Abstract
Fluorescent amplified-fragment length polymorphism (FAFLP) analysis was carried out for an outbreak of group A streptococcal (GAS) invasive disease. Streptococcal genomic DNAs were digested with endonucleases EcoRI and MseI, site-specific adaptors were ligated, and PCR amplification was carried out with an EcoRI adaptor-specific primer labelled with fluorescent dye. Amplified fragments of up to 600 bp in size were separated on a polyacrylamide sequencing gel which contained internal size markers in each lane. These data were automatically scanned and analyzed, fragments were precisely sized (±1 bp), and electropherograms were generated for each genome with GeneScan 2.1 software. All isolates were compared in this way. Among 27 GAS isolates examined, we found 18 FAFLP profiles, compared with 12 macrorestriction profiles by pulsed-field gel electrophoresis. FAFLP readily distinguished genotypes for two clones of GAS serotype M77 which were responsible for outbreaks of invasive disease in a care-of-the-elderly system. It provided an automated analysis of the whole genome of bacterial isolates. It was reproducible, more discriminatory, and capable of higher throughput than other molecular typing methods. Given agreed conditions, FAFLP would be reproducible between laboratories for rapid characterization of outbreak strains.
Streptococcus pyogenes (Lancefield group A streptococcus [GAS]) is a major causative agent of noninvasive and invasive human disease, ranging from sore throat to the progressively destructive tissue infection necrotizing fasciitis. Its emm gene encodes the filamentous M protein that is the antigenic basis for classical (Lancefield) serotyping. This distinguishes more than 90 M serotypes of GAS (5, 6).
Various molecular methods have been used to characterize GAS isolates. They include multilocus enzyme electrophoresis (8), restriction endonuclease analysis of genomic DNA (2), ribotyping (1, 12), random amplified polymorphic DNA (RAPD) fingerprinting (10), PCR-restriction fragment length polymorphism (PCR-RFLP) analysis of the emm gene (13), and pulsed-field gel electrophoresis (PFGE) (11, 12). The most reproducible of these methods, PFGE, is time-consuming and labor-intensive. While the PCR-based methods are more rapid, RAPD analysis is poorly reproducible and PCR-RFLP analysis is restricted to polymorphic analysis of regions of one to several kilobases. A combination of existing methods has been used for high-resolution epidemiological analysis of streptococcal outbreaks (13). Nonetheless, it would be greatly advantageous to have a single PCR-based technique which could rapidly and reproducibly analyze the whole genome for accurate strain genotyping.
Amplified-fragment length polymorphism (AFLP) analysis is a novel PCR-based technique (15) which has been used for DNA fingerprinting of plant genomes. It requires no prior knowledge of genomic DNA sequences and potentially offers better discriminatory power and speed than the existing molecular typing techniques described above. As originally proposed, AFLP used radioactively labelled primers for PCR amplification of small genomic fragments defined by known restriction sites and adaptors. Several bacterial genera have been studied by radioactive AFLP; they include Legionella (14), Aeromonas, and Xanthomonas (3, 4).
In the present study, we have used AFLP analysis with a fluorescently labelled primer (FAFLP) for molecular epidemiological investigation of S. pyogenes outbreaks. We evaluated the potential of FAFLP for achieving precise fragment amplification and sizing, compared it with PFGE, and examined its potential for defining outbreak strain genotypes and for subtyping. We evaluated the instrumentation (ABI Prism 377 DNA automated sequencer; Perkin-Elmer Applied Biosystems) and software (GeneScan 2.1) in terms of data capture and quality and investigated the systematic use of FAFLP for an outbreak analysis: in this case, the analysis of an outbreak of invasive disease in a district general hospital caused by S. pyogenes serotype M77.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Twenty-six isolates of S. pyogenes from patients with invasive or noninvasive streptococcal diseases were obtained from the Streptococcus and Diphtheria Reference Unit, Respiratory and Systemic Infection Laboratory. They included a number of isolates from two outbreaks of streptococcal disease in a care-of-the-elderly system; the identities of these isolates (see Table 1) were not revealed until after FAFLP analysis (see below) was completed. Isolates were subjected to conventional M serotyping (5, 6) before and after genotype analysis. The type strain of serotype M77, NCTC 12057, was obtained from the National Collection of Type Cultures (Central Public Health Laboratory, London, United Kingdom). Streptococci were cultured aerobically at 37°C for 18 to 24 h on horse blood agar plates and were preserved for reference in blood glycerol (16% [vol/vol]) broth (Oxoid, Basingstoke, United Kingdom) at −70°C.
