Abstract
Background:
Premature menopause is a risk factor for accelerated cardiovascular aging, but underlying mechanisms remain incompletely understood. This study investigated the role of leukocyte telomere length (LTL), a marker of cellular aging and genomic instability, in the association of premature menopause with cardiovascular disease.
Methods:
Participants from the UK Biobank and Women’s Health Initiative (WHI) with complete reproductive history and LTL measurements were included. Primary analyses tested the association between age at menopause and LTL using multivariable-adjusted linear regression. Secondary analyses stratified women by history of gynecologic surgery. Mendelian randomization was used to infer causal relationships between LTL and age at natural menopause. Multivariable-adjusted Cox regression and mediation analyses tested the joint associations of premature menopause and LTL with incident coronary artery disease (CAD).
Results:
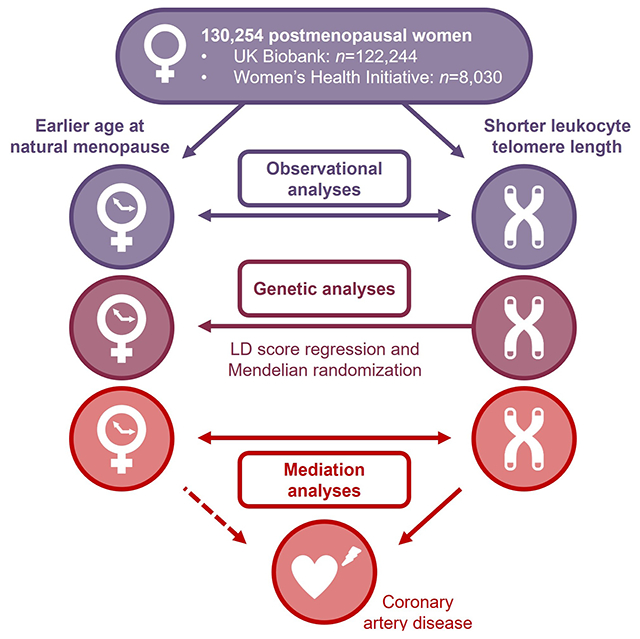
This study included 130,254 postmenopausal women (UK Biobank: n=122,224; WHI: n=8,030), of whom 4,809 (3.7%) had experienced menopause before age 40. Earlier menopause was associated with shorter LTL (meta-analyzed ß=−0.02 SD/5 years of earlier menopause [95% confidence interval, CI: −0.02 to −0.01], P=7.2×10−12). This association was stronger and significant in both cohorts for women with natural/spontaneous menopause (meta-analyzed ß=−0.04 SD/5 years of earlier menopause [95% CI: −0.04 to −0.03], P<2.2×10−16) and was independent of hormone therapy use. Mendelian randomization supported a causal association of shorter genetically predicted LTL with earlier age at natural menopause. LTL and age at menopause were independently associated with incident CAD, and mediation analyses indicated small but significant mediation effects of LTL in the association of menopausal age with CAD.
Conclusions:
Earlier age at menopause is associated with shorter LTL, especially among women with natural menopause. Accelerated telomere shortening may contribute to the heightened cardiovascular risk associated with premature menopause.
Keywords: Aging, Cardiovascular disease, Menopause, Telomeres, Women, Sex, Gender, Genetics
Graphical Abstract
INTRODUCTION
Menopause is a critical reproductive aging event that indicates the end of fertility in women and is associated with acceleration of cardiovascular disease risk.1,2 The median age at menopause is ~50-51 years,3 and age at menopause is increasingly recognized as a marker of biological aging and health.2,4 As earlier age at menopause has been consistently associated with increased risk of coronary artery disease (CAD) and other cardiovascular conditions,5,6 premature menopause (i.e., <40 years) is now incorporated as a “risk-enhancing factor” to guide allocation of primary-prevention statin therapy for women in midlife.7,8
The association between earlier age at menopause and cardiovascular disease is well-established. However, emerging evidence suggests that postmenopausal estrogen deficiency does not fully explain the increased cardiovascular risk in women with premature menopause.9 Genome-wide association studies of age at natural menopause indicate that DNA damage repair pathways play an important role in determining reproductive lifespan by influencing ovarian reserve early in life, as well as its subsequent rate of depletion.10 Additionally, recent research indicates that premature menopause is associated with clonal hematopoiesis of indeterminate potential (CHIP),9 an age-related condition characterized by the clonal expansion of hematopoietic stem cells with acquired mutations in leukemia-associated genes. CHIP has been associated with increased incidence of atherosclerotic cardiovascular disease, independent of traditional risk factors.9,11 These findings suggest that somatic (acquired) genomic phenomena may play a significant role in linking reproductive aging with cardiovascular disease.
Telomeres are nucleoprotein complexes that protect chromosomal DNA from shortening during successive cell divisions and prevent genomic instability.12 With aging, DNA damage repair mechanisms induce cellular senescence when telomeres become critically short,13 resulting in a “senescence-associated secretory phenotype” that promotes cardiovascular aging and disease.13 Leukocyte telomere length (LTL) has been proposed as a biomarker of biological aging, with epidemiologic and Mendelian randomization (MR) analyses supporting causal effects of shorter LTL on increased risk of cardiovascular disease.14,15 Recent data suggest that the development of CHIP leads to shorter LTL, possibly due to acceleration of the cell cycle in hematopoietic stem cells with CHIP driver mutations.15 However, the relationship between age at menopause and LTL remains unclear, with existing data being limited and inconsistent.16–18 It is currently unknown if LTL contributes to the heightened cardiovascular risk in women with premature menopause.
To gain insights into mechanisms of cardiovascular aging in women with premature menopause, this study tested the association of age at menopause with LTL among postmenopausal women in the UK Biobank and the Women’s Health Initiative (WHI). In addition, we inferred whether any associations between age at natural menopause and LTL were causal using MR. Finally, we tested the joint association of age at menopause and LTL with incident CAD and performed mediation analysis.
METHODS
Data availability
The UK Biobank data that support the findings of the present study can be accessed by application (https://www.ukbiobank.ac.uk/register-apply/), and the WHI data can be accessed by registered researchers through the WHI online resource (https://www.whi.org/datasets) or by other researchers through BioLINCC (https://biolincc.nhlbi.nih.gov/studies/whi_ctos/).
Study design
Briefly, we included postmenopausal women from the UK Biobank and WHI with complete reproductive history and LTL measurements. Primary analyses tested the associations between age at menopause and LTL using multivariable-adjusted linear regression models. Secondary analyses stratified women by history of gynecologic surgery (i.e., hysterectomy or bilateral oophorectomy) and tested premature menopause (i.e., <40 years) as well as natural and surgical premature menopause as separate exposures. We used MR and linkage disequilibrium (LD) score regression to infer causality in the observed associations between LTL and age at menopause. Multivariable-adjusted Cox regression and mediation analyses tested the role of LTL in the association of earlier age at menopause with incident coronary artery disease. The Supplemental Methods, Tables S1–4, and the Major Resources Table in the Supplemental Materials provide a detailed description of all methods used in this study.
Two-sided P<5.0×10−2 was considered statistically significant for the primary analysis. We did not apply correction for multiple testing; findings from secondary and MR analyses should be considered supportive and hypothesis-generating. All analyses were carried out using R version 4.1.3.
