Abstract
Introduction:
Acute mortality from carbon monoxide poisonings is 1-3%. The long-term mortality risk of survivors of carbon monoxide poisoning is doubled compared to age matched controls. Cardiac involvement also increases mortality risk. We built a clinical risk score to identify carbon monoxide poisoned patients at risk for acute and long-term mortality.
Methods:
We performed retrospective analysis. We identified 811 adult carbon monoxide poisoned patients in the derivation cohort, and 462 adult patients in the validation cohort. We utilized baseline demographics, laboratory values, hospital charge transactions, discharge disposition, clinical charting information in the electronic medical record in Stepwise Akaike’s Information Criteria with Firth logistic regression to determine optimal parameters.
Results:
In the derivation cohort , 5% had inpatient or 1-year mortality. Three variables following final Firth logistic regression minimized Stepwise Akaike’s Information Criteria: altered mental status, age, and cardiac complications. The following predict inpatient or 1-year mortality: age > 67, age > 37 with cardiac complications, age > 47 with altered mental status, or any age with cardiac complications and altered mental status. The sensitivity of the score was 82% (95% CI: 65-92%), the specificity was 80% (95% CI 77-83%), negative predictive value was 99% (95% CI 98-100%), positive predictive value 17% (95% CI 12-23%), and the area under the receiver operating curve was 0.81 (95% CI: 0.74-0.87). A score above the cut-off point of −2.9 was associated with an odds ratio of 18 (95% CI: 8-40). In the validation cohort (462 patients), 4% had inpatient death or 1-year mortality. The score performed similarly in the validation cohort: sensitivity was 72% (95% CI 47-90%), specificity was 69% (95% CI 63-73%), negative predictive value was 98% (95% CI 96-99%), positive predictive value was 9% (95% CI 5-15%) and and the area under the receiver operating curve was 0.70 (95% CI: 60%-81%).
Conclusions:
We developed and validated a simple, clinical-based scoring system, the Heart-Brain 346-7 Score, based on the following: age > 67, age > 37 with cardiac complications, age > 47 with altered mental status, or any age with cardiac complications and altered mental status. With further validation, this score will aid decision making to identify carbon monoxide poisoned patients with higher mortality risk.
Keywords: Complications of poisoning, Carbon monoxide, Heart, CNS/Psychological, Metabolic, Other
Introduction:
Over 50,000 carbon monoxide (CO) poisoning cases occur in the United States yearly [1]. Normobaric oxygen, hyperbaric oxygen therapy (HBOT), and supportive care are the main treatments [1–4]. The reported case fatality rate is 1-3% [1,2]. Characteristics associated with mortality include fire as CO source, age, syncope, Glasgow Coma Scale, endotracheal intubation, myocardial injury, carboxyhemoglobin level, white blood cell count, serum sodium, creatinine, and pH less than 7.20 [5–8].
Survivors of acute CO poisoning have increased long-term mortality [5,6,9], especially those with intentional exposure [6]. Major causes of death include alcohol use disorder, motor vehicle collisions, and intentional self-harm [3]. Patients with cardiovascular complications also have increased mortality compared to those without [5,10]. The quality of life for survivors is severely affected [9] and care should involve close outpatient follow up [2].
Beyond mortality, CO poisoned patients develop neurological and cardiovascular complications [1,5,11]. Between 15% and 40% of survivors of CO poisoning suffer from permanent neurocognitive and affective deficits [5,11,12]. Ventricular dysfunction, myocardial infarction, and dysrhythmias occur in patients with moderate to severe poisonings and are associated with increased mortality [5,6]. High carboxyhemoglobin levels are associated with both acute and future myocardial infarction [5,6]. In one study, over half of moderately to severely CO-poisoned patients developed left ventricular dysfunction [9].
While CO poisoning is a major cause morbidity and mortality, there is no universal mortality prediction score. Existing scores such as the poison severity score (PSS) [13], sequential organ failure assessment (SOFA) [14], the APACHE-II score [15], and Charlson Comorbidity Index [16] have imperfect capacity to identify CO poisoning patients at high-risk of acute or long-term mortality. A proposed score for carbon monoxide poisoning can identify high risk patients for: 1) inpatient assessment, and 2) close outpatient follow-up.
Rose et al. [4] previously reported a retrospective cohort of CO poisoning patients to identify and define patients receive who HBOT in clinical practice and characterize their acute and long-term mortality.
In this study, we have utilized the medical record database of a large regional health system to generate an initial cohort from 2000 to 2014 to develop a prediction score for combined in-hospital and 1-year mortality in CO poisoning utilizing deep learning techniques. We then generated a second cohort, from 2014 to 2018, to validate the prediction algorithm.
Methods
Study Design and identifying CO poisoned patients for the derivation and validation cohorts
We included the TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis) Statement for this study in the Supplemental Information.
Data from the electronic medical records of CO poisoned patient were used. For the derivation cohort, as previously reported [4], patients aged greater than or equal to 18 years with a CO poisoning diagnosis at hospital discharge between January 2000 to April 2014 were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) codes. Patients included in this study had ICD9-CM diagnosis codes 986, E868.3, E868.8, E868.9, E982.1, E982.1, E868.2, or E982.0. Patients were identified through an electronic medical record data repository that contains full-text medical records and integrates information from central transcription, laboratory, pharmacy, finance, administrative, and other departmental databases [17]. For the derivation cohort, we identified 2,825 encounters for 1,289 unique patients in 15 hospitals between January 2000 and April 2014 with defined ICD9-CM diagnosis codes. After excluding pediatric patients (190), we identified 1,099 unique adult patients with CO poisoning. Due to previous findings that HBOT significantly lowers both acute and 1-year mortality [4], we built the derivation algorithm from 811 of 1,099 patients who had no HBOT treatment documented as a method to minimize confounders (Table 1).
