Abstract
Extracranial germ cells tumors (GCT) are a biologically diverse group of tumors occurring in children, adolescents, and young adults. The majority of patients have excellent outcomes, but treatment-related toxicities impact their quality of survivorship. A subset of patients succumbs to the disease. Current unmet needs include clarifying which patients can be safely observed after initial surgical resection, refinement of risk-stratification to reduce chemotherapy burden in patients with standard-risk disease, and intensify therapy for patients with poor-risk disease. Furthermore, enhancing strategies for detection of minimal residual disease and early detection of relapse, particularly in serum tumor marker-negative histologies, is critical. Improving the understanding of the developmental and molecular origins of GCTs may facilitate discovery of novel targets. Future efforts should be directed towards assessing novel therapies in a biology-driven, biomarker-defined, histology-specific, risk-stratified patient population. Fragmentation of care between subspecialists restricts the unified study of these rare tumors. It is imperative that trials be conducted in collaboration with national and international cooperative groups, with harmonized data and biospecimen collection. Key priorities for the COG GCT Committee include a) better understanding the biology of GCTs with a focus on molecular targets and mechanisms of treatment resistance, b) strategic development of pediatric and young adult clinical trials, c) understanding late effects of therapy and identifying individuals most at risk, and d) prioritizing diversity, equity, and inclusion to reduce cancer health disparities and studying the impacts of social determinants of health on outcomes.
Keywords: extracranial, germ cell tumor, pediatric, children, adolescent, young adult
STATE OF THE DISEASE-CLINICAL
Overview
Extracranial germ cell tumors (GCTs) in children, adolescents, and young adults (AYAs) arise from molecular defects in totipotent primordial germ cells (PGC).1 GCTs are heterogenous tumors that occur in gonadal (ovarian or testicular) or extragonadal (mediastinal, sacrococcygeal, or other) sites. Histologic subtypes include seminoma (germinoma or dysgerminoma), non-seminoma (yolk sac tumor, choriocarcinoma, embryonal carcinoma) and teratoma (mature or immature). GCTs are classified in two subtypes: type I tumors in pre-pubertal children and type II tumors in AYAs (Figure 1).
Figure 1.
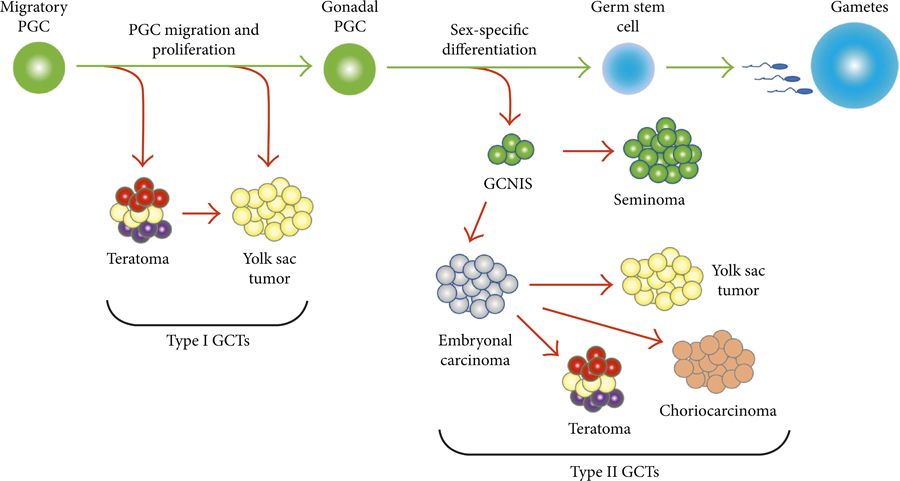
Biological development of germ cell tumors
Abbreviations: PGC, Primordial Germ Cell; GCTs, Germ Cell Tumors; GCNIS, Germinoma In Situ
Reproduced with permission from Hindawi.1
Incidence
GCTs account for 3% of malignancies in children under 15 years (y) of age and almost 14% of malignancies in adolescents aged 15–19y. Age-specific incidence rates follow a bimodal distribution with an initial peak in early childhood and a subsequent peak in adolescence (Figure 2).2 Incidence rates vary by race and ethnicity, with the highest rates in non-Hispanic white and Hispanic children.3 The annual percent change in incidence rates from 1992–2015 is not changing significantly for non-Hispanic whites, blacks, and Asians but is observed in Hispanics.3 The driving factors for these differences in incidence are not clearly understood.
Figure 2.
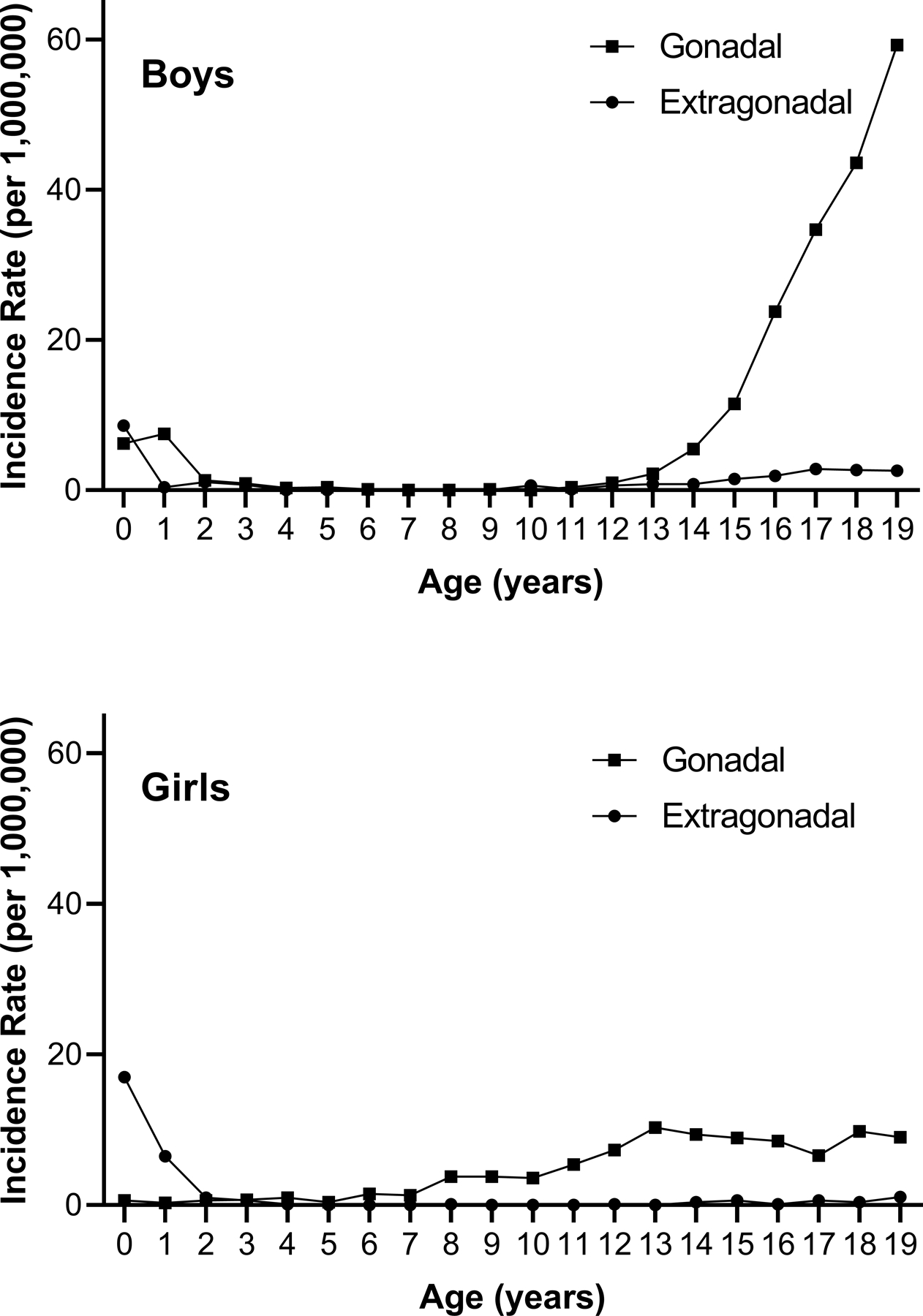
Age-specific incidence of germ cell tumors in boys and girls ages 0–19 year in the United States, 2015–2019. Source: National Childhood Cancer Registry.73
Staging and Risk Stratification
Pediatric and adult GCT staging systems vary significantly. The Children’s Oncology Group (COG) staging is based on post-operative findings.4 Adults with testicular tumors are characterized using the American Joint Committee on Cancer (AJCC) Tumor (T), Node (N), Metastasis (M) system and the International Germ Cell Cancer Collaborative Group (IGCCCG) risk-stratification system, which incorporates post-operative serum tumor markers (STM).5,6 Adults with ovarian tumors are staged using the International Federation of Gynecology and Obstetrics (FIGO).7 Additional work is necessary to facilitate consensus staging systems between pediatric and AYA patients with GCTs.