RESULTS
Description of the study cohorts
The final study sample included 130,254 postmenopausal women across the UK Biobank and WHI with LTL measurements (Figure S1). Among 122,224 women in the UK Biobank cohort (median age 61 [interquartile range, IQR: 57-64] years at blood draw), the median age at menopause was 50 (IQR: 48-53) years. Overall, 3,899 women (3.2%) in the UK Biobank experienced premature menopause (i.e., <40 years), including 1,686 (1.4%) with natural premature menopause and 2,213 (1.8%) with surgical premature menopause, and 9,959 (8.1%) experienced early menopause (i.e., 40-<45 years). Among 8,030 women in the WHI cohort (median age 69 [IQR: 64-74] years at blood draw), the median age at menopause was 50 (IQR: 45-52) years. In the WHI cohort, 910 women (11.3%) experienced premature menopause (239 [3.0%] natural and 671 [8.4%] surgical premature menopause), and 1,044 (13.0%) experienced early menopause. Premature menopause, especially surgical premature menopause, was more common in the WHI than the UK Biobank. History of gynecologic surgery was substantially more common in the WHI irrespective of premature menopause status (Table S5).
In both cohorts, women with vs. without premature menopause were more likely to be current or former smokers and had higher body mass index (BMI), higher prevalence of type 2 diabetes, and higher rates of antihypertensive and cholesterol-lowering medication use (Table S5). Prevalent CAD at baseline was more frequent in women with vs. without premature menopause. Pooled unadjusted prevalence of CHIP was 5.8% (n=262/4,543) among women with premature menopause and 4.2% (n=4,999/117,687) in those without (P=8.9×10−7). Among 5,261 women with CHIP, the most frequently mutated driver genes were DNMT3A (66.3%), TET2 (14.7%), and ASXL1 (7.6%). Table S6 presents the baseline characteristics of women with below-median vs. above-median LTL.
Association of earlier age at menopause with shorter LTL
As expected, LTL declined with increasing chronologic age (Figure 1). Every 5 years of earlier age at menopause was associated with shorter LTL by 0.02 standard deviations (SD; 95% confidence interval [CI]: −0.03 to −0.02) in minimally (P<2.2×10−16) (Table S7) and fully (P=1.1×10−13) (Table 1) adjusted models for the UK Biobank, whereas the same associations in the overall WHI cohort were null with significant heterogeneity between the UK Biobank and WHI (fully adjusted P[heterogeneity]=4.0×10−3). When stratified by history of gynecologic surgery, the association of age at menopause with LTL was stronger among women without prior hysterectomy or bilateral oophorectomy in both cohorts (UK Biobank: ß=−0.04 SD/5 years [95% CI: −0.04 to −0.03], P<2.2×10−16; WHI: ß=−0.03 SD/5 years [95% CI: −0.06 to 0.00], P=4.7×10−2; P[heterogeneity]=5.8×10−1). Among women with a history of natural menopause across cohorts, compared with menopause at age 50-54 years, ages at menopause <40 years, 40-<45 years, and 45-<50 years were associated with meta-analyzed adjusted differences in LTL by −0.08 SD (95% CI: −0.13 to −0.04, P=5.2×10−4), −0.07 SD (95% CI: −0.10 to −0.05, P=9.4×10−10), and −0.04 SD (95% CI: −0.06 to −0.03, P=6.2×10−9; P[heterogeneity]>7.0×10−1 for all categories), respectively; a similar graded relationship was not apparent among those with previous gynecologic surgery (Figure 2, Table S8). Compared with women who experienced menopause at age ≥40 years (i.e., without premature menopause), LTL was shorter in those with natural premature menopause (meta-analyzed adjusted difference: −0.07 SD [95% CI: −0.12 to −0.03], P=1.4×10−3) but not in those with surgical premature menopause (0.00 SD [95% CI: −0.04 to 0.04], P=9.9×10−1).
Figure 1. Leukocyte telomere length vs. chronologic age in the UK Biobank and Women’s Health Initiative by premature menopause status and history of gynecologic surgery.
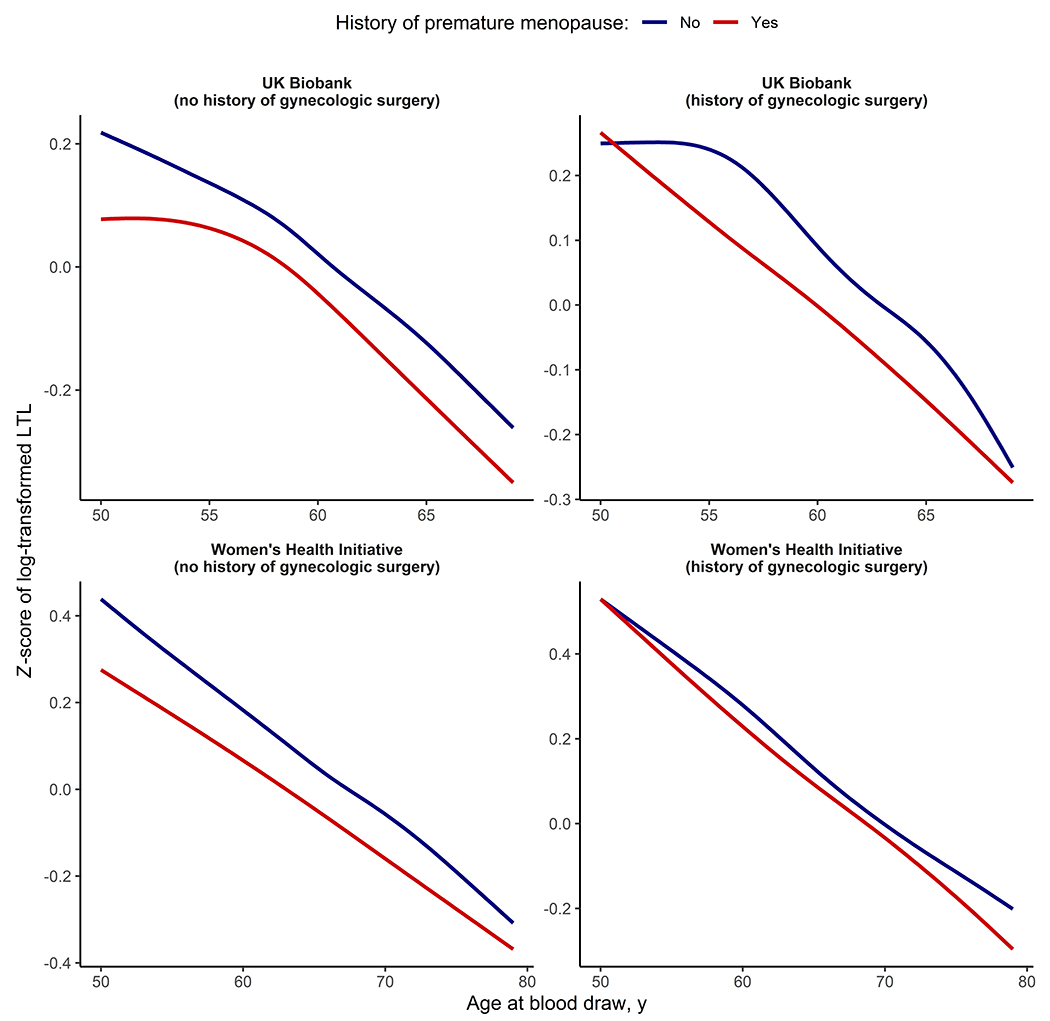
The colored lines represent the unadjusted Z-scores for leukocyte telomere length (LTL), plotted against age at blood draw and stratified by history of premature menopause. Plots were generated with the ggplot package in R version 4.1.3 using locally weighted polynomial smoothing.
Table 1.
