Summary
Background:
Heavy menstrual bleeding (HMB) occurs in 80% of females with von Willebrand disease (VWD) and is associated with iron deficiency and poor response to current therapies. International guidelines indicate low certainty regarding effectiveness of hormonal and nonhormonal therapy, including the nonhormonal standard tranexamic acid (TA). While VWF concentrate is approved for bleeds, no prospective trials guide its use in HMB. We hypothesized rVWF is superior to TA in reducing HMB.
Methods:
The aim of this multicenter (13-site), randomized, crossover, unblinded open-label trial was to determine the efficacy of recombinant VWF (Vonvendi®, rVWF) vs. tranexamic acid (Lysteda®, TA) in reducing HMB in VWD. Subjects 13–45 years of age with VWD, defined as VWF:RCo<0.50 IU/mL, and HMB, defined as a pictorial blood assessment chart (PBAC) score >100 in one of the last two cycles, were assigned in a 1:1 allocation ratio using \\\permuted block randomization to two consecutive cycles each of intravenous rVWF, 40 IU/kg on day 1, and oral TA 1,300 mg t.i.d. on days 1–5, the order determined by randomization. The primary outcome was a 40-point reduction in PBAC score by day 5 after two cycles per treatment. There was no washout. The primary endpoint was analyzed in all randomized patients with a least one post-baseline PBAC score, using a 4-period 2-group AABB/BBAA crossover design. The trial was registered at www.clinicaltrials.gov (NCT 02606045).
Findings:
36 subjects were enrolled between February 12, 2019 and November 16, 2021. Median follow-up was 23.97 weeks (IQR:21.81, 28.14). Mean PBAC score was significantly lower after 2 cycles with TA, than rVWF, 226 (95% CI: 188, 264) in TA-treated and 272 (95%CI: 230, 314) in rVWF-treated subjects, resulting in a mean treatment effect of 46 (95% CI: 2, 90), p=0.039. There were no serious adverse events or treatment-related deaths.
Interpretation:
rVWF is inferior to TA in reducing HMB in subjects with mild or moderate VWD, with neither treatment showed a clinically relevant effect, > 40 points difference in the score.
Funding:
National Heart Lung Blood Institute, NIH, Bethesda MD, NHLBI U01 HL133815-01A1
Keywords: Heavy Menstrual Bleeding, von Willebrand Disease, von Willebrand Factor, Women’s Health, Tranexamic Acid
Introduction
Von Willebrand disease (VWD) is the most common inherited bleeding disorder, with a symptomatic prevalence of 0.1% of the population.1 VWD results from deficient or defective von Willebrand factor (VWF), a multimeric protein which facilitates platelet binding to collagen at sites of injury, and is characterized by mucosal bleeding in the oropharyngeal, gastrointestinal, and genitourinary tracts.2 Among women and girls with VWD, up to 80% have heavy menstrual bleeding (HMB)3 defined as excess menstrual blood loss exceeding 80cc per cycle, which affects quality of life, with iron deficiency, and cognitive, physical, and psychological defects.4.5 Up to 30% of women who use hormonal or nonhormonal therapies for HMB find them ineffective or cannot tolerate them.6, The lack of effective therapies for HMB remains a major unmet healthcare need in VWD.6, Few randomized trials are available to guide treatment, and international guidelines indicate low certainty regarding the effectiveness of hormonal and nonhormonal therapy in HMB.7 Among the latter, tranexamic acid (TA, Lysteda®), a nonhormonal antifibrinolytic agent, is considered to have the least undesirable side effects as compared with hormonal therapy or desmopressin,7 but it requires three doses daily for five days each of the menstrual cycle. While VWF factor concentrate (pdVWF: Humate-P® or rVWF, Vonvendi®) safely reduces bleeding events in VWD,8,9 it is costly and requires intravenous infusion and few data are available regarding its use in HMB. In a summary of studies to date, 355 subjects with VWD receiving VWF for bleeds, 88 (24.8%) were women with HMB, of whom 84 received pdVWF for 1–6 days/cycle, and 4 received rVWF for 1–2 days/cycle, at a median dose of 43 IU/kg, with 95% reduction in bleeds and no adverse effects.10 Given its purity and prolonged half-life,11,12 rVWF represents a compelling alternative therapeutic for HMB that might be effective on 1 or 2 treatment days/cycle. We, therefore, conducted a randomized crossover trial comparing rVWF with the standard nonhormonal treatment, TA, in reducing HMB in women and girls with VWD.
Methods
Study design
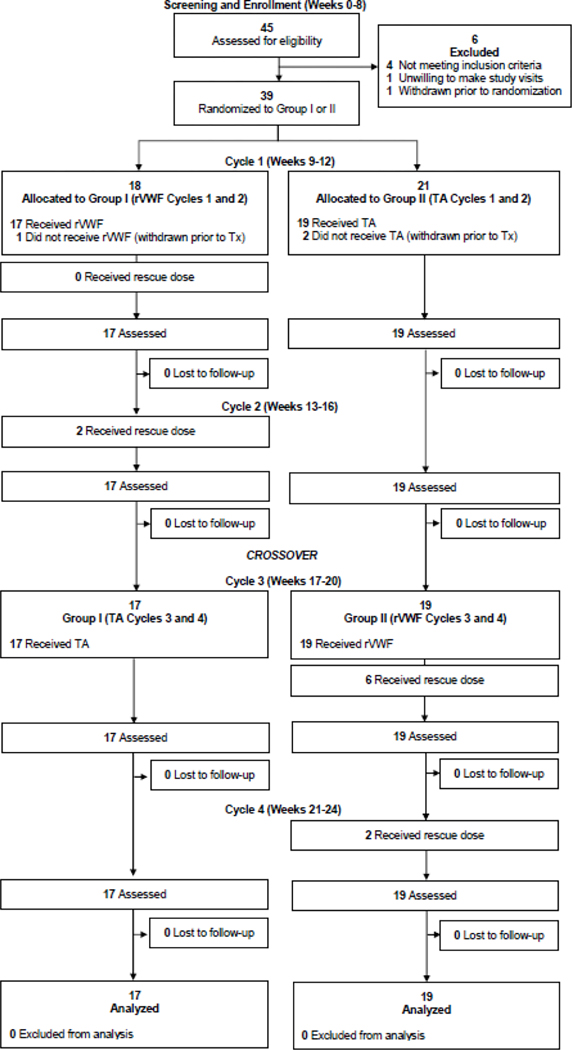
This phase 3, prospective outpatient, open-label, 1:1 randomized crossover NHLBI-funded U01 trial (ClinicalTrials.gov NCT0206045) evaluated HMB by PBAC score in subjects with VWD after 2 cycles each of intravenous recombinant von Willebrand factor (Vonvendi®, rVWF) or oral tranexamic acid (Lysteda®, TA). The research was approved by the relevant institutional review boards or ethics committees and all participants gave written informed consent. The work was funded by Grant NHLBI grant U01HL133815 from the Heart Blood and Lung Institute, National Institutes of Health, Bethesda, Maryland. Recombinant von Willebrand factor, Vonvendi®, was provided by Takeda Pharmaceuticals U.S.A. The trial was conducted at 18 U.S. sites, 13 of which enrolled subjects from February 12, 2019 to November 16, 2021. A flow diagram of the trial is provided in CONSORT format, Figure 1. The interim analysis reported in this paper was unplanned and performed at the request of the DSMB due to slow recruitment.
Figure 1. VWDMin Trial CONSORT Diagram.
The number of subjects screened, excluded, withdrawn, enrolled, randomized, with study arm allocation, and completing the trial are included.
