Abstract
Telomere attrition is a proposed hallmark of aging. To evaluate the association of telomere length (TL) with chronological age across the human lifespan, we conducted a systematic review and meta-analysis of 414 study samples comprising 743,019 individuals aged 0 to 112 years. We examined both cross-sectional and longitudinal data, and evaluated the impact of various biological and methodological factors including sex, health status, tissue types, DNA extraction procedures, and TL measurement methods. The pooled corrected correlation between TL and age from cross-sectional samples was −0.19 (95%CI: −0.22 to −0.15), which weakened with increased chronological age (β=0.003, p<0.001). Z-score change rates of TL across the lifespan showed a gradual decrease in shortening rate until around age 50 and remained at a relatively stable rate towards the elderly period. A greater attrition rate was observed in longitudinal than cross-sectional evaluations. For TL measured in base pairs, the median change rate of TL was −23 bp/year in cross-sectional samples and −38 bp/year in longitudinal samples. Methodological factors including TL measurement methods and tissue types impacted the TL-age correlation, while sex or disease status did not. This meta-analysis revealed the non-linear shortening trend of TL across the human lifespan and provides a reference value for future studies. Results also highlight the importance of methodological considerations when using TL as an aging biomarker.
Keywords: Telomere, Human, Meta-analysis
1. Introduction
Telomeres are nucleoprotein complexes that cap the end of chromosomes, assisting in end replication and protection (Blackburn, 1991). Telomere attrition occurs over the lifespan in dividing cells due to the inability of cells to fully replicate DNA ends during cell division. In addition to natural shortening due to cell division, telomere attrition can be accelerated through a myriad of internal and external stimuli (Hastings et al., 2017). Research implicates age-related telomere length (TL) with a broad range of risk factors that predict disease, disability, and early death (Arbeev et al., 2020; Haycock et al., 2014; Shalev and Hastings, 2017). Taken together, telomere attrition has been proposed as one of the hallmarks of aging that regulates human healthspan (Aviv, 2006; López-Otín et al., 2013).
Studies on TL dynamics in humans have reported decreases in TL as a function of increased age both in vitro (Harley et al., 1990) and in vivo (Aubert et al., 2012; Cowell et al., 2021; Frenck et al., 1998; Lapham et al., 2015; Rufer et al., 1999). Cross-sectional and longitudinal work suggests that TL shortens most rapidly in the first few years of life, with the shortening rate slowing in young adulthood and possibly speeding up or slowing down again at older ages (Aubert et al., 2012; Cowell et al., 2021; Frenck et al., 1998; Lapham et al., 2015). A 2013 systematic review of leukocyte TL and age using 124 cross-sectional and 5 longitudinal studies reported a significant inverse correlation between TL and chronological age, but acknowledged that research on the impacts of specific developmental periods, sex, and other methodological factors on the correlation between TL and age is sparse (Müezzinler et al., 2013). Importantly, the previous work has not explicitly assessed the TL-age correlation in children or in tissue types other than leukocytes, nor has it examined the potential moderating effects of health status or DNA extraction procedures, which limits our understanding of TL dynamics across the lifespan.
TL may be impacted by unfavorable exposures or diseases, but whether health status modulates the rate of telomere loss is unclear. Multiple studies have found that patients with certain conditions, such as Parkinson’s disease (Martin-Ruiz et al., 2020), Type 2 diabetes (AlDehaini et al., 2020), multiple sclerosis (Bühring et al., 2021), HIV-infection (Paghera et al., 2019), and stress-related mental disorders (i.e., depression, anxiety, post-traumatic stress disorder [PTSD]) (Pousa et al., 2021) have shorter TL than healthy controls. Moreover, shorter TL has been linked with increased risk of cardiovascular diseases (CVDs) (Weischer et al., 2012), certain cancers (Ma et al., 2011; Wentzensen et al., 2011), and more recently, severe COVID-19 (Anderson et al., 2022; Aviv, 2021). Possible mechanisms underlying the association between TL and disease status may include, as example, elevated levels of oxidative stress and inflammation, mitochondrial dysfunction (Pousa et al., 2021), increased tissue mitosis (Weischer et al., 2012), and DNA damage response (Rossiello et al., 2022). Although several systematic reviews have summarized the relationship between TL and specific diseases (Haycock et al., 2014; Li et al., 2018; Lin et al., 2016; Pousa et al., 2021; Schutte and Malouff, 2015; Zhang et al., 2017), these reviews restricted analyses to differences in TL between patients and controls rather than the rate of TL change within disease states.
Methodological factors can further contribute to variation in measured TL. To improve the reliability of TL assays, researchers have made efforts to optimize measurement methods and standardize reporting guidelines (Hastings et al., 2021; Lindrose et al., 2021; Morinha et al., 2020 There are several methods available to measure average TL across a mixture of cells (often reported as either length relative to a known single-copy gene or as an absolute length in kilobases), including Southern blot, quantitative polymerase chain reaction (qPCR), and fluorescence in-situ hybridization (FISH). Each method has its advantages and disadvantages, which have been reviewed elsewhere (Lai et al., 2018). In addition, tissue type can play a role in the observed variation in TL values. Although TL is correlated across different tissues within individuals, the difference in TL across tissues can still be relatively large (Daniali et al., 2013; Demanelis et al., 2020; Lin et al., 2019). Further variation can be introduced during DNA extraction as the size and quality of extracted DNA can vary based on each extraction kit’s specific protocols (Lin et al., 2019). Research investigating how these methodological factors moderate the relationship between TL and chronological age is limited.
To address these gaps, we conducted a systematic review and meta-analysis on the relationship between TL and chronological age. We aimed to determine the TL-age correlation in different developmental periods across the life course, and to examine potential differences by sex and health status. In addition, we extended our review to compare the TL-age correlation across different tissue types, DNA extraction procedures, and TL measurement methods, which can provide important context for optimizing future study designs and TL measurements.
2. Methods
The research protocol, data extraction guideline and template of this review were registered via Open Science Framework prior to data extraction (https://osf.io/chxs7/?view_only=7dcd0706906d4addb10e30567d79d241). The protocol was modified in terms of inclusion and exclusion criteria during the data extraction process to increase the feasibility of this review. This review adheres to the guidelines of Meta-analysis Of Observational Studies in Epidemiology (MOOSE).
2.1. Scope of review
The scope of this review included any original research articles that either reported age-related telomere attrition or correlation between TL and chronological age. Both longitudinal and cross-sectional studies reporting relevant statistics were included. We included study samples from the general population, healthy individuals, or patients with any of the following three categories of diseases: CVDs, cancers, and stress-related mental disorders (i.e., depression, anxiety, PTSD). The disease scope was based on the rationale that CVDs, cancers, and mental disorders were highly prevalent in the population, and they were all reported to be associated with changes in TL compared with healthy controls (Haycock et al., 2014; Pousa et al., 2021; Zhang et al., 2017). The selection of tissues included the most common tissue types in the TL literature, including blood leukocytes, peripheral blood mononuclear cells (PBMC), dried blood spots (DBS), saliva, and buccal cells. Likewise, the TL measurement covered the most common methods, including Southern blot, qPCR, qFISH, and flow-FISH.
2.2. Search strategy
PubMed and Web of Science databases were used to search publications with a combination of the following keywords: (“telomere decay”) OR (“telomere shortening”) OR (“telomere erosion”) OR (“telomere attrition”) OR (“telomere change”) OR ((“age”) AND (“telomere length”)) OR (“telomere dynamics”). Search results from the database inception until December 31st, 2021 were collected for screening. No search software was used. One reviewer (Q.Y.) performed all searches. Reference lists of obtained articles were not searched for additional data. Articles published in languages other than English were not included in this review. Non-peer reviewed articles and retracted articles were also excluded.