Table 1:
Characteristics of the derivation cohort and validation cohort populations
| Derivation cohort | Validation cohort | P valuea | |||
|---|---|---|---|---|---|
|
| |||||
| n | Results | n | Results | ||
|
| |||||
| Demographics | |||||
|
| |||||
| Age* | 811 | 43 (30-56) | 462 | 49 (35-60) | 0.003b |
|
| |||||
| Female | 811 | 417 (51%) | 462 | 94 (33%) | < 0.001 |
|
| |||||
| Race | 793 | 462 | 0.013 | ||
| White | 547 (69%) | 348 (75%) | |||
| African American | 194 (24%) | 80 (17%) | |||
| Other | 52 (7%) | 34 (7%) | |||
|
| |||||
| Admit charge | 811 | 148 (18%) | 462 | 127 (28%) | 0.001 |
|
| |||||
| Intensive care unit charge | 811 | 50 (6%) | 141 | 39 (28%) | < 0.001 |
|
| |||||
| Outside hospital transfer | 810 | 46 (5%) | 462 | 48 (10%) | 0.002 |
|
| |||||
| Predictors * * | |||||
|
| |||||
| Altered metal status | 811 | 145 (18%) | 431 | 105 (24%) | 0.007 |
|
| |||||
| Chest pain | 811 | 101 (12%) | 431 | 42 (10%) | 0.25 |
|
| |||||
| Syncope | 811 | 102 (13%) | 431 | 101 (23%) | < 0.001 |
|
| |||||
| Shortness of breath | 811 | 113 (14%) | 431 | 90 (21%) | 0.002 |
|
| |||||
| High carboxyhemoglobin (>25%) | 811 | 71 (9%) | 385 | 60 (16%) | < 0.001 |
|
| |||||
| Cardiac complication | 811 | 57 (7%) | 431 | 62 (14%) | < 0.001 |
|
| |||||
| Outcomes: | |||||
|
| |||||
| Inpatient death | 811 | 12 (1.5%) | 462 | 8 (1.7%) | 0.73 |
|
| |||||
| 1-year mortality*** | 811 | 26 (3.2%) | 462 | 11 (2.4%) | 0.57 |
|
| |||||
| Miscellaneous: | |||||
|
| |||||
| Received hyperbaric oxygen therapy**** | 811 | 0 (0%) | 141 | 35 (25%) | < 0.001 |
Median (IQR)
female gender as a predictor is included in demographics
inpatient mortality was excluded from 1-year mortality
patients receiving HBOT were excluded from derivation cohort, included in validation cohort due to the significant effect of HBOT on mortality as previously reported (19)
= chi square test for categorical variables except for age unless otherwise stated:
= Student’s t test for continuous variables.
For the validation cohort, patients aged greater than or equal to 18 years with a CO poisoning diagnosis at hospital discharge between May 2014 to April 2018 were identified using both the ICD9-CM codes or International Classification of Diseases, Tenth Revision, Clinical Modification (ICD10-CM) codes. Database based research findings utilizing ICD-9 codes are consistent with ICD-10 codes across various disciplines [18–20]. Patients included in this study had ICD9-CM diagnosis codes above or ICD10-CM diagnosis codes of: T58.2X1A-4A, T58.8X1A-4A, T58.91XA-94XA, T58.01XA-04XA, T58.11XA-14XA, T58.0-04, T58.1-14, T58.2, T58.2X-2X4, T58.8, T58.8X-8X4, and T58.9-94. Excluding pediatric patients, we identified 462 adult patients with CO poisoning in the validation cohort in 35 hospitals between May 2014 and April 2018 (Table 1). The validation cohort contained both patients who did and who did not receive HBOT therapy.
To maintain patient confidentiality, data were de-identified using De-ID Software through the use of an honest broker system [21]. The index hospitalization was defined as the first admission meeting all inclusion criteria for patients with multiple admissions.
Data collection
Baseline demographics, laboratory values, hospital charge transactions, emergency room physician documentation, and medical record discharge data were obtained from the electronic medical data repository. Two academic internal medicine physicians reviewed the de-identified emergency department reports to record the mechanism of poisoning, symptoms, cardiac involvement and carboxyhemoglobin levels in both derivation and validation cohorts. Acute mortality was determined by hospital discharge disposition. To determine 1-year mortality, we censored patients who died in-hospital, then examined patients with a health encounter greater than 1 year from the discharge date of the index CO poisoning. Patients who did not have a visit greater than 1 year from initial CO poisoning were compared with the United States Social Security Death Index to determine death.
We created the composite variable “cardiac complication” for any of the following recorded in the electronic medical record data repository [17] on the initial patient encounter: cardiac arrest, shock, dysrhythmia or myocardial infarction mentioned in clinical chart review, elevated serum troponin > 0.10 ng/mL, or shock requiring vasopressors or ionotropes.
Study Variables and Definitions
We used variables with minimal historical information to develop a model that would be readily available. These 10 variables were assessed: altered mental status, chest pain, syncope, shortness of breath, fire exposure, motor vehicle exposure, carboxyhemoglobin levels, sex, presence of any cardiac complication (described above), and age. We did not identify patients with domestic fuel exposure as the source of CO in the derivation cohort. We combined acute mortality and 1-year mortality as the primary outcome measure due to low rate of acute mortality alone and goal of identifying high risk patients.