The Malignant Germ Cell Tumors International Consortium (MaGIC) is a global network of pediatric and adult GCT experts focused on collaborative research. MaGIC developed a revised risk-classification system for pediatric and adolescent patients with extracranial GCTs using data collected over 25 years from COG and the Children’s Cancer and Leukemia Group (CCLG) in the United Kingdom (UK). Multivariable analysis identified that age ≥ 11y plus either ovarian stage IV disease, extragonadal stages III-IV disease, or testicular tumors with IGCCCG intermediate- or poor-risk (PR) had significantly worse long-term disease-free survival (LTDFS; Table 1).8 This validated model is the basis of risk-stratification in current COG trials.
TABLE 1.
| Risk Group | Age (y) | Site | COG Stage | IGCCCG (if applicable) |
Predicted LTDF Survival (%) |
95%CI (%) | |
|---|---|---|---|---|---|---|---|
| LR |
0–15 0–16 Any |
Testicular Ovarian Extragonadal |
I I I |
|
100* 96* unknown** |
- 74–99 - |
|
| SR1 |
<11 <11 <11 <11 <11 <11 |
Testicular Ovarian Testicular Ovarian Extragonadal Extragonadal |
II-III II-III IV IV II-III IV |
|
99 97 96 92 91 79 |
96–100 93–99 91–99 83–96 86–94 71–84 |
|
| SR2 |
≥11 ≥11 ≥11 ≥11 |
Testicular Ovarian Testicular Extragonadal |
II-III II-III IV II |
Good |
93 85 83 65 |
84–97 77–91 67–91 48–78 |
|
| PR |
≥11 ≥11 ≥11 ≥11 ≥11 |
Extragonadal Testicular Testicular Ovarian Extragonadal |
III II-IV II-IV IV IV |
Intermediate Poor |
65 75# 41# 67 40 |
48–78 71–79 35–47 49–80 24–56 |
|
Abbreviations: y, years; COG, Children’s Oncology Group; IGCCCG, International Germ Cell Cancer Collaborative Group;
MaGIC, Malignant Germ Cell International Consortium; LTDF, Long-term disease free survival; CI, Confidence Interval; LR, Low Risk; SR1, Standard Risk 1; SR2, Standard Risk 2; PR, Poor Risk
4 year Overall Survival used here
Currently being explored in COG AGCT 1531
5 year Event Free Survival used here
Bold Font: Risk Group, Age (y), Site, COG Stage, IGCCCG (if applicable), Predicted LTDF Survival (%), 95% CI (%)
Current Outcomes
The majority of GCTs have excellent long-term outcomes with surgical resection alone or in combination with platinum-based chemotherapy. Stage I gonadal disease has expected overall survival (OS) >98%. Individuals with standard-risk disease (SR) also have excellent outcomes of >90% OS with chemotherapy. However, patients in the MaGIC PR strata have an estimated LTDFS of <70%. IGCCCG intermediate and poor-risk disease remain challenging to treat with current outcomes approximating 60% OS (Table 1).6,8,9
Survivorship
The late effects of therapy in pediatric GCT survivors are not well known due to the lack of longitudinal studies on this group of patients. The Platinum Study is seminal in characterizing the late effects of therapy and their impact on quality of life in adult with testicular GCTs.10,11 Ototoxicity, neuropathy, second cancers, and cardiovascular disease are well-established late effects in adult testicular GCT survivors.12–15 Because children and adolescents are treated with the same chemotherapy agents, it is likely that similar late effects occur. The GCT Outcomes and Late effects Data (GOLD) study (APEI17N3) is a newly established cohort of patients recruited from the COG registry protocols to understand the late effects of therapy on pediatric and adolescent GCT survivors.
STATE OF THE DISEASE-BIOLOGICAL
Molecular Targets
GCTs are believed to arise from the embryonic PGC. Several key events need to occur to have a functional mature gamete including a) specification of the germline, b) migration along the genital ridge, c) proliferation, d) epigenetic reprograming, e) sex determination, and f) differentiation into mature gamete.1 Alterations in any of these steps can lead to a disease state. However, how PGCs transform into tumors is not clearly understood and the heterogeneity of GCTs raise questions on the timing of their developmental etiology. Currently, there are two proposed hypotheses: a) failure of determination wherein migratory PGCs fail to fully commit to the germline, allowing them to retain their inherent pluripotent state and contribute to the multiple histologic types observed in GCTs and b) the reprogramming model.16
Evidence supporting in utero origins of pediatric and adult GCT suggests that disruptions in normal germ cell development are likely highly relevant to disease etiology. To date, genome wide association studies (GWAS) have identified 78 independent susceptibility loci for testicular GCTs accounting for 44% of disease heritability.17 These loci are noteworthy because they are located in genetic regions highly relevant to germ cell development. For example, these genes are involved in survival (KITLG) or early differentiation of PGCs (DAZL), sex determination and gonadal development (DMRT1), and spermatogenesis (TEX14, RAD51C, PPM1E ).18–22 Associations with some of these genetic variants were recently confirmed in pediatric GCTs, suggesting that genetic risk factors may be common to all ages.23 An ongoing GWAS of >2000 pediatric GCT cases will provide additional insight into the genetic susceptibility in younger patients.
Similar to many tumors of embryonic origin, GCTs have low mutational burden. A recent study found a mutation rate of 0.23 non-silent mutations/Mb in both type I and II GCTs.24 Common genomic alteration in GCTs are primarily chromosomal gains/losses and activating mutations in cancer-related genes. Type I tumors show recurrent gains in 12p, 20q and 21 with losses in 1p and 6q, while type II have gains in 12p (commonly isochromosome i12p).24–27 Somatic point mutations, leading to gain of function in KIT, KRAS and NRAS have been reported in type I and type II tumors.25,27, 28–31 The Cancer Genome Atlas (TCGA) Research Network found KIT, KRAS and NRAS mutations in 18%, 14% and 4% of tumors respectively.25 Other signaling pathway alterations in GCTs include the WNT/CTNNB1 and PI3K/MTOR pathways. Due to the low frequency of actionable mutations, identifying targeted therapy for GCTs has had little success to date but continues to be an area of interest.
