Abstract
We have compared molecular, immunochemical, and cytotoxic assays for the detection of diphtheria toxin from 55 isolates of Corynebacterium diphtheriae and Corynebacterium ulcerans originally isolated in five different countries. The suitabilities and accuracies of these assays for the laboratory diagnosis of diphtheria were compared and evaluated against the “gold standard” in vivo methods. The in vivo and Vero cell cytotoxicity assays were accurate in their abilities to detect diphtheria toxin but were time-consuming; however, the cytotoxicity assay is a suitable in vitro alternative to the in vivo virulence test. There was complete concordance between all the phenotypic methods. Genotypic tests based upon PCR were rapid; however, PCR must be used with caution because some isolates of C. diphtheriae possessed toxin genes but failed to express a biologically active toxin. Therefore, phenotypic confirmation of toxigenicity for the microbiological diagnosis of diphtheria is recommended.
Pharyngeal or cutaneous diphtheria is caused by toxigenic strains of Corynebacterium diphtheriae. Therefore, the most significant test in the microbiological diagnosis of diphtheria is the detection of the potent and lethal exotoxin from a suspect clinical isolate as rapidly and accurately as possible. This is important, first, to confirm the diagnosis of clinical diphtheria and, second, to contain possible spread of the disease by identifying contacts who may be carriers (5). For many patients with advanced cases of infection, however, a clinical diagnosis of diphtheria would normally precede the microbiological diagnosis. It can often be difficult to diagnose respiratory diphtheria on clinical grounds, and the disease may be confused with other infections such as severe streptococcal tonsillitis or glandular fever. Hence, the laboratory diagnosis is important but should be regarded only as complementary to and not as a substitute for a clinical diagnosis (4).
Diphtheria is relatively uncommon within Western Europe and the United States; however, sporadic cases are still occurring, and the majority of these are usually imported from areas of endemicity (3, 18). For example, during 1997, there were two incidents of importation of toxigenic C. diphtheriae to the United Kingdom. The first occurred in a male patient who had returned to the United Kingdom from Indonesia with chronic skin lesions; toxigenic, genotypically indistinguishable isolates of C. diphtheriae var. mitis were isolated from the skin lesions of the index patient and a throat swab from an asymptomatic household contact. Both patients had been fully immunized; however, the potential implications of this situation are grave and serve to illustrate that skin lesions are reservoirs of transmission of pharyngeal diphtheria. The second incident was the first importation of a toxigenic C. diphtheriae isolate from Eastern Europe into the United Kingdom (18). This case of pharyngeal diphtheria occurred in an unimmunized 72-year-old female, who developed a sore throat during a Baltic cruise. In both instances, prompt clinical and microbiological action prevented the development of a more serious situation. These cases illustrate the importance of rapid diagnosis and laboratory confirmation of toxin-producing strains.
Diphtheria toxin (DT), the main virulence factor produced by the causative organism C. diphtheriae, is a protein molecule with a molecular mass of 58,350 Da. The protein consists of two functional domains; the enzymatically active, amino-terminal or A domain and the receptor-binding, carboxyl-terminal or B domain. It is an extremely potent bacterial toxin with a minimal lethal dose of less than 0.1 g/kg of body weight (11, 16, 17). Toxigenicity is currently determined in most laboratories by the Elek immunoprecipitation test (5), a method prone to misinterpretation, particularly in laboratories where it is performed infrequently. The ideal test for use in the diagnostic laboratory must be specific, sensitive, reliable, simple, and easy to perform and ultimately must be shown to correlate with the biological activity of DT.
We have performed a systematic evaluation of the toxigenicities of 55 potentially toxigenic isolates of corynebacteria by molecular, immunochemical, and cytotoxic methods so as to define their suitability for the microbiological diagnosis of diphtheria. The results obtained by in vitro methods were compared to those obtained by virulence and dermonecrosis bioassays with guinea pigs. This is the first comparative study examining a range of methodologies for the detection of toxigenicity among a diverse collection of C. diphtheriae and Corynebacterium ulcerans isolates from five different countries and three continents.
