Abstract
Intra-amniotic inflammation leading to preterm birth is one of the leading causes of neonatal morbidity and mortality. We recently reported that the mitochondrial levels of MNRR1 (Mitochondrial Nuclear Retrograde, Regulator 1; also called CHCHD2, AAG10, or PARK22), an important bi-organellar regulator of cellular function, are reduced in the context of inflammation and that genetic and pharmacological increases in MNRR1 levels can counter the inflammatory profile. Herein, we show that nitazoxanide, a clinically approved drug, is an activator of MNRR1 and abrogates preterm birth in a well-characterized murine model caused by intra-amniotic lipopolysaccharide (LPS) injection.
Keywords: CHCHD2, Decidua, Intra-amniotic infection, Intra-amniotic inflammation, Nitazoxanide (Alinia), Prematurity, Preterm labor
1. Introduction
Intra-amniotic inflammation leading to preterm birth is one of the primary causes of neonatal morbidity and mortality [1–3]. Intra-amniotic inflammation results from ascending microbial invasion from the lower genital tract to the amniotic cavity, termed intra-amniotic infection, or from endogenous danger signals (i.e., alar-mins) released upon cellular damage or stress, known as sterile intra-amniotic inflammation [3–5]. Although immune cells are known to play a key role in the response to inflammation at the fetal-maternal interface [6,7], the specific mechanisms underlying the response of cells in the placental tissues to inflammatory insults are largely unknown [8]. We recently identified a non-canonical, TLR4-independent signaling pathway involving mitochondria in placental cells that contributes to immune pathology [9]. Using in vitro and in vivo models, we found that the mitochondrial levels of MNRR1 (Mitochondrial Nuclear Retrograde, Regulator 1; also called CHCHD2, AAG10, or PARK22), an important bi-organellar regulator of cellular function [10–14], are reduced in the context of inflammation. However, whether MNRR1 is implicated in the pathophysiology of intra-amniotic inflammation-induced preterm labor and birth has not been well explored.
MNRR1 functions in two cellular compartments, the mitochondria and the nucleus. Mitochondrial MNRR1 binds to cytochrome c oxidase [11] or Bcl-xL [15] to regulate the mitochondrial roles of energy generation and apoptosis, respectively. Nuclear MNRR1 can function as a transcriptional regulator to modulate the activation of stress-responsive genes including MNRR1 itself [11,16]. This novel LPS-induced inflammatory signaling pathway was characterized in vitro by using a human trophoblast cell line (HTR8/SVneo) [9]. The pro-inflammatory pathway is initiated by activation of NOX2, generating radical oxygen species (ROS) that activate ATM kinase, which then phosphorylates and thereby stabilizes YME1L1, a mitochondrial intermembrane space protease that subsequently reduces mitochondrial MNRR1 levels. Furthermore, overexpression of MNRR1 was shown to dampen this inflammatory signaling pathway and to restore mitochondrial function.
Given the observed beneficial effects of restoring MNRR1 expression in vitro, we recently performed a screen of FDA-approved drugs to identify pharmacological activators of MNRR1 and identified nitazoxanide as an inducer of MNRR1 transcription. Moreover, we showed that nitazoxanide could rescue the trophoblast phenotype induced by LPS treatment [9]. Therefore, in the current study, we examined whether nitazoxanide could represent a viable strategy for preventing preterm birth induced by intra-amniotic inflammation caused by the microbial product LPS.
2. Results
2.1. The anti-inflammatory effects of nitazoxanide are mediated via transcriptional activation of MNRR1
Nitazoxanide was identified as an MNRR1 activator from a list of 2400 FDA-approved drugs and well-characterized small molecules and natural products (Fig. 1A). By using orthogonal assays with HTR8 cells, we found that the half-maximal effective concentration (EC50) of nitazoxanide is 0.115 μM (Fig. 1B). To evaluate whether the anti-inflammatory effects of this compound are mediated via activation of MNRR1, we tested the MNRR1 dependence of the ability of nitazoxanide to mitigate the LPS-induced inflammatory response in HTR8 cells. We found that activation of TNF transcription, a previously described effect of LPS exposure in HTR8 cells [9], was reduced to control levels in an MNRR1-dependent fashion (Fig. 1C). There was also a basal increase in TNF transcription after MNRR1 knockout that was restored to control levels upon reintroduction of MNRR1. Since the placental cells need to communicate this signal to activate an immune cell response, we analyzed the cytokine profile of HTR8/SVneo cells. We used a cytokine array to identify differences in control and LPS-treated cells. We found that the levels of the pro-inflammatory cytokine IL-6 are increased (Fig. 1D) as also observed by other studies [17,18]. We also identified that two important chemokines that can attract immune cells, IL-8 [19] and CXCL1 [20], are increased (Fig. 1D). Such findings may represent a novel mechanism by which placental cells can secrete immune modulators to attract immune cells.
