Abstract
Aims
To investigate the association of cardiac resynchronization therapy (CRT) on outcomes among participants with and without a history of atrial fibrillation (AF).
Methods
Individual-patient-data from four randomized trials investigating CRT-Defibrillators (COMPANION, MADIT-CRT, REVERSE) or CRT-Pacemakers (COMPANION, MIRACLE) were analyzed. Outcomes were time to a composite of heart failure hospitalization (HFH) or all-cause mortality or to all-cause mortality alone. The association of CRT on outcomes for patients with and without a history of AF was assessed using a Bayesian-Weibull survival regression model adjusting for baseline characteristics.
Results
Of 3,964 patients included, 586 (14.8%) had a history of AF; 2,245 (66%) were randomized to CRT. Overall, CRT reduced the risk of the primary composite endpoint (Hazard ratio [HR]: 0.69, 95% Credible Interval [CI]: 0.56–0.81). The effect was similar (posterior probability of no interaction = 0.26) in patients with (HR: 0.78, 95% CI: 0.55–1.10) and without a history of AF (HR: 0.67, 95% CI: 0.55–0.80). In these four trials, CRT did not reduce mortality overall (HR: 0.82, 95% CI: 0.66–1.01) without evidence of interaction (posterior probability of no interaction = 0.14) for patients with (HR: 1.09, 95% CI: 0.70–1.74) or without a history of AF (HR: 0.70, 95% CI: 0.60–0.97).
Conclusion
The association of CRT on the composite endpoint or mortality was not statistically different for patients with or without a history of AF, but this could reflect inadequate power. Our results call for trials to confirm the benefit of CRT recipients with a history of AF.
Keywords: Atrial fibrillation, heart failure, cardiac resynchronization therapy, CRT, trial, patient-level data, post hoc analysis
Introduction
Since 2001, several landmark trials have shown the benefits of cardiac resynchronization therapy (CRT) for appropriately selected patients with heart failure (HF) (1–5). However, atrial fibrillation or atrial flutter (AF) precludes coordination of atrio-ventricular contraction and may reduce the percentage of beats with effective biventricular pacing (6). Previous randomized trials of CRT included few patients in AF at randomization but most trials did include patients with a prior history of AF (1–5). Subgroup analysis has suggested that patients with a history of AF may not benefit from CRT (7).
Patients with a history of AF are at greater risk of further episodes, which may reduce or abolish the benefits of CRT. The evidence that CRT is effective in patients with AF is limited to observational data (8–11). In spite of this, administrative records and registries consistently show that up to 26% of patients who receive CRT have some form of AF (12). More evidence that CRT is effective for patients with a history of or actually in AF is clearly needed; this issue has been deemed to be of the highest importance by thought leaders(13).
In an individual-patient-data meta-analysis of four clinical trials of CRT-Defibrillators or CRT-Pacemakers that included patients with data on history of AF or flutter, we now describe the characteristics of patients with and without a history of AF (persistent, paroxysmal or atrial flutter) and determine whether the effect of CRT on morbidity and mortality varies according to a history of AF.
Methods
This analysis was a part of a National Heart, Lung, and Blood Institute funded project exploring evidence gaps in CRT.
Data sources
Prospective trials of CRT for patients with HF were considered for this analysis (2,4,5,14–19). The Resynchronization–Defibrillation for Ambulatory Heart Failure (RAFT), Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Multicenter InSync ICD II (MIRACLE ICD II), and Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial (18) were excluded because they either did not report prior history of AF or they excluded patients with AF altogether (5,16,17). It was not possible to obtain data from CARE-HF or European patients in REVERSE due to data-privacy regulations. Patient-level data from the following four prospective trials of CRT were combined: The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial (2), Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) (4), REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction (REVERSE) trial (14), and Multicenter InSync Randomized Clinical Evaluation (MIRACLE) (15). A full list of trial characteristics can be found in Supplementary Table 1.
Other exclusion criteria included missing data on left ventricular ejection fraction (LVEF) or QRS duration, LVEF >35% or QRS duration <120 ms and patients with unclear data on QRS morphology - for example being registered as having both left bundle branch block (LBBB) and right bundle branch block (RBBB), patients with AF at the time of randomization, and patients with missing data on AF. The trial flowchart is shown in Figure 1.
Figure 1. Flowchart of inclusion.
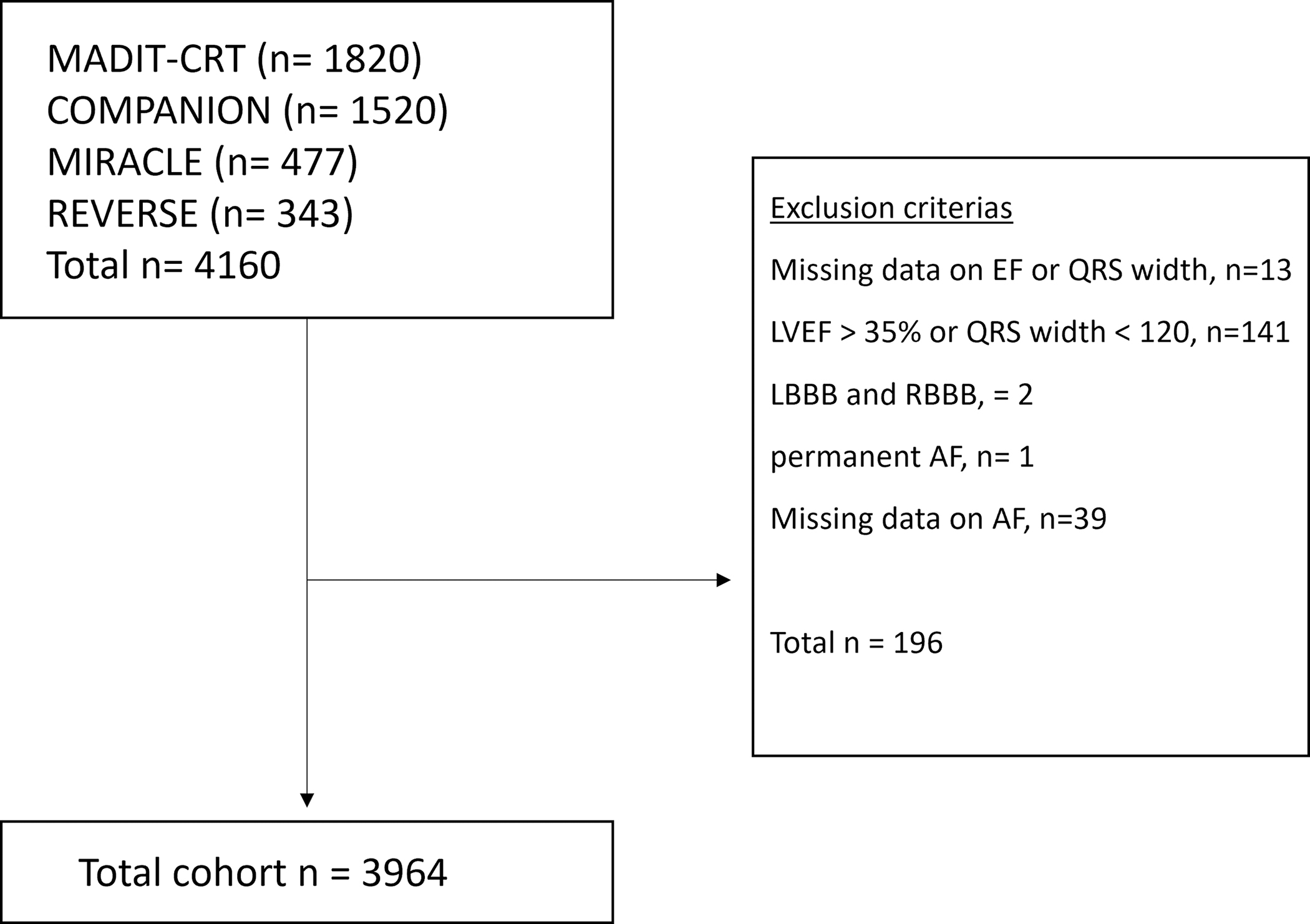
Flowchart of inclusion and exclusions of the trial.