TABLE 1.
Epidemiology and genotypes of GAS isolates
| Isolate no.a | Yr/source of isolationb | Disease | M serotype | mrpsc | FAFLP profilesd |
|---|---|---|---|---|---|
| SA10 | 1997/NPH | Cellulitis, septicemia | M77 | P1 | A1 |
| SA78 | 1997/NPH | Ulcer | M77 | P1 | A1 |
| SA800 | 1997/NPH | Pyrexia, septicemia | M77 | P2 | A2 |
| SA907 | 1997/NPH | Wound infection | M77 | P2 | A2 |
| SA978 | 1997/NPH | Ulcer | M77 | P2 | A2 |
| SA1010 | 1997/NPH | Chest infection | M77 | P2 | A2 |
| SA1046 | 1997/NPH | Pyrexia, septicemia | M77 | P2 | A2 |
| SA1047 | 1997/NPH | Pyrexia, septicemia | M77 | P2 | A2 |
| SA1048 | 1997/NPH | Pyrexia, septicemia | M77 | P2 | A2 |
| SA1049 | 1997/NPH | Wound infection | M77 | P2 | A2 |
| SA512 | 1997/Whittington Hospital | Fatal septicemia | M77 | P3 | A3 |
| SA779 | 1997/Carlisle | Abscess fluid | M77 | P4 | A4 |
| SA1024 | 1997/Bury | Septicemia | M77 | P5 | A5 |
| SA1036 | 1997/Harold Wood | Pyrexia, septicemia | M77 | P6 | A6 |
| SA1200 | 1997/Ireland | Necrotizing fasciitis | M77 | P6 | A7 |
| SA1393 | 1997/Cheltenham | Ulcers, septicemia | M77 | P6 | A8 |
| SA2158 | 1996/Hull | Septicemia | M77 | P6 | A9 |
| SA1558 | 1997/London | Cellulitis | M77 | P2 | A10 |
| SA2311 | 1996/Dorchester | Cellulitis | M77 | P6a | A11 |
| SA1339 | 1997/Orpington | Septicemia | M77 | P7 | A12 |
| SA2321 | 1996/Birch Hill | Chest infection | M77 | P8 | A13 |
| SA2367 | 1996/Surrey | Necrotizing fasciitis | M77 | P6 | A14 |
| NCTC 12057 | 1972/Blackpool | Unknown | M77 | P9 | A15 |
| SA990 | 1997/Nottingham | Wound infection | M5 | P10 | A16 |
| SA1050 | 1997/NPH | Joint pain | M4 | P11 | A17 |
| SA1051 | 1997/NPH | Skin infection | M4 | P11 | A17 |
| SA1052 | 1997/NPH | Sore throat | M6 | P12 | A18 |
Isolate numbers were assigned by the Streptococcus and Diphtheria Reference Unit.
NPH, Northwick Park Hospital, London, United Kingdom. Isolates SA10 and SA78 were involved in the first outbreak studied, and isolates SA800, SA907, SA978, SA1010, SA1046, SA1047, SA1048, and SA1049 were involved in the second outbreak studied.
Macrorestriction profiles obtained with SmaI after PFGE.
FAFLP profiles obtained with the EcoRI+0 and MseI+T primer pair.
Standard nucleic acid methods.
Genomic DNA was extracted from streptococcal plate cultures as described previously (12). The concentration of DNA was estimated with a spectrophotometer (Beckman DU 640) by standard methods (9). PFGE was carried out following SmaI macrorestriction as described previously (13).
FAFLP.