Participants
The study enrolled subjects who met all the following inclusion criteria; age 13–45 years of age with mild or moderate VWD; historical VWF:RCo < 0.50 IU/mL; past bleeding; HMB defined as PBAC > 100 in at least one of the previous two menstrual cycles; no prior history of allergic reaction or anaphylaxis to rVWF or TA; and willingness i) to have blood drawn; ii) to avoid ASA and nonsteroidal anti-inflammatory agents during study; iii) to comply with randomization to rVWF or TA study arms; iv) to keep a personal diary of HMB bleeding frequency duration and severity by PBAC, and any drugs or hemostatic agents taken; v) to make 4 visits, undergo blood sampling for coagulation studies, and accept randomization of two therapies for each of four consecutive menstrual cycles including an end-of-study visit; and vi) to use double-barrier method of contraception during the study. All subjects reported PBAC > 100 in both cycles and had other bleeding symptoms. Exclusion criteria were a bleeding disorder other than VWD; past thrombosis; pregnant or lactating; use of hormones (other than progesterone-only), combined oral contraceptives, or contraceptive implants in the past 3 months; platelet count < 100,000/μl; use of immunomodulatory or experimental drugs; surgery within the past 8 weeks; concomitant antiplatelet drugs, anticoagulants, dextran, aspirin or NSAIDs; treatment with DDAVP, cryoprecipitate, whole blood, plasma, or plasma derivatives containing VWF within 5 days of study; inability to comply with study requirements; hypothyroidism defined by elevated TSH; iron deficiency defined by low serum ferritin, unless iron replacement was initiated; or history of renal disease. Hormonal contraceptives were avoided due to a black box warning regarding thrombosis risk when the latter are used with TA. All patients provided written informed consent.
Randomisation and masking
Subjects were allocated to the two crossover groups in a 1:1 ratio using permuted block randomization with random block sizes of 4 and 6 to minimize the likelihood of knowing the assignment of subsequent participants. Th allocation sequence was generated by the unblinded statistician (SDR) prior to enrollment of any subjects and stored in a secure database at the Data Coordinating Center for concealment. Aside from the unblinded statistician, no members of the study team had access to the full sequence. Once eligibility was confirmed, clinical coordinators enrolled participants within 72 hours of screening and assigned them to their randomized study group via the electronic web portal hosted at the Data Coordinating Center. There was no blinding or masking.
Procedures
The order of study drugs was assigned to subjects in a 1:1 allocation ratio by permuted block randomization, either to IV infusion of rVWF 40 IU/kg day 1 or oral TA 1300 mg three times daily on days 1–5 in each of two consecutive menstrual cycles. There was no washout period as the half-life of the study drugs was shorter than the interval between cycles. The order of drugs was determined by randomization; in Group 1, rVWF was given in cycles 1 and 2, and TA in cycles 3 and 4; in Group 2, TA was given in cycles 1 and 2, and rVWF in cycles 3 and 4. A rescue dose of rVWF 40 IU/kg could be given, at the discretion of the participant, on day 2 of cycles in which rVWF was given. All drugs were given open label with no masking. Subjects were screened during routine clinic at participating U.S. hemophilia treatment centers (HTCs) (Appendix, page 80). Following confirmation of eligibility, subjects were randomized 1:1 by accession number linked with the randomization schema in the database, within 72 hours of screening. Subjects randomized to Group 1 received Arm A, rVWF 40 IU/kg intravenously on day 1 of cycles 1 and 2, and were crossed over to Arm B, TA 650 mg 2 tablets orally three times daily on days 1–5 of cycles 3 and 4. Subjects randomized to Group II received Arm B for each of two menstrual cycles, Cycles 1 and 2, and were crossed over to Arm A, for each of two menstrual cycles, Cycles 3 and 4. There were no planned interim analyses or stratification. Study drugs were prepared and shipped for each study subject by the contract research organization, McKesson Inc., Irving TX, to participating HTCs, where they were stored at 2–8°C until given to study subjects. Following HTC receipt of study drug shipment, the 24-week trial began with the first menstrual cycle following randomization. rVWF was given at 40 IU/kg by intravenous infusion over 5–10 minutes by standard intravenous technique into a vein on day 1 of menstrual bleeding. Study subjects were trained in intravenous technique by study nurses (or administered by home visiting nurse or HTC nurse). TA was given as two 650 mg tablets (1300 mg) orally three times daily on the first 5 days of menstrual bleeding during each of 2 consecutive cycles. All enrolled subjects were followed for up to 24 weeks, with follow-up visits at week 16 (post cycle 2) and 24 (post cycle 4, end-of-study. During the two cycles subjects were randomized to rVWF, an additional “rescue” dose was allowed the following day for bleeding not relieved after one dose. Each subject determined if her bleeding might require a “rescue” dose of rVWF. This was recorded in the patient diary and also verbally reported to the study team. There was no blinding and there was no washout period as the half-life of study drugs was shorter than the interval between cycles. The treatment schedule was two consecutive cycles on each drug, assessed after 5 days each. Protocol deviations included expansion of inclusion criteria to type 1 or type 2 VWD, age to 13–45, and clarification of allowance of progesterone-only hormonal agents. The interim analysis reported in this paper was unplanned and performed at the request of the DSMB due to slow recruitment. Stopping rules that, if reached, would halt the trial included 1) uncontrolled menstrual bleeding, 2) thrombosis, or 3) grade 2–5 allergic reactions (Appendix, page 40).
Outcomes
The primary outcome measure was the PBAC score,13,14 assessed by each subject using a PBAC chart and recorded in diary for each cycle of treatment (two cycles per treatment, per subject). To standardize measurement of menstrual blood loss, all subjects received a 4-month supply of Kotex super-plus tampons and Kotex super-plus maxi-pads for exclusive use on this trial. PBAC was evaluated after 2 cycles each on rVWF or TA, as 2 cycles provide as much information regarding PBAC change as 6 cycles.15
Secondary efficacy outcome measures included cycle severity (CS) and cycle length (CL), also measured for each cycle of treatment, as well as four quality-of-life (QoL) surveys, measured three times overall: at baseline, after completing two cycles of the first study treatment, and after completing two cycles of the second study treatment.
The QoL surveys included Short-Form-36 (SF-36),16 a 36-item general health survey in eight areas of physical and mental health, validated in women of reproductive age; the Ruta Menorrhagia Severity Score,17 a 15-item instrument measuring physical, psychological, and social effects of menorrhagia; the CDC Health-Related Quality of Life (HRQoL-14) Instrument,18,19 a 14-item instrument assessing the number of physically and mentally unhealthy days in the past 30 days, standardized for women of reproductive age; and the Center for Epidemiology Studies Depression (CES-D) Scale,20 a 20-item screen for depression assessing depressive symptoms over a range of ages and demographic groups. The Ruta Menorrhagia, CES-D, and SF-36 were each measured once after completion of two cycles on first study treatment and once after completion of two cycles on second study treatment, totaling 36 observations for each treatment, one per treatment, per participant.
Safety was assessed by 1) HMB blood loss unresponsive to study drugs, defined as a fall in hemoglobin >2 gm/dL from baseline, transfusion, or cardiopulmonary resuscitation, 2) thrombosis, 3) other bleeding, and 4) allergic reactions. Adverse events were graded by CTCAE Adverse Event Grading, v.4.21 VWF assays performed on baseline citrated samples included as previously described,22–25 including VWF:RCo by platelet agglutination (Chronolog Corp, Haverton PA) using a Chronolog aggregometer; VWF:Ag by “sandwich” ELISA using anti-VWF antibodies (DakoA0082, Carpintera CA); FVIII:C by chromogenic substrate assay (Coamatic, DiaPharma Group, Westchester OH); VWF:GPIbM by ELISA (Versiti, Milwaukee WI); and VWF multimer distribution visualized via hydrogel electrophoresis using Hydrasys 2 (Sebia, France). Historic VWF:RCo < 50 IU/dL were required before enrollment, while baseline VWF assays were obtained after enrollment while on the trial, and subject to stress-associated increase,15 including VWF:RCo > 50 IU/dL.
A Satisfaction Survey was completed after 2 cycles of rVWF, in which participants rated rVWF treatment with their usual treatment for HMB, the difficulty of rVWF use, and if rVWF would be considered for use in future menstrual cycles. Healthcare utilization measures included medication doses received, emergency department visits, hospitalizations, iron infusions and/or transfusions received, and days lost from school/work. Per patient direct and indirect costs were estimated using Federal Supply Schedule listings for medication costs, CMS data for emergency department visit costs, and Bureau of Labor Statistics wage data for cost of missed school or work.
