Abstract
Objective:
Focal cortical dysplasia (FCD) is the most common etiology of surgically-remediable epilepsy in children. 87% of FCD patients develop epilepsy (75% is pharmacoresistant (PRE)). Focal to bilateral tonic-clonic (FTBTC) seizures are associated with worse surgical outcomes. We hypothesized that children with FCD-related epilepsy with FTBTC seizures are more likely to develop PRE due to lesion interaction with restricted cortical neural networks.
Methods:
Patients were selected retrospectively from radiology and surgical databases from Children’s National Hospital. Inclusion criteria: 3T MRI-confirmed FCD from 1/2011–1/2020; ages 0-days to 22-years at MRI; 18 months of documented follow-up. FCD dominant network (Yeo 7-network parcellation) was determined. Association of FTBTC seizures with epilepsy severity, surgical outcome, and dominant network was tested. Binomial regression was used to evaluate predictors (FTBTC seizures, age of seizure onset, pathology, hemisphere, lobe) of pharmacoresistance and Engel outcome. Regression was used to evaluate predictors (age of seizure onset, pathology, lobe, percentage default mode network (DMN) overlap) of FTBTC seizures.
Results:
117 patients had median age of seizure onset 3.00y (IQR 0.42 – 5.59y). 83 patients had PRE (71%); 34 had PSE (29%). Twenty (17%) patients had FTBTC seizures. 73 patients underwent epilepsy surgery. Multivariate regression showed FTBTC seizures are associated with increased risk of PRE (OR 6.41 95%CI 1.21 – 33.98, p=0.02). FCD hemisphere/lobe were not associated with PRE. Percentage DMN overlap predicts FTBTC seizures. 72% (n=52) overall and 53% (n=9) of those with FTBTC seizures achieved Engel I outcome.
Significance:
In a heterogeneous population of surgical and non-operated patients with FCD-related epilepsy, the presence of FTBTC seizures is associated with a tremendous risk of PRE. This finding is a recognizable marker to help neurologists identify those children with FCD-related epilepsy at high risk of PRE, and can flag patients for earlier consideration of potentially curative surgery. FCD dominant network also contributes to FTBTC seizure clinical expression.
Keywords: FCD, pharmacoresistant epilepsy, drug resistant epilepsy, default mode network
Introduction
Focal cortical dysplasia (FCD) is the most common cause of surgically remediable epilepsy.1 Eighty-seven percent of FCD cases develop epilepsy.2 FCDs can occur in all lobes3, but it is FCD functional network co-localization4 rather than lobar location5 that determines age of seizure onset. FCD-related epilepsy can present with variable seizure expression despite similar cortical lesion location. Focal to bilateral tonic-clonic (FTBTC), previously known as “secondary generalized,” describes a focal-onset seizure which spreads bilaterally through brain networks, resulting in tonic clonic expansion.6 FTBTC seizures involve wider networks compared to non-FTBTC focal seizure types.7
FTBTC seizures are associated with worse clinical outcomes compared to other focal seizure types. They are associated with less favorable surgical outcomes in studies of temporal lobe epilepsy.8–12 A retrospective cohort study of 171 patients who underwent temporal lobe resection found that FTBTC seizures were associated with less favorable surgical outcomes at six month follow-up.11 In a retrospective study of 102 patients, FTBTC seizures were associated with long-term seizure recurrence following resection in Type II FCD.12 FTBTC seizures have worse health outcomes compared to other seizure types, including increased risk of SUDEP13, 14 and alteration of consciousness.15 Despite evidence that FTBTC seizures correlate with less favorable clinical outcomes, it remains unknown if the presence of FTBTC seizures affects pharmacoresistance.
FCD-related epilepsy is managed initially with monotherapy, and often requires polytherapy of antiseizure medications (ASMs).16, 17 Although a quarter of FCD epilepsy patients exhibit pharmacosensitive epilepsy (PSE), three quarters of FCD cases develop pharmacoresistant epilepsy (PRE), defined as seizures that do not respond to two appropriately selected antiseizure medication.2 Among FCD cases, pharmacoresistance is the most common indication for surgical intervention, typically with focal resection, lobectomy, neurostimulation, or re-resection.18 PRE is associated with earlier age of seizure onset.2, 19 Pharmacoresistance is a marker of epilepsy severity; however, predictors of pharmacoresistant epilepsy in FCD are not well understood.20
There is evidence that FTBTC seizures involve wider networks: they have faster cortico-cortical evoked potentials and broader ictal propagation compared to other seizure types.21, 22 We hypothesized that the wider network distribution of FTBTC seizures is associated with epilepsy severity, and that FTBTC seizures are a marker of PRE and poor surgical outcome. We also hypothesized that FCD co-localization to more widely connected functional cortical networks (e.g. default mode network (DMN)) would be associated with FTBTC seizures.