Genomic DNA (500 ng) was digested in a total volume of 22 μl, which consisted of 5 U of MseI (New England BioLabs [NEB], Hertfordshire, England), 2 μl of 10× MseI buffer (NEB), 0.2 μl of 10-μg/ml bovine serum albumin (NEB), and 1.0 μl of DNase-free RNase A (10 μg/μl) for 1 h at 37°C. To this digest was added 5 U of EcoRI (Life Technologies, Paisley, United Kingdom), 1.68 μl of 0.5 M Tris-HCl (pH 7.6), and 2.1 μl of 0.5 M NaCl; the reaction mixtures were incubated for a further hour at 37°C. Endonucleases were then inactivated (65°C for 10 min) prior to ligation.
To the double-digested DNA, a 25-μl solution containing 5 pmol of EcoRI adaptor (Genosys Biotechnologies, Cambridge, United Kingdom), 50 pmol of MseI adaptor (Genosys Biotechnologies), 40 U of T4 DNA ligase (NEB), and 5 μl of 10× T4 ligase buffer (NEB) was added. The reaction mixture was incubated at 12°C for 17 h, heated at 65°C for 10 min to inactivate the ligase, and stored at −20°C. The sequence of the EcoRI adaptor was as follows:
5′- CTCGTAGACTGCGTACC CATCTGACGCATGGTTAA-5′
The sequence of the MseI adaptor was as follows:
5′- TACTCAGGACTCATC GAGTCCTGAGTAGCAG-5′
The forward primer (EcoRI adaptor specific, termed EcoRI+O) was labelled with a blue fluorescent dye, 5-FAM (5-carboxyfluorescein). The reverse primer (MseI adaptor specific) contained an extra selective base (MseI+T). PCRs were performed in 25-μl volumes containing 2.5 μl of ligated DNA, 16.6 pmol of FAM-labelled EcoRI primer (5′-GACTGCGTACCAATTC-3′; Genosys Biotechnologies), 100 pmol of MseI+T primer (5′-GATGAGTCCTGAGTAAT-3′; Genosys Biotechnologies), 2.5 μl of 10× Taq polymerase buffer (Life Technologies), 1.5 mM MgCl2 (Life Technologies), each of the four deoxynucleoside triphosphates at a concentration of 10 mM (Life Technologies), 1 μl of 10-μg/ml bovine serum albumin (NEB), and 0.625 U of Taq DNA polymerase (Life Technologies). Touchdown PCR cycling conditions were used for amplification as follows: denaturation for 2 min at 94°C (1 cycle), followed by 30 cycles of denaturation at 94°C for 20 s, a 30-s annealing step (see below), and a 2-min extension step at 72°C. The annealing temperature for the first cycle was 66°C; for the next nine cycles, the temperature was decreased by 1°C at each cycle. The annealing temperature for the remaining 20 cycles was 56°C. This was followed by a final extension at 60°C for 30 min. PCR was performed in a PE-9600 thermocycler (Perkin-Elmer Corp., Norwalk, Conn.). FAFLP reaction mixtures were stored at −20°C.
Gel analysis.
Amplification products were separated on a 5% denaturing (sequencing) polyacrylamide gel on an ABI Prism 377 DNA automated sequencer (Perkin-Elmer Corp.). The gel was prepared from 5% acrylamide-bisacrylamide (19:1; Amresco, Solon, Ohio) and 6.0 M urea in 1× TBE (89 mM Tris, 89 mM boric acid, 2 mM EDTA). To 50 ml of gel solution was added 250 μl of 10% ammonium persulfate and 35 μl of N,N,N′,N′-tetramethylethylenediamine (Amresco). Spacers and sharks-tooth combs were 0.2 mm in thickness. Gels were poured with the ABI 377 gel cassette and gel injection device and were allowed to polymerize at room temperature for at least 2 h. FAFLP reaction mixtures were electrophoresed directly or were diluted; 1.5 μl of the reaction mixture was added to 1.5 μl of loading dye (a mixture containing 1.25 μl of formamide, 0.25 μl of loading solution [dextran blue in 50 mM EDTA], and 0.5 μl of the internal size marker [GeneScan-2500 labelled with the red fluorescent dye ROX {6-carboxy-x-rhodamine}; Perkin-Elmer]). The sample mixture was heated at 95°C for 2 min, cooled on ice, and immediately loaded onto the gel. Running buffer was 1× TBE buffer, and the electrophoresis conditions were 2.5 kV at 51°C for 5 h. The well-to-read distance was 36 cm.
