Abstract
At present, data independent acquisition (DIA) protein spectrum detection technology is one of the most attractive mass spectrometry acquisition techniques, which once led the new development of quantitative proteomics. Its application fields include the screening of clinical disease markers, the study of action mechanism, the study of drug targets and so on. DIA has been wide‐ranging used in clinic because of its high throughput, high resolution and high reproducibility. The occurrence of mental disorders in encephalitis is common, and such neurocognitive impairment has a dramatically impact on the disease progression and prognosis of patients, which undoubtedly increases the economic burden of sufferers' families and society. Under the circumstance that the mechanism of mental disorder of encephalitis is still unknown, this paper summarizes a large number of literatures of encephalitis, originates the possibility of the application of cerebrospinal fluid detection by DIA in the occurrence of mental disorder of encephalitis, seeks for biomarkers for the occurrence of mental disorder of encephalitis, and provides clinical guidance.
Keywords: DIA, EncephalitisL, Mental disorder, Biomarkers
Introduction
Encephalitis refers to the inflammatory disease rendered by pathogen invasion of brain parenchyma, with new level of and autoimmune infectious causes of encephalitis. In the pathogenesis, more than 50% of encephalitis cases remains unclear in countless studies, which brings additional challenges to prognosis and treatment (Gable M,et al. 2012; Glaser C,et al. 2006; Mailles A,et al. 2009). Psychiatric encephalitis is a shared infectious disease of the central nervous system and a manifestation of mental disorders in which pathogens invade the central system. Clinically, patients with influenza‐like prodromal infection manifest obvious behavioral and mental symptoms, usually accompanied by epilepsy, memory loss, language dysfunction, dyskinesia and consciousness disorder. In addition, this type of encephalitis is usually accompanied with autonomic nervous instability and hypopnea disorder (Dalmau J and Rosenfeld, 2008; Dalmau,et al. 2007). In spite of these symptoms have their own characteristics, misdiagnosis and delayed diagnosis are common, and up to 25% of patients may have adverse outcomes, such as persistent and severe neuropsychiatric disorders (Dalmau J,et al. 2008; Dalmau J,et al. 2011). On a global scale, data on the cost of encephalitis‐related sequelae are limited, it has been documented that the annual loss of disability adjusted life years (DALYS) of Japanese encephalitis alone in the world is as high as 1,859,170 years (Tarantola A,et al., 2014). A series of studies have found that about 95% of adult patients with N‐methyl‐D‐aspartate receptor (NMDAR) antibody encephalitis have clinical features of psychiatry, which usually appear at the onset of the disease, and some patients have long‐term isolated psychosis academic performance (Al‐Diwani A,et al., 2019). Mao Ling Y et al. analyzed the clinical information of 78 children with severe Japanese encephalitis (JE) in the Department of Neurology, Infectious Diseases, and Rehabilitation Department from 2014 to 2016, about 73% of severe JE cases were classified as patients with mental symptoms (Yang M,et al., 2019). Apart from the mental disorders caused by the above two kinds of encephalitis, tuberculous meningitis can also cause mental disorders. Although the incidence is much lower, Ursula K team find all their cases demonstrated neurodevelopmental deficits (Rohlwink U,et al., 2016).
Research status of the mechanism of mental disorders in encephalitis
Autoimmune encephalitis (AE)
AE generally refers to a kind of encephalitis mediated by autoimmune mechanism. Anti‐NMDAR encephalitis (ANE) has an excellent fatality rate in autoimmune encephalitis, featured by outstanding mental and neurological symptoms. Meanwhile, the incidence of mental disorders is the highest among encephalitis diseases. In recent years, through clinical and basic experimental studies, many scholars have found that the clinical situations and pathological changes of patients with ANE are different from those of other non‐inflammatory central system diseases. Some studies demonstrated that anti‐NMDAR antibodies can prompt innate immune response, facilitate aseptic neuroinflammation, and cause brain tissue damage (Bauer J,et al. 2012; Liu B,et al. 2018). Neuroinflammation and brain damage are considered to be the main causes of mental disorders. Nevertheless, the essence of inflammatory neuronal injury is still unclear and the mechanism has not been thoroughly elucidated.
The Jung‐Ick B team compared the levels of cytokines and chemokines in serum and cerebrospinal fluid (CSF) in 14 patients with anti‐NMDAR, 10 patients with anti‐leucine‐rich glioma inactivating 1 protein (LGI1) encephalitis and 10 patients with non‐inflammatory neurological diseases (control group). Compared with the non‐inflammatory neurological diseases group, the levels of IL‐17A, IL‐6, and CXCL13 in the cerebrospinal fluid of ANE patients were elevated, while those of anti‐LGI1 encephalitis patients did not. In the ANE, only IL‐2 was elevated, and there were more mental symptoms (Byun J,et al., 2016). Therefore, the increased levels of cytokines/chemokines observed in patients with ANE may be conducive to the pathogenesis of the disease, even the production of intrathecal antibodies. IL‐17A and IL‐6 are both pro‐inflammatory cytokines. IL‐17A can cause the expression of inflammatory genes in target cells, negatively regulate tight junction molecules, and further enable leukocytes to migrate across the blood‐brain barrier (Huppert J,et al., 2010). IL‐6 can stimulate B‐cell differentiation, improve the survival rate of plasma layer, and advance the generation of antibodies, and IL‐17A may activate the positive feedback loop of IL‐6 signal transduction through signal transduction and activator of transcription 3 (STAT3) and nuclear factor (NF)‐κ B (Chihara N,et al. 2011; Ogura H,et al. 2008). Hence, the synergistic activation of IL‐6 and IL‐17A may have significantly influences in the synthesis of intrathecal antibodies NMDAR encephalitis. Another team found CXCL13 was elevated in CSF in 70% of patients with ANE compared with patients with non‐inflammatory neurological diseases, contending that CXCL13 could be a potential biomarker for treatment response (Leypoldt F,et al., 2015). Zuzana L et al reported that there were remarkable changes in the levels of T and B cell‐related chemokines (CXCL10 and CXCL13) in CSF of patients with anti‐NMDAR. When clinical features (such as mental disorders, cognitive impairment, epilepsy, etc.) were most prominent, the levels of these chemokines increased critically in the preliminary stage of the disease, and the criterion of CXCL10 and CXCL13 were the highest during the rapid deterioration of the disease. It is considered that the increase of these chemokines and cytokines is closely related to the clinical manifestations of encephalitis, and the levels of T cell‐associated cytokines interferon‐γ (INF‐‐γ), tumor necrosis factor‐α (TNF‐ α) and interleukin‐17 (IL‐17A) in CSF of patients with ANE are increased, suggesting that T‐cell mechanism could also be ralated to the ANE (Liba,et al., 2016). Recently, mitochondrial DNA (mtDNA) is considered to be a damage‐associated molecular pattern molecule (DAMP), which is able to bring about inflammation.
