Abstract
Diesel exhaust has long been of health concern due to established toxicity including carcinogenicity in humans. However, the precise components of diesel engine emissions that drive carcinogenesis are still unclear. Limited work has suggested that nitrated polycyclic aromatic hydrocarbons (NPAHs) such as 1-nitropyrene and 2-nitrofluorene may be more abundant in diesel exhaust. The present study aimed to examine whether urinary amino metabolites of these NPAHs were associated with high levels of diesel engine emissions and urinary mutagenicity in a group of highly exposed workers including both smokers and nonsmokers. Spot urine samples were collected immediately following a standard work shift from each of the 54 diesel engine testers and 55 non-tester controls for the analysis of five amino metabolites of NPAHs, and cotinine (a biomarker of tobacco smoke exposure) using liquid chromatography–mass spectrometry. An overnight urine sample was collected in a subgroup of non-smoking participants for mutagenicity analysis using strain YG1041 in the Salmonella (Ames) mutagenicity assay. Personal exposure to fine particles (PM2.5) and more-diesel-specific constituents (elemental carbon and soot) was assessed for the engine testers by measuring breathing-zone concentrations repeatedly over several full work shifts. Results showed that it was 12.8 times more likely to detect 1-aminopyrene and 2.9 times more likely to detect 2-aminofluorene in the engine testers than in unexposed controls. Urinary concentrations of 1-aminopyrene were significantly higher in engine testers (p<0.001), and strongly correlated with soot and elemental carbon exposure as well as mutagenicity tested in strain YG1041 with metabolic activation (p<0.001). Smoking did not affect 1-aminopyrene concentrations and 1-aminopyrene relationships with diesel exposure. In contrast, both engine emissions and smoking affected 2-aminofluorene concentrations. The results confirm that urinary 1-aminopyrene may serve as an exposure biomarker for diesel engine emissions and associated mutagenicity.
Keywords: diesel exhaust, biomonitoring, inhalation exposure, Nitro-PAHs, cigarette smoking, amino-PAHs
Introduction
Diesel exhaust, as a whole, has been classified as carcinogenic to humans (Group 1) by IARC (IARC, 2014). Although the nitroarenes are recognized as a primary class of mutagens and carcinogens in diesel exhaust, further understanding is needed as to which compounds drive carcinogenesis. Diesel exhaust particles and elemental carbon (EC) have been used as a proxy of diesel exhaust in numerous toxicologic studies elucidating the biological mechanisms by which diesel exhaust exposure causes adverse health effects (Weitekamp et al., 2020). There is a long-standing interest in quantifying diesel exhaust exposure in order to assess the health risk attributable to this specific source of air pollution. This has motivated various research efforts attempting to distinguish diesel exhaust exposure from the air pollution mixture to which people are exposed in most urban areas. The effort has focused mainly on finding compounds present uniquely in diesel engine emissions. Certain nitrated polycyclic aromatic hydrocarbons (NPAHs) have been identified as a potential source signature for diesel exhaust as well as the likely key carcinogenic component of diesel engine exhaust driving lung cancer risk (Ross et al., 2015).
Sources of NPAHs in the atmosphere include thermal nitration of polycyclic aromatic hydrocarbons (PAHs) during combustion and atmospheric formation from the parent PAHs by hydroxyl radical reactions in the presence of sun light and by nitrate radical reactions in the absence of sun light (Gbeddy et al., 2020; Lin et al., 2016). Certain specific NPAHs (e.g., 1-nitropyrene) present in direct emissions are distinct from those (e.g., 2-nitropyrene) resulting from atmospheric reactions, reflecting their differing formation mechanisms. This has been demonstrated in the greater Los Angeles area in times when both diesel traffic and photochemical reactions were present (Reisen & Arey, 2005). Among reported measurements, elevated concentrations of several NPAHs (e.g. 1-nitropyrene, 2-nitrofluorene, 9-nitrophenanthrene, and/or 9-nitrofluorathene) were found in roadway tunnels (Dimashki et al., 2000), the ambient air of cities with a high diesel traffic density (Feilberg et al., 2001), and underground mines where diesel engines were in use (Scheepers et al., 2003). Among these NPAHs, 1-nitropyrene was found to be predominant in conventional diesel engine exhaust (Manzano et al., 2013). In contrast, 2-nitrofluorene and 1-nitropyrene were most and second most abundant in emissions of contemporary European diesel/biodiesel engines (Borillo et al., 2018).
Although concentrations of diesel-specific NPAHs in the atmosphere may be used to conduct source appointments, thereby estimating exposures attributable to diesel exhaust (Bamford et al., 2003; Zwirner-Baier & Neumann, 1999), the measurement of NPAHs has been hampered by technical challenges due to low NPAH concentrations and sampling artifacts (Dimashki et al., 2000; Lies et al., 1986; Reisen & Arey, 2005). Even at relatively higher concentrations, measuring NPAHs in the air requires a large sampling volume and high method sensitivity. Biomonitoring, on the other hand, may provide an alternative approach if a biomarker can capture diesel exhaust exposure with some degree of specificity.
Within the human body, NPAHs are metabolized either through ring oxidation or nitro reduction pathways (Figure S1) (Kielhorn et al., 2003). The reactive intermediates of nitro reduction pathway, nitroso-PAHs, could cause DNA and protein damages, while the final products of amino-PAHs can be excreted in urine (Laumbach et al., 2009). For instance, urinary concentrations of 1-aminopyrene were significantly increased in underground miners following the use of diesel-powered equipment and in adult nonsmoking volunteers after a 2-hour controlled exposure to diesel engine exhaust (Du et al., 2019; Laumbach et al., 2009). In nonsmoking adults living in London, UK, urinary 2-aminofluorene concentrations were significantly higher in those residing within 100 meter of a major roads and were significantly and positively associated with the nearby traffic flow of diesel-powered buses and heavy-duty vehicles (Yang et al., 2021). These limited number of published studies suggest the use of urinary 1-aminopyrene and 2-aminofluorene as promising exposure biomarkers for diesel and diesel plus biodiesel exhausts, respectively. More research is needed to replicate and confirm these suggestive results as well as to determine the extent to which tobacco smoke can affect urinary concentrations of these potential diesel exposure biomarkers.
The present study used a unique occupational population consisting of diesel engine testers exposed to high levels of diesel engine exhaust in a stable factory environment and comparable controls unexposed to diesel engine exhaust or other combustion products to assess and quantify the impact of personal diesel exhaust exposure on urinary concentrations of 1-aminopyrene and 2-aminofluorene, along with 1-aminonaphthalene, 2-aminonaphthalene, and 9-aminophenantherene. These compounds were selected based on the relative abundance of their parent NPAHs in diesel exhaust (Bandowe & Meusel, 2017) and their potential for source specificity (Gong et al., 2015; Reisen & Arey, 2005). We compared the results to the mutagenic potencies of the urine samples, which were determined previously (Wong et al., 2021). This is among the first studies to link urinary amino-PAH concentrations to exposure concentrations of diesel exhaust indicators (soot and EC) and is the first study, to the best of our knowledge, to examine the relationship between urinary amino-PAHs and urinary mutagenicity.