Next generation sequencing of VWF was performed on baseline sample genomic DNA, enriched for the complete coding regions and splice sites (±10 bp) using Sophia Clinical Exome Solution, a custom oligonucleotide-based capture method followed by next generation sequencing (NextSeq, Illumina) with 2×150 paired-end reads. Reads were mapped to human genome reference GRCh37(hg19), and sequence variants and copy number variants (CNVs) in VWF were detected using Sophia Genetics DDM software. Classification of variants was performed using the LOVD VWF Database https://databases.lovd.nl/shared/genes/VWF, Clinvar, and AMCG variant interpretation guidelines,26 with coverage of >100x for all variants identified. All pathogenic and likely pathogenic variants identified were previously identified and classified.
Statistical analysis
As rVWF is an intravenous medication and more costly, we considered it would have to demonstrate superiority to TA. We initially designed the trial to have sufficient statistical power to test for superiority of rVWF over TA with a presumed clinically important difference of 40 points in the PBAC score. A 40-point difference was determined to be the minimal clinically important difference, based on 1) data from a trial of TA in women with bleeding disorders which found that 40% had a 50-point reduction from baseline score of 100 in PBAC after 2 cycles,23 and 2) the greater burden of rVWF (IV route, cost) than TA, such that rVWF should improve PBAC by 40 points more than TA to be adopted into practice. The prespecified sample size was 60, adjusted to 66 for an estimated 10% dropout rate.
The analysis was planned as an intent-to-treat analysis in which all participants with any post-randomization data were included. We assumed a two-sided type 1 error rate of 0.05, a 4-period 2-group (AABB/BBAA) crossover design, an estimated between-subject standard deviation (SD) of 63 points, and a within-subject SD of 100 points. Under these assumptions, a sample size of 60 participants would have 84% power to detect a mean PBAC reduction of 40 points between rVWF and TA. To allow for potential dropout, recruitment of up to 66 participants was planned to allow for 10% dropout. There were no planned interim analyses for efficacy, but the Data and Safety Monitoring Board (DSMB) met every 6 months to review trial enrollment, participant completion, and safety data. The trial enrolled its first participant on February 12, 2019. By the January 26, 2022 DSMB meeting, 39 participants had been randomized, still short of the target of 60 participants, and thus the DSMB requested the unblinded statistician (SDR) to perform a conditional power calculation, in which data from the first 36 participants that had any outcome data were combined with additional simulated participants under varying assumptions about the within- and between-person variability while still assuming the original minimal important difference on yet-to-be-recruited participants. The conditional power simulations suggested a very low (<1%) probability that continuing to 60 participants would reach a conclusion of efficacy for the experimental agent (rVWF) versus the control agent (TA) (Appendix, page 75). Based on the slow enrollment and low conditional power, the DSMB recommended and NHLBI accepted that study enrollment cease, and this recommendation was communicated to the study team on February 15, 2022. At this time 39 participants had been randomized; 34 had completed the protocol, 2 were still active in the protocol, 2 had withdrawn from the study without ever starting study treatment, and 1 had been randomized but not yet started study drug. The latter two who withdrew were randomized to Study Group II had enrolled but had not started study drug at the time the NHLBI recommended study closure. The NHBLI advised that the 2 participants who had already initiated study drug could complete the protocol, but that the participant who had not yet started study drug be administratively withdrawn to expedite study closeout.
Safety stopping events included 1) uncontrolled menstrual bleeding defined as >2 gm% fall in hemoglobin from baseline, and/or requirement for RBC transfusion and/or cardiopulmonary resuscitation; 2) thrombosis, or 3) grade 2–5 allergic reactions (Appendix, page 40). These events were counted once only for a given subject, assessed by severity, frequency, and relationship of AEs to study intervention, start date, stop date, severity, relationship, expectedness, outcome, and duration, and were analyzed using descriptive statistics, by study subject and study arm, and evaluated for statistical significance using McNemar’s test for paired proportions.
Outcomes were analyzed using a modified intention-to-treat principle. Specifically, all subjects with at least one post-randomization data point were included in all endpoint and safety analyses. Descriptive characteristics are reported as mean (SD) (or median [interquartile range] as appropriate) for continuous variables and frequency (percentage) for categorical variables. The primary endpoint, PBAC score, was compared between rVWF and TA using a linear mixed-effects model fit via restricted maximum likelihood estimation. Fixed effects included treatment and menstrual cycle (both categorical) as well as average baseline PBAC score, with a random intercept for each participant (to account for the inclusion of multiple cycles on each treatment for each participant). Average baseline PBAC score was adjusted for, given that all participants had PBAC scores from two cycles pre-randomization. Due to the skewed distribution of PBAC scores, the natural logarithm of PBAC score was used as the outcome in the primary model with average baseline PBAC score similarly transformed. Prior to fitting the main effects model, we fit a model that also included a treatment-by-cycle interaction term; as the interaction was not significant, we assumed no carryover effects and proceeded with the main effects model as described above. In addition to the primary analysis on the transformed scale, we report results from a model using the raw (untransformed) PBAC scores. Least-squares means for treatment effects, and the difference between them, were estimated with 95% CIs to determine if significant differences in outcomes exist between rVWF and TA.
Continuous secondary outcomes (e.g., CL and QoL measures) were analyzed with linear mixed-effects models structured similarly to the primary outcome model (replacing the baseline PBAC score with the corresponding baseline measure of each outcome as a covariate in the model). Similar to the analysis of PBAC scores, two baseline values for CL and CS were obtained prior to randomization and were averaged prior to adjustment in statistical models. Categorical secondary outcomes are reported using generalized linear mixed-effects models (GLMMs) with a logit link function and again the same fixed effects and random effects as the primary outcome model; CS rating (0,1,2,3) was treated ordinally and analyzed using a GLMM with a cumulative logit link while adjusting for the average of the two baseline CS values. Results are presented as odds ratio (95% CI), where OR>1 indicates higher odds of greater severity. Cycle length>5 days, any clots, and any flooding (binary outcomes) were analyzed using a generalized linear mixed-effects model with a logit link function including fixed effects for treatment, menstrual cycle, baseline score, and random intercept for participant. Results are presented as odds ratio (95% CI), where OR>1 indicates higher odds of cycle length>5 days, any clots during cycles, and any flooding during cycles. All statistical analyses were performed using R version 4.1.3. The primary outcome (PBAC) was tested using a two-sided hypothesis test and alpha=0.05; secondary outcomes are reported without formal testing or adjustment for multiple comparisons. The trial was registered at was registered at www.clinicaltrials.gov (NCT 02606045).
For the prespecified, exploratory economic analysis, per subject medication costs for rWF (excluding infusion costs) and TA were estimated using medication unit costs, per the Federal Supply Schedule, multiplied by trial-based average per patient medication dosages. Total medication costs were compared between medication strategies without and with the addition of trial-based strategy-specific differences in per patient emergency visit and missed work costs under assumptions of either no significant differences between strategies or accounting for absolute observed differences between strategies respectively.
For the pre-specified, exploratory genetic analysis, genotype was compared with VWF:Ag to determine if genotype predicts bleeding severity; and with PBAC, baseline and after rVWF or TA, to determine if genotype predicts response to treatment.
Results
Of 39 enrolled, 36 subjects completed the trial, of whom 17 were randomized to Group 1 (rVWF, then TA) and 19 to Group 2 (TA, then rVWF) (Figure 1), with comparable differences between groups in baseline characteristics (Table 1). Median [IQR] follow-up time for the analyses presented was 23.97 weeks [21.81, 28.14]. Overall, the mean (standard deviation) age of subjects was 29.2 (8.9) years, race, 69.4% Caucasian; VWF:RCo, 0.39 (0.16) and GPIbM, 0.44 (0.20). Most had type 1 VWD (88.9%) and of 4 with type 2 VWD, three had type 2A and one had type 2M, defined by RCo/Ag < 0.7.27 The three patients with type 2A VWD had multimers confirming absence of HMW multimers and VWF:Ag levels of 0.10, 0.10, and 0.24 IU/mL, while the one patient with type 2M VWD had normal multimers and a VWF:Ag level of 0.10 IU/mL.
Table 1.
