Summary
Background
There are large disparities in mortality among racial–ethnic groups and by location in the USA, but the extent to which racial–ethnic disparities vary by location, or how these patterns vary by cause of death, is not well understood. This analysis estimated age-standardised mortality for five racial–ethnic groups, 3110 counties, and 19 causes of death over a 20-year period and describes the intersection between racial–ethnic and place-based disparities in mortality, comparing patterns across health conditions.
Methods
We applied small-area estimation models to death certificate data from the US National Vital Statistics system and population data from the US National Center for Health Statistics in order to estimate mortality by age, sex, county, and racial–ethnic group (non-Latino and non-Hispanic American Indian or Alaska Native [AIAN], non-Latino and non-Hispanic Asian or Pacific Islander [Asian], non-Latino and non-Hispanic Black [Black], Latino or Hispanic [Latino], and non-Latino and non-Hispanic White [White]) annually from 2000 to 2019 for 19 causes of death. We adjusted these mortality rates to correct for misreporting of race and ethnicity on death certificates and generated age-standardised results using direct standardisation to the 2010 Census population.
Findings
Racial–ethnic disparities in age-standardised mortality were noted for all causes of death considered. For most causes of death, AIAN and Black populations had substantially higher mortality than the White population, whereas Asian and Latino populations had substantially lower mortality. However, there are exceptions to this pattern, and the exact ordering among racial–ethnic groups, the magnitude of the disparity in both absolute and relative terms, and the change over time in this magnitude varied considerably by cause of death. Similarly, substantial geographic variation in mortality was observed for all causes of death, both overall and within each racial–ethnic group. Racial–ethnic disparities observed at the national level reflect widespread disparities at the county level, although the magnitude of these disparities varied widely among counties. Certain patterns of disparity were nearly universal among counties; for example, mortality was higher among the AIAN population than the White population in nearly all counties for skin and subcutaneous diseases (455 [97.8%] of 465) and HIV/AIDS and sexually transmitted infections (458 [98.5%]), and mortality was higher among the Black population than the White population in nearly all counties for diabetes and kidney diseases (1473 [99.1%] of 1486), maternal and neonatal disorders (1486 [100%]), and HIV/AIDS and sexually transmitted infections (1486 [100%]).
Interpretation
Disparities in mortality among racial–ethnic groups are ubiquitous, occurring across locations in the USA and for a wide range of health conditions. There is an urgent need to address the shared structural factors driving these widespread disparities.
Funding
National Institute on Minority Health and Health Disparities; National Heart, Lung, and Blood Institute; National Cancer Institute; National Institute on Aging; National Institute of Arthritis and Musculoskeletal and Skin Diseases; Office of Disease Prevention; and Office of Behavioral and Social Science Research, National Institutes of Health (contract #75N94019C00016).
Introduction
Racial–ethnic disparities in mortality have long been recognised in the USA.1–4 A recent study examining differences in life expectancy by racial–ethnic groups at the county level found that racial–ethnic disparities in life expectancy are widespread geographically, but the magnitude of these differences varies substantially among counties.5 These disparities in life expectancy represent the cumulative effect of disparities in mortality across a range of causes of death, and thus local, detailed, cause-specific data are crucial both for better understanding why differences in longevity are larger in some locations than others, as well as for pinpointing targets for interventions aimed at reducing and ultimately eliminating disparities while improving health and longevity for all.
Many studies have documented differences at the national level in mortality among racial–ethnic groups for specific causes of death.6,7 Similarly, a growing number of studies have documented geographic variation in mortality for select causes of death among counties or even more local geographic areas.8–12 However, studies that consider how mortality varies simultaneously by racial–ethnic group and county are less common,13–16 and typically focus only on a limited number of racial–ethnic groups, most frequently comparing outcomes between Black and White populations. Moreover, studies that do consider a wider range of racial–ethnic groups frequently do not address misclassification of racial–ethnic identity on death certificates (relative to self-identification), which has been shown to lead to biased mortality estimates for the Asian, Latino, and, especially, American Indian and Alaska Native populations.17 Additionally, these studies tend to focus on a single cause of death or several related causes. Thus, estimates are not available for all causes, and it can be difficult to compare across different causes due to varied methodological approaches across studies, as well as variation in the time periods, racial–ethnic groups, and locations considered.
In this study, we estimated cause-specific mortality in the USA annually from 2000 to 2019, stratified by county and racial–ethnic group, for 19 groups of causes of death. We used these estimates to describe spatial patterns in mortality and disparities in mortality among racial–ethnic groups for each cause, and to compare and contrast among different causes. This study provides the most comprehensive and detailed view available to date of local patterns of disparities in cause-specific mortality in the USA.
Methods
Unit of analysis
For this analysis of cause-specific mortality by county and racial–ethnic group, we adapted methods previously developed for estimating all-cause mortality and life expectancy.5 We estimated age-standardised mortality for 19 causes of death in Level 2 of the Global Burden of Diseases, Injuries, and Risk Factors (GBD) 2021 study cause hierarchy (appendix pp 34–49). Each cause was estimated by county, sex, combined race and Latino or Hispanic ethnicity, and year (2000–2019). Race and Latino or Hispanic ethnicity were combined into a single categorisation (“race–ethnicity”) with five mutually exclusive groups: non-Latino and non-Hispanic American Indian or Alaska Native (AIAN), non-Latino and non-Hispanic Asian or Pacific Islander (Asian), non-Latino and non-Hispanic Black (Black), Latino or Hispanic (Latino), and non-Latino and non-Hispanic White (White). These groups align with the standards for federal data collection on race and ethnicity issued by the Office of Management and Budget (OMB) in 1977.18 These standards were updated in 1997 to require that federal data collection systems provide separate Asian and Native Hawaiian and other Pacific Islander (NHPI) groups, and to allow individuals to identify as two or more races.19 However, these changes were not implemented on death certificates in all states until 2017,6 and estimates of misclassification of racial–ethnic group on death certificates are currently only available using the 1977 categorisation.17 Additionally, it is not possible to entirely disaggregate the Asian and NHPI populations in death certificate data prior to 2011, owing to the use of a combined Other Asian and Pacific Islander residual category for individuals who are Asian or NHPI but not one of the specific nationalities listed on death certificates (eg, Chinese or Hawaiian). Due to these data constraints, we combined the Asian and Pacific Islander populations for this analysis, however we refer to this combined group as “Asian,” recognising that our estimates for this combined group predominantly reflect the experience of the Asian population, which is much larger than the NHPI population (21.7 million non-Hispanic and non-Latino individuals identifying as Asian [either alone or in combination with other racial identities] compared with 1.2 million individuals identifying as NHPI in 2019).20
Some county boundaries changed over this period; therefore, we used a previously developed mapping of counties to temporally stable geographic units,9 which reduced the number of areas analysed from 3143 to 3110 counties or combined county units (appendix p 32). For simplicity, we refer to these 3110 areas as “counties.”
Data
We used deidentified death records from the US National Vital Statistics System and population estimates from the US National Center for Health Statistics (NCHS) for this analysis (appendix p 33). We tabulated these data by cause of death, county, age group (0 years, 1–4 years, 5-year age bands from 5–9 to 80–84, 85+ years), sex, racial–ethnic group, and year. The racial–ethnic groups used in this analysis are single-race groups, so for death certificates where the individual was identified as having multiple racial identities, we used the “primary” (or “bridged”) race imputed by NCHS.21 We utilised the cause list and hierarchy developed for the GBD 2021 study, and the associated mapping of ICD-10 codes22 to GBD causes (appendix pp 34–49). We also applied algorithms developed for the GBD study to reassign “garbage codes”—codes assigned as an underlying cause of death that refer to an intermediate or immediate cause of death, are otherwise implausible, or are insufficiently specific—to likely true underlying causes of death (appendix pp 8–9).23,24 This impacts the detailed cause of death assigned for 26.7% (13.6 million) of deaths; however, for the broader cause groups that are the focus of this paper, only 13.0% (6.64 million) of deaths are impacted by this reassignment. All causes in the GBD cause list with at least 10 000 deaths in total over the study period and at least 1000 deaths among males and females separately were analysed concurrently, however the focus of this paper is on 19 broad causes in Level 2 of the GBD cause hierarchy which accounted for 99.77% of all deaths in 2019.
We also incorporated data extracted from various sources on income and population density by county, and on post-secondary education, poverty, and birthplace (in the USA vs outside the USA) by county and race–ethnicity as covariates in the statistical model in order to better inform the estimates (appendix pp 9–12, 50–52). Finally, we utilised published estimates of race–ethnicity misclassification ratios, defined as the ratio of deaths among individuals of a particular racial–ethnic group as indicated by self-report to deaths among individuals of that same racial–ethnic group as indicated on death certificates.17
Statistical analysis
We carried out the statistical analysis in three stages. First, we used small area estimation models to estimate mortality rates by cause, county, racial–ethnic group, sex, age, and year, using the racial–ethnic group reported on death certificates. These models incorporate the covariates listed above, as well as a series of random intercepts by county, racial–ethnic group, year, and/or age. Models were fit separately for each cause of death using the Template Model Builder package25 in R (version 3.6.1),26 and 1000 draws of the mortality rate were generated from the approximated posterior distribution after fitting the models. Further details on model specification, model validation, and model performance are provided in the appendix (pp 12–24).
Second, we used race–ethnicity misclassification ratios to adjust draws of the mortality rate derived from the small area model. Extracted race–ethnicity misclassification ratios were combined across different stratifications (age and sex, census region, and low vs high co-ethnic density [a measure of the concentration of a particular racial or ethnic group within a given county]), and 1000 draws were generated for each combined misclassification ratio using the reported standard errors and assuming each of the misclassification ratios was log-normally and independently distributed. Draws of the unadjusted mortality rate and misclassification ratios were multiplied to generate adjusted mortality rate draws (appendix pp 24–26).
Third, to guarantee that the sum of estimated mortality across all causes is equal to estimated all-cause mortality and that adjustment for misclassification did not change the overall mortality rate estimated for a given county, we performed a post-hoc calibration on each of the 1000 draws from the approximated posterior distribution using a two-stage iterative proportional fitting algorithm (appendix pp 26–29).
Final point estimates were derived from the mean of the 1000 draws, and 95% uncertainty intervals (UIs) were derived from their 2.5th and 97.5th percentiles. We generated estimates for males and females combined and at aggregate geographic levels (ie, state and national) by population-weighting the age-specific mortality rates. Age-standardised mortality rates were calculated using age weights derived from the age structure of the USA population as recorded in the 2010 Census. When comparing any pair of age-standardised mortality estimates, we describe the difference as statistically significant when the posterior probability that the difference is greater than 0 was less than 2.5% or greater than 97.5%, akin to a two-tailed test with α = 0.05. Finally, we masked (ie, did not display) the modelled mortality rate estimates in every year for county and racial–ethnic group combinations that had a mean annual population of less than 1000 because model performance declined notably below this threshold (appendix pp 20–24). Over 97% of the population in each racial–ethnic group other than AIAN lived in counties with unmasked estimates; 82% of the AIAN population lived in counties with unmasked estimates (appendix pp 53–54).
This study complies with the Guidelines for Accurate and Transparent Health Estimates Reporting (appendix p 6).27 This research received institutional review board approval from the University of Washington. Informed consent was not required because the study used deidentified data and was retrospective.
Role of the funding source
Co-authors employed by the NIH contributed to data interpretation and to revising drafts of this report. Otherwise, the funders had no role in study design, data collection, data analysis, or the initial writing of the report.
Results
Estimates for all counties, race–ethnicity groups, and causes are available in an online visualisation tool (https://vizhub.healthdata.org/subnational/usa).
