Abstract
There has been considerable speculation regarding the function of the dentate gyrus (DG) — a subregion of the mammalian hippocampus — in learning and memory. In this Perspective article, we compare leading theories of DG function. We note that these theories all critically rely on the generation of distinct patterns of activity in the region to signal differences between experiences and to reduce interference between memories. However, these theories are divided by the roles they attribute to the DG during learning and recall and by the contributions they ascribe to specific inputs or cell types within the DG. These differences influence the information that the DG is thought to impart to downstream structures. We work towards a holistic view of the role of DG in learning and memory by first developing three critical questions to foster a dialogue between the leading theories. We then evaluate the extent to which previous studies address our questions, highlight remaining areas of conflict, and suggest future experiments to bridge these theories.
Introduction
The dentate gyrus (DG) sits at the gateway to the hippocampus as the first stop along a trisynaptic loop that receives inputs from the cortex1. In mammals such as rodents, the DG has garnered substantial attention as a site of adult neurogenesis — the continuous birth of neurons throughout adulthood — leading to exciting speculation as to the role of new neurons in learning and memory2-6. The primary function of the DG has been hotly debated, with numerous theories each differentially incorporating findings from anatomical, physiological and behavioural data. Research on DG function is currently fragmented as a consequence of this multidisciplinary approach, and there have been multiple major advances in the field that are challenging to integrate into a coherent theoretical framework. Surprisingly, few attempts have been made to consider points of overlap and differentiation among the leading theories of DG function. Can we leverage the distinguishing details of each theory to develop a more complete understanding of the features unique to the DG and a more holistic view of its cooperative role in larger medial temporal lobe networks?
In this Perspective article, we describe several of the leading theories of DG function (Fig. 1 and Table 1) and develop critical questions to highlight the shared and divergent features of the theories. We examine previous studies within the framework of these critical questions and identify future steps for integrating the theories, enabling a more cohesive view across approaches and facilitating more targeted tests of DG function. Our overarching goal is to provide a framework for a comprehensive view of DG function that bridges current theories.
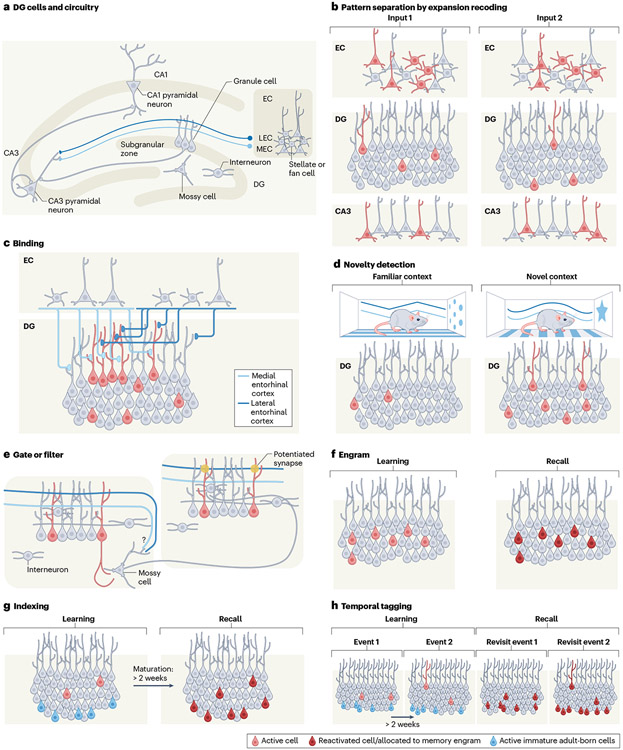
Fig. 1 ∣. Schematic of mechanisms driving each proposed theory of dentate gyrus function.
a, Schematic of anatomical connections and circuitry within the dentate gyrus (DG). Layer II of the entorhinal cortex (EC) sends excitatory projections to the outer two-thirds of the DG molecular layer and directly to CA3. Granule cells (GCs) in the DG GC layer send excitatory projections to CA3. The GC layer of the DG encloses a polymorph layer that contains many cell types, including mossy cells (MCs) that send excitatory projections to the inner one-third of the molecular layer. The subgranular zone is a site of adult neurogenesis. Multiple interneuron types can be found throughout all layers of the DG. b, Pattern separation by expansion recoding: similar patterns of activity in the EC (active pyramidal, stellate and fan cells in red) elicit distinct patterns of activity among a relatively larger number of GCs, which in turn activate distinct populations of cells in CA3. c, Binding: the convergence of inputs from the lateral and medial EC upon DG GCs facilitates the binding of information carried by each stream. d, Novelty detection: enhanced excitability in the DG during novel experiences facilitates the encoding of new information. e, Gate or filter: strong feedforward and feedback inhibition work together to filter inputs such that only the strongest and most consistent inputs pass through the DG. MC projections contribute to feedback inhibition and the potentiation of synapses with coincident entorhinal and MC input. f, Engram: representations of an experience are maintained by a distinct population of GCs, and reactivation of this population facilitates memory recall. g, Indexing (a recent variant that incorporates adult neurogenesis): immature adult-born neurons (blue) maintain activity that is selective for experiences that occurred during their development, and the reactivation of this unique population aids in indexing neocortical representations of those experiences. h, Temporal tagging: the continuous turnover of immature adult-born cells over time gives rise to distinct populations of later mature adult-born cells with activity that is selective to (and re-engaged by) experiences that occurred at a particular time point. LEC, lateral entorhinal cortex; MEC, medial entorhinal cortex.
Table 1 ∣.
Leading theories of dentate gyrus function
| Theory | Proposed function | Distinctive features | Mechanisms | Involvement during encoding and/or recall |
Refs. |
|---|---|---|---|---|---|
| Pattern separation by expansion recoding | Creates distinct population-level code for similar inputs | Orthogonalization of inputs | Expansion recoding | Both or just encoding | 9,76 |
| Binding | Creates highly selective conjunctions by binding incoming sensory information | Conjunctive information in spiking activity | Convergence of MEC and LEC inputs | Both | 21,31 |
| Novelty detection | Creates distinct population-level activity for new features of an experience | Enhanced responses to novelty | Neuromodulatory inputs | Encoding | 32,38,42 |
| Gate or filter | Permits selective information to pass given input strength, persistence and temporal coordination with other inputs | Contribution of interneurons and mossy cells within DG | Strong, inhibition, Hebbian or homeostatic synaptic plasticity | Both | 52 |
| Engram | Provides dedicated, distinct population-level cell activity for all experiences | Contribution of a stable, dedicated cell population | Strong, long-lasting synaptic modifications | Both | 53,58 |
| Indexing | Provides unique indices (for example, engrams) for memories stored in the cortex | Long-term contribution of adult-born neurons | Long-term integration of adult-born neurons | Both | 60 |
| Temporal tagging | Provides new, distinct and dedicated population-level code for temporally proximal experiences | Short-term and long-term contribution of adult-born neurons | Maturation and long-term integration of adult-born neurons | Both | 65,66,69 |
DG, dentate gyrus; LEC, Lateral entorhinal cortex; MEC, medial entorhinaL cortex.
DG anatomy
Before initiating a description of several of the leading theories of DG function, we first outline some key aspects of DG anatomy. The DG is composed of three layers: a molecular layer, a granule cell layer (GCL) and a polymorphic layer (also known as the hilus). In the trisynaptic loop, strong excitatory inputs from layer II of the entorhinal cortex (EC) terminate onto granule cell (GC) dendrites in the outer two-thirds of the molecular layer, and GCs send excitatory projections to CA3, which in turn sends excitatory projections to CA1 (ref. 1) (Fig. 1a). CA1 sends projections back to the deep layers of the EC (both directly and indirectly via the subiculum), which send projections that carry the output of hippocampal processing to the neocortex and back to the superficial layers7. The projection from CA1 thus completes a processing loop whereby the hippocampal output to the EC can affect the processing of the circuits that provide the input to the hippocampus. The hilus of the DG contains multiple cell types, including mossy cells (MCs). MCs receive input from GCs, backprojecting CA3 pyramidal cells and EC cells, and send excitatory projections primarily to the inner one-third of the molecular layer1. Multiple inhibitory interneuron types can be found in all three DG layers. Adult neurogenesis occurs within the subgranular zone at the base of the GCL, and the cell bodies of adult-born GCs integrate into the GCL8. Adult-born GCs undergo a transient developmental period in which they are more easily excited compared with mature GCs, and the continual generation and integration of new GCs in the region introduce a high degree of plasticity across the lifetime of the organism8.
Leading theories of DG function
Pattern separation by expansion recoding
Some of the first insights into possible roles for the DG were gleaned from studies that focused on the cerebellar cortex. In 1969, Marr9 proposed that the cerebellar GCL performs an expansion recoding (Fig. 1b) of its far fewer inputs from the pontine nuclei. Because each of the enormous number of GCs received only a small number of synaptic inputs (the input layer to GCL expansion ratio is approximately 1:600)10, Marr theorized that GCs would fire very sparsely and respond to an extremely narrow range of inputs. As a result, overlapping representations in the pontine nuclei would be transformed into independent (orthogonal) representations in the GCL. This transformation would result in an increase in the capacity of the GCs to learn and store, with minimal interference, the almost limitless contexts in which individual movements are made.
Years later, several hippocampal investigators applied the same idea to the GCL of the DG11-16. The numerical expansion of major excitatory inputs from the EC to GCs in the hippocampus is not nearly as strong as that of pontine inputs to GCs in the cerebellum (the expansion ratio is approximately 1:5 in rats)17, and there are a larger number of synaptic connections between the EC and DG GCs than between the pontine nuclei and cerebellar GCs. Nevertheless, the idea that the DG performs expansion recoding is incorporated into several computational theories.
Expansion recoding is but one mechanism by which the DG can perform pattern separation, which is defined computationally as a process by which different output representations are made less similar (less correlated) than their input representations. Multiple potential mechanisms (proposed by different theories subsequently) can perform this transformation. Of note, although we define pattern separation as an orthogonalization of activity at the cellular level, other researchers have utilized this term to describe behaviours that probably depend on distinct cellular representations in the hippocampus, such as the ability to distinctly recall highly similar experiences (explained thoroughly in previous reviews18). In this Perspective article, we use the term pattern separation to describe the recruitment of distinct patterns of activity across populations of GCs in the DG, through expansion recoding or the other possible mechanisms proposed subsequently.
Binding
The DG has also been cast as a site of convergence for separate information processing streams, suggesting a role for the DG in associating incoming sensory information. Entorhinal inputs to the DG can be divided into those from the medial EC (MEC) and the lateral EC (LEC) on the basis of differences in their anatomical connections and cytoarchitecture. Many researchers propose that these areas constitute anatomically segregated, parallel processing streams into the hippocampus19-22. Although there is substantial opportunity for communication23 between the streams before reaching the DG24,25, MEC and LEC activity in freely moving animals suggests that these regions may process different types of information26,27. Specifically, it is thought that LEC activity conveys information about, in the words of O’Keefe and Nadel26,28, “the items and events of an organism’s experience” — the content of an experience — from the first-person viewpoint of the organism26,29. By contrast, the MEC activity is thought to provide a framework for experience — the context of an experience — ordering and relating experiential information including (but not limited to) self-motion-based information about location and information about environmental landmarks26,30. The convergence of LEC and MEC inputs in the DG provides a locus for these streams to bind their information, and the binding theory (Fig. 1c) proposes that this is a major function of the DG21,31.
