Abstract
Primary pericardial mesotheliomas are extremely rare, accounting for <1% of all mesotheliomas, and their molecular genetic features and predisposing factors remain to be determined. Here, we report the clinicopathologic, immunohistochemical, and molecular genetic findings of three pericardial mesotheliomas without pleural involvement. Three cases diagnosed between 2004 and 2022 were included in the study and analyzed by immunohistochemistry and targeted next-generation sequencing (NGS); corresponding nonneoplastic tissue was sequenced in all cases. Two patients were female and one male, aged between 66 and 75 years. Two patients each had prior asbestos exposure and were smokers. Histologic subtypes were epithelioid in two cases and biphasic in one case. Immunohistochemical staining identified expression of cytokeratin AE1/AE3 and calretinin in all cases, D2–40 in two cases, and WT1 in one case. Staining for tumor suppressors revealed loss of p16, MTAP, and Merlin (NF2) expression in two cases, and loss of BAP1 and p53 in one case. Abnormal cytoplasmic BAP1 expression was observed in an additional case. Protein expression abnormalities correlated with NGS results, which showed concurrent complete genomic inactivation of CDKN2A/p16, CDKN2B, MTAP, and NF2 in two mesotheliomas and of BAP1 and TP53 in one mesothelioma each, respectively. In addition, one patient harbored a pathogenic BRCA1 germline mutation which resulted in biallelic inactivation in the mesothelioma. All mesotheliomas were mismatch repair proficient and showed several chromosomal gains and losses. All patients died from disease. In summary, our studies demonstrate that pericardial mesotheliomas share common morphologic, immunohistochemical, and molecular genetic features with pleural mesothelioma including recurrent genomic inactivation of canonical tumor suppressors. Our study adds new insights into the genetic landscape of primary pericardial mesothelioma and highlights BRCA1 loss as a potential contributing factor in a subset of cases, thereby contributing to refined precision diagnostics for this rare cancer.
Keywords: Mesothelioma, pericardium, asbestos, next-generation sequencing, BRCA1, germline, tumor suppressor, cell cycle regulator
Introduction
Mesothelioma most commonly arises in the pleura, accounting for ~90% of cases, followed by the peritoneum in ~10% of cases.1 Primary pericardial mesotheliomas are extremely rare and comprise <1% of all mesotheliomas, with an estimated annual incidence of 10–15 new cases in the United States.1,2 To date, only few cases of pericardial mesothelioma have been described, mostly as case reports3,4, with the largest single-site series including 12 cases.5 They most commonly affect adults ~60 years of age and patients usually present with dyspnea, chest pain, cough, signs of constrictive pericarditis, and heart failure.1,2,5–7 Given its nonspecific clinical presentation, pericardial mesothelioma is diagnosed a median of three months after clinical presentation and often post mortem.3,7 Pericardial mesothelioma spreads locally around the large vessels and into the myocardium and follows a very aggressive clinical course with a mean survival of 2.5–6 months.6,7 While asbestos exposure has long been established as a predisposing factor for pleural mesothelioma8, its role in the development of primary pericardial mesothelioma remains controversial. While some studies observed a significant association between occupational asbestos exposure and pericardial mesothelioma6,7 with detection of asbestos fibers in the lungs of affected patients9,10, other groups did not identify any such association.1,5
Most reported cases of pericardial mesothelioma exhibited epithelioid morphology5–7 and showed expression of immunohistochemical markers typically observed in pleural mesothelioma, such as keratins, calretinin, D2–40 (podoplanin), and WT1, and negativity for TTF1 and claudin-4.3 To date, only three cases of pericardial mesothelioma included in two different studies have been analyzed by next generation sequencing (NGS) and exhibited genomic aberrations that inactivated CDKN2A/B, BAP1, TP53, and NF25,11 representing aberrations typically observed in pleural mesothelioma.11,12 However, a possible role of tumor suppressor germline mutations, which have been identified as predisposing factor for the development of pleural mesothelioma (e.g., BAP1 mutations)13,14, remains to be determined in pericardial mesothelioma.
Here, we report the clinicopathologic, immunohistochemical, and molecular genetic findings in a series of three primary pericardial mesotheliomas.
Materials and Methods
Case Selection
This study was performed with the approval of the Institutional Review Board at Brigham and Women’s Hospital. Cases were retrieved from the surgical pathology files of Brigham and Women’s Hospital. In total, three epithelioid primary pericardial mesotheliomas without pleural involvement diagnosed between 2004 and 2022 were identified and examined postmortem (cases #1 and #3) or on surgical resection (case #2).
Immunohistochemistry
Immunohistochemistry was performed for all mesotheliomas on 4-μm-thick formalin-fixed paraffin-embedded whole tissue sections using the antibodies and staining condition listed in Table 1. Appropriate positive and negative controls were used throughout. All stains were independently evaluated by two anatomic pathologists (I.-M.S. and L.M.S.). For the tumor suppressors p16, MTAP, BAP1, Merlin (NF2), and p53, expression was considered lost (i.e., negative) when there was loss of nuclear (p16, BAP1, p53), cytoplasmic (MTAP), or membranous (Merlin) expression. In addition, loss of nuclear BAP1 expression and concurrent cytoplasmic BAP1 expression was considered abnormal. Strong nuclear expression of p53 in >95% of tumor cells was considered abnormal/overexpressed. Nonneoplastic cells (vascular endothelial cells and inflammatory cells) were used as internal positive control.
Table 1.
Immunohistochemical antibodies and staining conditions.
| Antibody | Clone | Dilution | Source | Pretreatment | Detection system |
|---|---|---|---|---|---|
| AE1/AE3 | AE1/AE3 | 1:200 | Agilent, Santa Clara, CA, USA | 10 mins protease | Envision Plus |
| Calretinin | Polyclonal | 1:200 | Invitrogen, Carlsbad, CA, USA | pH 6 citrate buffer, pressure cooker | Envision Plus |
| D2–40 | D2–40 | 1:100 | Biolegend, San Diego, CA, USA | None | Envision Plus |
| WT1 | 6F-H2 | 1:75 | Agilent, Santa Clara, CA, USA | pH 6 citrate buffer, pressure cooker | Envision Plus |
| TTF1 | 8G7G3/1 | 1:800 | Agilent, Santa Clara, CA, USA | pH 6 citrate buffer, pressure cooker | Envision Plus |
| Claudin-4 | 3E2C1 | 1:300 | Invitrogen, Carlsbad, CA, USA | pH 6 citrate buffer, pressure cooker | Envision Plus |
| BAP1 | C-4 | 1:100 | Santa Cruz Biotechnology, Dallas, TX, USA | pH 6 citrate buffer, pressure cooker | Envision Plus |
| p16 | BC42 | 1:50 | Biocare, Pacheco, CA, USA | pH 6 citrate buffer, pressure cooker | Envision Plus |
| MTAP | 42-T | 1:200 | Santa Cruz Biotechnology, Dallas, TX, USA | pH 6 citrate buffer, pressure cooker | Novolink |
| p53 | DO-7 | 1:500 | Agilent, Santa Clara, CA, USA | pH 6 citrate buffer, pressure cooker | Envision Plus |
| Merlin (NF2) | D1D8 | 1:250 | Cell Signaling Technology, Danvers, MA, USA | pH 6 citrate buffer, pressure cooker | Novolink |
Targeted Next-Generation Sequencing
Next-generation sequencing was performed using our institutional targeted sequencing platform, OncoPanel, which interrogates the exonic sequences of 447 cancer-associated genes for mutations and copy number variations, and 191 introns across 60 genes for gene rearrangements.15,16 DNA extraction from formalin-fixed paraffin-embedded tissue (QIAamp DNA mini kit, Qiagen, Valencia, CA, USA), construction of hybrid-capture libraries, sequencing using the Illumina HiSeq 2500 (Illumina, San Diego, CA, USA), and sequence data analysis was performed as previously described.16 All detected alterations (including single nucleotide variants, copy number alterations, and translocation calls) were reviewed and annotated manually.
