Abstract
Here we examine the effects of ambient red light on lens-induced myopia and diffuser-induced myopia in tree shrews, small diurnal mammals closely related to primates. Starting at 24 days of visual experience (DVE), seventeen tree shrews were reared in red light (624 ± 10 or 634 ± 10 nm, 527–749 human lux) for 12–14 days wearing either a −5D lens (RL-5D, n = 5) or a diffuser (RLFD, n = 5) monocularly, or without visual restriction (RL-Control, n=7). Refractive errors and ocular dimensions were compared to those obtained from tree shrews raised in broad-spectrum white light (WL-5D, n = 5; WLFD, n = 10; WL Control, n = 7). The RL-5D tree shrews developed less myopia in their lens-treated eyes than WL-5D tree shrews at the end of the experiment (−1.1 ± 0.9D vs. −3.8 ± 0.3D, p = 0.007). The diffuser-treated eyes of the RLFD tree shrews were near-emmetropic (−0.3 ± 0.6D, vs. −5.4 ± 0.7D in the WLFD group). Red light induced hyperopia in control animals (RL- vs. WL-Control, +3.0 ± 0.7 vs. +1.0 ± 0.2D, p = 0.02), the no-lens eyes of the RL-5D animals, and the no-diffuser eyes of the RLFD animals (+2.5 ± 0.5D and +2.3 ± 0.3D, respectively). The refractive alterations were consistent with the alterations in vitreous chamber depth. The lens-induced myopia developed in red light suggests that a non-chromatic cue could signal defocus to a less accurate extent, although it could also be a result of “form-deprivation” caused by defocus blur. As with previous studies in rhesus monkeys, the ability of red light to promote hyperopia appears to correlate with its ability to retard lens-induced myopia and form-deprivation myopia, the latter of which might be related to non-visual ocular mechanisms.
Keywords: light property, wavelength, emmetropization, refractive development, hyperopia, animal model
1. Introduction
To achieve good focus, early post-natal eye growth is controlled by a feedback mechanism called “emmetropization.” The result of this controlled process is that the refractive errors observed at infancy gradually reduce towards a near-zero “emmetropic” level. Emmetropization is vision-dependent, as removing meaningful visual feedback prevents this process and causes myopia (Howlett & McFadden, 2006; Schaeffel & Howland, 1991, p. 199; Siegwart Jr. & Norton, 1998; Smith III & Hung, 2000; Tejedor & de la Villa, 2003; Tkatchenko et al., 2010; Wallman & Adams, 1987; Wiesel & Raviola, 1977, 1979), which can be reversed if normal vision is restored in a timely manner (Howlett & McFadden, 2006; Qiao-Grider et al., 2004; Siegwart Jr. & Norton, 1998, 1998; Wallman & Adams, 1987). It has also been shown that eyes can determine their refractive state from visual feedback and use it to regulate ocular elongation. Specifically, rearing with negative lenses (imposing hyperopic defocus) increases the ocular elongation rate and causes myopia, whereas positive lenses slow ocular elongation (Barathi et al., 2008; Howlett & McFadden, 2009; Hung et al., 1995; Jiang et al., 2018; Metlapally & McBrien, 2008; Pardue et al., 2013; Siegwart, Jr. & Norton, 2010; Tkatchenko et al., 2010; Troilo et al., 2009). Using a tree shrew binocular lens wear model, Siegwert Jr. and Norton further showed that the eye could eliminate most of the defocus imposed by the lenses and reached the normal emmetropic level (Siegwart Jr. & Norton, 2010), suggesting that lens-compensation in essence is an emmetropization process over lenses (Khanal et al., 2023).
Other features of the visual environment can also influence refractive development. For example, rearing animals in narrowband ambient lighting can compromise emmetropization and cause refractive error. Specifically, rearing under narrowband “long” wavelength ambient light (peak wavelength 530nm – 760nm) caused axial myopia in chicks and guinea pigs (Foulds et al., 2013; Jiang et al., 2014; Long et al., 2009; Seidemann & Schaeffel, 2002), whereas rearing under “short” wavelength light (peak wavelength 430 – 530nm) caused axial hyperopic changes or axial hyperopia. These narrowband light effects have implications in understanding how eyes detect defocus. It has been proposed that the image degradations caused by longitudinal chromatic aberration (LCA) provide critical visual information that encodes defocus (Gawne et al., 2022; Gawne & Norton, 2020; Swiatczak & Schaeffel, 2022). For a recent review, see Sankaridurg et al., 2023). LCA is caused by the wavelength-dependent dispersion property of ocular media, which causes short wavelength light to focus before longer wavelength light. As reviewed by Rucker (2013), in narrowband light, eyes should hypothetically respond to the difference in focal plane location by reducing (in short wavelength light) or increasing (in long wavelength light) their elongation rates in order to maximize the contrast of the “monochromatic” image. However, long-wavelength light consistently produced progressive hyperopic instead of the predicted myopic changes in rhesus monkeys and tree shrews (Gawne, Siegwart, et al., 2017; Gawne, Ward, et al., 2017; Hung et al., 2018; Smith III et al., 2015), which was the opposite response as that predicted by LCA.
Narrowband ambient lighting also alters the development of visually induced ametropia. Chicks reared under short-wavelength light developed less visually induced myopia (Torii et al., 2017; Wang et al., 2018). For rhesus monkeys, narrowband red light caused progressive hyperopia in eyes viewing through lenses and diffusers (Hung et al., 2018). Here we report and discuss the observations of negative-lens-induced myopia and diffuser-induced myopia under ambient red light in tree shrews, a mammalian species phylogenetically close to primates (Cao et al., 2003). The issue is whether red light will not only promote hyperopia in this species, but also counteract negative-lens and form deprivation induced myopia, as it does in rhesus monkeys - although it must be noted that these two forms of experimental myopia might not reflect the mechanisms in human school-age onset myopia (Khanal et al., 2023).
2. Methods
2.1. Animal subjects
Seventeen northern tree shrews (Tupaia belangeri) were randomly assigned to three groups (red-light control or RL-Control, n = 7; red-light diffuser or RLFD, n = 5; red-light −5D or RL-5D, n = 5). We were careful not to assign littermates to the same experimental group. These animals were obtained from the Tree Shrew Core of the University of Alabama at Birmingham. The experiment began on the 24th day of visual experience (DVE, the number of days from the first day when both eyes were found open, typically at around three weeks of age) and ended on 35 DVE.
Northern tree shrews are diurnal animals. Their cone-dominated retina consist of short wavelength-sensitive (SWS, λmax ≈ 428nm) and long wavelength-sensitive cones (LWS cones, λ max ≈ 555nm) that are analogue to the S-cones (λmax ≈ 420nm) and L-cones (λmax ≈ 560) in humans (Bowmaker et al., 1980; Bowmaker & Dartnall, 1980). Tree shrew retinas do not have a medium-wavelength sensitive cone that is analogue to the human M-cones (Figure). Their LWS cone is the dominant photoreceptor type (Müller & Peichl, 1989; Sajdak et al., 2019), whereas SWS cones comprise about 4–10% of the total cone population (Müller & Peichl, 1989; Sajdak et al., 2019). This sparse SWS cone mosaic can in principle provide sufficient spatial resolution for the detection of the chromatic defocus cue (Gawne et al., 2021). On the other hand, rods only account for about 4% of the total photoreceptor population in the tree shrew retina (Sajdak et al., 2019) (1–14% depending the retinal region. Müller & Peichl, 1989). Similar to other mammals, rods in tree shrew are spectrally tuned to ~500nm wavelength (496nm, Petry & Harosi, 1990). Due to their role in vision for this diurnal species and their small fraction of the total tree shrew photoreceptor population (Müller & Peichl, 1989), rod photoreceptors were not considered important for the purpose of our study.
2.2. Narrowband, long-wavelength ambient lighting (“red light”)
Red light was produced using red light-emitting diodes (LED) (3.5*2.8mm surface-mounted diode LED strip, 60 LEDs/m; 634 ± 10 nm) arrays installed on the top of individual cage. Red light was maintained at a 14-hour-on, 10-hour-off cycle, which is standard for this colony. The illuminance of red light, measured on the cage floor, ranged between 527–749 human lux (Gawne, Ward, et al., 2017).