Materials and Methods
Subjects and sample collection
The present study used the data and banked urines of a previously published study of the impact of occupational exposure to diesel engine emissions on a variety of biological endpoints. The study design has been previously described in detail (Lan et al., 2015). The diesel engine manufacturing factory was selected because the engine testing process was stable and resulted in high levels of diesel engine exhaust exposure. Briefly, we enrolled 54 engine testers from a diesel engine manufacturing company and 55 age-matched controls with no occupational exposures to diesel exhaust. The fraction of smokers was also matched for both diesel testers and the control group. All engine testers were male. The protocol was approved by Institutional Review Boards at the US National Cancer Institute and the National Institute of Occupational Health and Poison Control, China CDC (Protocol number 13CN101). Participation was voluntary, and written informed consent was obtained. At the time of subject recruitment, a questionnaire was filled out by each subject that requested information on demographics, including age, height, and weight (used to calculate body mass index (BMI)), smoking history, and occupational history including number of years working in a diesel factory or the current factory. One urine sample was collected immediately at the end of a work shift from all subjects, and an overnight urine sample was collected from non-smokers (Wong et al., 2021). All the urine samples, in multiple aliquot tubes, were shipped on dry ice and stored at −80°C until sample analysis.
Urine sample analysis
One stored urine tube per subject was thawed for analysis of amino-PAHs, cotinine, and creatinine. In the analysis of amino-PAHs and cotinine, a 2 ml of urine aliquot was hydrolyzed by adding 20 μl of β-glucuronidase from Helix Pomatia Type H-2 (Sigma-Aldrich) and 2 ml of 0.1M sodium acetate buffer and incubated at 37 °C overnight. Following incubation, the solution was added with 25 μl of internal standards (100 ng/ml 1-aminonaphthalene-d7, and Cotinine D3, Sigma-Aldrich), 500 μl of 10M NaOH, and 6 ml of CH2Cl2/hexane mixture (1/2 v/v), and fully mixed by shaking for an hour and then centrifuged at 4000 rpm for 10 minutes. The supernatant was transferred to a tube and evaporated with high-purity nitrogen. The dried residue was re-dissolved and vortexed in an acetonitrile-water (1:4 by volume) solution. This final solution was analyzed for amino-PAHs and cotinine using an HPLC-MS/MS system consisting of an Accela Open Autosampler, an Accela 1250 pump, and a TSQ Quantum Access MAX triple quadrupole mass spectrometer (Thermo Fisher Scientific Inc., San Jose, CA, USA). A gradient mobile phase was run through a Phenomenex Luna 3 μm, C18 100 Å, 50×2.00 mm column to separate amino-PAHs and cotinine. The mobile phase involved the use of Solution A (0.1% formic acid in HPLC-grade water) and Solution B (0.1% formic acid in HPLC-grade acetonitrile) according to the following: 80% A and 20% B for the first 6 minutes, 10% A and 90% B for the next 2 minutes, and then 80% A and 20% B for the last 7 minutes. The mass spectrometer was set to the H-ESI positive mode with the spray voltage of 4000 V, vaporizer temperature and capillary temperature of 300 °C and 350 °C, respectively, and sheath gas pressure and aux gas pressure of 40 and 10 (arbitrary units), respectively. The injection volume was 20 μl. Standards were purchased from Sigma-Aldrich for cotinine and 5 target amino-PAHs including 1-aminonaphthalene, 2-aminonaphthalene, 2-aminofluorene, 9-aminophenathrene, and 1-aminopyrene. In addition, deuterium labeled 1-aminonaphthalene, and cotinine (Sigma-Aldrich) were used as an internal standard.
The limit of detection (LOD) was calculated as 3 times the standard deviation of 5 repeated injections of the standard with the lowest concentration. The LODs were 0.02 ng/mL for 1-aminopyrene and 2-aminonaphthalene, and 0.05 ng/mL for 9-aminophenanthrene and 2-aminofluorene. The recovery of each analyte was obtained by calculating the ratio of the detected amount in the urine spiked with the standard over the actual amount spiked. The recovery values were 92.0% for cotinine, 130.5% for 1-aminonaphthalene, 130% for 2-aminonaphthalene, 102% for 2-aminofluorene, 103% for 9-aminophenanthrene, and 144% for 1-aminopyrene. Using isotope-labeled internal standards for calibration, the HPLC-MS-MS methods had coefficient of variation of 6 repeated injections smaller than 10.0% for all the amino-PAHs and cotinine. The correlation coefficients R2 for all calibration curves were greater than 0.997.
Urinary mutagenicity was measured in a subgroup of 35 workers (20 engine testers and 15 non-testers) as reported previously (Wong et al., 2021). Briefly, 50 mL of urine were deconjugated enzymatically with β-glucuronidase for 16 hours at 37 °C and poured through a C-18 silica-gel column. The organics were eluted with 10 mL of methanol and solvent-exchanged into dimethyl-sulfoxide to generate a 150 × organic concentrate. The concentrate was tested in the Salmonella (Ames) plate-incorporation mutagenicity assay in strain YG1041 with metabolic activation (S9) made from Aroclor-induced Sprague-Dawley rat-liver S9 (Moltox, Boone, NC). The colonies were counted by an automatic colony counter after incubation at 37 °C for 3 days. The mutagenic potencies were the slopes calculated from the linear regressions using the initial linear portions of the dose-response curves.
The method of urinary creatinine measurement was modified from the method provided by Creatinine (urinary) Colorimetric Assay Kit (Cayman Chemical, item No. 500701). Briefly, 15 μl of creatinine standards and diluted samples were added to microplate wells, followed by the addition of a 150 μl of alkaline picrate solution. After 10-minute incubation, the microplates were put into a FlUOstar OPTIMA microplate reader (BMG LABTECH). Absorbance of mixtures was measured at 490–500 nm and converted into creatinine concentrations using standard calibration curves made for each batch of samples.
Personal inhalation exposure assessment
The method for assessing personal exposure to diesel engine emissions can be found in our previous publication (Lan et al., 2015). Briefly, several full-shift personal air samples of fine particles (PM2.5) were collected from engine testers onto a quartz fiber filter using a portable air sampler attached to the lapel near the breathing zone of each participating engine tester. This method captured average during-work-shift exposure for each subject. Exposure assessment was conducted for a subset of control workers and used to estimate exposure for the entire group, as described by (Lan et al., 2015). Concentrations of PM2.5 were determined gravimetrically. EC and organic carbon (OC) contents of PM2.5 were analyzed on the quartz filters using the NIOSH Method 5040 and their concentrations were determined by EC and OC mass divided by the sampling volume. Soot content of PM2.5 was determined using a smoke stain reflectometer (model M43D, diffusion Systems Ltd, London, UK) and and the soot concentration was measured as absorption coefficient in unit of 10−5/m.. Among these measurements, EC is considered the best proxy for diesel engine exhaust traffic emissions (Schauer, 2003).
