Abstract
Background:
Positron emission tomography (PET) scans for amyloid-β can aid in the early and accurate detection of Alzheimer’s disease. The results of amyloid PET scans could help people with cognitive impairment and caregivers better understand their diagnosis; however, there are concerns that they could also cause psychological harm.
Methods:
A systematic review of psychosocial and behavioral quantitative outcomes following the disclosure of an amyloid PET scan for persons living with cognitive impairment (subjective cognitive decline, mild cognitive impairment (MCI), Alzheimer’s Disease and other dementias) and caregivers.
Findings:
10 papers were identified from 7 studies. There was little evidence of an association between disclosure and depression. However, persons with MCI and their caregivers with elevated levels of amyloid had an increased risk of distress or anxiety compared with those without elevated amyloid. Participants correctly recalled the scan results; however, it is unclear whether this led to an increased understanding of their diagnosis. We did not identify any studies measuring behavioral outcomes.
Conclusions:
We found mixed evidence on the relationship between amyloid scans and psychosocial and behavioral outcomes in people with cognitive impairment and caregivers. These findings highlight the need for more methodologically rigorous research on this topic.
Keywords: amyloid PET scan, disclosure, biomarkers, mild cognitive impairment
Introduction
There has been rapid progress in the development of biomarkers for detecting the neuropathology associated with Alzheimer’s disease, potentially enhancing the accuracy of diagnoses, and transforming how Alzheimer’s disease and related dementias are diagnosed and treated1. Amyloid-β is a pathophysiological change which can be detected using positron emission tomography (PET) scans2. The results from amyloid scans are disclosed to recipients as a binary outcome, where a positive scan result indicates elevated levels of amyloid, consistent with the pathology of Alzheimer’s disease, and a negative scan does not indicate elevated amyloid3. This can have differing implications depending on the scan recipient’s level of cognitive impairment. For persons with Alzheimer’s disease and related dementias (ADRD), amyloid scans can be used to make a differential diagnosis4. For persons with Mild Cognitive Impairment (MCI), elevated amyloid may indicate an increased risk of developing dementia1. However, this relationship is not definitive. Elevated levels of amyloid may occur in persons with other neurodegenerative diseases or in cognitively healthy individuals5,6. In the US, amyloid scans have been limited to use for research purposes due to their limited predictive value at the individual level7. Evidence from the Imaging Dementia - Evidence for Amyloid Scanning (IDEAS) study shows amyloid scans are associated with changes in the clinical management of ADRD4, although the impact of disclosure on scan recipients and their caregivers is less understood.
Ethical debates regarding the disclosure of amyloid scan results in clinical practice center on the potential benefits and harms to the scan recipient and caregivers. Arguments in support of disclosure propose the scan recipient and caregiver would benefit from a more accurate diagnosis, allowing them to better understand their condition, receive appropriate treatments and engage in advance care planning8,9. Counter arguments cite concerns that scan recipients may experience psychological harm, workplace or social stigmatization9–11. Two previous systematic reviews have summarized the evidence of the impact of amyloid disclosure on cognitively healthy persons8,10 and found there was very little change in psychological outcomes following the disclosure of the scan result. However, previous research has yet to summarize caregiver outcomes following the disclosure of an amyloid scan. As amyloid scans can be used to assess suitability for amyloid targeting therapies (anti-amyloid monoclonal antibodies), it is becoming increasingly more likely that they will be incorporated into routine clinical care for people with dementia. Therefore, the objective of this systematic review is to update the findings from the two previous reviews on this topic and summarize quantitative psychosocial and behavioral outcomes following the disclosure of an amyloid PET scan for persons living with cognitive impairment and their caregivers.
Methods
This review was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines12,13 (eTable 1 in supplementary materials) and was registered in advance on PROSPERO (ID: CRD42022371876).
Eligibility criteria
Studies had to meet the following criteria: 1) studies included participants with subjective cognitive decline, MCI or ADRD, and/or their caregivers. Studies of cognitively healthy participants were included if subjective cognitive decline was explicitly stated as an inclusion criterion; 2) all study designs (with or without comparison groups) were included, however, outcomes were limited to those reported after the participant received the results from their own amyloid PET scan; 3) reported quantitative psychosocial and/or behavioral outcomes for patients or caregivers. Psychosocial outcomes were broadly defined as measures capturing mood, emotions, reaction to the scan, quality of life, and caregiver burden. Behavioral outcomes were broadly defined as changes in behavior, knowledge, or decision making following amyloid disclosure. 4) studies published in English, and 5) studies published in peer reviewed journals. Papers which reported outcomes from larger studies were included if outcomes were not duplicated.
Studies were excluded if they: 1) reported associations between amyloid positivity/ deposition without the disclosure of the scan result, 2) only reported qualitative data 3) disclosed hypothetical or fictitious results to participants, 4) tested other dementia-related biomarkers, or reported outcomes from plasma or cerebrospinal fluid biomarkers, 5) only include cognitively healthy participants without subjective cognitive decline, or 6) were reviews, discussion articles, case studies, pre-prints or conference abstracts.
Information sources, search strategy and selection process
We searched Embase, MEDLINE and PsychInfo, independently on OVID, on 3rd October 2022 with no limits on the search. The search terms included variations on the key words: amyloid, PET scan and dementia. MesH terms were also included for dementia, Alzheimer’s disease and PET scan. See eMethods 2 for the full search strategy.
The search results were exported to Covidence, where duplicates were removed. References were screened by title and abstract and then by full text. All articles were double screened by EC and MA and conflicts were resolved by MP.
Data Items and extraction
Relevant data items were extracted by EC using Covidence and a second author (WZ) checked the extracted items for accuracy. Extracted information included: study name, author names, year, country, study aims, design, participant diagnosis, details of amyloid disclosure, number of participants, participants average age, number and proportion of women, number and proportion of white participants, number and proportion of participants with elevated amyloid, outcomes and measures reported, analysis used, and results.
Study critical appraisal
Studies were critically assessed using the Joanna Briggs Institute (JBI) critical appraisal tool that corresponded to their study design14,15. EC conducted the critical appraisal, which was double checked for accuracy by WZ.
Synthesis
Due to the heterogeneity of designs, participants, and outcomes used by the included studies a meta-analysis would not be meaningful; therefore, results were summarized by a narrative synthesis16. Results for persons with cognitive impairment and caregivers were presented separately. We tabulated the key findings from each study by outcome.
