Abstract
An epidemiological survey on human calicivirus (HuCV) infections and associated gastroenteritis in infants was conducted to clarify the prevalence of HuCV infections in infants and adults in Kenya. Enzyme immunoassays (EIAs) for three genogroups of HuCVs, Norwalk virus (NV), Mexico virus (MXV), and Sapporo virus (SV), were used to detect antigen or antibody. We tested 1,431 stool samples obtained from children younger than 6 years old with acute gastroenteritis who visited outpatient clinics in three districts in Kenya from August 1991 to July 1994. Thirty-two (2.2%) of these stool samples were positive for SV antigen. Only one (0.1%) of 1,186 samples was positive for NV antigen and none of 246 samples was positive for MXV antigen. One hundred ninety-three serum samples were tested for antibodies to NV and MXV, and 64 of them were examined for antibody to SV. The pattern of the age-related prevalence of serum antibody to NV was different from that of antibodies to MXV and SV. The acquisition of serum antibodies to HuCVs in the three genogroups appeared in early childhood, at about 1 to 2 years of age. The prevalence of serum antibody to NV was low (about 60%) throughout adulthood compared with a high prevalence of antibody (∼80 to 90%) to MXV and SV. These data indicate that infections with viruses in the three genogroups of HuCVs are common in Kenya, and immunological responses to NV may be different from those to MXV and SV. The EIAs for the detection of NV and MXV antigens appear to be quite specific for prototype NV and MXV strains, respectively, so that they can detect only a few strains of HuCVs related to them. Alternatively, NV and MXV caused less severe infections that did not bring children to the outpatient clinics for gastroenteritis in Kenya.
Research work on viral gastroenteritis in Kenya has focused on only group A rotaviruses so far (4, 19, 23, 30), mainly because of the clinical importance of group A rotaviruses. Another reason is that a practical method like enzyme immunoassay (EIA) for the detection of other gastroenteritis viruses, especially for human caliciviruses (HuCVs), is not available in that country. HuCVs have been divided into at least three genogroups (genogroup I, represented by Norwalk virus [NV]; genogroup II, represented by Snow Mountain virus; and genogroup III, represented by Sapporo virus [SV] [2, 8, 25]) on the basis of genome analysis of the RNA-dependent RNA polymerase region and capsid protein region and also differences in antigenicity. Because of these antigenic differences in HuCVs, at least three kinds of EIA systems are needed to detect these HuCVs. Such EIAs have been limited to only a few research institutes in the world because of a limited supply of the reagents.
The recent success of NV and Mexico virus (MXV; genogroup II HuCV) gene cloning and the production of the recombinant NV (rNV) and recombinant MXV (rMXV) capsid proteins by the baculovirus expression system (11, 14) has resulted in the availability of an unlimited amount of rNV and rMXV antigen and high-titer hyperimmune sera to rNV and rMXV to enable large-scale epidemiological studies. The EIA for SV is available at the Department of Pediatrics, Sapporo Medical University, Sapporo, Japan (22). Previous seroepidemiological studies by these EIAs indicate that infection with NV, MXV, or SV is very common in the world (9, 10, 18, 20, 21, 24, 26–28).
Because a systematic survey of the HuCV infections and associated gastroenteritis in infants has not been conducted in Kenya, we conducted an epidemiological study to clarify the prevalence of HuCV infections in infants and adults in Kenya. Diarrheal stool samples obtained from infants who were mainly outpatients in two districts and in Nairobi, Kenya, were examined by the EIAs for viruses in the three genogroups of HuCVs to clarify the importance of HuCVs in causing infantile gastroenteritis in an outpatient setting. The age-related prevalence of serum antibody to three HuCVs was also examined by blocking EIAs (10, 22, 24).
MATERIALS AND METHODS
Clinical specimens.
