Abstract
Mechanotransduction is the ability of cells to “feel” or sense their mechanical microenvironment and to integrate and convert these physical stimuli into adaptive biochemical cellular responses. This phenomenon is vital for the physiology of numerous nucleated cell types to affect their various cellular processes. As the main drivers of hemostasis and clot retraction, the platelet also possesses this ability to sense the dynamic mechanical microenvironments of the circulation and convert those signals into biological responses integral to clot formation. Like other cell types, platelets leverage their “hands” or receptors/integrins to mechanotransduce important signals responding to vascular injury to achieve hemostasis. The clinical relevance of cellular mechanics and mechanotransduction is imperative as pathological alterations or aberrant mechanotransduction in platelets has been shown to lead to bleeding and thrombosis. As such, the aim of this review is to provide an overview of the most recent research related to platelet mechanotransduction, from platelet generation to platelet activation within the hemodynamic environment and clot contraction at the site of vascular injury, thereby covering the entire “life cycle” of the platelet. Additionally, we describe the key mechanoreceptors in platelets and discuss the new biophysical techniques that have enabled the field to understand how platelets sense and respond to their mechanical microenvironment via those receptors. Finally, the clinical significance and the importance of continued exploration of platelet mechanotransduction is discussed, as the key to better understanding both thrombotic and bleeding disorders lies with a more complete mechanistic understanding of platelet function by way of mechanotransduction.
Keywords: biomechanics, blood platelets, hemostasis, mechanotransduction, thrombosis
Introduction:
Mechanotranduction is the ability of cells to “feel”, sense, interact and convert mechanical stimuli from the physical microenvironment to biochemical signals that elicit adaptive cellular response. Over the last two decades, the field of mechanobiology has made substantial progress investigating how nucleated cells respond to their mechanical microenvironment. Specifically, how cells respond to the elastic (1, 2), viscous (3) and viscoelastic microenvironments(4-6). Studies have shown that mechanotransduction of the signals from these microenvironments effect cell adhesion(3), spreading (7), stem cell fate(1, 6) and cell migration(8). Most importantly it has been shown that mechanobiology and mechanotranduction in various cells have important clinical relevance (9, 10). Like nucleated cells, although less studied, platelets mechanotransduce signals from the mechanical microenvironment to adapt to their microenvironment, however, again like nucleated cells the challenge of translation is in creating tools able to closely mimic the in vivo microenvironment to undergo biomechanical studies with physiological relevance.
Hemostasis is a complex and inherently mechanical process involving both the coagulation cascade and platelets to form a clot at the site of injury to promote wound closure (11). Platelets in circulation are recruited to sites of vascular injury and activated though means of biochemical and biomechanical cues. Mechanically, during primary hemostasis, shear, and injury to endothelial cells on the blood vessel wall exposes the subendothelium, consequently allowing platelets to adhere to various proteins, such as collagen with the receptor GPVI and the integrin α2β1 and additionally adhere to von Willebrand Factor (vWF) with the receptor GP1b complex. Platelets then begin to accumulate and aggregate to each other with their surface integrin α2bβ3(GPIIb/IIIa) to form a platelet plug. Simultaneously, during secondary hemostasis, thrombin produced by the coagulation cascade converts fibrinogen to fibrin polymers while additionally further activating platelets, causing platelets to spread and contract, which reinforces and stabilizes the developing clot. As such, it is evident that the ability of platelets to sense and respond cues is of vital importance for thrombus formation.
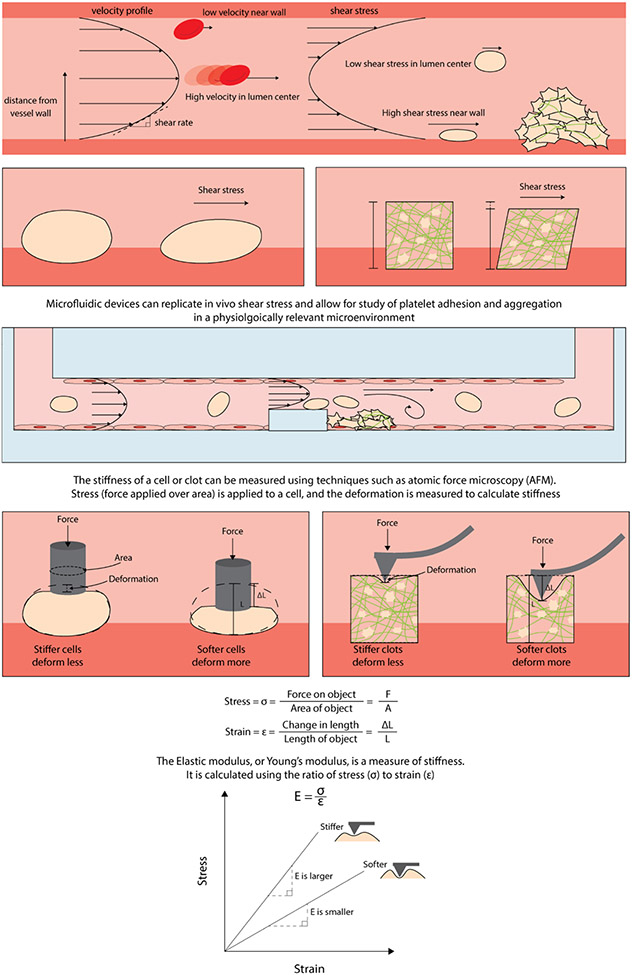
Many biophysical technologies have recently been developed that enable the investigation of these processes with quantitative rigor (Figure 1). One example is microfluidic devices that have been leveraged to experimentally mimic in vivo conditions of vascular flow. Numerous microfluidic technologies have led to key insights into platelet physiology as they allow for complete control of not only the biochemical microenvironment but the mechanical one as well. Because it is possible to control flow rate and shear conditions with these techniques, it has been shown these biophysical parameters are essential for thrombus formation and discoid platelet aggregation (12) and because it is possible to integrate various protein substrates that are expressed on the surface of the vascular endothelium, closely mimicking the in vivo microenvironment becomes possible(13) (Figure 1). Additionally, microfluidics allow for complete control of the geometry that platelets are exposed to and can be varied in order to mimic different blood vessels in the body, such as valves within the venous system(14) and stenosed blood vessels (15). The added ability to directly culture endothelial cells themselves (16, 17) within these devices has allowed for proper investigation into how platelets respond to blood vessel injury (18, 19). In regard to understanding platelet contraction and the final clot microenvironment, atomic force microscopy (AFM) has been used to measure cell length and force to characterize biomechanics of platelet contraction force and dynamics (20). Using AFM, it has been observed that platelets contract rapidly and generate high contraction and adhesive forces in a stiffness-dependent manner (Figure 1). This is suggestive of heterogeneity, with higher density of fibrin exhibiting higher stiffness. Platelet contraction may result in increases of force in such areas of increased stiffness (21). Consequently, it is evident that the development of in vitro technologies and methodologies are imperative for continued understanding of platelet mechanotransduction.
FIGURE 1.
Biophysics of shear flow and cell stiffness. Fluid flowing through a blood vessel reaches a steady state with a parabolic velocity profile. Shear rate is the change in velocity as the fluid distance from the vessel wall increases. Shear stress is a measure of how much force is acting on an object and is proportional to shear rate and fluid viscosity. Platelets aggregate with fibrin to form a network to develop a clot. The ability to control geometric constraints and endothelialize microfluidics has enabled in vitro studies that closely mimic the in vivo microenvironment. Clot stiffness changes over the course of contraction and deforms as platelets mechanically remodel the fibrin network.
As such, the objective of this review is to 1) discuss how mechanical forces and the mechanical microenvironment influence the initiation, propagation, and the stability of a developing clot, 2) discuss the mechanisms of how platelets leverage key receptors and integrins on their surface to sense and respond to their mechanical microenvironment 3) explore the new technologies and techniques that have enabled bulk and single cell investigations of these phenomena and 4) most importantly discuss why this matters, by highlighting the clinical relevance of platelet mechanotransduction for hemostasis and thrombosis.
Section 1: Platelet biomechanics spanning over the lifespan of the cell
1.1: Platelet production and circulation
With an average lifespan of 7-9 days and in a tightly controlled manner, 1 trillion platelets circulate the blood stream (22, 23). Although not as well characterized, mechanics not only play an imperative role in platelet response to the microenvironment within a growing thrombus, but also in the production of platelets themselves. Biochemically, it has been shown that agonists such as thrombopoietin(23), IL-α (24), CCL5(25) and many chemokine mediators(26) induce megakaryocyte production and/or thrombopoiesis. Mechanically, it has been made clear that the cytoskeleton, specifically microtubules play an essential role in the thrombopoietic process(27). More recently, it has been established that megakaryocytes are mechanically responsive and take in cues from the mechanical microenvironment to produce platelets. Specifically, megakaryocytes respond to shear (28, 29) as well as turbulent energy (30) in order to produce new platelets into circulation.
