pain Cue + Medium Heat” (LM) trials and “High Pain Cue + Medium Heat” (HM) trials, Low or High Pain cues, respectively, were followed by stimulation calibrated to elicit intensity ratings of 10 (Medium Pain). Thus, consistent with our prior work (Atlas et al., 2010), applied temperatures were identical in the critical LM and HM trials.
Participants underwent 8 blocks of the task (8 trials/block) and the thermode rotated across skin sites for each block (2 blocks/site). Participants first experienced two blocks of trials evenly divided between LL and HH trials, in pseudorandom order (see Fig. 3A). This Conditioning phase served primarily to reinforce the instructed cue–outcome relationships through associative learning. These were followed by six blocks of Test trials equally divided between LL, HH, LM, and HM trials, presented in pseudorandom order and counter-balanced across participants. Participants were not informed that medium heat stimulation would be delivered, and thus the comparison of HM and LM trials provides a measure of how cue-based expectations shape pain.
2.7. Pain expectancy ratings
Before the first block and after each subsequent block, participants heard the Low Pain and High Pain cues and were asked, after each tone, “When you hear this tone, how much pain do you expect?” Participants then rated both expected intensity and unpleasantness. The first such ratings were made prior to any pairing between predictive cue and thermal stimulation, thus providing a measure of cue effects on pain expectancy from instructions alone, unaffected by associative learning. The remaining eight ratings served as a manipulation check, allowing us to measure conscious cue-related expectancies and to test whether expectancies changed over the course of the experiment.
2.8. Heart rate analysis
To assess cardiac responses to thermal stimuli, we computed trial-wise estimates of heart rate change for each subject (Bradley et al., 2001). Peaks in the photoplethysmograph tracing, corresponding to cardiac R-waves in the QRS complex, were identified using in-house interactive beat detection software. Trials with ectopic beats, missed beats, or periods of noisy signal (where beat detection failed), were excluded from further analysis. R–R intervals were transformed into heart rate in beats per minute, in 500 msec bins. Changes in heart rate were determined by subtracting the mean heart rate for 2 sec preceding each thermal stimulation from the mean heart rate between 1 and 14 sec after thermal stimulus onset. Mean heart rate change was computed separately for each trial.
2.9. Skin conductance analysis
Skin conductance data were analyzed in Matlab (Mathworks, Natick, MA) using Ledalab (http://www.ledalab.de). Specifically, using Continuous Decomposition Analysis (Benedek & Kaernbach, 2010), the skin conductance data were deconvolved into phasic and tonic drivers of the skin conductance response (SCR). The response window for skin conductance fluctuations to be regarded as stimulus related was defined as 1–14 sec following heat onset. To correct for the skewed distribution of the skin conductance data, we square-root transformed SCR values (Boucsein et al., 2012; Schlosberg & Stanley, 1953). We analyzed both square-root transformed SCR and z-scored SCR within participants, which account for variations in amplitude between groups. We focus on the sum of amplitudes from Continuous Decomposition Analysis (CDA) as a measure of phasic heat-induced SCR in the main manuscript. We report additional phasic and tonic outcome measures derived from CDA (SCR, tonic mean) and trough-to-peak (TTP) scoring in Supplementary Tables S3, S4, and S5.
2.10. Statistical analysis
To test Hypothesis 1, that VMPFC lesions would alter pain sensitivity, pain thresholds, and pain tolerance, we evaluated QST measures of pain sensitivity derived from the adaptive staircase calibration. We used linear mixed effects models to test for group differences in pain sensitivity (i.e., the correspondence between temperature and pain intensity and pain unpleasantness ratings) across all trials during calibration, and during the experimental task. Details regarding linear mixed model specification are below (see Section 2.11: Linear Model Specification). We then used ANOVAs with Type III Sums of Squares, implemented using the car package (Fox, 2019) in R statistical software suite (Version 4.0.3) (R Core Team, 1996) to evaluate whether groups differed in the individually calibrated low, medium, and high temperatures that were applied during the subsequent experiment. Group differences in demographic variables and post-hoc comparisons of pain thresholds were assessed using Chi-square tests for categorical data and Mann-Whitney-Wilcoxon tests for continuous data, implemented in R.
To test Hypotheses 2 and 3, that VMPFC lesions would alter the impact of pain-predictive cues on expectations, subjective pain, and autonomic responses to painful stimuli, we examined effects of Group and Predictive Cue (Low or High) on 1) expectancy ratings collected at baseline and after each block of the experiment; 2) subjective pain ratings following critical medium heat trials; and 3) trial-wise estimates of autonomic reactivity during the medium heat trials. We used ANOVAs to examine group differences in expectancy ratings to each cue during each phase of the task, at baseline (following instructions), during conditioning, and during the test phase. To test the effects of expectancies on subjective pain ratings and autonomic responses to heat, we evaluated the interaction between Group and Cue on responses to medium heat trials using linear mixed models. Finally, to test the hypothesis that VMPFC lesions alter associations between subjective pain and autonomic responses to noxious stimuli, we performed similar linear mixed effects analyses with additional trial-wise regressors for SCR, mean centered for each subject, and examined the interaction between group and mean-centered ratings on each autonomic variable in the subset of medium temperature trials.
2.11. Linear Model Specification
All linear mixed models were implemented using the lme4 package (Bates et al., 2015) in the R statistical software suite (Version 4.0.3), and confirmed with the package nlme (Pinheiro et al., 2021) to account for autoregression, and with Bayesian models implemented in brms (Bürkner, 2017), where possible, to provide posterior estimates on the magnitude of the effects. We used the package bayestestR (Makowski, Ben-Shachar, & Lüdecke, 2019) to calculate posteriors and evaluate practical significance (i.e., our ability to accept or reject the null hypothesis (Makowski, BenShachar, & Lüdecke, 2019; Makowski, Ben-Shachar, Chen, & Lüdecke, 2019)). As discussed in Makowski et al. (Makowski, Ben-Shachar, & Lüdecke, 2019; Makowski, BenShachar, Chen, & Lüdecke, 2019), the probability of direction is comparable to a frequentist p-value to evaluate statistical significance; we therefore provide this value in all tables for comparison with frequentist approaches (i.e. LMER and NLME mixed models). “Practical significance” can be evaluated using the region of practical equivalence, or ROPE, which is defined as a range around a negligible parameter value (in our case the null hypothesis, 0) that depends on the standard deviation of the outcome “y” (ROPE = [−.1*SDy; .1*SDy]). We evaluated the percent of posterior estimates falling within the full ROPE range, and therefore defined practical significance (i.e. ability to reject the null hypothesis of no effect) as fewer than 2.5% of posterior estimates falling within the ROPE, and would accept the null if >97.5% of estimates fell within the ROPE (Makowski, Ben-Shachar, & Lüdecke, 2019; Makowski, Ben-Shachar, Chen, & Lüdecke, 2019). Terms that met frequentist criteria, but where 2.5%–97.5% of estimates fell within the ROPE, were considered to be of undetermined practical significance (i.e., neither supporting or refuting the null hypothesis) and interpreted with caution (Makowski, Ben-Shachar, Chen, & Lüdecke, 2019). Confidence intervals for LMER results were acquired using the “tab_model” function from the R package “sjPlot” (Lüdecke, 2021). Confidence intervals for NLME results were obtained using the ‘intervals’ function from the package “nlme” (Pinheiro et al., 2021).
Linear mixed models were consistent across analytic approaches (LMER (Bates et al., 2015), NLME (Pinheiro et al., 2021), and BRMS (Bürkner, 2017)) and across outcome measures, with few exceptions. For each analysis, we mean centered all predictors (Temperature, Cue, and Trial) and modeled Group as a mean-centered variable to aid with interpretation of the intercept. All models included fixed effects of Group with Subject modeled as random. Effects of time (Trial or Phase) were modeled as fixed in all models, while we included random slopes for other predictors unless models were unable to converge, indicating overfitting. Additional details about model specifications can be found in the Supplementary Methods and specific model parameters for each analysis are included in associated summary table legends.
3. Results
3.1. VMPFC lesion characteristics
The lesion group consisted of five adult neurosurgical patients with focal, bilateral parenchymal changes largely confined to the VMPFC, defined as the medial one-third of the orbitofrontal cortex (OFC) and the ventral one-third of the medial prefrontal cortex (Fig. 1A and B). All experimental data were collected at least 30 months after surgery (mean: 48.2, SD: 20.25). There were no differences in demographic or neuropsychological data between the VMPFC lesion group and the neurologically healthy comparison (HC) group (Table 1).