2.3. Lens and diffuser treatments
Tree shrews wore lenses or diffusers continuously throughout the red-light rearing period. All lenses and diffusers had a base curve of 7.5 mm and a diameter of 12 mm (Conforma Laboratories, Norfolk, VA). The lenses/diffusers were mounted on a goggle that was clipped onto a pedestal that was surgically installed on the animal’s skull (Siegwart Jr. & Norton, 1994).
2.4. Controls
Control data were obtained from tree shrews previously reared in white light with concurrent monocular −5D lens (WL-5D, n = 7) or monocular diffuser (WLFD, n = 10) treatments. In addition, tree shrews previously reared in white light without any visual restriction (WL Control, n = 7) were also included for all comparisons to represent the “colony normality” in refractive development (see Figures 2 – 5). Control lighting (white light, see Figure 2) was produced using fluorescent light bulbs (F34CW RS WM ECO, General Electronic Lighting) (Khanal et al., 2023). The illumination level ranged between 100~300 lux. The comparative red vs. white light levels were within a range that does not significantly affect emmetropization in tree shrews (Norton & Siegwart Jr., 2013).
Figure 2.
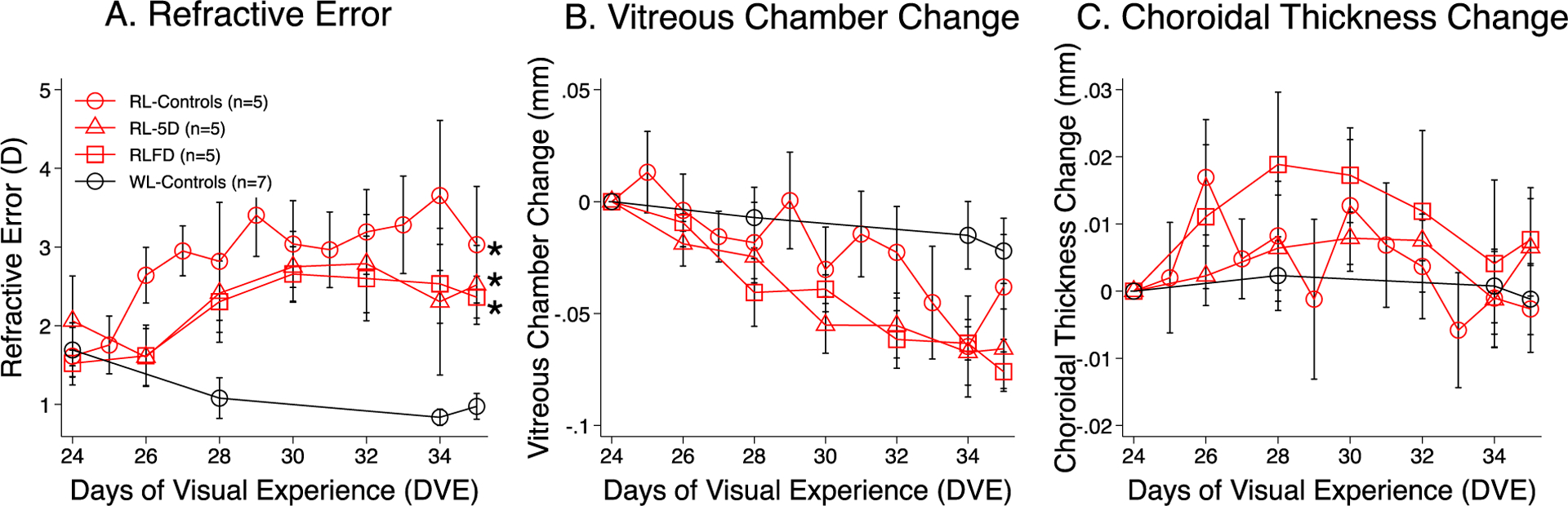
Comparing the normal-viewing eye refractive developments in white and red lighting. The three panels plot the mean ± SEM values for A. refractive error, B. vitreous chamber depth change, and C. choroidal thickness change, respectively, as a function of days of visual experience (DVE). Data presented were the binocular average of the RL- (open red circle) and WL-Control groups (white circle. Gawne et al., 2017), the no-lens eyes of the RL-5D group (open red triangle), and the no-diffuser eye of the RLFD group (open red square). Asterisk marks to the right of the trajectories indicate significant difference from white light. Red light produced significant hyperopia in the normal-viewing tree shrew eyes.
Figure 5.
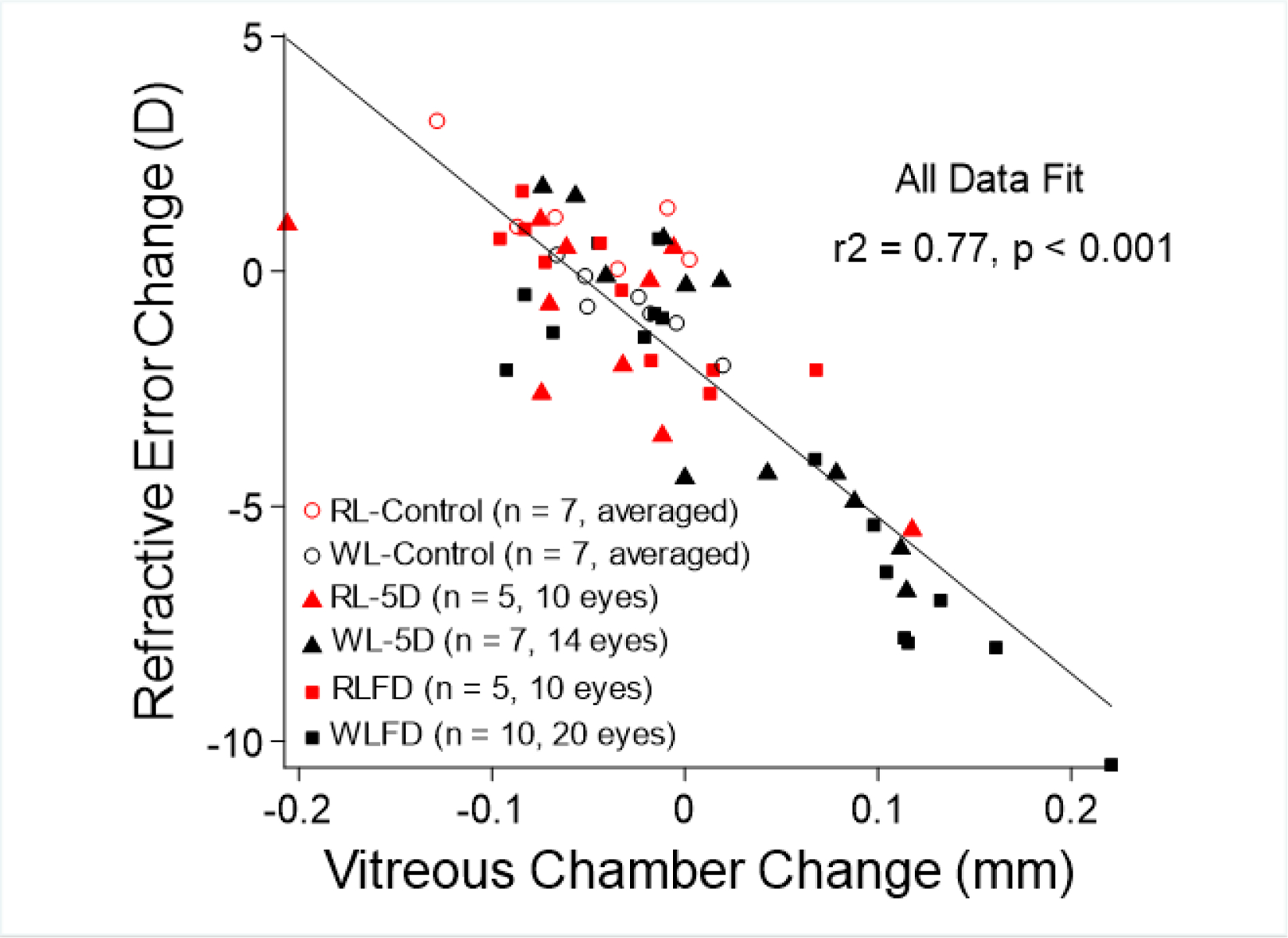
Correlation between refractive change and vitreous chamber depth change. Refractive error change at the end of the experiment (relative to baseline) was plotted against the change in vitreous chamber depth. Data presented were the binocular average of the RL- and WL-Control groups (red open circle and white circle), the lens-treated eyes and no-lens eyes of the RL-5D (red triangle) and WL-5D group (black triangle), and the diffuser-treated eyes and no-diffuser eyes of the RLFD (red square) and WLFD groups (black square). For the goggle-rearing groups, data for the two eyes were not distinguished by different symbols, because both lenses and diffusers produce axial ametropia in tree shrews. There was a strong, inverse correlation between refractive error change and vitreous chamber depth change (linear fit for all data, r2 = 0.77), suggesting that the red light produced an effect through alterations in the rate of axial elongation.
