Abstract
Background:
Calcineurin is highly enriched in immune T cells and in the nervous system. Calcineurin inhibitors, including cyclosporine and tacrolimus (FK506), are the cornerstone of immunosuppressive regimens for preserving transplanted organs and tissues. However, these drugs often cause persistent hypertension owing to excess sympathetic outflow, which is maintained by N-methyl-D-aspartate receptor (NMDAR)-mediated excitatory input to the hypothalamic paraventricular nucleus (PVN). It is unclear how calcineurin inhibitors increase NMDAR activity in the PVN to augment sympathetic vasomotor activity. α2δ−1 (encoded by the Cacna2d1 gene), known colloquially as a calcium channel subunit, is a newly discovered NMDAR-interacting protein. Here, we determined whether α2δ−1 plays a role in calcineurin inhibitor–induced synaptic NMDAR hyperactivity in the PVN and hypertension development.
Methods and Results:
Immunoblotting and coimmunoprecipitation assays revealed that prolonged treatment with FK506 in rats significantly increased protein levels of α2δ−1, GluN1 (the obligatory NMDAR subunit), and the α2δ−1–GluN1 complex in PVN synaptosomes. These effects were blocked by inhibiting α2δ−1 with gabapentin or interrupting the α2δ−1–NMDAR interaction with an α2δ−1 C-terminus peptide. Whole-cell recordings in brain slices showed that treatment with FK506 potentiated the activity of presynaptic and postsynaptic NMDARs in spinally projecting PVN neurons; such effects were abolished by gabapentin, Cacna2d1 knockout, or α2δ−1 C-terminus peptide. Furthermore, microinjection of α2δ−1 C-terminus peptide into the PVN diminished renal sympathetic nerve discharges and arterial blood pressure that had been increased by FK506 treatment. Remarkably, telemetry recording showed that concurrent administration of gabapentin prevented the development of FK506-induced hypertension in rats. Additionally, FK506 treatment induced sustained hypertension in wild-type mice but not in Cacna2d1 knockout mice.
Conclusions:
These findings indicate that α2δ−1 is essential for calcineurin inhibitor–induced increases in synaptic NMDAR activity in PVN presympathetic neurons and sympathetic outflow. Thus, α2δ−1 and α2δ−1–bound NMDARs represent new targets for treating calcineurin inhibitor–induced hypertension.
Keywords: autonomic nervous system, immunosuppressant, gabapentinoid, protein phosphatase, synaptic plasticity, sympathetic nervous system
Subject Terms: Animal Models of Human Disease, Basic Science Research, Hypertension, Physiology, Translational Studies
Graphical Abstract
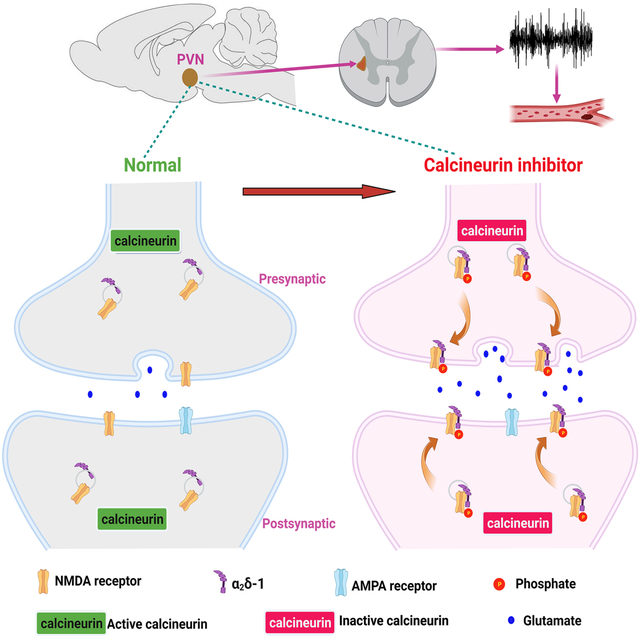
Introduction
Drug-induced hypertension can occur as a result of either the unintended consequences of a drug or its antagonistic effect on antihypertensive medications. Calcineurin inhibitors, such as cyclosporine and tacrolimus (FK506), are standard immunosuppressants used for minimizing rejection of transplanted organs and cells/tissues (i.e., bone marrow and hematopoietic stem cells) and for treating autoimmune diseases. However, long-term use of these drugs often causes uncontrolled, persistent hypertension.1–4 Sympathetic nerve discharges are substantially increased in calcineurin inhibitor–induced hypertension (CIH) in patients and in animal models.1,5–7 Widespread vasoconstriction