Fig. 1.
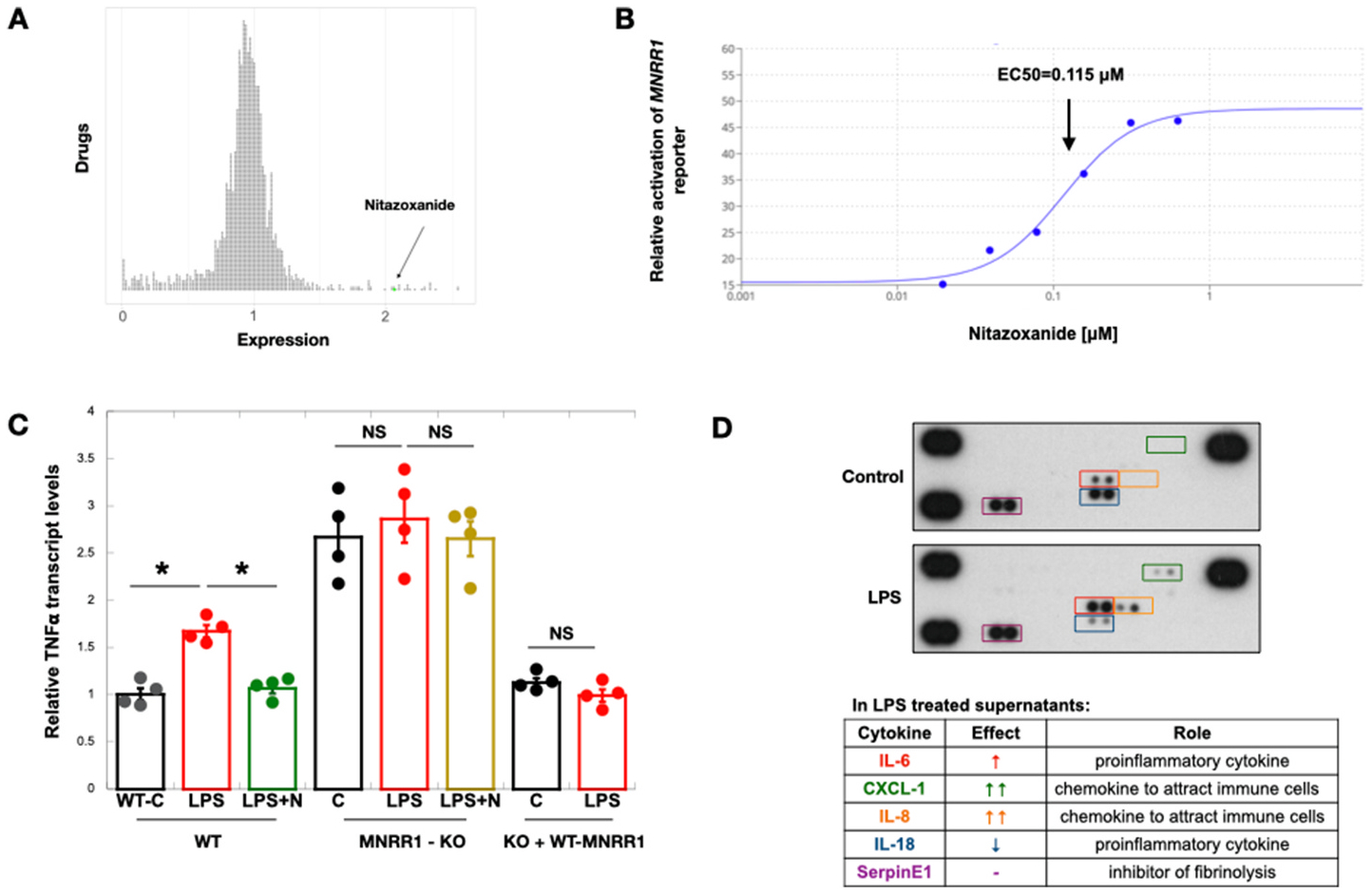
Nitazoxanide’s anti-inflammatory effects are via transcriptional activation of MNRR1.
A: Results of a screen of ~2400 FDA-approved drugs and natural compounds identified to transcriptionally activate (>1), inhibit (<1), or not affect (=1) MNRR1. Each circle represents one drug and the MNRR1 activator (nitazoxanide (N)) has been highlighted in green.
