Abstract
Autism in a broader sense is a neurodevelopmental disorder, which frequently occurs during early childhood and can last for a lifetime. This condition is primarily defined by difficulties with social engagement, with individuals displaying repetitive and stereotyped behaviors. Numerous neuroanatomical investigations on autistic children have revealed that their brains grow atypically, resulting in atypical neurogenesis, neuronal migration, maturation, differentiation, and degeneration. Special education programs, speech therapy, and occupational therapy have all been used to address autism‐related behavioral problems. While widely prescribed antidepressant drugs, antipsychotics, anticonvulsants, and stimulants have demonstrated response in autistic individuals. However, these medications do not fully reverse the core symptoms associated with autism spectrum disorder (ASD). The adverse reactions of ASD medicines and an increased risk of developing various other problems, such as obesity, dyslipidemia, diabetes mellitus, and thyroid disorders, prompted the researchers to investigate herbal medicines for the treatment of autistic individuals. Clinical trials are now being done to establish the efficacy of alternative techniques based on natural substances and to understand better the context in which they may be used to treat autism. This review of literature will look at crucial natural compounds derived from animals and plants that have shown promise as safe and effective autism treatment strategies.
Keywords: autism, dietary supplement, natural products, neurodevelopmental disorder, neurotherapeutics
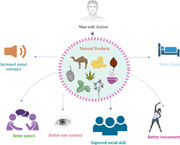
The figure explains that improvement like increased sound tolerance, better sleep pattern, better speech, improved social skills, and better eye contact and movement has been seen in patients with autism. An improvement in the symptoms can be observed by treating with natural products, such as camel's milk, luteolin, green tea, piperine, curcumin, cannabinoids, Ginkgo biloba, and Bacopa monnieri.
1. INTRODUCTION
In 1943, the term autism spectrum disorder (ASD) was coined by Leo Kanner, a child psychiatrist, when he came across 11 children who exhibited high detachment and an inability to build regular interactions with others. They were diagnosed with early infantile autism. Leo Kanner described those suffering from this illness as having an extreme desire for solitude and following a rigid schedule. They could easily spend hours distracting themselves with basic, repetitive activities and were easily agitated by the slightest break from their routine. Some autistic children could not speak or communicate. 1 A year following Kanner's discovery, Dr Hans Asperger separately recorded four of his clients who shared comparable features but showed considerable intellectual ability in science and mathematics. Asperger mentioned to his patients that they possess autism despite this distinction. 2 The symptoms frequently co‐occur with other comorbidities instead of appearing alone, including multiple psychiatric disorders, such as obsessive‐compulsive disorder, attention deficit hyperactive disorder and gastrointestinal conditions, and feeding disorders. In ASD, the term “spectrum” refers to the broad range of intensity of the symptoms, starting with minimally to seriously impaired autistic individuals who require long‐term expert support, frequently observed in affected children. ASD was reported to impact around 1%–2% of the overall population. 3 In 2010, the worldwide prevalence of ASD was 7.6 per 1000 or 1 in 132 persons. 4 From the late 1990s, ASD frequency has increased dramatically over time. 5 Numerous studies have claimed that changing case definitions and improved awareness account for the apparent increase. 6 Considering the rise in prevalence and detection of side effects of medicines, scientists have worked to bring out some of the natural products having the potential role in managing the clinical manifestations in individuals with autism. Thus, this literature review elaborates on some of the significant and effective naturally occurring products for managing the symptoms associated with ASD.
2. ENVIRONMENT AS A CAUSE FOR ASD
Vaccination, maternal smoking, thimerosal exposure, and, most likely, assisted reproductive technologies are all unrelated to the risk of ASD, according to current evidence. On the other hand, older parents are linked to a higher incidence of ASD. Birth problems linked to trauma, ischemia, and hypoxia have also been linked to ASD, although other pregnancy‐related variables, such as maternal obesity, diabetes, and caesarian section have demonstrated a less (but still substantial) link to ASD risk. The design of toxic element research has severely limited their findings; however, there is sufficient evidence for a link between specific heavy metals (most notably inorganic mercury and lead) and ASD to suggest further investigation. 7 Maximized possibility of autism is concerned with the exposure of the fetus to air pollution, poisons (thalidomide, retinoic acid, and valproic acid), and particulates. 8 Unhealthy lifestyle, prenatal stress, diet, and family history where the family members have suffered from several infectious diseases are all variables that contribute to an autistic newborn's behavioral abnormalities. 9 Autism is associated with perinatal conditions such as significantly low birth weight and hypoxia during birth or premature delivery. 10 Genetic variability may also increase due to environmental factors, which have been linked to enzymatic impairments in autism. Gene−environment interactions are complicated, and their mechanisms remain unknown at the molecular level. 10
2.1. Genetic makeup of ASD
The intricacy of ASD and its range of clinical manifestations may be explained by gene−gene interaction, as well as the influence of epigenetics, that is, exposure to environment‐associated modifiers or stressors that alter the expression of the gene. 11 , 12 Additionally, ASD has been linked to polygenic polymorphisms, single nucleotide variants, copy number variants, and uncommon inherited variants. 13 , 14 The uneven sex distribution, greater incidence in siblings, more concordance in monozygotic twins, and higher risk of ASD with more relatedness all imply a strong influence of genes on the occurrence of ASD. 14 , 15 , 16 Numerous studies have presented that male‐to‐male transmission occurs in several families, hence eliminating X‐linkage as the exclusive mode of inheritance. 17 Additionally, it was discovered that the frequency of ASD among siblings is more than the prevalence rate in the general population. 18 The relationship between clinical characteristics and particular genetic profiles is still being investigated. 19 , 20
3. CHANGES NOTICED IN AUTISTIC BRAIN
Neurobiological research in ASD patients, including neuroimaging, electrophysiology, and autopsy, has suggested that brain abnormalities, particularly aberrant neural connections, have a crucial significance in the occurrence of ASD. 21 , 22 Moreover, ASD children's heads perhaps expand more rapidly throughout infancy, and their overall brain size may be larger. 23 While comparing people having ASD with non‐ASD shows a significant difference in total gray and white matter volumes in some regions of the brain, altered brain neuromodulator concentrations, changed neural circuit anatomy, distorted gyral and sulcal anatomy, changed lateralization of the brain, and altered structure and anatomic organization of the cortex. 24 Additionally, aberrant neuronal differentiation during prenatal development appears to cause cortical abnormalities. 25 Furthermore, in comparison to people without ASD, patients with ASD use different neural pathways for cognitive processes, and specific brain regions process information during activities that require interaction in society (e.g., eye gazing, faces, speech). 26