caused by augmented sympathetic nerve activity impairs blood flow and perfusion to transplanted organs and tissues, which can severely limit the viability of grafts and increase recipient mortality.2–4,8 CIH is blunted by ganglionic blockade,7,9,10 α1-adrenergic receptor antagonists,9,11 surgical adrenalectomy,7 or chemical sympathectomy.11 Although a causal role for the sympathetic nervous system in the pathogenesis of CIH has been documented, it remains uncertain how excess central sympathetic outflow is generated at the molecular and cellular levels in CIH.
Calcineurin, also known as protein phosphatase-2B, is expressed abundantly not only in immune T cells but also in many brain regions, including the hypothalamic paraventricular nucleus (PVN), which plays a key role in generating elevated sympathetic output in hypertensive conditions.12–14 The presympathetic neurons in the PVN regulate vasomotor tone mainly via their projection to the rostral ventrolateral medulla and intermediolateral cell column in the spinal cord.15,16 Calcineurin is a Ca2+/calmodulin-dependent serine-threonine phosphatase that actively controls the phosphorylation status of N-methyl-D-aspartate receptors (NMDARs).5,17–19 Normal calcineurin activity in the brain is important for maintaining physiological neuronal activity and synaptic NMDAR plasticity.17,20,21 Systemic treatment with FK506 diminishes calcineurin activity in the PVN, which augments phosphorylation and activity of synaptic NMDARs in the PVN, leading to increased glutamatergic excitatory synaptic input and firing activity of presympathetic neurons and sympathetic outflow.5 Correspondingly, NMDAR antagonists are effective in reducing CIH in the animal model.5 However, it is unclear how synaptic NMDAR activity in the PVN is augmented in CIH. Furthermore, NMDARs in the central nervous system are involved in many physiological functions, including learning and memory.22 Because non-selectively blocking all NMDARs is associated with some serious adverse effects, such as dizziness, confusion, ataxia, and hallucination,23,24 long-term use of NMDAR antagonists is challenging. Therefore, there is a pressing need to identify a better therapeutic target for managing CIH.
α2δ−1, encoded by the Cacna2d1 gene, previously known as a subunit of voltage-gated calcium channels (VGCCs), is a newly discovered regulatory protein that preferentially interacts with phosphorylated NMDARs to promote synaptic trafficking of NMDARs in the spinal cord.25,26 In fact, NMDAR phosphorylation alone does not increase synaptic trafficking and activity of NMDARs unless α2δ−1 is present.26 Given the critical role of α2δ−1 in regulating NMDARs, we tested the hypothesis that calcineurin inhibition promotes α2δ−1–NMDAR interactions and their synaptic trafficking and activity in the PVN, thereby augmenting sympathetic outflow. Our study identifies for the first time the indispensable role of the α2δ−1 protein in calcineurin inhibitor–augmented synaptic NMDAR activity in the PVN and sympathetic vasomotor activity. Furthermore, our findings suggest that α2δ−1 or α2δ−1–bound NMDARs could be targeted for the prevention and treatment of CIH.
Materials and Methods
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Detailed methods can be found in the Supplemental Material.