2.3. Eligibility criteria
Duplicated publications were removed, and the remaining articles were screened based on the following exclusion criteria: 1) non-English articles; 2) not electronically available; 3) not original research articles (i.e., review articles, commentaries, perspectives, case reports, hypotheses, study protocols, abstract collections, or published errata); 4) non-peer reviewed or retracted articles; 5) non-human, in vitro, or in silico studies; 6) not within the review scope of disease, tissue types, or TL measurement methods; 7) twin studies; 8) studies of pregnant women; 9) studies of TL only in newborns, given the lack of age variation; 10) studies that did not measure TL or did not include TL data in the analysis; 11) longitudinal studies of patients who received intervention over a period of time; 12) studies without summary statistics of age or required statistics to obtain the correlation of TL and age or TL yearly change rate; 13) studies that only reported Spearman’s or Kendall’s correlation; 14) linear regression of TL on age adjusted for other factors; and 15) studies that reported the correlation based on log-transformed TL. For studies using the same sample or cohort, the statistics from the study with the larger sample size was included, while other studies were excluded. For articles that did not report required statistics, the authors of the articles were contacted (k=349).
The UK Biobank, the largest known cohort with TL data, was also included in our analyses (Codd et al., 2022). Although this study was published in a peer-review journal after Dec. 31st, 2021, considering its large sample size (N = 472,174), we extracted this study’s correlation data and presented meta-analysis results with and without the UK Biobank data.
2.4. Data extraction
Data from all eligible studies were extracted by one reviewer (Q.Y.). A second reviewer (A.A.) independently checked the extracted data. Unresolved disagreements were made by a third reviewer (I.S.). Detailed items for data extraction can be found on the Open Science Framework. Extracted data items included sample size, summary statistics (mean, median, standard deviation, range) of age and TL, number of males and females, tissue type used for TL measurement (blood leukocytes, PBMCs, DBS, saliva, or buccal cells), DNA extraction kit, TL measurement method (Southern blot, qPCR, qFISH, or flow FISH), Pearson’s correlation coefficient or univariate linear regression slope from cross-sectional studies, and yearly TL change rate from longitudinal studies. For DNA extraction kits, corresponding DNA extraction procedures were searched and used for further analyses. Detailed information on DNA extraction procedures is provided in Table S1.
Population-based studies not excluding individuals with certain health conditions were included as samples of the general population. Samples with exclusively healthy participants were included as samples of healthy individuals. Healthy participants were defined if the article explicitly reported that participants were healthy. Samples of participants with comorbidities in addition to the disease of interest were included with other samples of such disease.
2.5. Statistical analysis
Each paper was defined as one “study” throughout this systematic review report. The smallest unit of our analysis was “sample” because some studies included multiple samples. One sample corresponded to one effect size. Cross-sectional and longitudinal samples were analyzed separately. Descriptive analysis was conducted to summarize the numbers of samples, the number of subjects, the covered age range, and the follow-up duration. All analyses were conducted using R software (version 4.1.1). Two tailed p-values less than 0.05 were considered statistically significant.
2.5.1. Statistical analysis of cross-sectional samples
Cross-sectional studies usually reported Pearson’s correlation between TL and age, and/or unstandardized or standardized univariate linear regression slope. If only one statistic was reported, the other two were calculated using the following formula:
where, is the Pearson’s correlation coefficient; and are the standardized and unstandardized univariate linear regression slopes, respectively; and are the standard deviations (SD) of age and TL, respectively.
Since different samples had different age ranges, the TL-age correlation coefficient from each sample were corrected to reduce the noise caused by the heterogeneity of age range across samples using the following formula (Harrer et al., 2021; Hunter et al., 2006):
where, is the reported SD of restricted age of each sample; is the SD of unrestricted age; is the reported TL-age correlation in the original sample; and is the TL-age correlation corrected for age-range restriction. The SD of unrestricted age was set as 5 years to limit the theoretical age range width of a sample at around 18 years, close to the age range width of the youngest age group in our meta-analysis. For a normal distribution, a sample with a SD of age of 5 years would theoretically have a width of 95% age range of 19.7 years, whereas for a uniform distribution, a sample with a SD of age of 5 years would theoretically have a width of age range of 17.3 years.
Correlations were further transformed to Fisher’s for effect size pooling using the following formula:
where, is Fisher’s , and is the correlation.
The standard error of Fisher’s was obtained using the following formula:
where, is the sample size.
Meta-analyses (i.e., effect size pooling and meta-regression) were conducted using the ‘metafor’ package in R (Viechtbauer, 2010). Due to the expected between-study heterogeneity and the dependencies of effect sizes within each study, three-level mixed effect models were employed to pool the correlations from cross-sectional samples using a Restricted Maximum Likelihood (REML) procedure. Uncorrected and corrected correlations were analyzed separately. Caterpillar plots were drawn using individual effect sizes as well as pooled effect sizes. The difference in fits (DFFITS) value, the Cook’s distance, and the hat value were computed for each sample and used as model diagnostic metrics to identify potential outliers and influential cases. Specifically, the DFFITS value indicates how much the pooled effect size changes when one of the samples is removed. Cook’s distance value is similar to DFFITS value, indicating the squared difference in pooled effect size when one of the samples is removed. The hat value is equivalent to sample weight. A sample would be regarded an influential case if one of the following conditions was fulfilled (Harrer et al., 2021):
where, is the number of samples.
Meta-regression analyses were conducted to examine the moderation effects of age, sex, health status, tissue types, DNA extraction procedures, and TL measurement methods. Age was tested as both a continuous and a categorical variable. Specifically, when age was treated as a continuous variable, the age center (mean, median, or midrange) was included in the meta-regression models. When age was treated as a categorical variable, study samples were categorized into two (children [<18 years], adults [≥18 years]) or three age groups (children [<18 years], young and middle-aged adults [≥18 to <70 years], elderly adults [≥ 70 years]) based on the age range of the sample. Multiple comparisons in post-hoc analysis were adjusted using the Benjamini & Hochberg method. Between-study and between-sample heterogeneity was assessed using I2 (the percent of variability in effect sizes not due to sampling error) and 95% Prediction Interval (PI, the range where effects of future studies are expected to fall on, considering both the standard error of the pooled effect size and the between-study and between-sample heterogeneity variance). Potential publication bias was assessed using a funnel plot and Egger’s regression test.
TL change rate in base pairs per year (bp/year) was derived from the linear regression slope for TL measured in absolute unit (i.e., kb).
TL -score yearly change rate was calculated for TL measured in either relative (e.g., T/S ratio) or absolute unit (i.e., kb) using the following formula:
where, is the uncorrected TL-age correlation, and is the SD of age of each study sample.
Time-varying effect modeling (TVEM) (Lanza and Linden-Carmichael, 2021) was used to fit the change trend of TL-age correlation and TL -score change rate across chronological ages. In TVEM models, correlations or -score from different study samples were equally weighted. The following TVEM model equations were employed:
where, is the corrected correlation of sample , is the TL -score yearly change rate of sample , is an intercept term that changes as a nonparametric function of age center, and is the residual error.
2.5.2. Statistical analysis of longitudinal samples
For longitudinal samples, yearly TL change rate was derived from one of three ways: 1) mean or median of individual differences of TL at two time points divided by the follow-up duration; 2) difference of means or medians of TL at two time points divided by the follow-up duration; 3) coefficient of age in a linear mixed effect model. TL -score yearly change rate was calculated using the following formula:
where, and are the means of measured TL at follow-up and baseline; represents the time difference in years between the follow-up and baseline measurement; is the SD of measured TL at baseline.
3. Results
3.1. Overview of studies, samples, and subjects
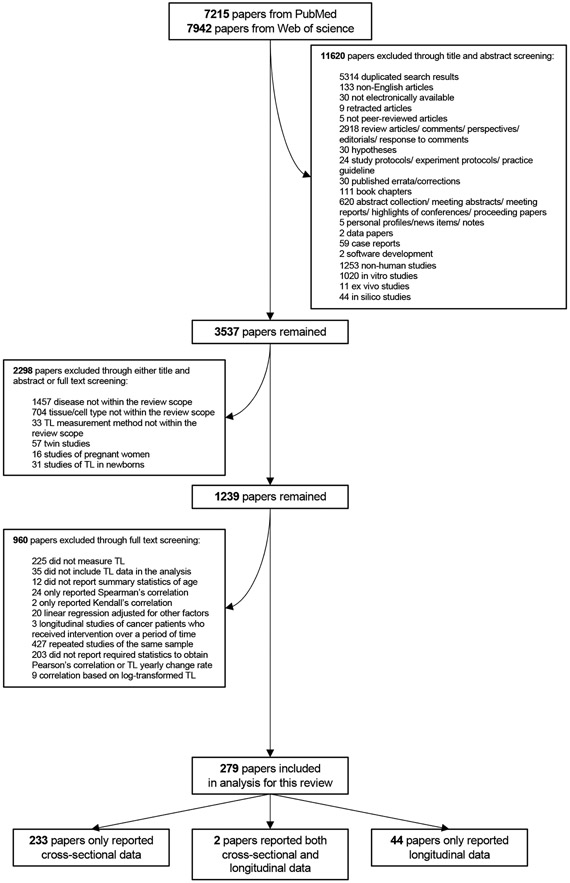
The literature search and screening process is illustrated in Figure 1. In total, 236 studies comprising 341 samples across 720,078 subjects were included as cross-sectional samples, and 46 studies comprising 73 samples across 22,941 subjects were included as longitudinal samples. The age ranged from 0 to 112 years for cross-sectional samples, and 0-97 years for longitudinal samples (median follow-up duration: 7 years). Among included samples, qPCR (72.7%) and blood leukocytes (76.8%) were the most common TL measurement method and tissue type, respectively. Descriptive statistics of all samples are shown in Table 1. The final extracted dataset used for analyses is reported in Supplementary Data File 1.
