SUMMARY
BACKGROUND:
Stereotactic ablative radiotherapy (SABR) is the standard treatment for medically inoperable early-stage non-small cell lung cancer (NSCLC), but regional/distant relapses are common. Immunotherapy reduces recurrence and improves survival in patients with stage III NSCLC after chemoradiotherapy. We conducted a randomized phase 2 trial of SABR alone vs SABR with immunotherapy (I-SABR) for patients with early-stage NSCLC.
METHODS:
Patients with histologically proven treatment-naïve T1–3N0M0 (stage IA-IB (tumor ≤4 cm, N0M0), stage IIA (≤5 cm, N0M0), or stage IIB (>5 cm & ≤7 cm, N0M0) by AJCC version 8) or isolated parenchymal recurrent (tumor ≤7 cm) NSCLC (TanyNanyM0 after definitive surgery or radiotherapy/chemotherapy) were included in this open-label trial conducted at 3 hospitals (NCT03110978). Patients were randomly assigned (Pocock & Simon methodology) to receive SABR with/without four 480mg cycles of nivolumab (every 4 weeks, first dose in the same day or within 36 hours after first SABR). The primary endpoint was 4-year event-free survival (EFS; local/regional/distant recurrence, second primary, or death). Analyses were both intention-to-treat (ITT) and per-protocol (PP).
FINDINGS:
From June 30, 2017 to March 22, 2022, 141 patients were randomized and received assigned therapy. At median 33 months’ follow-up, I-SABR significantly improved 4-year EFS from 53% to 77% (PP: hazard ratio [HR] 0.38, 95% confidence interval [CI] 0.19–0.75, P=0.006; ITT: HR 0.42, 95% CI 0.22–0.80, P=0.008). There were no grade 3+ adverse events associated with SABR. In the I-SABR arm, 15.2% patients had grade 3 immunologic adverse events related to nivolumab; none had grade 3 pneumonitis or grade 4+ toxicity.
INTERPRETATION:
Compared with SABR alone, I-SABR significantly improved EFS at 4-years in early-stage treatment-naïve or lung parenchymal recurrent node-negative NSCLC with tolerable toxicity.
FUNDING:
Bristol-Myers Squibb; NCI NIH through Cancer Center Core Support Grant (P30CA016672) and Clinical and Translational Science Award (UL1 RR024148) to MD Anderson.
Keywords: Non-small cell lung cancer, stereotactic ablative radiotherapy, stereotactic body radiotherapy, immunotherapy, recurrence
INTRODUCTION
For patients with early-stage non-small cell lung cancer (NSCLC), preliminary pooled randomized and propensity-matched prospective studies suggest that stereotactic ablative radiotherapy (SABR; also known as stereotactic body radiation therapy [SBRT]) could achieve similar overall survival (OS) with considerably reduced toxicity compared to standard-of-care surgery for operable stage IA (<3 cm) NSCLC.1,2 For inoperable early-stage NSCLC, SABR is the standard of care owing to improved clinical outcomes over conventionally fractionated radiotherapy (RT).3 SABR has also demonstrated efficacy for patients with lung parenchymal recurrences.4 As such, SABR is now a preferred option for patients deemed medically inoperable, who refuse surgery, or with isolated lung parenchymal recurrence.1–4
These successes notwithstanding, the incidence of any recurrence after SABR for early-stage NSCLC remains substantial with observed rates as much as 42%.5 The risk of relapse is higher for those with larger tumors6 or isolated locally recurrent parenchymal disease after SABR.7 Although local control rates in the irradiated field after SABR can exceed 90%, most failures are regional or distant recurrences outside the irradiated field (11–13% and 11–20% at 5 years, respectively).8–9 Randomized trials of cytotoxic chemotherapy have failed to improve distant failures and survival outcomes for stage I disease10–11, suggesting that improvements in systemic therapy are required. This is also true for isolated locally recurrent parenchymal disease, for which there remains a general dearth of randomized evidence-based approaches.
Emerging evidence indicates that RT and immunotherapy may have synergistic effects, particularly when the radiation is given as SABR, i.e., given to high biologically effective doses (BED) (>100 Gy) in relatively few (1–10) fractions. Ablative RT can transform tumors into an in situ cancer-specific “vaccine” by boosting the release of tumor-associated antigens, increasing the expression of programmed cell death ligand-1 (PD-L1), and better activating tumor-directed T lymphocytes to augment local tumor ablation and better eliminate occult micrometastatic disease.12–13
Compared with conventional chemotherapy, PD-(L)1 immunotherapy with or without chemotherapy has improved overall survival for patients with stage IV NSCLC.14 Neoadjuvant chemo/immunotherapy and adjuvant immunotherapy have shown potential for improving event-free survival15–18 and potentially overall survival in stage II-III NSCLC. For patients with medically inoperable stage III NSCLC treated with definitive chemoradiotherapy, consolidation immunotherapy has significantly improved progression-free and overall survival.19
In efforts to reduce recurrence and improve outcomes after SABR for treatment-naïve early-stage (node-negative) or isolated parenchymal recurrent NSCLC, and to test the theoretical advantages of combining SABR with immunotherapy (I-SABR), we conducted a phase 2 randomized trial of SABR alone vs I-SABR for early-stage or isolated parenchymal recurrent node-negative NSCLC.
MATERIALS AND METHODS
Study Design and Participants
The open-label randomized phase II I-SABR trial (NCT03110978) was approved by the MD Anderson Institutional Review Board and ethics committee, monitored by the institutional data and safety monitoring committee, and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent to participate. The trial was conducted at three different hospitals within the integrated MD Anderson network.