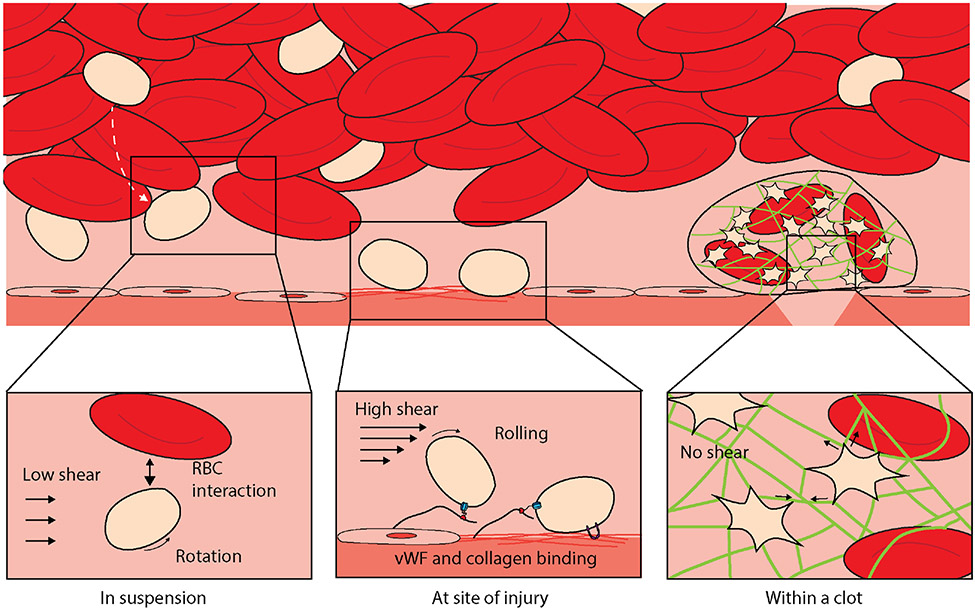
Once produced and in circulation, platelets experience mechanical forces from their surrounding microenvironment and throughout thrombus formation. Fluid shear stress is the most relevant force in platelet-meditated hemostasis and thrombosis (31) (Figure 1). In blood vessels, fluid shear stress is influenced by mechanical forces produced by pressure changes and influenced by the size of the microvasculature, fluid flow rate, and other rheological properties of blood (32). Inactivated platelets in circulation are subjected to these same mechanical forces under flow conditions in blood vessels. Platelets in circulation are pushed towards the periphery of flow nearer to maximal shear stresses generated at the vessel wall (Figure 2)(33).
FIGURE 2.
Biomechanical platelet interactions with the microenvironment. When platelets are in suspension in circulation, they not only move in the direction of the flow but also rotate and are subjected to mechanical interactions with red blood cells, which “push” them to the periphery. After vascular injury occurs, the extracellular matrix (collagen, laminin, and fibronectin) is exposed, and platelets in a high-shear environment bind and tether to von Willibrand factor and bind to collagen to begin thrombus formation. Following this, platelet activation and aggregation occur with signaling of mechanical and biochemical pathways. This subsequently leads to platelet plug formation and dot formation to dramatically shrink and stabilize the thrombus.
1.2: Platelets at the site of vascular injury
During vascular injury, convection and diffusion drive platelets to collide with the vascular surface, enabling interactions with the exposed subendothelium (Figure 2). Plasma vWF is immobilized onto the exposed subendothelial matrix and in doing so, vWF becomes a ligand for mechanopresentation binding to the mechanoreceptor GPIb(34). Under high shear rate flow conditions, vWF binds to its platelet receptor, GPIb-IX to form a “catch bond”. This bond allows transient platelet adhesion under high shear stress present in arteries and arterioles (35-37) (Figure 2).
Platelets express integrins that transduce signals from within the platelet and from the mechanical microenvironment. Under shear stress, vWF binds on both the platelet GPIb-IX-V complex and α2bβ3 (GPIIb/IIIa) to mediate aggregation (31). Shear-activated platelets release stored vWF to promote thrombus formation. The growth of the thrombus is further influenced by increasing shear rate as platelets on the surface of the thrombus are subject to greater activation and aggregation than those platelets within the core (38, 39). This demonstrates the effect of blood rheology in thrombus development as alterations in the microenvironment influence platelet adhesiveness onto thrombogenic surfaces and the rate of thrombus growth (40).
Stable platelet adhesion to the vessel wall is an essential first step in thrombus formation in response to vascular injury (41). Additionally, at sites of vascular injury and in response to changes in vessel geometry, it has been found that discoid platelets form aggregates due to rapid changes in the hemodynamic environment(12). To undergo this, platelets mechanosense shear microgradients by sensing sudden accelerations and decelerations in shear that lead to the formation and restructuring/strengthening of GPIb and α2bβ3 (GPIIb/IIIa) membrane tethers (42). These discoid platelet aggregates are then converted to stable platelet aggregates by the release of soluble platelet agonists such as ADP(42). Similarly, platelet activation mediated by GPVI and GPIb and subsequent biochemical secretion of platelet agonists reinforce αIIbβ3-dependent platelet aggregation. Fibrinogen is critical for formation of a stable thrombus and once the thrombus is formed, platelets within interact with a fibrin meshwork. Subsequent stable thrombus formation and clot retraction is driven by biochemical transduction mediated by integrin αIIbβ3 through outside-in signals following fibrinogen binding (43). Importantly, platelets mechanosense the stiffness of fibrin and fibrinogen as the fibrin meshwork forms in thrombus formation. The capability of platelets to mechanosense stiffness in the microenvironment in a growing thrombus allows for platelet mechanotransduction into further platelet aggregation and activation (20). Platelets are activated nonuniformly within the developing clot(44). As platelets contract and pull fibrin fibers together, secondary aggregates of platelets form, thereby remodeling the fibrin network and increasing the density and clot stiffness. Platelet contraction mechanically remodels the fibrin network of the clot over the course of clot contraction (45) (Figure 2).
Section 2: Overview of the mechanoreceptors in platelets
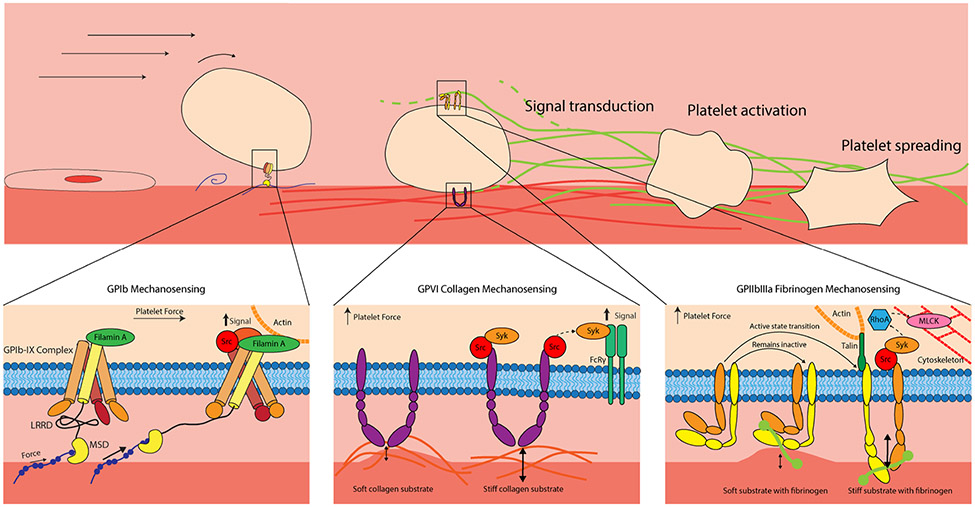
As noted previously mechanical forces play a major role in thrombus formation to stem bleeding. These processes all require platelet integrins or glycoproteins aka “hands” to interact, grab, and hold onto various proteins in the microenvironment such as vWF, collagen and fibrinogen (Figure 3). The major mechanoreceptors that we will discuss are the vWF receptor GPIb-IX complex, the fibrin(ogen) receptor α2bβ3 (GPIIb/IIIa) and the collagen receptors GPVI and α2β1.
FIGURE 3.
Platelet mechanotransduction occurs in all aspects of hemostasis after injury. Glycoprotein (GP)Ib mechanosensing: GPIb binds to von Willibrand factor and leverages its mechanosensory domain to activate platelets. GPVI collagen mechanosensing: GPVI interacts with collagen to activate platelets and support stable adhesion, responding to stiffness in the mechanical microenvironment. GPIIb/IIIa fibrinogen mechanosensing: The integrin αIIIb β3 (GPIIb/IIIa), the most abundant receptor on platelets, interacts with fibrin(ogen) and responds to changes in the developing clot microenvironment.