3.2. Quantitative sensory testing results
All participants first completed a quantitative sensory testing (QST) procedure (Amir et al., 2021; Atlas et al., 2010) to test whether VMPC lesions alter pain sensitivity, and to identify temperatures that elicit low, medium, and high pain to use for each individual in the main expectancy experiment (Fig. 2). Group means and comparisons are reported in Table 1.
To test the hypothesis that VMPC lesions alter pain sensitivity, we evaluated ratings of pain intensity and unpleasantness across all QST trials as a function of group and temperature using linear mixed effect (LME) models (Fig. 2B, and Table 2). We found an expected main effect of temperature for both types of ratings, indicating that higher temperatures were rated as more intense and unpleasant (intensity: βLMER = 1.17, P < .001; unpleasantness: βLMER = .84, P < .001). These effects were determined to be of practical significance in complementary Bayesian models (0% of posterior estimates in Region of Practical Equivalence [ROPE], see Section 2.11: Linear Model Specification (Makowski, Ben-Shachar, & Lüdecke, 2019; Makowski, Ben-Shachar, Chen, & Lüdecke, 2019)). We did not observe any main effect of Group nor any Group × Temperature interactions for either measure (all P’s > .1), and Bayesian models supported the null hypothesis of no group differences in temperature effects on pain intensity or unpleasantness (>99% in ROPE; Table 2). Thus, the VMPFC lesion group and the HC group showed similar associations between variations in temperature and pain ratings during the QST procedure.
Table 2 –
Temperature effects on pain during the adaptive calibration.a
| Estimates | Confidence Intervals | P-values/Probability of direction | Bayesian estimates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Outcome measure | Effect | LMERb | NLMEc | BRMSd | LMER | NLME | BRMS | LMER | NLME | BRMS | % in ROPE | Rhat | ESS |
| Intensity | (Intercept) | 8.475 | 8.428 | 8.49 | 7.17–9.78 | [7.08, 9.78] | [7.18, 9.90] | .000 | .000 | 100% | 0% | 1.001 | 2277 |
| Group | −1.113 | −1.115 | −.99 | −2.75 – .52 | [−2.90, .67] | [−2.58, .72] | .196 | .208 | 88.15% | 21.48% | 1 | 3487 | |
| Temperature | 1.165 | 1.242 | 1.16 | 1.02–1.32 | [1.08, 1.40] | [1.00, 1.33] | .000 | .000 | 100% | 0% | 1 | 5609 | |
| Group × Temperature | .078 | .067 | .07 | −.11 – .27 | [−.14, .27] | [−.14, .27] | .437 | .521 | 76.44% | 99.98% | 1.001 | 7078 | |
| Unpleasantness | (Intercept) | 5.507 | 5.447 | 5.53 | 4.45–6.57 | [4.37, 6.52] | [4.34, 6.62] | .000 | .000 | 100% | 0% | 1.002 | 2328 |
| Group | −.881 | −.887 | −.81 | −2.21 – .45 | [−2.31, .53] | [−2.16, .57] | .207 | .210 | 88.19% | 21.22% | 1 | 3397 | |
| Temperature | .842 | .872 | .84 | .71–.97 | [.74, 1.01] | [.69, .98] | .000 | .000 | 100% | 0% | 1.001 | 5904 | |
| Group × Temperature | .072 | .080 | .07 | −.10 – .24 | [−.09, .25] | [−.12, .26] | .412 | .364 | 77.88% | 99.85% | 1 | 6656 | |
This table presents results of linear mixed models predicting subjective pain as a function of mean-centered Temperature and Group (VMPFC versus Healthy Control) based on ratings during the Adaptive Staircase Calibration task prior to the main experiment. Separate models were conducted using Intensity ratings and Unpleasantness ratings. All predictors were dummy-coded and mean centered to facilitate interpretation of coefficients and interactions. In this and subsequent tables, we compared three types of linear mixed models: frequentist analysis using the “lmer” function of lme4 (Bates et al., 2015), frequentist analysis using the “lme” function of nlme (Pinheiro et al., 2021) accounting for autoregression, and Bayesian estimation using mildly informative conservative priors (i.e., centered on 0 for all effects) implemented in brms (Bürkner, 2017). Effects that are both statistically and practically significant are bolded, whereas effects that are statistically significant but not practically significant (i.e., >2.5% in the region of practical equivalence (ROPE) (Makowski, Ben-Shachar, Chen, & Lüdecke, 2019)) are italicized.
Estimates based on a linear mixed effects model implemented in the “lmer” function of lme4 (Bates et al., 2015) using the following code: lmer(Rating ~ Group*Temperature + (1 + Temperature|ubject)). Confidence intervals were obtained using the “tab_model” function from sjPlot (Lüdecke, 2021) and corresponds to the 95% confidence interval.
Estimates based on a linear mixed effects model implemented in the “lme” function of nlme (Pinheiro et al., 2021) including autoregression using the following code: lme(Rating ~ Group*Temperature, random = ~1 + Temperature |Subject, correlation = corAR1(*), na.action = na.exclude). Confidence intervals were obtained using the “intervals” function from nlme(Pinheiro et al., 2021) and corresponds to the 95% confidence interval.
Estimates based on Bayesian model linear mixed models using the “brms” function (Bürkner, 2017) using the following code: brm(Rating ~ Group*Temperature + (1 + Temperature | Subject, prior = set_prior(“normal(0,2.5)”, class = “b”), save_all_pars = TRUE, silent = TRUE, refresh = 0, iter = 4000, warmup = 1000). Posterior estimates, including the probable direction (which is roughly equivalent to [1− frequentist p-value), 95% confidence intervals, and the ROPE were obtained using the “describe_posterior” function from the package BayesTestR (Makowski, Ben-Shachar, & Lüdecke, 2019) and interpreted as in Makowski, Ben-Shachar, Chen, & Lüdecke, 2019) We report the median estimate for each parameter.
We then tested whether groups differed in the temperatures individually calibrated to elicit low pain (i.e., pain threshold), medium pain, and high pain (i.e., pain tolerance) for the main expectancy experiment using a 2 (Group: HC or VMPFC) × 3 (Pain Level: Low, Medium, or High) mixed ANOVA on predicted temperatures (Table 1, Fig. 2C). Consistent with LME models across all QST trials, there was an expected main effect of Pain Level (F(2, 69) = 64.06, P < .001) on predicted temperatures. Although temperature estimates for the VMPFC group were on average 1–3 °C higher than the HC group for each of the three estimated pain levels, there were no main effects of Group, nor Group × Pain Level interactions (all P’s > .1), indicating that temperatures used in the expectancy task were not significantly different between groups. Post-hoc non-parametric comparisons at each estimated temperature level were similarly not significant (Table 1, all P’s > .1), indicating that pain thresholds and pain tolerance were not significantly different between groups.
Thus, in contrast to prior case reports of patients with mixed frontal lobe lesions (Daum et al., 1995; Davis et al., 1994; Talbot et al., 1995), our analyses of pain ratings during the QST procedure indicate that pain sensitivity, pain thresholds, and pain tolerance are not significantly altered in individuals with focal VMPFC lesions. Further, our observation of similar, but slightly less variable, r2 values in the VMPFC group during QST indicates that sensory-discriminative function is intact across the range of temperatures used for the subsequent experiment (Table 1, Fig. 2B). Together, these findings indicate that any observed differences during the expectancy task cannot be explained by systematic group differences in pain sensitivity or applied temperatures.
3.3. Pain expectancy task summary
Following the QST procedure, participants completed a validated pain expectancy task (Atlas et al., 2010) in which Low or High Pain-predictive Cues were conditioned and reinforced with individually-calibrated Low or High temperatures (corresponding with calibrated pain intensity ratings of 5/20 and 15/20, respectively), then intermittently paired with Medium temperature stimuli (calibrated intensity rating of 10/20) during a Test Phase (see Fig. 3A). Participants were not informed that medium temperatures would be delivered, allowing us to test effects of unexpected sensory feedback (i.e., prediction errors) on expectancy ratings, and critically, to isolate expectancy effects on subjective ratings at a fixed temperature.
