Abstract
With the advancement of diagnostic molecular technology and the molecular classification of endometrial endometrioid carcinoma (EEC), it remains to be seen whether conventional International Federation of Gynecology and Obstetrics (FIGO) grading retains clinical significance in certain molecular subtypes of EECs. In this study, we explored the clinical significance of FIGO grade in microsatellite instability-high (MSI-H) and POLE-mutant EECs. A total of 162 cases of MSI-H EECs and 50 cases of POLE-mutant EECs were included in the analysis. Significant differences in tumor mutation burden (TMB), progression-free survival, and disease-specific survival were seen between the MSI-H and POLE-mutant cohorts. Within the MSI-H cohort, there were statistically significant differences in TMB and stage at presentation across FIGO grades, but not survival. Within the POLE-mutant cohort, there was significantly greater TMB with increasing FIGO grade, but there were no significant differences in stage or survival. In both the MSI-H and POLE-mutant cohorts, log-rank survival analysis showed no statistically significant difference in progression-free survival and disease-specific survival across FIGO grades. Similar findings were also seen when a binary grading system was utilized. Since FIGO grade was not associated with survival, we conclude that the intrinsic biology of these tumors, characterized by their molecular profile, may override the significance of FIGO grading.
Subject Ontology: Endometrioid carcinoma, International Federation of Gynecology and Obstetrics grade, microsatellite instability-high, MSI-H, POLE
Since its inception in 1988, the International Federation of Gynecology and Obstetrics (FIGO) grading system for endometrial carcinoma has been repeatedly shown to correlate with disease progression and relative survival.1,2 Based on these findings, it is currently standard of practice to assign a FIGO grade to endometrial endometrioid carcinomas (EECs). In 2013, a landmark analysis of The Cancer Genome Atlas (TCGA) data identified four genomic categories of endometrial carcinomas: 1) ultramutated polymerase epsilon (POLE)-mutant, 2) microsatellite instability-high (MSI-H), 3) copy number-high (CN-H), and 4) copy number-low (CN-L) tumors.3 The ultramutated POLE-mutant tumors, driven by mutations in the exonuclease domain of the DNA POLE gene, have a 100-fold greater tumor mutation burden (TMB) than non-POLE-mutant and microsatellite stable tumors, while MSI-H tumors, driven by defects in the mismatch repair (MMR) mechanism have a 10-fold greater TMB. The CN-H cluster primarily consists of serous and serous-like tumors with significant somatic copy number alterations (SCNAs), and the CN-L cluster consists of tumors with a lower mutation frequency compared to ultramutated and hypermutated tumors and fewer copy number alterations compared to CN-H tumors. Because of its lack of specific molecular alterations, the CN-L cluster is also referred to as the “no specific molecular profile” or NSMP group.
TCGA genomic clusters have been shown to display distinct biological behavior; CN-H tumors are associated with the poorest outcomes while POLE-mutant tumors are associated with a substantially more favorable prognosis.3–6 This is in contrast to predicted outcomes based on FIGO grading, as POLE-mutant tumors are generally associated with higher tumor grade.7,8 Indeed, studies have shown that POLE-mutant tumors maintain their favorable prognosis regardless of FIGO grade.9,10 Furthermore, MSI-H tumors more frequently harbor FIGO grade 3 adenocarcinomas compared to the CN-L group, yet they both have similar intermediate clinical risk profiles.3,9 These findings suggest that the interplay between traditional tumor grading and molecular subtyping is considerably complex, and that in certain molecular clusters, tumor grade may have limited utility in predicting outcome (Figure 1). It is, therefore, important to establish the significance of FIGO grading within molecular clusters to provide accurate prognostication and avoid under- or overtreatment. In this study, we sought to evaluate whether FIGO grade retains clinical significance in in MSI-H and POLE-mutant EECs.
Figure 1. International Federation of Gynecology and Obstetrics (FIGO) grading may be limited in predicting patient outcome.
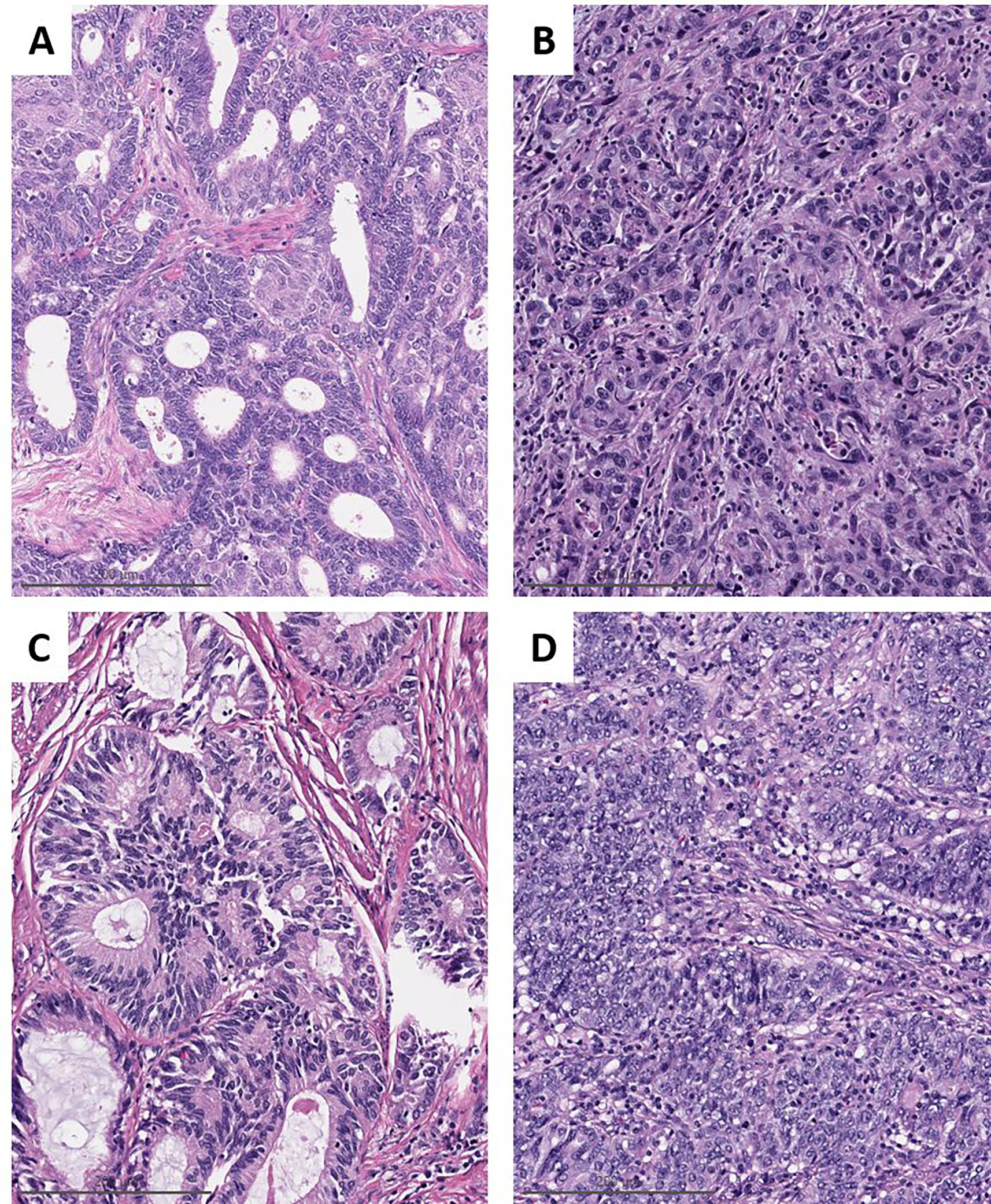
A) Microsatellite instability-high (MSI-H) endometrial endometrioid carcinoma (EEC), FIGO grade 1, high stage at diagnosis, and the patient died of disease. B) MSI-H EEC, FIGO grade 3, low stage at diagnosis with no evidence of disease at last follow-up. C and D) POLE-mutant EEC, high-stage FIGO grade 1 and low-stage FIGO grade 3, respectively. Neither patient had evidence of disease at last follow up.