2.1: Overview of GP1b-IX complex and mechanotransduction signaling mechanisms
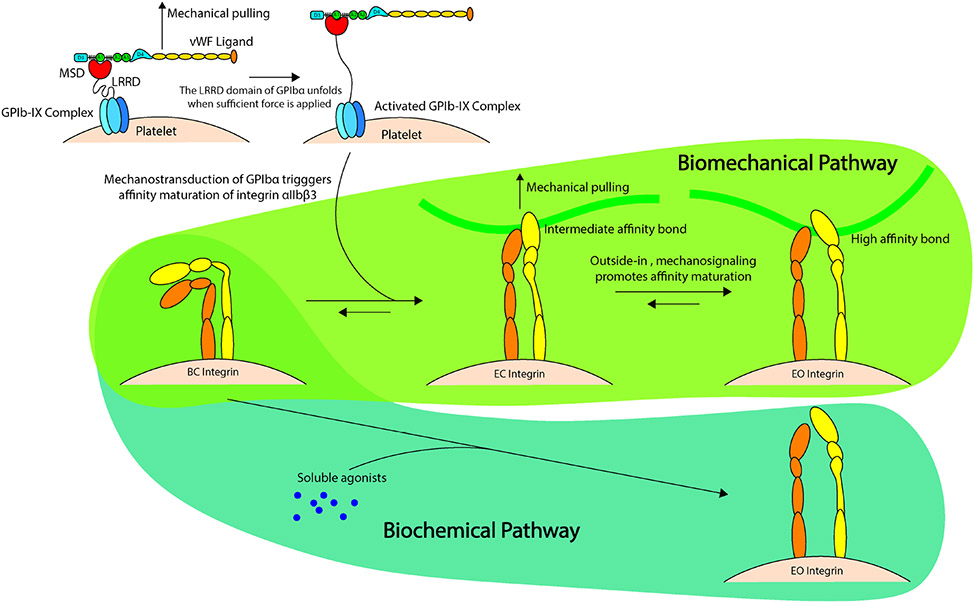
The GPIb-IX complex, with approximately 25,000 copies (46) is an essential platelet glycoprotein, where dysfunction and/or absence of this receptor leads to Bernard-Soulier syndrome, in which patients have a propensity to bleed(47). The GPIb-IX complex allows for platelets to sense mechanical force while responding to vascular injury through a mechanosensory domain (MSD) within the complex (Figure 3) (48). The MSD within GPIb acts as the mechanoreceptor, mechanotransmitter and mechanotransducer upon presentation of the vWF A1 domain. In physiological flow conditions, A1 is shielded in vWF and will undergo morphological changes under shear stress(49). For vWF to expose its A1 domain, it mechanosenses fluid shear to straighten the folded globular conformation of a vWF multimer. The MSD in GPIb is in a folded state until unfolded by vWF-mediated pulling which occurs in the presence of shear flow. Binding of vWF to MSD propagates force along the GPlb macroglycopeptide stalk to transmit platelet intracellular biochemical signaling by enhancing calcium triggering in platelets. Intracellular calcium triggering activates integrin α2bβ3 (GPIIb/IIIa) (49). Additionally, the MSD also plays a critical role in mechanotransduction via GPIb to induce a biomechanical pathway of integrin α2bβ3 (GPIIb/IIIa) affinity maturation and thrombus development which we will discuss further when discussing the integrin α2bβ3 (GPIIb/IIIa) (50).
The structure of GPIb-IX complex consists of GPIbα, GPIbβ and GPIX subunits (51). The GPIbα subunit N-terminal domain associates with vWF A1 domain via catch-bond to facilitate platelet tethering. There is a leucine-rich repeat domain (LRRD) within N-terminal domain of the ligand binding domain of GPIbα that is unfolded by this interaction with vWF (52). This ligand-receptor engagement further strengthens the vWF-A1 bond to prolong the bond lifetime and increases the likelihood of unfolding the MSD (53, 54) Fluorescence biomembrane force probe has enabled single-cell analysis of ligand binding kinetics to demonstrate the role of MSD unfolding in GPIb mechanosensing and has shown LRRD unfolding prolongs the bond vWF-A1 bond lifetime (55, 56). The C-terminal domain of GPIbα also plays an essential role by interacting with Filamin, an actin binding protein, and promoting vWF induced platelet activation(57, 58).
Several studies elucidated the mechanism by which MSD enables platelet mechanosensing. The use of optical tweezers enabled pulling of the N-terminal domain within GPIbα with recombinant vWF-A1 (48). This induced unfolding of a domain in the juxtamembrane stalk of GPIbα and thereby identified the MSD. Furthermore, it was identified that forces ranging from 5 to 20 pN were required to unfold the MSD which is comparable to the forces that induce catch-bonds in forming the complex of vWF-A1 with GPIbα. This unfolding force is lower than the drag force exerted on a platelet under physiologic shear conditions in circulation (49).
The ‘trigger’ model of GPIb-IX signaling further explained the shear requirement of the MSD to be induced and unfold from its resting folded state (49). The MSD was further established by studies altering the MSD regions in GPIb mutants and it was shown that no other region of the molecule served as an alternative mechanotransducer (59). Further it was identified that the unfolded MSD can refold with or without applied forces and is relatively unstable. Therefore, the MSD can refold and turn off the mechanosensory signaling of GPlb. The MSD requires a continuous pulling force of >15pN to fully active GPIb-IX (60). Thus, the microenvironment influences the conformation and stability of the MSD with downstream implications of platelet mechanotransduction. Pathologically, the clearance of platelets has more recently been linked to the MSD in type 2B von Willebrand’s Disease, where binding of vWF under physiological shear induces MSD unfolding and thereafter intracellular signaling that eventually leads to the exposure of β-galactose on the surface of the platelet causing enhanced clearance and thrombocytopenia(49).
Although the mechanotransductory mechanisms of the GPIb-IX complex are well characterized, one additional signaling mechanism that remains elusive and controversial is the role of GPIb-IX in thrombin induced platelet activation. Although some studies have shown little evidence that the GPIb-IX complex is associated with thrombin induced platelet activation(61), it has been shown that GPIb is necessary for full activation of platelets essentially working synergistically with protease-activated receptor (PAR)-1 as a co-receptor (62), but not PAR-4(63) (two key thrombin receptors). More recently, it has been shown that thrombin actually induces an independent pathway of signaling in GPIb(64) that works cooperatively with the PAR receptors leading to enhanced platelet activation(65). It is common for biological pathways to be redundant to intensify and strengthen a processes like hemostasis and as such, further investigation is necessary to determine the role of GPIb-IX complex in thrombin induced platelet activation.
2.3: Overview of α2bβ3 (GPIIb/IIIa) signaling and mechanotransduction mechanisms
The integrin α2bβ3 (GPIIb/IIIa), with approximately 80,000 copies per platelet, is the most abundant receptor on the platelet surface(66) and binds to arginine-glycine-aspartic acid (RGD) sites on fibrinogen, fibrin, vWF and fibronectin. The clinical relevance and the importance of α2bβ3 (GPIIb/IIIa) in hemostasis was established by the discovery of Glanzmann’s thrombasthenia, an autosomal recessive bleeding disorder that is caused by impaired synthesis and/or function of α2b and/or β3 subunits leading to impaired platelet function(67, 68). The conformation and affinity of α2bβ3 (GPIIb/IIIa) is tightly regulated and in resting platelets α2bβ3 (GPIIb/IIIa) adopts the inactive or bent confirmation and upon activation this low affinity integrin conformation adopts an active or extended conformation, possessing enhanced binding efficiency to fibrin(ogen)(69). Like other integrins, α2bβ3 (GPIIb/IIIa) bears a mechanical load to undergo mechanotransduction. This load can originate intracellularly from the acto-myosin machinery or inputs from the extracellular mechanical microenvironment (Figure 3). As such the signaling of α2bβ3 (GPIIb/IIIa) is bidirectional from either inside-out signaling and/or outside-in signaling with the end product leading to increased engagement of α2bβ3 (GPIIb/IIIa) to fibrin(ogen) and clot retraction. Inside-out signaling is a very redundant process as it can progress through vWF and/or collagen binding to glycoproteins at the site of injury, thrombin generated during secondary hemostasis, and/or ADP and TXA2 released granules within the platelet. The numerous signaling receptors that bind to these agonists cause triggering of downstream events leading to the activation of Rap1 (small GTPase) and recruitment of the mechanosensitive proteins talin and kindlin(70) which bind to the cytoplasmic tail of β3. This consequently causing a conformational change of α2bβ3 (GPIIb/IIIa) from the inactive bent conformation to the activated extended open conformation, allowing for connections between the intracellular cytoskeleton (acto-myosin machinery) and the extracellular matrix. This enabling strong interactions between α2bβ3 (GPIIb/IIIa and fibrin(ogen), allowing for force generation. Deficiencies in either talin or kindlin have been shown to be associated with bleeding diathesis and as such both proteins are essential for sufficient signaling and activation of α2bβ3 (GPIIb/IIIa) (71, 72). Additionally, it has been shown that the cytosolic protease, calpain, cleaves talin in order to increase platelet contraction of fibrin(73).
Recent advances in single platelet biophysical techniques have recognized a distinct intermediate state of α2bβ3 (GPIIb/IIIa) and as such a biomechanical pathway of inside-out signaling that is slightly different than what is known biochemically. Using biomembrane force probes (BFP)(50) it was shown that when triggered by mechanical stimulation, GP1b induces intracellular calcium release thereby promoting the intermediate state of α2bβ3 (GPIIb/IIIa), a state with affinity and bond lifetimes that are intermediate of both the inactive and active conformations. This in turn leading to outside-in signaling allowing for platelet aggregation and thrombus development(50) (Figure 4). Outside-in signaling occurs when α2bβ3 (GPIIb/IIIa) binds to and interacts with fibrin(ogen), here substrate stiffness plays an essential role as soft substrates result in weak α2bβ3 (GPIIb/IIIa) interactions with fibrinogen, while stiffer substrates lead to increased outside-in signaling, consequently causing increased platelet activation (Phosphytidyl serine exposure, granule release etc), adhesion, spreading and contraction(74, 75). This increased biophysical input from outside-in signaling alongside the biochemical input from inside-out signaling synergistically drive platelet aggregation and contraction. Similarly, utilizing subcellular techniques to investigate platelet integrin response to the mechanical microenvironment, it was shown that platelet integrins respond to tangential tension from the mechanical microenvironment (76) and that low level integrin tension is produced during adhesion while high level integrin tension is produced during contraction(77).
