Abstract
Polyunsaturated fatty acids (PUFAs) are essential fatty acids required for human health and are obtained primarily from food or synthesized in the body by highly regulated processes. The metabolites of these lipids, formed largely through the action of cyclooxygenase, lipoxygenase, or cytochrome P450 (CYP450) enzymes, are responsible for multiple biological functions including inflammation, tissue repair, cell proliferation, blood vessel permeability, and immune cell behavior. The role of these regulatory lipids in disease has been well studied since their discovery as druggable targets; however, the metabolites generated downstream of these pathways have only recently gained attention for regulating biology. Specifically, the biological activity of lipid vicinal diols formed from the metabolism of CYP450-generated epoxy fatty acids (EpFA) by epoxide hydrolases were previously thought to have little biological activity but increasingly are recognized as promoting inflammation and brown fat adipogenesis, and exciting neurons through the regulation of ion channel activity at low concentrations. These metabolites also appear to balance the action of the EpFA precursor. For example, EpFA demonstrate the ability to resolve inflammation and reduce pain, while some lipid diols, through opposing mechanisms, promote inflammation and pain. This review describes recent studies that highlight the role of regulatory lipids, focusing on the balance between EpFA and their diol metabolites in promoting or resolving disease.
Keywords: Eicosanoids, soluble epoxide hydrolase, regulatory lipids, epoxy fatty acids, vicinal diols
1. Introduction
“Regulatory lipids”, also described as oxylipins, comprise a class of omega-3 (n-3) and −6 (n-6) polyunsaturated fatty acids (PUFA) metabolites that are responsible for important biological functions, such as inflammation, tissue repair, cell proliferation, blood vessel permeability and immune cell behavior, among others. PUFA are essential fatty acids required for human health and are obtained primarily from food, but some are synthesized in the body by highly regulated processes. They are named by the number of carbon atoms and double bonds, with the most abundant lipids being linoleic acid (LA), an n-6 fatty acid with 18 carbons and two double bonds (LA, 18:2, n-6), alpha-linolenic acid (ALA, 18:3, n-3), arachidonic acid (AA, 20:4, n-6), eicosapentaenoic acid (EPA, 20:5, n-3), and docosahexaenoic acid (DHA, 22:6, n-3). PUFA are stored in cellular membranes and released in response to cellular signals, such as hormone levels, injury, or stress, and metabolized by the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP450) enzymes to form a cascade of regulatory lipids, also called oxylipins. This cascade has been widely described and published for ARA. Here, the formation of regulatory lipids from LA by these pathways is outlined in Figure 1. The metabolism of the other PUFAs undergo similar mechanisms, and their nomenclature is outlined in Table 1.
Figure 1. Regulatory lipid cascade, metabolism of linoleic acid.
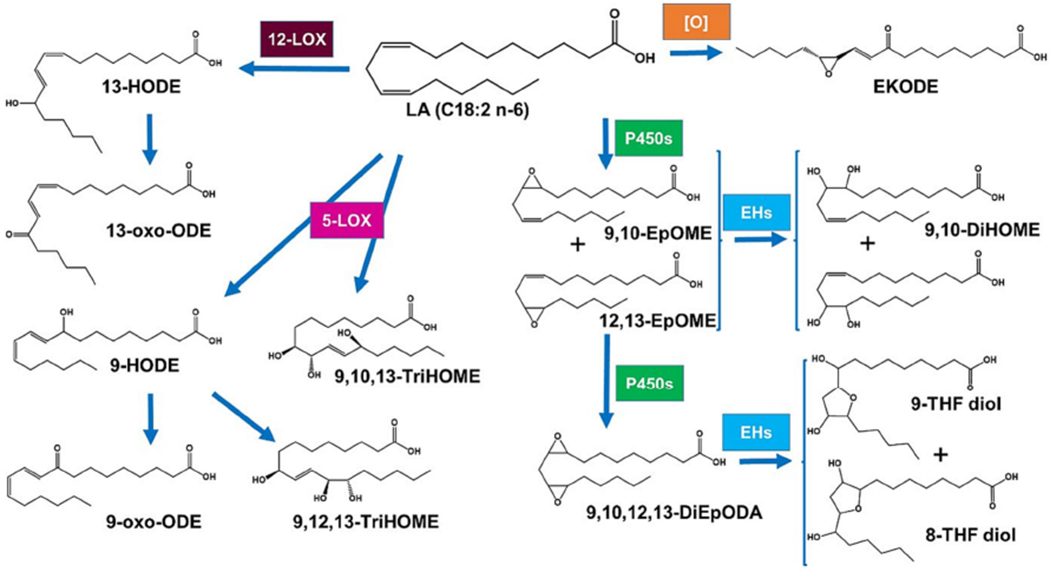
Like other PUFA, LA is metabolized to form a multitude of regulatory lipids; however, it is not oxidized by COX. From non-enzymatic oxidation, the pro-inflammatory EKODE can be formed. From the action of LOXs, a series of hydroxylated compounds are synthesized (left side of the figure), which have diverse biological activities(Szczuko, et al., 2020). The action of P450s results notably in the formation of monoepoxides (EpOMEs) that are further metabolized by sEH to vicinal diols (DiHOMEs), whose biological actions are detailed herein, or a diepoxide (DiEpODA), which leads to the formation of THF diols (right side of the figure). These latter compounds were shown to affect behavior and brain function in mice. (Villalon Landeros, et al., 2012)
Table 1.
Naming convention for EpFA, vicinal diols and their originating PUFA(Fahy, et al., 2009).
| PUFA | EpFA | Vicinal Diol |
|---|---|---|
| LA (18:2, n-6) | 9(10)- and 12(13)-EpOME, or leukotoxin and isoleukotoxin | 9,10- and 12,13- DiHOME, or leukotoxin diol and iso-leukotoxin diol, or batokine |
| ALA (18:3, n-3) | 9(10)-, 12(13)- and 15(16)-EpODE | 9,10-, 12,13- and 15,16-DiHODE |
| AA (20:4, n-6) | 5(6)-, 8(9)-, 11(12)- and 14(15)-EpETrE or x(y)-EET | 5,6-, 8,9-, 11,12- and 14,15-DiHETrE |
| EPA (20:5, n-3) | 8(9)-, 11(12)-, 14(15)- and 17(18)-EpETE or x(y)-EEQ | 8,9-, 11,12-, 14,15-, 17,18-DiHETE |
| DHA (22:6, n-3) | 4,5- 7(8)-, 10(11)-, 13(14)-, 16(17)- and 19(20)-EpDPE Or x(y)-EDP |
4,5- 7,8-, 10,11-, 13,14-, 16,17- and 19,20-DiHDPE |
The role of regulatory lipids in disease has been well studied since their discovery as druggable targets. Metabolism by the COX enzyme forms prostanoids, precursors to prostaglandins that are formed through prostaglandin synthase enzymes. In this pathway, most drugs focus on inhibiting COX enzymes to prevent the formation of pro-inflammatory prostaglandins. By mass these drugs remain the most widely used worldwide. The LOX pathway also generates inflammatory compounds, such as leukotrienes (LTs). Historically, this was the second regulatory lipid pathway to be therapeutically targeted. For example, ALOX5-inhibitors and LT-receptor antagonists have been developed for the treatment of asthma and seasonal allergies. Interestingly, they have recently become a target for liver disease (Liangpunsakul & Chalasani, 2019). The third pathway, oxidation by CYP450, forms both hydroxy and epoxy lipid metabolites, and this pathway is gaining attention as a potential therapeutic target. The epoxy fatty acids (EpFA) are unique among the regulatory lipids in that they are associated with beneficial properties, such as the ability to resolve inflammation as well tireduce endothelial dysfunction and endoplasmic reticulum (ER) stress. Unlike epoxides of some compounds, most epoxy fatty acids are chemically quite stable. Their low chemical reactivity and lack of chemical affinity for nucleic acids leads to low risk of forming mutagenic nucleic acid adducts or even protein adducts. Also the fatty acid backbone has little affinity for nucleic acids. The beneficial activity of EpFA is reduced by their rapid degradation by the soluble epoxide hydrolase (sEH) into corresponding and far more polar vicinal diols. These are an interesting group of chemical mediators where their concentration is modulated by release from membrane storage as well as reincorporation (Spector, 2009), changes in biosynthesis and also dramatic changes in rates of hydrolysis (C. McReynolds, Morisseau, Wagner, & Hammock, 2020).