Association of age at menopause with leukocyte telomere length (in standard deviations of log-transformed leukocyte telomere length) in the UK Biobank and Women’s Health Initiative.
| UK Biobank | Women’s Health Initiative | Meta-analysis | |||||
|---|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | P(heterogeneity) | |
| Per 5 years of earlier menopause, overall | −0.02 (−0.03 to −0.02) | 1.1×10−13 | 0.00 (−0.01 to 0.02) | 7.1×10−1 | −0.02 (−0.02 to −0.01) | 7.2×10−12 | 4.0×10−3 |
| Per 5 years of earlier natural menopause | −0.04 (−0.04 to −0.03) | <2.2×10−16 | −0.03 (−0.06 to 0.00) | 4.7×10−2 | −0.04 (−0.04 to −0.03) | <2.2×10−16 | 5.8×10−1 |
| Per 5 years of earlier menopause, history of gynecologic surgery | −0.02 (−0.03 to 0.00) | 2.6×10−2 | 0.00 (−0.02 to 0.02) | 9.3×10−1 | −0.01 (−0.02 to 0.00) | 5.9×10−2 | 2.4×10−1 |
| Premature menopausea | −0.03 (−0.07 to 0.00) | 3.6×10−2 | −0.01 (−0.08 to 0.06) | 7.9×10−1 | −0.03 (−0.06 to 0.00) | 4.4×10−2 | 5.2×10−1 |
| Natural premature menopausea | −0.08 (−0.13 to −0.03) | 1.9×10−3 | −0.05 (−0.18 to 0.07) | 4.2×10−1 | −0.07 (−0.12 to −0.03) | 1.4×10−3 | 7.3×10−1 |
| Surgical premature menopausea | 0.00 (−0.04 to 0.04) | 9.2×10−1 | 0.01 (−0.07 to 0.08) | 8.8×10−1 | 0.00 (−0.04 to 0.04) | 9.9×10−1 | 8.5×10−1 |
Analyses represent linear regression models adjusted for age, age², race/ethnicity, the first ten principal components of genetic ancestry, current/former smoking status, body mass index, diabetes status, current hormone therapy use, and prevalent coronary artery disease. The UK Biobank sample included 112,224 women, of whom 12,531 had a history of gynecologic surgery. The WHI sample included 8,030 women, of whom 3,170 had a history of gynecologic surgery.
Reference group is women with age at menopause ≥40 years. No corrections for multiple comparisons were made. CI indicates confidence interval.
Figure 2. Meta-analyzed adjusted differences in leukocyte telomere length (LTL) by age at menopause among women with and without a history of gynecologic surgery.
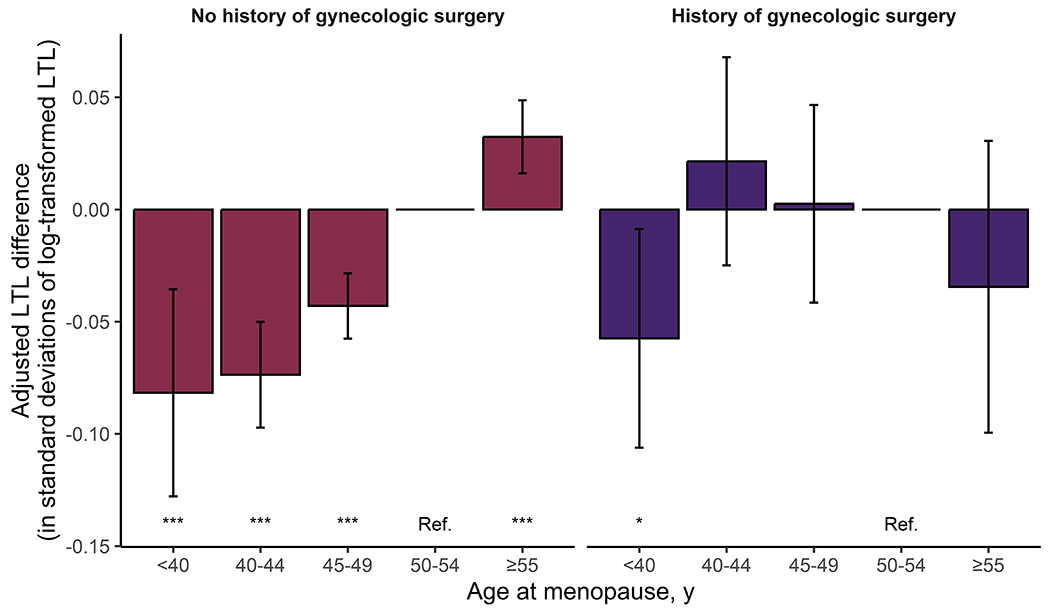
The bar graph represents the meta-analyzed effect size of each menopausal age category on LTL, calculated using linear regression models adjusted for age, age2, race/ethnicity, the first ten principal of genetic ancestry, current/former smoking status, body mass index, diabetes status, current hormone therapy use, and prevalent coronary artery disease. Analyses in the WHI were further adjusted for inverse probability of sampling weights, while those in the UK Biobank were further adjusted for Townsend deprivation index. Age at menopause was incorporated as a categorical, nonordered variable with age 50-54 years as the reference category. The error bars indicate 95% confidence intervals. No corrections for multiple comparisons were made. ***P<1.0×10−3, *P<5.0×10−2.
Associations of age at menopause with shorter LTL were consistent across multiple sensitivity analyses. These included exclusion of individuals with pre-existing CAD (Table S9), exclusion of individuals with a history of cancer (Table S10), exclusion of individuals with a history of hysterectomy only without bilateral oophorectomy (Table S11), and stratification by age at blood collection (<65 vs. ≥65 years) (Table S12). While associations of earlier age at natural menopause with shorter LTL were consistent for women who self-reported as Black and those who self-reported as White, exploratory analyses suggested that premature natural menopause was associated with a more pronounced decrease in LTL in women who self-reported as Black vs. White (P[interaction]=5.9×10−2) (Table S13). Associations of age at menopause with shorter LTL were consistent across additional sensitivity analyses that accounted for reproductive characteristics, such as those that further adjusted for history of hormone therapy (Table S14), stratified by history of hormone therapy (Table S15), or further adjusted for age at menarche (Table S16). Women with menarche at ≤11 years had shorter LTL (−0.03 SD [95% CI: −0.05 to −0.02], P=1.7×10−4) vs. those with menarche at 13 years (Table S17); this significant association persisted after further adjustment for age at menopause (−0.03 SD [95% CI: −0.05 to −0.01], P=3.7×10−4). There was a graded relationship between shorter reproductive lifespan and shorter LTL, with the lowest estimates observed in women with a reproductive lifespan of <33 vs. 36-38 years (−0.03 SD [95% CI: −0.05 to −0.01], P=7.8×10−4).
Previous research has established that earlier menopause is associated with an increased prevalence of CHIP9 and that CHIP may lead to reduced LTL.15 While presence of CHIP was significantly associated with LTL after multivariable adjustment in both the UK Biobank (ß=−0.08 SD [95% CI: −0.10 to −0.05], P=4.2×10−7) and WHI (ß=−0.14 SD [95% CI: −0.22 to −0.06], P=4.4×10−4), adjustment for CHIP did not attenuate the associations between age at menopause and LTL (Table S18). In analyses stratified by CHIP status, continuous age at menopause and natural premature menopause were significantly associated with lower LTL, irrespective of CHIP status (Table S19). When evaluating the associations of combined menopause and CHIP status with LTL, the largest magnitude of telomere attrition was observed for women with natural premature menopause and CHIP (ß=−0.34 SD [95% CI: −0.53 to −0.16], P=2.9×10−4; Figure S2) vs. those without a history of premature menopause and no CHIP.