FIGURE 4.
Platelet mechanotransduction demonstrated using the “trigger” model of glycoprotein GPIb-IX signaling and integrin αIIIb β3 affinity maturation. First, mechanoreception occurs by mechanical pulling force applied by von Willibrand factor on GPIbα to unfold. Mechanoreception is demonstrated by leucine-rich repeat domain engaging with GPIbα. This leads to mechanotransmission along the GPIbα macroglycopeptide stalk and transfers this signal via mechanotransduction to induce platelet activation via the biochemical pathway (soluble-agonist dependent) and biomechanical pathway. The biomechanical pathway upregulates integrin αIIIb β3 from the inactive state (bent closed) to the intermediate state (extended closed). Outside-in signaling further promotes affinity maturation to the high-affinity state (extended open integrin conformation).
Unlike the GPIb-IX complex, the mechanosensitive domains/epitopes of α2bβ3 (GPIIb/IIIa) are not as well characterized. What is known is that structurally, the domains of integrins are highly conserved within the integrin family(78). One example of a mechanosensitive domain is the plexin-semaphorin-integrin (PSI) domain, located on the β subunit of α2bβ3 (GPIIb/IIIa). It has been shown to be an important domain for integrin transition from the inactive to the active state(79) similar to its role in activation for the β2 subunit of other integrins(80). Although there is evidence that this domain shows some importance in the transition from the inactive to active confirmation, other domains of α2bβ3 (GPIIb/IIIa) need to be explored in order to determine the epitopes essential for affinity maturation.
2.4: Overview of mechanotransduction on collagen surfaces
One of the most abundant subendothelial proteins for platelet adhesion and aggregation at the injured vessel wall is collagen. Consequently, on their surface, platelets express two collagen receptors, GPVI and the integrin α2β1 in order to trigger platelet activation and aid in thrombus formation. GPVI has been thought to be the main receptor involved in platelet activation, weak adhesion to collagen by activating the integrin α2β1 to promote strong platelet adhesion to collagen (81) and activating the integrin α2bβ3 (GPIIb/IIIa) further leading to platelet activation (82). As such, clinically speaking absence of these receptors is known to cause a mild bleeding phenotype(83). Although GPVI has been known to bind collagen leading to platelet activation, recent evidence has indicated that GPVI also plays an important role in platelet aggregation and development of a thrombus through its interactions with both fibrinogen and fibrin(84) leading to thrombin generation(85). GPVI specifically interacts with fibrinogen in its dimeric form(84) close to its collagen binding site(86) to further support adhesion and enhance platelet activation. Fibrin formation then leads to increased avidity of GPVI and further promotes platelet activation and platelet spreading through mechanisms independent of α2bβ3 (GPIIb/IIIa)(87).
Like α2bβ3 (GPIIb/IIIa), the true mechanotransductory domains of these receptors have not been well characterized, but our group specifically has shown that platelets do mechanosense collagen surfaces of different stiffnesses and spread further on stiffer substrates (Figure 3)(88) and this mechanotransduction is mediated through actin polymerization and the myosin light chain kinase (MLCK), similar to platelet response on fibrinogen coated surfaces(75). Together, knowing GPVI is thought to be involved in early platelet adhesion to collagen(82) and studies showing no difference in the intensity of αIIbβ3 activation on collagen substrates of different stiffnesses, it was postulated that αIIbβ3 activation is mediated through initial binding of GPVI to collagen thereby activating αIIbβ3, while increased spreading area on collagen coated surfaces of different stiffnesses is mediated by α2β1 through outside-in mechanisms(88). Additionally, other studies have elucidated the fact that platelets reorganize their cytoskeleton on collagen surfaces to regulate their spreading behavior depending on the surrounding geometric constraints further highlighting the ability of platelets to “feel” and mechanosense their microenvironment on collagen surfaces (89, 90).
Section 3: Investigating platelet response to the mechanical microenvironment
When thinking of the platelet mechanical microenvironment, it is imperative to discuss whole blood clot contraction and platelet interactions with fibrin(ogen). In brief, during clot contraction, thrombin produced by the coagulation cascade converts fibrinogen to form fibrin polymers and platelets perform many different biophysical actions during this process, they adhere to, they spread to, and contract these fibrin polymers, dramatically stiffening the clot (91, 92). Like most biological tissues, the clot in itself is viscoelastic containing both viscous and elastic properties (93), and this viscoelasticity can vary greatly during the clotting process(94). Historically, research has focused on understanding macroscale platelet mechanical behavior by measuring bulk clot forces(95) and clot viscoelasticity(96). The challenge however with understanding mechanotransduction and single platelet biomechanical behavior with bulk clot assays lies within the complexity of its components and the constantly changing mechanical microenvironment (97). This consequently, making it nearly impossible to decouple the influence of individual components. Additionally, although a necessary step, an issue with decoupling individual components lies with simplifying complex interactions that might influence behavior. Substantial progress has been made to decouple the role of fibrin in this process (96, 98), and more recently progress has been made on the influence the mechanical microenvironment has on platelets due to new single platelet technologies. For example, decoupling the viscous component or fluidity of a developing blood clot and focusing on the contributions of the elastic component of a stiffening clot, single platelet assays have provided key information regarding how single platelets respond to increased and decreased stiffness using standard adhesion assays on hydrogels of varying stiffness (75) and how they respond to different geometries(89) in order to spread and release granules(90).
Understanding how individual platelets integrate into the myriad of the clot environment and generate platelet forces has been a more recent and important area of exploration. Although low throughput, our own lab has leveraged atomic force microscopy (AFM) to measure the contractile forces of single platelets (21) which allowed for the force measurements of platelets in various mechanical microenvironments. Building on this work we developed a microfluidic system, coined the “Platelet Contraction Cytometer” that has increased the throughput of the measurements of single platelet forces from tens to hundreds of platelets(74). This recent advance in our single platelet contraction technology has allowed for a more thorough investigation into platelet biomechanical behavior to different substrates, consequently allowing for key insights into how platelet force is influenced by the stiffness of the mechanical microenvironment (74). Mechanistically our system showed that the Rho kinase and not the Myosin Light Chain Kinase pathway (MLCK) is required for mechanosensitive platelet contraction in various mechanical microenvironments. This was mechanistically interesting as our group had previously shown that other biophysical parameters such as adhesion and spreading were primarily associated with the MLCK pathway(75, 88). This eluding to the concept that different biophysical parameters are associated with different mechanistic pathways.
Although our group has focused its efforts on leveraging fibrin(ogen) as the protein of choice for both our AFM and microfluidic systems, other groups have used collagen or vWF as the protein of choice for their platelet contraction systems and have provided key insights on how platelets behave on these surfaces. Using microfluidics and high shear gradients, it was found that platelet forces of a growing platelet aggregate are sensitive to various platelet inhibitors (99). Similarly, one common technique many groups have utilized to measure platelet forces at the single cell level is traction force microscopy. Mechanistically these techniques have been beneficial by showing the importance of the GPIb complex interactions with the A1 domain of vWF for platelet force generation and highlighted the protein filamin as the intermediary that transmits forces from the cytoskeleton within the platelet to vWF (Figure 3) (100). Additionally, we know from previous work that within a clot, the platelet population is heterogenous and this heterogeneity is advantageous (44) and it is known that the forces a single platelet can generate has a wide range from 1 nN – 100 nN(74, 92, 97), however another elusive question is what makes one platelet highly contractile while another weakly contractile. Recently, using traction force microscopy one group has begun to answer this question, they showed that highly contractile platelets exhibit increased spreading area, and are morphologically more circular with uniformly distributed F-actin(101). As such, it is evident that single platelet biophysical assays especially regarding contraction have provided key mechanistic insights and as such have shown the importance of investigating cellular mechanics at the single cell. More recently, leveraging our knowledge of platelet mechanical behavior at the bulk clot level and the more recent developments of how platelets behave at the single platelet level, computational models have aided in connecting the macroscale to the microscale (44, 97) by helping us understand the importance of platelet heterogeneity in isovolumetric contraction (44) and the connection between single platelet force and bulk clot force(97). Although substantial progress has been made, more work is necessary to definitively translate platelet behavior at the single cell level to collective platelet behavior at the bulk clot level, an area complicated by the complexity of the microenvironment.