LVEF; left ventricular ejection fraction, RV; right ventricle, LBBB; left bundle branch block, RBBB; right bundle branch block, AF; atrial fibrillation.
All original trials obtained the approval of institutional review committees, and all enrolled patients provided informed consent. The current analysis was approved by the Duke Institutional Review Board.
Trial population and covariates
All trials (COMPANION, MADIT-CRT, MIRACLE, and REVERSE trial) required patients to be in sinus rhythm at enrollment. For MADIT-CRT extended follow-up data was included. The different trial definitions of a prior history of AF or flutter are shown in Supplemental Table 2. The MIRACLE trial included patients with a history of paroxysmal AF (however 1 permanent AF was excluded), the MADIT-CRT trial included patients with a history of non-chronic AF (both paroxysmal and persistent) and atrial flutter. The COMPANION trial included patients with a history of paroxysmal AF or atrial flutter. The REVERSE trial included patients with a history of paroxysmal AF and persistent AF. Additional variables of interest included diabetes, hypertension, ischemic cardiomyopathy, creatinine level (mg/dl), LVEF, NYHA class, QRS duration and QRS morphology, presence of an ICD, use of diuretics, and rate- and rhythm controlling drugs.
Outcomes
Outcome data were captured by each individual trial. The primary outcome for this analysis was the combined endpoint of time to heart failure hospitalization (HFH) or to all-cause mortality. The secondary outcome was time to all-cause mortality. Incident AF was not captured.
Statistical Analyses
Baseline was defined as the time of randomization. Baseline characteristics were compared between participants with and without a prior history of AF using a t-test that allows for heteroscedasticity if the covariate was numerical or using a chi-square test for homogeneity if it was categorical. Baseline characteristics were similarly compared between participants receiving and not receiving CRT within each subgroup defined by history of AF.
The unadjusted association (all-cause mortality/HFH-free survival, survival time) between CRT versus no CRT within each subgroup (with and without a history of AF) is presented using Kaplan-Meier survival curves and compared using the log-rank test. The proportional hazard assumption was verified for each model via the scaled Schoenfeld residuals from the corresponding adjusted Cox proportional hazard mixed effects model with a random baseline hazard function and a random treatment effect at the trial level.
The adjusted association between CRT versus no CRT for all outcomes for patients with AF and without a history of AF was assessed using a Bayesian-Weibull survival regression model with random effects terms for the trial-specific treatment effects, baseline hazard functions, and interactions between history of AF and CRT (20). CRT hazard ratio estimates are presented with 95% credible intervals (CI). All analyses were adjusted for selected baseline characteristics (age, sex, NYHA class, ejection fraction, QRS width, presence of LBBB, diabetes, hypertension, ischemic heart disease, use of antiarrhythmic drugs, use of beta-blockers, use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and the presence of an ICD). To evaluate if the CRT hazard ratio differs between patients with and without a history of AF, we computed the 2-sided posterior probability that the mean of the interaction term between CRT and AF is zero (null interaction). All priors are non-informative. The priors used for the fixed effects and the mean components of the random effect distributions were normal distributions, the priors for the variance components of the random effect distributions were half-normal distributions, and the prior for the shape parameter of the Weibull model was a log-normal distribution. Similar models were fitted to assess the CRT association with the composite outcome of HFH and all-cause mortality and all-cause mortality individually. The adjusted relationship (adjusted hazard ratios) between CRT versus no CRT within each subgroup (with and without AF) is shown using forest plots. Finally, a subgroup analysis including only patients randomized to CRT was conducted by presence of history of AF.
Results
A total of 3964 patients were included, 586 (14.8%) of whom had a history of AF. All of them had a history of paroxysmal or persistent AF. Of patients with a history of AF, 397 (68%) were assigned to CRT. During a median follow-up of 20.8 months (IQR 11.4 – 37.1 months), a total of 818 patients were hospitalized for HF and 528 patients died.
Baseline Characteristics
Compared with patients with no AF, patients with a history of AF were older (median [IQR] age 70 [62 – 76] years versus 65 [57 – 72], p<0.001 years), were more often men (82% versus 70%, p<0.001), had a higher proportion of ischemic heart disease (68% versus 53%, p<0.001), and a lower baseline glomerular filtration rate (GFR) 65 ml/min/1.73m2 versus 70 ml/min/1.73m2 p<0.001). Patients with a history of AF also had worse NYHA class (NYHA IV, 10% versus 6%), and statistically lower but clinically similar LVEF (25% versus 27%, p<0.001) (Table 1) than patients with no history of AF.
Table 1.
Baseline characteristics by history of AF
| Characteristics | History of AF (N = 586) | No AF (N = 3,378) | P value† |
|---|---|---|---|
| CRT recipient | 397 (68%) | 2,245 (66%) | 0.5 |
| ICD recipient | 352 (60%) | 2,227 (66%) | 0.006 |
| Median age, years (IQR) | 70 (62, 76) | 65 (57, 72) | <0.001 |
| Men | 479 (82%) | 2,356 (70%) | <0.001 |
| Diabetes | 193 (33%) | 1,178 (35%) | 0.4 |
| Hypertension | 317 (54%) | 1,929 (57%) | 0.2 |
| Ischemic heart disease | 401 (68%) | 1,786 (53%) | <0.001 |
| GFR, ml/min/1.73m2* (IQR) | 65 (50, 78) | 70 (57, 85) | <0.001 |
| LVEF, % (IQR) | 25 (20, 30) | 27 (21, 30) | <0.001 |
| NYHA | <0.001 | ||
| I | 40 (7%) | 251 (7%) | |
| II | 201 (34%) | 1,501 (44%) | |
| III | 287 (49%) | 1,418 (42%) | |
| IV | 58 (10%) | 208 (6%) | |
| QRS duration, ms (IQR) | 160 (142, 176) | 160 (142, 172) | 0.084 |
| LBBB | 396 (68%) | 2,440 (72%) | 0.021 |
| Anti-arrhythmic¥ | 115 (39%) | 78 (4%) | <0.001 |
| RAS-inhibitor | 517 (88%) | 3,154 (93%) | <0.001 |
| Beta-blocker | 386 (66%) | 2,759 (82%) | <0.001 |
| Digoxin | 319 (54%) | 1,692 (50%) | 0.052 |
| Diuretics | 514 (88%) | 2,742 (81%) | <0.001 |
Summaries presented as in median (IQR), or n (%).