This binding theory is distinct from (but not mutually exclusive with) the aforementioned role of the DG in pattern separation via expansion recoding, in that it specifies the item or event and contextual information entering the DG. As a consequence, this theory proposes that an additional function of the DG is to provide highly selective responses to the co-occurrence of items or events in specific contexts, termed conjunctive activity, which can be leveraged by downstream networks to form distinct memories for similar experiences.
Novelty detection
The novelty detection theory, which has a long history32, posits that the DG exerts a particularly strong influence on downstream networks when organisms encounter new experiences or need to incorporate new information into previous experiences (Fig. 1d). In addition to a large excitatory input from the EC, the DG also receives many neuromodulatory influences33,34, which alter local responses to inputs under certain conditions, such as a fearful experience (via norepinephrine) or the initiation of rapid eye movement sleep (via acetylcholine). Both norepinephrine and acetylcholine have been shown to influence GCs and MCs during new experiences, through mechanisms that include enhancing excitatory responses to entorhinal inputs, decreasing feedforward inhibition, raising the resting membrane potential of GCs and enhancing long-term potentiation of EC–GC synapses35-40. The enhanced excitability during new experiences41,42 supports the theory that DG has a role in novelty detection.
Gate or filter
The gate or filter hypothesis proposes that intricate connectivity among GCs, MCs and interneurons restricts responses to entorhinal inputs and prevents overactivation. A major mechanism for the gate or filter function is inhibition of GCs by local interneurons that are engaged through feedforward inputs from the EC and feedback inputs from GCs and MCs43-49 (Fig. 1e). In addition, GCs have an action potential threshold greatly depolarized compared with the resting membrane potential50. Therefore, only the strongest entorhinal inputs activate GCs. GCs that overcome this inhibition send excitatory inputs to both local interneurons (contributing to the aforementioned feedback inhibition51) and MCs in addition to their long-range CA3 targets. MCs send excitatory projections to a diverse array of local interneuronal and GC targets, with ipsilateral and contralateral projections that spread across the hippocampus such that they target GCs in other planes throughout the septotemporal axis. In the gate or filter theory, complex adjustments of EC (perforant path) and inhibitory synaptic strength also arise depending on the pattern of presynaptic input52. Strong, consistent activation of GCs leads to potentiation of EC–GC synapses by several mechanisms, including MC excitatory feedback onto the GCL. Relatively weaker inputs are scaled down as a consequence of homeostatic synaptic plasticity observed in the region. A consequence of these physiological features is that the history of activity at perforant path synapses has the ability to both strengthen and weaken future attempts to re-engage the network through the same inputs in a manner that is dependent on input timing and strength (metaplasticity).
A major distinguishing feature of the gate or filter theory is that it provides an intrinsic mechanism for reactivating a specific subset of GCs for revisited experiences or recruiting distinct populations of active cells (that is, performing pattern separation) for similar or repeated experiences. DG inputs to downstream networks thus have the potential to flexibly shift between engaging an exact, previously active network and providing an orthogonal code, perhaps to meet different behavioural demands.
Engram
Some researchers have focused on plasticity within the DG during learning and the ability to reliably engage populations of cells in the region upon the recurrence of an experience (Fig. 1f). One theory proposes that the DG generates engrams, or enduring physical changes in the brain that support recall53, through local biochemical and physiological changes during learning. Several relatively recent studies have demonstrated that reactivation of subpopulations of GCs that are active during an initial experience is sufficient to re-engage previously active downstream neuronal networks and to instigate behavioural expressions of memory (for example, freezing in response to learned fear of an environment)54-57.
A major distinguishing feature of this theory is the view that learned experiences re-engage dedicated or previously active populations of cells in the DG and that the DG exerts a domino effect or chain reaction in the brain that can be powerfully leveraged for memory recall58. Similar to other theories, however, this theory proposes that different learned experiences recruit distinct populations of DG GCs.
Indexing
Another major theory proposes that the hippocampus forms and retains indices of neocortical representations activated by experiences59. A recent variant of this theory60 proposed a unique role for the DG that incorporates its ability to continually give rise to new neurons throughout adulthood, termed adult neurogenesis. Immature adult-born neurons undergo a period of higher intrinsic and synaptic excitability as well as greater synaptic plasticity than their mature GC counterparts61-64. As a consequence, adult-born neurons become potentiated to inputs experienced during their development and exhibit selective and dedicated activity to future instantiations of these experiences65 (Fig. 1g). The dedicated activity or persistent activation of adult-born cells across instantiations of specific experiences is thought to index learned memories during recall, jumpstarting a chain reaction of downstream networks upon their reactivation in a manner similar to the proposed role of engrams in the DG60.
A major distinguishing feature of this theory is the incorporation of adult-born neurons into engrams within the DG. This theory integrates physiological properties of adult-born neurons into hypotheses of DG function and, similar to other theories, proposes that a distinct population-level code in DG for different experiences is leveraged by downstream networks.
Temporal tagging
The DG is also thought to incorporate temporal information into representations of experience by demonstrating dynamic activity over time, whether on the order of hours, days or weeks. This activity is thought to facilitate distinct representations of information occurring at different times66,67 and possibly contribute to a drift in the active population of cells that is ideal for updating and forming new memories68. One theory proposes a short-term and long-term role for adult-born neurons in temporally tagging new memories69,70. This theory proposes that the transiently hyperexcitable immature adult-born neurons are broadly tuned to experiences during their development, resulting in a commonly active population of immature cells for temporally proximal events. Over time, new populations of immature GCs arise and mature, creating distinct populations of adult-born GCs that exhibit selective and dedicated activity to experiences that occurred during their development (Fig. 1h). The constant turnover and physiological development of adult-born neurons are thus thought to facilitate an enhanced ability to engage distinct populations of GCs for experiences that occur far apart in time. A major distinguishing feature of this theory is the representation of separate temporal epochs by adult-born GC activity.
Critical questions
The theories discussed earlier all have features that render them unique from the others, with some aspects that render them at least partially incompatible with other theories and other components that are highly synergistic. Here, we identify three critical questions that tackle key points of convergence and divergence across these theories. Addressing these questions will allow researchers to determine the extent to which combinations of theories are mutually exclusive or synergistic. We recognize that our consideration of seven of the current leading theories of DG function does not fully capture the wide range of perspectives on the topic (for example, sequence generation71, memory resolution72 and other proposed DG functions73-75). However, we present the following critical questions in the hope of providing a framework for collaborative dialogue across different perspectives moving forward:
How does the role of the DG differ between learning and recall?
How do specific cell types and connectivity within the DG shape its processing?
What information does the DG impart to downstream structures?
The role of the DG in memory recall is a major point of controversy across the theories. Although the theories all propose that the DG minimizes interference by recruiting distinct populations of GCs across experiences during learning, this role potentially hinders the re-engagement of populations across experiences during recall. A subset of the theories (binding, gate or filter, engram, indexing and temporal tagging) propose that plasticity in the DG during an initial experience facilitates the recruitment of the same unique population of GCs during subsequent recurrences, which can be leveraged for re-engaging the initially active downstream networks to jumpstart recollection. Although there are theoretical advantages to engaging distinct populations of GCs during learning and re-engaging dedicated populations of GCs during recall, the extent to which (and behavioural circumstances determining when) the DG flexibly engages one, the other or both is currently unknown and a subject of debate.
The specific contributions of interneurons, MCs and adult-born GCs to DG function are a major focus of several theories. In particular, the gate or filter hypothesis suggests that inhibitory interneuron and MC feedback shape GC responses to current and future inputs. Other theories such as indexing or temporal tagging suggest that the heightened plasticity of adult-born neurons during their immature state shapes their long-term responsiveness to inputs. The short-term and long-term contributions of these different cells to the DG network are not currently well understood.
Each theory suggests different mechanisms for recruiting distinct populations of GC across experiences (Fig. 1), changing the information that the DG is thought to impart to downstream structures. The theory of pattern separation by expansion recoding focuses primarily upon the fact that dentate GCs outnumber entorhinal projection neurons by a ratio of 5:1 (refs. 17,76), resulting in the engagement of non-overlapping populations of GCs and orthogonalized outputs from DG, given similar inputs from EC. The theory of binding also adds that the DG is in an ideal location for integrating item or event and contextual information, as information processed by parallel input streams undergoes a major point of convergence in the DG. Thus, the mechanistic focus of the latter theory specifies that the DG provides unique conjunctive information to downstream structures. Theories regarding the role of the DG in novelty detection, indexing and temporal tagging note enhanced excitability in the region as a potential mechanism for recruiting distinct populations of cells for new experiences. In the case of novelty detection, this enhanced excitability occurs specifically during novel or changing experiences, constraining the DG to the processing of new information. By contrast, indexing and temporal tagging theories posit that this enhanced excitability results from the continuous recruitment of transiently hyperexcitable adult-born cells over time, allowing the DG to incorporate the passage of time in its code. The gate or filter theory suggests that the connectivity within the DG filters out weak or inconsistent input patterns, allowing only strong or persistent input patterns to pass through and highlighting a role for DG in conveying information about repetition or consistency. Although the engram theory does not currently subscribe to a particular mechanism, it requires mechanisms for maintaining and re-engaging stable representations of experience in the DG for long periods of time to support its proposed role in jumpstarting recall downstream. Of note, although each theory proposes different mechanisms for recruiting distinct populations of GC across experiences, it is possible for all mechanisms to coexist harmoniously. Together, they highlight the many possible routes for achieving distinct population-level codes across experiences and potential functions of the DG. The extent to which any of the aforementioned mechanisms are at play in the mammalian DG (or in non-mammalian species to achieve a similar function (Box 1)) is not yet known.
Box 1. How might other species accomplish similar functions with dissimilar structures?
Previous studies have identified elements of continuity between presumed quasi-reptilian ancestors and the mammalian hippocampus219,220. Importantly, innovations in mammals appear different from those introduced in its avian homologue (reviewed elsewhere221). In particular, the bird hippocampus lacks a clear trisynaptic circuit and an obvious dentate gyrus (DG) equivalent. This suggests, in combination with the spectacular spatial memory abilities of other species, that we should stop seeing the mammalian phenotype as the unique solution, or even the best one, to a predefined computational challenge. Evolution is blind and proceeds mainly by mistake. Yet, despite this mistake-studded history, the key traits of the mammalian DG are clear, such as its relation to CA3 (despite substantial species-to-species variability in the quantitative proportions of the components) and its preserved capacity for adult neurogenesis222,223. The critical questions identified in this Perspective can provide a framework for integrating work conducted across species into research on mammalian hippocampal function224 and on any particular DG role in it. To what extent do other species use alternative mechanisms to recruit distinct populations of hippocampal cells to encode different experiences and re-engage the same population during recall? Are subsets or combinations of elements in the mammalian DG (for example, converging input streams, vast inhibitory inputs and the continuous addition of neurons in adulthood) leveraged by hippocampal homologues in other species? Why is adult hippocampal neurogenesis restricted to the mammalian DG and widespread in other lineages225? What can we learn from fish that lack a cortex and wear their hippocampal homologue laterally not medially, but still use it to remember the spatial location of an exit route226? Addressing these questions in other species will help to identify the design elements that support hypothesized functions of the mammalian DG.
Although supporting evidence for each theory is often considered in isolation, here we discuss how previous modelling, behavioural, lesion, physiology and imaging studies address the critical questions we have identified as central to all theories.
Critical question 1: how does the role of the DG differ between learning and recall?