Materials and Methods
This study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSK).
We reviewed all cases of EEC with targeted next-generation sequencing performed at our institution indicating MSI-H or POLE somatic exonuclease domain hotspot mutation.11 Only cases of EECs with resection material reviewed at our institution were included in the final analysis. Cases of other histologic types, including mixed endometrial carcinoma, serous carcinoma, clear cell carcinoma, and carcinosarcoma, were excluded. Histopathologic and morphologic data, including tumor histotype, FIGO grade, depth of invasion, and presence of lymphovascular invasion (LVSI) were extracted from pathology reports. To mitigate the effect of suboptimal interobserver concordance, the study was conducted within a single institution with a group of experienced gynecologic pathologists. Biweekly diagnostic consensus conferences encouraged a uniform diagnostic approach within the group, as did frequent review of cases for tumor board and quality assurance.
Tumor grade was assigned following the FIGO grading system for endometrioid carcinoma, which is based on the amount of non-squamous solid growth (grade 1: up to 5%, grade 2: greater than 5% and up to 50%, and grade 3: greater than 50%). In cases with heterogeneous architecture, the grade is based on the overall architecture. In addition to the use of the traditional three-tiered FIGO grading system, we explored the effect of a binary grading system in which cases were reassigned into a “low-grade” or “high-grade” category, the former composed of FIGO grades 1 and 2 EECs while FIGO grade 3 EECs make up the high-grade category.
Results of immunohistochemistry (IHC) for MMR proteins performed as part of initial case work-up were reviewed. IHC studies were performed either within our institution (MLH1, clone ES05, Leica Biosystems; MSH2, clone G219–1129, Cell Marque; MSH6, clone EP49, Agilent/Dako; and PMS2, clone A16.4, BD Bioscience) or at an outside laboratory.
Molecular analysis was performed through MSK-IMPACT (MSK–Integrated Mutation Profiling of Actionable Cancer Targets), a molecular profiling platform using next-generation sequencing to detect somatic alterations using a 468-gene panel.12 EECs with somatic mutations in the POLE exonuclease domain (residues 268–471) were identified. These include mutations that have been described as hotspots or pathogenic mutations and those that are recurrent and/or associated with an ultramutated phenotype.11,13,14 The detected protein changes are listed in Table 1. Tumor MSI status was obtained by MSIsensor, which interrogates the number and length of all available genomic microsatellites covered by MSK-IMPACT within tumor samples against matched normal DNA.15 An MSI score is then assigned with MSI-H defined as a score of 10 or greater. MLH1 promoter hypermethylation status was determined by the bisulfite mediated detection of methylated cytosines, as described previously.16
Table 1.
POLE protein change and characteristics of all cases with POLE exonuclease domain mutation.
| Protein change | Number of cases | TMB (mean ± stdev) | MSIsensor score | MMR expression | p53 expression |
|---|---|---|---|---|---|
| A456P | 5 (9%) | 98.24 ± 37.11 | MSS | Lost (1) Retained (4) |
Wild type (4) NA (1) |
| F367S | 1 (2%) | 542.4 | MSI-High | Lost | NA |
| F367V | 2 (4%) | 365.60 ± 137.18 | MSI-Indeterminate (1) MSS (1) |
Retained (2) | Wild type (1) NA (1) |
| M471V | 1 (2%) | 228.2 | MSS | Retained | Wild type |
| P286R | 18 (34%) | 132.77 ± 81.09 | MSS | Retained | Wild type (16) Mixed (1) NA (1) |
| P436R | 1 (2%) | 113.2 | MSS | Retained | Wild type |
| S297F | 2 (4%) | 45.65 ± 29.77 | MSS | Lost (1) Retained (1) |
Mixed (1) NA (1) |
| S459F | 2 (4%) | 432.7 ± 11.17 | Indeterminate (2) | Equivocal (1) NA (1) |
Aberrant (1) NA (1) |
| V411L | 17 (32%) | 223.6 ± 145.00 | MSI-Indeterminate (3) MSS (14) |
Lost (4)* Retained (13) |
Aberrant (2) Mixed (1) Wild type (9) NA (5) |
| V411M | 1 (2%) | 52.7 | MSI-High | Retained | NA |
| A509V** | 1 (2%) | 30.5 | MSI-High | Lost | Wild type |
| D368N** | 1 (2%) | 301.9 | MSI-High | Lost | Wild type |
| E1767K** | 1 (2%) | 32.5 | MSI-Indeterminate | Retained | Wild type |
TMB: Tumor mutational burden; MSS: Microsatellite stable; MSI: Microsatellite instability; MMR: mismatch repair proteins; NA: Not available
Loss of MMR expression was seen in two cases with MSI-Indeterminate and two MSS cases. The additional MSI-Indeterminate case showed retained MMR expression.