Association of genetic predisposition to shorter LTL with earlier age at natural menopause
LD score regression was used to evaluate the genetic correlation between LTL and age at natural menopause. We found a significant and positive genetic correlation between both traits (Rg=0.13 [95% CI, 0.06 to 0.20]; P=2.0×10−4), suggesting shared genetic architecture between shorter LTL and earlier age at natural menopause. In addition, we performed two-sample MR to infer causality in the observed associations between LTL and age at natural menopause. We identified 43 uncorrelated, genome-wide significant single-nucleotide variants associated with LTL (Table S20) and 35 with age at natural menopause (Table S21). While MR analysis revealed no significant effect of genetically predicted age at menopause on LTL (ß=0.04 SD/5 years [95% CI: −0.03 to 0.11], P=2.4×10−1), each SD decrease in genetically predicted LTL was significantly associated with 0.60 years of earlier natural menopause (95% CI: 0.07 to 1.14, P=2.8×10−2). The genetic association of shorter LTL with earlier natural menopause was highly consistent across sensitivity analyses (Figure 3), and there was no evidence of directional pleiotropy affecting these results (Egger intercept test: P=6.4×10−1).
Figure 3. Mendelian randomization (MR) analyses showing genetic associations between leukocyte telomere length and age at natural menopause.
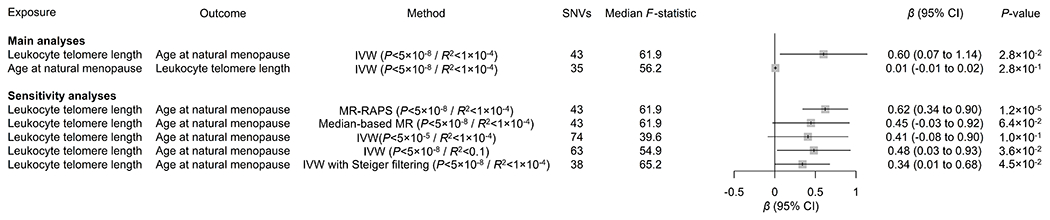
Main MR analyses used the inverse-variance-weighted (IVW) method, using genome-wide significant (P<5×10−8) single-nucleotide variants (SNVs) clumped into independent loci using a linkage disequilibrium R2 threshold of 1×10−4. Sensitivity analyses used the MR with robust adjusted profile score (MR-RAPS) and median-based MR methods, as well as the IVW method using more lenient P-value (5.0×10−5) or linkage disequilibrium R² (1.0×10−1) thresholds. Genetic data were expressed per standard deviation of (increased) leukocyte telomere length and per year of (older) age at natural menopause. No corrections for multiple comparisons were made. CI indicates confidence interval.
Association of shorter LTL with incident CAD events
Given previous work suggesting that shorter LTL is associated with accelerated cardiovascular aging,14,15 we further tested the role of LTL in the association of age at menopause with CAD. Follow-up for incident CAD events occurred over a median 11.1 (IQR: 10.4-11.8) years in the UK Biobank and 13.1 (IQR: 6.8-18.8) years in the WHI. Over the course of follow-up, 4.4% (n=5,224/119,600) and 15.7% (n=1,130/7,183) of women in the UK Biobank and WHI cohorts, respectively, experienced incident CAD events. Consistent with the previous literature,5 women with premature menopause more often experienced incident CAD events during follow-up (Figure S3), and earlier age of continuous menopause and premature menopause were independently associated with incident CAD in multivariable-adjusted models (Table S22).
Women with below-average LTL had a higher cumulative incidence of CAD across cohorts (Figure S4). After multivariable adjustment, associations between LTL and incident CAD were similar among women enrolled in the UK Biobank (HR: 1.07 per SD decrease in LTL [95% CI: 1.04-1.10], P=2.2×10−6) and the WHI (HR: 1.09 per SD decrease in LTL [95% CI: 1.03-1.16], P=5.4×10−3), yielding a meta-analyzed HR of 1.07 per SD decrease in LTL (95% CI: 1.05-1.10, P=5.0×10−8; P[heterogeneity]=5.1×10−1). The association of LTL with incident CAD events was robust to sensitivity analyses stratifying by age at blood collection (<65 vs. ≥65 years), further adjusted for history of hormone therapy use, stratified by history of hormone therapy use, or excluded participants with imputed covariates (Table S23). Additional adjustment for CHIP status did not alter the association between shorter LTL and incident CAD events (Table S24).
When evaluating the combined impact of age at menopause and LTL on incident CAD events, we found that CAD incidence was highest among women with premature menopause and below-median LTL, regardless of history of gynecologic surgery (Figure 3 and Figures S5–7). Further stratification by LTL suggested a dose-response relationship between LTL and incident CAD, with the highest risks observed in women with the shortest LTL across premature menopause categories (Figure S8).
LTL mediates a small but significant portion of CAD risk in women with natural menopause
Mediation analysis showed that approximately 3% of the association of menopausal age with incident CAD was attributable to LTL among those with natural menopause (UK Biobank: 2.8% [95% CI: 1.6 to 4.7%], P<2.2×10−16; WHI: 2.6% [95% CI: −0.1 to 12.3%], P=6.6×10−2). C-reactive protein, HDL cholesterol, and current/former smoking were other significant mediators of this association in women with natural menopause, with proportions mediated ranging from 1.7% for C-reactive protein (95% CI, 0.9 to 3.1%; P<2.2×10−16) to 6.1% for smoking (95% CI, 3.9 to 10.3%; P<2.2×10−16) in the UK Biobank (Table S25). LTL was not a statistically significant mediator in the association of age at menopause with incident CAD for those with previous gynecologic surgery (UK Biobank: 0.1% [−0.7 to 1.2%], P=6.7×10−1; WHI: 0.2% [95% CI: −33.8 to 46.0%], P=9.1×10−1; Table S26). Additional analyses testing for effect modification by premature menopause status revealed similar directions and magnitudes of effects for most risk factors, including LTL, in women with vs. without a history of premature menopause (Table S27). The only risk factor with evidence of effect modification by history of premature menopause was systolic blood pressure (P[interaction]=4.1×10−4), which was associated with a higher risk of incident CAD in women without, but not in those with, a history of premature menopause (Table S27).
DISCUSSION
In this study of two large cohorts of postmenopausal women with detailed information on reproductive history and LTL measurements, earlier continuous age at menopause and natural premature menopause were independently associated with shorter LTL. The association between premature menopause and shorter LTL was stronger and more consistently observed among women with a history of natural menopause and substantially attenuated among women with surgical menopause, implying that postmenopausal sex hormone deficiency was not responsible for observed differences in LTL. Additionally, findings from this study suggest that accelerated telomere shortening contributes causally to earlier natural menopause and highlight the mediating role of LTL in the association of earlier age at menopause with incident CAD. These results may have important implications for understanding the cardiovascular consequences of early menopause, underlying mechanisms, and preventive strategies for this population.