One thousand four hundred thirty-one stool samples were collected from children younger than 6 years old with acute gastroenteritis who were visiting outpatient clinics in Nanyuki, Kitui, and Nairobi, Kenya, from August 1991 to July 1994. Fifty-three percent of the stool samples were obtained from infants younger than 11 months old, 34% were from children 12 to 24 months old, 7% were from children 25 to 36 months old, and 6% were from children 37 to 72 months old. These samples had been examined by conventional EIA for group A rotavirus and by EIA with monoclonal antibodies to either type 40 or type 41 enteric adenoviruses (23). These samples were also tested by the antigen EIA for SV (genogroup III human calicivirus) (22). One thousand one hundred eighty-six stool specimens were examined by the antigen EIA for NV (genogroup I human calicivirus) (24), and 246 stool specimens were examined by the EIA for MXV (genogroup II human calicivirus) (10, 13). Stool samples were prepared as a 10% (wt/vol) suspension in 10 mM phosphate-buffered saline (PBS; pH 7.4) and clarified by centrifugation at 7,000 × g for 20 min. The aqueous phase was stored at 4°C until it was tested.
Serum specimens.
Eighty serum specimens were collected from adult patients (ages, 20 to more than 50 years) with liver diseases in Mombasa, Machakos, and Nairobi, Kenya, from 1991 to 1995. One hundred thirteen serum specimens were obtained from children (ages, 0 months to 19 years) without gastroenteritis who were chronic hepatitis B virus carrier cases in Maragua in Kenya from 1986 to 1989 (32). For testing, these sera were divided into 10 groups according to the ages of the donors (0 to 3, 4 to 11, and 12 to 23 months and 2 to 5, 6 to 11, 12 to 19, 20 to 29, 30 to 39, 40 to 49, and older than 50 years), and 18, 18, 19, 19, 19, 20, 20, 20, 20, and 20 samples from individuals in each age group, respectively, were tested for antibodies to NV and MXV. For the detection of antibody to SV, 5, 10, 8, 13, 11, and 11 samples from individuals in each of the age groups consisting of individuals younger than 19 years, respectively, and 6 serum samples from adults ages 20 to 29 years were tested. All serum specimens were stored at −20°C until they were used and were tested without knowledge of the age of the subjects.
Baculovirus-expressed NV or MXV capsid antigen.
NV and MXV capsid antigens (rNV and rMXV, respectively), which were produced by the baculovirus expression system, were obtained as described previously (11, 14). rNV and rMXV were diluted to a concentration of 1 μg/ml and were used for the following experiments.
EIA for detection of three genogroups of caliciviruses.
The EIAs for SV antigen and for NV antigen in stools were done as described previously (22, 24). The EIA for MXV antigen was performed in the same way as described above for the EIAs for SV and NV antigens, but a modified EIA which was different from the original EIA for MXV antigen described previously (13) was used. The sensitivity and specificity of the EIA for MXV antigen have been described elsewhere (10). Briefly, PBS containing 10% fetal calf serum, 1% bovine serum albumin (BSA), and 0.05% Tween 20 was used as the dilution buffer for stool samples, blocking serum, and detector antibody. The peroxidase-conjugated goat antibody to rabbit immunoglobulin G was diluted in PBS containing 5% normal guinea pig serum, 1% BSA, and 0.05% Tween 20. Duplicate wells of the 96-well polyvinyl chloride flat-bottom microtiter plates (Dynatech Laboratories, Inc., Alexandria, Va.) were each coated with 100 μl of a 1:10,000 dilution of either hyperimmune guinea pig serum to MXV or preimmune guinea pig serum diluted in 10 mM PBS (pH 7.4), and the plates were incubated at 4°C overnight. The plates were then washed five times with PBS containing 0.05% Tween 20 (PBS-T) and were blocked with 1% BSA in PBS for 60 min at 37°C. The residual blocking fluid was then removed, 50 μl of the dilution buffer was added to all wells, and then 25 μl of each test sample (10% stool suspension) was added. The plates were incubated at 4°C overnight. After five washings in PBS-T, 50 μl of a 1:5,000 dilution of rabbit serum hyperimmune to MXV was added, and the plates were incubated for 90 min at 37°C. After five washings in PBS-T, 50 μl of a 1:10,000 dilution of peroxidase-conjugated goat antibody to rabbit immunoglobulin G (Seikagaku Corporation, Tokyo, Japan) was added. The plates were incubated for 90 min at 37°C and washed five times in PBS-T. A 100-μl portion of o-phenylenediamine dihydrochloride (0.4 mg/ml; Wako Pure Chemical Industries, Ltd., Osaka, Japan) in 0.15 M citric acid buffer (pH 4.0) containing 0.4 μl of 30% H2O2 per ml was added to each well, and the plates were incubated for 30 min at room temperature. The reaction was stopped with 100 μl of 1 M H2SO4, and the A492 was measured with an EIA reader (Easy Reader EAR400; SLT-Labinstruments).