Case excluded from analysis.
Clinical data were obtained via electronic medical records.
Statistical Analysis
One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was used to compare continuous variables across FIGO groups. Nominal categorical data were analyzed using the chi-square test. Ordinal categorical variables were analyzed using the Kruskal-Wallis test. Analysis in the context of binary grading and between MSI-H and POLE-mutant cohorts was performed using Welch’s t test. Log-rank (Mantel-Cox) test was used for progression-free survival (PFS) and disease-specific survival (DSS) analyses. Multivariable Cox regression analysis was performed to include age, FIGO grade, FIGO stage, TMB, presence of LVSI, and adjuvant treatment. Statistical significance was set at p<0.05. Data were analyzed using GraphPad Prism 9.2.0 (GraphPad Software, San Diego, CA).
Results
Clinicopathologic Features
There were 53 cases of EECs with somatic mutations in the POLE exonuclease domain (Table 1). Of these, 50 cases were included in the analysis, including 47 cases harboring mutations previously identified as a pathogenic11, two case with F367S, which has been reported as “likely oncogenic”17, and one case with M471V protein change, which, though not previously described, showed a POLE-ultramutated signature with retained MMR and microsatellite stable by MSIsensor. Three cases with the following protein changes: A509V, D368N, and E1767K, were excluded from analysis as these alterations were not previously described as pathogenic and the tumors were either MSI-H or MSI-Indeterminate by MSIsensor. Additionally, 162 MSI-H EECs fulfilled the above criteria and were included in the analysis.
The clinicopathologic features are summarized in Table 2. The average follow-up is 1165 days (39 months). Within the MSI-H cohort, 55 cases (34%) were classified as FIGO grade 1, 72 (44%) as FIGO grade 2, and 35 (22%) as FIGO grade 3. Within the POLE-mutant cohort, 18 (36%) were classified as FIGO grade 1, 11 (22%) as FIGO grade 2, and 21 (42%) as FIGO grade 3. There were no significant differences in the evaluated patient characteristics, including age, body mass index (BMI), and race distribution between the cohorts and across FIGO grades. There was no significant correlation in the frequency of LVSI across FIGO grades in both the MSI-H and POLE-mutant cohorts. However, there was a significant association between FIGO grade and stage at presentation in the MSI-H cohort (p=0.0011) with higher stage seen more frequently with higher grade tumors (Supplemental Figure 1A). Grade was not significantly associated with stage in POLE-mutant EECs, as the majority of cases presented with low-stage disease across all FIGO grades (p=0.5363, Supplemental Figure 1B).
Table 2.
Clinicopathologic features by molecular subgroup and grade.
| Characteristic | MSI-High (N=162) |
POLE-mutated (N=50) |
||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | p | Grade 1 | Grade 2 | Grade 3 | p | |
|
| ||||||||
| n (%) | 55 (34) | 72 (44) | 35 (22) | - | 18 (36) | 11 (22) | 21 (42) | - |
|
| ||||||||
| Age at diagnosis, years | 0.6049 | 0.6941 | ||||||
| Mean | 62 | 61 | 60 | 53 | 56 | 55 | ||
| Range | 37–81 | 31–81 | 37–93 | 38–66 | 35–81 | 39–75 | ||
|
| ||||||||
| Body mass index, kg/m 2 | 0.8567 | 0.7368 | ||||||
| Mean | 32 | 31 | 31 | 28 | 29 | 29 | ||
| Range | 19–68 | 17–49 | 15–52 | 18–52 | 21–38 | 21–38 | ||
|
| ||||||||
| Race distribution, n (%) | 0.3421 | 0.5411 | ||||||
| White | 48 (87) | 56 (78) | 29 (83) | 15 (83) | 9 (83) | 17 (81) | ||
| African American | 1 (2) | 4 (6) | 2 (6) | 0 | 0 | 1 (5) | ||
| Spanish/Hispanic/Latino | 1 (2) | 2 (3) | 0 | 0 | 0 | 1 (5) | ||
| Asian-Far East/Indian | 4 (7) | 6 (8) | 3 (9) | 3 (17) | 1 (8) | 1 (5) | ||
| Other/Unknown | 1 (2) | 4 (6) | 1 (3) | 0 | 1 (8) | 1 (5) | ||
|
| ||||||||
| Stage, n (%) | 0.0011 | 0.5363 | ||||||
| I | 43 (78) | 43 (60) | 14 (40) | 16 (89) | 8 (72) | 18 (86) | ||
| II | 1 (2) | 3 (4) | 3 (9) | 0 | 0 | 2 (9) | ||
| III | 9 (16) | 19 (26) | 11 (31) | 2 (11) | 1 (9) | 1 (5) | ||
| IV | 2 (4) | 7 (10) | 7 (20) | 0 | 2 (18) | 0 (0) | ||
|
| ||||||||
| LVSI, n (%) | 0.2150 | 0.1014 | ||||||
| Absent | 31 (56) | 31 (43) | 14 (40) | 15 (83) | 6 (55) | 11 (52) | ||
| Present | 24 (44) | 41 (57) | 21 (60) | 3 (17) | 5 (45) | 10 (48) | ||
|
| ||||||||
| Adjuvant treatment, n (%) | 0.0015 | <0.0001 | ||||||
| No adjuvant treatment | 25 (45) | 15 (21) | 6 (17) | 14 (78) | 2 (18) | 1 (5) | ||
| Radiotherapy only | 19 (35) | 27 (38) | 8 (23) | 3 (17) | 5 (46) | 14 (67) | ||
| Chemotherapy only | 2 (4) | 10 (14) | 4 (11) | 0 | 4 (36) | 0 | ||
| Chemoradiotherapy | 9 (16) | 20 (28) | 17 (49) | 1 (5) | 0 | 6 (28) | ||
MSI-High: microsatellite instability-high; LVSI, lymphovascular space invasion
As expected, TMB in the POLE-mutant cohort was significantly greater than that of the MSI-H cohort in each corresponding FIGO grade group (p<0.01, Supplemental Figures 1C and 1D). Within each cohort, there was also significantly greater TMB in FIGO grade 3 tumors compared to FIGO grades 1 and 2 while the difference in TMB between FIGO grades 1 and 2 tumors was not statistically significant.