4. CROSSTALK BETWEEN DIETARY SUPPLEMENT DEFICIENCY AND ASD
Experiments and population‐based investigations have demonstrated that the pathogenic alterations associated with ASD appear to begin during fetal development. The behavioral and neurological aspects of ASD in the fetus have been hypothesized to be acquired due to maternal metabolic disorders. Kawicka et al. previously demonstrated that metabolic problems, such as obesity and diabetes, during pregnancy might be one of the factors that fetus develop ASD. 27 Appropriate nutritional intake is necessary for brain growth and maturation during pregnancy. 28 Certain associative measures of children's nutritional and dietary status have suggested that a deficiency of some of the supplements, vitamins, and minerals, such as pyridoxine (vitamin B6), magnesium, calcium, folic acid (vitamin B9), omega‐3 fatty acids, potassium, iron, cholecalciferol (vitamin D), tocopherols (vitamin E), and zinc may be potential risk factors for ASD. 29 Additionally, abnormally high amounts of copper, folic acid, iron, and calcium have impaired zinc absorption. 30 Pregnant women who use excessive calcium and iron‐rich supplements may have a zinc absorption deficit. Zinc and iron deficiency appear to be a part of alteration in the expression of genes involved in neuroplasticity and neurogenesis during the prenatal period, including BDNF, SDF‐1, CamKIIa, and PSD‐95. Certain pharmacological drugs, such as angiotensin‐converting enzyme (ACE) inhibitors, which are extensively used to treat hypertension, may also cause a drop in blood zinc levels. 31 Additionally, folic acid is required for normal erythropoiesis and neural tube formation. 32 While some studies have indicated that folic acid intake effectively treats ASD, problems in folate metabolism and folic acid overload during pregnancy have been associated with the development of ASD symptoms in progenies. 33 Vitamin D is found in less amounts in the diet; it is generally absorbed through skin exposure to sunlight. 34 Vitamin D deficiency caused by certain environmental conditions, especially the weather, has been linked to ASD. 35 Vitamin E has long been recognized as a potent antioxidant protecting the body from oxidative stress. According to research, children with vitamin E deficiency frequently exhibit autistic‐like behavioral abnormalities. 36 The polyunsaturated fatty acids (omega‐3 and omega‐6 fatty acids) appear critical for brain development and neuroplasticity regulation. Consumption of omega fatty acids appears to decrease due to lifestyle changes. As a result, omega fatty acid deficiency has been identified as a risk factor for ASD. 37 Gastrointestinal (GI) issues have been recognized as a frequent symptom in individuals. 38
On the one hand, changes in the gut microbiome and gastrointestinal illnesses may impair the digestion of dietary supplements, resulting in vitamin, mineral, and other essential nutrient deficiencies. However, it may disrupt the gut−brain axis. 39 It is well established that the gut−brain axis influences brain development and behavior via modulation of neurogenesis, neuroplasticity, neuroendocrine, and neuroimmune activities. 40 Dairy and gluten‐containing diets significantly affect the equilibrium of the gut microbial environment, impairing the gut−brain axis and further impairing neuronal processes. 41 Thus, disruption of the gut−brain axis, which is frequently observed in individuals with aberrant behavioral patterns, may also play a significant role in the development of ASD. 42 Additionally, food allergens, 42 and the accumulation of some toxic metals, such as cadmium, arsenic, and mercury, have been implicated in the development of ASD. 43
5. CURRENT THERAPEUTIC INTERVENTIONS FOR THE TREATMENT AND MANAGEMENT OF AUTISM
While some psychosocial therapies are helpful, there is no effective therapeutic plan for ASD. Affected individuals have a wide variety of symptoms that vary significantly. 44 Treatment approaches are customized for every patient. Specialized training, educational programmers, and behavioral therapies may aid in the development of job skills, self‐care, and maturity, while drugs may help alleviate anxiety and irritation. 45 Applied behavior analysis is a beneficial intervention that relies on unique one‐on‐one training assignments based on behaviorist principles of reward, stimulus, and response. 46 Discrete trial training teaches essential skills like imitation, attention, and compliance through a somewhat different technique. In autistic persons, pivotal response training promotes self‐management and social bonding. Anticonvulsants, stimulants, antidepressants, and antipsychotics, such as risperidone or aripiprazole, are typically provided to diagnosed children. 47 However, the long‐term consequences of such medications must be thoroughly researched, as each individual reacts differently to them. 48
6. ROLE OF DIFFERENT POTENTIAL NATURAL PRODUCTS IN ASD
The requirement for safe and effective drugs for the efficient management of autism has resulted in exploring a variety of natural plant‐based products with therapeutic possibilities. Effective herbal treatments may reduce clinical manifestations with fewer side effects. 47 The causes, symptoms, and potent natural products for the management of ASD are shown in Figure 1. Table 1 shows the effect of natural products on animal models and Table 2 shows the effect of natural products on children and adults with autism.
Figure 1.
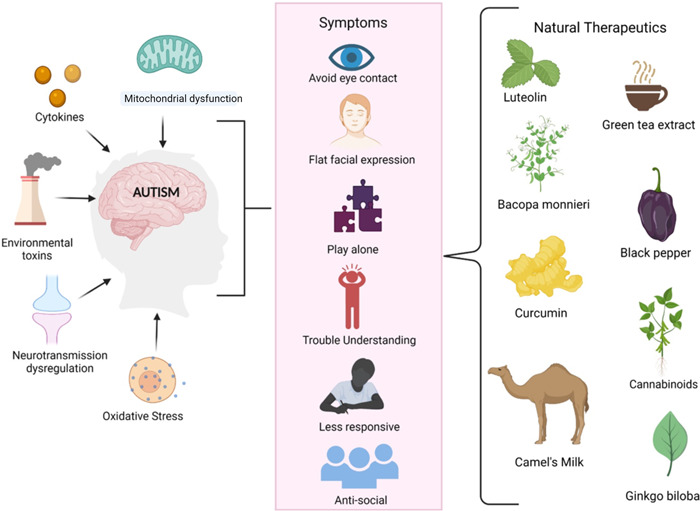
The figure shows the potential risk factor associated with ASD, such as the release of cytokines, mitochondrial dysfunction, oxidative stress, neurotransmission dysregulation, and environmental toxins. It has been seen that the release of pro‐inflammatory cytokines, which causes inflammation, influences the development of ASD. The elevated levels of oxidative stress lead to mitochondrial dysfunction through disrupted energy regulation. Impaired mitochondrial function, neurotransmission, and prolonged exposure to environmental toxins are linked with abnormal brain development, causing ASD. ASD, autism spectrum disorder. [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Effect of natural products on animal model
| Natural product | Nonclinical model name and method | Method | Result | References |
|---|---|---|---|---|
| Luteolin | Murine model | Valproic acid induced mouse | Improved nonsocial and social behavior | [49] |
| Green tea extract | Young mice (both male and female) | Dose: Valproate (400 mg/kg subcutaneously) was given to newborns on postnatal Day 14. Green tea extract (75 and 300 mg/kg) was given to newborns up to postnatal Day 40. | Neuronal cytoprotective effect and behavioral improvement at 300 mg/kg of green tea extract | [50] |
| Curcumin | Male black and Tan Brachyury (BTBR) mice | CUR (25,50, and 100 mg/kg, i.p) | Alleviates ASD‐like symptoms in BTBR mice and mitigates oxidative stress | [51] |
| Curcumin | Forty neonatal male Western Albino rats | Four rat groups, third group received valproic acid with curcumin, fourth group only curcumin | Improved delayed maturation and abnormal weight, corrected dysfunction of IL‐6, CYP450, glutamate, and oxidized glutathione | [52] |
| Piperine | BALB/c mice 13 days, 3 males 3 females | Five groups, behavioral test up to PND 40 | Mice killed, improved behavioral alterations, lowered oxidative stress markers | [53] |
| Bacopa monnieri | 12.5 days female pregnant rats | Control and valproic acid (600 mg/kg i.p)‐treated groups | Animals killed but ameliorate autistic symptoms | [54] |
Table 2.