All control data have been previously published and discussed (El Hamdaoui et al., 2021; Gawne, Ward, et al., 2017; He et al., 2014). We consider these historical data valid for use as reference because the normal emmetropization pattern illustrated in these data was consistent between groups and stable over time. For example, the refractive development trajectory in the no-lens eyes of the WL-5D (He et al., 2014) and the no-diffuser eyes of the WLFD group (El Hamdaoui et al, 2021) (Figures 3B and 4B) both conformed with the developmental pattern in the WL-Control group (Gawne, Ward, et al., 2017), as illustrated in the same figures. In the supplementary material, we provided additional data to support our use of the historical control (see Supplementary Material B).
Figure 3.
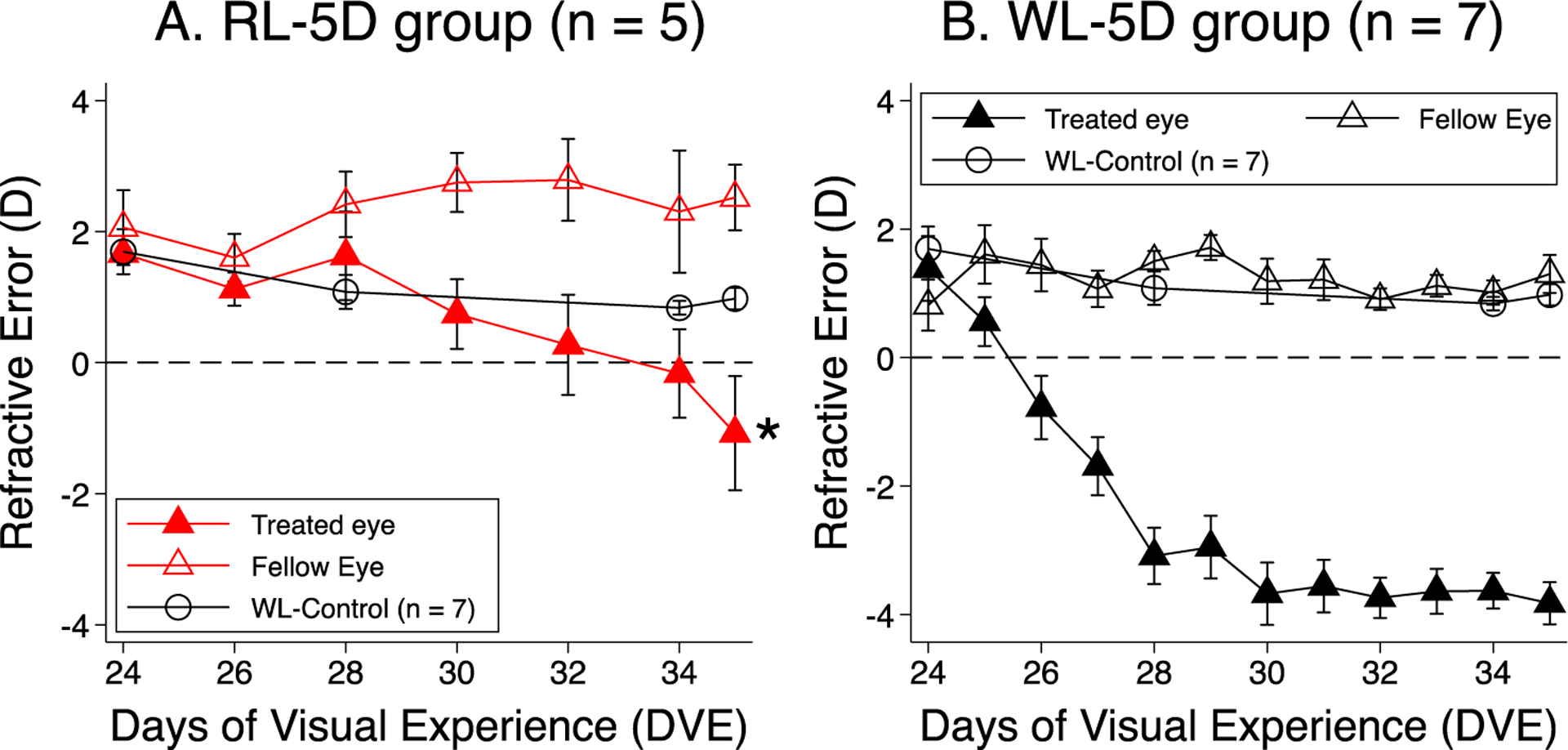
Comparison of lens-induced myopia between red and white light. Panels A and B plot the mean ± SEM refractive error as a function of DVE the lens-treated (solid symbols) and no-lens eyes (open symbols) of the RL-5D and WL-5D group (He et al., 2014), respectively. White circles represent WL-Control (Gawne et al., 2017). Asterisk mark indicates significant difference from white light. Red light significantly retarded lens-induced myopia and caused moderate hyperopia in the no-lens eye.
Figure 4.
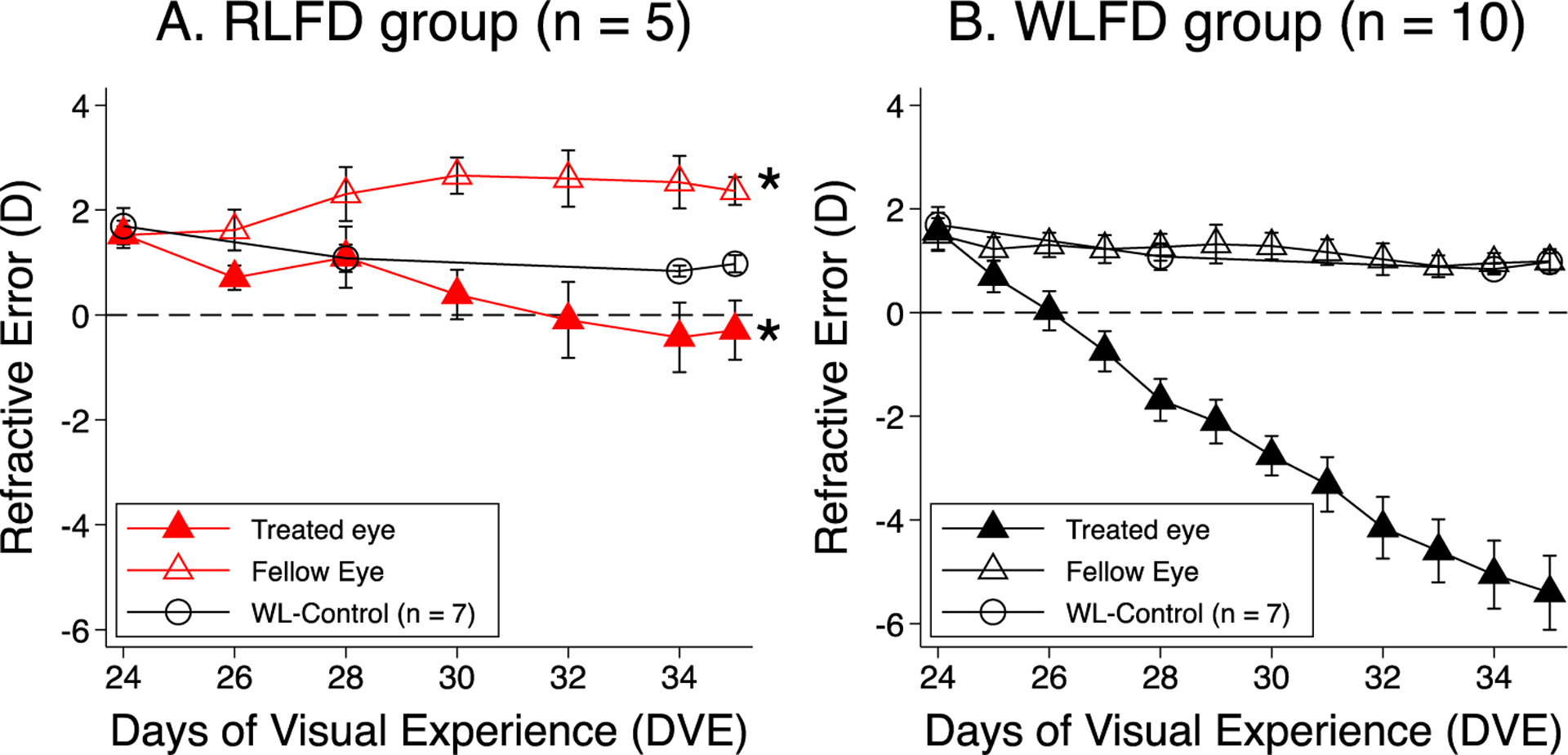
Comparison of the development of form-deprivation myopia in red and white light. Panels A and B plot the mean ± SEM of the refractive error as a function of DVE the diffuser-treated (solid symbols) and no-diffuser eyes (open symbols) of the RLFD and WLFD group (El Hamdaoui et al., 2021), respectively. White circles represent WL-Control (Gawne et al., 2017). The asterisk mark indicates a significant difference from white light. Red light prevented form-deprivation myopia and caused moderate hyperopia in the no-diffuser eye.