Section 4: Clinical Implications of Platelet Biomechanics and Mechanotransduction
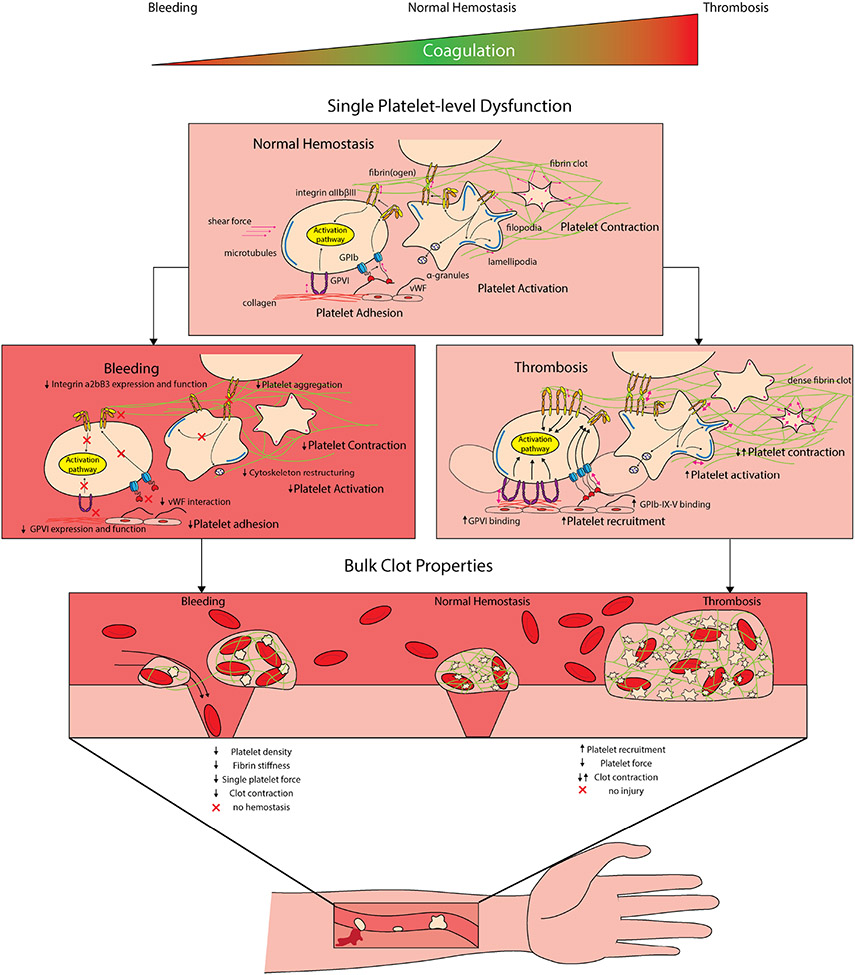
Our understanding of platelet mechanics and how the mechanical microenvironment shapes platelet behavior has improved with the advancement of tools such as in-vitro flow-based microfluidics, volumetric bulk clot contraction assays, single platelet assays and assays investigating subcellular integrin mechanics in addition to the improved imaging techniques to further allow single and aggregate platelet exploration(102). Numerous bulk, aggregate and single platelet experimental techniques have linked platelet mechanics to not only hematological clinical disorders but numerous others (Table 1). Leveraging volumetric bulk clot contraction assays it has been shown that impaired bulk clot contraction is implicated in numerous disease states such as Sickle Cell Disease (103), asthma (104), lupus(105), stroke(106) and trauma(107) to name a few (Figure 5). Similarly in microclots, patients with von Willibrand’s Disease show impaired clot contraction(108). However enhanced clot contraction has been implicated in disorders such as in Thromboangiitis obliterans(109), Polycythemia Vera(110), severe coronary artery disease(111) and chest pain associated coronary artery disease (112). The difficulty however is in understanding whether these changes at the bulk clot contraction are due to the platelets themselves or due to changes in the biochemical or mechanical microenvironments. Specifically, in these disease states is this impairment or enhancement of contraction due to increases in platelet count, alterations in the fibrin content or stiffness, and/or due to abnormal concentrations of coagulation factors. As such, new single and aggregate platelet technologies have started to decouple disorders that are due to impaired platelet mechanics. As a start, and unsurprisingly measuring single platelet, we and other groups have shown that patients with cytoskeletal disorders such as May Hegglin Disorder(74, 113) and Wiskot Aldrich Syndrome(74) have impaired single platelet contraction forces. Additionally with our high throughput single platelet contraction system, we showed that patients with symptomatic bleeding but completely normal clinical hemostatic tests have impaired platelet contraction force, this in turn connecting impaired platelet nanomechanics to a bleeding phenotype(74) (Figure 5).
Table 1:
Disorders linked to mechanical platelet dysfunction
| Disorder | Platelet Mechanical Insights | Reference |
|---|---|---|
| Sickle Cell Disease | Decreased bulk contraction force | (103) |
| Asthma | Decreased bulk contraction force | (104) |
| Lupus | Decreased bulk contraction force | (105) |
| Acute ischemic stroke | Decreased bulk contraction force | (106) |
| Covid-19 | Decreased bulk contraction force | (114) |
| Trauma | Decreased aggregate and bulk contraction force | (99) (107) |
| Thromboangiitis obliterans | Increased bulk contraction force | (109) |
| Polycythemia Vera | Increased bulk contraction force | (110) |
| Severe coronary artery disease | Increased bulk contraction force | (111) |
| Chest pain associated coronary artery disease | Increased bulk contraction force and increased clot elastic modulus | (112) |
| May Hegglin Disorder | Decreased single platelet contractile force | (74, 113) |
| Wiskot Aldrich Syndrome | Decreased single platelet contractile force | (74) |
| Symptomatic Bleeding | Decreased single platelet contraction force | (74) |
| type 2B von Willebrand’s Disease | Enhanced mechanical activation of GP1b leading to platelet clearance | (49) |
| Glanzmann’s thrombasthenia | Impaired platelet adhesion to fibrinogen | (67, 68) |
| Bernard-Soulier syndrome | Impaired adhesion to vWF | (47) |
| GPVI deficiency | Impaired platelet response to collagen | (115) |
| α2β1 deficiency | Impaired platelet response to collagen | (83) |
FIGURE 5.
Aberrant hemostasis occurs when there is too little clotting (bleeding) or too much clotting thrombosis, wherein platelet dysfunction can lead to both disease processes. At the single-platelet level, decreased platelet activation or interactions with exposed extracellular matrix proteins can lead to deficiencies at the bulk clot level, causing decreases in platelet density and decreases in contraction force, leading to impaired hemostasis. Alternatively, platelet preactivation leads to enhanced recruitment, leading to thrombosis. Interestingly, both increases and decreases in bulk clot contraction force have been implicated in thrombosis. Consequently, more thorough exploration is necessary to determine the reasoning as to why in some disorders, both increased and decreased forces leads to thrombosis.
Leveraging our knowledge of platelet contraction, our group has leveraged the ability of platelets to generate forces to break open fibrinogen coated microcapsules to deliver hemostatic agents with implications for patients with hemophilia(116). Additionally, decreased platelet aggregate forces have been shown to be found in trauma patients that require blood transfusions (99). This further highlighting the clinical importance of decoupling platelet mechanical behavior from bulk clots. Not only have these systems allowed for advancements in our knowledge of platelet influence on disease pathophysiology, but also on how various drugs can alter platelet mechanics such as in patients taking aspirin and abciximab(99). More recently, the importance of Piezo receptors (117) as a mechanosensitive ion channel that responds to stretching and shear stress has shown to have relevance in platelets(118, 119) particularly clinical relevance. Specifically, Piezo1 has been shown to mediate a thrombotic pathway in the platelets of patients suffering from diabetes(120) as well as in hypertension(121). As such, it is evident that new platelet mechano-pathways need to be explored as they may have important clinical relevance. Additionally and important to note when interpreting results from animal disease models and anti-platelet drugs, that platelet biophysical behavior varies between animal species, noting that each species possess a unique single platelet biophysical signature (92).
Concluding Remarks
Platelets demonstrate biomechanical properties that are integral to hemostasis and thrombosis. The platelet microenvironment is influenced by rheological properties that platelets sense through biomechanical cues of the surrounding milieu. Altered hemodynamic forces, shear stress, vascular injury, stiffness of the microenvironment and receptor/integrin and matrix protein interactions influence how platelets sense and respond to their mechanical microenvironment. Although we have made substantial progress in how individual platelets integrate in the myriad of the clot environment, sense their surroundings, and contract to restore hemostasis, many questions remain unanswered. Several groups have shown that platelet force ‘correlates’ with bleeding and/or thrombosis and as such are linking clinical symptomology to mechanics (Table 1, Figure 4). However, what is important to confirm is whether impaired platelet force is correlated to bleeding or is actually causing symptomatic bleeding and what are the mechanisms behind the impairment in cellular force.
Another area of important exploration is the role of viscosity in a developing clot, the field has made substantial progress on how platelets respond to varying elasticity but the role of viscosity in the viscoelastic clot microenvironment remains poorly explored. What key advantage does the continuously changing viscosity give to a developing clot and how are platelets responding to these changes? This will be important to investigate and will lead to a more accurate understanding of how platelets are interacting with a visoelastic microenvironment that is constantly changing/remodeling. Additionally, and subcellularly, the identification of the biomechanical pathway leading to the intermediate state of the integrin α2bβ3 (GPIIb/IIIa) has provided us with an initial understanding of integrin affinity maturation, however further studies are necessary to help us understand the biological and clinical importance of this intermediate state. Are integrin conformation states affected or altered in clinical disorders? Knowing that antibodies and platelet inhibitors can stabilize integrins into specific conformations(122) and that termination of integrin α2bβ3 (GPIIb/IIIa) tension is coupled with the exposure of phosphatidylserine and cessation of contraction(76), do certain disorders stabilize integrin confirmations and do these stabilized conformations lead to impaired platelet function and abberant mechanotransduction? Finally, in every contractile cell including platelets, the role of MLCK and Rho kinase pathways has been well characterized, but platelets still produce contraction forces and still adhere to matrix protein coated surfaces while these pathways are inhibited, and as such these pathways are not telling the complete story. Similarly to the recent Piezo1 discoveries (118, 119), it will be important to investigate what other pathways are driving the initiation and propagation of platelet contraction and mechanics.