Results
Study selection
We identified 16,534 references (Figure 1). After duplicates were removed, we screened 10,495 references for inclusion. We screened the full text of 59 papers and included 10 in this systematic review. Reasons for exclusion included wrong publication type (N=25), outcomes were not collected post-disclosure (N=9), participants were cognitively normal (N=7), scan results were hypothetical or fictitious (N=3), only qualitative data were reported (N=3), and papers did not include psychosocial or behavioral outcomes (N=2).
Figure 1.
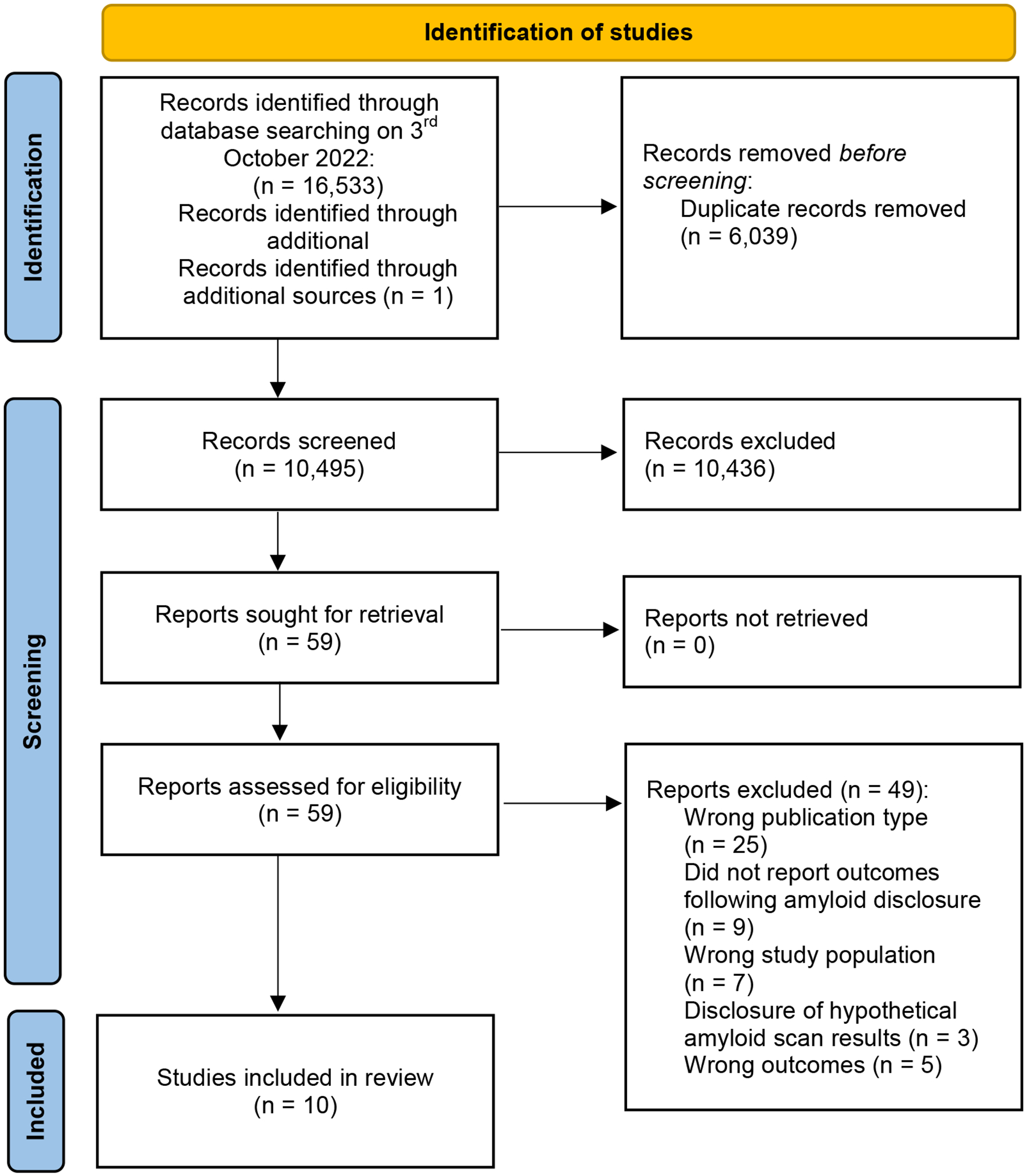
PRISMA Diagram
Study and participant characteristics
We identified ten papers from seven studies. Table 1 presents an overview of the study characteristics. Three studies presented data from the CARE-IDEAS study, a cross-sectional survey of people with MCI, ADRD and their caregivers17–19, and two studies presented data from the RAISR randomized controlled trial (RCT), comparing amyloid PET scan disclosure with psychoeducation20,21. Four papers presented findings from pre/post designs, two with participants with subjective cognitive impairment22,23 and one with participants with early onset ADRD24, and one with participants with MCI and mild ADRD25. One study presents findings from a cross-sectional study of caregivers26. Sample sizes ranged from 11 to 3,690 (1,845 caregiving dyads). The quality of the included studies varied greatly (see eTables 1, 2 and 3). Although most studies aimed to understand the participant’s emotional responses to amyloid PET scan disclosure, 6/9 reported associations between the scan result and outcomes. Another key weakness was the lack of a comparison group among the included studies. Only the RAISR study compared outcomes between scan recipients and non-scan recipients20. All studies included in this review reported psychosocial outcomes. We did not identify any behavioral outcomes.
Table 1.