B: Equal numbers of HTR cells overexpressing the MNRR1-luciferase reporter were plated on a 96 well plate and treated with increasing amounts of nitazoxanide or vehicle (DMSO) for 24 h. EC50 was calculated using the activation of MNRR1 reporter relative to vehicle treated cells using the Quest Graph™ EC50 Calculator.
C: TNF transcript levels were measured in human placental cells. Actin was used as a housekeeping gene for normalization. Abbreviations: WT, MNRR1-WT; MNRR1-KO, knockout of MNRR1; C, control (water); LPS, lipopolysaccharide; N, nitazoxanide; KO + WT-MNRR1, knockout cells + MNRR1-WT overexpression. n = 4 biological replicates; significance: *, p-value<0.05; ns is non-significant.
D: Cell culture supernatants from control (water) or LPS-treated cells were tested as described in Materials and Methods for multiple cytokines using a membrane array. The sample in the bottom right corner is a blank negative control and the remaining
2.2. Activation of MNRR1 using nitazoxanide prevents preterm birth in vivo
We next tested the effects of nitazoxanide in a well-characterized murine model of intra-amniotic LPS injection that results in inflammation and preterm birth [21–24]. Dams received nitazoxanide orally and ultrasound-guided intra-amniotic injection of LPS as shown in Fig. 2A. Nitazoxanide extended the gestational age, preventing LPS-induced preterm birth [delivery <18.5 days post coitum (dpc)] in all pregnant dams (Fig. 2B). Furthermore, neonatal mortality at birth was also significantly reduced in dams treated with nitazoxanide compared to controls (Fig. 2C). When we assessed neonatal mortality for up to 3 weeks, all neonates from the LPS-injected dams died within the first week of life, whereas almost one-half of the neonates born to dams treated with nitazoxanide survived up to 3 weeks (Fig. 2D). Finally, we used a separate group of mice to show that nitazoxanide treatment restored the LPS-induced reduction of MNRR1 at the transcript level in the decidua (Fig. 2E) and at the protein level in the placenta (Fig. 2F). To assess MNRR1 transcript and protein levels, separate animals were used from those where we observed outcomes until delivery. We also assessed the transcript levels of several inflammatory factors such as Il6, Il1b, Tnf, and Tlr4, among others, in the decidual samples and found an increased expression in dams intra-amniotically injected with LPS that was abrogated upon treatment with nitazoxanide (Supplementary Figs. 1A and B).
Fig. 2.
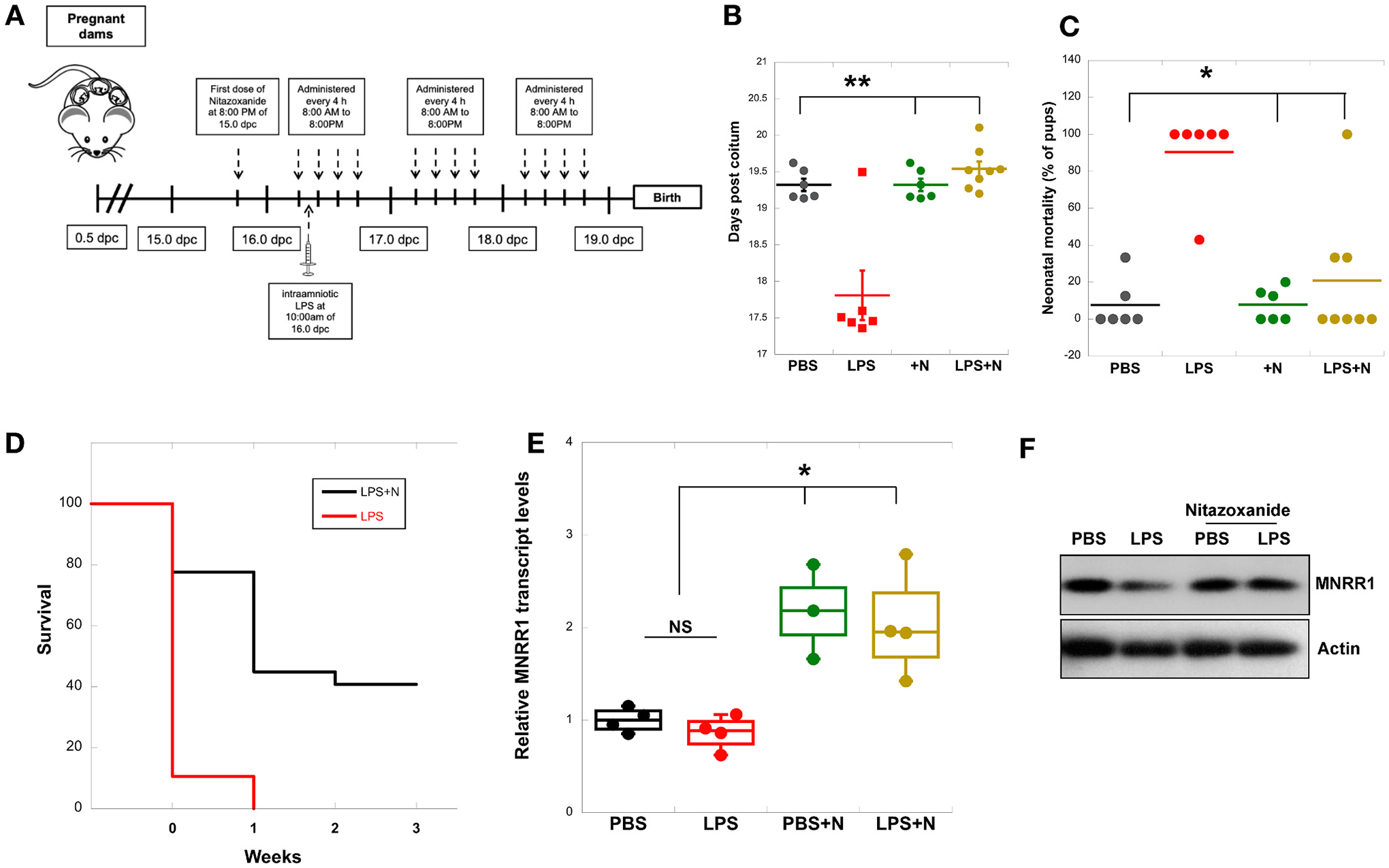
Nitazoxanide prevents preterm delivery and improves neonatal mortality in vivo.
A: Schematic illustration depicting the administration of nitazoxanide (Alinia) and LPS in the murine model of preterm birth.
B: Gestational length of mice was obtained using video-monitoring until delivery (n = 6–8 animals).
C: Neonatal mortality was determined by calculating the number of dead pups over total litter size (n = 6–8 animals).