Baseline Characteristics of Trial Population
| Group 1 (rVWF 1,2; TA 3,4) |
Group 2 (TA 1,2; rVWF 3,4) |
Total | |
|---|---|---|---|
| No. of Participants | 17 | 19 | 36 |
| Age (years) | 31.9 (6.3) | 26.7 (10.3) | 29.2 (8.9) |
| Age < 18 | 0 (0%) | 4 (21.1%) | 4 (11.1%) |
| Age >= 18 | 17 (100%) | 15 (78.9%) | 32 (88.9%) |
| Race | |||
| White or Caucasian | 12 (70.6%) | 13 (68.4%) | 25 (69.4%) |
| Black or African American | 1 (5.9%) | 2 (10.5%) | 3 (8.3%) |
| Asian | 2 (11.8%) | 0 (0%) | 2 (5.6%) |
| Other/Unknown | 2 (11.8%) | 4 (21.1%) | 6 (16.7%) |
| Ethnicity | |||
| Hispanic or Latino | 2 (11.8%) | 5 (26.3%) | 7 (19.4%) |
| Not Hispanic or Latino | 15 (88.2%) | 13 (68.4%) | 28 (77.8%) |
| Unknown | 0 (0%) | 1 (5.3%) | 1 (2.8%) |
| Height (cm) | 165.0 (6.8) | 164.6 (7.4) | 164.8 (7.0) |
| Weight (kg) | 72.4 [63.2, 94.3] | 67.2 [62.1, 82.8] | 70.9 [62.3, 90.6] |
| BMI (kg/m2) | 28.7 [22.8, 33.2] | 25.0 [21.7, 31.4] | 26.8 [22.1, 32.1] |
| BMI >30 | 7 (41.2%) | 6 (31.6%) | 13 (36.1%) |
| BMI <=30 | 9 (52.9%) | 13 (68.4%) | 22 (61.1%) |
| Type VWD | |||
| Type 1 | 15 | 17 | 32 |
| Type 2 | 2 | 2 | 4 |
| Type 2A | 1 | 2 | 3 |
| Type 2M | 1 | 0 | 1 |
| VWF:RCo (IU/mL)* | 0.37 (0.17) | 0.40 (0.15) | 0.39 (0.16) |
| VWF:RCo <0.30 IU/mL | 5 (29.4%) | 5 (26.3%) | 10 (27.8%) |
| VWF:RCo 0.30–0.50 IU/mL | 9 (52.9%) | 9 (47.4%) | 18 (50.0%) |
| VWF:RCo >0.50 IU/mL | 3 (17.6%) | 5 (26.3%) | 8 (22.2%) |
| VWF:GPIbM (IU/mL)* | 0.44 (0.23) | 0.45 (0.18) | 0.44 (0.20) |
| VWF:Ag (IU/mL)* | 0.35 (0.14) | 0.40 (0.16) | 0.38 (0.15) |
| FVIII Activity (IU/mL)* | 0.86 (0.36) | 0.80 (0.27) | 0.83 (0.32) |
| PBAC Baseline (past 2 cycles) | 597 (389) | 483 (270) | 537 (331) |
| PBAC >100 in 1 cycle | 0 (0%) | 0 (0%) | 0 (0%) |
| PBAC >100 in 2 cycles | 17 (100%) | 19 (100%) | 36 (100%) |
| Average baseline clots | 18 [5, 29] | 11 [5.5, 17.8] | 11 [4.9, 25.9] |
| No. baseline clots | 17 (100%) | 17 (89.5%) | 34 (94.4%) |
| Average baseline flooding | 3 [0.5, 5] | 1 [0, 3.5] | 1.8 [0, 4.6] |
| No. baseline flooding | 14 (82.4%) | 11 (57.9%) | 25 (69.4%) |
| Cycle severity score | 1.8 (0.6) | 2.1 (0.5) | 1.9 (0.6) |
| Cycle length (days) | 7.4 (2.1) | 7.8 (4.1) | 7.6 (3.3) |
| No. cycle length > 5 days | 15 (88.2%) | 13 (68.4%) | 28 (77.8%) |
| Hemoglobin (g/dL) | 13.5 (1.7) | 13.4 (1.1) | 13.4 (1.4) |
| No. anemia* (Hb<11.7 g/dL) | 2 (11.8%) | 1 (5.3%) | 3 (8.3%) |
| No. abnormally low ferritin* | 3 (17.6%) | 1 (5.3%) | 4 (11.1%) |
| No. abnormally low iron* | 2 (11.8%) | 2 (10.5%) | 4 (11.1%) |
| No. Hypothyroid* (TSH>4.5 mlU/L) | 0 (0%) | 0 (0%) | 0 (0%) |
| Quality-of-Life Measure | |||
| Ruta Menorrhagia Score | 43.2 (12.4) | 45.0 (14.0) | 44.2 (13.1) |
| CES-D Score | 8.5 (7.5) | 14.7 (12.7) | 11.8 (10.9) |
| SF-36 Score | |||
| Physical functioning | 87.1 (21.5) | 84.2 (22.4) | 85.6 (21.7) |
| Role limitations due to physical health | 66.2 (41.4) | 60.5 (41.1) | 63.2 (40.8) |
| Role limitations due to emotional problems | 76.5 (36.8) | 57.9 (41.3) | 66.7 (39.8) |
| Energy/fatigue | 57.7 (19.8) | 36.6 (24.2) | 46.5 (24.4) |
| Emotional well-being | 76.2 (16.8) | 65.5 (22.3) | 70.6 (20.4) |
| Social functioning | 77.2 (17.8) | 70.4 (25.8) | 73.6 (22.3) |
| Pain | 72.1 (22.8) | 65.1 (27.5) | 68.4 (25.3) |
| General health | 67.1 (18.1) | 57.1 (22.1) | 61.8 (20.7) |
| CDC HRQoL | |||
| Physically unhealthy days | 2.4 (3.9) | 2.7 (6.2) | 2.5 (5.1) |
| Mentally unhealthy days | 3.8 (5.0) | 6.9 (6.4) | 5.3 (5.9) |
Variables are reported as mean (standard deviation), or number (percentage). Median [interquartile range] are reported for Weight, BMI, Baseline Clots, and Baseline Flooding given skewed distributions.
VWF:RCo, VWF:Ag, VWF:GPIbM, VIII activity are baseline (not historic) values, subject to stress-induced increase.
Anemia is defined as Hgb < 11.7 g/dL
Abnormally low ferritin is defined as <6 mcg/L for ages 16–19, <10 mcg/L for ages 20 and up.
Abnormally low iron is defined as <27 micromol/L for ages 16–19, <40 micromol/L for ages 20–29, <45 micromol/L for ages 30 and above.
The PBAC was significantly lower during 2 cycles with TA than during 2 cycles with rVWF, 225 (207) vs. 273 (227), estimated mean treatment effect (95% CI), 46 (2, 90), p=0.039, (Table 2, Figure 2), with less frequent flooding, 44.4% vs. 59.7%, estimated odds ratio (95% CI), 3.8 (1.3, 10.7), p=0.014, but no significant difference in cycle severity or cycle length. While neither rVWF nor TA completely corrected PBAC, there was a reduction in PBAC and in the frequency of flooding. There was no significant difference in treatment response by VWF:RCo group (< 0.50 IU/mL vs. > 0.50 IU/mL), p=0.443. Specifically, among those with VWF:RCo < 0.50 IU/mL, PBAC after TA was 221 (212) as compared with 274 (242) after rVWF, adjusted mean difference (95% CI), 53 (−1, 107); and among those with VWF:RCo ≥ 0.50 IU/ml, PBAC after TA 241 (193) as compared with 266 (140) after rVWF, adjusted mean difference (95% CI), 11 (−59, 81). There was also no detectable difference in 5-day PBAC trajectories between rVWF and TA, p=0.686, as determined by fitting a linear mixed effects model with fixed effects for day, treatment, day-by-treatment interaction, and random effects for patient and slope with an unstructured covariance matrix.
Table 2.
