Abstract
A commercially available slide agglutination test (SAT) for the diagnosis of human leptospirosis was evaluated by comparing it to an immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA) and to the microscopic agglutination test (MAT). For all 108 patients, leptospirosis was diagnosed on the basis of a fourfold or greater increase in titer by MAT (seroconversion), and all but 1 of 245 controls were MAT negative (titers, <1:100). Both SAT and the IgM ELISA failed to detect one case of infection (sensitivity, 99%). Only 3 of 145 blood donors and none of the 100 patients with other illnesses were SAT positive (specificity, 99%). The overall results were similar for the three tests; however, SAT and ELISA were statistically more sensitive as initial screening tests. For 22% of the patients, the diagnosis of leptospirosis was made earlier by SAT than by MAT. SAT detected 27 (44%) of 62 MAT-negative patients with the first serum sample. ELISA and SAT had very similar results. Follow-up of patients for 1 year after the onset of symptoms showed a decreasing rate of positivity by SAT from the third month on. The rate of positivity by ELISA decreased more slowly, to about 67% by the end of the study. By MAT all patients were persistently reactive. SAT and ELISA seem to be convenient methods for the rapid and early screening for leptospirosis and could replace the less sensitive MAT. ELISA gives less subjective results than SAT and provides information on IgM kinetics, but it can be performed only by the more sophisticated laboratories. SAT is inexpensive, can be performed more quickly and more easily than ELISA, and could be used by the less well equipped laboratories.
Leptospirosis is a worldwide zoonosis caused by pathogenic Leptospira species, for which humans are accidental hosts. Humans can become infected through contact with an environment contaminated by the urine of a shedder host, such as rodents.
In Brazil, leptospirosis is endemic in most of the large urban areas, where epidemic outbreaks occur after flooding caused by heavy seasonal rainfall in the summer. Since 1982, it was designated a notifiable illness in the state of São Paulo.
The severity of this acute febrile illness varies considerably from mild to rapidly fatal, and the wide spectrum of symptoms makes clinical diagnosis very unreliable. Laboratory confirmation is therefore of the utmost importance, and up to the present, serology has been the cornerstone of differential laboratory diagnosis (6).
The “gold standard” serodiagnostic method, the microscopic agglutination test (MAT), is specific, but its inherent complexity limits its use to experienced personnel in central laboratory facilities (8). To overcome this problem, some potentially useful screening tests for use in all routine laboratories have been proposed (2, 8, 10–14).
This study compares MAT and an immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA) with an easy-to-perform, inexpensive, and rapid slide macroagglutination test for the screening of large numbers of human sera, available as a commercial kit called Simples Teste (BioManguinhos, FIOCRUZ, Rio de Janeiro, Rio de Janeiro, Brazil) (1) and further referred to as the slide agglutination test (SAT).
MATERIALS AND METHODS
Patients’ sera.
The study was conducted at the Instituto Adolfo Lutz, São Paulo, where MAT is currently used for the serological diagnosis of human leptospirosis.
A total of 108 patients for whom at least two blood samples had been submitted to the Leptospira Laboratory for routine diagnosis were analyzed. The first serum sample was negative by MAT for each of 62 patients and positive by MAT (titer, ≥1:100) for 46 patients. In order to determine the long-term results of SAT, patients, assisted by one of the authors (M. V. Silva), were asked if they accepted to be followed up monthly over a period of up to 1 year after the onset of symptoms.
MAT was repeated for all sera from this group, and leptospirosis was diagnosed in the laboratory on the basis of a fourfold or greater rise in MAT titers. All the cases were reported to the “Centro de Vigilância Epidemiológica” (Epidemiological Surveillance Center) of the State of São Paulo as being clinically compatible with leptospirosis.
Control group sera.
