Abstract
Introduction:
Allogeneic hematopoietic cell transplant (alloHCT) provides cure for older patients with acute myeloid leukemia (AML); however, disease relapse remains a major concern. Based on recent data suggesting that younger donor age confers the greatest benefit among matched unrelated donors (MUD), we attempted to answer a practical question: which donor type provides the best outcomes when an older patient with AML has a matched sibling donor (MSD, also older) vs the best MUD?
Methods:
This retrospective cohort registry study accessed data from Center for International Blood and Marrow Transplant Research database (CIBMTR) in patients with AML 50 years or older undergoing alloHCT from older MSD (aged≥50) or younger MUD (aged≤35) between 2011 and 2018. The study included common allograft types, conditioning regimens, and graft-versus-host-disease (GVHD) prophylaxis. The primary outcome was relapse risk. Secondary outcomes included non-relapse mortality (NRM), GVHD, disease-free survival (DFS), and overall survival (OS).
Results:
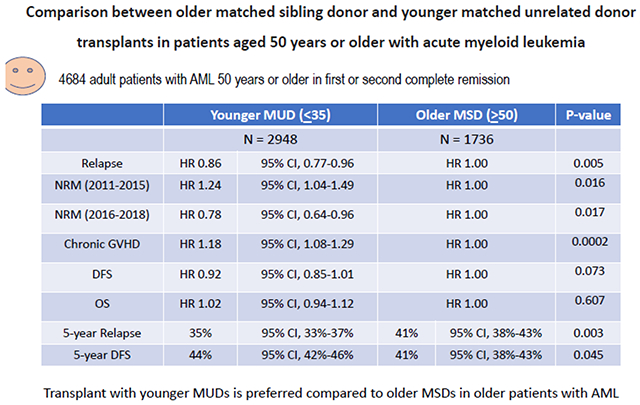
Among 4684 eligible patients, 1736 underwent alloHCT with an older MSD whereas 2948 received transplant from a younger MUD. In multivariable analysis, compared to an alloHCT from older MSDs, younger MUDs conferred a decreased relapse risk (HR 0.86; p=.005) and a significantly lower adjusted 5-year cumulative incidence of relapse (35% vs 41%; p=.003), but was associated with an increased risk for chronic GVHD (HR 1.18; 95% CI, 1.08-1.29; p=.0002) and greater NRM only in the earlier period from 2011-2015 (HR 1.24; p=.016). The corresponding NRM rates were significantly lower in the more recent period from 2016-2018 (HR 0.78; p=.017). The adjusted 5-year DFS probability was 44% (95% CI, 42%-46%) with an alloHCT from younger MUDs compared to 41% (95% CI, 38%-43%) with an older MSD (p=.04).
Conclusion:
In older patients with AML undergoing alloHCT, the use of younger MUDs is associated with a decreased relapse risk and improved DFS compared to older MSDs.
Keywords: Acute myeloid leukemia, Stem cell transplantation, Donor, Age, Allogeneic hematopoietic cell transplant, Donor type, Relapse
Graphical Abstract
INTRODUCTION:
An aging population has resulted in an increase in the incidence of acute myeloid leukemia (AML) (Surveillance, Epidemiology, and End Results [SEER] Program database)1. Allogeneic hematopoietic cell transplantation (alloHCT) is a curative modality for patients with AML, however, disease relapse remains a major issue. Human leukocyte antigen (HLA)-matched sibling donors (MSD) are considered the preferred donor type in clinical practice. With an increasing median age of patients, sibling donors for these patients are likely to be older, and can often have increased comorbidities2. Older donor age impacts alloHCT outcomes via several mechanisms. Senescence is associated with shorter leukocyte telomere lengths that impact post-transplant non-relapse mortality (NRM)3. Additionally, ageing is associated with a higher risk of clonal hematopoiesis of indeterminate potential (CHIP), stem cell exhaustion, impaired regenerative potential of stem cells, and age-related gut dysbiosis impact T-cell subtypes and offset the balance between graft-versus-host (GVH) and graft-versus-leukemia (GVL)4–8.
With a median age at diagnosis approaching 70 years (SEER database), an older AML population remains the most common indication for alloHCT1. Prior registry studies have demonstrated that donor-recipient HLA disparity and older donor age confer inferior survival9,10. An earlier analysis by the European Society for Blood and Marrow Transplantation (EBMT), examining older AML (aged>55) alloHCT who were recipients from older MSD and younger matched unrelated donors (MUD), showed no difference in outcomes11. A more recent Center for International Blood and Marrow Transplant Registry (CIBMTR) study examining 822 older patients with AML (age: 50-75) receiving alloHCT from alternative donor sources revealed a superior 5-year OS with younger MUD (aged<40) compared to haploidentical HCT (haploHCT) (42% [95% CI, 38%-47%] vs 32% [95% CI, 23%-42%])12. AlloHCT from a young MUDs was associated with lower relapse risk compared to an alloHCT from an older MSD among patients with myelodysplastic syndrome (MDS)13,14. Given the lack of uniform and contemporary data related to alloHCT outcomes for patients with AML, coupled with effective induction therapy rendering an increasing number of older AML patients eligible for alloHCT, there is a critical need to evaluate outcomes in this rapidly growing population of high-risk patients in a larger sample size. We hypothesized that alloHCT from a younger MUD would be associated with better disease control compared to older MSD for older adults with AML, resulting in superior survival.
METHODS:
Study objectives:
The aim of the study was to compare relapse rates, NRM, graft-versus-host disease (GVHD), overall survival (OS), and DFS in older patients with AML (aged≥50) undergoing alloHCT either from older MSDs or younger MUDs.
Data sources:
CIBMTR is a working group of more than 500 hematopoietic cell transplantation centers worldwide that contribute detailed data on HCT to a statistical center at the Medical College of Wisconsin (MCW). Participating centers are required to report all transplantations consecutively and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The MCW and National Marrow Donor Program’s (NMDP) Institutional Review Boards approved this study. Protected health information used in conducting such research is collected and maintained in the capacity of the CIBMTR as a public health authority under the Health Insurance Portability and Accountability Act Privacy Rule.