Results
Clinicopathologic Findings
Table 2 summarizes the clinicopathologic findings of the three primary pericardial mesotheliomas. The cases included one male and two female patients aged 75, 66, and 68 years, respectively. The tumors were located in the pericardium without pleural involvement by imaging studies and/or examination at autopsy.
Table 2.
Clinicopathologic features of three cases of primary pericardial mesothelioma.
| Case #1 | Case #2 | Case #3 | |
|---|---|---|---|
| Age/Sex | 75/M | 66/F | 68/F |
| Clinical presentation | Hypoxia, pericardial effusion, and biventricular dysfunction | Dyspnea and intermittent epigastric pain | Chest pain, abdominal pain, and complete heart block |
| Pleural involvement | No | No | No |
| Prior asbestos exposure | Yes | Yes | N/A |
| Smoker | No | Yes (40 Py) | Yes (N/A) |
| Treatment | Partial pericardial resection | Extensive pericardial resection | None |
| Follow-up status | DOD (<1 week) | DOD (15 months) | DOD (<1 week) |
| Histologic subtype | Epithelioid | Epithelioid | Biphasic (sarcomatoid component: 60%) |
| Histologic grade | High | Low | - |
| Immunohistochemistry | |||
| AE1/AE3 | Positive | Positive | Positive |
| Calretinin | Positive | Positive | Positive |
| D2–40 | Positive | Negative | Negative |
| WT1 | Negative | Positive | Negative |
| TTF1 | Negative | Negative | Negative |
| Claudin-4 | Negative | Negative | Negative |
DOD: Died of disease; F: Female; M: Male; N/A: Not available; Py: Pack years
Patient #1 was a 75-year-old male with a history of cardiac arrhythmias (right and left bundle branch block, atrial flutter, and atrial fibrillation) who presented with hypoxia and recurrent pericardial effusion with evidence of pericardial thickening leading to biventricular dysfunction. He had a history of asbestos exposure and was a non-smoker. The patient underwent an open mediastinotomy with attempted pericardiectomy which demonstrated an irregular, thickened, nodular pericardium plastered to and encasing the heart and ascending aorta. Intraoperative frozen section examination was highly suggestive of mesothelioma. The tumor was partially removed but was ultimately deemed unresectable. The patient expired the same day in cardiogenic shock and underwent autopsy examination limited to the chest and abdomen which revealed a tan to white solid mass which diffusely replaced the pericardium extending from the base of the heart to the apex (Figure 1A). Locoregional lymph nodes were involved by metastatic spread. Given the absence of pleural involvement, a diagnosis of primary pericardial mesothelioma was made.
Figure 1. Gross and radiologic findings of primary pericardial mesothelioma.
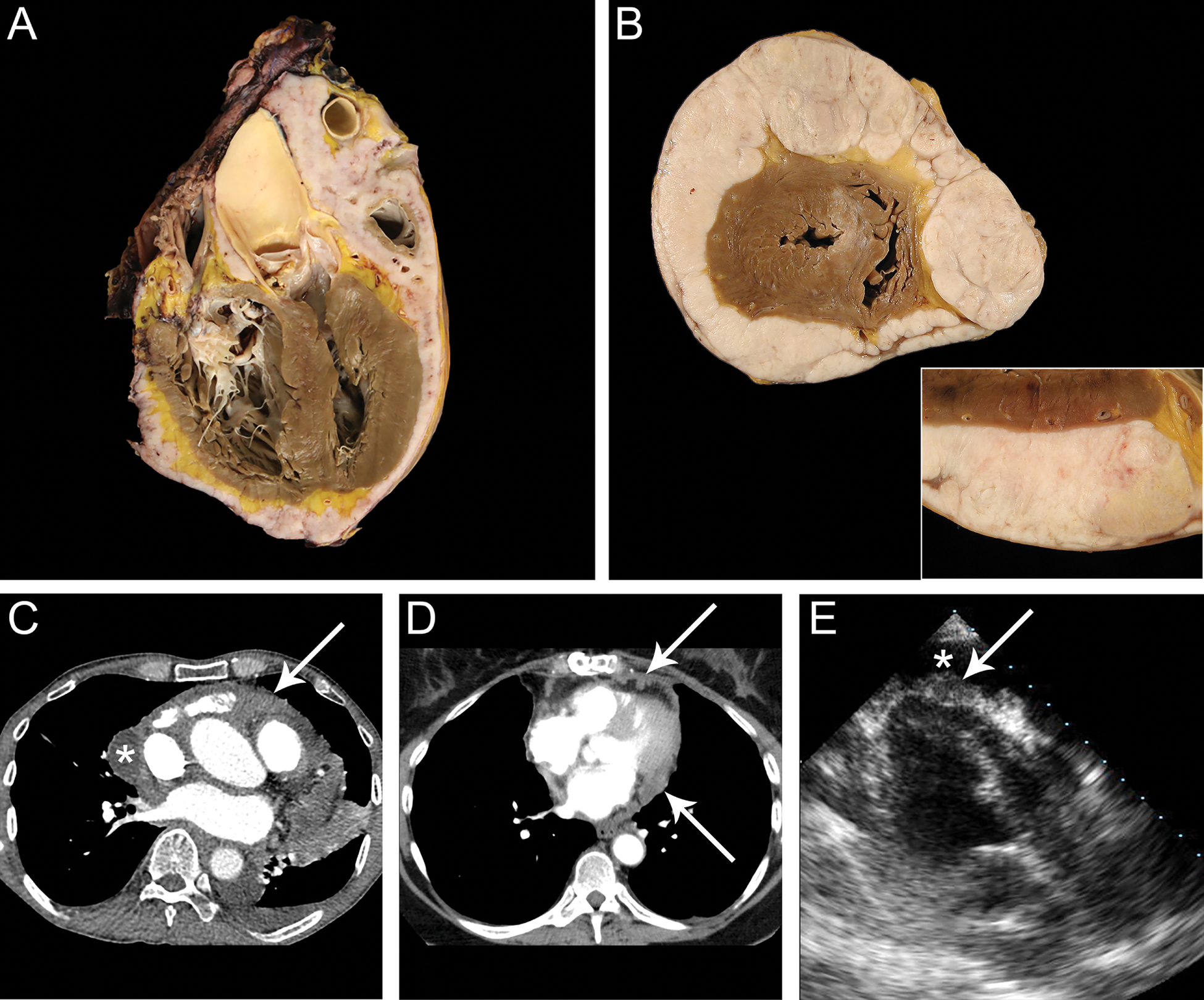
Gross examination at autopsy in cases #1 (A) and #3 (B; B, inset) revealed a solid tan to white mass replacing the pericardium and completely encasing the heart from the base to the apex. C Contrast-enhanced chest CT imaging in case #1 showed a loculated pericardial effusion (*) with soft tissue in the superior pericardial recess and anterior pericardial space as well as circumferential thickening and enhancement of the visceral and parietal pericardium (arrow). D In case #2, contrastenhanced chest CT imaging displayed nodular pericardial thickening with nodules invading the epicardial fat (arrows). E Three-chamber echocardiography imaging revealed a pericardial effusion (*) with nodular soft tissue along the visceral pericardium (arrow).