Adults ≥18 years of age with adequate performance status (Eastern Cooperative Oncology Group [ECOG] score 0–2) and organ function to receive nivolumab, and pathologically confirmed early-stage NSCLC who were unable or unwilling to undergo surgery were eligible for enrollment. Early-stage disease was defined as stage IA-IB (tumor size ≤4 cm, N0M0), stage IIA (≤5 cm, N0M0), or stage IIB (>5 cm & ≤7 cm, N0M0) (per the AJCC Version 8 staging system). Pathologic confirmation was required, and the eligible histologies were as follows: squamous cell carcinoma, adenocarcinoma with or without bronchioloalveolar features, large cell carcinoma with or without neuroendocrine features, neuroendocrine carcinoma (NSCLC with neuroendocrine features or atypical carcinoids, but not small cell carcinoma) or NSCLC not otherwise specified. Multiple primary lung cancers were allowed. Isolated parenchymal recurrences (tumor size <7 cm) of initially TanyNanyM0 disease were also allowed because their natural history after a second SABR course is similar to that after primary SABR.20 For these cases, prior therapy was to have been definitive surgery or RT/chemotherapy, completed prior therapy at least 6 months prior to protocol therapy, and the recurrent lesion had to be amenable to SABR. Of note, there were no specific inclusion criteria based on operability; both medically inoperable patients as well as those who refused surgery were eligible. Key exclusion criteria were tumors ≥7 cm or ineligible for SABR based on location (e.g. involving mediastinal organs-at-risk), previous receipt of immune checkpoint inhibitors, nodal or metastatic disease discovered on protocol-specified workup studies, any additional planned upfront local or systemic therapy, cases not meeting minimal requirements for SABR dose-volume constraints, and immunotherapy-related contraindications.
Within 12 weeks of study entry, requirements included evaluation by a medical and radiation oncologist, complete blood count, complete metabolic panel, and pulmonary function testing. Whole body 18F-fluorodeoxyglucose positron emission tomography – computed tomography (PET-CT) was required for all patients. Endoscopic or endobronchial ultrasound, cervical mediastinoscopy, or other nodal staging techniques (sampling bilateral stations 4 and station 7 at minimum) were strongly recommended for all patients, and were required for cases with clinical suspicion or any mediastinal lymph node measuring >1.0 cm (short axis diameter) or having maximum standard uptake value above that of the mediastinal blood pool. Brain CT or magnetic resonance imaging was required in case of symptoms suggestive of central nervous system (CNS) metastases.
Randomization and Masking
Randomization to the SABR arm or the I-SABR arm, carried out by the MD Anderson Department of Biostatistics, followed the methods of Pocock and Simon21 with a minimization probability parameter of 0.90. The 4 stratification variables were ECOG performance status (0–1 vs 2), tumor size (≤3 cm vs 3.1–5 cm vs 5.1–7 cm), histology (squamous vs non-squamous), and lung cancer history (primary vs recurrent disease). This trial was not masked to the investigators or patients.
Procedures
The first dose of nivolumab (480 mg per dose via intravenous infusion) was to occur in the same day of the first SABR fraction or within 36 hours after the first fraction, and every 4 weeks (± 1 week) thereafter for a total of 4 planned cycles. In the first few months of the trial, nivolumab was delivered as 240 mg every 2 weeks for a total of 7 planned cycles, prior to approval of the amended regimen. In this trial, a short-term nivolumab regimen was chosen based on the trial hypothesis owing to potentially stronger immune stimulation by the concurrent I-SABR regimen, the lack of standard adjuvant therapy after definitive SABR, cost-effectiveness considerations, and the presence of a highly comorbid patient population.
Complete details pertaining to SABR are described elsewhere2,4,9,22, along with the Supplement and the study protocol. All patients underwent four-dimensional CT simulation–based motion management. The prescribed 4-fraction (daily) SABR dose was 50 Gy to the planning target volume (PTV), with simultaneous integrated boost to the internal gross tumor volume to 60 Gy for peripheral or selected central lesions if dose-volume constraints for critical normal structures could be met. For all other central lesions, 10 daily fractions were delivered, with a PTV dose of 70 Gy and simultaneous integrated boost to the internal gross tumor volume to 80 Gy if dose-volume constraints could be achieved.22 Ultra-central lesions, defined as within 0.5 cm of critical structures (trachea, tracheobronchial tree, esophagus, heart, major vessels, brachial plexus) were not allowed.
Follow-up visits as well as clinical examination and CT or PET-CT imaging, were scheduled every 3 months for the first 2 years, every 6 months for another 3 years, and annually thereafter. Any findings on imaging suspected of being recurrent disease were evaluated by a multidisciplinary team, and biopsy with molecular profiling was strongly recommended to confirm recurrence.
Archived available baseline tumor-tissue samples were tested for PD-L1 and molecular profiling with standard procedures14 and those described in the Supplement. However, enrollment was not restricted to any threshold for PD-L1 expression level or driver mutation status.
Outcomes
The primary endpoint was 4-year event-free survival (EFS) rate for patients who had been randomized and received assigned therapy (Figure 1), which encompassed local recurrence (in-field regrowth or new disease anywhere in the same lobe), regional recurrence (any intrathoracic lymph node), distant metastasis (all extrathoracic areas, as well as lung disease in any separate lobe), second primary lung cancer (SPLC)9,20, or death from any cause. Any recurrence and SPLC were analyzed per protocol design metrics, and subgroup analysis was intended to be exploratory.
Figure 1.