Information available only for 1,982 patients.
Information available only for 2,542 patients.
t-test or Pearson’s Chi-squared test.
AF: atrial fibrillation, CRT: cardiac resynchronization therapy, ICD: implantable cardioverter defibrillator, LBBB: left bundle branch block, LVEF: left ventricular ejection fraction, NYHA: New York Heart Association, RAS: renin-angiotensin system. SD: Standard deviation. IQR: Interquartile range. MS: milliseconds.
Baseline characteristics by history of AF and randomization (CRT versus no CRT) are shown in Table 2. For patients with a history of AF, most baseline characteristics were similar for those assigned to CRT or control (no CRT). Among patients with a history of AF, compared with patients with no CRT, patients in the CRT group were more likely to have an ICD (64% assigned to CRT, 52% assigned no CRT, p=0.005) and to have worse NYHA class (NYHA III, 54% for CRT patients versus 38% for controls, p<0.001).
Table 2.
Baseline characteristics by treatment (CRT versus no-CRT) and history of AF
| History of AF (N = 586) | No AF (N = 3,378) | |||||
|---|---|---|---|---|---|---|
| Characteristics | CRT (N = 397) | No CRT (N = 189) | P value† | CRT (N = 2,245) | No CRT (N = 1,133) | P value† |
| ICD recipient | 254 (64%) | 98 (52%) | 0.005 | 1,545 (69%) | 682 (60%) | <0.001 |
| Median age, years (IQR) | 70 (62, 76) | 70 (63, 76) | 0.9 | 65 (57, 73) | 65 (57, 72) | 0.8 |
| Men | 334 (84%) | 145 (77%) | 0.030 | 1,541 (69%) | 815 (72%) | 0.049 |
| Diabetes | 133 (34%) | 60 (32%) | 0.6 | 789 (35%) | 389 (34%) | 0.7 |
| Hypertension | 208 (53%) | 109 (58%) | 0.3 | 1,300 (58%) | 629 (56%) | 0.2 |
| Ischemic heat disease | 275 (69%) | 126 (67%) | 0.5 | 1,172 (52%) | 614 (54%) | 0.3 |
| GFR, ml/min/1.73m2* (IQR) | 275 (69%) | 126 (67%) | 0.5 | 1,172 (52%) | 614 (54%) | 0.3 |
| LVEF, % (IQR) | 25 (20, 30) | 27 (21, 30) | 0.017 | 26 (20, 30) | 28 (23, 31) | <0.001 |
| NYHA | 0.001 | <0.001 | ||||
| I | 22 (6%) | 18 (10%) | 148 (7%) | 103 (9%) | ||
| II | 121 (30%) | 80 (42%) | 920 (41%) | 581 (51%) | ||
| III | 216 (54%) | 71 (38%) | 1,031 (46%) | 387 (34%) | ||
| IV | 38 (10%) | 20 (11%) | 146 (7%) | 62 (5%) | ||
| QRS duration, ms (IQR) | 160 (140, 174) | 160 (144, 180) | 0.3 | 160 (142, 172) | 160 (142, 170) | >0.9 |
| LBBB | 255 (64%) | 141 (75%) | 0.012 | 1,623 (72%) | 817 (72%) | >0.9 |
| Anti-arrhythmic¥ | 65 (39%) | 50 (39%) | >0.9 | 44 (3.5%) | 34 (3.8%) | 0.7 |
| RAS-inhibitor | 352 (89%) | 165 (87%) | 0.6 | 2,083 (93%) | 1,071 (95%) | 0.054 |
| Beta-blocker | 256 (64%) | 130 (69%) | 0.3 | 1,826 (81%) | 933 (82%) | 0.5 |
| Digoxin | 225 (57%) | 94 (50%) | 0.11 | 1,176 (52%) | 516 (46%) | <0.001 |
| Diuretics | 356 (90%) | 158 (84%) | 0.036 | 1,859 (83%) | 883 (78%) | <0.001 |
Summaries presented as median (IQR), or n (%).
Information available only for 1,982 patients.
Information available only for 2,542 patients.
Welch Two Sample t-test or Pearson’s Chi-squared test.
AF: atrial fibrillation, CRT: cardiac resynchronization therapy, ICD: implantable cardioverter defibrillator, LBBB: left bundle branch block, LVEF: left ventricular ejection fraction, NYHA: New York Heart Association, RAS: renin-angiotensin system. SD: Standard deviation. IQR: interquartile range.
For patients without AF, most baseline characteristics were evenly distributed, except for patients on digoxin (52% for the CRT group, 46% for the no-CRT group, p<0.001).
Outcomes
The HRs for all outcomes and the interaction terms are shown in Table 3. For the overall population, CRT was associated with a significantly longer time to HFH or all-cause mortality (adjusted HR: 0.69, 95% CI: 0.56 – 0.81, p<0.001) but was not significantly associated with a longer time to all-cause mortality (adjusted HR: 0.82, 95% CI: 0.66 – 1.01, p=0.067).
Table 3.
Primary and secondary outcomes associated with CRT for patients with and without a history of AF
| Estimate | 95% Credible interval | Posterior probability | |
|---|---|---|---|
| Time to all-cause mortality or HFH | |||
| HR for CRT overall† | 0.69 | 0.56 – 0.81 | < 0.001 |
| By AF status¥ | |||
| HR for CRT in history of AF | 0.78 | 0.55 – 1.10 | 0.17 |
| HR for CRT in no history of AF | 0.67 | 0.55 – 0.80 | <0.001 |
| Ratio of hazard ratios (History of AF/No AF) | 1.17 | 0.83 – 1.64 | 0.26 |
| Estimate | 95% Credible interval | Posterior probability | |
| Time to all-cause mortality | |||
| HR for CRT overall† | 0.82 | 0.66 – 1.01 | 0.067 |
| By AF status¥ | |||
| HR for CRT in history of AF | 1.09 | 0.70 – 1.74 | 0.70 |
| HR for CRT in no history of AF | 0.76 | 0.60 – 0.97 | 0.024 |
| Ratio of hazard ratios (History of AF/No AF) | 1.45 | 0.89 – 2.27 | 0.14 |
The hazard rate for each outcome in AF subgroups with CRT compared to no CRT. All models are adjusted for age, sex, NYHA class, ejection fraction, QRS width, presence of LBBB, diabetes, hypertension, ischemic etiology, use of antiarrhythmic drugs, use of beta-blockers, use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and the presence of an ICD
Estimates obtained from model with an overall CRT effect.