Patient #2 was a 66-year-old female with a history of systemic hypertension, hypothyroidism, and gastroesophageal reflux disease who was hospitalized for dyspnea and intermittent epigastric pain. She had a history of asbestos exposure and a smoking history of 40 pack-years. During her hospitalization, she was found to have a large pericardial effusion and nodularity of the pericardium. Imaging studies demonstrated an ill-defined soft tissue mass along the anterior and the inferior pericardium immediately adjacent to the diaphragm and along the ascending aorta with the most prominent nodular area of pericardial enhancement noted near the cardiac apex. The pleura was not involved. Histopathologic examination of a pericardial biopsy revealed mesothelioma. She subsequently underwent radical pericardiectomy with radical left lymphadenectomy and heated intraoperative chemotherapy. Histopathologic examination of the resected specimens revealed mesothelioma extensively involving the pericardium and abutting the diaphragmatic skeletal muscle. Given the absence of pleural involvement, a diagnosis of primary pericardial mesothelioma was made. She was subsequently discharged and died 15 months later from disease recurrence.
Patient #3 was a 68-year-old female with a history of gastric carcinoma (status post partial gastrectomy 7 years earlier), diffuse atherosclerosis, and peripheral vascular disease with remote myocardial infarction and stroke who presented with chest pain, abdominal pain, and complete heart block. The patient was a smoker with no known asbestos exposure. Physical exam revealed decompensated congestive heart failure and pericardial and pleural effusions. Cardiac catheterization identified severe three-vessel disease, and the patient was admitted to undergo cardiac surgery for coronary bypass grafting which was ultimately cancelled after an angiogram showed complete atherosclerotic occlusion of the distal aorta at the level of the renal arteries. The patient developed ventricular tachycardia and subsequently expired. She underwent unrestricted autopsy examination which revealed a tan to white solid mass and diffusely replacing the pericardium extending from the base to the apex (Figure 1B). Given the absence of pleural involvement, a diagnosis of primary pericardial mesothelioma was made.
Radiologic Findings
Figure 1C–E summarizes the radiologic findings for the three primary pericardial mesotheliomas. In patient #1, contrast-enhanced chest CT images showed a loculated pericardial effusion with soft tissue in the superior pericardial recess and anterior pericardial space as well as circumferential thickening and enhancement of the visceral and parietal pericardium (Figure 1C). In patient #2, contrast-enhanced chest CT images displayed nodular pericardial thickening with nodules invading the epicardial fat (Figure 1D). In patient #3, CT imaging was not available. Three-chamber echocardiography imaging revealed a pericardial effusion with nodular soft tissue along the visceral pericardium (Figure 1E).
Histopathologic Features
Histopathologic examination revealed high-grade epithelioid mesothelioma in case #1, lowgrade epithelioid mesothelioma in case #2 with atypical tumor cells growing in sheets and strands and forming glandular structures (Figure 2A–D). Case #3 represented a biphasic mesothelioma with sarcomatoid components accounting for 60% of the tumor, consisting of moderately to severely atypical tumor cells growing in solid sheets with scattered glandular structures (Figure 2E, F). The tumor cells showed a higher degree of nuclear pleomorphism than cases #1 and #2. Geographic necrosis was present.
Figure 2. Histopathologic features of primary pericardial mesothelioma.
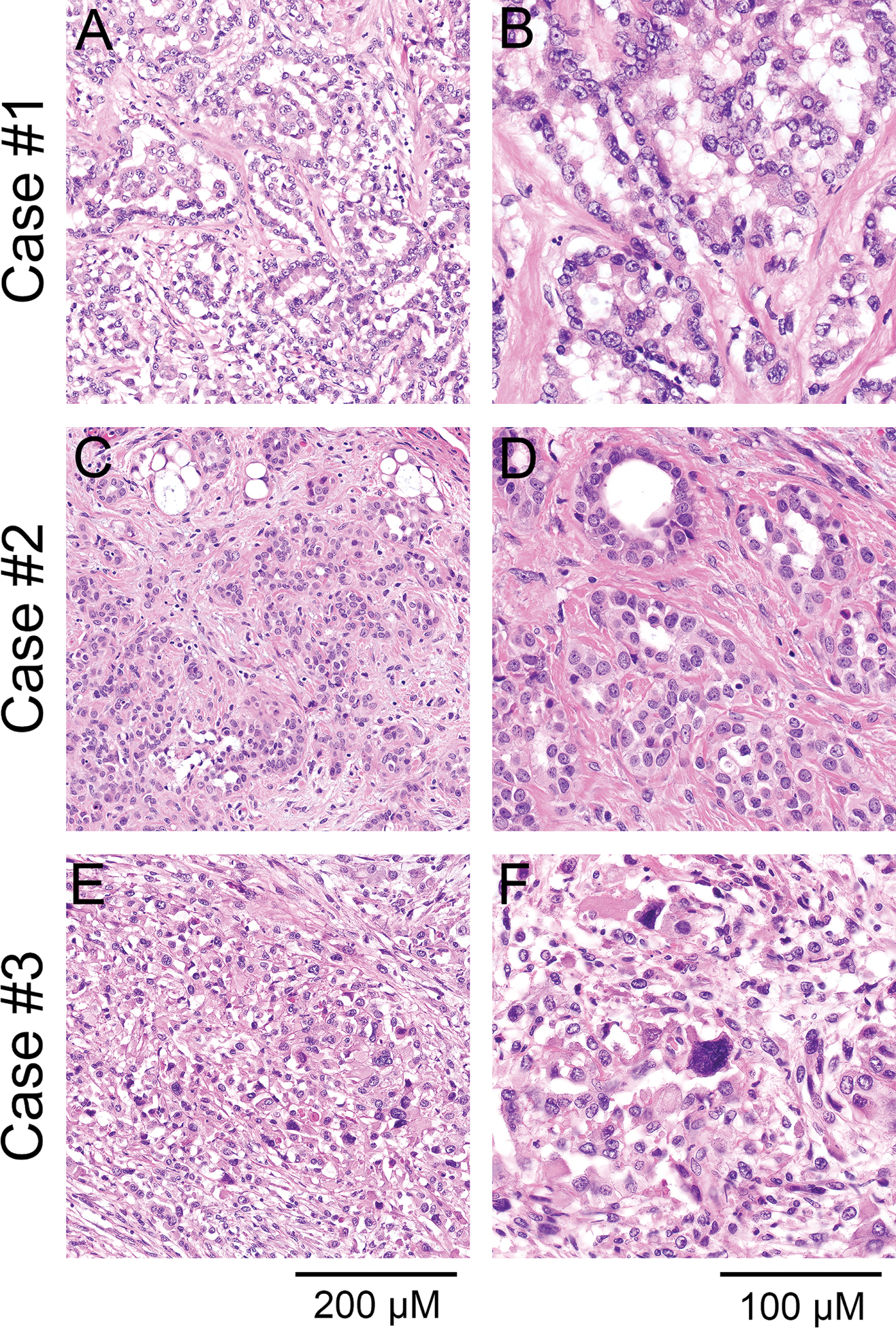
Histopathologic examination revealed epithelioid mesothelioma in cases #1 (A, x200; B, x400) and #2 (C, x200; D, x400) with moderately atypical tumor cells growing in sheets and strands and forming glandular structures. E (x200), F (x400) Case #3 represented a biphasic mesothelioma consisting of moderately to severely atypical tumor cells growing in solid sheets with scattered glandular structures; hematoxylin-eosin stain.