D: Survival rates of neonates born to dams intra-amniotically injected with LPS (red line) or intra-amniotically injected with LPS and treated with nitazoxanide (black line) up to 3 weeks of age are displayed as Kaplan-Meier survival curves. No pups from LPS-injected dams survived beyond week 0 of birth. The statistical comparison for week 0 is shown in Fig. 1C.
E: MNRR1 transcript levels were measured in mouse decidual tissue (17.5 dpc). Actin was used as a housekeeping gene to normalize expression (n = 4 animals for all except PBS + N, where n = 3).
F: Equal amounts of whole placental lysates were separated on an SDS-PAGE gel and probed for MNRR1 levels. Actin was used as a loading control. Gel is representative of data obtained from 2 animals.
Figure labels for B, C, and E are: PBS, phosphate buffered saline injection; LPS, lipopolysaccharide injection; PBS + N, PBS injection with oral gavage of nitazoxanide; LPS + N, LPS injection with oral gavage of nitazoxanide. Significance: *, p < 0.05; **, p < 0.01.
3. Discussion
We show here that nitazoxanide, a clinically approved drug sold commercially as Alinia, can abrogate preterm birth in an LPS-based mouse model of intra-amniotic inflammation by stimulating the transcription of MNRR1. We previously showed that LPS treatment produces an inflammatory phenotype in a trophoblast cell line that includes reduced mitochondrial respiration and increased production of ROS and inflammatory cytokines such as TNF [9]. This phenotype included reduced expression of the mitochondrial regulator MNRR1, and stimulation of this factor, either genetically or pharmacologically, with nitazoxanide reversed the inflammatory profile. Consistently, we show here that the anti-inflammatory effects of nitazoxanide in pregnant mice are also mediated through transcriptional activation of MNRR1 since there is no nitazoxanide-mediated benefit in MNRR1 knockout cells (Fig. 1C). There was also a basal increase in TNF transcription after MNRR1 knockout that was restored to control levels upon reintroduction of MNRR1.
Nitazoxanide is currently used as a treatment for several infectious organisms including protozoa, helminths, bacteria, and viruses [25,26]. The molecular target of nitazoxanide is known in protozoa [27] but not in humans. Inflammation-induced preterm birth leads to significant adverse effects on maternal and fetal health [28]. Administration of nitazoxanide in pregnant mice injected with LPS prevented preterm birth and reduced neonatal mortality up to 3 weeks. It also significantly reversed the inflammation induced by LPS in these animals. Hence, this clinically approved drug appears to be worthy of further evaluation for potential use in high-risk pregnancies that may result in preterm birth. Specifically, although recent studies have shown that intra-amniotic infection can be treated with appropriate antibiotics [29–31], sterile intra-amniotic inflammation currently lacks treatment [28], thus nitazoxanide could potentially serve as a therapeutic strategy for this condition.