Characteristics of included studies by study design
| Author (Year) | Study name | Country | Sample size (n) | Details of amyloid PET scan disclosure | Study aim | Level of comparison(s) | Outcomes |
|---|---|---|---|---|---|---|---|
| Randomized controlled trials | |||||||
| Lingler (2020) | RAISR | USA | MCI (N = 82); Caregivers (N = 82) | Disclosure followed standardized script: including verbal and visual presentations of scan results; short-term risk estimates for conversion to AD; brain health information; and follow-up monitoring instructions. | To determine the effect of receiving amyloid PET results on understanding of, and perceived efficacy to cope with MCI, compared with psychoeducation | Amyloid PET scan vs psychoeducation. Sub-group analysis comparing elevated vs non-elevated amyloid amongst scan recipients | Understanding of scan result/diagnosis; self-efficacy for coping; depression; anxiety; test related distress |
| Mattos (2019) | RAISR | USA | MCI (N = 24) | To examine the impact of disclosing amyloid PET results to individuals with MCI | Adverse events following amyloid PET scan disclosure | ||
| Pre/Post studies | |||||||
| Lim (2016) | USA | Subjective Cognitive Decline (N = 11) | Scan results were mailed to participant’s board-certified neurologists | To explore impact of amyloid PET scan disclosure on psychological outcomes | Elevated vs non-elevated amyloid (all participants received a scan) | Depression; anxiety; stress; test related distress | |
| Wake (2018) | Japan | Subjective Cognitive Decline (N = 42) | Two study psychiatrists disclosed the PET results to each participant | To examine the short-term psychological impact of delivering amyloid PET results to asymptomatic Japanese elderly with SCD | Elevated vs non-elevated amyloid (all participants received a scan) | Depression; anxiety; stress; test related distress | |
| van der Doelen (2022) | The Netherlands | Young onset ADRD (N = 154) | Participants underwent the full diagnostic process including amyloid PET scans | To explore the association between diagnostic outcome and clinician’s level of certainty with quality of life (QoL) after amyloid PET results were disclosed in young onset dementia patients in a memory clinic cohort | Pre scan vs post scan (all participants received a scan) | Quality of life | |
| Taswell (2017) | Australia | MCI or ADRD (N = 133) | Scan results were initially disclosed by the referring physician followed by a more in-depth disclosure by a member of the study team. | To assess the psychological impact of disclosing amyloid PET scan results on people with MCI or mild ADRD | Pre scan vs post scan, elevated vs non-elevated amyloid, and MCI vs ADRD (all participants received a scan) | Depression; Anxiety | |
| Cross sectional studies | |||||||
| Bensaidane (2016) | Canada | Caregivers (N = 23) | Scan result was disclosed by a member of the study team to the patient and caregiver. Patients and caregivers were shown the scan. | To explore the clinical utility of amyloid PET scans in the differential diagnosis of dementia and its impact on caregivers | Elevated vs non-elevated amyloid (all participants received a scan) | Anxiety; depression; understanding of the disease; expectation of the future; quality of life | |
| Jutkowitz (2020) | CARE-IDEAS | USA | MCI or ADRD (N = 1551); Caregivers (N = 1551) | Scans were performed and interpreted at each participating PET facility. Results were then provided to the ordering provider. Study protocol specified that providers disclose the PET scan results, preferably in person, to patients and care partners as a part of clinical care with no specified timeframe | To evaluate determinants of willingness to accept a treatment to return memory to normal among persons with cognitive impairment who received an amyloid PET scan and their care partner | Elevated vs non-elevated amyloid (all participants received a scan) | Willingness to accept risky treatment |
| James (2020) | CARE-IDEAS | USA | MCI (N = 1349); ADRD (N = 496); Caregivers (N = 1845) | To understand how accurately patients with MCI or dementia and their care partners report results of amyloid PET scans and the concordance between their reports, and identify factors that influence correct reporting of scan results and concordance within the patient-care partner dyad | Elevated vs non-elevated amyloid (all participants received a scan) | Understanding of scan result/ diagnosis | |
| Belanger (2022) | CARE-IDEAS | USA | Caregivers (N = 1872) | To explore caregiver emotional responses to amyloid PET Scans | Elevated vs non-elevated amyloid (all participants received a scan) | Anxiety; depression | |
Note: MCI = Mild cognitive Impairment; ADRD = Alzheimer’s disease and related dementias; PET = Positron Emission Tomography; RAISR = The return of amyloid imaging scan results study; CARE-IDEAS = The caregivers’ reactions and experience supplemental study of the imaging dementia evidence for amyloid scanning study.
Table 2 summarizes the characteristics of included participants. The average age of persons with cognitive impairment was 69.6 and 46.8% were female. The proportion of scan recipients with elevated amyloid ranged from 23.8% - 78.1% (mean = 43.7%). Caregivers had an average age of 65 and 64% were female. Only the CARE-IDEAS and RAISR studies presented information on the participants race/ethnicity. Most persons with cognitive impairment and caregivers were white, 94.1% and 93.0% respectively. Persons with cognitive impairment and caregivers in the included studies were highly educated in terms of degrees earned and number of years in education.
Table 2.
Characteristics of included participants
| Persons with cognitive impairment | Caregivers | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Age (mean) | % women | % white participants | % Amyloid Positive | Level of education | Age (mean) | % women | % white participants | Level of education |
| RAISR (Lingler 2020 & Mattos 2019) | 72.6 | 40.0 | 92.0 | 33.3 | 82.9% bachelor’s degree or above | 66.8 | 76.0 | 90.0 | 80.5% bachelor’s degree or above |
| CARE-IDEAS (Belanger 2022, James 2020, Jutkowitz 2020) | 74.5 | 38.8 | 96.1 | 67.6 | 58.9% bachelor’s degree or above | 70.0 | 68.3 | 96.1 | 57.7% bachelor’s degree or above |
| Lim (2016) | 63.0 | 62.0 | NR | 16.0 | 17 years of education on average | - | - | - | - |
| Wake (2018) | 74.8 | 52.3 | NR | 23.8 | Average age on leaving education 15 years old | - | - | - | - |
| Van Der Doelen (2022) | 62.0 | 42.0 | NR | NR | 59 % high levels of education | - | - | - | - |
| Taswell (2017) | 70.9 | 45.9 | NR | 78.1 | NR | - | - | - | - |
| Bensaidane (2016) * | 59.3 | 46.4 | NR | 50.0 | 13.3 years of education on average | NR | NR | NR | NR |
| Average | 69.6 | 46.8 | 94.1 | 43.76 | N/A | 68.4 | 72.2 | 93.0 | 69.1% bachelor’s degree or above |
Note: RAISR = The return of amyloid imaging scan results study; CARE-IDEAS = The caregivers’ reactions and experience supplemental study of the imaging dementia evidence for amyloid scanning study; NR = Not reported.
Authors present characteristics of scan recipients but present outcomes from caregivers. This data is not included in the averages.
Results of individual studies
The association between amyloid scan disclosure and outcomes for persons with cognitive impairment
Eight studies assessed the association between amyloid PET scan disclosure and psychosocial and behavioral outcomes for persons with cognitive impairment (Table 3).
Table 3.