Assay cross-reactivity and SAT specificity were assessed by testing sera from 145 apparently healthy individuals and also from a total of 100 patients with the following illnesses: Chagas’ disease (n = 8), dengue (n = 8), hepatitis B and C (n = 15), mucocutaneous and visceral leishmaniasis (n = 16), Plasmodium falciparum and Plasmodium vivax malaria (n = 18 plasma samples), rheumatic fever (n = 8), syphilis (n = 17), and typhoid fever (n = 10). The diagnosis of these diseases was made on the basis of serological tests, and hepatitis, malaria, and typhoid fever were confirmed by, in addition to serology, testing for hepatitis B virus surface antigen, visualization of P. vivas or P. falciparum by thick smears, and isolation of Salmonella enterica subsp. enterica serovar Typhi strains, respectively. Only one sample from each individual was evaluated, and those presenting with MAT titers of ≥1:100 were excluded from this study unless we had access to the patient’s clinical and epidemiological history.
MAT.
The micro-MAT technique (4) was performed with the following 22 serovars of Leptospira as antigen: andamana, australis, autumnalis, bataviae, butembo, canicola, castellonis, celledoni, copenhageni, cynopteri, djasiman, grippotyphosa, hebdomadis, icterohaemorrhagiae, javanica, panama, patoc, pomona, pyrogenes, shermani, tarassovi, and wolffi. Each of the 6- to 9-day-old cultures in bovine albumin polysorbate medium (EMJH medium; Difco Laboratories) were adjusted with phosphate-buffered saline (PBS; 5 mM, pH 7.2) to an optical density equivalent to that of a McFarland no. 0.5 turbidity unit and were used as antigens. Sera were screened at a 1:100 dilution by adding 50 μl of each antigen to 50 μl of serum diluted to 1:50 in PBS and pipetted into separate microtiter plate wells. The plates were covered and were incubated for 2 h at 28 to 30°C. Each well was then directly examined for agglutination with a dark-field microscope equipped with a long-working-distance objective. Those sera showing at least 50% agglutination with one or more serovars were further titrated in serial twofold dilutions. Titers from 1:100 upward were recorded.
Macroscopic SAT.
The antigen provided with the commercial kit (Simples Teste; BioManguinhos, FIOCRUZ) (1) was prepared by the technique of Galton et al. as reported by Faine (6), with modifications. The antigen suspension was added in volumes of 55 μl to 10 μl of undiluted serum placed on a glass slide divided into squares (2.5 by 2.5 cm). The serum and antigen drops were thoroughly mixed with an applicator stick, and then the plate was left in an orbital shaker at 120 rpm for 4 to 6 min at room temperature. The reaction was immediately read under direct light (shaded from the eyes) against a dark background and was recorded as positive or negative. The positive agglutination reaction appeared as fine clumps to coarse particles beginning at the edges of the drop. Both positive and negative control sera provided with the kit were incorporated into each assay. When negative or lipemic, the sera were reexamined after dilution to 1:2 in PBS. Lipemic sera were also filtered through 0.45-μm-pore-size membrane filters.
IgM ELISA.
Specific IgM antibodies were detected by an indirect technique incorporating a sonicated mixture of Leptospira serovars brasiliensis, canicola, cynopteri, hebdomadis, and icterohaemorrhagiae as the antigen, as described by Silva et al. (9). However, peroxidase-conjugated immunoglobulin (Biolab Diagnóstica, São Paulo, Brazil), o-phenylenediamine (Sigma Chemical Co., St. Louis, Mo.), and hydrogen peroxide were used as the indicator system. This leptospiral antigen was prepared by the method described by Adler et al. (2) and modified by Silva et al. (9, 10) at the Instituto Adolfo Lutz, where it is being introduced as a method for the laboratory diagnosis of leptospirosis.
All sera from patients with leptospirosis and those from the control group that were positive by SAT were subjected to this assay and were used at a dilution of 1:400. Reactions were recorded as positive when their absorbance values, measured at 495 nm with a microplate reader (Titertek Multiskan MCC; Flow Laboratories), were above the determined cutoff level of 0.470. Each assay included positive control sera (from two patients with leptospirosis caused by serovar icterohaemorrhagiae, as confirmed clinically and by MAT) and negative control sera (from two apparently healthy individuals who had no clinical or epidemiological antecedents of leptospirosis and who were negative by MAT).