Some researchers have clarified the content of free mtDNA and inflammation‐related cytokines in CSF of invalids with ANE are thoroughly higher than those of patients with non‐inflammatory nervous system diseases. There was a positive correlation between free mtDNA content in CSF and Modified Rankin Scale (MRS) score (evaluation of neurological deficit) when ANE patients were admitted to hospital and followed up for 6 months. It is proffered that the content of free mtDNA in CSF shows the potential neuroinflammatory process in patients with ANE, even related to clinical MRS score (Peng,et al., 2019).
Viral encephalitis (VE)
VE is a brain inflammation caused by virus directly invading the brain parenchyma. The acute effects of central nervous system virus infection give rise to typical adaptive disease behaviors, including mental disorders, and its incidence is second only to ANE. Researches have contended that the clinical manifestations of mental disorders may be rendered by the injury or loss of neurons or the continuous activation of inflammatory cascade reaction, which have negative effects on the homeostasis or function of nerve cells, respectively, but the mechanism is not toughly understood.
VE is mainly caused by enteroviruses, while enterovirus 71 (EV71) is a neurotropic virus, and the incidence of neurological complications in EV71‐related cases is high. The imbalance of abnormal expression of inflammatory cytokines and chemokines brings about the development and severity of EV71‐related diseases. The Yingchun Xu team purported that the levels of IL‐8, IL‐1b, IL‐6 and IL‐10 in CSF of children with enterovirus 71‐associated encephalitis were higher than those of children with febrile convulsion alone (Xu,et al., 2019). Moreover, some studies have demonstrated that astrocyte dysfunction in VE gives rise to the increase of permeability of the blood‐brain barrier (BBB), and the BBB permeability increases critically 3 to 4 days after virus infection, which brings about the increase of virus permeability and / or the infiltration of immune cytokines (Daniels B,et al. 2014; Pfefferkorn C,et al. 2016). Furthermore, Neurocognitive impairment and memory impairment also appear in subgroups of patients with West Nile virus (WNV), the main marker of neurogenesis of WNV encephalitis is also neuroinflammation, neurons are the underlying sources of pro‐inflammatory cytokines in the brain after WNV infection, these neuron‐derived cytokines participate in WNV‐induced neurotoxicity, and the activation of astrocytes is also mediated by cytokines which released by neurons. The analysis of cytokines produced by microglia and astrocytes isolated in vitro manifests that the latter is the main source of IL‐1 (Garber C,et al., 2018). More than that, after WNV infected human neuroblastoma cell line (SK‐N‐SH), the expression of IL‐1 β, IL‐6, IL‐8 and TNF‐ α was dose‐and time‐dependence, which was consistent with the increase of cell death prompted by virus. Treating cells with anti‐IL‐1 β or anti‐TNF‐ α could dramatically reduce the neurotoxicity of WNV (Kumar M,et al., 2010).
In the inflammatory mechanism, Charles L et al. infected the brain with Taylor's mouse encephalomyelitis virus (TMEV), thus rapidly triggering the expression of CCL2 in neurons, which is the core of CCR2‐dependent inflammatory monocytes infiltrating into the brain in the acute phase of encephalitis. Some studies have contended that the biomarkers in neurodegenerative diseases such as Alzheimer's disease (AD) are associated to the central nervous system infections rendered by bacteria and viruses (RF, 2017; Zhan X, 2017), and in the past decades, many infectious sources, including herpes viruses, have been considered to be related to the morbidity of AD (Itzhaki, 2017; Louveau A,et al. 2016). At present, S100B is a new biomarker of BBB permeability and central nervous system injury. Kacper T et al. studied the neuroinflammatory characteristics of non‐polio enteroviruses by evaluating the concentration of biomarkers in CSF associated to the neuropathological pathway of neurodegenerative diseases, the concentrations of Aβ42, t‐tau and S100B in 42 children with enteroviral meningitis (EM) were measured and compared with those without central nervous system infection. The results documented that EM could reduce the content of Aβ42 in CSF, while the concentrations of t‐tau and S100B in CSF were not affected by EM, and the absolute number of mononuclear cells in CSFwas related with t‐tau (Toczylowski K,et al., 2019).
Tuberculous meningitis (TBM)
TBM is a non‐suppurative inflammatory disease of meninges and spinal meninges caused by mycobacterium tuberculosis. About half of patients with TBM died or suffered from severe neurological and mental disorders. Yet compared with ANE and VE, the incidence of mental disorders is lower. Randall et al. have proved that Mycobacterium tuberculosis (M.tb) directly infects neurons, but its influence on neuronal function and the process of intercellular interaction are still unclear (Randall P,et al., 2014).