EpFA can be formed from CYP450 oxidation at each double bond of PUFAs, and subsequent hydrolysis forms the corresponding vicinal diols (Table 1). Although other hydrolases can form diols from EpFA, sEH is the most efficient enzyme responsible hydrolysis for all but 5(6) EpETrE which is a poor substrate for sEH and is more efficiently metabolized by COX (Spector & Norris, 2007) (Morisseau, et al., 2021). In most tissues the sEH has a higher concentration and higher specific activity than the microsomal epoxide hydrolase (mEH), but there are situations where the mEH play an important or even the major role in hydrolysis (Edin, et al., 2018; Morisseau, et al., 2021). Considering the numerous EpFA and diol chain length, regioisomers, optical isomers and metabolites formed from the various PUFA and their double bonds, published literature needs to be carefully reviewed to determine if biological activity assigned to compounds represent a class or are result of being the only oxylipin included in the analysis. To further complicate data interpretation, often analytical methods do not monitor all the EpFA or diols formed, nor do they quantify other oxylipins. Lack of quantification becomes increasingly important if select compounds have concentration dependent different actions, such as dihydroxyoctadecenoic acids (DiHOMEs), the diols derived from linoleic acid. The field is further complicated by an often high background of other lipids in matrices analyzed, instability of the analytes, as well as a high cost and complexity of analytical tools used.
DiHOMEs have beneficial metabolic and cardioprotective effects at lower concentrations by stimulating brown fat adipogenesis (Macêdo, Muñoz, Cintra, & Pauli, 2022); however, at higher concentrations, these compounds activate neutrophils and promote a hyperinflammatory response (Bergmann, et al., 2022), as well as disrupt endocrine function in rats (Markaverich, et al., 2007). Interestingly, the biological activity of other lipid diols has not been well characterized although biological roles in pain and inflammation have been described for the arachidonic acid formed DiHETrEs by impairing neutrophil response (Bergmann, et al., 2021) and blocking potassium channel activation (Nandkishor Kisanrao Mule, 2018). As bioactive lipid metabolism is being recognized as an important process in health and disease, more studies are incorporating this analysis in their research platforms; thus, standardization of methods and reporting standards are important in interpreting study results. To help address these inconsistencies in future studies, there are current efforts to unify both naming conventions and rigor in both analytical methods and reporting (McDonald, et al., 2022). Despite this, there is an abundance of literature identifying that an increase in lipid diols is correlated with disease. Until recently, the lipid diols were considered biologically inactive and probably short lived (Spector & Norris, 2007). Vicinal diols are surprisingly polar, are targets for conjugation reactions, and even without conjugation tend to move out of cells while EpfA concentrate in cells. However, recent studies provide evidence that lipid diols promote inflammation, pain and endothelial dysfunction through opposing actions of the EpFA.
It is critical for survival that animals can turn on inflammation pathways quickly to counter infections and other maladies. However, these same inflammatory pathways and associated cell death pathways can lead to severe damage to the organism or death. Thus, it is not a surprise that we have systems to balance acute inflammation involving an active resolution of inflammation. It appears that long chain EpFA are involved in the resolution of inflammation acting on the ER stress response and in part through other downstream inflammation resolving lipids (Abdalla 1369). For example, the monoepoxides from AA (EET) and omega-3 lipids (EDP and EEQ)s ca help to resolve the inflammatory response. However, the average western diet is now exceptionally high in linoleate. This omega −6 octadecanoid fatty acid is in a way a biproduct of the corn industry that has been driven by economics into most cooking oils and a variety of processed food. This high body burden of linoleate coupled with the decrease in long chain omega 3 fatty acids whose epoxides are particularly potent at resolving inflammation can lead to production of high levels of linoleate epoxides, which unlike the pro-resolving EETs, EDPs, and EEQs, can be highly inflammatory and cytotoxic as their diols. Thus, western society’s dramatic shift toward linoleate as a dietary component which normally is tolerated as an energy source, becomes the most abundant substrate for epoxidation leading to enhancement of ER stress, inflammation, cell death and other biological effects at a time when our bodies need to actively resolve inflammation. This review describes recent studies that highlight the balance between the epoxy fatty acids and their sEH formed diol metabolites in promoting disease.
2. Regulatory role in inflammation
The beneficial effects of EpFAs have been demonstrated in numerous studies, and their anti-inflammatory action attributed primarily to inhibiting the nuclear translocation of NF-κB (Node, et al., 1999) (Xu, et al., 2006). The epoxides of linoleic acid (EpOMEs) appeared to be an exception; they were originally described as ‘leukotoxins’ because of their cytotoxic effects on leukocytes (Hu, et al., 1988). In several case studies, the risk of mortality in burn patients was correlated with increased leukotoxin; however, the corresponding diols were not measured in these studies because of an early bias that assumed the more water-soluble diol metabolites lacked biological activity due to rapid (Spector & Kim, 2015) and the common assumption that all epoxides are chemically reactive as electrophiles. Moghaddam et al. were the first to demonstrate a biological role of LA-diols (DiHOMEs) by demonstrating that the cytotoxic effect of ‘leukotoxin’ was eliminated when the sEH was genetically absent or inhibited (Moghaddam, et al., 1997). These findings established that DiHOMEs, and not their parent epoxides, called ‘leukotoxin’, are the actual major cause for the cytotoxic effect. Further studies determined that lipid diols were pro-inflammatory lipid mediators responsible for monocyte chemotaxis (Kundu, et al., 2013). Thus, the metabolism of regulatory lipids can either resolve inflammation through regulation of NF-κA by EpFA, or promote chronic inflammation through the formation of lipid diols that are required for chemotaxis of leukocytes (Bergmann, et al., 2021) (Kundu, et al., 2013) (Figure 2).
Figure 2. Mechanism for regulatory lipids controlling the immune response.
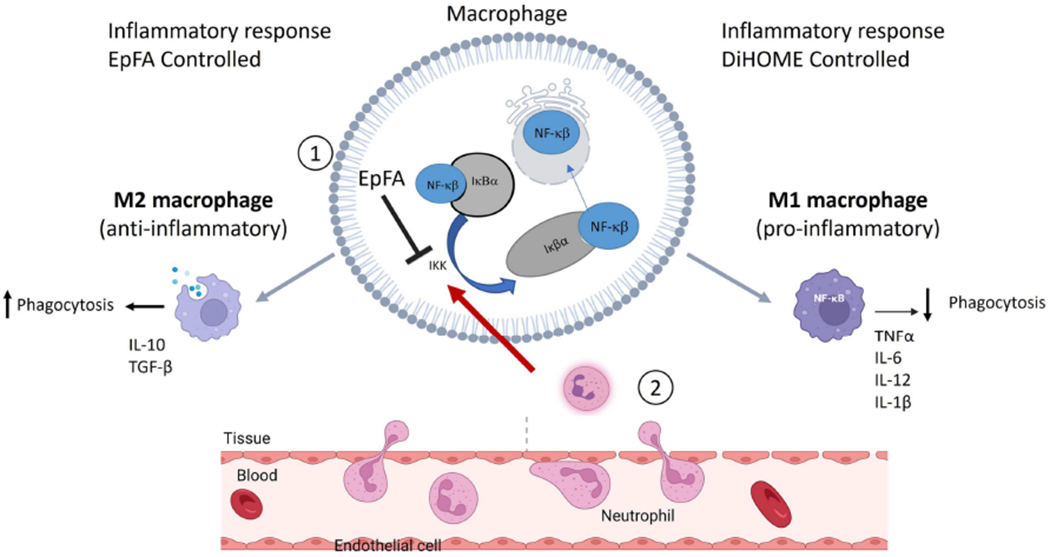
Background: NFκβ is a transcription factor that is regulated by binding to the cellular protein I-kappa-beta-alpha (Iκβα). Binding to Iκβα prevents translocation of NFκβ into the nucleus. Inflammatory cytokines activate IκB kinase (IKK) to degrade Iκβα and release NFκβ, allowing for nuclear translocation and further initiation of inflammatory cytokines (Castro-Alcaraz, Miskolci, Kalasapudi, Davidson, & Vancurova, 2002). Neutrophils are one of the first leukocytes to migrate to the inflammatory site and activate IKK to initiate an inflammatory cascade. These actions result in an inflammatory phenotype (M1 polarization of macrophages). In contract, suppression of NFκβ leads to an anti-inflammatory phenotype (M2 polarization of macrophages). Effects of regulatory lipids on inflammation: 1. EpFA (e.g. EpETrE formed from AA) blocks inflammatory signaling by inhibiting IKK to prevent subsequent nuclear translocation of NFκβ. EpFA preserve M2 polarization and resolution of inflammation, phagocytosis and anti-inflammatory cytokines. (Dai, et al., 2015) {Abdalla, 2023 #3055}. In contrast, DiHOMEs activate neutrophils, signal IKK to degrade Iκβα and allow for NFκβ to initiate an inflammatory cascade. Continued activation by neutrophils induce M1 polarization of macrophages, reduce phagocytosis and promote chronic, dysregulated inflammation. (Lin, et al., 2022). Furthermore, continued signaling from neutrophils will continue the inflammation resulting in inflamed endothelial cells, and disruption of vascular integrity (Martin-Fernandez, et al., 2021). Image adapted from BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates
The immunological role of DiHOMEs was not followed in great detail until the 2020 COVID-19 pandemic. To gain a better understanding of the pandemic, the research community deployed multiple metabolomic analyses to understand the pathophysiology of COVID-19, including plasma oxylipin analysis. In multiple studies, the bioactive LA metabolites showed significant correlations with worsening COVID-19 outcomes. The first of these studies was conducted in a small subset of hospitalized COVID-19 patients in early 2020. This first study analyzed only 6-subjects; however, the increase in LA-derived diols was significantly higher in severe COVID-19 patients compared to healthy controls (C. McReynolds, et al., 2021). Further studies with larger cohorts of patients confirmed the correlation of DiHOMEs with severe COVID-19 (Ripon, Bhowmik, Amin, & Hossain, 2021). In a follow-up study designed to investigate oxylipin profiles in ICU-admitted COVID-19 patients (n=7) compared to hospitalized COVID-19 patients not admitted to the ICU (n=37) in early 2020, investigators confirmed that several inflammatory oxylipins, including DiHOMEs, were upregulated in COVID-19 patients admitted to the ICU compared to COVID-19 patients hospitalized but not in the ICU. The increase in DiHOMEs also correlated with increased neutrophils and inflammatory cytokines (Karu, et al., 2022).