Statistical analysis.
The specificity and sensitivity of the tests were determined with the Epi Info Statcalc and Epitable programs (5). Data analysis was also carried out by McNemar’s test (7), with significance levels set at 0.05 and 0.01.
RESULTS
SAT, IgM ELISA, and MAT were performed with a total of 359 blood samples obtained from 108 patients with confirmed leptospirosis (95 males and 13 females); their ages ranged from 6 to 67 years (mean age, 32 years).
According to the MAT titers, serogroup Icterohaemorrhagiae (serovar icterohaemorrhagiae and/or copenhageni) accounted for 64 cases, serogroup Autumnalis (serovar autumnalis) accounted for 8 cases, serogroup Grippotyphosa accounted for 6 cases, serogroup Canicola accounted for 4 cases, serogroup Sejroe accounted for 3 cases, serogroups Australis and Bataviae accounted for 2 cases each, and serogroups Castellonis, Cynopteri, and Pyrogenes accounted for 1 case each. The probable infecting serogroup could not be assessed for 16 patients due to the presence of coagglutinins in their sera. Among the 46 patients whose first serum sample was positive by MAT, sera from 21 had borderline titers of 1:100 (n = 10) and 1:200 (n = 11) and sera from 25 patients had titers ranging from 1:400 to 1:12,800. Among the 62 patients whose first sample was negative, the second serum sample was positive for 57 patients and the third serum sample was positive for 5 patients. Among the individuals in the control group, only one MAT-positive individual was not excluded from this study, since although a fourfold increase in titer for serogroup Autumnalis was observed, S. enterica subsp. enterica serovar Typhi was isolated by hemoculture. This SAT- and IgM ELISA-negative patient from the control group was assisted by one of the authors (M. V. Silva), who confirmed the diagnosis of typhoid fever.
Among the 108 patients with leptospirosis, 107 were reactive by SAT. The SAT-negative patient was positive by the IgM ELISA and seroconverted to positivity by MAT, with the maximum titer being to serovar icterohaemorrhagiae (1:400). Among the control group, 242 of the 245 individuals were SAT negative. The three apparently healthy, SAT-positive individuals tested negative both by MAT and by IgM ELISA. Thus, overall, SAT showed 99% (107 of 108 individuals) sensitivity, 99% (242 of 245) specificity, a positive predictive value of 97% (107 of 110), and a negative predictive value of 99% (242 of 243).
The IgM ELISA also had a 99% sensitivity. The ELISA-negative patient was SAT positive and seroconverted to positivity for serovars castellonis and cynopteri at the same titer (1:200). Both SAT and the IgM ELISA failed to detect leptospirosis in one patient each, but by using the tests together, leptospirosis was diagnosed in all 108 patients. The results of both tests were highly concordant with those of MAT for the overall diagnosis of leptospirosis in the 108 patients confirmed to have leptospirosis. However, SAT and IgM ELISA were more sensitive than MAT at the beginning of the acute phase.
Table 1 presents the positive results of the three tests with serum samples collected at different times after the onset of symptoms of the disease. SAT showed 57% positivity with sera obtained in the first 6 days of illness, and this reached 99% by the 3rd week and then decreased markedly from the 3rd month on to about 20% with sera obtained during the 6th to 12th months. The IgM ELISA had a sensitivity of 53% in the first week, and the sensitivity reached a maximum with sera collected from the 3rd week up to the end of the 2nd month (mean, 99%), and from then on the sensitivity slowly decreased to about 70% with sera obtained from the 6th to the 12th months. MAT was less sensitive with early blood samples (34% in the first 6 days), reaching 100% with sera collected by the end of the 2nd month up to the end of the study. By applying the McNemar χ2 test, SAT (χ2 = 10.22) and ELISA (χ2 = 6.86) were shown to be more sensitive than MAT (P < 0.01) with sera obtained during the first 6 days of illness. SAT becomes less sensitive than MAT from the fourth month on and less sensitive than the IgM ELISA from the sixth month on. Furthermore, MAT was more sensitive than the IgM ELISA from the sixth month on (P < 0.05).