Effect of natural products in children and adults with ASD
| Natural product | ASD (children or adults) | Outcomes | References |
|---|---|---|---|
| Luteolin | Children | Thirty‐seven children were given luteolin for 4 months, which shows 10% improvement in speech, 25% improvement in social skills, 50% improvement in eye contact, and 75% improvement in gastrointestinal symptoms | [55] |
| Luteolin | Children | After 26 weeks of treatment with luteolin, TNF and IL‐6 levels reduced, which finally improved behavior | [56] |
| Luteolin | Children | Fifty children were given one capsule having 10 kg of weight per day along with the food and this dose reduced almost all symptoms with no major adverse effect | [57] |
| Cannabidiol | Adults | Thirty‐four adult men in which 50% are diagnosed with autism were given 600 mg cannabidiol, which alters the fractional amplitude of low‐frequency fluctuations | [58] |
| Cannabidiol | Children and adults (both) | One hundred and fifty participants from the age of 5−21 years were given whole plant extract, which shows 49% improvement in behavior with no major adverse effect | [59] |
| Camel milk | Children | 500 ml camel's milk was given to around 45 children on daily basis for 2 weeks, the serum level of activation‐regulated chemokines decreased and the childhood autism rating scale score improved. | [60] |
6.1. Luteolin for ASD
Microglia are a form of macrophage found in the CNS that perform comprehensive scanning and activation in response to stressors like damage, disease, or infection. 61 Their activation is also associated with CNS inflammatory responses. 62 Autism is connected with maternal immunological stimulation and the consequent microglial dysfunction in the development of the brain. 63 Numerous etiological ideas with varying degrees of evidence suggest that targeting microglial activation to modulate these inflammatory cascades may benefit the treatment of autism. 64 , 65 Luteolin is a flavonoid that occurs naturally in food plants. Lutein therapy effectively inhibited the increase in glial fibrillary acidic protein (GFAP) in astrocytes induced by IL‐6 in a cell‐based human maternal immunological activation model. 65 GFAP is typically overexpressed in proliferating glial scars. 65 A significant decrease in the phosphorylated transcription factor STAT3 was observed. 65 Excessive phosphorylation of STAT3 indicates increased cytokine and growth factor activity, frequently resulting in inflammation. Additionally, luteolin treatment decreases TBR1‐ and CTIP2‐positive cells. 66 The expression of TBR1 and CTIP2 is required for proper cortical development during the earliest phases. 67 Bertolino et al. demonstrated that the flavonoid luteolin combined with the fatty acid palmitoylethanolamide was neuroprotective and anti‐inflammatory. Neuroinflammation is one of the characteristic features of autism; elevated levels of interleukin‐6 (IL‐6) and tumor necrosis factor (TNF) are also detected in the serum of affected individuals. 68 However, autistic youngsters who took a luteolin dietary supplement regularly demonstrated enhanced social bonding and behavior. Likewise, serum levels of IL‐6, TNF, and other cytokines significantly decreased with luteolin consumption. 56
6.2. Green tea (Camellia sinensis) for ASD
Increased oxidative stress has been associated with autism development. 43 Children with autism exhibit higher levels of lipid peroxidation, significant antioxidant serum proteins, altered glutathione status, and levels of critical antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, and catalase. 69 C. sinensis contains a significant amount of caffeine, polyphenol, and flavonoids, with well‐established antioxidant properties. Experiments have demonstrated that green tea has many good health impacts. 70 Flavonoids may cross the blood−brain barrier and possess a range of neuroprotective properties. 71 The daily ingestion of green tea extract (75−300 mg/kg) is recommended for the production of neuroprotective effects in the brain. 50 The bioactive components of green tea have been shown to impact the level of neurotransmitters in the brain directly, most importantly dopamine and serotonin in specific brain areas. 72 l‐theanine, an amino acid found in tea, has antistress qualities and increases long‐term potentiation in an NMDA‐independent way; hence, it helps in improving memory. 72 Autism is characterized by a decline in the functioning of Purkinje cells in the cerebellar region. 73 Histological findings of naive mouse models who received 300 mg/kg of green tea extract consistently exhibited progressive regeneration of the Purkinje layer and cells, showing that green tea extract may well have neuroprotective qualities to treat autism. Moreover, green tea's cytoprotective activity on brain cells has been established, and it may be useful in managing symptoms of autism through early dietary intervention. 74
6.3. Piperine for ASD
Piperine, chemically an N‐acylpiperidine, is the primary alkaloid extracted from black pepper and long pepper. The chemical can activate pain‐sensing nerve cells' heat and acidity‐sensing ion channels. Specifically, they are called nociceptors. 75 It has a vital effect on the nervous system. Historically, it has been used widely in treating epileptic disorders, exhibits significant antioxidative properties, and helps in memory enhancement and cognition. 76 Piperine pretreatment protected cultured hippocampus neurons against cell viability loss caused by a glutamatergic increase. The mechanism through which it operates action has been linked to the control of Ca2+ ion entry. 77 Twenty mg/kg of sodium valproate was used experimentally to treat autistic Balb/C mice, after which they were evaluated behaviorally, histopathologically, and biochemically on postnatal Day 14. The piperine can elicit beneficial effects, as evidenced by its antioxidant activity, cognitive enhancement, and neuroprotective characteristics. 53 Additionally, the chemical has anxiolytic properties, for which it acts as an antistress and relaxing medicine. Thus, clinical studies, including piperine research, are going toward elucidating its potential benefits for autistic children. 76
6.4. Curcumin for ASD
Curcumin is the primary curcuminoid found in turmeric (Curcuma longa), a spice known for its neuroprotective qualities. It has been shown to target several signaling pathways inside the cell and play a role in controlling nitrosative or oxidative stress, mitochondrial function, and protein aggregation. 78 Curcumin possesses a broad spectrum of anti‐inflammatory properties and can quickly cross the blood−brain barrier. 79 It was discovered in a study that curcumin supplements have been shown to significantly increase the concentration of antioxidant enzymes. 80 Curcumin at a dose of up to 200 mg/kg given to male Sprague–Dawley rats exhibiting autistic phenotypes has been shown to reduce oxidative stress, mitochondrial defect, tumor necrosis factor (TNF‐) release, and matrix degradation metalloproteinases. Thus, it has been observed that curcumin acts as a neuro‐psycho‐pharmacotherapeutic substance in treating ASD. 81 As a direct result, curcumin can lower numerous inflammatory indicators in various disorders and has consistently exhibited antioxidant radical scavenging activity in vitro and in vivo. 82 Increased synaptic plasticity, which results from regular consumption of curcumin in the diet, has been shown to improve cognition. However, there are no compelling data on clinical studies demonstrating the efficacy of clinical experiments on humans; evidence supporting curcumin's neuroprotective properties is enough for it to be used in upcoming autism research and additionally linked illnesses. 83