For each sample, the results were expressed as the ratio of the A492 for wells coated with hyperimmune serum (positive) to the A492 for wells coated with preimmune serum (negative) (P/N ratio). The cutoff value of this system was obtained by testing 20 stool suspensions from patients with group A rotavirus gastroenteritis. The mean P/N ratio of these samples plus 3 standard deviations was 1.7. A P/N ratio of >1.7 and an A492 of >0.075 were considered to indicate a positive reaction. All tests were performed in duplicate, and the results were averaged. The optimal dilutions of reagents were determined by checkerboard titrations with rMXV capsid protein and 10% stool suspensions containing group A rotavirus. Positive controls consisting of rNV, rMXV, and SV antigens were run in each plate.
Blocking EIA for detection of antibodies for three genogroups of caliciviruses.
Antibodies to NV, MXV, and SV were measured by a blocking EIA (10, 22, 24). This format was used in this laboratory (Department of Pediatrics, Sapporo Medical University) to measure antibody to a variety of enteric viruses because of the advantages described previously (24). The sensitivity and specificity of this EIA are described elsewhere (10).
Statistical analysis.
Student’s t test or chi-square analysis was used where appropriate.
RESULTS
Prevalence of gastroenteritis viruses in Kenya.
The prevalence of gastroenteritis viruses in stool samples obtained from children younger than 6 years old who had acute gastroenteritis from August 1991 to July 1994 was as follows: group A rotavirus was the main virus detected every year and had a prevalence of 22.2% (range, 20.9 to 23.7%), followed by HuCVs at 2.2% (range, 0.7 to 4.9%) and enteric adenoviruses (type 40 and type 41) at 1.4% (range, 1.2 to 1.6%) (data not shown).
Detection of three genogroups of caliciviruses in diarrheal stool samples.
One thousand one hundred eighty-six stool specimens were tested for NV antigen by the EIA. Only one (0.1%) of them was positive for NV antigen. The patient was a 6-month-old female who had a clinically mild case of diarrhea of 2 days’ duration and 1 day of vomiting. Although this positive sample was tested by reverse transcription-PCR with the 35-36 primer set for the RNA polymerase region (12), no PCR product was obtained, probably because of the presence of inhibitors in the stool sample. The presence of inhibitors was confirmed by inhibition of amplification of an internal standard to this sample (data not shown).
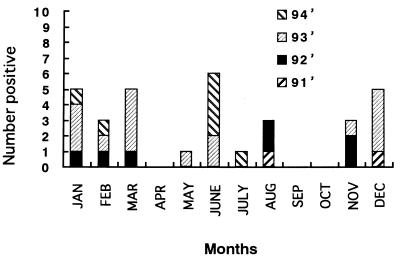
Thirty-two (2.2%) of 1,431 stool samples were positive for SV by the EIA for the SV antigen. The age distribution of the patients infected with SV ranged from 5 months to 6 years and 11 months (mean, 17.7 months), and the sex ratio (number of males/number of females) was 1.75. The seasonal shedding of SV from August 1991 to July 1994 is shown in Fig. 1. In a tropical climate like Kenya, there are two seasons, dry and wet periods. In Kenya, the wet period usually lasts from April to July and from November to December and the rest of the year is the dry period. SV was constantly detected every year without regard to the climate in Kenya.
