Abstract
Opioid abuse and overdose have risen to epidemic proportions in the United States. Oxycodone is the most abused prescription opioid. Treatments for opioid use disorder (OUD) seek to reduce vulnerability to relapse by reducing sources of reinforcement to seek drug (i.e., acute drug effects or drug withdrawal/craving). Accumulating evidence that glutamate release elicits drug-seeking behaviors has generated interest in pharmacotherapies targeting the glutamate system. Agonists and positive allosteric modulators of the metabotropic glutamate 2 (mGlu2) receptor decrease glutamate activity, reducing drug taking and seeking. The present study tested whether the mGlu2 receptor positive allosteric modulator ADX106772 reduces oxycodone self-administration and the conditioned reinstatement of oxycodone seeking without affecting behaviors directed toward a highly palatable non-drug reinforcer (sweetened condensed milk). Male Wistar rats were trained to self-administer oxycodone (0.15 mg/kg/infusion, i.v.,12 h/day) or sweetened condensed milk (SCM; diluted 2:1 v/v in H2O, orally, 30 min/day) for 13 days in the presence of a contextual/discriminative stimulus (SD), and the ability of ADX106772 (0, 0.3, 1, 3 and −10 mg/kg, s.c.) to decrease self-administration was tested. The rats then underwent extinction training, during which oxycodone, SCM, and the SD were withheld. After extinction, the ability of ADX106772 to prevent SD-induced conditioned reinstatement of oxycodone and SCM seeking was tested. ADX106772 reduced oxycodone self-administration and conditioned reinstatement without affecting SCM self-administration or conditioned reinstatement. ADX106772 reduced oxycodone taking and seeking and did not affect the motivation for the palatable conventional reinforcer, suggesting that activating mGlu2 receptors with a positive allosteric modulator is a potential approach for prescription OUD treatment.
Keywords: prescription opioid use disorder, reinstatement, metabotropic glutamate 2 receptor, positive allosteric modulator, oxycodone
INTRODUCTION
Over 75,000 individuals in the United States overdosed on opioids between July 2019 and 2021, with almost 14,000 of these deaths attributed to semi-synthetic opioids like oxycodone (Ahmad, 2022). Moreover, approximately 10.1 million Americans reported misusing a prescription opioid in 2019 (USDHHS, 2020). Chronic drug use drives the recruitment of antireward systems, which contributes to drug withdrawal, enhanced sensitivity to stress, and pervasive drug seeking despite prolonged abstinence (Koob & Bloom, 1988; Koob & Le Moal, 1997; Koob, 2020). Understanding the neurobiological mechanisms that drive negative reinforcement is particularly critical to reduce negative health consequences that are associated with opioids. Pharmacological interventions may reduce negative reinforcement by providing relief from a dependence-induced allosteric state among individuals with substance use disorders (Koob & Le Moal, 1997; Koob, 2020, 2021), allowing them to make behavioral and environmental changes toward recovery that symptoms of such a state preclude.
The glutamatergic system has arisen as a potential therapeutic target for the treatment of substance use disorders. Preclinical evidence suggests that glutamate (Glu) transmission influences behaviors and symptoms that are commonly associated with addiction (McFarland & Kalivas, 2001; Kalivas, 2004; Kenny & Markou, 2004; Moran et al., 2005; Shen et al., 2014; Guo et al., 2009; Kalivas, 2009; Kalivas et al., 2009). Drugs of abuse elicit the release of Glu (Kalivas et al., 2005), which enhances mesolimbic dopamine release that is associated with reinforcement processing (Tzschentke, 2001). Glutamate release from the prefrontal cortex to the nucleus accumbens has been specifically reported to enhance reinforcing effects of cocaine and the reinstatement of cocaine seeking (Cornish et al., 1999; Cornish & Kalivas, 2000; Di Ciano & Everitt, 2001; McFarland & Kalivas, 2001; Capriles et al., 2003; McFarland et al., 2003;). The prefrontal cortex also provides glutamatergic inputs to the ventral tegmental area and has reciprocal glutamatergic connections with the thalamus and amygdala; all of these regions are known to be impacted by addiction (Kalivas & Volkow, 2005). The prefrontal cortex also exhibits an increase in metabolic activity during drug craving that is elicited by exposure to drug-conditioned stimuli, stress, and drug primes (Kilts et al., 2001; Wilson et al., 2004; Schacht et al., 2013).
Metabotropic glutamate (mGlu) receptors are a family of G protein-coupled receptors that are activated by Glu. The mGlu family is composed of eight members (mGlu1–8), which are further divided into three groups: Group I, Group II, and Group III (Pin & Acher, 2002). Group II includes the mGlu2 receptor, which acts on Gi/Go proteins to inhibit Glu release (Cartmell & Shoepp, 2000; Gass & Olive, 2008), and are expressed in regions that are associated with addiction (i.e., prefrontal cortex, hippocampus, striatum, thalamus, and hypothalamus; Moussawi & Kalivas, 2010; Justinova et al., 2015; Yuan et al., 2021). Deletion of mGlu2 receptors increases various responses to opioids including accumbal dopamine release, locomotor activity, self-administration behaviors, analgesia, and withdrawal (Gao et al., 2018). The administration of mGlu2 receptor positive allosteric modulators (PAM) and agonists can block the acquisition and expression of morphine-induced conditioned place preference (Baharlouei et al., 2015) and reduce nicotine self-administration and conditioned reinstatement of nicotine (Justinova et al., 2015; Li et al., 2016), cocaine (Jin et al., 2010), and alcohol (Augier et al., 2016) seeking. Such actions of stimulating mGlu2 may influence the motivation for drug by elevating intracranial self-stimulation thresholds and/or blocking drug-induced reductions of intracranial self-stimulation thresholds (Jin et al., 2010). Although mGlu2 receptor activation plays a role in the induction of long-term depression (Cho and Bashir, 2002), repeated nicotine (Liechti et al., 2007), cocaine (Morishima et al., 2005) and morphine (Qian et al., 2019) use impair the ability of Group II mGlu receptors to provide negative glutamatergic feedback. Lower mGlu2 receptor expression in the infralimbic cortex has been reported with alcohol dependence in both humans and rats (Meinhardt et al., 2013) and with morphine exposure in rats (Qian et al., 2019). Likewise, restoring mGlu2 receptor function reduced alcohol self-administration in alcohol-dependent rats (Meinhardt et al., 2013). Methamphetamine craving (Caprioli et al., 2015), morphine withdrawal (Zhu & Barr, 2004), and opioid withdrawal-induced synaptic plasticity (Wu et al., 2012) were shown to be reduced by mGlu2 receptor agonists. The influence of mGlu2 receptor activation by an agonist or a PAM on opioid self-administration and the conditioned reinstatement of opioid seeking has not yet been reported clinically, but significant preclinical evidence suggests that mGlu2 receptors are a promising therapeutic target to reduce both the intake and seeking of various drug classes and thus may be promising for the treatment of opioid use disorder (OUD).
Neural systems that are involved in processing natural rewards and drugs of abuse overlap, and neuroplasticity that is caused by drug exposure may be responsible for maladaptive, compulsive, and addictive behavior (Kelley & Berridge, 2002; Aston-Jones & Harris, 2004; Kalivas & O’Brien, 2008; Wanat et al., 2009). Therefore, when exploring potential treatments for substance use disorder, it is crucial to identify therapeutic strategies that selectively reduce the motivation to obtain drug and do not cause nonspecific side effects that can reduce, for example, the motivation to obtain a nondrug reinforcer (e.g., a food reward). For example, the pharmacological manipulation of Group II mGlu receptors selectively disrupted drug- vs. nondrug-seeking behavior (e.g., Baptista et al., 2004), and Glu release during the conditioned reinstatement of alcohol and cocaine seeking was more pronounced than during the reinstatement of nondrug reinforcers (Gass et al., 2011; McFarland et al., 2003). These findings indicate that targeting Group II mGlu receptors could be an effective strategy to treat substance use disorders. Likewise, mGlu2 receptor PAMs that reduced the self-administration and conditioned reinstatement of nicotine (AZD8529; Justinova et al., 2015), cocaine (BINA; Jin et al., 2010), and alcohol (AZD8529; Augier et al., 2016) had less of an effect on food-seeking behavior (Jin et al., 2010; Justinova et al., 2015; Augier et al., 2016). Additionally, although the restoration of mGlu2 receptor expression in the infralimbic cortex in alcohol-dependent rats reduced the conditioned reinstatement of alcohol seeking, the reinstatement of seeking a highly palatable conventional reinforcer (i.e., sweetened condensed milk [SCM]) was relatively unaltered (Meinhardt et al., 2013). Collectively, the literature suggests that reducing Glu release via mGlu2 receptor activation can selectively reduce drug-directed behavior.
Therefore, to determine whether mGlu2 receptor activation selectively interferes with drug reinforcement, the present study investigated the effects of ADX106772, a selective mGlu2 receptor PAM (described as compound 27 in Cid et al., 2016), on oxycodone vs. SCM self-administration. A secondary objective of the present study was to expand our understanding of the role of mGlu2 receptors in addiction-relevant conditioned effects of oxycodone and the potential to target this receptor as an approach to prevent relapse. To establish whether mGlu2 receptor activation preferentially modifies drug-directed behavior and exerts general suppressant effects on motivated behavior, the effects of ADX106772 on responding that was induced by stimuli that were conditioned to SCM were also tested.