RESULTS
Epidemiology of the outbreak.
Two clusters of S. pyogenes infections affected elderly patients in North London in 1997. Cluster 1 occurred in January and involved two residents of a nursing home. The first patient presented with signs of cellulitis and septicemia, and blood cultures yielded GAS (isolate SA10, Table 1). Subsequent screening of staff and another resident yielded GAS (isolate SA78, Table 1) from that resident’s leg ulcer. Both isolates were serotyped as T13 M77.
Cluster 2 occurred over a 6-week period, from April to June, and involved 10 inpatients on three of four care-of-the-elderly wards at the local hospital (Northwick Park Hospital, London, United Kingdom). After the first four patients were identified, all staff, other patients, and the environment were screened, but no reservoirs or sources of infection could be identified.
Three new isolates were obtained on 1 day, one via the general practitioner of a patient who had been recently hospitalized and two from inpatients. All four wards were then closed for admissions or transfers, and sampling and screening of all patients, staff, and the environment were undertaken. Patients recently discharged from any of the relevant wards were identified, and samples for screening cultures were requested via their general practitioners. Four further S. pyogenes isolates were obtained, including one from a member of the staff. All the patient isolates were serotyped as T13 M77, while that from the staff member was T12 M12 (not included in this study).
There was regular transfer of patients between the nursing home involved in cluster 1 and the care-of-the-elderly wards at Northwick Park Hospital, raising the possibility that the two clusters could be linked.
Macrorestriction and PFGE.
Twenty-six S. pyogenes isolates and the type strain for serotype M77 were analyzed in a “blinded” manner, with their identities concealed until completion of genotypic analysis. The discriminatory power of PFGE was compared with that of FAFLP. Macrorestriction with SmaI generated 10 to 13 genomic fragments ranging in size from 40 to 500 kbp. However, because of their large size and the limitations of sizing from agarose gels, these fragments could only be indirectly and approximately sized.
Twelve PFGE profiles were observed among the 27 isolates; 2 of these shared the same profile, profile P1. Profile P2 was shared by nine isolates, while profile P6 was shared by six isolates (22%). Comparative data for PFGE and FAFLP analysis for the 27 isolates are presented in Table 1.
FAFLP.
FAFLP gel data consisted of precisely sized amplified fragments in the size range of 90 to 600 bp. Sequencing gel data were transformed with GeneScan 2.1 software (ABI) into electropherograms; these were color coded, overlaid, and visually inspected for polymorphisms. Two kinds of experiments were carried out to test the reproducibilities of the FAFLP profiles and the precision of sizing of amplified fragments. First, the same DNA preparation was used in different FAFLP reaction mixtures and was then analyzed on the same gel and also on different gels. Second, different DNA preparations from the same strain or isolate were subjected to the same FAFLP reaction (conditions) and were analyzed as described above. Under all these experimental conditions, the characteristic amplified fragment profile (size range, 90 to 600 bp) was reproducibly detected, and none of the fragment sizes varied in any instance by more than 1.0 bp. The FAFLP procedure was rapid and easy to use and could discriminate diverse strain genotypes among isolates.