Racial–ethnic inequalities in cause-specific mortality at the national level
Figure 1 depicts national-level trends in age-standardised all-cause mortality and mortality due to 19 groups of causes of death by racial–ethnic group. There were large disparities among racial–ethnic groups in all-cause mortality throughout the study period, with the lowest mortality rates observed for the Asian population, followed by the Latino and then the White populations. Mortality was highest among the AIAN and Black populations, though the ordering of these two groups relative to each other switched during the study period, such that the Black population had the highest estimated mortality rate in 2000 but the AIAN population had the highest estimated mortality rate in 2019.
Figure 1: Estimated age-standardised mortality in the USA, 2000–2019, by cause, year, and racial–ethnic group.
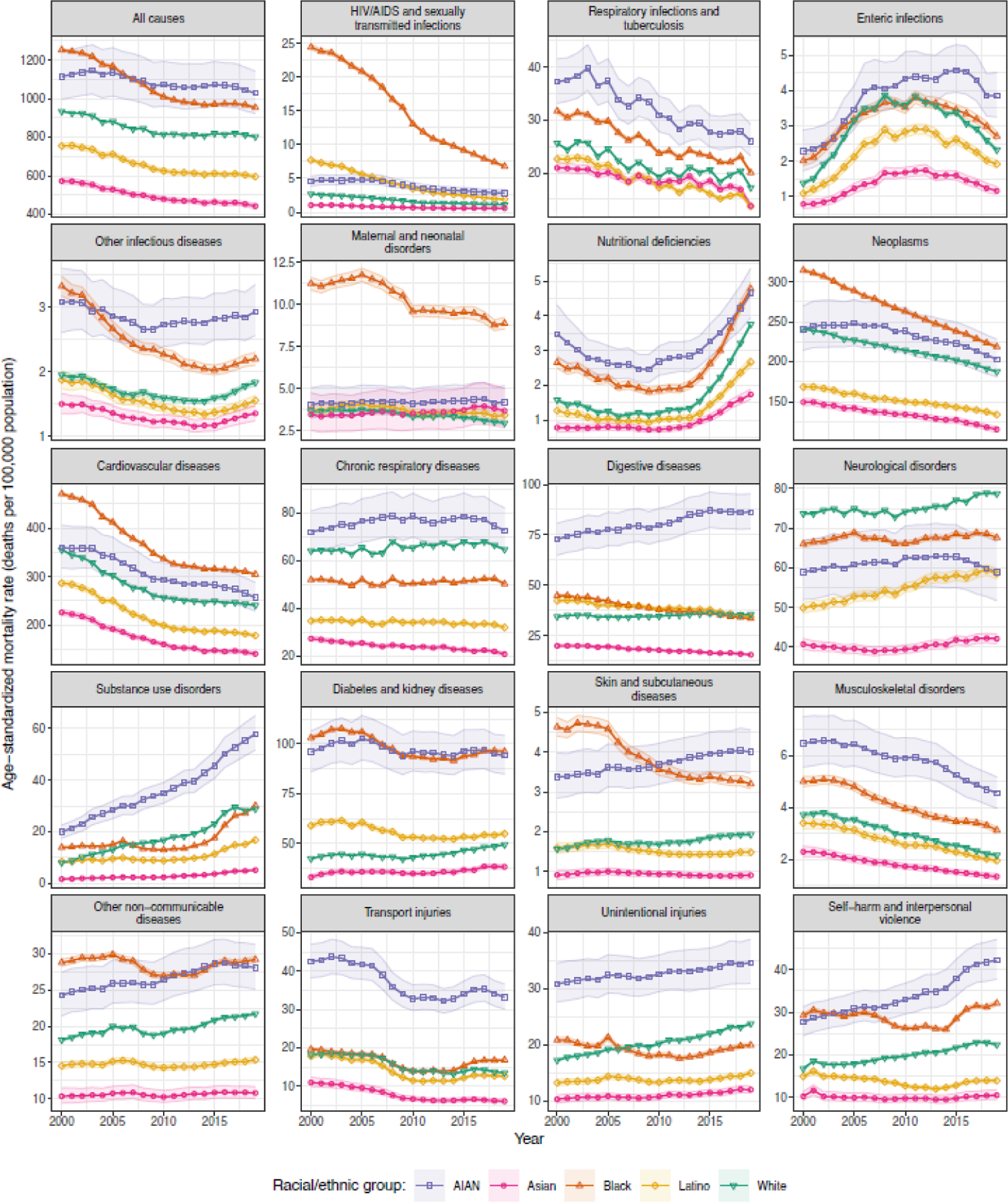
Shaded areas indicate the 95% uncertainty intervals.
Although the mortality rates, trends in mortality rates over the study period, and size of the racial–ethnic disparities in mortality rates varied meaningfully among causes, certain patterns were noticeable across a wide range of causes. The Asian population consistently experienced low mortality rates relative to other racial–ethnic groups: for every cause group except respiratory infections and tuberculosis, and maternal and neonatal disorders, the estimated mortality rate for the Asian population was the lowest among racial–ethnic groups in every year. Mortality among the Latino population was also low relative to other racial–ethnic groups (apart from Asian) across many causes, though there are exceptions to this pattern, most notably for HIV/AIDS and sexually transmitted infections and for diabetes and kidney diseases, where the estimated mortality rate for the Latino population exceeded that for the White population in all years. As was the case for all-cause mortality, the estimated mortality rate was higher among the AIAN and Black populations compared to other racial–ethnic groups for most causes. The most notable exceptions were for neurological disorders (where the highest estimated mortality rate in all years was among the White population), chronic respiratory diseases (where mortality was lower among the Black population than among the White population in all years), and unintentional injuries (where mortality was lower among the Black population than among the White population from 2007 onward). For some causes, the AIAN population experienced extremely high mortality relative to all other groups, including digestive diseases (2.4 [95% UI: 2.2–2.7] times higher than for the group with next highest mortality rate in 2019), substance use disorders (1.9 [1.7–2.2] times higher), transport injuries (2.0 [1.8–2.2] times higher), musculoskeletal disorders (1.5 [1.3–1.7] times higher), and unintentional injuries (1.5 [1.3–1.6] times higher). The same was true for the Black population for a smaller number of causes including HIV/AIDS and sexually transmitted infections (2.4 [2.0–2.8] times higher than for the group with the next highest rate in 2019) and maternal and neonatal disorders (2.1 [1.7–2.6] times higher).
Table 1 ranks causes in terms of their mortality rate nationally and in terms of the degree of racial–ethnic inequality in 2019. Cardiovascular diseases and neoplasms were the first and second leading cause of death, respectively, overall and for every racial–ethnic group. Beyond these two causes, the ranking among causes varies by racial–ethnic group although there are still similarities: for example, diabetes and kidney diseases is within the top five causes for every racial–ethnic group; chronic respiratory diseases is within the top five causes for all groups except Latino; and neurological disorders is ranked in the top five causes for all groups except AIAN. The degree of absolute inequality was strongly correlated with the mortality rate among causes (rank correlation: 0.92 and 0.93 for the difference between the highest and lowest mortality rates and the standard deviation, respectively), and thus cardiovascular diseases and neoplasms had the highest degree of absolute inequality among racial–ethnic groups. In contrast, the degree of relative inequality was moderately negatively correlated with the mortality rate (rank correlation: −0.33 and −0.34 for the ratio of the highest to lowest mortality rates and the coefficient of variation, respectively). The causes with the highest degree of relative inequality among racial–ethnic groups were HIV/AIDS and sexually transmitted infections, substance use disorders, digestive diseases, transport injuries, and skin and subcutaneous diseases.
Table 1:
National age-standardised mortality rates by racial–ethnic group in 2019, and associated ranks and inequality metrics.
| Cause of Death | National Age-Standardised Mortality Rate and Rank, 2019 | Inequality Measures, 2019 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | AIAN | Asian | Black | Latino | White | Max. - Min. | Std. Deviation | Max. / Min. | Coef. of Variation | |||||||||||
| All causes | 783.4 (782.2–784.6) | - | 1028.2 (922.2–1142.3) | - | 442.3 (429.3–455.0) | - | 953.5 (947.5–958.8) | - | 595.6 (583.7–606.8) | - | 802.5 (800.3–804.7) | - | 585.8 | - | 244.5 | - | 2.32 | - | 0.32 | - |
| HIV/AIDS and sexually transmitted infections | 1.9 (1.9–1.9) | 18 | 2.8 (2.4–3.3) | 19 | 0.6 (0.5–0.7) | 19 | 6.8 (6.6–7.0) | 14 | 1.9 (1.8–2.0) | 16 | 1.1 (1.1–1.1) | 19 | 6.2 | 13 | 2.5 | 13 | 11.58 | 1 | 0.93 | 1 |
| Respiratory infections and tuberculosis | 17.2 (17.1–17.3) | 11 | 26.0 (23.2–29.2) | 12 | 13.8 (13.3–14.3) | 7 | 20.1 (19.8–20.4) | 10 | 13.7 (13.3–14.1) | 11 | 17.3 (17.1–17.4) | 11 | 12.3 | 12 | 5.1 | 12 | 1.9 | 17 | 0.28 | 17 |
| Enteric infections | 2.3 (2.2–2.3) | 15 | 3.9 (3.2–4.5) | 17 | 1.2 (1.1–1.3) | 17 | 2.7 (2.6–2.8) | 18 | 1.9 (1.8–2.0) | 17 | 2.3 (2.3–2.4) | 15 | 2.7 | 18 | 1 | 18 | 3.31 | 9 | 0.42 | 11 |
| Other infectious diseases | 1.9 (1.8–1.9) | 19 | 2.9 (2.6–3.4) | 18 | 1.4 (1.2–1.5) | 15 | 2.2 (2.1–2.3) | 19 | 1.6 (1.5–1.6) | 18 | 1.8 (1.8–1.9) | 18 | 1.6 | 19 | 0.6 | 19 | 2.16 | 16 | 0.31 | 15 |
| Maternal and neonatal disorders | 4.1 (4.0–4.1) | 13 | 4.2 (3.4–5.2) | 15 | 3.7 (2.7–5.0) | 13 | 8.9 (8.5–9.3) | 13 | 3.4 (3.2–3.7) | 13 | 3.0 (2.9–3.1) | 14 | 5.9 | 14 | 2.4 | 14 | 2.99 | 10 | 0.52 | 7 |
| Nutritional deficiencies | 3.7 (3.6–3.7) | 14 | 4.7 (4.0–5.4) | 13 | 1.7 (1.6–1.9) | 14 | 4.8 (4.6–5.0) | 15 | 2.7 (2.5–2.8) | 14 | 3.8 (3.7–3.8) | 13 | 3 | 17 | 1.3 | 15 | 2.74 | 12 | 0.37 | 14 |
| Neoplasms | 182.4 (181.9–182.8) | 2 | 203.0 (180.8–228.2) | 2 | 115.3 (111.7–118.7) | 2 | 218.7 (217.1–220.3) | 2 | 133.8 (131.1–136.4) | 2 | 187.5 (186.9–188.2) | 2 | 103.3 | 2 | 44.9 | 2 | 1.9 | 18 | 0.26 | 18 |