6.5. Cannabinoids for ASD
Cannabis is currently being studied medically for various neurological illnesses, with success shown with its use. 84 When used in sufficient amounts, tetrahydrocannabinol (THC), the phytocannabinoid that is the primary psychoactive component of Cannabis sativa, can worsen various neurological disorders. The study done by Salgado et al. 85 states that cannabidiol (CBD) use can be efficacious in decreasing autistic behavior. The substance has an impact on immunomodulation, antioxidant defense, and neuroprotection and offers promising therapeutic alternatives with negligible or no side effects. 86 Additionally, cannabidivarin (CBDV) has been demonstrated a favorable potential for ameliorating behavioral changes, and clinical trials with this chemical demonstrated significant improvement in autistic patients. 87
Moreover, an additional 12 weeks of CBDV at a dose of 10 mg/kg/day has been approved as an assessment to ensure that it is tolerable and safe. 88 The endogenous cannabinoid (EC) system is a crucial neuromodulatory mechanism. It can regulate emotional reactions and behavioral reactivity to a desirable degree of interpersonal communication. In most cases, ASD patients' EC systems are found to be compromised. 88 Endogenous substances such as signaling chemicals produced from arachidonic acid and related enzymes can bind to and activate EC receptors, resulting in increased RNA and protein levels. 89 But, when this mechanism fails, it disrupts normal metabolic pathways, resulting in neuroinflammation. Therefore, activating the EC system with natural cannabis phytoproducts may be beneficial in modulating the immunological responses, possess antioxidant properties, and aid in ameliorating the autism spectrum's symptoms. 88
6.6. Ginkgo biloba for ASD
The standardized extract of G. biloba leaves contains around 24% flavone glycosides (mostly quercetin, kaempferol, and isorhamnetin) and 6% terpene lactones (2.8%–3.4% ginkgolides A, B, and C, respectively and 2.6%–3.2% bilobalide). 89 Nearly 0.8% and 0.1%, respectively, were ginkgolide B and bilobalide. Additionally, proanthocyanidins, glucose, and organic acids are present, as well as rhamnose, d‐glucaric acid, and ginkgolic acid. 90 The presence of terpenoids, organic acids, and flavonoids in the extract contributes to its efficacy. It protects against ischemic stroke, Parkinson's disease, and Alzheimer's disease. 91 In an observational study, 100 mg/kg of G. biloba extract taken twice daily was beneficial in improving autistic people's symptoms and odd behavior. The extract has been demonstrated to resolve behavioral problems effectively; impatience, hyperactivity, poor eye contact, and improper speech are all characteristics of autism. 92 G. biloba extract is used to treat autism with other drugs. The treatment group reported fewer adverse events compared to the placebo group. There is a knowledge gap about the pharmacokinetics and bioavailability of medicines in the CNS. More research is required to evaluate the efficiency of G. biloba in the treatment of neurological diseases, including autism. 93
6.7. Bacopa monnieri for ASD
Bacosides are widely utilized medicinally by the tribes of India and are the primary bioactive constituents of B. monnieri (L.) West. 94 This herb has long been recognized for its intellect‐ and cognition‐enhancing effects and its nerve tonic abilities. 95 B. monnieri's pharmacological activities have been linked to its alkaloids, saponins, and sterols constituents. 96 B. monnieri considerably reduced behavioral changes in a BTBR T+ tf/J mouse model of oxidative stress, decreased pain threshold, normalized locomotor deficits, and anxiety autism model. The increased locomotive activity was clarified to B. monnieri's antianxiety qualities and its capacity to reduce glutamate accumulation and restore the architecture of the cerebellum. 54
6.8. Camel milk for ASD
The use of camel milk has lately been revealed to have potential treatment benefits for several ailments. 97 In individuals with ASD, it was related to decreased plasma GSH and cysteine levels and has been demonstrated to have a favorable effect on the behavior. Autistic children showed significant improvements in the scale for evaluating autism in childhood (CARS) after camel milk consumption. Camel milk is unique in its composition and cannot be found in other ruminants' milk. In comparison to the udder of a cow, camel's milk has a greater concentration of elements (calcium, iron, magnesium, copper, zinc, and potassium) and vitamins (A, B2, E, and C), less fat, cholesterol, salt, and lactose. 98
Additionally, it lacks beta‐lactoglobulin and beta‐casein, two critical active ingredients found in cow's milk intolerance. Camel milk has several protective proteins and enzymes that are antibacterial, antiviral, and immunologic. 98 Immunoglobulins, lysozymes, lactoperoxidase, N‐acetylglucosaminidase, and peptidoglycan recognition protein are all crucial peptidoglycan‐recognition proteins for preventing and curing food allergies. 99 Camel milk owes its origins to its anti‐inflammatory proteins, hypoallergenic properties, and smaller nanobodies. 97 It can alleviate specific main autistic symptoms due to its hypoallergenic qualities and antibodies identical to those found in antibodies against humans. 100 Nanobodies are present in milk. Due to their small size, camel milk nanobodies exhibit unique structural properties, including enhanced tissue penetration, 101 and the ability to detect not immediately apparent epitopes. These qualities may aid in infection prevention and may confer extra advantages—the immune system's strength. 97
Furthermore, the structure of camel milk nanobodies is strikingly similar to that of human immunoglobulins (IgG3). This reveals that camel antibodies are identical to human antibodies. The unique composition of camel milk has been demonstrated to positively impact the condition of children with ASD by increasing superoxide dismutase levels and myeloperoxidase levels, plasma GSH levels, and oxidative stress. According to some studies, camel milk was utilized for 2 weeks as a potential treatment approach. There are significant differences in CARS, SRS, and ATEC scores among individuals with ASD. 97 The trial's findings have shown that antioxidant enzymes and nonenzymatic antioxidant substances found in camel milk may have a vital component in the process of normalizing ASD behaviors. Tests on a broader scale that focuses on the dosage of the camel milk samples are necessary to look into its effect on oxidative stress markers and hence its antioxidant properties in ASD treatment. 97 Figure 2 shows the impact of natural products on the symptoms associated with ASD.