Patient selection:
Adult patients with AML 50 years or older undergoing first alloHCT from older MSDs (donor age≥50) or younger (donor age≤35) MUDs between 2011 and 2018 were included. The MUD arm was restricted to those with donor age≤35 based on recent evidence indicating that MUDs younger than 35 confer the greatest benefit9,10,14–16. The study included common stem cell (SC) sources (peripheral blood [PB] vs bone marrow [BM]), conditioning regimens (RIC/non-myeloablative conditioning [NMA] vs myeloablative conditioning [MAC]), and GVHD prophylaxis strategies (tacrolimus-based vs cyclosporin-based vs others). Main exclusion criteria were recipients of ex vivo T-cell depleted or CD34-selected allografts, post-transplant cyclophosphamide (PTCy) recipients, and mismatched unrelated donor, haploidentical donor, cord blood or identical twin transplants. The cohort selection process is included in table S1.
Definitions and study endpoints:
The primary outcome was relapse rate. Secondary outcomes included NRM, GVHD, DFS, and OS. CIBMTR defines MUD as an unrelated donor who is 8/8 fully HLA-allele matched (matched at HLA-A, HLA-B, HLA-C, and HLA-DRB1) and MSD as a sibling donor who is 8/8 HLA-matched (allele level matching of HLA-A, B, C, and DRB1)17. GVHD was defined per the NIH consensus criteria18. NRM was defined as death without relapse/progression with relapse accounted as the competing event. Correspondingly, NRM was the competing event for relapse. DFS was defined as survival following alloHCT without relapse or progression. Relapse/progression of disease, or death were considered events. Death from any cause was considered an event and surviving patients were censored at the time of last follow-up. The causes of death were described.
Statistical analysis:
Baseline characteristics of the study population were summarized using descriptive statistics with median and range for continuous variables and proportions for categorical variables. Cumulative incidence (C.I.) estimates were calculated for competing risks outcomes. The Kaplan-Meier method was used to estimate the probabilities for survival. To evaluate potential risk factors, multivariable cox regression was used. Interactions between the main effect (donor age group) and significant risk factors were tested. Fine and Gray model was used for GVHD, NRM and relapse. In multivariable regression models, various covariates (patient age, race/ethnicity, gender match, CMV match, ELN risk group 201719, AML subtype, CR and measurable residual disease (MRD) status at HCT, comorbidities score (HCT-CI), Karnofsky performance status (KPS), interval between diagnosis and transplant, intensity of the conditioning regimen, SC source, GVHD prophylaxis, in vivo T cell depletion [anti-thymocyte globulin (ATG)/ alemtuzumab], transplant year, and center effect) were considered. Adjusted probabilities of DFS and OS and adjusted C.I. estimates were generated from the final regression models. These adjusted probabilities estimate the likelihood of outcomes in populations with similar prognostic factors. Given the multicenter nature of the analysis, the influence of center effect was tested on the main effect for all outcomes and adjusted accordingly. All analyses were performed at a significance level p<0.05 using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS:
Baseline characteristics:
Among 4684 eligible patients in the study cohort, 1736 underwent alloHCT from an older MSD whereas 2948 received alloHCT from a younger MUD. Baseline characteristics of patients, disease, and transplant according to the donor type are summarized in Table 1. Median recipient age was 61 years (range: 50-80) in the older MSD arm and 63 years (range: 50-84) in the younger MUD arm. Study arms were balanced in terms of performance status, comorbidity burden, ELN risk group 2017, AML subtype (de novo, transformed, therapy-related), remission and MRD status (for those in CR1).
Table 1 –
Baseline characteristics
| Characteristic | Matched sibling donor | Matched unrelated donor | P Value |
|---|---|---|---|
| No. of patients | 1736 | 2948 | |
| Patient-related | |||
|
| |||
| Age at HCT - median (min-max) | 61 (50-80) | 63 (50-84) | <.01b |
| Age at HCT - no. (%) | <.01a | ||
| 50-59 | 735 (42) | 1000 (34) | |
| 60-69 | 862 (50) | 1526 (52) | |
| >=70 | 139 (8) | 422 (14) | |
| Karnofsky score at HCT - no. (%) | <.01a | ||
| 90-100 | 918 (53) | 1631 (55) | |
| <90 | 783 (45) | 1287 (44) | |
| Missing | 35 (2) | 30 (1) | |
| HCT-CI - no. (%) | <.01a | ||
| 0 | 293 (17) | 508 (17) | |
| 1 | 268 (15) | 349 (12) | |
| 2 | 273 (16) | 440 (15) | |
| 3+ | 886 (51) | 1630 (55) | |
| Missing | 16 (1) | 21 (1) | |
| Race and Ethnicity - no. (%) | <.01a | ||
| White non-Hispanic | 1431 (82) | 2767 (94) | |
| Black or African American non-Hispanic | 66 (4) | 20 (1) | |
| Asian non-Hispanic | 70 (4) | 46 (2) | |
| Hispanic | 132 (8) | 79 (3) | |
| Other | 13 (1) | 6 (0) | |
| Missing | 24 (1) | 30 (1) | |
| Disease-related | |||
|
| |||
| ELN risk group - no. (%) | <.01a | ||
| Normal | 129 (7) | 178 (6) | |
| Favorable | 270 (16) | 391 (13) | |
| Intermediate | 507 (29) | 763 (26) | |
| Poor | 478 (28) | 742 (25) | |
| Missing | 352 (20) | 874 (30) | |
| Subtype of AML - no. (%) | 0.42a | ||
| De-novo | 1350 (78) | 2286 (78) | |
| Transformed from MDS | 222 (13) | 408 (14) | |
| Therapy-related | 164 (9) | 254 (9) | |
| Disease status at time of HCT - no. (%) | 0.02a | ||