Primary and Secondary Outcomes
| Outcome | TA | rVWF | Adjusted Mean Diff* (95% CI) | P-Value (Trt Diff) |
|---|---|---|---|---|
| Primary Outcome | ||||
| Pictorial Blood Assessment Chart (PBAC) across 2 cycles, mean (SD) | 225 (207) | 272 (223) | 46 (2, 90) | 0.039 |
| Cycle 1 | 217 (207) | 311 (263) | ||
| Cycle 2 | 233 (209) | 234 (168) | ||
| ln(PBAC), mean (SD) | 5.1 (0.9) | 5.3 (0.8) | 0.3 (0.1, 0.4) | 0.004 |
| Secondary Outcomes & Quality of Life | ||||
| Continuous, mean (SD) | ||||
| Cycle Length | 7.1 (4.5) | 7.0 (3.9) | 0.0 (-0.7, 0.7) | 0.998 |
| Ruta Menorrhagia | 32.4 (13.5) | 34.8 (13.0) | 2.4 (-1.0, 5.8) | 0.168 |
| CES-D | 13.5 (10.6) | 11.0 (10.2) | -2.5 (-4.9, 0.1) | 0.055 |
| SF-36 | ||||
| Physical functioning | 84.4 (19.6) | 83.6 (20.0) | -0.8 (-4.8, 3.2) | 0.681 |
| Role limitations of physical health | 70.1 (39.1) | 67.4 (37.7) | -2.4 (-12.4,7.6) | 0.633 |
| Role limitations of emotional health | 63.9 (41.7) | 68.5 (39.0) | 4.8 (-5.7, 15.3) | 0.362 |
| Energy/fatigue | 50.3 (20.6) | 47.9 (22.8) | -2.3 (-7.4, 2.8) | 0.375 |
| Emotional well-being | 69.2 (17.7) | 72.9 (20.0) | 3.6 (-0.3, 7.4) | 0.068 |
| Social functioning | 76.4 (21.9) | 80.9 (20.2) | 4.5 (-2.3, 11.3) | 0.189 |
| Pain | 71.2 (23.5) | 68.8 (24.2) | -2.4 (-9.2, 4.5) | 0.489 |
| General health | 58.8 (19.9) | 59.3 (21.2) | 0.5 (-3.2, 4.3) | 0.786 |
| CDC HRQoL | ||||
| Physically Unhealthy Days | 3.1 (4.0) | 1.9 (3.2) | -1.0 (-2.3, 0.2) | 0.098 |
| Mentally Unhealthy Days | 4.7 (5.1) | 5.7 (7.0) | 0.7 (-1.6, 3.0) | 0.557 |
| Categorical, frequency (%) | ||||
| Cycle Severity Rating | 1.4 (0.8, 2.7)* | 0.266 | ||
| 0: Mild bleeding, much less than usually experienced | 25 (34.7%) | 17 (23.6%) | ||
| 1: Moderate bleeding, less than usually experienced | 29 (40.3%) | 33 (45.8%) | ||
| 2: Moderately severe bleeding, but not as bad as the worst menstrual bleeding experienced | 14 (19.4%) | 22 (30.6%) | ||
| 3: Severe bleeding, as bad as the worst menstrual bleeding ever experienced | 4 (5.6%) | 0 (0%) | ||
| No. with Clots | 69 (95.8%) | 67 (93.1%) | 0.2 (0,1174) * | 0.724 |
| No. with Flooding | 32 (44.4%) | 43 (59.7%) | 3.8 (1.3, 10.7)* | 0.014 |
| No. Cycle Length > 5 days | 34 (47.2%) | 39 (54.2%) | 1.8 (0.7, 5.0)* | 0.221 |
| Healthcare Utilization | ||||
| Days lost from school/work in last 2 cycles | NA | NA | ||
| 0 | 28 (77.8%) | 29 (80.6%) | ||
| 1 | 3 (8.3%) | 3 (8.3%) | ||
| 2 | 3 (8.3%) | 2 (5.6%) | ||
| 3 | 2 (5.6%) | 0 (0.0%) | ||
| 4 or more | 0 (0.0%) | 2 (5.6%) | ||
| Received iron infusion(s) in last 2 cycles | NA | NA | ||
| 0 | 34 (94.4%) | 33 (91.7%) | ||
| 1 | 1 (2.8%) | 3 (8.3%) | ||
| 2 | 1 (2.8%) | 0 (0.0%) | ||
| Received RBC transfusion(s) in last 2 cycles | ||||
| 0 | 36 (100%) | 36 (100%) | ||
| ER visits for heavy periods in last 2 cycles | NA | NA | ||
| 0 | 34 (94.4%) | 36 (100%) | ||
| 1 | 1 (2.8%) | 0 (0.0%) | ||
| 2 | 1 (2.8%) | 0 (0.0%) | ||
| Hospitalizations in last 2 cycles | ||||
| 0 | 36 (100%) | 36 (100%) | ||
| Satisfaction Survey | ||||
| Rating rVWF vs past treatment | ||||
| Much more satisfied | NA | 18 (50.0%) | NA | NA |
| Somewhat more satisfied | NA | 8 (22.2%) | NA | NA |
| About as satisfied | NA | 5 (13.9%) | NA | NA |
| Somewhat less satisfied | NA | 4 (11.1%) | NA | NA |
| Much less satisfied | NA | 1 (2.8%) | NA | NA |
| Difficulty of rVWF administration | ||||
| Not difficult at all | NA | 13 (36.1%) | NA | NA |
| Somewhat difficult | NA | 14 (38.9%) | NA | NA |
| No more difficult than past treatment | NA | 4 (11.1%) | NA | NA |
| Somewhat more difficult than past treatment | NA | 3 (8.3%) | NA | NA |
| Much more difficult than past treatment | NA | 2 (5.6%) | NA | NA |
| Likelihood to use rVWF in the future | ||||
| Very likely | NA | 14 (38.9%) | NA | NA |
| Somewhat likely | NA | 8 (22.2%) | NA | NA |
| About as likely | NA | 3 (8.3%) | NA | NA |
| Somewhat unlikely | NA | 6 (16.7%) | NA | NA |
| Very unlikely | NA | 5 (13.9%) | NA | NA |
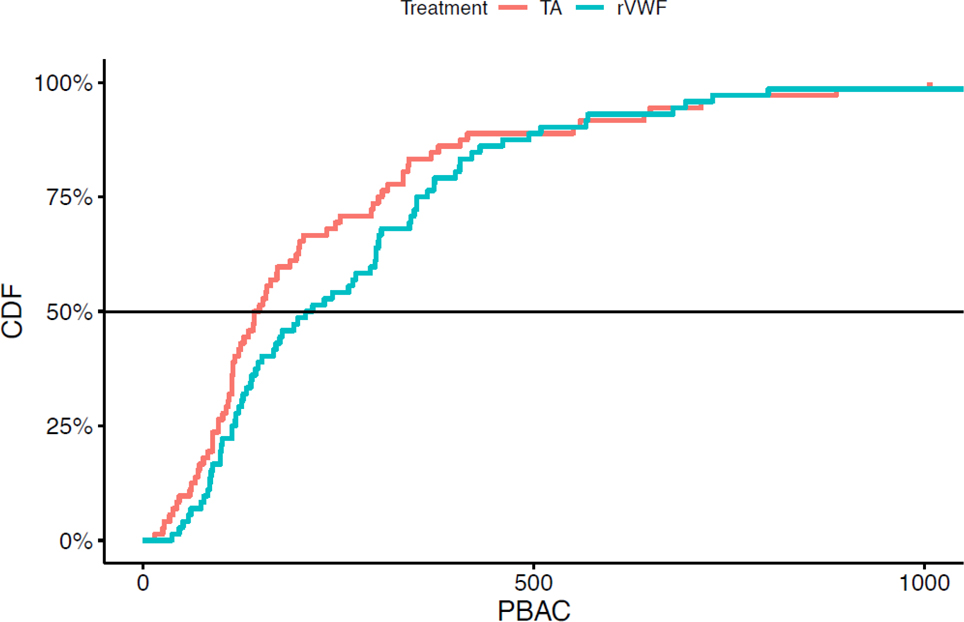
Figure 2. PBAC Blood Loss by Treatment.
Cumulative distribution function (CDF), comparing PBAC score after tranexamic acid, TA,  , vs. recombinant VWF, rVWF,
, vs. recombinant VWF, rVWF,  , p=0.039. The cumulative distribution function is on the y axis (scaled from 0 to 100%), and the pictorial blood assessment chart (PBAC) score is on the x axis. PBAC scores after cycles on TA are considerably lower than PBAC scores after cycles on rVWF. For example, the median PBAC score on TA (i.e. where the curve for TA crosses the horizontal 50% reference line) is 146, while the median PBAC score on rVWF (where the curve for rVWF crosses the horizontal 50% reference line) is 213.