MATERIALS AND METHODS
Animals
Male Wistar rats (n = 32; Charles River, Wilmington, MA, USA), weighing 150–175 g upon arrival, were housed two per cage in a temperature- and humidity-controlled vivarium on a reverse 12 h/12 h light/dark cycle with ad libitum access to food and water throughout the experiments. All procedures were performed during the rats’ active (dark) phase and were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs
ADX106772 (Addex Therapeutics, Geneva, Switzerland) was dissolved in a 40% hydroxypropyl-β-cyclodextrin (HPbCD; Sigma, St. Louis, MO, USA) solution on a heated plate (~70°C), and the pH was adjusted to 4. Sterile water was added so that the dosing solution had a final concentration of 20% HPbCD. The solution was administered at doses of 0, 0.3, 1, 3, and 10 mg/kg (s.c.) 30 min before testing. Oxycodone hydrochloride (Spectrum Chemicals, St. Louis, MO, USA) for intravenous (i.v.) administration was dissolved in 0.9% sodium chloride (Hospira, Lake Forest, IL, USA) and administered at a dose of 0.15 mg/kg/0.1 ml (Wade et al., 2015; Matzeu & Martin-Fardon, 2020). Sweetened condensed milk (Nestlé, Solon, OH, USA) was diluted 2:1 (v/v) in water and delivered in a 0.1 ml volume (e.g., Martin-Fardon et al., 2009; Matzeu et al., 2018).
Experimental procedure
The timeline of the procedures for the oxycodone and SCM groups is presented in Fig. 1A and B, respectively. Rats in the oxycodone group (n = 16) were surgically implanted with jugular catheters under general anesthesia (isoflurane, 5% for induction, 1–3% for maintenance). Catheters were made by attaching Micro-Renathane tubing (0.037 diameter; Braintree Scientific, Braintree, MA, USA) to a guide cannula (Plastics One, Roanoke, VA, USA) that was anchored to a 2 cm circular mesh, all secured with dental acrylic cement. As previously described (Caine & Koob, 1993), the tubing was aseptically inserted and secured in the right jugular vein. The catheter port was placed within a small incision on the back and closed with a small plastic cap and metal cover cap to keep the inside of the catheter clean and protected. Rats in the oxycodone group were allowed 7–10 days to recover from surgery before self-administration training. Rats in the SCM group did not undergo surgery. Catheter patency was maintained with 15 unit/0.05ml heparin flushes immediately prior to and following self-administration sessions and was confirmed weekly (unless otherwise needed) with an injection of 0.1–0.2 ml of Brevital sodium solution (10 mg/ml; Par Pharmaceutical, Woodcliff Lake, NJ, USA). Catheter patency was assumed if the immediate loss of reflexes was observed.
Figure 1.
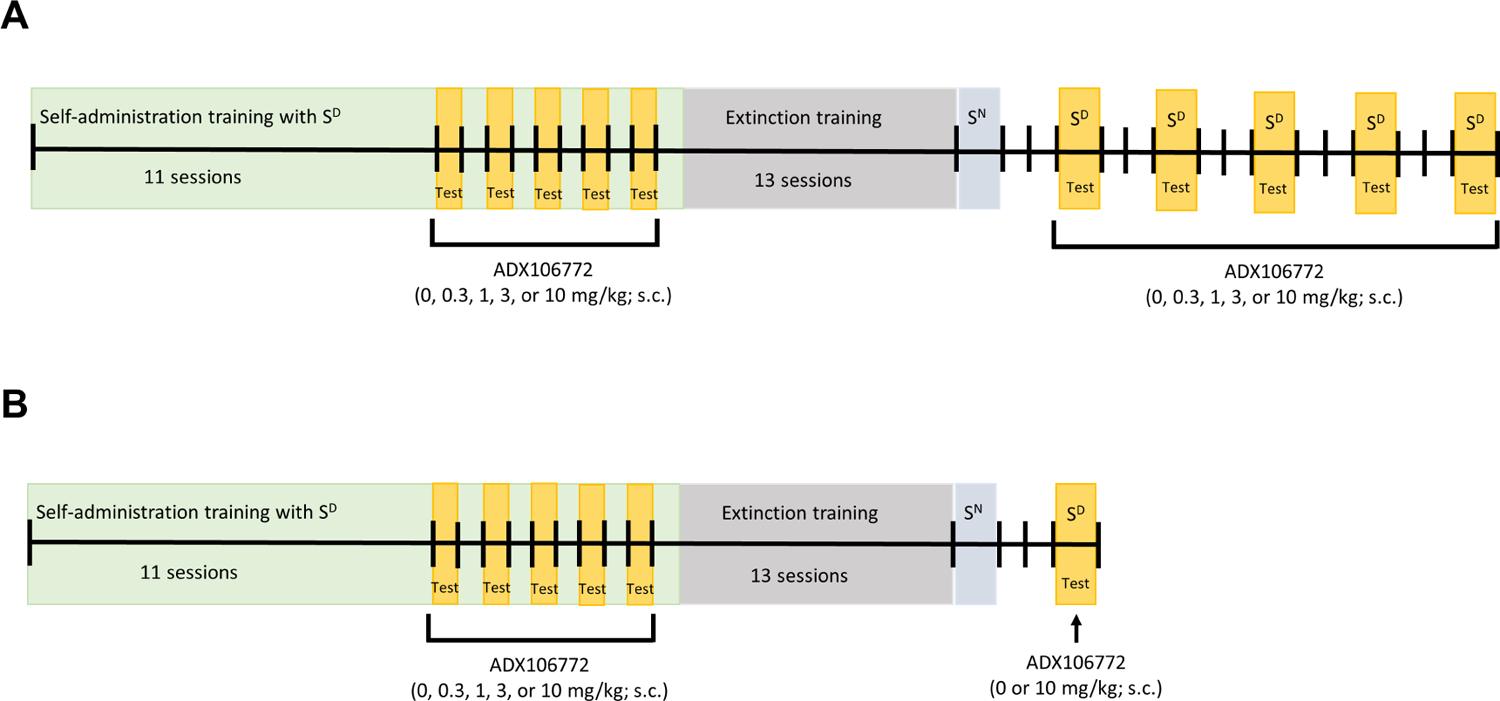
Timeline of experimental procedures.
Oxycodone self-administration training occurred in daily 12 h (extended access) sessions, 5 days/week (Monday to Friday), interspersed by 48 h withdrawal periods (Saturday and Sunday) when rats remained in their home cage (Matzeu & Martin-Fardon, 2020). Self-administration training for SCM occurred in daily 30 min sessions to prevent satiation by excessive SCM ingestion. All operant sessions were initiated by the extension of two retractable levers into the operant chamber (29 cm × 24 cm × 19.5 cm, Med Associates, St. Albans, VT, USA), and the commencement of the contextual/discriminative stimulus (SD; constant 70 dB white noise) signaled self-administration sessions. Responses on the right “active” lever were reinforced on a fixed-ratio 1 schedule by the infusion of oxycodone (0.15 mg/0.1 ml/infusion, i.v.) over 4 s or delivery of 0.1 ml SCM (diluted 2:1 v/v) through a drinking receptacle. All oxycodone or SCM reinforcers were followed by a 20-s timeout (TO20s) period and paired with the illumination of a cue light above the active lever throughout the timeout period. Responses on the left “inactive” lever were recorded but had no scheduled consequences.
Oxycodone withdrawal signs (i.e., jumps, paw tremors, teeth chattering, wet dog shakes, piloerection, and ptosis; Wei et al., 1973; Schulteis et al., 1999; Rehni et al., 2013; Matzeu & Martin-Fardon, 2020) were scored once per week, 1 h before the training session (before self-administration sessions 4, 9, 14, and 19 and the first extinction session [EXT 1]), corresponding to an 11-h abstinence period. Each withdrawal measure was assigned a score of 0–2, based on the following severity scale: 0 = no sign, 1 = moderate, 2 = severe. The sum of the six observation scores was used as a quantitative measure of withdrawal severity to verify the development of oxycodone dependence. Once the rats acquired oxycodone or SCM self-administration and oxycodone dependence was verified, the effect of ADX106772 (0, 0.3, 1, 3, and 10 mg/kg, s.c.) on oxycodone and SCM self-administration was tested. The rats were administered ADX106772 30 min before the initiation of oxycodone and SCM self-administration in a within-subjects Latin-square design every other day (sessions 12, 14, 16, 18, and 20) to control for possible order effects of ADX106772 dosing. On alternate days (sessions 13, 15, 17, 19, and 21), the rats underwent regular self-administration sessions to control for possible carryover effects of ADX106772 (Fig. 1A, B).