| Cardiovascular diseases | 237.8 (237.2–238.4) | 1 | 256.7 (228.9–288.2) | 1 | 140.3 (136.2–144.2) | 1 | 304.8 (302.7–306.9) | 1 | 178.3 (174.5–182.0) | 1 | 240.9 (240.2–241.7) | 1 | 164.4 | 1 | 65.1 | 1 | 2.17 | 15 | 0.29 | 16 |
| Chronic respiratory diseases | 58.6 (58.4–58.8) | 4 | 72.7 (64.4–82.2) | 5 | 20.7 (20.0–21.4) | 5 | 50.3 (49.7–50.9) | 5 | 32.0 (31.2–32.7) | 6 | 64.8 (64.5–65.0) | 4 | 52 | 6 | 21.8 | 5 | 3.51 | 7 | 0.45 | 9 |
| Digestive diseases | 34.5 (34.3–34.6) | 6 | 86.2 (78.0–95.3) | 4 | 15.6 (15.0–16.1) | 6 | 33.6 (33.1–34.0) | 6 | 34.4 (33.7–35.2) | 5 | 35.5 (35.3–35.7) | 6 | 70.7 | 3 | 26.6 | 4 | 5.54 | 3 | 0.65 | 3 |
| Neurological disorders | 74.2 (74.0–74.5) | 3 | 58.9 (51.7–66.6) | 6 | 42.1 (40.8–43.3) | 3 | 67.6 (66.9–68.4) | 4 | 58.5 (57.2–59.9) | 3 | 78.7 (78.4–78.9) | 3 | 36.6 | 7 | 13.5 | 7 | 1.87 | 19 | 0.22 | 19 |
| Substance use disorders | 25.2 (25.0–25.4) | 7 | 57.8 (51.6–65.0) | 7 | 5.2 (4.8–5.6) | 12 | 30.1 (29.7–30.5) | 8 | 16.8 (16.3–17.3) | 7 | 28.8 (28.6–29.1) | 7 | 52.7 | 5 | 19.6 | 6 | 11.19 | 2 | 0.71 | 2 |
| Diabetes and kidney diseases | 54.4 (54.2–54.6) | 5 | 94.1 (84.8–104.3) | 3 | 38.3 (37.0–39.5) | 4 | 96.1 (95.4–97.0) | 3 | 54.8 (53.8–56.0) | 4 | 49.4 (49.2–49.6) | 5 | 57.8 | 4 | 26.7 | 3 | 2.51 | 14 | 0.4 | 12 |
| Skin and subcutaneous diseases | 2.0 (1.9–2.0) | 17 | 4.0 (3.5–4.6) | 16 | 0.9 (0.8–1.0) | 18 | 3.2 (3.1–3.3) | 16 | 1.5 (1.4–1.6) | 19 | 1.9 (1.9–2.0) | 17 | 3.1 | 16 | 1.3 | 16 | 4.39 | 5 | 0.55 | 5 |
| Musculoskeletal disorders | 2.3 (2.2–2.3) | 16 | 4.6 (4.0–5.2) | 14 | 1.3 (1.2–1.4) | 16 | 3.1 (3.0–3.2) | 17 | 1.9 (1.9–2.1) | 15 | 2.2 (2.1–2.2) | 16 | 3.2 | 15 | 1.3 | 17 | 3.43 | 8 | 0.48 | 8 |
| Other non-communicable diseases | 21.5 (21.4–21.6) | 10 | 28.1 (25.1–31.3) | 11 | 10.8 (10.0–11.7) | 9 | 29.2 (28.8–29.6) | 9 | 15.4 (15.0–15.8) | 8 | 21.7 (21.5–21.9) | 10 | 18.4 | 11 | 8 | 11 | 2.7 | 13 | 0.38 | 13 |
| Transport injuries | 13.5 (13.4–13.6) | 12 | 33.2 (30.1–36.6) | 10 | 6.1 (5.6–6.7) | 11 | 16.8 (16.5–17.2) | 12 | 12.6 (12.2–13.0) | 12 | 13.5 (13.3–13.7) | 12 | 27.1 | 9 | 10.1 | 9 | 5.45 | 4 | 0.62 | 4 |
| Unintentional injuries | 22.2 (22.0–22.4) | 8 | 34.6 (31.1–38.5) | 9 | 12.0 (11.4–12.6) | 8 | 19.9 (19.5–20.3) | 11 | 15.0 (14.5–15.4) | 8 | 23.7 (23.5–23.9) | 8 | 22.6 | 10 | 8.8 | 10 | 2.89 | 11 | 0.42 | 10 |
| Self-harm and interpersonal violence | 22.0 (21.8–22.2) | 9 | 42.1 (37.8–47.1) | 8 | 10.6 (9.6–11.8) | 10 | 32.1 (31.5–32.7) | 7 | 14.0 (13.5–14.4) | 10 | 22.4 (22.1–22.6) | 9 | 31.6 | 8 | 13 | 8 | 3.99 | 6 | 0.54 | 6 |
Age-standardised mortality rates are presented as estimate (95% Uncertainty Interval) and the units are deaths per 100 000 population.
The difference between the maximum rate and minimum rate (Max. – Min.) and the standard deviation (Std. Deviation) are both measures of absolute inequality and the units are deaths per 100 000 population. The ratio of the maximum rate to the minimum rate (Max. / Min.) and the coefficient of variation (Coef. of Variation) are both measures of relative inequality and are unitless. Italicised numbers are rankings among causes. AIAN = non-Latino and non-Hispanic American Indian or Alaska Native.
Geographic inequalities in cause-specific mortality at the county level
For every cause of death, mortality varied substantially among counties with unmasked estimates, both overall and within each racial–ethnic group. Figure 2 depicts the ratio of county to national age-standardised mortality rate for each cause of death for the total (all racial–ethnic groups combined) population. Areas depicted in increasingly dark shades of purple had lower mortality rates compared to the national average, whereas areas depicted in increasingly dark shades of orange had higher mortality rates compared to the national average. While there was considerable variation among counties for every cause of death, both the degree of variation as well as the specific spatial pattern varied by cause. Age-standardised all-cause mortality in 2019 was higher than the national rate in large swaths of the Southeast, South, Midwest, and Appalachia in addition to other locations throughout the country. Mortality was lower than the national rate in and around many urban centres throughout the country, as well as in some more rural counties in the northern half of the USA to the west of the Great Lakes. We observe a broadly similar pattern for many other causes, including most of the largest causes of death (eg, cardiovascular diseases, neoplasms). However, some causes have different and distinctive spatial patterns. For example, higher-than-average mortality rates due to HIV/AIDS and sexually transmitted infections are concentrated in counties located in states along the Gulf and Atlantic coasts from Texas to North Carolina and in many large metropolitan areas in other parts of the country. For substance use disorders, counties with the highest mortality rates are concentrated in Appalachia, although there are many other counties in the Eastern USA, Oklahoma, the Southwest, and parts of the Pacific coast including Alaska that also have relatively high mortality rates. There are also distinctive spatial patterns for transport injuries, whereby more urbanised areas have lower mortality rates than more rural areas. A similar pattern, though with less extreme differences, is present for unintentional injuries.
Figure 2: Estimated age-standardised mortality rate ratio compared with the national population in 2019 by cause and county.
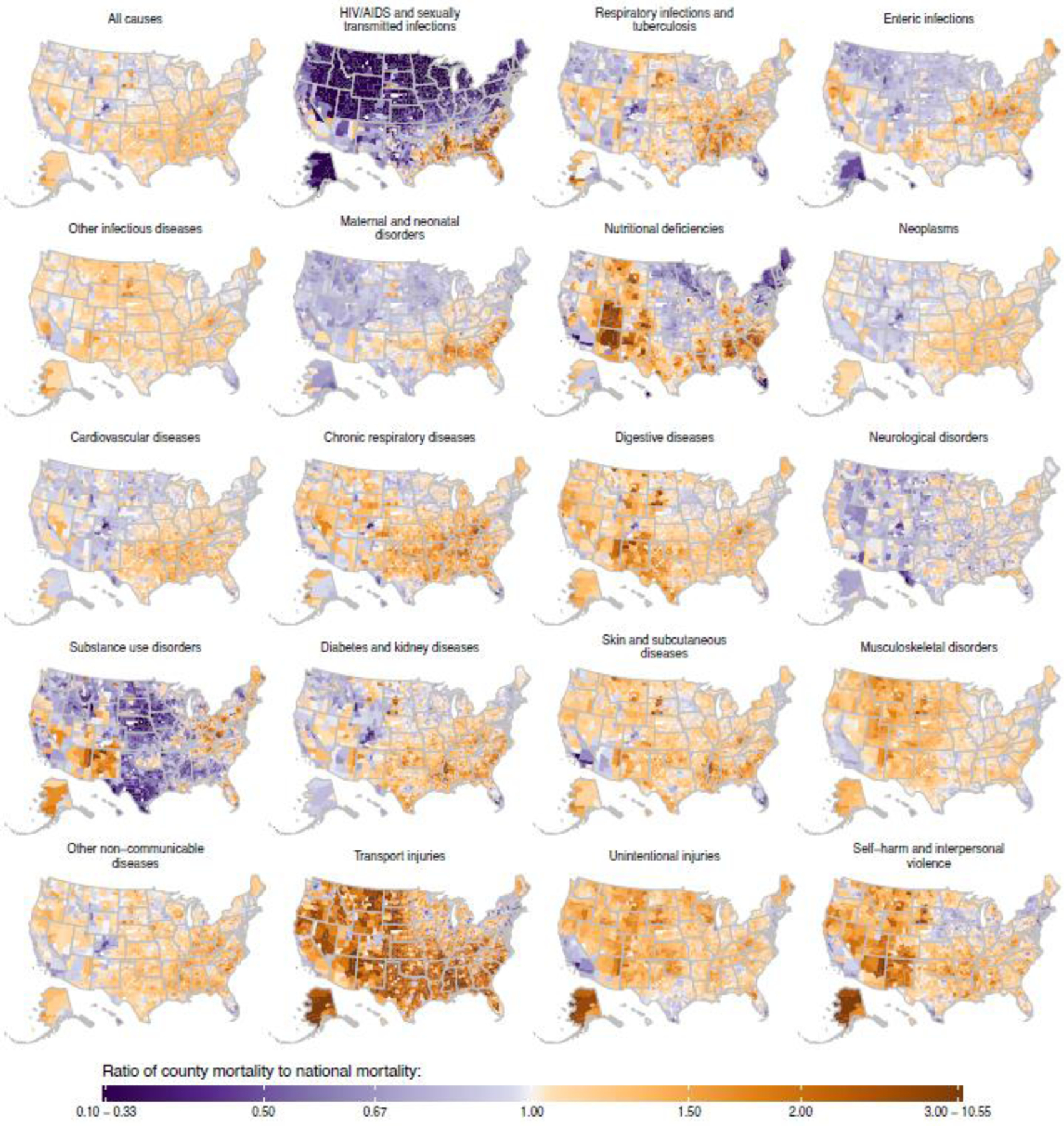
Estimates have been masked for county and racial–ethnic groups with a mean annual population fewer than 1000 people because model performance declined notably below this threshold.
Table 2 ranks causes in terms of geographic inequality in age-standardised mortality in 2019. As was the case for racial–ethnic inequality at the national level, causes with higher mortality rates also tended to have higher absolute geographic inequality. The rankings in terms of geographic inequality and racial–ethnic inequality, when measured in absolute terms, were consequently similar. The results with respect to relative inequality were more variable. Some top causes of racial–ethnic inequality at the national level were also top causes of geographic inequality—both overall and separately by racial–ethnic group—including HIV/AIDS and sexually transmitted infections, substance use disorders, and transport injuries. In contrast, several causes that ranked high in terms of relative racial–ethnic inequality at the national level ranked much lower in terms of relative geographic inequality (overall, and for some racial–ethnic groups separately), including digestive diseases and musculoskeletal disorders, whereas the opposite was true for other causes such as nutritional deficiencies and respiratory infections and tuberculosis.