Inhibition of the sEH has been explored as a therapeutic strategy based on the hypothesis that resulting increased in the concentrations of biological active EpFA will be beneficial in dysregulated inflammatory conditions. However, analysis of COVID-19 data suggests that the potentially deleterious effects of DiHOMEs may have a more important role in the pathophysiology of pathological inflammatory states. Thus, reducing these diols through sEH inhibition may be more important in that case than the resulting increases in their corresponding epoxides. To better understand the pathophysiologic role of diols, a mouse burn model was utilized to correlate oxylipin profiles with immunological function. Two separate studies confirmed that lipid diols have biological activity promoting chronic inflammation. The first study (Bergmann, et al., 2021) identified that the AA-derived DiHETrE increased in tissue surrounding the wound, and the second study recognized that plasma levels of LA-derived DiHOMEs were increased 6-hours post burn injury (Bergmann, et al., 2022). Mouse splenocytes and bone-marrow incubated with plasma samples from burn injured mice, which have increased DiHOME concentrations, resulted in impaired neutrophil function, reduced capacity for phagocytosis, and failed macrophage activation. These changes are hypothesized to result in a fatigued immune system that is unable to respond to additional challenges and could explain increased mortality in burn patients with secondary sepsis, or the incidence of secondary infections in COVID-19 patients.
The hypothesis that DiHOMEs drive a prolonged neutrophil response that fatigues the immune system and results in failed resolution was indirectly tested in a clinical trial of n-3 lipid supplementation in hospitalized COVID-19 patients (Arnardottir, et al., 2022). Hospitalized COVID-19 patients were supplemented intravenously with either a high n-3 PUFA emulsion containing fish oil or saline for 5-days. Patients supplemented with fish oil had significantly less DiHOMEs compared to the saline treated group. In addition, the decreased DiHOMEs correlated with increased phagocytosis and decreased neutrophil to lymphocyte ratio, essentially resolving the immune dysfunctions observed with DiHOMEs, such as decreased phagocytosis and increased neutrophil to lymphocyte ratio (Bergmann, et al., 2022).
While the role of lipid diols in driving dysregulation of the immune system is being investigated more intensely, there are still many knowledge gaps in this area of research as it pertains to conditions with acutely dysregulated inflammatory responses, such as sepsis. In fact, only one study had included DiHOMEs analysis in patients with sepsis (Hamaguchi, et al., 2019) prior to the COVID pandemic. In this small study, 5 septic patients were enrolled for oxylipin analysis. There was a single fatality due to septic shock. This patient had 100-200-fold increases in 12,13- and 9,10-DiHOMEs, respectively, on the first day admitted to ICU compared to the patients that survived. Although this sample size is very small and the only known study analyzing lipid vicinal diols in sepsis, these data support future efforts in exploring if the previous hypothesis resulting from the rodent study that suggests DiHOMEs impair neutrophil activation and prevent monocyte phagocytosis, resulting in fatigue of the immune system, or immunoparesis, and eventually, increased risk of death in sepsis can translate to human patients. In fact, immunoparesis could be considered a failure to actively resolve inflammation (Serhan & Savill, 2005), further supporting a role for bioactive EpFA and diols in septic shock.
More recent studies are being conducted to analyze DiHOME fatty acids as they relate to the pathophysiology of severe sepsis. In pet dogs with sepsis, plasma lipidomic analyses identified 12,13-DiHOME as one of the top 3 metabolites that predicted severity of disease at an early stage, based on its increase compared to healthy dogs (Montague, et al., 2022). In contrast, a human study with analysis of global lipidomics in early sepsis determined that plasma oxylipins (including DiHOMEs) failed to discriminate which septic patients would develop acute respiratory distress syndrome (ARDS). Notably, the authors determined that severity of disease and increased risk of mortality at an early stage, independent of ARDS, correlate with plasma oxylipins (Rogers, et al., 2021).
3. Regulatory role of vicinal diols in pain.
Following multiple rodent studies (Inceoglu, et al., 2006) (Rose, et al., 2010) (K. Wagner, Inceoglu, Gill, & Hammock, 2011), interest in sEH inhibitors as drug development candidates for their potential as a novel therapeutic class of safe, effective, and non-addictive analgesics after Guedes et al. (2017) showed that sEH activity levels positively correlated with the severity of painful laminitis in horses. Treatment of these refractory cases with a sEH inhibitor, t-TUCB (A. Guedes, et al., 2017; A. G. Guedes, et al., 2013), significantly reduced pain and even cured this inflammatory condition in some cases. Since this initial success, multiple additional studies have reported pain relief in rodents (Y. Wang, Wagner, Morisseau, & Hammock, 2021), dogs (C. B. McReynolds, et al., 2019), cats (C. B. McReynolds, et al., 2021) and additional painful conditions in horses (A. Guedes, et al., 2017) (Tucker, et al., 2021) (A. G. P. Guedes, et al., 2018). Importantly, an sEH inhibitor is currently being developed for treatment of neuropathic and inflammatory pain, and is already undergoing human clinical trials (Hammock, et al., 2021).
In humans, perturbations in the sEH pathway are observed in osteoarthritis (OA) (Phillips, 2022). Valdes et al. identified increased AA-derived diols, 14,15-DiHETrE, 11,12-DiHETrE and 8,9-DiHETrE, in the synovial fluid of unilateral OA knee compared to the normal knee in the same individual after adjusting for age, sex, BMI and use of NSAIDs. These oxylipins were also associated with increased risk of tibiofemoral OA progression. In plasma, 8,9-DiHETrE was the only oxylipin significantly associated with OA (Gowler, et al., 2021; Valdes, et al., 2018). Multiple publications have reported protection of chondrocytes with sEH inhibition or directly by adding EpETrEs in cell models after an inflammatory challenge that mimics OA. (C. B. McReynolds, et al., 2019; Walters, Trumble, Wendt-Hornickle, Kennedy, & Guedes, 2022). Because chondrocyte loss is a hallmark of OA progression (Hwang & Kim, 2015), one proposed mechanism for the negative association of sEH activity in OA could be related to a failure to protect chondrocytes from inflammation-induced cytotoxicity. In the first study, McReynolds et al. prevented cytotoxicity by treating in vitro a canine chondrocyte cell line with a mixture of EpETrEs before or after IL1β challenge. Meloxicam (a COX-2 inhibitor with anti-inflammatory and analgesic properties) was added as a positive control and only prevented cytotoxicity if added prior to IL1β exposure (C. B. McReynolds, et al., 2019). In the second study, Walters et al. investigated the cytotoxic protection of an sEH inhibitor, t-TUCB, on primary horse chondrocytes challenged with an ER-stress inducing compound, tunicamycin. In this study, a COX inhibitor, phenylbutazone, and a selective COX-2 inhibitor, firocoxib, both significantly increased apoptosis associated with tunicamycin exposure, as would be expected because COX-inhibition increases ER-stress and apoptosis (Tsutsumi, et al., 2004). Interestingly, coadministration of these drugs with t-TUCB prevented apoptosis presumably by decreasing ER-stress (Walters, et al., 2022).