Statistical analysis
Amino-PAHs in relation to occupational status.
Amino-PAHs were expected to be present in the human urine at low levels (Gong et al., 2015). Hence, we assessed the detectability of amino-PAHs in relation to occupational status. We used logistic regression models to calculate odds ratios (ORs) for the testers having amino-PAH concentrations above the LOD values in reference to the non-testers, controlling for smoking status, alcohol consumption, age, and BMI. We assigned below LOD amino-PAH concentrations with (LOD/√2). Considering that amino-PAH concentrations were highly skewed towards the lower end of the range (LOD), we used linear regression to model the log10 concentrations of amino-PAH as a function of occupational status, controlling for smoking status, alcohol usage, BMI, and age.
Amino-PAHs in relation to pollutant exposures.
Because personal exposure concentrations of PM2.5 and diesel emission pollutants (EC and soot) were available only for the engine testers, we used Pearson Correlation and linear regression to examine the relationship between urinary concentrations and personal exposure (i.e., breathing zone concentrations) in this subgroup of subjects. Amino-PAH concentrations were log10-transformed. Smoking status (or log10 cotinine concentrations), alcohol consumption, age, and BMI were controlled for in the linear regression analysis. In an exploratory analysis, we introduced an interaction term of pollutant concentration by smoking status to examine potential effect modifications by smoking.
Amino-PAHs in relation to smoking status and cotinine concentrations.
To examine the impact of smoking status on urinary amino-PAH concentrations, we used the same methods described for assessing the impact of occupational status to compare the detection rates in smokers versus nonsmokers. We also estimated ORs for the smokers having amino-PAH concentrations above the LOD values in reference to the nonsmokers, controlling for occupational status, alcohol consumption, age, and BMI. We also replaced “smoking status” with cotinine concentrations in the models described above to examine the relationships between amino-PAH concentrations and cotinine concentrations.
Amino-PAHs in relation to urinary mutagenicity.
We used Pearson Correlation to examine the relationships between urinary amino-PAHs concentrations and urinary mutagenicity. We also used linear regression to examine the exposure-response relationship between amino-PAH concentration and mutagenicity. All statistical analyses were conducted using the R software (version 3.3.2).
Results
Amino-PAHs by occupational status
The 54 diesel engine testers and the 55 non-testers were comparable in terms of age, BMI, smoking status (Table 1). Among current smokers, the mean (SD) cigarettes smoked per day was 13.2 (6.4) and 12.3 (7.3) in diesel testers and controls, respectively. Although a lower fraction of the engine testers reported to drink alcohol than the non-testers (72.2% versus 85.5%), the difference was statistically nonsignificant. The engine testers had higher detection rates of 1-aminopyrene and 2-aminofluorene than the engine non-testers (Table 1). The two groups did not show noticeable differences in detection rate for the other 3 amino-PAHs (Table 1). After adjusting for the demographic variables shown in Table 1, we found that it was approximately 12.8 times more likely to detect 1-aminopyrene and 2.9 times more likely to detect 2-aminofluorene in the engine testers than in the non-testers (Figure 1A). Consistently shown in Figure 2B, the engine testers had significantly higher concentrations of 1-aminopyrene and marginally higher concentrations of 2-aminofluorene (p=0.068). The two groups did not show differences in concentrations of the other 3 amino-PAHs. In addition to the boxplot data distributions shown in Figure 1B, ranges and central tendencies of amino-PAHs concentrations are also presented in Table S1 and Figure S2 of the Supplemental Material which also show clearly higher concentrations of 1-aminopyrene among the engine testers as compared with non-testers. Among the other variables examined in the multivariable models, smoking status had a significant effect on the detection rate of 2-aminonaphthalene, and concentrations of 2-aminofluorene and 2-aminonaphthalne. BMI had a significant effect on 9-aminophenanthrene and 2-aminofluorene (Table S2).
Table 1.
Demographic characteristics and detection frequencies of urinary amino-PAHs among diesel engine testers and non-testers (controls)
| Engine Testers (n=54)a | Non-testers (n=55) | p-valueb | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years | 42.0 ± 6.8 | 42.1 ± 7.4 | 0.96 |
| Body mass index, kg/m2 | 24.7 ± 3.4 | 25.2 ± 3.8 | 0.44 |
| Smoking status | 0.95 | ||
| Current smokers | 34 (63.0%) | 35 (63.6%) | |
| Former smokers | 11 (20.4%) | 12 (21.8%) | |
| Never smokers | 9 (16.7%) | 8 (14.5%) | |
| Alcohol use | 0.091 | ||
| Yes | 39 (72.2%) | 47 (85.5%) | |
| No | 15 (27.8%) | 8 (14.5%) | |
| Detection rate | |||
| 1-Aminopyrene | 43 (79.6%) | 13 (23.6%) | 1.5 × 10−8 |
| 9-Aminophenanthrene | 20 (37.0%) | 22 (40.0%) | 0.90 |
| 2-Aminofluorene | 45 (83.3%) | 35 (63.6%) | 0.035 |
| 1-Aminonaphthalene | 51 (94.4%) | 49 (89.1%) | 0.50 |
| 2-Aminonaphthalene | 33 (61.1%) | 28 (50.9%) | 0.38 |
Data are mean ± standard deviations or count (percentage);
Between-group differences are tested by one-way ANOVA or chi-square test.
Figure 1.
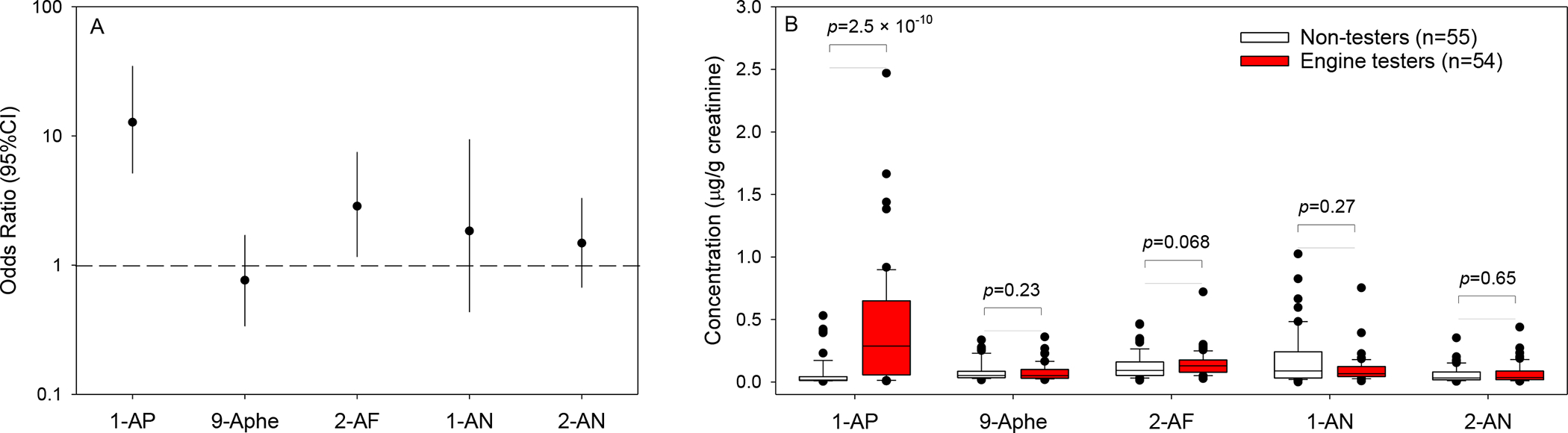
Odds ratios for being detectable (concentrations > LOD values, panel A) and concentrations of amino-PAHs (panel B) in relation to occupation status among all study participants. ORs for the diesel engine tester having amino-PAH concentrations above LOD in reference to the non-testers were tested by logistic regressions. Differences in concentrations were tested by linear regressions. Both analyses were adjusted for current smoking, alcohol use, age, and BMI. 1-AP = 1-aminopyrene, 9-Aphe = 9-aminophenanthrene, 2-AF = 2-aminofluorene, 1-AN = 1-aminonaphthalene, and 2-AN = 2-aminonaphthalene.