All rearing, intervention, and data collection procedures adhered to the guidelines by the Association for Research in Vision and Ophthalmology for the use of animals in ophthalmic and visual research and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
2.5. Outcome measures and data collection
Refractive error and axial dimension components were always measured on the first and last day of treatment. They were also measured during the intervening days, sometimes every day, and sometimes every two days, depending on the condition. Measurements took place between 9AM – 12PM to reduce the influence of ocular circadian rhythms and were performed on awake animals without cycloplegia (non-cycloplegic refraction in tree shrews are consistently +0.4D more hyperopic than “wet” refraction with atropine/phenylephrine. Norton et al., 2003). To prevent exposing our subjects to non-treatment light, tree shrews were placed in an opaque container for transportation. In addition, the measurement room was dimly illuminated with either white (for white light subjects) or red LED (for red light subject) during data collection. Finally, the white central fixation light of the autorefractor and the white flash used by the LenStar for corneal power measurement were both disabled.
Refractive error was defined as the spherical equivalent of the corneal-plane refractive correction. It was reported as the average of five measurements obtained using an autorefractor (Nidek ARK-700A, NIDEK USA, Inc., San Jose, CA) and was corrected for the “small-eye artifact” in this species, of which the magnitude (+4D) was determined by examining the difference between the autorefractor readings and the corrective prescription that maximized visually evoked potential (Norton et al., 2003). Axial dimension components (anterior chamber depth, lens thickness, vitreous chamber depth, and axial length) were measured using an optical biometer (LenStar LS 900, Haag-Streit AG, Koeniz, Switzerland) and processed with custom software. Anterior chamber depth was defined as the axial distance between the anterior corneal surface to the anterior lens surface. Vitreous chamber depth was measured from the posterior lens surface to the vitreous-retinal interface, whereas axial length was measured from the anterior corneal surface to the vitreous-retinal interface (i.e., the sum of the anterior chamber depth, lens thickness and vitreous chamber depth). The LenStar assumes human refractive indices; here the ocular dimension values were corrected for the optical properties of tree shrew ocular media, as described previously (El Hamdaoui et al., 2019).
Choroidal thickness changes were derived from the LenStar using the software mentioned above. We first manually delineated the retina-RPE and choroidoscleral borders in the optical A-scan. The software will automatically calculate the thickness of the retinal pigment epithelium–Bruch’s membrane–choroid complex (RPE-BM-Ch complex) using the delineation and the refractive index of the human retina. Then, we used the endpoint-baseline difference in RPE-BM-Ch-complex thickness as a surrogate observation for choroidal thickness under the assumption that the thicknesses of the RPE (El Hamdaoui et al., IOVS 2020;61: ARVO Abstract 3412) and Bruch’s membrane do not change during the experimental period. The “choroidal thickness change” obtained using this approach does not represent a completely precise and accurate estimate of true tissue thickness, however it is sufficient for estimating the extent to which choroidal thickness alteration contributes to vitreous chamber depth changes, which was subject to the influence of choroidal thinning/thickening.
2.6. Statistical analysis
We compared the time course of refractive development between the red-light groups and the corresponding white light groups using a mixed effect model. In comparison to variance-based methods, such as repeated-measures ANOVA, a mixed effect model does not require data to be obtained at symmetric time points, and can handle heterogeneity of variance between groups (Rabe-Hesketh & Skrondal, 2012). Refractive error and ocular dimensions at the end of the experiment were also compared using two-tail, paired (for between-eye comparisons) and unpaired t-tests (for between-group comparisons). One-sample t-tests were used to examine for non-zero changes.
All statistical procedures were performed using STATA (17SE, STATA CORP, College Station, TX) at an α = 0.05.
3. Results
3.1. Red light prevented the maintenance of emmetropia
Figure 2 illustrates the response to red light in eyes that have otherwise unrestricted visual experience. As seen in Fig.2A, the RL-Control eyes (red circles) developed progressive hyperopic changes that were not seen in WL-Control eyes (black circles) (p<0.05). The mean refraction of the RL-Controls at the end of the experiment was +3.0 ± 0.7D, about +2D more hyperopic than the WL-Controls (+1.0 ± 0.2D, p = 0.02). The untreated eyes in the RL-5D and RLFD groups also developed hyperopia. The time course of refractive development in the no-lens eyes of the RL-5D group and the no-diffuser eyes of RLFD group were both similar to the RL-Control and differed significantly from the WL-Control (mixed effect model analysis, p < 0.05). As shown Fig.2B, the development of hyperopia in red light was accompanied by a synchronized reduction in vitreous chamber depth. Fig.2C illustrates the change in the choroidal thickness over time. Although there was a trend of initial choroidal thickening in red light, most notably in the RL-Control group, the differences were not statistically significant.
3.2. Red light reduced negative-lens-induced myopia
Figure 3 illustrates the refractions as a function of time for the lens-treated and no-lens eyes in both red (A) and white (B) light. The rate of lens-treated eye myopia development was significantly slower in red light than in white light (mixed effect model analysis, p < 0.01). Lens-treated eyes in the RL-5D group were less myopic (−1.1 ± 0.9D) than those of the WL-5D group at the end of the experiment (−3.8 ± 0.3D, p = 0.007). The no-lens eyes in the RL-5D group appeared more hyperopic than that of the WL-5D group, but the difference was not significant (+2.5 ± 0.5D vs. +1.5 ± 0.3D, p = 0.051). Despite the reduced myopic change in the lens-treated eyes and the relative hyperopic change in the no-lens eyes, the interocular difference in refraction remained statistically significant in the RL-5D group (p = 0.001).
3.3. Red light prevented form-deprivation myopia
Figure 4 illustrates the refractions as a function of time for the diffuser-treated and no-diffuser eyes in both red (A) and white (B) light. A mixed effect model analysis showed that diffuser-treated-eye myopia development was significantly slower in the RLFD group (p<0.0001). These eyes were near emmetropic at the end of the experiment (−0.3 ± 0.6D) and less hyperopic than their no-diffuser fellow eyes (+2.3 ± 0.3D, p = 0.003). Both diffuser-treated and no-diffuser eyes of the RLFD tree shrews were less myopic/relatively hyperopic in comparison to those of the WLFD tree shrews (−5.4 ± 0.7D and +1.0 ± 0.2D for diffuser-treated and no-diffuser eyes, respectively, p < 0.05).
3.4. Refraction changes were correlated with vitreous chamber depth changes
We did not observe alterations in anterior chamber depth and lens thickness that could explain the refractive differences (see Supplementary Material A for a summary table of refractive error and ocular biometry). The less myopic/more hyperopic refractive state in the treated eyes of goggle-wearing RL tree shrews were consistent with the shorter vitreous chamber depth (RL vs. WL-5D, 2.81 ± 0.03mm vs. 2.98 ± 0.03mm, p = 0.02; RL vs. WLFD, 2.84 ± 0.03mm vs. 3.00 ± 0.03mm, p = 0.005). In addition, there was a strong, inverse correlation between refractive error changes and vitreous chamber depth changes (Pearson correlation, r = −0.86, p < 0.001. Figure 5), but not choroidal thickness changes (r = −0.19, p = 0.34).