MATERIALS AND METHODS
Bacterial isolates.
The study was undertaken with 50 clinical isolates of pathogenic corynebacteria referred to the Streptococcus and Diphtheria Reference Unit from United Kingdom and overseas (Australia, Canada, Europe, and the United States) laboratories. The collection also included five C. diphtheriae reference strains from the National Collection of Type Cultures (NCTC), and three of these were used as controls for all tests: NCTC 10648 (strong toxin producer), NCTC 3984 (weak toxin producer), and NCTC 10356 (nontoxigenic). Isolates were cultured initially onto Columbia agar with horse blood (5% [vol/vol]) and were stored in blood glycerol (16% [vol/vol]) broth at −70°C. Biotypes were determined by conventional tests as described previously (5).
Elek immunoprecipitation tests.
All isolates were screened by the conventional Elek test (5) and the modified Elek test (7) as described previously.
Detection of DT by immunoblotting.
Isolates were grown in Elek broth medium for 48 h at 37°C (9), and bacterial cells were harvested by centrifugation at 4,000 × g for 10 min and washed once in physiological saline prior to lysis with lysozyme (200 μg/ml; Sigma Chemical Company, Poole, Dorset, United Kingdom). Whole-cell lysates were treated with sodium dodecyl sulfate (SDS) and reducing agent (β-mercaptoethanol) and were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in 12.5% acrylamide gels. The proteins were transferred onto nitrocellulose membranes and were detected with a monoclonal antibody specific for the catalytic domain of the toxin (9) and a goat anti-mouse immunoglobulin conjugated to horseradish peroxidase, as described previously (10). Purified DT (2 μg; Sigma) was also included on the gels to confirm the location of the toxin protein.
Cytotoxicity assay for DT.
The cytotoxicity assays were performed as described previously by Murphy et al. (13) and Miyumara et al. (12) with the modification, described by Hoy and Sesardic (10), of incorporating the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) to enable colorimetric determination of cell survival as the endpoint. Vero cells (African Green monkey kidney cells; National Institute of Public Health and Environmental Protection, Bilthoven, The Netherlands) were grown in modified Eagle’s medium supplemented with HEPES (15 mM), sodium bicarbonate (0.13% [vol/vol]), lactalbumin hydrolysate (4% [vol/vol]), glucose (1% [vol/vol]), fetal calf serum (5% [vol/vol]), penicillin and streptomycin (1% [vol/vol]), and amphotericin B (1% [vol/vol]) (all reagents were obtained from Sigma). A suspension of 2.5 × 105 live trypsinized cells/ml was used in the assay. Isolates were grown in Elek broth (5 ml) at 37°C for 48 h. Bacterial cells were removed by microfiltration with a 0.2-μm-pore-size filter, and the culture filtrate was diluted 20-fold. Serial 50-μl dilutions of the diluted culture filtrates or purified DT (12.5 ng/ml or 0.625 ng per assay; Wellcome Biotechnology Research Laboratories, Beckenham, United Kingdom) were aseptically dispensed into sterile microtiter plates prior to the addition of the Vero cells. The plates were sealed and incubated for 4 days at 37°C. A 20-μl volume of MTT (5 mg/ml) was added to each well as a marker for cell viability. Following a 2-h incubation at 37°C, the colored formazan product and cells were solubilized with 100 μl of SDS (20% [wt/vol]) and N,N-dimethylformamide (50% [vol/vol]) prior to measurement of the absorbance at 570 nm. The cytotoxicity of purified DT in culture supernatants was determined as the concentration of culture supernatant killing 50% of the cells in the assay (Vero CD50/ml).
PCR for detection of the DT gene.