Both network modelling and experimental results have provided strong evidence that the DG contributes greatly to learning, but there is currently a lack of consensus as to its involvement in recall (Fig. 2). This creates conflict between theories that propose that the DG exerts its greatest impact only during learning (novelty detection; Fig. 1d) and those that propose a strong influence during both learning and recall (engram, indexing and temporal tagging; Fig. 1f-h). For this reason, we subsequently focus our coverage on evidence that suggests a minimal or strong DG influence on memory recall.
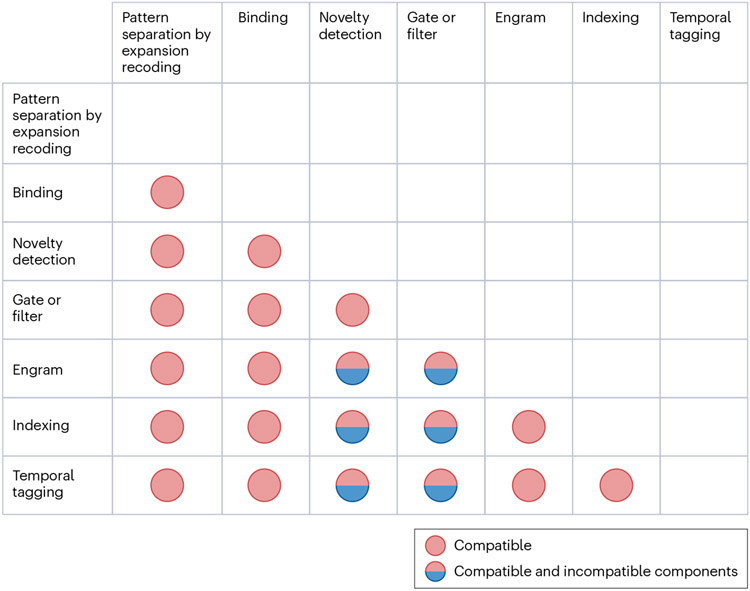
Fig. 2 ∣. Leading theory compatibility.
For each pair of leading theories, coloured circles indicate whether the theories have compatible (red circles) or both compatible and incompatible components (red and blue circles).
Network models have been used to test the hypothesis that the architecture of the DG minimizes interference between memories specifically during learning. A few key aspects of DG inputs and outputs were considered in these models. The major excitatory input to the DG originates from layer II of the EC, which also projects to the CA3 subregion of the hippocampus1. GCs in the rat DG form powerful synapses onto the proximal dendrites of an estimated 15 pyramidal cells in CA3, its primary output structure17,77-79. These synapses have been described as detonators, a description supported by the massive boutons of the GCs observed initially in the 1960s80, the ability of GCs to reliably induce spiking in downstream neurons in vivo81 and the glutamate toxicity produced by excessive GC firing in seizures82. Conceptually, the nature of their effect was captured with the simplified notion of all-or-none detonator synapses by McNaughton and Morris12 and then quantitatively assessed by Treves and Rolls83 within the framework laid out by Marr et al.84. In these models, CA3 receives two distinct input streams: directly through associatively modifiable (the so-called Hebbian) synapses from layer II of the EC and indirectly through strong synapses from the DG. The lack of associative modifications in the latter stream (but see the ‘Gate or filter’ section and ref. 71) makes it unsuitable to relay a partial cue83, but it can induce relatively different patterns of activity in CA3 across experiences. The direct perforant path input re-engages previously stored patterns through CA3 recurrent collateral activity. Whether the direct input expresses plasticity (possibly non-associative83) has been argued83 not to significantly impact CA3 function, as its DG inputs would tend to be irrelevant during retrieval, when long-term plasticity would eventually be expressed. The DG would thus afford downstream CA3 with a quantitatively enhanced ability to distinctly represent new information, contrasting its tendency to reinstate previously acquired information. The information–theoretic analysis was later extended to spatial representations85, leading to the same prediction that dentate activity should not be required for the reactivation of memories already stored in CA3.
Other models such as the complementary learning systems (CLS) model86 characterize the effects of the DG and downstream circuits on larger networks outside the hippocampus. Specifically, the CLS model proposes that the hippocampus rapidly stores memories as highly segregated representations of experience and allows for a slow integration with representations stored in neocortical systems in a manner that minimizes interference. Later reflections on this model also posited a specific role for the DG in recruiting unique representations of experience specifically during learning87, again arguing along with Marr84 that recall would require overcoming the sparse and non-overlapping patterns imposed by the DG in a cascade effect jumpstarted by recurrent collateral activity in CA3. This notion is in conflict with engram, indexing and temporal tagging theories (Figs. 1 and 2). Future modelling could explicitly test the effect of recruiting a dedicated population of GCs during recall, although such a role would arguably interfere with its ability to provide information about the distinct aspects of a recurring experience (but some studies have suggested that this can be accomplished by distinct portions of CA3 along the transverse axis88).
Behavioural, lesion and inactivation studies provide insights into when and how selective damage to the DG affects memory. These studies parameterize aspects of memory processes (for example, learning versus recall), testing for specific deficits that arise when the integrity of the DG has been compromised. Testing the specific function of the DG had not been possible until a neurotoxin called colchicine was introduced to allow selective lesions in the DG while leaving other subfields of the hippocampus such as the CA1 and CA3 relatively unaffected89-103. Since then, additional methods for selectively and reversibly inactivating the DG104, or GCs specifically105,106, have been developed. Several studies in both rats and mice have used these methods to explicitly test whether the integrity of the DG is required during learning versus recall, with some studies observing deficits only during learning96,102,105 and others observing deficits during both learning and recall104. The mixed results in the literature for the involvement of the DG in recall of hippocampal memory might be attributable to different training protocols and environmental settings among different studies. The necessity of this input from the DG during both encoding and recall may increase with higher similarity between cues or environments107. During recall, the DG may still be required if the original cues or environments are similar to each other or modified to become more similar to each other100,104. Other conditions such as the amount of training, the nature of task demands (especially regarding whether it can be learned with non-hippocampal strategies) and working memory requirements may determine the involvement of the DG in the encoding and/or recall of memory.
Several theories (engram, indexing and temporal tagging theories; Fig. 1f-h) explicitly predict that (at least a subset of) the same cells that are active during the encoding of an experience should be active when recalling a memory. This notion would predict that GCs fire similarly during initial exposures to novel cues or environments (when encoding is presumably prioritized) and during subsequent re-exposure to those cues or environments (when recall processes may be enhanced). The DG contains place cells108 — neurons that exhibit activity when an animal is in a particular region of a spatial environment — and their activity is thought to help map both absolute and relative spatial relationships29,109. Many research groups investigate how spatial representations in the rodent hippocampus transform across experiences to gain insight into the larger contributions of the hippocampus to human episodic memory21. For example, the cognitive map theory suggests that place cells form a spatial framework that is used to organize and interrelate the items and events of experience to allow storage and subsequent recall of an episodic memory29. Relational learning theory suggests a similar role for place cells but regards the spatial tuning of these cells as a reflection of more general, not necessarily spatial, relational mapping functions of the hippocampus109. Several studies have shown that at least a subset of GCs show similar place field representations across repeated exposures to the same environment54,110-117, showing that the DG is capable of re-engaging stable representations of space beyond initial encoding (see discussion of ‘Critical question 3’ for a review of responses across changing or different environments). Other studies have shown that DG activity is dynamic across time and with increasing experience. Multiple imaging and physiological recording studies have indicated heightened GC and MC responses upon the introduction of novel stimuli42,118-121. In a paradigm in which head-fixed mice traverse along a treadmill, the introduction of new objects by affixing them to a point along the treadmill causes a reconfiguration of GC and MC spatial representations, with MCs exhibiting earlier and larger changes in response to the introduction of novel cues118. These studies provide support for the novelty detection theory (Fig. 1d), which predicts a strong DG influence during learning and exposure to novelty. Exposing mice repeatedly (over 27 days) to the same sequence of objects on a treadmill belt results in a refinement of GC (but not MC) spatial (including landmark vector) representations over time, resulting in more single-fielded place cells122. This refinement of activity with increased experience also occurs in response to odours in odour–reward association tasks123. GCs (but not LEC neurons) initially exhibit responses to multiple odours before developing more selective responses to single odours (more often rewarded odours) over the course of learning, resulting in the evolution of minimally overlapping representations between odours. Thus, the DG may require time or experience to establish refined representations for complex stimuli or environments. Together, these findings suggest that neurons in the DG show spatial responses, with evidence of conjunctive representations among multiple cell types in the DG that evolves with time or experience. The exact influence of time or experience on the ability of DG neurons to re-engage initial (as proposed by the engram, indexing and temporal tagging theories; Fig. 1f-h) or refined patterns of activity during recall is not well understood.
Studies using genetic methods to label active neurons during circumscribed periods of time suggest that largely different populations of neurons are active during the initial and subsequent exposures to stimuli or environments. These studies suggest that approximately 6% of GCs are active during a single exposure to an environment or memory encoding124. On the timescale of days, although 6% of the neurons appear to be active during memory formation and 6% of the neurons are active during subsequent re-exposure to an environment (which may be considered as a new encoding event or recall), there is only a partial re-engagement of the 6% of initially active neurons (approximately 2%; that is, one-third of the initially active neurons are reactivated)124-126. This suggests that although approximately one-third of the 6% of the initially active neurons appear to be stably involved in encoding and recall, the remaining two-thirds of these neurons are active during either encoding or recall. Notably, if the timescale between the initial contextual fear conditioning and re-exposure is stretched to a month, the populations of neurons labelled as active at each time point appear not to overlap127,128. Nevertheless, optogenetic stimulation of either the population of neurons active during encoding or the population of cells active during recall (after days or months) elicits freezing responses, suggesting that both populations have the ability to drive context-specific behavioural responses124,127-129. The original population of tagged cells maintains this ability even under circumstances in which anisomycin is administered to block learning-related protein synthesis130. Even after multiple bouts of learning (for example, fear conditioning and extinction, which elicit activity in two non-overlapping DG ensembles), the original ensemble maintains its capacity to modulate contextual fear behaviour57. Of note, optogenetic silencing of the population of cells active during initial contextual fear encoding disrupts context-selective freezing responses 2 weeks later131, implying some degree of necessity for observing contextual fear behaviour on the scale of weeks. The function of this persistent influence upon contextual fear behaviour in the face of a large natural turnover of the active population across experiences is still being investigated. Taken together, these results suggest that there are both enduring changes in the DG that provide a stable population of GCs with a long-term ability to jumpstart memory recall and a continually evolving landscape of connections that can recruit new populations of GCs and imbue them with this same capability. Although the existence of a stable population of cells with a long-term influence on contextual fear behaviour supports the engram, indexing and temporal tagging theories (Fig. 1f-h), the large turnover of cells with this influence supports more recent notions of memory storage in supple synapses or ephemeral networks132,133.
There are several remaining areas of ambiguity regarding the role of the DG during recall. Specifically, the degree to which the cells active during learning are naturally reactivated and necessary during successful recall is still unknown. Future studies will need to address this issue to resolve conflicts across subsets of leading theories (Figs. 1d,f-h and 2). Our review of previous literature suggests that important strides can be made towards this goal by redeploying different methodological approaches to further insight gained by a single method. For example, previous network modelling studies have explicitly tested the effect of an orthogonalized code in the DG upon CA3, but mechanisms for reinstating representations in CA3 using a stable, reactivated population or newly recruited population of cells can also be explored in greater detail using this method. Studies in humans have the ability to explicitly prompt encoding and recall during specific intervals and even probe memory for recall events to examine the extent to which encoding and recall functions co-occur during repeated exposures134. Behavioural studies in rodents indicate that the DG may contribute to successful memory recall when organisms are tasked with discerning relationships between cues or environments and initial experiences. The thoughtful design of these behavioural studies can be leveraged by physiology and imaging experiments to characterize the nature of DG activity under such circumstances and specifically during isolated learning and recall phases. Together, these studies can help to unify the theories regarding the contributions of the DG to memory recall.