Key findings of the impact of amyloid disclosure on patient outcomes
| Outcome | Measure | Author (year) | Level of Cognitive Impairment | Study design | Timing of outcome | Analysis | Key Findings |
|---|---|---|---|---|---|---|---|
| Depression | Patient health questionnaire (PHQ-4) | Mattos (2019) | MCI | RCT | Each day, for 14 days after amyloid PET scan disclosure | Random coefficient modelling | No significant difference in depressive symptoms by scan result. Those with positive scans showed greater daily variability in depressive symptoms over the follow-up period compared to those with negative scans |
| Center for Epidemiological Studies Depression Scale (CESD) | Lingler (2020) | MCI | RCT | Before and 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | No significant difference in depressive symptoms between those who received and amyloid scan and those who received psychoeducation | |
| Taswell (2017) | MCI & ADRD | Pre/Post | Before the scan and approximately 57 days after the scan | Paired-samples t-test | No significant difference before and after scan. No significant difference between amyloid positive and amyloid negative groups. No significant differences between participants with MCI and participants with ADRD. | ||
| Depression subscale of depression anxiety and stress scale (DASS) | Lim (2016) | SCD | Pre/Post | Before disclosure and 9 or 18 months post scan | Not reported | Amyloid positive participants showed little change in DASS scores | |
| Beck Depression Inventory (BDI-II) | Wake (2018) | SCD | Pre/Post | Before disclosure and 6 week follow-up | ANOVA | Depression scores did not change over time, nor were there any differences in depressive symptoms between amyloid positive and amyloid negative groups | |
| Geriatric Depression Scale (GDS) | Taswell (2017) | MCI & ADRD | Pre/Post | Before the scan and approximately 57 days after the scan | Paired-samples t-test | No significant difference before and after scan. No significant difference between amyloid positive and amyloid negative groups. No significant differences between participants with MCI and participants with ADRD. | |
| Hospital Anxiety and Depression Scales (HADS-D) | Taswell (2017) | MCI & ADRD | Pre/Post | Before the scan and approximately 57 days after the scan | Paired-samples t-test | No significant difference before and after scan. No significant difference between amyloid positive and amyloid negative groups. No significant differences between participants with MCI and participants with ADRD. | |
| Anxiety | Spielberger State Anxiety Inventory (STAI) | Lingler (2020) | MCI | RCT | Before and 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | All participant’s symptoms of anxiety remained stable over the entire follow-up period |
| Wake (2018) | SCD | Pre/Post | Before disclosure and 6 week follow-up | ANOVA | Anxiety scores did not change over time, nor were there any differences in anxiety scores between amyloid positive and amyloid negative groups | ||
| Taswell (2017) | MCI & ADRD | Pre/Post | Before the scan and approximately 57 days after the scan | Paired-samples t-test | No significant difference before and after scan. No significant difference between amyloid positive and amyloid negative groups. No significant differences between participants with MCI and participants with ADRD. | ||
| Anxiety subscale of patient health questionnaire (PHQ-4) | Mattos (2019) | MCI | RCT | Each day, for 14 days after amyloid PET scan disclosure | Random coefficient modelling | No significant difference in anxious symptoms by scan result. Those with positive scans showed greater daily variability in anxious symptoms over the follow-up period compared to those with negative scans | |
| Hospital Anxiety and Depression Scales (HADS-A) | Taswell (2017) | MCI & ADRD | Pre/Post | Before the scan and approximately 57 days after the scan | Paired-samples t-test | No significant difference before and after scan. No significant difference between amyloid positive and amyloid negative groups. No significant differences between participants with MCI and participants with ADRD. | |
| Test related distress | Impact of Event Scale (IES) | Lingler (2020) | MCI | RCT | 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | Amyloid positive participants scored higher on the IES compared to amyloid negative. Although IES scores declined for amyloid positive patients over the study period, scores remained greater than for amyloid negative participants |
| Lim (2016) | SCD | Pre/Post | 9 or 18 months post scan | Not reported | Learning the PET scan results had low impact on participants IES scores | ||
| Wake (2018) | SCD | Pre/Post | At 6 week follow-up | ANOVA | IES scores were below the cut off for determining high levels of distress. There were no significant differences between IES scores between amyloid positive and amyloid negative participants | ||
| Distress and Positive Impact subscales of the Impact of Genetic Testing-Alzheimer’s Disease (IGT-AD) | Lingler (2020) | MCI | RCT | 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | Amyloid Positive participants scored higher on the IGT-AD than amyloid positive patients 4 weeks after disclosure and remained stable over time. However, scores for amyloid negative gradually increased at each follow-up time point. | |
| Quality of Life | Quality of Life- Alzheimer’s Disease (QOL-AD) | van der Doelen (2022) | ADRD | Pre/Post | Before disclosure and at 3 month follow-up | Multivariate regression models | Change in diagnosis was associated with quality of life, when adjusting for other variables |
| EuroQoL EQ-5D | van der Doelen (2022) | ADRD | Pre/Post | Before disclosure and at 3 month follow-up | Multivariate regression models | Change in clinician confidence in diagnosis was associated with a small change in quality of life, when adjusting for other variables | |
| Self efficacy for coping | Self efficacy for coping scale (CSE) | Lingler (2020) | MCI | RCT | Before and 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | There were no significant differences in self efficacy between participants who received an amyloid scan and those who received psychoeducation. There was no significant difference in scores over time |
| Understanding of scan result or diagnosis | Accurate reporting of scan result | James (2020) | MCI & ADRD | Cross-sectional | Approximately 4.5 months following disclosure | Chi-squared test | 83% of participants correctly reported the result of the amyloid PET scan. Participants with dementia were more likely to correctly recall the scan result than participants with MCI. |
| MCI/AD Knowledge Assessment | Lingler (2020) | MCI | RCT | Before and 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | No significant difference in knowledge scores between time points or between those who received an amyloid PET scan and those who received psychoeducation. | |
| Illness coherences subscale of the revised illness perception questionnaire (IPQ-R) | Lingler (2020) | MCI | RCT | Before and 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | No significant difference in understanding of MCI between amyloid PET scan and psychoeducational group. However, sub-group analyses showed increased perceived ambiguity amongst amyloid positive participants compared with amyloid negative. | |
| Willingness to accept risky treatment | The following question: Suppose there is a new technology that can return your memory to normal but has a risk of death. What is the highest risk of death, if any, that you would be willing to accept for this treatment? The number you give can be anywhere between 0% and 100% | Jutkowitz (2020) | MCI & ADRD | Cross-sectional | Approximately 4.5 months following disclosure | Marginal effects estimated through logistic and linear regression models | Patients on average were willing to accept a treatment that carried a 27.94% risk of death. Receiving a positive scan result was associated with an increased risk taking willingness to accept risky treatments. Scan recipients with a positive result were willing to accept 5.62 percentage points of a risk of death than those with a negative scan result. |
Note: SCD = subjective cognitive decline; MCI = Mild cognitive Impairment; ADRD = Alzheimer’s disease and related dementias; RCT = randomized controlled trial; PET = Positron Emission Tomography.