TABLE 1.
Rates of positivity of sera from 108 patients with leptospirosis by SAT, IgM ELISA, and MAT according to time of illnessa
| Time interval | No. of serum samples | No. (%) of sera positive by the following:
|
||
|---|---|---|---|---|
| SAT | IgM ELISA | MAT | ||
| 0–6 days | 68 | 39 (57) | 36 (53) | 23 (34) |
| 7–14 days | 86 | 71 (83) | 71 (83) | 64 (74) |
| 15 days–2 mo | 131 | 129 (99) | 130 (99) | 130 (99) |
| 3 mo | 15 | 10 (67) | 14 (93) | 15 (100) |
| 4–5 mo | 12 | 6 (50) | 11 (92) | 12 (100) |
| 6–8 mo | 23 | 4 (17) | 17 (74) | 23 (100) |
| 9–12 mo | 24 | 6 (25) | 17 (71) | 24 (100) |
Significant differences by the McNemar test were as follows: for 0 to 6 days, SAT × MAT, χ2 = 10.22 (P < 0.01); ELISA × MAT, χ2 = 6.86 (P < 0.01); for 4 to 5 months, SAT × MAT, χ2 = 4.17 (P < 0.05); for 6 to 8 months, SAT × MAT, χ2 = 17.05 (P < 0.01); SAT × ELISA, χ2 = 11.08 (P < 0.01); ELISA × MAT, χ2 = 4.17 (P < 0.05); and for 9 to 12 months, SAT × MAT, χ2 = 16.06 (P < 0.01); SAT × ELISA, χ2 = 9.09 (P < 0.01); ELISA × MAT, χ2 = 5.14 (P < 0.05).
The ability of the tests to detect antibodies in each of the first three serum samples submitted for laboratory analysis is presented in Table 2. Both SAT and the IgM ELISA were more sensitive with the first serum sample than the MAT. On initial testing, with sera collected on the sixth (mean) day after the onset of symptoms, 70 (65%) and 68 (63%) patients were positive by SAT and the IgM ELISA, respectively. Comparing the results to those of MAT (by which 46 patients were positive and 62 patients were negative), SAT and the IgM ELISA enabled the earlier detection of 22% (24 of 108) and 20% (22 of 108) of the confirmed cases, respectively. The correlation between SAT and ELISA was 93% (100 of 108) with the first serum sample, 97% (105 of 108) with the second serum sample, and 96% (44 of 46) with the third serum sample. A similar correlation was observed between SAT and MAT with the second (95%) and third (98%) blood samples. For the first sample (72% correlation), the differences were statistically significant (P < 0.01) according to the McNemar χ2 test (SAT compared with MAT, χ2 = 17.63; ELISA compared with MAT, χ2 = 14.70).
TABLE 2.
Comparison of SAT to IgM ELISA and MAT for the detection of antibodies in serial serum samples from 108 patients with leptospirosis
| SAT results | No. of serum samples with the indicated result by the following test:
|
|||
|---|---|---|---|---|
| IgM ELISA
|
MAT
|
|||
| Positive | Negative | Positivea | Negative | |
| First serum sampleb | ||||
| Positive | 65 | 5 | 43 | 27 |
| Negative | 3 | 35 | 3 | 35 |
| Second serum samplec | ||||
| Positive | 100 | 2 | 100 | 2 |
| Negative | 1 | 5 | 3 | 3 |
| Third serum sampled | ||||
| Positive | 44 | 1 | 45 | 0 |
| Negative | 1 | 0 | 1 | 0 |
Titer, ≥1:100.
Collected at between 1 and 35 days after the onset of infection (mean, 6 days).
Collected at between 3 and 77 days after the onset of infection (mean, 17 days).
Collected at between 4 and 110 days after the onset of infection (mean, 28 days).