Estimates obtained from a model CRT effect by AF status (that is a model with an interaction between CRT and AF).
AF: atrial fibrillation, CRT: cardiac resynchronization therapy, HFH: heart failure hospitalization, HR: hazard ratio.
For patients without a history of AF, the association of CRT with longer time to HFH or all-cause mortality (adjusted HR: 0.67, 95% CI: 0.55 to 0.80, p<0.001) and longer time to all-cause mortality (adjusted HR: 0.76, 95%CI: 0.60–0.97, p=0.024) were both statistically significant.
For patients with a history of AF, the association of CRT with a longer time to HFH or all-cause mortality (adjusted HR: 0.78, 95% CI: 0.55 to 1.10, p=0.17) and a longer time to all-cause mortality (adjusted HR: 1.09, 95%CI: 0.70–1.74, p=0.70) was not statistically significant. The interaction (estimate shown as a ratio of HRs) between AF and CRT was not significant for any of the outcomes (p=0.26 for the combined endpoint and p=0.14 for all-cause mortality suggesting that CRT may not result in different outcomes based on the presence or absence of a history of AF.
The HRs with 95% CI for each trial included for the overall population, and for those with and without a history of AF are shown in Figure 3 for time to the combined endpoint and in Figure 4 for the endpoint of time to all-cause mortality alone.
Figure 3. Forest plot for time to heart failure hospitalization or all-cause mortality.
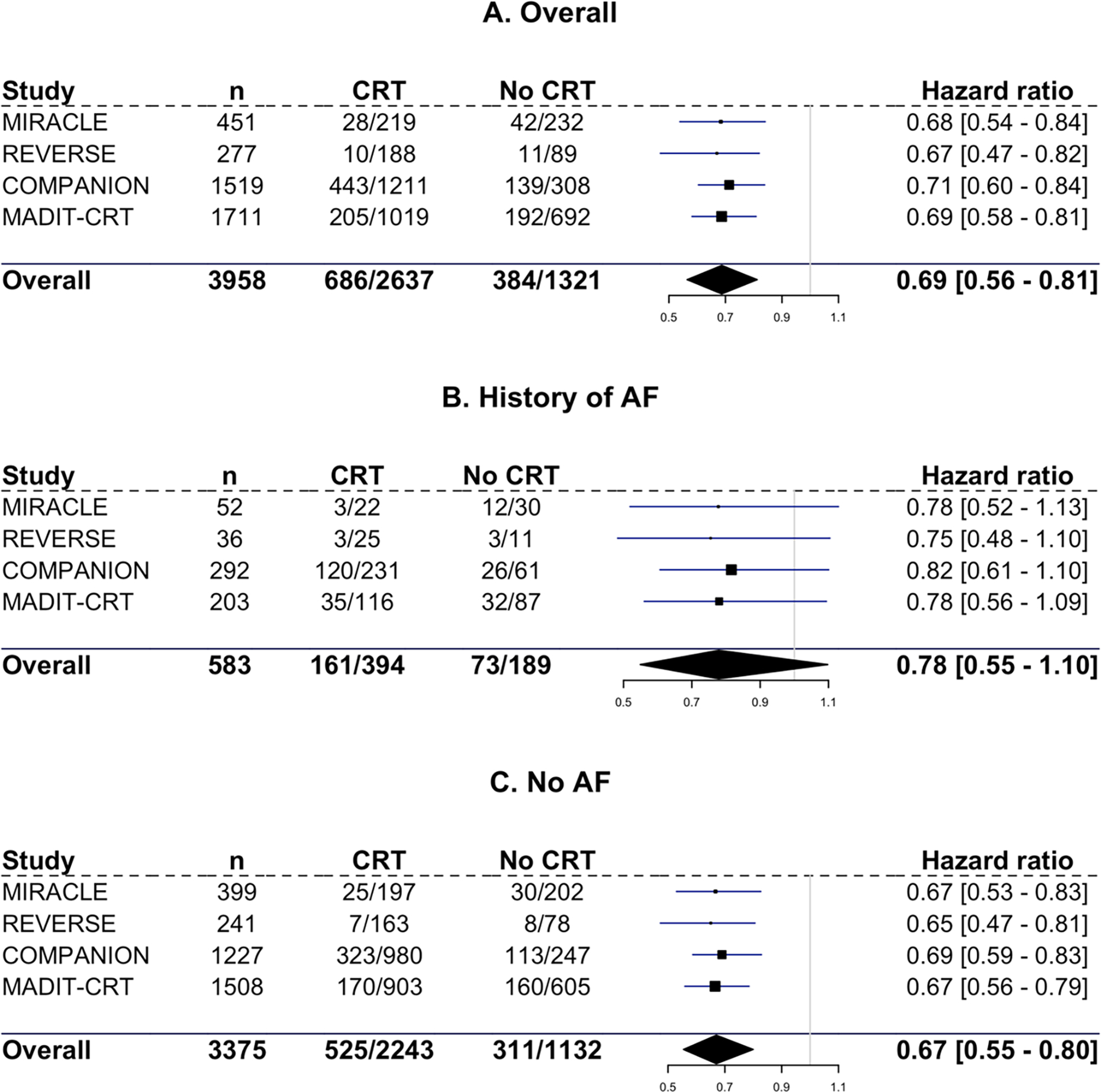
Hazard ratios with 95% credible interval for heart failure hospitalization or all-cause mortality for all three subgroups and for each individual trial. A: overall population. B: Patients with a history of AF. C: Patients without AF.
Figure 4. Forest plot for time to all-cause mortality.