| 1st complete remission | 1472 (85) | 2421 (82) | |
| 2nd complete remission | 264 (15) | 527 (18) | |
| MRD (for CR1 only) - no. (%) | <.01a | ||
| Negative | 759 (44) | 1323 (45) | |
| Positive | 612 (35) | 886 (30) | |
| N/A, Disease status not in CR1 | 264 (15) | 527 (18) | |
| Missing | 101 (6) | 212 (7) | |
| Time from diagnosis to HCT (months) for CR1 cases - median (min-max) | 4 (0-140) | 5 (1-691) | <.01b |
| Transplant-related | |||
|
| |||
| Graft type - no. (%) | <.01a | ||
| Bone marrow (BM) | 64 (4) | 337 (11) | |
| Peripheral blood (PB) | 1672 (96) | 2611 (89) | |
| Conditioning regime - no. (%) | <.01a | ||
| MAC | 812 (47) | 1162 (39) | |
| RIC/NMA | 874 (50) | 1704 (58) | |
| Missing | 50 (3) | 82 (3) | |
| Donor/recipient sex match - no. (%) | <.01a | ||
| M-M | 507 (29) | 1266 (43) | |
| M-F | 397 (23) | 905 (31) | |
| F-M | 449 (26) | 333 (11) | |
| F-F | 383 (22) | 429 (15) | |
| Missing | 0 (0) | 15 (1) | |
| Donor/recipient CMV serostatus - no. (%) | <.01a | ||
| +/+ | 728 (42) | 775 (26) | |
| +/− | 184 (11) | 255 (9) | |
| −/+ | 481 (28) | 1200 (41) | |
| −/− | 331 (19) | 699 (24) | |
| Missing | 12 (1) | 19 (1) | |
| Donor age - median (min-max) | 60 (50-85) | 25 (18-35) | <.01b |
| Donor age - no. (%) | <.01a | ||
| 1-19 | 0 (0) | 178 (6) | |
| 20-29 | 0 (0) | 2249 (76) | |
| 30-39 | 0 (0) | 477 (16) | |
| 50-59 | 859 (49) | 0 (0) | |
| 60-69 | 766 (44) | 0 (0) | |
| 70-79 | 110 (6) | 0 (0) | |
| 80+ | 1 (0) | 0 (0) | |
| Missing | 0 (0) | 44 (1) | |
| GVHD-Prophylaxis - no. (%) | 0.03a | ||
| FK-based | 1486 (86) | 2574 (87) | |
| CSA-based | 231 (13) | 327 (11) | |
| Other | 19 (1) | 47 (2) | |
| In-vivo T-cell depletion (ATG/alemtuzumab) - no. (%) | <.01a | ||
| No | 1568 (90) | 1849 (63) | |
| Yes | 168 (10) | 1099 (37) | |
| Year of HCT - no. (%) | <.01a | ||
| 2011 | 65 (4) | 254 (9) | |
| 2012 | 83 (5) | 247 (8) | |
| 2013 | 150 (9) | 322 (11) | |
| 2014 | 310 (18) | 406 (14) | |
| 2015 | 307 (18) | 393 (13) | |
| 2016 | 309 (18) | 412 (14) | |
| 2017 | 268 (15) | 425 (14) | |
| 2018 | 244 (14) | 489 (17) | |
| Follow-up - median (range) | 50 (6-113) | 51 (3-113) | |
Hypothesis testing:
Pearson chi-square test
Kruskal-Wallis test
The median donor age was significantly lower in the MUD cohort compared to the MSD cohort (25 years vs 60 years, respectively, p<.01). Other differences in the MUD cohort included longer median interval from diagnosis to transplant (5 vs 4 months, p<.01), more BM allografts (11% vs 4%, p<.01), fewer MAC recipients (39% vs 47%, p<.01), fewer female-donor-male-recipient pairs (11% vs 26%, p<.01), greater D−/R+ CMV serostatus (41% vs 28%, p<.01), and greater utilization of ATG/alemtuzumab (37% vs 10%, p<.01) compared to the older MSD cohort. The median follow-up in MSD and MUD cohorts was 51 months (range, 6-113) and 50 months (range, 3-113), respectively (p<.01).
Relapse:
In multivariable analysis, alloHCT from younger MUDs was associated with a significantly decreased risk of relapse (HR 0.86; 95% CI, 0.77-0.96; p=.005) compared to older MSDs (Table 2). The adjusted cumulative incidence of relapse at 5 years was significantly lower with younger MUDs (35%; 95% CI, 33-37%) vs older MSDs (41%; 95% CI, 38%-43%) (p=.003) (Table 3) (Figure 1a).
Table 2:
Multivariable analyses of the impact of donor age (MUD vs MSD) on alloHCT outcomes
| Outcome | No. of patients | HR (95% CI) | P-value |
|---|---|---|---|
|
| |||
| Relapse: | |||
| MSD | 1720 | 1.00 (Reference) | 0.005 |
| MUD | 2924 | 0.86 (0.77-0.96) | |
|
| |||
| NRM 2011-2015: | |||
| MSD | 907 | 1.00 (Reference) | 0.016 |
| MUD | 1609 | 1.24 (1.04-1.49) | |
|
| |||
| NRM 2016-2018: | |||
| MSD | 813 | 1.0 (Reference) | 0.017 |
| MUD | 1315 | 0.78 (0.64-0.96) | |
|
| |||
| Chronic GVHD | |||
| MSD | 1730 | 1.00 (Reference) | 0.0002 |
| MUD | 2939 | 1.18 (1.08-1.29) | |
|
| |||
| DFS: | |||
| MSD | 1720 | 1.0 (Reference) | 0.073 |
| MUD | 2924 | 0.92 (0.85-1.01) | |
|
| |||
| OS: | |||
| MSD | 1736 | 1.0 (Reference) | 0.607 |
| MUD | 2948 | 1.02 (0.94-1.12) | |
|
| |||
| OS (Center effect): | |||
| MSD | 1736 | 1.0 (Reference) | 0.653 |
| MUD | 2948 | 1.02 (0.93-1.12) | |
Abbreviations:
AlloHCT, allogeneic hematopoietic cell transplant; AML, acute myeloid leukemia; MSD, HLA-matched sibling donors; MUD, matched unrelated donor; NRM, Non-relapse mortality; DFS, disease-free survival; OS, overall survival.
Table 3:
5-year adjusted CI of relapse, NRM, GVHD, and survival probability.
| Outcome | No. of patients at risk | % (95% CI) | P-value |
|---|---|---|---|
|
| |||
| Relapse: | |||
| MSD | 327 | 41% (38%-43%) | 0.003 |
| MUD | 557 | 35% (33%-37%) | |
|
| |||
| NRM 2011-2015: | |||
| MSD | 304 | 24% (20%-27%) | 0.045 |
| MUD | 530 | 28% (25%-30%) | |
|
| |||
| NRM 2016-2018: | |||
| MSD | 23 | 20% (17%-24%) | 0.147 |
| MUD | 27 | 17% (15%-20%) | |
|
| |||
| Chronic GVHD (1-year CI) | |||
| MSD | 169 | 41% (39%-43%) | 0.07 |
| MUD | 326 | 44% (42%-46%) | |
|
| |||
| Chronic GVHD (3-year CI) | |||
| MSD | 169 | 51% (49%-53%) | 0.005 |
| MUD | 326 | 56% (54%-57%) | |
|
| |||
| DFS: | |||
| MSD | 327 | 41% (38%-43%) | 0.045 |
| MUD | 557 | 44% (42%-46%) | |
|
| |||
| OS: | |||
| MSD | 287 | 47% (44%-49%) | 0.953 |
| MUD | 523 | 47% (45%-49%) | |
Abbreviations:
AlloHCT, allogeneic hematopoietic cell transplant; CI, cumulative incidence; AML, acute myeloid leukemia; MSD, HLA-matched sibling donors; MUD, matched unrelated donor; NRM, Non-relapse mortality; DFS, disease-free survival; OS, overall survival.