Materials and Methods
This was an IRB-approved retrospective cohort study from Children’s National Hospital (CNH) in Washington, DC. The IRB determined informed consent was waived. Patients were identified from an electronic medical record search of centralized radiology database (Nuance mPower) containing all imaging reports for the hospital system for the term “focal cortical dysplasia,” and from a central epilepsy surgical database. We included patients with 3T MRI-confirmed FCD who were treated at CNH from January, 2011 to January, 2020, with ages 0 day to 22 years. Clinical notes from Cerner PowerChart were reviewed by authors PC and NTC to determine if the patient met inclusion criteria. MRIs were all reported by board-certified pediatric neuroradiologists with significant experience in pediatric epilepsy surgery (including LGV) and independently reviewed by NTC. Children were included if they had at least 18 months of documented follow-up from MRI identification of FCD, unless they had single seizure in which case these patients were all included. Children were excluded if they had dual pathology (except for mesial temporal sclerosis viewed as secondary to the FCD), hemimegalencephaly, or tuberous sclerosis complex. The following data were abstracted manually from clinical notes into a database: seizure semiology, age at seizure onset; demographic data; epilepsy diagnosis; date of MRI; MRI result; duration of follow-up; and if applicable, epilepsy surgery date, side, procedure, pathologic diagnosis, and surgical outcome.
Definitions and outcomes:
Pharmacoresistant epilepsy (PRE) was defined by either starting a third antiseizure medication (ASM) (because of inefficacy of two prior adequately-dosed ASM, not including intolerance due to adverse reaction following the ILAE criteria23) or having undergone epilepsy surgery (in cases where failure of two ASMs documented but specific date of third ASM initiation could not be found). Time to PRE (in years) was defined as date the first ASM was prescribed subtracted from either the date the third ASM was prescribed, or date of initial epilepsy surgery. Pharmacosensitive epilepsy (PSE) was defined as drug-sensitive (epilepsy with seizures controlled on one or two appropriately dosed ASM). Any patient with epilepsy who was not seizure-free at last follow-up but who had not yet met criteria for pharmacoresistance (either on one inadequately dosed ASM or two ASMs with one inadequately dosed ASM) was included in the pharmacosensitive epilepsy group for purposes of analysis. Patients with single lifetime unprovoked or recurrent provoked seizures (e.g. febrile seizures) were also included. Seizure type was determined by health record review using the ILAE definitions for seizure types.6 Surgery type was defined as lesion resection, lobectomy, or other. Surgical outcomes were reported as Engel score from the most recent neurology or neurosurgery clinic follow-up.24 Favorable surgical outcome was defined as Engel I, and unfavorable outcome as Engel II, III, or IV. FCD lobe was defined as frontal, occipital, parietal, temporal, or cingulate cortex. FCD pathology was defined as Type I, Type II, or Type III, or unknown.
Image analysis:
Volumetric analysis of FCD lesion size was performed by collecting 3T MRI images from the hospital MRI database. Lesion masks were drawn by NTC on preoperative T1 or T2 image using the FMRIB Software Library (FSL). Lesion masks were then coregistered to the native spoiled gradient-echo (SPGR) image and then warped to Montreal Neurological Institute (MNI) space. Schaefer atlas, a 400 parcel map that has been used to localize functional connectivity networks,25 was applied to the MNI warped image to identify the FCD dominant functional network using the Yeo 7-network parcellation (somatomotor, visual, default mode, dorsal attention, ventral attention, frontoparietal and limbic).26 The image was then warped back into native space with the Schaefer parcels and modified lesion mask mapped to the native space.
Dominant network was defined as the network with the largest percent of the total lesion. Data collected were the size (voxels) of the lesion in the dominant and secondary networks, and the percentage overlap with those networks. For analytic purposes, total lesion size was evaluated as total number of vertices representing the projected lesion surface area on the parcellation map.
Statistical Analysis:
Chi-square test was used for categorical comparison (seizure type vs. PRE or PSE; seizure type vs. Engel Outcome; seizure type vs. FCD dominant network). Given FCDs may colocalize preferentially to the DMN5, independent samples t-test was used to evaluate the association of percentage overlap with DMN and presence of FTBTC seizures. Binomial regression was used to analyze the relationship between epilepsy severity (PRE vs. PSE) and the following predictors: presence of FTBTC seizures, age of seizure onset, lesion hemisphere (right or left), FCD lobe, and lesion size. Binomial regression was also used to analyze the relationship between the Engel outcome (Engel I vs. not Engel I) and the following predictors: presence of FTBTC seizures, pathology, age at surgery, age of seizure onset, and FCD lobe. Binomial regression was used to evaluate the following predictors of FTBTC seizures: age at seizure onset, percentage overlap of DMN, FCD lobe, pathology, and lesion size. In all regression models, FCD lobe was analyzed by comparison against frontal lobe lesions. Odds ratio was calculated with 95% confidence interval. Significance threshold was p < 0.05 for all testing. Statistical analysis was performed using Jamovi (https://www.jamovi.org/).
Standard Protocol Approvals, Registrations, and Patient Consents:
This study was approved by the Children’s National Institutional Review Board as exempt.