The mechanisms underlying the analgesic effects of sEH inhibition involve both its anti-inflammatory activity as well as the ability to reduce ER-stress. Epoxy fatty acids have anti-inflammatory properties that promote the resolution of inflammation and reduce inflammatory pain. However, inhibiting sEH also decreases PGE2 concentrations by reducing prostaglandin synthase, COX-2 and even prostaglandin receptor levels, which provides further anti-inflammatory benefits and also reduces ER-stress. Chopra et al. (2019) determined that ER-stress is responsible for upregulating COX-2 and mPGES-1 to promote the synthesis of PGE2, which drives the inflammatory and painful response (Chopra, et al., 2019). EpFA have been shown to reduce ER-stress (Inceoglu, et al., 2015), downregulate COX-2 either through unknown direct mechanisms (Schmelzer, et al., 2005) or indirectly by decreasing ER-stress, and act up-stream of PGE2 (Inceoglu, et al., 2011); all three being mechanisms that partially can explain the analgesic properties of sEH inhibitors. Interestingly, recent data have shown increases in lipid diols in correlation with painful conditions. In patients with early onset gout, a subset of oxylipins, both EpFA and corresponding diols, were observed at higher concentrations compared to healthy controls (C. Wang, et al., 2022). A separate study in a small cohort (n=22) of patients with Achilles tendinopathy, 12,13-DiHOME was significantly increased compared to healthy controls (Gouveia-Figueira, et al., 2015). Higher 12,13-DiHOME concentrations are also associated with chronic post-traumatic headache and lower life satisfaction in humans post traumatic brain injury (Domenichiello, et al., 2020). In rodents, 12,13-DiHOME is associated with heightened painful response through TRPV1 and TRPA1 signaling (Green, et al., 2016; Zimmer, et al., 2018), suggesting a possible independent role of diols in the pathogenesis of pain. Zimmer et al. demonstrated that 12,13-DiHOME, but not 9,10-DiHOME, injected into the hind paw of mice resulted in thermal hypersensitivity up to 1-hr post injection. The authors also demonstrated that 12,13-DiHOME, but not other sEH derived diols from LA or AA, were able to activate the transient receptor potential vanilloid 1 (TRPV1) dependent calcium influx in sensory neurons (Zimmer, et al., 2018).
Transient receptor potential vanilloid 1 (TRPV1) is a calcium permeable, temperature sensitive ion channel involved in pain modulation and responsible for the burning sensation associated with heat or the well-known agonist with analgesic properties, capsaicin, originally found in hot peppers. These channels are regulated through coupling with large-conductance calcium- and voltage-activated potassium (BK) channels. BK channels will hyperpolarize and consequently decrease membrane potentials to counteract the depolarization effects from TRPV1 opening (Wu, et al., 2013). EPA (EpETEs) and AA-derived EpFAs (EpETrEs) activate BK channels (Lauterbach, et al., 2002) (Yamaura, et al., 2006). Thus, under normal conditions, the EpFA and diols would provide a balance between the activation of TRPV1 by DiHOMEs and subsequent hyperpolarization by BK channel activation from EpETEs or EpETrEs to suppress this signal. However, the current food supply chain and dietary choices have resulted in an overabundance of LA that could shift the balance towards TRPV1 activation and hypersensitivity if DiHOMEs are in higher concentrations than EpFAs. Other circumstances where sEH activity is increased would further imbalance this pathway towards lower concentrations of EpFA available to activate BK channels, resulting in neuronal hyperexcitability and increased synaptic transmission. These pathways are outlined in Figure 3.
Figure 3. Mechanisms for regulatory lipids in pain signaling in neurons.
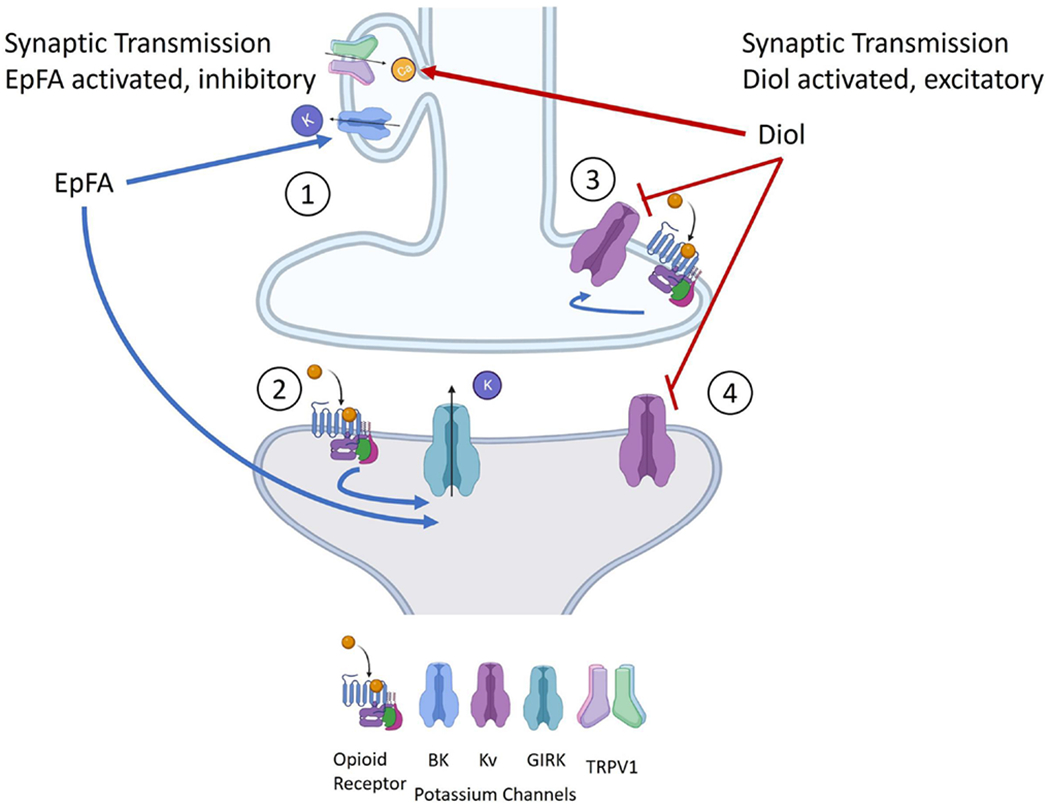
The following four pathways are proposed mechanisms for how EpFA and vicinal diols activate and regulate pain: 1) 12,13-DiHOMEs directly activate TRPV1 receptors, increasing neurotransmission. EpFA activate BK channels (Yamaura, et al., 2006) and complex with TRP channels to hyperpolarize the neuron and downregulate neurotransmission; 2) In postsynaptic neurons, agonists of mu opioid receptors activate GIRK channels resulting in K+ release and hyperpolarization of the neuron. EpFA also activate GIRK channels in postsynaptic neurons and could help in understanding why preventing EpFA formation, either genetically or pharmaceutically, reduces morphine analgesia; 3) 11,12-DiHETrE blocks Kv1.2, downstream of opioid signaling, although the effects of increased diols on morphine efficacy have not been studied; and 4) 11,12-DiHETrE significantly blocks Kv4.2, an important potassium channel in pain relief.