Critical question 2: how do specific cell types and connectivity within the DG shape its processing?
Several network models have tested the role that specific DG cell subtypes may have in recruiting distinct populations of GCs across experiences. Of these, a network model that includes CA3 projections back to the DG suggests that this recurrent connectivity recruits feedback inhibition that is necessary for sparse coding in the DG15. In this network model, CA3 projections to MCs also have an important role in exciting GCs in distant lamellae, describing a mechanism for “inhibition of recently active GCs while depolarizing a different subset of GCs — making the latter more likely to respond to the next entorhinal input”15. In addition, network models that incorporate extrinsic γ-rhythmic inhibitory inputs and lateral inhibition through parvalbumin-expressing interneurons in the DG reveal that both contribute substantially to the generation of distinct DG output patterns given similar EC inputs135. These studies augment and update the theory of pattern separation by expansion recoding, providing additional mechanisms for pattern separation that rely heavily on cells intrinsic to the DG in ways similar to the gate or filter hypothesis (Fig. 1b,e). Several network models that incorporate adult neurogenesis have noted that an increased number of neurons in the DG enhances its capacity to store information about new experiences in synaptic modifications across non-overlapping populations of cells136-138, although some models note that this comes at the expense of accelerating the forgetting of old experiences136,139-141. In networks of DG neurons that have already undergone some degree of learning, the ability to eliminate cells in the network through simulated apoptosis and add new GCs with random connectivity additionally enhanced the ability to separate new representations136,137. Other models have emphasized the transient high excitability or enhanced plasticity of adult-born GCs relative to mature GCs during their development69,70,138. In these network models, mature GCs are able to maintain their responsiveness to old experiences, whereas young GCs contribute a distinct output for new experiences. Of note, one network model predicted that the transient window of elevated excitability and plasticity in adult-born GCs would allow them not only to bind experiences that occur close in time but also to enhance the separation of events that occur far apart in time through the constant turnover of new GCs entering and maturing out of this transient stage70. In this case, different subpopulations of adult-born GCs would contribute distinct outputs for new experiences that occurred far apart in time. Together, the network models incorporating adult neurogenesis suggest that new GCs enhance the coding capacity of the DG, in many instances by distinctly representing new experiences in a manner that supports the indexing and temporal tagging theories and to some extent the novelty detection theory142 (Fig. 1d,g,h). Overall, network models that incorporate multiple cell types within the DG suggest that they strongly support the pattern separation abilities of the DG for new experiences.
Several more recent studies have attempted to selectively lesion or inhibit specific cell types within the DG and ascertain their contributions to behavioural performance in learning and memory tasks. One such study selectively lesioned MCs within the DG, with the lesioned animals having an impaired ability to exhibit appropriate context-specific behavioural responses in non-fearful environments following fear conditioning training143. This behavioural finding combined with evidence from the same study and many others47,144 that MCs substantially recruit local inhibitory interneuron activity in the DG provide support for the gate or filter hypothesis (Fig. 1e). Other studies that selectively inhibited MCs also found effects on context-specific memory145. Generally, tasks that involve disambiguating similar experiences are impaired without adult-born GCs146,147, the ability to disambiguate experiences is supported when adult neurogenesis is increased148, and the age of adult-born cells at the time of an experience influences their contribution64,149. These studies support indexing and temporal tagging theories, both of which propose an important role for the adult-born GC population in DG function (Fig. 1g,h). One notable exception includes a study in which ablating adult neurogenesis in rats improved their performance in a spatial working memory task that required them to ignore conflicting non-relevant information from previous trials150, potentially supporting the hypotheses that adult-born neurons introduce memory interference (or temporally integrate) in their immature stage of development (Fig. 1h). A more explicit test of the role of the DG in temporal tagging found that the DG is necessary to link spatial events that are presented close in time151. This finding supports the indexing and temporal tagging theories and, to some extent, the binding theory (Fig. 1c,g,h). Of note, multiple studies suggest that increasing adult neurogenesis facilitates forgetting152-158, whereas decreasing the levels of such neurogenesis promotes retention152,153 (reviewed elsewhere159). These studies once again challenge the proposed existence and usefulness of stable and enduring engrams in the DG, creating areas of conflict for the engram theory (particularly in the case of older memories) while partially supporting the indexing and temporal tagging theories in relation to newly formed memories (Fig. 1f-h).
The intrinsic properties of neurons within the DG and their physiological responses during behaviour reveal multiple potential mechanisms for generating distinct patterns of activity or sparse firing in the DG. Several physiological recording studies indicate that mature GCs are hard to activate. One reason is that their resting potential is more hyperpolarized than other hippocampal neurons50. GCs also have strong spike frequency adaptation, such that given persistent excitatory input, they fire initially and then cease160-162. GCs also receive strong inhibition49,51. Together, these studies provide a strong foundation for the gate or filter hypothesis (Fig. 1e), indicating that intrinsic GC properties and strong synaptic inhibition combine to allow only the strongest excitatory inputs from the EC to be sent through the DG to CA3. MCs are also thought to support a gating function of the DG through the activation of DG interneurons that inhibit GCs143. The broader spatial tuning of MCs relative to GCs111,112,163 could provide them with a profound inhibitory impact. However, MCs can excite the GCs strongly, depending on the temporal pattern of inputs to the region164. MCs also show a strong response to novelty41,42. To date, few studies have examined the spiking dynamics of DG interneurons in behaving rats, with one showing that DG interneurons have highly dynamic responses during exposure to novelty that are well coordinated with shifts in principal cell activity165. This suggests that interneurons have a powerful role in dynamically sculpting principal cell activity in response to novelty, partially supporting both the novelty detection and gate or filter theories (Fig. 1d,e). Another study found that changing spatial avoidance task contingencies results in changing subsecond (within 133 ms) patterns of activity between pairs of DG neurons, including increased co-firing of excitatory place cell and interneuron pairs, in arena locations with higher memory discrimination demand110. This study suggests a strong or more consistent inhibitory influence of interneurons upon GC activity when mice perform spatial discriminations that rely upon the DG, which again supports the gate or filter theory (Fig. 1e). The hippocampus, and the DG in particular, also exhibits rich local oscillatory activity166-169, which can provide a powerful mechanism for selectively engaging interactions between excitatory and inhibitory cells. The extent to which rhythmic coordination in the DG facilitates its distinct population-level code, changing subsecond interactions among DG ensembles or interactions with other structures, is largely theoretical.
The physiological contributions of adult-born GCs are still under investigation, but several lines of evidence suggest that their influence upon the local DG network and downstream CA3 is unique from their mature counterparts. Early in development, they have direct excitatory or inhibitory influences on mature GCs, depending on the origin and timing of inputs170. Adult-born GCs additionally innervate local GABAergic interneurons, which in turn hyperpolarize GCs, placing them in a powerful position to influence the inhibitory tone and sparse firing in the network171-177 in a manner that provides some degree of support to the gate or filter theory (Fig. 1e). Of note, adult-born GC proliferation, development and integration into existing networks are highly regulated by their local environment and experience65,66,121,139,173,178, with evidence indicating that mature adult-born GC activation is shaped by experiences occurring during their development. In particular, imaging evidence suggests that re-exposure to environments experienced during immature adult-born GC development reactivates the same adult-born GCs in their mature state65, providing strong support for both the indexing and temporal tagging theories (Fig. 1g,h). In addition, the activation of distinct populations of cells across environments is influenced by temporal separation and adult-neurogenesis levels. Environments introduced at largely different times (2 or 3 weeks apart) engage largely distinct populations of GCs in a manner dependent on adult neurogenesis, whereas environments introduced at the same time recruit similar populations66. Such activity might be necessary to encode similar, yet temporally distinct, episodes as proposed by the temporal tagging theory (Fig. 1h). Of note, the existence of adult-born neurons is not always beneficial, as ectopic populations of adult-born neurons develop abnormally in cases of epilepsy, and promote chronic seizure development in a manner that provides some support to the gate or filter theory179,180 (Fig. 1e).
From the many studies discussed in this section, it seems clear that there are multiple complementary mechanisms for generating distinct spatiotemporal patterns of activity in the DG across experiences, many of which involve specific contributions from MCs, interneurons or adult-born neurons (or all three). To further our understanding of the interactions within the DG that support its proposed functions, it is imperative to develop more methods for identifying and characterizing distinct cell types of the DG in electrophysiological recording experiments (for example, see refs. 111,112,181). Such methods allow explicit tests of hypotheses regarding the roles of GCs, MCs, interneurons and adult-born GCs in dentate network operations by characterizing their potentially unique physiological contributions during the parameterized experiments described earlier.
Critical question 3: what information does the DG impart to downstream structures?
One of the fundamental questions facing those who study the DG is why the hippocampus needs it. Many leading theories suggest that the DG contributes computations that are not unique to the DG. For example, multiple areas in the brain are capable of performing pattern separation by expansion recoding9,76, and engrams have been shown to exist in many brain regions53. Features such as pattern separation may be important in different structures for different reasons (reviewed elsewhere182). In this section, we consider the proposed computations of the DG in the context of its input and output structures and review what is known or highlight areas of ambiguity regarding its impact on the hippocampal network and hippocampal-dependent behaviours.
A limited number of network models aim to understand how representations of experience specific to the EC are transformed by the DG. The CLS model emphasized the convergence of object category and identity information from the ventral visual stream and spatial information from the dorsal visual stream in the hippocampus, outlining a mechanism for the formation of highly specific conjunctions in DG. It argued that unique representations for different combinations of co-occurring stimuli across distinct populations of GCs can be leveraged by downstream networks to minimize interference86,87. This model thus supports aspects of the binding theory well (Fig. 1c), particularly during learning. Another model specifically incorporated the convergence of spatial and nonspatial inputs in the DG and predicted that strong LEC inputs would boost existing spatial firing preferences of GCs and thus reduce the multiplicity of fields183. This model also supports the binding theory well (Fig. 1c), as this boost is specific to GCs that receive a convergence of MEC and LEC input. Notably, findings from subsequent experimental studies suggest that such multifielded activity is more often a property of MCs rather than GCs111,112,163. Another model incorporated adult neurogenesis into a DG network receiving both MEC and LEC inputs and predicted that immature adult-born GCs would yield similar activity for events that occur close in time (within a transient window in their development when they exhibit high excitability and high degrees of plasticity), but separate subpopulations of adult-born GCs would be active for events that occur far apart in time70. This model supports some aspects of the binding theory (Fig. 1c), as adult-born cells combine information from both input streams. It also supports engram, indexing and temporal tagging theories (Fig. 1f-h), proposing that enduring changes across the adult-born GC population allow their re-engagement during subsequent exposures to environments experienced during a critical period in their development. In this case, the model outlines a mechanism through which adult neurogenesis contributes to the formation of temporal associations in DG, linking events that occur close in time69. Advances in our understanding of the specific inputs that the DG receives from the MEC and LEC will inform future modelling work. For example, recent work suggests that the LEC and MEC encode information in egocentric and allocentric coordinate frames, respectively184, calling for modelling work to investigate how these fundamentally different ways of representing the world can be bound into a coherent representation of experience.