Figure 2.
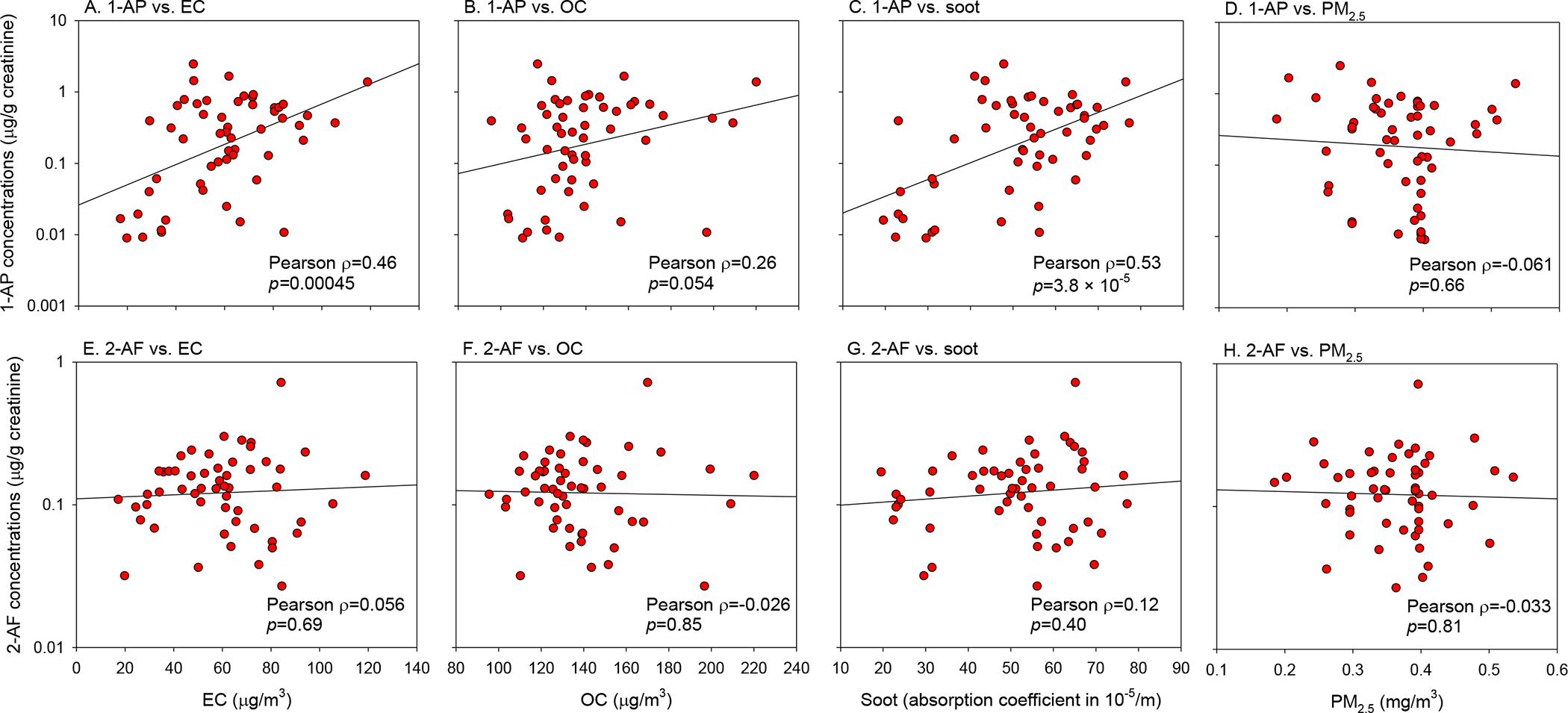
Pearson correlation coefficients between pollutant exposures and amino-PAHs among engine testers. Concentrations of amino-PAHs were log10-transformed.
Amino-PAHs in relation to cigarette smoking
Sixty-nine out of the 109 subjects were self-reported current smokers. After adjusting for the demographic variables and occupational status, we found that it was 2.4 times more likely to detect 2-aminonaphthalene in the current smokers than in the non-smokers. However, smoking status did not significantly affect detection rates of the other 4 amino-PAHs (Figure S3A). Likewise, after adjusting for the demographic variables and occupational status, smoking was significantly associated with higher concentrations of 2-aminonaphthalene and 2-aminofluorene. The concentrations for the other 3 amino-PAHs were not significantly different between smokers and non-smokers (Figure S3B).
In addition to using self-reported smoking status, urinary concentrations of cotinine (with a half-life of approximately 15 hours) were measured to capture short-term exposure to both active and passive smoking. Creatinine-normalized cotinine concentrations ranged from 0.03 to 151.2 (median of 30.5) μg/g for the nonsmokers and from 107.9 to 8228 (median of 2636) μg/g for the smokers. Cotinine concentrations in our subjects were within the ranges reported for population-based samples including smokers and nonsmokers with or without exposure to second hand smoke (Kim & Jung, 2013). We found that increasing cotinine concentrations were associated with an increased odds for detecting 2-aminonaphthalene. We also found a significant positive association of cotinine concentration with 2-aminofluorene and with 2-aminonaphthalene, respectively. Further, adjusting cotinine concentration generated the same findings on the effect of occupational status on amino-PAH detection rate and the effect on amino-PAH concentrations as observed in the models adjusting for smoking status (Table S3.)
Correlations among urinary amino-PAHs
Among all subjects, the correlations among the five amino-PAHs were low, with the highest Pearson correlation coefficient (ρ) being 0.31 between 2-aminofluorene and 2-aminonaphthalene and lowest ρ between 1-aminonaphthalene and 1-aminopyrene (Table S4). Significant weak to moderate correlations were found between 1-aminopyrene and 2-aminofluorene, between 9-aminophenanthrene and 2-aminofluorene, and between 9-aminophenanthrene and 2-aminonaphthalene in the engine testers but not in the non-testers (Table S4). In contrast, the correlations between 2-aminofluorene and 2-aminonaphthalene and between 1-aminopyrene and 9-aminophenanthrene were only significant in the non-testers and not in the engine testers.