Immunohistochemistry Results
All three primary pericardial mesotheliomas expressed cytokeratin AE1/AE3 and calretinin by immunohistochemistry (Table 2, Figure 3A–C). Staining for D2–40 was positive in case #1, whereas WT1 was negative in cases #1 and #3 (cytoplasmic staining) and positive in case #2 (nuclear staining) (Figure 3A–C). TTF1 and claudin-4 were negative in all three cases (Table 2, Figure 3A–C). Staining for tumor suppressors frequently dysregulated in mesothelioma revealed concurrent loss of p16 and MTAP expression in cases #1 and #2, loss of BAP1 expression in case #2, loss of Merlin expression in cases #2 and #3, and loss of p53 expression in case #3 (Figure 4A–C). In addition, case #3 showed loss of nuclear BAP1 expression and abnormal cytoplasmic expression (Figure 4A–C).
Figure 3. Results of immunohistochemical staining for mesothelioma markers in primary pericardial mesothelioma.
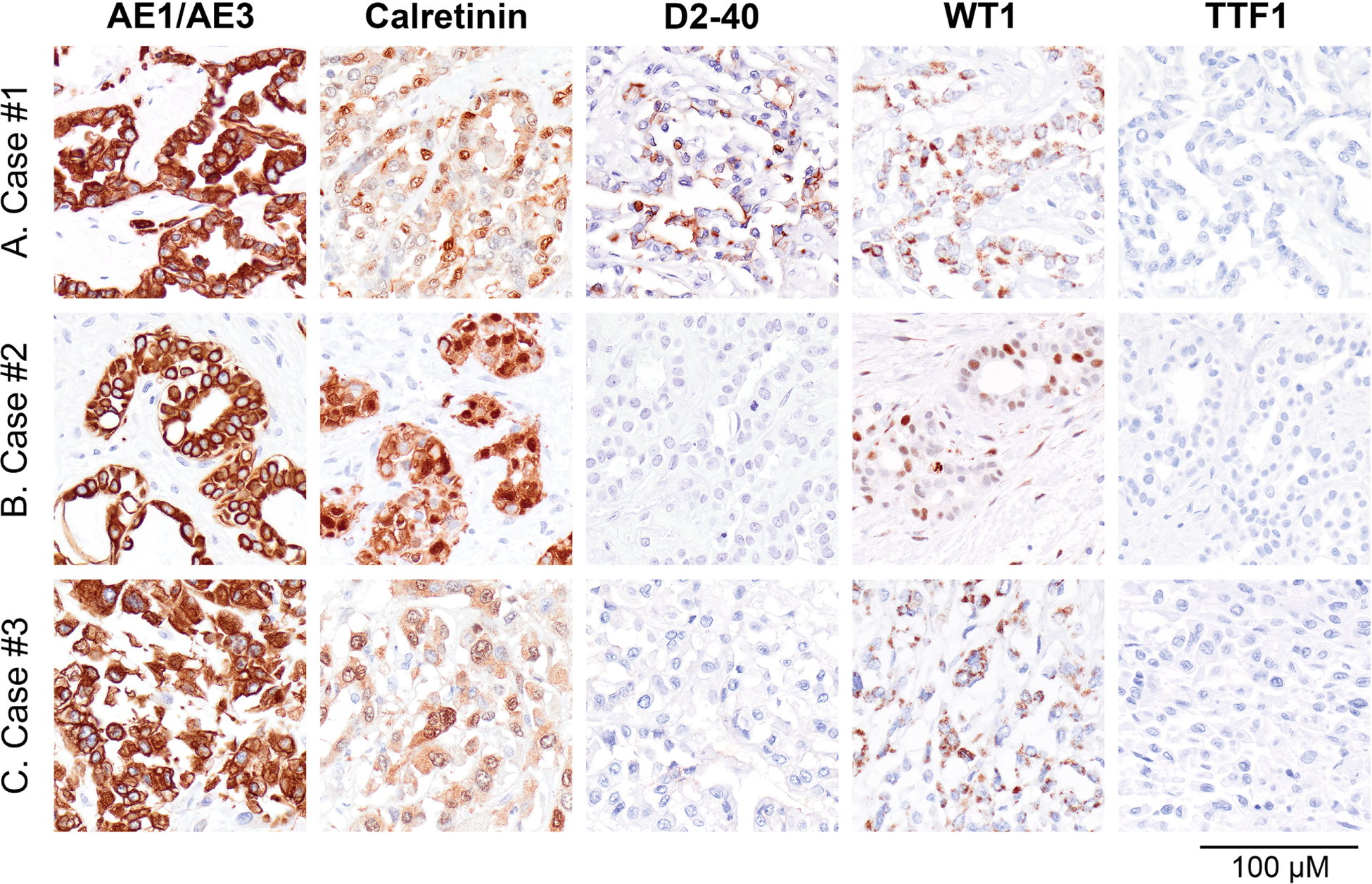
A-C All three cases expressed cytokeratin AE1/AE3 and calretinin by immunohistochemistry. A-C Staining for D2–40 was positive in case #1, whereas WT1 was negative in cases #1 and #3 (cytoplasmic staining) and positive in case #2 (nuclear staining). A-C TTF1 and claudin-4 stains were negative in all three cases.
Figure 4. Results of immunohistochemical staining for tumor suppressor abnormalities in primary pericardial mesothelioma.