After testing effects of ADX106772 on oxycodone and SCM self-administration, extinction and reinstatement session length was held constant at 2 hours in order to be comparable across reinforcers. Therefore, for both the oxycodone and SCM groups, the rats’ behavior was extinguished in daily 2-h extinction sessions, during which responses on the previously active lever had no programmed consequences (i.e., no oxycodone, no SCM, and no cue presentation) for 13 sessions. After the last extinction session, all rats were presented with a neutral stimulus (SN) in a 2-h session to control for specificity of the SD to reinstate extinguished oxycodone or SCM seeking (Fig. 1A, B). During the SN session, the illumination of a 2.8 W house light at the top of the chamber’s front panel served in place of the SD. Responding on the right active lever was followed by a 70 dB (70 kHz) intermittent beeping tone, during which the lever remained inactive for 20 s. After the SN session, the rats were returned to their regular home cages. Two days after SN testing, the rats in the oxycodone group were administered ADX106772 (0, 0.3, 1, 3, and 10 mg/kg, s.c.) 30 min before being presented with the SD. Tests of the reinstatement of oxycodone seeking were conducted every third day (with 2 days off) for a total of five reinstatement tests (Fig. 1A), allowing ADX106772 doses to be tested in a within-subjects Latin-square design. The conditioned reinstatement of SCM seeking was tested only once with 0 or 10 mg/kg ADX106772 (Fig. 1B) in a between-subjects design because behavior that is controlled by stimuli that are associated with conventional rewards (i.e., SD) has been shown to extinguish rapidly in the absence of primary reinforcement (Martin-Fardon & Weiss, 2017).
Statistical analysis
Statistical analyses were conducted separately in the oxycodone and SCM groups. The acquisition of oxycodone self-administration was analyzed using a two-way repeated-measures analysis of variance (RMANOVA), with session and lever (active vs. inactive) as factors. Withdrawal scores were analyzed using the nonparametric Friedman test. Nonparametric Spearman correlations were used to establish linear dependence between withdrawal scores and the number of oxycodone infusions that were earned in sessions 3, 8, 13, 18, and 21. The effects of ADX106772 on oxycodone self-administration and conditioned reinstatement were analyzed using two-way RMANOVAs, with dose or session (extinction, SN, and SD for conditioned reinstatement) and lever as factors. A one-way ANOVA was conducted to ensure that conditioned reinstatement of oxycodone-seeking under the vehicle condition (0 mg/kg ADX106772) was not influenced by the session during which testing occurred. Subsequent interrogation of the time course of ADX106772’s effects on self-administration and conditioned reinstatement were analyzed using two-way RMANOVAs, with time and dose as within-subjects factors. Similar to the acquisition of oxycodone self-administration, SCM self-administration was analyzed using two-way RMANOVAs, with session and lever as factors. The effect of ADX106772 on SCM self-administration was analyzed using two-way RMANOVAs, with dose or session and lever as factors. Finally, the time course of effects of ADX106772 on SCM self-administration and conditioned reinstatement was analyzed using two-way RMANOVAs, with time and dose as factors. Significant effects were followed up by Bonferroni or Dunnett multiplecomparison post hoc tests. The results are expressed as the mean ± SEM. The statistical analyses were performed using GraphPad Prism 9.3.1 software. Values of p < 0.05 were considered statistically significant.
RESULTS
Effect of ADX106772 on oxycodone self-administration and conditioned reinstatement
All 16 rats assigned to the oxycodone group completed all testing procedures and are represented throughout these data. Rats acquired oxycodone self-administration over the 11 sessions of training (Fig. 2A), reflected by a significant increase in the number of responses on the active lever vs. session 1 by session 6 (p ≤ 0.01, Bonferroni post hoc test following two-way RMANOVA, session: F15,225 = 7.87, p ≤ 0.0001; lever: F1,15 = 26.89, p = 0.0001; session × lever: F15,225 = 11.91, p ≤ 0.0001). The number of responses on the inactive lever did not change significantly across training (p > 0.05, Bonferroni post hoc test following two-way RMANOVA). The number of responses on the active lever remained elevated in sessions 13, 15, 17, 19, and 21 after ADX106772 testing (i.e., sessions 12, 14, 16, 18, and 20; p ≤ 0.0001, vs. session 1, Bonferroni post hoc test following two-way RMANOVA; Fig. 2A). Somatic signs of oxycodone withdrawal were significantly more pronounced by session 14 of oxycodone self-administration (p = 0.0174, vs. session 4, Dunn’s test following Friedman test: 46.16, p ≤ 0.0001; Fig. 2B). The number of infusions during oxycodone self-administration sessions significantly correlated with somatic signs of withdrawal that were measured at 11 h of abstinence (Spearman R = 0.3471, p = 0.0016; Fig. 2C).
Figure 2.
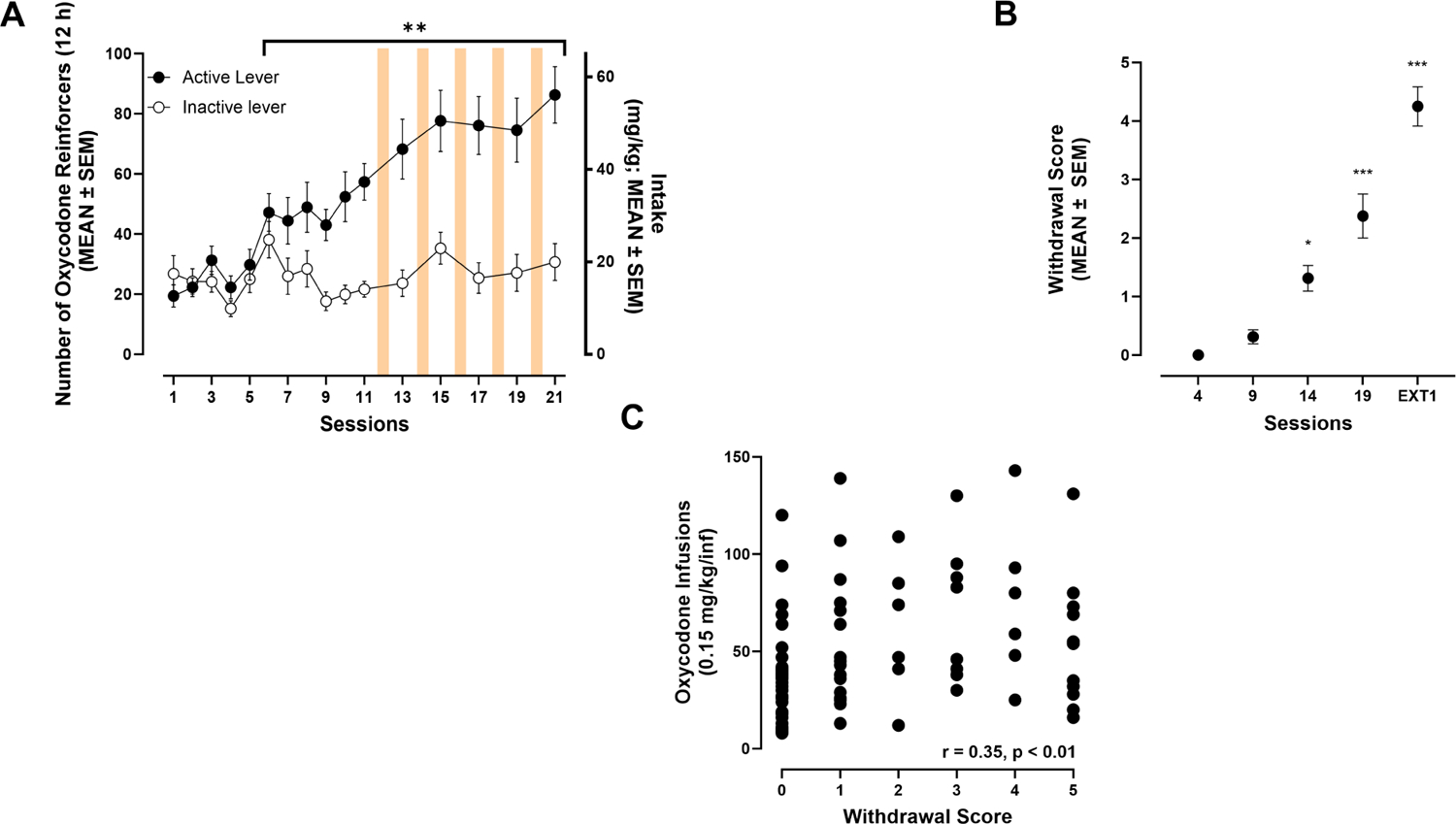
(A) Oxycodone self-administration over the 21 sessions (12 h/day; Bonferroni: **p ≤ 0.001, vs. session 1). (B) Somatic withdrawal symptoms across self-administration sessions (Dunn’s: *p ≤ 0.05, ***p ≤ 0.001, vs. session 4). (C) Correlation plot between the somatic withdrawal scores and the number of oxycodone infusions.
ADX106772 (1–10 mg/kg) reduced oxycodone self-administration (p ≤ 0.0001, vs. 0 mg/kg ADX106772, Bonferroni post hoc test following two-way RMANOVA, session: F4,60 = 8.40, p ≤ 0.0001; lever: F1,15 = 27.54, p ≤ 0.0001; session × lever: F4,60 = 8.15, p ≤ 0.0001; Fig. 3A) without modifying responses on the inactive lever (p > 0.05, vs. 0 mg/kg). Further scrutiny of the cumulative number of responses during the 12 h of oxycodone self-administration (Fig. 3B) showed that the decrease in oxycodone self-administration occurred sooner when the dose of ADX106772 was increased. At 3 and 10 mg/kg ADX106772, the cumulative number of oxycodone infusions began to significantly decrease 2 h after initiation of the self-administration session (p ≤ 0.05, vs. 0 mg/kg ADX106772, Dunnett’s post hoc test following two-way RMANOVA, time: F11,165 = 54.73, p ≤ 0.0001; dose: F4,60 = 9.85, p ≤ 0.0001; time × dose: F44,660 = 8.73, p ≤ 0.0001). At 1 mg/kg ADX106772, the reduction was observed 3 h after initiation of the self-administration session (p ≤ 0.05, vs. 0 mg/kg ADX106772).