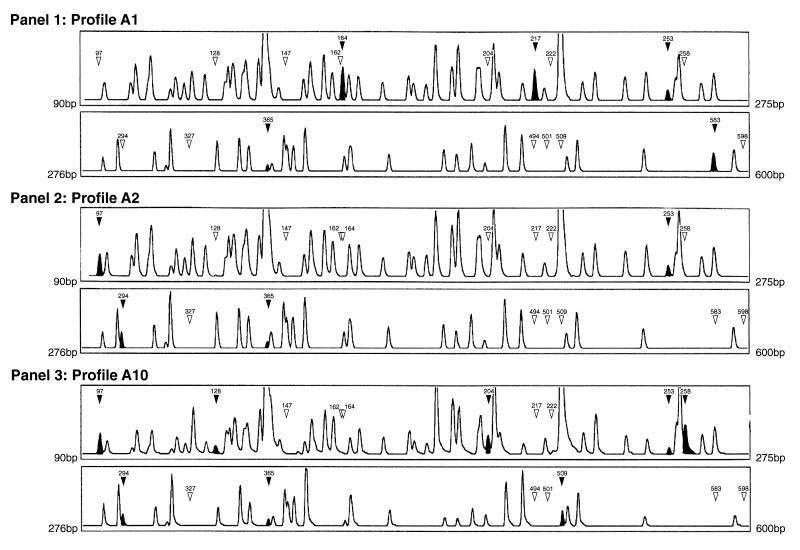
FAFLP generated 100 to 120 fragments upon amplification with the selective primers EcoRI+0 and MseI+T. The sizes of the amplified fragments ranged from 60 to 600 bp; fragments of less than 90 bp were discounted from GeneScan 2.1 analysis due to inadequate resolution in this size range. Peak height (Fig. 1) indicates the relative fluorescence of the detected fragments. Peak height thresholds were set at 125; any peak heights of less than this value were not included in the analysis. Peak height was not found to vary between replicate runs with identical DNAs. Examples of the electropherograms derived by GeneScan 2.1 analysis for three EcoRI+0 plus MseI+T amplifications (i.e., FAFLP profiles for three different strains) are shown in Fig. 1.
FIG. 1.
GeneScan 2.1 software-derived electropherograms (FAFLP profiles) for EcoRI+0 plus MseI+T amplifications of three GAS genomes. Panel 1, profile A1; the first outbreak strain (Table 1); the two sections represent FAFLP fragments from 90 to 275 bp and from 276 to 600 bp, respectively. Panel 2, profile A2; the second outbreak strain. Panel 3, profile A10; a non-outbreak-related isolate which had the same PFGE profile as that of the second outbreak strain shown in panel 2. The solid arrowheads and peaks indicate a fragment characteristic of that profile (sizes are indicated in base pairs). Open arrowheads indicate the absence of a polymorphic fragment from that profile.
The GeneScan 2.1 software could overlay up to 16 electropherograms for comparison between isolates. We thereby identified two genotypes, to which we could assign two (Fig. 1, panel 1) and eight (Fig. 1, panel 2) isolates, respectively. The first FAFLP genotype (profile A1; Fig. 1, panel 1) was characterized by a unique combination of 18 polymorphic fragments. It contained 5 characteristic fragments of 164, 217, 253, 365, and 583 bp (solid arrowheads and peaks, Fig. 1) and lacked 13 polymorphic fragments (open arrowheads, Fig. 1) found in other profiles. We assigned eight isolates to a second FAFLP genotype (profile A2; Fig. 1, panel 2). This contained four fragments of 97, 253, 294, and 365 bp (solid arrowheads and peaks, Fig. 1) and did not contain any of 14 polymorphic fragments (open arrowheads, Fig. 1) found in other profiles.
When the experiment was unblinded, profiles A1 and A2 were found to include 10 serotype M77 isolates from the two North London outbreaks. Profile A1 was shared by two isolates from the first outbreak, while profile A2 was shared by eight isolates from the second outbreak. Thirteen non-outbreak-related serotype M77 isolates also included in the study were circulating isolates found in diverse geographical areas of the United Kingdom. In Table 2 we summarize the polymorphisms observed in the FAFLP profiles for all serotype M77 isolates studied. The discriminatory power of FAFLP exceeded that of PFGE (80 to 90 FAFLP fragments versus 10 to 13 PFGE fragments). For example, the circulating serotype M77 isolate SA1558 showed the same PFGE profile as the second outbreak strain but exhibited a distinct FAFLP profile, profile A10 (Fig. 1, panel 3). It contained a unique combination of eight fragments of 97, 128, 204, 253, 258, 294, 365, and 509 bp and did not contain 10 polymorphic fragments found in other profiles. Similarly, six circulating isolates of serotype M77 sharing the same PFGE profile were each assigned a unique FAFLP profile (Table 1).
TABLE 2.