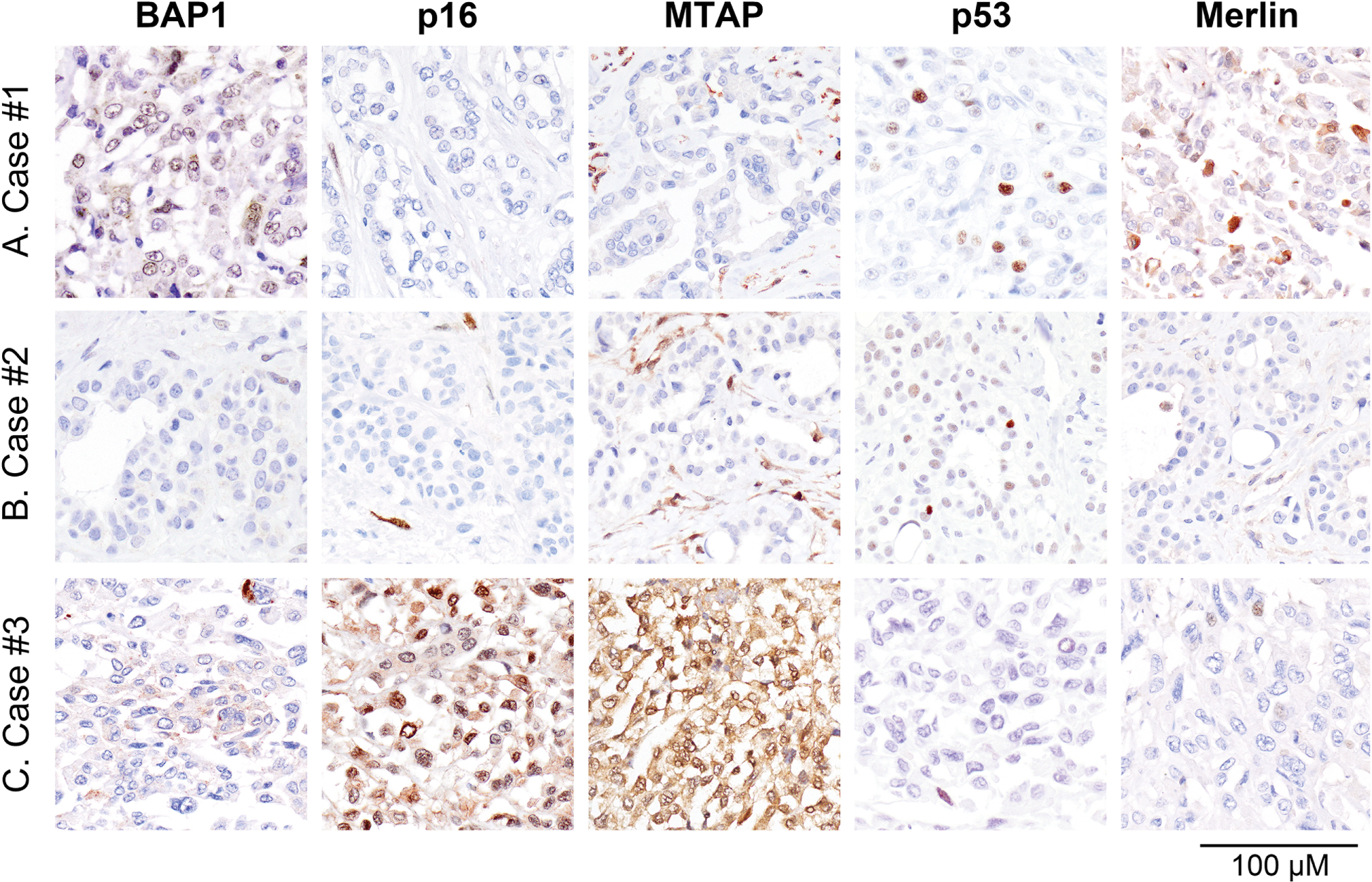
A-C Immunohistochemical staining revealed loss of BAP1 expression in case #2, concurrent loss of p16 and MTAP expression in cases #1 and #2, loss of p53 expression in case #3, and loss of Merlin expression in cases #2 and #3. For each marker, vascular endothelial cells and inflammatory cells served as internal control.
Targeted Next-Generation Sequencing Results
All three mesotheliomas and corresponding non-neoplastic tissues (liver in cases #1 and #3, lymph node in case #2) underwent successful targeted next-generation sequencing. The overall mean target coverage (MTC) was 208 (range, 27–484); one case (the mesothelioma of patient #3) had a low MTC of 27 which is likely attributable to post-mortem DNA degradation. Figure 5A demonstrates that the protein expression abnormalities of p16, MTAP, BAP1, Merlin, and p53 correlated with genomic aberrations involving CDKN2A, CDKN2B, MTAP, BAP1, NF2, and TP53, respectively. The frequency and mutational pattern of NGS genomic results for the three pericardial mesotheliomas are summarized in Figure 5B and largely corresponded to the genomic aberrations reported by the cBioPortal for Cancer Genomics (project GENIE v13.0-public, N=167,423 samples total; N=963 mesotheliomas, including N=800 pleural, N=154 peritoneal, and N=9 testicular mesotheliomas)17,18, shown in Figure 5C, with CDKN2A, CDKN2B, and MTAP generally being inactivated through homozygous deletions, and BAP1, NF2, TP53, and BRCA1 usually targeted by inactivating mutations.
Figure 5. Results of targeted next-generation sequencing for tumor suppressor aberrations in primary pericardial mesothelioma.
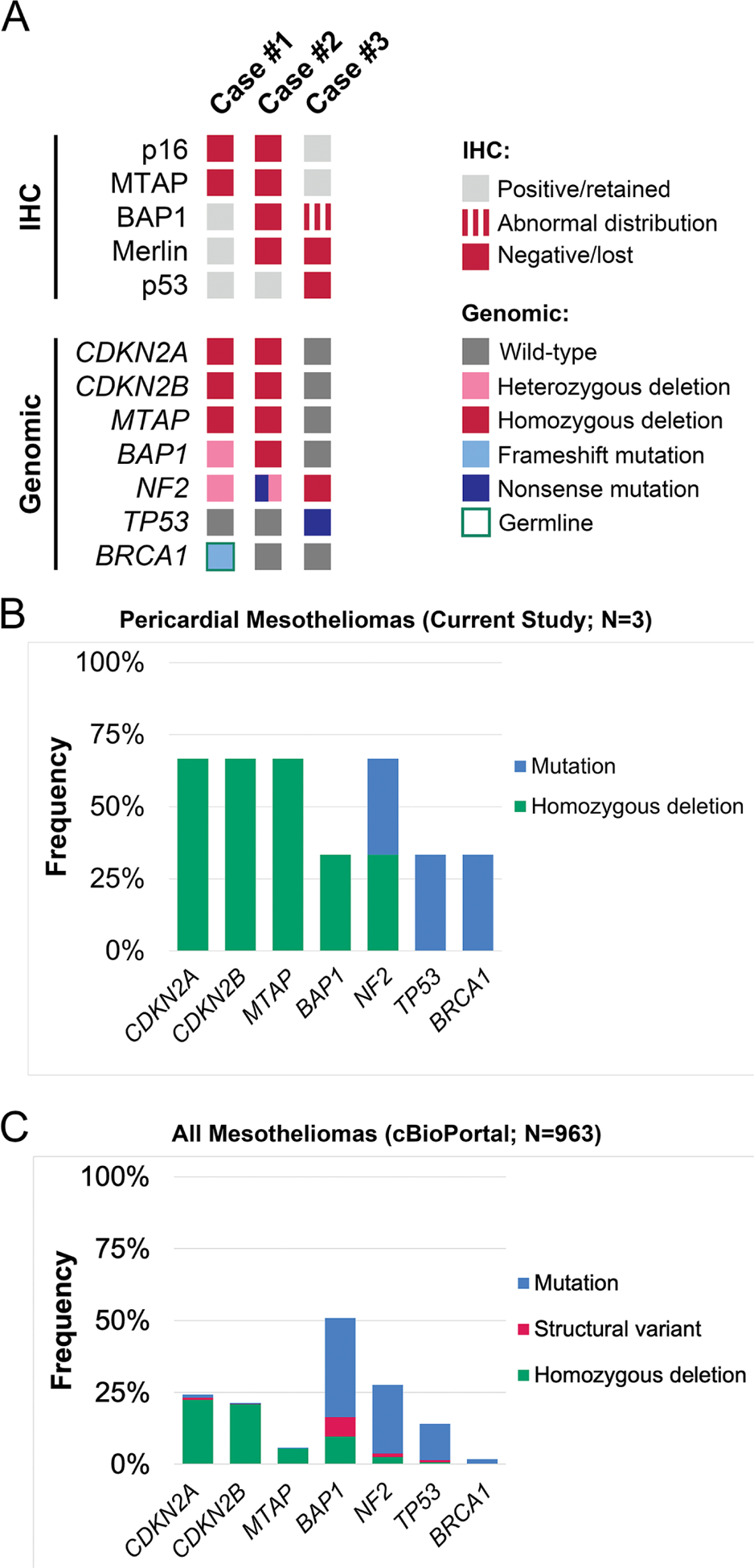
A Heat map displaying results of protein expression analysis by immunohistochemistry and targeted next-generation sequencing in primary pericardial mesotheliomas. Frequency of recurrent genomic aberrations and inactivating mechanism for CDKN2A, CDKN2B, MTAP, BAP1, NF2, TP53, and BRCA1 in three pericardial mesotheliomas (B) and in all mesotheliomas included in the cBioPortal for Cancer Genomics (project GENIE v13.0-public, N=167,423 samples total; N=963 mesotheliomas, including N=800 pleural, N=154 peritoneal, and N=9 testicular mesotheliomas) (C).