First, natural premature menopause may promote development of CAD via multiple pathways linked to genomic instability. Large-scale genome-wide association studies have identified variants in DNA damage repair genes as the primary genetic contributors to accelerated ovarian aging, implicating genomic instability in the etiology of premature natural menopause.10,19 Genetic variants in TERT (i.e., the gene that encodes telomerase reverse transcriptase) have been associated with accelerated epigenetic aging and earlier age at natural menopause,20 as well as with CHIP.21 As recent data indicate that women with premature natural menopause have a higher prevalence of CHIP vs. those without,9 with CHIP representing an independent risk factor for CAD,9,11 CHIP likely contributes to the heightened cardiovascular risk in this population. Recent work by Nakao et al.15 revealed a complex interaction between LTL and CHIP, with longer LTL predisposing individuals to develop CHIP, which, in turn, accelerates LTL shortening. Similarly, previous work indicates that germline mutations in POT1 causing excessively long telomeres can lead to a familial predisposition to CHIP,22 and that longer LTL is associated with the development of various cancers.23 The current study builds upon these findings by showing that the association between LTL and menopausal age is independent of CHIP and by identifying a potentially causal association of accelerated telomere attrition with earlier natural menopause. Consistent with a causal association of LTL on age at natural menopause, research in mice suggests that defective telomere elongation and accelerated telomere shortening contribute to oocyte dysfunction, which in turn may lead to accelerated reproductive aging.24 These findings, together with previous evidence suggesting an association of natural premature menopause with CHIP during midlife,9 highlight the importance of genomic instability in the etiology of accelerated reproductive aging as well as its association with CAD.
Second, the mechanisms driving cardiovascular risk in individuals with surgical premature menopause may differ from those associated with natural premature menopause. While deprivation of endogenous estrogen has been proposed as a key mechanism underlying the association of both surgical and natural premature menopause with cardiovascular disease,25 women who undergo menopause prematurely also have adverse cardiovascular risk profiles before the menopausal transition.26 Furthermore, in a recent analysis of ~144,000 postmenopausal women, use of hormone therapy did not appear to offset the risk associated with premature menopause.5 Collectively, these findings suggest that mechanisms other than estrogen depletion play a role in the association between earlier age at menopause and heightened cardiovascular risk. The current study found no association between age at menopause and LTL in women with surgical menopause, underscoring that mechanisms of excess cardiovascular risk may differ between natural and surgical premature menopause. A study of NHANES showed that the association between surgical menopause and cardiovascular disease attenuated after adjusting for family history, suggesting that susceptibility genes that increase the risk of both outcomes may explain part of this association.27 It is also possible that disease and treatment effects may contribute to the excess risk of CAD observed in women who underwent gynecologic surgery due to malignancy.28–30 Understanding the mechanistic differences across menopause groups is crucial for developing precision medicine approaches to mitigate cardiovascular risk in postmenopausal women, warranting further research into mechanisms underlying the higher rates of cardiovascular disease in women with prior surgical premature menopause.
Third, women with premature menopause may derive particular benefit from intensive primordial and primary prevention strategies. Indeed, current guidelines incorporate history of premature menopause as a risk-enhancing factor to help inform allocation of primary-prevention statin therapy.7,8 Nonpharmacologic interventions may also be particularly important in those who experienced early menopause.5 Animal experiments and randomized controlled trials in humans have demonstrated that endurance exercise interventions can increase telomerase activity and LTL.31–33 Nonrandomized clinical trial data suggest that other modifiable lifestyle factors such as diet, stress management, and social support may also favorably affect LTL.34 Importantly, MR analyses suggest a causal role of LTL in the development of CAD,14,35 which implies that reversing LTL shortening may lead to improved cardiovascular outcomes in women with premature menopause. Furthermore, in a substudy of WOSCOPS, a randomized controlled trial of statin therapy in the primary prevention setting, individuals in the lowest tertile of LTL had a twofold risk of developing CAD vs. those in the highest tertile over a five-year follow-up period.36 However, allocation to statin treatment completely abrogated LTL-attributable cardiovascular risk. This clinical benefit of statins among individuals with low LTL is supported by previous research, both in animals and in vitro, demonstrating a positive effect of statins on telomere biology.37,38 These data, along with the findings from the current study, indicate that individuals with premature menopause, especially those with short LTL, may derive particular benefit from primary-prevention statin therapy. In addition, telomere-directed therapeutics are emerging as potential tools for preventing and treating cardiovascular disease. For instance, the ongoing TACTIC trial aims to investigate the effects of a small-molecule telomerase activator on telomere length, immune function, and endothelial function in patients with acute coronary syndromes.39
Study limitations
Strengths of this study include a large sample size of postmenopausal women and the use of state-of-the-art methods to estimate LTL. However, this study has limitations. First, most study participants were White, implicating that findings from this study may not be generalizable to women from other races/ethnicities. As exploratory analyses suggested that natural premature menopause was associated with a more pronounced decrease in LTL in women who self-reported as Black vs. White, further research is warranted into mechanisms of genomic and reproductive aging in women from non-White racial and/or ethnic groups. Second, the UK Biobank and WHI cohorts differ with respect to age, gynecologic surgery and hormone therapy practices, LTL measurement methods, and DNA sequencing methods. Despite this heterogeneity, associations of earlier age at natural menopause with LTL, and of LTL with incident CAD, were highly concordant across cohorts. Third, to avoid introducing bias by categorically excluding women who underwent hysterectomy alone without oophorectomy,40 we retained such individuals in primary analyses and labeled them as having had surgical premature menopause when the hysterectomy occurred before age 40 years, which may have introduced misclassification in this group; however, null findings among women who underwent surgical premature menopause were consistent in sensitivity analyses excluding those who underwent hysterectomy with ovarian conservation. Furthermore, hysterectomy with ovarian preservation is associated with earlier age of menopause.41 Finally, age at menopause was ascertained by participant self-report in both cohorts, which may lead to misclassification. However, any such misclassification would be expected to bias results toward the null.9 Furthermore, the use of self-reported age at menopause in this study may reflect its utility in clinical practice.
CONCLUSIONS
Earlier age at menopause is independently associated with LTL, especially among women with natural menopause, with MR analyses supporting causal effects of shorter LTL on earlier age of natural menopause. Furthermore, LTL mediates a proportion of the excess cardiovascular risk associated with premature natural menopause. These findings extend our understanding of the link between premature menopause and heightened cardiovascular risk and highlight opportunities for cardiovascular prevention in this population.
Supplementary Material
Figure 4. Pooled cumulative incidence and multivariable-adjusted associations of menopause and leukocyte telomere length (LTL) categories with incident coronary artery disease (CAD) events.