FAFLPs characterizing serotype M77 strains
| Strain no. | FAFLP profile | Presence or absence of fragments of the following sizes (bp)a
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 97 | 128 | 147 | 162 | 164 | 204 | 217 | 222 | 253 | 258 | 294 | 327 | 365 | 494 | 501 | 509 | 583 | 598 | ||
| SA10b | A1 | − | − | − | − | • | − | • | − | • | − | − | − | • | − | − | − | • | − |
| SA800c | A2 | • | − | − | − | − | − | − | • | − | • | − | • | − | − | − | − | − | |
| SA512 | A3 | − | − | • | − | • | − | − | − | − | • | • | • | − | − | − | • | − | • |
| SA779 | A4 | − | − | • | − | • | − | − | − | • | − | • | − | • | − | − | • | − | − |
| SA1024 | A5 | • | • | − | − | • | • | − | − | • | • | • | • | • | − | − | • | − | • |
| SA1036 | A6 | • | • | − | − | − | • | − | • | • | − | • | − | − | − | − | − | − | − |
| SA1200 | A7 | • | − | − | − | • | − | − | − | • | − | • | − | • | − | − | • | − | − |
| SA1393 | A8 | • | • | − | − | • | − | − | − | − | − | • | − | − | − | − | − | − | − |
| SA2158 | A9 | − | − | − | − | − | • | • | − | • | • | − | − | − | − | − | • | • | − |
| SA1558 | A10 | • | • | − | − | − | • | − | − | • | • | • | − | • | − | − | • | − | − |
| SA2311 | A11 | • | − | − | • | − | − | − | − | • | − | • | − | − | • | • | − | − | − |
| SA1339 | A12 | • | − | − | − | − | − | − | • | − | − | − | − | − | − | − | − | − | − |
| SA2321 | A13 | • | − | − | − | • | • | − | − | • | • | • | − | − | − | − | • | − | − |
| SA2367 | A14 | • | − | − | − | • | − | − | − | − | • | − | − | − | − | − | − | − | − |
| NCTC 12057 | A15 | − | − | • | − | • | − | − | − | • | − | • | − | • | − | − | − | − | − |
The presence or absence of differential fragments is indicated. •, a fragment characteristically present in that FAFLP profile; −, characteristic absence of fragment from that profile.
FAFLP profile found for two isolates.
FAFLP profile found for eight isolates.
Single isolates of three other GAS serotypes which had been included generated distinct FAFLP profiles. The polymorphisms observed between the isolates of other serotypes (serotypes M4, M5, and M6) were different from those observed between isolates of serotype M77. Two contemporaneous isolates of serotype M4, also from Northwick Park Hospital and with identical PFGE profiles, had the same FAFLP profile, profile A17.
DISCUSSION
We have demonstrated that FAFLP can define the genotype of an outbreak strain by reproducibly detecting and precisely sizing amplified fragments unique to that genome. In this respect, FAFLP qualifies as a true “typing” technique, since different laboratories using the same reagents and instrumentation could determine that FAFLP genotypes are identical, even when the isolates are from different sources and times. The FAFLP data presented here are intrinsically more reproducible than those obtained by other PCR-based methodologies, such as RAPD analysis, that are based on amplification with degenerate primers and that do not generate fingerprints reproducible enough for interlaboratory comparison (7).
We found that the discriminatory power of FAFLP was considerably higher than that of PFGE. For example, FAFLP could differentiate the genotypes of the two outbreak strains from those of the circulating isolates of the same serotype. Among 13 circulating isolates of serotype M77, FAFLP could assign unique profiles to each, whereas PFGE could not differentiate between 6 of them. The number of datum points (fragments) available for comparison and definition of strain genotype is almost eight times more for FAFLP than for PFGE. The sizing of the fragments by FAFLP is also much more precise (±1 bp) than that by PFGE. FAFLP was less labor intensive, could be performed more rapidly (2 to 2.5 days for FAFLP versus 3.5 to 4 days for PFGE from pure culture), and was easier to perform than PFGE.
As well as subtyping isolates within an M serotype (M77), FAFLP distinguished between different M serotypes of GAS. This application is documented by other preliminary studies in our laboratory (data not shown) and may allow organisms with homogeneous serotypes to be rapidly grouped together.