The theories all suggest that distinct spatiotemporal patterns of activity in the DG should minimize interference between memories and support disambiguation between similar experiences. Of note, rats with DG lesions can successfully discriminate between odours and environmental contexts, but they demonstrate an impaired ability to associate odours or object cues with the contexts in which they are rewarded185. This suggests that the DG is not important for sensory discrimination per se, but is involved in linking specific sensory cues with distinct outcomes. Many studies suggest that the integrity of the DG is necessary for learning or retrieving associations among exact spatial locations, environments or contexts with different objects or outcomes94,97-100,102,107. These behavioural and lesion studies support aspects of the binding theory well (Fig. 1c) and generally support all theories to some extent. In addition, a few studies have shown that lesions of the DG result in an impaired ability to link temporally proximal events, providing support for the temporal tagging theory (Fig. 1h). Taken together, these studies suggest that the pattern separation abilities of the DG may be necessary when behaviours depend on learning or recalling distinct associations between complex stimuli (for example, scenes and objects) and spatiotemporal contexts.
Many physiological recording and imaging studies have reported distinct activity patterns in GCs under conditions in which there are subtle changes or even no change to an environment across multiple exposures. These distinct activity patterns can manifest as changes to the firing rate and spatial preferences of GCs with place fields186,187 and as the recruitment of activity across distinct populations of cells66,115,117,188. Some of these studies contrast activity patterns in the GCs with those in CA3 pyramidal cells, noting that the GCs exhibit representations of different experiences according to both metrics that are far less similar than those in CA3 (refs. 115,186). These findings support the unified argument across theories that the DG conveys distinct representations of experience (Fig. 1 and Table 1). This contribution may be particularly important when there is a high similarity between experiences, as increasing the ambiguity of different visual patterns linked to spatial choices elicits orthogonal place cell firing patterns in CA3 when the DG is intact but fails to do so in CA3 place cells when there is a lesion in the DG100. Notably, one study found that 10% of GCs fire in both familiar and novel virtual environments, with similar place fields in each that remain relatively more stable across days than spatial representations in CA3 and CA1 (ref. 117). This finding suggests that a small minority of GCs exhibit stable representations that generalize across different virtual environments, even as the large majority of such cells discriminates the environments. The authors of this study suggested that this subpopulation of cells conveys stable engrams to downstream networks117 (see also ref. 189). These results provide some support to the engram theory and, to some extent, the indexing and temporal tagging theories (Fig. 1f-h). The extent to which both changing and stable patterns of activity are leveraged by downstream networks across experiences is not yet known (see discussion of ‘Critical question 1’).
To fully understand the computational capabilities of a region, future studies will need to better characterize its firing properties within the context of its inputs. With this knowledge, it is possible to quantify input–output transformations and deduce the computational processing behind those transformations. For the DG, its main excitatory inputs are the MEC and LEC. Although much is known about the physiological properties of the MEC, based on a huge amount of effort inspired by the discovery of the remarkable grid cells in 2005 (ref. 190), much less is known about the properties of the LEC. Recent work suggests that the LEC may be critically involved in representing the egocentric bearing of the rat (and perhaps distance) towards specific landmarks, boundaries or other locations in an environment184,191,192. Other findings suggest that the LEC and fasciola cinereum (a region that receives LEC input and projects exclusively to the crest of the DG) convey information about the temporal duration or order of an experience, over a wide range of timescales, which might allow different memories to be segregated over time67,193. To date, few studies have performed simultaneous recordings in the DG and its upstream structures169,194,195. These studies have provided insight into the behavioural circumstances surrounding strong influences from the MEC or LEC as well as oscillatory mechanisms for coordinating the integration of information in the DG from multiple sources. In future studies, computational models of the DG (whether they be models of pattern separation or other hypothesized functions) can incorporate the different types of information conveyed to the DG from its major inputs and provide further explanations for how these two sets of input may be combined and transformed in the DG to support both the spatial mapping functions of the hippocampus and its role in episodic memory183.
A unified theory of DG function will need to include information about different connectivities and activities along the longitudinal axis (the septal, dorsal, or posterior hippocampus compared with temporal, ventral, or anterior hippocampus)196, the upper versus lower blades of the DG197, and the proximal (closest to the DG) versus distal regions of CA3 (refs. 88,198). In rodents, septal DG (also called dorsal DG in rodents and posterior DG in primates) receives input from the lateral band of the MEC and LEC, and temporal DG (also called ventral DG in rodents and anterior DG in primates) receives input from the medial band of the MEC and LEC199. In the MEC, the gradient from the lateral to medial band is associated with an increasing scale of the spacing of firing fields of grid cells, with a corresponding increase in the size of place fields in the topographic projections to the dorsal and ventral hippocampus200-202. The authors have suggested that the dorsal hippocampus encodes spatial maps at a higher resolution than the ventral hippocampus200-202, although the population decoding of ventral hippocampus place fields can track the position of an animal as accurately as decoding of the dorsal place fields203. Septal and temporal GCs also differ in expression of GluR2–GluR3 AMPA-type glutamate receptors and glutamate decarboxylase 67 (ref. 204), serotonin receptor subtype HTR1A205 and other gene expression patterns206,207. The temporal hippocampus is more associated with areas such as the amygdala and prefrontal cortex than the septal hippocampus208,209. These connectivity patterns suggest a functional dissociation along the longitudinal axis207, although complex associational pathways along this axis suggest that there is more functional crosstalk210. The upper (or enclosed) blade of the DG expresses a greater proportion of ARC-expressing neurons following open-field foraging tasks than the lower (exposed) blade197. Little is known about the functional differences between the upper and lower blades of the DG197,211, and this is an area ripe for investigation. Proximal CA3 receives input from the upper and lower blades of the DG, receives relatively little direct input from the EC, sends the majority of backprojections to DG MCs and is similar to the DG in the remapping properties of its place fields88,212,213. Proximal CA3 cells are also similar to MCs in several ways214. Distal CA3 differs from proximal CA3 because it receives input primarily from the upper blade212,215, has more inputs from the EC and has a higher density of recurrent collaterals212,216,217, and its place fields are more likely to show pattern completion, generalization or error correction functions than proximal CA3 and the DG88. More generally, little is known regarding how CA3 operates as it combines input from the DG, the EC and its own recurrent connections. Future studies will need to target understanding the information that is processed across these multiple regions.
Concluding remarks
In this Perspective article, we have harnessed insights from the modelling, lesion, behavioural, physiology and imaging literature to address critical questions central to multiple theories of DG function. In doing so, we have identified several points of continued ambiguity as to the distinct phases of memory that may engage DG function, the mechanisms within the DG that enable it to perform its many likely functions and the information it relays to downstream structures. In particular, although all theories can agree that distinct spatiotemporal patterns of activity across experiences are probable and highly useful in the DG, there is minimal agreement on when and how these patterns might be leveraged, which has implications for what is thought to be its primary role. Although there are numerous challenges currently faced by DG researchers (Box 2), we encourage future research to include the following to help resolve identified areas of conflict between theories: systematic parameterization and manipulation of experiences in DG-dependent behavioural paradigms, considerations of the computations performed by its intricate microcircuitry, and comparisons of DG activity with the activity within its input and output structures.
Box 2. Current challenges in studying and understanding DG function.
Research investigating dentate gyrus (DG) function must overcome several major challenges. It is often acknowledged that large-scale electrophysiological recordings of neural activity in the DG are technically challenging. The extreme sparsity of granule cell (GC) firing in freely moving animals presents a major impediment to studying the properties of these cells as part of the hippocampal place cell or episodic memory network. On the basis of estimates that only 2–10% (more likely the lower end) of GCs have a place field in a given environment54, on average, one must record between 10 and 50 well-isolated GCs to record the spatial firing properties of a single one of them. The remaining 9–49 of the cells will be mostly silent in that environment.
Additional challenges are faced when attempting to characterize cell-type-specific activity. It is essential that researchers adopt methods to properly characterize cells as GCs or mossy cells from single-unit electrophysiology111,112. One study estimates that the large majority of recordings purported to be of GCs in the literature were really recordings from mossy cells114. Large-scale Ca2+ imaging may help in this regard117,123,163,227,228, but one must be on guard against the possibility (especially with one-photon miniscopes implanted on freely behaving animals) of substantial damage to the perforant path inputs to the DG and to the distal-most part of the GC dendritic arbor caused by the need to implant a lens close enough to the GC layer to observe the optical signals. Furthermore, Ca2+ imaging has limited temporal resolution, and it is not clear whether it can always capture the activity of single spikes229.
Another area of research that has been technically challenging to investigate and thus largely unexplored surrounds the continual birth of new cells in DG throughout the lifetime of an organism, called adult neurogenesis. Although there have been computational models and experimental evidence suggesting that newborn GCs link experiences that occur close in time and afford a distinct population-level code for experiences that occur at distant time points66,70, much is still unknown regarding their transient and long-term influences on the larger DG circuit230. Another challenge comes from distinguishing adult-born GCs from developmentally born GCs; of the former, it is also critical to identify whether adult-born GCs are immature or have reached full maturity. Currently, there are no methods to distinguish these cell types using extracellular unit recordings alone, yet studying the behavioural correlates of their firing is critical for understanding the computational importance of adult neurogenesis in the DG. Do newborn GCs have place fields, and if so, when do they show such firing? Are they more active than the sparsely active developmentally born GCs? If so, their effect on downstream CA3 cells may be disproportionate to their numbers. When adult-born GCs become mature, do they have the same physiological properties as developmentally born GCs, or do they maintain a distinct physiological signature that suggests a different computational function231? Some work has been performed on these questions using Ca2+ imaging in head-fixed animals232. However, recordings from extracellular electrodes of identified adult-born GCs are extremely difficult, given that they are a small percentage of the total GC population, recordings must be performed within specific maturation windows, and their maturation is regulated by experience61,233.
Future behavioural paradigms will need to provide a framework for comparing the degree of change to a sensory stimulus or environment (inputs to the DG) with the magnitude of activity changes across DG neuronal populations. This requires the parametrization and systematic change of primary variables such as the geometry of the recording arena186, the spatial relationships between distal and local cue sets113,114,218 or the level of noise in visual contexts100,102. The combined use of in vivo electrophysiology or imaging methods with novel behavioural designs that systematically parameterize and manipulate aspects of natural experience will provide a clearer understanding of the range of circumstances that critically engage the DG, while also allowing quantification of its physiological responses given systematic change.
The most impactful research moving forward will probably be interdisciplinary, leveraging findings and tools across methodological approaches to tackle the central questions and bridge findings across communities. Many of these approaches have worked synergistically in the past, with examples of physiological recording studies designed to explicitly test specific modelling predictions66. Research that combines multiple techniques can also in some cases overcome the shortcomings of a single method of investigation and place researchers in a pivotal position to address discrepancies across different approaches. The pursuit of interdisciplinary approaches, but more importantly an increased dialogue between researchers across modalities, will hopefully expedite a more holistic understanding of DG function. The combined efforts will markedly improve knowledge of when the DG has the largest influence on learning and memory systems, how the DG performs its proposed functions and what information DG conveys to downstream structures.