Figure 3.
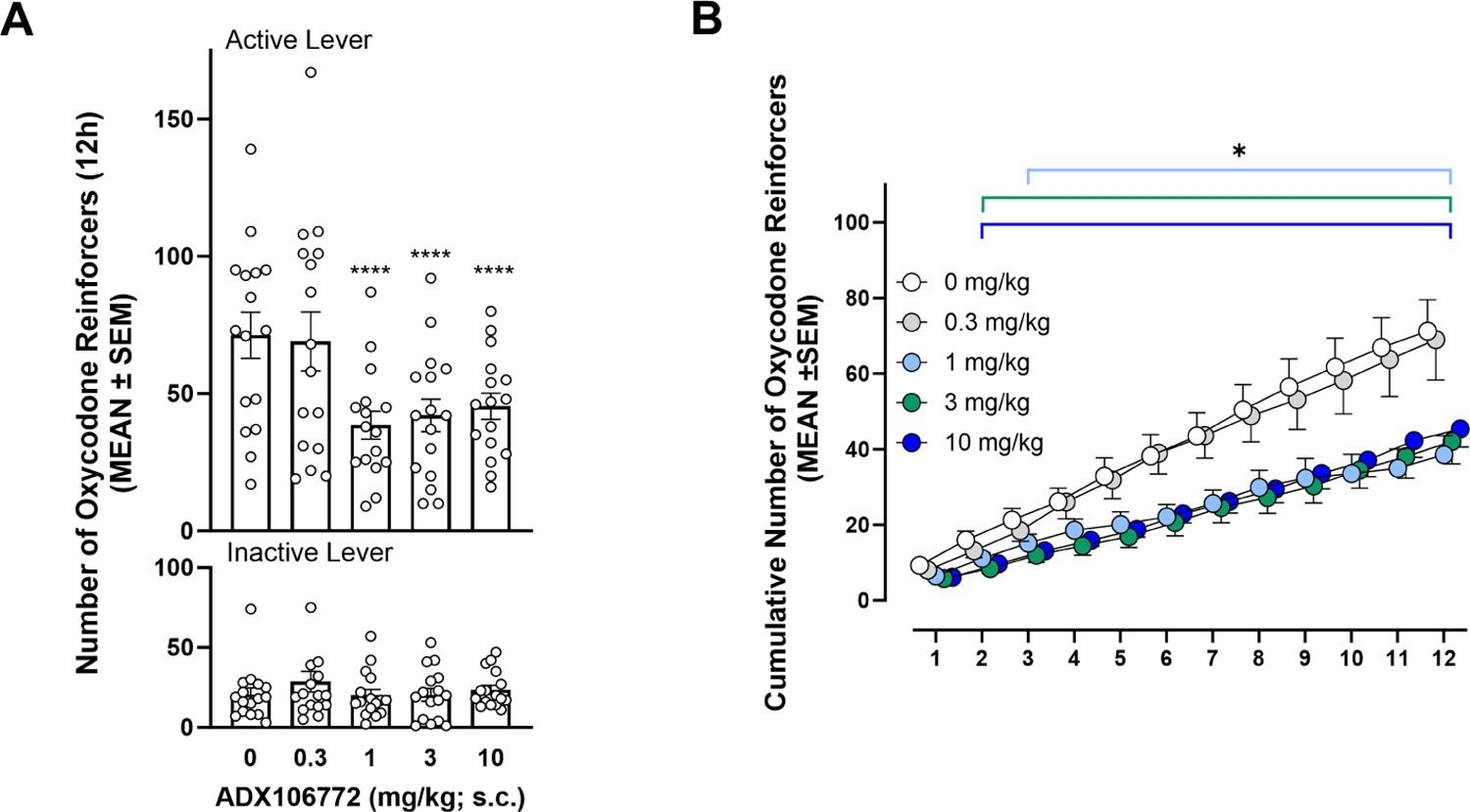
(A) Effect of ADX106772 on oxycodone self-administration (Bonferroni: ****p ≤ 0.0001, active lever responses vs. 0 mg/kg active lever responses). (B) Time course of effects of ADX106772 on oxycodone self-administration (Dunnett’s: *p ≤ 0.05, vs. 0 mg/kg).
At the end of the 13 sessions of extinction training, the rats reached an average of 9.88 ± 1.21 responses on the active lever. Following extinction training, presentation of the SD elicited the reinstatement of oxycodone seeking (p ≤ 0.0001, 0 mg/kg vs. EXT13, Bonferroni post hoc test following two-way RMANOVA, session: F6,90 = 4.78, p = 0.0003; lever: F1,15 = 43.82, p ≤ 0.0001; session × lever: F6,90 = 6.68, p ≤ 0.0001; Fig. 4A) and reinstatement was not affected by multiple presentations of the SD during which 0 mg/kg (i.e., ADX106772 vehicle solution) was tested (one-way ANOVA, session: F4,11 = 0.2786, p = 0.8857). The total number of active and inactive lever responses at the end of the 2-h conditioned reinstatement sessions was not significantly reduced by ADX106772 administration (p ≤ 0.05, vs. EXT13; Fig. 4A). However, scrutiny of the time course of responses revealed that ADX106772 time-dependently reduced the cumulative number of responses (p ≤ 0.05, vs. 0 mg/kg, Dunnett’s post hoc test following two-way RMANOVA, time: F23,345 = 32.90, p ≤ 0.0001; dose: F4,60 = 2.99, p = 0.0256; time × dose: F92,1380 = 2.13, p ≤ 0.0001). At 1 mg/kg ADX106772, the cumulative number of active lever responses began to significantly decrease 15 min after initiation of the reinstatement session and lasted for the remainder of the session (p ≤ 0.05, vs. 0 mg/kg; Fig. 4B). At 3 and 10 mg/kg ADX106772, the reduction was observed starting at 30 and 35 min, respectively, after initiation of the reinstatement sessions and lasted for the remainder of the session (p ≤ 0.05, vs. 0 mg.kg).
Figure 4.
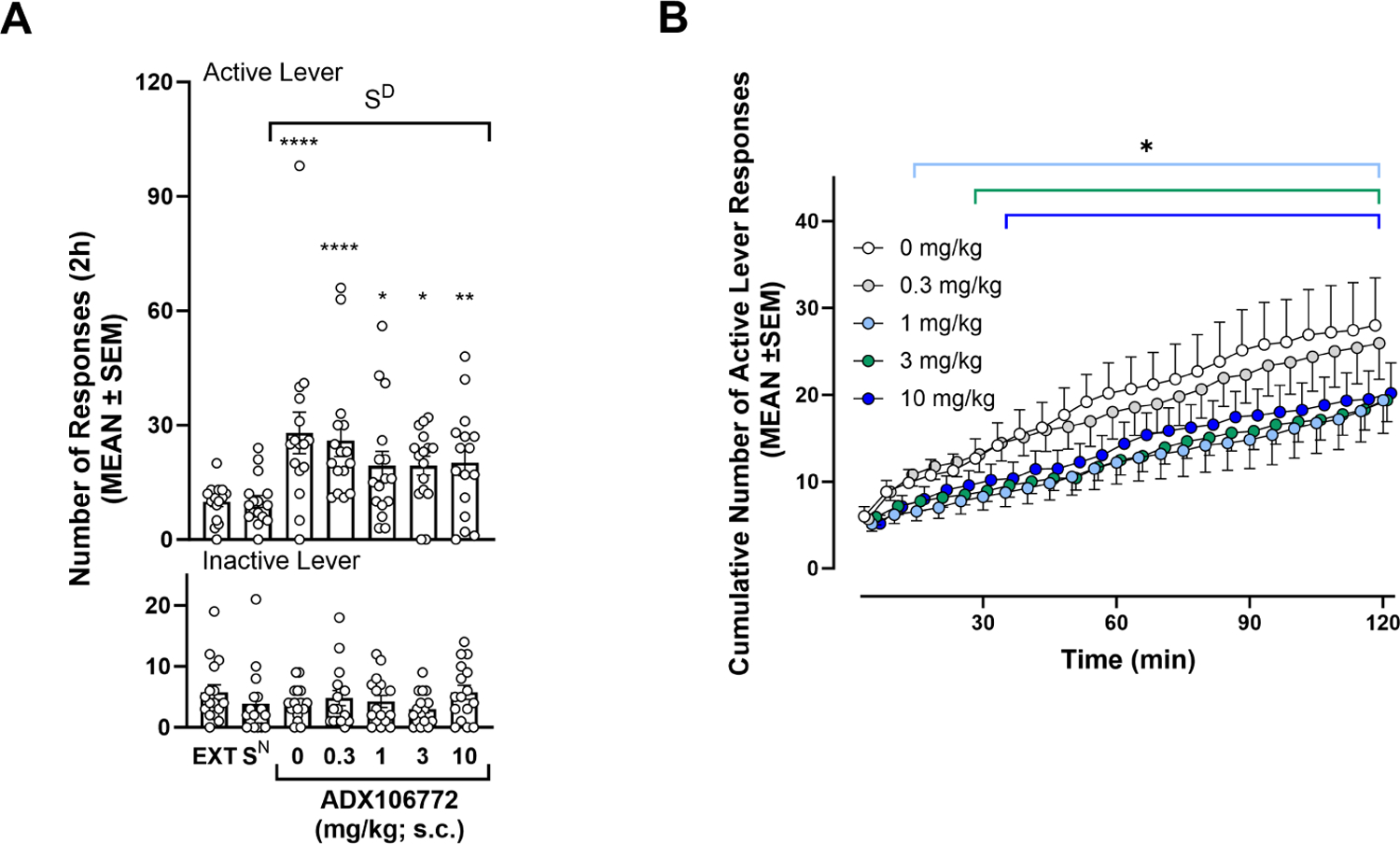
(A) Effect of ADX106772 on conditioned reinstatement of oxycodone seeking (Bonferroni: *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001, active lever responses vs. EXT13). (B) Time course of effects of ADX106772 on the conditioned reinstatement of oxycodone seeking (Dunnett’s: *p ≤ 0.05, vs. 0 mg/kg ADX106772).