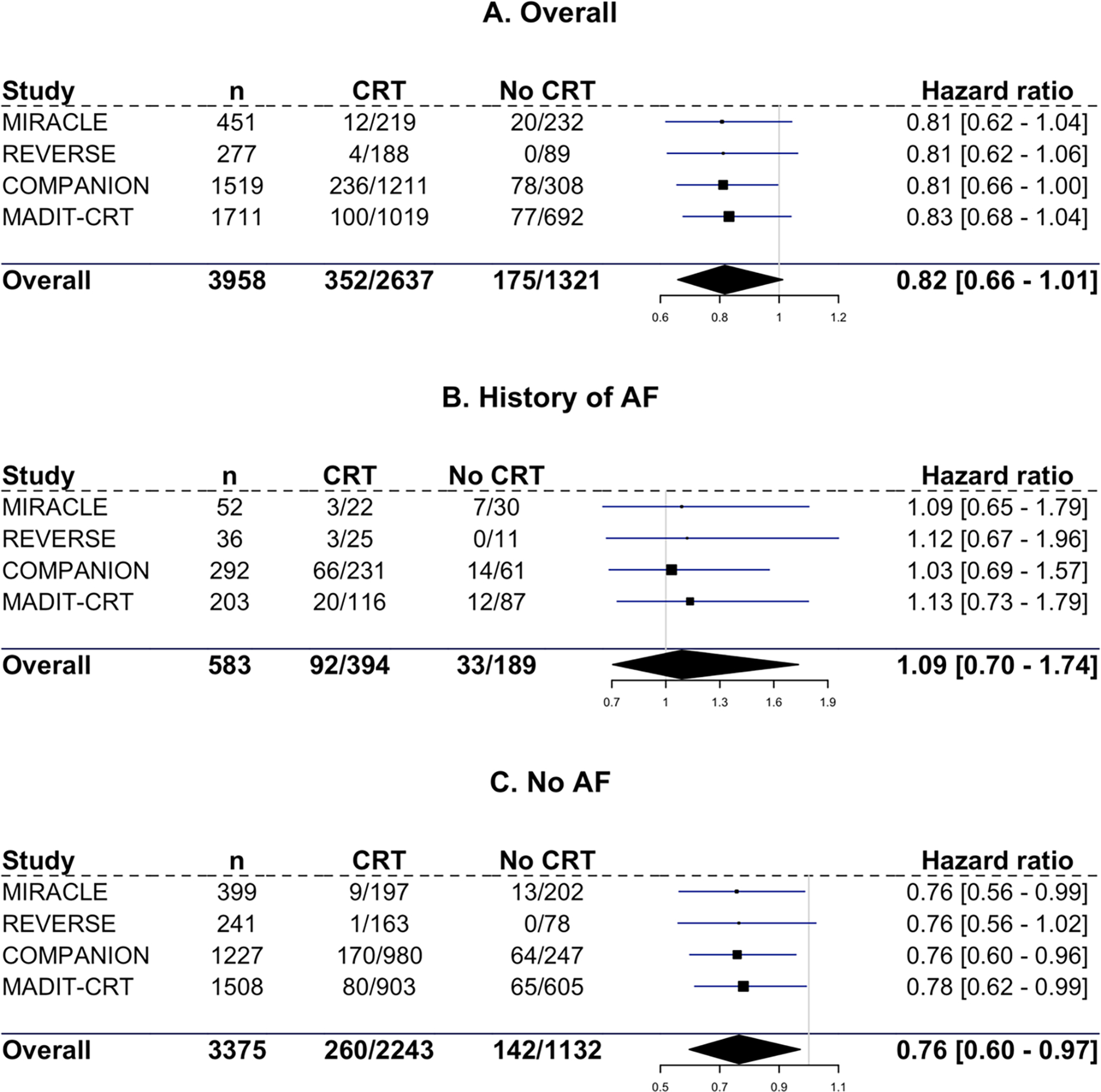
Hazard ratios with 95% credible interval for all-cause mortality for all three subgroups and for each individual trial. A: overall population. B: Patients with a history of AF. C: Patients without AF.
Subgroup analysis
In the subgroup analysis, we analyzed the outcomes in patiens with and without a history of AF in those assigned to CRT (n=2642) (Table 4). In CRT recipients, a history of AF was associated with a significantly shorter time to HFH and all-cause mortality (HR 1.43, 95% CI: 1.15–1.78, p=0.007) and a similar significantly shorter time to all-cause mortality (HR 1.45 95% CI: 1.09–1.99, p=0.013).
Table 4.
Primary and secondary outcomes by history of AF in those assigned to CRT (n=2642).
| Outcome | Total events | Events / history of AF | Events / No AF | HR (history of AF/No AF) | 95% Credible interval | Posterior probability |
|---|---|---|---|---|---|---|
| Time to all-cause mortality or HFH | 689 | 164 | 525 | 1.43 | 1.15 – 1.78 | 0.007 |
| Time to all-cause mortality | 353 | 93 | 260 | 1.45 | 1.09 – 1.99 | 0.013 |
The hazard rate for each outcome in patients with history of AF compared to no history of AF in those assigned to CRT (n=2642). All models are adjusted for age, sex, NYHA class, ejection fraction, QRS width, presence of LBBB, diabetes, hypertension, ischemic etiology, use of antiarrhythmic drugs, use of beta-blockers, use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and the presence of an ICD. AF: atrial fibrillation, CRT: cardiac resynchronization therapy, HFH: heart failure hospitalization, HR: hazard ratio.
Discussion
In this first-to-date individual patient-level data meta-analysis of four clinical trials of CRT in patients with and without a history of AF, we found that 1) few patients were reported to have a history of AF, and these patients were older and had a higher number of comorbidities than those without AF; 2) overall and in patients without a history of AF, CRT was associated with increased time to HFH or mortality; 3) For patients with a history of AF, CRT was not associated with improved outcomes; however, there was no statistically significant interaction between CRT and a history of AF for any outcome suggesting that CRT may not result in different outcomes based on the presence or absence of a history of AF, and 4) in patients with a CRT, a history of AF appeared to be associated with worse outcomes.
According to the European CRT survey, 54.5% of patients upgraded to CRT and 26% of de novo CRT implants are in patients with AF (12,21). In addition, paroxysmal atrial tachyarrhythmias have been found in up to 20% of CRT recipients (22). Therefore, patients with AF or a history of AF constitute a large group for whom little data on CRT efficacy are available. Not surprisingly, and consistent with previous trials, our meta-analysis indicates that CRT patients with AF are older and have more comorbidities including ischemic heart disease and worse kidney function, than those without AF (23). That patients with AF are older and have more comorbidities may mitigate any potential benefit from CRT in heart failure. We did not find a significant association between CRT and outcomes in patients with a history of AF (Table 3). However, even in this meta-analysis of 4 clinical trial, we only had 14.8% patients with a history of AF and thus may have lacked power to discern CRT benefit in this cohort. However, some trials have shown patients with permanent AF benefit from CRT. For example, the MUSTIC (MUltisite STimulation In Cardiomyopathies) AF trial, which recruited 59 patients with HF and a broad QRS, permanent AF and a bradyarrhythmic indication for RV pacing, showed a significant sustained improvement in exercise tolerance (as measured by 6-minute walk distance and VO2 uptake) with CRT compared with RV pacing alone [24]. In a post-hoc analysis of the Resynchronization for Ambulatory Heart Failure Trial (RAFT), patients with permanent AF and CRT-Defibrillator had a trend towards a lower risk of HFH when compared with those receiving implantable cardioverter defibrillator (ICD) alone (HR 0.58; 95% Confidence Interval 0.38 – 1.01; p = 0.052).