When cases were reclassified using a binary grading system, the results were similar to those of the three-tiered grading system. In the MSI-H cohort, significantly greater TMB and higher stage at presentation were seen in high-grade tumors (p=0.0002 and p=0.0022, respectively). There were no significant differences in age, BMI, race distribution, and presence of LVSI. Similarly, in the POLE-mutant cohort, significantly greater TMB was seen in high-grade tumors (p=0.0009); however, there were no significant differences in age, BMI, race distribution, presence of LVSI, and stage at presentation.
Correlation with Mismatch Repair Protein and p53 Expression
IHC for MMR proteins was available for 151 MSI-H cases and 49 POLE-mutant cases. IHC for p53 was available for 93 MSI-H cases and 39 POLE-mutant cases (Table 3). Loss of MMR staining was seen in 95% of MSI-H tumors with 82% showing MLH1/PMS2 loss and 13% showing MSH2/MSH6 loss.
Table 3.
Mismatch repair protein and p53 expression by immunohistochemistry.
| MSI-High N=151 |
POLE-mutated N=49 |
|||||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
|
| ||||||
| Total MMR IHC, n | 55 | 64 | 32 | 18 | 11 | 20 |
| MLH1/PMS2 Loss, n (%) | 43 (78) | 59 (92) | 22 (69) | 0 | 0 (0) | 1 (5) |
| MSH2/MSH6 Loss, n (%) | 9 (16) | 3 (5) | 7 (22) | 1 (6) | 2 (18) | 5 (25) |
| MMR Retained, n (%) | 3 (5) | 2 (3) | 3 (9) | 17 (94) | 9 (82) | 14 (70) |
|
| ||||||
|
MSI-High N=93
|
POLE-mutated N=39
|
|||||
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
|
| ||||||
| Total p53 IHC, n | 32 | 39 | 22 | 12 | 10 | 17 |
| Aberrant, n (%) | 1 (3) | 0 | 2 (9) | 0 | 2 (20) | 4 (24) |
| Wildtype, n (%) | 31 (97) | 39 (100) | 20 (91) | 12 (100) | 8 (80) | 13 (76) |
MSI-High: microsatellite instability-high; MMR: mismatch repair; IHC: immunohistochemistry
Discordant MMR protein expression and aberrant p53 staining were confirmed by review of slides. Eight (5%) of the 151 MSI-H cases with IHC for MMR proteins had discrepancies between DNA MMR protein staining and MSIsensor results. Secondary review of the IHC slides showed focal/geographic loss of expression in 6 cases and fully retained expression in 2. Three (3%) of the 93 MSI-H cases with IHC for p53 had focal/geographic aberrant p53 expression, suggestive of a subclonal process.
The majority of POLE-mutated tumors showed intact MMR protein expression; however, 9 (18%) of the 49 POLE-mutant cases with IHC for MMR proteins showed a loss of one or more MMR protein expression. Similarly, wild-type pattern of p53 expression was seen in the majority of cases; however, 6 (15%) of the 39 POLE-mutant cases with IHC for p53 showed aberrant expression. In 6 cases (66%) with loss of MMR staining and 5 cases (83%) with aberrant p53 staining, the pattern was focal/geographic, suggestive of a subclonal process.
Of the 124 MSI-H cases with MLH1/PMS2 loss, MLH1 promoter methylation analysis was available for 114, of which hypermethylation was detected in 105 cases (92%).
Patient Outcomes
In the MSI-H cohort, 76 patients (47%) recurred and 22 (14%) died of disease. In the POLE-mutant cohort, eight patients (15%) recurred, one of whom died of disease. Log-rank survival analysis showed statistically significant differences in PFS (p=0.006) and DSS (p=0.0358) between the MSI-H and POLE-mutant cohorts (Figures 2A and 2B).
Figure 2. Patient outcomes analysis.
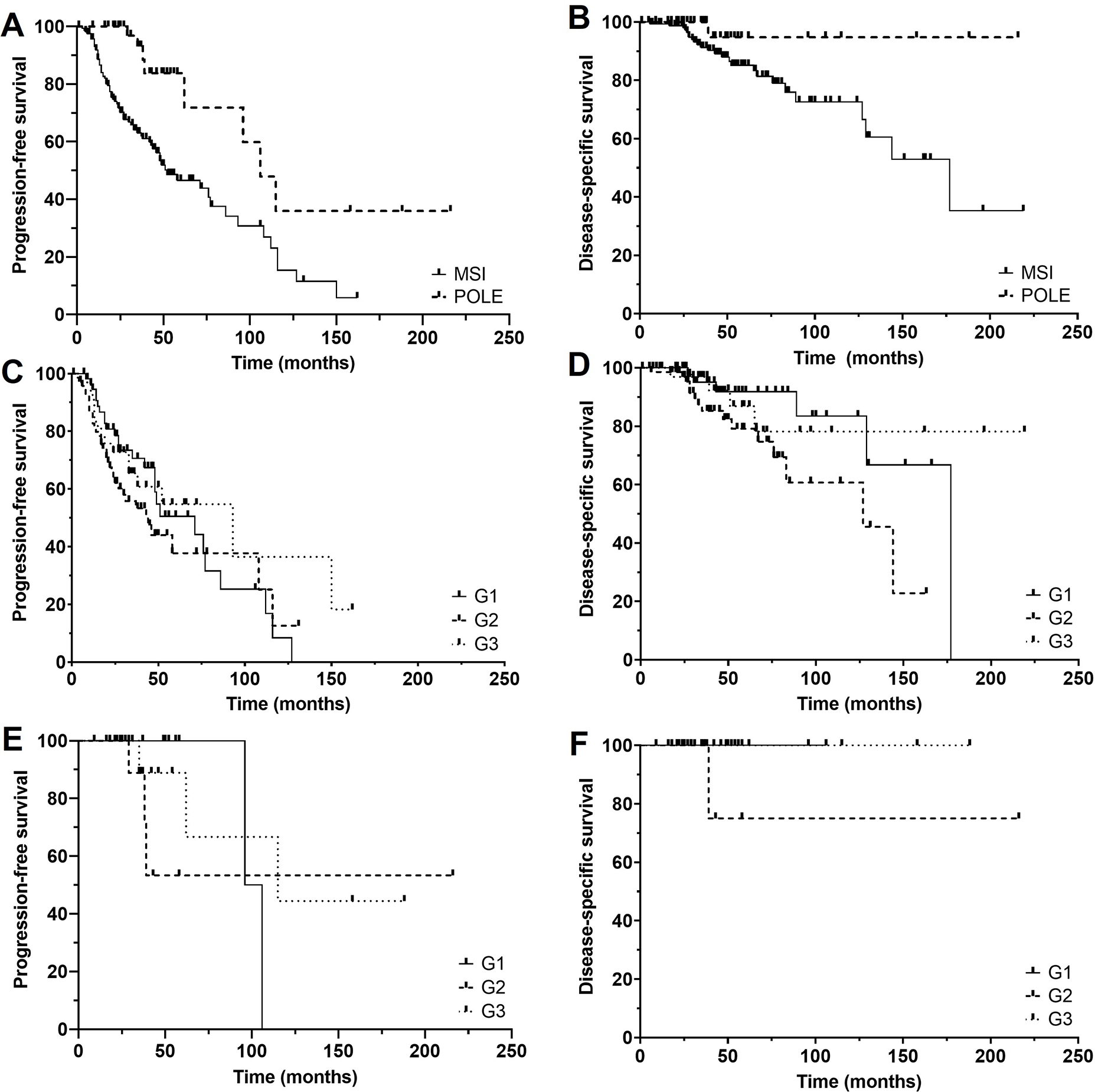
Kaplan-Meier curves of progression-free survival (A) and disease-specific survival (B) of microsatellite instability-high (MSI-H) and POLE-mutant tumors showing significantly better survival in the POLE-mutant cohort. No significant differences were seen in progression-free survival (C) and disease-specific survival (D) across grade groups in the MSI-H cohort. Similarly, no significant differences were seen in the progression-free survival (E) and disease-specific survival (F) across grade groups in the POLE-mutant cohort.