Two sets of primers targeting the entire toxin gene and the fragment A region of the gene were assessed. The method of Pallen and colleagues (15) was used for the detection of the fragment A portion of the toxin gene (248 bp). Primers for the detection of the entire toxin gene (1,600 bp; (5′-GTTTGCGTCAATCTTAATAGGG-3′ [nucleotide positions 15 to 36] and 5′-ACCTTGGTGTGATCTACTGTTT3′ [nucleotide positions 1622 to 1643]) were synthesized by British Biotechnology, Products, Abingdon, Oxon, United Kingdom. The method was performed as previously described by Pallen et al. (15), with the following modifications to the cycling times and electrophoresis; the PCR mixture was denatured for 1.5 min at 96°C, followed by 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 3 min and a final 10-min extension at 72°C; a 20-μl aliquot of the amplified product was electrophoresed on a 0.8% agarose gel at 150 V for 30 min. An artificial template was added to each reaction mixture as an internal control. The control template contains an internal 42-bp deletion that allows it to be distinguished from the natural product by electrophoretic mobility. The presence of a 206-bp product in the negative reactions showed that the PCR had worked and that false-negative results were absent (15).
In vivo assays.
All in vivo procedures were performed as specified by the United Kingdom Home Office. Female Dunkin Hartley guinea pigs (weight, 450 to 500 g; Harlan Olac, Bicester, United Kingdom) were shaved on the flanks and injected intradermally with 0.2 ml of 50-fold dilutions of Elek broth culture filtrates from C. diphtheriae strains or with purified DT (62.5 ng/ml, 2 ng per assay) in parallel with the same preparations of culture filtrates previously reacted with diphtheria antitoxin (0.01 IU/ml). The culture supernatants were prepared as described above for the cytotoxicity assay. The animals were observed for 48 h, and a positive reaction was assessed by the presence of specific dermonecrotic lesions which were absent from the animals administered preparations treated with diphtheria antitoxin. The subcutaneous test for virulence was performed as described previously with female Dunkin Hartley guinea pigs (5). The guinea pigs were observed on a daily basis for clinical manifestations and systemic effects associated with the production of DT. If the test isolate produced DT, the unprotected animal died within 2 to 5 days; postmortem examination revealed the presence of hemorrhagic and swollen adrenal glands. Due to the severity limits of this test, the time factor to achieve a result, and the costs involved, this procedure is no longer undertaken within the Public Health Laboratory Service.
RESULTS
The results of the toxigenicity tests are summarized in Table 1.
TABLE 1.
Detection of DT among isolates of pathogenic corynebacteria
| Species | No. of isolates examined | No. of toxigenic isolates determined by the following assay:
|
||||||
|---|---|---|---|---|---|---|---|---|
| In vivo | Vero cell | Immunoblotting | Elek
|
PCR
|
||||
| Conventional | Modified | Fragment A gene | Entire gene | |||||
| C. diphtheriae var. mitis | 31 | 18 | 18 | 18 | 18 | 18 | 24 | 22 |
| C. diphtheriae var. gravis | 15 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| C. diphtheriae var. belfanti | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. diphtheriae var. intermedius | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| C. ulcerans | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Total | 55 | 26 | 26 | 26 | 26 | 26 | 32 | 30 |
In vivo toxin detection methods.
The dermonecrosis assay and the subcutaneous test for virulence were in complete agreement and identified 26 toxigenic and 29 nontoxigenic isolates of pathogenic corynebacteria.
Detection of DT by cytotoxicity assay.
Twenty-six of 55 isolates were found to be toxigenic by the cytotoxicity assay. The 29 nontoxigenic isolates were not cytotoxic to cultured Vero cells. These results are in complete concordance with those of the guinea pig assays. The amount of toxin produced by the isolates varied and is shown in Table 2; the strong toxigenic control strain (NCTC 10648) produced 5,120 Vero CD50/ml of DT, and the weakly toxigenic control strain (NCTC 3984) produced 1,280 Vero CD50/ml of DT. Five isolates, four of biotype C. diphtheriae var. mitis and one of C. diphtheriae var. intermedius, were identified as particularly low-level toxin producers (80 to 320 Vero CD50/ml). The highest-level toxin-producing strains were isolates of C. diphtheriae var. gravis (>5,200 Vero CD50/ml) isolated from a patient who imported diphtheria into the United Kingdom in 1989 and C. ulcerans from the throat of a patient presenting with clinical diphtheria (Table 2).
TABLE 2.