For CRT recipients with prior history of AF, the data are also scarce, and no randomized trial has yet compared patients with a history of AF with and without CRT. A subgroup analysis from the COMPANION trial, which was also part of the present meta-analysis, showed that patients with a history of AF (n=293) did not derive significant benefit from CRT in relation to time to mortality or HFH (HR 1.16 (95% CI: 0.83 to 1.63); (p=0.38) compared with those without CRT [7]. Like our analysis, the COMPANION substudy did not have sufficient power to show a clear treatment effect of CRT in patients with history of AF.
Previous meta-analyses have shown conflicting results in patients with AF. One meta-analysis of retrospective studies suggested that CRT benefit may be attenuated in patients with AF (9). The Spanish Atrial Fibrillation and Resynchronization [SPARE] Trial, a large retrospective trial, found no differences in clinical response and LV remodeling between patients with sinus rhythm and patients with AF; however, AF was a significant risk factor for heart failure related mortality (24). A previous non case-based meta-analysis conducted more than a decade ago showed no significant mortality-difference by CRT at 1-year follow-up between patients with and without AF (8). Others have reported higher mortality in CRT recipients with AF than those without AF (9) (25,26). To summarize, the evidence for clinical benefit of CRT in patients with AF is conflicting.
There are several reasons why effectiveness of CRT in patients with a history of AF may be reduced. Firstly, AF is generally associated with poorer outcomes in patients with HF regardless of CRT. The reasons for this include loss of atrial systole and decreased cardiac output (27). Secondly, for CRT recipients, AF has detrimental negative effect on biventricular pacing percentage which is associated with poorer outcomes and a higher spontaneously conducted ventricular rates leading to deterioration of LV function (28,29). A recent retrospective study confirmed the importance of biventricular pacing percentage in patients with AF such that biventricular pacing percentage ≤98% had a higher risk of heart transplantation or all-cause mortality whereas patients with AF and a biventricular pacing percentage >98% did not diminish CRT benefit compared with patients without AF (30). Interestingly, other studies have found that even when biventricular pacing exceeds 98% in patients with AF compared to sinus rhythm, worse outcomes are still observed indicating other potential deteriorating factors in AF (29). We speculate if such factors could be the overestimation of the degree of effective biventricular pacing in patients with AF and CRT therapy (31). Unfortunately, biventricular pacing percentage was not available in our study. Hence, in this study we can only speculate upon the relative contribution of biventricular pacing to our results. Additionally, more patients with a history of AF were treated with anti-arrhythmic drugs which may have also negatively influenced outcomes. It is possible that the non-significant association between history of AF and CRT outcomes could be due to the number of patients with a history of AF in the study was relatively small (n=586), limiting the power to detect a statistically significant improvement in outcomes among patients with a history of AF.
Overall, randomized data regarding CRT in patients with heart failure and a history of AF are sorely needed to provide evidence on the role of CRT in this growing patient population.
Strengths and limitations
To our knowledge, this is the largest study of patients with a history of AF and CRT using patient-level data from clinical trials of CRT. We also included patients across all NYHA classes. However, several limitations are noteworthy. Firstly, the number of patients with a history of AF was still relatively small, limiting the statistical power of analyses of all outcomes. There were also no available data on rhythm monitoring, AF burden, biventricular pacing percentage, or frequency of AV junction ablation after randomization, nor AF burden pre-randomization, limiting our ability to delineate the specific association of having a history of AF with outcomes. Patients with permanent AF were not included, and all included patients were in sinus rhythm at the time of enrollment. The control group (no CRT) included both pharmacotherapy and ICDs and therefore had some heterogeneity. Finally, this was a post hoc analysis, and it is possible that unmeasured confounders across trials could have impacted the associations of interest.
Conclusions
In this first patient-level meta-analysis of clinical trials of CRT with and without a history of AF, there is evidence of benefits of CRT in the overall population in relation to time to HFH and mortality. The interaction between a history of AF, CRT, and outcomes was not statistically significant, demonstrating overall similar CRT benefit among patients with versus without a history of AF. However, due to small number of patients included with history of AF, the power to detect a statistically significant improvement in outcomes among patients with AF was limited. This uncertainty regarding history of AF and CRT benefit calls for randomized trials to evaluate the treatment effect of CRT in patients with a history of AF.
Supplementary Material
Figure 2. Kaplan-Meier curves for all outcomes.
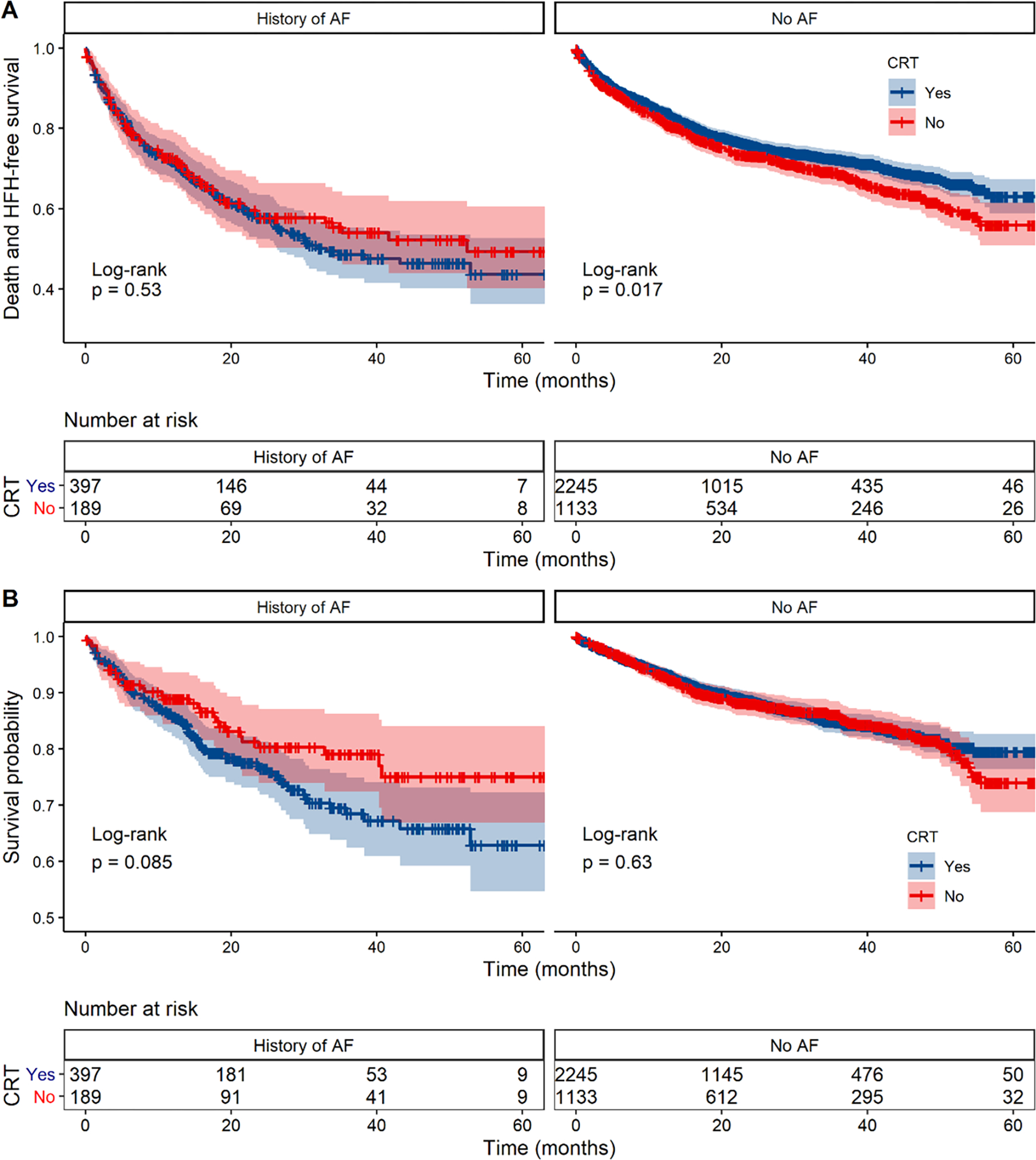
A. Kaplan-Meier survival curves for the combined endpoint of time to HFH or all-cause mortality in patients with and without a history of AF stratified by CRT treatment (blue line: CRT, red line: No CRT). B. Kaplan-Meir survival curves for time to all-cause mortality in patients with and without a history of AF stratified by CRT treatment. The Kaplan-Meier survival curves and log-rank tests are for unadjusted analyses.