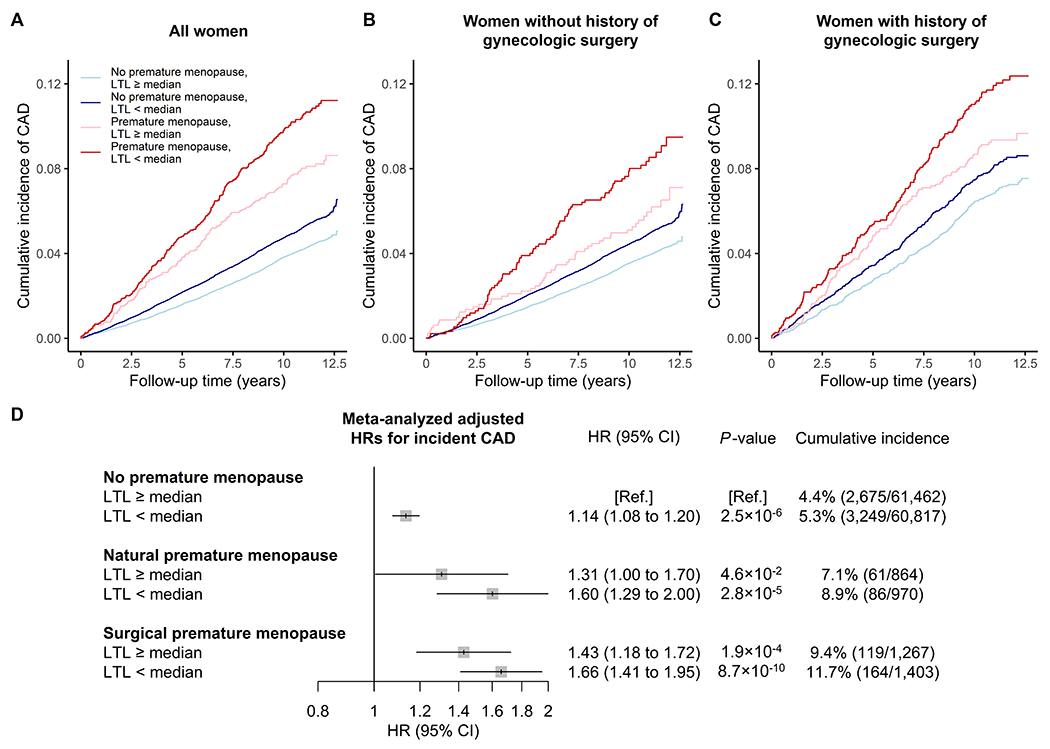
The upper panels show the pooled cumulative incidence of CAD events among (A) all postmenopausal women, (B) women without a history of gynecologic surgery, and (C) women with a history of gynecologic surgery during follow-up truncated at 12.7 years. The lower panel (D) shows the adjusted HRs for incident CAD events during follow-up meta-analyzed across cohorts. P-values correspond to Cox regression models adjusted for age, age², the first ten principal components of genetic ancestry, race/ethnicity, current/former smoking status, body mass index, diabetes status, current hormone therapy use, antihypertensive medication use, cholesterol-lowering medication use, systolic blood pressure, total cholesterol, and high-density lipoprotein cholesterol. Analyses in the WHI were further adjusted for inverse probability of sampling weights, while those in the UK Biobank were further adjusted for Townsend deprivation index. Menopause status and LTL were included as combined nonordered, categorical variables, with women without premature menopause and above-median LTL constituting the reference category. No corrections for multiple comparisons were made.
Novelty and Significance.
What is known?
Women who undergo menopause prematurely are at heightened risk of cardiovascular disease through partially unknown mechanisms.
Whether age at menopause is associated with telomere attrition, and whether this contributes to the heightened cardiovascular risk in those with premature menopause, is unclear.
What new information does this article contribute?
Earlier age at menopause, particularly natural menopause, was associated with shorter leukocyte telomere length.
Leukocyte telomere length was a mediator in the association of earlier age at natural menopause with incident coronary artery disease. Highest risks were observed in women with premature menopause and below-average telomere length.
These findings suggest that somatic genomic phenomena play a role in the association of premature natural menopause with cardiovascular disease.
In two large cohorts collectively including 130,254 postmenopausal women, earlier age at menopause was independently associated with shorter leukocyte telomere length (LTL), especially among women with natural menopause. Mendelian randomization analyses revealed a significant association of genetically predicted LTL with age at natural menopause, suggesting that accelerated telomere attrition contributes causally to natural premature menopause. Analyses of incident coronary artery disease indicated that LTL was a mediator of excess cardiovascular risk in women with premature natural menopause, and the highest event rates were observed in those who underwent menopause prematurely and had below-average LTL. These findings highlight the role of somatic genomic phenomena in the association of premature natural menopause with coronary artery disease and support intensive lifestyle interventions and primary prevention strategies in women who experience premature menopause.
SOURCES OF FUNDING
This research was conducted under UK Biobank application number 7089. The WHI is funded by the U.S. National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), and Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, and 75N92021D00005. Molecular data gathering for the TOPMed program was supported by the U.S. NHLBI. Genome sequencing for the WHI TOPMed cohort (phs001237.v2.p1) was performed at the Broad Institute Genomics Platform (contract HH-SN268201500014C). Core support including centralized genomic read mapping and genotype calling, along with variant quality metrics and filtering, were provided by the TOPMed Informatics Research Center (3R01HL-11762602S1; contract HHSN268201800002I). Core support including phenotype harmonization, data management, sample-identity quality control, and general program coordination was provided by the TOPMed Data Coordinating Center (grant R01HL-120393; grant U01HL-120393; contract HHSN268201800001I). The authors thank the studies and participants who provided biological samples and data.
SOURCES OF FUNDING
Mr. Schuermans is supported by the Belgian American Educational Foundation. Dr. Nakao is supported by the Japan Society for the Promotion of Science. Dr. Mathias is supported by the U.S. NHLBI (R01HL141944) and receives support as the Sarah Miller Coulson Scholar in the Johns Hopkins Center for Innovative Medicine. Dr. Reiner is supported by the U.S. NHLBI (R01HL146500, R01HL130733). Dr. Bick is supported by a Burroughs Wellcome Foundation Career Award for Medical Scientists and the NIH Director’s Early Independence Award (DP5-OD029586). Dr. Natarajan is supported by the U.S. NHLBI (R01HL148050). Dr. Honigberg is supported by the U.S. NHLBI (K08HL166687) and American Heart Association (940166, 979465).
DISCLOSURES
Dr. Bick is a founding scientific advisor to and shareholder in TenSixteen Bio. Dr. Manson reports grant support from Mars Edge. Dr. Natarajan reports grants from Amgen, Apple, Boston Scientific, AstraZeneca, Allelica, Novartis, and Genentech; consulting income from GV, Blackstone Life Sciences, Foresite Labs, Apple, AstraZeneca, Allelica, Novartis, HeartFlow, and Genentech; is a scientific advisor to Esperion Therapeutics, Preciseli, and TenSixteen Bio; is a scientific co-founder of TenSixteen Bio; and reports spousal employment at Vertex; all unrelated to the present work. Dr. Honigberg reports consulting fees from CRISPR Therapeutics, advisory board service for Miga Health, and grant support from Genentech, all unrelated to this work.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- CAD
coronary artery disease
- CHIP
clonal hematopoiesis of indeterminate potential
- CI
confidence interval
- HR
hazard ratio
- IQR
interquartile range
- LD
linkage disequilibrium
- LTL
leukocyte telomere length
- MR
Mendelian randomization
- SD
standard deviation
- WHI
Women’s Health Initiative
REFERENCES
- 1.O’Kelly AC, Michos ED, Shufelt CL, Vermunt J v., Minissian MB, Quesada O, Smith GN, Rich-Edwards JW, Garovic VD, el Khoudary SR, et al. Pregnancy and Reproductive Risk Factors for Cardiovascular Disease in Women. Circ Res. 2022;130:652–672. doi: 10.1161/CIRCRESAHA.121.319895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.el Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation. 2020;142:E506–E532. doi: 10.1161/CIR.0000000000000912 [DOI] [PubMed] [Google Scholar]