Within the MSI-H cohort, there were no statistically significant differences in PFS (p=0.2654) or DSS (p=0.0617) across FIGO grade groups (Figures 2C and 2D). Correspondingly, no statistically significant differences in PFS (p=0.2523) or DSS (p=0.2564) were seen when the cases were sorted into a binary grading system (Supplemental Figures 2A and 2B).
Similarly, there were no statistically significant differences in PFS (p=0.8495) or DSS (p=0.3247) across FIGO grade groups within the POLE-mutant cohort (Figures 2E and 2F). In the setting of a binary grading system, there were also no statistically significant differences in PFS (p=0.5932) or DSS (p=0.4669) (Supplemental Figures 2C and 2D).
Multivariable analysis performed on the MSI-H cohort showed that recurrence and DSS were independent of FIGO grade, FIGO stage, age, the presence of LVSI, and TMB (Table 4). The analysis could not be performed in the POLE-mutant cohort due to the low number of events.
Table 4.
Multivariable analysis of microsatellite instability-high tumors.
| Recurrence | Disease-specific survival | |||
|---|---|---|---|---|
|
| ||||
| Hazard ratio | 95% CI | Hazard ratio | 95% CI | |
|
| ||||
| Age | 1.017 | 0.9960–1.039 | 1.024 | 0.9858–1.067 |
|
| ||||
| Grade | ||||
| 1 | 1 | 1 | ||
| 2 | 1.288 | 0.7514–2.242 | 2.332 | 0.8565–7.526 |
| 3 | 0.8935 | 0.4275–1.804 | 0.7991 | 0.1790–3.343 |
|
| ||||
| Stage | ||||
| 1 | 1 | 1 | ||
| 2 | 2.797 | 0.8174–7.267 | 1.257 | 0.1713–5.357 |
| 3 | 1.238 | 0.6621–2.276 | 0.5398 | 0.1641–1.531 |
| 4 | 1.960 | 0.9080–3.861 | 2.105 | 0.5536–6.465 |
|
| ||||
| LVSI | 1.226 | 0.7258–2.097 | 2.612 | 0.9929–7.739 |
|
| ||||
| Adjuvant therapy | 0.6652 | 0.3658–1.223 | 0.9242 | 0.3050–3.085 |
|
| ||||
| TMB | 0.9927 | 0.9773–1.003 | 0.9833 | 0.9436–1.008 |
MSI-High: microsatellite instability-high; LVSI: lymphovascular space invasion; TMB: tumor mutational burden
Characteristics of the eight recurrent cases of POLE-mutant tumors are summarized in Table 5. One of the cases showed MSI-H by MSIsensor and two were indeterminate. However, all eight cases showed retained MMR expression by IHC. Out of the four cases with available p53 IHC, one showed aberrant overexpression. In the one case of death from disease, the tumor was found to be microsatellite stable by molecular analysis with retained MMR protein expression and wild-type p53 expression.
Table 5.
POLE protein change and tumor characteristics of recurrent POLE-mutant cases.
| Protein change | FIGO grade | TMB | MSIsensor score | MMR expression | p53 expression |
|---|---|---|---|---|---|
| V411L | 1 | 448.6 | MSS | Retained | NA |
| V411M | 1 | 52.7 | MSI-High | Retained | NA |
| V411L* | 2 | 186.9 | MSS | Retained | Wild type |
| V411L | 2 | 154.5 | MSS | Retained | Aberrant |
| F367V | 2 | 462.6 | MSI-Indeterminate | Retained | NA |
| P286R | 3 | 272.1 | MSS | Retained | Wild type |
| V411L | 3 | 342.3 | MS-Indeterminate | Retained | NA |
TMB: Tumor mutational burden; MSS: Microsatellite stable; MSI: Microsatellite instability; MMR: mismatch repair proteins, NA: Not available
Patient died of disease
As per clinical guidelines, there was a significantly greater proportion of patients with high-grade EECs who received adjuvant therapy in both the MSI-H and POLE-mutant cohorts (Table 2). Nonetheless, multivariable analysis showed that adjuvant therapy was not associated with PFS or DSS in the MSI-H cohort (HR: 0.6652 [95% CI: 0.3658–1.223] and HR: 0.9242 [95% CI: 0.3050–3.085], respectively; Table 4). In the POLE-mutant cohort, 33 patients (66%) received adjuvant therapy. Statistical analysis could not be performed in this cohort due to the low number of events. Five of the 8 patients who recurred received adjuvant therapy, including the patient who died from disease; this patient had a FIGO grade 2, stage IV tumor.