Before turning to planned expectancy analyses, we first analyzed pain intensity and unpleasantness ratings across all experimental trials using linear mixed models including Group (HC or VMPFC), Cue (Low and High), Temperature (Low, Medium, and High), and Time (Trial) to validate findings from QST analyses. Complete results are reported in Supplementary Table S1, with strong main effects of Temperature, Cue, and Time, regardless of analytic approach (all P’s < .001). Effects of Temperature were in line with QST results and Temperature and Cue effects were both practically significant (0% in ROPE). Ratings generally decreased over time, suggesting potential habituation, although the effect of time was of questionable practical significance (>80% in ROPE). Consistent with the fact that noxious stimuli were individually calibrated prior to the experimental phase, we did not observe a main effect of Group, nor any interactions between Group and Temperature (all P’s > .1) for either type of pain rating. The only difference between groups that was practically significant was a Group × Cue interaction in unpleasantness (all P’s < .001; 1.1% in ROPE) suggesting that predictive cues influenced unpleasantness ratings and that these effects differed by group. We therefore turned to analyses of self-reported expectations and subjective pain ratings during the critical medium heat trials to evaluate expectancy effects.
3.4. Individuals with VMPFC lesions report larger cue effects on pain expectations
To test whether VMPFC lesions alter the acquisition and evolution of explicit cue-based expectations about pain, we analyzed group differences in ratings of expected pain for each Cue (Low Pain Cue versus High Pain Cue) at Baseline (i.e., after verbal instruction but before any pairings between cue and heat), in response to reinforcement with predicted heat levels during a Conditioning Phase, and after the covert introduction of unexpected sensory feedback (i.e., Medium temperatures) during a Test Phase (see Fig. 3C and D, Supplementary Table S2). At baseline, individuals with VMPFC lesions reported larger differences in expected pain intensity between low and high cues than individuals without lesions (t(14.44) = −2.57, P = .022). There were no group differences in expected unpleasantness at baseline (P = .27).
For both types of ratings, a 2 Group (HC, VMPFC) × 2 Cue (Low, High) × 3 Phase (Baseline, Conditioning, Test) mixed ANOVA revealed significant main effects of Cue (Intensity: F(1,364) = 829.4, P < .001; Unpleasantness: F(1,364) = 640.2, P < .001) and significant Group × Cue interactions (Intensity: F(1,364) = 23.4, P < .001; Unpleasantness: F(1,364) = 24.1, P < .001), indicating that explicit expectations about pain were sensitive to predictive cues, and that patients with VMPFC lesions had larger differences in expected pain between low and high pain cues than HC adults. For ratings of pain unpleasantness, there were significant Cue × Phase (F(2,364) = 3.06, P = .048) and Group × Cue × Phase (F(2,364) = 3.36, P = .036) interactions, suggesting group differences in expectancy updating during the task. This effect was driven by a significant reduction in expected pain unpleasantness in the HC group between the Conditioning and Test Phases (Group × Cue × (Test − Conditioning): βLMER = −3.33, P = .011), corresponding with the covert introduction of medium pain trials (Fig. 3C and D). A similar pattern was observed for ratings of pain intensity but did not meet frequentist criteria for statistical significance (see Supplementary Table S2).
Thus, we found that VMPFC lesions enhance explicit cue-related expectations about pain. Although group differences are present after verbal instruction alone, violation of established cue-pain associations during the Test phase (i.e., introduction of covert medium temperature stimuli) serves to enhance group differences, particularly for ratings of expected pain unpleasantness. We suggest that these differences are driven by impaired on-line updating of established cue-pain associations in response to expectancy violations (i.e., the introduction of medium trials between Conditioning and Test blocks) in individuals with VMPFC lesions relative to HC participants.
3.5. VMPFC lesions enhance cue effects on pain unpleasantness ratings
To test whether VMPFC lesions alter the impact of cue-based expectations on subjective pain ratings during thermal stimulation, we next analyzed pain ratings in response to the critical Medium heat trials crossed with Low and High pain-predictive cues (i.e., LM and HM conditions). Across analytic approaches, there were practically significant main effects of Cue on pain intensity and unpleasantness, indicating that ratings were sensitive to cue effects (all P’s < .001; 0% in ROPE). Although ratings generally decreased over time, there were no significant interactions between Time × Group or Cue, and as with models across all trial types, main effects of Time were of undecided practical significance (>5.5% in ROPE, see Table 3). Individuals with VMPFC lesions showed stronger effects of pain-predictive cues on subjective unpleasantness ratings (see Fig. 3E and F), as indexed by a practically significant Group × Cue interaction (all P’s < .001; .5% in ROPE). We observed Group × Cue interactions on intensity ratings in the same direction, although this term was marginal in frequentist analyses and of undecided significance based on Bayesian models (P > .05, 10% in ROPE). There was no main effect of Group in any model (all P’s > .3), indicating that the impact of VMPFC lesions was specific to expectations with no overall differences in ratings of medium temperature stimuli.
Table 3 –
Pain-predictive cue effects on pain and SCR evoked by medium heat.e
| Estimates | Confidence Intervals | P-values/Probability of direction | Bayesian estimates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Outcome measure | Predictors | LMER | NLME | BRMS | LMER | NLME | BRMS | LMER | NLME | BRMS | % in ROPE | Rhat | ESS |
| Intensity | (Intercept) | 7.98 | 8.00 | 7.98 | [7.25, 8.72] | [7.25, 8.74] | [7.322, 8.618] | <.001 | 0 | 100.00% | 0 | 1.001 | 1957.107 |