, p=0.039. The cumulative distribution function is on the y axis (scaled from 0 to 100%), and the pictorial blood assessment chart (PBAC) score is on the x axis. PBAC scores after cycles on TA are considerably lower than PBAC scores after cycles on rVWF. For example, the median PBAC score on TA (i.e. where the curve for TA crosses the horizontal 50% reference line) is 146, while the median PBAC score on rVWF (where the curve for rVWF crosses the horizontal 50% reference line) is 213.
The PBAC in ten cycles in which a “rescue dose” of rVWF was used, did not differ from that during the 62 cycles without a “rescue dose,” 308 (150) vs. 267 (233), p=0.591. There was also no significant difference between PBAC on Day 1 of cycles in which a “rescue dose” was given, compared to PBAC on Day 1 of cycles when a “rescue dose” was not given, 77 (74) vs. 56 (51), p=0.252. There were no significant differences in quality of life (QoL) between treatment groups by Ruta, p=0.254, CES-D, p=0.055, HRQoL-14 physical, p=0.098, or mental measures, p=0.557; or by any of eight SF-36 measures, including general health, p=0.786 (Table 2).
The study drugs were well tolerated with no serious adverse events, specifically no excessive HMB bleeding despite study drugs, no thrombosis, other bleeding or allergic reactions. and only infrequent mucosal or traumatic bleeding unrelated to study drugs (Table 3). There were no dose reductions, no drug-related toxicity, and no treatment-related deaths. Health care utilization did not differ between TA and rVWF arms, in days lost from school/work, 22.2% vs 19.4%; iron infusion, 5.6% vs 8.3%; or emergency visits for HMB, 0% vs. 2.8%, respectively. No subjects required RBC blood transfusion or hospitalization. Three subjects received DDAVP for bleeding unrelated to menses and not during menstrual cycle bleeding.
Table 3.
Adverse Events During Trial
| Adverse Event | Grades 1 or 2 | Grades 3, 4, or 5 | ||
|---|---|---|---|---|
| Cycles | During 2 Cycles of TA | During 2 Cycles of rVWF | During 2 Cycles of TA | During 2 Cycles of rVWF |
| Bleeding Unresponsive to rVWF or TA | 0 /72 (0%) | 0/72 (0%) | 0/72 (0%) | 0/72 (0%) |
| Thrombosis | 0/72 (0%) | 0/72 (0%) | 0/72 (0%) | 0/72 (0%) |
| Other Bleeding | 6/72 (8.3%) | 3/72 (4.2%) | 0/72 (0%) | 0/72 (0%) |
| Mucosal Bleeding | 4/72 (5.6%) | 0/72 (0%) | 0/72 (0%) | 0/72 (0%) |
| Injury/Trauma | 0/72 (0%) | 1/72 (1.4%) | 0/72 (0%) | 0/72 (0%) |
| Other | 4/72 (5.6%) | 2/72 (2.8%) | 0/72 (0%) | 0/72 (0%) |
| Hematoma | 0/72 (0%) | 1/72 (1.4%) | 0/72 (0%) | 0/72 (0%) |
| Allergic Reaction | 0/72 (0%) | 0/72 (0%) | 0/72 (0%) | 0/72 (0%) |
Adverse events are reported as the number of cycles in which an event occurred (two cycles per treatment per participant, totaling 72 cycles on each treatment.) There were no serious adverse events observed.
Defined as >2 gm% fall in hemoglobin from baseline, requiring red cell transfusion or cardiopulmonary resuscitation.
Comparing rVWF with past treatment for HMB, 72.2% were much more or somewhat more satisfied; 86.1% rated rVWF treatment not difficult, somewhat more difficult, or no more difficult than past treatment; and 79.4% were very, somewhat, or about as likely to use rVWF in the future (Table 2). From an economic perspective, TA was associated with lower costs than rVWF, even when non-significant differences in emergency department visits and missed workdays between treatments were considered (Table 4, Appendix, page 78).
VWF sequencing identified pathogenic/likely pathogenic (P/LP) variants in eight subjects, variants of uncertain significance (VUS) in six subjects, and nonpathogenic variants/none (NP/N) in 22 subjects (Table 5, Appendix, Page 79). Those with P/LP variants had significantly lower VWF:Ag levels, 0.24 (0.14) IU/ml, than those with VUS, 0.45 (0.14) IU/ml, p=0.017, or than those with NP/N, 0.41 (0.14) IU/ml, p=0.007. VWF:Ag levels did not differ significantly in the latter two groups, VUS vs. NP/N, p=0.531. Mean and 95% CI for baseline PBAC score, estimated using linear mixed effects models with a random intercept (Table 5, Appendix, Page 79), did not differ between VWF variants, with 490 (249, 731) in those with P/LP variants, 570 (291, 848) in those with VUS, and 545 (399, 691) in those with NP/N. Adjusted mean differences and 95% CI for treatment effect, estimated using linear mixed-effects models, including fixed effects for treatment, cycle, baseline score, and random intercept for participant revealed no differences between VWF variants, respectively, 10 (−92, 112) in those with P/LP variants, 66 (−94, 226) in those with VUS, and 51 (−4, 105) in those with NP/N.
In nine subjects the p.Asp1472His variant was detected, including one with a P/LP variant, in one with VUS, and seven with NP/N (data not shown). Among the p.Asp1472His positive, the mean VWF:Ag was 0.41 (0.16) IU/ml (range: 0.10–0.60 IU/ml), the mean VWF:RCo was 0.41 (0.16) IU/ml (range: 0.13–0.74 IU/ml), and the mean GPIbM was 0.48 (0.22) IU/ml (range: 0.04–0.86 IU/ml). The mean VWF:Ag did not differ by VWF variant between those with and those without the p.Asp1472His variant, 0.10 IU/ml vs. 0.26 (0.14) IU/ml in P/LP; 0.42 IU/ml vs. 0.46 (0.15) IU/ml in VUS; and 0.45 (0.12) IU/ml vs. 0.39 (0.14) IU/ml in NP/N, respectively.
Discussion
Among women with VWD, HMB is a major burden of disease associated with poor quality of life, with iron deficiency, cognitive, physical, and psychological defects. Current hormonal and nonhormonal therapies for HMB are limited by ineffectiveness and intolerance, and few trials are available to guide treatment.7 Although rVWF has not been previously evaluated for HMB, its prolonged half-life, 19.3 hours,11,12 suggests a single dose per menstrual cycle may reduce HMB. While only a few women in clinical trials of VWF bleed prophylaxis incidentally found VWF was effective and safe in reducing HMB, it is generally used as a last line agent after hormonal agents, DDAVP, and TA fail,10 and, moreover, the dose and frequency for general bleeds do not apply to HMB.8,10
We believe this is the first study to assess the efficacy and safety of rVWF in women with mild and moderate type 1 and 2 VWD and HMB. In this randomized crossover trial, in which each subject served as her own control, rVWF resulted in lesser reduction in HMB by absolute PBAC score than TA. While neither drug corrected PBAC score to the normal range, each drug reduced PBAC and the frequency of flooding. There was no improvement in PBAC after a “rescue” dose of rVWF. As quality of life and healthcare utilization did not differ between treatment groups, and TA is less costly per Federal Supply Schedule pricing, it appears to be a more effective approach to HMB.
It is noteworthy, however, that a significant improvement in PBAC scores and bleed control did occur with rVWF, and, importantly, after only a single dose of rVWF, although the treatment PBAC scores still remained well within the HMB range. Treatment with a single dose of factor for a bleeding event is rarely seen. As none of the subjects in this study had type 3 and only a few had type 2 VWD, future studies will be needed to determine whether rVWF has a role in HMB in women with more severe disease. While the PBAC reduction with TA is well-recognized in non-bleeding disorders, it is of interest that PBAC scores were reduced in all subjects by rVWF, with no difference by enrollment VWF:RCo ≥50 IU/mL vs. <50 IU/mL. Whether the beneficial role of rVWF in HMB is attributable to a general pro-hemostatic effect or to a more specific VWF-platelet interaction is unknown, or whether TA and rVWF could provide complimentary effects will require further study.
Pathogenic VWF variants were associated with lower VWF:Ag levels than in subjects with variants of unknown significance or nonpathogenic variants, consistent with previous studies.28 However, pathogenic variants did not predict bleeding severity, either by baseline PBAC scores or by reduction in PBAC scores after either intervention, rVWF or TA. This finding is limited by the subjective nature of the PBAC score, the well-known lack of VWF levels or biomarkers to predict bleeding severity,29 and the likelihood of modifier genes not accounted for in these genetic data. Better predictors of bleeding severity are needed.