Relationships between urinary amino-PAHs and personal inhalation exposures
Personal air concentrations of EC, OC, soot, and PM2.5 were reported previously (Lan et al., 2015). Briefly, concentration ranges and mean ± standard deviations were 17.2 – 118.8 and 59.6 ± 22.1 μg/m3 for EC, 95.6 – 220.1 and 138.9 ± 25.9 μg/m3 for OC, 19.6– 77.3 and 50.7 ± 15.3 ×10−5/m for soot, and 0.2 – 0.5 and 0.4 ± 0.1 mg/m3 for PM2.5. Considering only 1-aminopyrene and 2-aminofluorene were significantly affected by occupational status, we conducted correlation analyses for these two amino-PAHs among the testers only. As shown in Figure 2, 1-aminopyrene was significantly correlated with EC, OC, and soot, respectively, with the highest Pearson correlation coefficient of 0.53 observed for soot. In contrast, 2-aminofluorene was not correlated with any pollutant. In the Pearson Correlation analysis, we log10-transformed amino-PAH concentrations due to the skewed data distribution. We also used a nonparametric analysis (Spearman Correlation), generating the same finding of significant correlations of 1-aminopyrene with EC (ρ=0.37), OC (ρ=0.29), and soot (ρ=0.37), respectively. The association between 1-aminopyrene and air pollutants remains robust after the adjustment of smoking status or urinary cotinine levels (Figure S4).
Among the engine testers, there were similar levels of EC in the current smokers and combined never-smokers and former smokers (median, 10th-90th percentiles: 61.7, 35.8– 84.1 vs 59.8, 22.1–89.2 μg/m3, respectively). There was a similar relationship between EC and urinary 1-aminopyrene among never/former smokers (n=20) and current smokers (n=34) (beta, SE, p value: 0.016, 0.006, 0.010 and 0.012, 0.005, 0.022, respectively) and the test for multiplicative interaction was not significant (p = 0.69). No significant interactions were observed for other air pollutants or 2-aminofluorene (Figure S5).
Relationships between urinary amino-PAH concentrations and urinary mutagenicity
Urinary mutagenicity was measured in 20 engine testers and 15 non-testers. As reported previously, the mutagenicity levels were significantly higher among engine testers (13.0 ± 10.1 rev/mL-eq) as compared with non-testers (5.6 ± 4.4 rev/mL-eq, p=0.01) (Wong et al., 2021). In this study, we further found that the urinary mutagenicity was significantly correlated with urinary levels of 1-aminopyrene and 2-aminofluorene but not the other amino-PAHs (Figure 3). Of note, the urinary mutagenicity exhibited a stronger correlation with 1-aminopyrene (ρ=0.79, p=1.5*10−8) than with 2-aminofluorene (ρ=0.53, p=0.0012). Among the testers only, urinary mutagenicity is also strongly correlated with 1-aminopyrene (ρ=0.81, p=1.5*10−5) and 2-aminofluorene (ρ=0.54, p=0.015).
Figure 3.
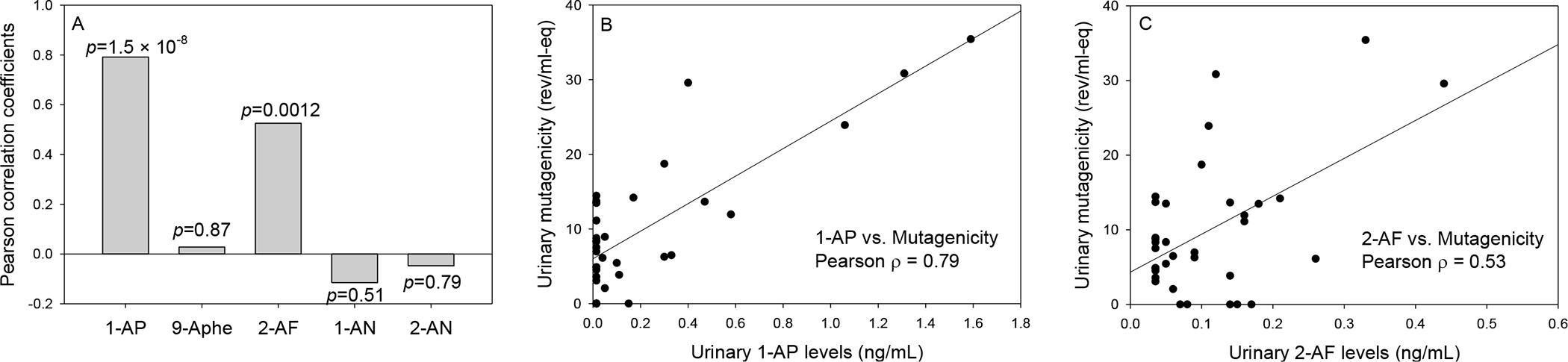
Pearson correlation between urinary mutagenicity and amino-PAHs concentrations (panel A) and the exposure-response relationships between urinary mutagenicity and 1-AP (panel B) and 2-AF (panel C).
Discussion
Our study used a cross-sectional design to assess multiple amino-PAHs as well as other types of biomarkers in relation to diesel exhaust exposure by comparing an occupational exposure group with a wide range of exposure and a control group. We also assessed the exposure-response relationship between personal exposure to (breathing-zone concentrations of multiple diesel engine exhaust constituents. In 54 diesel engine testers and 55 non-tester controls, we have provided evidence that urinary 1-aminopyrene was a better biomarker of diesel exhaust compared with other amino-PAHs because it was not influenced by exposure to tobacco smoke, a prevalent pollution sources in occupational settings, or by alcohol consumption, age, and BMI. This was further supported by significant associations between 1-aminopyrene concentrations and personal exposures to EC and soot particles in the diesel engine testers. EC in particular has been considered a good surrogate of diesel exhaust (Schauer, 2003). In addition, we found a strong and significant correlation between 1-aminopyrene and urinary mutagenicity and a significant correlation between 2-aminofluorene and mutagenicity (Figure 3).
Our 1-aminopyrene findings provide strong confirmation of its relationship with diesel engine exhaust given that our study population had a wide range of exposures with subjects exposed to relatively high levels and no occupational exposure to other sources of nitro-PAHs. In underground and ground miners who used diesel-powered equipment but were also exposed to other airborne pollutants (e.g., dust, machine debris, oil residues), 1-aminopyrene concentrations were higher in the urine samples collected post-work shift than in those collected prior to the work shift (Du et al., 2019; Seidel et al., 2002). In nonsmoking adult volunteers, a 1-hour exposure to diluted diesel engine exhaust in a controlled environmental facility resulted in elevated 1-aminopyrene concentrations in post-exposure urines compared to pre-exposure urines (Laumbach et al., 2009). Our finding on higher detectability of 1-aminopyrene in the diesel engine testers than non-testers suggests that urinary 1-aminopyrene has the potential to be used to help quantify potential occupational exposure to diesel engine exhaust.