| Group | −.81 | −.85 | −.71 | [−2.56, .93] | [−2.73, 1.02] | [−2.119, .745] | .361 | .354 | 78.18% | 21.992 | 1 | 2608.068 | |
| Cue | 2.58 | 2.59 | 2.55 | [1.96, 3.20] | [1.96, 3.21] | [2.036, 3.054] | <.001 | 0 | 100.00% | 0 | 1 | 6480.134 | |
| Trial | −.44 | −.42 | −.44 | [−.54, −.33] | [−.52, −.32] | [−.526, −.350] | <.001 | 0 | 100.00% | 5.917 | 1 | 12483.224 | |
| Group*Cue | 1.28 | 1.27 | 1.20 | [−.18, 2.75] | [−.23, 2.77] | [.055, 2.368] | .086 | .0958 | 94.65% | 10.083 | 1.001 | 6599.099 | |
| Group*Trial | −.15 | −.19 | −.15 | [−.41. .10] | [−.42, .04] | [−.354, .059] | .232 | .1044 | 88.33% | 93.642 | 1 | 14314.655 | |
| Cue*Trial | −.03 | −.08 | −.03 | [−.24, .18] | [−.25, .09] | [−.194, .153] | .791 | .3663 | 60.02% | 99.883 | 1 | 13010.665 | |
| (Group*Cue)*Trial | .12 | .05 | .12 | [−.39, .62] | [−.35, .46] | [−.275, .538] | .649 | .793 | 67.13% | 78.558 | 1 | 13453.963 | |
| Unpleasantness | (Intercept) | 5.42 | 5.43 | 5.40 | [4.66, 6.18] | [4.67, 6.20] | [4.756, 6.055] | <.001 | 0 | 100.00% | 0 | 1.001 | 2307.889 |
| Group | −.09 | −.12 | −.10 | [−1.90, 1.72] | [−2.05, 1.81] | [−1.507, 1.350] | .924 | .9006 | 54.48% | 25.392 | 1.001 | 3741.232 | |
| Cue | 1.48 | 1.47 | 1.46 | [1.13, 1.83] | [1.13, 1.80] | [1.140, 1.771] | <.001 | 0 | 100.00% | 0 | 1 | 11086.081 | |
| Trial | −.32 | −.31 | −.32 | [−.40, −.24] | [−.39, −.23] | [−.388, −.252] | <.001 | 0 | 100.00% | 20.75 | 1 | 16085.791 | |
| Group*Cue | 1.59 | 1.58 | 1.54 | [.75, 2.42] | [.78, 2.39] | [.782, 2.278] | <.001 | .0001 | 99.88% | 0.5 | 1 | 10754.967 | |
| Group*Trial | −.07 | −.07 | −.07 | [−.27, .13] | [−.26, .12] | [−.233, .089] | .491 | .4627 | 75.87% | 98.308 | 1 | 16393.391 | |
| Cue*Trial | −.04 | −.07 | −.04 | [−.21, .13] | [−.21, .08] | [−.176, .095] | .649 | .3664 | 68.16% | 99.792 | 1 | 15911.796 | |
| (Group*Cue)*Trial | .05 | .03 | .05 | [−.35, .45] | [−.31, .36] | [−.264, .380] | 0.8 | .8763 | 60.16% | 83.25 | 1 | 16260.922 | |
| Skin conductance response (CDA Ampsum, Z–scored) | (Intercept) | −.216 | −.215 | −.216 | [−.29, −.14] | [−.30, −.13] | [−.278, −.152] | .000 | 0 | 100.00% | .033 | 1 | 10232.784 |
| Group | −.021 | −.021 | −.021 | [−.20, .15] | [−.24, .20] | [−.165, .129] | .821 | .8401 | 59.20% | 58.458 | 1 | 9689.083 | |
| Cue | .132 | .137 | .132 | [.00, .26] | [.03, .24] | [.028, .242] | .047 | .0109 | 97.62% | 20.467 | 1 | 17008.287 | |
| Trial | −.054 | −.058 | −.054 | [−.08, −.02] | [−.09, −.03] | [−.079, −.029] | .001 | .0001 | 99.98% | 92.767 | 1 | 17267.529 | |
| Group*Cue | .027 | −.002 | .026 | [−.28, .34] | [−.25, .25] | [−.233, .267] | .863 | .9864 | 56.73% | 36.283 | 1 | 14843.577 | |
| Group*Trial | −.031 | −.033 | −.031 | [−.10, .04] | [−.10, .04] | [−.092, .030] | .408 | .3476 | 79.61% | 88.325 | 1 | 16533.377 | |
| Cue*Trial | −.024 | −.027 | −.023 | [−.09, .04] | [−.08, .03] | [−.073, .029] | .449 | .3093 | 77.38% | 95.1 | 1 | 15037.218 | |
| (Group*Cue)*Trial | −.059 | −.068 | −.059 | [−.21, .09] | [−.19, .06] | [−.179, .058] | .432 | .2862 | 78.56% | 56.292 | 1 | 14678.671 | |
This table presents results of linear mixed models predicting subjective pain (intensity or unpleasantness) and skin conductance response (Z-scored summary of amplitudes) as a function of Cue (High versus Low), Group (VMPFC versus Healthy Control), and Trial. Separate models were conducted for each outcome measure. All predictors were dummy-coded and mean centered to facilitate interpretation of coefficients and interactions. As in Table 2, effects that are both statistically and practically significant are bolded, whereas effects that are statistically significantbut not practically significant (i.e., >2.5% in the region of practical equivalence (ROPE)) are italicized. Models specified as follows. LMER: lmer(Rating ~ Group*Cue*Trial + (1 + Cue|Subject)); NLME: lme(Rating ~ Group*Cue*Trial, random = ~1 + Temperature*Cue|Subject, correlation = corAR1(*), na.action = na.exclude); BRMS: brm(Rating ~ Group*Cue*Trial + (1 + Cue|Subject, prior = set_prior(“normal(0,2.5)”, class = “b”), save_all_pars = TRUE, silent = TRUE, refresh = 0, iter = 4000, warmup = 1000). Additional details regarding model interpretation can be found in Table 2 legend. A complete summary of separate analyses for square-root-transformed and transformed/z-scored values for CDA Ampsum and other phasic SCR measures derived from Ledalab’s Continuous Decomposition Analysis (CDA), including SCR and tonic mean, as well as the sum of amplitudes derived from trough-to-peak (TTP) analyses (Benedek & Kaernbach, 2010) can be found in Supplementary Table S4.
None of the remaining interaction terms were significant (all P’s > .1; see Table 3). Thus, in line with group differences in explicit expectancy ratings, the VMPFC group exhibited significantly stronger cue-based modulation of subjective unpleasantness ratings at a constant level of thermal stimulation (see Fig. 3E and F).
3.6. No impact of VMPFC lesions on modulation of physiological arousal
To determine whether a failure to generate autonomic responses during the expectancy task contributed to group differences in expectancy and cue-based pain modulation, we evaluated Temperature, Cue, and Group effects on autonomic responses to thermal stimuli. Whereas we observed practically significant effects of temperature on SCR amplitude regardless of statistical approach (all P’s < .001), there was no influence of temperature on heat-evoked mean HR change (see Fig. 4A; Supplementary Table S3). We therefore focused on SCR for the remainder of our autonomic analyses.
Fig. 4 –
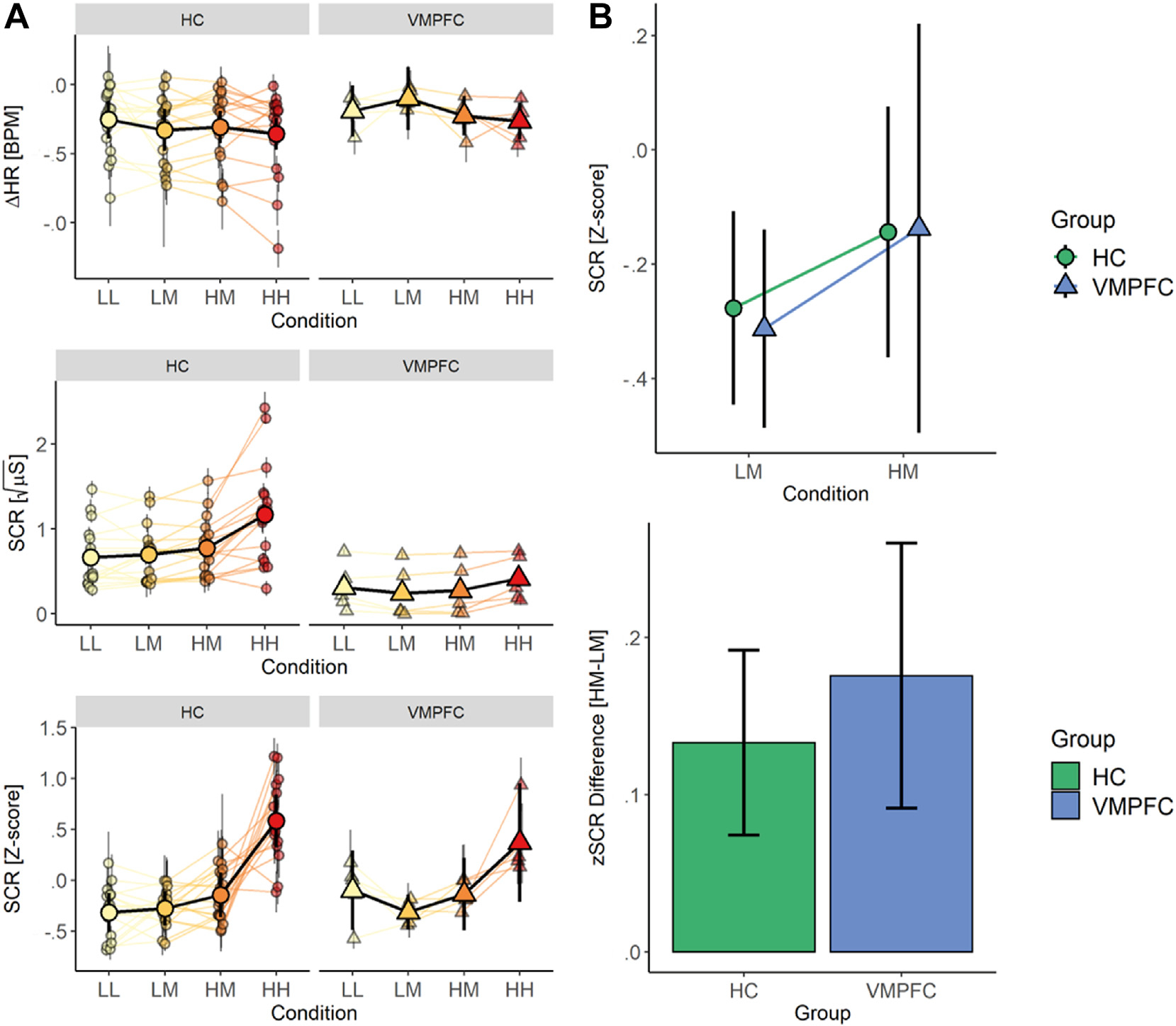
Physiologic Responses to Pain Stimuli. (A) Line graph showing parameter estimates for change in heart rate (top), square root-normalized Skin Conductance Response (middle), and z-transformed Skin Conductance Response (bottom). Patients with VMPFC lesions exhibit blunted SCRs overall, but z-transformed SCR showed amplitude modulation by temperature and cue. (B) Group summary plots showing greater SCR amplitudes for medium trials preceded by high (HM) relative to low (LM) pain cues (top) in both groups and no differences between groups in the difference in SCR amplitude between HM and LM trials.