The frequency of iron deficiency,11%, in participants at screening was lower than reported rates of 50% or higher,3,4 and are likely related to the requirement of iron replacement pre-study. Thus, it is not possible to assess the effect of iron replacement on subjects’ quality of life or cognitive, physical, and psychological health.30 Whether lower rates of iron deficiency in study subjects was responsible for the lack of change in the four quality of life tools assessed, or whether two cycles is too short a time to see an improvement in quality of life is not known.
So, how do these findings impact how we treat women with VWD and HMB? While international guidelines suggest VWF for those with severe and frequent bleeding,7 and rVWF does reduce other mucosal bleeding,9 until this study, rVWF was not considered a standard treatment modality for HMB. While rVWF was not superior to TA, it did reduce PBAC blood loss and may provide another approach to HMB management in those for whom TA, hormones, or DDAVP are ineffective or poorly tolerated, and, as such, adds evidence to the ASH/ISTH/NHF/WFH international guidelines.7 These findings support the importance of discussing treatment options for HMB with patients based on their preferences and lived experience.
Future non-factor VWD agents are in development including the bispecific monoclonal FVIII mimic, emicizumab; the FVIII fusion variant independent of VWF, efanesoctocog alfa (BIVV001); the siRNA silencing mutant but not wild-type VWF expression; the aptamer blocking VWF clearance, rondoraptivon pegol (BT 200); and the dual AAV-vector VWF gene therapy under an endothelial cell promoter. Whether these agents will be safe and effective in preventing bleeds, including HMB, remains unknown, but, at minimum, will require careful monitoring of women for thrombosis and future offspring for teratogenic effects.
There are several limitations of this study. Enrollment was slow in this randomized trial in a rare disease, and further slowed by concomitant COVID restrictions, including study site closures and hesitancy by patients to enroll for personal and family health concerns. As a result, the study was stopped before the target number of participants was reached, which may limit the validity of the results of the trial. Yet, the cross-over design provided sufficient data and power to analyze the primary endpoint. A second limitation was assessment of the primary efficacy endpoint by PBAC score, which, although widely used in clinical practice as well as in clinical trials, is a subjective tool with high variability, also potentially limiting its validity. A third limitation was the inclusion of only patients with type 1 and 2 VWD, thus limiting generalizability of the findings. A fourth limitation was the use of pre-study VWF levels to establish a VWD diagnosis in study subjects; however, when VWF levels performed at enrollment of studies may be erroneous due study-related stress.15 Further, neither the p.Asp1472His polymorphism nor the GPIbM assay was available at trial initiation to confirm VWD diagnosis.27 Study strengths include the completeness and quality of data collected, gene variant analysis, and the quality-of-life assessments.
Supplementary Material
Panel: Research in Context.
Evidence before this study
Among women with von Willebrand disease (VWD), up to 80% have heavy menstrual bleeding (HMB). Current treatment of HMB is limited by ineffectiveness and intolerance in up to 30% of patients. Few randomized trials are available to guide treatment, and the ASH/ISTH/NHF/WFH international guidelines indicate low certainty regarding the effectiveness of hormonal and nonhormonal therapy in HMB. The lack of effective HMB treatment remains a major unmet healthcare need for women with HMB. We determined this by conducting a literature review using medical subject heading (MeSH) search terms ‘von Willebrand factor’, menorrhagia, and von Willebrand disease to assess the use of VWF in menorrhagia.10 Further, In a survey of 16 hemophilia treatment centers, VWF concentrate was used as a third-line treatment for menorrhagia, only after first- and second-line treatment failed: in all 13 subjects receiving VWF there was reduction in heavy menstrual bleeding.10 In a summary of published studies to date,10 including two prospective trials, two retrospective trials, and two observational network studies, a total of 455 VWD subjects were treated with plasma-derived (pd) VWF or rVWF concentrate. Of these, one-third or 88 (19.2%) were women with type 1, 2, or 3 VWD and menorrhagia treated with pdVWF at a dose of 36–50 IU/kg for 1–6 days of menstrual cycle bleeding.10 In these studies, 95–100% of these women reported reduction in menorrhagia, with no reported adverse effects. While VWF is approved to treat and prevent bleeds, few data and no prospective trials guide its use in HMB. We, therefore, conducted a randomized crossover trial comparing recombinant VWF with the nonhormonal standard, tranexamic acid, 1:1 in women with VWD and HMB.
Added value of this study
We believe this is the first study to assess the efficacy and safety of recombinant VWF (rVWF) in women with VWD and HMB. In this crossover trial in which each subject served as her own control, TA was superior and less costly than rVWF in reducing HMB by absolute PBAC score, and in reducing frequency of flooding.
Implications of all the available evidence
How do these findings impact how we treat women with VWD and HMB? While international guidelines suggest VWF for those with severe and frequent bleeding, until this study, rVWF was not considered a standard treatment modality for HMB. While rVWF was not superior to TA, it did reduce PBAC blood loss and may provide another approach to HMB management in those for whom TA, hormones, or DDAVP are ineffective or poorly tolerated, and as such, adds evidence to the ASH/ISTH/NHF/WFH international guidelines. These findings support the importance of discussing treatment options for HMB with patients based on their preferences and lived experience.
KEY POINTS:
rVWF was inferior to TA in reducing HMB in VWD by absolute PBAC score, with more flooding but no differences in cycle severity or length.
Quality-of-life measures by Ruta, CES-D, SF-36, CDC HRQoL-14, and healthcare utilization showed no differences between treatments.
Acknowledgements
The authors thank the patients, caregivers, and investigators who took part in this randomized trial (NCT020606045, https://clinicaltrials.gov/ct2/show/NCT020606045). This study was funded by the National Heart Lung Blood Institute, National Institutes of Health, Bethesda MD, NHLBI U01 HL133815–01A1. Recombinant VWF (Vonvendi ®) was kindly provided by Takeda Pharmaceuticals, USA. We also acknowledge the expertise of Gynecologic Consultant, Andra James, MD, Duke University, Durham NC; and the two dedicated Medical Monitors, Sarah O’Brien, MD, Nationwide Children’s Hospital, Columbus OH, and John Adamson, MD, University Southern California San Diego, CA. We also acknowledge Collaborators: Diana Gilligan MD, State University New York, Syracuse NY; Philip Kuriakose MD, Henry Ford Hospital, Detroit MI; Elaine Majerus MD, Washington University, St. Louis, MO; George Rogers MD, University of Utah, Salt Lake City UT; and Bethany Samuelson Bannow, MD, University Oregon Health Sciences Center, Portland OR. We acknowledge the expertise and oversite of Doris Rubio, PhD, the initial Data Coordinating Center principal investigator, currently Assistant Vice Chancellor for Clinical Research Education and Training, Health Sciences, and Director, Institute for Clinical Research Education, University of Pittsburgh. Finally, we acknowledge the participation of the study subjects, even during the COVID pandemic, without whom we could not have conducted this trial.