Funding:
Primary funding was provided by the National Heart, Lung, and Blood Institute (1R01HL131754). NHLBI did not participate in the literature search, determination of trial eligibility criteria, data analysis or interpretation, or preparation or approval of the manuscript for publication.
Footnotes
Disclosures
Dr. Friedman has received: research support from American Heart Association, Boston Scientific, Biosense Webster, Merit Medical, Medtronic, the National Institutes of Health, and Abbott, and consulting fees from Abbott, AtriCure, Microport, NI Medical, and Sanofi. Dr. Al-Khatib receives research funding from Medtronic and Boston Scientific through grants to her institution. Dr. Cleland reports grants and personal fees from Pharmacosmos, personal honoraria from Abbott, Astra Zeneca, Idorsia, Myokardia, NI Medical, Novartis, Servier anmd Torrent pharmaceuticals; grants and personal honoraria from Amgen/Cytokinetics, Bayer, Bristol Myers Squibb, Johnson & Johnson, Medtronic, Vifor and Viscardia; personal honoraria and non-financial support from Boehringer-Ingelheim outside the submitted work. Dr Fudim was supported by the National Heart, Lung, and Blood Institute (NHLBI (K23HL151744), the American Heart Association (20IPA35310955), Bayer, Bodyport, BTG Specialty Pharmaceuticals and Verily; he receives consulting fees from Abbott, Alleviant, Audicor, AxonTherapies, Bayer, Bodyguide, Bodyport, Boston Scientific, Coridea, CVRx, Daxor, Deerfield Catalyst, Edwards LifeSciences, Feldschuh Foundation, Fire1, Gradient,Intershunt, Medtronic, NXT Biomedical, Pharmacosmos, PreHealth, Shifamed, Splendo, Vironix, Viscardia, Zoll. Dr Linde has received research support to her institution from Swedish Heart-Lung Foundation, Swedish Royal Academy of Science, Roche Diagnostics, Astra Zeneca and Stockholm County Council and speaker honoraria from Medtronic, Impulse Dynamics, Bayer, Boeringer Ingelheim, Novartis, Vifor Pharma and Microport. Dr. Gold serves on a medical advisory board for Medtronic and EBR, receives research support to his institution from Boston Scientific, Abbott and Medtronic, and is a consultant to Boston Scientific and Medtronic. Dr. Curtis serves on medical advisory boards for Janssen Pharmaceuticals, Medtronic, Inc., Abbott, Sanofi Aventis, Milestone Pharmaceuticals, and Eagle Pharmaceuticals; she has received honoraria for speaking from Abbott and Medtronic. All other authors have no conflict of interest to report.
Data availability statement
The datasets analyzed during the current study are not publicly available due to data use agreements with the individual clinical trials but are available from the corresponding author on reasonable request following review by the principal investigator leadership group
References
- 1.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, et al. Effects of Multisite Biventricular Pacing in Patients with Heart Failure and Intraventricular Conduction Delay. New England Journal of Medicine. 2001. Mar 22;344(12):873–80. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. New England Journal of Medicine. 2004. May 20;350(21):2140–50. [DOI] [PubMed] [Google Scholar]
- 3.Ruwald MH, Solomon SD, Foster E, Kutyifa V, Ruwald AC, Sherazi S, et al. Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: results from the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT-CRT) trial. Circulation. 2014. Dec 23;130(25):2278–86. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. New England Journal of Medicine. 2009. Oct 1;361(14):1329–38. [DOI] [PubMed] [Google Scholar]
- 5.Tang ASL, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure. New England Journal of Medicine. 2010. Dec 16;363(25):2385–95. [DOI] [PubMed] [Google Scholar]
- 6.Barold SS, Herweg B. Cardiac Resynchronization in Patients with Atrial Fibrillation. J Atr Fibrillation. 2015. Dec;8(4):1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalscheur MM, Saxon LA, Lee BK, Steinberg JS, Mei C, Buhr KA, et al. Outcomes of cardiac resynchronization therapy in patients with intermittent atrial fibrillation or atrial flutter in the COMPANION trial. Heart Rhythm. 2017. Jun 1;14(6):858–65. [DOI] [PubMed] [Google Scholar]
- 8.Upadhyay GA, Choudhry NK, Auricchio A, Ruskin J, Singh JP. Cardiac resynchronization in patients with atrial fibrillation: a meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2008. Oct 7;52(15):1239–46. [DOI] [PubMed] [Google Scholar]
- 9.Wilton SB, Leung AA, Ghali WA, Faris P, Exner DV. Outcomes of cardiac resynchronization therapy in patients with versus those without atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2011;8(7):1088–94. [DOI] [PubMed] [Google Scholar]
- 10.Healey JS, Hohnloser SH, Exner DV., Birxnie DH, Parkash R, Connolly SJ, et al. Cardiac resynchronization therapy in patients with permanent atrial fibrillation: Results from the Resynchronization for Ambulatory Heart Failure Trial (RAFT). Circ Heart Fail. 2012. Sep;5(5):566–70. [DOI] [PubMed] [Google Scholar]
- 11.Khazanie P, Hammill BG, Qualls LG, Fonarow GC, Hammill SC, Heidenreich PA, et al. Clinical effectiveness of cardiac resynchronization therapy versus medical therapy alone among patients with heart failure: analysis of the ICD Registry and ADHERE. Circ Heart Fail. 2014. Nov 1;7(6):926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickstein K, Normand C, Auricchio A, Bogale N, Cleland JG, Gitt AK, et al. CRT Survey II: a European Society of Cardiology survey of cardiac resynchronisation therapy in 11 088 patients-who is doing what to whom and how? Eur J Heart Fail. 2018. Jun 1;20(6):1039–51. [DOI] [PubMed] [Google Scholar]