Taken collectively, investigations into platelet mechanotranduction will provide important insights into numerus clinical and biological phenomena. Specifically, further investigation at not only the single cell level, but the subcellular integrin level will improve our knowledge of the pathways that govern mechanical translation, the clinical disorders that are affected by abberant mechanotransdunction, the link between single platelet biomechanics and the mechanics that are occurring within the bulk clot microenvironments.
Acknowledgements
The authors are grateful to colleagues and collaborators for critical comments on this review and contributions to concepts presented. Financial support for this work was provided by a National Institutes of Health grant F31HL160210 (to O.O), a National Institutes of Health grants T32HL139443-3 (to S. A..), R01 HL130918 (to W.A.L.), and R35HL145000 (to W.A.L.)
Footnotes
Conflict of interest
None of the authors declare any conflict of interest with regards to this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Oluwamayokun Oshinowo, Emory University School of Medicine, Division of Pediatric Hematology/Oncology, Department of Pediatrics, Atlanta, GA, USA; Emory University and Georgia Institute of Technology, The Wallace H. Coulter Department of Biomedical Engineering, Atlanta, GA, USA; Children's Healthcare of Atlanta Inc Aflac Cancer and Blood Disorders Center, Atlanta, GA, USA.
Sally S. Azer, Emory University School of Medicine, Division of Pediatric Hematology/Oncology, Department of Pediatrics, Atlanta, GA, USA; Emory University and Georgia Institute of Technology, The Wallace H. Coulter Department of Biomedical Engineering, Atlanta, GA, USA; Children's Healthcare of Atlanta Inc Aflac Cancer and Blood Disorders Center, Atlanta, GA, USA.
Jessica Lin, Emory University and Georgia Institute of Technology, The Wallace H. Coulter Department of Biomedical Engineering, Atlanta, GA, USA.
Wilbur A. Lam, Wilbur Lam, Department of Pediatrics, 2015 Uppergate Dr NE, Room 448, Atlanta, GA 30322, USA.
References
- 1.Discher DE, Janmey P, Wang Y-l. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science. 2005;310(5751):1139–43. [DOI] [PubMed] [Google Scholar]
- 2.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. [DOI] [PubMed] [Google Scholar]
- 3.Bennett M, Cantini M, Reboud J, Cooper JM, Roca-Cusachs P, Salmeron-Sanchez M. Molecular clutch drives cell response to surface viscosity. Proceedings of the National Academy of Sciences. 2018;115(6):1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, et al. Substrate stress relaxation regulates cell spreading. Nat Commun. 2015;6:6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020;584(7822):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15(3):326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo JH, Kakinoki S, Inoue Y, Nam K, Yamaoka T, Ishihara K, et al. The significance of hydrated surface molecular mobility in the control of the morphology of adhering fibroblasts. Biomaterials. 2013;34(13):3206–14. [DOI] [PubMed] [Google Scholar]
- 8.Adebowale K, Gong Z, Hou JC, Wisdom KM, Garbett D, Lee H-p, et al. Enhanced substrate stress relaxation promotes filopodia-mediated cell migration. Nat Mater. 2021;20(9):1290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35(8):564–77. [DOI] [PubMed] [Google Scholar]
- 10.Tse HT, Gossett DR, Moon YS, Masaeli M, Sohsman M, Ying Y, et al. Quantitative diagnosis of malignant pleural effusions by single-cell mechanophenotyping. Sci Transl Med. 2013;5(212):212ra163. [DOI] [PubMed] [Google Scholar]
- 11.Gale AJ. Continuing education course #2: current understanding of hemostasis. Toxicol Pathol. 2011;39(1):273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, et al. A shear gradient–dependent platelet aggregation mechanism drives thrombus formation. Nature Medicine. 2009;15(6):665–73. [DOI] [PubMed] [Google Scholar]
- 13.Rana K, Timmer BJ, Neeves KB. A combined microfluidic-microstencil method for patterning biomolecules and cells. Biomicrofluidics. 2014;8(5):056502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann M, Schoeman RM, Krohl PJ, Wallbank AM, Samaniuk JR, Jandrot-Perrus M, et al. Platelets Drive Thrombus Propagation in a Hematocrit and Glycoprotein VI–Dependent Manner in an In Vitro Venous Thrombosis Model. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38(5):1052–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin MT, Kim D, Ku DN. Shear-induced platelet aggregation: 3D-grayscale microfluidics for repeatable and localized occlusive thrombosis. Biomicrofluidics. 2019;13(5):054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122(1):408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu Y, Ahn B, Sakurai Y, Hansen CE, Tran R, Mimche PN, et al. Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nature Biomedical Engineering. 2018;2(6):453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakurai Y, Hardy ET, Ahn B, Tran R, Fay ME, Ciciliano JC, et al. A microengineered vascularized bleeding model that integrates the principal components of hemostasis. Nature Communications. 2018;9(1):509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poventud-Fuentes I, Kwon KW, Seo J, Tomaiuolo M, Stalker TJ, Brass LF, et al. A Human Vascular Injury-on-a-Chip Model of Hemostasis. Small. 2021;17(15):2004889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu Y, Brown AC, Myers DR, Sakurai Y, Mannino RG, Tran R, et al. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proceedings of the National Academy of Sciences. 2014;111(40):14430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam WA, Chaudhuri O, Crow A, Webster KD, Li T-D, Kita A, et al. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nature Materials. 2011;10(1):61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of "hypersplenic" thrombocytopenia. J Clin Invest. 1966;45(5):645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaushansky K Determinants of platelet number and regulation of thrombopoiesis. Hematology Am Soc Hematol Educ Program. 2009:147–52. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura S, Nagasaki M, Kunishima S, Sawaguchi A, Sakata A, Sakaguchi H, et al. IL-1α induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J Cell Biol. 2015;209(3):453–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machlus KR, Johnson KE, Kulenthirarajan R, Forward JA, Tippy MD, Soussou TS, et al. CCL5 derived from platelets increases megakaryocyte proplatelet formation. Blood. 2016;127(7):921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nature Medicine. 2004;10(1):64–71. [DOI] [PubMed] [Google Scholar]
- 27.Tablin F, Castro M, Leven RM. Blood platelet formation in vitro. The role of the cytoskeleton in megakaryocyte fragmentation. Journal of Cell Science. 1990;97(1):59–70. [DOI] [PubMed] [Google Scholar]
- 28.Soves CP, Miller JD, Begun DL, Taichman RS, Hankenson KD, Goldstein SA. Megakaryocytes are mechanically responsive and influence osteoblast proliferation and differentiation. Bone. 2014;66:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blin A, Le Goff A, Magniez A, Poirault-Chassac S, Teste B, Sicot G, et al. Microfluidic model of the platelet-generating organ: beyond bone marrow biomimetics. Scientific Reports. 2016;6(1):21700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito Y, Nakamura S, Sugimoto N, Shigemori T, Kato Y, Ohno M, et al. Turbulence Activates Platelet Biogenesis to Enable Clinical Scale Ex Vivo Production. Cell. 2018;174(3):636–48.e18. [DOI] [PubMed] [Google Scholar]
- 31.Kroll M, Hellums J, McIntire L, Schafer A, Moake J. Platelets and shear stress. Blood. 1996;88(5):1525–41. [PubMed] [Google Scholar]
- 32.Myers DR, Lam WA. Vascularized Microfluidics and Their Untapped Potential for Discovery in Diseases of the Microvasculature. Annual Review of Biomedical Engineering. 2021;23(1):407–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao R, Kameneva MV, Antaki JF. Investigation of platelet margination phenomena at elevated shear stress. Biorheology. 2007;44(3):161–77. [PubMed] [Google Scholar]
- 34.Ju L, Qian J, Zhu C. Transport regulation of two-dimensional receptor-ligand association. Biophys J. 2015;108(7):1773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doggett TA, Girdhar G, Lawshe A, Miller JL, Laurenzi IJ, Diamond SL, et al. Alterations in the intrinsic properties of the GPIbα–VWF tether bond define the kinetics of the platelet-type von Willebrand disease mutation, Gly233Val. Blood. 2003;102(1):152–60. [DOI] [PubMed] [Google Scholar]