Figure 1. Flowchart of the paper screening process.
The paper using the UK biobank cohort (Codd et al., 2022) was included in a later process.
Table 1.
Descriptive statistics of all the study samples.
| Study design | Cross- sectional |
Cross- sectional |
Longitudinal | Longitudinal |
|---|---|---|---|---|
| Available statistics | Correlation available |
Corrected correlation available |
TL yearly change rate available |
TL yearly -score change rate available |
| Number of study samples | 341 | 286 | 73 | 49 |
| Number of subjects | 720,078 | 626,744 | 22,941 | 20,276 |
| Number of males | 335,093 | 293,070 | 10,820 | 9,743 |
| Number of females | 383,418 | 333,168 | 11,560 | 10,529 |
| Age range (years) | 0-112 | 0-112 | 0-97 | 0-94 |
| Follow-up duration (years)‡ | / | / | 7 (1-41) | 6 (1-41) |
| Sex included† | ||||
| Only males | 43 (12.6%) | 38 (13.3%) | 17 (23.3%) | 9 (18.4%) |
| Only females | 48 (14.1%) | 39 (13.6%) | 16 (21.9%) | 8 (16.3%) |
| Both males and females | 233 (68.3%) | 200 (69.9%) | 38 (52.0%) | 31 (63.3%) |
| Not reported | 17 (5.0%) | 9 (3.2%) | 2 (2.7%) | 1 (2.0%) |
| Health status† | ||||
| Healthy | 141 (41.4%) | 121 (42.3%) | 16 (21.9%) | 12 (24.5%) |
| General population | 112 (32.8%) | 93 (32.5%) | 53 (72.6%) | 33 (67.4%) |
| CVDs | 25 (7.3%) | 24 (8.4%) | 1 (1.4%) | 1 (2.0%) |
| Cancer | 51 (15.0%) | 36 (12.6%) | 1 (1.4%) | 1 (2.0%) |
| Depression/Anxiety/PTSD | 12 (3.5%) | 12 (4.2%) | 2 (2.7%) | 2 (4.1%) |
| TL measurement method† | ||||
| Southern blot | 76 (22.3%) | 56 (19.6%) | 23 (31.5%) | 11 (22.4%) |
| qPCR | 248 (72.7%) | 216 (75.5%) | 50 (68.5%) | 38 (77.6%) |
| qFISH | 12 (3.5%) | 9 (3.2%) | 0 (0%) | 0 (0%) |
| Flow FISH | 5 (1.5%) | 5 (1.8%) | 0 (0%) | 0 (0%) |
| Tissue type used for TL measurement† | ||||
| Blood leukocytes | 262 (76.8%) | 222 (77.6%) | 47 (64.4%) | 38 (77.6%) |
| Dry blood spots | 4 (1.2%) | 4 (1.4%) | 2 (2.7%) | 0 (0%) |
| PBMCs | 50 (14.7%) | 37 (12.9%) | 16 (21.9%) | 3 (6.1%) |
| Saliva | 14 (4.1%) | 14 (4.9%) | 7 (9.6%) | 7 (14.3%) |
| Buccal cells | 11 (3.2%) | 9 (3.2%) | 1 (1.4%) | 1 (2.0%) |
| DNA extraction procedure† | ||||
| Salting out | 65 (19.1%) | 59 (20.6%) | 23 (31.5%) | 21 (42.9%) |
| Silica membrane column | 90 (26.4%) | 76 (26.6%) | 32 (43.8%) | 12 (24.5%) |
| Magnetic bead | 15 (4.4%) | 14 (4.9%) | 1 (1.4%) | 1 (2.0%) |
| Phenol-chloroform | 18 (5.3%) | 14 (4.9%) | 1 (1.4%) | 1 (2.0%) |
| Anion exchange | 1 (0.3%) | 1 (0.4%) | 0 (0%) | 0 (0%) |
| Not reported | 152 (44.6%) | 122 (42.7%) | 16 (21.9%) | 14 (28.6%) |
CVD, cardiovascular disease; PTSD, post-traumatic stress disorder; qPCR, quantitative polymerase chain reaction; qFISH, quantitative fluorescence in situ hybridization; PBMC, peripheral blood mononuclear cells.
Data are presented as number of study samples (percentage)
Data are presented as median (range)
3.2. Findings from cross-sectional samples
For the sections below, we note that TL and age is inversely correlated, so “stronger correlations” means “more negative” estimates of the values and “weaker correlation” means “less negative or more positive” estimates of the values.
3.2.1. Pooled correlation of cross-sectional samples
Pearson’s correlations between TL and age were extracted as effect sizes and corrected for age range restriction. We corrected the TL-age correlations based on a SD of unrestricted age of 5 years to limit the theoretical age range width of a sample at around 18 years (see Methods section 2.5.1). The pooled raw TL-age correlation was −0.23 (95% CI: −0.26 to −0.20). Between-study and between-sample heterogeneity was substantial, with total I2 statistic of 98.4%. The 95% PI was −0.59 to 0.22. After correcting for age range restriction, the pooled corrected correlation reduced to −0.17 (95% CI: −0.21 to −0.12), with I2 statistic of 99.0%, and 95% PI of −0.67 to 0.45. The caterpillar plot of the corrected correlations is shown in Figure S1. To further examine the impact of age range restriction correction, a sensitivity analysis of the pooled corrected correlation was conducted by changing the SD of unrestricted age (Figure S2). As the SD of unrestricted age increased, the pooled corrected correlation became more negative, meaning, the TL-age correlation became stronger when age-range increased. The corrected correlations were used to test the moderation effect of biological and methodological factors.
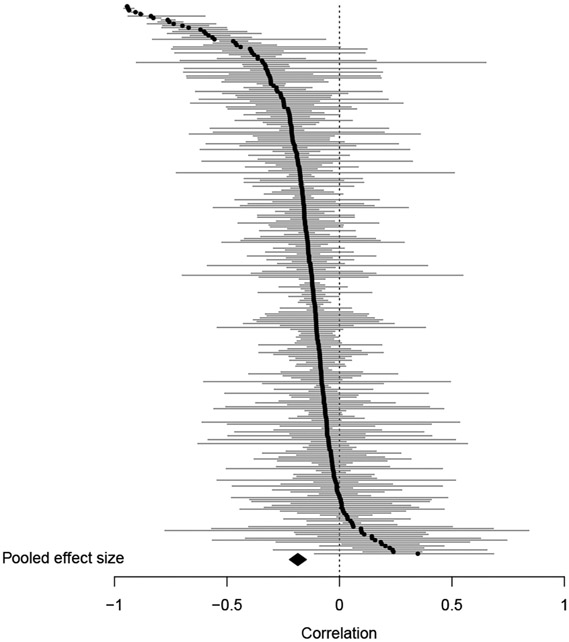
To examine the influence of outliers, DFFITS values, Cook’s distances, and hat values were used as criteria for identifying influential cases among the cross-sectional samples. Two samples (Nelson et al., 2020; Osler et al., 2016) were identified as influential cases based on DFFITS values (Figure S3). After excluding the two samples, the pooled corrected correlation increased to −0.19 (95% CI, −0.22 to −0.15, Figure 2), with I2 statistic of 98.6%, and 95% PI of −0.62 to 0.34. The moderation effects reported below were based on samples without the influential cases.