MAJOR FINDINGS
Clinical Achievements
Over the last decade, the GCT community has focused on identifying prognostic factors to allow reduction in therapy (and subsequently long-term toxicity) for a subset of patients while also identifying which patients need intensified therapy to improve outcomes (Figure 3).
Figure 3.
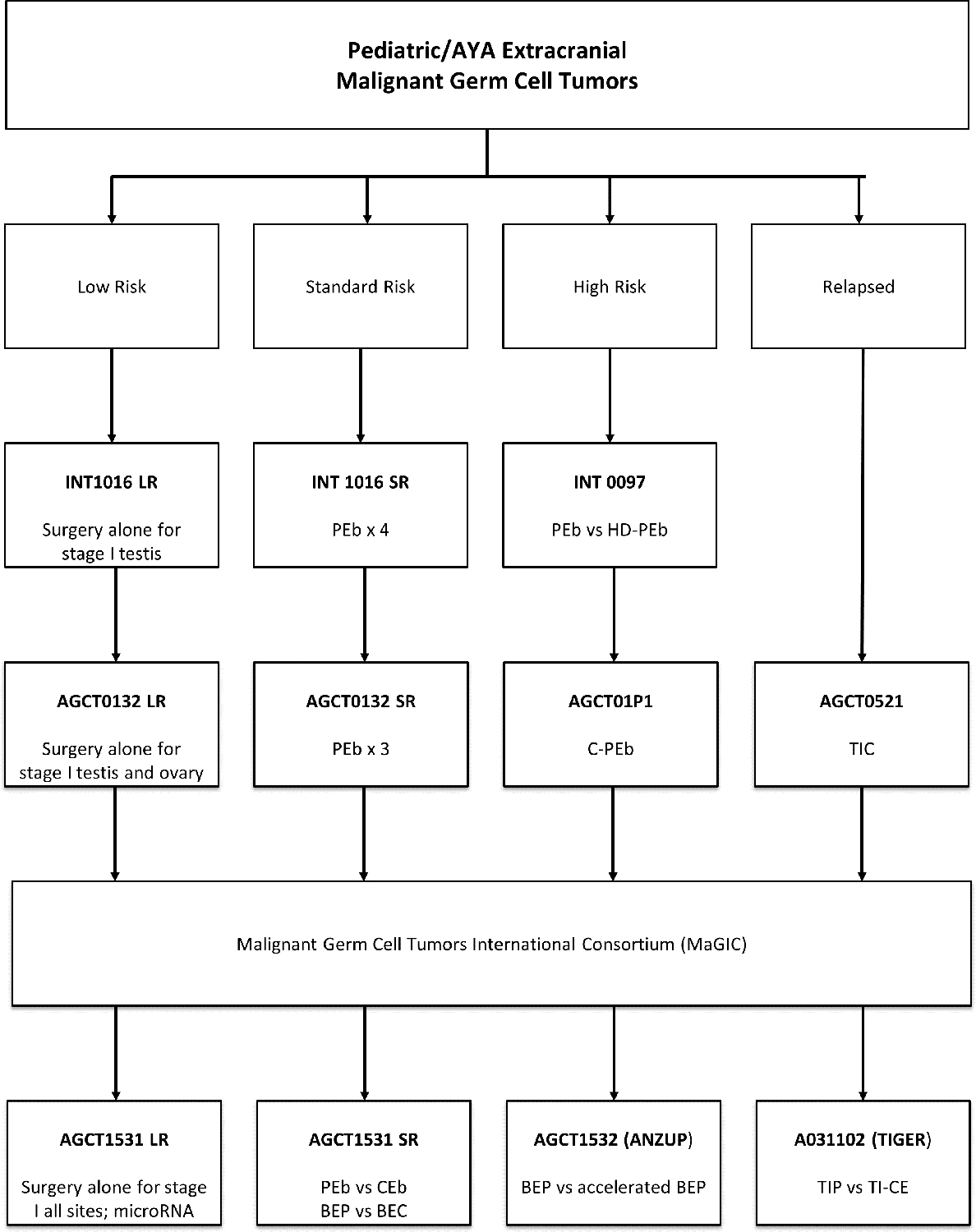
Past and current COG GCT trials
Abbreviations: AYA, Adolescent and Young Adult; LR, Low risk; SR, standard risk; PEb, cisplatin, etoposide, bleomycin once per cycle; HD-PEb, high dose-PEb; C-PEb, cyclophosphamide-PEb; TIC, paclitaxel, ifosfamide, carboplatin; BEP, bleomycin weekly, etoposide, cisplatin; BEC, bleomycin weekly, etoposide, carboplatin; CEb, carboplatin, etoposide, bleomycin once per cycle; TIP, paclitaxel, ifosfamide, cisplatin; TI-CE, paclitaxel, ifosfamide, carboplatin, etoposide
Low-Risk
Intergroup studies and AGCT0132 successfully demonstrated that stage I gonadal GCTs can be treated with surgical resection and observation alone.32–33 AGCT0132 followed patients with stage I gonadal tumors with surveillance alone; 4-year event free survival (EFS) and OS in boys was 74% and 100%, respectively. Age ≥11, presence of lymphovascular invasion, and mixed histology were associated with statistically significant decreased 4-year EFS.32 Singla et al. confirmed outstanding OS of 100% in boys with clinical stage I testicular GCT despite increased rates of relapse with older age and pT AJCC staging.33 These findings support surveillance in prepubertal patients with low-risk (LR) testicular tumors. Management in AYA men with testicular GCTs is more controversial because several treatment options including surveillance, one cycle of BEP (bleomycin weekly, etoposide, cisplatin), or retroperitoneal lymph node dissection (RPLND) deliver similar outcomes.
Stage I malignant ovarian GCTs can also be treated with surveillance alone. AGCT0132 followed 25 girls treated with surgery only; EFS and OS were 52% and 96%, respectively. Despite lower EFS compared to stage I testicular GCTs, ovarian GCTs maintained high salvage rates at the time of recurrence.34 Thus, surgery followed by observation is being further evaluated in a larger sample as part of AGCT1531 [NCT03067181].
Standard-Risk
SR patients require chemotherapy but have excellent survival outcomes; however, late effects of chemotherapy may negatively impact quality of life.35 Thus, current efforts focus on minimizing therapy and associated toxicity without sacrificing excellent outcomes.
AGCT0132 attempted to reduce therapy from 4- to 3-cycles of PEb (cisplatin, etoposide, and bleomycin once per cycle) for SR patients. The EFS after 3-cycles of PEb (89%) was significantly lower than the prespecified parametric model (92%). Thus, 4-cycles of PEb remains the standard of care for pediatric SR patients. The EFS was significantly associated with stage (stage I, 100%; stage II, 92%; stage III, 85%; and stage IV, 54%; P < .001).36 Future trials may seek to reduce cycle numbers for lower stage, younger patients.
Another strategy to reduce toxicity of treatment for SR patients is to use carboplatin, a cisplatin-analogue known to have fewer late effects. In pediatric studies using doses of carboplatin higher than given in adult trials, 5-year EFS was 88%.37 The development of MaGIC allowed for ongoing comparisons between both treatment strategies given the UK’s primary use of carboplatin in pediatric and AYA GCTs. In multivariate analysis, the regimens were not significantly different. Cisplatin-based regimens had overall 4-year EFS of 85% while carboplatin regimens had 4-year EFS of 86%.There was no difference detected in any of the subset analyses by age, gender, site, or risk group.37,38 Such comparisons are the basis of the actively enrolling clinical trial AGCT1531 which randomizes SR patients to cisplatin vs. carboplatin.