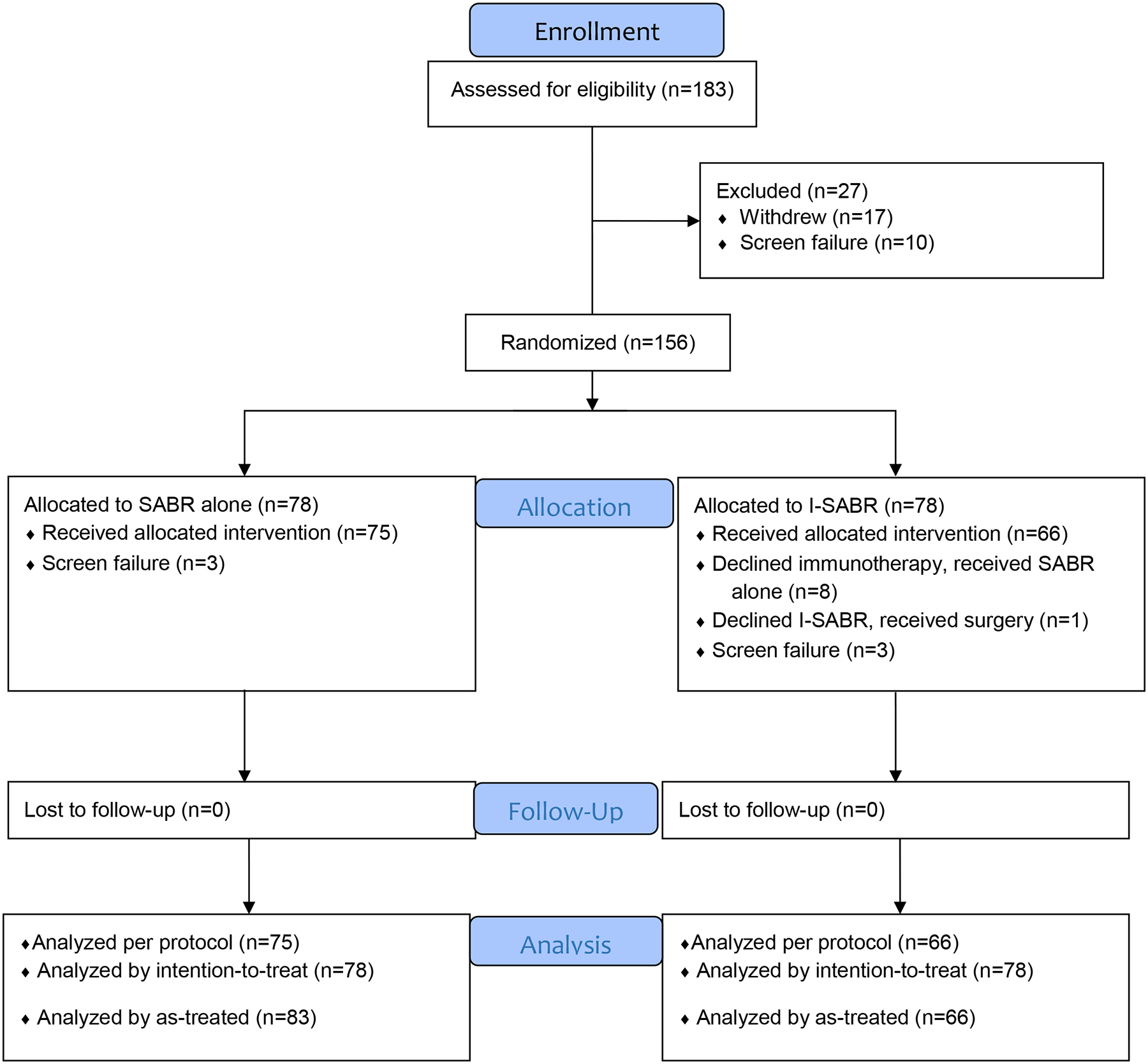
I-SABR CONSORT diagram.
Although the study was not blinded, potential recurrences as demonstrated by chest CT and PET/CT were reviewed by independent radiologists who were not part of the research team or treating physicians, and were not aware of treatment assignment. Histological confirmation of recurrence could not be mandated but was strongly recommended after image-based identification of recurrence.
Patients who were alive without recurrence were censored at the time of most recent follow-up or 4 years after randomization, whichever occurred first. Key secondary analyses were EFS rate in the intention-to-treat (ITT) population and adverse events (per the Common Terminology Criteria for Adverse Events version 4.0). All time-to-event endpoints were calculated from the randomization date. At the time of publication, overall survival data are not yet mature, and the secondary endpoints of immune-related biomarkers and image-based radiomics analyses will be reported in the near future.
Statistical Analysis
From prior prospective data, the historical 4-year cumulative event rate for SABR alone was 46%.9 For the I-SABR trial, an event rate of 23% was considered clinically significant; based on the assumption that the time-to-event followed an exponential distribution, the corresponding hazard ratio (HR) was calculated as 0.424 for patients who were randomized and received assigned therapy. With a one-sided type I error rate of 0.05, an accrual rate of 3.5 patients per month, and an additional 20 months of follow-up, a sample size of 140 patients (70 in each arm) would have 85% power to detect a 23% difference in 4-year EFS. Based on the statistical design using our historical data9, the final efficacy analysis was planned when the number of events (recurrence and/or death) reached 41, which was anticipated to happen by the 4-year timepoint.
An interim analysis test was 0.303, which was not less than or equal to 0.006 (the cutoff p-value for stopping the trial due to superiority), and not larger than or equal to 0.417 (the cutoff p-value for stopping the trial due to futility). The boundary was therefore not crossed, and the MD Anderson Data Safety Committee approved the study to continue as planned.
Toxicity (per the Common Terminology Criteria for Adverse Events, version 4.0) was monitored for the incidence of grade ≥2 adverse effects related to SABR or nivolumab. Bayesian stopping boundaries, calculated based on the beta-binomial distribution, were implemented to prematurely cease accrual if excessive toxicity (defined as a cumulative probability >14%) was detected as accrual progressed.
The chi-squared test (for categorical variables) or Wilcoxon rank sum test (for continuous variables) was used to assess differences in baseline characteristics between arms. The Kaplan-Meier method was used to estimate EFS, which was compared with log-rank tests. Univariable and multivariable Cox proportional hazards models were used to determine the effects of I-SABR on EFS without or with adjustment for other prognostic factors. Analyses were performed in both the per-protocol population as well as the intention-to-treat (ITT) population. All tests were two-sided, and P values less than 0.05 were considered statistically significant. Statistical software utilized for all analyses were SAS 9.4 (SAS, Cary, NC), R version 4.2.2 (R Foundation for Statistical Computing), and S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA).
Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication. All of the authors participated in writing the manuscript and provided approval to submit the manuscript for publication.
RESULTS
From June 30, 2017 to March 22, 2022, 156 patients were randomized (ITT population: n=78 each) and 141 patients (per-protocol population: n=75 SABR, n=66 I-SABR) from 19 states of the USA treated at three different facilities were enrolled to the I-SABR trial (Figure 1). No significant differences were found in age, sex, performance status, tumor histology, tumor size, smoking status, or recurrent vs newly diagnosed disease between the two arms (Table 1; Supplemental Table 1). There were 28 patients with isolated locally recurrent disease, and the median time from initial diagnosis to recurrence was 46.4 months (range, 15.6–111.1 months). Of note, 32 patients were potential surgical candidates but declined further evaluation for surgery and were then enrolled onto this trial (16 I-SABR, 16 SABR).
Table 1.