Effect of ADX106772 on SCM self-administration and conditioned reinstatement
All 16 rats assigned to the SCM group completed all testing procedures and are represented throughout these data. Rats acquired SCM self-administration by session 2 of training (p ≤ 0.01, vs. session 1, Bonferroni post hoc test following two-way RMANOVA, session: F15,225 = 29.46, p ≤ 0.0001; lever: F1,15 = 373.20, p ≤ 0.0001; session × lever: F10,150 = 72.20, p ≤ 0.0001; Fig. 5A), reflected by a significant increase in the number of active lever responses across training sessions. The number of responses on the inactive lever did not change significantly across training (p > 0.05, Bonferroni post hoc test following two-way RMANOVA).
Figure 5.
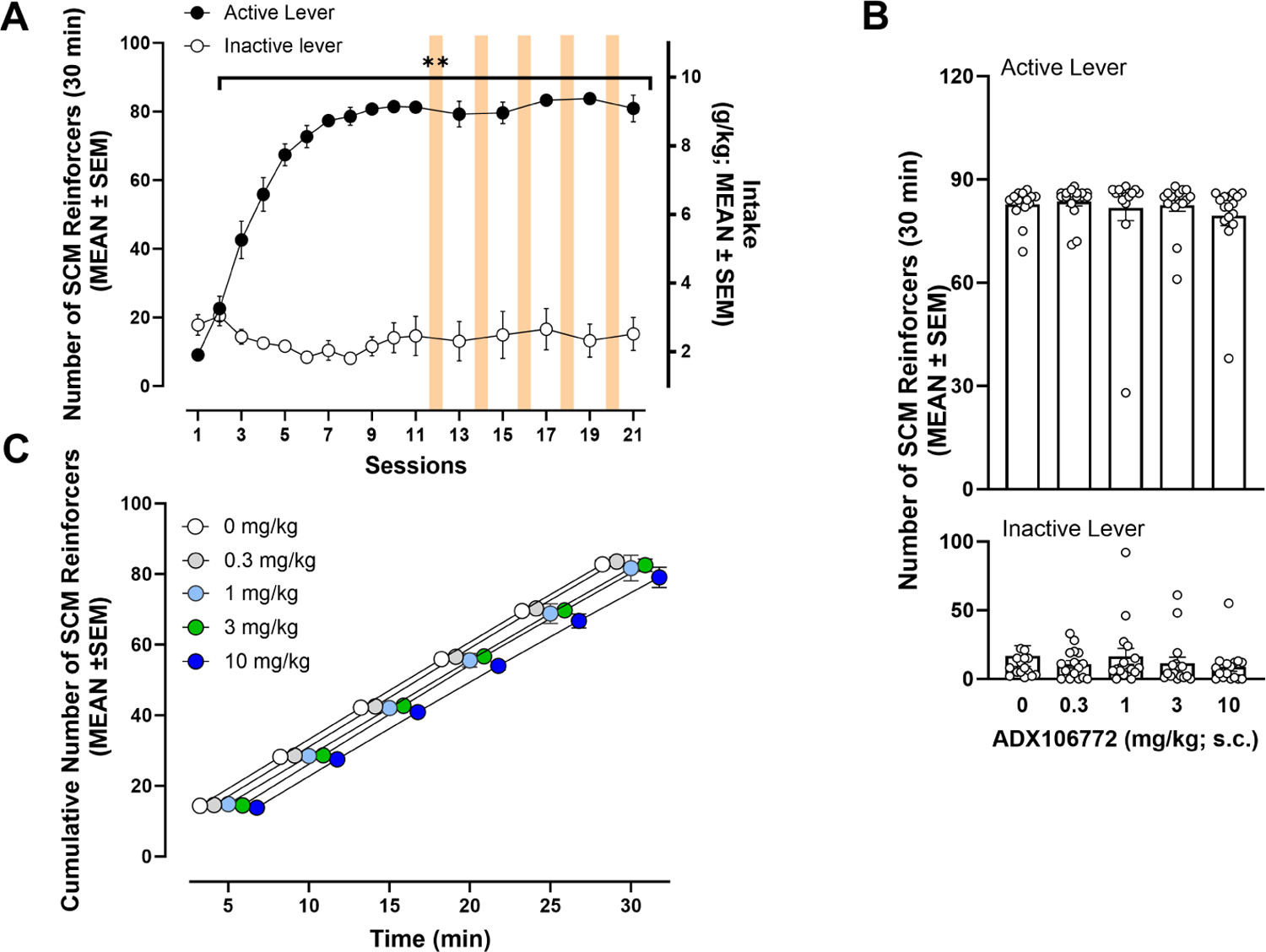
(A) Sweetened condensed milk self-administration over 21 sessions (30 min/day; Bonferroni: **p ≤ 0.01, vs. session 1). (B) Effect of ADX106772 on SCM self-administration. (C) Time course of effects of ADX106772 on SCM self-administration.
In contrast to results of oxycodone self-administration, ADX106772 did not affect overall SCM self-administration (p > 0.05, vs. 0 mg/kg ADX106772, Bonferroni post hoc test following two-way RMANOVA, session: F4,60 = 1.75, p = 0.1512; lever: F1,15 = 249.60, p ≤ 0.0001; session × lever: F4,60 = 0.97, p = 0.4266; Fig. 5B). Responses on the inactive lever were also not influenced by ADX106772 administration (p > 0.05, vs. 0 mg/kg ADX106772, Bonferroni post hoc test following two-way RMANOVA). Further scrutiny of the cumulative number of responses during the 30-min SCM self-administration session (Fig. 5C) confirmed that ADX106772 did not significantly affect SCM self-administration (two-way RMANOVA, time: F5,75 = 1300, p ≤ 0.0001; dose: F4,60 = 1.54, p = 0.1987; time × dose: F20,300 = 0.78, p = 0.7399).
At the end of the 13 sessions of extinction training, the rats reached an average of 9.75 ± 1.59 responses on the active lever. Presentation of the SD elicited the reinstatement of SCM seeking (p ≤ 0.0001, 0 mg/kg vs. EXT13, Bonferroni post hoc test following two-way RMANOVA, session: F3,44 = 29.18, p ≤ 0.0001; lever: F1,44 = 85.84, p ≤ 0.0001; session × lever: F3,44 = 20.27, p ≤ 0.0001; Fig. 6A), but the overall conditioned reinstatement of SCM seeking was unaffected by 10 mg/kg (p ≤ 0.0001, vs. EXT13; p ≤ 0.0001, vs. 0 mg/kg, Bonferroni post hoc test following two-way RMANOVA; Fig. 6A). Responses on the inactive lever did not change with reinstatement or ADX106772 administration (p > 0.05, all inactive lever pairwise comparisons, Bonferroni post hoc test following two-way RMANOVA). Scrutiny of the cumulative number of responses during the 2-h SCM conditioned reinstatement session (Fig. 6B) indicated no significant effects of 10 mg/kg ADX106772 (two-way RMANOVA, time: F23,322 = 13.58, p ≤ 0.0001; dose: F1,14 = 1.18, p = 0.2967; time × dose: F23,322 = 0.93, p = 0.9310). Even though there was no significant effect of 10 mg/kg ADX106772 on the cumulative number of responses observed during the 2-h SCM conditioned reinstatement session, a modest decrease was noted during the first 30 minutes (matching the length of the self-administration training) of the session. However, a separate statistical analysis of this 30-min period still did not reveal any significant effect of ADX106772 on reinstatement of SCM seeking (two-way RMANOVA, time: F5,70 = 13.56, p ≤ 0.0001; dose: F1,14 = 4.175, p = 0.0603; time × dose: F5.70 = 0.1453, p = 0.9808; Fig. 6B).
Figure 6.