Data Availability Statement:
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Participants:
117 subjects (58% male) met inclusion criteria. The age of seizure onset for all participants ranged from birth to 17 years (median 3.00y (IQR 0.42 – 5.59y). Twenty patients (17%) had at least one episode of FTBTC seizure. FCD lobar distribution was as follows: frontal, 50 (43%); temporal, 38 (32%); parietal, 16 (14%); occipital, 9 (8%); cingulate, 4 (3%). Eighty-three patients (71%) had PRE and 34 patients (29%) had PSE. Of the PSE group, 24 patients (70%) had trialed first ASM monotherapy; of these patients, 19 (79%) were seizure-free and 5 (21%) had ongoing seizures on first ASM monotherapy at last follow-up. Median total lesion size was 23987 vertices (IQR 7909–144,077).
Pharmacoresistance:
Using binomial regression, PRE was predicted by presence of FTBTC (OR 6.46 95%CI 1.22 – 34.17, p=0.028). Lesion hemisphere, FCD lobe, and lesion size were not associated with PRE. Age at seizure onset was inversely related to PRE (OR = 0.84, 95% CI = 0.76 – 0.94, p = 0.002) (Table 2).
Table 2:
Binomial logistic regression of predictors of epilepsy severity (pharmacoresistant vs. pharmacosensitive epilepsy)
| Model Fit Measures | ||||||
|---|---|---|---|---|---|---|
| Overall Model Test | ||||||
| Model | Deviance | AIC | R2McF | χ2 | df | p |
| 1 | 121 | 139 | 0.145 | 20.4 | 8 | 0.009 |
| 95% Confidence Interval | |||||||
|---|---|---|---|---|---|---|---|
| Predictor | Estimate | SE | Z | p | Odds ratio | Lower | Upper |
| Intercept | 1.027 | 0.4752 | 2.161 | 0.031 | 2.793 | 1.100 | 7.088 |
| FTBTC seizures: | |||||||
| yes – no | 1.865 | 0.8500 | 2.194 | 0.028 | 6.457 | 1.220 | 34.168 |
| Hemisphere: | |||||||
| L – R | 0.428 | 0.4625 | 0.925 | 0.355 | 1.534 | 0.620 | 3.797 |
| Age seizure onset | −0.173 | 0.0554 | −3.115 | 0.002 | 0.842 | 0.755 | 0.938 |
| FCD lobe: | |||||||
| parietal – frontal | 0.357 | 0.6608 | 0.540 | 0.589 | 1.429 | 0.391 | 5.218 |
| occipital – frontal | −0.300 | 0.8320 | −0.360 | 0.719 | 0.741 | 0.145 | 3.786 |
| temporal – frontal | 0.779 | 0.5544 | 1.405 | 0.160 | 2.179 | 0.735 | 6.459 |
| cingulate – frontal | 0.356 | 1.2991 | 0.274 | 0.784 | 1.428 | 0.112 | 18.216 |
| Size | 9.70e-8 | 2.50e-7 | 0.389 | 0.698 | 1.000 | 1.000 | 1.000 |
Note. Estimates represent the log odds of “epilepsy_severity = PRE” vs. “epilepsy_severity = PSE”
Surgical Outcomes:
Of the 83 PRE patients, 72 underwent surgery: 71 resection, one ablation, one responsive neurostimulation. Median follow-up after surgery was 3 years (IQR 1.5–5.8 years). Pathology was available for 71 patients: Type I (n=22, 31%), Type II (n=43, 60.6%), Type III (n=5, 7.0%), and not clearly classified (n=1, 1.4%). In surgical patients, Engel I outcomes were achieved in 52 patients (71.2%); the remaining outcomes were Engel II (n=10, 13.7%), Engel III (n=9, 12.3%), and Engel IV (n=2, 2.7%) (Table 1). Of the 17 patients with FTBTC seizures who underwent surgery, Engel I outcome was achieved in nine patients (53%); Engel II (n=3, 18%); Engel III (n=4, 23%); Engel IV (n=1, 6%). Of the FTBTC patients who had surgery, pathology was Type I (n=7, 41%); Type II (n=7, 41%); Type III (n=2, 12%), and unknown (n=1, 2.7%). Pathology was not associated with favorable Engel outcome (χ2 = 3.96, p=0.266). χ2 analysis shows a trend that absence of FTBTC seizures is correlated with Engel I outcome (χ2 = 3.62, OR 0.34, 95% CI 0.11–1.06, p = 0.057).
Table 1:
Clinical characteristics
| Variable | FTBTC (n=20) | non-FTBTC (n=97) | Total (n=117) |
|---|---|---|---|
| Mean (SD) | |||
| Age of seizure onset (years) | 4.47 (4.74) | 4.21 (4.03) | 4.25 (4.14) |
| n (column %) | |||
| Male | 11 (55) | 57 (59) | 68 |
| Pathology | |||
| Type I | 7 (35) | 15 (15) | 22 |
| Type II | 7 (35) | 36 (37) | 43 |
| Type III | 2 (35) | 3 (3) | 5 |
| Lobe | |||
| Frontal | 9 (45) | 41 (42) | 50 |
| Temporal | 8 (40) | 30 (31) | 38 |
| Parietal | 0 | 16 (17) | 16 |
| Occipital | 2 (10) | 7 (7) | 9 |
| Other (Cingulate) | 1 (5) | 3 (3) | 4 |
| Epilepsy Severity | |||
| PRE | 18 (90) | 65 (67) | 83 |
| PSE | 2 (10) | 32 (33) | 34 |
Network Analysis:
The distribution of FCD dominant networks is shown in Figure 1. For the 20 patients with FTBTC seizures, the DMN (n=8) and limbic (n=6) were most common; but there was no dominant network association with FTBTC seizures (χ2 = 6.44, p = 0.37). However, a FCD may reside in two networks; when considering this overlap, the median percentage DMN overlap of FCDs with FTBTC seizures was 39.6% vs. 10.8% for FCD without FTBTC seizures (Mann-Whitney U 631, p=0.013).