4. Discussion
We found that red light reduced lens-induced myopia and prevented form-deprivation myopia. We have previously shown that red light induces hyperopic changes in tree shrews (Gawne, Siegwart, et al., 2017; Gawne, Ward, et al., 2017), and here also show that red light induces hyperopia in the untreated eyes of goggle-wearing tree shrews. The refractive alterations in the goggle-wearing tree shrews were correlated with changes in vitreous chamber depth.
Our observation that rearing in ambient red light promoted relative hyperopic changes in control animals and in the untreated eyes of goggle-wearing animals, an effect that prevented the development of normal emmetropization pattern, agreed with our previous observations (Gawne, Siegwart, et al., 2017; Gawne, Ward, et al., 2017) and the observations in rhesus monkeys (Hung et al., 2018; Smith III et al., 2015). However, it did not corroborate with the study of Liu et al, in which rhesus monkeys underwent extended red-light rearing (610 ± 10 nm, 50 – 407 days of age) became 1.43D more myopic than those reared in white light, in large part attributed to two subjects that developed absolute myopia (mean ± SD: −2.75 ± 0.35D while other red-light monkeys remained mild hyperopic) (Liu et al., 2014). Except for the Liu et al. study, the red light effects observed in tree shrews and rhesus monkeys were opposite to those reported for chicks (Foulds et al., 2013; Seidemann & Schaeffel, 2002) and guinea pigs (Jiang et al., 2014; Liu et al., 2011; Long et al., 2009; Wang et al., 2011) in which long wavelength light induced myopic instead of hyperopic changes. The reason for the between-species discrepancy in red-light effect remains unclear.
The two main findings in our lens experiment were that both eyes of RL-5D tree shrews became less myopic/more hyperopic than the WL-5D tree shrews, and that a lens-induced myopia and interocular difference in refraction still developed. The first aspect was consistent with the observations that tree shrews with otherwise unrestricted vision developed hyperopia in red light (Gawne et al., 2017), and was similar to those observed in lens-wearing rhesus monkeys, which developed progressive hyperopia in the lens-treated eye in red light regardless of the sign of the treatment lenses (Hung et al., 2018; Smith III et al., 2015). These findings suggest that rearing in red light promotes relative axial hyperopic changes both for primates and for (at least some) mammals closely related to primates. The second aspect, on the other hand, did not corroborate those in rhesus monkeys, in which red light also eliminated lens-induced interocular differences in refraction, which suggested that red light produced an erroneous myopic signal that overwrote the lens-imposed hyperopic defocus (Hung et al., 2018). However, it agreed with early observations that eyes could respond to lenses in narrowband light (Norton et al., 2021; Rohrer et al., 1992; Schaeffel & Howland, 1991; Seidemann & Schaeffel, 2002; Wildsoet et al., 1993). For example, Rohrer et al. found that rearing chicks in narrowband red light did not prevent lens compensation (Rohrer et al., 1992). Implicated in these previous and our current observations is that eyes could determine defocus when chromatic cues are weak or unavailable (Gawne et al., 2021; Gawne & Norton, 2020), possibly by maximizing the luminance contrast of the retinal image (i.e., “wavelength defocus” or synonymously “wavelength target”) (Gawne, Ward, et al., 2017; Rucker, 2013), although “non-chromatic” cues by themselves do not appear to be sufficiently strong to allow accurate compensation to occur. However, given that chromatic cues were absent and the visual image was compromised by defocus, one should not rule out that negative lenses triggered “form-deprivation” myopia in our RL-5D animals.
Fewer studies have examined the effects of narrowband light on form-deprivation myopia. For the chicks, narrowband “ultra violet” (375 nm, half bandwidth: 5 nm) (Torii et al., 2017) and blue light (465 nm, half bandwidth 15 nm) (Wang et al., 2018), instead of red light, also reduced diffuser-induced myopia. A previous study in rhesus monkeys showed that red light causes progressive hyperopic changes in the diffuser-treated eyes, which ended up equally hyperopic as their no-diffuser eyes (Hung et al., 2018). In the present study, we showed that red light prevented form-deprivation myopia in the diffuser-treated eyes, but these eyes remained less hyperopic than the no-diffuser eyes. Using the no-diffuser eyes as reference, red light appeared to have a stronger effect in the diffuser-treated eyes for rhesus monkeys than for tree shrews. The reason for this difference is unclear.
As mentioned previously, it has been hypothesized that eyes might increase their axial elongation rate in long wavelength narrowband light to maximize the luminance contrast of the quasi-monochromatic retinal image (Rucker 2013). In this respect, our finding that red light protected tree shrews against form-deprivation myopia joined the previous studies in chicks (Torii et al., 2017; Wang et al., 2018) and rhesus monkeys (Hung et al., 2018), suggesting that narrowband lights might influence refractive development through non-visual ocular mechanisms, such as relieving scleral hypoxia (Wu et al., 2018) or directly affecting mitochondrial functioning (Eells et al., 2004; Karu, 2008). A consideration here is that effects of this kind could potentially synergize with (such as red light for the tree shrews) or antagonize the effects of LCA (such as blue light for the chicks), making it difficult to identify their respective contribution. Further studies are required to elucidate how narrowband light influences refractive development under different visual conditions.
The role of choroidal thickness in the refractive effect under narrowband light varied between studies. In the current study, choroidal thickness changes did not appear to be a significant contributor to the final refractive state. However, previous studies have found that narrowband red light induced significant choroidal thickening in tree shrews (Gawne et al., 2017) and rhesus monkeys (40 – 60 μm) (Hung et al., 2018), which might contribute to red light-induced hyperopia. For the chicks, although there was no direct measure of choroidal thickness, the reported axial length differences induced by narrowband light (Torii et al., 2017; Wang et al., 2018) were within the reported range of vision-induced choroidal thickness alterations (Wildsoet & Wallman, 1995), therefore a prominent role of choroidal thickness in final refractive state cannot be ruled out. Based on the current evidence, the relationship between visual environment-induced choroidal thickness changes and refractive development remained unclear (for a review, see Ostrin et al., 2023). Further studies are required to understand the role and the underlying mechanisms of choroidal thickening in narrowband light.
Melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs) play an important role in determining pupillary diameter (Hung et al., 2018; Keenan et al., 2016), pupillary response (Chen et al., 2011; Gamlin et al., 2007; Güler et al., 2008; Lou et al., 2021; Sand et al., 2012), photoentrainment (Berson et al., 2002; Chen et al., 2011), and possibly refractive development (Chakraborty et al., 2022; Liu et al., 2022. For reviews, see Nickla et al., 1998, and Chakraborty et al., 2018). ipRGCs are also present in the tree shrew retina (Johnson et al., 2019). In our study, the direct activation level of ipRGCs are expected to be low in red light due to their short-wavelength-tuned spectral sensitivity (λmax ≈479nm) (Bailes & Lucas, 2013). Khanal et al. previously showed that rearing tree shrews under diurnal narrowband red light did not significantly alter circadian activity rhythm, nor did it prevent light-entrained phase-advance in circadian activity pattern (Khanal et al., 2021. Optom Vis Sci, program#: 210006), suggesting that some circadian features are not significantly affected by a low direct ipRGC activation level (although ipRGCs can be activated by inputs from cones). However, we cannot exclude that the very low ipRGC activation level in red light contributed to our refractive observations through its roles in other circadian biological rhythms, especially those that are more closely related to refractive development (e.g., ocular circadian rhythm) (Nickla et al., 1998).
5. Limitations
We used small sample sizes due to the limited abundance of tree shrews, which might affect the statistical power of our study. In addition, we were unable to measure the corneal radii of tree shrews.
6. Conclusions and implications
We have demonstrated here that narrowband red light can significantly reduce myopia created by either negative lens wearing or form deprivation in tree shrews, diurnal mammals closely related to primates. The observed effectiveness of red light against form-deprivation myopia joins the existing literature in other species and suggested that the refractive effects of narrowband light might be mediated by non-visual ocular mechanisms. We also showed that the tree shrew’s emmetropization mechanism can still respond to visual cues in narrow-band red light, where chromatic cues are not available. It is possible that this is just a reaction to the reduced image quality in both minus lens and form deprivation. Regardless, even though emmetropization can react to changes in the image using non-chromatic visual cues, these non-chromatic cues by themselves did not appear sufficient for eyes to accurately achieve sharp focus.