Analyses of square-root normalized SCR amplitude revealed group differences in the intercept that were practically significant, indicating that SCR was blunted overall in the VMPFC lesion group (see Fig. 4A; Supplementary Table S3), consistent with prior reports (Bechara et al., 2000; Damasio & Carvalho, 2013a). We therefore z-scored responses within participants to account for group differences in mean SCR amplitude. Importantly, there were no group differences in the magnitude of temperature effects on z-scored SCR amplitudes, suggesting that although responses are blunted in the VMPFC group, they continue to scale with stimulus intensity (all P’s > .1; see Fig. 4A). A comprehensive summary of temperature effects on z-scored and square-root-transformed SCR values across a range of phasic and tonic estimates can be found in Supplementary Results and Supplementary Table S3.
Next, we explored whether VMPFC lesions impacted expectancy effects on autonomic responses akin to observed effects on subjective pain ratings. Analyses of z-scored SCR amplitude during medium heat revealed a main effect of Cue (all P’s < .05; see Table 3), such that SCR amplitude was higher in response to medium heat paired with High Pain Cues relative to Low Pain Cues, although this effect was of undecided practical significance (20.47% in ROPE). Interestingly, there were no differences between Groups, nor any interactions with Group (all P’s > .2), suggesting that groups did not differ in the magnitude of cue effects on SCR (Fig. 4B) despite group differences in cue effects on subjective pain ratings. Thus, across outcome measures, we observed larger heat-evoked SCRs when medium heat was preceded by high pain cues relative to low pain cues. Surprisingly, after accounting for the overall reduction in SCR amplitude, the VMPFC group exhibited similar cue effects on SCR relative to the HC group, indicating that a failure to generate autonomic responses alone is unlikely to account for observed differences in pain ratings. A comprehensive summary of Cue effects on z-scored and square-root-transformed SCR values across a range of phasic and tonic estimates can be found in Supplementary Table S4.
Finally, we evaluated whether the relationship between SCR and subjective pain differed between groups in the subset of medium heat trials. We observed significant positive associations with intensity and unpleasantness ratings for all SCR outcomes (all P’s < .1; see Supplementary Table S5) indicating that heat stimuli rated as more painful were associated with larger amplitude SCRs. There were no group differences in the association between SCR amplitude and pain ratings (all P’s > .3). Thus, VMPFC lesions were not associated with differences in the correspondence between subjective pain and physiologic arousal.
4. Discussion
Using a combination of subjective pain ratings and physiologic measures of autonomic reactivity in a population of neurosurgical patients with focal, bilateral damage to VMPFC from a common etiology, we present evidence supporting a key contribution of this region to processing conscious expectations about pain. We provide the first evidence that VMPFC lesions enhance explicit expectations about painful stimuli as well as the magnitude of resulting expectation effects on the conscious perception (and subjective ratings) of pain. Pain ratings were driven more strongly by expectations in patients with VMPFC lesions than in neurologically healthy adults. These differences were particularly strong for pain unpleasantness, which reflects pain’s affective and motivational qualities. Although patients with VMPFC lesions reported larger differences in expected pain between Low and High Pain predictive cues based on verbal instructions alone, group differences were enhanced following the covert introduction of unexpected medium temperature stimuli during the Test Phase (Fig. 3D). We interpret this result as evidence of impaired updating of cue-pain associations during experiential learning in the VMPFC group; whereas HC adults adjusted expected pain estimates such that Low Pain predictive cues were associated with slightly higher pain ratings and High Pain predictive cues were associated with slightly lower expectation ratings (i.e., both closer to the predicted medium temperature rating of 10/20), expectations in the VMPFC group remained stable throughout the experiment. Notably, observed differences were not linked to altered pain sensitivity overall, nor to altered modulation of autonomic responses, indicating that lesion effects are specific to expectancy-based pain modulation. Although these findings suggest that VMPFC is required for modulating expectations about pain based on experience, conclusions about the specificity of findings to VMPFC are limited by the absence of a lesion comparison group to account for non-specific effects of a shared clinical history (i.e., neurosurgical patients with meningioma resection). With this key caveat in mind, we discuss our findings and their implications.
4.1. VMPFC is involved in generating and updating expectations about pain
Neuroimaging meta-analyses indicate that expectations based on pain-predictive cues or placebo administration shape responses to noxious stimuli in pain-related regions, including the insula, thalamus, and anterior cingulate cortex (ACC) (Atlas & Wager, 2014). Our previous work suggests that cue effects on these regions are mediated by cue-evoked responses in the VMPFC, as well as the striatum (Atlas et al., 2010). Other studies have also linked expectations and contextual modulation of pain to the VMPFC (for review, see (Koban et al., 2017; Roy et al., 2012)). Based on this prior work, we hypothesized that VMPFC lesions would alter expectancy effects. Although the presence of fMRI activations in VMPFC in prior studies might suggest that lesions should abolish or attenuate expectancy effects, we found evidence that VMPFC lesions enhance expectancy effects. Specifically, lesions prevent cue-related expectations established with explicit verbal instruction from being revised and updated based on experience. We initially hypothesized that VMPFC lesions might also impact the acquisition of cue-pain associations (i.e., conditioning), especially if lesions were associated with impaired perceptual discrimination between low and high temperature stimuli. However, we found that sensory discriminative processing was intact in patients with VMPFC lesions and that cue-pain associations were established and maintained with verbal instruction alone. Thus, our findings suggest that an intact VMPFC is specifically required to integrate cognitive context and prior experience with somatosensory information to modulate and update expectations (Roy et al., 2012).
A primary role of VMPFC in updating expectancies is consistent with studies of affective learning that implicate VMPFC subregions, particularly medial OFC, in processing expected value and learning latent (i.e., hidden or unconscious) rules to guide decision-making (Jones et al., 2012; Schuck et al., 2016; Wilson et al., 2014). Our results provide new evidence that VMPFC also contributes to explicit predictions about outcomes (i.e., expectancy ratings). Although it is not clear whether pain expectations are specifically enhanced, the observed pattern of results suggests that patients with VMPFC lesions relied more on established expectations than new sensory information when considering the pain predictive value of each cue. Thus, our findings suggest that VMPFC is not only involved in generating latent predictions, but also in updating explicit expectations. These findings are consistent with previous views of the OFC/VMPFC and its involvement in generating and updating expected value and abstract rules, but highlight that acquisition of expectations through verbal instruction does not require intact VMPFC.
Our findings of enhanced cue-based expectations and expectancy-based modulation in the VMPFC group suggest that VMPFC lesions promote greater reliance on verbal instruction and impair the updating of previously established associations. This interpretation accounts for our observation that group differences in the Test Phase are driven by a failure of VMPFC patients to adjust expectations following the introduction of medium heat trials, likely reflecting impaired integration of new sensory feedback and or inadequate generation of prediction error signals to update cue-related expectancies. A possible alternative explanation is that receiving a temperature lower than their estimated high pain temperature (i.e., those with high pain >49 °C) led two of five patients in the VMPFC group to rely more on verbal instruction when rating pain. However, the observed expectancy effect was stronger in the three patients that received their estimated high pain stimulus, suggesting that lower ‘high pain’ temperatures in two patients with predicted values >49 °C may have suppressed the observed group effect.
Previous fMRI studies indicate that instructions about contingencies interact with learning-related responses in the VMPFC/OFC and that interactions with dorsolateral prefrontal cortex (DLPFC)—a region implicated in instructed learning (Atlas et al., 2016; Doll et al., 2009; Li et al., 2011), cognitive control (MacDonald, 2000), and expectancy-based pain modulation (Atlas et al., 2010; Krummenacher et al., 2010; Wager, 2004)—likely account for changes in VMPFC/OFC activity following verbal instruction (Atlas et al., 2016, 2021; Li et al., 2011). Thus, spared regions of DLPFC in our sample could explain the successful acquisition of instructed contingencies, with VMPFC lesions primarily interfering with the extent to which actual reinforcement reduces the effects of verbal instructions, consistent with known contributions of VMPFC to reversal (Fellows & Farah, 2005; Schiller et al., 2008) and extinction learning (Dunsmoor et al., 2019; Gottfried & Dolan, 2004; Kalisch, 2006; Rudebeck et al., 2013). Future work could incorporate instructed reversals (Atlas, 2019; Costa et al., 2015; Grings, 1973) or compare instructed versus learned expectancies to determine more conclusively whether VMPFC patients rely more strongly on instructions or associative learning. In addition, future studies in other species can provide further insight as to whether the integration of context, learning, and sensory experience depends on neurons within the VMPFC or fibers passing through the region (e.g., Rudebeck et al., 2013), although the impact of verbal instructions can only be tested in human studies.