MNRR1 is known to function in mitochondria and in the nucleus [10–14], and, in a recent cellular model, its nuclear function alone as a transcriptional activator was sufficient to dampen LPS-induced inflammation [9]. However, in animals, a more detailed understanding of its action in activating multiple homeostatic mechanisms [16] may well uncover additional genes of interest as well as its potential utility for treating other inflammatory conditions.
4. Materials and methods
4.1. Methods details
4.1.1. Cell culture and reagents
Cell lines:
All cell media were supplemented with 5% fetal bovine serum (FBS) (Sigma Aldrich, St. Louis, MO, USA) plus Penicillin-Streptomycin (HyClone, Logan, UT, USA). HTR8/SVneo (HTR) cells were cultured in Roswell Park Memorial Institute Medium (RPMI) (HyClone, Logan, UT, USA).
Chemicals: Nitazoxanide was obtained from Selleckchem (Houston, TX, USA) and solubilized in DMSO (used as vehicle control in all cell culture nitazoxanide experiments). Alinia was obtained from All Care Pharmacy (Allen Park, MI, USA). Ultrapure LPS for cell culture experiments (lipopolysaccharide from Escherichia coli 0111: B4) was purchased from Invivogen (San Diego, CA, USA).
Plasmids:
The MNRR1 promoter firefly luciferase reporter plasmid and pRL-SV40 (Renilla) luciferase plasmids (for normalization of firefly luciferase) have been described previously [11]. All plasmids were purified by using the EndoFree plasmid purification kit from Qiagen (Germantown, MD, USA).
4.1.2. Transfections and luciferase reporter assays
HTR cells were transfected with the indicated plasmids, using TransFast transfection reagent (Promega, Madison, WI, USA) according to the manufacturer’s protocol as previously described [9]. Luciferase assays were performed with the dual-luciferase reporter assay kit (Promega, Madison, WI, USA). Transfection efficiency for the MNRR1 reporter was normalized with the co-transfected pRL-SV40 Renilla luciferase expression plasmid [10,12,13]. EC50 was calculated by using the Quest Graph™ EC50 Calculator (AAT Bioquest, Inc., https://www.aatbio.com/tools/ec50-calculator).
4.1.3. Real-time polymerase chain reaction
Total cellular RNA was extracted from mouse placental tissues or HTR8 cells by using a RNeasy Plus Mini Kit (Qiagen, Germantown, MD, USA), according to the manufacturer’s instructions. Complementary DNA (cDNA) was generated by reverse transcriptase polymerase chain reaction (PCR) with the ProtoScript® II First Strand cDNA Synthesis Kit (New England Biolabs, Ipswich, MA, USA). Transcript levels were measured by real-time PCR by using SYBR green on an ABI 7500 system. Real-time analysis was performed by the ΔΔCt method as previously described [12]. The primer sequences were as follows: MNRR1 mouse (forward) 5′-ATGGCCCAGATGGCTACC-3′, MNRR1 mouse (reverse) 3′-CTGGTTCTGAGCACACTCCA-5’; Actin mouse (forward) 5′-TCCTCCCTGGAGAAGAGCTA3′, Actin mouse (reverse) 3′-ACGGATGTCAACGTCACACT-5′, TNF human (forward) 5′-TGTAGCAAACCCTCAAGCTG-3’
TNF human (reverse) 3′-GAGGTTGACCTTGGTCTGGT-5′, Actin human (forward) 5′-CATTAAGGAGAAGCTGTGCT-3′, Actin human (reverse) 3′-GTTGAAGGTAGTTTCGTGGA-5’
4.1.4. Immunoblotting
Immunoblotting was performed as previously described [10,11]. Tissue lysates were prepared by using 50 mM Tris-HCl (pH 8.0) with 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS. All lysis buffers included a protease and phosphatase inhibitor cocktail (Sigma, PPC1010).
4.1.5. Cytokine array
Cell culture supernatants were centrifuged at 1200 rpm to pellet cells and supernatants were used with a membrane array to measure cytokines and chemokines. To do so, the supernatant was collected, mixed with fresh 100% trichloroacetic acid solution in a 4:1 ratio, and incubated for 1 h at 4 °C. After centrifugation at 14,000 rpm for 30 min at 4 °C, the pellet was collected, washed twice with acetone, and centrifuged as above but for only 10 min. The pellet was air-dried, resuspended in array buffer, and again collected by centrifugation. The supernatant was then used for the array per manufacturer’s instructions (Proteome Profiler Array Catalog # ARY005B, Biotechne, Minneapolis, MN, USA). The sample in the bottom right corner is blank (a negative control), and the remaining three corner samples are proprietary positive controls.