Analysis of more than one blood sample from each patient increased the detection level by SAT or the IgM ELISA. Upon testing of a second serum sample, the rates of detection of positive patients increased from 65% (SAT) and 63% (ELISA) to 94% for both tests; i.e., 32 and 33 patients, respectively, became positive when a second sample of their sera was analyzed. Testing of a third serum sample confirmed the diagnosis for the remaining patients.
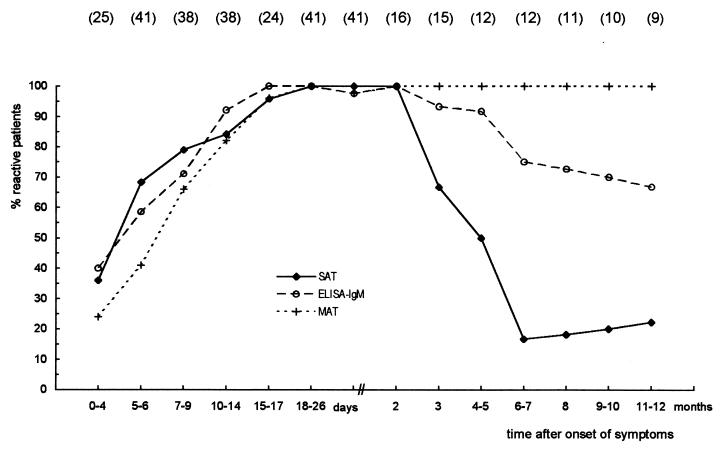
The results of the follow-up study are presented in Fig. 1. At the end of the first month after the onset of symptoms, 20 patients agreed to be followed up; this decreased to 16 patients in the 3rd month, 13 patients in the 4th month, 12 patients from the 5th to the 7th months, 11 patients in the 8th month, and 10 patients in the 9th and 10th months. Nine patients continued to be followed up to the end of the 12-month follow-up period.
FIG. 1.
Longitudinal comparison of SAT, IgM ELISA, and MAT for patients with leptospirosis who were followed up. Values in parentheses are number of patients examined.
SAT was positive for 9 of 25 patients (36%) from whom sera were collected as early as 0 to 4 days after the onset of the disease. The number of SAT-positive patients increased during the acute phase of illness, reaching 100% in the period from the 18th day to the 2nd month of follow-up. The number of positive patients decreased to 10 of 15 (67%) in the third month. During the fourth and fifth months, 6 of 12 patients were positive, and only 2 patients continued to be positive from the sixth month until the end of this study.
IgM ELISA results were very similar to those of SAT during the acute phase. All except one of the patients were reactive from the 15th day to the end of the 2nd month. The patient who was the exception became negative on the 27th day of follow-up. The rate of positivity by this test declined slowly thereafter, and six of nine patients (67%) were reactive at the end of the 1-year study.
Six of 25 patients (24%) had titers of ≥1:100 by MAT in the first 4 days of leptospirosis. From the 18th day up to the end of the 12-month follow-up, all except one of the patients from whom the first serum sample was collected in the first month presented with a fourfold rise in antibody titer by MAT.
DISCUSSION
Laboratory tests are necessary to confirm the diagnosis of clinically suspected leptospirosis due to its varied symptoms. Moreover, leptospirosis must always be considered during the differential diagnosis of other illnesses. Laboratory analysis relies mainly on serological methods, and the most widely used reference standard method, MAT, has many disadvantages (3, 8, 12–14), at least for screening purposes.
In São Paulo, a heavily populated metropolitan area, leptospirosis is endemic but outbreaks occur as a consequence of severe flooding. During the rainy months more than 30 blood samples are referred daily to the Instituto Adolfo Lutz for leptospiral serology. MAT is time-consuming and needs specialized personnel for its execution. Moreover, examination of such a large number of serum specimens by a complex technique like MAT may compromise the quality of the results. Under these conditions, a rapid test is needed for the screening of large numbers of serum specimens. In addition, an easy screening test could be performed at the less specialized routine clinical laboratories, thus enabling more rapidly available results.