The mesothelioma of patient #1 showed a homozygous deletion involving CDKN2A, CDKN2B, and MTAP, a heterozygous deletion of BAP1, and heterozygous deletion of NF2, gain of WT1, and a pathogenic frameshift mutation of BRCA1 (p.E1210Rfs*) (allele fraction [AF] 0.8; PolyPhen-2 score: 0.995) (Figure 6A). The same mutation was detected as a heterozygous mutation in the patient’s corresponding non-neoplastic tissue (AF 0.5) (Figure 6B) thereby credentialing it as a germline variant. The mesothelioma of patient #2 displayed a homozygous deletion involving CDKN2A, CDKN2B, and MTAP, a homozygous deletion involving BAP1, a nonsense mutation of NF2 (p.Y144*, AF 0.11) with loss of one copy of NF2, and gain of WT1. No germline mutations were identified in the non-neoplastic tissue of patient #2. The mesothelioma of patient #3 displayed a two-copy loss of NF2, homozygous TP53 nonsense mutation (p.Q331*; AF 0.74), and gain of WT1 (Figure 6C). No genomic abnormality involving BAP1 was identified upon manual review of the sequencing results. No germline mutations were identified in the non-neoplastic tissue of patient #3. All three mesotheliomas were MMR proficient. The mean tumor mutational burden per Megabase was 4.31 (range, 3–6) which is comparable to an average of 4.56 (range, 1–56) mutations per Megabase for all mesotheliomas included in project GENIE (N=831).
Figure 6. Select single nucleotide variants and copy number alterations in primary pericardial mesotheliomas.
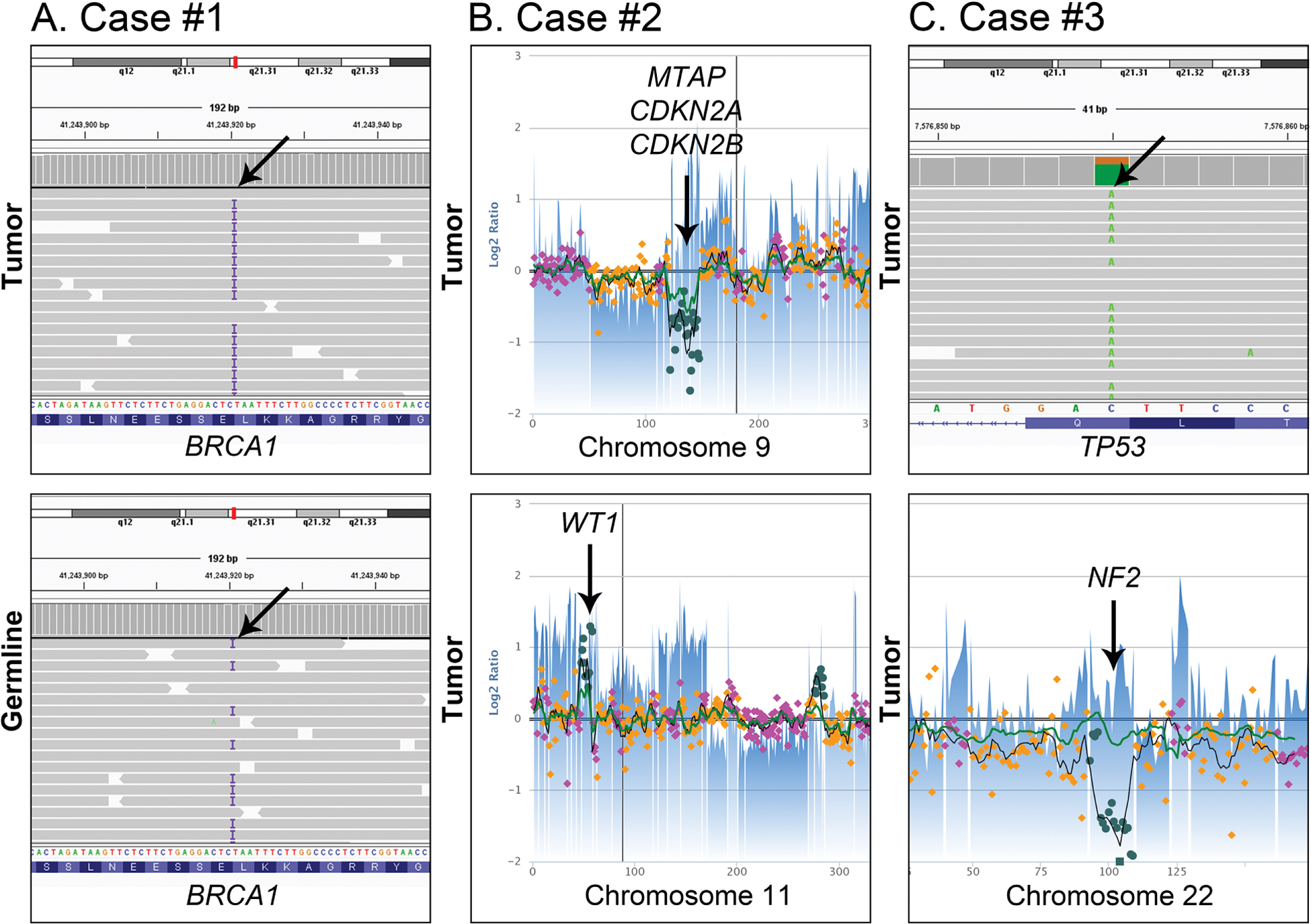
A The mesothelioma of case #1 showed a homozygous BRCA1 frameshift mutation (p.E1210Rfs*) (allele fraction [AF] 0.8) (top). The same mutation was detected as a heterozygous mutation in the patient’s corresponding non-neoplastic tissue (AF 0.5) thereby credentialing it as a germline variant (bottom). B The mesothelioma of case #2 had a homozygous deletion involving MTAP, CDKN2A and CDKN2B on chromosome 9p. (top). In addition, this tumor had gain of WT1 on chromosome 11p (bottom). C The mesothelioma of case #3 displayed a homozygous TP53 nonsense mutation (p.Q331*; AF 0.74) (top) and two-copy loss of NF2 of chromosome 22 (bottom).
Overall, copy number analysis revealed several chromosomal gains and losses in all three mesotheliomas, whereas no copy number alterations were identified in the corresponding nonneoplastic tissues (Figure 7).
Figure 7. Copy number alterations in primary pericardial mesotheliomas.