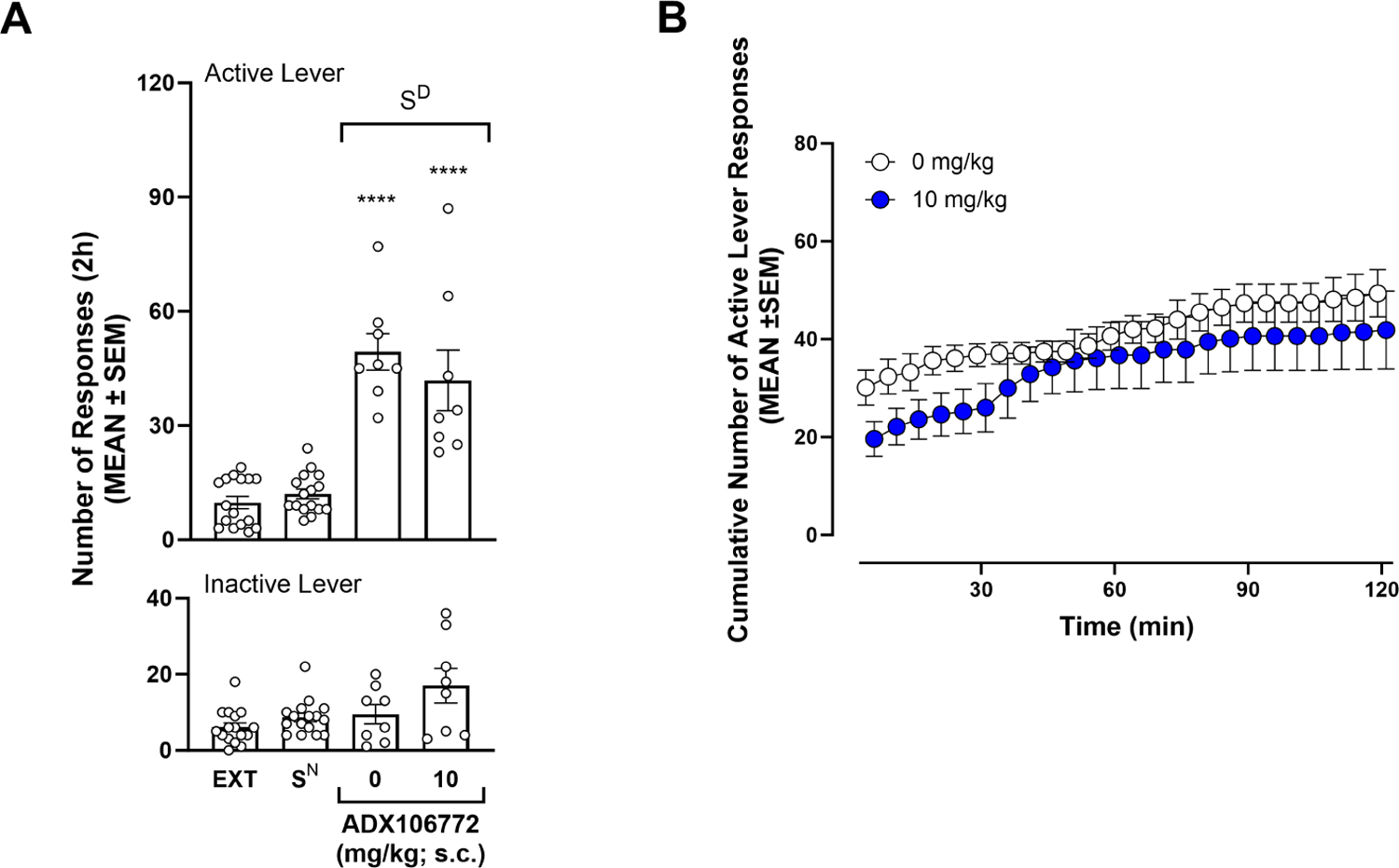
(A) Effect of ADX106772 on conditioned reinstatement of SCM seeking (Bonferroni: ****p ≤ 0.0001, vs. EXT13). (B) Time course of effects of ADX106772 on conditioned reinstatement of SCM seeking.
DISCUSSION
The present study confirmed earlier findings (Wade et al., 2015; Nguyen et al., 2019; Blackwood et al., 2019; Matzeu & Martin-Fardon, 2020; Kimbrough et al., 2020), in which rats with 12-h access readily acquired oxycodone self-administration and exhibited significant signs of dependence. The present study tested whether ADX106772, an mGlu2 receptor PAM, reduces oxycodone self-administration and the conditioned reinstatement of oxycodone seeking without affecting behaviors that are directed toward SCM. The data showed that ADX106772 decreased oxycodone intake and reduced oxycodone-seeking behavior that was induced by oxycodone-related contextual stimuli but had no effect on either the consumption of a palatable conventional reinforcer (SCM) or responding that was elicited by a stimulus that was conditioned to it. These observations suggest that the pharmacological activation of mGlu2 receptors may be a good approach to treat prescription OUD without producing nonspecific side effects.
Previous studies reported that the acute administration of other compounds that increase mGlu2 activity reduced the intake and seeking of nicotine in monkeys (Justinova et al., 2015) and rats (Li et al., 2016), cocaine in rats (Jin et al., 2010), and alcohol in rats (Augier et al., 2016). The present study was the first to demonstrate such effects of an mGlu2 receptor PAM on oxycodone intake and seeking. Faster onset of action of ADX106772 on oxycodone self-administration was observed with increasing doses of ADX106772. For example, 10 mg/kg ADX106772 reduced oxycodone self-administration within 2.5 h after s.c. administration, and self-administration remained lowered throughout the 12-h self-administration session. This time course was similar to findings with BINA, an alternative mGlu2 receptor PAM (Jin et al., 2010), showing that systemic administration (20–40 mg/kg, i.p.) in rats significantly decreased cocaine self-administration within the first 2 h post-treatment, and responding remained lowered when examining the entire 6 h self-administration session. The time course of effects of mGlu2 receptor PAMs on the conditioned reinstatement of drug seeking was also similar to self-administration. Thirty-five minutes after the administration of ADX106772 (1–10 m/kg), the reinstatement of oxycodone-seeking behavior decreased in a 2 h session. Likewise, BINA (10–40 mg/kg) was reported to significantly reduce cocaine-seeking behavior in a 1-h session that was initiated 1 h post-treatment (Jin et al., 2010). Notably, the effectiveness of mGlu2 receptor PAMs in reducing the conditioned reinstatement of drug-seeking behavior has been reported following 8 days (Jin et al., 2010), 14 days (present study), and 30 days (Augier et al., 2016) of abstinence, suggesting that these compounds remain effective long after drug intake has terminated. Collectively, these findings indicate that targeting the Glu system, particularly mGlu2 receptors, can influence the intake and seeking of various classes of drugs throughout the cycle of drug dependence, without affecting a normal motivated behavior.
ADX106772 selectively reduced oxycodone- vs. nondrug-seeking behavior, adding to the existing literature that shows that mGlu2 receptor PAMs can be used to prevent the reinstatement of drug-seeking behavior (nicotine: Justinova et al., 2015; Li et al., 2016; cocaine: Jin et al., 2010; alcohol: Augier et al., 2016). However, chronic or repeated treatment with mGlu receptor PAMs may be required to effectively manage OUD because this condition is characterized by drug craving that persists despite drug abstinence (Koob, 2021). Furthermore, the influence of higher and repeated doses of ADX106772 on non-drug seeking and other off-target behaviors should be evaluated. For example, chronic treatment with AZD8418 and AZD8529 (i.e., other mGlu2 receptor PAMs) in rats reduced food self-administration (Li et al., 2016). However, tolerance to reductions of food but not drug self-administration developed after day 6 of AZD8529 treatment, suggesting that the beneficial effect of reducing drug self-administration may outlast some off-target effects on other consummatory behaviors (Li et al., 2016). In the present study, the highest dose of ADX106772 tested (10 mg/kg, s.c.) did not significantly alter responses on the inactive lever, consistent with a previous study that found that distance travelled was not significantly influenced in the 30 min after a higher (20 mg/kg, s.c.) dose of acute ADZ8529 in rats (Augier et al., 2016). Nevertheless, in addition to excluding off-target side effects of chronic ADX106772 treatment, future studies should assess effects of chronic ADX106772 to identify the most effective dosing regimen to promote lasting drug abstinence.