Recent clinical trials found that Repeated Low-Level Red Light (RLRL) slowed the progression of myopia in children (Dong et al., 2023; Jiang et al., 2021; Zhou et al., 2022. For a review, see Tang et al., 2023). Although these studies showed the potential of red light for myopia treatment, little is known about RLRL’s therapeutic parameters, underlying mechanism, and particularly long-term safety. Of note, it has been reported that RLRL therapy caused structural damage in the fovea and functional loss in the macular and paramacular region (Liu et al., 2023). Because in these studies red light was irradiated into the eyes via a collimated beam, it is plausible that the local light intensities during RLRL therapy are much greater than previous red light experiments using rhesus monkeys and tree shrews. In this respect, tree shrews and rhesus monkeys, both of which respond to red light by developing hyperopic changes, could be especially appropriate as animal models for such investigations.
Supplementary Material
Figure 1.
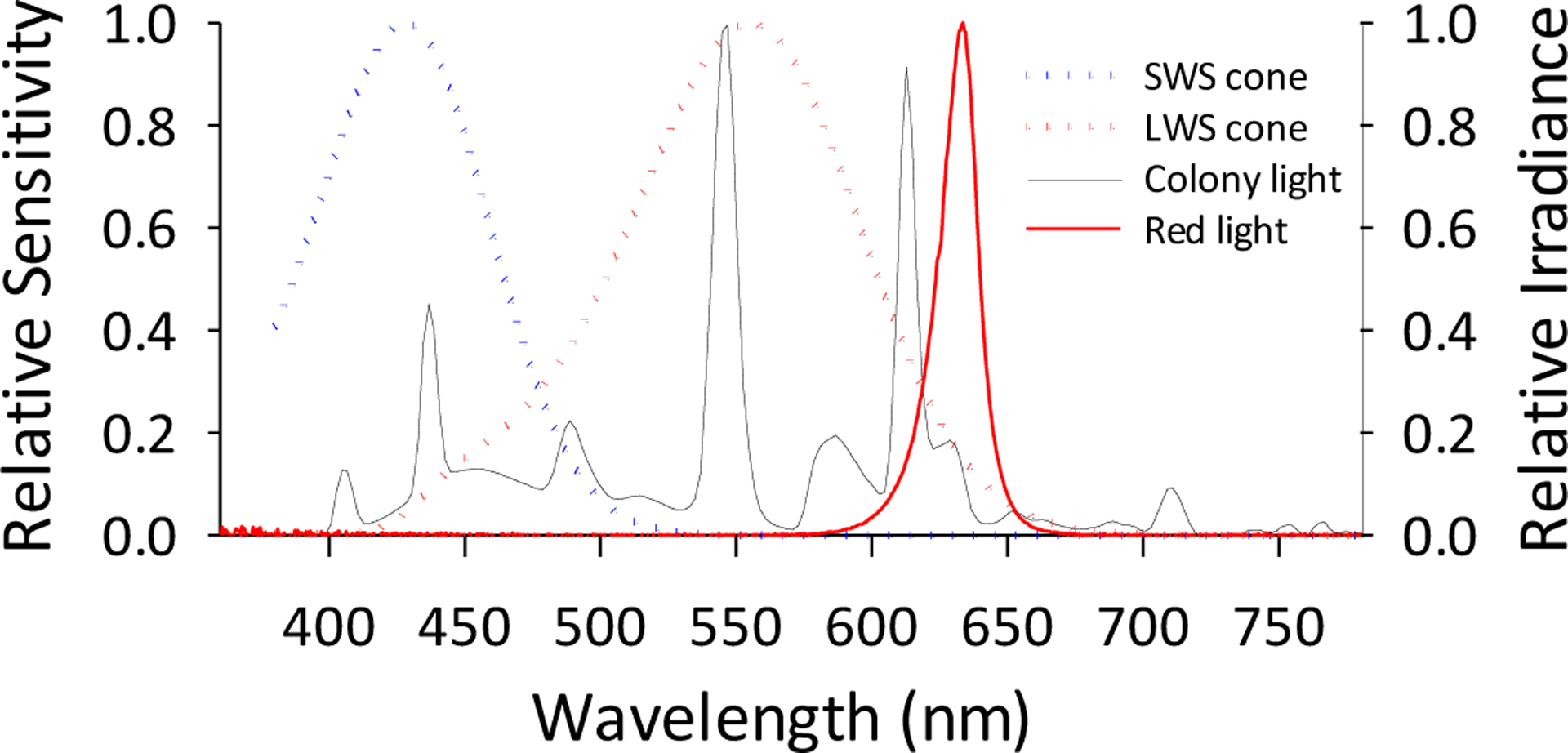
Comparing tree shrew photoreceptor spectral sensitivity with the spectral emission profiles of our experimental lighting. In this figure, the normalized spectral sensitivity of the tree shrew SWS cone (blue dotted line) and LWS cone (red dotted line) (Petry & Harosi, 1990) are plotted along with the normalized spectral irradiance of the white light (black solid line) and narrowband red light (red LED, shown as solid red line, 634 ± 10 nm). Normalized irradiances were based on the data measured in the cage (STS-VIS miniature spectrometer, Ocean Insight, Orlando FL). This figure shows that the red light in our study was at the far end of the LWS sensitivity range and out of the sensitivity range of SWS cones, thereby selectively stimulating tree shrew LWS-cones.
Highlights.
Narrowband red light reduced lens-induced and form-deprivation myopia in tree shrews
The vitreous chamber became shorter, but choroidal thickness did not differ
Red light’s ability to retard myopia and promote hyperopia in tree shrews appear correlated
Acknowledgements
The authors acknowledge the professional assistance and insightful input of Dr. Thomas Norton.
The authors acknowledge the technical assistance of Russell Veal and Johanna Henry.
Funding support:
National Eye Institute R01EY028578 (TJG) and P30EY003039 (UAB core) Commercial relationships disclosure: Z. She: None; A.H. Ward: None; T.J. Gawne: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
TJG has provisional patents for myopia control treatments using alternative optical methods.