4.2. VMPFC lesions disrupt judgements about pain affect
Case reports of patients with frontal lobotomy indicate that frontal lesions can drastically alter affective components of pain without impacting sensory/discriminative judgements about stimulus location and intensity (Foltz & White, 1962). Consistent with a proposed role in generating pain affect, the impact of VMPFC lesions on cue-based expectations was most pronounced for ratings of pain unpleasantness. However, we did not observe more general deficits in pain affect across the full range of temperatures as might be expected from the prior preclinical and fMRI literature, suggesting that the causal contribution of VMPFC is likely specific to the link between expected value and affective processing, rather than simply mediating subjective judgements about unpleasantness, per se.
We propose that the absence of more profound alterations of pain affect reflects the focality and homogeneity of VMPFC lesions in our sample; all subjects had bilateral VMPFC damage from growth and ultimate surgical resection of orbital meningiomas, leaving adjacent structures like DLPFC (described above) and ACC largely intact. In rodents, inactivating regions homologous to ACC elicits significant reductions in pain avoidance behavior, a common proxy for the affective domain of pain (Fuchs et al., 2014; Gu et al., 2015; Johansen et al., 2001). Notably, in all prior human neurosurgical cases, lesions of ACC and VMPFC, ACC alone, or the cingulum bundle connecting ACC to more posterior brain regions were necessary for the observed reductions in pain affect (Daum et al., 1995; Davis et al., 1994; Foltz & White, 1962; Freeman & Watts, 1947; Talbot et al., 1995). Further, in both humans and animal models, individual neurons within the ACC (but not VMPFC or rodent homologue infralimbic cortex) have been shown to be pain responsive (Hutchison et al., 1999; Johansen et al., 2001). The absence of more profound effects on pain processing and pain thresholds suggests that spared regions in our sample (e.g., ACC) are more likely than VMPFC to mediate these effects.
It is also possible that our failure to observe group differences in pain sensitivity, pain thresholds, and pain tolerance stems from limitations in our experimental design. For safety purposes, the thermal stimulator cannot exceed 49 °C at the stimulus duration we used, which was lower than the computed subjective pain tolerance for two of the five VMPFC lesion patients and four of twenty HC adults (with four additional HC adults at the 49 °C threshold). Therefore, the computed pain tolerance may underestimate the true tolerance for VMPFC lesion patients. Indeed, the three patients who did receive their estimated high pain temperature had a substantially greater difference between LM and HM ratings. Both patients who had an estimate high temperature >49 °C exhibited a pattern of ratings more similar in magnitude to the HC group, suggesting that our maximum temperature limitation may have actually suppressed more robust group differences. Further, three HC participants with an r2 > .4 at the end of the calibration procedure were included in calibration/QST analyses but excluded from task analyses to maintain consistency with prior work (Amir et al., 2021; Atlas et al., 2010). When we exclude these participants from pain sensitivity analyses, we do find a main effect of group (P = .046) in the ANOVA across Pain Levels, suggesting that “noisy” calibration values among excluded HC participants may be artificially suppressing a true group difference in pain processing in patients with VMPFC lesions. However, post-hoc tests at each temperature level remain non-significant even after removing the three HC participants, suggesting that their overall contribution is modest. Considering the small sample size of patients with lesions, this negative result should be interpreted with caution. Future studies involving larger samples of patients with lesions involving multiple subregions of PFC, higher intensities of noxious stimulation, or alternative pain modalities are needed to more conclusively determine whether focal VMPFC lesions significantly increase pain thresholds.
4.3. VMPFC lesions are associated with blunted autonomic responses
Previous work indicates that patients with VMPFC lesions fail to mount normal skin conductance responses as uncertain choices evolve to become more risky and less rewarding, which corresponds with a failure to update their decision-making strategy toward more favorable outcomes (Bechara et al., 1996, 2005). In addition, individuals with VMPFC lesions show blunted SCRs in response to emotionally salient images (Damasio et al., 1990) and during Pavlovian fear conditioning (Battaglia et al., 2020). Consistent with these findings, heat-evoked skin conductance responses were indeed blunted in the VMPFC lesion group. However, once differences in mean amplitude were accounted for, both groups showed similar effects of temperature and pain-predictive cues, and no differences in the correspondence between autonomic responses and subjective decisions (i.e., pain report). These findings diverge from previous findings of expectancy driven effects on physiological outcomes published in our lab (Motzkin et al., 2014), and suggest that differences in autonomic responses alone are unlikely to account for the differences in explicit cue-related expectations or expectancy effects on subjective pain. However, it remains possible that significant overall blunting of SCRs in the VMPFC group limits the utility of this autonomic signal and could account for the observed failure of expectancy updating. This latter interpretation would suggest that whereas robust physiologic responses to prediction errors are required to update behavior during decision-making (or subjective appraisals of stimuli), physiologic responses are not required to make affective judgements about pain, which remain intact in the VMPFC group (see Figs. 2B and 3E). Future work should explore whether the intact modulation of SCR by expectations in the VMPFC group may be explained by the inherently increased averseness of pain as compared to previously studied aversive stimuli (e.g., emotional pictures, monetary loss), or by specific neuroanatomical substrates that were not damaged in our sample.
4.4. Limitations and additional considerations
The lack of a lesion comparison group with similar clinical characteristics to the VMPFC group but without damage to VMPFC significantly limits our ability to draw strong conclusions about the specificity of observed findings to VMPFC. It is possible that nonspecific effects of brain damage or other unique clinical characteristics of the VMPFC group (e.g., prophylactic anti-seizure medication use in 3/5 patients and SSRI use in 2/5 patients) could have contributed to our findings. However, the specificity of group differences to expectancy with otherwise intact sensory-discriminative and affective function, together with a lack of published evidence supporting alterations in acute pain processing with Keppra and SSRIs, suggests that these non-specific effects are less likely. However, future studies including a lesion comparison group will be essential to rule out these possibilities and support the necessity of VMPFC for updating expectations about pain.
An additional feature of this study that warrants consideration is the small sample size of VMPFC lesion patients (n = 5). We used extremely stringent selection criteria for our target group; lesions had to involve substantial portions of the VMPFC but could not extend significantly outside the VMPFC and could not involve other brain regions implicated in pain. Therefore, although our sample size may be small by conventional VMPFC lesion patient standards, it is unique with respect to the homogeneity, uniformity, and focality of VMPFC lesions. In addition, all lesions were bilateral, in contrast to other studies that include only lateralized lesions (Daum et al., 1995). Future work in larger samples of lesion patients with voxel-based lesion-symptom mapping (Bates et al., 2003) or in nonhuman primates (Vaidya et al., 2019) are required to test our predictions about the specific contributions of other regions like DLPFC and ACC to individual components of expectancy, learning, and pain.
To address the statistical challenges posed by our small sample of patients with VMPFC lesions, we used a mixed effects modeling approach designed to improve inferential power in small and unbalanced populations. Compared to traditional methods that use single parameter estimates from each individual for group-level inference, mixed models include data from all experimental trials and simultaneously model all factors that may influence the outcome variables of interest, which improves model power and the precision of resulting estimates (Baayen et al., 2008). We combine these powerful methods with a convergent analysis approach across identically specified linear, nonlinear, and Bayesian models that maintain low type-1 error rates in small samples (Ukyo et al., 2019; van de Schoot et al., 2015) to infer the practical significance of our findings. Thus, although we are cautious in our interpretation of negative findings (e.g., the absence of group differences in pain sensitivity and in cue effects on SCR), our modeling strategy addresses several key limitations posed by our small sample size and yields convergent estimates across models that increase our confidence in the central findings of enhanced expectancy effects in the VMPFC lesion group.
5. Conclusions
Taken together, our results highlight the specific contribution of VMPFC to affective pain processing. Our findings of enhanced modulation of explicit expectations and subjective pain ratings in the lesion group suggests that damage to VMPFC promotes greater reliance on explicit instructions and established conscious expectations than incoming sensory information to guide appraisals of noxious thermal stimuli. This finding is remarkably consistent with a substantial literature that proposes a central role for VMPFC in integrating sensory information with contextual information to guide action tendencies during reward learning and decision-making (Rushworth et al., 2007). Our findings extend this literature to demonstrate a key role of VMPFC in modulating and updating expectations in aversive contexts and highlight that other processes commonly ascribed to VMPFC, including autonomic integration and generation of affective responses to pain, likely rely on neural substrates outside of the region of damage in our sample.