Figure 2. Caterpillar plot of corrected correlations from cross-sectional samples.
The black dots and the gray horizontal lines are effect sizes and 95% confidence intervals from individual samples. The black diamond is the pooled correlation. The two influential cases were excluded from this plot. Correlations were corrected for the age range restriction to reduce the noise caused by the heterogeneity of age range across different samples.
3.2.2. Moderation effect of age
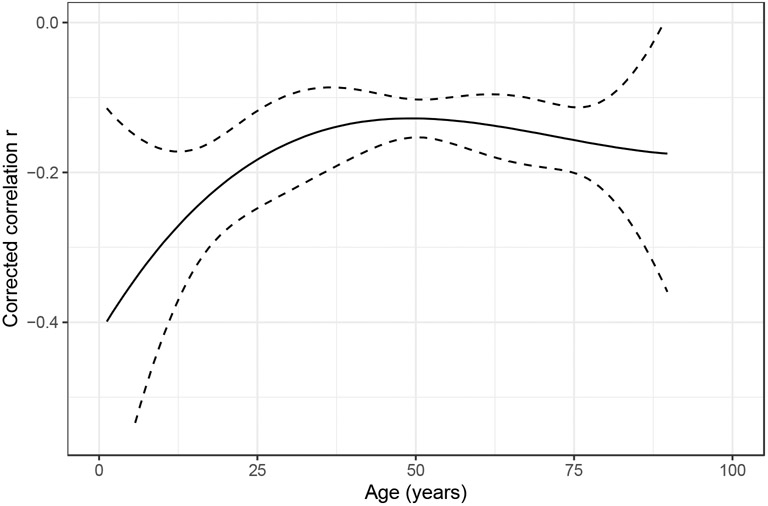
The TL-age correlation at different developmental periods was examined by including chronological age as a variable in meta-regression. Age had a significant moderation effect on the corrected TL-age correlation. Specifically, the correlation weakened with increased chronological age across the life course (β=0.003, p<0.001, Table 2). When separating study samples into two age groups (children: <18 years; adults: ≥18 years), the correlation in adults was significantly weaker compared with children (β=0.337, p<0.001). When separating study samples into three age groups (children: <18 years; young and middle-aged adults: ≥18 to <70 years; elderly adults: ≥70 years), the correlation was not significantly different between the elderly adults and the young and middle-aged adults (β=−0.010, p=0.916). Examination of the effect of age using TVEM demonstrated that the TL-age correlation became gradually weaker from age 0 to 50 and remained relatively weak at older ages, though there were large variations in both younger and older ages (Figure 3). The moderation effects of sex, health status, and methodological factors reported below were adjusted for age.
Table 2.
Meta-regression examining the moderation effects of biological and methodological factors on the corrected correlation between telomere length and chronological age in cross-sectional samples excluding the two influential cases.
| Moderator | Number of study samples |
β estimate | 95% CI | P value |
|---|---|---|---|---|
| Age center | 284 | 0.003 | 0.002 to 0.005 | <0.001*** |
| Age group | ||||
| Children (<18 years) | 21 | Reference | ||
| Adults (≥18 years) | 212 | 0.337 | 0.204 to 0.471 | <0.001*** |
| Sex † | ||||
| Only male | 37 | Reference | ||
| Only female | 39 | 0.023 | −0.098 to 0.144 | 0.711 |
| Both males and females | 199 | −0.003 | −0.109 to 0.102 | 0.950 |
| Health status ‡ | ||||
| Healthy | 120 | Reference | ||
| General population | 92 | −0.095 | −0.181 to −0.009 | 0.031* |
| CVDs | 24 | 0.010 | −0.119 to 0.138 | 0.883 |
| Cancer | 36 | 0.009 | −0.123 to 0.140 | 0.897 |
| Depression/Anxiety/PTSD | 12 | 0.044 | −0.130 to 0.219 | 0.620 |
| TL measurement method ‡ | ||||
| Southern blot | 56 | Reference | ||
| qPCR | 214 | 0.030 | −0.072 to 0.132 | 0.559 |
| qFISH | 9 | −0.280 | −0.557 to −0.004 | 0.047* |
| Flow FISH | 5 | −0.135 | −0.429 to 0.159 | 0.367 |
| Tissue type ‡ | ||||
| Blood leukocytes | 221 | Reference | ||
| Dry blood spots | 4 | −0.280 | −0.557 to −0.004 | 0.047* |
| PBMCs | 37 | 0.055 | −0.059 to 0.169 | 0.343 |
| Saliva | 13 | 0.151 | −0.025 to 0.327 | 0.093 |
| Buccal cells | 9 | −0.204 | −0.406 to −0.001 | 0.049* |
| DNA extraction procedure‡ | ||||
| Salting out | 59 | Reference | ||
| Silica membrane column | 76 | 0.065 | −0.065 to 0.194 | 0.324 |
| Magnetic bead | 12 | 0.129 | −0.104 to 0.362 | 0.275 |
| Phenol-chloroform | 14 | 0.086 | −0.157 to 0.330 | 0.485 |
| Anion exchange | 1 | 0.161 | −0.480 to 0.801 | 0.621 |
CVD, cardiovascular disease; PTSD, post-traumatic stress disorder; qPCR, quantitative polymerase chain reaction; qFISH, quantitative fluorescence in situ hybridization; PBMC, peripheral blood mononuclear cells; CI, confidence interval. Correlations were corrected for the age range restriction to reduce the noise caused by the heterogeneity of age range across different samples.
Meta-regression models were controlled for age center.
Meta-regression models were controlled for age center and sex.
p < 0.05
p < 0.01
p < 0.001
Figure 3. Change trend of corrected correlations across age fitted by a time-varying effect model in cross-sectional samples.
The solid black line is the point estimate. The dashed lines are the boundaries of the 95% confidence interval. The two influential cases were excluded from this plot. Correlations were corrected for the age range restriction to reduce the noise caused by the heterogeneity of age range across different samples.
3.2.3. Moderation effect of sex
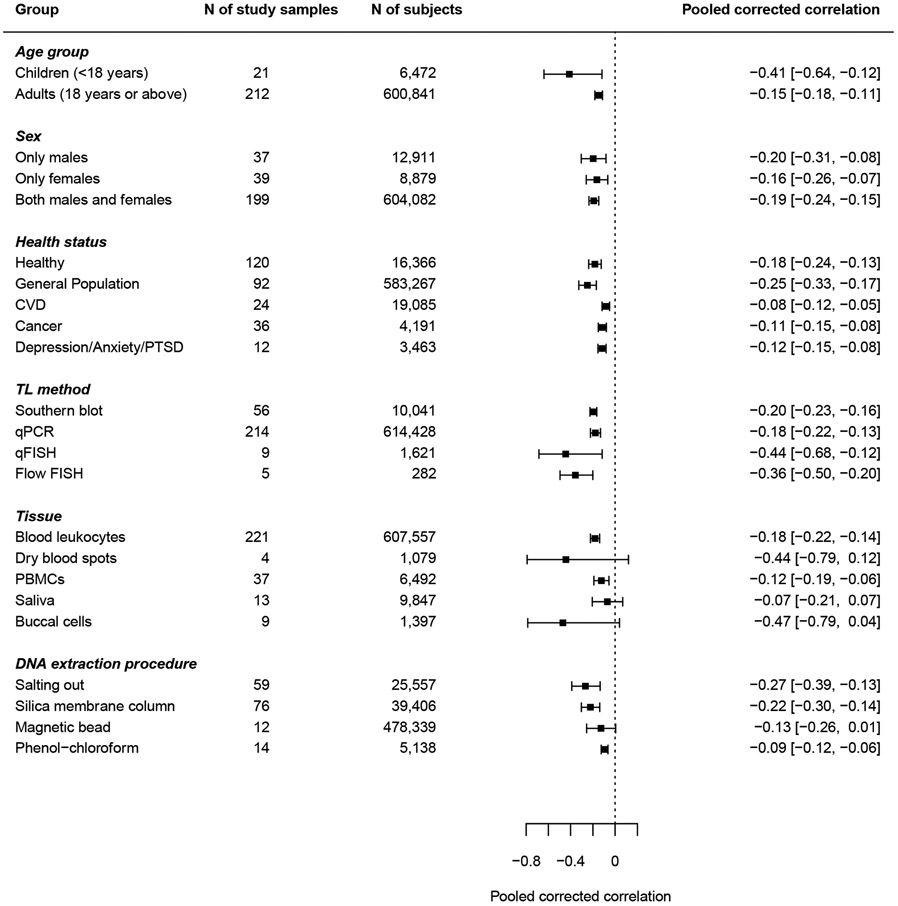
Sex did not significantly impact the TL-age correlation (Table 2). Study samples including exclusively females had comparable correlations with those including only males. Most samples (k=199) included both males and females in their analyses. Compared with samples including only males or females, samples including both sexes did not have significantly different TL-age correlations (Table S2). The pooled corrected correlation for samples including only males was −0.20 (95% CI: −0.31 to −0.08), and for samples including only females was −0.16 (95% CI: −0.26 to −0.07, Figure 4).