Baseline Characteristics of the Trial Population.
| Characteristics | SABR, No. (%) | I-SABR, No. (%) |
|---|---|---|
| Sex | ||
| Female | 41 (55) | 46 (70) |
| Male | 34 (45) | 20 (30) |
| Race | ||
| White | 64 (85) | 62 (94) |
| Non-White | 11 (15) | 4 (6) |
| Age, years | ||
| Median (IQR) | 72 (66–78) | 72 (66–75) |
| Smoking Status | ||
| Never | 7 (9) | 7 (11) |
| Current/Previous | 68 (91) | 59 (89) |
| ECOG Performance Status Score | ||
| 0–1 | 68 (91) | 62 (94) |
| 2 | 7 (9) | 4 (6) |
| Tumor Histology | ||
| Non-squamous carcinoma | 61 (81) | 55 (83) |
| Squamous cell carcinoma | 14 (19) | 11 (17) |
| Tumor Size, cm | ||
| Median (IQR) | 1.7 (1.3–2.2) | 2.0 (1.4–2.6) |
| ≤2 cm | 51 (68) | 35 (53) |
| 2–3 cm | 16 (21) | 22 (33) |
| 3–5 cm | 8 (11) | 9 (14) |
| Volume of GTV, cc | ||
| Median (IQR) | 4.2 (2.4–9.1) | 6.4 (2.5–15.1) |
| Lung Cancer History | ||
| Newly diagnosed | 63 (84) | 50 (76) |
| Recurrent | 12 (16) | 16 (24) |
| Single lesion | 74 (99) | 62 (94) |
| Two lesions | 1 (1) | 4 (6) |
| SABR Regimen | ||
| 50 Gy in 4 fractions | 63 (84) | 59 (89) |
| 70 Gy in 10 fractions | 12 (16) | 7 (11) |
| No of Nivolumab Cycles, | N/A | |
| median (IQR) | 4 (4–4) | |
| ≤ 2 | 11 (17) | |
| >2 | 55 (83) | |
| PD-L1 status | ||
| <1% | 34 (45) | 27 (41) |
| ≥1% | 16 (21) | 15 (23) |
| Unknown | 25 (33) | 24 (36) |
| EGFR status | ||
| Wild-type | 22 (29) | 25 (38) |
| Mutated | 3 (4) | 1 (2) |
| Unknown | 50 (67) | 40 (61) |
| Underwent EBUS | ||
| Yes | 50 (65) | 43 (65) |
| No | 25 (35) | 23 (35) |
| Received Brain MRI | ||
| Yes | 40 (53) | 34 (52) |
| No | 35 (47) | 32 (48) |
Abbreviations: SABR, stereotactic ablative radiotherapy; I-SABR, immunotherapy + stereotactic ablative radiotherapy; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; GTV, gross tumor volume; Gy, Gray; N/A, not applicable; PD-L1, programmed cell death ligand-1; EGFR, epidermal growth factor receptor
The median follow-up time was 33 months (95% confidence interval [CI] 28.7–38.1). In the per-protocol analysis, the 4-year EFS rate in the SABR arm was 53% (95% CI 42%–67%) and 77% in the I-SABR arm (95% CI 66%–91%) with an HR of 0.38 (95% CI 0.19–0.75), a statistically significant difference (P=0.006) (Figure 2A). After adjusting for the 4 stratification variables (ECOG performance status, tumor size, lung cancer history, and histology) used in the minimisation procedure, the EFS benefit of I-SABR remained significant (HR 0.36, 95% CI 0.18–0.74, p=0.005, Supplemental Table 2). On Cox univariable analysis, receiving nivolumab and female gender were associated with longer EFS (P<0.05; Supplemental Table 3); after adjustment for both variables in Cox multivariable analysis, both remained significantly associated with EFS (P<0.05 for both; Supplemental Table 4). Post-hoc subgroup analysis revealed that EFS time was longer after I-SABR than after SABR, particularly for men, patients aged ≤72 years (median age), with ECOG performance status 0–1, newly diagnosed disease, squamous cell histology, and tumors ≤2 cm (Figure 3). Further analysis of the primary endpoint, I-SABR vs. SABR, in the ITT population revealed confirmatory results (HR 0.42, 95% CI 0.22–0.80, P=0.008, Figure 2B). An exploratory as-treated analysis (83 SABR, 66 I-SABR) demonstrated similar results (HR 0.43, 95% CI 0.23–0.81, P=0.01 [Supplemental Figure 1]).
Figure 2. Kaplan-Meier Event-Free Survival (EFS).