Tumor necrosis factor (TNF) is the core of tuberculosis in the central nervous system (Mastroianni C,et al. 1997; Tobin D,et al. 2012; Wilson M,et al. 2019). It plays a safeguarding role in the immune response to mycobacteria (Kaplan and Freedman, 1996). Local production of TNF‐α in the central nervous system will also fortify the permeability of the blood‐brain barrier, so that allowing other immune mediators to flow into the central nervous system. In the mouse model of central nervous system tuberculosis, Tsenova et al. clarified the correlation between TNF‐α level and brain pathological deterioration through CSF leukocytosis, protein accumulation, meningitis, persistent bacterial load and clinical manifestations (Tsenova L,et al., 1999). A research from Rock, it was manifested that the pro‐inflammatory cytokines secreted by microglia in response to TBM include not only TNF‐ α, IFN‐ γ and IL‐6, but also IL‐1 β, CCL2, CCL5 and CXCL10. Compared with microglia, astrocytes produce only a moderate amount of CXCL10 (Rock R,et al., 2005). In the clinical experiment, Ursula K examined the CSF and serum of 44 children with TBM complicated with hydrocephalus and the control group [(1)11 children undergoing elective surgery for spinal cord adipose fibrosis (a congenital abnormality unrelated to nerve injury or inflammation), (2) 9 children clinically diagnosed and treated with pulmonary tuberculosis (PTB)]. The results contended that neural biomarkers in CSF (S100B, neuron specific enolase (NSE), glial fibrillary acidic protein (GFAP) and inflammatory markers [IL‐1 β, IL‐1 receptor antagonist (RA), IL‐6, IL‐10, IL‐12 p40, tumor TNF‐α, IFN‐γ, IL‐8, growth regulatory oncogene (GRO), monocyte chemoattractant protein 1 (MCP‐1), interferon inducible protein 10(IP‐10)]. Macrophage inflammatory protein 1 α (MIP‐1α) and vascular endothelial growth factor (VEGF) increased at admission and within 3 weeks. The initial and maximum concentrations of S100B and NSE in the first week are correlated to unfavorable prognosis, and the curve that the maximum concentrations of S100B, NSE and GFAP increase with time is also correlated to dreadful prognosis. Thus, neural biomarkers in CSF of TBM patients can help us comprehend persistent brain injury and prognosis (Rohlwink U,et al., 2017). Meanwhile, other clinical studies used voxel‐based morphometry (VBM) to anatomically register different forms of TBM. Seventeenpatients with TBM who had stopped anti‐tuberculosis treatment for more than 6 months and 17 healthy subjects to determine whether gray matter defect in TBM was associated to acute manifestations and chronic cognitive impairment. The results demonstrated that patients with chronic TBM may still have multi‐domain cognitive impairment after proper treatment, and the severity of the initial disease may give rise to brain tissue damage, followed by neuropsychological consequences (Chen H,et al., 2015). Although TBM has mental disorder and neurocognitive dysfunction, few studies have reported neurocognitive function of adult TBM. A retrospective study in New Zealand contends that during the median follow‐up of 18 months (ranging from 1 to 197 months), 12% of adult TBM survivors had cognitive impairment, but the method of cognitive assessment was still unclear (Anderson,et al., 2010). In two cohort studies from India (nasty 30 and nasty 65), sufferers were evaluated with a 30‐point mini‐mental state examination (MMSE) at 6 months and 1 year after TBM diagnosis. According to the level of education, more than half of the patients (54% and 55%, respectively) were impaired using a cut‐off score of 22 to 29 (Folstein,et al. 1975; Kalita,et al. 2007; Ranjan,et al. 2003).
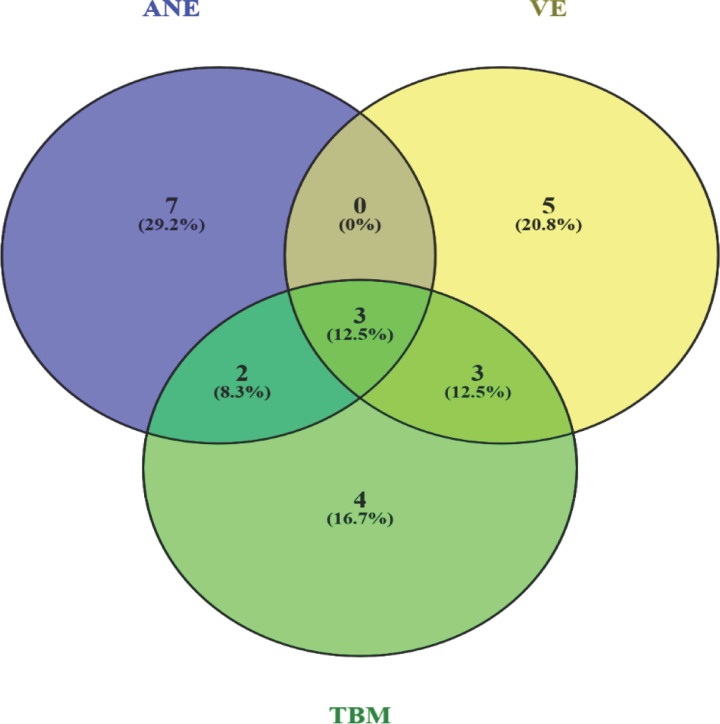
To sum up, the research on encephalitis is mainly focused on neuroinflammation rendered by neurotoxic environment after pathogen infection and neurological dysfunction rendered by central nervous system injury. As for its biomarkers, (1) Anti‐NMDAR encephalitis includes: IL‐17A, IL‐6, IL15, IL‐2, IL‐10, IL‐4, IL‐12, CXCL13, CXCL10, INF‐ γ, TNF‐ α, mtDNA and the like. (2) viral meningitis includes IL‐8, IL‐1 β, IL‐6, IL‐10, IL‐33, IFN‐ γ, TNF‐ α, A β 42, t‐tau, S100B, CCL‐2 and the like. (3) Tuberculous meningitis includes IL‐6, IL‐8, IL‐10, IL‐12, IL‐12p40, IL‐1 β, TNF‐ α, IFN‐ γ, CCL5, CXCL10, S100, GRO and the like. The shared biomarkers of the three types of encephalitis were found by Venn intersection analysis, mainly INF‐ γ, TNF‐ α and IL‐6 (Figure 1 ).