In addition to 1-aminopyrene, we found that 2-aminofluorene also had higher concentrations among engine testers as compared to non-testers. However, 2-aminofluorene concentration was not correlated with any external exposure estimate of diesel exhaust. This is at least partially explained by our findings that smoking significantly contributed to 2-aminofluorene concentrations independent of diesel exhaust. Additionally, it should also be noted that we did not assess gaseous phase pollutant emitted from diesel engines. Previous studies have found that a greater proportion of 2-nitrofluorene existed in the gaseous phase as compared to 1-nitropyrene (Chen et al., 2017), which may be another reason why urinary 2-aminofluorene levels were not correlated with PM2.5 mass and components (EC, OC, soot) measurements.
This study presents a novel HPLC-MS-MS method to simultaneously measure urinary amino-PAHs and cotinine. The method sensitivity was adequate to measure trace levels of cotinine in the majority of the nonsmoking subjects. As cotinine is a well-established biomarker of tobacco smoke exposure, the method can be used to characterize exposures to the two combustion sources (tobacco and diesel), especially after 1-aminopyrene has been further validated as a diesel exhaust exposure biomarker. However, few studies have measured urinary amino-PAHs in a sample of the general population.
In a panel study relating within-person temporal variations in urinary amino-PAH concentration to changes in pollutant sources and levels brought by traffic controls during the 8-week period of the 2008 Beijing Summer Olympics and Paralympics, decreases in urinary concentrations of 1- and 2-aminonaphthalene, but not concentrations of 1-aminopyrene, were associated with the general traffic control. In contrast, during the post-Olympic period when ambient EC concentrations increased substantially, 1-aminopyrene concentrations also increased substantially (Gong et al., 2015). The panel study was conducted in a group of nonsmoking young adults (medical students and nurses) living and working in a hospital located in central Beijing. The substantial post-Olympic increase in EC concentration was attributed to both diesel emissions and coal combustion (Zhang et al., 2013). This previous study did not measure NPAH concentrations. However, our literature search indeed found that 1-nitropyrene was the most abundant NPAHs emitted from bituminous coal combustion (Figure S6) (Huang et al., 2014). The moderately elevated 1-aminopyrene levels in some subjects from control workplaces in our study could potentially reflect coal combustion as well as diesel engine exhaust exposure (Figure 1).
Our literature search also identified two additional emission sources with relatively abundant 1-nitropyrene. One was Chinese ships fueled by diesel or heavy fuel oil (Zhao et al., 2019) and the other was European engines using diesel/biodiesel blends (Figure S6) (Borillo et al., 2018). It is worth noting that although 1-nitropyrene was the single most abundant NPAH in conventional diesel exhaust (hence used in the standard reference material for diesel exhaust particles), 2-nitrofluorene appeared to be most abundant in diesel and biodiesel blend emissions even though these measurements were based on particle samples that did not account for the emission of gaseous 2-nitrofluorene. In a recent study conducted in adult participants residing in a large European city (London of UK), urinary concentrations of the same five amino-PAHs measured in the present study were assessed in relation to residential proximity road type, road length, traffic flow and traffic volume (Yang et al., 2021). The study reported that participants living within 100 m of a major road had significantly higher concentrations of 2-aminofluorene and that 2-aminofluorene concentrations were significantly associated with the traffic flow of nearby buses, and heavy-duty vehicles, but not motorbikes, taxis, or coaches. Given that the diesel/biodiesel blend was the common fuel used by European diesel engines, it was plausible to see 2-aminofluorene (a metabolite of 2-nitrofluorene) associated with indicators of diesel traffic in the city. In the present study, we observed higher detection rate and higher concentration of 2-aminofluorene in the diesel engine testers than in the non-testers. However, the difference in 2-aminofluorene was much less profound than the difference in 1-aminopyrene. Combining this finding with the London study finding suggests the potential for using these two amino-PAHs to differentiate exposure to the conventional diesel exhaust from exposure to diesel/biodiesel exhaust (in the absence of coal combustion exposure). However, the present study showed that 2-aminofluorene was affected by smoking status. Hence, tobacco smoke exposure needs to be considered when using 2-aminofluorene to assess exposure to diesel/biodiesel blend exhaust.
In addition to 1-aminopyrene and 2-aminofluorene, three other amino-PAHs were also examined in the present study. No significant differences in these amino-PAHs (detection rate and concentration) were found between the engine testers and their controls. Their precursor NPAHs may be abundant in other combustion sources. For example, it has been shown that combustion of solid cooking fuels (wood and coal) contributed to elevated kitchen air concentrations of 1-nitronaphthalene and 2-nitronaphthalene (Chen et al., 2017). These two amino-PAHs were abundant in anthracite coal combustion and in tobacco smoke (Chen et al., 2017; Huang et al., 2014). 9-nitrophenanthrene appeared to be most abundant in wood combustion (Shen et al., 2013) and Chinese ships burning diesel or heavy fuel oil (Figure S6) (Zhao et al., 2019). Future studies are recommended to evaluate whether these other amino-PAHs may be used as biomarkers with some degree of specificity to other pollution sources. Considering the weak correlations among the 5 amino-PAHs measured in the present study, it is likely that different amino-PAHs may reflect different combustion sources.
PAHs have been studied extensively because they are generated universally from incomplete combustion of fuels and wastes and during certain cooking processes such as grilling and char broiling of meats and vegetables. Exposure to PAHs has shown a range of toxic effects including mutagenesis, teratogenesis, and carcinogenesis. Derivative PAHs, such as NPAHs, are often more toxic (especially mutagenic or carcinogenic) than their parent PAHs (IARC, 2014; Pitts et al., 1978). Compared to knowledge about PAHs, much less is known about sources of and human exposures to NPAHs, largely due to technical challenges in measuring NPAHs that are present in the atmosphere at lower concentrations than their parent PAHs. The measurement of atmospheric NPAHs has been long recognized to be challenging due to the instability of these compounds in sampling media (filters for particle-phase and adsorbents for gas-phase NPAHs) (Bamford et al., 2003; Lies et al., 1986). Because NPAHs concentrations are typically orders of magnitude lower than their parent PAHs (Reisen & Arey, 2005), a large sampling volume is required to collect sufficient quantities for laboratory analysis of NPAHs. This can be logistically prohibitive in human exposure assessment studies. After entering the human body, NPAHs can be enzymatically reduced into amino-PAHs in the liver (Laumbach et al., 2009), which forms the basis for urine biomonitoring of amino-PAHs. The present study showed that 5 selected amino-PAHs can be measured in urine samples stored for years. This biomonitoring method can be useful in future assessment of health effects and disease risks associated with exposure to NPAHs.
Urinary mutagenicity has been thought to reflect total exposure to genotoxic chemicals and has been correlated with tumor development (Peters et al., 2003). In our previous study in the same population, urinary mutagenicity was significantly associated with personal EC exposure (Wong et al., 2021). In the current study, we showed that urinary mutagenicity was significantly associated with urinary 1-aminopyrene and 2-aminofluorene. The finding supports the long-recognized role that of the parent NPAHs as the primary class of mutagens in diesel exhaust (IARC, 2014). Thus, 1-aminopyrene could be a good surrogate for diesel exhaust mutagenicity in well characterized occupational settings.