Poor-Risk
PR disease is an area of ongoing research with a goal to improve outcomes. Intergroup study INT-0097 between the Pediatric Oncology Group (POG-9049) and Children’s Cancer Group (CCG-8882) showed that PR patients classified as stage III-IV gonadal and all stages of extragonadal disease had 4-year EFS of 80.5% with PEb. On further analysis, children with advanced stage extragonadal tumors had 6-year EFS of 73.1%. This study assessed if intensified therapy with high-dose cisplatin (200 mg/m2/cycle) improved outcomes in PR patients. While an improvement in EFS was seen (89.6% vs. 80.5%), it was at the cost of increased morbidity with severe ototoxicity in most patients.4 AGCT01P1 attempted to intensify standard PEb therapy with escalating dose cyclophosphamide for patients with stage III-IV extragonadal GCT, but the results of the trial did not demonstrate any clear benefit in EFS.39
Teratoma
Mature teratomas are benign tumors managed with surgical resection due to the risk of benign or malignant recurrence as well as the rare occurrence of somatic malignant transformation.40 Overall, 5-year EFS and OS for mature teratoma is excellent at 92.2% and 99%respectively.41
Immature teratomas (IT) are also treated with complete surgical resection but carry a higher risk of recurrence compared to mature teratomas. Data from UK Childhood Cancer Study Group show 5-year EFS 85.9% and OS 95.1%.41 The role of systemic chemotherapy in ovarian ITs remains controversial. Pooled data from MaGIC confirmed that pediatric patients with ovarian IT do very well with surgical resection alone. Five-year EFS and OS were 91% and 99%, respectively. Children with grade 3, stage III ITs treated with surgery alone were more likely to have disease recurrence compared to stage I-II (5-year EFS 92% vs. 52%). Administration of post-operative chemotherapy did not decrease risk of relapse and thus, is not routinely recommended. In adult women with ovarian IT greater than stage I, grade I disease in which the standard of care is to receive chemotherapy, OS was similar to pediatric patients.42 In the current trial AGCT1531, a surveillance-alone strategy for low stage, higher grade IT is being investigated.
Germinoma
Germinomatous tumors are rare in children and adolescents but the most common subtype in young adults. Localized pure ovarian dysgerminoma (FIGO stages IA and IB) is treated with surgical resection alone, while advanced stage disease (FIGO stages IC-IV) is treated with adjuvant chemotherapy with excellent outcomes regardless of age or stage. A recent analysis from the MaGIC database demonstrated no difference in EFS or OS between cisplatin- and carboplatin-based chemotherapy regimens: 5-year EFS was 93.2% using cisplatin and 96% with carboplatin, supporting the use of carboplatin-based regimens in advanced disease.43
Biological Achievements
MicroRNA (miRNA) clusters expressed in embryonic stem cells have been identified as universal biomarkers for malignant GCTs.44–46 The identification of circulating miR-371a-3p is one of the biggest recent biological breakthroughs for patients with testicular tumors. Quantifying circulating serum miR-371a-3p using reverse transcription-polymerase chain reaction (RT-PCR) shows high sensitivity (90.1%) and specificity (94%) for testicular malignant GCT diagnosis, with a positive predictive value of 97.2%.47 Detection of circulating microRNAs are being evaluated for use in diagnosis, monitoring response, detection of minimal residual disease, and early detection of relapse (Figure 4).48
Figure 4:
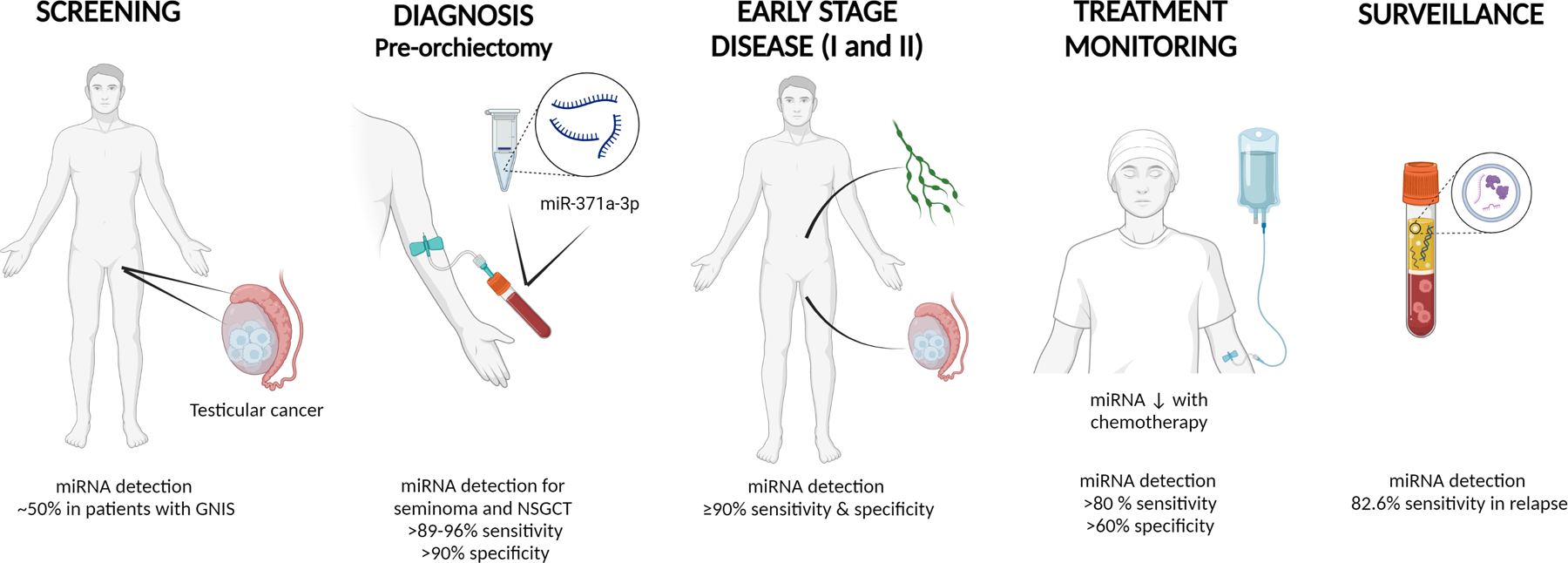
Use of miRNA in testicular germ cell tumor screening, diagnosis, treatment response monitoring, and surveillance 48,74
Created with Biorender.com
Recently, two oncofetal antigens have been identified as biomarkers for GCTs. Oncofetal antigens are proteins expressed during normal fetal development and aberrantly expressed by tumors. The utility of Claudin-6 (CLDN6) as a biomarker was evaluated in a Pan-Cancer analysis and was found to have high expression in testicular GCTs.49 These findings have led to the generation of a chimeric antigen receptor (CAR)-T cells to CLDN6 as a potential therapeutic target [NCT04503278]. Glypican-3 (GPC3) is a cell-surface glycoprotein found on fetal liver with high expression in yolk sac tumors and choriocarcinomas.50 A phase 1 study of codrituzumab, a recombinant humanized monoclonal antibody to GPC3, is being studied in relapsed/refractory solid tumors expressing GPC3 [NCT04928677].