Outcome analysis of MSI-H cases with MLH1/PMS2 loss was performed between cases with and without MLH1 promoter hypermethylation. The results suggest better PFS (p=0.0740) and DSS (p=0.2809) in cases without MLH1 promoter hypermethylation(Supplemental Figures 3A and 3B). However, statistical significance could not be established due to the low number of cases of cases without MLH1 promoter hypermethylation. There was no significant difference in TMB between cases with and without MLH1 promoter hypermethylation (35.05 ± 29.39 Muts/Mb and 36.62± 13.21 Muts/Mb, respectively), and no significant differences in mutational profile were seen between MSI-H tumors with and without MLH1 promoter hypermethylation.
Discussion
The current standard of care for the management of EEC includes FIGO grade designation, which is incorporated into several risk stratification models and therapy guidelines for clinical practice18,19. While the FIGO grading system has been a historically useful tool in prognostication, it is not without shortcomings. Though the system appears straightforward, in practice, the distinction between grades is not always precise. Several studies have shown there is significant interobserver variability in assigning FIGO grade, with overall kappa statistics of 0.41–0.65, indicating only moderate levels of interobserver agreement.20–22
Recent data have shown that molecular clustering of EECs plays an important role in predicting outcome.3–6 This was further reinforced by the findings of the PORTEC-3 trial, which showed that molecular classification plays a significant role in predicting response to therapy in high-risk endometrial carcinoma.23 While a correlation between clinical outcome and FIGO grade group has been shown historically, it was unclear whether this grading system held the same significance when tumors were stratified into TCGA genomic clusters.
In our study, we validated the expected differences in patient outcome between the MSI-H and POLE-mutant cohorts, with the latter associated with significantly better PFS and DSS. In the POLE-mutant cohort, we saw a recurrence rate of 15%, which is higher than the previously reported rate of 2–6%.7,23 While generally associated with good prognosis, recurrences in POLE-mutant tumors have been previously reported, including one patient who died of disease in the PORTEC-3 trial.23 The high rate of recurrences in our study may reflect an element of selection bias, as many patients are referred to our institution at the development of a recurrence. Additionally, on our initial review of cases with POLE exonuclease domain mutation, we found that four tumors showed microsatellite instability by MSIsensor, three of which also showed loss of MMR protein expression. Although none of the three cases recurred, it raises the possibility that a POLE exonuclease mutation may be a secondary event to microsatellite instability rather than the reverse. Furthermore, mutational signature analysis revealed that four cases with POLE hotspot mutations showed a DNA MMR deficiency signature, of which one case showed MSI with loss of MMR, one with MSI and retained MMR, one MS stable with loss of MMR, and one MS stable with retained MMR. Again, no recurrences were seen in these four cases. However, a further look into POLE-mutant tumors with an aggressive clinical course is warranted.
While there were significant differences in TMB and stage at presentation across the FIGO grade groups in each cohort, these differences did not translate to patient outcomes. Indeed, we found that in both the MSI-H and POLE-mutant cohorts, there were no significant differences in PFS or DSS across FIGO grades. FIGO grade might continue to guide decisions to perform a staging procedure in the MSI-H group, but this would only be applicable to practices in which sentinel lymph node evaluation was not planned and when the determination of MSI-H precedes hysterectomy. With this one exception, we conclude that genomic classification of EECs is a more robust discriminator in predicting tumor behavior than traditional FIGO grading in MSI-H and POLE-mutant cases.
Several classification and regression tree statistical analyses have previously demonstrated that reclassifying EECs using a binary system (consolidating FIGO grades 1 and 2 tumors as “low grade” and FIGO grade 3 tumors as “high grade”) is the next most informative prognostic factor after stage.24,25 Prior studies have also shown that the binary system performed as well as or better than the three-tiered system, with a superior interobserver variability kappa score.26 In our study, statistical analyses performed using both the three-tiered and the binary system showed similar findings, demonstrating that, in this context, both systems result in comparable categorization. Nevertheless, molecular clustering remains a superior predictor of clinical outcome in MSI-H and POLE-mutant EECs compared to both the three-tiered and binary grading systems.
As the importance of molecular clustering in prognostication becomes increasingly evident, it is crucial to establish practical methods to determine EEC genotypic cluster. A number of studies have shown MMR protein IHC as an accurate and accessible surrogate for detecting MSI-H tumors.4,9,27,28 The utility of IHC in this context was also demonstrated in our data set, with loss of one or more MMR protein expression in 95% of cases with MSI by molecular testing. Review of slides from discordant cases showed that in the majority, the loss of MMR protein expression was geographic or focal, suggesting a subclonal process. Similar discordant IHC has been previously reported,27,28 demonstrating molecular testing is indicated despite retained MMR protein expression in cases in which histologic or clinical suspicion is high.
Loss of MMR protein expression also does not categorically indicate MSI. In our data set, 22% of POLE-mutant EECs displayed a single or multiple loss of MMR protein expression, often geographic or focal. Studies have shown that in these so-called multiple classifier cases, the POLE mutation event precedes the MMR defect, and these tumors are best classified as POLE-mutant rather than MSI-H.29,30 However, it has also been shown that a shift from POLE- to MSI-related mutational processes can occur in progression from primary to recurrent/metastatic endometrial carcinoma.31 Thus, while loss of MMR protein expression can be seen in POLE-mutant tumors, there is currently no categorical method to predict whether a shift to MSI-related mutational processes and its associated prognosis may occur and whether the tumor should be considered in the MSI-H cluster based on MSIsensor, MMR protein expression, and mutational signature analysis.
Similarly, aberrant p53 expression was seen in three MSI-H and six POLE-mutant EECs. Studies have shown that TP53 mutation in the context of MSI-H and POLE mutation does not affect clinical outcome and is best considered a passenger mutation, with the affected carcinomas having low levels of CNAs unlike tumors in the CN-H cluster.30 In fact, three of the cases with aberrant p53 staining (two diffuse and one heterogeneous) did not show any TP53 mutations by next-generation sequencing. Thus, while a generally accurate and useful tool, IHC for MMR protein and p53 is not infallible for the detection of MSI-H or CN-H tumors, respectively, and should be used with its sensitivity and specificity in mind, particularly in cases with patchy or focal staining.