Charlson Comorbidity Index
We used ICD-9 and ICD-10 codes of each patient to determine medical comorbidities. We assigned a Charlson Comorbidity Index score derived from comorbidities [22].
Statistical Analysis
Results were expressed as median and inter-quartile ranges (continuous variables) or as percentages (categorical variables). We compared variable distribution using Student’s t-tests or Wilcoxon rank sum tests, as appropriate, for continuous variables, and using chi-square tests for categorical variables. All statistical analyses were performed with Stata 17.0 (StataCorp LP, College Station, TX, USA).
Model derivation and comparison to the Charlson Comorbidity Index
We used deep learning to identify a desirable model from the data set with Stepwise Akaike’s Information Criterion with backward model selection [23]. We calibrated for outliers and separation in the final model with Firth logistic regression [24]. The model was compared to the Charlson Comorbidity Index using chi square binomial test to assess sensitivity, specificity, positive predictive value, and negative predictive value. Likelihood ratio test was used to compare odds ratios.
Model validation
By the TRIPOD guidelines, we conducted internal validation of the score using a bootstrapping method in validation cohort utilizing (Supplementary Table 1) [25].
Results
Patient characteristics in derivation and validation cohorts
For the derivation cohort, we identified 2825 encounters for 1,289 unique patients in 15 hospitals between January 2000 and April 2014 with defined ICD9-CM diagnosis codes. After excluding pediatric patients (190), we identified 1,099 unique adult patients with CO poisoning. Due to previous findings that HBOT significantly lowers both acute and 1-year mortality [4], we built the derivation algorithm from 811 of 1,099 patients who had no HBOT treatment documentation to minimize confounders. Excluding pediatric patients, we identified 462 adult patients with CO poisoning in the validation cohort in 35 hospitals between May 2014 and April 2018 (Table 1). The validation cohort contained both patients who did and who did not receive HBOT therapy.
In the derivation cohort of 811 patients, the median age was 43 years, 51% were female sex, 69% were Caucasian, 24% African American, and 7% other race. Eighteen percent were admitted to the hospital, 6% required intensive care unit (ICU) level care, and 5% were transferred from outside or University of Pittsburgh Medical Center (UPMC) affiliated hospital. For predictors of interest, 18% had altered mental status, 12% had chest pain, 13% had syncope, 14% had shortness of breath, 9% had a carboxyhemoglobin level > 25%, and 7% had a cardiac complication. Twelve of 811 (1.5%) patients died while inpatients, and amongst survivors of initial CO poisoning, 3.2% (26 of 811) patients died within 1 year for a combined 38 fatalities (4.7%) (Table 1).
For the validation cohort, we identified 462 unique patients. The median age was 49 years, 33% were female sex, 75 % Caucasian, 17% African American, and 7% other race. 28% were admitted inpatient, 28% required ICU level care, and 10% were directly transferred from outside or University of Pittsburgh Medical Center (UPMC) affiliated hospital. For predictors of interest, 24% had altered mental status, 10% had chest pain, 23% had syncope, 21% had shortness of breath, 16% had a carboxyhemoglobin level > 25%, and 14% had a cardiac complication. Eight of 462 (1.7%) patients died will inpatients, and amongst survivors of initial CO poisoning, 2.4% (11 of 462) patients died within 1 year, for a combined 19 (4.1%) fatalities . Thirty-five of 141 patients (25%) were known to receive HBOT (Table 1).
Deriving a model to predict inpatient or 1-year mortality
Ten variables that would be immediately available to an accepting triage provider, with no laboratory results other than the reported carboxyhemoglobin level or potentially point of care troponin level, were included in model selection: altered mental status, chest pain, syncope, shortness of breath, fire exposure source of CO, motor vehicle exposure of CO, carboxyhemoglobin level, sex, presence of cardiac complication, and age. Through stepwise model selection, four variables were selected to minimize the Akaike’s Information Criteria: syncope, age, altered mental status, and cardiac complications. To calibrate the model using firth logistic regression and control for separation, three variables were selected: altered mental status, age, and cardiac complications. The final regression model is described in Table 2 with the “constant” parameter, or intercept, representing the output of the derived equation when all independent variables are equal to zero.
Table 2:
Final regression of the Heart-Brain 346-7 Score determined from the derivation cohort using Stepwise Akaike Information Criterion (AIC) with backward model selection, calibrated with Firth Logistic Regression to an optimized bet cut-point of −2.9.