Figure 4. Forest plot of pooled corrected correlations stratified by demographic, disease, and methodological factors.
CVD, cardiovascular disease; PTSD, post-traumatic stress disorder; qPCR, quantitative polymerase chain reaction; qFISH, quantitative fluorescence in situ hybridization; PBMC, peripheral blood mononuclear cells. The two influential cases were excluded from this plot. Correlations were corrected for the age range restriction to reduce the noise caused by the heterogeneity of age range across different samples.
3.2.4. Moderation effect of health status
Study samples were grouped into five categories based on the reported health status of the participants. The categories were: healthy individuals, general population, patients with CVDs, patients with cancers, and patients with stress-related disorders (see Methods 2.1-2.4 section for details on inclusion criteria and data extraction). Study samples with participants recruited from the population regardless of health status were treated as samples of general population. The TL-age correlation was stronger in the general population than in healthy individuals (β=−0.095, p=0.031, Table 2), though it was not significant after adjusting for multiple comparisons in post-hoc analyses (Table S2). CVDs, cancer and stress-related disorders did not significantly moderate the TL-age correlation. The pooled corrected correlations stratified by health status is shown in Figure 4.
3.2.5. Moderation effect of methodological factors
TL measurement method and tissue type significantly moderated the correlation between TL and age (Table 2). For TL measurement method, the TL-age correlation was stronger for TL measured using qFISH than Southern blot (β=−0.280, p=0.047), though the moderation effect was not significant in post-hoc pairwise comparisons (Table S2). For tissue type, the TL-age correlation was stronger in dry blood spots (DBS, β=−0.280, p=0.047) and buccal cells (β=−0.204, p=0.049) than in blood leukocytes (Table 2), which was reduced to non-significance in post-hoc analyses (Table S2). In addition, the TL-age correlation was weaker in saliva than in DBS or buccal cells (Table S2). No significant moderation effect of DNA extraction procedures was found for the TL-age correlation after excluding the two influential cases. The pooled correlations stratified by methodological factors are presented in Figure S4.
3.2.6. TL yearly change rate in base pairs and -score
TL change rate in base pairs per year (bp/year) was derived from the linear regression slope for TL measured in base pairs. For the 87 cross-sectional samples that reported TL in base pairs, the median TL change rate was −23 bp/year (range, −170 to 46 bp/year; interquartile range [IQR], −42 to −15 bp/year, Table 3).
Table 3.
Telomere length yearly change rate in base pairs.
| Study design |
TL method | Number of study samples |
Number of subjects |
TL yearly change rate (bp/year) | ||
|---|---|---|---|---|---|---|
| Median | Range | Interquartile range |
||||
| Cross-sectional | Any TL method | 87 | 20,042 | −23 | −170 to 46 | −42 to −15 |
| Southern blot (TRF) | 65 | 12,690 | −24 | −100 to 38 | −38 to −19 | |
| qPCR (aTL) | 8 | 5,564 | −12 | −46 to 30 | −24 to −3 | |
| qFISH | 10 | 1,718 | −30 | −170 to 46 | −53 to −16 | |
| Flow FISH | 4 | 70 | −71 | −81 to 3 | −77 to −50 | |
| Longitudinal | Southern blot (TRF) & qPCR (aTL) | 32 | 6,068 | −38 | −2900 to 740 | −65 to −19 |
| Southern blot (TRF) | 23 | 2,220 | −27 | −2900 to 740 | −40 to −14 | |
| qPCR (aTL) | 9 | 3,848 | −67 | −150 to 44 | −75 to −58 | |
TRF, terminal restriction fragment; qPCR, quantitative polymerase chain reaction; qFISH, quantitative fluorescence in situ hybridization; aTL, absolute telomere length
TL yearly change rate in -score () was calculated for 284 cross-sectional study samples (excluding influential cases) using the uncorrected correlation and SD of age for each sample. The median was −0.02 per year (range, −0.57 to 0.07; IQR, −0.04 to −0.01). The distribution of of the cross-sectional samples is reported in Figure S5(A). Descriptive statistics of stratified by TL measurement method, sex, health status, tissue type, and DNA extraction procedures can be found in Table S3-S7, where the median of cross-sectional samples remained relatively stable across different categories. When stratified by TL measurement method, median was similar between Southern blot and qPCR, but slightly larger in samples using qFISH and flow-FISH (Table S5). The change of across age (Figure S6) followed a similar trend as the change of the corrected TL-age correlation (Figure 3).
3.2.7. Sensitivity analyses excluding the UK Biobank
The UK Biobank (Codd et al., 2022), the largest known cohort with TL data (N = 472,174), was included in our main analyses. To examine the potential impact of the UK Biobank cohort we conducted sensitivity analyses by excluding this sample. The pooled correlations (without the UK biobank and the two influential cases) remained similar (pooled uncorrected correlation: −0.23, 95% CI: −0.26 to −0.20; pooled corrected correlation: −0.19, 95% CI: −0.22 to −0.15). Likewise, the moderation effect of age, sex, health status, and methodological factors remained similar after excluding the UK biobank cohort (Table S8).
3.2.8. Publication bias
Egger’s regression test of the funnel plot (Figure S7) did not indicate significant asymmetry (z=0.943, p=0.346), indicating that no publication bias due to small-study effects was detected.
3.3. Findings from longitudinal samples
Among the 49 longitudinal samples with TL yearly -score change rate () available, ranged from −0.74 to 0.36 per year, with a median of −0.06 and an IQR of −0.19 to −0.03. When compared with the from cross-sectional samples, longitudinal samples had more negatively shifted values, meaning, TL shortening rate was generally reported to be faster based on longitudinal results than based on cross-sectional results. The distribution of the from longitudinal samples is shown in Figure S5(B).
The median was similar between male and female samples (Table S3), and across different TL measurement methods (Table S5). The median was more negative in samples of healthy individuals (−0.19 per year) than in samples of the general population (−0.04 per year) and patients with CVD (−0.06 per year) or cancer (−0.04 per year, Table S4). The median also varied across different tissue types; PBMC samples had the most negative median (−0.26 per year), followed by buccal cells (−0.23 per year), saliva (−0.15 per year) and blood leukocytes (−0.05 per year, Table S6). When stratified by DNA extraction procedures, the median was similar between salting out and silica membrane column procedures, but smaller in magnitude for phenol-chloroform and larger for magnetic bead procedures (Table S7). These latter results could be confounded by the limited number of longitudinal samples and the non-linear shortening trend of TL across the lifespan.