A limitation of the present study was that it was conducted only with male rats. Future studies should test whether the present findings can be replicated in females and other populations that are vulnerable to drug dependence, such as adolescents. Indeed, an earlier study reported that female rats exhibited significantly higher Glu levels in the nucleus accumbens in response to morphine injections (7 mg/kg, s.c.) compared with male rats (Mousavi et al., 2007), suggesting greater sensitivity of the Glu system to the effects of opioids in females. Behaviorally, males exhibited significantly greater acute morphine-induced antinociception than females, but females developed tolerance to these effects sooner than males (Mousavi et al., 2007). Tolerance to antinociceptive effects of morphine in female rats was accompanied by an increase in Glu release in the nucleus accumbens 20 min after a morphine injection, an effect that was not observed in males (Mousavi et al., 2007). These results indicate that sex influences Glu release and behavioral responses to morphine. Future studies should investigate whether effects of ADX106772 on oxycodone taking and seeking can be generalized to females. Finally, the exact mechanisms underlying ADX106772 effects on oxycodone self-administration and conditioned reinstatement are not currently clear. It is known that acute morphine can suppress Glu-evoked neuronal responses (e.g., Coutinho-Neito et al., 1980; Martin et al., 1999; Hao et al., 2005), but repeated (≥ 7 days treatment) morphine exposure can result in tolerance to this effect, which can eventually result in a significant increased neuronal sensitivity to Glu (e.g., Satoh et al., 1976; Fry et al., 1980; Haberny & Young, 1994). Moreover, morphine withdrawal is characterized by overflow of Glu (Sepúlveda et al., 2004) and reduced mGlu2-dependent long-term depression (Robbe et al., 2002) in regions such as the nucleus accumbens. Therefore, mGlu2 PAMs may possibly reduce oxycodone taking and seeking by reducing the overall effect of chronic oxycodone and withdrawal on Glu release (for review see Gass & Olive, 2008). This warrants further investigation on the mechanism of action of ADX106772 to fully explain the effects observed here on oxycodone taking and seeking.
In conclusion, the present results add to the existing literature that shows that targeting mGlu2 receptors can selectively influence the intake and seeking of various drugs (e.g., nicotine, cocaine, alcohol, and oxycodone) over motivated behavior toward a nondrug reward. Effects of acute ADX106772 treatment on drug intake and seeking were observed within 1–2 h of administration and lasted at least 12 h after administration. Future studies should assess the effectiveness of acute vs. chronic ADX106772 treatment in reducing drug intake and seeking without increasing the risk of negative side effects. The present findings indicate that enhancing mGlu2 receptor activity by positive allosteric modulation may be promising treatment option for reducing excessive drug use and relapse.
ADX106772, an mGlu2 receptor PAM, reduced intake and seeking of oxycodone.
ADX106772 did not affect intake or seeking of sweetened condensed milk.
Positive modulation of mGlu2 receptors could treat prescription opioid use disorder.
Acknowledgements
This is publication number 30225 from The Scripps Research Institute. The authors thank Michael Arends for proofreading the manuscript. This work was supported by NIH/NIAAA grants AA026999, AA028549, AA006420 (to RM-F) and T32 AA007456 (to FJF-R and JMI) and NIH/NIDA grant DA053443 (to RM-F).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Statement
Jessica Illenberger: Formal analysis, Writing, Visualization. Francisco Flores-Ramirez: Investigation, Writing. Alessandra Matzeu: Investigation. Robert Lütjens: Conceptualization. Rémi Martin-Fardon: Conceptualization, Writing, Supervision, Funding acquisition.
Declaration of Interest
Declaration of Interest: none
REFERENCES
- Ahmad FB, Cisewski JA, Rossen LM, & Sutton P (2022). Provisional drug overdose death counts. National Center for Health Statistics. [Google Scholar]
- Aston-Jones G, Harris GC, (2004). Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology, 47 (Suppl 1), 167–179. doi: 10.1016/j.neuropharm.2004.06.020 [DOI] [PubMed] [Google Scholar]
- Augier E, Dulman RS, Rauffenbart C, Augier C, Cross AJ, & Heiling M (2016). The mGluR2 positive allosteric modulator, AZD8529, and cue-induced relapse to alcohol seeking in rats. Neuropsychopharmacol, 41(12): 2932–2940. doi: 10.1038/npp.2016.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharlouei N, Sarihi A, Komaki A, Shahidi S, Haghparast A (2015). Blockage of acquisition and expression of morphine-induced conditioned place preference in rats due to activation of glutamate receptors type II/III in nucleus accumbens. Pharmacol Biochem Behav, 135: 192–198. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, & Weiss F (2004). Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci, 24: 4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood CA, Hoerle R, Leary M, Schroeder J, Job MO, McCoy MT, Ladenheim B, Jayanthi S, Cadet JL (2019). Molecular adaptations in the rat dorsal striatum and hippocampus following abstinence-induced incubation of drug seeking after escalated oxycodone-self-administration. Mol Neurobiol, 56(5): 3606–3615. doi: 10.1007/s12035-018-1318-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Koob GF (1993). Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science, 260(5115):1814–1816. doi: 10.1126/science.8099761 [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, & Stewart J (2003). A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacol (Berl), 168: 66–74. doi: 10.1007/s00213-002-1283-z [DOI] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, Marchant NJ, Lucantonio F, Schoenbaum G, Bossert JM, & Shaham Y (2015). Effect of novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry, 78:463–473. doi: 10.1016/j.biopsych.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD (2000). Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem, 75(3): 889–907. doi: 10.1046/j.1471-4159.2000.0750889.x [DOI] [PubMed] [Google Scholar]
- Cho K, Bashir ZI (2002). Cooperation between mglu receptors: a depressing mechanism? Trends Neurosci, 25(8): 405–411. doi: 10.1016/s0166-2236(02)02228-2 [DOI] [PubMed] [Google Scholar]
- Cid JM, Tresadern G, Vega JA, De Lucas AI, Del Cerro A, Matesanz E, Linares ML, Garcia A, Iturrino L, Perez-Benito L, Macdonald GJ, Oehlrich D, Lavreysen H, Peeters L, Ceusters M, Ahnaou A, Drinkenburg W, Mackie C, Somers M, & Trabanco AA (2016). Discovery of 8-Trifluoromethyl-3-cyclopropylmethyl-7-[(4-(2,4-difluorophenyl)-1-piperazinyl)me thyl]-1,2,4-triazolo[4,3-a]pyridine (JNJ-46356479), a Selective and Orally Bioavailable mGlu2 Receptor Positive Allosteric Modulator (PAM). J Med Chem, 59, 8495–507. doi: 10.1021/acs.jmedchem.6b00913 [DOI] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, & Kalivas PW (1999). A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neurosci, 93: 1359–1367. doi: 10.1016/s0306-4522(99)00214-6 [DOI] [PubMed] [Google Scholar]
- Cornish JL, & Kalivas PW (2000). Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci, 20: RC89 (1–5). doi: 10.1523/JNEUROSCI.20-15-j0006.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho-Neito J, Abdul-Ghani A, Bradford HF (1980). Suppression of evoked and spontaneous release of neurotransmitters in vivo by morphine. Biochem Pharmacol, 29(20): 2777–2780. doi: 10.1016/0006-2952(80)90011-8 [DOI] [PubMed] [Google Scholar]
- Di Ciano P, & Everitt BJ (2001). Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacol, 25: 341–360. doi: 10.1016/S0893-133X(01)00235-4 [DOI] [PubMed] [Google Scholar]
- Fry JP, Herz A, Zieglgänsberger W, (1980). A demonstration of naloxone-precipitated opiate withdrawal on single neurones in the morphine-tolerant/dependent rat brain. Br J Pharmacol, 68: 585–592. doi: 10.1111/j.1476-5381.1980.tb14574.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Jordan CJ, Bi G, He Y, Yang H, Gardner EL, Xi Z (2018). Deletion of the type 2 metabotropic glutamate receptor increases heroin abuse vulnerability in transgenic rats. Neuropsychopharmacol, 43(13): 2615–2626. doi: 10.1038/s41386-018-0231-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, & Olive MF (2008). Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol, 75: 218–265. doi: 10.1016/j.bcp.2007.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Sinclair CM, Cleva RM, Widholm JJ, & Olive MF (2011). Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol, 16(2): 215–228. doi: 10.1111/j.1369-1600.2010.00262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wang H, Xiang X, & Zhao Y (2009). The role of glutamate and its receptors in mesocorticolimbic dopaminergic regions in opioid addiction. Neurosci Biobehav Rev, 33: 864–873. doi: 10.1016/j.neubiorev.2009.02.005 [DOI] [PubMed] [Google Scholar]
- Haberny KA, Young GA, (1994). Interactive effects of MK-801and morphine on EEG, EEG power spectra and behavior in rats 1. Morphine tolerance development. Eur J Pharmacol, 261(1–2): 1–9. doi: 10.1016/0014-2999(94)90293-3 [DOI] [PubMed] [Google Scholar]
- Hao Y, Yang JY, Guo M, Wu CF, Wu M,F (2005). Morphine decreases extracellular levels of glutamate in the anterior cingulate cortex: an in vivo microdialysis study in freely moving rats. Brain Res, 1040(1–2): 191–196. doi: 10.1016/j.brainres.2005.01.072 [DOI] [PubMed] [Google Scholar]