Reference
- Bailes HJ, & Lucas RJ (2013). Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm) supporting activation of Gq/11 and Gi/o signalling cascades. Proceedings of the Royal Society B: Biological Sciences, 280(1759), 20122987. 10.1098/rspb.2012.2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barathi VA, Boopathi VG, Yap EPH, & Beuerman RW (2008). Two models of experimental myopia in the mouse. Vision Research. 10.1016/j.visres.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, & Takao M (2002). Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science, 295(5557), 1070–1073. 10.1126/science.1067262 [DOI] [PubMed] [Google Scholar]
- Bowmaker JK, & Dartnall HJ (1980). Visual pigments of rods and cones in a human retina. The Journal of Physiology, 298(1), 501–511. 10.1113/jphysiol.1980.sp013097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker JK, Dartnall HJ, & Mollon JD (1980). Microspectrophotometric demonstration of four classes of photoreceptor in an old world primate, Macaca fascicularis. The Journal of Physiology, 298(1), 131–143. 10.1113/jphysiol.1980.sp013071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Yang E-B, Su J-J, Li Y, & Chow P (2003). The tree shrews: Adjuncts and alternatives to primates as models for biomedical research. Journal of Medical Primatology, 32(3), 123–130. 10.1034/j.1600-0684.2003.00022.x [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Landis EG, Mazade R, Yang V, Strickland R, Hattar S, Stone RA, Iuvone PM, & Pardue MT (2022). Melanopsin modulates refractive development and myopia. Experimental Eye Research, 214, 108866. 10.1016/j.exer.2021.108866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Ostrin LA, Nickla DL, Iuvone MP, Pardue MT, & Stone RA (2018). Circadian rhythms, refractive development, and myopia. Ophthalmic and Physiological Optics, 38(3), 217–245. 10.1111/opo.12453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-K, Badea TC, & Hattar S (2011). Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature, 476(7358), Article 7358. 10.1038/nature10206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Zhu Z, Xu H, & He M (2023). Myopia Control Effect of Repeated Low-Level Red-Light Therapy in Chinese Children: A Randomized, Double-Blind, Controlled Clinical Trial. Ophthalmology, 130(2), 198–204. 10.1016/j.ophtha.2022.08.024 [DOI] [PubMed] [Google Scholar]
- Eells JT, Wong-Riley MTT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, & Whelan HT (2004). Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion, 4(5), 559–567. 10.1016/j.mito.2004.07.033 [DOI] [PubMed] [Google Scholar]
- El Hamdaoui M, Gann DW, Norton TT, & Grytz R (2019). Matching the LenStar optical biometer to A-Scan ultrasonography for use in small animal eyes with application to tree shrews. Experimental Eye Research, 180, 250–259. 10.1016/j.exer.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hamdaoui M, Levy AM, Gaonkar M, Gawne TJ, Girkin CA, Samuels BC, & Grytz R (2021). Effect of Scleral Crosslinking Using Multiple Doses of Genipin on Experimental Progressive Myopia in Tree Shrews. Translational Vision Science & Technology, 10(5), 1–1. 10.1167/TVST.10.5.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds WS, Barathi VA, & Luu CD (2013). Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Investigative Ophthalmology and Visual Science. 10.1167/iovs.13-12476 [DOI] [PubMed] [Google Scholar]
- Gamlin PDR, McDougal DH, Pokorny J, Smith VC, Yau K-W, & Dacey DM (2007). Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Research, 47(7), 946–954. 10.1016/j.visres.2006.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Grytz R, & Norton TT (2021). How chromatic cues can guide human eye growth to achieve good focus. Journal of Vision, 21(5), 11. 10.1167/jov.21.5.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, & Norton TT (2020). An opponent dual-detector spectral drive model of emmetropization. Vision Research, 173, 7–20. 10.1016/j.visres.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, She Z, & Norton TT (2022). Chromatically simulated myopic blur counteracts a myopiagenic environment. Experimental Eye Research, 222, 109187. 10.1016/j.exer.2022.109187 [DOI] [PubMed] [Google Scholar]
- Gawne TJ, Siegwart JT, Ward AH, Norton TT, Siegwart JT Jr., Ward AH, & Norton TT (2017). The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Experimental Eye Research, 155, 75–84. 10.1016/j.exer.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Ward AH, & Norton TT (2017). Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Research. 10.1016/j.visres.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao H-W, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau K-W, & Hattar S (2008). Melanopsin cells are the principal conduits for rod–cone input to non-image-forming vision. Nature, 453(7191), Article 7191. 10.1038/nature06829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Frost MR, Siegwart JT, & Norton TT (2014). Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Experimental Eye Research, 123, 56–71. 10.1016/J.EXER.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett MHC, & McFadden SA (2006). Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Research, 46(1–2), 267–283. 10.1016/j.visres.2005.06.036 [DOI] [PubMed] [Google Scholar]
- Howlett MHC, & McFadden SA (2009). Spectacle lens compensation in the pigmented guinea pig. Vision Research. 10.1016/j.visres.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Hung L-F, Arumugam B, She Z, Ostrin L, & Smith III EL (2018). Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Experimental Eye Research, 176(June 2018), 147–160. 10.1016/j.exer.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L-F, Crawford MLJ, & Smith III EL (1995). Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Medicine, 1(8), 765. 10.1038/nm0895-761 [DOI] [PubMed] [Google Scholar]
- Jiang L, Zhang S, Schaeffel F, Xiong S, Zheng Y, Zhou X, Lu F, & Qu J (2014). Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus). Vision Research, 94, 24–32. 10.1016/j.visres.2013.10.020 [DOI] [PubMed] [Google Scholar]
- Jiang X, Kurihara T, Kunimi H, Miyauchi M, Ikeda SI, Mori K, Tsubota KK, Torii H, & Tsubota KK (2018). A highly efficient murine model of experimental myopia. Scientific Reports. 10.1038/s41598-018-20272-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zhu Z, Tan X, Kong X, Zhong H, Zhang J, Xiong R, Yuan Y, Zeng J, Morgan IG, & He M (2021). Effect of Repeated Low-Level Red-Light Therapy for Myopia Control in Children: A Multicenter Randomized Controlled Trial. Ophthalmology. 10.1016/j.ophtha.2021.11.023 [DOI] [PubMed] [Google Scholar]
- Johnson EN, Westbrook T, Shayesteh R, Chen EL, Schumacher JW, Fitzpatrick D, & Field GD (2019). Distribution and diversity of intrinsically photosensitive retinal ganglion cells in tree shrew. Journal of Comparative Neurology, 527(1), 328–344. 10.1002/cne.24377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu TI (2008). Mitochondrial Signaling in Mammalian Cells Activated by Red and Near-IR Radiation. Photochemistry and Photobiology, 84(5), 1091–1099. 10.1111/j.1751-1097.2008.00394.x [DOI] [PubMed] [Google Scholar]
- Keenan WT, Rupp AC, Ross RA, Somasundaram P, Hiriyanna S, Wu Z, Badea TC, Robinson PR, Lowell BB, & Hattar SS (2016). A visual circuit uses complementary mechanisms to support transient and sustained pupil constriction. ELife, 5, e15392. 10.7554/eLife.15392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal S, Norton TT, & Gawne TJ (2023). Limited bandwidth short-wavelength light produces slowly-developing myopia in tree shrews similar to human juvenile-onset myopia. Vision Research, 204, 108161. 10.1016/j.visres.2022.108161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A-L, Liu Y-F, Wang G, Shao Y-Q, Yu C-X, Yang Z, Zhou Z-R, Han X, Gong X, Qian K-W, Wang L-Q, Ma Y-Y, Zhong Y-M, Weng S-J, & Yang X-L (2022). The role of ipRGCs in ocular growth and myopia development. Science Advances, 8(23), eabm9027. 10.1126/sciadv.abm9027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yang Y, Guo J, Peng J, & Zhao P (2023). Retinal Damage After Repeated Low-level Red-Light Laser Exposure. JAMA Ophthalmology. 10.1001/jamaophthalmol.2023.1548 [DOI] [PubMed] [Google Scholar]
- Liu R, Hu M, He JC, Zhou XT, Dai JH, Qu XM, Liu H, & Chu RY (2014). The effects of monochromatic illumination on early eye development in rhesus monkeys. Investigative Ophthalmology and Visual Science. 10.1167/iovs.13-12276 [DOI] [PubMed] [Google Scholar]
- Liu R, Qian YF, He JC, Hu M, Zhou XT, Dai JH, Qu XM, & Chu RY (2011). Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Experimental Eye Research, 92(6), 447–453. 10.1016/j.exer.2011.03.003 [DOI] [PubMed] [Google Scholar]
- Long Q, Chen D, & Chu RY (2009). Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutaneous and Ocular Toxicology, 28(4), 176–180. 10.3109/15569520903178364 [DOI] [PubMed] [Google Scholar]