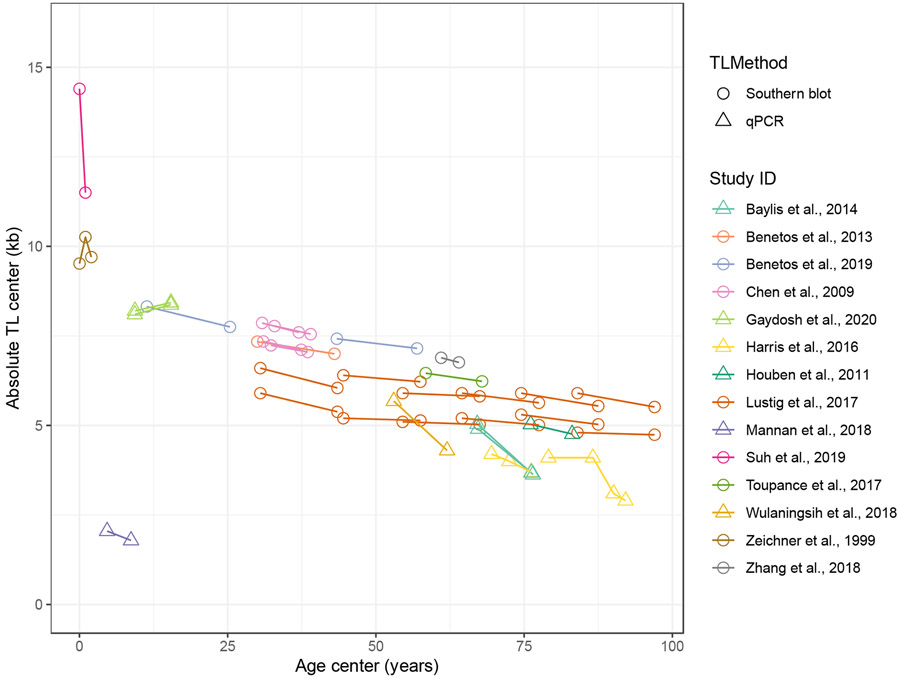
For TL measured using Southern blot, the TL yearly change rate in bp ranged from −2900 to 740 bp/year, with a median of −27 bp/year and an IQR of −40 to −14 bp/year. For TL measured in kb using qPCR, the median TL yearly change rate was −67 bp/year, with a range of −150 to 44 bp/year and an IQR of −75 to −58 bp/year (Table 3). Results remained similar after excluding longitudinal samples with follow-up duration less than 5 years (Table S9). The change trend of TL in kb measured by Southern blot and qPCR across different ages is presented as a line plot in Figure 5, with TL decreasing with increased age from 0 to 100 years.
Figure 5. Absolute telomere length change trend across the lifespan in longitudinal studies.
Age and TL center values (mean, median, or midrange) at baseline and follow-up from longitudinal studies were connected for each study in the line plot to show the change trend of TL as a function of age.
4. Discussion
This systematic review and meta-analysis investigated the dynamics of TL across the human lifespan. We examined the moderation effect of age, sex, health status, TL measurement methods, tissue type, and DNA extraction procedures based on meta data from published cross-sectional and longitudinal studies. Results provide a comprehensive overview of the TL-age correlation in humans and highlights methodological considerations when using TL as an aging biomarker. As expected, TL decreased with advancing chronological age, albeit in a non-linear fashion. Based on 341 cross-sectional study samples including 720,078 subjects, and for populations with a SD of age of 5 years, the pooled corrected correlation was −0.19 (95% CI: −0.22 to −0.15), which was similar to the correlation reported in the UK biobank sample (−0.185, without technical adjustment; −0.195, adjusted for technical parameters in TL measurement) (Codd et al., 2022). For TL measured in base pairs, the median change rate of TL was −23 bp/year in cross-sectional samples and −38 bp/year in longitudinal samples.
In addition to demonstrating the negative TL-age correlation, we found that the strength of the correlation varied by developmental periods across the life course. The finding that TL-age correlation was weaker in adults (r = −0.15) than in children (r = −0.41) indicates that age is a less dominant factor in explaining the TL attrition as individuals get older. The cross-sectional changes in z score and the longitudinal changes in base pairs across the lifespan are consistent with findings from previous studies (Aubert et al., 2012; Cowell et al., 2021; Frenck et al., 1998) where TL shortens more rapidly during the first few years of life. This could be explained by the relatively high levels of cell proliferation in the first few years after birth wherein each cycle of cell proliferation leads to TL shortening due to the end replication problem. In addition, long telomeres have relatively low concentration of stabilizing proteins (i.e., shelterin complex), which may lead to more rapid attrition rate early in life (Li et al., 2017).
Biological sex did not have a significant moderation effect on the TL-age correlation. A previous systematic review and meta-analysis (Gardner et al., 2014) on the association between gender and TL synthesizing findings from 36 cohorts across 36,230 participants reported that females had longer TL than males after adjusting for age. Further, there was little evidence that the TL-sex association varied by age group, indicating that females have consistently longer TL than males across different age groups. Our meta-analysis similarly indicated that the TL-age correlation did not vary significantly by sex across the life course. It has been suggested that the longer TL in females might be due to slower TL attrition rate (Okuda et al., 2002) or sex-dependent differences in TL starting from birth (Gardner et al., 2014). Although there are inconsistent findings of TL difference between female and male newborns (Aubert et al., 2012; Okuda et al., 2002), our results do not support different TL attrition rates between males and females.
The TL-age correlation was not significantly moderated by health status (CVDs, cancer, and stress-related disorders). This could indicate that diseases affecting TL may contribute to changes in TL equally across the lifespan. Alternatively, between-study heterogeneity or grouping of diverse disease subtypes under each disease category might have influenced our ability to detect significant moderation effects. Moreover, disease onset and duration relative to the timing of sample collection for TL measurement can further influence the impact of disease status on the correlation between TL and age. The potential difference in TL-age correlation between the general population and healthy individuals could be due to unidentified risk or protective factors other than the disease categories included in this meta-analysis. Those unidentified factors could include psychological stress (Shalev and Hastings, 2017), diet (e.g., daily calorie intake) (Kark et al., 2012), and lifestyle (e.g., walking pace) (Dempsey et al., 2022), all of which have been found to be significantly associated with TL. Despite being non-significant, the overall pooled correlation without adjustment for age in patients with CVDs, cancer, or stress-related disorders tended to be weaker than the pooled correlation in the general population or healthy individuals. One explanation could be the difference in disease prevalence in different age groups, where younger individuals were likely to be healthier and older individuals had increased chances of being affected by chronic diseases. Our results indicated that the TL-age correlation was weaker in older individuals and thus, weaker TL-age correlation could be observed in patients with disease. On the other hand, it is also possible that telomere attrition associated with certain diseases is greater than the attrition due to the increase of chronological age. In this case, morbidity at a younger chronological age could be associated with advanced biological age at diagnosis, leading to a diminished correlation between TL and age in patients with disease. Longitudinal studies within young populations prior to disease onset may provide further insight on the dynamics of TL attrition in disease patients versus healthy individuals. Further, the association between cancer and TL is also more complex than other diseases. Previous research reported that shorter TL was associated with increased risk of bladder and gastric cancer whereas longer TL was associated with increased risk of melanoma, soft tissue sarcoma, and lung cancer adenocarcinoma (Barrett et al., 2015). As shortened TL can trigger cellular senescence or apoptosis, TL maintenance and lengthening are often critical barriers cancer cells must overcome to maintain cell survival and proliferation (Barrett et al., 2015), which could be presented as diminished TL-age association in cancer patients.
For TL measurement methods, qPCR and Southern blot were the most common, followed by FISH-based methods such as qFISH and flow FISH. In our analysis, Southern blot and qPCR did not significantly differ in TL-age correlation, but there was suggestive evidence for stronger TL-age correlation in studies using qFISH methods. This could be related to differences in measurement procedures and precision between protocols (for a review see (Lai et al., 2018)). However, there were notably fewer samples using qFISH than those using qPCR or Southern blot, which may lead to inaccurate estimates of differences between methods.
In terms of the moderation effect of tissue type, the TL-age correlation has been reported to be tissue-specific with a correlation of −0.35 in whole blood in donors aged 20-70 years, a magnitude that is slightly smaller than in stomach and aorta tissues, but larger than in post-mitotic tissues (i.e., brain and muscle) (Demanelis et al., 2020). Our analyses extended these results with evidence that the correlation between TL and age in saliva was smaller than that in DBS and buccal cells. One explanation could be the larger variation in cell proportions in saliva. Saliva is a mixture of buccal cells and leukocytes. Tissues collected via buccal swabs have a more uniform cell type that are predominantly buccal epithelial cells with less leukocytes, whereas saliva collected through passive drool contains less buccal cells (Lin et al., 2019). Results further revealed stronger TL-age correlation in DBS versus blood leukocytes, which could be potentially due to differences in collection and processing procedures. However, these findings should be taken cautiously considering the large variability and smaller number of DBS studies included in this meta-analysis.