Footnotes
Conflict of Interest Disclosures
MVR reports research funding to her institution from BioMarin, Sanofi, SPARK, and Takeda Pharmaceuticals, and service on advisory boards of BeBio, BioMarin, Hemab Therapeutics, Sanofi, SPARK, and Takeda Pharmaceuticals; DN reports research funding from Bayer; service on advisory boards for Alnylam, Aptevo, Bioverativ, Genentech, Octapharma; and service on speaker’s bureaus for Octopharma, American Porphyria Foundation, BPL/Soleo Health, and Alnylam; AL reports research funding from BioMarin and Pfizer, and service on advisory boards including BioMarin, CSL, Dova, and Pfizer; RS reports research funding from Takeda and Octapharma, and consulting with Sanofi/Sobi, Takeda, Octapharma, NovoNordisk, Bayer, Pfizer, Hema Biologics, Guardian Therapeutics, and Hemab Therapeutics; CP serves on advisory boards for Takeda, HEMA Biologics, and Coagulant Therapeutics; RP receives honoraria from CSL Behring, Genentech Inc, Bayer Healthcare AG, Hema Biologics, Instrumentation Laboratory, and Merck; APW serves on advisory boards of NovoNordisk, HEMA Biologics, Takeda, Sanofi, Genentech, Octapharma, Pfizer, Bioverativ, Bayer, and Spark; RK serves on advisory boards of CSL Behring, Sanofi, NovoNordisk, and Pfizer; CS serves as a consultant to Genentech, Hema Biologics, Novo Nordisk, and Takeda; KJS reports research funding from Sanofi Pasteur; TCN reports research funding from Pfizer and Spark; and ADA is currently an employee of Medtronic. SDR, RF, LM, EK, JL, DA, NM, FX, MM, DB, GH, EPM, DI, DV, and GK declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing
All datasets and biologic specimens from the VWD Min trial will be made available through BioLINCC at https://biolincc.nhlbi.nih.gov within 3 months of the initial request to researchers who provide a methodologically sound proposal. The data will include deidentified participant data, a data dictionary, protocol, informed consent form, and statistical analysis plan. Biologic specimens and datasets from the VWD MINIMIZE trial will be available through BioLINCC to any researcher or investigator who makes formal application request and is formally approved by NHLBI, as summarized at https://biolincc.nhlbi.nih.gov.This repository has data access policies and procedures in place that will provide data access to qualified researchers, consistent with NIH data sharing policies and applicable laws and regulations. Once an investigator application request is approved by NHLBI, and upon receipt of a Research Materials Distribution Agreement, data will be transferred by secure transfers through the BioLINCC website. For biologic specimen requests, upon NHLBI approval of an investigator application request, and with evidence of funding and adequate facilities and expertise to perform the proposed research, BioLINCC will request that the repository prepare and ship the requested biologic specimens.
References
- 1.Rodeghiero F, Castaman G, Dini E. Epidemiologic investigation of the prevalence of von Willebrand disease. Blood. 1987;69(2):454–459. [PubMed] [Google Scholar]
- 2.Leebeek FW, Eikenboom JC. Von Willebrand disease. N Engl J Med. 2016;375(2):2067–2080. [DOI] [PubMed] [Google Scholar]
- 3.Ragni MV, Bontempo FA, Cortese-Hassett AL. von Willebrand disease and bleeding in women. Haemophilia. 1999;5(5):313–317. [DOI] [PubMed] [Google Scholar]
- 4.Govorov I, Ekelund L, Chaireti R, et al. Heavy menstrual bleeding and health-associated quality of life in women with von Willlebrand disease. Exp Ther Med. 2016;11(5):1923–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadir RA, Edlund M, von Mackensen S. The impact of menstrual disorders on quality of life i in women with inherited bleeding disorders. Haemophilia. 2010;16(5):832–839. [DOI] [PubMed] [Google Scholar]
- 6.People Healthy 2010.2003:17:3–19. http://www.health.gov/_healthy_people. [Google Scholar]
- 7.Connell NT, Flood VH, Brignardello-Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021;5(1):301–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill J, Castaman G, Windyga J, et al. Safety, efficacy, and pharmacokinetics of a recombinant von Willebrand factor in patients with severe von Willebrand disease. Blood. 2015;126(17):2038–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leebeek FWG, Peyvandi F, Escobar M, et al. Recombinant von Willebrand factor prophylaxis in patients with severe von Willebrand disease: phase 3 study results. Blood. 2022;140(2):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragni MV, Machin N, Malec LM, et al. Von Willebrand factor for menorrhagia: a survey and literature review. Haemophilia. 2016;22(3):397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turecek PL, Schrenk G, Rotensteiner H, et al. Structure and function of a recombinant von Willebrand factor drug candidate. Semin Thromb Hemost. 2020;36(5):510–521. [DOI] [PubMed] [Google Scholar]
- 12.Mannucci PM, Kempton C, Miller C, et al. Pharmacokinetics and safety of a novel recombinant human von Willebrand factor manufactured with a plasma-free method: a multicenter prospective clinical trial. Blood. 2013:122(5):648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Haematol. 1990;97(8):734–739. [DOI] [PubMed] [Google Scholar]
- 14.Reid PC, Coker A, Coltart R. Assessment of menstrual blood loss using a pictorial chart: a validation study. BJOG. 2000;107(3):320–322. [DOI] [PubMed] [Google Scholar]
- 15.Ragni MV, Jankowitz RC, Merricks EP, Kloos M, Nichols TM. Phase II prospective open-label trial of recombinant interleukin-11 (rhIL-11, Neumega®) in women with von Willebrand disease and menorrhagia. Thromb Haemost. 2011;106(4):641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware JE. SF-36 Health survey update. Spine. 2000;25(24):3130–3139. [DOI] [PubMed] [Google Scholar]
- 17.Ruta DA, Garratt AM, Chadha YC, Flett M, Hall MH, Russell IT. Assessment of patients with menorrhagia: how valid is a structured clinical history as a measure of health status? Qual Life Res. 1995;4(1):33–40. [DOI] [PubMed] [Google Scholar]
- 18.Dewee EM, Mauser-Bunschoten EP, van der Bom JG, et al. ; WIN Study Group. Health-related quality of life among adult patients with moderate and severe von Willebrand disease. J Thromb Haemost. 2010;8(7):1492–1499. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). Health related quality-of-life and activity limitation – eight states. MMWR. 1998;47(7):137–140. [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychol Measure. 1977;1(3):385–401. [Google Scholar]
- 21.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), most recent version. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm [Google Scholar]
- 22.Haberichter SL, Merricks EP, Fahs SA, et al. Re-establishment of VWF-dependent Weibel-Palade bodies in VWD endothelial cells. Blood. 2005;105(1):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson RE, Catalfamo JL, Dodds WJ. A multispecies enzyme-linked immunosorbent assay for von Willebrand factor. J Lab Clin Med. 1992;119(4):420–427. [PubMed] [Google Scholar]
- 24.Brouland JP, Egan T, Roussi J, et al. In vivo regulation of von Willebrand factor synthesis: von Willebrand factor production in endothelial cells after lung transplantation between normal pigs and von Willebrand factor-deficient pigs. Arterioscler Thromb Vasc Biol. 1999;19(12):3055–3062. [DOI] [PubMed] [Google Scholar]
- 25.Nichols TC, Bellinger DA, Reddick RL, et al. The role of von Willebrand factor and factor VIII in arterial thrombosis: studies in canine on Willebrand disease and hemophilia A. Blood. 1993;81(10):2644–2651. [PubMed] [Google Scholar]
- 26.Richards S, Bale S, Bick D, Das S, Gastier-Foster J. Standards and guidelines for the Interpretation of sequence variants: A joint consensus recommendations of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James PD, Connell NT, Ameer B, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5(1):280–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadler B, Christopherson PA, Heller G, Montgomery RR, Di Paola J, and the Zimmerman Program Investigators. Von Willebrand factor antigen levels are associated with burden of rare nonsynonymous variants in the VWF gene. Blood. 2021;137(23):3277–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavin M, Aguila S, Schneppenheim S, et al. Novel insights into the clinical phenotype and pathophysiology underlying low VWF levels. Blood. 2017;130(21):2344–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruner AB, Hoffe A, Duggan AK, Casella JF, Brandt J. Randomized study of cognitive effects of iron supplementation in non-anemic iron deficient girls. Lancet. 1996;348(9033):992–996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets and biologic specimens from the VWD Min trial will be made available through BioLINCC at https://biolincc.nhlbi.nih.gov within 3 months of the initial request to researchers who provide a methodologically sound proposal. The data will include deidentified participant data, a data dictionary, protocol, informed consent form, and statistical analysis plan. Biologic specimens and datasets from the VWD MINIMIZE trial will be available through BioLINCC to any researcher or investigator who makes formal application request and is formally approved by NHLBI, as summarized at https://biolincc.nhlbi.nih.gov.This repository has data access policies and procedures in place that will provide data access to qualified researchers, consistent with NIH data sharing policies and applicable laws and regulations. Once an investigator application request is approved by NHLBI, and upon receipt of a Research Materials Distribution Agreement, data will be transferred by secure transfers through the BioLINCC website. For biologic specimen requests, upon NHLBI approval of an investigator application request, and with evidence of funding and adequate facilities and expertise to perform the proposed research, BioLINCC will request that the repository prepare and ship the requested biologic specimens.