Acknowledgements
This work was partially supported by the NSF DMS-1042134, NIH R03MH120406, NIH R01 NS039456, NRF 2021R1A4A2001803, NRF 2019R1A2C2088799, NRF 2022M3E5E8017723, the NIH Institute of Neural Computation/Biology T32 MH020002 and the Kavli Institute for Brain and Mind. The authors gratefully thank D. Nitz, L.K. Quinn, J.W. Rueckemann, T. Johnson, P. Riviere and C. Heyman for insightful discussions and editorial assistance.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Amaral DG, Scharfman HE & Lavenex P The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res 163, 3–22 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron H, Woolley C, McEwen B & Gould E Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56, 337–344 (1993). [DOI] [PubMed] [Google Scholar]
- 3.Eriksson PS et al. Neurogenesis in the adult human hippocampus. Nat. Med 4, 1313–1317 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Kuhn HG, Toda T & Gage FH Adult hippocampal neurogenesis: a coming-of-age story. J. Neurosci 38, 10401–10410 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paredes MF et al. Does adult neurogenesis persist in the human hippocampus? Cell Stem Cell 23, 780–781 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempermann G. et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell 23, 25–30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohara S. et al. Local projections of layer Vb-to-Va are more prominent in lateral than in medial entorhinal cortex. eLife 10, e67262 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ming G & Song H Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marr D. A theory of cerebellar cortex. J. Physiol 202, 437–470 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eccles J, Ito M & Szentagothai J The Cerebellum as a Neuronal Machine 335 (Springer, 1967). [Google Scholar]
- 11.Rolls ET in Neural Models of Plasticity 240–265 (Elsevier, 1989). [Google Scholar]
- 12.McNaughton BL & Morris RG Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 10, 408–415 (1987). [Google Scholar]
- 13.McNaughton BL & Nadel L in Neuroscience and Connectionist Theory (eds Gluck MA & Rumelhart DE) 1–63 (Lawrence Erlbaum, 1990). [Google Scholar]
- 14.O’reilly RC & McClelland JL Hippocampal conjunctive encoding, storage, and recall:avoiding a trade-off. Hippocampus 4, 661–682 (1994). [DOI] [PubMed] [Google Scholar]
- 15.Myers CE & Scharfman HE Pattern separation in the dentate gyrus: a role for the CA3 backprojection. Hippocampus 21, 1190–1215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers CE & Scharfman HE A role for hilar cells in pattern separation in the dentate gyrus: a computational approach. Hippocampus 19, 321–337 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amaral D, Ishizuka N & Claiborne B Neurons, numbers and the hippocampal network. Prog. Brain Res 83, 1–11 (1990). [DOI] [PubMed] [Google Scholar]
- 18.Yassa MA & Stark CE Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishkin M, Suzuki WA, Gadian DG & Vargha-Khadem F Hierarchical organization of cognitive memory. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 352, 1461–1467 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manns JR & Eichenbaum H Evolution of declarative memory. Hippocampus 16, 795–808 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Knierim JJ, Lee I & Hargreaves EL Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus 16, 755–764 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Reagh ZM & Yassa MA Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc. Natl Acad. Sci. USA 111, E4264–E4273 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doan TP, Lagartos-Donate MJ, Nilssen ES, Ohara S & Witter MP Convergent projections from perirhinal and postrhinal cortices suggest a multisensory nature of lateral, but not medial, entorhinal cortex. Cell Rep. 29, 617–627 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Nilssen ES, Doan TP, Nigro MJ, Ohara S & Witter MP Neurons and networks in the entorhinal cortex: a reappraisal of the lateral and medial entorhinal subdivisions mediating parallel cortical pathways. Hippocampus 29, 1238–1254 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Burwell RD & Amaral DG Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J. Comp. Neurol 391, 293–321 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Knierim JJ, Neunuebel JP & Deshmukh SS Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local–global reference frames. Philos. Trans. R. Soc. B Biol. Sci 369, 20130369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neunuebel JP, Yoganarasimha D, Rao G & Knierim JJ Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. J. Neurosci 33, 9246–9258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Keefe JO & Nadel L The Hippocampus as a Cognitive Map (Clarendon, 1978). [Google Scholar]
- 29.O’Keefe J & Nadel L Précis of O’Keefe & Nadel’s The hippocampus as a cognitive map. Behav. Brain Sci 2, 487–494 (1979). [Google Scholar]
- 30.Rueckemann JW, Sosa M, Giocomo LM & Buffalo EA The grid code for ordered experience. Nat. Rev. Neurosci 22, 637–649 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JW & Jung MW Separation or binding? Role of the dentate gyrus in hippocampal mnemonic processing. Neurosci. Biobehav. Rev 75, 183–194 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Vinogradova O. The hippocampus and the orienting reflex. Neuronal Mech. Orient. Reflex 128, 154 (1975). [Google Scholar]
- 33.Amaral DG & Campbell M Transmitter systems in the primate dentate gyrus. Hum. Neurobiol 5, 169–180 (1986). [PubMed] [Google Scholar]
- 34.Prince LY, Bacon TJ, Tigaret CM & Mellor JR Neuromodulation of the feedforward dentate gyrus-CA3 microcircuit. Front. Synaptic Neurosci 8, 32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harley CW Norepinephrine and the dentate gyrus. Prog. Brain Res 163, 299–318 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Straube T, Korz V, Balschun D & Uta Frey J Requirement of β-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J. Physiol 552, 953–960 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown RA, Walling SG, Milway JS & Harley CW Locus ceruleus activation suppresses feedforward interneurons and reduces β-γ electroencephalogram frequencies while it enhances θ frequencies in rat dentate gyrus. J. Neurosci 25, 1985–1991 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitchigina V, Vankov A, Harley C & Sara SJ Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur. J. Neurosci 9, 41–47 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Ogando MB et al. Cholinergic modulation of dentate gyrus processing through dynamic reconfiguration of inhibitory circuits. Cell Rep. 36, 109572 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Hofmann ME & Frazier CJ Muscarinic receptor activation modulates the excitability of hilar mossy cells through the induction of an afterdepolarization. Brain Res. 1318, 42–51 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duffy AM, Schaner MJ, Chin J & Scharfman HE Expression of c-fos in hilar mossy cells of the dentate gyrus in vivo. Hippocampus 23, 649–655 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein HL, Lu Y-L, Botterill JJ & Scharfman HE Novelty and novel objects increase c-Fos immunoreactivity in mossy cells in the mouse dentate gyrus. Neural Plast. 10.1155/2019/1815371 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ewell LA & Jones MV Frequency-tuned distribution of inhibition in the dentate gyrus. J. Neurosci 30, 12597–12607 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buzsàki G & Eidelberg E Commissural projection to the dentate gyrus of the rat: evidence for feed-forward inhibition. Brain Res. 230, 346–350 (1981). [DOI] [PubMed] [Google Scholar]
- 45.Li Y. et al. Molecular layer perforant path-associated cells contribute to feed-forward inhibition in the adult dentate gyrus. Proc. Natl Acad. Sci. USA 110, 9106–9111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sloviter R & Brisman J Lateral inhibition and granule cell synchrony in the rat hippocampal dentate gyrus. J. Neurosci 15, 811–820 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scharfman HE Electrophysiological evidence that dentate hilar mossy cells are excitatory and innervate both granule cells and interneurons. J. Neurophysiol 74, 179–194 (1995). [DOI] [PubMed] [Google Scholar]
- 48.Scharfman H, Kunkel D & Schwartzkroin PA Synaptic connections of dentate granule cells and hilar neurons: results of paired intracellular recordings and intracellular horseradish peroxidase injections. Neuroscience 37, 693–707 (1990). [DOI] [PubMed] [Google Scholar]
- 49.Otis T & Mody I Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience 49, 13–32 (1992). [DOI] [PubMed] [Google Scholar]
- 50.Staley KJ, Otis TS & Mody I Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J. Neurophysiol 67, 1346–1358 (1992). [DOI] [PubMed] [Google Scholar]
- 51.Espinoza C, Guzman SJ, Zhang X & Jonas P Parvalbumin + interneurons obey unique connectivity rules and establish a powerful lateral-inhibition microcircuit in dentate gyrus. Nat. Commun 9, 4605 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog. Brain Res 163, 601–613 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Josselyn SA, Köhler S & Frankland PW Finding the engram. Nat. Rev. Neurosci 16, 521–534 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Hainmueller T & Bartos M Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat. Rev. Neurosci 21, 153–168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun X. et al. Functionally distinct neuronal ensembles within the memory engram. Cell 181, 410–423 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramirez S. et al. Activating positive memory engrams suppresses depression-like behaviour. Nature 522, 335–339 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lacagnina AF et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat. Neurosci 22, 753–761 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denny CA, Lebois E & Ramirez S From engrams to pathologies of the brain. Front. Neural Circuits 11, 23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teyler TJ & DiScenna P The hippocampal memory indexing theory. Behav. Neurosci 100, 147 (1986). [DOI] [PubMed] [Google Scholar]
- 60.Miller SM & Sahay A Functions of adult-born neurons in hippocampal memory interference and indexing. Nat. Neurosci 22, 1565–1575 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piatti VC, Espósito MS & Schinder AF The timing of neuronal development in adult hippocampal neurogenesis. Neuroscientist 12, 463–468 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Espósito MS et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci 25, 10074–10086 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt-Hieber C, Jonas P & Bischofberger J Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Gu Y. et al. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat. Neurosci 15, 1700–1706 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tashiro A, Makino H & Gage FH Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J. Neurosci 27, 3252–3259 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rangel L. et al. Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nat. Commun 5, 3181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park S-B et al. The fasciola cinereum subregion of the hippocampus is important for the acquisition of visual contextual memory. Prog. Neurobiol 210, 102217 (2022). [DOI] [PubMed] [Google Scholar]
- 68.Mau W, Hasselmo ME & Cai DJ The brain in motion: how ensemble fluidity drives memory-updating and flexibility. eLife 9, e63550 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aimone JB, Wiles J & Gage FH Potential role for adult neurogenesis in the encoding of time in new memories. Nat. Neurosci 9, 723–727 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Aimone JB, Wiles J & Gage FH Computational influence of adult neurogenesis on memory encoding. Neuron 61, 187–202 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lisman JE Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate–CA3 interactions. Neuron 22, 233–242 (1999). [DOI] [PubMed] [Google Scholar]
- 72.Aimone JB, Deng W & Gage FH Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70, 589–596 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nolan CR, Wyeth G, Milford M & Wiles J The race to learn: spike timing and STDP can coordinate learning and recall in CA3. Hippocampus 21, 647–660 (2011). [DOI] [PubMed] [Google Scholar]
- 74.Legéndy CR On the ‘data stirring’ role of the dentate gyrus of the hippocampus. Rev. Neurosci 28, 599–615 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Sahay A & Hen R Adult hippocampal neurogenesis in depression. Nat. Neurosci 10, 1110–1115 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Cayco-Gajic NA & Silver RA Re-evaluating circuit mechanisms underlying pattern separation. Neuron 101, 584–602 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamlyn L. Electron microscopy of mossy fibre endings in Ammon’s horn. Nature 190, 645–646 (1961). [DOI] [PubMed] [Google Scholar]
- 78.Chicurel ME & Harris KM Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J. Comp. Neurol 325, 169–182 (1992). [DOI] [PubMed] [Google Scholar]