FIG. 1.
Seasonal shedding of SV in Kenya from 1991 to 1994.
Complete clinical information was available for 1,286 of 1,431 patients. Although the mean duration of diarrhea due to SV (6.22 days; 22 samples) was longer than that due to group A rotaviruses (3.98 days; 284 samples), enteric adenoviruses (4.13 days; 20 samples), and no virus (4.55 days; 960 samples), there was no statistically significant difference among them (t test). However, the prevalence of persistent diarrhea which continued for more than 14 days was significantly higher in patients with diarrhea due to SV (5 of 22) than in patients with diarrhea due to group A rotaviruses (7 of 284) and no virus (48 of 960) (chi-square test).
Two hundred forty-six stool specimens were tested for the MXV antigen by EIA. None of them was positive for the MXV antigen.
Prevalence of antibodies to three genogroups of caliciviruses.
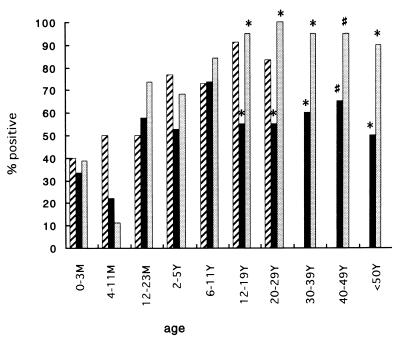
The age-related prevalence of serum antibodies to NV, MXV, and SV in Kenya is shown in Fig. 2. Six (33.3%) or seven (38.9%) of 18 infants below the age of 3 months had antibody to NV or MXV, respectively. The prevalence of serum antibody decreased thereafter and reached a minimum in the group consisting of 4- to 11-month-old children (22.2 and 11.1%, respectively). The rate of positivity for antibody to MXV steeply increased among preschool-age children, reached a maximum of more than 90% in the group consisting of 12- to 19-year-olds, and was maintained thereafter. On the other hand, the prevalence of serum antibody to NV increased to 58% in infants ages 12 to 23 months and was maintained thereafter at between 50 and 70%. The prevalence of serum antibodies to NV and MXV was statistically different (P < 0.01 and P < 0.05, respectively [chi-square test]) in the groups consisting of individuals older than 12 years of age (Fig. 2). Although the sample number was smaller for SV-infected patients than those for patients infected with the other two viruses, the pattern of acquisition of serum antibody was similar to those for NV and MXV, except that no clear decrease in antibody prevalence was seen in the group consisting of individuals 4 to 11 months of age. The rate of positivity for antibody to SV was maintained at between 70 and 90% from infants to adults.
FIG. 2.
Age-related prevalence of antibody to NV (■), MXV ( ), and SV (▨) in Kenya. *, P < 0.01; #, P < 0.05 (chi-square test). M, month; Y, year.
DISCUSSION
This is the first systematic study to investigate the role of three genogroups of HuCVs in causing gastroenteritis in an outpatient setting. SV-related strains were detected every year in 1 to 4% of diarrheal stool samples from infants younger than 6 years old, whereas NV and MXV were rarely detected or were not detected. However, the seroepidemiological study in Kenya suggested that acquisition of antibodies to three genogroups of HuCVs starts in early childhood and that infections with these three viruses are common in Kenya.
Several possibilities may explain the low rate of detection or lack of detection of NV and MXV compared to the high prevalence of antibodies to these viruses. First, the EIA for rNV or rMXV antigen detection is quite specific for prototype NV or MXV strains and may detect only a few strains of genogroup I or II HuCVs which are antigenically and genetically close to those prototype viruses (5, 24). Actually, the EIA for rMXV, subgenogroup 2 of HuCV genogroup II, detects only a subset of subgenogroup 1 of HuCV genogroup II-positive samples (13). More broadly reactive EIAs are required to overcome the possible explanation for the low rate of detection of NV and MXV antigen in stool samples described above. Second, the positive reactions for NV or MXV antibody by the EIA may reflect separate infections with HuCVs of the same genogroup. Serological responses can detect the antigenic relatedness among viruses of the same genogroup more broadly than the antigen detection method (16). Third, NV and MXV gastroenteritis might be milder than SV infections and may not require visits to outpatient clinics. Finally, these viruses may cause diseases other than acute gastroenteritis. Low rates of detection of NV and MXV in infantile gastroenteritis were also demonstrated in Japan (10, 24) and the United Kingdom (1, 26), so that practical methods for the detection of the antigen more broadly and the examination of large numbers of samples are needed.