- 3.Zhu D, Chung HF, Pandeya N, Dobson AJ, Cade JE, Greenwood DC, Crawford SL, Avis NE, Gold EB, Mitchell ES, et al. Relationships between intensity, duration, cumulative dose, and timing of smoking with age at menopause: A pooled analysis of individual data from 17 observational studies. PLoS Med. 2018;15:e1002704. doi: 10.1371/JOURNAL.PMED.1002704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shadyab AH, MacEra CA, Shaffer RA, Jain S, Gallo LC, Gass MLS, Waring ME, Stefanick ML, LaCroix AZ. Ages at menarche and menopause and reproductive lifespan as predictors of exceptional longevity in women: The Women’s Health Initiative. Menopause. 2017;24:35–44. doi: 10.1097/GME.0000000000000710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, Januzzi JL, Scott NS, Natarajan P. Association of Premature Natural and Surgical Menopause With Incident Cardiovascular Disease. JAMA. 2019;322:2411–2421. doi: 10.1001/JAMA.2019.19191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu D, Chung HF, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. 2019;4:e553–e564. doi: 10.1016/S2468-2667(19)30155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/J.JACC.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 8.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honigberg MC, Zekavat SM, Niroula A, Griffin GK, Bick AG, Pirruccello JP, Nakao T, Whitsel EA, Farland L v., Laurie C, et al. Premature Menopause, Clonal Hematopoiesis, and Coronary Artery Disease in Postmenopausal Women. Circulation. 2021;143:410–423. doi: 10.1161/CIRCULATIONAHA.120.051775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruth KS, Day FR, Hussain J, Martínez-Marchal A, Aiken CE, Azad A, Thompson DJ, Knoblochova L, Abe H, Tarry-Adkins JL, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. 2021;596:393–397. doi: 10.1038/s41586-021-03779-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. New England Journal of Medicine. 2017;377:111–121. doi: 10.1056/NEJMOA1701719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Meyer T, Nawrot T, Bekaert S, de Buyzere ML, Rietzschel ER, Andrés V. Telomere Length as Cardiovascular Aging Biomarker: JACC Review Topic of the Week. J Am Coll Cardiol. 2018;72:805–813. doi: 10.1016/J.JACC.2018.06.014 [DOI] [PubMed] [Google Scholar]
- 13.Childs BG, Li H, van Deursen JM. Senescent cells: a therapeutic target for cardiovascular disease. J Clin Invest. 2018;128:1217–1228. doi: 10.1172/JCI95146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Stoma S, Lotta LA, Warner S, Albrecht E, Allione A, Arp PP, Broer L, Buxton JL, Da Silva Couto Alves A, et al. Genome-wide Association Analysis in Humans Links Nucleotide Metabolism to Leukocyte Telomere Length. Am J Hum Genet. 2020;106:389–404. doi: 10.1016/J.AJHG.2020.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakao T, Bick AG, Taub MA, Zekavat SM, Uddin MM, Niroula A, Carty CL, Lane J, Honigberg MC, Weinstock JS, et al. Mendelian randomization supports bidirectional causality between telomere length and clonal hematopoiesis of indeterminate potential. Sci Adv. 2022;8:eabl6579. doi: 10.1126/sciadv.abl6579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna CW, Bretherick KL, Gair JL, Fluker MR, Stephenson MD, Robinson WP. Telomere length and reproductive aging. Human Reproduction. 2009;24:1206–1211. doi: 10.1093/HUMREP/DEP007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenassa ED, Rossen LM. Telomere length and age-at-menopause in the US. Maturitas. 2015;82:215–221. doi: 10.1016/J.MATURITAS.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray KE, Schiff MA, Fitzpatrick AL, Kimura M, Aviv A, Starr JR. Leukocyte telomere length and age at menopause. Epidemiology. 2014;25:139. doi: 10.1097/EDE.0000000000000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, Stolk L, Finucane HK, Sulem P, Bulik-Sullivan B, et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet. 2015;47:1294–1303. doi: 10.1038/ng.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, Stolk L, Finucane HK, Sulem P, Bulik-Sullivan B, et al. GWAS of epigenetic aging rates in blood reveals a critical role for TERT. Nat Commun. 2018;9:1–13. doi: 10.1038/s41467-017-02697-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bick AG, Weinstock JS, Nandakumar SK, Fulco CP, Bao EL, Zekavat SM, Szeto MD, Liao X, Leventhal MJ, Nasser J, et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature. 2020;586:763–768. doi: 10.1038/s41586-020-2819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBoy EA, Tassia MG, Schratz KE, Yan SM, Cosner ZL, McNally EJ, Gable DL, Xiang Z, Lombard DB, Antonarakis ES, et al. Familial Clonal Hematopoiesis in a Long Telomere Syndrome. New England Journal of Medicine. 2023;388:2422–2455. doi: 10.1056/NEJMOA2300503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Codd V, Wang Q, Allara E, Musicha C, Kaptoge S, Stoma S, Jiang T, Hamby SE, Braund PS, Bountziouka V, et al. Polygenic basis and biomedical consequences of telomere length variation. Nat Genet. 2021;53:1425–1433. doi: 10.1038/s41588-021-00944-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Blasco MA, Trimarchi JR, Keefe DL. An Essential Role for Functional Telomeres in Mouse Germ Cells during Fertilization and Early Development. Dev Biol. 2002;249:74–84. doi: 10.1006/DBIO.2002.0735 [DOI] [PubMed] [Google Scholar]
- 25.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke. Menopause. 2012;19:1081–1087. doi: 10.1097/GME.0B013E3182517BD0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kok HS, van Asselt KM, van der Schouw YT, van der Tweel I, Peeters PHM, Wilson PWF, Pearson PL, Grobbee DE. Heart Disease Risk Determines Menopausal Age Rather Than the Reverse. J Am Coll Cardiol. 2006;47:1976–1983. doi: 10.1016/J.JACC.2005.12.066 [DOI] [PubMed] [Google Scholar]
- 27.Appiah D, Winters SJ, Allison MA, Baumgartner RN, Groves FD, Myers JA, Hornung CA. Cardiovascular disease among women with and without diabetes mellitus and bilateral oophorectomy. Diabetes Res Clin Pract. 2015;108:473–481. doi: 10.1016/J.DIABRES.2015.02.017 [DOI] [PubMed] [Google Scholar]
- 28.Coughlin SS, Datta B, Guha A, Wang X, Weintraub NL. Cardiovascular conditions and obesity among gynecologic cancer survivors: Results from the 2020 behavioral risk factor surveillance system survey. Gynecol Oncol. 2022;165:405–409. doi: 10.1016/J.YGYNO.2022.03.025 [DOI] [PubMed] [Google Scholar]
- 29.Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol. 2012;126:176–179. doi: 10.1016/J.YGYNO.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 30.Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR, dos-Santos-Silva I, Smeeth L, Bhaskaran K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. The Lancet. 2019;394:1041–1054. doi: 10.1016/S0140-6736(19)31674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werner CM, Hecksteden A, Morsch A, Zundler J, Wegmann M, Kratzsch J, Thiery J, Hohl M, Bittenbring JT, Neumann F, et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur Heart J. 2019;40:34–46. doi: 10.1093/EURHEARTJ/EHY585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werner C, Fürster T, Widmann T, Pöss J, Roggia C, Hanhoun M, Scharhag J, Büchner N, Meyer T, Kindermann W, et al. Physical Exercise Prevents Cellular Senescence in Circulating Leukocytes and in the Vessel Wall. Circulation. 2009;120:2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005 [DOI] [PubMed] [Google Scholar]
- 33.Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Pöss J, Bauersachs J, Thum T, Pfreundschuh M, Müller P, et al. Effects of Physical Exercise on Myocardial Telomere-Regulating Proteins, Survival Pathways, and Apoptosis. J Am Coll Cardiol. 2008;52:470–482. doi: 10.1016/J.JACC.2008.04.034 [DOI] [PubMed] [Google Scholar]
- 34.Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, Marlin R, Frenda SJ, Magbanua MJM, Daubenmier J, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112–1120. doi: 10.1016/S1470-2045(13)70366-8 [DOI] [PubMed] [Google Scholar]