| Coefficient | Standard error | Z | P value | 95% Confidence interval | |
|---|---|---|---|---|---|
| Age | 0.0528 | 0.00989 | 5.34 | 0.00 | 0.0334 to 0.0722 |
| Altered mental status | 1.11 | 0.371 | 2.93 | 0.003 | 0.369 to 1.86 |
| Cardiac complications | 1.64 | 0.399 | 4.10 | 0.000 | 0.855 to 2.42 |
| Constant | −6.53 | 0.682 | −9.57 | 0.000 | −7.87 to −5.19 |
The optimal cut-point for the regression to predict combined inpatient or 1-year mortality was −2.9. The following combinations of age (integer cut-off’s) and symptoms produced regression output greater than −2.9, to predict that a patient would be at risk for acute or 1-year mortality: age > 68 without other features, age > 37 with cardiac complication, age > 47 with altered mental status, and both cardiac complication and altered mental status at any age. For ease of use, functionally, the cut-off of age > 68 was changed to age > 67 to create the “Heart Brain 346-7 Score”. This simple age-category definition of the score was what we tested for performance as it is what would be used clinically. We termed the score “Heart Brain 346-7 Score” as it involves assessing if a given patient for cardiac manifestations, a neurocognitive complaint (altered mental status), and age at given cut-off points (37, 47, and 67 years old). To determine if a patient is at risk for inpatient or 1-year mortality, the three variables are input into the logistic regression to determine if the output is greater than the optimized cut-point of −2.9. For example, a 55-year-old patient with altered mental status and no cardiac complication has an output of −2.5, thus increased risk of death. Using this score, the following scenarios would be examples of a positive Heart Brain 346-7 Score: All adults with altered mental status and cardiac complication, age > 37 with cardiac complication, age > 47 with altered mental status, and all adults ages > 67. The distribution of the Heart-Brain 346-7 Score in the derivation cohort is shown in Supplementary Figure 1.
The performance of the Heart-Brain Score was assessed in the derivation cohort. The sensitivity was 82% (95% CI: 66-92%), specificity 80% (95% CI: 77-83%), and area under the receiver operating curve (AUC ROC) was 0.81 (95% CI: .74-.87). The positive predictive value (PPV) was 17% (12-23%) and the negative predictive value (NPV) was 99% (98-99%). A Heart Brain 346-7 Score greater than the cut-off point has an odds ratio of 18 (7.8-40) for inpatient or 1-year mortality (Table 3).
Table 3:
Performance of the Heart-Brain 346-7 Score in the derivation cohort to predict inpatient death or 1-year mortality
| Heart-Brain 346-7 Score positive (n) | Heart-Brain 346-7 Score negative (n) | Total (n) | |
|---|---|---|---|
| Inpatient or 1-year mortality (n) | 31 | 7 | 38 |
| No mortality (n) | 155 | 618 | 773 |
| Total (n) | 186 | 625 | 811 |
| Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
OR (95% CI) |
AUC ROC (95%CI) |
|---|---|---|---|---|---|
| 82% (66-92%) |
80% (77-83%) |
17% (12-23%) |
99% (98-99%) |
18 (7.8-40) |
0.81 (0.71-0.87) |
CI = confidence interval: PPV = positive predictive value: NPV = negative predictive value: OR = odds ratio: AUC ROC = Area under curve for receiver operating characteristic curve.
Comparing the Heart Brain 346-7 Score to the Charlson Comorbidity Index in the derivation cohort
We compared the performance of the Heart Brain 346-7 Score to a commonly used prognostic tool, the Charlson Comorbidity Index. The Charlson Comorbidity Index is validated for predicting 1-year or in-hospital mortality [26,27]. The Charlson Comorbidity Index was easily obtainable with retrospective, EMR based data (e.g. ICD-9 or ICD-10 codes) [26]. We determined the cut-off of a Charlson Comorbidity Index of 2 has the highest AUC ROC to predict inpatient or 1-year death in this cohort. 791 patients in the derivation cohort have a Charlson Comorbidity Index of 2. The Charlson Comorbidity Index of 2 was associated with a sensitivity was 25% (95% CI: 12-42%), specificity 96% (95% CI: 94-97%), PPV 21% (10-36%), and NPV 96% (95-98%). A CCI of 2 has an odds ratio of 7.1 (3.1-16) for the combined outcome measure. The Heart Brain 346-7 Score was superior to a Charlson Comorbidity Index of 2 for sensitivity (P < 0.0001), NPV (P = 0.003), and diagnostic odds ratio (P < 0.001) (Table 3). The Heart Brain 346-7 Score was inferior to the Charlson Comorbidity Index for specificity (P < 0.0001) and similar in PPV (p=0.51) (Figure 1).