Supplementary Material
Acknowledgments
This work is supported by National Institutes of Health Neuroscience Research Training Grant T32GM007507 (JCM), National Institutes of Mental Health Emotion Research Training Grant T32MH018931–21 (JCM), National Institutes of Neurological Disorders and Stroke R25 Grant NS070680 (JCM); National Institutes of Mental Health grant R01MH101162 (MK); and by support from the Intramural Research Program of the National Center for Complementary and Integrative Health (ZIA-AT000030 (LYA)). The authors would like to thank Maia Pujara for assistance with data collection, Joe Weilgosz for assistance with technical implementation, and Geoff Schoenbaum, Allan Basbaum, and Howard Fields for their feedback on a prior version of this manuscript. Data, analysis code, and experiment materials are available at https://osf.io/79y3a/?view_only=9a0859ebc36c447caa62e9ad6a8a4497.
Footnotes
Declaration of competing interest
Authors declare that they have no competing interests.
Open practices section
The study in this article earned Open Data and Open Material badges for transparent practices. The data and material for this study are available at: https://osf.io/79y3a/?view_only=9a0859ebc36c447caa62e9ad6a8a4497.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cortex.2023.04.017.
REFERENCES
- Amir C, Rose-McCandlish M, Weger R, Dildine TC, Mischkowski D, Necka EA, Lee I, Wager T, Pine D, & Atlas L (2021). Test-retest reliability of an adaptive thermal pain calibration procedure in healthy volunteers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY (2019). How instructions shape aversive learning: Higher order knowledge, reversal learning, and the role of the amygdala. Current Opinion in Behavioral Sciences, 26, 121–129. [Google Scholar]
- Atlas LY (2021). A social affective neuroscience lens on placebo analgesia. Trends in Cognitive Sciences, 25, 992–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, & Wager TD (2010). Brain mediators of predictive cue effects on perceived pain. Journal of Neuroscience, 30, 12964–12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Dildine TC, Palacios-Barrios EE, Yu Q, Reynolds RC, Banker LA, Grant SS, & Pine DS (2021). Instructions and experiential learning have similar impacts on pain and pain-related brain responses but produce dissociations in value-based reversal learning. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Doll BB, Li J, Daw ND, & Phelps EA (2016). Instructed knowledge shapes feedback-driven aversive learning in striatum and orbitofrontal cortex, but not the amygdala. eLife, 5. Available at: https://elifesciences.org/articles/15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, & Wager TD (2014). A meta-analysis of brain mechanisms of placebo analgesia: Consistent findings and unanswered questions. In Handbook of experimental pharmacology (pp. 37–69). Berlin, Heidelberg: Springer Berlin Heidelberg. Available at: 10.1007/978-3-662-44519-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Wielgosz J, Whittington RA, & Wager TD (2013). Specifying the non-specific factors underlying opioid analgesia: Expectancy, attention, and affect. Psychopharmacology, 231, 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, & Gee JC (2004). Geodesic estimation for large deformation anatomical shape averaging and interpolation. Neuroimage, 23, S139–S150. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, & Bates DM (2008). Mixed-effects modeling with crossed random effects for subjects and items. Journal of Memory and Language, 59, 390–412. [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67. Available at: http://www.jstatsoft.org/v67/i01/ Accessed November 20, 2019. [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, & Dronkers NF (2003). Voxel-based lesion–symptom mapping. Nature Neuroscience, 6, 448–450. [DOI] [PubMed] [Google Scholar]
- Battaglia S, Garofalo S, di Pellegrino G, & Starita F (2020). Revaluing the role of vmPFC in the acquisition of Pavlovian threat conditioning in humans. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 40, 8491–8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, & Damasio AR (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex, 10, 295–307. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, & Damasio AR (2005). The Iowa Gambling task and the somatic marker hypothesis: Some questions and answers. Trends in Cognitive Sciences, 9, 159–162. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, & Damasio AR (1996). Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex, 6, 215–225. [DOI] [PubMed] [Google Scholar]
- Benedek M, & Kaernbach C (2010). A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods, 190, 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT, Dawson ME, Filion DL, & Measures S for PRAHC on E. (2012). Publication recommendations for electrodermal measurements. University of Wuppertal, Wuppertal, Germany: Blackwell Publishing Ltd. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=22680988&retmode=ref&cmd=prlinkspapers3://publication/doi/10.1111/j.1469-8986.2012.01384.x http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=22680988&. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, & Lang PJ (2001). Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion, 1, 276. [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, & Ashburner J (2001). Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage, 14, 486–500. [DOI] [PubMed] [Google Scholar]
- Büchel C, Geuter S, Sprenger C, & Eippert F (2014). Placebo analgesia: A predictive coding perspective. Neuron, 81, 1223–1239. [DOI] [PubMed] [Google Scholar]
- Bürkner P-C (2017). brms: An R package for Bayesian multilevel models using Stan. Journal of statistical software, 80, 1–28. [Google Scholar]
- Colloca L, Sigaudo M, & Benedetti F (2008). The role of learning in nocebo and placebo effects. Pain, 136, 211–218. [DOI] [PubMed] [Google Scholar]
- Colloca L, Tinazzi M, Recchia S, Le Pera D, Fiaschi A, Benedetti F, & Valeriani M (2008). Learning potentiates neurophysiological and behavioral placebo analgesic responses. Pain, 139, 306–314. [DOI] [PubMed] [Google Scholar]
- Costa VD, Bradley MM, & Lang PJ (2015). From threat to safety: Instructed reversal of defensive reactions. Psychophysiology, 52, 325–332. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1996). The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 351, 1413–1420. [DOI] [PubMed] [Google Scholar]
- Damasio A, & Carvalho GB (2013a). The nature of feelings: Evolutionary and neurobiological origins. Nature Reviews Neuroscience, 14, 143–152. [DOI] [PubMed] [Google Scholar]
- Damasio A, & Carvalho GB (2013b). The nature of feelings: Evolutionary and neurobiological origins. Nature Reviews Neuroscience, 14, 143–152. [DOI] [PubMed] [Google Scholar]
- Damasio A, Tranel D, & Damasio H (1990). Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research, 41, 81–94. [DOI] [PubMed] [Google Scholar]
- Daum I, Braun C, Riesch G, Miltner W, Ackermann H, Schugens MM, & Birbaumer N (1995). Pain-related cerebral potentials in patients with frontal or parietal lobe lesions. Neuroscience Letters, 197, 137–140. [DOI] [PubMed] [Google Scholar]
- Davis KD, Hutchison WD, Lozano AM, & Dostrovsky JO (1994). Altered pain and temperature perception following cingulotomy and capsulotomy in a patient with schizoaffective disorder. Pain, 59, 189–199. [DOI] [PubMed] [Google Scholar]
- Dildine TC, Necka EA, & Atlas LY (2020). Confidence in subjective pain is predicted by reaction time during decision making. Scientific Reports, 10, Article 21373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Jacobs WJ, Sanfey AG, & Frank MJ (2009). Instructional control of reinforcement learning: A behavioral and neurocomputational investigation. Brain Research, 1299, 74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Kroes MCW, Li J, Daw ND, Simpson HB, & Phelps EA (2019). Role of human ventromedial prefrontal cortex in learning and recall of enhanced extinction. The Journal of Neuroscience, 39, 3264–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, & Farah MJ (2005). Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex, 15, 58–63. [DOI] [PubMed] [Google Scholar]
- Foltz EL, & White LE (1962). Pain “relief” by frontal cingulumotomy. Journal of neurosurgery, 19, 89–100. [DOI] [PubMed] [Google Scholar]
- Forsberg JT, Martinussen M, & Flaten MA (2017). The placebo analgesic effect in healthy individuals and patients: A meta-analysis. Psychosomatic Medicine, 79, 388–394. [DOI] [PubMed] [Google Scholar]
- Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, & Venables PH (1981). Committee report. Publication recommendations for electrodermal measurements. Psychophysiology, 18, 232–239. [DOI] [PubMed] [Google Scholar]
- Fox J (2019). John Fox and Sanford Weisberg. An R companion to applied regression (3rd ed..). Sage Publications; [Google Scholar]. [Google Scholar]
- Freeman W, & Watts JW (1947). Retrograde degeneration of the thalamus following prefrontal lobotomy. Journal of Comparative Neurology, 86, 65–93. [DOI] [PubMed] [Google Scholar]
- Fuchs PN, Peng YB, Boyette-Davis JA, & Uhelski ML (2014). The anterior cingulate cortex and pain processing. Frontiers in Integrative Neuroscience, 8, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh A, Ambroggi F, Odean N, & Fields HL (2012). Prefrontal cortex mediates extinction of responding by two distinct neural mechanisms in Accumbens Shell. Journal of Neuroscience, 32, 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, & Dolan RJ (2004). Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nature Neuroscience, 7, 1144–1152. [DOI] [PubMed] [Google Scholar]