Table 2:
Inequality in age-standardised mortality rates among counties in 2019.
| Cause of Death | Total | AIAN | Asian | Black | Latino | White | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std. Dev. | Coef. of Var. | Std. Dev. | Coef. of Var. | Std. Dev. | Coef. of Var. | Std. Dev. | Coef. of Var. | Std. Dev. | Coef. of Var. | Std. Dev | Coef. of Var. | |||||||||||||
| All causes | 153.3 | - | 0.17 | - | 412.3 | - | 0.39 | - | 92.2 | - | 0.20 | - | 185.4 | - | 0.19 | - | 157.6 | - | 0.29 | - | 155.4 | - | 0.18 | - |
| HIV/AIDS and sexually transmitted infections | 1.3 | 14 | 0.92 | 1 | 1.7 | 15 | 0.59 | 3 | 0.2 | 19 | 0.46 | 1 | 3.1 | 13 | 0.58 | 2 | 0.7 | 15 | 0.51 | 2 | 0.6 | 16 | 0.58 | 1 |
| Respiratory infections and tuberculosis | 6.3 | 10 | 0.31 | 5 | 10.3 | 11 | 0.41 | 12 | 2.6 | 11 | 0.24 | 12 | 6.5 | 11 | 0.30 | 6 | 3.9 | 12 | 0.31 | 13 | 6.6 | 10 | 0.33 | 5 |
| Enteric infections | 0.7 | 16 | 0.27 | 10 | 1.5 | 17 | 0.38 | 16 | 0.3 | 18 | 0.24 | 14 | 0.6 | 18 | 0.21 | 13 | 0.4 | 18 | 0.21 | 17 | 0.7 | 15 | 0.29 | 7 |
| Other infectious diseases | 0.3 | 19 | 0.14 | 19 | 1.1 | 19 | 0.37 | 17 | 0.3 | 17 | 0.22 | 16 | 0.3 | 19 | 0.15 | 19 | 0.3 | 19 | 0.19 | 19 | 0.3 | 19 | 0.14 | 19 |
| Maternal and neonatal disorders | 1.2 | 15 | 0.29 | 8 | 1.5 | 16 | 0.39 | 14 | 1.1 | 14 | 0.26 | 10 | 2 | 15 | 0.23 | 11 | 0.8 | 14 | 0.21 | 18 | 0.8 | 14 | 0.22 | 11 |
| Nutritional deficiencies | 2.1 | 13 | 0.48 | 3 | 2.2 | 13 | 0.52 | 5 | 1.3 | 13 | 0.45 | 2 | 2.2 | 14 | 0.40 | 3 | 1.6 | 13 | 0.48 | 3 | 2.1 | 13 | 0.50 | 3 |
| Neoplasms | 32.5 | 2 | 0.16 | 18 | 83.6 | 2 | 0.38 | 15 | 21.5 | 2 | 0.19 | 18 | 41.3 | 2 | 0.18 | 18 | 31.1 | 2 | 0.26 | 15 | 32.9 | 2 | 0.16 | 18 |
| Cardiovascular diseases | 55.2 | 1 | 0.21 | 13 | 121.3 | 1 | 0.44 | 9 | 32.3 | 1 | 0.23 | 15 | 72 | 1 | 0.23 | 10 | 51.7 | 1 | 0.34 | 10 | 55.6 | 1 | 0.21 | 13 |
| Chronic respiratory diseases | 20.4 | 3 | 0.28 | 9 | 35.2 | 6 | 0.44 | 10 | 5.7 | 5 | 0.25 | 11 | 13.1 | 6 | 0.23 | 9 | 10.2 | 7 | 0.34 | 11 | 22.7 | 3 | 0.29 | 6 |
| Digestive diseases | 10 | 7 | 0.24 | 11 | 43 | 4 | 0.51 | 7 | 5.3 | 6 | 0.29 | 9 | 7.6 | 9 | 0.21 | 14 | 14.6 | 5 | 0.43 | 5 | 9.2 | 7 | 0.23 | 10 |
| Neurological disorders | 14.6 | 5 | 0.21 | 15 | 23.4 | 7 | 0.40 | 13 | 6.9 | 4 | 0.18 | 19 | 14.3 | 5 | 0.22 | 12 | 16.5 | 4 | 0.36 | 8 | 15.6 | 4 | 0.21 | 12 |
| Substance use disorders | 12.3 | 6 | 0.53 | 2 | 35.7 | 5 | 0.63 | 1 | 2.3 | 12 | 0.44 | 3 | 15.3 | 4 | 0.60 | 1 | 10.7 | 6 | 0.68 | 1 | 13.1 | 6 | 0.54 | 2 |
| Diabetes and kidney diseases | 18.9 | 4 | 0.30 | 7 | 56.9 | 3 | 0.58 | 4 | 12.2 | 3 | 0.30 | 7 | 28.6 | 3 | 0.27 | 8 | 20.7 | 3 | 0.40 | 6 | 15.5 | 5 | 0.26 | 8 |
| Skin and subcutaneous diseases | 0.5 | 17 | 0.23 | 12 | 1.5 | 18 | 0.36 | 18 | 0.4 | 16 | 0.37 | 5 | 1 | 16 | 0.28 | 7 | 0.6 | 17 | 0.35 | 9 | 0.4 | 18 | 0.18 | 17 |
| Musculoskeletal disorders | 0.5 | 18 | 0.18 | 17 | 2 | 14 | 0.43 | 11 | 0.5 | 15 | 0.30 | 8 | 0.6 | 17 | 0.19 | 17 | 0.7 | 16 | 0.32 | 12 | 0.5 | 17 | 0.18 | 16 |
| Other non-communicable diseases | 5.2 | 12 | 0.20 | 16 | 9.7 | 12 | 0.35 | 19 | 2.9 | 10 | 0.24 | 13 | 6.5 | 10 | 0.21 | 15 | 4 | 11 | 0.26 | 14 | 5.1 | 12 | 0.20 | 14 |
| Transport injuries | 8.8 | 8 | 0.39 | 4 | 18.2 | 9 | 0.62 | 2 | 3.1 | 9 | 0.39 | 4 | 9 | 8 | 0.40 | 4 | 7.6 | 8 | 0.44 | 4 | 8.4 | 8 | 0.38 | 4 |
| Unintentional injuries | 5.7 | 11 | 0.21 | 14 | 15.3 | 10 | 0.46 | 8 | 3.1 | 9 | 0.21 | 17 | 4.6 | 12 | 0.20 | 16 | 4.7 | 10 | 0.25 | 16 | 5.3 | 11 | 0.19 | 15 |
| Self-harm and interpersonal violence | 8.1 | 9 | 0.31 | 6 | 20.4 | 8 | 0.52 | 6 | 4.1 | 7 | 0.34 | 6 | 9.8 | 7 | 0.35 | 5 | 6 | 9 | 0.38 | 7 | 6.8 | 9 | 0.26 | 9 |
The standard deviation (Std. Dev.) is a measure of absolute inequality and the units are deaths per 100 000 population. The coefficient of variation (Coef. of Var.) is a measure of relative inequality and is unitless. Italicised numbers are the rankings among causes. AIAN = non-Latino and non-Hispanic American Indian or Alaska Native.
Intersection of racial–ethnic and geographic inequalities in cause-specific mortality at the county level
Detailed maps depicting age-standardised mortality by county and racial–ethnic group for each cause are available in the appendix (pp 109–188) and results for all causes are also available in an online visualisation tool (https://vizhub.healthdata.org/subnational/usa). Figure 3 summarises this information for 2019, showing age-standardised mortality rates among counties with unmasked estimates for each racial–ethnic group and by cause of death. For every cause of death, there was considerable variation in mortality within each racial–ethnic group. The differences in mortality among racial–ethnic groups observed at the national level are also evident at the county level, however due to the variation in mortality within each racial–ethnic group, there is nearly always some overlap in mortality when comparing across counties—eg, for HIV/AIDS and sexually transmitted infections, the distribution of mortality rates for the Black population is clearly shifted towards higher mortality rates compared to the White population, but the lowest mortality rates observed for the Black population are nonetheless substantially lower than the highest mortality rates observed for the White population. To further illustrate this point and as an example of the complex interactions between patterns of racial–ethnic and geographic disparities in mortality, Figures 4, 5, and 6 show age-standardised mortality due to HIV/AIDS and sexually transmitted infections, maternal and neonatal disorders, and chronic respiratory diseases, respectively, by county and racial–ethnic group. These maps underscore the large degree of variation in age-standardised mortality among counties, both across and within racial–ethnic groups. They also highlight the sometimes different patterns observed for different causes of death.
Figure 3: Estimated age-standardised mortality in 2019 by cause, county, and racial–ethnic group.
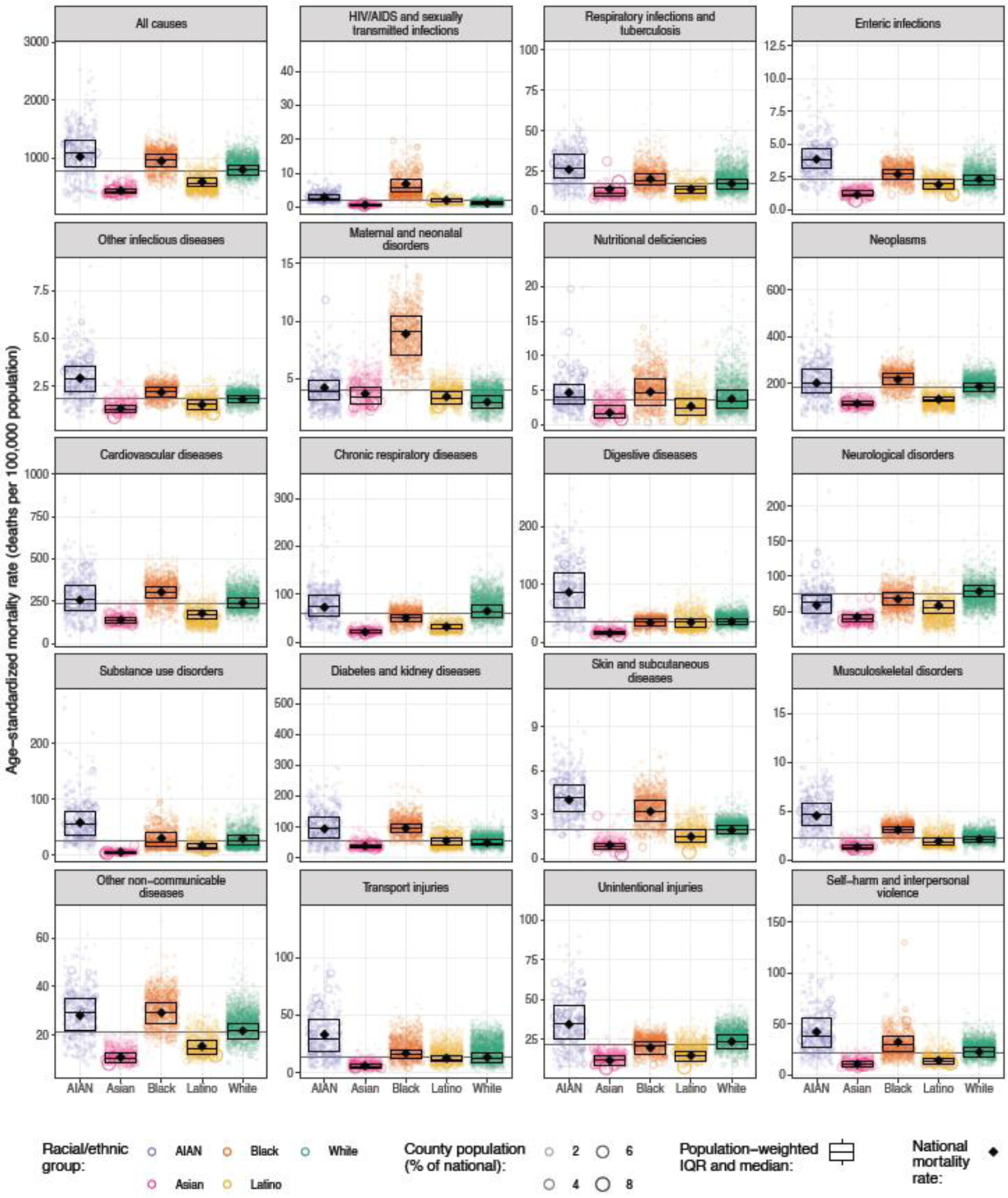
Each circle corresponds to one county, and the size of the circle is proportional to the population of a given racial–ethnic group in that county. The boxes indicate the population-weighted median and interquartile range of the county-level mortality rates—ie, a quarter of the population lives in counties where mortality is lower than the level indicated by the bottom of the box, another quarter of the population lives in counties where mortality is between the level indicated by the bottom of the box and the level indicated by the middle bar, and so on. The diamond indicates the national mortality rate. Estimates have been masked for county and racial–ethnic groups with a mean annual population fewer than 1000 people because model performance declined notably below this threshold.