Figure 1:
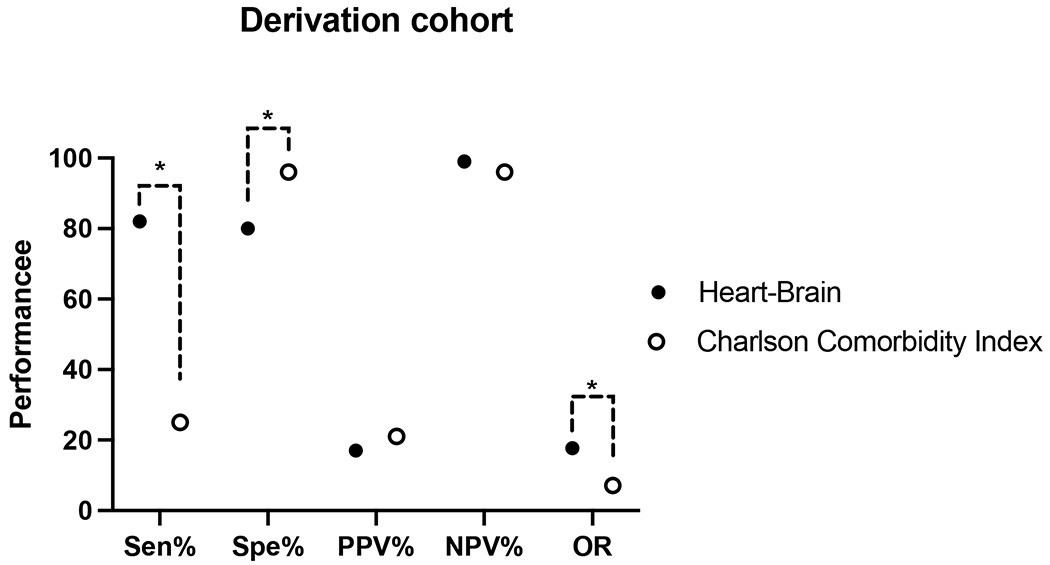
Comparison of the Heart Brain 346-7 Score with the Charlson Comorbidity Index of 2 in the Derivation cohort. A Charlson Comorbidity Index of 2 has the highest performance to predict inpatient or 1-year death in this cohort. The sensitivity, specificity, negative predictive value, positive predictive value expressed in percentage and odds ratio expressed as unitless. PPV = positive predictive value: NPV = negative predictive value; OR = diagnostic odds ratio. * = P value < 0.01
Heart Brain 346-7 Score performance in the validation cohort
In the validation cohort of 462 patients, 19 (4.1%) experienced the combined inpatient death or death within 1 year of CO poisoning. The Heart-Brain 346-7 Score was able to be calculated in 431 patients. Due to missing retrospective chart review data, the Heart-Brain 346-7 Score could not be calculated in one survivor of inpatient hospitalization who died within 1 year and 30 patients who survived to 1 year and thus were not included in the analysis. One hundred forty-three of the 431 patients had a positive Heart-Brain 346-7 Score (33%). Of those 143 patients with a positive Heart-Brain 346-7 Score, 13 (9.1%) had the combined outcome of inpatient or 1-year mortality. Patients with a negative Heart-Brain 346-7 Score (5 of 288) had a 1.7% combined outcome. The distribution of the Heart-Brain 346-7 Score in the validation cohort is shown in Supplementary Figure 2.The performance of the score in the validation cohort consisted the following: sensitivity 72% (95% CI: 47-90%), specificity 69% (95% CI 64-73%), PPV 9.0% (95% CI: 5.0-15%), NPV 98% (95% CI: 96-99%), odds ratio 5.7 (95% CI 2.0-16), and AUC ROC 0.70 (95% CI 60%-81%) (Table 4).
Table 4:
Performance of the Heart-Brain 346-7 Score in the validation cohort to predict inpatient death or 1-year mortality
| Heart-Brain 346-7 Score positive (n) | Heart-Brain 346-7 Score negative (n) | Total (n) | |
|---|---|---|---|
| Inpatient or 1-year mortality (n) | 13 | 5 | 18 |
| No mortality (n) | 130 | 283 | 413 |
| Total (n) | 143 | 288 | 431 |
| Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
OR (95% CI) |
AUC ROC (95%CI) |
|---|---|---|---|---|---|
| 72% (47-90%) |
69% (64-73%) |
9.0% (5.0-15%) |
98% (96-99%) |
5.7 (2.0-16) |
0.70 (0.60-0.81) |
CI = confidence interval: PPV = positive predictive value: NPV = negative predictive value: OR = odds ratio: AUC ROC = Area under curve for receiver operating characteristic curve.
As a sensitivity analysis, we evaluated Heart Brain 346-7 score’s performance on acute, one-year, and three-year mortality separately in the validation cohort. We censored acute mortality when evaluating one-year and three-year morality. When evaluating for inpatient mortality alone, the Heart-Brain 346-7 Score performed similarly as combined acute and one-year mortality, sensitivity 100% (63-100%), specificity 68% (63-73%), PPV 5.6% (2.5-11%), NPV 100% (99-100%) (Supplementary Table 2). When evaluating for one-year mortality, the Heart-Brain 346-7 Score had higher specificity while retaining high NPV compared to the combined outcome, sensitivity 50% (19-81%), specificity 97% (95-99%), PPV 29% (10-56%), NPV 99% (97-100%) (Supplementary Table 3). When evaluating for three-year mortality alone, the Heart-Brain 346-7 Score had higher specificity and PPV while retaining high NPV compared to the combined outcome, sensitivity 65% (44-83%), specificity 100% (99-100%), PPV 100% (81-100%), NPV 98% (96-99%) (Supplementary Table 4).
Charlson Comorbidity Index performance in the validation cohort
We compared the performance of Heart Brain 346-7 Score in the validation cohort to the Charlson Comorbidity Index. We determined the Charlson Comorbidity Indexfor 431 patients. We determined the cut-off of CCI of 2 has the highest AUC ROC to predict inpatient or 1-year death. The CCI of 2 had the following performance: sensitivity 39% (95% CI: 16-62%), specificity 85% (95% CI: 82-89%), PPV 10% (95% CI: 4-20%), NPV 97% (95% CI: 95-98%), and odds ratio of 5.74. The Heart Brain 346-7 Score was superior to a CCI of 2 for sensitivity (P < 0.05) and odds ratio (P < 0.02) (Figure 2), similar for PPV (P =0.81) and NPV (P = 0.29), and inferior for specificity (P < 0.001) (Figure 2).