Lastly, the interpretation of POLE mutations may, at times, pose a difficulty. While the majority of cases in our cohort (47/50) harbored alterations that have been previously reported as pathogenic11, the approach to a novel variant is far from straightforward. We excluded three cases with previously unreported variants that were also MSI-H or MSI-Indeterminate. In contrast, we included a case with M471V alteration, which, although has not been previously described, was microsatellite stable by MSIsensor, MMR proficient by immunohistochemistry, and had a POLE mutational signature with very high tumor mutational burden, indicating a POLE-ultramutated genotype. Similarly, two cases with F367V alteration were included. This variant has been classified as “likely oncogenic”, and the cases in our cohort were MMR proficient and had a POLE mutational signature. Taken together, although the findings from our study suggest that these variants result in a POLE ultramutated genotype, they have not been independently verified as pathogenic. In the clinical setting, the presence of a POLE mutation must be interpreted carefully, taking into account the variant and mutational signature, as well as the possibility that a POLE exonuclease mutation may be a secondary event to microsatellite instability, as described above. To avoid misclassification and mismanagement, continual studies confirming the pathogenicity of novel variants are necessary.
One factor that must be considered in interpreting the results of our study is the effect of adjuvant treatment. Based on current guidelines, adjuvant therapy is recommended for patients with high-stage or high-grade disease. Accordingly, there is a significantly greater proportion of patients with FIGO grade 3 EECs who received adjuvant therapy in our study. Although the extent of treatment effect on recurrence rate and DSS cannot be determined with precision, multivariable regression analysis showed no association between adjuvant therapy and PFS or DSS in MSI-H tumors. In the POLE-mutant cohort, patients appear to do well regardless of adjuvant treatment, consistent with published data.32,33 Furthermore, recent studies also suggest that currently used therapeutics for endometrial carcinoma are either not effective (platinum-based therapy in POLE-mutant, MSI-H, and CN-L carcinomas)23,34 or marginally effective (checkpoint inhibitors in MSI-H carcinomas).35,36 In summary, reported therapeutic data and our multivariable regression analysis suggest that adjuvant therapy did not, or should not, have significantly improved PFS or DSS in histologically high-grade POLE-mutated or MSI-H carcinomas. These conclusions would be strengthened with more conclusive evidence, for which studies may be difficult or impossible to accrue.
One factor that may be of importance in prognostication is MLH1 promoter hypermethylation. Several studies have previously shown that MSI-H tumors with MLH1 promoter hypermethylation have poorer survival and show greater resistance to immune checkpoint inhibitors compared to those without MLH1 promoter hypermethylation (Lynch or Lynch-like tumors).37–40 Our study showed similarly worse outcomes in cases with MLH1 promoter hypermethylation; although due to the low number of cases without MLH1 promoter hypermethylation, the trend did not reach statistical significance. As MLH1 promoter hypermethylation status may confer significant prognostic information, it may be prudent to perform the analysis in all MSI-H cases or in cases identified as DNA MMR-deficient by loss of expression of MLH1 and PMS2.
Finally, the question remains as to whether FIGO grading is of clinical significance in any of the EEC molecular subgroups. Our data have shown FIGO grading of EECs is not associated with prognosis in MSI-H and POLE-mutant tumors, while the discussion of FIGO grading in the CN-H cluster is moot, as the preponderance of these tumors are histologically high-grade and have been shown to follow a similar or mostly similar biological course as serous carcinoma.3,5,41 Furthermore, interobserver reproducibility is poor in the diagnosis of high-grade EEC, highlighting the need for molecular analysis rather than sole reliance on histological subtyping.42 Lastly, EECs without a specific molecular profile (absence of MMR deficiency, POLE hotspot mutation, and TP53 mutation) cluster into the CN-L molecular subtype. These tumors have an intermediate prognosis and span across FIGO grade groups and genotype.3,6,41 Therefore, in the CN-L cluster, FIGO grading is likely to reflect prognosis. Indeed, a recent publication confirms the importance of FIGO grade in CN-L tumors, leading us to conclude FIGO grading may only be prognostically informative in CN-L EECs.32,41
In summary, we have shown that molecular profile overrides FIGO grade in predicting the biologic behavior of EECs in MSI-H and POLE-mutant EECs. Nevertheless, many current risk stratification models have yet to incorporate molecular subtyping. This discrepancy is most apparent in POLE-mutant EECs. Currently, a FIGO grade 3 POLE-mutant EEC would be categorized clinically in at least the intermediate-high-risk group, with treatment decisions determined accordingly. However, as has been repeatedly shown, POLE hotspot mutations portend an excellent prognosis regardless of other factors, such as grade and stage. Indeed, studies have proposed a treatment de-escalation for patients with POLE-mutant EECs.43,44 On the other end of the spectrum, CN-H EECs are intrinsically high risk regardless of FIGO grade or stage, and while MSI-H EECs span all three FIGO grades, they collectively have an intermediate prognosis. Therefore, to better predict tumor behavior and improve therapy guidance, it would be prudent to incorporate molecular subtyping into risk stratification models rather than retaining a reliance on conventional tumor grade unless the tumor has a non-specific molecular profile.
Supplementary Material
Funding:
This study was supported by the NIH/NCI Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748), the Molecular Diagnostics Service in the Department of Pathology, and the Marie-Josee and Henry R. Kravis Center for Molecular Oncology.
Footnotes
Conflict of Interests: Dr. Abu-Rustum reports grant support paid to the institution from GRAIL. The other author authors have no potential conflicts of interest to disclose.
Ethics Approval and Consent to Participate: This study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSK).
Supplementary information is available at Modern Pathology’s website.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
The materials described in the manuscript, including all relevant raw data, will be freely available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality.