Five studies assessed the association between amyloid PET scan disclosure and depressive symptoms in persons with MCI (N = 2), MCI & ADRD (N = 1), and subjective cognitive decline (N = 2)20–23,25. These studies assessed depressive symptoms at a wide range of time points following the scan. The shortest timepoint was 14 days and the longest timepoint was 52 weeks. None of these studies detected any significant changes in depressive symptoms at any timepoint after the scan. Similarly, four studies assessed levels of anxiety at multiple timepoints ranging from 14 days to 52 weeks after the scan. None of these studies found significant changes in levels of anxiety after the scan at any timepoint20–22,25. However, one study found greater variation in anxious and depressive symptoms among persons with MCI and elevated amyloid compared to those without elevated amyloid in the 2 weeks following disclosure21.
The association between disclosure and test-related distress varied depending on the participant’s level of cognitive impairment and the scan result. Two studies of participants with subjective cognitive decline found there was no differences in test-related distress between those with elevated and non-elevated amyloid22,23. One study measured test-related distress 6 weeks after post-disclosure22 and the other measured distress 9 or 18 months post-disclosure23. However, a RCT comparing amyloid PET scan disclosure to psychoeducation in persons with MCI found those with elevated amyloid experienced greater levels of distress on two separate measures compared to those without elevated amyloid20. This study measured test-related distress 4, 24, and 52 weeks post-scan. Participants with elevated amyloid’s distress scores did decline over time, however they remained consistently higher than those without elevated amyloid.
One study assessed the impact disclosing scans as part of the diagnostic process on quality of life measures in persons with young onset AD24. They found a change in diagnosis was associated with improvement in Alzheimer’s related quality of life and increased clinician confidence following the scan was associated with improvement in a generic measure of quality of life. Although, the size of this effect may not constitute a clinically meaningful difference. Lingler et al20 also assessed the impact of amyloid scan disclosure on person’s with MCI’s self-efficacy for coping and found no significant difference in scores between those randomized to receive the scan and those randomized to receive psychoeducation.
Two studies assessed patient’s understanding of the scan result or diagnosis following disclosure. James et al18 found 83% of persons with MCI or ADRD correctly recalled their scan result 4.5 months after disclosure. Lingler et al20 found scan recipient’s knowledge of MCI/Alzheimer’s disease or perceived ambiguity of MCI did not change between baseline and 4, 24 or 52 weeks after disclosure. Furthermore, knowledge scores did not differ between those randomized to receive an amyloid scan and those who received psychoeducation. However, a subgroup analysis found that participants who received a result for non-elevated amyloid reported greater perceived ambiguity about MCI. The authors suggest that participants correctly understood that their symptoms remained unexplained.
Jutkowitz et al19 assessed the impact of amyloid PET scan disclosure on participant’s willingness to accept risky treatments to restore their memory. They found on average, participants were willing to accept a 27.94% risk of death to restore their memory to normal and participants with elevated amyloid were willing to accept more 5.6% risk than those without elevated amyloid.
The association between amyloid scan disclosure and caregiver outcomes
Five studies included in this review reported caregiver outcomes following the disclosure of an amyloid scan (Table 4).Three studies assessed the effect of disclosure on depressive and anxious symptoms among caregivers17,20,26. There were no significant differences in depressive symptoms17,20,24. Likewise, Bensaidane et al26 did not find an association between the scan result and caregiver anxiety, however the authors did not use a validated scale. Belanger and Lingler17,20 both assessed caregiver anxiety using some form of the Spielberger State Anxiety Inventory. Lingler et al found anxiety increased in the 4 and 24 weeks after disclosure but returned to baseline levels at week 52 for all caregivers3. They did not detect differences in levels of anxiety by scan result20. However, they noted that the increase in anxiety was below the cut off for clinical significance. On the other hand, Belanger et al stratified their analysis by the scan recipient’s level of impairment and found that elevated amyloid was associated with increased anxiety for caregivers to persons with MCI but not ADRD approximately 4.5 months following disclosure17.
Table 4.