Figure 2:
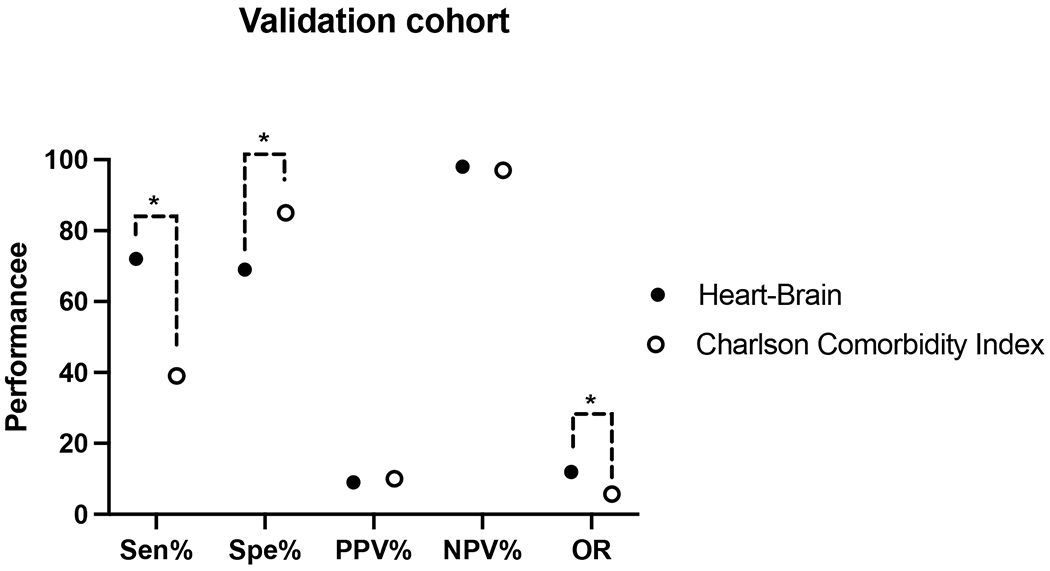
Comparison of the Heart Brain 346-7 Score with the Charlson Index of 2 in the Validation cohort. A Charlson Comorbidity Index of 2 has the highest performance to predict inpatient or 1-year death in this cohort. The sensitivity, specificity, negative predictive value, positive predictive value expressed in percentage and odds ratio expressed as unitless. PPV = positive predictive value: NPV = negative predictive value; OR = diagnostic odds ratio. * = P value < 0.01
Discussion:
We developed a novel mortality prediction score utilizing machine learning termed the Heart-Brain 346-7 Score. This score utilizes altered mental status, age, and cardiac complications, to predict inpatient or 1-year mortality. The score performed similarly across two different derivation and validation populations. The Heart-Brain Score performed “good” in the derivation cohort and “fair” in the validation cohort when examining the ROC score [28,29]. Diagnostic tools generated from machine learning have limitations in real-world validation [30,31]. The Heart-Brain 346-7 Score’s similar performance in a separate, real-world data validation cohort demonstrates its potential usefulness for an AI-derived diagnostic tool. We also demonstrated the score is a useful tool when assessing acute, 1-year, and 3-year mortality independently (not acute and long-term mortality combined) in the derivation cohort. While the negative predictive value is high and the positive predictive value is low, one would potentially accept this level of performance as this is a tool to guide triage, observation, and follow-up decisions.
Both derivation and validation cohorts enrolled diverse patients with varying age, race, and hospital disposition. The validation cohort was older, more male dominant, and had more Caucasians compared to the derivation cohort. The validation cohort had more severe CO poisoning patients with higher proportion of outside hospital transfer, hospital admission, ICU admission. Another critical difference between the cohorts was whether they received HBOT. Timely administration of HBOT is associated with improved acute and inpatient mortality [4]. We excluded patients who received HBOT in the derivation cohort due to this known effect in the cohort [4]. The addition of patients who eventual received HBOT could factor into the lower sensitivity and specificity of the Heart-Brain 346-7 score in the validation cohort. In both cohorts, the Heart-Brain 346-7 score had high negative predictive value, which speaks to this unique score to differentiate CO poisoning patients at low vs. high risk.
The Heart-Brain-346-7 score has advantages over other validated scores, albeit in different populations. The poison severity score (PSS) is a 5 grade severity grading system based in 12 organ systems with features requiring laboratory values (e.g., musculoskeletal requires CPK concentrations) [13,32]. The sequential organ failure assessment (SOFA) and the Acute Physiology and Chronic Health disease Classification System II (APACHE-II) score predicts ICU mortality using laboratory results and clinical data [14,15,33]. The Heart-Brain 346-7 score does not require laboratory results that are necessary for the PSS, APACHE-II, and SOFA [15,33]. The Charlson Comorbidity Index was developed from inpatients to predict long-term mortality [16]. Co-morbidities from ICD-9 codes are prerequisites for the Charlson Comorbidity Index [16,34]. This is not readily available at clinical presentation when many CO-poisoned patients present with acute mental status changes and have non-reliable histories. The Charlson Comorbidity Index is also not validated in CO poisoning [16]. In contrast, the Heart Brain 346-7 Score is specifically designed for CO poisoning patients. In this study, we demonstrated that the Heart-Brain-346-7 score is superior to the Charlson Comorbidity Index in terms of sensitivity and odds ratio. Finally, the Heart Brain 346-7 score is more easily implemented due to its simplicity similar to other mortality prediction scores[35] [36]. The Heart Brain 346-7 only requires three clinical inputs that should be readily available to even a triaging initial provider without needing laboratory criteria and thus has the potential to have widespread use.