SV seemed to be an important gastroenteritis virus along with group A rotavirus as a cause of infantile viral gastroenteritis in Kenya. This virus was constantly detected in Kenya every year without seasonality, which was similar to the findings for developed countries like Japan (17). Interestingly, the prevalence of persistent diarrhea continuing for 14 days or longer was significantly higher in patients infected with calicivirus than in patients infected with group A rotavirus and patients who were virus negative. A combination of SV infection with other factors in Kenya might play a role in this phenomenon because similar findings were not found in Japan. Two reports have suggested a relationship between enteric adenovirus gastroenteritis and persistent diarrhea (7, 29), but another report does not (31). Further investigation is required to clarify the causes of persistent diarrhea, which increases the risk of malnutrition and mortality among children in developing countries.
Generally, NV infection is more prevalent in developing countries than in developed countries, and differences in hygienic conditions between those countries may be one of the factors responsible for this difference (15). However, a high prevalence of antibody to NV was reported even in developed countries after the introduction of sensitive immunoassays like radioimmunoassay and EIA (3, 6, 24), whereas a lower prevalence has been reported in the other developed countries like the United Kingdom (26) and Norway (20). In contrast, even adults in developing countries, like Kenya in this study, show a low prevalence of antibody to NV. One possible explanation may be that there is a difference in sensitivity to NV infection among individuals of different races. Some individuals remained resistant to NV infection even after virus challenge in volunteer studies, whereas others sensitive to NV have been repeatedly infected with NV following sequential challenge (16). Further investigation of the sensitivities of black African and Scandinavian individuals to NV is required to clarify these possibilities. The other possibility is that a low population density may result in a low rate of infection with NV (3, 26). The sera from adults in Kenya used in this study were collected from adults residing in rural areas.
Whereas EIAs for the detection of antigens of and antibodies to the three genogroups of human caliciviruses are now available, the development of more broadly reactive EIAs is necessary to clarify more precisely the natural history and epidemiology of human calicivirus infections throughout the world.
ACKNOWLEDGMENTS
This study was supported in part by grant 044543878 from the Ministry of Education, Science, and Culture of Japan, by grants from the U.S. Public Health Service (grants HD-13021 and AI 28855), by the Jeffress Research Grant Foundation (grant J-303), and by U.S. Public Health Service grant AI 38036.