- 36.Doggett TA, Girdhar G, Lawshé A, Schmidtke DW, Laurenzi IJ, Diamond SL, et al. Selectin-Like Kinetics and Biomechanics Promote Rapid Platelet Adhesion in Flow: The GPIbα-vWF Tether Bond. Biophysical Journal. 2002;83(1):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar RA, Dong J-F, Thaggard JA, Cruz MA, López JA, McIntire LV. Kinetics of GPIbα-vWF-A1 Tether Bond under Flow: Effect of GPIbα Mutations on the Association and Dissociation Rates. Biophysical Journal. 2003;85(6):4099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinar IP, Arthur JF, Andrews RK, Gardiner EE, Ryan K, Carberry J. Methods to Determine the Lagrangian Shear Experienced by Platelets during Thrombus Growth. PLOS ONE. 2015;10(12):e0144860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi X, Yang J, Huang J, Long Z, Ruan Z, Xiao B, et al. Effects of different shear rates on the attachment and detachment of platelet thrombi. Molecular Medicine Reports. 2016;13(3):2447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson SP, Nesbitt WS, Westein E. Dynamics of platelet thrombus formation. Journal of Thrombosis and Haemostasis. 2009;7:17–20. [DOI] [PubMed] [Google Scholar]
- 41.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific Synergy of Multiple Substrate–Receptor Interactions in Platelet Thrombus Formation under Flow. Cell. 1998;94(5):657–66. [DOI] [PubMed] [Google Scholar]
- 42.Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007;109(2):566–76. [DOI] [PubMed] [Google Scholar]
- 43.Durrant TN, Van Den Bosch MT, Hers I. Integrin αIIbβ3 outside-in signaling. Blood. 2017;130(14):1607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Myers DR, Nikolov SV, Oshinowo O, Baek J, Bowie SM, et al. Platelet heterogeneity enhances blood clot volumetric contraction: An example of asynchrono-mechanical amplification. Biomaterials. 2021;274:120828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colace TV, Muthard RW, Diamond SL. Thrombus Growth and Embolism on Tissue Factor-Bearing Collagen Surfaces Under Flow. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(6):1466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coller BS, Peerschke EI, Scudder LE, Sullivan CA. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983;61(1):99–110. [PubMed] [Google Scholar]
- 47.López JA, Andrews RK, Afshar-Kharghan V, Berndt MC. Bernard-Soulier syndrome. Blood. 1998;91(12):4397–418. [PubMed] [Google Scholar]
- 48.Zhang W, Deng W, Zhou L, Xu Y, Yang W, Liang X, et al. Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein Ib-IX complex. Blood. 2015;125(3):562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng W, Xu Y, Chen W, Paul DS, Syed AK, Dragovich MA, et al. Platelet clearance via shear-induced unfolding of a membrane mechanoreceptor. Nature Communications. 2016;7(1):12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Ju LA, Zhou F, Liao J, Xue L, Su QP, et al. An integrin αIIbβ3 intermediate affinity state mediates biomechanical platelet aggregation. Nat Mater. 2019;18(7):760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du X, Beutler L, Ruan C, Castaldi P, Berndt M. Glycoprotein Ib and glycoprotein IX are fully complexed in the intact platelet membrane. Blood. 1987;69(5):1524–7. [PubMed] [Google Scholar]
- 52.Ju L, Chen Y, Zhou F, Lu H, Cruz MA, Zhu C. Von Willebrand factor-A1 domain binds platelet glycoprotein Ibα in multiple states with distinctive force-dependent dissociation kinetics. Thrombosis Research. 2015;136(3):606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ju L, Chen Y, Li K, Yuan Z, Liu B, Jackson SP, et al. Dual Biomembrane Force Probe enables single-cell mechanical analysis of signal crosstalk between multiple molecular species. Scientific Reports. 2017;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ju L, Dong J-F, Cruz MA, Zhu C. The N-terminal Flanking Region of the A1 Domain Regulates the Force-dependent Binding of von Willebrand Factor to Platelet Glycoprotein Ibα. Journal of Biological Chemistry. 2013;288(45):32289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ju L, Qian J, Zhu C. Transport Regulation of Two-Dimensional Receptor-Ligand Association. Biophysical Journal. 2015;108(7):1773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ju L, Lou J, Chen Y, Li Z, Zhu C. Force-Induced Unfolding of Leucine-Rich Repeats of Glycoprotein Ibα Strengthens Ligand Interaction. Biophysical Journal. 2015;109(9):1781–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrews RK, Fox JE. Identification of a region in the cytoplasmic domain of the platelet membrane glycoprotein Ib-IX complex that binds to purified actin-binding protein. J Biol Chem. 1992;267(26):18605–11. [PubMed] [Google Scholar]
- 58.Williamson D, Pikovski I, Cranmer SL, Mangin P, Mistry N, Domagala T, et al. Interaction between platelet glycoprotein Ibalpha and filamin-1 is essential for glycoprotein Ib/IX receptor anchorage at high shear. J Biol Chem. 2002;277(3):2151–9. [DOI] [PubMed] [Google Scholar]
- 59.Ruggeri ZM. Platelet GPIb: sensing force and responding. Blood. 2015;125(3):423–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang XF, Zhang W, Quach ME, Deng W, Li R. Force-Regulated Refolding of the Mechanosensory Domain in the Platelet Glycoprotein Ib-IX Complex. Biophysical Journal. 2019;116(10):1960–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest. 1999;103(6):879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Candia E, Hall SW, Rutella S, Landolfi R, Andrews RK, De Cristofaro R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par-1 on intact platelets. J Biol Chem. 2001;276(7):4692–8. [DOI] [PubMed] [Google Scholar]
- 63.Adam F, Verbeuren TJ, Fauchère JL, Guillin MC, Jandrot-Perrus M. Thrombin-induced platelet PAR4 activation: role of glycoprotein Ib and ADP. J Thromb Haemost. 2003;1(4):798–804. [DOI] [PubMed] [Google Scholar]
- 64.Lova P, Canobbio I, Guidetti GF, Balduini C, Torti M. Thrombin induces platelet activation in the absence of functional protease activated receptors 1 and 4 and glycoprotein Ib-IX-V. Cell Signal. 2010;22(11):1681–7. [DOI] [PubMed] [Google Scholar]
- 65.Estevez B, Kim K, Delaney MK, Stojanovic-Terpo A, Shen B, Ruan C, et al. Signaling-mediated cooperativity between glycoprotein Ib-IX and protease-activated receptors in thrombin-induced platelet activation. Blood. 2016;127(5):626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, Jordan RE. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood. 1996;88(3):907–14. [PubMed] [Google Scholar]
- 67.Phillips DR, Agin PP. Platelet membrane defects in Glanzmann's thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest. 1977;60(3):535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.George JN, Caen JP, Nurden AT. Glanzmann's Thrombasthenia: The Spectrum of Clinical Disease. Blood. 1990;75(7):1383–95. [PubMed] [Google Scholar]
- 69.Luo BH, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006;18(5):579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Z, Delaney MK, O'Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30(12):2341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204(13):3103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moser M, Nieswandt B, Ussar S, Pozgajova M, ssler R. Kidlin3 is essential for integrin activation and platelet aggregation. Nature Medicine. 2008;14:325–30. [DOI] [PubMed] [Google Scholar]
- 73.Fong KP, Molnar KS, Agard N, Litvinov RI, Kim OV, Wells JA, et al. Cleavage of talin by calpain promotes platelet-mediated fibrin clot contraction. Blood Adv. 2021;5(23):4901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Myers DR, Qiu Y, Fay ME, Tennenbaum M, Chester D, Cuadrado J, et al. Single-platelet nanomechanics measured by high-throughput cytometry. Nat Mater. 2017;16(2):230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu Y, Brown AC, Myers DR, Sakurai Y, Mannino RG, Tran R, et al. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proceedings of the National Academy of Sciences. 2014;111(40):14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, Qiu Y, Blanchard AT, Chang Y, Brockman JM, Ma VP, et al. Platelet integrins exhibit anisotropic mechanosensing and harness piconewton forces to mediate platelet aggregation. Proc Natl Acad Sci U S A. 2018;115(2):325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, LeVine DN, Gannon M, Zhao Y, Sarkar A, Hoch B, et al. Force-activatable biosensor enables single platelet force mapping directly by fluorescence imaging. Biosens Bioelectron. 2018;100:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. [DOI] [PubMed] [Google Scholar]
- 79.Zhu G, Zhang Q, Reddy EC, Carrim N, Chen Y, Xu XR, et al. The integrin PSI domain has an endogenous thiol isomerase function and is a novel target for antiplatelet therapy. Blood. 2017;129(13):1840–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiong JP, Stehle T, Goodman SL, Arnaout MA. A novel adaptation of the integrin PSI domain revealed from its crystal structure. J Biol Chem. 2004;279(39):40252–4. [DOI] [PubMed] [Google Scholar]
- 81.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102(2):449–61. [DOI] [PubMed] [Google Scholar]
- 82.Lecut C, Schoolmeester A, Kuijpers MJ, Broers JL, van Zandvoort MA, Vanhoorelbeke K, et al. Principal role of glycoprotein VI in alpha2beta1 and alphaIIbbeta3 activation during collagen-induced thrombus formation. Arterioscler Thromb Vasc Biol. 2004;24(9):1727–33. [DOI] [PubMed] [Google Scholar]