Figure 4: Estimated age-standardised mortality due to HIV/AIDS and sexually transmitted infections in 2019 by county and racial–ethnic group.
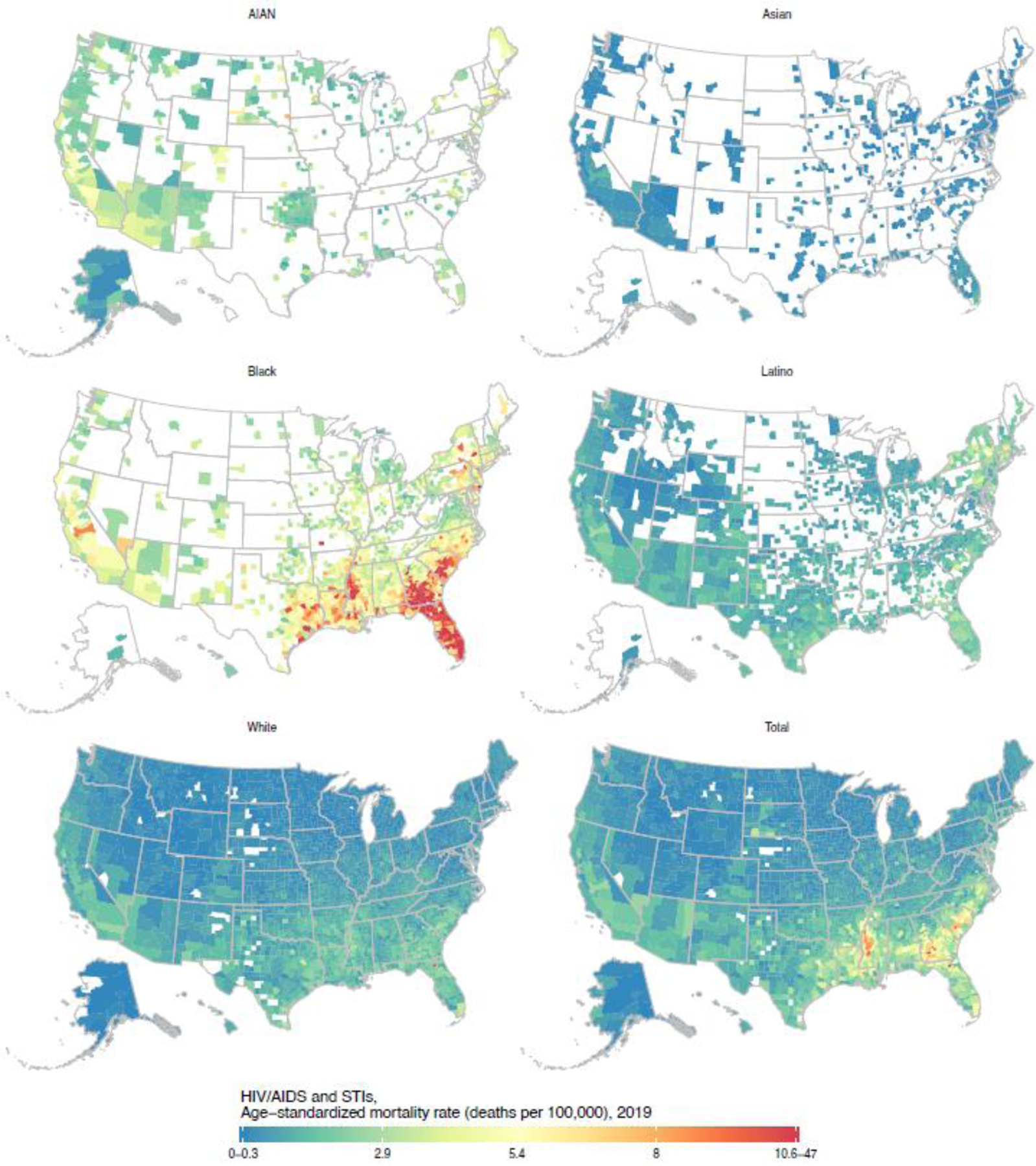
Estimates have been masked for county and racial–ethnic groups with a mean annual population fewer than 1000 people because model performance declined notably below this threshold.
Figure 5: Estimated age-standardised mortality due to maternal and neonatal disorders in 2019 by county and racial–ethnic group.
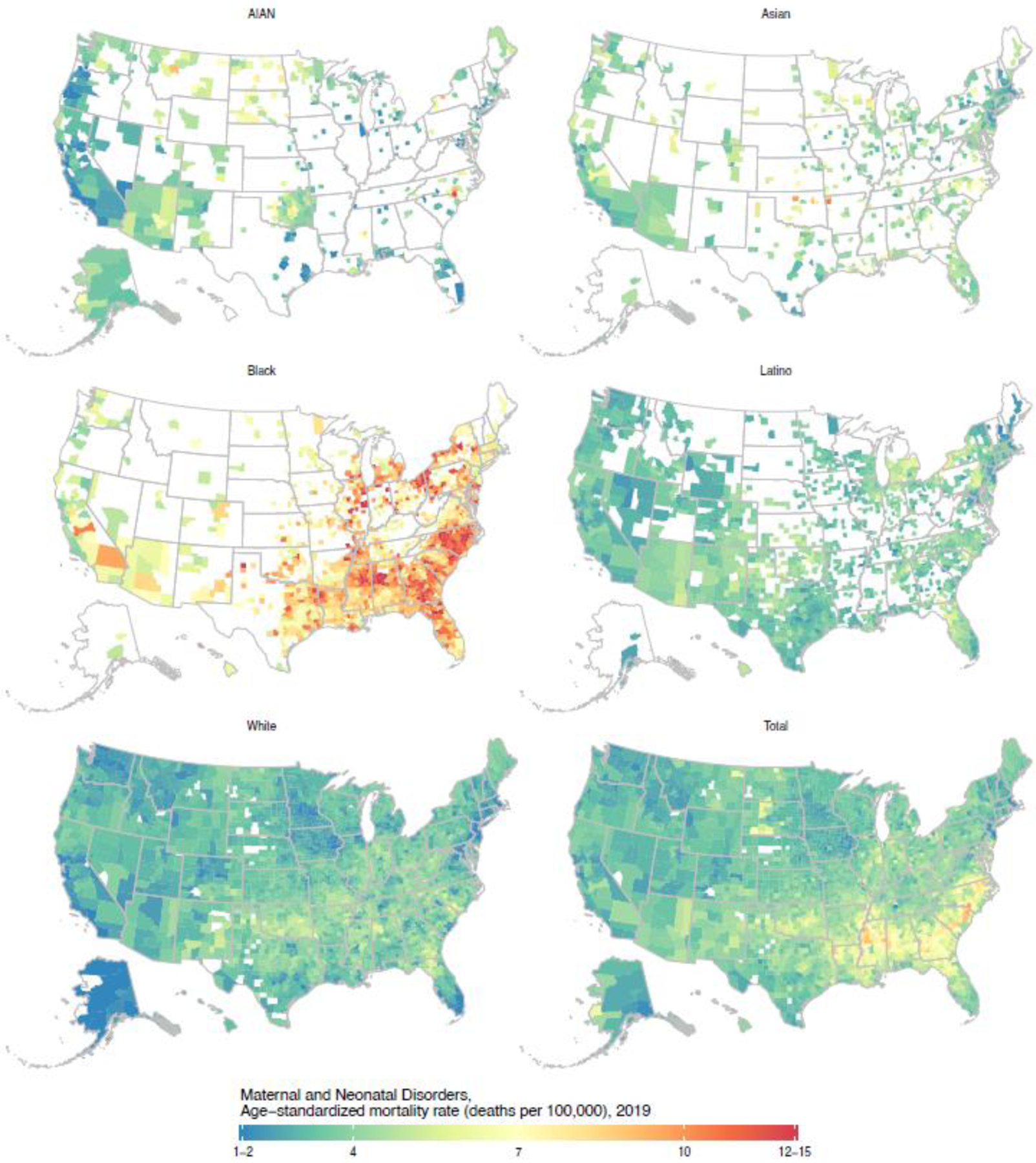
Estimates have been masked for county and racial–ethnic groups with a mean annual population fewer than 1000 people because model performance declined notably below this threshold.
Figure 6: Estimated age-standardised mortality due to chronic respiratory diseases in 2019 by county and racial–ethnic group.
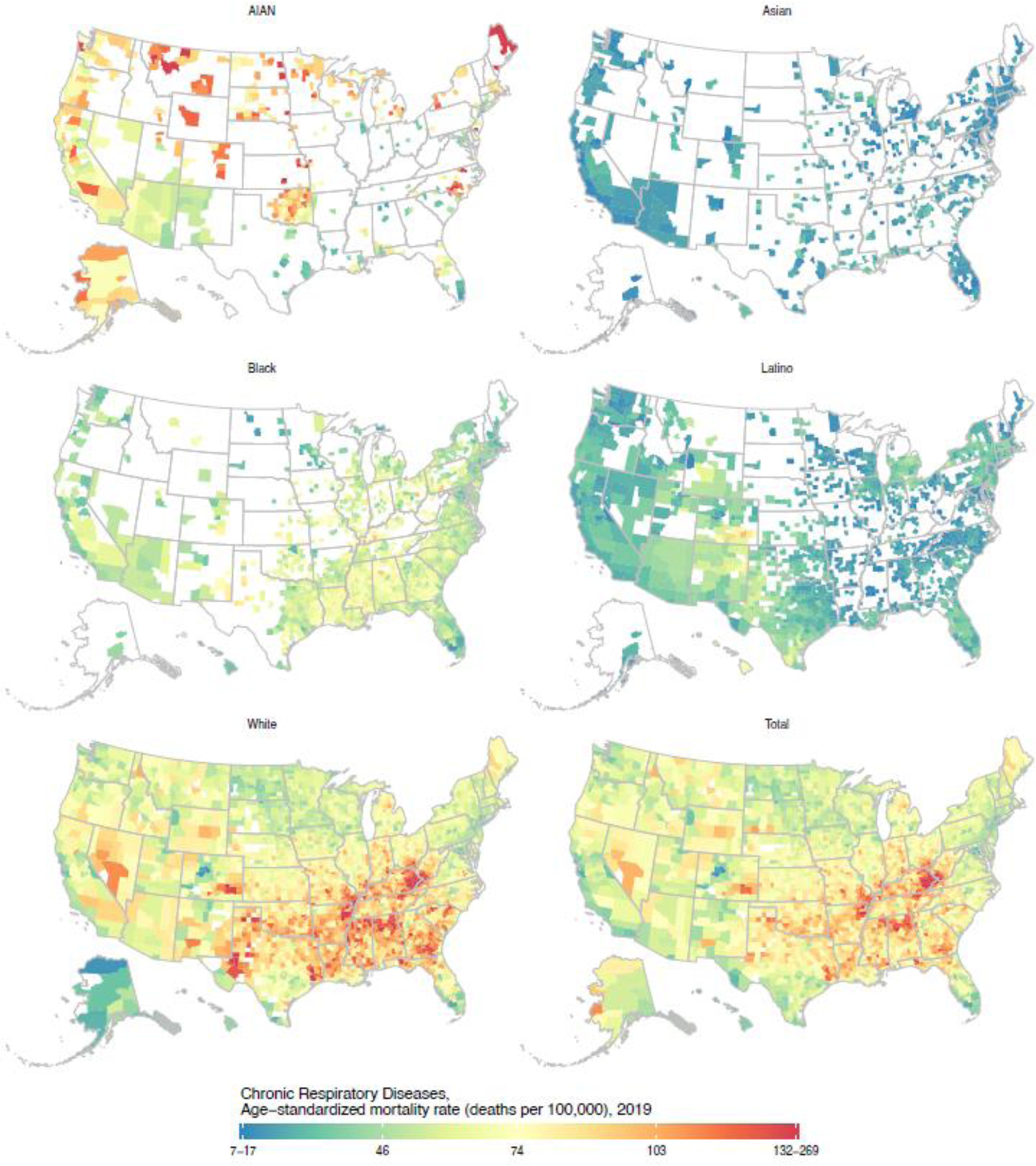
Estimates have been masked for county and racial–ethnic groups with a mean annual population fewer than 1000 people because model performance declined notably below this threshold.