Figure 1:
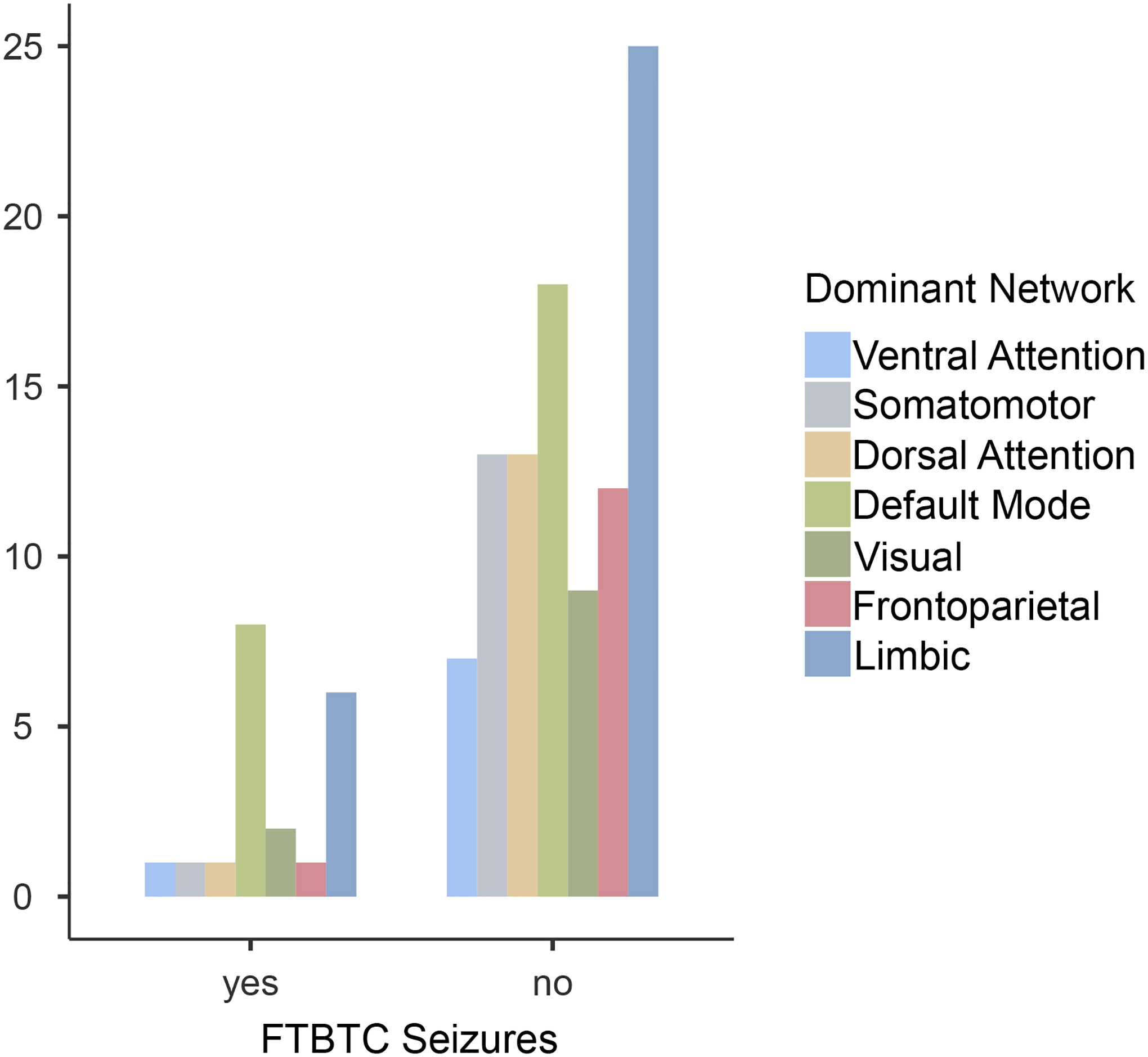
FCD Dominant Network and the Presence of FTBTC Seizures
| Dominant Network | ||||||||
|---|---|---|---|---|---|---|---|---|
| FTBTC seizures | Ventral Attention | Somatomotor | Dorsal Attention | Default Mode | Visual | Frontoparietal | Limbic | Total |
| yes | 1 | 1 | 1 | 8 | 2 | 1 | 6 | 20 |
| no | 7 | 13 | 13 | 18 | 9 | 12 | 25 | 97 |
| Total | 8 | 14 | 14 | 26 | 11 | 13 | 31 | 117 |
Given the findings of the association of DMN with FTBTC seizures, we performed a binomial regression to evaluate predictors of FTBTC seizures including age at seizure onset, percentage DMN overlap, FCD lobe, pathology, and lesion size. In this multivariate model, percentage DMN overlap is a significant predictor of FTBTC seizures (OR 1.04 95%CI 1.01–1.07, p=0.006). Type I FCD pathology also predicts FTBTC seizures (OR 6.36 95%CI 1.15–35.21, p=0.03). Age at seizure onset and FCD lobe do not predict FTBTC seizures (Table 3).