- Lou L, Arumugam B, Hung L-F, She Z, Beach KM, Smith EL, & Ostrin LA (2021). Long-Term Narrowband Lighting Influences Activity but Not Intrinsically Photosensitive Retinal Ganglion Cell-Driven Pupil Responses. Frontiers in Physiology, 12, 711525. 10.3389/fphys.2021.711525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlapally S, & McBrien NA (2008). The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. Journal of Vision. 10.1167/8.3.1 [DOI] [PubMed] [Google Scholar]
- Müller B, & Peichl L (1989). Topography of cones and rods in the tree shrew retina. Journal of Comparative Neurology, 282(4), 581–594. 10.1002/cne.902820409 [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, & Wallman J (1998). Visual Influences on Diurnal Rhythms in Ocular Length and Choroidal Thickness in Chick Eyes. Experimental Eye Research, 66(2), 163–181. 10.1006/exer.1997.0420 [DOI] [PubMed] [Google Scholar]
- Norton TT, Khanal S, & Gawne TJ (2021). Tree shrews do not maintain emmetropia in initially-focused narrow-band cyan light. Experimental Eye Research, 206, 108525. 10.1016/j.exer.2021.108525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, & Siegwart JT Jr. (2013). Light levels, refractive development, and myopia–a speculative review. Experimental Eye Research, 114(205), 48–57. 10.1016/j.exer.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Wu WW, & Siegwart JT Jr (2003). Refractive State of Tree Shrew Eyes Measured with Cortical Visual Evoked Potentials. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 80(9), 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin LA, Harb E, Nickla DL, Read SA, Alonso-Caneiro D, Schroedl F, Kaser-Eichberger A, Zhou X, & Wildsoet CF (2023). IMI—The Dynamic Choroid: New Insights, Challenges, and Potential Significance for Human Myopia. Investigative Ophthalmology & Visual Science, 64(6), 4. 10.1167/iovs.64.6.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue MT, Stone RA, & Iuvone MP (2013). Investigating mechanisms of myopia in mice. Experimental Eye Research, 114, 96–105. 10.1016/j.exer.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry HM, & Harosi FI (1990). Visual pigments of the tree shrew (Tupaia belangeri) and greater galago (Galago crassicaudatus): A microspectrophotometric investigation. Vision Research, 30(6), 839–851. 10.1016/0042-6989(90)90053-N [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung L-F, Kee C, Ramamirtham R, & Smith III EL (2004). Recovery from form-deprivation myopia in rhesus monkeys. Investigative Ophthalmology and Visual Science, 45(10), 3361–3372. 10.1167/iovs.04-0080 [DOI] [PubMed] [Google Scholar]
- Rabe-Hesketh S, & Skrondal A (2012). Multilevel and longitudinal modeling using Stata—Volume I: Continious Responses. In Stata Press. [Google Scholar]
- Rohrer B, Schaeffel F, & Zrenner E (1992). Longitudinal chromatic aberration and emmetropization: Results from the chicken eye. The Journal of Physiology. 10.1113/jphysiol.1992.sp019090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker FJ (2013). The role of luminance and chromatic cues in emmetropisation. Ophthalmic and Physiological Optics. 10.1111/opo.12050 [DOI] [PubMed] [Google Scholar]
- Sajdak BS, Salmon AE, Cava JA, Allen KP, Freling S, Ramamirtham R, Norton TT, Roorda A, & Carroll J (2019). Noninvasive imaging of the tree shrew eye: Wavefront analysis and retinal imaging with correlative histology. Experimental Eye Research, 185, 107683. 10.1016/j.exer.2019.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand A, Schmidt TM, & Kofuji P (2012). Diverse types of ganglion cell photoreceptors in the mammalian retina. Progress in Retinal and Eye Research, 31(4), 287–302. 10.1016/j.preteyeres.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaridurg P, Berntsen DA, Bullimore MA, Cho P, Flitcroft I, Gawne TJ, Gifford KL, Jong M, Kang P, Ostrin LA, Santodomingo-Rubido J, Wildsoet C, & Wolffsohn JS (2023). IMI 2023 Digest. Investigative Ophthalmology & Visual Science, 64(6), 7. 10.1167/iovs.64.6.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffel F, & Howland HC (1991). Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Research, 31(4), 717–734. 10.1016/0042-6989(91)90011-S [DOI] [PubMed] [Google Scholar]
- Seidemann A, & Schaeffel F (2002). Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Research. 10.1016/S0042-6989(02)00262-6 [DOI] [PubMed] [Google Scholar]
- Siegwart JT Jr., & Norton TT (1994). Goggles for controlling the visual environment of small animals. Laboratory Animal Science, 44(3), 292–294. [PubMed] [Google Scholar]
- Siegwart JT Jr., & Norton TT (1998). The susceptible period for deprivation-induced myopia in tree shrew. Vision Research. 10.1016/S0042-6989(98)00053-4 [DOI] [PubMed] [Google Scholar]
- Siegwart JT Jr., & Norton TT (2010). Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Experimental Eye Research, 91(5), 660–669. 10.1016/j.exer.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith III EL, & Hung L-F (2000). Form-deprivation myopia in monkeys is a graded phenomenon. Vision Research, 40(4), 371–381. 10.1016/S0042-6989(99)00184-4 [DOI] [PubMed] [Google Scholar]
- Smith EL III, Hung L-F, Arumugam B, Holden BA, Neitz M, Neitz J, Smith EL, Hung LF, Arumugam B, Holden BA, Neitz M, & Neitz J (2015). Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Investigative Ophthalmology and Visual Science, 56(11), 6490–6500. 10.1167/iovs.15-17025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatczak B, & Schaeffel F (2022). Myopia: Why the retina stops inhibiting eye growth. Scientific Reports, 12(1), Article 1. 10.1038/s41598-022-26323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Liao Y, Yan N, Dereje SB, Wang J, Luo Y, Wang Y, Zhou W, Wang X, & Wang W (2023). Efficacy of repeated low-level red-light therapy for slowing the progression of childhood myopia: A systematic review and meta-analysis. American Journal of Ophthalmology. 10.1016/j.ajo.2023.03.036 [DOI] [PubMed] [Google Scholar]
- Tejedor J, & de la Villa P (2003). Refractive Changes Induced by Form Deprivation in the Mouse Eye. Investigative Ophthalmology & Visual Science, 44(1), 32–36. 10.1167/iovs.01-1171 [DOI] [PubMed] [Google Scholar]
- Tkatchenko TV, Shen Y, & Tkatchenko AV (2010). Mouse experimental myopia has features of primate myopia. Investigative Ophthalmology and Visual Science, 51(3), 1297–1303. 10.1167/iovs.09-4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii H, Kurihara T, Seko Y, Negishi K, Ohnuma K, Inaba T, Kawashima M, Jiang X, Kondo S, Miyauchi M, Miwa Y, Katada Y, Mori K, Kato K, Tsubota K, Goto H, Oda M, Hatori M, & Tsubota K (2017). Violet Light Exposure Can Be a Preventive Strategy Against Myopia Progression. EBioMedicine, 15, 210–219. 10.1016/j.ebiom.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Totonelly K, & Harb E (2009). Accommodation and the Visual Regulation of Refractive State in Marmosets. Optom Vis Sci. 10.1097/OPX.0b013e318194072e.Accommodation [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, & Adams IJ (1987). Developmental aspects of experimental myopia in chicks: Susceptibility, recovery and relation to emmetropization. Vision Research. 10.1016/0042-6989(87)90027-7 [DOI] [PubMed] [Google Scholar]
- Wang F, Zhou J, Lu Y, & Chu RY (2011). Effects of 530 nm green light on refractive status, melatonin, MT1 receptor, and melanopsin in the guinea pig. Current Eye Research, 36(2), 103–111. 10.3109/02713683.2010.526750 [DOI] [PubMed] [Google Scholar]
- Wang M, Schaeffel F, Jiang B, & Feldkaemper M (2018). Effects of Light of Different Spectral Composition on Refractive Development and Retinal Dopamine in Chicks. Investigative Ophthalmology & Visual Science, 59(11), 4413–4424. 10.1167/iovs.18-23880 [DOI] [PubMed] [Google Scholar]
- Wiesel TN, & Raviola E (1977). Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature, 266(5597), Article 5597. 10.1038/266066a0 [DOI] [PubMed] [Google Scholar]
- Wiesel TN, & Raviola E (1979). Increase in axial length of the macaque monkey eye after corneal opacification. Investigative Ophthalmology and Visual Science, 18(12), 1232–1236. [PubMed] [Google Scholar]
- Wildsoet CF, Howland HC, Falconer S, & Dick K (1993). Chromatic aberration and accommodation: Their role in emmetropization in the chick. Vision Research, 33(12), 1593–1603. 10.1016/0042-6989(93)90026-s [DOI] [PubMed] [Google Scholar]
- Wildsoet C, & Wallman J (1995). Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Research, 35(9), 1175–1194. 10.1016/0042-6989(94)00233-C [DOI] [PubMed] [Google Scholar]
- Wu H, Chen W, Zhao F, Zhou Q, Reinach PS, Deng L, Ma L, Luo S, Srinivasalu N, Pan M, Hu Y, Pei X, Sun J, Ren R, Xiong Y, Zhou Z, Zhang S, Tian G, Fang J, … Zhou X (2018). Scleral hypoxia is a target for myopia control. Proceedings of the National Academy of Sciences, 115(30), E7091–E7100. 10.1073/pnas.1721443115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Xing C, Qiang W, Hua C, & Tong L (2022). Low-intensity, long-wavelength red light slows the progression of myopia in children: An Eastern China-based cohort. Ophthalmic and Physiological Optics, 42(2), 335–344. 10.1111/opo.12939 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