Figure 1.
Encephalitis‐related biomarkers. In the study of various biomarkers related to encephalitis, the biomarker of the intersection of anti‐NMDAR encephalitis and viral encephalitis was IL‐10. The intersecting biomarkers of viral encephalitis and tuberculous meningitis were IL‐8, IL‐1 β and S100B. The intersecting biomarker of tuberculous meningitis and anti‐NMDAR encephalitis was IL‐12. The common biomarkers of the three encephalitis were IL‐6, INF γ and TNF‐ α.
The current paradigm for diagnosing encephalitis relies on the physician formulating a differential diagnosis based on patient’s history, clinical manifestations, and imaging findings, followed by serial laboratory testing. Through the above explanation of the mechanism of encephalitis, there are many methods involved in the laboratory testing of CSF, include enzyme‐linked immunosorbent assay (ELISA), polymerase chain reaction (PCR), flow cytometry (FCM), Immunofluorescence technique (IF) and so on. Among them, next generation sequencing (NGS) can be used for early and accurate identification of various encephalitis through cerebrospinal fluid. In a 1‐year multicenter prospective study, M.R. The Wilson team studied the usefulness of the cerebrospinal fluid metagenomic NGS in the diagnosis of infectious meningitis and encephalitis in hospitalized patients. In this study, the giant genome NGS in the CSF of patients with meningitis or encephalitis improved the diagnosis of nervous system infections (Wilson M,et al., 2019). NGS is also known as high‐throughput sequencing and deep sequencing, because this technology can simultaneously determine millions or even hundreds of millions of DNA or RNA sequences, which can save a lot of clinical time and cost (Levy and Boone, 2019). At the same time, NGS can quickly and accurately determine infectious pathogens through unbiased “full pathogen” screening (Wilson M, et al., 2018). However, precisely because of its high‐throughput advantages, when obtaining gene sequences of multiple suspected pathogens, NGS results that are completely inconsistent with clinical manifestations often appear. Secondly, CSF NGS needs to compare the genomes of known pathogens in the database to get the results. However, for pathogen genomes that do not exist in the database or are currently unidentified, CSF NGS cannot find these pathogens, which is the second limitation of NGS (Park SJ,et al., 2019).
Meanwhile, through the above research report, it can be seen that in the clinical and basic research of encephalitis, numerous researchers focus on encephalitis group and non‐inflammatory central nervous system disease group, but in encephalitis group, it is not clear whether patients manifest mental disorders, in addition, there are few important biomarkers in CSF and plasma that utilize immunophenotyping, systematic mRNA spectrum, DNA and protein microarray to distinguish mental disorders of encephalitis patients. Hence, it is of remarkable clinical significance and practical value to study the various mechanisms of mental disorders caused by encephalitis and promote early diagnosis and treatment, which is worthy of further exploration.
Application prospect of DIA protein spectroscopy in molecular research of clinical diseases
Advantages of DIA protein spectroscopy
Mass spectrometry‐based proteomics (Hu A,et al., 2016) is a powerful technique that can analyze proteomes, discover biomarkers, and study protein degradation through post‐translational modification, protein‐protein interactions and protein‐ligand interactions or target deconvolution to go into biological regulation (Feng Y,et al. 2014; Frei A,et al. 2012; Savitski M,et al. 2014). The principle is that during the mass spectrometry data acquisition, all the parent ions and their daughter ions in each acquisition window are fragmented at high speed and circularly and then scanned completely, rather than the secondary fragments are scanned again after screening according to the signal strength of the parent ions (Egertson J,et al., 2015). In all these methods, proteome coverage, repeatability and quantitative accuracy are the key to obtain comprehensive and accurate biological images. Technically, proteome coverage has increased dramatically in recent years (Hu A,et al. 2016; Christoforou A,et al. 2016). Therefore, its advantages are: (1) high requirements for stability and repeatability of liquid chromatography‐tandem mass spectrometry(LC‐MS), high repeatability of identification and quantitative results; (2) Expensive isotope labels are not needed, and the experiment cost is low; (3) The pretreatment process is less, so that the sample is closest to the original state and is not limited by the sample conditions; (4) The flux is high, which is not limited by the number of samples, and can be used for large‐scale sample number detection; (5) The loss of low‐abundance parent ions and fragment ions is reduced, and the flux of secondary quantification of high‐resolution mass spectrometry is thoroughly improved; (6) The panoramic and accurate quantification is realized.