Table 3:
Binomial logistic regression of predictors of FTBTC seizures
| Model Fit Measures | ||||||
|---|---|---|---|---|---|---|
| Overall Model Test | ||||||
| Model | Deviance | AIC | R2McF | χ2 | df | p |
| 1 | 56.3 | 78.3 | 0.279 | 21.8 | 10 | 0.016 |
| 95% Confidence Interval | |||||||
|---|---|---|---|---|---|---|---|
| Predictor | Estimate | SE | Z | p | Odds ratio | Lower | Upper |
| Intercept | −3.8344 | 1.2066 | −3.17787 | 0.001 | 0.0216 | 0.00203 | 0.230 |
| Age seizure onset | 0.1449 | 0.0963 | 1.50549 | 0.132 | 1.1560 | 0.95719 | 1.396 |
| Default mode perc | 0.0415 | 0.0152 | 2.73708 | 0.006 | 1.0424 | 1.01186 | 1.074 |
| FCD lobe: | |||||||
| parietal – frontal | −16.3362 | 2142.1857 | −0.00763 | 0.994 | 8.04e-8 | 0.00000 | Inf |
| occipital – frontal | 1.9554 | 1.5607 | 1.25291 | 0.210 | 7.0665 | 0.33172 | 150.535 |
| temporal – frontal | 0.7439 | 0.9307 | 0.79934 | 0.424 | 2.1042 | 0.33954 | 13.040 |
| cingulate – frontal | −1.2703 | 1.5911 | −0.79840 | 0.425 | 0.2807 | 0.01242 | 6.348 |
| path: | |||||||
| Type I – Type II | 1.8499 | 0.8732 | 2.11856 | 0.034 | 6.3594 | 1.14854 | 35.212 |
| Type III – Type II | 1.7728 | 1.2294 | 1.44200 | 0.149 | 5.8872 | 0.52899 | 65.520 |
| unknown – Type II | 19.2776 | 6522.6387 | 0.00296 | 0.998 | 2.36e+8 | 0.00000 | Inf |
| Size | −3.43e−6 | 4.42e-6 | −0.77723 | 0.437 | 1.0000 | 0.99999 | 1.000 |
Note. Estimates represent the log odds of “FTBTC_yn = yes” vs. “FTBTC_yn = no”
Discussion
FCDs are highly epileptogenic lesions known to be associated with a high rate of pharmacoresistance. Patients with FCD-related epilepsy may experience transient periods of seizure control only to later develop bouts of explosive seizure recurrences. The factors contributing to these state shifts remain poorly defined. FTBTC seizures are a known risk factor for sudden unexplained death in epilepsy13, 14 and are reported to be associated with unfavorable surgical outcomes.8–12 This study demonstrates that the presence of FTBTC seizures is associated with a marked increased risk of pharmacoresistance in patients with FCD-related epilepsy. We found a trend that seizure-free surgical outcome may be more likely in FCD-related epilepsy in patients without FTBTC seizures but over 50% of patients with FTBTC seizures achieve seizure freedom with surgery. Furthermore, we found an association between FCD overlap with the DMN and FTBTC seizures.
There is an expanding understanding of FCD-related epilepsy as a network disorder. We showed previously that FCD co-localization to functional networks determines the age of seizure onset: earliest in sensorimotor network and latest in limbic network.4 Here we observe there is an association of FCD overlap with the DMN and the presence of FTBTC seizures. In multivariate binomial regression, we demonstrate that the percentage overlap of the FCD with the DMN is a modest but significant predictor of FTBTC seizures, which is supported by data from other functional studies. A SPECT cerebral blood flow study of 53 patients showed that during and following FTBTC seizures, there was decreased blood flow in default mode regions, as well as increases in thalamic, basal ganglionic and cerebellar blood flow during generalization.27 Other studies have shown, in temporal lobe epilepsy, that FTBTC seizures alter thalamocortical and subcortical networks.28, 29 A diffusion MRI study of 83 temporal lobe epilepsy patients with FTBTC seizures found nodal structural connectivity alterations subcortically and in parietal regions, and global whole-brain connectivity alterations compared to controls.7 There is increasing evidence of a network view of epilepsy severity. Structural connectivity studies have correlated more widespread network nodal abnormality with worse surgical outcome in mesial temporal lobe epilepsy.30 In a rsfMRI connectivity study of 25 patients with pharmacoresistant epilepsy of heterogeneous etiology, seven patients had abnormal nodal connectivity alterations in the DMN.31 DMN hub redistributions in TLE are associated with seizure freedom. Interestingly, no difference was found in global or nodal organization of the DMN in patients with ongoing seizures and healthy controls. The authors posit that there may be transient ictal shifts in network efficiency characteristics (e.g. local, regional, and global connectivity changes) that allow for seizure propagation.32 The presence of FTBTC seizures may imply a more widespread epileptogenic network, or may be the result of propagation or other factors not directly explored in this study. In light of our findings and these results, we hypothesize that there may be a network basis of epilepsy severity that incorporates both cortical and subcortical functional connectivity alterations which will be the topic of future studies.