Potassium channels play a broader role in regulating pain (Tsantoulas & McMahon, 2014) that are regulated by EpFA and diols. In addition to activity of EpFA on BK channels (Lauterbach, et al., 2002) (Yamaura, et al., 2006), other sEH products influence potassium channel signaling in mechanisms that may contribute to the biological effects observed with these lipid mediators. For example, 11,12-DiHETrE, enhance excitatory synaptic transmission in mouse hippocampal neurons by blocking voltage gated potassium channel Kv1.2 and Kv4.2 (Nandkishor Kisanrao Mule, 2018). EpETrEs also activate G protein-gated inwardly rectifying potassium (GIRK) channels in mouse hippocampal cells to regulate neurotransmission (N. K. Mule, Orjuela Leon, Falck, Arand, & Marowsky, 2017). GIRK channels are necessary for opioid-induced analgesia (Nockemann, et al., 2013), and recent studies implicate EpFA as a possible downstream signaling molecule necessary for opioid analgesia. In fact, eliminating EpFA formation by knocking out the CYP-reductase to prevent CYP activity attenuates morphine-mediated analgesia in mice (Conroy, et al., 2010; Hough, et al., 2011) independent of opioid receptor activation (Terashvili, et al., 2008). In addition, wild-type mice treated with a CYP450 inhibitor, CC12 also attenuated morphine-mediated anti-nociception (Conroy, et al., 2010). EpFA are formed primarily from CYP-2C but not −2D or 3A. Genetic ablation of the CYP-2C cluster but not CYP2D or 3A significantly reduced morphine-mediated anti-nociception (Hough, Nalwalk, Ding, & Scheer, 2015), suggesting that EpFA are required for opioid-mediated analgesia.
4. Dysregulated regulatory lipids in disease.
The beneficial effects of EpFA revolve around their anti-inflammatory properties, ability to reduce ER-stress and endothelial dysfunction. The regulatory role of EpFA on endothelial cells is associated with BK channel signaling, and EpFA were originally described as endothelial derived hyperpolarizing factors for their ability to signal KATP channels, hyperpolarize membrane potentials resulting in vasodilation of blood vessels (Campbell, Gebremedhin, Pratt, & Harder, 1996). Although a direct role of lipid-diols on the endothelium has not been reported, there are many cardiovascular diseases associated with dysregulated lipids.
Heart Disease:
Cardiovascular disease is a general term that describes a number of vascular pathologies, many of which identify with dysregulated oxylipins in human patients. In peripheral heart disease, patients display an increase in 8,9 DiHETrE (Caligiuri, et al., 2017), and in coronary artery disease, a decrease in EpETrEs (Theken, et al., 2012), and genetic polymorphisms resulting in increased sEH activity correlates with increased risk of atherosclerosis. A detailed review of the role of regulatory lipids in cardiovascular disease has been reviewed extensively (Imig, Cervenka, & Neckar, 2022). In more recent studies, DiHOMEs have been implicated in a rare inflammatory heart disease, arrhythmogenic cardiomyopathy (ACM), a genetic heart condition resulting in myocardial inflammation and often presenting as sudden cardiac arrest in young athletes. In a small cohort of patients, 9,10 and 12-13 DiHOME were significantly increased compared to healthy controls (Jeffrey E. Saffitz, 2021). In this disease, NF-κB is associated with disease progression (Chelko, et al., 2019), and therapies targeting its reduction are being considered for this patient population (Saffitz, 2019).
Cerebrovascular disease:
Small vessel disease results in reduced blood flow to the brain and has been associated with numerous pathologies, including stroke, dementia, and Alzheimer’s disease (AD) (Morris, et al., 2019). An increased diol/epoxide ratio was observed in patients with small vessel disease compared to healthy subjects (Yu, et al., 2019), and although this does not identify a causative effect, numerous studies report increased diols associated with poor outcomes in diseases associated with cerebrovascular disease. In a study analyzing effects after mild to moderate stroke, increased ratio of 12,13 DiHOME to 12,13 EpOME in small vessel, but not large vessel, stroke was associated with higher white matter free water diffusion in the temporal lobe, and 9,10- DiHOME/9(10)- EpOME ratios were associated with temporal lobe atrophy (Yu, et al., 2023). White matter free water diffusion has recently become an interesting diagnostic tool for monitoring cerebral vascular disease in stroke and dementia (Alber, et al., 2019), suggesting that altered lipid ratios correlates with worsening outcomes after stroke. Genetic polymorphisms also link increased sEH activity to worsening stroke outcomes and increased neuronal cell death, while reduced sEH activity was associated with improved outcomes and reduced cell death after ischemic injury (Koerner, et al., 2007). Clinically, inhibition of the sEH improves outcomes after sub arachnoid hemorrhage. Patients receiving a sEH inhibitor demonstrated positive effects in reducing the length of ICU and hospital stay, reduced inflammatory cytokines in the synovial fluid, as well as improved outcomes over time compared to patients not given the inhibitor (Martini, et al., 2021).
Cerebrovascular disease contributes to the structural neuropathologies, such as brain atrophy, white matter free water diffusion, and accumulation of β-amyloid that overlap or exacerbate AD. In patients with vascular cognitive impairment, sEH activity was associated with reduced psychomotor processing speed, attention and executive function (Yu, et al., 2023). In further support of a mechanistic role of sEH in AD pathology, sEH protein is upregulated in AD patient brain tissue compared to age-matched controls without dementia (Ghosh, et al., 2020), possibly due to polymorphisms in the non-coding region. For example, sEH is regulated by an enhancer element, rs2279590, (Padhy, Kapuganti, Hayat, Mohanty, & Alone, 2023) which has been identified as a high-risk variant associated with AD (Padhy, Hayat, Nanda, Mohanty, & Alone, 2017).
5. Conclusion
The beneficial effects of EpFA and at least some understanding of their mechanisms of action are well described in multiple reviews (Atone, Wagner, Hashimoto, & Hammock, 2020; Spector & Norris, 2007; K. M. Wagner, McReynolds, Schmidt, & Hammock, 2017); however, a bioactive role for regulatory lipid diols has only been recently appreciated (Table 2). Under healthy conditions, where inflammation and ER-stress are not upregulated, EpFA and diols provide a balance to help maintain health; however, epigenetic factors can increase the sEH resulting in an imbalance of increased diols and decreased epoxides. This imbalance would drive inflammation when diols promote neutrophils to stimulate NF-κB activation without the concentrations of EpFA needed to resolve this inflammation through inhibiting nuclear translocation of NF-κB. Similarly, in pain signaling, an increased concentration of diols would depolarize membrane potentials through TRPV1 resulting in neuronal hyper-excitability without EpFA to counterbalance this through potassium channel activation to decrease neuronal transmission. Potassium signaling is also important in regulating cardiovascular tissue and considered responsible for maintaining endothelial hyperpolarization.EpFA are described as endothelium derived hyperpolarizing factors due to their ability to activate KATP channels, release potassium and vasodilate small blood vessels (Campbell, et al., 1996). Although increases in diol concentrations have been associated with numerous cardiovascular diseases, a direct action of lipid diols in cardiovascular tissue has not been determined, and some suggest a protective effect from DiHOMEs resulting from brown fat adipogenesis at low concentrations. It is unknown if these effects are maintained at higher concentrations, or if the inflammatory actions of DiHOMEs would counteract any benefits from brown-fat adipogenesis. Therapeutic strategies to prevent diol formation with an sEH inhibitor or treat disease by supplementing with a metabolically stable regioisomer of EpFA are being explored both pre-clinically and clinically (C. McReynolds, et al., 2020). A more comprehensive assessment on the biological effects of the different lipid regioisomers is needed to determine if EpFA mimics would be a successful target drug. Furthermore, additional research is needed to understand the role of lipid diols and if the observations in the studies described above can translate to a pharmaceutical target to treat disease.
Table 2.