Given the presence of a subset of patients who have relapsed/refractory disease with poor prognosis, research into identification of PR factors and exploration into the mechanism of cisplatin resistance is ongoing. Several groups have reported TP53 alterations and MDM2 amplification as features of cisplatin resistant testicular and mediastinal type II GCTs.28,29,51–53 Timmerman, et al. recently implicated a gain in chromosome 3p25.3 as a predictor of poor outcome associated with cisplatin resistance in type II GCTs.54 However, the role these alterations play in type I tumors and ovarian GCTs needs to be further explored. The proposed PANTHER trial (Phase 3 Randomized trial of Adaptive Dose Intense Treatment for High Risk GCTs) will evaluate for TP53 alteration as part of its treatment assignment.
Alterations in epigenetic states have been shown to correlate with cisplatin resistance. DNA methylation status defines different histologic states in GCTs, modeling the timepoints in development they closest represent and correlating with the level of cisplatin sensitivity. When comparing GCT cell lines that become cisplatin-resistant, there is an increase in global DNA methylation.55–58 In in vitro and in vivo studies, pretreatment with DNA methyltransferase (DNMT) inhibitors restores cisplatin sensitivity.59–61 A phase 1b trial of cisplatin-refractory GCTs combined the DNMT inhibitor guadecitabine and cisplatin and proved tolerability with 3 patients each with partial stable disease [NCT02429466].62 Another phase 1 trial of guadecitabine combined with cisplatin and gemcitabine in solid malignancies had GCT patients with improvement in STM and stable disease. Thus, DNMT inhibitors are being evaluated as a potential therapeutic for relapse/refractory GCTs.63
Xu L., et al. further defined the molecular landscape of childhood and adolescent GCTs, identifying alterations in the WNT pathway. Alterations were seen in DNA copy-number, promoter methylation status, and gene expression. Patients whose tumors had ≥5 changes in the WNT pathway had higher beta-catenin expression and lower EFS and OS. These findings were validated in a separate testicular GCT data set.24 The WNT pathway inhibitor tegavivint is being tested in a phase 1/2 study of children and AYA with recurrent or refractory solid tumors including GCTs with WNT pathway aberrations [NCT04851119].
STRATEGIC APPROACH TO THERAPY
Surgical Guidelines
Surgical guidelines for GCTs focus on accurate intra-operative staging to guide therapy while preserving fertility when possible. The primary goal is to evaluate the extent of disease with appropriate surgical staging, maximize safe and complete tumor resection without violation of the tumor capsule, and spare uninvolved organs. Testicular GCTs undergo radical orchiectomy while ovarian tumors are resected with complete oophorectomy, with or without removal of the fallopian tube based on extent of disease. Imaging characteristics and intraoperative frozen pathology are neither sensitive nor specific of tumor histology and should not replace pre-operative STM measurement and comprehensive surgical staging.64,65 Further efforts utilizing radiomics and artificial intelligence to improve pre-operative predictive accuracy are being explored.
Low-Risk
Patients with COG localized resectable GCTs should have upfront resection when possible, followed by observation if confirmed to be stage I. Eligible patients are offered enrollment on LR arm of AGCT1531. AGCT1531 assesses observation in all stage I extragonadal tumors and reserves chemotherapy for patients with residual or relapsed disease. AGCT1531 will also prospectively assess the significance of serum miRNA in all stage I tumors in children and AYAs for the first time.
Standard-Risk
SR GCTs are managed based on age and stage at diagnosis. Using the revised MaGIC risk-stratification, all prepubertal patients (<11y) beyond stage I are considered SR and are offered enrollment on the SR1 arm of AGCT1531. Patients enrolled on SR1 are randomized to 4-cycles of chemotherapy with PEb vs. CEb (carboplatin, etoposide, bleomycin once per cycle).
Post-pubertal patients ≥11y should receive systemic therapy similar to adult guidelines. Testicular stage II-IV with IGCCCG good-risk, ovarian stage II-III, and extragonadal stage II disease are considered SR and should be offered enrollment on the SR2 arm of AGCT1531. Patients are randomized to 3-cycles of systemic therapy with BEP vs. BEC (bleomycin weekly, etoposide, carboplatin).
Poor-Risk
PR patients ages 11–45y with testicular stage II-IV plus IGCCCG intermediate or poor-risk, ovarian stage IV, or extragonadal stage III-IV disease are offered enrollment on AGCT1532 [NCT02582697], a randomized phase 3 clinical trial comparing 4-cycles of standard BEP (3-week cycles) vs. accelerated BEP (2-week cycles).
Relapsed/Refractory Disease
A randomized phase 3 trial (AO31102; NCT02375204; TIGER) comparing standard-dose paclitaxel, ifosfamide, cisplatin (TIP) vs. high-dose paclitaxel, ifosfamide, carboplatin, etoposide (TI-CE) in patients older than 14 years at first relapse has recently completed accrual. Findings from this trial will establish a new standard of care for patients with relapsed malignant GCTs. Cancer-related mortality exceeds 90% in patients who have failed second- and third-line chemotherapy. Therefore, discovery and development of novel therapeutic strategies is imperative. Table 2 summarizes the ongoing trials of interest for patients with relapsed/refractory GCT
TABLE 2.
Ongoing Trials for Relapsed/Refractory Extracranial Malignant Germ Cell Tumors
| Novel Target | Novel Agent | Clinical Trial | ClinicalTrials.gov Identifier |
|---|---|---|---|
| WNT | Tegavivint (BC2059) | A Phase 1/2 Study of Tegavivint (IND#156033, NSC#826393) in Children, Adolescents, and Young Adults with Recurrent or Refractory Solid Tumors, Including Lymphomas and Desmoid Tumors | NCT04851119 |
| c-MET VEGFR1–3 KIT | Cabozantinib | A Phase II Trial Evaluating the Efficacy of Cabozantinib in the Treatment of Incurable Patients with Refractory Germ Cell Tumors | NCT04876456 |
| Glypican 3 | Codrituzumab | A Multi-center Phase 1 Study of Codrituzumab in Pediatric Patients with Relapsed or Refractory Glypican 3 (GPC3) Expressing Extracranial Solid Tumors | NCT04928677 |
| Glypican 3 | Glypican 3 CAR-T | Interleukin-15 Armored Glypican-3-specific Chimeric Antigen Receptor Expressed in T Cells for Pediatric Solid Tumors | NCT04377932 |
| Claudin-6 | BNT142 | Trial in progress: First-in-human, Open-label, Multicenter, Phase I/IIa, Dose Escalation Trial with Expansion Cohorts to Evaluate Safety and Preliminary Efficacy of BNT142 in Patients with CLDN6-positive Advanced Solid Tumors | NCT05262530 |
| Claudin-6 | DS-9606a | Trial in progress: A Phase 1, First-in-Human Study of DS-9606a in Patients with Tumor Types Known to Express CLDN6 | NCT05394675 |
| Claudin-6 | Claudin-6 (CLDN6) CAR-T +/− CLDN6 RNA-LPX (BNT211) | A Phase 1/2 trial to Evaluate Safety and Efficacy of CLDN6 CAR-T cells and CARVac-mediated in vivo Expansion in Patients with CLDN6+ Advanced Solid Tumors | NCT04503278 |
| Claudin-6 | CLDN6 CAR-NK Cell | Phase I/IIa Trial to Evaluate Safety and Preliminary Efficacy of CLDN6-CAR-NK in Patients With CLDN6-positive Advanced Solid Tumors | NCT05410717 |
| CD30 | CD30 CAR-T | Administration of T Lymphocytes Expressing the CD30 Chimeric Antigen Receptor (CAR) for Patients with CD30+ Nonseminomatous Germ Cell Tumors (NSGCT) | NCT05634785 |
Abbreviations: CAR-T, Chimeric Antigen Receptor-T cell
Bold: Novel Target, Novel Agent, Clinical Trial, Clinicaltrials.gov Identifier
KEY TRIALS TO BE PURSUED
Current unmet needs include refinement of risk-stratification, intensified therapies for patients with poor-risk disease, enhanced strategies for detection of minimal residual disease, and early detection of relapse (particularly in STM-negative histologies). Fragmentation of care of patients between multiple specialists restricts consistent study of these rare tumors. Therefore, it is imperative that trials be conducted in collaboration with national and international cooperative groups, with harmonized data and specimen collection. Incorporation of GCT data into the Pediatric Cancer Data Commons, now called Data for the Common Good, is already underway and will allow for streamlined data analyses. The creation of a global biobank is also of interest to similarly allow for effective basic science research on pediatric and AYA GCTs.66
Age at diagnosis and race/ethnicity impact outcomes. AYAs have significantly decreased EFS compared to children and older adults.67 In the United States, 5-year survival rates do not differ by race/ethnicity in females diagnosed with a GCT <20y. In contrast, there are significantly higher risks of death in Asian/Pacific Islander and Hispanic males not mediated by socioeconomic status.68,69 Understanding these disparities and determining the impact of social determinants of health on outcomes remains a priority for the GCT Committee. Efforts are underway to expand age of enrollment for pediatric patients on adult therapeutic clinical studies to improve inclusion and accessibility to novel therapies.