- Jin X, Semenova S, Yang L, Ardeky R, Sheffler DJ, Dahl R, Conn PJ, Cosford NDP, Markou A (2010). The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacol, 35(10): 2021–2036. doi: 10.1038/npp.2010.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Panlilio LV, Secci ME, Redhi GH, Schindler CW, Cross AJ., Mrzlijak L, Medd A, Shaham Y, Goldberg SR (2015). The novel metabotropic glutamate receptor 2 positive allosteric modulator, AZD8529, decreases nicotine self-administration and relapse in squirrel monkeys. Biol Psychiatry, 78: 452–462. doi: 10.1016/j.biopsych.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2004). Glutamate systems in cociane addiction. Curr Opin Pharmacol, 4: 23–29. doi: 10.1016/j.coph.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Kalivas PW (2009). The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci, 10(8): 561–572. doi: 10.1038/nrn2515 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, LaLumiere RT, Knackstedt L, & Shen H (2009). Glutamate transmission in addiction. Neuropharmacology, 56:169–173. doi: 10.1016/j.neuropharm.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C (2007). Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacol, 33(1), 166–180. doi: 10.1038/sj.npp.1301564 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, & Volkow ND (2005). The Neural Basis of Addiction: a Pathology of Motivation and Choice. Am J Psychiatry, 162(8): 1403–1413. doi: 10.1176/appi.ajp.162.8.1403 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, & Seamans J (2005). Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron, 45(5): 647–650. doi: 10.1016/j.neuron.2005.02.005 [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC (2002). The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci, 22(9), 3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, & Markou A (2004). The ups and downs of addiction: role of metabotropic glutamate receptors. TRENDS Pharmacol Sci, 25(5): 265–272. doi: 10.1016/j.tips.2004.03.009 [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Muhammad F, Hofamnn J, & Drexler K (2001). Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry, 58: 334–341. doi: 10.1001/archpsyc.58.4.334 [DOI] [PubMed] [Google Scholar]
- Kimbrough A, Kononoff J, Simpson S, Kallupi M, Sedighim S, Palomino K, Conlisk D, Momper JD, Guglielmo G, George O (2020). Oxycodone self-administration and withdrawal behaviors in male and female Wistar rats. Psychopharmacol, 237, 1545–1555. doi: 10.1007/s00213-020-05479-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2020). Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry, 87(1): 44–53. doi: 10.1016/j.biopsych.2019.05.023 [DOI] [PubMed] [Google Scholar]
- Koob GF, (2021). Drug addiction: hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev, 73(1): 163–201. doi: 10.1124/pharmrev.120.000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Bloom FE (1988). Cellular and Molecular Mechanisms of Drug Dependence. Science, 242: 715–723. doi: 10.4103/0019-5545.33253 [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (1997). Drug Abuse: hedonic homeostatic dysregulaion. Science (278): 52–58. doi: 10.1126/science.278.5335.52 [DOI] [PubMed] [Google Scholar]
- Li X, D’Souza MS, Nino AM., Doherty J, Cross A, Markou A (2016). Attenuation of nicotine-taking and nicotine-seeking behavior by the mGlu2 receptor positive allosteric modulators AZD8418 and AZD8529 in rats. Psychopharmacol, 233: 1801–1814. doi: 10.1007/s00213-016-4220-2 [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, & Markou A (2007). Metabotropic glutamate receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci, 27(34):9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Przewlocki R, Siggins GR (1999). Chronic morphine treatment selectively augments metabotropic glutamate receptor-induced inhibition of N-methyl-D-aspartate receptor mediated neurotransmission in nucleus accumbens. J Pharmacol Exp Ther, 288(1): 30–35. [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MAS, Dayas CV, Weiss F (2009). Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther, 329(3): 1084–1090. doi: 10.1124/jpet.109.151357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R & Weiss F (2017). Perseveration of craving: effects of stimuli conditioned to drugs of abuse versus conventional reinforcers differing in demand. Addict Biol, 22, 923–932. doi: 10.1111/adb.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Kallupi M, George O, Schweitzer P, Martin-Fardon R (2018). Dynorphin counteracts orexin in the paraventricular nucleus of the thalamus: cellular and behavioral evidence. Neuropsychopharmacol, 43(5), 1010–1020. doi: 10.1038/npp.2017.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A & Martin-Fardon R (2020). Targeting the orexin system for prescription opioid use disorder: Orexin-1 receptor blockade prevents oxycodone taking and seeking in rats. Neuropharmacology, 164, 107906. doi: 10.1016/j.neuropharm.2019.107906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, & Kalivas PW (2003). Prefrontal Glutamate Release into the Core of the Nucleus Accumbens Mediates Cocaine-Induced Reinstatement of Drug-Seeking Behavior. J Neurosci, 23(8):3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, & Kalivas PW (2001). The Circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci, 21(21): 8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH (2013). Rescue of Infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci, 33(7): 2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, & Seamans JK (2005). Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci, 25(27): 6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S (2005). Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci USA, 102(11): 41704175. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi Z, Shafaghi B, Kobarfard F, & Jorjani M (2007). Sex differences and role of gonadal hormones on glutamate level in the nucleus accumbens in morphine tolerant rats: a microdialysis study. Eur J Pharmacol, 554: 145–149. doi: 10.1016/j.ejphar.2006.10.010 [DOI] [PubMed] [Google Scholar]
- Moussawi K, & Kalivas PW (2010). Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol, 639: 115–122. doi: 10.1016/j.ejphar.2010.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Hwang CS, Vandewater SA, Janda KD, Cole M, Taffe MA (2019). Δ9-tetrahydrocannabinol attenuates oxycodone self-administration under extended access conditions. Neuropsychopharmacol, 151: 127–135. doi: 10.1016/j.neuropharm.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Wu X, Qiao Y, Shi M, Liu Z, Ren W, Han J, Zheng Q (2019). Downregulation of mGluR2/3 receptors during morphine withdrawal in rats impairs mGluR2/3- and NMDA receptor-dependent long-term depression in the nucleus accumbens. Neurosci Letters, 690: 76–82. doi: 10.1016/j.neulet.2018.10.018 [DOI] [PubMed] [Google Scholar]
- Pin JP, Acher F (2002). The metabotropic glutamate receptors: structure, activation mechanism and pharmacology. CNS Neurol Disord Drug Targets, 1(3), 297–317. doi: 10.2174/1568007023339328 [DOI] [PubMed] [Google Scholar]
- Rehni AK, Jaggi AS & Singh N (2013). Opioid withdrawal syndrome: emerging concepts and novel therapeutic targets. CNS Neurol Disord Drug Targets, 12, 112–25. doi: 10.2174/1871527311312010017 [DOI] [PubMed] [Google Scholar]
- Robbe D, Bockaert J, Manzoni OJ (2002). Metabotropic glutamate receptor 2/3-dependent long-term depression in the nucleus accumbens is blocked in morphine withdrawn mice. Eur J Neurosci, 16(11): 2231–2235. doi: 10.1046/j.1460-9568.2002.02273.x [DOI] [PubMed] [Google Scholar]
- Satoh M, Zieglgansberger W, Herz A (1976). Supersensitivity of cortical-neurons of rat to acetylcholine and L-glutamate following chronic morphine treatment. Naunyn-Schmiedeb Arch Pharmacol, 293(1): 101–103. doi: 10.1007/BF00498877 [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, & Myrick H (2013). Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol, 18: 121–133. doi: 10.1111/j.1369-1600.2012.00464.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, & Koob GF (1999). Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol, 10(3): 235–242. doi: 10.1097/00008877-199905000-00001 [DOI] [PubMed] [Google Scholar]
- Sepúlveda J, Oliva P, Contreras E (2004). Neurochemical changes of the extracellular concentrations of glutamate aspartate in the nucleus accumbens of rats after chronic administration of morphine. Eur J Pharmacol, 483: 249–258. doi: 10.1016/j.ejphar.2003.10.037 [DOI] [PubMed] [Google Scholar]
- Shen H, Scofield MD, Boger H, Hensley M, & Kalivas PW (2014). Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci, 34: 5649–5657. doi: 10.1523/JNEUROSCI.4564-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM (2001). Pharmacology and behavioral pharmacoogy of the mesocortical dopamine system. Prog Neurobiol, 63(3): 241–320. doi: 10.1016/s0301-0082(00)00033-2 [DOI] [PubMed] [Google Scholar]
- United States Department of Health & Human Services (USDHHS) (2020). 2019 NSDUH Annual National Report. Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF (2015). Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacol, 40(2), 421–428. doi: 10.1038/npp.2014.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PEM (2009). Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev, 2(2), 195–213. doi: 10.2174/1874473710902020195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei E, Loh HH, & Way EL (1973). Quantitative aspects of precipitated abstinence in morphine-dependent rats. J Pharmacol Exp Ther(184): 398–403. [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, & Fiez JA (2004). Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci, 7: 211–214. doi: 10.1038/nn1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Shi M, Wei C, Yang M, Liu Y, Liu Z, Zhang X, Ren W (2012). Potentiation of synaptic strength and intrinsic excitability in the nucleus accumbens after 10 days of morphine withdrawal. J Neurosci Res, 90(6):1270–83. doi: 10.1002/jnr.23025 [DOI] [PubMed] [Google Scholar]
- Yuan G, Guehl NJ, Zheng B, Qu X, Moon S, Dhaynaut M, Shoup TM, Afshar S, Kang HJ, Zhang Z, Fakhri GE, Normandin MD, & Brownell A (2021). Synthesis and Characterization of [18F]JNJ-46356479 as the First 18F-Labeled PET Imaging Ligand for Metabotropic Glutamate Receptor 2. Mol Imaging Biol, 23: 527–536. doi: 10.1007/s11307-021-01586-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Barr GA (2004). The role of AMPA and metabotropic glutamate receptors on morphine withdrawal in infant rats. Int J Devl Neuroscience, 22: 379–395. doi: 10.1016/j.ijdevneu.2004.06.005 [DOI] [PubMed] [Google Scholar]