- 83.Nieuwenhuis HK, Akkerman JW, Houdijk WP, Sixma JJ. Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature. 1985;318(6045):470–2. [DOI] [PubMed] [Google Scholar]
- 84.Xu R-G, Gauer JS, Baker SR, Slater A, Martin EM, McPherson HR, et al. GPVI (Glycoprotein VI) Interaction With Fibrinogen Is Mediated by Avidity and the Fibrinogen αC-Region. Arteriosclerosis, Thrombosis, and Vascular Biology. 2021;41(3):1092–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mammadova-Bach E, Ollivier V, Loyau S, Schaff M, Dumont B, Favier R, et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126(5):683–91. [DOI] [PubMed] [Google Scholar]
- 86.Induruwa I, Moroi M, Bonna A, Malcor J-D, Howes J-M, Warburton EA, et al. Platelet collagen receptor Glycoprotein VI-dimer recognizes fibrinogen and fibrin through their D-domains, contributing to platelet adhesion and activation during thrombus formation. Journal of Thrombosis and Haemostasis. 2018;16(2):389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alshehri OM, Hughes CE, Montague S, Watson SK, Frampton J, Bender M, et al. Fibrin activates GPVI in human and mouse platelets. Blood. 2015;126(13):1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kee MF, Myers DR, Sakurai Y, Lam WA, Qiu Y. Platelet mechanosensing of collagen matrices. PLoS One. 2015;10(4):e0126624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kita A, Sakurai Y, Myers DR, Rounsevell R, Huang JN, Seok TJ, et al. Microenvironmental geometry guides platelet adhesion and spreading: a quantitative analysis at the single cell level. PLoS One. 2011;6(10):e26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakurai Y, Fitch-Tewfik JL, Qiu Y, Ahn B, Myers DR, Tran R, et al. Platelet geometry sensing spatially regulates α-granule secretion to enable matrix self-deposition. Blood. 2015;126(4):531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jen CJ, McIntire LV. The structural properties and contractile force of a clot. Cell Motil. 1982;2(5):445–55. [DOI] [PubMed] [Google Scholar]
- 92.Oshinowo O, Copeland R, Sakurai Y, Fay ME, Petrich BG, Leong T, et al. Significant differences in single-platelet biophysics exist across species but attenuate during clot formation. Blood Advances. 2021;5(2):432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem. 2004;112(2-3):267–76. [DOI] [PubMed] [Google Scholar]
- 94.Weisel JW. Structure of fibrin: impact on clot stability. J Thromb Haemost. 2007;5 Suppl 1:116–24. [DOI] [PubMed] [Google Scholar]
- 95.Cohen I, De Vries A. Platelet Contractile Regulation in an Isometric System. Nature. 1973;246(5427):36–7. [DOI] [PubMed] [Google Scholar]
- 96.Shah JV, Janmey PA. Strain hardening of fibrin gels and plasma clots. Rheologica Acta. 1997;36(3):262–8. [Google Scholar]
- 97.Sun Y, Oshinowo O, Myers DR, Lam WA, Alexeev A. Resolving the missing link between single platelet force and clot contractile force. iScience. 2022;25(1):103690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Collet JP, Shuman H, Ledger RE, Lee S, Weisel JW. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci U S A. 2005;102(26):9133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ting LH, Feghhi S, Taparia N, Smith AO, Karchin A, Lim E, et al. Contractile forces in platelet aggregates under microfluidic shear gradients reflect platelet inhibition and bleeding risk. Nature Communications. 2019;10(1):1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feghhi S, Munday AD, Tooley WW, Rajsekar S, Fura AM, Kulman JD, et al. Glycoprotein Ib-IX-V Complex Transmits Cytoskeletal Forces That Enhance Platelet Adhesion. Biophys J. 2016;111(3):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beussman KM, Mollica MY, Leonard A, Miles J, Hocter J, Song Z, et al. Black dots: High-yield traction force microscopy reveals structural factors contributing to platelet forces. Acta Biomaterialia. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oshinowo O, Lambert T, Sakurai Y, Copeland R, Hansen CE, Lam WA, et al. Getting a good view: in vitro imaging of platelets under flow. Platelets. 2020;31(5):570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tutwiler V, Litvinov RI, Protopopova A, Nagaswami C, Villa C, Woods E, et al. Pathologically stiff erythrocytes impede contraction of blood clots. Journal of Thrombosis and Haemostasis. 2021;19(8):1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tomasiak-Lozowska MM, Misztal T, Rusak T, Branska-Januszewska J, Bodzenta-Lukaszyk A, Tomasiak M. Asthma is associated with reduced fibrinolytic activity, abnormal clot architecture, and decreased clot retraction rate. Allergy. 2017;72(2):314–9. [DOI] [PubMed] [Google Scholar]
- 105.Le Minh G, Peshkova AD, Andrianova IA, Sibgatullin TB, Maksudova AN, Weisel JW, et al. Impaired contraction of blood clots as a novel prothrombotic mechanism in systemic lupus erythematosus. Clin Sci (Lond). 2018;132(2):243–54. [DOI] [PubMed] [Google Scholar]
- 106.Tutwiler V, Peshkova AD, Andrianova IA, Khasanova DR, Weisel JW, Litvinov RI. Contraction of Blood Clots Is Impaired in Acute Ischemic Stroke. Arterioscler Thromb Vasc Biol. 2017;37(2):271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jones WL, Ramos CR, Banerjee A, Moore EE, Hansen KC, Coleman JR, et al. Apolipoprotein A-I, elevated in trauma patients, inhibits platelet activation and decreases clot strength. Platelets. 2022;33(8):1119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Z, Lu J, Zhang C, Hsia I, Yu X, Marecki L, et al. Microclot array elastometry for integrated measurement of thrombus formation and clot biomechanics under fluid shear. Nature Communications. 2019;10(1):2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carr ME Jr., Hackney MH, Hines SJ, Heddinger SP, Carr SL, Martin EJ. Enhanced platelet force development despite drug-induced inhibition of platelet aggregation in patients with thromboangiitis obliterans--two case reports. Vasc Endovascular Surg. 2002;36(6):473–80. [DOI] [PubMed] [Google Scholar]
- 110.Rusak T, Piszcz J, Misztal T, Brańska-Januszewska J, Tomasiak M. Platelet-related fibrinolysis resistance in patients suffering from PV. Impact of clot retraction and isovolemic erythrocytapheresis. Thromb Res. 2014;134(1):192–8. [DOI] [PubMed] [Google Scholar]
- 111.Greilich PE, Carr ME, Zekert SL, Dent RM. Quantitative assessment of platelet function and clot structure in patients with severe coronary artery disease. Am J Med Sci. 1994;307(1):15–20. [DOI] [PubMed] [Google Scholar]
- 112.Krishnaswami A, Carr ME Jr., Jesse RL, Kontos MC, Minisi AJ, Ornato JP, et al. Patients with coronary artery disease who present with chest pain have significantly elevated platelet contractile force and clot elastic modulus. Thromb Haemost. 2002;88(5):739–44. [PubMed] [Google Scholar]
- 113.Baumann J, Sachs L, Otto O, Schoen I, Nestler P, Zaninetti C, et al. Reduced platelet forces underlie impaired hemostasis in mouse models of MYH9-related disease. Science Advances.8(20):eabn2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Litvinov RI, Evtugina NG, Peshkova AD, Safiullina SI, Andrianova IA, Khabirova AI, et al. Altered platelet and coagulation function in moderate-to-severe COVID-19. Sci Rep. 2021;11(1):16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dumont B, Lasne D, Rothschild C, Bouabdelli M, Ollivier V, Oudin C, et al. Absence of collagen-induced platelet activation caused by compound heterozygous GPVI mutations. Blood. 2009;114(9):1900–3. [DOI] [PubMed] [Google Scholar]
- 116.Hansen CE, Myers DR, Baldwin WH, Sakurai Y, Meeks SL, Lyon LA, et al. Platelet–Microcapsule Hybrids Leverage Contractile Force for Targeted Delivery of Hemostatic Agents. ACS Nano. 2017;11(6):5579–89. [DOI] [PubMed] [Google Scholar]
- 117.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ilkan Z, Wright JR, Goodall AH, Gibbins JM, Jones CI, Mahaut-Smith MP. Evidence for shear-mediated Ca(2+) entry through mechanosensitive cation channels in human platelets and a megakaryocytic cell line. J Biol Chem. 2017;292(22):9204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu L, Zhang Q, Xiao S, Sun Z, Ding S, Chen Y, et al. Inhibition of Shear-Induced Platelet Aggregation by Xueshuantong via Targeting Piezo1 Channel-Mediated Ca(2+) Signaling Pathway. Front Pharmacol. 2021;12:606245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu W, Guo S, Homilius M, Nsubuga C, Wright SH, Quan D, et al. PIEZO1 mediates a mechanothrombotic pathway in diabetes. Science Translational Medicine. 2022;14(626):eabk1707. [DOI] [PubMed] [Google Scholar]
- 121.Zhao W, Wei Z, Xin G, Li Y, Yuan J, Ming Y, et al. Piezo1 initiates platelet hyperreactivity and accelerates thrombosis in hypertension. Journal of Thrombosis and Haemostasis. 2021;19(12):3113–25. [DOI] [PubMed] [Google Scholar]
- 122.Lin FY, Li J, Xie Y, Zhu J, Huong Nguyen TT, Zhang Y, et al. A general chemical principle for creating closure-stabilizing integrin inhibitors. Cell. 2022;185(19):3533–50.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]