4.1.6. Mice
C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA), and bred in the animal care facility at the C.S. Mott Center for Human Growth and Development at Wayne State University (Detroit, MI, USA). Mice were housed under a circadian cycle (12 h light:12 h dark). Eight- to 12-week-old females were mated with males of proven fertility. Female mice were examined daily between 8:00 a.m. and 9:00 a.m. for the presence of a vaginal plug, which indicated 0.5 days post coitum (dpc). Female mice with a vaginal plug were removed from the mating cages and housed separately. A weight gain of ≥2 g confirmed pregnancy at 12.5 dpc. All animal experiments were approved by the Institutional Animal Care and Use Committee at Wayne State University (Protocol No. 21-04-3506).
4.1.7. Animal model of intra-amniotic LPS-induced preterm labor and birth
Nitazoxanide (Alinia; ROMARK, Tampa, FL, USA) was dissolved in sterile injectable water. The nitazoxanide volume was mouse weight dependent (100 μL of nitazoxanide solution at 200 mg/kg was administered for every mouse weight of 10 g). Control mice did not receive carrier solution based on previous validation studies showing no effect of gavage intervention on preterm birth or neonatal mortality. Dams were anesthetized on 16.5 dpc by inhalation of 2–3% (Fluriso™ (Isoflurane, USP), Vetone Boise, ID, USA) and 1–2 L/min of oxygen in an induction chamber. Anesthesia was maintained with a mixture of 1.5–2% isoflurane and 1.5–2 L/min of oxygen. Dams were positioned on a heating pad and stabilized with adhesive tape. Fur removal from the abdomen and thorax was performed by applying Nair cream (Church & Dwight Co., Inc., Ewing, NJ, USA) to those areas. Body temperature was detected with a rectal probe (VisualSonics Inc., Toronto, Ontario, Canada) and maintained in the range of 37 ± 1 °C. Respiratory and heart rates were monitored by electrodes embedded in the heating pad. An ultrasound probe was fixed and mobilized with a mechanical holder, and the transducer was slowly moved toward the abdomen. Ultrasound-guided intra-amniotic injection of lipopolysaccharide of Escherichia coli O111:B4 (LPS; Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 100 ng dissolved in 25 μL of sterile 1x PBS was performed into each amniotic sac by using a 30 G needle (BD PrecisionGlide Needle), and controls were injected with 25 μL of sterile 1x PBS alone. The syringe was stabilized by a mechanical holder (VisualSonics Inc.). Following ultrasound, dams were placed under a heat lamp for recovery (dam regaining normal activity, such as walking and responding), which occurs 10–15 min after removal from anesthesia. Following injection, dams were monitored by using a video camera with infrared light (Sony) until delivery. Video monitoring allowed for determination of gestational length, which was calculated from the presence of the vaginal plug (0.5 dpc) until the observation of the first pup in the cage bedding. Preterm birth was defined as delivery before 18.5 dpc, and its rate was represented by the percentage of dams delivering preterm among the total number of mice injected. The rates of neonatal mortality at birth were calculated as the number of pups found dead among the total litter size.
4.1.8. Nitazoxanide treatment
Dams were treated with 200 mg/kg of nitazoxanide (Alinia, ROMARK, Tampa, FL, USA) via oral gavage at 8 p.m. on 15.5 dpc, again at 8 a.m., 12 p.m., 4 p.m., and 8 p.m. on 16.5 dpc, and then the tissues were collected the morning of 17.5 dpc for molecular experiments. These dams were intra-amniotically injected with either LPS or PBS control at 10 a.m. on 16.5 dpc.
4.1.9. High-throughput RT qPCR
This was performed as previously described [24]. Briefly, total RNA was isolated from decidua with RNeasy Mini Kits (Qiagen), according to the manufacturer’s instructions. RNA concentrations, purity, and integrity were evaluated with the NanoDrop 8000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and the Bioanalyzer 2100 (Agilent Technologies, Wilmington, DE, USA). cDNA was synthesized by using SuperScript IV VILO Master Mix (Invitrogen by Thermo Fisher Scientific Baltics UAB, Vilnius, Lithuania). Gene expression profiling of the tissues was performed on the BioMark System for high-throughput RT-qPCR (Fluidigm, San Francisco, CA, USA) or the ABI 7500 Fast real-time PCR system (Applied Biosystems, Life Technologies Corporation, Pleasanton, CA, USA) with the TaqMan gene expression assays (Applied Biosystems, Life Technologies Corporation). This list has been provided as Supplementary Table 1. Data were normalized by using multiple reference genes (Gusb, Hsp90ab1, Gapdh, and Actb) averaged within each sample, and relative changes in transcript levels were calculated by the ΔΔCt method. Heatmaps were plotted in Excel (Microsoft, Redmond, WA, USA).