MAT showed low sensitivity in the early days of illness, as has also been reported from other studies (3, 8, 13, 14), but once antibodies were detected in subsequent serum samples from all patients, none of these became negative (titer, <1:100) during the follow-up study. The nine patients studied 1 year after infection presented with titers of from 1:800 to 1:3,200 (median titer, 1:1,600). This relatively late, high, and persistent sensitivity presented by MAT, at least in areas of endemicity where background leptospiral antibodies may be present, requires that paired serum specimens be examined to confirm a recent infection. Leptospirosis can be rapidly fatal; therefore, it is important that a laboratory diagnosis be readily available to ensure that patients receive effective therapy.
SAT seems to be a convenient test for the initial diagnosis of leptospirosis. It detected 65% of the cases of illness with the admission sample and 94% with a second serum sample collected on about the 17th day of symptoms. SAT was able to detect 44% of 62 patients whose first serum sample was MAT negative. It showed high sensitivity (99%), failing to detect illness in only 1 of 108 patients, and good overall specificity (99%). None of the 17 patients with other spirochetal infections or 75 patients with diseases commonly confused with leptospirosis were reactive by SAT, nor was SAT affected by nonspecific anti-immunoglobulins (8 patients). Only 3 of 145 healthy blood donors were SAT positive. We did not have access to these individuals to try to explain their reactivities, but the possibility that they had mild forms of the disease cannot be discarded.
The follow-up study revealed that SAT is an excellent screening test for the acute phase of leptospirosis. Its sensitivity is high at the beginning of the infection and low in the convalescent phase. By the sixth month after the onset of symptoms, only two patients continued to be positive. These patients and another patient who was positive by ELISA presented with polyneuropathy, probably as sequelae of infection, and are under investigation.
SAT results were very similar to those of the IgM ELISA for the detection of infection early in the course of the illness. Both tests may thus be an alternative to the current MAT as an initial screen for leptospirosis.
MAT remains very useful for epidemiologic studies, identification of strains, assessment of the probable infecting serogroup, and confirmation of illness for public health surveillance. In the last case, MAT should be performed with paired serum specimens preferably run at the same time in a specialized reference laboratory.
The IgM ELISA gives less subjective interpretation of results than SAT and provides information on the kinetics of IgM, which was shown to be a good marker of the acute phase of leptospirosis (9, 12). On the other hand, SAT is less expensive and does not require special equipment, reagents, or training. It can therefore be performed by less well equipped routine diagnostic laboratories.
Thus, on the basis of the results presented above, we conclude that SAT should be considered an alternative to MAT or even a replacement for MAT for laboratory screening during the acute phase of leptospirosis, since it requires fewer laboratories facilities, is less prone to giving positive results with background leptospiral antibodies, and enables a faster diagnosis and earlier therapeutic intervention.
ACKNOWLEDGMENTS
We thank Ciro Rossetti of CVE for providing data on patients with leptospirosis, Fernando Ghilardi of the “Banco de Sangue São Paulo” for kindly providing blood donors’ sera, Silvia M. F. di Santi from SUCEN for providing plasma samples from patients with malaria, Candida C. Souza and Eliete C. Romero of IAL for supplying the antigens used in the MAT, and all laboratories of IAL that provided sera from patients with illnesses other than leptospirosis. We are grateful to Maria Alice S. Telles for kindly reviewing the manuscript, especially the linguistic aspects.