The discriminatory power of FAFLP can be systematically varied by performing the amplification with primers of specified selectivity to produce different numbers and sizes of amplified fragments. The technique can thereby be tailored to the level of typing discrimination required. FAFLP profiles are suitable for rapid electronic transmission for interlaboratory comparison and are well suited for storage in epidemiological databases for future comparison. The capacity of the automated equipment to scan amplified fragments in gels is high: 36 lanes can be loaded with different FAFLP reaction mixtures, and fragments of from 50 to 600 bp can be read in 5 h. We consider that these features make FAFLP an excellent tool for the rapid and definitive analysis of outbreaks, even some due to apparently clonal subtypes that are not susceptible to subdivision by existing phenotypic techniques or by other currently used molecular techniques.
ACKNOWLEDGMENT
We thank Philip Mortimer for critical reading of the manuscript.
REFERENCES
- 1.Bingen E, Denamur E, Lambert-Zechovsky N, Boissinot C, Brahimi N, Aujard Y, Blot P, Elion J. Mother-to-infant vertical transmission and cross-colonization of Streptococcus pyogenes confirmed by DNA restriction fragment length polymorphism analysis. J Infect Dis. 1992;165:147–150. doi: 10.1093/infdis/165.1.147. [DOI] [PubMed] [Google Scholar]
- 2.Cleary P P, Kaplan E L, Handley J P, Wlazlo A, Kim M H, Hauser A R, Schlievert P M. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet. 1992;339:518–521. doi: 10.1016/0140-6736(92)90339-5. [DOI] [PubMed] [Google Scholar]
- 3.Huys G, Kersters I, Coopman R, Janssen P, Kersters K. Genotypic diversity among Aeromonas isolates recovered from drinking water production plants as revealed by AFLPTM analysis. Syst Appl Microbiol. 1996;19:428–435. [Google Scholar]
- 4.Janssen P, Coopman R, Huys G, Swings J, Bleeker M, Vos P, Zabeau M, Kersters K. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology. 1996;142:1881–1893. doi: 10.1099/13500872-142-7-1881. [DOI] [PubMed] [Google Scholar]
- 5.Johnson D R, Kaplan E L, Sramek J, Bicova R, Havlicek J, Havlickova H, Motlova J, Kriz P. Laboratory diagnosis of group A streptococcal infections. Geneva, Switzerland: Oriental Press; 1996. [Google Scholar]
- 6.Lancefield R C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962;89:307–313. [PubMed] [Google Scholar]
- 7.Meunier J R, Grimont P A. Factors affecting reproducibility of random amplified polymorphic DNA fingerprinting. Res Microbiol. 1993;144:373–379. doi: 10.1016/0923-2508(93)90194-7. [DOI] [PubMed] [Google Scholar]
- 8.Musser J M, Hauser A R, Kim M H, Schlievert P M, Nelson K, Selander R K. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci USA. 1991;88:2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 10.Seppala H, He Q, Osterblad M P, Huovinen P. Typing of group A streptococci by random amplified polymorphic DNA analysis. J Clin Microbiol. 1994;32:1945–1948. doi: 10.1128/jcm.32.8.1945-1948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Single L A, Martin D R. Clonal differences within M-types of the group A Streptococcus revealed by pulsed field gel electrophoresis. FEMS Microbiol Lett. 1992;70:85–89. doi: 10.1016/0378-1097(92)90567-8. [DOI] [PubMed] [Google Scholar]
- 12.Stanley J, Linton D, Desai M, Efstratiou A, George R. Molecular subtyping of prevalent M serotypes of Streptococcus pyogenes causing invasive disease. J Clin Microbiol. 1995;33:2850–2855. doi: 10.1128/jcm.33.11.2850-2855.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanley J, Desai M, Xerry J, Tanna A, Efstratiou A, George R. High-resolution genotyping elucidates the epidemiology of group A streptococcal outbreaks. J Infect Dis. 1996;174:500–506. doi: 10.1093/infdis/174.3.500. [DOI] [PubMed] [Google Scholar]
- 14.Valsangiacomo C, Baggi F, Gaia V, Balmelli T, Peduzzi R, Piffaretti J. Use of amplified fragment length polymorphism in molecular typing of Legionella pneumophila and application to epidemiological studies. J Clin Microbiol. 1995;33:1716–1719. doi: 10.1128/jcm.33.7.1716-1719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kulper M, Zabeau M. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]