The GCT Committee has several concepts in development focusing on improving outcomes by intensifying therapy for certain subsets of patients, identifying novel agents to be used in patients with relapsed/refractory disease, and minimizing late effects of therapy.
Patients with unresectable teratomas are challenging to care for given lack of standardized management. While surgical resection is ideal, sometimes it is not feasible or highly morbid based on location or number of prior recurrences and surgeries. A clinical trial is in development to evaluate the natural history and biological similarities of mature, immature, and growing teratomas in children and AYAs starting at resection. Assessing the role of CDK4/6 inhibition for unresectable teratoma will also be explored based on strong pre-clinical data.70,71
With the identification of serum miR-371a-3p as a sensitive and specific marker for testicular GCTs, a trial is in development to identify stage I testicular GCT patients who are more likely to have recurrence with active surveillance and thus warrant upfront intensified therapy. Individuals with an elevated miR-371a-3p post-orchiectomy, regardless of STM status, will be recommended to undergo RPLND. Pathology from RPLND will be used to verify the sensitivity and specificity of the miRNA test. Patients without elevated miRNA will be recommended to follow a standard surveillance schedule.
Ototoxicity from cisplatin can have significant impacts on quality of life for survivors of childhood GCTs. Hearing loss and/or tinnitus can be progressive and irreversible in some patients. Thus, the GCT Committee is exploring the role of a randomized clinical trial incorporating sodium thiosulfate into standard therapy.
For PR GCT, the GCT Committee is invested in opening the PANTHER trial to identify a favorable and unfavorable cohort of PR patients using STM decline and tumor genetics. The unfavorable group will receive intensified therapy (randomization between high-dose therapy versus the intensive GETUG-13 regimen) with the primary objective to improve EFS.72
Over the last decade, the GCT Committee has made tremendous progress in understanding pediatric and AYA GCTs and identifying treatment strategies with excellent outcomes. However, more work is needed to continue to learn about the biology of the disease and further risk-stratify patients to allow for de-escalation of care in some subsets and improvement of outcomes in others.
Acknowledgments:
The authors would like to express their deep gratitude to Drs. James F. Amatruda, Deborah F. Billmire, Thomas A. Olson, and Marcio H. Malogolowkin for their leadership and mentorship, and to all members of the Children’s Oncology Group Germ Cell Tumor Committee for their significant contributions to the research and care of patients with GCTs. Their pioneering efforts, visionary leadership, scientific critique, mentorship, and ongoing support has been instrumental to our evolution, success, and sustainability.
Funding support:
Grant support from the National Institute of Health, U10CA180886, U10CA180899, U10CA098543, U10CA098413, U24CA196173, U24CA114766.
Abbreviations Key:
- AJCC
American Joint Committee on Cancer
- AYA
Adolescents and Young Adults
- BEC
Bleomycin weekly, Etoposide, Carboplatin
- BEP
Bleomycin weekly, Etoposide, Cisplatin
- CAR
Chimeric Antigen Receptor
- CCG
Children’s Cancer Group
- CCLG
Children’s Cancer and Leukemia Group
- CEb
Carboplatin, Etoposide, and Bleomycin once per cycle
- CLDN6
Claudin-6
- COG
Children’s Oncology Group
- DNMT
DNA methyltransferase
- EFS
Event-Free Survival
- FIGO
The International Federation of Gynecology and Obstetrics
- GOLD
GCT Outcomes and Late effects Data
- GCT
Germ Cell Tumor
- GPC3
Glypican-3
- GWAS
Genome Wide Association Studies
- IGCCCG
International Germ Cell Cancer Collaborative Group
- IT
Immature Teratoma
- LR
Low-Risk
- LTDFS
Long-Term Disease-Free Survival
- MaGIC
Malignant Germ Cell Tumors International Consortium
- miRNA
micro-ribonucleic acid
- OS
Overall Survival
- PANTHER
Phase 3 Randomized trial of Adaptive Dose Intense Treatment for High Risk GCTs
- PEb
Cisplatin, Etoposide, and Bleomycin once per cycle
- POG
Pediatric Oncology Group
- PGC
Primordial Germ Cell
- PR
Poor Risk
- RPLND
Retroperitoneal Lymph Node Dissection
- RT-PCR
Reverse transcription-polymerase chain reaction
- STM
Serum Tumor Marker
- SR
Standard-Risk
- TCGA
The Cancer Genome Atlas
- TI-CE
Paclitaxel, Ifosfamide, Carboplatin, Etoposide
- TIP
Paclitaxel, Ifosfamide, Cisplatin
- TNM
Tumor, Node, Metastasis
- UK
United Kingdom
- Y
Years
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
REFRENCES
- 1.Pierce JL, Frazier AL, Amatruda JF. Pediatric Germ Cell Tumors: A Developmental Perspective. Adv Urol 2018;2018:9059382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surveillance Epidemiology, and Results End (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 8 Registries, Nov 2021 Sub (1975–2020) - Linked To County Attributes - Time Dependent (1990–2020) Income/Rurality, 1969–2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2023, based on the November 2022 submission
- 3.Poynter JN, Amatruda JF, Ross JA. Trends in incidence and survival of pediatric and adolescent patients with germ cell tumors in the United States, 1975 to 2006. Cancer 2010;116(20):4882–4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cushing B, Giller R, Cullen JW, et al. Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: a pediatric intergroup study--Pediatric Oncology Group 9049 and Children’s Cancer Group 8882. J Clin Oncol 2004;22(13):2691–2700. [DOI] [PubMed] [Google Scholar]
- 5.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 6.Mead GM, Stenning SP. The International Germ Cell Consensus Classification: a new prognostic factor-based staging classification for metastatic germ cell tumours. Clin Oncol (R Coll Radiol) 1997;9(4):207–209. [DOI] [PubMed] [Google Scholar]
- 7.Mutch DG, Prat J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol 2014;133(3):401–404. [DOI] [PubMed] [Google Scholar]
- 8.Frazier AL, Hale JP, Rodriguez-Galindo C, et al. Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J Clin Oncol 2015;33(2):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaikh F, Murray MJ, Amatruda JF, et al. Paediatric extracranial germ-cell tumours. Lancet Oncol 2016;17(4):e149–e162. [DOI] [PubMed] [Google Scholar]
- 10.Kerns SL, Fung C, Monahan PO, et al. Cumulative Burden of Morbidity Among Testicular Cancer Survivors After Standard Cisplatin-Based Chemotherapy: A Multi-Institutional Study. J Clin Oncol 2018;36(15):1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung C, Sesso HD, Williams AM, et al. Multi-Institutional Assessment of Adverse Health Outcomes Among North American Testicular Cancer Survivors After Modern Cisplatin-Based Chemotherapy. J Clin Oncol 2017;35(11):1211–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisina RD, Wheeler HE, Fossa SD, et al. Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer. J Clin Oncol 2016;34(23):2712–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolan ME, El Charif O, Wheeler HE, et al. Clinical and Genome-Wide Analysis of Cisplatin-Induced Peripheral Neuropathy in Survivors of Adult-Onset Cancer. Clin Cancer Res 2017;23(19):5757–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milano MT, Dinh PC, Yang H, et al. Solid and Hematologic Neoplasms After Testicular Cancer: A US Population-Based Study of 24 900 Survivors. JNCI Cancer Spectr 2020;4(3):pkaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubberts S, Groot HJ, de Wit R, et al. Cardiovascular Disease in Testicular Cancer Survivors: Identification of Risk Factors and Impact on Quality of Life. J Clin Oncol 2023:JCO2201016. [DOI] [PMC free article] [PubMed]