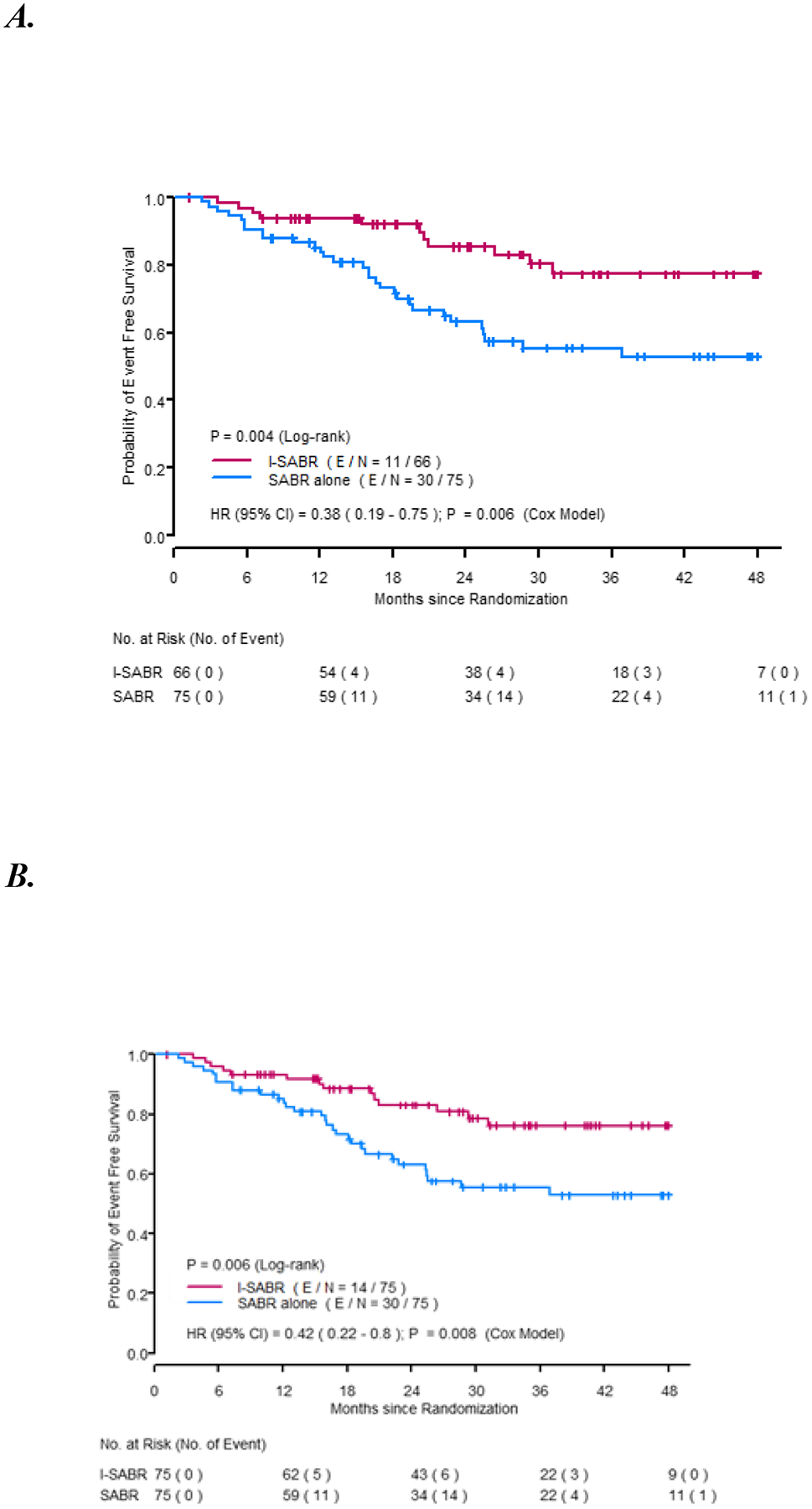
A. EFS for patients who were randomized and treated based on protocol (n=141). B. EFS for randomized Intent-To-Treat population (n=150).
Figure 3.
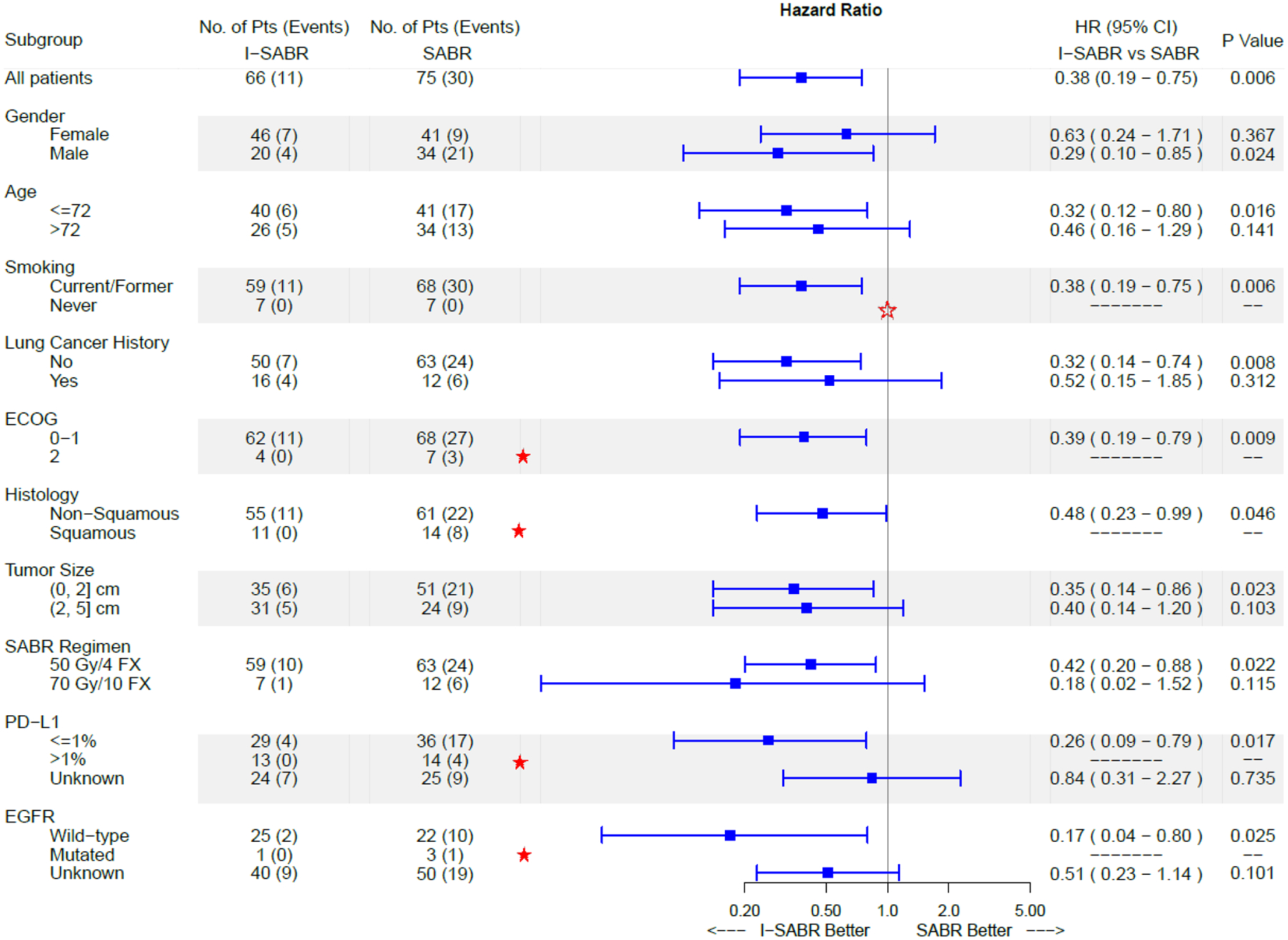
Forest Plot of I-SABR vs SABR for EFS in Subgroup Analysis.