Key findings of the impact of amyloid disclosure on caregiver outcomes
| Outcome | Measure | Study | Study design | Timing of outcome | Analysis | Key Findings |
|---|---|---|---|---|---|---|
| Depression | Patient Health Questionnaire (PHQ-2) | Belanger (2022) | Cross-sectional | Approximately 4.5 months following disclosure | Multivariate logistic regression | No significant difference in odds of depressive symptoms by scan result |
| Center for Epidemiological Studies Depression Scale (CESD) | Lingler (2020) | RCT | 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | No significant difference in depressive symptoms between caregivers to scan recipients and those who received psychoeducation. There was no significant difference in scores over time | |
| 4 questions developed by authors Scored on a likert scale | Bensaidane (2016) | Cross-sectional | At least 30 days after amyloid disclosure | Independent samples t-test | There was no significant difference between amyloid positive and amyloid negative groups | |
| Anxiety | Spielberger State Anxiety Inventory (STAI) | Lingler (2020) | RCT | 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | Caregivers’ anxiety levels increased from baseline at both 4 and 24 weeks of follow-up, returning to baseline at week 52. However, anxiety levels remained below the cut-off for clinical significance |
| Belanger (2022) | Cross-sectional | Approximately 4.5 months following disclosure | Multivariate logistic regression | Elevated amyloid PET scan results were associated with higher levels of anxiety among caregivers of persons with MCI, even after controlling for covariates | ||
| 4 questions developed by authors Scored on a likert scale | Bensaidane (2016) | Cross-sectional | At least 30 days after amyloid disclosure | Independent samples t-test | There was no significant difference between amyloid positive and amyloid negative groups | |
| Test related distress | Impact of Event Scale (IES) | Lingler (2020) | RCT | 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | No significant differences between caregivers to scan recipients and those who received psychoeducation. No significant differences in IES scores over time |
| Distress and Positive Impact subscales of the Impact of Genetic Testing - Alzheimer’s Disease (IGT-AD) | Lingler (2020) | RCT | 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | Caregivers of persons with elevated amyloid reacted more negatively to test results on the distress and positive subscales | |
| Quality of life | 3 questions developed by authors Scored on a likert scale | Bensaidane (2016) | Cross-sectional | At least 30 days after amyloid disclosure | Independent samples t-test | There was no significant difference between amyloid positive and amyloid negative groups |
| Self efficacy for coping | Self efficacy for coping scale (CSE) | Lingler (2020) | RCT | 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | Decreased self-efficacy for coping was most pronounced among caregivers of persons with elevated amyloid at 4 weeks post disclosure and those of amyloid negative patients at 24 weeks post disclosure |
| Understanding of scan result or diagnosis | Accurate reporting of scan result | James (2020) | Cross-sectional | Approximately 4.5 months following disclosure | Chi-squared test | 85% of care partners correctly reported the results of the amyloid PET scan; In 75% of dyads, both patient and care partner correctly reported results, while in 7% of dyads, both incorrectly reported results; The remaining 18% of dyads were discordant in their reporting of scan results. Caregivers to people with dementia were more likely to correctly recall the results than caregivers to people with MCI. |
| MCI/AD Knowledge Assessment | Lingler (2020) | RCT | 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | Objective knowledge of MCI/AD increased from baseline among caregivers to scan recipients and those who received psychoeducation. There was no significant difference between intervention groups | |
| Illness coherences subscale of the revised illness perception questionnaire (IPQ-R) | Lingler (2020) | RCT | 4, 24, and 52 weeks post-disclosure | Intention to treat using linear mixed modelling | There was no significant difference in perceived ambiguity of MCI between caregivers of scan recipients and those who received psychoeducation. There were no significant differences between caregivers to persons with positive and negatives scans | |
| 6 questions developed by authors Scored on a likert scale | Bensaidane (2016) | Cross-sectional | At least 30 days after amyloid disclosure | Independent samples t-test | There was no significant difference between amyloid positive and amyloid negative groups | |
| Expectations for future | 4 questions developed by authors Scored on a likert scale | Bensaidane (2016) | Cross-sectional | At least 30 days after amyloid disclosure | Independent samples t-test | There was no significant difference between amyloid positive and amyloid negative groups |
| Willingness to accept risky treatment | The following question: Suppose there is a new technology that can return your memory to normal but has a risk of death. What is the highest risk of death, if any, that you would be willing to accept for this treatment? The number you give can be anywhere between 0% and 100% | Jutkowitz (2020) | Cross-sectional | Approximately 4.5 months following disclosure | Marginal effects estimated through logistic and linear regression models | On average, care partners believed the patient would accept a treatment that carried a 29.68% risk of death; 24% of dyads agreed precisely on the amount risk that would be accepted, 35% of care partners underestimated and 41% of care partners overestimated the amount of risk the patient would accept by an average of 1.65 percentage points. |
Note: RCT = randomized controlled trial; PET = Positron Emission Tomography.
Bensaidane et al26 assessed the impact of amyloid PET scan disclosure on caregiver quality of life and found no significant difference between elevated and non-elevated groups. Lingler et al20 found caregivers to scan recipients experienced decreased self-efficacy for coping compared to those who received psychoeducation. For caregivers to persons with elevated amyloid this decline occurred at 4 weeks post disclosure, whereas the decline occurred at 24 weeks for caregivers to persons without elevated amyloid.
Three studies assess caregivers’ understanding of the diagnosis or scan result18,20,26. James et al18 found that 85% of caregivers correctly recalled the scan result 4.5 months after disclosure. Lingler et al20 found caregiver knowledge of MCI/Alzheimer’s disease improved for all caregivers between baseline and 52 week follow-up. However, there was no significant difference in knowledge scores between caregivers to persons who received an amyloid scan and those who received psychoeducation. Similarly, there was no difference in knowledge scores between caregivers to persons with elevated amyloid compared to non-elevated amyloid. Bensaidane et al26 found no significant difference in understanding of the scan result between caregivers to persons with elevated and non-elevated amyloid on an author-developed measure. Similarly, they found no significant difference in expectations for the future between caregivers to persons with elevated and non-elevated amyloid26.
Jutkowitz et al19 assessed caregiver perceptions of the scan recipient’s willingness to accept risky treatment to return their memory to normal. On average, caregivers believed scan recipients would accept at 29.68% risk of death. Approximately one quarter of caregivers were in exact agreement with the amount of risk the scan recipient was willing to accept. However, 35% underestimated and 41% over-estimated the amount of risk they were willing to accept.
Discussion
The aim of this review was to summarize the association between the disclosure of amyloid PET scan results and psychosocial and behavioral outcomes for persons with cognitive impairment and caregivers. We identified ten papers from seven studies. All but one of the included studies used observational designs and two thirds were conducted in the US. We found some evidence that the psychological impact of receiving an amyloid scan on psychological responses varied by the scan recipients’ level of impairment. Participants were able to correctly recall the scan result, however, it is not clear whether this is associated with increased knowledge of their diagnosis. We did not identify any studies reporting behavioral outcomes. The small number of papers included and the heterogeneity of study design, participant characteristics and outcomes limit the strength of the conclusions we can draw.
Amyloid scan disclosure had little association with measures of anxiety, depression and self-efficacy for coping for persons with cognitive impairment, which is consistent with findings from two systematic reviews of amyloid disclosure on cognitively healthy participants8,10. However, test-related distress may vary depending on the participant’s level of impairment and scan result. One study found persons with MCI and elevated amyloid reported greater levels of test-related distress on two separate measures (the Impact of Events Scale (IES) and Impact of Genetic Testing – Alzheimer’s Disease (IGT-AD)20. Similar effects were not found in those with subjective cognitive decline as measured by the IES22,23 however, neither of these studies stratified their analysis by the scan result and the timing of the assessments differed greatly. Therefore, it is not possible to be sure if the observed differences at due to the participant’s level of cognitive impairment or are a result of differing methodologies. A more recent study of persons with subjective cognitive decline, found a positive amyloid scan was associated with increased test-related distress27. Similarly, two studies in this review found caregivers to persons with elevated amyloid and MCI may experience increased anxiety post-disclosure17,20. The observed increases in distress among persons with MCI and elevated amyloid and their caregivers may be due to the implication of the scan result. A scan showing elevated amyloid among people with MCI indicates a strong risk of developing Alzheimer’s disease in the future. Persons with MCI and elevated amyloid and their caregivers may require additional post-diagnostic support following the disclosure of an amyloid PET scan.