- 16.Nicholls PK, Page DC. Germ cell determination and the developmental origin of germ cell tumors. Development 2021;148(8). [DOI] [PubMed] [Google Scholar]
- 17.Pluta J, Pyle LC, Nead KT, et al. Identification of 22 susceptibility loci associated with testicular germ cell tumors. Nat Commun 2021;12(1):4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Runyan C, Schaible K, Molyneaux K, et al. Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development 2006;133(24):4861–4869. [DOI] [PubMed] [Google Scholar]
- 19.Kee K, Angeles VT, Flores M, et al. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 2009;462(7270):222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krentz AD, Murphy MW, Kim S, et al. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc Natl Acad Sci U S A 2009;106(52):22323–22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenbaum MP, Yan W, Wu MH, et al. TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci U S A 2006;103(13):4982–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuznetsov S, Pellegrini M, Shuda K, et al. RAD51C deficiency in mice results in early prophase I arrest in males and sister chromatid separation at metaphase II in females. J Cell Biol 2007;176(5):581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcotte EL, Pankratz N, Amatruda JF, et al. Variants in BAK1, SPRY4, and GAB2 are associated with pediatric germ cell tumors: A report from the children’s oncology group. Genes Chromosomes Cancer 2017;56(7):548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Pierce JL, Sanchez A, et al. Integrated genomic analysis reveals aberrations in WNT signaling in germ cell tumors of childhood and adolescence. Nat Commun 2023;14(1), 2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen H, Shih J, Hollern DP, Wang L, et al. Integrated Molecular Characterization of Testicular Germ Cell Tumors. Cell Rep 2018;23(11): 3392–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geurts van Kessel A, van Drunen E, de Jong B, et al. Chromosome 12q heterozygosity is retained in i(12p)-positive testicular germ cell tumor cells. Cancer Genet Cytogenet 1989;40(1):129–134. [DOI] [PubMed] [Google Scholar]
- 27.Litchfield K, Summersgill B, Yost S, et al.Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nat Commun 2015;6:5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loveday C, Litchfield K, Proszek PZ, et al. Genomic landscape of platinum resistant and sensitive testicular cancers. Nat Commun 2020;11(1):2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor-Weiner A, Zack T, O’Donnell E, et al. Genomic evolution and chemoresistance in germ-cell tumours. Nature 2016;540(7631):114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutcutache I, Suzuki Y, Tan IB, et al. Exome-wide Sequencing Shows Low Mutation Rates and Identifies Novel Mutated Genes in Seminomas. Eur Urol 2015;68(1): 77–83. [DOI] [PubMed] [Google Scholar]
- 31.Kraggerud SM, Hoei-Hansen CE, Alagaratnam S, et al. Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: implications for pathogenesis. Endocr Rev 2013;34(3): 339–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rescorla FJ, Ross JH, Billmire DF, et al. Surveillance after initial surgery for Stage I pediatric and adolescent boys with malignant testicular germ cell tumors: Report from the Children’s Oncology Group. J Pediatr Surg 2015;50(6):1000–1003. [DOI] [PubMed] [Google Scholar]
- 33.Singla N, Wong J, Singla S, et al. Clinicopathologic predictors of outcomes in children with stage I testicular germ cell tumors: A pooled post hoc analysis of trials from the Children’s Oncology Group. J Pediatr Urol 2022;18(4):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billmire DF, Cullen JW, Rescorla FJ, et al. Surveillance after initial surgery for pediatric and adolescent girls with stage I ovarian germ cell tumors: report from the Children’s Oncology Group. J Clin Oncol 2014;32(5):465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fung C, Fossa SD, Williams A, Travis LB. Long-term Morbidity of Testicular Cancer Treatment. Urol Clin North Am 2015;42(3):393–408. [DOI] [PubMed] [Google Scholar]
- 36.Shaikh F, Cullen JW, Olson TA, et al. Reduced and Compressed Cisplatin-Based Chemotherapy in Children and Adolescents With Intermediate-Risk Extracranial Malignant Germ Cell Tumors: A Report From the Children’s Oncology Group. J Clin Oncol 2017;35(11):1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaikh F, Nathan PC, Hale J, Uleryk E, Frazier L. Is there a role for carboplatin in the treatment of malignant germ cell tumors? A systematic review of adult and pediatric trials. Pediatr Blood Cancer 2013;60(4):587–592. [DOI] [PubMed] [Google Scholar]
- 38.Frazier AL, Stoneham S, Rodriguez-Galindo C, et al. Comparison of carboplatin versus cisplatin in the treatment of paediatric extracranial malignant germ cell tumours: A report of the Malignant Germ Cell International Consortium. Eur J Cancer 2018;98:30–37. [DOI] [PubMed] [Google Scholar]
- 39.Malogolowkin MH, Krailo M, Marina N, Olson T, Frazier AL. Pilot study of cisplatin, etoposide, bleomycin, and escalating dose cyclophosphamide therapy for children with high risk germ cell tumors: a report of the children’s oncology group (COG). Pediatr Blood Cancer 2013;60(10):1602–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terenziani M, D’Angelo P, Bisogno G, et al. Teratoma with a malignant somatic component in pediatric patients: the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) experience. Pediatr Blood Cancer 2010;54(4):532–537. [DOI] [PubMed] [Google Scholar]
- 41.Mann JR, Gray ES, Thornton C, et al. Mature and immature extracranial teratomas in children: the UK Children’s Cancer Study Group Experience. J Clin Oncol 2008;26(21):3590–3597. [DOI] [PubMed] [Google Scholar]
- 42.Pashankar F, Hale JP, Dang H, et al. Is adjuvant chemotherapy indicated in ovarian immature teratomas? A combined data analysis from the Malignant Germ Cell Tumor International Collaborative. Cancer 2016;122(2):230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah R, Xia C, Krailo M, et al. Is carboplatin-based chemotherapy as effective as cisplatin-based chemotherapy in the treatment of advanced-stage dysgerminoma in children, adolescents and young adults? Gynecol Oncol 2018;150(2):253–260. [DOI] [PubMed] [Google Scholar]
- 44.Fankhauser CD, Nuno MM, Murray MJ, et al. Circulating MicroRNAs for Detection of Germ Cell Tumours: A Narrative Review. Eur Urol Focus 2022;8(3):660–662. [DOI] [PubMed] [Google Scholar]