Figure 1A:
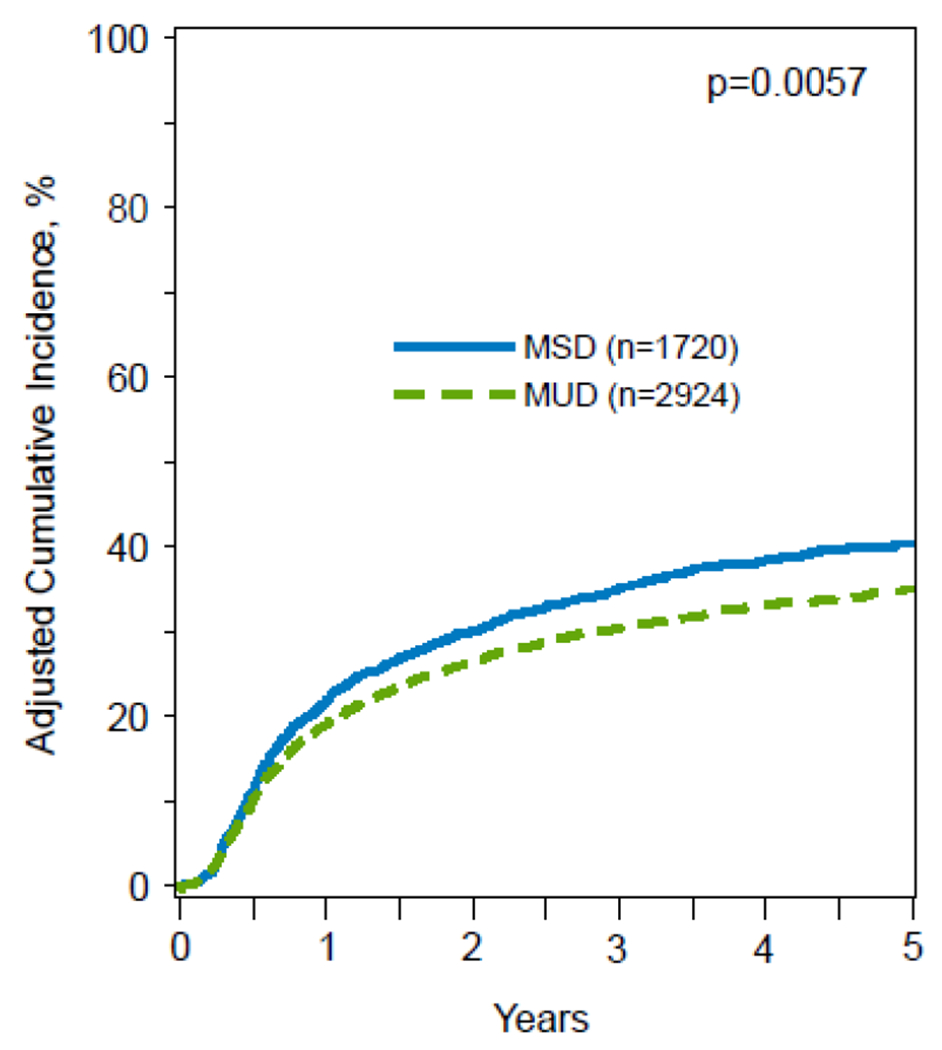
5-year adjusted CI of relapse, stratified by donor type.
Other significant predictors of increased relapse included poor risk disease, transformed and therapy-related AML, in vivo T-cell depletion with ATG/alemtuzumab, disease not in CR1, and RIC/NMA conditioning. Factors associated with the reduced relapse included a longer interval between diagnosis and alloHCT beyond 6 months, PBSC source, and transplant in the recent years (2016-2018) compared to earlier years (2011-2015) (table S3).
Non-relapse Mortality:
In multivariate analysis, NRM was reported in 2 subgroups of transplant years (2011-2015 and 2016-2018) as there was significant interaction between the main effect (MUD vs MSD) and alloHCT year (p=.003). AlloHCT from a younger MUD was associated with an increased NRM (HR 1.24; 95% CI, 1.04-1.49; p=.016) compared to older MSDs in the earlier period of 2011-2015, whereas NRM was lower with younger MUDs in the latter period, 2016-2018 (HR, 0.78, 95% CI, 0.64-0.96; p=.017) (Table 2; Figure 1b). While there was no difference in the adjusted cumulative incidence of NRM at 5 years in the latter period (17% [95% CI, 15%-20%] vs 20% [95% CI, 17%-24%]) (p=.147), NRM was significantly higher in patients with AML who underwent alloHCT between 2011 and 2015 from a younger MUD (28%; 95% CI, 25-30%) compared to older MSDs (24%; 95% CI, 20%-27%) (p=.045) (Table 3).
Figure 1B:
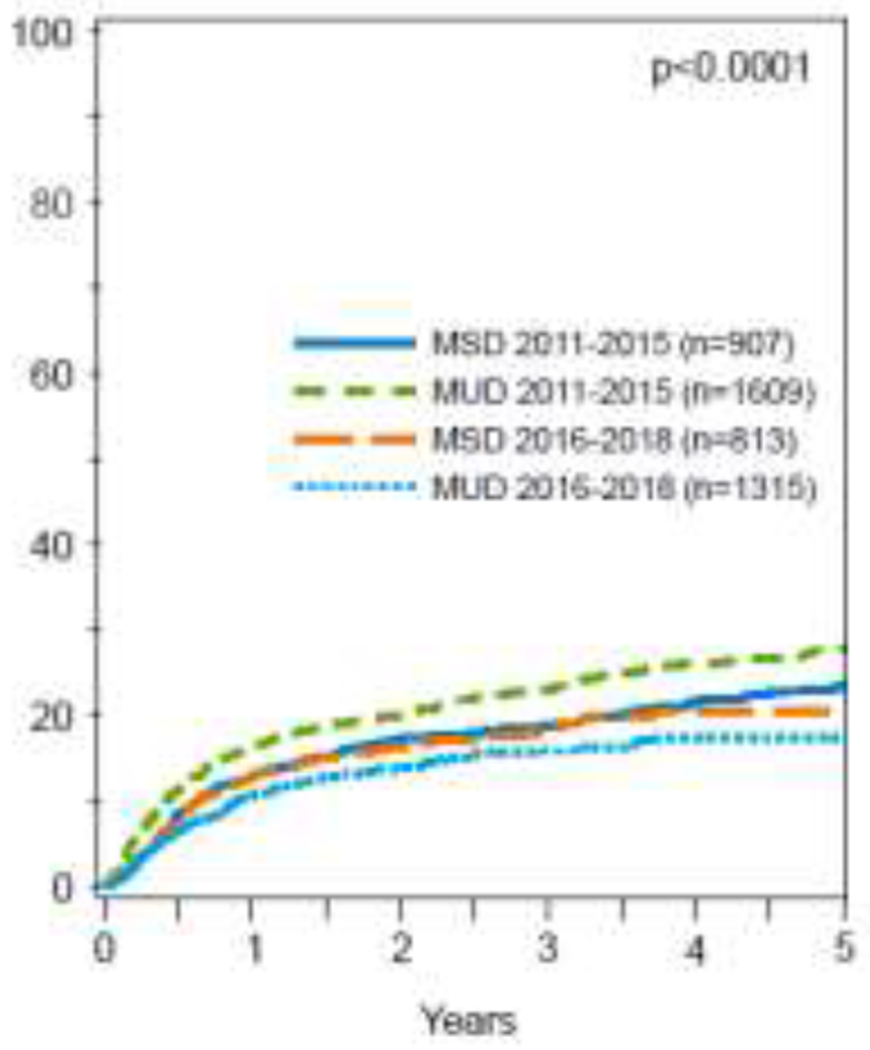
5-year adjusted CI of non-relapse mortality, stratified by donor type.