- 35.Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN, Bowden J, Wade KH, Timpson NJ, Evans DM, Willeit P, et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017;3:636–651. doi: 10.1001/JAMAONCOL.2016.5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. The Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3 [DOI] [PubMed] [Google Scholar]
- 37.Bennaceur K, Atwill M, al Zhrany N, Hoffmann J, Keavney B, Breault D, Richardson G, von Zglinicki T, Saretzki G, Spyridopoulos I. Atorvastatin induces T cell proliferation by a telomerase reverse transcriptase (TERT) mediated mechanism. Atherosclerosis. 2014;236:312–320. doi: 10.1016/J.ATHEROSCLEROSIS.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 38.Spyridopoulos I, Haendeler J, Urbich C, Brummendorf TH, Oh H, Schneider MD, Zeiher AM, Dimmeler S. Statins Enhance Migratory Capacity by Upregulation of the Telomere Repeat-Binding Factor TRF2 in Endothelial Progenitor Cells. Circulation. 2004;110:3136–3142. doi: 10.1161/01.CIR.0000142866.50300.EB [DOI] [PubMed] [Google Scholar]
- 39.Maier R, Bawamia B, Bennaceur K, Dunn S, Marsay L, Amoah R, Kasim A, Filby A, Austin D, Hancock H, et al. Telomerase Activation to Reverse Immunosenescence in Elderly Patients With Acute Coronary Syndrome: Protocol for a Randomized Pilot Trial. JMIR Res Protoc. 2020;9:e19456. doi: 10.2196/19456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howard B V, Kuller L, Langer R, Manson JAE, Allen C, Assaf A, Cochrane BB, Larson JC, Lasser N, Rainford M, et al. Risk of Cardiovascular Disease by Hysterectomy Status, With and Without Oophorectomy. Circulation. 2005;111:1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD [DOI] [PubMed] [Google Scholar]
- 41.Moorman PG, Myers ER, Schildkraut JM, Iversen ES, Wang F, Warren N. Effect of hysterectomy with ovarian preservation on ovarian function. Obstetrics and Gynecology. 2011;118:1271–1279. doi: 10.1097/AOG.0B013E318236FD12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the women’s health initiative study design. Ann Epidemiol. 2003;13:S5–S17. doi: 10.1016/S1047-2797(03)00043-7 [DOI] [PubMed] [Google Scholar]
- 44.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The women’s health initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S78–S86. doi: 10.1016/S1047-2797(03)00045-0 [DOI] [PubMed] [Google Scholar]
- 45.Taliun D, Harris DN, Kessler MD, Carlson J, Szpiech ZA, Torres R, Taliun SAG, Corvelo A, Gogarten SM, Kang HM, et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature. 2021;590:290–299. doi: 10.1038/s41586-021-03205-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav K, Lewis RJ. Immortal Time Bias in Observational Studies. JAMA. 2021;325:686–687. doi: 10.1001/JAMA.2020.9151 [DOI] [PubMed] [Google Scholar]
- 47.Mishra SR, Chung HF, Waller M, Dobson AJ, Greenwood DC, Cade JE, Giles GG, Bruinsma F, Simonsen MK, Hardy R, et al. Association Between Reproductive Life Span and Incident Nonfatal Cardiovascular Disease: A Pooled Analysis of Individual Patient Data From 12 Studies. JAMA Cardiol. 2020;5:1410–1418. doi: 10.1001/JAMACARDIO.2020.4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Backman JD, Li AH, Marcketta A, Sun D, Mbatchou J, Kessler MD, Benner C, Liu D, Locke AE, Balasubramanian S, et al. Exome sequencing and analysis of 454,787 UK Biobank participants. Nature. 2021;599:628–634. doi: 10.1038/s41586-021-04103-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlasschaert C, Mack T, Heimlich JB, Niroula A, Uddin MM, Weinstock JS, Sharber B, Silver AJ, Xu Y, Savona MR, et al. A practical approach to curate clonal hematopoiesis of indeterminate potential in human genetic datasets. Blood. Published online January 18, 2023. doi: 10.1182/BLOOD.2022018825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703–1719. doi: 10.1038/S41375-022-01613-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES, Getz G. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benjamin D, Sato T, Cibulskis K, Getz G, Stewart C, Lichtenstein L. Calling Somatic SNVs and Indels with Mutect2. bioRxiv. Published online December 2, 2019:861054. doi: 10.1101/861054 [DOI] [Google Scholar]
- 53.Welsh S, Peakman T, Sheard S, Almond R. Comparison of DNA quantification methodology used in the DNA extraction protocol for the UK Biobank cohort. BMC Genomics. 2017;18:1–7. doi: 10.1186/S12864-016-3391-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21–e21. doi: 10.1093/NAR/GKN1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Codd V, Denniff M, Swinfield C, Warner SC, Papakonstantinou M, Sheth S, Nanus DE, Budgeon CA, Musicha C, Bountziouka V, et al. Measurement and initial characterization of leukocyte telomere length in 474,074 participants in UK Biobank. Nat Aging. 2022;2:170–179. doi: 10.1038/s43587-021-00166-9 [DOI] [PubMed] [Google Scholar]
- 56.Ding Z, Mangino M, Aviv A, Spector T, Durbin R. Estimating telomere length from whole genome sequence data. Nucleic Acids Res. 2014;42:e75–e75. doi: 10.1093/NAR/GKU181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taub MA, Conomos MP, Keener R, Iyer KR, Weinstock JS, Yanek LR, Lane J, Miller-Fleming TW, Brody JA, Raffield LM, et al. Genetic determinants of telomere length from 109,122 ancestrally diverse whole-genome sequences in TOPMed. Cell Genomics. 2022;2:100084. doi: 10.1016/J.XGEN.2021.100084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Honigberg MC, Zekavat SM, Pirruccello JP, Natarajan P, Vaduganathan M. Cardiovascular and Kidney Outcomes Across the Glycemic Spectrum: Insights From the UK Biobank. J Am Coll Cardiol. 2021;78:453–464. doi: 10.1016/J.JACC.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, et al. Estrogen plus Progestin and the Risk of Coronary Heart Disease. New England Journal of Medicine. 2003;349:523–534. doi: 10.1056/NEJMOA030808 [DOI] [PubMed] [Google Scholar]
- 60.Breslow NE, Amorim G, Pettinger MB, Rossouw J. Using the Whole Cohort in the Analysis of Case-Control Data: Application to the Women’s Health Initiative. Stat Biosci. 2013;5:232–249. doi: 10.1007/S12561-013-9080-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R Package for Causal Mediation Analysis. J Stat Softw. 2014;59:1–38. doi: 10.18637/JSS.V059.10526917999 [DOI] [Google Scholar]
- 62.Lee H, Herbert RD, McAuley JH. Mediation Analysis. JAMA. 2019;321:697–698. doi: 10.1001/JAMA.2018.21973 [DOI] [PubMed] [Google Scholar]
- 63.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prevention Science. 2000;1:173–181. doi: 10.1023/A:1026595011371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, Duncan L, Perry JRB, Patterson N, Robinson EB, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Stat. 2018;48:1742–1769. doi: 10.48550/arxiv.1801.09652 [DOI] [Google Scholar]
- 66.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7. doi: 10.7554/ELIFE.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The UK Biobank data that support the findings of the present study can be accessed by application (https://www.ukbiobank.ac.uk/register-apply/), and the WHI data can be accessed by registered researchers through the WHI online resource (https://www.whi.org/datasets) or by other researchers through BioLINCC (https://biolincc.nhlbi.nih.gov/studies/whi_ctos/).