Our results indicate that FTBTC seizures mark a high risk of pharmacoresistance in children with FCD-related epilepsy. The presence of FTBTC seizures should flag these patients for early consideration of epilepsy surgery. First, although our results show a trend for surgical outcomes to be better in FCD patients without FTBTC seizures, 53% of FCD patients with FTBTC seizures experienced Engel I outcome. Second, we have shown previously that children with FCD-related epilepsy who fail just one antiseizure medication are at a huge risk of developing pharmacoresistance and have recommended surgical consideration before waiting for the second antiseizure medication to fail the child.2 Others have advocated similarly for early surgical consideration of FCD-related epilepsy.33, 34 Patients with FCD-related epilepsy often face long delays to surgical consideration which is associated with worse outcomes.35 Third, the presence of FTBTC seizures may be a clinical marker of widespread connectivity alterations which may not respond as well to traditional resective techniques, and rather may be targeted better through network-based neurostimulation strategies.36 This hypothesis will need to be confirmed in larger prospective functional connectivity studies.
This study has limitations. There may be referral bias because of the single-center study design. However, Children’s National is a large, urban pediatric hospital system with broad regional primary, secondary, and tertiary care practice. CNH provides the primary epilepsy care (from seizure onset) for the majority (>90%) of pediatric epilepsy patients in this region. Our study design minimizes referral bias as patients by identifying all FCD patients through the hospital system’s radiology database including all patients imaged for new onset seizure as well as epilepsy surgical candidates. Given the catchment of our hospital system, this is a close approximation to a population-based study. Our study is limited by its retrospective design. It is possible that patients included in the pharmacosensitive group may have ultimately developed pharmacoresistant epilepsy. This population may represent the small portion of FCD patients who undergo epilepsy surgery as adults, with possible late conversion to PRE.35 Given that 18/20 (90%) of the patients with FTBTC seizures had PRE, if the 2/20 (10%) with FTBTC seizures and PSE were included in the PRE group, this would strengthen our findings that FTBTC predict PRE outcome. The size of the FTBTC group prevents subgroup analyses to determine differences among FCD with FTBTC seizures to explain epilepsy severity and surgical outcomes. The study design does not allow for causal inference to be made between the association of FTBTC seizures and pharmacoresistance. Our technique is insufficient to fully define epileptogenic networks37, but the FCD lesions are demonstrably linked to the epileptogenic network, as 71% of the cohort gained seizure freedom with lesion removal. Our surgical results are from a single quaternary, Level IV epilepsy center, and may not generalize broadly to all populations but are similar to other reports.12, 38–40 The focus of the present study is limited to cortical networks, but FCD with FTBTC seizures likely involve both cortical and subcortical connectivity alterations which will be the topic of future investigations. We did not track quantitatively the frequencies of FTBTC seizures. This should be addressed in future prospective studies to stratify the impact and timing of FTBTC seizures on FCD-related epilepsy.
Conclusion
We found that FTBTC seizures are associated with high odds of pharmacoresistance in FCD-related epilepsy. Dysplasia overlap with the DMN predicts modest but significant odds of expression of FTBTC seizures. More than half of children with FCDs with FTBTC seizures had Engel I outcome. FTBTC seizures represent a clinical marker that should alert the managing neurologist to refer the child for expedited surgical evaluation in FCD-related epilepsy.
Key Points:
Focal to bilateral tonic-clonic seizures predict pharmacoresistance in focal cortical dysplasia-related epilepsy.
Focal cortical dysplasia overlap with the default mode network is associated with focal to bilateral-tonic clonic seizure development.
Focal cortical dysplasia-related epilepsy is associated with high rates of Engel I outcome after resective surgery.
Acknowledgements:
NTC is supported by the Pediatric Epilepsy Research Foundation/Child Neurology Foundation Shields Research Grant, the Children’s National Research Institute Chief Research Officer Award. This work was also supported by DC-IDDRC NICHD NIH P50 HD105328. This publication was supported by Award Number UL1TR001876 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Ethics Approval and Patient Consents: This study was approved by the Children’s National Institutional Review Board as exempt.
Author Contributions:
Phat Chang: conceptualization, formal analysis, investigation, writing- original draft preparation, revision & editing; Hua Xie: formal analysis, investigation, revision & editing; Venkata Sita Priyanka Illapani: formal analysis, investigation, revision & editing; Xiaozhen You: formal analysis, investigation, revision & editing; Tayyba Anwar: investigation, revision & editing; Archana Pasupuleti: data curation, revision & editing; Thuy-Anh Vu: data curation, revision & editing; L. Gilbert Vezina: investigation, revision & editing; Taha Gholipour: formal analysis, investigation, revision & editing; Chima O. Oluigbo: data curation, revision & editing; Anqing Zhang: formal analysis, investigation, revision & editing; William Davis Gaillard: conceptualization, formal analysis, investigation, writing- original draft preparation, revision & editing; Nathan T. Cohen: conceptualization, formal analysis, funding acquisition, investigation, writing- original draft preparation, revision & editing
Conflict of Interest/Ethical Publication Statement: None of the authors has any conflicts of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Data Availability Statement:
Anonymized data not published within this article will be made available by request from any qualified investigator.