Most participants with MCI, ADRD and their caregivers correctly recalled the scan result18. Participants included in this review were highly educated, however, James et al18 found neither scan recipient nor caregiver levels of education affected the likelihood of correctly recalling the scan result. It is unclear what impact accurate recall has on participants’ understanding of their diagnosis. Lingler et al20 found generally scan recipients’ knowledge of their condition did not change after disclosure and did not differ significantly from the control group who received psychoeducation for MCI, although a subgroup analysis found persons with MCI and elevated amyloid may correctly understand their symptoms remain unexplained. Furthermore, caregiver’s knowledge of the condition did improve but a similar improvement was also observed in participants randomized to receive psychoeducation20. Findings from the analysis of qualitative data indicate scan recipients and caregivers can be confused by the language used when disclosing the scan result18. The included studies reported using a variety of protocols for disclosing the amyloid PET scan results. In most cases, the results were disclosed by a member of the study team, which may not reflect clinical practice. Future research should explore how the delivery of amyloid scan results influences the patient and caregiver’s understanding of the diagnosis.
Ethical debates regarding the use of amyloid PET scan during the dementia diagnosis process focus on the potential benefits and the risk of harm. The findings of this review indicate there may be a small risk of psychological harm among persons with MCI and elevated amyloid and their caregivers. However, this relationship is not conclusive. Similarly, there is no clear benefit of amyloid PET scan disclosure in terms of enhancing persons with cognitive impairments and caregivers understanding of the diagnosis. Despite concerns held by clinicians and researchers, people with cognitive impairment may wish to know their amyloid status. Future research should explore how to lessen the impact of potential “bad news” when disclosing amyloid PET scan results, especially in the case of elevated amyloid among persons with MCI or subjective cognitive decline where there are no disease modifying treatments available. Researchers have proposed this could be done through pre-disclosure counselling and by providing follow-up support post-disclosure28.
Strengths and limitations
This review draws together the evidence on the impact of amyloid disclosure on persons with cognitive impairment and caregivers and indicates a nuanced and mixed experience of amyloid disclosure by the scan recipients’ level of impairment and scan result. Patient and caregivers experiences of amyloid PET scan disclosure is a growing area of research - four new studies have been published on this topic since running our search27,29–31. We used broad search terms and did not include search terms for outcomes to capture as many studies in this area as possible. We identified a small number of studies, which varied greatly in terms of participants characteristics, sample size, study design, and outcomes, limiting the strength of the evidence on this topic. Only three studies included participants with ADRD, two of which also included participants with MCI. It is possible that psychosocial and behavioral responses may differ between those with MCI and ADRD due to the differing implications of the scan result. A scan result indicating elevated amyloid can be used to make a differential diagnosis for persons with ADRD but can only be used to determine the risk of developing Alzheimer’s disease in the future for persons with MCI. This is an important area for further investigation. Although we aimed to explore the association between receiving an amyloid scan on psychosocial and behavioral outcomes, most studies compared associations between the scan result and outcomes, making it difficult to determine if the observed effects are from getting the scan or from the recipient’s amyloid status. Additionally, we did not identify any behavioral outcomes. Therefore, we cannot determine whether an amyloid scan leads to changes in behaviors for persons with cognitive impairment or their care partners. Future research should examine if receiving an amyloid scan is associated with changes to lifestyle, employment, health service utilization or advanced care planning. Furthermore, only one study included in this review used a comparison group meaning changes in outcomes cannot be attributed to the scan alone. Finally, only two out of seven studies reported the race/ethnicity of their participants, who were predominantly white, limiting the generalizability of the findings from this review. Future research should be methodologically rigorous, with a comparison group and ethno-culturally diverse samples.
Conclusions
It is becoming increasingly important to understand the impact of amyloid PET scan disclosure on persons with cognitive impairment and caregivers. In this review, we identified a small number of studies assessing the psychosocial and behavioral impact of amyloid disclosure. The scan recipient’s level of distress and caregiver’s levels of anxiety post-disclosure may vary depending on the scan recipient’s level of cognitive impairment and the scan result. Scan recipients and caregivers could correctly recall the scan result, but it is not clear this is associated with a better understanding of their diagnosis. More rigorous research with diverse samples of cognitively impaired participants and their caregivers is needed before firm conclusions can be drawn regarding the potential harms and benefits of disclosing amyloid PET scan results.
Supplementary Material
Acknowledgements:
We thank Dr Emmanuelle Belanger for providing feedback on a draft of this manuscript.
Funding:
EC was supported by an AHRQ National Research Service Award T32 (Grant 5T32 HS000011-37. MTA was supported by the National Institutes of Health’s National Institute on Aging Ruth L. Kirschstein Postdoctoral Individual National Research Service Award F32 (Grant F32AG072730-01).