- 79.Rollenhagen A. et al. Structural determinants of transmission at large hippocampal mossy fiber synapses. J. Neurosci 27, 10434–10444 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laatsch RH & Cowan W Electron microscopic studies of the dentate gyrus of the rat. I. Normal structure with special reference to synaptic organization. J. Comp. Neurol 128, 359–395 (1966). [DOI] [PubMed] [Google Scholar]
- 81.Henze DA, Wittner L & Buzsáki G Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat. Neurosci 5, 790–795 (2002). [DOI] [PubMed] [Google Scholar]
- 82.Scharfman HE The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep 7, 348–354 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Treves A & Rolls ET Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 2, 189–199 (1992). [DOI] [PubMed] [Google Scholar]
- 84.Marr D. Simple memory: a theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci 262, 23–81 (1971). [DOI] [PubMed] [Google Scholar]
- 85.Cerasti E & Treves A How informative are spatial CA3 representations established by the dentate gyrus? PLoS Computat. Biol 6, e1000759 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McClelland JL, McNaughton BL & O’Reilly RC Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev 102, 419 (1995). [DOI] [PubMed] [Google Scholar]
- 87.O’Reilly RC, Bhattacharyya R, Howard MD & Ketz N Complementary learning systems. Cogn. Sci 38, 1229–1248 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Lee H, GoodSmith D & Knierim JJ Parallel processing streams in the hippocampus. Curr. Opin. Neurobiol 64, 127–134 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jarrard LE, Okaichi H, Steward O & Goldschmidt RB On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav. Neurosci 98, 946 (1984). [DOI] [PubMed] [Google Scholar]
- 90.McLamb RL, Mundy WR & Tilson HA Intradentate colchicine impairs acquisition of a two-way active avoidance response in a Y-maze. Neurosci. Lett 94, 338–342 (1988). [DOI] [PubMed] [Google Scholar]
- 91.Mcnaughton BL, Barnes CA, Meltzer J & Sutherland R Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp. Brain Res 76, 485–496 (1989). [DOI] [PubMed] [Google Scholar]
- 92.Nanry KP, Mundy WR & Tilson HA Colchicine-induced alterations of reference memory in rats: role of spatial versus non-spatial task components. Behav. Brain Res 35, 45–53 (1989). [DOI] [PubMed] [Google Scholar]
- 93.Xavier GF, Oliveira-Filho FJ & Santos AM Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: difficulties in ‘place strategy’ because of a lack of flexibility in the use of environmental cues? Hippocampus 9, 668–681 (1999). [DOI] [PubMed] [Google Scholar]
- 94.Gilbert PE, Kesner RP & Lee I Dissociating hippocampal subregions: a double dissociation between dentate gyrus and CA1. Hippocampus 11, 626–636 (2001). [DOI] [PubMed] [Google Scholar]
- 95.Lee I & Kesner RP Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behav. Neurosci 117, 1044 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Lee I & Kesner RP Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus 14, 66–76 (2004). [DOI] [PubMed] [Google Scholar]
- 97.Lee I & Kesner RP Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus 14, 301–310 (2004). [DOI] [PubMed] [Google Scholar]
- 98.Lee I, Hunsaker MR & Kesner RP The role of hippocampal subregions in detecting spatial novelty. Behav. Neurosci 119, 145 (2005). [DOI] [PubMed] [Google Scholar]
- 99.Hernández-Rabaza V. et al. The hippocampal dentate gyrus is essential for generating contextual memories of fear and drug-induced reward. Neurobiol. Learn. Mem 90, 553–559 (2008). [DOI] [PubMed] [Google Scholar]
- 100.Lee C-H & Lee I Impairment of pattern separation of ambiguous scenes by single units in the CA3 in the absence of the dentate gyrus. J. Neurosci 40, 3576–3590 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee I & Solivan F The roles of the medial prefrontal cortex and hippocampus in a spatial paired-association task. Learn. Mem 15, 357–367 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahn J & Lee I Intact CA3 in the hippocampus is only sufficient for contextual behavior based on well-learned and unaltered visual background. Hippocampus 24, 1081–1093 (2014). [DOI] [PubMed] [Google Scholar]
- 103.Walsh TJ, Schulz DW, Tilson HA & Schmechel DE Cochicine-induced granule cell loss in rat hippocampus: selective behavioral and histological alterations. Brain Res. 398, 23–36 (1986). [DOI] [PubMed] [Google Scholar]
- 104.Bernier BE et al. Dentate gyrus contributes to retrieval as well as encoding: evidence from context fear conditioning, recall, and extinction. J. Neurosci 37, 6359–6371 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lassalle J-M, Bataille T & Halley H Reversible inactivation of the hippocampal mossy fiber synapses in mice impairs spatial learning, but neither consolidation nor memory retrieval, in the Morris navigation task. Neurobiol. Learn. Mem 73, 243–257 (2000). [DOI] [PubMed] [Google Scholar]
- 106.Madroñal N. et al. Rapid erasure of hippocampal memory following inhibition of dentate gyrus granule cells. Nat. Commun 7, 10923 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee I & Solivan F Dentate gyrus is necessary for disambiguating similar object-place representations. Learn. Mem 17, 252–258 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Keefe J & Dostrovsky J The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971). [DOI] [PubMed] [Google Scholar]
- 109.Eichenbaum H, Dudchenko P, Wood E, Shapiro M & Tanila H The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23, 209–226 (1999). [DOI] [PubMed] [Google Scholar]
- 110.van Dijk MT & Fenton AA On how the dentate gyrus contributes to memory discrimination. Neuron 98, 832–845 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Senzai Y & Buzsáki G Physiological properties and behavioral correlates of hippocampal granule cells and mossy cells. Neuron 93, 691–704 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.GoodSmith D. et al. Spatial representations of granule cells and mossy cells of the dentate gyrus. Neuron 93, 677–690 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Knierim JJ & Neunuebel JP Tracking the flow of hippocampal computation: pattern separation, pattern completion, and attractor dynamics. Neurobiol. Learn. Mem 129, 38–49 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.GoodSmith D, Lee H, Neunuebel JP, Song H & Knierim JJ Dentate gyrus mossy cells share a role in pattern separation with dentate granule cells and proximal CA3 pyramidal cells. J. Neurosci 39, 9570–9584 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Neunuebel JP & Knierim JJ CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 81, 416–427 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Neunuebel JP & Knierim JJ Spatial firing correlates of physiologically distinct cell types of the rat dentate gyrus. J. Neurosci 32, 3848–3858 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hainmueller T & Bartos M Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 558, 292–296 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jung D. et al. Dentate granule and mossy cells exhibit distinct spatiotemporal responses to local change in a one-dimensional landscape of visual-tactile cues. Sci. Rep 9, 9545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fredes F & Shigemoto R The role of hippocampal mossy cells in novelty detection. Neurobiol. Learn. Mem 183, 107486 (2021). [DOI] [PubMed] [Google Scholar]
- 120.Fredes F et al. Ventro-dorsal hippocampal pathway gates novelty-induced contextual memory formation. Curr. Biol 31, 25–38 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kirschen GW et al. Active dentate granule cells encode experience to promote the addition of adult-born hippocampal neurons. J. Neurosci 37, 4661–4678 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim S, Jung D & Royer S Place cell maps slowly develop via competitive learning and conjunctive coding in the dentate gyrus. Nat. Commun 11, 4550 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Woods NI et al. The dentate gyrus classifies cortical representations of learned stimuli. Neuron 107, 173–184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramirez S. et al. Creating a false memory in the hippocampus. Science 341, 387–391 (2013). [DOI] [PubMed] [Google Scholar]
- 125.Guo N. et al. Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nat. Med 24, 438–449 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kitamura T. et al. Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Josselyn SA & Tonegawa S Memory engrams: recalling the past and imagining the future. Science 367, eaaw4325 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guskjolen A. et al. Recovery of ‘lost’ infant memories in mice. Curr. Biol 28, 2283–2290 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Ryan TJ, Roy DS, Pignatelli M, Arons A & Tonegawa S Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Denny CA et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Richards BA & Frankland PW The conjunctive trace. Hippocampus 23, 207–212 (2013). [DOI] [PubMed] [Google Scholar]
- 133.Routtenberg A. Lifetime memories from persistently supple synapses. Hippocampus 23, 202–206 (2013). [DOI] [PubMed] [Google Scholar]
- 134.Stark CE & Okado Y Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J. Neurosci 23, 6748–6753 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guzman SJ et al. How connectivity rules and synaptic properties shape the efficacy of pattern separation in the entorhinal cortex–dentate gyrus–CA3 network. Nat. Comput. Sci 1, 830–842 (2021). [DOI] [PubMed] [Google Scholar]
- 136.Chambers RA, Potenza MN, Hoffman RE & Miranker W Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology 29, 747–758 (2004). [DOI] [PubMed] [Google Scholar]
- 137.Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus 15, 722–738 (2005). [DOI] [PubMed] [Google Scholar]
- 138.Wiskott L, Rasch MJ, & Kempermann G A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus 16, 329–343 (2006). [DOI] [PubMed] [Google Scholar]
- 139.Deisseroth K. et al. Excitation–neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42, 535–552 (2004). [DOI] [PubMed] [Google Scholar]
- 140.Weisz VI & Argibay PF Neurogenesis interferes with the retrieval of remote memories: forgetting in neurocomputational terms. Cognition 125, 13–25 (2012). [DOI] [PubMed] [Google Scholar]
- 141.Tran LM, Josselyn SA, Richards BA & Frankland PW Forgetting at biologically realistic levels of neurogenesis in a large-scale hippocampal model. Behav. Brain Res 376, 112180 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chambers RA & Conroy SK Network modeling of adult neurogenesis: shifting rates of neuronal turnover optimally gears network learning according to novelty gradient. J. Cogn. Neurosci 19, 1–12 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jinde S. et al. Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation. Neuron 76, 1189–1200 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scharfman HE The enigmatic mossy cell of the dentate gyrus. Nat. Rev. Neurosci 17, 562–575 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Botterill JJ et al. Bidirectional regulation of cognitive and anxiety-like behaviors by dentate gyrus mossy cells in male and female mice. J. Neurosci 41, 2475–2495 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakashiba T. et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149, 188–201 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Clelland C. et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sahay A. et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Masachs N. et al. The temporal origin of dentate granule neurons dictates their role in spatial memory. Mol. Psychiatry 26, 7130–7140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saxe MD et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl Acad. Sci. USA 103, 17501–17506 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morris AM, Curtis BJ, Churchwell JC, Maasberg DW & Kesner RP Temporal associations for spatial events: the role of the dentate gyrus. Behav. Brain Res 256, 250–256 (2013). [DOI] [PubMed] [Google Scholar]
- 152.Akers KG et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344, 598–602 (2014). [DOI] [PubMed] [Google Scholar]
- 153.Epp JR et al. Neurogenesis-mediated forgetting minimizes proactive interference. Nat. Commun 7, 10838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cuartero MI et al. Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice. J. Clin. Investig 129, 1536–1550 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ishikawa R, Fukushima H, Frankland PW & Kida S Hippocampal neurogenesis enhancers promote forgetting of remote fear memory after hippocampal reactivation by retrieval. eLife 5, e17464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gao A. et al. Elevation of hippocampal neurogenesis induces a temporally graded pattern of forgetting of contextual fear memories. J. Neurosci 38, 3190–3198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ishikawa R, Uchida C, Kitaoka S, Furuyashiki T & Kida S Improvement of PTSD-like behavior by the forgetting effect of hippocampal neurogenesis enhancer memantine in a social defeat stress paradigm. Mol. Brain 12, 68 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Scott GA et al. Adult neurogenesis mediates forgetting of multiple types of memory in the rat. Mol. Brain 14, 97 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ryan TJ & Frankland PW Forgetting as a form of adaptive engram cell plasticity. Nat. Rev. Neurosci 23, 173–186 (2022). [DOI] [PubMed] [Google Scholar]