References
- 1.Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery The New England journal of medicine. 2017;377:1648–1656. [DOI] [PubMed] [Google Scholar]
- 2.Cohen NT, Chang P, You X, Zhang A, Havens KA, Oluigbo CO, et al. Prevalence and Risk Factors for Pharmacoresistance in Children With Focal Cortical Dysplasia-Related Epilepsy Neurology. 2022. Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagstyl K, Whitaker K, Raznahan A, Seidlitz J, Vértes PE, Foldes S, et al. Atlas of lesion locations and postsurgical seizure freedom in focal cortical dysplasia: A MELD study Epilepsia. 2021. Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen NT, You X, Krishnamurthy M, Sepeta LN, Zhang A, Oluigbo C, et al. Networks Underlie Temporal Onset of Dysplasia-Related Epilepsy: A MELD Study Annals of neurology. 2022. Sep;92:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiwattanadittakul N, Suwannachote S, You X, Cohen NT, Tran T, Phuackchantuck R, et al. Spatiotemporal distribution and age of seizure onset in a pediatric epilepsy surgery cohort with cortical dysplasia Epilepsy research. 2021. May;172:106598. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology Epilepsia. 2017. Apr;58:522–530. [DOI] [PubMed] [Google Scholar]
- 7.Sinha N, Peternell N, Schroeder GM, de Tisi J, Vos SB, Winston GP, et al. Focal to bilateral tonic-clonic seizures are associated with widespread network abnormality in temporal lobe epilepsy Epilepsia. 2021. Mar;62:729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Englot DJ, Lee AT, Tsai C, Halabi C, Barbaro NM, Auguste KI, et al. Seizure types and frequency in patients who “fail” temporal lobectomy for intractable epilepsy Neurosurgery. 2013. Nov;73:838–844; quiz 844. [DOI] [PubMed] [Google Scholar]
- 9.Barba C, Rheims S, Minotti L, Guénot M, Hoffmann D, Chabardès S, et al. Temporal plus epilepsy is a major determinant of temporal lobe surgery failures Brain (London, England : 1878). 2016;139:444–451. [DOI] [PubMed] [Google Scholar]
- 10.Ryvlin P, Rheims S. Predicting epilepsy surgery outcome Current opinion in neurology. 2016;29:182–188. [DOI] [PubMed] [Google Scholar]
- 11.Janszky J, Janszky I, Schulz R, Hoppe M, Behne F, Pannek HW, et al. Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome Brain. 2005;128:395–404. [DOI] [PubMed] [Google Scholar]
- 12.Rácz A, Becker AJ, Quesada CM, Borger V, Vatter H, Surges R, et al. Post-Surgical Outcome and Its Determining Factors in Patients Operated on With Focal Cortical Dysplasia Type II-A Retrospective Monocenter Study Front Neurol. 2021;12:666056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, et al. Do antiepileptic drugs or generalized tonic-clonic seizure frequency increase SUDEP risk? A combined analysis Epilepsia (Copenhagen). 2012;53:249–252. [DOI] [PubMed] [Google Scholar]
- 14.Sveinsson O, Andersson T, Mattsson P, Carlsson S, Tomson T. Clinical risk factors in SUDEP: A nationwide population-based case-control study Neurology. 2020. Jan 28;94:e419–e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nie L, Jiang Y, Lv Z, Pang X, Liang X, Chang W, et al. A study of brain functional network and alertness changes in temporal lobe epilepsy with and without focal to bilateral tonic–clonic seizures BMC Neurology. 2022. 2022/01/07;22:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shorvon SD, Perucca E, Engel J, Adjei P. The treatment of epilepsy. Fourth edition. ed. Chichester, England: Wiley Blackwell; 2016. [Google Scholar]
- 17.Fattore C, Perucca E. Novel Medications for Epilepsy Drugs. 2011. Nov 2011 2014-12-09;71:2151–2178. [DOI] [PubMed] [Google Scholar]
- 18.Cohen NT, Ziobro JM, Depositario-Cabacar DF, Havens K, Kao A, Schreiber JM, et al. Measure thrice, cut twice: On the benefit of reoperation for failed pediatric epilepsy surgery Epilepsy research. 2020. Mar;161:106289. [DOI] [PubMed] [Google Scholar]
- 19.Maynard LM, Leach JL, Horn PS, Spaeth CG, Mangano FT, Holland KD, et al. EPILEPSY PREVALENCE AND SEVERITY PREDICTORS IN MRI-IDENTIFIED FOCAL CORTICAL DYSPLASIA Epilepsy research. 2017;132:41–49. [DOI] [PubMed] [Google Scholar]
- 20.Wahab A Difficulties in Treatment and Management of Epilepsy and Challenges in New Drug Development Pharmaceuticals (Basel). 2010;3:2090–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enatsu R, Jin K, Elwan S, Kubota Y, Piao Z, O’Connor T, et al. Correlations between ictal propagation and response to electrical cortical stimulation: A cortico-cortical evoked potential study Epilepsy research. 2012;101:76–87. [DOI] [PubMed] [Google Scholar]