4.2. Statistical analyses
Statistical analyses were performed with MSTAT version 6.1.1 (N. Drinkwater, University of Wisconsin, Madison, WI). The two-sided Wilcoxon rank-sum test was applied to determine statistical significance for p-values. Data were considered statistically significant with * for p < 0.05 and ** for p < 0.01. Error bars represent standard error of mean.
Supplementary Material
Acknowledgements
This work was supported by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS) under Contract No. HHSN275201300006C. We thank Dr. Valeria Garcia-Flores for help with the high throughput RT-PCR. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the NIH. This study was conducted at the Perinatology Research Branch, NICHD/NIH/DHHS, in Detroit, Michigan; the Branch has since been renamed as the Pregnancy Research Branch, NICHD/NIH/DHHS.
Footnotes
Declaration of competing interest
The authors state that they have no conflicts to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.placenta.2023.07.005.
References
- [1].Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK, Inflammation in preterm and term labour and delivery, Semin. Fetal Neonatal Med 11 (2006) 317–326, 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR, Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years, Am. J. Obstet. Gynecol 182 (2000) 675–681, 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- [3].Villamor-Martinez E, Alvarez-Fuente M, Ghazi AMT, Degraeuwe P, Zimmermann LJI, Kramer BW, Villamor E, Association of Chorioamnionitis with Bronchopulmonary dysplasia among preterm infants: a systematic review, meta-analysis, and metaregression, JAMA Netw. Open 2 (2019), e1914611, 10.1001/jamanetworkopen.2019.14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, et al. , Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes, Am. J. Reprod. Immunol 72 (2014) 458–474, 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, et al. , Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes, J. Matern. Fetal Neonatal Med 28 (2015) 1394–1409, 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Miller D, Garcia-Flores V, Romero R, Galaz J, Pique-Regi R, Gomez-Lopez N, Single-cell immunobiology of the maternal-fetal interface, J. Immunol 209 (2022) 1450–1464, 10.4049/jimmunol.2200433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M, Immune cells in term and preterm labor, Cell. Mol. Immunol 11 (2014) 571–581, 10.1038/cmi.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cobo T, Kacerovsky M, Jacobsson B, Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes, Am. J. Obstet. Gynecol 211 (2014) 708, 10.1016/j.ajog.2014.06.060. [DOI] [PubMed] [Google Scholar]
- [9].Purandare N, Kunji Y, Xi Y, Romero R, Gomez-Lopez N, Fribley A, Grossman LI, Aras S, Lipopolysaccharide induces placental mitochondrial dysfunction in murine and human systems by reducing MNRR1 levels via a TLR4-independent pathway, iScience 25 (2022), 105342, 10.1016/j.isci.2022.105342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Aras S, Pak O, Sommer N, Finley R Jr., M. Huttemann, N. Weissmann, L. I. Grossman, Oxygen-dependent expression of cytochrome c oxidase subunit 4–2 gene expression is mediated by transcription factors RBPJ, CXXC5 and CHCHD2, Nucleic Acids Res 41 (2013) 2255–2266, 10.1093/nar/gks1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Aras S, Bai M, Lee I, Springett R, Huttemann M, Grossman LI, MNRR1 (formerly CHCHD2) is a bi-organellar regulator of mitochondrial metabolism, Mitochondrion 20 (2015) 43–51, 10.1016/j.mito.2014.10.003. [DOI] [PubMed] [Google Scholar]
- [12].Purandare N, Somayajulu M, Huttemann M, Grossman LI, Aras S, The cellular stress proteins CHCHD10 and MNRR1 (CHCHD2): partners in mitochondrial and nuclear function and dysfunction, J. Biol. Chem 293 (2018) 6517–6529, 10.1074/jbc.RA117.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aras S, Arrabi H, Purandare N, Huttemann M, Kamholz J, Zuchner S, Grossman LI, Abl2 kinase phosphorylates Bi-organellar regulator MNRR1 in mitochondria, stimulating respiration, Biochim. Biophys. Acta Mol. Cell Res 1864 (2017) 440–448, 10.1016/j.bbamcr.2016.11.029. [DOI] [PubMed] [Google Scholar]