Although county-level mortality rates are generally positively correlated across racial–ethnic groups—ie, a county that has relatively high mortality for one group typically has relatively high mortality for others as well—the strength of these correlations is often moderate (appendix p 189), which leads to substantial variation in the degree of racial–ethnic disparity across counties. Racial–ethnic disparity among all groups is difficult to quantify at the county level, as unmasked estimates for all groups are not available in most counties, so for this purpose we instead consider the mortality rate ratio for each racial–ethnic group relative to the White population (the majority group nationally and in most counties) living in the same county. Figure 7 shows these mortality rate ratios for counties with unmasked estimates for each racial–ethnic group and by cause of death (maps of these mortality rate ratios for each cause of death are available in the appendix, pp 109–188). These mortality rate ratios vary substantially at the county level, and in some counties are dramatically larger than what is observed at the national level. For any given cause and racial–ethnic group, a large majority of counties typically have mortality rate ratios that are in the same direction (ie, greater than 1 or less than 1) as what is observed at the national level, however there are usually also a minority of counties where the rate ratio is reversed compared to the national level. Several causes are notable for their consistency: mortality is nearly universally higher among the AIAN population compared to the White population for skin and subcutaneous diseases (97.8% [455 counties] of 465 counties with unmasked estimates; statistically significant in 69.9% [325 counties]) and HIV/AIDS and sexually transmitted infections (98.5% [458]; statistically significant in 67.3% [313]). Similarly, mortality is nearly universally higher among the Black population compared to the White population for skin and subcutaneous diseases (96.6% [1436] of 1486 counties with unmasked estimates; statistically significant in 78.9% [1172]), diabetes and kidney diseases (99.1% [1473]; statistically significant in 93.3% [1386], maternal and neonatal disorders (100% [1486]; statistically significant in 96.6% [1436]), and HIV/AIDS and sexually transmitted infections (100% [1486]; statistically significant in 99.3% [1476]). In the other direction, mortality is lower for the Asian population compared to the White population in 95% or more of counties with unmasked estimates for all causes combined (99.7% [665] of 667; statistically significant in 97.6% [651]) and for 13 individual causes (respiratory infections and tuberculosis, transport injuries, cardiovascular diseases, digestive diseases, skin and subcutaneous diseases, enteric infections, other non-communicable diseases, unintentional injuries, self-harm and interpersonal violence, neoplasms, neurological disorders, chronic respiratory diseases, and substance use disorders). The same is observed for the Latino population compared to the White population for four individual causes: cardiovascular diseases, neoplasms, self-harm and interpersonal violence, and chronic respiratory diseases.
Figure 7: Estimated age-standardised mortality rate ratio compared with the White population residing in the same county in 2019 by cause, county, and racial–ethnic group.
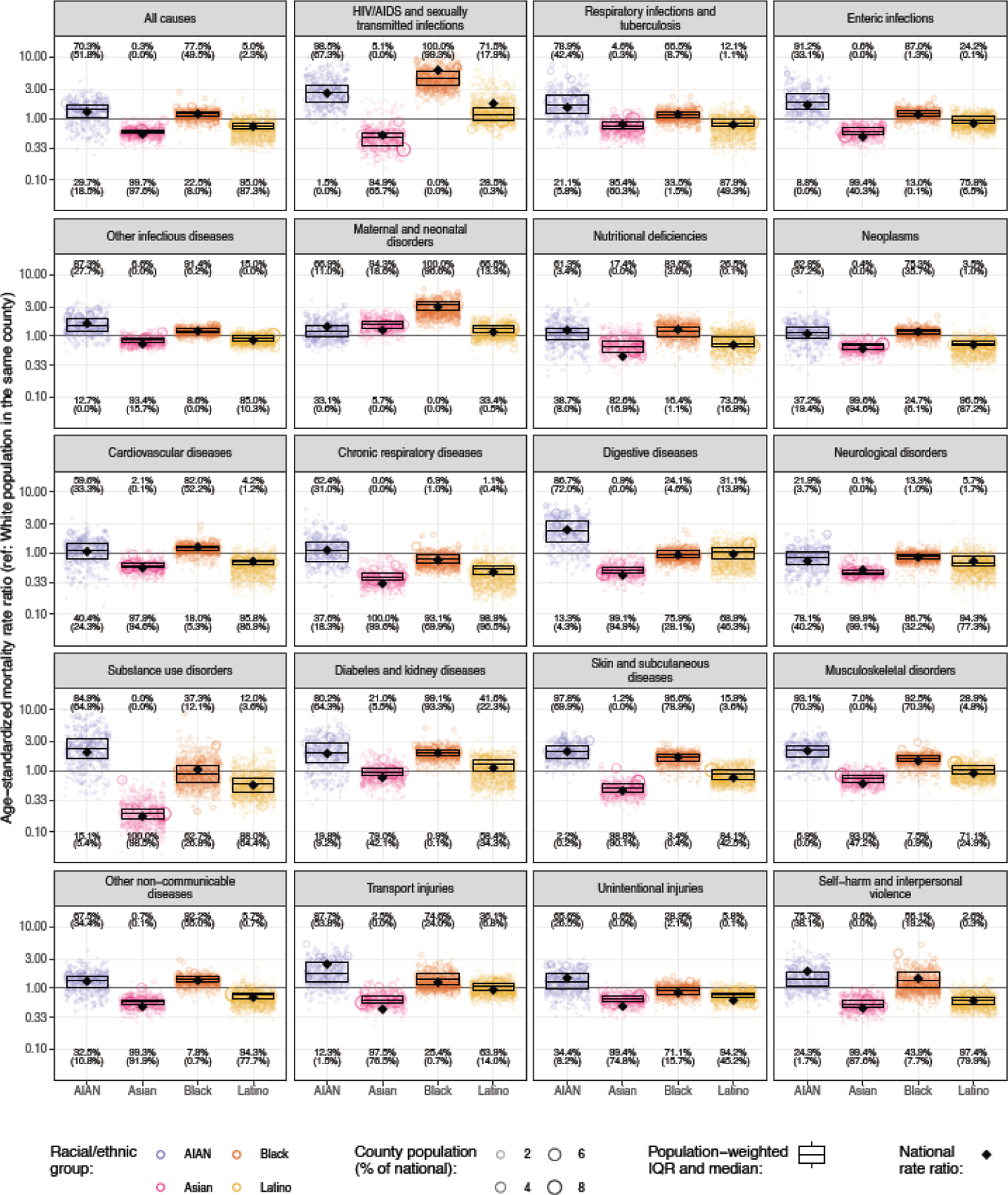
Each circle corresponds to one county, and the size of the circle is proportional to the population of a given racial–ethnic group in that county. The boxes indicate the population-weighted median and interquartile range of the county-level mortality rate ratios—ie, a quarter of the population lives in counties where the mortality rate ratio is lower than the level indicated by the bottom of the box, another quarter of the population lives in counties where the mortality rate ratio is between the level indicated by the bottom of the box and the level indicated by the middle bar, and so on. The diamond indicates the national mortality rate. Numbers at the top of each panel indicate the percentage of counties with unmasked estimates where the mortality rate ratio is >1, with the percentage where this is statistically significant shown in parentheses. Similarly, numbers at the bottom of each panel indicate the percentages where the rate ratio is <1. Estimates have been masked for county and racial–ethnic groups with a mean annual population fewer than 1000 people because model performance declined notably below this threshold.
For some causes and racial–ethnic groups, the degree of racial–ethnic disparity—as measured by the rate ratio with the White population as the reference group—is systematically different at the county level than at the national level. For example, the national rate ratio for Black compared to White populations for HIV/AIDS and sexually transmitted infections is 6.2 (95% UI 6.0–6.4), but the population-weighted median rate ratio among counties was 4.5 (IQR 3.4–5.9), and 77.8% of the Black population (33.9 million people) lives in counties where the rate ratio was less than that observed at the national level. This apparent discrepancy occurs because a much higher proportion of the Black population than the White population lives in regions such as the Southeast where mortality due to HIV/AIDS and sexually transmitted infections is relatively high for both groups, and thus the national mortality rate for the Black population is weighted more heavily toward the high rates in these regions, further exacerbating at the aggregate level the disparities observed locally. There are also examples of this phenomenon operating in the opposite direction. For example, the national rate ratio for Asian compared to White populations for maternal and neonatal disorders is 1.2 (95% UI 0.9–1.7), but the population-weighted median rate ratio among counties was 1.5 (IQR 1.4–1.7), and 84.7% of the Asian population (17.7 million people) lives in counties where the rate ratio was higher than that observed at the national level. Thus, in this case, the rate ratio observed at the national level tends to understate the degree of disparity observed locally.
Discussion
In this study, we present estimates of mortality for 19 causes of death by county and racial–ethnic group from 2000 to 2019. These estimates provide a far more complete and detailed view than previously available of racial–ethnic and geographic inequalities in mortality for a nearly exhaustive set of causes of death. We find that racial–ethnic disparities in mortality are ubiquitous, occurring across a range of causes of death and across locations in the USA. At the same time, our results demonstrate that there is remarkable heterogeneity in mortality by cause, by racial–ethnic group, by location, and over time. Collectively, these results underscore the pressing need to address widespread disparities in mortality and longevity in the USA, as well the importance of detailed local data for informing efforts to reduce or eliminate these disparities.