- 45.Murray MJ, Scarpini CG, Coleman N. A Circulating MicroRNA Panel for Malignant Germ Cell Tumor Diagnosis and Monitoring. Methods Mol Biol 2021;2195:225–243. [DOI] [PubMed] [Google Scholar]
- 46.Palmer RD, Murray MJ, Saini HK, et al. Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res 2010;70(7):2911–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dieckmann KP, Radtke A, Geczi L, et al. Serum Levels of MicroRNA-371a-3p (M371 Test) as a New Biomarker of Testicular Germ Cell Tumors: Results of a Prospective Multicentric Study. J Clin Oncol 2019;37(16):1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leao R, Albersen M, Looijenga LHJ, et al.Circulating MicroRNAs, the Next-Generation Serum Biomarkers in Testicular Germ Cell Tumours: A Systematic Review. Eur Urol 2021;80(4):456–466. [DOI] [PubMed] [Google Scholar]
- 49.Cava C, Bertoli G, Colaprico A. Integration of multiple networks and pathways identifies cancer driver genes in pan-cancer analysis. BMC Genomics 2018;19(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ortiz MV, Roberts SS, Glade Bender J, Shukla N, Wexler LH. Immunotherapeutic Targeting of GPC3 in Pediatric Solid Embryonal Tumors. Front Oncol 2019;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bagrodia A, Lee BH, Lee W, et al. Genetic Determinants of Cisplatin Resistance in Patients With Advanced Germ Cell Tumors. J Clin Oncol 2016;34(33):4000–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Necchi A, Bratslavsky G, Chung J, et al. Genomic Features for Therapeutic Insights of Chemotherapy-Resistant, Primary Mediastinal Nonseminomatous Germ Cell Tumors and Comparison with Gonadal Counterpart. Oncologist 2019;24(4):e142–e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Necchi A, Bratslavsky G, Corona RJ, et al. Genomic Characterization of Testicular Germ Cell Tumors Relapsing After Chemotherapy. Eur Urol Focus 2020;6(1):122–130. [DOI] [PubMed] [Google Scholar]
- 54.Timmerman DM, Eleveld TF, Sriram S, et al. Chromosome 3p25.3 Gain Is Associated With Cisplatin Resistance and Is an Independent Predictor of Poor Outcome in Male Malignant Germ Cell Tumors. J Clin Oncol 2022;40(26):3077–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fazal Z, Singh R, Fang F, et al. Hypermethylation and global remodelling of DNA methylation is associated with acquired cisplatin resistance in testicular germ cell tumours. Epigenetics 2021;16(10):1071–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobsen C, Honecker F. Cisplatin resistance in germ cell tumours: models and mechanisms. Andrology 2015;3(1):111–121. [DOI] [PubMed] [Google Scholar]
- 57.Lobo J, Constancio V, Leite-Silva P, et al. Differential methylation EPIC analysis discloses cisplatin-resistance related hypermethylation and tumor-specific heterogeneity within matched primary and metastatic testicular germ cell tumor patient tissue samples. Clin Epigenetics 2021;13(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wermann H, Stoop H, Gillis AJ, et al. Global DNA methylation in fetal human germ cells and germ cell tumours: association with differentiation and cisplatin resistance. J Pathol 2010;221(4):433–442. [DOI] [PubMed] [Google Scholar]
- 59.Albany C, Hever-Jardine MP, von Herrmann KM, et al. Refractory testicular germ cell tumors are highly sensitive to the second generation DNA methylation inhibitor guadecitabine. Oncotarget 2017;8(2):2949–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beyrouthy MJ, Garner KM, Hever MP, et al. High DNA methyltransferase 3B expression mediates 5-aza-deoxycytidine hypersensitivity in testicular germ cell tumors. Cancer Res 2009;69(24):9360–9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biswal BK, Beyrouthy MJ, Hever-Jardine MP, et al. Acute hypersensitivity of pluripotent testicular cancer-derived embryonal carcinoma to low-dose 5-aza deoxycytidine is associated with global DNA Damage-associated p53 activation, anti-pluripotency and DNA demethylation. PLoS One 2012;7(12):e53003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Albany C, Fazal Z, Singh R, et al. A phase 1 study of combined guadecitabine and cisplatin in platinum refractory germ cell cancer. Cancer Med. 2021;10(1):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crabb SJ, Danson S, Catto JWF, et al. Phase I Trial of DNA Methyltransferase Inhibitor Guadecitabine Combined with Cisplatin and Gemcitabine for Solid Malignancies Including Urothelial Carcinoma (SPIRE). Clin Cancer Res 2021;27(7):1882–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Billmire D, Dicken B, Rescorla F, et al. Imaging Appearance of Nongerminoma Pediatric Ovarian Germ Cell Tumors Does Not Discriminate Benign from Malignant Histology. J Pediatr Adolesc Gynecol 2021;34(3):383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dicken BJ, Billmire DF, Rich B, et al. Utility of frozen section in pediatric and adolescent malignant ovarian nonseminomatous germ cell tumors: A report from the children’s oncology group. Gynecol Oncol 2022;166(3):476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fonseca A, Lobo J, Hazard FK, et al. Advancing clinical and translational research in germ cell tumours (GCT): recommendations from the Malignant Germ Cell International Consortium. Br J Cancer 2022;127(9):1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fonseca A, Frazier AL, Shaikh F, et al. Germ Cell Tumors in Adolescents and Young Adults. JCO 2019;15(8):433–41. [DOI] [PubMed] [Google Scholar]
- 68.Williams LA, Frazier AL, Poynter JN. Survival differences by race/ethnicity among children and adolescents diagnosed with germ cell tumors. Int J Cancer 2020;146(9):2433–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kehm RD, Spector LG, Poynter JN, et al. Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer 2018;124(20):4090–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaugh DJ, Hwang WT, Rosen MA, et al. Phase 2 trial of the cyclin-dependent kinase 4/6 inhibitor Palbociclib in patients with retinoblastoma protein-expressing germ cell tumors. Cancer 2015;121 (9):1463–8. [DOI] [PubMed] [Google Scholar]
- 71.Narayan V, Hwang WT, Lal P, et al. Cyclin-Dependent Kinase 4/6 Inhibition for the Treatment of Unresectable Mature Teratoma: Long-Term Follow-Up of a Phase II Study. Clin Genitourin Cancer 2016. 14(6):504–510. [DOI] [PubMed] [Google Scholar]
- 72.Fizazi K, Pagliaro L, Laplanche A, et al. Personalized chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): a phase 3, multicentre, randomised trial. Lancet Oncol 2014;15(13):1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.NCCR*Explorer: An interactive website for NCCR cancer statistics [Internet]. National Cancer Institute [Cited May 19, 2023]. Available from https://NCCRExplorer.ccdi.cancer.gov/
- 74.Piao J, Lafin JT, Scarpini CG, et al. A Multi-institutional Pooled Analysis Demonstrates That Circulating miR-371a-3p Alone is Sufficient for Testicular Malignant Germ Cell Tumor Diagnosis. Clin Genitourin Cancer 2021;19(6):469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]