Opposing effects of EpFA and vicinal diols on different diseases
| Effect | Mechanism | EpFA | Vicinal Diol |
|---|---|---|---|
| Inflammation | Regulation of NF-κB through IKK | Prevent nuclear translocation of NF-κB by blocking degradation of Iκβα (Node, et al., 1999) (Xu, et al., 2006) |
Activated neutrophils signal IKK to degrade Iκβα, initiating nuclear translocation and activation of NF-κB signaling (Bergmann, et al., 2021) |
| Pain | Ion Channels | Activate BK channels to counteract TRPV1 signaling, Activate GIRK channels downstream of mu opioid receptor (Lauterbach, et al., 2002) (Yamaura, et al., 2006) (N. K. Mule, et al., 2017) |
Activate TRPV1 Block Kv1.2 downstream of opioid signaling and Kv4.2, an important potassium channel in pain relief. (Zimmer, et al., 2018) (Nandkishor Kisanrao Mule, 2018) |
| Cardiovascular Disease | Endothelial dysfunction | EETs act as endothelial derived hyperpolarizing factors to increase vasodilation (Campbell, et al., 1996) |
No known effects |
| Autoimmune Disease | Macrophage polarization | Reduce inflammatory cytokines, maintain M2 macrophage polarization, reduce neutrophil activation (Dai, et al., 2015) (Abdalla, et al., 2023) |
Activate neutrophils and maintain M1 macrophage inflammatory phenotype (Lin, et al., 2022) |
Financial Disclosure:
Partial support was provided by the NIEHS/Superfund Research Program (P42 ES004699), the NIEHS RIVER Grant (R35 ES030443), and the NHLBI Grant (R43HL164226)
Abbreviations:
- ARDS
acute respiratory distress syndrome
- AD
Alzheimer’s disease
- AA
arachidonic acid
- ACM
arrhythmogenic cardiomyopathy
- KATP
ATP-sensitive potassium channel
- BK
big conductance potassium channels
- COX
cyclooxygenase
- CYP450
cytochrome P450
- DiHDPE
dihydroxy-docosapentaenoic acid
- DiHETE
dihydroxy-eicosatetraenoic acid
- DiHETrE
Dihydroxy-eicosatrienoic acid
- DiHODE
dihydroxy-octadecadienoic acid
- DiHOME
dihydroxy-octadecenoic Acid
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- EpDPE/EDP
epoxy-docosapentaenoic acid
- EpETE/EEQ
epoxy-eicosatetraenoic acid
- EpETrE/EET
Epoxy-eicosatrienoic acid
- ER
endoplasmic reticulum
- EpFA
epoxy fatty acids
- EpODE
epoxy-octadecadienoic acid
- EpOME
epoxy-octadecenoic acid
- GIRK
G protein-gated inwardly rectifying potassium
- LTs
leukotrienes
- LA
linoleic acid
- ALA
linolenic acid
- LOX
lipoxygenase
- mEH
microsomal epoxide hydrolase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- n-3
omega-3
- n-6
omega-6
- OA
osteoarthritis
- PUFA
polyunsaturated fatty acid
- sEH
soluble epoxide hydrolase
- TRPV1
Transient receptor potential vanilloid 1
- Kv
voltage gated potassium channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Dr. McReynolds is employed by, and Dr. Hammock is partly employed by EicOsis, which is developing soluble epoxide hydrolase inhibitors for the treatment of diseases caused by chronic inflammation.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
BD Hammock and CB McReynolds are cofounders and employees of EicOsis L.L.C., a startup company advancing sEH inhibitors as potential therapeutics. The University of California holds patents on the sEH inhibitors used in this study as well as their use to treat inflammation, inflammatory pain, and neuropathic pain.
6. References
- Alber J, Alladi S, Bae HJ, Barton DA, Beckett LA, Bell JM, Berman SE, Biessels GJ, Black SE, Bos I, Bowman GL, Brai E, Brickman AM, Callahan BL, Corriveau RA, Fossati S, Gottesman RF, Gustafson DR, Hachinski V, Hayden KM, Helman AM, Hughes TM, Isaacs JD, Jefferson AL, Johnson SC, Kapasi A, Kern S, Kwon JC, Kukolja J, Lee A, Lockhart SN, Murray A, Osborn KE, Power MC, Price BR, Rhodius-Meester HFM, Rondeau JA, Rosen AC, Rosene DL, Schneider JA, Scholtzova H, Shaaban CE, Silva N, Snyder HM, Swardfager W, Troen AM, van Veluw SJ, Vemuri P, Wallin A, Wellington C, Wilcock DM, Xie SX, & Hainsworth AH (2019). White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement (N Y), 5, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnardottir H, Pawelzik SC, Sarajlic P, Quaranta A, Kolmert J, Religa D, Wheelock CE, & Bäck M (2022). Immunomodulation by intravenous omega-3 fatty acid treatment in older subjects hospitalized for COVID-19: A single-blind randomized controlled trial. Clin Transl Med, 12, e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atone J, Wagner K, Hashimoto K, & Hammock BD (2020). Cytochrome P450 derived epoxidized fatty acids as a therapeutic tool against neuroinflammatory diseases. Prostaglandins Other Lipid Mediat, 147, 106385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CB, Hammock BD, Wan D, Gogolla F, Goetzman H, Caldwell CC, & Supp DM (2021). TPPU treatment of burned mice dampens inflammation and generation of bioactive DHET which impairs neutrophil function. Sci Rep, 11, 16555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CB, McReynolds CB, Wan D, Singh N, Goetzman H, Caldwell CC, Supp DM, & Hammock BD (2022). sEH-derived metabolites of linoleic acid drive pathologic inflammation while impairing key innate immune cell function in burn injury. Proc Natl Acad Sci U S A, 119, e2120691119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri SPB, Aukema HM, Ravandi A, Lavallee R, Guzman R, & Pierce GN (2017). Specific plasma oxylipins increase the odds of cardiovascular and cerebrovascular events in patients with peripheral artery disease. Can J Physiol Pharmacol, 95, 961–968. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, & Harder DR (1996). Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res, 78, 415–423. [DOI] [PubMed] [Google Scholar]
- Chelko SP, Asimaki A, Lowenthal J, Bueno-Beti C, Bedja D, Scalco A, Amat-Alarcon N, Andersen P, Judge DP, Tung L, & Saffitz JE (2019). Therapeutic Modulation of the Immune Response in Arrhythmogenic Cardiomyopathy. Circulation, 140, 1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra S, Giovanelli P, Alvarado-Vazquez PA, Alonso S, Song M, Sandoval TA, Chae CS, Tan C, Fonseca MM, Gutierrez S, Jimenez L, Subbaramaiah K, Iwawaki T, Kingsley PJ, Marnett LJ, Kossenkov AV, Crespo MS, Dannenberg AJ, Glimcher LH, Romero-Sandoval EA, & Cubillos-Ruiz JR (2019). IRE1alpha-XBP1 signaling in leukocytes controls prostaglandin biosynthesis and pain. Science, 365. [DOI] [PubMed] [Google Scholar]
- Conroy JL, Fang C, Gu J, Zeitlin SO, Yang W, Yang J, VanAlstine MA, Nalwalk JW, Albrecht PJ, Mazurkiewicz JE, Snyder-Keller A, Shan Z, Zhang SZ, Wentland MP, Behr M, Knapp BI, Bidlack JM, Zuiderveld OP, Leurs R, Ding X, & Hough LB (2010). Opioids activate brain analgesic circuits through cytochrome P450/epoxygenase signaling. Nature Neuroscience, 13, 284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenichiello AF, Jensen JR, Zamora D, Horowitz M, Yuan ZX, Faurot K, Mann JD, Mannes AJ, & Ramsden CE (2020). Identifying oxidized lipid mediators as prognostic biomarkers of chronic posttraumatic headache. Pain, 161, 2775–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin ML, Hamedani BG, Gruzdev A, Graves JP, Lih FB, Arbes SJ 3rd, Singh R, Orjuela Leon AC, Bradbury JA, DeGraff LM, Hoopes SL, Arand M, & Zeldin DC (2018). Epoxide hydrolase 1 (EPHX1) hydrolyzes epoxyeicosanoids and impairs cardiac recovery after ischemia. J Biol Chem, 293, 3281–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Comerota MM, Wan D, Chen F, Propson NE, Hwang SH, Hammock BD, & Zheng H (2020). An epoxide hydrolase inhibitor reduces neuroinflammation in a mouse model of Alzheimer’s disease. Sci Transl Med, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia-Figueira S, Nording ML, Gaida JE, Forsgren S, Alfredson H, & Fowler CJ (2015). Serum levels of oxylipins in achilles tendinopathy: an exploratory study. PLoS One, 10, e0123114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowler PRW, Turnbull J, Shahtaheri M, Gohir S, Kelly T, McReynolds C, Jun Y, Jha RR, Fernandes GS, Zhang W, Doherty M, Walsh DA, Hammock BD, Valdes AM, Barrett DA, & Chapman V (2021). Clinical and preclinical evidence for roles of soluble epoxide hydrolase in osteoarthritis knee pain. Arthritis Rheumatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D, Ruparel