- 22.Sinha N, Peternell N, Schroeder GM, Tisi J, Vos SB, Winston GP, et al. Focal to bilateral tonic–clonic seizures are associated with widespread network abnormality in temporal lobe epilepsy Epilepsia (Copenhagen). 2021;62:729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies Epilepsia. 2010. Jun;51:1069–1077. [DOI] [PubMed] [Google Scholar]
- 24.Rodgers WP, Durnford AJ, Kirkham FJ, Whitney A, Mullee MA, Gray WP. Interrater reliability of Engel, International League Against Epilepsy, and McHugh seizure outcome classifications following vagus nerve stimulator implantation: Clinical article Journal of Neurosurgery: Pediatrics PED. 2012. 01 Sep. 2012;10:226–229. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, et al. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI Cerebral cortex (New York, NY : 1991). 2018. Sep 1;28:3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity Journal of neurophysiology. 2011. Sep;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, et al. Cortical and subcortical networks in human secondarily generalized tonic–clonic seizures Brain (London, England : 1878). 2009;132:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He X, Chaitanya G, Asma B, Caciagli L, Bassett DS, Tracy JI, et al. Disrupted basal ganglia-thalamocortical loops in focal to bilateral tonic-clonic seizures Brain : a journal of neurology. 2020. Jan 1;143:175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caciagli L, Allen LA, He X, Trimmel K, Vos SB, Centeno M, et al. Thalamus and focal to bilateral seizures: A multiscale cognitive imaging study Neurology. 2020. Oct 27;95:e2427–e2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha N, Wang Y, Moreira da Silva N, Miserocchi A, McEvoy AW, de Tisi J, et al. Structural Brain Network Abnormalities and the Probability of Seizure Recurrence After Epilepsy Surgery Neurology. 2021. Feb 2;96:e758–e771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakai Y, Nishibayashi H, Donishi T, Terada M, Nakao N, Kaneoke Y. Regional abnormality of functional connectivity is associated with clinical manifestations in individuals with intractable focal epilepsy Scientific reports. 2021. Jan 15;11:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ofer I, LeRose C, Mast H, LeVan P, Metternich B, Egger K, et al. Association between seizure freedom and default mode network reorganization in patients with unilateral temporal lobe epilepsy Epilepsy & behavior : E&B. 2019. Jan;90:238–246. [DOI] [PubMed] [Google Scholar]
- 33.Ben Zvi I, Enright N, D’Arco F, Tahir MZ, Chari A, Cross JH, et al. Children with seizures and radiological diagnosis of focal cortical dysplasia: can drug-resistant epilepsy be predicted earlier? Epileptic disorders : international epilepsy journal with videotape. 2021. Nov 8. [DOI] [PubMed] [Google Scholar]
- 34.Hale AT, Chari A, Scott RC, Cross JH, Rozzelle CJ, Blount JP, et al. Expedited epilepsy surgery prior to drug resistance in children: a frontier worth crossing? Brain : a journal of neurology. 2022. Jul 27. [DOI] [PubMed] [Google Scholar]
- 35.Wagstyl K, Whitaker K, Raznahan A, Seidlitz J, Vértes PE, Foldes S, et al. Atlas of lesion locations and postsurgical seizure freedom in focal cortical dysplasia: A MELD study Epilepsia (Copenhagen). 2022;63:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piper RJ, Richardson RM, Worrell G, Carmichael DW, Baldeweg T, Litt B, et al. Towards network-guided neuromodulation for epilepsy Brain : a journal of neurology. 2022. Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartolomei F, Lagarde S, Wendling F, McGonigal A, Jirsa V, Guye M, et al. Defining epileptogenic networks: Contribution of SEEG and signal analysis Epilepsia. 2017. Jul;58:1131–1147. [DOI] [PubMed] [Google Scholar]
- 38.Willard A, Antonic-Baker A, Chen Z, O’Brien TJ, Kwan P, Perucca P. Seizure Outcome After Surgery for MRI-Diagnosed Focal Cortical Dysplasia: A Systematic Review and Meta-analysis Neurology. 2022. Jan 18;98:e236–e248. [DOI] [PubMed] [Google Scholar]
- 39.Kwon HE, Eom S, Kang HC, Lee JS, Kim SH, Kim DS, et al. Surgical treatment of pediatric focal cortical dysplasia: Clinical spectrum and surgical outcome Neurology. 2016. Aug 30;87:945–951. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Lizana E, Fauser S, Brandt A, Schuler E, Wiegand G, Doostkam S, et al. Long-term seizure outcome in pediatric patients with focal cortical dysplasia undergoing tailored and standard surgical resections Seizure. 2018. Nov;62:66–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.
Anonymized data not published within this article will be made available by request from any qualified investigator.