The Heart Brain 346-7 Score utilizes age, cardiac complication, and altered mental status as the main mortality predictors. Heart-Brain Score specifically defines age cutoffs of less than 37, 37 to 47, and more than 67 years old for mortality risk that may become more accepted with further validation. Additionally, the score suggests cardiac and neurological involvement in CO poisoning correlates with poor outcomes. Prior investigation has similar findings. For a 1 mg/m3 increase of average environmental carbon monoxide concentration on the present day versus previous day, there was a significant increase in daily mortality from cardiovascular disease, from coronary artery disease, and from stroke. = [37]. A low initial Glasgow Coma Scale is associated with delayed neurological sequelae [38]. Additionally, CO poisoning mortality proportionally correlates with age and peaks in patients older than 80 years old [39]. However, age may be confounding as it is correlated with poorer cardiovascular health and neurological comorbidities [40,41]. Nevertheless, it suggests that laboratory values such as carboxyhemoglobin, a well-established test for CO poisoning, may not have a simple linear relationship with mortality.
Rose et al. [4] found a significant impact of receiving HBOT on mortality, we could not derive the score from those patients that received HBOT in the derivation cohort. To make the score have broader generalizability in all patients presenting to the emergency department with CO poisoning, the validation cohort included patients who would and would not receive HBOT. This could have impacted the performance of the score. The Heart Brain 346-7 Score differentiates only mortality risk with limited stratification. Thus, it would be valuable as a screening mortality tool rather than a sophisticated mortality prognosticator. Significant morbidities of CO poisoning stem from neurocognitive deficits [11,12]. We did not assess neurocognitive impact, in regard to survivor function or healthcare utilization. The score was developed and validated in a state-wide health system. This could diminish global applicability of the score. We did not record intention on patients due to lack of reliable data. Intentional poisonings have been reported to have higher mortality than non-intentional poisonings [3,42].
Conclusions:
We developed and validated a simple scoring system to risk stratify carbon monoxide poisoned patients. Further validation of this score at other sites would help in evaluating its performance. The Heart-Brain 346-7 score can be used to triage high risk carbon monoxide poisoned patients at initial presentation and identify high risk survivors upon hospital discharge
Supplementary Material
Acknowledgements:
Dr. Jason J. Rose and Dr. Michael S. Zhang contributed equally to this manuscript. The authors acknowledge Dr. Michael Donahoe and Dr. Mark Gladwin for their contributions.
Financial Support:
National Institutes of Health, Parker B Francis Foundation, Robin B. Martin Family Foundation, U.S. Department of Defense. These funding sources were not involved in the collection, management, analysis or interpretation of the data or the preparation, review, or approval of the manuscript.
Declaration of Interests:
Dr. Rose is a coinventor on patents and applications related to using carbon monoxide scavenging molecules as therapies for carbon monoxide poisoning, licensed to Globin Solutions. Globin Solutions have a license for technologies using sodium nitrite as a therapeutic against cardiovascular disease from the National Institutes of Health and the University of Pittsburgh. Dr. Rose is a coinventor on a patent of using nitrite as a treatment for halogen gas inhalation and smoke inhalation injuries. Dr. Rose is a co-founder, officer, and director of Globin Solutions. Dr. Rose’s role in Globin Solutions disclosed above does not have direct conflicts of interests with the contents of this manuscript. Globin Solutions is developing a carbon monoxide poisoning antidote. Globin Solutions did not provide financial support to this study. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References:
- [1].Rose JJ, Wang L, Xu Q, et al. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am J Respir Crit Care Med. 2017;195:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hampson NB, Piantadosi CA, Thom SR, et al. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am J Respir Crit Care Med. 2012;186:1095–1101. [DOI] [PubMed] [Google Scholar]
- [3].Hampson NB. U.S. Mortality Due to Carbon Monoxide Poisoning, 1999-2014. Accidental and Intentional Deaths. Ann Am Thorac Soc. 2016;13:1768–1774. [DOI] [PubMed] [Google Scholar]
- [4].Rose JJ, Nouraie M, Gauthier MC, et al. Clinical Outcomes and Mortality Impact of Hyperbaric Oxygen Therapy in Patients With Carbon Monoxide Poisoning. Crit Care Med. 2018;46:e649–e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Henry CR, Satran D, Lindgren B, et al. Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA. 2006;295:398–402. [DOI] [PubMed] [Google Scholar]
- [6].Hampson NB, Rudd RA, Hauff NM. Increased long-term mortality among survivors of acute carbon monoxide poisoning. Crit Care Med. 2009;37:1941–1947. [DOI] [PubMed] [Google Scholar]
- [7].Kao H-K, Lien T-C, Kou YR, et al. Assessment of myocardial injury in the emergency department independently predicts the short-term poor outcome in patients with severe carbon monoxide poisoning receiving mechanical ventilation and hyperbaric oxygen therapy. Pulm Pharmacol Ther. 2009;22:473–477. [DOI] [PubMed] [Google Scholar]
- [8].Amirabadizadeh A, Nakhaee S, Jahani F, et al. Prognostic indicators in critically ill poisoned patients: development of a risk-prediction nomogram. Drug Metab Pers Ther. 2020;/j/dmdi.ahead-of-print/dmdi-2020-0108/dmdi-2020-0108.xml. [DOI] [PubMed] [Google Scholar]