Figure 2.
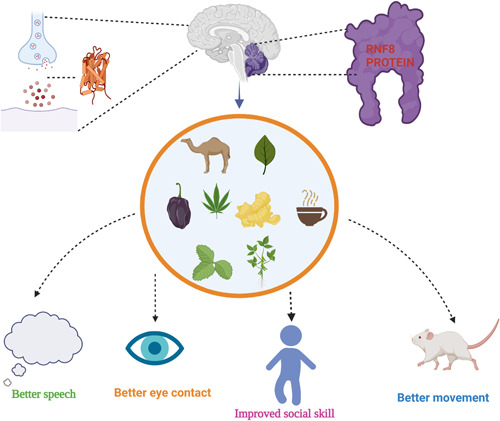
Autism affects the cerebellum, marked by blue color, and in the cerebellum, RNF8 protein is found. It has been seen that the absence of the RNF8 gene leads to impaired learning skills. GABA neurotransmitter is involved in autism, which natural products can balance. In preclinical and clinical trials reduced the negative symptoms and showed positive responses in mice (movement) and better speech, eye contact, and improved social skills in children and adults. [Color figure can be viewed at wileyonlinelibrary.com]
7. FUTURE DIRECTION AND CONCLUSION
Natural products have demonstrated a plausible therapy for various conditions, including neurodevelopmental disorders such as autism. Numerous years of study have determined that the advantages and negative impacts of the above‐mentioned natural products have not been identified, established, or recommended in the near future. These naturally occurring chemicals discovered as possible therapeutic candidates can be used as chemical models or templates to synthesize or modify novel compounds for the treatment of autism. Plant resources, in particular, have the potential to be immensely valuable. There are FDA‐approved medications to address autistic persons' behavioral issues. However, the core symptoms of these drugs are not treated. Over half of patients get psychoactive drugs or anticonvulsants, particularly synthetic antidepressants, stimulants, and antipsychotics, all of which have a variety of harmful consequences when used indefinitely. While advanced learning approaches and alternative therapies are accessible nowadays, herbal medications remain a dependable option. All that remains is to determine their efficacy against autism. Enduring and unshakable, as evidenced by further investigations.
AUTHOR CONTRIBUTIONS
Punya Sachdeva: Conceptualization; writing; editing; drawing figures; and reviewing. Intizaar Mehdi: Writing; editing; and reviewing. Rohit Kaith: Writing; editing; reviewing. Faizan Ahmad: Drawing figures and tables. Md Sheeraz Anwar: Reviewing; editing.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENT
The acknowledgment information is not applicable. This study did not receive any funding.
Sachdeva P, Mehdi I, Kaith R, Ahmad F, Anwar MS. Potential natural products for management of autism spectrum disorder. ibrain. 2022;8:365‐376. 10.1002/ibra.12050
DATA AVAILABILITY STATEMENT
Data generated during the study are mentioned in the paper.
REFERENCES
- 1. Kanner L. Etude de l'évolution de onze enfants autistes initialement rapportée en 1943 [Follow‐up study of eleven autistic children originally reported in 1943. 1971]. Psychiatr Enfant. 1995;38(2):421‐461. [PubMed] [Google Scholar]
- 2. Dell'Osso L, Luche RD, Gesi C, Moroni I, Carmassi C, Maj M. From Asperger's Autistischen Psychopathen to DSM‐5 autism spectrum disorder and beyond: a subthreshold autism spectrum model. Clin Pract Epidemiol Ment Health. 2016;12:120‐131. 10.2174/1745017901612010120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elsabbagh M, Divan G, Koh YJ, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5(3):160‐179. 10.1002/aur.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45(3):601‐613. 10.1017/S003329171400172X [DOI] [PubMed] [Google Scholar]
- 5. Kogan MD, Vladutiu CJ, Schieve LA, et al. The prevalence of parent‐reported autism spectrum disorder among US children. Pediatrics. 2018;142(6):e20174161. 10.1542/peds.2017-4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65(6):591‐598. 10.1203/PDR.0b013e31819e7203 [DOI] [PubMed] [Google Scholar]
- 7. Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence‐based review of systematic reviews and meta‐analyses. Mol Autism. 2017;8:13. 10.1186/s13229-017-0121-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kinney DK, Munir KM, Crowley DJ, Miller AM. Prenatal stress and risk for autism. Neurosci Biobehav Rev. 2008;32(8):1519‐1532. 10.1016/j.neubiorev.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lyall K, Schmidt RJ, Hertz‐Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. 2014;43(2):443‐464. 10.1093/ije/dyt282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zachariah SM, Oommen SP, Koshy B. Clinical features and diagnosis of autism spectrum disorder in children. Curr Med Issues. 2017;15:6‐16. [Google Scholar]
- 11. Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):e472‐e486. 10.1542/peds.113.5.e472 [DOI] [PubMed] [Google Scholar]
- 12. Bacchelli E, Maestrini E. Autism spectrum disorders: molecular genetic advances. Am J Med Genet, Part C. 2006;142C(1):13‐23. 10.1002/ajmg.c.30078 [DOI] [PubMed] [Google Scholar]
- 13. Robinson EB, Neale BM, Hyman SE. Genetic research in autism spectrum disorders. Curr Opin Pediatr. 2015;27(6):685‐691. 10.1097/MOP.0000000000000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandin S, Lichtenstein P, Kuja‐Halkola R, Hultman C, Larsson H, Reichenberg A. The heritability of autism spectrum disorder. JAMA. 2017;318(12):1182‐1184. 10.1001/jama.2017.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095‐1102. 10.1001/archgenpsychiatry.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, Law PA. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med. 2009;163(10):907‐914. 10.1001/archpediatrics.2009.98 [DOI] [PubMed] [Google Scholar]
- 17. Cheng Y, Qin G, Dai X, Zhao Y. NPY1, a BTB‐NPH3‐like protein, plays a critical role in auxin‐regulated organogenesis in Arabidopsis. Proc Natl Acad Sci USA. 2007;104(47):18825‐18829. 10.1073/pnas.0708506104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bolton P, Macdonald H, Pickles A, et al. A case−control family history study of autism. J Child Psychol Psychiatry. 1994;35(5):877‐900. 10.1111/j.1469-7610.1994.tb02300.x [DOI] [PubMed] [Google Scholar]
- 19. Piven J, Gayle J, Chase GA, et al. A family history study of neuropsychiatric disorders in the adult siblings of autistic individuals. J Am Acad Child Adolesc Psychiatry. 1990;29(2):177‐183. 10.1097/00004583-199003000-00004 [DOI] [PubMed] [Google Scholar]
- 20. Yin J, Schaaf CP. Autism genetics—an overview. Prenat Diagn. 2017;37(1):14‐30. 10.1002/pd.4942 [DOI] [PubMed] [Google Scholar]