- 160.Fricke RA & Prince DA Electrophysiology of dentate gyrus granule cells. J. Neurophysiol 51, 195–209 (1984). [DOI] [PubMed] [Google Scholar]
- 161.Scharfman HE Blockade of excitation reveals inhibition of dentate spiny hilar neurons recorded in rat hippocampal slices. J. Neurophysiol 68, 978–984 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Staley KJ & Mody I Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J. Neurophysiol 68, 197–212 (1992). [DOI] [PubMed] [Google Scholar]
- 163.Danielson NB et al. In vivo imaging of dentate gyrus mossy cells in behaving mice. Neuron 93, 552–559 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Botterill JJ et al. An excitatory and epileptogenic effect of dentate gyrus mossy cells in a mouse model of epilepsy. Cell Rep. 29, 2875–2889 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nitz D & McNaughton B Differential modulation of CA1 and dentate gyrus interneurons during exploration of novel environments. J. Neurophysiol 91, 863–872 (2004). [DOI] [PubMed] [Google Scholar]
- 166.Rangel LM, Chiba AA & Quinn LK Theta and beta oscillatory dynamics in the dentate gyrus reveal a shift in network processing state during cue encounters. Front. Syst. Neurosci 9, 96 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Trimper JB, Galloway CR, Jones AC, Mandi K & Manns JR Gamma oscillations in rat hippocampal subregions dentate gyrus, CA3, CA1, and subiculum underlie associative memory encoding. Cell Rep. 21, 2419–2432 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sasaki T. et al. Dentate network activity is necessary for spatial working memory by supporting CA3 sharp-wave ripple generation and prospective firing of CA3 neurons. Nat. Neurosci 21, 258–269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sullivan D. et al. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J. Neurosci 31, 8605–8616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Luna VM et al. Adult-born hippocampal neurons bidirectionally modulate entorhinal inputs into the dentate gyrus. Science 364, 578–583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Piatti VC, Ewell LA & Leutgeb JK Neurogenesis in the dentate gyrus: carrying the message or dictating the tone. Front. Neurosci 7, 50 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ikrar T. et al. Adult neurogenesis modifies excitability of the dentate gyrus. Front. Neural Circ 7, 204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Trinchero MF, Giacomini D & Schinder AF Dynamic interplay between GABAergic networks and developing neurons in the adult hippocampus. Curr. Opin. Neurobiol 69, 124–130 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Drew LJ et al. Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons. Hippocampus 26, 763–778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lacefield CO, Itskov V, Reardon T, Hen R & Gordon JA Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus 22, 106–116 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Berdugo-Vega G. et al. Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat. Commun 11, 135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McHugh SB et al. Adult-born dentate granule cells promote hippocampal population sparsity. Nat. Neurosci 25, 1481–1491 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Aimone JB et al. Regulation and function of adult neurogenesis: from genes to cognition. Physiol. Rev 94, 991–1026 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Scharfman HE, Goodman JH & Sollas AL Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J. Neurosci 20, 6144–6158 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cho K-O et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun 6, 6606 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.GoodSmith D. et al. Flexible encoding of objects and space in single cells of the dentate gyrus. Curr. Biol 32, 1088–1101 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sahay A, Wilson DA & Hen R Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70, 582–588 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Si B & Treves A The role of competitive learning in the generation of DG fields from EC inputs. Cogn. Neurodyn 3, 177–187 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang C. et al. Egocentric coding of external items in the lateral entorhinal cortex. Science 362, 945–949 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Morris AM, Weeden CS, Churchwell JC & Kesner RP The role of the dentate gyrus in the formation of contextual representations. Hippocampus 23, 162–168 (2013). [DOI] [PubMed] [Google Scholar]
- 186.Leutgeb JK, Leutgeb S, Moser M-B & Moser EI Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966 (2007). [DOI] [PubMed] [Google Scholar]
- 187.Alme CB et al. Place cells in the hippocampus: eleven maps for eleven rooms. Proc. Natl Acad. Sci. USA 111, 18428–18435 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Deng W, Mayford M & Gage FH Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife 2, e00312 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kim SH et al. Global remapping in granule cells and mossy cells of the mouse dentate gyrus. Cell Rep. 42, 112334 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hafting T, Fyhn M, Molden S, Moser M-B & Moser EI Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005). [DOI] [PubMed] [Google Scholar]
- 191.Keene CS et al. Complementary functional organization of neuronal activity patterns in the perirhinal, lateral entorhinal, and medial entorhinal cortices. J. Neurosci 36, 3660–3675 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang C, Chen X & Knierim JJ Egocentric and allocentric representations of space in the rodent brain. Curr. Opin. Neurobiol 60, 12–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tsao A. et al. Integrating time from experience in the lateral entorhinal cortex. Nature 561, 57–62 (2018). [DOI] [PubMed] [Google Scholar]
- 194.Dvorak D, Chung A, Park EH & Fenton AA Dentate spikes and external control of hippocampal function. Cell Rep. 36, 109497 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fernández-Ruiz A. et al. Gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies. Science 372, eabf3119 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jung MW, Wiener SI & McNaughton BL Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci 14, 7347–7356 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chawla M. et al. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus 15, 579–586 (2005). [DOI] [PubMed] [Google Scholar]
- 198.Lu L, Igarashi KM, Witter MP, Moser EI & Moser M-B Topography of place maps along the CA3-to-CA2 axis of the hippocampus. Neuron 87, 1078–1092 (2015). [DOI] [PubMed] [Google Scholar]
- 199.Van Strien N, Cappaert N & Witter M The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat. Rev. Neurosci 10, 272–282 (2009). [DOI] [PubMed] [Google Scholar]
- 200.Royer S, Sirota A, Patel J & Buzsáki G Distinct representations and theta dynamics in dorsal and ventral hippocampus. J. Neurosci 30, 1777–1787 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jung MW, Wiener SI & McNaughton BL Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci 14, 7347–7356 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kjelstrup KB et al. Finite scale of spatial representation in the hippocampus. Science 321, 140–143 (2008). [DOI] [PubMed] [Google Scholar]
- 203.Keinath AT et al. Precise spatial coding is preserved along the longitudinal hippocampal axis. Hippocampus 24, 1533–1548 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jinno S & Kosaka T Stereological estimation of numerical densities of glutamatergic principal neurons in the mouse hippocampus. Hippocampus 20, 829–840 (2010). [DOI] [PubMed] [Google Scholar]
- 205.Tanaka KF, Samuels BA & Hen R Serotonin receptor expression along the dorsal–ventral axis of mouse hippocampus. Philos. Trans. R. Soc. B: Biol. Sci 367, 2395–2401 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Christensen T, Bisgaard C, Nielsen HB & Wiborg O Transcriptome differentiation along the dorso–ventral axis in laser-captured microdissected rat hippocampal granular cell layer. Neuroscience 170, 731–741 (2010). [DOI] [PubMed] [Google Scholar]
- 207.Fanselow MS & Dong H-W Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Witter MP & Amaral DG in The Rat Nervous System 635–704 (Elsevier, 2004). [Google Scholar]
- 209.Paxinos G. The Rat Nervous System (Gulf Professional Publishing, 2004). [Google Scholar]
- 210.Amaral DG & Witter MP The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31, 571–591 (1989). [DOI] [PubMed] [Google Scholar]
- 211.Scharfman HE, Sollas AL, Smith KL, Jackson MB & Goodman JH Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. J. Comp. Neurol 454, 424–439 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Witter MP Intrinsic and extrinsic wiring of CA3: indications for connectional heterogeneity. Learn. Mem 14, 705–713 (2007). [DOI] [PubMed] [Google Scholar]
- 213.Lee H, Wang C, Deshmukh SS & Knierim JJ Neural population evidence of functional heterogeneity along the CA3 transverse axis: pattern completion versus pattern separation. Neuron 87, 1093–1105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Scharfman HE Spiny neurons of area CA3c in rat hippocampal slices have similar electrophysiological characteristics and synaptic responses despite morphological variation. Hippocampus 3, 9–28 (1993). [DOI] [PubMed] [Google Scholar]
- 215.Claiborne BJ, Amaral DG & Cowan WM A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J. Comp. Neurol 246, 435–458 (1986). [DOI] [PubMed] [Google Scholar]
- 216.Ishizuka N, Weber J & Amaral DG Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J. Comp. Neurol 295, 580–623 (1990). [DOI] [PubMed] [Google Scholar]
- 217.Li X, Somogyi P, Ylinen A & Buzsáki G The hippocampal CA3 network: an in vivo intracellular labeling study. J. Comp. Neurol 339, 181–208 (1994). [DOI] [PubMed] [Google Scholar]
- 218.Lee I, Yoganarasimha D, Rao G & Knierim JJ Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature 430, 456–459 (2004). [DOI] [PubMed] [Google Scholar]
- 219.Tosches MA et al. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 360, 881–888 (2018). [DOI] [PubMed] [Google Scholar]
- 220.Bingman VP & Muzio RN Reflections on the structural–functional evolution of the hippocampus: what is the big deal about a dentate gyrus. Brain Behav. Evol 90, 53–61 (2017). [DOI] [PubMed] [Google Scholar]
- 221.Treves A, Tashiro A, Witter MP & Moser EI What is the mammalian dentate gyrus good for? Neuroscience 154, 1155–1172 (2008). [DOI] [PubMed] [Google Scholar]
- 222.Amrein I, Slomianka L & Lipp H Granule cell number, cell death and cell proliferation in the dentate gyrus of wild-living rodents. Eur. J. Neurosci 20, 3342–3350 (2004). [DOI] [PubMed] [Google Scholar]
- 223.Amrein I. Adult hippocampal neurogenesis in natural populations of mammals. Cold Spring Harb. Perspect. Biol 7, a021295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fiedler J, De Leonibus E & Treves A Has the hippocampus really forgotten about space? Curr. Opin. Neurobiol 71, 164–169 (2021). [DOI] [PubMed] [Google Scholar]
- 225.Augusto-Oliveira M, Arrifano GP, Malva JO & Crespo-Lopez ME Adult hippocampal neurogenesis in different taxonomic groups: possible functional similarities and striking controversies. Cells 8, 125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rodríguez F. et al. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J. Neurosci 22, 2894–2903 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Allegra M, Posani L, Gómez-Ocádiz R & Schmidt-Hieber C Differential relation between neuronal and behavioral discrimination during hippocampal memory encoding. Neuron 108, 1103–1112 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stefanini F. et al. A distributed neural code in the dentate gyrus and in CA1. Neuron 107, 703–716 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lin B, Chen T & Schild D Cell type-specific relationships between spiking and [Ca2+] i in neurons of the Xenopus tadpole olfactory bulb. J. Physiol 582, 163–175 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kropff E, Yang SM & Schinder AF Dynamic role of adult-born dentate granule cells in memory processing. Curr. Opin. Neurobiol 35, 21–26 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Snyder JS Recalibrating the relevance of adult neurogenesis. Trends Neurosci. 42, 164–178 (2019). [DOI] [PubMed] [Google Scholar]
- 232.Danielson NB et al. Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron 90, 101–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Alvarez DD et al. A disynaptic feedback network activated by experience promotes the integration of new granule cells. Science 354, 459–465 (2016). [DOI] [PubMed] [Google Scholar]