DT production and quantification in C. diphtheriae and C. ulcerans isolates by Vero cell cytotoxicity assays
| Strain and strain no. | Origin | Source | Cytotoxicity titer (Vero CD50/ml) | Relative cytotoxicitya |
|---|---|---|---|---|
| C. diphtheriae var. gravis | ||||
| C89/59 | UKb | Throat | >5,200 | >52 |
| NCTC 10648 | UK | Throat | 5,120 | 51.2 |
| NCTC 3984 | UK | Throat | 1,280 | 12.8 |
| C. diphtheriae var. mitis | ||||
| C93/4 | Australia | Throat | 1,560 | 15.6 |
| NCTC 11327 | UK | Throat | 2,048 | 20.5 |
| C89/340 | UK | Throat | 1,280 | 12.8 |
| C89/346 | UK | Throat | 1,024 | 10.2 |
| C88/311 | UK | Throat | 2,048 | 20.4 |
| C88/313 | UK | Throat | 512 | 5.1 |
| C89/350 | UK | Throat | 512 | 5.1 |
| C89/351 | UK | Throat | 1,024 | 10.2 |
| C92/85 | UK | Throat | 640 | 6.4 |
| C88/309 | UK | Throat | 2,048 | 20.5 |
| C88/310 | UK | Throat | 512 | 5.1 |
| C90/55 | UK | Throat | 512 | 5.1 |
| C90/56 | UK | Throat | 512 | 5.1 |
| C90/54 | UK | Skin | 256 | 2.6 |
| C88/261 | Canada | Throat | 320 | 3.2 |
| C92/48 | UK | Skin | 160 | 1.6 |
| C92/83 | UK | Skin | 320 | 3.2 |
| NCTC 10681 | UK | Throat | 80 | 0.8 |
| C. diphtheriae var. intermedius C88/299 | Canada | Throat | 100 | 1.0 |
| C. ulcerans | ||||
| C91/90 | UK | Sinus | 2,040 | 20.4 |
| C91/18 | UK | Throat | 512 | 5.1 |
| C92/37 | UK | Throat | >5,200 | >52 |
| C89/372 | UK | Throat | 1,024 | 10.2 |
| C92/45 | UK | Skin | 640 | 6.4 |
Relative to the low-level-toxin-producing isolate of C. diphtheriae (C88/299).
UK, United Kingdom.
Elek immunoprecipitation test.
The results of the conventional and modified Elek tests determined at 48 and 24 h, respectively, were in excellent agreement with those of the bioassays. Of the 55 isolates examined, 26 isolates produced visible immunoprecipitin lines and were identified as toxigenic. Five of the 26 toxigenic isolates produced weak precipitin lines in both the conventional and the modified Elek tests (Table 1).
Immunoblot detection of DT.
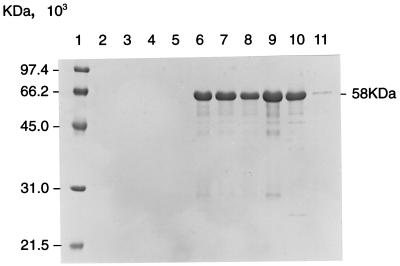
Twenty-six of the 55 isolates were identified as toxigenic by immunoblotting with a monoclonal antibody which recognized the catalytic domain (fragment A; molecular mass, 21 kDa) of DT (Table 1). A specific band within the 58-kDa region was present in whole-cell lysates of all the toxigenic strains but was absent from the nontoxigenic strains (Fig. 1). There was no evidence of the presence of the DT translocation or receptor binding domains (fragment B molecule; 37 kDa) because the monoclonal antibody was selective for the catalytic domain (9). The whole-cell lysates contained unnicked DT that has fragments A and B fused together. The antibody appeared to be selective for fragment A but also detected this fragment in the whole-protein monomer of DT. Five strains produced bands of weak intensity by this assay, which correlated with their weak activities in the Elek tests and the cytotoxicity assay. The limit of detection of the immunoblot assay is in the range of 0.1 to 1.0 μg/ml; the assay is qualitative and therefore cannot be used for quantitative determination of toxin production.