As for DNA extraction procedures, previous studies (Cunningham et al., 2013; Raschenberger et al., 2016) indicated that different procedures may influence TL measurement. For example, Raschenberger et al.(2016) found that DNA extracted using a magnetic bead procedure (Qiagen EZ1 kit) had significantly shorter relative TL than DNA extracted using a salting out procedure (INVISORB kit), and the association of TL with CVD was stronger when using the salting out compared with the magnetic bead procedure. In this meta-analysis, we found that the TL-age correlation was weaker in samples using magnetic bead procedures when including the two influential cases (Nelson et al., 2020; Osler et al., 2016). However, analysis excluding the two influential cases did not show any significant moderation effect of magnetic bead procedures on the TL-age correlation. A further examination into the two cases revealed that the TL-age correlations were derived from participant samples with very narrow age range (less than one-year age difference between participants), which could have caused the potential artifact of the moderation effect. No significant differences were observed between other DNA extraction procedures.
We acknowledge limitations. First, due to a wide age coverage in most study samples, we were unable to examine the moderation effect of a finer age group classification when using age as a categorical variable. Second, correlations may differ in different disease subtypes, and factors such as psychological stress, diet, and lifestyle can further affect the rate of TL attrition across the lifespan, but we did not collect detailed information about disease subtypes or non-disease factors given substantial study heterogeneity. Future studies are encouraged to examine the unique effects of psychological stress, diet, and psychological stress on TL and its change rate. Third, many studies did not report the DNA extraction kit used (i.e., 44.6% of cross-sectional and 21.9% of longitudinal samples), which limited the power to test the moderation effect of DNA extraction procedures. Fourth, tissue types, DNA extraction procedures, and TL measurement methods were not randomly distributed across study samples, which might introduce confounding effects and limit the generalizability of the results. Additionally, we were less powered to test the moderation effect of sex because most samples included both males and females. Similarly, we were also unable to test the potential moderation effect of race and ethnicity given most samples included participants of diverse race and ethnicity. Further, because not all study samples reported SD of age, fewer cross-sectional samples were included in meta-regression after correcting the correlation for age range restriction. Similarly, not all longitudinal study samples reported SD of TL, which prevented the calculation of -score change rate of TL for those samples. Lastly, there are inherent limitations of using TL as an aging biomarker. Length is only one of many parameters of the telomere structure. DNA replication stress can cause aberrant telomeric structures such as fragile telomeres (i.e., presence of diffuse telomeric signals across a single chromatid arm), sister telomere loss, and sister telomere chromatid fusions, which have been found to change with chronological age (Boccardi et al., 2020). The formation of aberrant telomeric structures could not be fully reflected by the change of TL and may carry valuable information about biological aging. We further note that telomere regulation is more complex than initially assumed and can involve telomere regulatory shelterin proteins that contributes to aging via telomere-dependent and independent functions (Wolf and Shalev, 2023). Characterizing a comprehensive profile of telomere change across the lifespan will be a focus of future studies.
5. Conclusions
In conclusion, this systematic review and meta-analysis provides a comprehensive overview of TL and chronological age across the human lifespan while accounting for the impact of biological and methodological factors. The overall trend of TL shortening with increased chronological age was demonstrated based on the synthesized evidence. Further, the strength of the correlation was significantly different at different developmental periods across the life course, wherein children had a stronger TL-age correlation than adults. While biological factors including sex and disease status may not be influential moderators, methodological factors such as TL measurement method and tissue type may moderate the correlation between TL and age. Moreover, there was a large amount of heterogeneity between and within studies. Future TL studies should follow consistent methodology and reporting guidelines as recommended by the Telomere Research Network (Lindrose and Drury, 2020) to help improve the reliability of using TL as an aging biomarker in diverse populations and settings. Finally, results could be used as a benchmark to evaluate TL-age correlation in future telomere-based studies.
Supplementary Material
Highlights.
We conducted a meta-analysis to evaluate the association of telomere length with age.
Telomere length shortens in a non-linear manner and more rapidly in early life.
A greater telomere attrition rate was observed in longitudinal evaluations.
Telomere measurement methods and tissue types impacted the telomere-age correlation.
Sex or disease status did not impact the telomere-age correlation.
Acknowledgements
We thank all the authors of the original studies for their contribution of data.
Funding
This work was supported by the National Institute of Environmental Health Sciences (U01ES030949 to I.S.). L.E., S.E.W. and A.T.A were supported by National Institute on Aging T32 AG049676 to The Pennsylvania State University. W.J.H. is supported by the Telomere Research Network (U24AG066528). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Competing Interest
The corresponding author (I.S.) is a Steering Committee member of Telomere Research Network.
Data Availability Statement
All data generated or analyzed during this review are included in this article and its supplementary files.
Reference
- AlDehaini DMB, Al-Bustan SA, Ali ME, Malalla ZHA, Sater M, Giha HA, 2020. Shortening of the leucocytes' telomeres length in T2DM independent of age and telomerase activity. Acta Diabetol 57, 1287–1295. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Susser E, Arbeev KG, Yashin AI, Levy D, Verhulst S, Aviv A, 2022. Telomere-length dependent T-cell clonal expansion: A model linking ageing to COVID-19 T-cell lymphopenia and mortality. EBioMedicine 78, 103978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeev KG, Verhulst S, Steenstrup T, Kark JD, Bagley O, Kooperberg C, Reiner AP, Hwang S-J, Levy D, Fitzpatrick AL, Christensen K, Yashin AI, Aviv A, 2020. Association of Leukocyte Telomere Length With Mortality Among Adult Participants in 3 Longitudinal Studies. JAMA Network Open 3, e200023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM, 2012. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet 8, e1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A., 2006. Telomeres and human somatic fitness. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 61, 871–873. [DOI] [PubMed] [Google Scholar]
- Aviv A., 2021. Short telomeres and severe COVID-19: The connection conundrum. EBioMedicine 70, 103513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JH, Iles MM, Dunning AM, Pooley KA, 2015. Telomere length and common disease: study design and analytical challenges. Human Genetics 134, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH, 1991. Structure and function of telomeres. Nature 350, 569–573. [DOI] [PubMed] [Google Scholar]
- Boccardi V, Cari L, Nocentini G, Riccardi C, Cecchetti R, Ruggiero C, Arosio B, Paolisso G, Herbig U, Mecocci P, 2020. Telomeres Increasingly Develop Aberrant Structures in Aging Humans. J Gerontol A Biol Sci Med Sci 75, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühring J, Hecker M, Fitzner B, Zettl UK, 2021. Systematic Review of Studies on Telomere Length in Patients with Multiple Sclerosis. Aging Dis 12, 1272–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd V, Denniff M, Swinfield C, Warner SC, Papakonstantinou M, Sheth S, Nanus DE, Budgeon CA, Musicha C, Bountziouka V, Wang Q, Bramley R, Allara E, Kaptoge S, Stoma S, Jiang T, Butterworth AS, Wood AM, Di Angelantonio E, Thompson JR, Danesh JN, Nelson CP, Samani NJ, 2022. Measurement and initial characterization of leukocyte telomere length in 474,074 participants in UK Biobank. Nature Aging 2, 170–179. [DOI] [PubMed] [Google Scholar]