Predictors of increased NRM included older recipient age, inferior KPS, higher HCT-CI score, and AML not in CR1. Recipient’s CMV seronegative status, RIC/NMA conditioning, and male-donor-female-recipient pairs decreased NRM risk (table S3).
Chronic graft-versus-host disease:
Multivariable analysis showed that alloHCT from a younger MUDs were associated with a greater risk for chronic GVHD (HR 1.18; 95% CI, 1.08-1.29; p=.0002) compared to older MSDs (Table 2; Figure S1). The adjusted 1-year (44% [95% CI, 42%-46%] vs 41% [95% CI, 39%-43%]; p=.07) and 3-year (56% [95% CI, 54%-57%] vs 51% [95% CI, 49%-53%]; p=.005) incidence of chronic GVHD was also significantly greater with younger MUDs compared to older MSDs (Table 3). Other factors significantly associated with increased chronic GVHD included PB allografts, whereas longer time from diagnosis to alloHCT (>6 months) and in vivo T-cell depletion were associated with decreased chronic GVHD risk (table S3).
Survival:
In multivariable analysis, there was no difference in OS or DFS between alloHCT from younger MUDs vs older MSDs (OS: HR 1.02; 95% CI, 0.94-1.12; p=.607; DFS: HR 0.92; 95% CI, 0.85-1.01; p=.073) (Table 2; Figure 2a&b). While there was no difference in 5-year OS probability between the two groups, the adjusted 5-year DFS probability was higher with younger MUDs compared to older MSDs (44% (95% CI, 42%-46%) vs. 41% (95% CI, 38%-43%; p=.04) (Table 3). Other significant predictors of DFS included recipient’s age, HCT-CI score, ELN risk group 2017, AML subtype, ATG/alemtuzumab usage, remission status, and alloHCT year (table S3). OS was significantly impacted by recipient’s age, KPS, HCT-CI score, ELN risk group 2017, AML subtype, donor-recipient sex matches, and year of alloHCT (table S3).
Figure 2A:
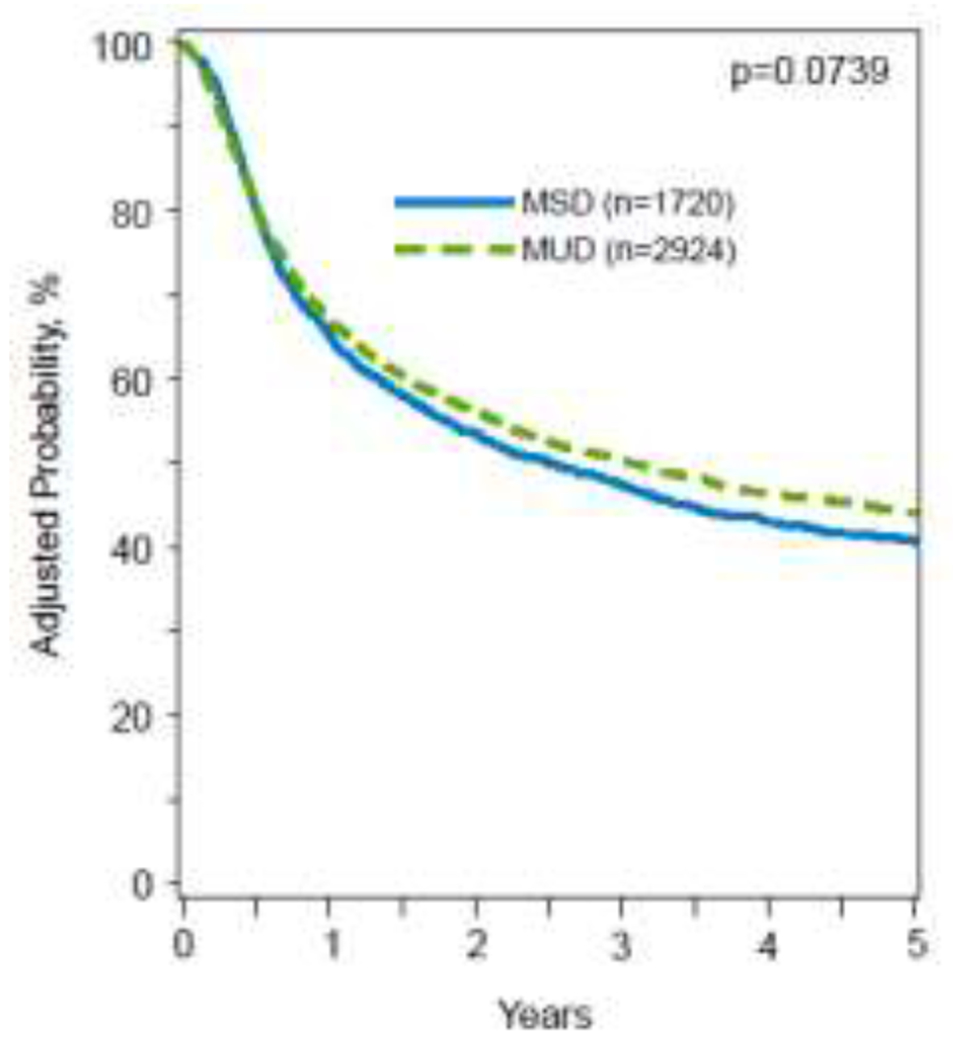
5-year adjusted CI of disease-free survival, stratified by donor type.