FIG. 1.
Immunoblot detection of DT from clinical isolates of C. diphtheriae. Lane 1, low-molecular-mass marker; lanes 2 to 5, whole-cell protein lysates of non-toxin-producing strains of C. diphtheriae; lanes 6 to 10, strong toxin producers; lane 11, very weak toxin producer. The toxin protein (58 kDa) was detected with a monoclonal antibody specific for the catalytic domain.
Detection of DT gene by PCR.
Of the 55 strains analyzed by PCR, 32 possessed the fragment A portion of the DT gene, and of these, 30 possessed the entire DT structural gene. Only 26 of the PCR-positive strains were confirmed to be toxigenic by in vivo and in vitro methods, as summarized in Table 1. The phenotypically nontoxigenic strains that possessed the entire gene or a portion of the DT toxin gene were isolated from Canada and the United States (8). There were no false-negative results by the genotypic tests.
DISCUSSION
The microbiological diagnosis of diphtheria has traditionally relied upon assays that either are technically demanding, are greatly prone to misinterpretation, or have not been fully evaluated against a diverse collection of isolates (9, 19). In this study, we have compared genotypic, immunologic, and cytotoxic assays for the detection of DT among clinical isolates of corynebacteria.
An in vitro assay capable of detecting biologically active DT based upon the cytotoxicity of DT to cultured Vero cells has been described. This bioassay was evaluated with the tetrazolium compound MTT as a marker for cell viability and the assessment of toxigenicity. The limits of detection of purified DT were estimated to be 60 pg/ml of biologically active DT, or 3 pg of DT per assay. The assay is specific, accurate, and reliable for the quantification of biologically active DT produced by isolates of C. diphtheriae. The limitations of the Vero cell assay which hinder its use in the diagnostic laboratory are the length of time required for determination of a positive or a negative result and the need for specialized tissue culture facilities. The results of the assay were in complete agreement with those of the in vivo methods, and, as such, it is a suitable replacement for in vivo virulence assays, which have always been regarded as the “gold standard” tests for toxigenicity.
The majority of diphtheria cases are due to infection caused by toxigenic C. diphtheriae strains; however, C. ulcerans, a bovine zoonotic agent, can also carry the same corynephage that encodes DT. Sporadic incidents of human infection caused by C. ulcerans have been reported (1, 20). Most cases are associated with the consumption of unpasteurized dairy products and/or contact with farm animals. More recently, in the United States a case of respiratory diphtheria caused by C. ulcerans was reported in Indiana (1). These isolates were associated with minor clinical manifestations of disease, which in these cases were either mild forms of pharyngitis or superficial skin lesions.
The most widely used methods for the microbiological diagnosis of diphtheria are those based upon immunologic techniques. The Elek immunoprecipitin test is still used in many laboratories worldwide; however, this test is prone to misinterpretation, particularly when it is performed infrequently. In this study, 5 of 26 toxigenic strains produced very weak immunoprecipitin lines in Elek tests, and the results for these strains could be misinterpreted. In addition, the clarity and accuracy of the test are dependent upon the constituents of the medium, the concentration of antitoxin, and the use of appropriate control strains (2). Variability in the Elek basal medium has been documented, and it is essential that a medium with a low ash content be used (2). The excellent correlation between the results of the Elek tests and the in vivo and cytotoxicity assays described here was due to the very carefully controlled medium and test conditions used; the Elek basal medium described by Colman and colleagues (2) is recommended.
Immunoblotting with a monoclonal antibody specific for the catalytic domain (fragment A) of the toxin was used to assess the presence of toxin in whole-cell lysates of pathogenic corynebacteria. There was complete concordance between immunoblot detection of the fragment A domain and toxigenicity as determined by functional assays. This monoclonal antibody was previously used in an enzyme-linked immunosorbent assay for the detection of DT (9); those investigators documented occasional false-positive results, presumably due to nonspecific binding of monoclonal antibody to defective toxin. In contrast, the detection of DT by immunoblotting in this study with the same monoclonal antibody did not result in any false-positive results. This was because the proteins were separated by SDS-PAGE before detection, thus avoiding any interaction with defective and/or nondenatured toxin. In view of the lengthy time factor and specialized reagents required for immunoblotting, it is not recommended for routine diagnostic use.