REFERENCES
- 1.Cubitt W D, Jiang X. Study on occurrence of human calicivirus (Mexico strain) as cause of sporadic cases and outbreaks of calicivirus-associated diarrhoea in the United Kingdom, 1983–1995. J Med Virol. 1996;48:273–277. doi: 10.1002/(SICI)1096-9071(199603)48:3<273::AID-JMV10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 2.Estes M K, Hardy M E. Norwalk virus and other enteric caliciviruses. In: Blaser M J, et al., editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 1009–1034. [Google Scholar]
- 3.Gary G W, Kaplan J E, Stine S E, Anderson L J. Detection of Norwalk virus antibodies and antigen with a biotin-avidin immunoassay. J Clin Microbiol. 1985;22:274–278. doi: 10.1128/jcm.22.2.274-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatheru Z, Kobayashi N, Adachi N, Chiba S, Muli J, Ogaja P, Nyangao J, Kiplagat E, Tukei P M. Characterization of human rotavirus strains causing gastroenteritis in Kenya. Epidemiol Infect. 1993;110:419–423. doi: 10.1017/s0950268800068357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham D Y, Jiang X, Tanaka T, Opekun A R, Madore H P, Estes M K. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis. 1994;170:34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg H B, Wyatt R G, Valdesuso J, Kalica A R, London W T, Chanock R M, Kapikian A Z. Solid-phase microtiter radioimmunoassay for detection of the Norwalk strain of acute non-bacterial, epidemic gastroenteritis virus and its antibodies. J Med Virol. 1979;2:97–108. doi: 10.1002/jmv.1890020204. [DOI] [PubMed] [Google Scholar]
- 7.Grimwood K, Carzino R, Barnes G L, Bishop R F. Patients with enteric adenovirus gastroenteritis admitted to an Australian pediatric teaching hospital from 1981 to 1992. J Clin Microbiol. 1995;33:131–136. doi: 10.1128/jcm.33.1.131-136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy M E, Kramer S F, Treanor J J, Estes M K. Human calicivirus genogroup II capsid sequence diversity revealed by analyses of the prototype Snow Mountain agent. Arch Virol. 1997;142:1469–1479. doi: 10.1007/s007050050173. [DOI] [PubMed] [Google Scholar]
- 9.Hinkula J, Ball J M, Lofgren S, Estes M K, Svensson L. Antibody prevalence and immunoglobulin IgG subclass pattern to Norwalk virus in Sweden. J Med Virol. 1995;47:52–57. doi: 10.1002/jmv.1890470111. [DOI] [PubMed] [Google Scholar]
- 10.Honma S, Nakata S, Kogawa K, Numata K, Yamashita T, Oseto M, Jiang X, Chiba S. Epidemiological study of genogroup II human calicivirus (Mexico virus) infections in Japan and Southeast Asia as determined by enzyme-linked immunosorbent assays. J Clin Microbiol. 1998;36:2481–2484. doi: 10.1128/jcm.36.9.2481-2484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang X, Wang M, Graham D Y, Estes M K. Expression, self-assembly, and antigenicity of Norwalk virus capsid protein. J Virol. 1992;66:6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang X, Wang M, Graham D Y, Estes M K. Detection of Norwalk virus in stool by polymerase chain reaction. J Clin Microbiol. 1992;30:2529–2534. doi: 10.1128/jcm.30.10.2529-2534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, Cubitt W D, Hu J, Dai X, Treanor J, Matson D O, Pickering L K. Development of an ELISA to detect MX virus, a human calicivirus in the Snow Mountain agent genogroup. J Gen Virol. 1995;76:2739–2747. doi: 10.1099/0022-1317-76-11-2739. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Matson D O, Ruiz-Palacios G M, Hu J, Treanor J, Pickering L K. Expression, self-assembly, and antigenicity of a Snow Mountain agent-like calicivirus capsid protein. J Clin Microbiol. 1995;33:1452–1455. doi: 10.1128/jcm.33.6.1452-1455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, et al., editors. Virology. New York, N.Y: Raven Press; 1996. pp. 1657–1708. [Google Scholar]
- 16.Kapikian A Z, Estes M K, Chanock R M. Norwalk group of viruses. In: Fields B N, et al., editors. Virology. New York, N.Y: Raven Press; 1996. pp. 783–810. [Google Scholar]