S, Gao X, Ruparel N, Patil M, Akopian A, & Hargreaves K (2016). Central activation of TRPV1 and TRPA1 by novel endogenous agonists contributes to mechanical allodynia and thermal hyperalgesia after burn injury. Mol Pain, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes A, Galuppo L, Hood D, Hwang SH, Morisseau C, & Hammock BD (2017). Soluble epoxide hydrolase activity and pharmacologic inhibition in horses with chronic severe laminitis. Equine Vet J, 49, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes AG, Morisseau C, Sole A, Soares JH, Ulu A, Dong H, & Hammock BD (2013). Use of a soluble epoxide hydrolase inhibitor as an adjunctive analgesic in a horse with laminitis. Vet Anaesth Analg, 40, 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes AGP, Aristizabal F, Sole A, Adedeji A, Brosnan R, Knych H, Yang J, Hwang SH, Morisseau C, & Hammock BD (2018). Pharmacokinetics and antinociceptive effects of the soluble epoxide hydrolase inhibitor t-TUCB in horses with experimentally induced radiocarpal synovitis. J Vet Pharmacol Ther, 41, 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M, Wu HN, Tanaka M, Tsuda N, Tantengco OAG, Matsushima T, Nakao T, Ishibe T, Sakata I, & Yanagihara I (2019). A case series of the dynamics of lipid mediators in patients with sepsis. Acute Med Surg, 6, 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock BD, McReynolds CB, Wagner K, Buckpitt A, Cortes-Puch I, Croston G, Lee KSS, Yang J, Schmidt WK, & Hwang SH (2021). Movement to the Clinic of Soluble Epoxide Hydrolase Inhibitor EC5026 as an Analgesic for Neuropathic Pain and for Use as a Nonaddictive Opioid Alternative. J Med Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Ding X, & Scheer N (2015). Opioid Analgesia in P450 Gene Cluster Knockout Mice: A Search for Analgesia-Relevant Isoforms. Drug Metab Dispos, 43, 1326–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Yang J, Conroy JL, VanAlstine MA, Yang W, Gargano J, Shan Z, Zhang SZ, Wentland MP, Phillips JG, Knapp BI, Bidlack JM, Zuiderveld OP, Leurs R, & Ding X (2011). Brain P450 epoxygenase activity is required for the antinociceptive effects of improgan, a nonopioid analgesic. Pain, 152, 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JN, Taki F, Sugiyama S, Asai J, Izawa Y, Satake T, & Ozawa T (1988). Neutrophil-derived epoxide, 9,10-epoxy-12-octadecenoate, induces pulmonary edema. Lung, 166, 327–337. [DOI] [PubMed] [Google Scholar]
- Hwang HS, & Kim HA (2015). Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int J Mol Sci, 16, 26035–26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD, Cervenka L, & Neckar J (2022). Epoxylipids and soluble epoxide hydrolase in heart diseases. Biochem Pharmacol, 195, 114866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Bettaieb A, Trindade da Silva CA, Lee KS, Haj FG, & Hammock BD (2015). Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci U S A, 112, 9082–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, & Hammock BD (2006). Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci, 79, 2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, Ulu A, Hegedus C, Rose T, Brosnan R, & Hammock BD (2011). Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc Natl Acad Sci U S A, 108, 5093–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffitz Jeffrey E., B. D. H, Hwang Sung Hee. (2021). SOLUBLE EPOXIDE HYDROLASE (sEH) INHIBITORS AND DUAL COX/sEH INHIBITORS FOR THE TREATMENT OF ARRHYTHMOGENIC CARDIOMYOPATHY. In Beth I Israel Deaconess Medical Center, The Regents Of The University Of California (Ed.). Worldwide: Beth Israel Deaconess Medical Center, Inc., The Regents Of The University Of California. [Google Scholar]
- Karu N, Kindt A, Lamont L, van Gammeren AJ, Ermens AAM, Harms AC, Portengen L, Vermeulen RCH, Dik WA, Langerak AW, van der Velden VHJ, & Hankemeier T (2022). Plasma Oxylipins and Their Precursors Are Strongly Associated with COVID-19 Severity and with Immune Response Markers. Metabolites, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, & Alkayed NJ (2007). Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci, 27, 4642–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Roome T, Bhattacharjee A, Carnevale KA, Yakubenko VP, Zhang R, Hwang SH, Hammock BD, & Cathcart MK (2013). Metabolic products of soluble epoxide hydrolase are essential for monocyte chemotaxis to MCP-1 in vitro and in vivo. J Lipid Res, 54, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach B, Barbosa-Sicard E, Wang MH, Honeck H, Kärgel E, Theuer J, Schwartzman ML, Haller H, Luft FC, Gollasch M, & Schunck WH (2002). Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension, 39, 609–613. [DOI] [PubMed] [Google Scholar]
- Liangpunsakul S, & Chalasani N (2019). Lipid mediators of liver injury in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol, 316, G75–g81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macêdo APA, Muñoz VR, Cintra DE, & Pauli JR (2022). 12,13-diHOME as a new therapeutic target for metabolic diseases. Life Sci, 290, 120229. [DOI] [PubMed] [Google Scholar]
- Markaverich BM, Alejandro M, Thompson T, Mani S, Reyna A, Portillo W, Sharp J, Turk J, & Crowley JR (2007). Tetrahydrofurandiols (THF-diols), leukotoxindiols (LTX-diols), and endocrine disruption in rats. Environ Health Perspect, 115, 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini RP, Siler D, Cetas J, Alkayed NJ, Allen E, & Treggiari MM (2021). A Double-Blind, Randomized, Placebo-Controlled Trial of Soluble Epoxide Hydrolase Inhibition in Patients with Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care. [DOI] [PubMed] [Google Scholar]
- McDonald JG, Ejsing CS, Kopczynski D, Holčapek M, Aoki J, Arita M, Arita M, Baker ES, Bertrand-Michel J, Bowden JA, Brügger B, Ellis SR, Fedorova M, Griffiths WJ, Han X, Hartler J, Hoffmann N, Koelmel JP, Köfeler HC, Mitchell TW, O’Donnell VB, Saigusa D, Schwudke D, Shevchenko A, Ulmer CZ, Wenk MR, Witting M, Wolrab D, Xia Y, Ahrends R, Liebisch G, & Ekroos K (2022). Introducing the Lipidomics Minimal Reporting Checklist. Nat Metab, 4, 1086–1088. [DOI] [PubMed] [Google Scholar]
- McReynolds C, Cortes-Puch I, Ravindran R, Khan IH, Hammock BG, Shih P.-a. B., Hammock BD, & Yang J (2021). Plasma Linoleate Diols Are Potential Biomarkers for Severe COVID-19 Infections. Front Physiol, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds C, Morisseau C, Wagner K, & Hammock B (2020). Epoxy Fatty Acids Are Promising Targets for Treatment of Pain, Cardiovascular Disease and Other Indications Characterized by Mitochondrial Dysfunction, Endoplasmic Stress and Inflammation. Adv Exp Med Biol, 1274, 71–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds CB, Hwang SH, Yang J, Wan D, Wagner K, Morisseau C, Li D, Schmidt WK, & Hammock BD (2019). Pharmaceutical Effects of Inhibiting the Soluble Epoxide Hydrolase in Canine Osteoarthritis. Front Pharmacol, 10, 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds CB, Yang J, Guedes A, Morisseau C, Garcia R, Knych H, Tearney C, Hamamoto B, Hwang SH, Wagner K, & Hammock BD (2021). Species Differences in Metabolism of Soluble Epoxide Hydrolase Inhibitor, EC1728, Highlight the Importance of Clinically Relevant Screening Mechanisms in Drug Development. Molecules, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, & Hammock BD (1997). Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med, 3, 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague B, Summers A, Bhawal R, Anderson ET, Kraus-Malett S, Zhang S, & Goggs R (2022). Identifying potential biomarkers and therapeutic targets for dogs with sepsis using metabolomics and lipidomics analyses. PLoS One, 17, e0271137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Kodani SD, Kamita SG, Yang J, Lee KSS, & Hammock BD (2021). Relative Importance of Soluble and Microsomal Epoxide Hydrolases for the Hydrolysis of Epoxy-Fatty Acids in Human Tissues. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Piccolo BD, John CS, Green ZD, Thyfault JP, & Adams SH (2019). Oxylipin Profiling of Alzheimer’s Disease in Nondiabetic and Type 2 Diabetic Elderly. Metabolites, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mule NK (2018). The role of epoxygenated arachidonic acid-derived metabolites in neuronal transmission. University of Zurich. [Google Scholar]