Across 19 causes of death, 3110 counties, and five racial–ethnic groups, our study reveals certain repeated patterns, especially with respect to racial–ethnic disparities in mortality. For nearly all of the causes considered in this analysis, the AIAN and Black populations experienced substantially higher mortality rates than the White population nationally. The same is true in most counties, although the magnitude of disparity typically varies substantially. This repeated pattern of racial–ethnic disparities across causes of death and across locations strongly suggests shared root causes.28 An extensive body of evidence links systemic racism to poor health and increased risk of early death,29,30 in large part via the impact of systemic racism on minoritised individuals’ and populations’ socioeconomic status,28,30 but also via other pathways including residential segregation,31 mass incarceration,32 chronic stress,33,34 and discrimination in health care,35,36 among others. Given these multiple pathways, the magnitude of the impact of systemic racism on health and longevity likely varies by cause of death, by local context, and by affected population group, which may explain some of the variation we observed in this study in the magnitude of racial–ethnic disparities across causes and locations. The estimates that we present here may be useful for future research aimed at better understanding the impact of systemic racism in a local context over time, and to support developing strategies to mitigate the resulting and persistent harms. However, mitigating the effects of systemic racism can only go so far, and dismantling systemic racism will ultimately be required to eliminate racial–ethnic disparities in mortality.28.37 To this end, there is a pressing need for better measurement of various domains of racism. Research in this area is ongoing, however data availability is a substantial challenge, and domains of racism that are more easily quantified using readily available data sources—such as residential segregation, which can be quantified using census data38—are the most thoroughly studied.39,40 Thus, it is critical that future research focus on data collection and operationalisation of measures of various forms of racism that identify mechanisms that increase risk of poor health outcomes and premature mortality, as well as salient intervention points.
Mortality for the Asian and Latino populations, both nationally and in many counties, is lower than for the White population for most causes of death. The reasons for this finding are complex, and not fully understood, but previous research has highlighted the central role of migration in explaining these differences.41,42 Approximately two-thirds of the Asian population and one-third of the Latino population residing in the USA was born outside of the USA, compared to 13.7% of all Americans (all racial–ethnic groups combined).43 Explanations for lower mortality rates among foreign-born individuals include positive selection for emigration (ie, individuals in good health are more likely to emigrate than those in poor health)41,42 and differences in certain health risk factors between foreign-born and USA-born individuals (eg, lower cigarette smoking prevalence among foreign-born individuals).44 It is also possible that some foreign-born individuals who experience a decline in health may return to their country of origin prior to death and thus not be included in USA mortality statistics; however, research focused on the Latino population has found that this phenomenon only accounts for a small part of the mortality difference between Latino and White populations.45 The generally lower mortality rates observed for Asian and Latino populations should not be construed as indicating that these two populations do not experience or are not harmed by racism, as there is plentiful evidence to the contrary,46,47 although the impacts of racism and particularly structural or institutional forms of racism are understudied for these populations.39,48 Other factors, including the mortality advantage observed among foreign-born individuals, may offset the negative impact of systemic racism to a degree, which could explain why these populations have generally lower mortality rates despite being impacted by systemic racism. Moreover, previous research has highlighted important differences in mortality within the Asian and Latino groups—for example, within the Latino population by racial identity3 and within both groups by national origin.49–52 Our study similarly demonstrates that there is considerable variation across counties in mortality within the Asian and Latino groups, and that the mortality rate for each of these populations compares less favourably to that among the White population in certain locations for select causes. This spatial variation is likely related to these other differences that have been noted among populations within the Asian and Latino groups. For instance, NHPI populations are known to have higher mortality and worse health outcomes than Asian populations across a range of health conditions;49,51,53,54 it is consequently unsurprising that in this study, we find that counties in Hawaii—where the size of the NHPI population relative to the Asian population is much higher than in the USA overall—often have relatively high mortality for the combined Asian and NHPI population, such that for many causes, mortality is higher for this population than for the White population, in contrast to the national pattern.
While there are some repeated patterns of racial–ethnic and geographic disparity observed across causes of death, the differences are equally striking, and underscore the need to consider mortality by cause in addition to measures of all-cause mortality such as life expectancy. Similarly, the large degree of variation among counties in overall mortality and in racial–ethnic disparity highlights the utility of considering these trends at a local level. These differences are particularly important for the purposes of planning interventions to reduce mortality and to reduce racial–ethnic disparities, since the relative importance of different causes of death, and the room for improvement in terms of reducing any given cause of death, clearly varies by county and among racial–ethnic groups. Thus, local, detailed, cause-specific data such as the estimates presented here can be used to identify for a particular population and location the specific causes where there is the greatest need for intervention, or the biggest opportunities for improvements in achieving health equity. Similarly, for any given cause of death, these data can be used to identify the populations that are most negatively impacted and the factors leading to these disproportionately negative effects, and to design and implement interventions and programmes with these populations in mind. Retrospectively, these types of data may also be useful for evaluating the effectiveness of programmes or policies in terms of both their impact on mortality overall, as well as their impact on disparities in mortality. In this study we focused on 19 relatively broad causes of death; however, the framework we developed is readily extended to more detailed causes of death, and we intend to use this approach to produce estimates for additional, more detailed causes. We hope that these even more detailed cause-specific estimates will be useful for researchers and policymakers interested in particular causes of death.
This analysis is subject to several limitations. First, the underlying deaths, population, and covariates data for this analysis are subject to error, which may in turn lead to errors in the estimated mortality rates. We implemented adjustments for two prominent sources of bias in the death certificate data—namely misclassification of racial–ethnic identity (relative to self-report) and the use of “garbage codes” for underlying cause of death—however, these adjustments rely on several assumptions and generalisations, which are difficult to validate, and violation of these assumptions may lead to errors in the resulting estimates. With additional research, it may be possible to further refine and improve the methods for addressing these biases; however, fully eliminating the error and uncertainty related to these biases requires addressing the problem at its source and thus improving the reliability of data collected on death certificates is critically important. Second, the small area estimation model that we used for this analysis was validated using an empirical validation framework and shown to perform well for populations as small as 1000 people. However, this validation framework relies on data from large populations as a “gold standard,” and consequently White populations and more urban areas are over-represented, whereas other racial–ethnic groups (particularly AIAN and Asian) as well as rural areas are under-represented, limiting the generalisability of the results of the validation exercise. Third, this small area model smooths over space, time, age group, and racial–ethnic group. While this smoothing allows us to produce more reliable estimates in general, there may be particular cases where this smoothing is inappropriate and leads to errors. For example, this is likely the case when there are sudden, temporary increases in mortality, such as caused by natural disasters. Fourth, the estimated mortality rates are associated with uncertainty, as indicated by the 95% uncertainty intervals. This uncertainty is typically greater for counties and racial–ethnic groups with smaller populations and also for racial–ethnic groups where the adjustment required for racial–ethnic misclassification on death certificates is larger and more uncertain. Consequently, estimates for the White population are typically the most certain, and estimates for other groups, especially the AIAN population, are frequently less certain. Additionally, because we mask estimates based on population size, estimates are unavailable for a larger number of counties for the AIAN, Asian, Black, and Latino populations compared to the White population. Fifth, although this study uses a larger set of racial–ethnic groups than is typical for subnational analyses, these groups are still relatively broad and there is considerable heterogeneity within each group. Due to data constraints, we produced estimates for a combined Asian and Pacific Islander group (labelled “Asian” in this analysis), rather than separate estimates for Asian and for NHPI populations. Studies that report separately for these groups generally find substantially poorer health outcomes among NHPI populations compared to Asian populations.49,51,53,54 Since the Asian population is much larger than the Pacific Islander population overall in the USA and in nearly all counties, our estimates for the combined group primarily reflect outcomes in the Asian population and likely conceal worse outcomes for the NHPI population. Additionally, only “primary” race was considered, despite a growing population of individuals in the USA who identify as more than one race.55 We are actively working on developing methods for addressing the data constraints noted here—in particular, estimating misclassification for NHPI and multiracial populations—with the goal of producing separate estimates for NHPI and multiracial populations in future analyses. Sixth, the estimates presented here are cross-sectional measures that reflect the population living in a given county in a given year. Migration between counties in the USA is relatively common, and so exposures in one county could impact later mortality in another county, which in turn may have an impact on trends in geographic disparities in mortality. Finally, this analysis covers the 20-year period preceding the COVID-19 pandemic, and patterns of racial–ethnic and geographic inequalities in mortality have likely changed substantially in some cases as a result.
Our study also has a number of unique strengths. For most individual causes of death, the estimates reported here are far more detailed than what has previously been available, allowing inspection of both racial–ethnic and geographic inequalities in mortality, and the intersection thereof. Moreover, since we applied a consistent methodology across a nearly exhaustive set of causes of death, the results are fully comparable across causes and internally consistent. We believe this detail, comprehensiveness, and comparability make these estimates far more useful to a range of audiences, including for future research. Additionally, the 20-year time period of this analysis provides unique opportunities to assess trends in mortality and disparities at a local level. Although this time period is entirely before the COVID-19 pandemic, which has indelibly altered the landscape of health and longevity in the USA, an understanding of how patterns of mortality and disparities have changed—or, in some cases, not changed—over this two-decade span provides important context for future efforts to reduce mortality and eliminate disparities.
When it comes to mortality in the USA, inequality is the rule, not the exception. For every cause of death, mortality is much higher in some racial–ethnic groups and in some locations than in others, representing substantial preventable loss of life. Revealing the substantial overlap of racial–ethnic and geographical inequalities allows for identification of local population groups and areas at highest risk that can be prioritised for targeted public health and health care interventions. Ultimately, examining and addressing the shared underlying social, structural, and environmental factors, as well the factors that are specific to each cause of death which lead to these differences in mortality among different populations, is crucial for reducing and ultimately eliminating inequalities in mortality and longevity in the USA.
Supplementary Material
Research in Context.
Evidence before this study
Large, persistent differences in mortality among racial–ethnic groups in the USA have been observed, with American Indian or Alaska Native and Black populations generally experiencing higher mortality rates, and Asian and Latino populations generally experiencing lower mortality rates compared to the White population. Large geographic differences in mortality have also been noted, including at the regional, state, and county level. The exact magnitude and pattern of racial–ethnic or geographic disparity in mortality varies by health condition and can change over time.
Added value of this study
We estimated age-standardised mortality by year, county, and racial–ethnic group (non-Latino and non-Hispanic American Indian or Alaska Native [AIAN], non-Latino and non-Hispanic Asian or Pacific Islander [Asian], non-Latino and non-Hispanic Black [Black], Latino or Hispanic [Latino], and non-Latino and non-Hispanic White [White]) in the USA from 2000 to 2019 for 19 causes of death that collectively account for 99.77% of all deaths in 2019 in the USA. Many of the causes of death in this study are presented for the first in a time-series analysis of racial–ethnic disparities in mortality at the county level. This is also the first county-level time-series analysis of racial–ethnic disparities in mortality to consider a nearly exhaustive set of causes of death, and to incorporate corrections for misreporting of racial and ethnic identity on death certificates. These estimates make it possible to examine geographic variation in racial–ethnic disparities in mortality in unprecedented detail, and to make comparisons across different health conditions. These data are also made available publicly to enable additional research. Finally, the estimation framework developed here can be further extended to more detailed causes of death.
Implications of all available evidence
Racial–ethnic disparities in mortality are ubiquitous, occurring across causes of death, and across locations within the USA. For most causes, and in most locations, mortality is substantially higher for the AIAN and Black populations, and lower for the Asian and Latino populations, compared to the White population, although there are exceptions for some causes and locations. In some cases, racial–ethnic disparities in mortality are essentially universal among counties—eg, mortality was higher for the AIAN population than the White population in nearly all counties for skin and subcutaneous diseases and HIV/AIDS and sexually transmitted infections, while mortality was higher among the Black population than the White population in nearly all counties for diabetes and kidney diseases, maternal and neonatal disorders, and HIV/AIDS and sexually transmitted infections. The consistency of these patterns strongly suggests shared root causes and highlights the widespread, persistent, and substantial negative impact of systemic racism on health. This consistency notwithstanding, there is remarkable variation in both the magnitude of mortality and the degree of racial–ethnic disparity in mortality by cause, by county, and over time. This heterogeneity underscores the need for detailed, local, and timely data that can be used to identify the most pressing needs for specific communities and support plans of action to meet these needs. Moreover, detailed cause-specific estimates of racial–ethnic and geographic disparities in mortality provide an opportunity to better understand the underlying drivers of these disparities, such as systemic racism and social determinants of health, and how these drivers may function differently for different causes of death and by location. It is crucial that we use every tool available to reduce unnecessary loss of life, and ultimately eliminate all kinds of health inequities.