- 21. Sato W, Uono S. The atypical social brain network in autism: advances in structural and functional MRI studies. Curr Opin Neurol. 2019;32(4):617‐621. 10.1097/WCO.0000000000000713 [DOI] [PubMed] [Google Scholar]
- 22. Boddaert N, Zilbovicius M, Philipe A, et al. MRI findings in 77 children with non‐syndromic autistic disorder. PLoS One. 2009;4(2):e4415. 10.1371/journal.pone.0004415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hazlett HC, Gu H, Munsell BC, et al. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542(7641):348‐351. 10.1038/nature21369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang G, Gudsnuk K, Kuo SH, et al. Loss of mTOR‐dependent macroautophagy causes autistic‐like synaptic pruning deficits. Neuron. 2014;83(5):1131‐1143. 10.1016/j.neuron.2014.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bauman ML, Kemper TL. The neuropathology of the autism spectrum disorders: what have we learned? Novartis Found Symp. 2003;251:112‐122. [PubMed] [Google Scholar]
- 26. Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and 'mirror neuron' functioning in autistic spectrum disorder. Neuropsychologia. 2006;44(4):610‐621. 10.1016/j.neuropsychologia.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 27. Kawicka A, Regulska‐Ilow B. How nutritional status, diet and dietary supplements can affect autism. A review. Rocz Panstw Zakl Hig. 2013;64(1):1‐12. [PubMed] [Google Scholar]
- 28. Hyman SL, Stewart PA, Schmidt B, et al. Nutrient intake from food in children with autism. Pediatrics. 2012;130(Suppl 2):S145‐S153. 10.1542/peds.2012-0900L [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fujiwara T, Morisaki N, Honda Y, Sampei M, Tani Y. Chemicals, nutrition, and autism spectrum disorder: a mini‐review. Front Neurosci. 2016;10:174. 10.3389/fnins.2016.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yasuda H, Yoshida K, Yasuda Y, Tsutsui T. Infantile zinc deficiency: association with autism spectrum disorders. Sci Rep. 2011;1:129. 10.1038/srep00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagmeyer S, Sauer AK, Grabrucker AM. Prospects of zinc supplementation in autism spectrum disorders and shankopathies such as Phelan McDermid Syndrome. Front Synaptic Neurosci. 2018;10:11. 10.3389/fnsyn.2018.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun C, Zou M, Zhao D, Xia W, Wu L. Efficacy of folic acid supplementation in autistic children participating in structured teaching: an open‐label trial. Nutrients. 2016;8(6):337. 10.3390/nu8060337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wiens D, DeSoto M. Is high folic acid intake a risk factor for autism?—a review. Brain Sci. 2017;7(12):149. 10.3390/brainsci7110149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saad K, Abdel‐Rahman AA, Elserogy YM, et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr Neurosci. 2016;19(8):346‐351. 10.1179/1476830515Y.0000000019 [DOI] [PubMed] [Google Scholar]
- 35. Cannell JJ. Vitamin D and autism, what's new? Rev Endocr Metab Disord. 2017;18(2):183‐193. 10.1007/s11154-017-9409-0 [DOI] [PubMed] [Google Scholar]
- 36. Krajcovicova‐Kudlackova M, Valachovicova M, Mislanova C, Hudecova Z, Sustrova M, Ostatnikova D. Plasma concentrations of selected antioxidants in autistic children and adolescents. Bratisl Lek Listy. 2009;110(4):247‐250. [PubMed] [Google Scholar]
- 37. Parletta N, Niyonsenga T, Duff J. Omega‐3 and Omega‐6 polyunsaturated fatty acid levels and correlations with symptoms in children with attention deficit hyperactivity disorder, autistic spectrum disorder and typically developing controls. PLoS One. 2016;11(5):e0156432. 10.1371/journal.pone.0156432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leader G, Tuohy E, Chen JL, Mannion A, Gilroy SP. Feeding problems, gastrointestinal symptoms, challenging behavior and sensory issues in children and adolescents with autism spectrum disorder. J Autism Dev Disord. 2020;50(4):1401‐1410. 10.1007/s10803-019-04357-7 [DOI] [PubMed] [Google Scholar]
- 39. van De Sande MM, van Buul VJ, Brouns FJ. Autism and nutrition: the role of the gut−brain axis. Nutr Res Rev. 2014;27(2):199‐214. 10.1017/S0954422414000110 [DOI] [PubMed] [Google Scholar]
- 40. Tognini P. Gut microbiota: a potential regulator of neurodevelopment. Front Cell Neurosci. 2017;11:25. 10.3389/fncel.2017.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanctuary MR, Kain JN, Angkustsiri K, German JB. Dietary considerations in autism spectrum disorders: the potential role of protein digestion and microbial putrefaction in the gut−brain axis. Front Nutr. 2018;5:40. 10.3389/fnut.2018.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jyonouchi H. Food allergy and autism spectrum disorders: is there a link. Curr Allergy Asthma Rep. 2009;9(3):194‐201. 10.1007/s11882-009-0029-y [DOI] [PubMed] [Google Scholar]
- 43. Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry. 2014;4(2):e360. 10.1038/tp.2014.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bent S, Hendren RL. Complementary and alternative treatments for autism part 1: evidence‐supported treatments. AMA J Ethics. 2015;17(4):369‐374. 10.1001/journalofethics.2015.17.4.sect1-1504 [DOI] [PubMed] [Google Scholar]
- 45. Myers SM, Johnson CP, American Academy of Pediatrics Council on Children With Disabilities . Management of children with autism spectrum disorders. Pediatrics. 2007;120(5):1162‐1182. 10.1542/peds.2007-2362 [DOI] [PubMed] [Google Scholar]
- 46. Coury DL, Anagnostou E, Manning‐Courtney P, et al. Use of psychotropic medication in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130(Suppl 2):S69‐S76. 10.1542/peds.2012-0900D [DOI] [PubMed] [Google Scholar]
- 47. Casano AM, Peri F. Microglia: multitasking specialists of the brain. Dev Cell. 2015;32(4):469‐477. 10.1016/j.devcel.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 48. Bang M, Lee SH, Cho SH, et al. Herbal medicine treatment for children with autism spectrum disorder: a systematic review. Evid‐based Complement Alternat Med. 2017;2017:8614680. 10.1155/2017/8614680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bertolino B, Crupi R, Impellizzeri D. Beneficial effects of co‐ultramicronized palmitoylethanolamide/luteolin in a mouse model of autism and in a case report of autism. CNS Neurosci Therapeut. 2017;23:87‐98. 10.1111/cns.12648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Banji D, Banji OJ, Abbagoni S, Hayath MS, Kambam S, Chiluka VL. Amelioration of behavioral aberrations and oxidative markers by green tea extract in valproate induced autism in animals. Brain Res. 2011;1410:141‐151. 10.1016/j.brainres.2011.06.063 [DOI] [PubMed] [Google Scholar]