Figure 2B:
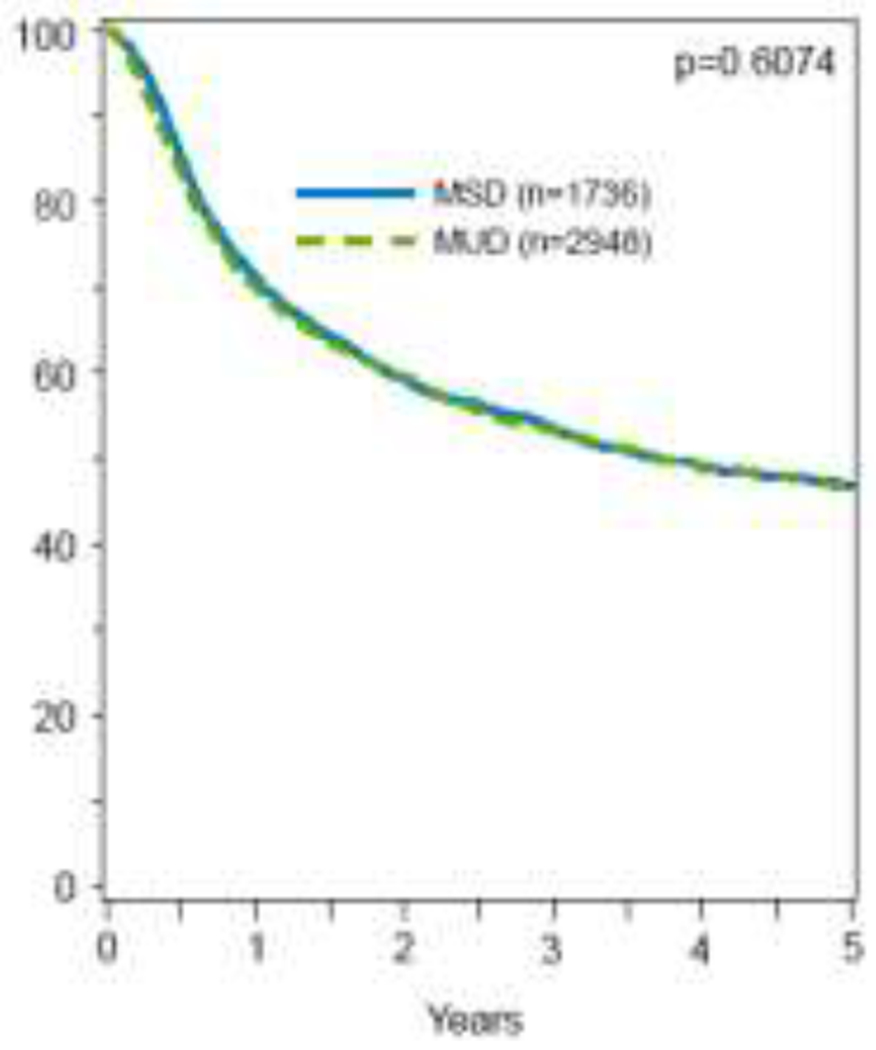
5-year adjusted CI of overall survival, stratified by donor type.
Cause of death:
Common causes of death included primary disease, GVHD, infections, and organ failure and are described in table S4.
DISCUSSION:
The primary intent of alloHCT in patients with AML in CR is to cure disease by preventing relapse20,21. In this study including 4684 patients with AML older than 50 years, we found that alloHCT from a younger MUD was associated with a significantly reduced relapse risk compared to an older MSD. The relapse advantage was sustained at 5 years on adjusted analysis and translated into an improved DFS.
AlloHCT offers cure for patients with AML and is the only curative modality for patients with high-risk leukemia2,21. Longitudinal trend analyses in more than 300,000 patients reported to CIBMTR demonstrated a decline in NRM and GVHD over the last 4 decades but an increase in relapse22. AML relapse remains the primary cause of transplant failure and continues to account for at least half of the deaths in HCT recipients22. Further, outcomes of patients who relapse after alloHCT are poor21,23,24. Prior registry studies have demonstrated that donor-recipient HLA-disparity and older donor age portend poor survival. While younger MUD remains a plausible alternative, it is unclear if younger MUDs are better than older MSDs in older patients with AML. Results of prior single-center and registry studies, often including heterogenous group of hematologic malignancies, yielded conflicting results and the impact of donor age on alloHCT outcomes in AML remains unclear9-11,25. A CIBMTR analysis in 10,000 alloHCT recipients from MUDs showed that younger MUDs conferred superior survival. However, this analysis was not disease-specific, included patients with all myeloid malignancies, and did not directly compare MUDs to MSDs9. Other CIBMTR and EBMT analyses, either examined the impact of donor type in all myeloid malignancies (not AML-specific) or examined the impact in all adults (not in older adults). The results either showed no difference between the 2 donor types or favored MSD over MUD10,25,11. Further, these registry studies pre-dated the contemporary transplant era of modern therapeutics and supportive care.
Although the conventional donor choice is MSD, these donors are likely to be older and have age-related issues such as T-cell exhaustion resulting in a diminished GVL effect26,27. In the current study, in addition to significant relapse reduction with younger MUD, HCT-associated NRM considerably decreased in recent years, which is likely due to enhanced GVHD prophylaxis, infection preemption and preventative strategies, and overall improved supportive care measures. However, these changes in practice likely helped improve outcomes in both the study arms over time. This is in congruence with the existing data28,29. Although there was a trend in DFS in favor of younger MUDs, the difference did not reach statistical significance (HR 0.92; 95% CI, 0.85-1.01; p=.07). However, the adjusted 5-year DFS probability was superior with younger MUDs compared to older MSDs. A greater proportion of patients in the earlier years likely experienced a higher NRM from younger MUDs, which could have diminished the impact of relapse reduction with younger MUDs on OS benefit in the overall study cohort. However, with the lack of data on aGVHD and severity of cGVHD in the current analysis, this potential association will need to be validated in future analyses.