- [14].Grossman LI, Purandare N, Arshad R, Gladyck S, Somayajulu M, Huttemann M, Aras S, MNRR1, a Biorganellar regulator of mitochondria, Oxid. Med. Cell. Longev 2017 (2017), 6739236, 10.1155/2017/6739236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu Y, Clegg HV, Leslie PL, Di J, Tollini LA, He Y, Kim TH, Jin A, Graves LM, Zheng J, et al. , CHCHD2 inhibits apoptosis by interacting with Bcl-x L to regulate Bax activation, Cell Death Differ 22 (2015) 1035–1046, 10.1038/cdd.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aras S, Purandare N, Gladyck S, Somayajulu-Nitu M, Zhang K, Wallace DC, Grossman LI, Mitochondrial Nuclear Retrograde Regulator 1 (MNRR1) rescues the cellular phenotype of MELAS by inducing homeostatic mechanisms, Proc. Natl. Acad. Sci. U. S. A 117 (2020) 32056–32065, 10.1073/pnas.2005877117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fan M, Li X, Gao X, Dong L, Xin G, Chen L, Qiu J, Xu Y, LPS induces preeclampsia-like phenotype in rats and HTR8/SVneo cells dysfunction through TLR4/p38 MAPK pathway, Front. Physiol 10 (2019) 1030, 10.3389/fphys.2019.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anton L, Brown AG, Parry S, Elovitz MA, Lipopolysaccharide induces cytokine production and decreases extravillous trophoblast invasion through a mitogen-activated protein kinase-mediated pathway: possible mechanisms of first trimester placental dysfunction, Hum. Reprod 27 (2012) 61–72, 10.1093/humrep/der362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moore BB, Kunkel SL, Attracting attention: discovery of IL-8/CXCL8 and the birth of the chemokine field, J. Immunol 202 (2019) 3–4, 10.4049/jimmunol.1801485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N, Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation, Blood 121 (2013) 4930–4937, 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- [21].Gomez-Lopez N, Romero R, Arenas-Hernandez M, Panaitescu B, Garcia-Flores V, Mial TN, Sahi A, Hassan SS, Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change, J. Matern. Fetal Neonatal Med 31 (2018) 439–446, 10.1080/14767058.2017.1287894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Faro J, Romero R, Schwenkel G, Garcia-Flores V, Arenas-Hernandez M, Leng Y, Xu Y, Miller D, Hassan SS, Gomez-Lopez N, Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome, Biol. Reprod 100 (2019) 1290–1305, 10.1093/biolre/ioy261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Garcia-Flores V, Romero R, Miller D, Xu Y, Done B, Veerapaneni C, Leng Y, Arenas-Hernandez M, Khan N, Panaitescu B, et al. , Inflammation-induced adverse pregnancy and neonatal outcomes can Be improved by the immunomodulatory peptide exendin-4, Front. Immunol 9 (2018) 1291, 10.3389/fimmu.2018.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Motomura K, Romero R, Galaz J, Tao L, Garcia-Flores V, Xu Y, Done B, Arenas-Hernandez M, Miller D, Gutierrez-Contreras P, et al. , Fetal and maternal NLRP3 signaling is required for preterm labor and birth, JCI Insight (2022) 7, 10.1172/jci.insight.158238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shakya A, Bhat HR, Ghosh SK, Update on nitazoxanide: a multifunctional Chemotherapeutic agent, Curr. Drug Discov. Technol 15 (2018) 201–213, 10.2174/1570163814666170727130003. [DOI] [PubMed] [Google Scholar]
- [26].Al-Kuraishy HM, Al-Gareeb AI, Elekhnawy E, Batiha GE, Nitazoxanide and COVID-19: a review, Mol. Biol. Rep 49 (2022) 11169–11176, 10.1007/s11033-022-07822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG, Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni, Antimicrob. Agents Chemother 51 (2007) 868–876, 10.1128/AAC.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gomez-Lopez N, Galaz J, Miller D, Farias-Jofre M, Liu Z, Arenas-Hernandez M, Garcia-Flores V, Shaffer Z, Greenberg JM, Theis KR, et al. , The immunobiology of preterm labor and birth: intra-amniotic inflammation or breakdown of maternal-fetal homeostasis, Reproduction 164 (2022) R11–R45, 10.1530/REP-22-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yoon BH, Romero R, Park JY, Oh KJ, Lee J, Conde-Agudelo A, Hong JS, Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes, Am. J. Obstet. Gynecol 221 (2019) 142 e141–e142 e122, 10.1016/j.ajog.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Oh KJ, Romero R, Park JY, Lee J, Conde-Agudelo A, Hong JS, Yoon BH, Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency, Am. J. Obstet. Gynecol 221 (2019) 140 e141–e140 e118, 10.1016/j.ajog.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee J, Romero R, Kim SM, Chaemsaithong P, Yoon BH, A new antibiotic regimen treats and prevents intra-amniotic inflammation/infection in patients with preterm PROM, J. Matern. Fetal Neonatal Med 29 (2016) 2727–2737, 10.3109/14767058.2015.1103729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