Acknowledgments
The views expressed are those of the authors and should not be construed to represent those of the US National Institutes of Health (NIH) or the federal government. This study was funded by the NIH’s National Institute on Minority Health and Health Disparities, National Heart, Lung, and Blood Institute, National Cancer Institute, National Institute on Aging, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Office of Disease Prevention, and Office of Behavioral and Social Science Research (contract 75N94019C00016).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
We declare no competing interests.
Data sharing
Estimates of mortality by cause, county, racial–ethnic group, year, age, and sex are available for download from the Global Health Data Exchange (https://ghdx.healthdata.org/record/ihme-data/united-states-causes-death-life-expectancy-by-county-race-ethnicity-2000-2019) and via a user-friendly data visualisation: https://vizhub.healthdata.org/subnational/usa. Information about the underlying data sources is available in the appendix (pp 33, 50–52). The code used for this analysis is available on GitHub: https://github.com/ihmeuw/USHD.
References
- 1.Du Bois WEB. The Philadelphia Negro: a social study. Philadelphia: University of Pennsylvania Press, 1899. [Google Scholar]
- 2.Harper S, MacLehose RF, Kaufman JS. Trends in the black-white life expectancy gap among US states, 1990–2009. Health Aff (Millwood) 2014; 33: 1375–82. [DOI] [PubMed] [Google Scholar]
- 3.Arias E, Johnson NJ, Vera BT. Racial disparities in mortality in the adult Hispanic population. SSM - Popul Health 2020; 11: 100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arias E, Xu J, Curtin S, Bastian B, Tejada-Vera B. Mortality profile of the non-Hispanic American Indian or Alaska Native population, 2019. Natl Vital Stat Rep 2021; 70. DOI: 10.15620/cdc:110370. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer-Lindgren L, Kendrick P, Kelly YO, et al. Life expectancy by county, race, and ethnicity in the USA, 2000–19: a systematic analysis of health disparities. The Lancet 2022; 400: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Murphy SL, Kochanek KD, Arias E. Deaths: final data for 2019. Natl Vital Stat Rep 2021; 70. [PubMed] [Google Scholar]
- 7.Howard G, Peace F, Howard VJ. The contributions of selected diseases to disparities in death rates and years of life lost for racial/ethnic minorities in the United States, 1999–2010. Prev Chronic Dis 2014; 11. DOI: 10.5888/pcd11.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossen LM, Khan D, Warner M. Hot spots in mortality from drug poisoning in the United States, 2007–2009. Health Place 2014; 26: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. US county-level trends in mortality rates for major causes of death, 1980–2014. JAMA 2016; 316: 2385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dwyer-Lindgren L, Stubbs RW, Bertozzi-Villa A, et al. Variation in life expectancy and mortality by cause among neighbourhoods in King County, WA, USA, 1990–2014: a census tract-level analysis for the Global Burden of Disease Study 2015. Lancet Public Health 2017; 2: e400–10. [DOI] [PubMed] [Google Scholar]
- 11.Rossen LM, Hedegaard H, Khan D, Warner M. County-level trends in suicide rates in the U.S., 2005–2015. Am J Prev Med 2018; 55: 72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughan AS, Flynn A, Casper M. The where of when: geographic variation in the timing of recent increases in US county-level heart disease death rates. Ann Epidemiol 2022; 72: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickle LW, Mungiole M, Jones GK, White AA. Atlas of United States mortality. Hyattsville, MD: National Center for Health Statistics, 1996. http://www.cdc.gov/nchs/products/other/atlas/atlas.htm (accessed May 10, 2016). [Google Scholar]
- 14.Chien L-C, Yu H-L, Schootman M Efficient mapping and geographic disparities in breast cancer mortality at the county-level by race and age in the U.S. Spat Spatio-Temporal Epidemiol 2013; 0: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rust G, Zhang S, Yu Z, et al. Counties eliminating racial disparities in colorectal cancer mortality. Cancer 2016. DOI: 10.1002/cncr.29958. [DOI] [PubMed] [Google Scholar]
- 16.Vaughan AS, Coronado F, Casper M, Loustalot F, Wright JS. County‐level trends in hypertension‐related cardiovascular disease mortality—United States, 2000 to 2019. J Am Heart Assoc 2022; 11: e024785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arias E, Heron M, Hakes J. The validity of race and Hispanic-origin reporting on death certificates in the United States: an update. Vital Health Stat 2016; 2. https://www.cdc.gov/nchs/data/series/sr_02/sr02_172.pdf. [PubMed] [Google Scholar]
- 18.Office of Management and Budget. Race and ethnic standards for federal statistics and administrative reporting. Statistical Policy Directive 15. Washington, DC, 1977. https://wonder.cdc.gov/wonder/help/populations/bridged-race/directive15.html (accessed Dec 13, 2020). [PubMed] [Google Scholar]
- 19.Office of Management and Budget. Revisions to the standards for the classification of federal data on race and ethnicity. Fed Regist 1997; 62: 58782–90. [Google Scholar]
- 20.US Census Bureau. County Population by Characteristics: 2010–2019. https://www.census.gov/data/tables/time-series/demo/popest/2010s-counties-detail.html (accessed Aug 3, 2020).
- 21.Ingram D, Parker J, Schenker N, et al. United States Census 2000 population with bridged categories. Vital Health Stat 2003; 2. [PubMed] [Google Scholar]
- 22.World Health Organization. International statistical classification of diseases and related health problems, 10th revision. Geneva, Switzerland: World Health Organization, 1992. [Google Scholar]
- 23.Naghavi M, Makela S, Foreman K, O’Brien J, Pourmalek F, Lozano R. Algorithms for enhancing public health utility of national causes-of-death data. Popul Health Metr 2010; 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet 2020; 396: 1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kristensen K, Nielsen A, Berg CW, Skaug H, Bell B. TMB: automatic differentiation and Laplace approximation. J Stat Softw 2016; 70: 1–21. [Google Scholar]
- 26.R: The R Project for Statistical Computing. https://www.r-project.org/ (accessed April 6, 2021).
- 27.Stevens GA, Alkema L, Black RE, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. The Lancet 2016; 388: e19–23. [DOI] [PubMed] [Google Scholar]
- 28.Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Annu Rev Sociol 2015; 41: 311–30. [Google Scholar]
- 29.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. The Lancet 2017; 389: 1453–63. [DOI] [PubMed] [Google Scholar]
- 30.Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health 2019; 40: 105–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep 2001; 116: 404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wildeman C, Wang EA. Mass incarceration, public health, and widening inequality in the USA. The Lancet 2017; 389: 1464–74. [DOI] [PubMed] [Google Scholar]
- 33.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. Am J Public Health 2006; 96: 826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger M, Sarnyai Z. ‘More than skin deep’: stress neurobiology and mental health consequences of racial discrimination. Stress 2015; 18: 1–10. [DOI] [PubMed] [Google Scholar]
- 35.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal treatment: confronting racial and ethnic disparities in health care. Washington (DC): National Academies Press (US), 2003. http://www.ncbi.nlm.nih.gov/books/NBK220358/ (accessed Aug 11, 2022). [PubMed] [Google Scholar]
- 36.Howell EA. Reducing disparities in severe maternal morbidity and mortality. Clin Obstet Gynecol 2018; 61: 387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braveman PA, Arkin E, Proctor D, Kauh T, Holm N. Systemic and structural racism: definitions, examples, health damages, and approaches to dismantling. Health Aff (Millwood) 2022; 41: 171–8. [DOI] [PubMed] [Google Scholar]
- 38.Massey DS, Denton NA. The dimensions of residential segregation. Soc Forces 1988; 67: 281–315. [Google Scholar]
- 39.Groos M, Wallace M, Hardeman R, Theall K. Measuring inequity: a systematic review of methods used to quantify structural racism. J Health Disparities Res Pract 2018; 11: 190–206. [Google Scholar]
- 40.Alson JG, Robinson WR, Pittman L, Doll KM. Incorporating measures of structural racism into population studies of reproductive health in the United States: a narrative review. Health Equity 2021; 5: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riosmena F, Wong R, Palloni A. Migration selection, protection, and acculturation in health: a binational perspective on older adults. Demography 2013; 50: 1039–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riosmena F, Kuhn R, Jochem WC. Explaining the immigrant health advantage: self-selection and protection in health-related factors among five major national-origin immigrant groups in the United States. Demography 2017; 54: 175–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.US Census Bureau. American Community Survey, 2019 American Community Survey 1-Year Estimates, Tables B05003A-B05003I. https://data.census.gov/cedsci/ (accessed Aug 9, 2022).
- 44.Fenelon A, Blue L. Widening life expectancy advantage of Hispanics in the United States: 1990–2010. J Immigr Minor Health 2015; 17: 1130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turra CM, Elo IT. The impact of salmon bias on the Hispanic mortality advantage: new evidence from social security data. Popul Res Policy Rev 2008; 27: 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gee GC, Ro A, Shariff-Marco S, Chae D. Racial discrimination and health among Asian Americans: evidence, assessment, and directions for future research. Epidemiol Rev 2009; 31: 130–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLOS ONE 2015; 10: e0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acevedo-Garcia D, Lochner KA, Osypuk TL, Subramanian SV. Future directions in residential segregation and health research: a multilevel approach. Am J Public Health 2003; 93: 215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park CB, Braun KL, Horiuchi BY, Tottori C, Onaka AT. Longevity disparities in multiethnic Hawaii: an analysis of 2000 life tables. Public Health Rep 2009; 124: 579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fenelon A, Chinn JJ, Anderson RN. A comprehensive analysis of the mortality experience of hispanic subgroups in the United States: Variation by age, country of origin, and nativity. SSM - Popul Health 2017; 3: 245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quint JJ. Disaggregating data to measure racial disparities in COVID-19 outcomes and guide community response — Hawaii, March 1, 2020–February 28, 2021. Morb Mortal Wkly Rep 2021; 70: 1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah NS, Palaniappan LP, Khan SS. Proportional mortality from ischemic heart disease among Asian American subgroups, from 2018 to 2020. JAMA Intern Med 2022; 182: 1101–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braun KL, Kim BJ, Ka’opua LS, Mokuau N, Browne CV. Native Hawaiian and Pacific Islander elders: what gerontologists should know. The Gerontologist 2015; 55: 912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taparra K, Qu V, Pollom E. Disparities in survival and comorbidity burden between Asian and Native Hawaiian and other Pacific Islander patients with cancer. JAMA Netw Open 2022; 5: e2226327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones N, Marks R, Ramirez R, Ríos-Vargas M. Improved race and ethnicity measures reveal U.S. population is much more multiracial. U. S. Census Bur. 2021. https://www.census.gov/library/stories/2021/08/improved-race-ethnicity-measures-reveal-united-states-population-much-more-multiracial.html (accessed Sept 28, 2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Estimates of mortality by cause, county, racial–ethnic group, year, age, and sex are available for download from the Global Health Data Exchange (https://ghdx.healthdata.org/record/ihme-data/united-states-causes-death-life-expectancy-by-county-race-ethnicity-2000-2019) and via a user-friendly data visualisation: https://vizhub.healthdata.org/subnational/usa. Information about the underlying data sources is available in the appendix (pp 33, 50–52). The code used for this analysis is available on GitHub: https://github.com/ihmeuw/USHD.