- Cowell W, Tang D, Yu J, Guo J, Wang S, Baccarelli AA, Perera F, Herbstman JB, 2021. Telomere dynamics across the early life course: Findings from a longitudinal study in children. Psychoneuroendocrinology 129, 105270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JM, Johnson RA, Litzelman K, Skinner HG, Seo S, Engelman CD, Vanderboom RJ, Kimmel GW, Gangnon RE, Riegert-Johnson DL, Baron JA, Potter JD, Haile R, Buchanan DD, Jenkins MA, Rider DN, Thibodeau SN, Petersen GM, Boardman LA, 2013. Telomere length varies by DNA extraction method: implications for epidemiologic research. Cancer Epidemiol Biomarkers Prev 22, 2047–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A, 2013. Telomeres shorten at equivalent rates in somatic tissues of adults. Nature Communications 4, 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demanelis K, Jasmine F, Chen LS, Chernoff M, Tong L, Delgado D, Zhang C, Shinkle J, Sabarinathan M, Lin H, Ramirez E, Oliva M, Kim-Hellmuth S, Stranger BE, Lai TP, Aviv A, Ardlie KG, Aguet F, Ahsan H, Doherty JA, Kibriya MG, Pierce BL, 2020. Determinants of telomere length across human tissues. Science 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey PC, Musicha C, Rowlands AV, Davies M, Khunti K, Razieh C, Timmins I, Zaccardi F, Codd V, Nelson CP, Yates T, Samani NJ, 2022. Investigation of a UK biobank cohort reveals causal associations of self- reported walking pace with telomere length. Commun Biol 5, 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenck RW Jr., Blackburn EH, Shannon KM, 1998. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A 95, 5607–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, Martin-Ruiz C, Shiels P, Sayer AA, Barbieri M, Bekaert S, Bischoff C, Brooks-Wilson A, Chen W, Cooper C, Christensen K, De Meyer T, Deary I, Der G, Diez Roux A, Fitzpatrick A, Hajat A, Halaschek-Wiener J, Harris S, Hunt SC, Jagger C, Jeon H-S, Kaplan R, Kimura M, Lansdorp P, Li C, Maeda T, Mangino M, Nawrot TS, Nilsson P, Nordfjall K, Paolisso G, Ren F, Riabowol K, Robertson T, Roos G, Staessen JA, Spector T, Tang N, Unryn B, van der Harst P, Woo J, Xing C, Yadegarfar ME, Park JY, Young N, Kuh D, von Zglinicki T, Ben-Shlomo Y, 2014. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol 51, 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW, 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460. [DOI] [PubMed] [Google Scholar]
- Harrer M, Cuijpers P, Furukawa TA, Ebert DD, 2021. Doing Meta-Analysis With R: A Hands-On Guide, 1st ed. Chapman & Hall/CRC Press, Boca Raton, FL and London. [Google Scholar]
- Hastings WJ, Eisenberg DTA, Shalev I, 2021. Impact of Amplification Efficiency Approaches on Telomere Length Measurement via Quantitative-Polymerase Chain Reaction. Front Genet 12, 728603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings WJ, Shalev I, Belsky DW, 2017. Translating Measures of Biological Aging to Test Effectiveness of Geroprotective Interventions: What Can We Learn from Research on Telomeres? Frontiers In Genetics 8, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P, 2014. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. Bmj 349, g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Schmidt FL, Le H, 2006. Implications of direct and indirect range restriction for meta-analysis methods and findings. J Appl Psychol 91, 594–612. [DOI] [PubMed] [Google Scholar]
- Kark JD, Goldberger N, Kimura M, Sinnreich R, Aviv A, 2012. Energy intake and leukocyte telomere length in young adults. The American Journal of Clinical Nutrition 95, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai TP, Wright WE, Shay JW, 2018. Comparison of telomere length measurement methods. Philos Trans R Soc Lond B Biol Sci 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Linden-Carmichael AN, 2021. Time-Varying Effect Modeling for the Behavioral, Social, and Health Sciences, 1 ed. Springer; Cham. [Google Scholar]
- Lapham K, Kvale MN, Lin J, Connell S, Croen LA, Dispensa BP, Fang L, Hesselson S, Hoffmann TJ, Iribarren C, Jorgenson E, Kushi LH, Ludwig D, Matsuguchi T, McGuire WB, Miles S, Quesenberry CP Jr., Rowell S, Sadler M, Sakoda LC, Smethurst D, Somkin CP, Van Den Eeden SK, Walter L, Whitmer RA, Kwok PY, Risch N, Schaefer C, Blackburn EH, 2015. Automated Assay of Telomere Length Measurement and Informatics for 100,000 Subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) Cohort. Genetics 200, 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Feng C, Li L, Yang S, Chen Y, Hui R, Zhang M, Zhang W, 2018. The association of telomere attrition with first-onset stroke in Southern Chinese: a case-control study and meta-analysis. Sci Rep 8, 2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JSZ, Miralles Fusté J, Simavorian T, Bartocci C, Tsai J, Karlseder J, Lazzerini Denchi E, 2017. TZAP: A telomere-associated protein involved in telomere length control. Science (New York, N.Y.) 355, 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Smith DL, Esteves K, Drury S, 2019. Telomere length measurement by qPCR - Summary of critical factors and recommendations for assay design. Psychoneuroendocrinology 99, 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Huang YC, Hung CF, 2016. Shortened telomere length in patients with depression: A meta-analytic study. J Psychiatr Res 76, 84–93. [DOI] [PubMed] [Google Scholar]
- Lindrose A, Drury S, 2020. Minimum Reporting Recommendations for PCR-based Telomere Length Measurement., OSF Preprints. [Google Scholar]
- Lindrose AR, McLester-Davis LWY, Tristano RI, Kataria L, Gadalla SM, Eisenberg DTA, Verhulst S, Drury S, 2021. Method comparison studies of telomere length measurement using qPCR approaches: A critical appraisal of the literature. PLoS One 16, e0245582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, Dunning AM, Svenson U, Roos G, Hosgood HD 3rd, Shen M, Wei Q, 2011. Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One 6, e20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ruiz C, Williams-Gray CH, Yarnall AJ, Boucher JJ, Lawson RA, Wijeyekoon RS, Barker RA, Kolenda C, Parker C, Burn DJ, Von Zglinicki T, Saretzki G, 2020. Senescence and Inflammatory Markers for Predicting Clinical Progression in Parkinson's Disease: The ICICLE-PD Study. J Parkinsons Dis 10, 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinha F, Magalhães P, Blanco G, 2020. Standard guidelines for the publication of telomere qPCR results in evolutionary ecology. Mol Ecol Resour 20. [DOI] [PubMed] [Google Scholar]
- Müezzinler A, Zaineddin AK, Brenner H, 2013. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev 12, 509–519. [DOI] [PubMed] [Google Scholar]
- Nelson BW, Wright DB, Allen NB, Laurent HK, 2020. Maternal stress and social support prospectively predict infant inflammation. Brain Behav Immun 86, 14–21. [DOI] [PubMed] [Google Scholar]
- Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G, Aviv A, 2002. Telomere length in the newborn. Pediatric Research 52, 377–381. [DOI] [PubMed] [Google Scholar]
- Osler M, Bendix L, Rask L, Rod NH, 2016. Stressful life events and leucocyte telomere length: Do lifestyle factors, somatic and mental health, or low grade inflammation mediate this relationship? Results from a cohort of Danish men born in 1953. Brain Behav Immun 58, 248–253. [DOI] [PubMed] [Google Scholar]
- Paghera S, Quiros-Roldan E, Sottini A, Properzi M, Castelli F, Imberti L, 2019. Lymphocyte homeostasis is maintained in perinatally HIV-infected patients after three decades of life. Immun Ageing 16, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pousa PA, Souza RM, Melo PHM, Correa BHM, Mendonca TSC, Simoes ESAC, Miranda DM, 2021. Telomere Shortening and Psychiatric Disorders: A Systematic Review. Cells 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschenberger J, Lamina C, Haun M, Kollerits B, Coassin S, Boes E, Kedenko L, Kottgen A, Kronenberg F, 2016. Influence of DNA extraction methods on relative telomere length measurements and its impact on epidemiological studies. Sci Rep 6, 25398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiello F, Jurk D, Passos JF, d'Adda di Fagagna F, 2022. Telomere dysfunction in ageing and age-related diseases. Nature Cell Biology 24, 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM, 1999. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 190, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, 2015. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety 32, 229–238. [DOI] [PubMed] [Google Scholar]
- Shalev I, Hastings WJ, 2017. Psychological stress and cellular aging, Oxford Research Encyclopedia of Psychology. [Google Scholar]
- Viechtbauer W., 2010. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 36, 1–48. [Google Scholar]
- Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Nordestgaard BG, 2012. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol 32, 822–829. [DOI] [PubMed] [Google Scholar]
- Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA, 2011. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 20, 1238–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SE, Shalev I, 2023. The shelterin protein expansion of telomere dynamics: Linking early life adversity, life history, and the hallmarks of aging. Neuroscience and Biobehavioral Reviews 152, 105261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhao Q, Zhu W, Liu T, Xie SH, Zhong LX, Cai YY, Li XN, Liang M, Chen W, Hu QS, Zhang B, 2017. The Association of Telomere Length in Peripheral Blood Cells with Cancer Risk: A Systematic Review and Meta-analysis of Prospective Studies. Cancer Epidemiol Biomarkers Prev 26, 1381–1390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this review are included in this article and its supplementary files.