- 13.Fudim M, Dalgaard F, Al-Khatib SM, J. Friedman D, Lallinger K, Abraham WT, et al. Future research prioritization in cardiac resynchronization therapy. Am Heart J. 2020. May 1;223:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linde C, Abraham WT, Gold MR, St. John Sutton M, Ghio S, Daubert C, et al. Randomized Trial of Cardiac Resynchronization in Mildly Symptomatic Heart Failure Patients and in Asymptomatic Patients With Left Ventricular Dysfunction and Previous Heart Failure Symptoms. J Am Coll Cardiol. 2008. Dec 2;52(23):1834–43. [DOI] [PubMed] [Google Scholar]
- 15.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined Cardiac Resynchronization and Implantable Cardioversion Defibrillation in Advanced Chronic Heart Failure. JAMA. 2003. May 28;289(20):2685. [DOI] [PubMed] [Google Scholar]
- 16.Abraham WT, Young JB, León AR, Adler S, Bank AJ, Hall SA, et al. Effects of Cardiac Resynchronization on Disease Progression in Patients With Left Ventricular Systolic Dysfunction, an Indication for an Implantable Cardioverter-Defibrillator, and Mildly Symptomatic Chronic Heart Failure. Circulation. 2004. Oct 18;110(18):2864–8. [DOI] [PubMed] [Google Scholar]
- 17.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac Resynchronization in Chronic Heart Failure. New England Journal of Medicine. 2002. Jun 13;346(24):1845–53. [DOI] [PubMed] [Google Scholar]
- 18.Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, et al. Biventricular Pacing for Atrioventricular Block and Systolic Dysfunction. New England Journal of Medicine. 2013. Apr 25;368(17):1585–93. [DOI] [PubMed] [Google Scholar]
- 19.Cleland JGF, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. New England Journal of Medicine. 2005. Apr 14;352(15):1539–49. [DOI] [PubMed] [Google Scholar]
- 20.Lunn D, Jackson C, Best N, Thomas A, Spiegelhalter D. The BUGS Book Statistics The BUGS Book. 2012. [Google Scholar]
- 21.Linde CM, Normand C, Bogale N, Auricchio A, Sterlinski M, Marinskis G, et al. Upgrades from a previous device compared to de novo cardiac resynchronization therapy in the European Society of Cardiology CRT Survey II. Eur J Heart Fail. 2018. Oct 1;20(10):1457–68. [DOI] [PubMed] [Google Scholar]
- 22.Leclercq C, Padeletti L, Cihák R, Ritter P, Milasinovic G, Gras D, et al. Incidence of paroxysmal atrial tachycardias in patients treated with cardiac resynchronization therapy and continuously monitored by device diagnostics. Europace. 2010;12(1):71–7. [DOI] [PubMed] [Google Scholar]
- 23.Gasparini M, Leclercq C, Lunati M, Landolina M, Auricchio A, Santini M, et al. Cardiac Resynchronization Therapy in Patients With Atrial Fibrillation: The CERTIFY Study (Cardiac Resynchronization Therapy in Atrial Fibrillation Patients Multinational Registry). JACC Heart Fail. 2013. Dec 1;1(6):500–7. [DOI] [PubMed] [Google Scholar]
- 24.Tolosana JM, Hernandez Madrid A, Brugada J, Sitges M, Garcia Bolao I, Fernandez Lozano I, et al. Comparison of benefits and mortality in cardiac resynchronization therapy in patients with atrial fibrillation versus patients in sinus rhythm (Results of the Spanish Atrial Fibrillation and Resynchronization [SPARE] Study). Am J Cardiol. 2008. Aug 15;102(4):444–9. [DOI] [PubMed] [Google Scholar]
- 25.Smit MD, Maass AH, Hillege HL, Wiesfeld ACP, Van Veldhuisen DJ, Van Gelder IC. Prognostic importance of natriuretic peptides and atrial fibrillation in patients receiving cardiac resynchronization therapy. Eur J Heart Fail. 2011. May;13(5):543–50. [DOI] [PubMed] [Google Scholar]
- 26.Van Bommel RJ, Borleffs CJW, Ypenburg C, Marsan NA, Delgado V, Bertini M, et al. Morbidity and mortality in heart failure patients treated with cardiac resynchronization therapy: influence of pre-implantation characteristics on long-term outcome. Eur Heart J. 2010. Nov;31(22):2783–90. [DOI] [PubMed] [Google Scholar]
- 27.Chouairi F, Pacor J, Miller PE, Fuery MA, Caraballo C, Sen S, et al. Effects of Atrial Fibrillation on Heart Failure Outcomes and NT-proBNP Levels in the GUIDE-IT Trial. Mayo Clin Proc Innov Qual Outcomes [Internet]. 2021. Apr [cited 2023 May 26];5(2):447. Available from: /pmc/articles/PMC8105522/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ousdigian KT, Borek PP, Koehler JL, Heywood JT, Ziegler PD, Wilkoff BL. The epidemic of inadequate biventricular pacing in patients with persistent or permanent atrial fibrillation and its association with mortality. Circ Arrhythm Electrophysiol. 2014;7(3):370–6. [DOI] [PubMed] [Google Scholar]
- 29.Hayes DL, Boehmer JP, Day JD, Gilliam FR, Heidenreich PA, Seth M, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm [Internet]. 2011. [cited 2023 May 26];8(9):1469–75. Available from: https://pubmed.ncbi.nlm.nih.gov/21699828/ [DOI] [PubMed] [Google Scholar]
- 30.Jacobsson J, Reitan C, Carlson J, Borgquist R, Platonov PG. Atrial fibrillation incidence and impact of biventricular pacing on long-term outcome in patients with heart failure treated with cardiac resynchronization therapy. BMC Cardiovasc Disord. 2019. Aug 13;19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamath GS, Cotiga D, Koneru JN, Arshad A, Pierce W, Aziz EF, et al. The Utility of 12-Lead Holter Monitoring in Patients With Permanent Atrial Fibrillation for the Identification of Nonresponders After Cardiac Resynchronization Therapy. J Am Coll Cardiol. 2009. Mar 24;53(12):1050–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to data use agreements with the individual clinical trials but are available from the corresponding author on reasonable request following review by the principal investigator leadership group