References
- 1.Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973–87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 1994;10(1):31–46. [DOI] [PubMed] [Google Scholar]
- 2.Zaino RJ, Kurman RJ, Diana KL, Morrow CP. The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer. 1995;75(1):81–86. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research N, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kommoss S, McConechy MK, Kommoss F, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol. 2018;29(5):1180–1188. [DOI] [PubMed] [Google Scholar]
- 5.Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017;123(5):802–813. [DOI] [PubMed] [Google Scholar]
- 6.Bosse T, Nout RA, McAlpine JN, et al. Molecular Classification of Grade 3 Endometrioid Endometrial Cancers Identifies Distinct Prognostic Subgroups. Am J Surg Pathol. 2018;42(5):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107(1):402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church DN, Briggs SE, Palles C, et al. DNA polymerase ε and δ exonuclease domain mutations in endometrial cancer. Hum Mol Genet. 2013;22(14):2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer. 2015;113(2):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng B, Hoang LN, McIntyre JB, et al. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol. 2014;134(1):15–19. [DOI] [PubMed] [Google Scholar]
- 11.Leon-Castillo A, Britton H, McConechy MK, et al. Interpretation of somatic POLE mutations in endometrial carcinoma. J Pathol. 2020;250(3):323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Church DN, Briggs SE, Palles C, et al. DNA polymerase epsilon and delta exonuclease domain mutations in endometrial cancer. Hum Mol Genet. 2013;22(14):2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niu B, Ye K, Zhang Q, et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics. 2014;30(7):1015–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benhamida JK, Hechtman JF, Nafa K, et al. Reliable Clinical MLH1 Promoter Hypermethylation Assessment Using a High-Throughput Genome-Wide Methylation Array Platform. J Mol Diagn. 2020;22(3):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korkmaz V, Meydanli MM, Yalçın I, et al. Comparison of three different risk-stratification models for predicting lymph node involvement in endometrioid endometrial cancer clinically confined to the uterus. J Gynecol Oncol. 2017;28(6):e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh WJ, Abu-Rustum NR, Bean S, et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(2):170–199. [DOI] [PubMed] [Google Scholar]
- 20.Kapucuoglu N, Bulbul D, Tulunay G, Temel MA. Reproducibility of grading systems for endometrial endometrioid carcinoma and their relation with pathologic prognostic parameters. Int J Gynecol Cancer. 2008;18(4):790–796. [DOI] [PubMed] [Google Scholar]
- 21.Nedergaard L, Jacobsen M, Andersen JE. Interobserver agreement for tumour type, grade of differentiation and stage in endometrial carcinomas. APMIS. 1995;103(7–8):511–518. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen AL, Thomsen HK, Nyholm HC. Evaluation of the reproducibility of the revised 1988 International Federation of Gynecology and Obstetrics grading system of endometrial cancers with special emphasis on nuclear grading. Cancer. 1991;68(10):2303–2309. [DOI] [PubMed] [Google Scholar]
- 23.Leon-Castillo A, de Boer SM, Powell ME, et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J Clin Oncol. 2020;38(29):3388–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barlin JN, Zhou Q, St Clair CM, et al. Classification and regression tree (CART) analysis of endometrial carcinoma: Seeing the forest for the trees. Gynecol Oncol. 2013;130(3):452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlin JN, Soslow RA, Lutz M, et al. Redefining stage I endometrial cancer: incorporating histology, a binary grading system, myometrial invasion, and lymph node assessment. Int J Gynecol Cancer. 2013;23(9):1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conlon N, Leitao MM Jr., Abu-Rustum NR, Soslow RA. Grading uterine endometrioid carcinoma: a proposal that binary is best. Am J Surg Pathol. 2014;38(12):1583–1587. [DOI] [PubMed] [Google Scholar]
- 27.McConechy MK, Talhouk A, Li-Chang HH, et al. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol. 2015;137(2):306–310. [DOI] [PubMed] [Google Scholar]
- 28.Stelloo E, Jansen AML, Osse EM, et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann Oncol. 2017;28(1):96–102. [DOI] [PubMed] [Google Scholar]
- 29.Haradhvala NJ, Kim J, Maruvka YE, et al. Distinct mutational signatures characterize concurrent loss of polymerase proofreading and mismatch repair. Nat Commun. 2018;9(1):1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leon-Castillo A, Gilvazquez E, Nout R, et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J Pathol. 2020;250(3):312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashley CW, Da Cruz Paula A, Kumar R, et al. Analysis of mutational signatures in primary and metastatic endometrial cancer reveals distinct patterns of DNA repair defects and shifts during tumor progression. Gynecol Oncol. 2019;152(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leon-Castillo A, Horeweg N, Peters EEM, et al. Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol Oncol. 2022;164(3):577–586. [DOI] [PubMed] [Google Scholar]
- 33.McAlpine JN, Chiu DS, Nout RA, et al. Evaluation of treatment effects in patients with endometrial cancer and POLE mutations: An individual patient data meta-analysis. Cancer. 2021;127(14):2409–2422. [DOI] [PubMed] [Google Scholar]
- 34.Randall ME, Filiaci V, McMeekin DS, et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J Clin Oncol. 2019;37(21):1810–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oaknin A, Gilbert L, Tinker AV, et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J Immunother Cancer. 2022;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasanen A, Loukovaara M, Butzow R. Clinicopathological significance of deficient DNA mismatch repair and MLH1 promoter methylation in endometrioid endometrial carcinoma. Mod Pathol. 2020;33(7):1443–1452. [DOI] [PubMed] [Google Scholar]
- 38.Cosgrove CM, Cohn DE, Hampel H, et al. Epigenetic silencing of MLH1 in endometrial cancers is associated with larger tumor volume, increased rate of lymph node positivity and reduced recurrence-free survival. Gynecol Oncol. 2017;146(3):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shikama A, Minaguchi T, Matsumoto K, et al. Clinicopathologic implications of DNA mismatch repair status in endometrial carcinomas. Gynecol Oncol. 2016;140(2):226–233. [DOI] [PubMed] [Google Scholar]
- 40.Bellone S, Roque DM, Siegel ER, et al. A phase 2 evaluation of pembrolizumab for recurrent Lynch-like versus sporadic endometrial cancers with microsatellite instability. Cancer. 2022;128(6):1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Momeni-Boroujeni A, Nguyen B, Vanderbilt CM, et al. Genomic landscape of endometrial carcinomas of no specific molecular profile. Mod Pathol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol. 2013;37(6):874–881. [DOI] [PubMed] [Google Scholar]
- 43.León-Castillo A, de Boer SM, Powell ME, et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J Clin Oncol. 2020;38(29):3388–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Gool IC, Rayner E, Osse EM, et al. Adjuvant Treatment for. Clin Cancer Res. 2018;24(13):3197–3203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The materials described in the manuscript, including all relevant raw data, will be freely available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality.