Footnotes
Supplemental Content
eMethods 1. PRISMA Checklist
eMethods 2. Full search strategy
eTable 1. JBI Critical appraisal of randomized controlled trials
eTable 2. JBI Critical appraisal of pre/post studies
eTable 3. JBI Critical appraisal of cross-sectional studies
Conflicts of interest: None to declare
References
- 1.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 3.Lingler JH, Butters MA, Gentry AL, et al. Development of a Standardized Approach to Disclosing Amyloid Imaging Research Results in Mild Cognitive Impairment. J Alzheimers Dis. 2016;52(1):17–24. doi: 10.3233/JAD-150985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinovici GD, Gatsonis C, Apgar C, et al. Association of Amyloid Positron Emission Tomography With Subsequent Change in Clinical Management Among Medicare Beneficiaries With Mild Cognitive Impairment or Dementia. JAMA. 2019;321(13):1286–1294. doi: 10.1001/jama.2019.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924–1938. doi: 10.1001/jama.2015.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the Clinical Diagnosis of Alzheimer Disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71(4):266–273. doi: 10.1097/NEN.0b013e31824b211b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Medicare & Medicaid Services. Final Decision Memorandum for: CAG-00431N Beta Amyloid Positron Emission Tomography in Dementia and Neurodegenerative Disease. Published online September 27, 2013. Accessed December 16, 2022. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=265
- 8.de Wilde A, van Buchem MM, Otten RHJ, et al. Disclosure of amyloid positron emission tomography results to individuals without dementia: a systematic review. Alzheimers Res Ther. 2018;10(1):72. doi: 10.1186/s13195-018-0398-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leuzy A, Zimmer ER, Heurling K, Rosa-Neto P, Gauthier S. Use of amyloid PET across the spectrum of Alzheimer’s disease: clinical utility and associated ethical issues. Amyloid. 2014;21(3):143–148. doi: 10.3109/13506129.2014.926267 [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Lingler JH. Disclosure of amyloid PET scan results: A systematic review. Prog Mol Biol Transl Sci. 2019;165:401–414. doi: 10.1016/bs.pmbts.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 11.Erickson CM, Clark LR, Ketchum FB, Chin NA, Gleason CE, Largent EA. Implications of preclinical Alzheimer’s disease biomarker disclosure for US policy and society. Alzheimers Dement. 2022;14(1):e12339. doi: 10.1002/dad2.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tetzlaff J, Altman DG, Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. Published online 2009. https://www.acpjournals.org/doi/abs/10.7326/0003-4819-151-4-200908180-00135 [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu P-F. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E M Z, ed. JBI Manual for Evidence Synthesis. JBI; 2020. [Google Scholar]
- 15.Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In: Aromataris E M Z, ed. JBI Manual for Evidence Synthesis. JBI; 2020. [Google Scholar]
- 16.Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version. 2006;1(1):b92. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=ed8b23836338f6fdea0cc55e161b0fc5805f9e27 [Google Scholar]
- 17.Belanger E, D’Silva J, Carroll MS, et al. Reactions to Amyloid PET Scan Results and Levels of Anxious and Depressive Symptoms: CARE IDEAS Study. Gerontologist. 2022;((Belanger, D’Silva, Carroll, Wetle) Center for Gerontology and Healthcare Research, Brown University, School of Public Health, Providence, RI, USA(Belanger, Wetle) Department of Health Services, Policy&Practice, Brown University, School of Public Health). doi: 10.1093/geront/gnac051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James HJ, Van Houtven CH, Lippmann S, et al. How Accurately Do Patients and Their Care Partners Report Results of Amyloid-beta PET Scans for Alzheimer’s Disease Assessment? J Alzheimers Dis. 2020;74(2):625–636. doi: 10.3233/JAD-190922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jutkowitz E, Van Houtven CH, Plassman BL, Mor V Willingness to Undergo a Risky Treatment to Improve Cognition among Persons with Cognitive Impairment Who Received an Amyloid PET Scan. Alzheimer Dis Assoc Disord. 2020;34(1):1–9. doi: 10.1097/WAD.0000000000000338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lingler JH, Sereika SM, Butters MA, et al. A randomized controlled trial of amyloid positron emission tomography results disclosure in mild cognitive impairment. Alzheimer’s and Dementia. 2020;16(9):1330–1337. doi: 10.1002/alz.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattos MK, Sereika SM, Beach SR, et al. Research Use of Ecological Momentary Assessment for Adverse Event Monitoring Following Amyloid-β Results Disclosure. J Alzheimers Dis. 2019;71(4):1071–1079. doi: 10.3233/JAD-190091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wake T, Tabuchi H, Funaki K, et al. The psychological impact of disclosing amyloid status to Japanese elderly: A preliminary study on asymptomatic patients with subjective cognitive decline. Int Psychogeriatr. 2018;30(5):635–639. doi: 10.1017/S1041610217002204 [DOI] [PubMed] [Google Scholar]
- 23.Lim YY, Maruff P, Getter C, Snyder PJ Disclosure of positron emission tomography amyloid imaging results: A preliminary study of safety and tolerability. Alzheimer’s and Dementia. 2016;12(4):454–458. doi: 10.1016/j.jalz.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 24.Van Der Doelen DM, Handels RLH, Zwan MD, et al. The Impact of Amyloid PET Disclosure on Quality of Life in Patients with Young Onset Dementia. Alzheimer Dis Assoc Disord. 2022;36(1):1–6. doi: 10.1097/WAD.0000000000000470 [DOI] [PubMed] [Google Scholar]
- 25.Taswell C, Donohue CL, Mastwyk M, et al. Safety of Disclosing Amyloid Imaging Results to MCI and AD Patients. Am J Geriatr Psychiatry. 2017;25(3):S129. doi: 10.1016/j.jagp.2017.01.147 [DOI] [Google Scholar]
- 26.Bensaidane MR, Beauregard J-M, Poulin S, et al. Clinical Utility of Amyloid PET Imaging in the Differential Diagnosis of Atypical Dementias and Its Impact on Caregivers. J Alzheimers Dis. 2016;52(4):1251–1262. doi: 10.3233/JAD-151180 [DOI] [PubMed] [Google Scholar]
- 27.Caprioglio C, Ribaldi F, Visser LNC, et al. Analysis of Psychological Symptoms Following Disclosure of Amyloid–Positron Emission Tomography Imaging Results to Adults With Subjective Cognitive Decline. JAMA Netw Open. 2023;6(1):e2250921–e2250921. doi: 10.1001/jamanetworkopen.2022.50921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Largent EA, Grill J, O’Brien K, Wolk D, Harkins K, Karlawish J. Testing for Alzheimer Disease Biomarkers and Disclosing Results Across the Disease Continuum. Neurology. Published online January 31, 2023. doi: 10.1212/WNL.0000000000206891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bélanger E, Couch E, Carroll MS, et al. Advance directives among cognitively impaired persons who had an amyloid PET scan and their care partners: a mixed-methods study. BMC Palliat Care. 2022;21(1):194. doi: 10.1186/s12904-022-01082-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepherd-Banigan M, Ford CB, DePasquale N, et al. Making the Informal Formal: Discussing and Completing Advance Care Plans in Care Dyads with Cognitive Impairment. J Palliat Care. 2022;37(3):289–297. doi: 10.1177/08258597211063047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Couch E, Belanger E, Gadbois EA, DePasquale N, Zhang W, Wetle T. “I know that my role is going to change”: a mixed-methods study of the relationship between amyloid-β PET scan results and caregiver burden. Aging Clin Exp Res. Published online December 9, 2022:1–11. doi: 10.1007/s40520-022-02314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