★ Because no events occurred in the I-SABR group in the subgroups with ECOG=2, Histology=squamous, PD-L1≥1%, and EGFR=mutated, the hazard ratios and 95% confidence intervals could not be estimated. However, events did take place in the SABR group, suggesting that I-SABR has benefit over SABR in these subgroups.
Notably, the estimated benefit was more reliable in subgroups with adequate sample sizes (e.g., those with squamous histology and PD-L1≥1%).
☆ Because no events occurred in either the I-SABR or the SABR groups in never-smokers, the hazard ratio and 95% confidence interval could not be estimated.
We then conducted an additional exploratory analysis to verify the benefit of I-SABR vs. SABR alone based on tumor size and disease status (Supplemental Tables 5–8). The results showed significant EFS improvement in patients with tumors ≤2 cm (HR 0.35, 95% CI 0.14–0.86, P=0.023). However, for patients with tumor >2 cm, a nonsignificant trend towards improvement was observed (HR 0.40, 95% CI 0.14–1.20, P=0.103). There was no impact on size of the gross tumor volume on EFS (HR 0.99, 95% CI 0.98–1.02, P=0.895). Additionally, there was a significant improvement in EFS for patients with treatment-naïve early-stage disease (HR 0.32, 95% CI 0.14–0.74, P=0.008); however, in patients with isolated parenchymal recurrent NSCLC, there was no discernible different on account of low case numbers (n=28, HR 0.52, 95% CI 0.15–1.85, P=0.312).
SABR was not associated with any grade ≥3 adverse events; 10 (15.2%) patients in the I-SABR group had grade 3 events, the most common of which was fatigue (p<0.001, Table 2). Fatigue was also the most common toxicity that led to discontinuation of nivolumab because patients declined to come back for additional cycles. Three (4%) patients in the SABR arm had grade 2 events. No patient in either cohort prematurely discontinued SABR. Toxicity necessitated withholding or delaying nivolumab for the subsequent cycle in 5 (7.6%) patients; nivolumab was discontinued in the other 5 (7.6%) due to toxicity concerns. All patients recovered from grade 3 toxicities. Sixteen (24.2%) patients experienced grade 2 events; among them, nivolumab was withheld or delayed for 2 (3%) patients and discontinued for 1 (1.5%) patient at the physician’s discretion. No grade ≥3 pneumonitis was experienced in the I-SABR group. No instances of grade 4+ toxicities were reported in either arm.
Table 2.
Grade ≥2 Adverse Events Possibly, Probably, or Definitively Related to Therapy.
| Adverse Event | Grade 2 | Grade 3 | ||
|---|---|---|---|---|
| SABR, No. of Events | I-SABR, No. of Events | SABR, No. of Events | I-SABR, No. of Events | |
| Acute kidney injury | 1 | |||
| Adrenal insufficiency | 1 | |||
| Anorexia | 1 | |||
| Arthralgia | 2 | |||
| Blurred vision | 1 | |||
| Conjunctivitis | 1 | |||
| Diarrhea | 1 | |||
| Dyspnea | 1 | |||
| Fatigue | 1 | 7 | 2 | |
| Hyperthyroidism | 1 | |||
| Hypoxia | 1 | |||
| Hepatitis (acute) | 1 | |||
| Myalgia | 1 | |||
| Oral mucositis | 1 | |||
| Oral dysesthesia | 1 | |||
| Pneumonia (infectious) | 1 | |||
| Pneumonitis | 1 | 2 | ||
| Pruritus | 2 | |||
| Rash | 2 | 1 | ||
| Xeroophthalmia | 1 | |||
| Xerostomia | 1 | |||
Abbreviations: SABR, stereotactic ablative radiotherapy; I-SABR, immunotherapy + stereotactic ablative radiotherapy.
No grade 4–5 adverse events occurred.
Overall, the crude rates of recurrence were lower in the I-SABR arm, and Supplemental Table 9 shows a complete tabulation of patterns of failure in both arms. Recurrence of any type as the first event occurred in 36.0% (27/75) of patients in the SABR arm compared with 12.1% (8/66) of the I-SABR arm. Local recurrences were observed in 13.3% (10/75) with SABR vs. 0% (0/66) with I-SABR. Regional recurrences were observed in 10.7% (8/75) and 6.1% (4/66), respectively. Distant recurrences occurred in 16.0% (12/75) and 3.0% (2/66), respectively. With SABR, 10.7% (8/75) had more than one type of recurrence (e.g. local, regional and distant), compared to 0% (0/66) with I-SABR. SPLC was observed in 8.0% (6/75) and 3.0% (2/66), respectively. 13 patients had died (9 SABR and 4 I-SABR).
PD-L1 expression status and EGFR mutation status were available for 92/141 (65.2%) and 51/141 (36.2%) of patients, respectively. In an exploratory subgroup analysis, the EFS benefit of I-SABR was more pronounced for tumor PD-L1-positive patients, and no such patient developed recurrence (0/15 I-SABR vs 5/16 SABR, Figure 3); however, the EFS benefit from I-SABR remained statistically significant in PD-L1-negative cases (n=61, HR 0.27, 95% CI 0.09–0.81, P=0.012). No conclusions regarding the relative benefit of I-SABR in patients bearing EGFR mutations could be drawn due to the small size of this subgroup caused by limited tissue amount from fine-needle aspiration in this patient population (Figure 3). The subgroup analyses were performed for exploratory purposes and were not pre-specified in the protocol analysis; therefore, they should be interpreted with caution.
DISCUSSION
I-SABR is the first randomized trial to evaluate the therapeutic strategy of combining immunotherapy and SABR for newly diagnosed early-stage or recurrent NSCLC. I-SABR led to significant improvements in EFS over SABR alone with an EFS rate of 77% vs 53% at 4 years, and a HR of 0.38 (95% CI 0.19–0.75, P=0.006) in the primary study population, corresponding to a 62% reduction in the risk of recurrence, disease progression, or death. Benefit appeared to occur across major subgroups, including for PD-L1-negative cases. Considering the emerging role of targeted/immunotherapy as less toxic and more effective for resected early-stage NSCLC15–18,23, the current study adds the highest quality data thus far for immunotherapy in the setting of early-stage NSCLC patients treated with SABR as well as for isolated pulmonary recurrences.