Clinical application of DIA protein spectroscopy
Based on the above advantages, this technique has been widely used in the molecular research of clinical diseases. Clinically, the Melissa Steam quantified 341 proteomes in two independent cohorts and one longitudinal cohort using DIA technology, these include Parkinson's disease and healthy control samples from three different sources. The first cohort analysis was conducted on 53 patients with Parkinson's disease and 72 healthy controls. Compared with healthy controls, 53 proteins with obvious changes were identified, and several new protein changes in cerebrospinal fluid of Parkinson's disease may be employed to comprehend the etiology of the disease and to develop biomarkers (Rotunno M,et al., 2020). Likewise, Meng X et al. developed a new serum proteomics platform combining DIA and customizable antibody microarray data, which can analyze about 10 orders of magnitude of serum proteomics. Taking psoriasis as a conceptual disease model, 50 proteomes were screened from the sera of healthy groups and psoriatic patients pretherapy and post‐treatment on the researcher's serum proteomics platform. The results indicated that the researchers identified 106 differentially expressed proteins in patients with psoriasis, which are participated in psoriasis‐related biological processes, such as coagulation, inflammation, apoptosis and angiogenesis signaling pathways. In this way, their in‐depth serum proteomics platform has clinical application value in the specific diagnosis and predictive biomarker identification of psoriasis and other immune‐mediated diseases (Xu Y,et al., 2019). Richard J team measured the changes of cerebrospinal fluid proteome correlated to Alzheimer's disease (AD). The results manifested that four new AD CSF markers (NrCAM, YKL‐40, chromogranin A, carnosine I) could enhance the diagnostic rate of Aβ 42 and tau. Six biomarkers (NrCAM, YKL‐40, Chromogranin A, carnosine I, transthyretin, cystatin C) described six clinicopathological stages from normal cognition to mild dementia, including the stage defined by the increased risk of cognitive decline (Perrin R,et al., 2011). Thus, it suggested that the prospect of clinical application of DIA protein spectroscopy is very promising.
Conclusions and prospects
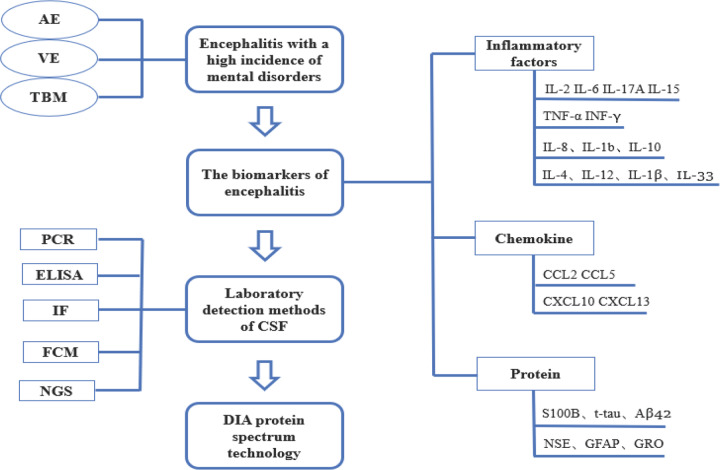
At the moment, the detection techniques of CSF biomarkers are mainly focused on immunofluorescence and immunohistochemical methods, but there are few reports on the detection of CSF biomarkers in patients with encephalitis by DIA protein spectrum. Compared with the traditional biomarker discovery method, DIA can combine the biomarker discovery stage with the verification stage because of its high repeatability in identifying proteins in hundreds of samples, which eliminates the need for a large number of independent samples for verification, and improves the detection efficiency of biomarkers (Figure 2 ).
Figure 2.
The flow chart of this article. AAE, autoimmune encephalitis. VE, viral encephalitis. TBM, tuberculous meningitis. CSF, cerebrospinal fluid. PCR, polymerase chain reaction. ELISA, enzyme linked immunosorbent assay. FCM, flow cytometry. IF, immunofluorescence. NGS, next generation sequencing.
DIA protein spectrum was employed to detect the changes of protein associated to disease in CSF samples of patients with mental disorders and patients without mental disorders in encephalitis patients, so as to deeply understand the causes of mental disorders in encephalitis, and may be helpful to identify biomarkers of diseases. Such findings can classify disease stages, monitor pathological progress, and predict cognitive decline, which provides a good reference value for clinical practice.
Ethical statement
Not applicable.
Conflict of interest
No conflicts of interest were declared.
Funding
This study was supported by grant from the National Natural Science Foundation of China (Grant Nos. 82001604, 82060243 and 81960214) and Joint Fund of Zunyi Science and Technology Bureau‐Affiliated Hospital of Zunyi Medical University (No. HZ2020250).
Transparency statement
The authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Author's contribution
Lin Zhou delivered the main conception and drafted of the work; Zheng‐Meng Wang and Song Wen supported the work with multiple files ; Juan Li and Liu‐Lin Xiong revised the manuscript; Zhao‐Qiong Zhu approved the final verison.
Acknowledgments
We would like to thank Professor Ting‐Hua Wang (Institute of Neurobiological Disease, West China Hospital, Sichuan University) for his careful guidance.
Contributor Information
Liu‐Lin Xiong, Email: 499465010@qq.com.
Zhao‐Qiong Zhu, Email: ganzhuzhaoqiong@sina.com.