- 51. jayaprakash P, Isaev D, Shabbir W, Lorke DE, Sadek B, Oz M. Curcumin potentiates α7 nicotinic acetylcholine receptors and alleviates autistic‐like social deficits and brain oxidative stress status in mice. Int J Mol Sci. 2021;22(14):7251. 10.3390/ijms22147251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhong H, Xiao R, Ruan R, et al. Neonatal curcumin treatment restores hippocampal neurogenesis and improves autism‐related behaviors in a mouse model of autism. Psychopharmacology. 2020;237(12):3539‐3552. 10.1007/s00213-020-05634-5 [DOI] [PubMed] [Google Scholar]
- 53. Pragnya B, Kameshwari JS, Veeresh B. Ameliorating effect of piperine on behavioral abnormalities and oxidative markers in sodium valproate induced autism in BALB/C mice. Behav Brain Res. 2014;270:86‐94. 10.1016/j.bbr.2014.04.045 [DOI] [PubMed] [Google Scholar]
- 54. Sandhya T, Sowjanya J, Veeresh B. Bacopa monniera (L.) Wettst ameliorates behavioral alterations and oxidative markers in sodium valproate induced autism in rats. Neurochem Res. 2012;37(5):1121‐1131. 10.1007/s11064-012-0717-1 [DOI] [PubMed] [Google Scholar]
- 55. Theoharides TC, Asadi S, Panagiotidou S. A case series of a luteolin formulation (NeuroProtek®) in children with autism spectrum disorders. Int J Immunopathol Pharmacol. 2012;25(2):317‐323. 10.1177/039463201202500201 [DOI] [PubMed] [Google Scholar]
- 56. Tsilioni I, Taliou A, Francis K, Theoharides TC. Children with autism spectrum disorders, who improved with a luteolin‐containing dietary formulation, show reduced serum levels of TNF and IL‐6. Transl Psychiatry. 2015;5(9):e647. 10.1038/tp.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taliou A, Zintzaras E, Lykouras L, Francis K. An open‐label pilot study of a formulation containing the anti‐inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin Ther. 2013;35(5):592‐602. 10.1016/j.clinthera.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 58. Pretzsch CM, Voinescu B, Mendez MA, et al. The effect of cannabidiol (CBD) on low‐frequency activity and functional connectivity in the brain of adults with and without autism spectrum disorder (ASD). J Psychopharmacol. 2019;33(9):1141‐1148. 10.1177/0269881119858306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aran A, Harel M, Cassuto H, et al Cannabinoid treatment for autism: a proof‐of‐concept randomized trial. Mol Autism. 2021;12:6. 10.1186/s13229-021-00420-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bashir S, Al‐Ayadhi LY. Effect of camel milk on thymus and activation‐regulated chemokine in autistic children: double‐blind study. Pediatr Res. 2014;75(4):559‐563. 10.1038/pr.2013.248 [DOI] [PubMed] [Google Scholar]
- 61. Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1(1):14. 10.1186/1742-2094-1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim JW, Hong JY, Bae SM. Microglia and autism spectrum disorder: overview of current evidence and novel immunomodulatory treatment options. Clin Psychopharmacol Neurosci. 2018;16(3):246‐252. 10.9758/cpn.2018.16.3.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gottfried C, Bambini‐Junior V, Francis F, Riesgo R, Savino W. The impact of neuroimmune alterations in autism spectrum disorder. Front Psychiatry. 2015;6:121. 10.3389/fpsyt.2015.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marchezan J, Winkler Dos Santos E, Deckmann I, Riesgo R. Immunological dysfunction in autism spectrum disorder: a potential target for therapy. Neuroimmunomodulation. 2018;25(5‐6):300‐319. 10.1159/000492225 [DOI] [PubMed] [Google Scholar]
- 65. Zuiki M, Chiyonobu T, Yoshida M, et al. Luteolin attenuates interleukin‐6‐mediated astrogliosis in human iPSC‐derived neural aggregates: a candidate preventive substance for maternal immune activation‐induced abnormalities. Neurosci Lett. 2017;653:296‐301. 10.1016/j.neulet.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 66. Xu N, Li X, Zhong Y. Inflammatory cytokines: potential biomarkers of immunologic dysfunction in autism spectrum disorders. Mediators Inflamm. 2015;2015:531518. 10.1155/2015/531518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bahmani M, Sarrafchi A, Shirzad H, Rafieian‐Kopaei M. Autism: pathophysiology and promising herbal remedies. Curr Pharm Des. 2016;22(3):277‐285. 10.2174/1381612822666151112151529 [DOI] [PubMed] [Google Scholar]
- 68. Bertolino B, Crupi R, Impellizzeri D, et al. Beneficial effects of co‐ultramicronized palmitoylethanolamide/luteolin in a mouse model of autism and in a case report of autism. CNS Neurosci Ther. 2017;23(1):87‐98. 10.1111/cns.12648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology. 2006;13(3):171‐181. 10.1016/j.pathophys.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 70. Schimidt HL, Garcia A, Martins A, Mello‐Carpes PB, Carpes FP. Green tea supplementation produces better neuroprotective effects than red and black tea in Alzheimer‐like rat model. Food Res Int. 2017;100(Pt 1):442‐448. 10.1016/j.foodres.2017.07.026 [DOI] [PubMed] [Google Scholar]
- 71. Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea—a review. J Am Coll Nutr. 2006;25(2):79‐99. 10.1080/07315724.2006.10719518 [DOI] [PubMed] [Google Scholar]
- 72. Takeda A, Sakamoto K, Tamano H, et al. Facilitated neurogenesis in the developing hippocampus after intake of theanine, an amino acid in tea leaves, and object recognition memory. Cell Mol Neurobiol. 2011;31(7):1079‐1088. 10.1007/s10571-011-9707-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sundberg M, Sahin M. Cerebellar development and autism spectrum disorder in tuberous sclerosis complex. J Child Neurol. 2015;30(14):1954‐1962. 10.1177/0883073815600870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Urdaneta KE, Castillo MA, Montiel N, Semprún‐Hernández N, Antonucci N, Siniscalco D. Autism spectrum disorders: potential neuro‐psychopharmacotherapeutic plant‐based drugs. Assay Drug Dev Technol. 2018;16(8):433‐444. 10.1089/adt.2018.848 [DOI] [PubMed] [Google Scholar]