In the SABR arm, rates of first relapse were 13.3% local, 10.7% regional, and 16.0% distant, and the corresponding rates for the I-SABR arm were 0%, 6.1%, and 3.0%. With the addition of just 4 cycles of immunotherapy, I-SABR reduced the rate of any type of recurrence from 36.0% to 12.1%, and mortality from 12.0% to 6.1% at 4 years. There was also a lower rate of second primary lung cancers, which were observed in 8% and 3% in the SABR and I-SABR arms, respectively. Although the patterns of recurrence in the SABR arm are consistent with published literature, the failure rates are much lower in the I-SABR cohort than previously reported SABR literature.1–5,8–9,20 This is in keeping with the finding of a “tail” on the Kaplan-Meier curve of the I-SABR arm (Figure 2A–B) herein, indicating not only durable disease control but also cure in some patients (without further recurrences after the 2–3 year mark). Despite differing patient populations, these results appear lower than those for patients with lower-risk NSCLC undergoing lobectomy or segmentectomy and mediastinal nodal dissection at our institution.1,2 In a prospective randomized study comparing lobectomy vs segmentectomy/wedge resection in ≤2 cm (T1aN0M0) NSCLC, overall recurrence was about 30% with an additional 18% SPLC at 5 years.24 These findings support our hypothesis that the I-SABR strategy of combining immunotherapy with the immune-galvanizing effects of SABR can reduce recurrence and increase cure rates.13 As such, it may be interesting to explore the effectiveness of I-SABR compared to immunotherapy and surgery for patients with resectable, early-stage NSCLC.
Most patients in the current study were not eligible for surgery due to comorbid conditions. Initially, the primary concern for I-SABR was immune-mediated toxicity, which was the main reason why some patients randomized to I-SABR declined immunotherapy at the initial phase (Figure 1). However, our findings showed good tolerance of I-SABR, with no grade 4+ toxicity, 9.1% toxicity-related discontinuation of immunotherapy, and no grade 3+ pneumonitis, which had been a major concern for concurrent SABR and immunotherapy. This toxicity profile is comparable to that of PD-1 immunotherapy alone for stage IV NSCLC.14
In the current study, patients with PD-L1-negative tumors appeared to benefit from the addition of nivolumab, although PD-1 or PD-L1 inhibitor monotherapy has limited efficacy in the PD-L1 negative stage IV NSCLC population.14 One plausible explanation is that SABR can induce PD-L1 expression in some patients, thereby rendering combinations of PD-(L)1 inhibitors plus SABR beneficial for some PD-L1-negative tumors, as was shown in this study and corroborated by translational work,12 the neoadjuvant setting of SABR plus durvalumab,25 and SABR plus pembrolizumab for stage IV NSCLC.26–27 These randomized phase II data in stage IV NSCLC suggests that combining immunotherapy with SABR (with 50 Gy in 4 fractions) may improve clinical outcomes and objective tumor responses, even outside the radiation field (i.e. the abscopal effect).26–27 However, owing to the post-hoc nature of the PD-L1 analysis herein, and the lack of mandatory PD-L1 level testing in this trial, no firm conclusions can be made, and further randomized data to corroborate the efficacy in PD-L1 negative cases is still needed.
Despite just 3 months of immunotherapy, we observed about a 60% reduction of the likelihood of EFS (HR 0.38). While direct comparison among trials should be hypothesis-generating at best, of note, the HR for EFS benefit for adjuvant PD-L1 (Impower01017) was 0.79, 0.76 for PD-1 immunotherapy after surgical resection in stage II/IIIA NSCLC (KeyNote-09118), 0.63 for neoadjuvant immunotherapy in operable stage IB-IIIA NSCLC (CheckMate 81616), and 0.52 for PD-L1 immunotherapy for medically inoperable stage III NSCLC treated with definitive concurrent chemotherapy and conventionally fractionated radiotherapy (PACIFIC19). One potential explanation for the large magnitude of benefit observed for the I-SABR regimen may be the strong immune stimulation resulting from ablative RT dosing.12,13 Another explanation could be that the delivery of SABR within 10 days could minimize lymphopenia compared to 30 daily RT sessions28; irradiating smaller target volumes, in particular avoiding irradiation of lymph nodes, may also be important for improving the CD8 T-cell response to cancer immunotherapy.29 Another possibility is the timing of immunotherapy and RT; in this trial, nivolumab was started during SABR, as opposed to several weeks after RT in the PACIFIC trial.19 These aspects may carry important points for future studies, including for stage IV disease.
The current study provides the first clinical evidence that nivolumab could be an effective treatment for early-stage lung cancer when combined with ablative SABR dosing to all visible disease. Our study also supports the I-SABR regimen for patients with recurrent disease, another relatively common clinical scenario for which SABR is standard treatment. These promising results support the proposal that ablative doses (BED>100 Gy) should be considered to achieve optimal clinical outcomes12–13 and provide effective immune stimulation.30
The current study had a few limitations. First, it had limited sample size, and while SABR was considered a standard treatment for the study population, there was some heterogeneity in terms of tumor size, prior therapy, multiple lesions, and histologies. Second, it was a phase 2 study performed at a limited number of hospitals, was not double-blinded or placebo-controlled, did not utilize blinded independent central review of imaging, and therefore does not obviate the need for large-volume multicenter phase 3 studies (e.g., NCT03833154, NCT03924869, NCT04214262). Third, our study does not have adequate statistical power to address the efficacy of the I-SABR regimen for patients bearing EGFR mutations or other oncogenic drivers for which targeted agents are known to be effective in the metastatic setting. Given the efficacy of osimertinib in the adjuvant setting after surgery in patients with stage IB-IIIA NSCLC bearing EGFR mutations,23 future trials investigating the use of targeted agents in conjunction with SABR for NSCLC patients bearing such oncogenic drivers merit consideration. Fourth, PD-L1 testing was not mandated, thereby limiting interpretation of the impact of PD-L1 levels on efficacy. Lastly, overall survival data were not mature at the time of publication and will be reported separately.