References
- Al‐Diwani, A. , A. Handel , L. Townsend , T. Pollak , M. Leite , P. Harrison , B. Lennox , D. Okai , S. Manohar , Irani S. J. T. l. P.. The psychopathology of NMDAR‐antibody encephalitis in adults: a systematic review and phenotypic analysis of individual patient data. 2019; 6 (3): 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, N. E. , J. Somaratne , Mason D. F., D. Holland , Thomas M.G.. Neurological and systemic complications of tuberculous meningitis and its treatment at Auckland City Hospital, New Zealand. Journal of Clinical Neuroscience 2010; 17 (9): 1114–1118. [DOI] [PubMed] [Google Scholar]
- Bauer, J. , A. Vezzani , Bien C. J. B. p.. Epileptic encephalitis: the role of the innate and adaptive immune system. 2012; 22 (3): 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun, J. , S. Lee , J. Moon , K. Jung , J. Sunwoo , J. Lim , T. Kim , Y. Shin , K. Lee , J. Jun , H. Lee , W. Lee , Y. Kim , S. Kim , D. Jeon , K. Park , K. Jung , M. Kim , K. Chu , Lee S. J. J. o. n.. Distinct intrathecal interleukin‐17/interleukin‐6 activation in anti‐N‐methyl‐d‐aspartate receptor encephalitis. 2016; 297 (141–147. [DOI] [PubMed] [Google Scholar]
- Chen, H. , C. Lu , C. Chang , P. Chen , M. Chen , N. Hsu , K. Chou , W. Lin , C. Lin , Lin W. J. B. i. d.. Structural deficits and cognitive impairment in tuberculous meningitis. 2015; 15 (279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara, N. , T. Aranami , W. Sato , Y. Miyazaki , S. Miyake , T. Okamoto , M. Ogawa , T. Toda , Yamamura T. J. P. o. t. N. A. o. S. o. t. U. S. o. A.. Interleukin 6 signaling promotes anti‐aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. 2011; 108 (9): 3701–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforou, A. , C. Mulvey , L. Breckels , A. Geladaki , T. Hurrell , P. Hayward , T. Naake , L. Gatto , R. Viner , A. Arias , Lilley Martinez K. J. N. c.. A draft map of the mouse pluripotent stem cell spatial proteome. 2016; 7 (8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau, J. , A. Gleichman , E. Hughes , J. Rossi , X. Peng , M. Lai , S. Dessain , M. Rosenfeld , R. Balice‐Gordon , Lynch D. J. T. L. N.. Anti‐NMDA‐receptor encephalitis: case series and analysis of the effects of antibodies. 2008; 7 (12): 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau, J. , E. Lancaster , E. Martinez‐Hernandez , M. Rosenfeld , Balice‐Gordon R. J. T. L. N.. Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. 2011; 10 (1): 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau, J. , E. Tuzun , Wu H. Y., J. Masjuan , Rossi J. E., A. Voloschin , Baehring J. M., H. Shimazaki , R. Koide , D. King , W. Mason , Sansing L. H., Dichter M. A., Rosenfeld M. R., Lynch D. R.. Paraneoplastic anti‐N‐methyl‐D‐aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007; 61 (1): 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau, J. , Rosenfeld M. J. T. L. N.. Paraneoplastic syndromes of the CNS. 2008; 7 (4): 327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, B. , D. Holman , L. Cruz‐Orengo , H. Jujjavarapu , D. Durrant , Klein R. J. m.. Viral pathogen‐associated molecular patterns regulate blood‐brain barrier integrity via competing innate cytokine signals. 2014; 5 (5): e01476–01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertson, J. , B. MacLean , R. Johnson , Y. Xuan , MacCoss M. J. N. p.. Multiplexed peptide analysis using data‐independent acquisition and Skyline. 2015; 10 (6): 887–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y. , G. De Franceschi , A. Kahraman , M. Soste , A. Melnik , P. Boersema , P. de Laureto , Y. Nikolaev , A. Oliveira , Picotti P. J. N. b.. Global analysis of protein structural changes in complex proteomes. 2014; 32 (10): 1036–1044. [DOI] [PubMed] [Google Scholar]
- Folstein, M. , S. Folstein , McHugh P. J. J. o. p. r.. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. 1975; 12 (3): 189–198. [DOI] [PubMed] [Google Scholar]
- Frei, A. , O. Jeon , S. Kilcher , H. Moest , L. Henning , C. Jost , A. Plц╪ckthun , J. Mercer , R. Aebersold , E. Carreira , Wollscheid B. J. N. b.. Direct identification of ligand‐receptor interactions on living cells and tissues. 2012; 30 (10): 997–1001. [DOI] [PubMed] [Google Scholar]
- Gable, M. , H. Sheriff , J. Dalmau , D. Tilley , Glaser C. J. C. i. d. a. o. p. o. t. I. D. S. o. A.. The frequency of autoimmune N‐methyl‐D‐aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. 2012; 54 (7): 899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber, C. , M. Vasek , L. Vollmer , T. Sun , X. Jiang , Klein R. J. N. i.. Astrocytes decrease adult neurogenesis during virus‐induced memory dysfunction via IL‐1. 2018; 19 (2): 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser, C. , S. Honarmand , L. Anderson , D. Schnurr , B. Forghani , C. Cossen , F. Schuster , L. Christie , Tureen J. J. C. i. d. a. o. p. o. t. I. D. S. o. A.. Beyond viruses: clinical profiles and etiologies associated with encephalitis. 2006; 43 (12): 1565–1577. [DOI] [PubMed] [Google Scholar]
- Hu, A. , Noble W. S., Wolf‐Yadlin A.. Technical advances in proteomics: new developments in data‐independent acquisition. F1000Res 2016; 5( [DOI] [PMC free article] [PubMed]