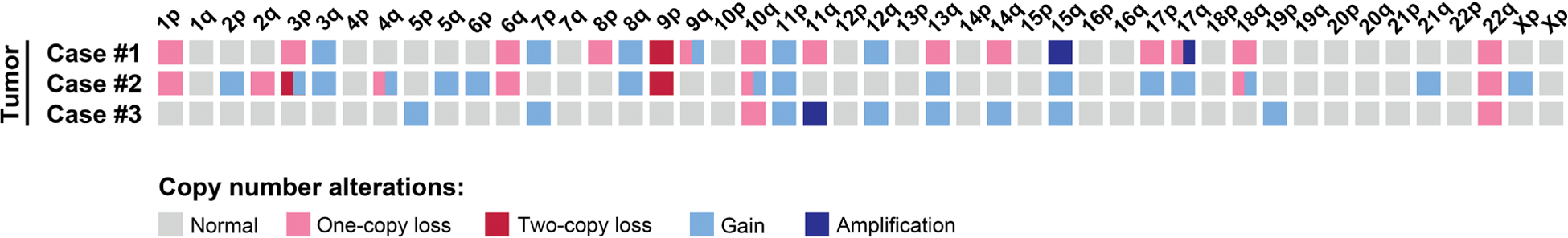
Heat map displaying copy number losses, gains, and amplification events in cases #1–3.
Discussion
Primary pericardial mesotheliomas are extremely rare and unlike pleural mesotheliomas, their etiologic factors remain elusive. The three cases of pericardial mesothelioma reported herein largely reflect the clinicopathologic features described for patients with pleural mesotheliomas, including a patient age of ~70 years as well as prior asbestos exposure and smoking history reported in two patients each. While it is commonly accepted that tobacco smoking and asbestos exposure interact synergistically for the causation of lung cancer, and asbestos exposure has been long been established as risk factor for pleural mesothelioma19, their role for the development of pericardial mesothelioma has not been definitively determined. Some studies observed a significant association between occupational asbestos exposure and pericardial mesothelioma6,7 whereas others have not.1,5 While two of the patients reported in our study each had a history of smoking and asbestos exposure, our study is limited by small case numbers and our observations do not allow for conclusions about causation.
Histopathologic examination revealed epithelioid or biphasic histomorphology as reported previously for pericardial mesotheliomas.5,20 In addition, we observed immunohistochemical expression of markers commonly expressed in mesothelioma, such as cytokeratin AE1/AE3, calretinin, D2–40, and WT1 as well as negativity for TTF1 and claudin-4.
The results of our immunohistochemical analyses point towards frequent tumor suppressor inactivation in pericardial mesotheliomas, including expression abnormalities of p16, MTAP, BAP1, Merlin, and p53 resulting from genomic inactivation of CDKN2A/p16, CDKN2B, MTAP, BAP1, NF2, and TP53, respectively, which are also frequently dysregulated in pleural mesothelioma.21 Our findings are in line with those reported by Offin et al. and Kato et al. who previously described genomic aberrations inactivating CDKN2A/B, BAP1, TP53, and NF2, in altogether three patients’ pericardial mesotheliomas.5,11
Cytoplasmic expression of BAP1 with concurrent loss of nuclear expression observed in case #3 has been reported in pleural mesothelioma by De Rienzo and colleagues.22 This staining pattern is considered abnormal since BAP1 is normally localized in the nucleus. Aberrant cytoplasmic expression suggests an underlying mutation affecting the nuclear localization signal of BAP1.22 However, we were not able to identify a BAP1 genomic abnormality in case #3, which might be explained by technical limitations of our NGS assay or the low MTC in this case.
The results of our analyses indicate that pericardial mesotheliomas share genomic aberrations with pleural mesotheliomas and might therefore be driven by the same biologic pathways. Similarly, mesothelioma of the tunica vaginalis, another rare subtype of mesothelioma, was found to exhibit a similar mutational profile comparable to those of the pleura/peritoneum; however, CDKN2A and BAP1 alterations appeared to be less common.23 A subset of cases had potentially targetable alterations in driver genes (PTCH1 and TSC1) that are unusual in mesotheliomas at other anatomical sites23 and were not identified in the three pericardial mesotheliomas reported here.
Comparison with the genomic data from our institution included revealed that the three pericardial mesotheliomas had a mutational burden comparable to all mesotheliomas included in project GENIE. In addition, one patient with pericardial mesothelioma harbored a pathogenic BRCA1 germline mutation (p.E1210Rfs*) that has previously been reported 17 times in ClinVar as a germline variant in patients with hereditary breast and ovarian carcinoma (VCV000037534.35).24 Mesothelioma patients have been reported to harbor germline mutations in CDKN2A and BAP1 (encoding BAP1 or BRCA1 associated protein-1)13,14,25, as well as other cancer susceptibility genes, such as ATM, CHEK2, TP53, BRCA1, and BRCA2.26 As discussed by Panou et al., these findings suggest that predominantly aberrations in genes involved in DNA damage response pathways play important roles in the development of pleural mesothelioma. The identification of a BRCA1 germline mutation in one of our patients indicates that deficient DNA damage response pathways might give rise to pericardial mesothelioma as well. While BRCA1 germline mutations have initially been reported in patients with hereditary breast and ovarian cancer syndrome, additional tumor types have been added to the list of neoplasms associated with BRCA1 germline variants, including gastric cancer, pancreatic cancer, and biliary tract cancer.27 In breast, pancreatic, and ovarian cancers, BRCA1 germline mutations are associated with failure of DNA double-strand break-repair by homologous recombination.28 Importantly, homologous recombination pathway alterations can occur concurrently with canonical driver mutations, as reported in lung cancer29 and pancreatic cancer.30 Our studies provide evidence of biallelic BRCA1 inactivation in the mesothelioma of patient #1 which occurred concurrently with mutations in mesothelioma driver genes (e.g., biallelic inactivation of CDKN2A, CDKN2B, and MTAP) indicating that BRCA1 inactivation is being selected for in the tumor cells and likely contributed to tumor development and/or progression.
BRCA1 mutations have been reported to render patients with ovarian or breast cancer particularly sensitive to cisplatin chemotherapy31 and poly-ADP ribose polymerase (PARP) inhibitors32,33, which induce synthetic lethality of alternate DNA repair pathways and thereby lead to cell death. Based on its interaction with BRCA1, BAP1 has been shown to increase sensitivity to PARP inhibitors in patients with mesothelioma. The three cases of pericardial mesothelioma reported herein behaved aggressively and the patients died between 0 and 15 months after diagnosis. Offin et al., reported that trimodality therapy (i.e., surgical resection, adjuvant chemotherapy, and radiation) may improve outcomes in selected patients with primary pericardial mesothelioma.5 Ongoing studies are exploring the efficacy of targeting DNA damage repair pathway deficiencies in patients with mesothelioma34 and it remains to be determined whether such approaches might also benefit patients with pericardial mesothelioma in the future.
In summary, we report three pericardial mesotheliomas which showed morphologic, immunohistochemical, and molecular genetic features typical of pleural mesothelioma including recurrent genomic inactivation of CDKN2A/p16, CDKN2B, MTAP, BAP1, NF2, and TP53. Our study adds new insights into the genetic landscape of primary pericardial mesothelioma and highlights BRCA1 loss as a potential contributing factor in a subset of cases, thereby contributing to refined precision diagnostics for this rare cancer.
Acknowledgements
The authors would like to thank the Center for Advanced Molecular Diagnostics in the Department of Pathology at Brigham and Women’s Hospital, for supporting this project.