REFERENCES
- 1.Abrão R V, da Silva E D. Anais da IV Jornada Científica da Fundação Oswaldo Cruz. 70 anos do Hospital Evandro Chagas (1921–1991), Rio de Janeiro, Brazil. 1991. Leptoteste-S: diagnóstico da leptospirose por reação única de macroaglutinação em placa, abstr; pp. B62–63. [Google Scholar]
- 2.Adler B, Murphy A M, Locarnini S A, Faine S. Detection of specific anti-leptospiral immunoglobulins M and G in human serum by solid-phase enzyme-linked immunosorbent assay. J Clin Microbiol. 1980;11:452–457. doi: 10.1128/jcm.11.5.452-457.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appassakij H, Silpapojakul K, Wansit R, Woodtayakorn J. Evaluation of the immunofluorescent antibody test for the diagnosis of human leptospirosis. Am J Trop Med Hyg. 1995;52:340–343. doi: 10.4269/ajtmh.1995.52.340. [DOI] [PubMed] [Google Scholar]
- 4.Cole J R, Jr, Sulzer C R, Pursell A R. Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol. 1973;25:976–980. doi: 10.1128/am.25.6.976-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean A G, Dean J A, Coulombier D, Brendel K A, Smith D C, Burton A H, Dicker R C, Sullivan K, Fagan R F, Arner T G. Epi Info, version 6: a word processing, database, and statistics program for public health on IBM-compatible microcomputers. Atlanta, Ga: Centers for Disease Control and Prevention; 1995. [Google Scholar]
- 6.Faine S, editor. Guidelines for the control of leptospirosis. WHO offset publication no. 67. Geneva, Switzerland: World Health Organization; 1982. [PubMed] [Google Scholar]
- 7.Fleiss J L. Statistical methods for rates and proportions. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1981. [Google Scholar]
- 8.Pappas M G, Ballow R W, Gray M R, Takafuji E T, Miller R N, Hockmeyer W T. Rapid serodiagnosis of leptospirosis using the IgM specific dot-ELISA: comparison with the microscopic agglutination test. Am J Trop Med Hyg. 1985;34:346–354. doi: 10.4269/ajtmh.1985.34.346. [DOI] [PubMed] [Google Scholar]
- 9.Silva M V, Camargo E D, Batista L, Vaz A J, Brandão A P, Nakamura P M, Negrão J M. Behaviour of specific IgM, IgG and IgA class antibodies in human leptospirosis during the acute phase of the disease and during convalescence. J Trop Med Hyg. 1995;98:268–272. [PubMed] [Google Scholar]
- 10.Silva M V, Camargo E D, Vaz A J, Souza A M C, Chieffi P P, Sakata E E. Imunodiagnóstico da leptospirose humana através do teste ELISA-IgM, empregando-se diferentes preparações antigênicas a partir de sorotipos prevalentes de Leptospira interrogans. Rev Inst Med Trop São Paulo. 1990;32:233–239. doi: 10.1590/s0036-46651990000400001. [DOI] [PubMed] [Google Scholar]
- 11.Silva M V, Nakamura P M, Camargo E D, Batista L, Vaz A J, Brandão A P, Ferreira A W. Dot-ELISA-IgM in saliva for the diagnosis of human leptospirosis using polyester fabric-resin as support (preliminary report) Rev Inst Med Trop São Paulo. 1994;36:475–478. doi: 10.1590/s0036-46651994000500014. [DOI] [PubMed] [Google Scholar]
- 12.Silva M V, Nakamura P M, Camargo E D, Batista L, Vaz A J, Romero E C, Brandão A P. Immunodiagnosis of human leptospirosis by dot-ELISA for the detection of IgM, IgG, and IgA antibodies. Am J Trop Med Hyg. 1997;56:650–655. doi: 10.4269/ajtmh.1997.56.650. [DOI] [PubMed] [Google Scholar]
- 13.Watt G, Alquiza L M, Padre L P, Tuazon M L, Laughlin L W. The rapid diagnosis of leptospirosis: a prospective comparison of the dot-enzyme linked immunosorbent assay and the genus-specific microscopic agglutination test at different stages of illness. J Infect Dis. 1988;157:840–842. doi: 10.1093/infdis/157.4.840. [DOI] [PubMed] [Google Scholar]
- 14.Winslow W E, Merry D J, Pirc M L, Devine P L. Evaluation of a commercial enzyme-linked immunosorbent assay for detection of immunoglobulin M antibody in diagnosis of human leptospiral infection. J Clin Microbiol. 1997;35:1938–1942. doi: 10.1128/jcm.35.8.1938-1942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]