The limitation of current immunologic assays is their inability to differentiate between biologically active and inactive toxin. Their specificity and sensitivity are dependent upon the reactivity profiles of the antibodies used. Polyclonal antibodies directed at multiple epitopes on the toxin molecule are unlikely to differentiate between intact active toxin and biologically inactive toxin, whereas a panel of well-defined monoclonal or antipeptide antibodies specific for DT functional domains might be more suitable for toxin detection.
Genotypic methods, based upon PCR, offer many advantages over phenotypic techniques; they are rapid, simple, and easy to interpret and facilities are becoming increasingly available in many laboratories. The detection of the DT structural gene by PCR provides a rapid detection method with good sensitivity; results are available within 4 h from the time of selection of only a few bacterial colonies. This method, however, does not provide information on the ability of the organism to express fully functional DT. Any defects or mutations either in the structural gene or in genes coding for regulatory elements required for DT expression may not be detected by this method. In this study, 6 of 55 isolates (11%) were found to be phenotypically nontoxigenic by all methods but possessed a portion or the entire DT structural gene. For diagnostic purposes, these strains would be designated nontoxigenic. These isolates were obtained from outbreaks of pharyngitis which occurred within closed communities in the northern United States and from sporadic incidents in Canada. Two predominant “clones” were observed among the isolates from the United States; their molecular typing patterns were quite distinct from the molecular typing patterns produced by the Canadian isolates (6). Such isolates are uncommon (4, 8, 15); however, their distribution in nature is unknown, and it is therefore advisable to use PCR only as an adjunct to phenotypic tests, such as the Elek test. However, an accurate, negative PCR result is useful for the rapid exclusion of toxigenicity. PCR has been used for the detection of the DT gene directly from clinical specimens with two sets of primers for the detection of both the A and B subunits of the DT structural gene, in which sensitivity levels of 50 and 500 CFU/PCR mixture were achieved (14). However, optimal conditions for PCR, in addition to the collection, transportation, and storage of clinical specimens, were found to be crucial (14).
Given the immense public health implications associated with the isolation of a toxigenic strain of C. diphtheriae, the delay between the time of isolation of a suspicious organism and the time that the results of toxigenicity tests are available can provoke great anxiety among laboratory staff, clinicians, and public health officials. The procedures for undertaking toxigenicity tests in a microbiology laboratory will vary and are dependent upon the facilities and resources available, the expertise of personnel, and the availability of a diphtheria reference laboratory for that country. It is particularly important that all countries have the laboratory capability for the identification of toxigenic isolates of corynebacteria, particularly in view of the reemergence of the disease in Eastern Europe and its continued endemicity in other areas of the world (21).
ACKNOWLEDGMENTS
We are grateful to Robert George and Michael Corbel for helpful advice and critical review of the manuscript and also to Barry Cookson for useful discussion during the early stages of this study.