The present study has several strengths. It was conducted in a population of workers exposed to a wide range of diesel engine exhaust levels that extended to relatively high levels in a stable workplace, with relatively uniform within-person exposure substantial inter-individual variation (Lan et al., 2015) with negligible exposure to other sources of air pollutants. There was also an extensive exposure assessment component with repeat sampling and modeling of multiple components of diesel engine exhaust. It also had a number of limitations. Although it was conducted in a relatively highly exposed group of workers that had adequate power to detect a wide range of relatively strong biomarker signals (Lan et al., 2015; Drizik et al., 2020; Wong et al., 2021), the sample size was relatively small, and power to detect interactions was limited. Also, only male workers were available at the diesel engine testing facility, so we could not evaluate if sex modifies the NPAH to amino-PAH metabolism. Finally, we did not have air NPAH data to directly relate metabolites to their parent compounds. As noted, it is extremely challenging to measure these directly, and EC is considered a good surrogate for diesel engine exhaust exposure based on many studies (Schauer, 2003). However, in absence of gaseous phase data, we cannot evaluate to what extent the gaseous NPAHs emitted from diesel exhaust would contribute to the urinary amino-PAHs levels.
Conclusions
Detection rates and concentrations of urinary 1-aminopyrene were significantly and substantially higher in diesel engine testing workers than in non-engine testers. Across the engine testers, urinary 1-aminopyrene concentrations were significantly correlated with breathing-zone concentrations of diesel exhaust pollutants (EC and soot particles) measured during a work shift prior to urine collection. Neither smoking status nor urinary cotinine concentrations affected 1-aminopyrene concentrations and 1-aminopyrene relationships with the diesel exhaust pollutants. Further, urinary 1-aminopyrene was more strongly correlated with urinary mutagenicity than 2-aminofuorene in diesel testers. These findings contribute to the limited previous data supporting urinary 1-aminopyrene as an exposure biomarker for diesel exhaust and associated mutagenicity in both smokers and nonsmokers. In contrast, 2-aminofuorene, a previously suggested exposure biomarker for the diesel/biodiesel traffic in nonsmoking adults, was contributed by both diesel engine emissions and cigarette smoking. Future work should focus on larger studies of populations with a wide range of diesel engine exhaust, including lower environmental exposures as well as in occupational settings, to further explore the relationships shown here.
Supplementary Material
Acknowledgements and Disclaimer
Funding was supported by intramural funds from the National Cancer Institute (NCI) and the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agency. Further, NCI does not endorse the purchase of any commercial products or services mentioned in the publication. We wish to thank Dr. Jinpu Jia and Ms. Peijia Yan for their assistance in analyzing urinary amino-PAHs.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Bamford HA, Bezabeh DZ, Schantz MM, Wise SA, & Baker JE (2003). Determination and comparison of nitrated-polycyclic aromatic hydrocarbons measured in air and diesel particulate reference materials. Chemosphere, 50(5), 575–587. [DOI] [PubMed] [Google Scholar]
- Bandowe BAM, & Meusel H (2017). Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) in the environment - A review. Sci Total Environ, 581–582, 237–257. 10.1016/j.scitotenv.2016.12.115 [DOI] [PubMed] [Google Scholar]
- Borillo GC, Tadano YS, Godoi AFL, Pauliquevis T, Sarmiento H, Rempel D, Yamamoto CI, Marchi MRR, Potgieter-Vermaak S, & Godoi RHM (2018). Polycyclic Aromatic Hydrocarbons (PAHs) and nitrated analogs associated to particulate matter emission from a Euro V-SCR engine fuelled with diesel/biodiesel blends. Sci Total Environ, 644, 675–682. 10.1016/j.scitotenv.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Chen Y, Du W, Shen G, Zhuo S, Zhu X, Shen H, Huang Y, Su S, Lin N, Pei L, Zheng X, Wu J, Duan Y, Wang X, Liu W, Wong M, & Tao S (2017). Household air pollution and personal exposure to nitrated and oxygenated polycyclic aromatics (PAHs) in rural households: Influence of household cooking energies. Indoor Air, 27(1), 169–178. 10.1111/ina.12300 [DOI] [PubMed] [Google Scholar]
- Dimashki M, Harrad S, & Harrison RM (2000). Concentrations and Phase Distribution of Nitro-PAH in the Queensway Road Tunnel in Birmingham, United Kingdom. Polycyclic Aromat Compd, 20(1–4), 205–223. 10.1080/10406630008034786 [DOI] [Google Scholar]
- Du M, Mullins BJ, Franklin P, Musk AW, Elliot NSJ, Sodhi-Berry N, Junaldi E, de Klerk N, & Reid A (2019). Measurement of urinary 1-aminopyrene and 1-hydroxypyrene as biomarkers of exposure to diesel particulate matter in gold miners. Sci Total Environ, 685, 723–728. 10.1016/j.scitotenv.2019.06.242 [DOI] [PubMed] [Google Scholar]
- Feilberg A, Poulsen B, W M, Nielsen T, & Henrik S. (2001). Occurrence and sources of particulate nitro-polycyclic aromatic hydrocarbons in ambient air in Denmark. Atmos Environ, 35(2), 353–366. 10.1016/s1352-2310(00)00142-4 [DOI] [Google Scholar]
- Gbeddy G, Goonetilleke A, Ayoko GA, & Egodawatta P (2020). Transformation and degradation of polycyclic aromatic hydrocarbons (PAHs) in urban road surfaces: Influential factors, implications and recommendations. Environ Pollut, 257, 113510. 10.1016/j.envpol.2019.113510 [DOI] [PubMed] [Google Scholar]
- Gong J, Zhu T, Kipen H, Rich DQ, Huang W, Lin WT, Hu M, & Zhang JJ (2015). Urinary polycyclic aromatic hydrocarbon metabolites as biomarkers of exposure to traffic-emitted pollutants. Environ Int, 85, 104–110. 10.1016/j.envint.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Liu M, Bi X, Chaemfa C, Ren Z, Wang X, Sheng G, & Fu J (2014). Phase distribution, sources and risk assessment of PAHs, NPAHs and OPAHs in a rural site of Pearl River Delta region, China. Atmospheric Pollution Research, 5(2), 210–218. 10.5094/apr.2014.026 [DOI] [Google Scholar]
- IARC. (2014). Diesel and Gasoline Engine Exhausts and Some Nitroarenes. Iarc Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC Monogr Eval Carcinog Risks Hum, 105, 9–699. https://www.ncbi.nlm.nih.gov/pubmed/26442290 [PMC free article] [PubMed] [Google Scholar]