- Huppert, J. , D. Closhen , A. Croxford , R. White , P. Kulig , E. Pietrowski , I. Bechmann , B. Becher , H. Luhmann , A. Waisman , Kuhlmann C. J. F. j. o. p. o. t. F. o. A. S. f. E. B.. Cellular mechanisms of IL‐17‐induced blood‐brain barrier disruption. 2010; 24 (4): 1023–1034. [DOI] [PubMed] [Google Scholar]
- Itzhaki, R. F. Herpes simplex virus type 1 and Alzheimer's disease: possible mechanisms and signposts. Faseb j 2017; 31 (8): 3216–3226. [DOI] [PubMed] [Google Scholar]
- Kalita, J. , U. Misra , Ranjan P. J. E. j. o. n.. Predictors of long‐term neurological sequelae of tuberculous meningitis: a multivariate analysis. 2007; 14 (1): 33–37. [DOI] [PubMed] [Google Scholar]
- Kaplan, G. , Freedman V. J. R. i. i.. The role of cytokines in the immune response to tuberculosis. 1996; 147 (565–572. [DOI] [PubMed] [Google Scholar]
- Kumar, M. , S. Verma , Nerurkar V. J. J. o. n.. Pro‐inflammatory cytokines derived from West Nile virus (WNV)‐infected SK‐N‐SH cells mediate neuroinflammatory markers and neuronal death. 2010; 7 (73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, S. , Boone B. J. C. S. H. p. i. m.. Next‐Generation Sequencing Strategies. 2019; 9 (7): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypoldt, F. , R. Hц╤ftberger , M. Titulaer , T. Armangue , N. Gresa‐Arribas , H. Jahn , K. Rostц║sy , W. Schlumberger , T. Meyer , K. Wandinger , M. Rosenfeld , F. Graus , Dalmau J. J. J. n.. Investigations on CXCL13 in anti‐N‐methyl‐D‐aspartate receptor encephalitis: a potential biomarker of treatment response. 2015; 72 (2): 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liba, Z. , J. Kayserova , M. Elisak , P. Marusic , H. Nohejlova , J. Hanzalova , V. Komarek , Sediva A. J. J. o. n.. Anti‐N‐methyl‐D‐aspartate receptor encephalitis: the clinical course in light of the chemokine and cytokine levels in cerebrospinal fluid. 2016; 13 (1): 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, B. , Z. Xie , G. Liu , Y. Gu , S. Pan , Wang H. J. C. c. a., i. j. o. c. chemistry . Elevated neuron‐specific enolase and S100 calcium‐binding protein B concentrations in cerebrospinal fluid of patients with anti‐N‐methyl‐d‐aspartate receptor encephalitis. 2018; 480 (79–83. [DOI] [PubMed] [Google Scholar]
- Louveau, A. , S. Da Mesquita , Kipnis J. J. N.. Lymphatics in Neurological Disorders: A Neuro‐Lympho‐Vascular Component of Multiple Sclerosis and Alzheimer's Disease? 2016; 91 (5): 957–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailles, A. , J. Stahl , J. C. i. d. a. o. p. o. t. I. D. S. o. A.. Infectious encephalitis in france in 2007: a national prospective study. 2009; 49 (12): 1838–1847. [DOI] [PubMed] [Google Scholar]
- Mastroianni, C. , F. Paoletti , M. Lichtner , C. D'Agostino , V. Vullo , S. J. C. i., Delia immunopathology. Cerebrospinal fluid cytokines in patients with tuberculous meningitis. 1997; 84 (2): 171–176. [DOI] [PubMed] [Google Scholar]
- Ogura, H. , M. Murakami , Y. Okuyama , M. Tsuruoka , C. Kitabayashi , M. Kanamoto , M. Nishihara , Y. Iwakura , Hirano T. J. I.. Interleukin‐17 promotes autoimmunity by triggering a positive‐feedback loop via interleukin‐6 induction. 2008; 29 (4): 628–636. [DOI] [PubMed] [Google Scholar]
- Park, S. J. , S. Onizuka , M. Seki , Y. Suzuki , T. Iwata , Nakai K.. A systematic sequencing‐based approach for microbial contaminant detection and functional inference. BMC Biol 2019; 17 (1): 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y. , D. Zheng , X. Zhang , S. Pan , T. Ji , J. Zhang , H. Shen , Wang H. J. F. i. i.. Cell‐Free Mitochondrial DNA in the CSF: A Potential Prognostic Biomarker of Anti‐NMDAR Encephalitis. 2019; 10 (103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin, R. , R. Craig‐Schapiro , J. Malone , A. Shah , P. Gilmore , A. Davis , C. Roe , E. Peskind , G. Li , D. Galasko , C. Clark , J. Quinn , J. Kaye , J. Morris , D. Holtzman , R. Townsend , Fagan A. J. P. o.. Identification and validation of novel cerebrospinal fluid biomarkers for staging early Alzheimer's disease. 2011; 6 (1): e16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn, C. , C. Kallfass , S. Lienenklaus , J. Spanier , U. Kalinke , M. Rieder , K. Conzelmann , T. Michiels , Staeheli P. J. J. o. v.. Abortively Infected Astrocytes Appear To Represent the Main Source of Interferon Beta in the Virus‐Infected Brain. 2016; 90 (4): 2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall, P. , N. Hsu , D. Lang , S. Cooper , B. Sebesho , N. Allie , R. Keeton , N. Francisco , S. Salie , A. Labuschagnц╘ , V. Quesniaux , B. Ryffel , L. Kellaway , M. J. I., Jacobs immunity. Neurons are host cells for Mycobacterium tuberculosis. 2014; 82 (5): 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan, P. , J. Kalita , Misra U. J. N.. Serial study of clinical and CT changes in tuberculous meningitis. 2003; 45 (5): 277–282. [DOI] [PubMed] [Google Scholar]
- RF, I. Herpes simplex virus type 1 and Alzheimer's disease: possible mechanisms and signposts. 2017; 31 (8): 3216–3226. [DOI] [PubMed] [Google Scholar]