REFERENCES
- 1.Centers for Disease Control and Prevention. Respiratory diphtheria caused by Corynebacterium ulcerans—Terra Haute, Indiana, 1996. Morbid Mortal Weekly Rep. 1997;46:330–332. [PubMed] [Google Scholar]
- 2.Colman G, Weaver E, Efstratiou A. Screening tests for pathogenic corynebacteria. J Clin Pathol. 1992;45:46–48. doi: 10.1136/jcp.45.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dittmann S. Epidemic diphtheria in the Newly Independent States and the Baltic countries. Working Paper CMDS01. Expanded Programme on Immunization, World Health Organization Regional Office for Europe. Seventh Meeting of National Programme Managers. 1997. [Google Scholar]
- 4.Efstratiou A, George R C. Microbiology and epidemiology of diphtheria. Rev Med Microbiol. 1996;7:31–42. [Google Scholar]
- 5.Efstratiou A, Maple P A C. Manual for the laboratory diagnosis of diphtheria. ICP/EPI038(C). Expanded Programme on Immunization in the European Region of WHO, Copenhagen, Denmark. 1994. [Google Scholar]
- 6.Efstratiou A, Sangrador A, Pallen M J, Cookson B D. Abstracts of the European Congress of Clinical Microbiology and Infectious Diseases. 1993. Molecular epidemiology of unusual strains of Corynebacterium diphtheriae from the UK and overseas. [Google Scholar]
- 7.Engler K H, Glushkevich T, Mazurova I K, George R C, Efstratiou A. A modified Elek test for detection of toxigenic corynebacteria in the diagnostic laboratory. J Clin Microbiol. 1997;35:495–498. doi: 10.1128/jcm.35.2.495-498.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groman N, Cianciotto N, Bjorn M, Rabin M. Detection and expression of DNA homologous to the tox gene in non-toxigenic isolates of Corynebacterium diphtheriae. Infect Immun. 1983;42:48–56. doi: 10.1128/iai.42.1.48-56.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallas G, Harrison T G, Samuel D, Colman G. Detection of diphtheria toxin in culture supernates of Corynebacterium diphtheriae and C. ulcerans by immunoassay with monoclonal antibody. J Med Microbiol. 1990;32:247–253. doi: 10.1099/00222615-32-4-247. [DOI] [PubMed] [Google Scholar]
- 10.Hoy C S, Sesardic D. In vitro assays for detection of diphtheria toxin. Toxicol In Vitro. 1994;8:694–695. doi: 10.1016/0887-2333(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 11.Mekada E, Okada Y, Uchida T. Identification of diphtheria toxin receptor and a nonproteinous diphtheria toxin-binding molecule in vero cell membrane. J Cell Biol. 1988;107:511–519. doi: 10.1083/jcb.107.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamura K, Nishio S, Ito A, Muata R, Kono R. Micro cell culture method for determination of diphtheria toxin and antitoxin titre using vero cells. J Biol Stand. 1974;2:189–201. doi: 10.1016/0092-1157(74)90015-8. [DOI] [PubMed] [Google Scholar]
- 13.Murphy J R, Bacha P, Teng M. Determination of Corynebacterium diphtheriae toxigenicity by a colorimetric tissue culture assay. J Clin Microbiol. 1978;7:91–96. doi: 10.1128/jcm.7.1.91-96.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakao H, Popovic T. Development of a direct PCR for detection of the diphtheria toxin gene. J Clin Microbiol. 1997;7:1651–1655. doi: 10.1128/jcm.35.7.1651-1655.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pallen M J, Hay A, Puckey L H, Efstratiou A. Polymerase chain reaction for screening clinical isolates of corynebacteria for the production of diphtheria toxin. J Clin Pathol. 1994;47:353–356. doi: 10.1136/jcp.47.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pappenheimer A M. The diphtheria bacillus and its toxin: a model system. J Hyg Camb. 1984;93:397–404. doi: 10.1017/s0022172400064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappenheimer A M. The story of a toxic protein 1888–1992. Protein Sci. 1993;2:292–298. doi: 10.1002/pro.5560020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Public Health Laboratory Service. Diphtheria acquired during a cruise in the Baltic Sea. Commun Dis Rep CDR Weekly. 1997;7:207. [PubMed] [Google Scholar]
- 19.Toma C, Sisvath L, Iwanaga M. Reversed passive latex agglutination assay for detection of toxigenic Corynebacterium diphtheriae. J Clin Microbiol. 1997;35:3147–3149. doi: 10.1128/jcm.35.12.3147-3149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White J, Efstratiou A. Incidence of diphtheria and other infections caused by Corynebacterium diphtheriae and C. ulcerans in England and Wales: 1986–1993. Proceedings of the First International Meeting of the European Laboratory Working Group on Diphtheria. PHLS Microbiol Digest. 1995;12:23–24. [Google Scholar]
- 21.World Health Organization. EPI information system, Global summary. Global Programme for Vaccines and Immunization, Expanded Programme on Immunization, World Health Organization, Geneva, Switzerland. 1997. [Google Scholar]