- Kielhorn J, Wahnschaffe U, Mangelsdorf I, & International Programme on Chemical, S. (2003). Selected nitro and nitro-oxy-polycyclic aromatic hydrocarbons. In. Geneva: World Health Organization. [Google Scholar]
- Kim S, & Jung A (2013). Optimum cutoff value of urinary cotinine distinguishing South Korean adult smokers from nonsmokers using data from the KNHANES (2008–2010). Nicotine Tob Res, 15(9), 1608–1616. 10.1093/ntr/ntt027 [DOI] [PubMed] [Google Scholar]
- Lan Q, Vermeulen R, Dai Y, Ren D, Hu W, Duan H, Niu Y, Xu J, Fu W, Meliefste K, Zhou B, Yang J, Ye M, Jia X, Meng T, Bin P, Kim C, Bassig BA, Hosgood HD 3rd, . . . Rothman N. (2015). Occupational exposure to diesel engine exhaust and alterations in lymphocyte subsets. Occup Environ Med, 72(5), 354–359. 10.1136/oemed-2014-102556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumbach R, Tong J, Zhang L, Ohman-Strickland P, Stern A, Fiedler N, Kipen H, Kelly-McNeil K, Lioy P, & Zhang J (2009). Quantification of 1-aminopyrene in human urine after a controlled exposure to diesel exhaust. J Environ Monit, 11(1), 153–159. 10.1039/b810039j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies KH, Hartung A, Postulka A, Gring H, & Schulze J (1986). Composition of diesel exhaust with particular reference to particle bound organics including formation of artifacts. Dev Toxicol Environ Sci, 13, 65–82. https://www.ncbi.nlm.nih.gov/pubmed/2435507 [PubMed] [Google Scholar]
- Lin Y, Qiu X, Ma Y, Wang J, Wu Y, Zeng L, Hu M, Zhu T, & Zhu Y (2016). A novel approach for apportionment between primary and secondary sources of airborne nitrated polycyclic aromatic hydrocarbons (NPAHs). Atmos Environ, 138, 108–113. 10.1016/j.atmosenv.2016.05.017 [DOI] [Google Scholar]
- Manzano C, Hoh E, & Simonich SL (2013). Quantification of complex polycyclic aromatic hydrocarbon mixtures in standard reference materials using comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry. J Chromatogr A, 1307, 172–179. 10.1016/j.chroma.2013.07.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters U, DeMarini DM, Sinha R, Brooks LR, Warren SH, Chatterjee N, & Rothman N (2003). Urinary mutagenicity and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev, 12(11 Pt 1), 1253–1256. https://www.ncbi.nlm.nih.gov/pubmed/14652290 [PubMed] [Google Scholar]
- Pitts JN, Van Cauwenberghe KA, Grosjean D, Schmid JP, Fitz DR, Belser WL, Knudson G, & Hynds PM (1978). Atmospheric reactions of polycyclic aromatic hydrocarbons: facile formation of mutagenic nitro derivatives. Science, 202(4367), 515–519. [DOI] [PubMed] [Google Scholar]
- Reisen F, & Arey J (2005). Atmospheric reactions influence seasonal PAH and nitro-PAH concentrations in the Los Angeles basin. Environ Sci Technol, 39(1), 64–73. https://www.ncbi.nlm.nih.gov/pubmed/15667076 [PubMed] [Google Scholar]
- Ross JA, Nelson GB, Mutlu E, Warren SH, Gilmour MI, & DeMarini DM (2015). DNA adducts induced by in vitro activation of extracts of diesel and biodiesel exhaust particles. Inhal Toxicol, 27(11), 576–584. 10.3109/08958378.2015.1068892 [DOI] [PubMed] [Google Scholar]
- Schauer JJ (2003). Evaluation of elemental carbon as a marker for diesel particulate matter. J Expo Anal Environ Epidemiol, 13(6), 443–453. 10.1038/sj.jea.7500298 [DOI] [PubMed] [Google Scholar]
- Scheepers PT, Micka V, Muzyka V, Anzion R, Dahmann D, Poole J, & Bos RP (2003). Exposure to dust and particle-associated 1-nitropyrene of drivers of diesel-powered equipment in underground mining. Ann Occup Hyg, 47(5), 379–388. 10.1093/annhyg/meg036 [DOI] [PubMed] [Google Scholar]
- Seidel A, Dahmann D, Krekeler H, & Jacob J (2002). Biomonitoring of polycyclic aromatic compounds in the urine of mining workers occupationally exposed to diesel exhaust. Int J Hyg Environ Health, 204(5–6), 333–338. 10.1078/1438-4639-00116 [DOI] [PubMed] [Google Scholar]
- Shen G, Tao S, Wei S, Chen Y, Zhang Y, Shen H, Huang Y, Zhu D, Yuan C, Wang H, Wang Y, Pei L, Liao Y, Duan Y, Wang B, Wang R, Lv Y, Li W, Wang X, & Zheng X (2013). Field measurement of emission factors of PM, EC, OC, parent, nitro-, and oxy- polycyclic aromatic hydrocarbons for residential briquette, coal cake, and wood in rural Shanxi, China. Environ Sci Technol, 47(6), 2998–3005. 10.1021/es304599g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitekamp CA, Kerr LB, Dishaw L, Nichols J, Lein M, & Stewart MJ (2020). A systematic review of the health effects associated with the inhalation of particle-filtered and whole diesel exhaust. Inhal Toxicol, 32(1), 1–13. 10.1080/08958378.2020.1725187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JYY, Vermeulen R, Dai Y, Hu W, Martin WK, Warren SH, Liberatore HK, Ren D, Duan H, Niu Y, Xu J, Fu W, Meliefste K, Yang J, Ye M, Jia X, Meng T, Bassig BA, Hosgood HD, . . . Lan Q. (2021). Elevated urinary mutagenicity among those exposed to bituminous coal combustion emissions or diesel engine exhaust. Environ Mol Mutagen, 62(8), 458–470. 10.1002/em.22455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Lin Y, Wang S, Liu X, Cullinan P, Chung KF, & Zhang J (2021). Urinary Amino-Polycyclic Aromatic Hydrocarbons in Urban Residents: Finding a Biomarker for Residential Exposure to Diesel Traffic. Environ Sci Technol, 55(15), 10569–10577. 10.1021/acs.est.1c01549 [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhu T, Kipen H, Wang G, Huang W, Rich D, Zhu P, Wang Y, Lu SE, Ohman-Strickland P, Diehl S, Hu M, Tong J, Gong J, Thomas D, & Committee, H. E. I. H. R. (2013). Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Res Rep Health Eff Inst(174), 5–174. https://www.ncbi.nlm.nih.gov/pubmed/23646463 [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang Y, Wang T, Sun L, Yang Z, Lin Y, Chen Y, & Mao H (2019). Characterization of PM2.5-bound polycyclic aromatic hydrocarbons and their derivatives (nitro-and oxy-PAHs) emissions from two ship engines under different operating conditions. Chemosphere, 225, 43–52. 10.1016/j.chemosphere.2019.03.022 [DOI] [PubMed] [Google Scholar]
- Zwirner-Baier I, & Neumann H-G (1999). Polycyclic nitroarenes (nitro-PAHs) as biomarkers of exposure to diesel exhaust. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 441(1), 135–144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