Funding
This work is supported by the NCI K08CA241085 grant (I.-M.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics Approval
Patient sample collection and analysis were conducted following protocols approved by the Dana-Farber/Brigham and Women’s Hospital Institutional Review Board. The study was performed in accordance with the Declaration of Helsinki.
Competing interests: The authors declare no conflict of interest.
Data Availability
As per genomic data sharing guidelines, somatic sequence and assay data are deposited in a publicly available database sponsored by the American Association for Cancer Research (AACR) Project GENIE. Variant calls and a limited clinical dataset from patients are sent to the Synapse platform, developed by Sage Bionetworks, where the data are harmonized and protected health information (PHI) removed in a secure Health Insurance Portability and Accountability Act (HIPAA)-compliant environment that provides data governance.
References
- 1.Mezei G, Chang ET, Mowat FS, Moolgavkar SH. Epidemiology of mesothelioma of the pericardium and tunica vaginalis testis. Ann Epidemiol. May 2017;27(5):348–359 e11. [DOI] [PubMed] [Google Scholar]
- 2.Delgermaa V, Takahashi K, Park EK, Le GV, Hara T, Sorahan T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ. Oct 1 2011;89(10):716–24, 724A-724C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oka N, Orita Y, Oshita C, Nakayama H, Teragawa H. Primary malignant pericardial mesothelioma with difficult antemortem diagnosis: A case report. World J Clin Cases. Nov 26 2022;10(33):12380–12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui F, Hu Y, Li Y. A Rare Case of Primary Malignant Pericardial Mesothelioma Diagnosed with Pericardiotomy. Heart Surg Forum. Dec 19 2022;25(6):E840–E842. [DOI] [PubMed] [Google Scholar]
- 5.Offin M, De Silva DL, Sauter JL, et al. Multimodality Therapy in Patients With Primary Pericardial Mesothelioma. J Thorac Oncol. Dec 2022;17(12):1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marinaccio A, Consonni D, Mensi C, et al. Association between asbestos exposure and pericardial and tunica vaginalis testis malignant mesothelioma: a case-control study and epidemiological remarks. Scand J Work Environ Health. Nov 1 2020;46(6):609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGehee E, Gerber DE, Reisch J, Dowell JE. Treatment and Outcomes of Primary Pericardial Mesothelioma: A Contemporary Review of 103 Published Cases. Clin Lung Cancer. Mar 2019;20(2):e152–e157. [DOI] [PubMed] [Google Scholar]
- 8.Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. Oct 1960;17(4):260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzardi C, Barresi E, Brollo A, Cassetti P, Schneider M, Melato M. Primary pericardial mesothelioma in an asbestos-exposed patient with previous heart surgery. Anticancer Res. Apr 2010;30(4):1323–5. [PubMed] [Google Scholar]
- 10.Fujiwara H, Kamimori T, Morinaga K, et al. An autopsy case of primary pericardial mesothelioma in arc cutter exposed to asbestos through talc pencils. Ind Health. Apr 2005;43(2):346–50. [DOI] [PubMed] [Google Scholar]
- 11.Kato S, Tomson BN, Buys TP, Elkin SK, Carter JL, Kurzrock R. Genomic Landscape of Malignant Mesotheliomas. Mol Cancer Ther. Oct 2016;15(10):2498–2507. [DOI] [PubMed] [Google Scholar]
- 12.Hiltbrunner S, Fleischmann Z, Sokol ES, Zoche M, Felley-Bosco E, Curioni-Fontecedro A. Genomic landscape of pleural and peritoneal mesothelioma tumours. Br J Cancer. Nov 2022;127(11):1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. Aug 28 2011;43(10):1022–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan R, Morrow B, Thomas A, et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci U S A. Apr 30 2019;116(18):9008–9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagle N, Berger MF, Davis MJ, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. Jan 2012;2(1):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia EP, Minkovsky A, Jia Y, et al. Validation of OncoPanel: A Targeted Next-Generation Sequencing Assay for the Detection of Somatic Variants in Cancer. Arch Pathol Lab Med. Jun 2017;141(6):751–758. [DOI] [PubMed] [Google Scholar]
- 17.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. May 2012;2(5):401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. Apr 2 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klebe S, Leigh J, Henderson DW, Nurminen M. Asbestos, Smoking and Lung Cancer: An Update. Int J Environ Res Public Health. Dec 30 2019;17(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosso F, Cerbone L, Pasello G. Pericardial Mesothelioma, a Disease for Brave Hearts. J Thorac Oncol. Dec 2022;17(12):1333–1334. [DOI] [PubMed] [Google Scholar]
- 21.Chapel DB, Hornick JL, Barlow J, Bueno R, Sholl LM. Clinical and molecular validation of BAP1, MTAP, P53, and Merlin immunohistochemistry in diagnosis of pleural mesothelioma. Mod Pathol. Oct 2022;35(10):1383–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Rienzo A, Chirieac LR, Hung YP, et al. Large-scale analysis of BAP1 expression reveals novel associations with clinical and molecular features of malignant pleural mesothelioma. J Pathol. Jan 2021;253(1):68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson WJ, Sholl LM, Fletcher CDM, et al. Molecular and immunohistochemical characterisation of mesothelioma of the tunica vaginalis. Histopathology. Jul 2022;81(1):65–76. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Biotechnology Information. ClinVar; [VCV000037534.35], https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000037534.35 (Accessed April 26, 2023).
- 25.Betti M, Aspesi A, Biasi A, et al. CDKN2A and BAP1 germline mutations predispose to melanoma and mesothelioma. Cancer Lett. Aug 10 2016;378(2):120–30. [DOI] [PubMed] [Google Scholar]
- 26.Panou V, Gadiraju M, Wolin A, et al. Frequency of Germline Mutations in Cancer Susceptibility Genes in Malignant Mesothelioma. J Clin Oncol. Oct 1 2018;36(28):2863–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Momozawa Y, Sasai R, Usui Y, et al. Expansion of Cancer Risk Profile for BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol. Jun 1 2022;8(6):871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexandrov LB, Kim J, Haradhvala NJ, et al. The repertoire of mutational signatures in human cancer. Nature. Feb 2020;578(7793):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsang ES, Csizmok V, Williamson LM, et al. Homologous recombination deficiency signatures in gastrointestinal and thoracic cancers correlate with platinum therapy duration. NPJ Precis Oncol. Mar 24 2023;7(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntyre CA, Lawrence SA, Richards AL, et al. Alterations in driver genes are predictive of survival in patients with resected pancreatic ductal adenocarcinoma. Cancer. Sep 1 2020;126(17):3939–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. Oct 12 2011;306(14):1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. Jul 24 2010;376(9737):245–51. [DOI] [PubMed] [Google Scholar]
- 33.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. Jul 24 2010;376(9737):235–44. [DOI] [PubMed] [Google Scholar]
- 34.Perera ND, Mansfield AS. The Evolving Therapeutic Landscape for Malignant Pleural Mesothelioma. Curr Oncol Rep. Nov 2022;24(11):1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As per genomic data sharing guidelines, somatic sequence and assay data are deposited in a publicly available database sponsored by the American Association for Cancer Research (AACR) Project GENIE. Variant calls and a limited clinical dataset from patients are sent to the Synapse platform, developed by Sage Bionetworks, where the data are harmonized and protected health information (PHI) removed in a secure Health Insurance Portability and Accountability Act (HIPAA)-compliant environment that provides data governance.