- 75. McNamara FN, Randall A, Gunthorpe MJ. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1). Br J Pharmacol. 2005;144(6):781‐790. 10.1038/sj.bjp.07060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wattanathorn J, Chonpathompikunlert P, Muchimapura S, Priprem A, Tankamnerdthai O. Piperine, the potential functional food for mood and cognitive disorders. Food Chem Toxicol. 2008;46(9):3106‐3110. 10.1016/j.fct.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 77. Ornoy A, Weinstein‐Fudim L, Ergaz Z. Prevention or amelioration of autism‐like symptoms in animal models: will it bring us closer to treating human ASD. Int J Mol Sci. 2019;20(5):1074. 10.3390/ijms20051074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem‐Biol Interact. 2008;174(1):27‐37. 10.1016/j.cbi.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 79. Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197‐212. 10.1007/978-0-387-46401-5_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Al‐Askar M, Bhat RS, Selim M, Al‐Ayadhi L, El‐Ansary A. Postnatal treatment using curcumin supplements to amend the damage in VPA‐induced rodent models of autism. BMC Complement Altern Med. 2017;17(1):259. 10.1186/s12906-017-1763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bhandari R, Kuhad A. Neuropsychopharmacotherapeutic efficacy of curcumin in experimental paradigm of autism spectrum disorders. Life Sci. 2015;141:156‐169. 10.1016/j.lfs.2015.09.012 [DOI] [PubMed] [Google Scholar]
- 82. Panahi Y, Saadat A, Beiraghdar F, Sahebkar A. Adjuvant therapy with bioavailability‐boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double‐blind placebo‐controlled trial. Phytother Res. 2014;28(10):1461‐1467. 10.1002/ptr.5149 [DOI] [PubMed] [Google Scholar]
- 83. Solimini R, Rotolo MC, Pichini S, Pacifici R. Neurological disorders in medical use of cannabis: an update. CNS Neurol Disord Drug Targets. 2017;16(5):527‐533. 10.2174/1871527316666170413105421 [DOI] [PubMed] [Google Scholar]
- 84. Shen LL, Jiang ML, Liu SS, et al. Curcumin improves synaptic plasticity impairment induced by HIV‐1gp120 V3 loop. Neural Regen Res. 2015;10(6):925‐931. 10.4103/1673-5374.158358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Salgado CA, Castellanos D. Autism spectrum disorder and cannabidiol: have we seen this movie before. Glob Pediatr Health. 2018;5:2333794X18815412. 10.1177/2333794X18815412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Cannabinoids as novel anti‐inflammatory drugs. Future Med Chem. 2009;1(7):1333‐1349. 10.4155/fmc.09.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Perucca E. Cannabinoids in the treatment of epilepsy: hard evidence at last? J Epilepsy Res. 2017;7(2):61‐76. 10.14581/jer.17012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zamberletti E, Gabaglio M, Parolaro D. The endocannabinoid system and autism spectrum disorders: insights from animal models. Int J Mol Sci. 2017;18(9):1916. 10.3390/ijms18091916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brigida AL, Schultz S, Cascone M, Antonucci N, Siniscalco D. Endocannabinod signal dysregulation in autism spectrum disorders: a correlation link between inflammatory state and neuro‐immune alterations. Int J Mol Sci. 2017;18(7):1425. 10.3390/ijms18071425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ude C, Schubert‐Zsilavecz M, Wurglics M. Ginkgo biloba extracts: a review of the pharmacokinetics of the active ingredients. Clin Pharmacokinet. 2013;52(9):727‐749. 10.1007/s40262-013-0074-5 [DOI] [PubMed] [Google Scholar]
- 91. Fang W, Deng Y, Li Y, et al. Blood brain barrier permeability and therapeutic time window of Ginkgolide B in ischemia‐reperfusion injury. Eur J Pharmaceut Sci. 2010;39(1‐3):8‐14. 10.1016/j.ejps.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 92. Niederhofer H. First preliminary results of an observation of Ginkgo biloba treating patients with autistic disorder. Phytother Res. 2009;23(11):1645‐1646. 10.1002/ptr.2778 [DOI] [PubMed] [Google Scholar]
- 93. Hasanzadeh E, Mohammadi MR, Ghanizadeh A, et al. A double‐blind placebo controlled trial of Ginkgo biloba added to risperidone in patients with autistic disorders. Child Psychiatry Hum Dev. 2012;43(5):674‐682. 10.1007/s10578-012-0292-3 [DOI] [PubMed] [Google Scholar]
- 94. Malishev R, Shaham‐Niv S, Nandi S, Kolusheva S, Gazit E, Jelinek R. Bacoside‐A, an Indian traditional‐medicine substance, inhibits β‐Amyloid cytotoxicity, fibrillation, and membrane interactions. ACS Chem Neurosci. 2017;8(4):884‐891. 10.1021/acschemneuro.6b00438 [DOI] [PubMed] [Google Scholar]
- 95. Russo A, Borrelli F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine. 2005;12(4):305‐317. 10.1016/j.phymed.2003.12.008 [DOI] [PubMed] [Google Scholar]
- 96. Hoffman EJ, Turner KJ, Fernandez JM, et al. Estrogens suppress a behavioral phenotype in Zebrafish mutants of the autism risk gene, CNTNAP2. Neuron. 2016;89(4):725‐733. 10.1016/j.neuron.2015.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Al‐Ayadhi LY, Elamin NE. Camel milk as a potential therapy as an antioxidant in autism spectrum disorder (ASD). Evid‐Based Complement Alternat Med. 2013;2013:602834. 10.1155/2013/602834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kappeler S, Farah Z, Puhan Z. Sequence analysis of Camelus dromedarius milk caseins. J Dairy Res. 1998;65(2):209‐222. 10.1017/s0022029997002847 [DOI] [PubMed] [Google Scholar]
- 99. Zafra O, Fraile S, Gutiérrez C, et al. Monitoring biodegradative enzymes with nanobodies raised in Camelus dromedarius with mixtures of catabolic proteins. Environ Microbiol. 2011;13(4):960‐974. 10.1111/j.1462-2920.2010.02401.x [DOI] [PubMed] [Google Scholar]
- 100. Shabo Y, Barzel R, Margoulis M, Yagil R. Camel milk for food allergies in children. Isr Med Assoc J. 2005;7(12):796‐798. [PubMed] [Google Scholar]
- 101. Tillib SV, Ivanova TI, Vasilev LA. Fingerprint‐like analysis of “nanoantibody” selection by phage display using two helper phage variants. Acta Naturae. 2010;2(3):85‐93. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated during the study are mentioned in the paper.