- Rock, R. , S. Hu , G. Gekker , W. Sheng , B. May , V. Kapur , Peterson P. J. T. J. o. i. d.. Mycobacterium tuberculosis‐induced cytokine and chemokine expression by human microglia and astrocytes: effects of dexamethasone. 2005; 192 (12): 2054–2058. [DOI] [PubMed] [Google Scholar]
- Rohlwink, U. , K. Donald , B. Gavine , L. Padayachy , J. Wilmshurst , G. Fieggen , Figaji A. J. D. m., Neurology C.. Clinical characteristics and neurodevelopmental outcomes of children with tuberculous meningitis and hydrocephalus. 2016; 58 (5): 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlwink, U. , K. Mauff , K. Wilkinson , N. Enslin , E. Wegoye , R. Wilkinson , Figaji A. J. C. i. d. a. o. p. o. t. I. D. S. o. A.. Biomarkers of Cerebral Injury and Inflammation in Pediatric Tuberculous Meningitis. 2017; 65 (8): 1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotunno, M. , M. Lane , W. Zhang , P. Wolf , P. Oliva , C. Viel , A. Wills , R. Alcalay , C. Scherzer , L. Shihabuddin , K. Zhang , Sardi S. J. S. r.. Cerebrospinal fluid proteomics implicates the granin family in Parkinson's disease. 2020; 10 (1): 2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitski, M. , F. Reinhard , H. Franken , T. Werner , M. Savitski , D. Eberhard , D Martinez. Molina , R. Jafari , R. Dovega , S. Klaeger , B. Kuster , P. Nordlund , M. Bantscheff , Drewes G. J. S.. Tracking cancer drugs in living cells by thermal profiling of the proteome. 2014; 346 (6205): 1255784. [DOI] [PubMed] [Google Scholar]
- Tarantola, A. , F. Goutard , P. Newton , X. de Lamballerie , O. Lortholary , J. Cappelle , Buchy P. J. P. n. t. d.. Estimating the burden of Japanese encephalitis virus and other encephalitides in countries of the mekong region. 2014; 8 (1): e2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin, D. , F. Roca , S. Oh , R. McFarland , T. Vickery , J. Ray , D. Ko , Y. Zou , N. Bang , T. Chau , J. Vary , T. Hawn , S. Dunstan , J. Farrar , G. Thwaites , M. King , C. Serhan , Ramakrishnan L. J. C.. Host genotype‐specific therapies can optimize the inflammatory response to mycobacterial infections. 2012; 148 (3): 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toczylowski, K. , M. Wojtkowska , A. Sulik . Enteroviral meningitis reduces CSF concentration of Aн╡42, but does not affect markers of parenchymal damage. Eur J Clin Microbiol Infect Dis 2019; 38 (8): 1443–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsenova, L. , A. Bergtold , V. Freedman , R. Young , Kaplan G. J. P. o. t. N. A. o. S. o. t. U. S. o. A.. Tumor necrosis factor alpha is a determinant of pathogenesis and disease progression in mycobacterial infection in the central nervous system. 1999; 96 (10): 5657–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, M. , B. O'Donovan , J. Gelfand , H. Sample , F. Chow , J. Betjemann , M. Shah , M. Richie , M. Gorman , R. Hajj‐Ali , L. Calabrese , K. Zorn , E. Chow , J. Greenlee , J. Blum , G. Green , L. Khan , D. Banerji , C. Langelier , C. Bryson‐Cahn , W. Harrington , Lingappa J., Shanbhag N., Green A., Brew B., Soldatos A., Strnad L., Doernberg S., Jay C., Douglas V., Josephson S., DeRisi J. J. J. N.. Chronic Meningitis Investigated via Metagenomic Next‐Generation Sequencing. 2018; 75 (8): 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, M. , H. Sample , K. Zorn , S. Arevalo , G. Yu , J. Neuhaus , S. Federman , D. Stryke , B. Briggs , C. Langelier , A. Berger , V. Douglas , S. Josephson , F. Chow , B. Fulton , J. DeRisi , J. Gelfand , S. Naccache , J. Bender , Bard J Dien., J. Murkey , Carlson M., Vespa P., Vijayan T., Allyn P., Campeau S., Humphries R., Klausner J., Ganzon C., Memar F., Ocampo N., Zimmermann L., Cohen S., Polage C., DeBiasi R., Haller B., Dallas R., Maron G., Hayden R., Messacar K., Dominguez S., Miller S., Chiu C. J. T. N. E. j. o. m.. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. 2019; 380 (24): 2327–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. , J. Deng , K. Xu , T. Zhu , L. Han , Y. Yan , D. Yao , H. Deng , D. Wang , Y. Sun , C. Chang , X. Zhang , J. Dai , L. Yue , Q. Zhang , X. Cai , Y. Zhu , H. Duan , Y. Liu , D. Li , Y. Zhu , Radstake T., Balak D., Xu D., Guo T., Lu C., Yu X. J. T.. In‐depth serum proteomics reveals biomarkers of psoriasis severity and response to traditional Chinese medicine. 2019; 9 (9): 2475–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. , S. Li , C. Cai , J. Liu , Y. Wang , Y. Jiang , L. Du , Z. Chen . Characterization of inflammatory cytokine profiles in cerebrospinal fluid of hand, foot, and mouth disease children with enterovirus 71‐related encephalitis in Hangzhou, Zhejiang, China. Medicine (Baltimore) 2019; 98 (52): e18464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , N. Xiao , S. Chen , W. J. B., Jiang development. Clinical analysis of psychiatric symptoms of Japanese encephalitis during the convalescent Period: A single center study in Chongqing, China. 2019; 41 (7): 614–617. [DOI] [PubMed] [Google Scholar]
- Zhan, X. J. N. Author response: Gram‐negative bacterial molecules associate with Alzheimer disease pathology. 2017; 88 (24): 2338. [DOI] [PubMed] [Google Scholar]