- Gracely RH, McGrath P, & Dubner R (1978). Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: Manipulation of affect by diazepam. Pain, 5, 19–29. [DOI] [PubMed] [Google Scholar]
- Grings WW (1973). Cognitive factors in electrodermal conditioning. Psychological Bulletin, 79, 200–210. [DOI] [PubMed] [Google Scholar]
- Gu L, Uhelski ML, Anand S, Romero-Ortega M, Kim Y, Fuchs PN, & Mohanty SK (2015). Pain inhibition by optogenetic activation of specific anterior cingulate cortical neurons. Plos One, 10, Article e0117746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison WD, Davis KD, Lozano AM, Tasker RR, & Dostrovsky JO (1999). Pain-related neurons in the human cingulate cortex. Nature Neuroscience, 2, 403–405. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, & Manning BH (2001). The affective component of pain in rodents: Direct evidence for a contribution of the anterior cingulate cortex. Proceedings of the National Academy of Sciences, 98, 8077–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston NE, Atlas LY, & Wager TD (2012). Opposing effects of expectancy and somatic focus on pain. Plos One, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Esber GR, McDannald MA, Gruber AJ, Hernandez A, Mirenzi A, & Schoenbaum G (2012). Orbitofrontal cortex supports behavior and learning using inferred but not cached values. Science, 338, 953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R (2006). Context-dependent human extinction memory is mediated by a ventromedial prefrontal and Hippocampal Network. Journal of Neuroscience, 26, 9503–9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Jepma M, Geuter S, & Wager TD (2017). What’s in a word? How instructions, suggestions, and social information change pain and emotion. Neuroscience and Biobehavioral Reviews, 81, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher P, Candia V, Folkers G, Schedlowski M, & Schönbächler G (2010). Prefrontal cortex modulates placebo analgesia. Pain, 148, 368–374. [DOI] [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Youell P, Watson A, Derbyshire SWG, Frackowiak RSJ, Friston KJ, & Jones AKP (2005). Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. European Journal of Neuroscience, 21, 3133–3142. [DOI] [PubMed] [Google Scholar]
- Li J, Delgado MR, & Phelps E (2011). How instructed knowledge modulates the neural systems of reward learning. Proceedings of the National Academy of Sciences, 108, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdecke D (2021). sjPlot: Data visualization for statistics in social Science. Available at: https://CRAN.R-project.org/package=sjPlot. [Google Scholar]
- MacDonald AW (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288, 1835–1838. [DOI] [PubMed] [Google Scholar]
- Makowski D, Ben-Shachar MS, Chen SHA, & Lüdecke D (2019). Indices of effect existence and significance in the bayesian Framework. Frontiers in Psychology, 10, 2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski D, Ben-Shachar M, & Lüdecke D (2019). bayestestR: Describing effects and their uncertainty, existence and significance within the Bayesian Framework. JOSS, 4, 1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, & Wall PD (1988). The challenge of pain. Penguin; London. [Google Scholar]
- Michalska KJ, Feldman JS, Abend R, Gold AL, Dildine TC, Palacios-Barrios EE, Leibenluft E, Towbin KE, Pine DS, & Atlas LY (2018). Anticipatory effects on perceived pain: Associations with development and Anxiety. Psychosomatic Medicine, 80, 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischkowski D, Palacios-Barrios EE, Banker L, Dildine TC, & Atlas LY (2019). Pain or nociception? Subjective experience mediates the effects of acute noxious heat on autonomic responses - corrected and republished. Pain, 160, 1469–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, & Koenigs M (2014). Ventromedial prefrontal cortex lesions alter neural and physiological correlates of anticipation. Journal of Neuroscience, 34, 10430–10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, O’Doherty JP, Schoenbaum G, O’Doherty JP, & Schoenbaum G (2007). What we know and do not know about the functions of the orbitofrontal cortex after 20 Years of cross-species studies. Journal of Neuroscience, 27, 8166–8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzke F, Harris RE, Williams DA, Clauw DJ, & Gracely RH (2005). Differences in unpleasantness induced by experimental pressure pain between patients with fibromyalgia and healthy controls. European Journal of Pain, 11. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, Debroy S, Sarkar D, & R Core Team. (2021). nlme: Linear and nonlinear mixed effects models. Available at: https://CRAN.R-project.org/package=nlme. [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, & Lebron K (2000). The role of ventromedial prefrontal cortex in the recovery of extinguished fear. Journal of Neuroscience, 20, 6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (1996). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: https://www.R-project.org/. [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, & Bushnell MC (1997). Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science, 277, 968–971. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, & Wager TD (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, & Murray EA (2013). Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature Neuroscience, 16, 1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ, Rudebeck PH, & Walton ME (2007). Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends in Cognitive Sciences, 11, 168–176. [DOI] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, Ledoux JE, & Phelps E (2008). From fear to safety and back: Reversal of fear in the human brain. Journal of Neuroscience, 28, 11517–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosberg H, & Stanley WC (1953). A simple test of the normality of twenty-four distributions of electrical skin conductance. Science, 117, 35–37. [DOI] [PubMed] [Google Scholar]
- Schneider B, & Koenigs M (2017). Human lesion studies of ventromedial prefrontal cortex. Neuropsychologia, 107, 84–93. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, & Stalnaker TA (2007). Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Annals of the New York Academy of Sciences, 1121, 320–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Schoot R, Broere JJ, Perryck KH, Zondervan-Zwijnenburg M, & van Loey NE (2015). Analyzing small data sets using bayesian estimation: The case of posttraumatic stress symptoms following mechanical ventilation in burn survivors. European Journal of Psychotraumatology, 6, Article 25216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck NW, Cai MB, Wilson RC, & Niv Y (2016). Human orbitofrontal cortex represents a cognitive map of state Space. Neuron, 91, 1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, Miller C, & Fields HL (2017). Cortico-Accumbens regulation of approach-avoidance behavior is modified by experience and chronic pain. Cell Reports, 19, 1522–1531. [DOI] [PubMed] [Google Scholar]
- Spalding KN, Schlichting ML, Zeithamova D, Preston AR, Tranel D, Duff MC, & Warren DE (2018). Ventromedial prefrontal cortex is necessary for normal associative inference and memory integration. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 38, 3767–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JD, Villemure JG, Bushnell MC, & Duncan GH (1995). Evaluation of pain perception after anterior capsulotomy: A case report. null, 12, 115–126. [DOI] [PubMed] [Google Scholar]
- Tinnermann A, Geuter S, Sprenger C, Finsterbusch J, & Büchel C (2017). Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science, 358, 105–108. [DOI] [PubMed] [Google Scholar]
- Ukyo Y, Noma H, Maruo K, & Gosho M (2019). Improved small sample inference methods for a mixed-effects model for repeated measures approach in incomplete longitudinal data analysis. Stats, 2, 174–188. [Google Scholar]
- Vaidya AR, Pujara MS, Petrides M, Murray EA, & Fellows LK (2019). Lesion studies in contemporary neuroscience. Trends in Cognitive Sciences, 23, 653–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD (2004). Placebo-induced changes in fMRI in the anticipation and experience of pain. Science, 303, 1162–1167. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, & Zubieta J-K (2007). Placebo effects on human m-opioid activity during pain. Proceedings of the National Academy of Sciences, 104, 11056–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K (2016). Deconstructing the sensation of pain: The influence of cognitive processes on pain perception. Science, 354, 584–587. [DOI] [PubMed] [Google Scholar]
- Wikenheiser AM, & Schoenbaum G (2016). Over the river, through the woods: Cognitive maps in the hippocampus and orbitofrontal cortex. Nature Reviews. Neuroscience, 17, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, & Niv Y (2014). Orbitofrontal cortex as a cognitive map of task Space. Neuron, 81, 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, Vlaev I, Seymour B, Chater N, & Dolan RJ (2014). Relative valuation of pain in human orbitofrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience, 34, 14526–14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, & Preston AR (2012). Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron, 75, 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunhammer M, Spisák T, Wager TD, Bingel U, & The Placebo Consortium. (2021). Meta-analysis of neural systems underlying placebo analgesia from individual participant fMRI data. Nature Communications, 12, 1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