The intent of this analysis was to compare the current standard-of-care, an older MSD, with the best MUD (younger than 35)9,10,14,16. While it is not possible to analyze the differential impact of age vs unrelatedness on improved disease control with the current design, we postulate that relapse reduction with younger MUDs could be due to a combination of factors, namely more robust donor-derived immunity, lesser CHIP, greater germline single nucleotide polymorphisms discordance, lower secondary events or inherited susceptibility to myeloid malignancies resulting in decreased early relapse compared to older donors8,30. While the time to alloHCT is more important than donor matching in high-risk patients, it is imperative that the time involved in finding a younger MUD does not compromise alloHCT outcomes in racial/ethnic minority groups31. Use of haploHCT is bridging the alloHCT access gap for patients from racial and ethnic groups other than non-Hispanic whites32,33. Expectedly, the interval between diagnosis to alloHCT was longer with younger MUDs in our analysis. Nonetheless, the difference in median time from diagnosis to transplant between the 2 cohorts was only 1 month (4 vs 5 months), consistent with prior studies34. As CIBMTR does not capture data on physicians/centers’ discretion and preferences, it is plausible that this decision could impact the results. However, the time from diagnosis to alloHCT and center effect were adjusted in the multivariable model. Additionally, the homogeneity of AML population in CR1 and CR2 further minimizes that chance of physicians/centers’ discretion may have impacted the results in a significant way. According to recent NMDP data, the median time to find an MUD donor is 88 days (range: 17-183 days)35, which is considerably shorter than the median time of 4-5 months in each of our 2 study arms. Notably, our results may not be applicable in rare situations when the search for MUD might take longer than the current projected NMDP timelines. Similarly, the possibilities of AML risk, subtype, and MRD status (for patients in CR1) affecting the alloHCT timing and donor choice were thoroughly assessed in multivariable models. No significant associations were found.
Results have practical implications in view of an aging AML population. There has been a dramatic increase in the number of older patients undergoing HCT in recent years22. According to CIBMTR trend analyses, 26% of alloHCT recipients were older than 65 years in 2019 compared to only 2% in the year 200022, in sharp contrast to stable transplant trends in other age groups. Additionally, AML remains the most common indication for alloHCT in the US. A trend is further observed towards transplantation while in remission (CR1/CR2), as in our study’s inclusion criteria22. The absolute relapse reduction with DFS benefit was homogeneous across subgroups in the current study with no discernible subgroup benefiting more than others.
Despite the largest sample size to date, limitations of the current analysis include lack of information on genomic mutations, donor clonal hematopoiesis, T-cell repertoire kinetics, physicians’ decisions, and the interval between donor search initiation and identification. Secondly, granular information was unavailable on acute GVHD or the severity of chronic GVHD due to data transitions within the registry. While the limitations inherent with retrospective studies remain, the sample size and strengths associated with the CIBMTR registry (prospective collection of high-quality data on all consecutive alloHCT patients) mitigate some of these limitations, particularly given that a prospective randomized clinical trial comparing the two common donor types is not feasible. Thirdly, the longer-term effect of using younger stem cells was not evaluated and whether they may be more protective against developing secondary malignancies or MDS post-transplant remains unanswered36. Fourthly, while the variability in practice across centers (decisions related to diagnostic patterns, treatment decisions, and transplant referral practices, etc.) were adjusted in the analysis via the center effect, there remains a possibility of unmeasured variables. However, this also is a key strength of the current analysis as the results provide real-world evidence across centers. Finally, PTCy is likely to become the standard GVHD prophylaxis given the recent Blood and Marrow Transplant Clinical Trials Network (BMTCTN) study results. Hence, future analyses will be needed once the PTCy practice is widely accepted37.
This large study conducted in the contemporary transplant era found significant relapse reduction in older patients with AML who underwent alloHCT with younger MUDs compared to older MSDs. The results highlight that a younger MUD donor type exerts a stronger GVL effect and should be preferred when available. The benefit of relapse reduction was likely abrogated by higher NRM in the MUD arm in the earlier years and drove the DFS benefit lower, as DFS is a composite endpoint of NRM and relapse. However, the results provide a roadmap for the field indicating the need for reducing NRM with younger MUDs (e.g., as shown in BMTCTN 1703). Overall, the results aid in donor selection and guide physicians and transplant centers in decision making in real-world clinical scenarios when both donor types are available.
Supplementary Material
HIGHLIGHTS:
Younger MUDs (donor age ≤35) are associated with a significantly decreased relapse risk (HR 0.86; p=.005) and a significantly lower adjusted 5-year cumulative incidence of relapse (35% vs 41%; p=.003) compared to older MSDs (donor age ≥50).
Compared to older MSDs, younger MUDs are associated with a greater NRM in the earlier years (2011-2015) but a significantly lower NRM in the more recent years (2016-2018).
The adjusted 5-year DFS probability is higher with younger MUDs compared to older MSDs.
Combining the use of younger MUD donor type with improved strategies to reduce GVHD is worth further exploration to improve outcomes.
ACKNOWLEDGMENTS:
The authors thank Ms. Renee Dunn (CIBMTR) for her assistance with preparation of the manuscript figures.
Funding Source Statement:
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Angiocrine; Anthem; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc.; CRISPR; CSL Behring; CytoSen Therapeutics, Inc.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medexus Pharma; Merck & Co.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Pluristem; PPD Development, LP; Sanofi; Sanofi-Aventis U.S. Inc.; Sobi, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV. This work was also supported in part by the Intramural Research Program of the National Heart, Lung, and Blood Institute (CSH).
Conflict of Interest:
The National Heart, Lung, and Blood Institute receives research funding for the laboratory of Dr. Hourigan from Sellas and from the Foundation of the NIH AML MRD Biomarkers Consortium.
Dr. Badar reports advisory board participation for Pfizer and Takeda.
Dr. Sabloff reports research support from Abbvie, Astellas, Celgene, BMS, JAZZ, Taiho, Astex, Takeda, Geron, and Actinium; and Advisory Board participation for Abbvie, Astellas, Pfizer, BMS, Taiho, and Celgene.
Dr. Litzow reports compensation (research funding) from Abbvie, Astellas, Amgen, Actinium, Pluristem, and Syndax; Advisory Board participation for Jazz; and Data Monitoring Committee participation for Biosight.
Dr. Allan is a paid medical consultant in Stem Cells at Canadian Blood Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the American Society of Hematology Annual Meeting in December 2022 and the American Society of Transplantation & Cellular Therapy in February 2023.