- Mule NK, Orjuela Leon AC, Falck JR, Arand M, & Marowsky A (2017). 11,12 -Epoxyeicosatrienoic acid (11,12 EET) reduces excitability and excitatory transmission in the hippocampus. Neuropharmacology, 123, 310–321. [DOI] [PubMed] [Google Scholar]
- Nockemann D, Rouault M, Labuz D, Hublitz P, McKnelly K, Reis FC, Stein C, & Heppenstall PA (2013). The K(+) channel GIRK2 is both necessary and sufficient for peripheral opioid-mediated analgesia. EMBO Mol Med, 5, 1263–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, & Liao JK (1999). Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science, 285, 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhy B, Hayat B, Nanda GG, Mohanty PP, & Alone DP (2017). Pseudoexfoliation and Alzheimer’s associated CLU risk variant, rs2279590, lies within an enhancer element and regulates CLU, EPHX2 and PTK2B gene expression. Hum Mol Genet, 26, 4519–4529. [DOI] [PubMed] [Google Scholar]
- Padhy B, Kapuganti RS, Hayat B, Mohanty PP, & Alone DP (2023). Wide-spread enhancer effect of SNP rs2279590 on regulating epoxide hydrolase-2 and protein tyrosine kinase 2-beta gene expression. Gene, 854, 147096. [DOI] [PubMed] [Google Scholar]
- Phillips R (2022). Inhibition of epoxide hydrolysis targets pain in osteoarthritis. Nat Rev Rheumatol, 18, 5. [DOI] [PubMed] [Google Scholar]
- Ripon MAR, Bhowmik DR, Amin MT, & Hossain MS (2021). Role of arachidonic cascade in COVID-19 infection: A review. Prostaglandins Other Lipid Mediat, 154, 106539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers AJ, Leligdowicz A, Contrepois K, Jauregui A, Vessel K, Deiss TJ, Belzer A, Liu T, Lippi M, Ke S, Ross E, Zhou H, Hendrickson C, Gomez A, Sinha P, Kangelaris KN, Liu KD, Calfee CS, & Matthay MA (2021). Plasma Metabolites in Early Sepsis Identify Distinct Clusters Defined by Plasma Lipids. Crit Care Explor, 3, e0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose TE, Morisseau C, Liu JY, Inceoglu B, Jones PD, Sanborn JR, & Hammock BD (2010). 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem, 53, 7067–7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffitz J (2019). Anti-inflammatory therapy in arrhythmogenic cardiomyopathy (acm). In Beth I Israel Deaconess Medical Center (Ed.): Beth Israel Deaconess Medical Center, Inc. [Google Scholar]
- Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, & Hammock BD (2005). Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A, 102, 9772–9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, & Savill J (2005). Resolution of inflammation: the beginning programs the end. Nat Immunol, 6, 1191–1197. [DOI] [PubMed] [Google Scholar]
- Spector AA (2009). Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res, 50 Suppl, S52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA, & Kim HY (2015). Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim Biophys Acta, 1851, 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA, & Norris AW (2007). Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol, 292, C996–1012. [DOI] [PubMed] [Google Scholar]
- Terashvili M, Tseng LF, Wu HE, Narayanan J, Hart LM, Falck JR, Pratt PF, & Harder DR (2008). Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of beta-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol Exp Ther, 326, 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theken KN, Schuck RN, Edin ML, Tran B, Ellis K, Bass A, Lih FB, Tomer KB, Poloyac SM, Wu MC, Hinderliter AL, Zeldin DC, Stouffer GA, & Lee CR (2012). Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease. Atherosclerosis, 222, 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsantoulas C, & McMahon SB (2014). Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci, 37, 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Gotoh T, Tomisato W, Mima S, Hoshino T, Hwang HJ, Takenaka H, Tsuchiya T, Mori M, & Mizushima T (2004). Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ, 11, 1009–1016. [DOI] [PubMed] [Google Scholar]
- Tucker L, Trumble TN, Groschen D, Dobbs E, Baldo CF, Wendt-Hornickle E, & Guedes AGP (2021). Targeting Soluble Epoxide Hydrolase and Cyclooxygenases Enhance Joint Pain Control, Stimulate Collagen Synthesis, and Protect Chondrocytes From Cytokine-Induced Apoptosis. Front Vet Sci, 8, 685824.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Ravipati S, Pousinis P, Menni C, Mangino M, Abhishek A, Chapman V, Barrett DA, & Doherty M (2018). Omega-6 oxylipins generated by soluble epoxide hydrolase are associated with knee osteoarthritis. J Lipid Res, 59, 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Inceoglu B, Gill SS, & Hammock BD (2011). Epoxygenated fatty acids and soluble epoxide hydrolase inhibition: novel mediators of pain reduction. J Agric Food Chem, 59, 2816–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KM, McReynolds CB, Schmidt WK, & Hammock BD (2017). Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol Ther, 180, 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters B, Trumble TN, Wendt-Hornickle E, Kennedy M, & Guedes A (2022). Effects of cyclooxygenase and soluble epoxide hydrolase inhibitors on apoptosis of cultured primary equine chondrocytes. Res Vet Sci, 147, 44–49. [DOI] [PubMed] [Google Scholar]
- Wang C, Lu J, Sun W, Merriman TR, Dalbeth N, Wang Z, Wang X, Han L, Cui L, Li X, Ji A, Li H, Ji X, He Y, Li C, & Liu Z (2022). Profiling of Serum Oxylipins Identifies Distinct Spectrums and Potential Biomarkers in Young People with Very Early Onset Gout. Rheumatology (Oxford). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wagner KM, Morisseau C, & Hammock BD (2021). Inhibition of the Soluble Epoxide Hydrolase as an Analgesic Strategy: A Review of Preclinical Evidence. J Pain Res, 14, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Liu Y, Hou P, Yan Z, Kong W, Liu B, Li X, Yao J, Zhang Y, Qin F, & Ding J (2013). TRPV1 channels are functionally coupled with BK(mSlo1) channels in rat dorsal root ganglion (DRG) neurons. PLoS One, 8, e78203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, Kim IH, Tuteja D, Mateo RK, Singapuri A, Davis BB, Low R, Hammock BD, & Chiamvimonvat N (2006). Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A, 103, 18733–18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaura K, Gebremedhin D, Zhang C, Narayanan J, Hoefert K, Jacobs ER, Koehler RC, & Harder DR (2006). Contribution of epoxyeicosatrienoic acids to the hypoxia-induced activation of Ca2+ -activated K+ channel current in cultured rat hippocampal astrocytes. Neuroscience, 143, 703–716. [DOI] [PubMed] [Google Scholar]
- Yu D, Hennebelle M, Sahlas DJ, Ramirez J, Gao F, Masellis M, Cogo-Moreira H, Swartz RH, Herrmann N, Chan PC, Pettersen JA, Stuss DT, Black SE, Taha AY, & Swardfager W (2019). Soluble Epoxide Hydrolase-Derived Linoleic Acid Oxylipins in Serum Are Associated with Periventricular White Matter Hyperintensities and Vascular Cognitive Impairment. Transl Stroke Res, 10, 522–533. [DOI] [PubMed] [Google Scholar]
- Yu D, Liang N, Zebarth J, Shen Q, Ozzoude M, Goubran M, Rabin JS, Ramirez J, Scott CJM, Gao F, Bartha R, Symons S, Haddad SMH, Berezuk C, Tan B, Kwan D, Hegele RA, Dilliott AA, Nanayakkara ND, Binns MA, Beaton D, Arnott SR, Lawrence-Dewar JM, Hassan A, Dowlatshahi D, Mandzia J, Sahlas D, Casaubon L, Saposnik G, Otoki Y, Lanctôt KL, Masellis M, Black SE, Swartz RH, Taha AY, & Swardfager W (2023). Soluble Epoxide Hydrolase Derived Linoleic Acid Oxylipins, Small Vessel Disease Markers, and Neurodegeneration in Stroke. J Am Heart Assoc, 12, e026901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B, Angioni C, Osthues T, Toewe A, Thomas D, Pierre SC, Geisslinger G, Scholich K, & Sisignano M (2018). The oxidized linoleic acid metabolite 12,13-DiHOME mediates thermal hyperalgesia during inflammatory pain. Biochim Biophys Acta Mol Cell Biol Lipids, 1863, 669–678. [DOI] [PubMed] [Google Scholar]