- 17.Kogawa K, Nakata S, Ukae S, Adachi N, Numata K, Matson D O, Estes M K, Chiba S. Dot blot hybridization with a cDNA probe derived from the human calicivirus Sapporo 1982 strain. Arch Virol. 1996;141:1949–1959. doi: 10.1007/BF01718206. [DOI] [PubMed] [Google Scholar]
- 18.Lew J F, Valdesuso J, Vesikari T, Kapikian A Z, Jiang X, Estes M K, Green K Y. Detection of Norwalk virus or Norwalk-like virus infections in Finnish infants and young children. J Infect Dis. 1994;169:1364–1367. doi: 10.1093/infdis/169.6.1364. [DOI] [PubMed] [Google Scholar]
- 19.Mutanda L N. Epidemiology of acute gastroenteritis in early childhood in Kenya: aetiological agents. Trop Geogr Med Hyg. 1980;46:272–277. [PubMed] [Google Scholar]
- 20.Myrmel M, Rimstad E, Estes M K, Skjerve E, Wasteson Y. Prevalence of serum antibodies to Norwalk virus among Norwegian military recruits. Int J Food Microbiol. 1996;29:233–240. doi: 10.1016/0168-1605(95)00033-x. [DOI] [PubMed] [Google Scholar]
- 21.Nakata S, Chiba S, Terashima H, Nakao T. Prevalence of antibody to human calicivirus in Japan and Southeast Asia determined by radioimmunoassay. J Clin Microbiol. 1985;22:519–521. doi: 10.1128/jcm.22.4.519-521.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakata S, Estes M K, Chiba S. Detection of human calicivirus antigen and antibody by enzyme-linked immunosorbent assays. J Clin Microbiol. 1988;26:2001–2005. doi: 10.1128/jcm.26.10.2001-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakata, S., Z. Gatheru, S. Ukae, N. Adachi, N. Kobayashi, J. Muli, P. Ogaja, J. Nyangao, E. Kiplagat, P. M. Tukei, and S. Chiba. Epidemiological study of the G serotype distribution of group A rotaviruses in Kenya from 1991 to 1994. Submitted for publication. [DOI] [PubMed]
- 24.Numata K, Nakata S, Jiang X, Estes M K, Chiba S. Epidemiological study of Norwalk virus infections in Japan and Southeast Asia by enzyme-linked immunosorbent assays with Norwalk virus capsid protein produced by the baculovirus expression system. J Clin Microbiol. 1994;32:121–126. doi: 10.1128/jcm.32.1.121-126.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Numata K, Hardy M E, Nakata S, Chiba S, Estes M K. Molecular characterization of morphologically typical human calicivirus Sapporo. Arch Virol. 1997;142:1537–1552. doi: 10.1007/s007050050178. [DOI] [PubMed] [Google Scholar]
- 26.Parker S P, Cubitt W D, Jiang X, Estes M K. Seroprevalence studies using a recombinant Norwalk protein enzyme immunoassay. J Med Virol. 1994;42:146–150. doi: 10.1002/jmv.1890420209. [DOI] [PubMed] [Google Scholar]
- 27.Parker S P, Cubitt W D, Jiang X. Enzyme immunoassay using baculovirus-expressed human calicivirus (Mexico) for the measurement of IgG responses and determining its seroprevalence in London, UK. J Med Virol. 1995;46:194–200. doi: 10.1002/jmv.1890460305. [DOI] [PubMed] [Google Scholar]
- 28.Taylor M B, Parker S, Grabow W O, Cubitt W D. An epidemiological investigation of Norwalk virus infection in South Africa. Epidemiol Infect. 1996;116:203–206. doi: 10.1017/s0950268800052444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uhnoo I, Wadell G, Svensson L, Johansson M. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984;20:365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urasawa T, Urasawa S, Chiba Y, Taniguchi K, Kobayashi N, Mutanda L N, Tukei P M. Antigenic characterization of rotaviruses isolated in Kenya from 1982 to 1983. J Clin Microbiol. 1987;25:1891–1896. doi: 10.1128/jcm.25.10.1891-1896.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van R, Wun C C, O’Ryan M L, Matson D O, Jackson L, Pickering L K. Outbreaks of human enteric adenovirus types 40 and 41 in Houston day care centers. J Pediatr. 1992;120:516–521. doi: 10.1016/s0022-3476(05)82477-1. [DOI] [PubMed] [Google Scholar]
- 32.Yamanaka T, Takayanagi N, Nakao T, Kobayashi M, Baba K. Seroepidemiological study of hepatitis B (HBV) infection in the rural community of Kenya—changing pattern of transmission mode of HBV in Kenya. Kansenshogakuzasshi. 1991;65:26–34. doi: 10.11150/kansenshogakuzasshi1970.65.26. . (In Japanese.) [DOI] [PubMed] [Google Scholar]