Data Use Statement:
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
REFERENCES:
- 1.The Surveillance, Epidemiology, and End Results (SEER) Program Database. [Google Scholar]
- 2.Lin RJ, Artz AS. Allogeneic hematopoietic cell transplantation for older patients. Hematology 2021; 2021:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myllymäki M, Redd R, Reilly CR et al. Short telomere length predicts nonrelapse mortality after stem cell transplantation for myelodysplastic syndrome. Blood 2020; 136:3070–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaiswal S, Fontanillas P, Flannick J et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014; 371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol 2007; 5:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abid MB. Could the menagerie of the gut microbiome really cure cancer? Hope or hype. J Immunother Cancer 2019; 7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taur Y, Jenq RR, Perales MA et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014; 124:1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese G, Kähler AK, Handsaker RE et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. New England Journal of Medicine 2014; 371:2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollman C, Spellman SR, Zhang MJ et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood 2016; 127:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alousi AM, Le-Rademacher J, Saliba RM et al. Who is the better donor for older hematopoietic transplant recipients: an older-aged sibling or a young, matched unrelated volunteer? Blood 2013; 121:2567–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peffault de Latour R, Labopin M, Cornelissen J et al. In patients older than 55 years with AML in first CR, should we search for a matched unrelated donor when an old sibling donor is available? Bone Marrow Transplantation 2015; 50:1411–1415. [DOI] [PubMed] [Google Scholar]
- 12.Miguel-Angel P, Benjamin T, Mei-Jie Z et al. Alternative donor transplantation for acute myeloid leukemia in patients aged ≥50 years: young HLA-matched unrelated or haploidentical donor? Haematologica 2020; 105:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroger N, Zabelina T, de Wreede L et al. Allogeneic stem cell transplantation for older advanced MDS patients: improved survival with young unrelated donor in comparison with HLA-identical siblings. Leukemia 2013; 27:604–609. [DOI] [PubMed] [Google Scholar]
- 14.Guru Murthy GS, Kim S, Hu ZH et al. Relapse and Disease-Free Survival in Patients With Myelodysplastic Syndrome Undergoing Allogeneic Hematopoietic Cell Transplantation Using Older Matched Sibling Donors vs Younger Matched Unrelated Donors. JAMA Oncol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kollman C, Howe CW, Anasetti C et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood 2001; 98:2043–2051. [DOI] [PubMed] [Google Scholar]
- 16.Shaw BE, Logan BR, Spellman SR et al. Development of an Unrelated Donor Selection Score Predictive of Survival after HCT: Donor Age Matters Most. Biol Blood Marrow Transplant 2018; 24:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisdorf D, Spellman S, Haagenson M et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant 2008; 14:748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitko CL, Pidala J, Schoemans HM et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group Report. Transplantation and Cellular Therapy 2021; 27:545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Döhner H, Estey E, Grimwade D et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129:424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horowitz M, Schreiber H, Elder A et al. Epidemiology and biology of relapse after stem cell transplantation. Bone Marrow Transplant 2018; 53:1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. New England Journal of Medicine 2015; 373:1136–1152. [DOI] [PubMed] [Google Scholar]
- 22.Phelan R, Chen M, Bupp C et al. Updated Trends in Hematopoietic Cell Transplantation in the United States with an Additional Focus on Adolescent and Young Adult Transplantation Activity and Outcomes. Transplantation and Cellular Therapy 2022; 28:409.e401–409.e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ossenkoppele GJ, Janssen JJ, van de Loosdrecht AA. Risk factors for relapse after allogeneic transplantation in acute myeloid leukemia. Haematologica 2016; 101:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavletic SZ, Kumar S, Mohty M et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on the Epidemiology and Natural History of Relapse following Allogeneic Cell Transplantation. Biol Blood Marrow Transplant 2010; 16:871–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saber W, Opie S, Rizzo JD, Zhang MJ, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood 2012; 119:3908–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeiser R, Beelen DW, Bethge W et al. Biology-Driven Approaches to Prevent and Treat Relapse of Myeloid Neoplasia after Allogeneic Hematopoietic Stem Cell Transplantation. Biology of Blood and Marrow Transplantation 2019; 25:e128–e140. [DOI] [PubMed] [Google Scholar]
- 27.Rimando JC, Christopher MJ, Rettig MP, DiPersio JF. Biology of Disease Relapse in Myeloid Disease: Implication for Strategies to Prevent and Treat Disease Relapse After Stem-Cell Transplantation. Journal of Clinical Oncology 2021; 39:386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev 2019; 36:70–87. [DOI] [PubMed] [Google Scholar]
- 29.McDonald GB, Sandmaier BM, Mielcarek M et al. Survival, Nonrelapse Mortality, and Relapse-Related Mortality After Allogeneic Hematopoietic Cell Transplantation: Comparing 2003-2007 Versus 2013-2017 Cohorts. Annals of internal medicine 2020; 172:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersdorf EW, Stevenson P, Malkki M et al. Patient HLA Germline Variation and Transplant Survivorship. J Clin Oncol 2018; 36:2524–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SJ, Klein J, Haagenson M et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 2007; 110:4576–4583. [DOI] [PubMed] [Google Scholar]
- 32.Abid MB, Hamadani M, Szabo A et al. Severity of Cytokine Release Syndrome and Its Association with Infections after T Cell-Replete Haploidentical Related Donor Transplantation. Biol Blood Marrow Transplant 2020; 26:1670–1678. [DOI] [PubMed] [Google Scholar]
- 33.Marks DI, Abid MB. A Stem Cell Donor for Every Adult Requiring an Allograft for Acute Lymphoblastic Leukemia? Biol Blood Marrow Transplant 2017; 23:182–183. [DOI] [PubMed] [Google Scholar]
- 34.Visram A, Aziz J, Bryant A et al. Effect of Donor Age and Donor Relatedness on Time to Allogeneic Hematopoietic Cell Transplantation in Acute Leukemia. Biol Blood Marrow Transplant 2018; 24:2466–2470. [DOI] [PubMed] [Google Scholar]
- 35.Dehn J, Chitphakdithai P, Shaw BE et al. Likelihood of Proceeding to Allogeneic Hematopoietic Cell Transplantation in the United States after Search Activation in the National Registry: Impact of Patient Age, Disease, and Search Prognosis. Transplantation and Cellular Therapy 2021; 27:184.e181–184.e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majhail NS. Long-term complications after hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther 2017; 10:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtan SG, Hamadani M, WU J et al. Post-Transplant Cyclophosphamide, Tacrolimus, and Mycophenolate Mofetil As the New Standard for Graft-Versus-Host Disease (GVHD) Prophylaxis in Reduced Intensity Conditioning: Results from Phase III BMT CTN 1703. Blood 2022; 140:LBA-4–LBA-4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.