- [9].Huang C-C, Chung M-H, Weng S-F, et al. Long-term prognosis of patients with carbon monoxide poisoning: a nationwide cohort study. PLoS One. 2014;9:e105503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Garg J, Krishnamoorthy P, Palaniswamy C, et al. Cardiovascular Abnormalities in Carbon Monoxide Poisoning. Am J Ther. 2018;25:e339–e348. [DOI] [PubMed] [Google Scholar]
- [11].Weaver LK, Hopkins RO, Churchill SK, et al. Neurological outcomes 6 years after acute carbon monoxide poisoning. Undersea Hyperb Med. 2008;35. [Google Scholar]
- [12].Hopkins RO, Weaver LK. Cognitive outcomes 6 years after acute carbon monoxide poisoning. Undersea Hyperb Med. 2008;35. [Google Scholar]
- [13].Schwarz ES, Kopec KT, Wiegand TJ, et al. Should We Be Using the Poisoning Severity Score? J Med Toxicol. 2017;13:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. [DOI] [PubMed] [Google Scholar]
- [15].Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- [16].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- [17].Yount RJ, Vries JK, Councill CD. The medical archival system: an information retrieval system based on distributed parallel processing. Information processing & management. 1991;27:379–389. [Google Scholar]
- [18].Cheng D, DuMontier C, Yildirim C, et al. Updating and Validating the U.S. Veterans Affairs Frailty Index: Transitioning From ICD-9 to ICD-10. J Gerontol A Biol Sci Med Sci. 2021;76:1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hernandez-Ibarburu G, Perez-Rey D, Alonso-Oset E, et al. ICD-10-CM extension with ICD-9 diagnosis codes to support integrated access to clinical legacy data. Int J Med Inform. 2019;129:189–197. [DOI] [PubMed] [Google Scholar]
- [20].Ohnuma T, Raghunathan K, Fuller M, et al. Trends in Comorbidities and Complications Using ICD-9 and ICD-10 in Total Hip and Knee Arthroplasties. J Bone Joint Surg Am. 2021;103:696–704. [DOI] [PubMed] [Google Scholar]
- [21].Gupta D, Saul M, Gilbertson J. Evaluation of a deidentification (De-Id) software engine to share pathology reports and clinical documents for research. Am J Clin Pathol. 2004;121:176–186. [DOI] [PubMed] [Google Scholar]
- [22].Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- [23].Akaike H A new look at the statistical model identification. IEEE transactions on automatic control. 1974;19:716–723. [Google Scholar]
- [24].Puhr R, Heinze G, Nold M, et al. Firth’s logistic regression with rare events: accurate effect estimates and predictions? Stat Med. 2017;36:2302–2317. [DOI] [PubMed] [Google Scholar]
- [25].Moons KGM, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–73. [DOI] [PubMed] [Google Scholar]
- [26].Charlson ME, Carrozzino D, Guidi J, et al. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. PPS. 2022;91:8–35. [DOI] [PubMed] [Google Scholar]
- [27].Bannay A, Chaignot C, Blotière P-O, et al. The Best Use of the Charlson Comorbidity Index With Electronic Health Care Database to Predict Mortality. Med Care. 2016;54:188–194. [DOI] [PubMed] [Google Scholar]
- [28].DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- [29].Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol. 2022;75:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vazquez-Zapien GJ, Mata-Miranda MM, Garibay-Gonzalez F, et al. Artificial intelligence model validation before its application in clinical diagnosis assistance. World J Gastroenterol. 2022;28:602–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Domalpally A, Channa R. Real-world validation of artificial intelligence algorithms for ophthalmic imaging. The Lancet Digital Health. 2021;3:e463–e464. [DOI] [PubMed] [Google Scholar]
- [32].Persson HE, Sjöberg GK, Haines JA, et al. Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36:205–213. [DOI] [PubMed] [Google Scholar]
- [33].Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- [34].Brusselaers N, Lagergren J. The Charlson Comorbidity Index in Registry-based Research. Methods Inf Med. 2017;56:401–406. [DOI] [PubMed] [Google Scholar]
- [35].Dzeshka MS, Lane DA, Lip GYH. Stroke and bleeding risk in atrial fibrillation: navigating the alphabet soup of risk-score acronyms (CHADS2 , CHA2 DS2 -VASc, R2 CHADS2 , HAS-BLED, ATRIA, and more). Clin Cardiol. 2014;37:634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Proietti M, Farcomeni A, Romiti GF, et al. Association between clinical risk scores and mortality in atrial fibrillation: Systematic review and network meta-regression of 669,000 patients. Eur J Prev Cardiol. 2020;27:633–644. [DOI] [PubMed] [Google Scholar]
- [37].Liu C, Yin P, Chen R, et al. Ambient carbon monoxide and cardiovascular mortality: a nationwide time-series analysis in 272 cities in China. Lancet Planet Health. 2018;2:e12–e18. [DOI] [PubMed] [Google Scholar]
- [38].Sert ET, Kokulu K, Mutlu H. Clinical predictors of delayed neurological sequelae in charcoal-burning carbon monoxide poisoning. Am J Emerg Med. 2021;48:12–17. [DOI] [PubMed] [Google Scholar]
- [39].Mattiuzzi C, Lippi G. Worldwide epidemiology of carbon monoxide poisoning. Hum Exp Toxicol. 2020;39:387–392. [DOI] [PubMed] [Google Scholar]
- [40].Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016;594:2061–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Novy J, Sander JW. Age, Comorbidity, Frailty in Observational and Analytic Studies of Neurological Diseases. Front Neurol Neurosci. 2016;39:71–80. [DOI] [PubMed] [Google Scholar]
- [42].Simonsen C, Thorsteinsson K, Mortensen RN, et al. Carbon monoxide poisoning in Denmark with focus on mortality and factors contributing to mortality. PLoS One. 2019;14:e0210767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