In summary, this randomized phase II trial demonstrated that for newly diagnosed early-stage or isolated parenchymal recurrent node-negative NSCLC, I-SABR significantly improved EFS and may be a treatment option for such patients. The results of phase III investigations are anticipated in order to confirm the findings presented herein.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence Before this Study
We searched PubMed for published studies, without date or language restrictions, regarding the role of immunotherapy following stereotactic ablative radiotherapy (SABR; also known as SBRT) for early-stage non-small cell lung cancer (NSCLC) or for isolated parenchymal recurrence (node-negative) NSCLC. Searches were intentionally broad and contained the terms “lung cancer” AND “stereotactic radiation” OR “stereotactic radiotherapy” AND “immunotherapy” OR “immune checkpoint” OR “nivolumab” OR “pembrolizumab” OR “durvalumab” OR “atezolizumab” OR “ipilimumab” OR “avelumab”. Although retrospective studies and a phase I investigation were found, no single-arm phase II or randomized trials were found.
Added Value of this Study
To our knowledge, no randomized data evaluating SABR vs immunotherapy plus SABR exist to date. Results of this trial are the first to show that the addition of immunotherapy to SABR improve outcomes for treatment-naïve early-stage or isolated parenchymal recurrent NSCLC. This trial also provides additional randomized evidence supporting the efficacy and safety of combined immunotherapy and radiotherapy.
Implications of All the Available Evidence
Collectively, the results of this trial imply that combined-modality I-SABR may represent an enhanced therapeutic option for early-stage NSCLC or lung parenchymal recurrences, and provide an important precedent as several phase III trials in this population continue to accrue.
ACKNOWLEDGEMENTS
We thank Ms. Christine Wogan, MS, ELS, of MD Anderson’s Division of Radiation Oncology for editorial help. We thank Ms. Rachel C Maguire and Ms. Luyang Yao for their help of patient screening, enrollment, and data collection. This research was supported by Bristol-Myers Squibb-MD Anderson Cancer Center Alliance, the National Cancer Institute at the National Institutes of Health through Cancer Center Core Support Grant (Grant No. P30CA016672, used the Clinical Trials Support Resource) and through Clinical and Translational Science Award (Grant No. UL1 RR024148) to MD Anderson Cancer Center. The principal investigator of I-SABR is a recipient of Texas 4000 Distinguished Professorship at MD Anderson Cancer Center and the Joan and Herb Kelleher Charitable Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
There are no direct conflicts of interest (COIs) related to this project. Other potential COIs are listed in the COI forms.
Conflicts of Interest: COI forms attached.
DATA SHARING
De-identified patient data from this study along with a data dictionary can be made available after a signed data access agreement upon reasonable request to the principal investigator of the trial.
REFERENCES
- 1.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang JY, Mehran RJ, Feng L, et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol 2021;22:1448–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 2019;20:494–503. [DOI] [PubMed] [Google Scholar]
- 4.Sun B, Brooks ED, Komaki R, et al. Long-term outcomes of salvage stereotactic ablative radiotherapy for isolated lung recurrence of Non-Small Cell Lung Cancer: A phase II clinical trial. J Thorac Oncol 2017;12:983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmerman RD, Hu C, Michalski JM, et al. Long-term results of stereotactic body radiation therapy in medically inoperable stage I non-small cell lung cancer. JAMA Oncol 2018;4:1287–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma V, Shostrom VK, Kumar SS, et al. Multi-institutional experience of stereotactic body radiotherapy for large (≥5 centimeters) non-small cell lung tumors. Cancer 2017;123:688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trakul N, Harris JP, Le QT, et al. Stereotactic ablative radiotherapy for reirradiation of locally recurrent lung tumors. J Thorac Oncol 2012;7:1462–1465. [DOI] [PubMed] [Google Scholar]
- 8.Senthi S, Lagerwaard FJ, Haasbeek CJA, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802–809. [DOI] [PubMed] [Google Scholar]
- 9.Sun B, Brooks ED, Komaki R, et al. 7-year Follow-Up Outcomes after Stereotactic Ablation Radiotherapy for Stage I NSCLC: Results of a Phase II Clinical Trial. Cancer 2017;123:3031–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719–727. [DOI] [PubMed] [Google Scholar]
- 11.Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010;28:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nature Communication 2017;8:15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol 2016;13:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819–1830. [DOI] [PubMed] [Google Scholar]
- 15.Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022;386:1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage Non-Small-Cell Lung Cancer. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398:1344–1357. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien M, Paz-Ares L, Marreaud S, et al. : Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022; 23:1274–1286. [DOI] [PubMed] [Google Scholar]
- 19.Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC trial: Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. J Clin Oncol 2022;40:1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks ED, Sun B, Feng L, et al. Association of long-term outcomes and survival with multidisciplinary salvage treatment for local and regional recurrence after stereotactic ablative radiotherapy for early-stage lung cancer. JAMA Netw Open 2018;1:e181390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–115. [PubMed] [Google Scholar]
- 22.Chang JY, Bezjak A, Mornex F, et al. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. J Thorac Oncol 2015;10:577–585. [DOI] [PubMed] [Google Scholar]
- 23.Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711–1723. [DOI] [PubMed] [Google Scholar]
- 24.Altorki N, Wang X, Kozono D, et al. Lobar or sublobar resection for peripheral stage IA non–small-cell lung cancer. N Engl J Med 2023;388:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altorki NK, McGraw TE, Borczu AC, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol 2021;22:824–835. [DOI] [PubMed] [Google Scholar]
- 26.Welsh J, Menon H, Chen D, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer 2020;8:e001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med 2021;9:467–475. [DOI] [PubMed] [Google Scholar]
- 28.Abravan A, Faivre-Finn C, Kennedy J, McWilliam A, van Herk M. Radiotherapy-related lymphopenia affects overall survival in patients with lung cancer. J Thorac Oncol 2020;36:1624–1635. [DOI] [PubMed] [Google Scholar]
- 29.Rahim MK, Okholm TLH, Jones KB, et al. Dynamic CD8+ T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell 2023;186:1127–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demaria S, Guha C, Schoenfeld J, et al. Radiation dose and fraction in immunotherapy: one-size regimen does not fit all settings, so how does one choose? J Immunother Cancer 2021;9:e002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified patient data from this study along with a data dictionary can be made available after a signed data access agreement upon reasonable request to the principal investigator of the trial.