Animal Models
All experiments were approved by Institutional Animal Care and Use Committee of MD Anderson Cancer Center. Sprague-Dawley rats were purchased from Envigo. Cacna2d1 knockout (KO, Cacna2d1−/−) mice and wild-type (WT, Cacna2d1+/+) littermates were obtained by breeding Cacna2d1+/− mice.25 For induction of CIH, FK506 was injected intraperitoneally at a dose of 3 mg/kg once daily for 14 consecutive days in rats and mice.5 Final experiments were conducted 5–7 days after the last FK506 injection. Animals received a same amount of dimethyl sulfoxide as the vehicle group.
PVN Synaptosome Preparation, Coimmunoprecipitation, and Immunoblotting
Synaptosomes from PVN tissues were prepared, and coimmunoprecipitation and immunoblotting were performed as described previously13,27. The protein samples were incubated with Protein G bead prebound to a rabbit anti-GluN1 antibody. Protein samples on the beads were separated by electrophoresis and then transferred to a polyvinylidene difluoride membrane. The membrane was incubated with a mouse anti–α2δ−1 antibody, a mouse anti–PSD-95 antibody, or a mouse anti-GluN1 antibody.
Arterial Blood Pressure Measurement with Telemetry
Arterial blood pressure (ABP) in rats and mice was measured using a Millar telemetry system and a DSI telemetry system, respectively.5 Heart rate (HR) values were derived from ABP pulse signals.
Electrophysiological Recording in Brain Slices
Spinally projecting PVN neurons were retrogradely labeled and recorded as described previously.34–36 Whole-cell configurations were used to record miniature excitatory postsynaptic currents (mEPSCs) and puff NMDAR currents in labeled PVN neurons. The mEPSCs were recorded in the presence of 1 μM tetrodotoxin and 20 μM bicuculline at a holding potential of −60 mV. To record postsynaptic NMDAR activity, we puffed NMDA (100 μM) directly to labeled PVN neurons at a holding potential of −60 mV in the presence of tetrodotoxin.5,36
Recording of Renal Sympathetic Nerve Activity and PVN Microinjection
Renal sympathetic nerve activity (RSNA) was recorded as descried previously.5,27 Rats were anesthetized with intraperitoneal injection of a mixture of α-chloralose (60–75 mg/kg) and urethane (800 mg/kg), and RSNA was recorded from a branch of the left renal nerve. PVN microinjections were performed as reported previously.12,29 A glass pipette was advanced into the PVN, and the drug solution was pressure-ejected via a calibrated microinjector.
Statistical Analysis
All data are expressed as mean ± SEM. Student t test was used to determine difference between 2 groups, and one-way or two-way ANOVA followed by Dunnett’s or Bonferroni’s post hoc test was used to determine differences among 3 or more groups.
Results
Calcineurin inhibition promotes the α2δ−1–NMDAR interaction and synaptic trafficking of α2δ−1–bound NMDARs in the PVN
Systemic treatment with the calcineurin inhibitor FK506 diminishes calcineurin activity in the forebrain and increases NMDAR phosphorylation in the PVN.5 α2δ−1 is a phospho-binding protein and preferentially interacts with phosphorylated NMDARs independently of VGCCs in the cell line and spinal cord.26 We first determined whether calcineurin inhibition affects the synaptic protein levels of α2δ−1 and NMDARs in the PVN. For induction of CIH, rats were systemically treated with FK506 (3 mg/kg per day) for consecutive 14 days, which gradually and persistently increased mean arterial blood pressure (MAP) and heart rate (HR), lasting more than 7 days after FK506 treatment was discontinued.5 Because treatment with FK506 for 14 days caused a similar degree of increases in MAP and HR in male and female rats (n = 6 rats per group; Figure S1), we mainly used male rats for the following experiments.
Treatment with FK506 increases phosphorylation of GluN1, but not GluN2A or GluN2B, in the PVN.5 GluN1 is the obligatory subunit of NMDARs, and other NMDAR subunits require GluN1 to function and traffic together with GluN1.22 Immunoblotting showed that treatment with FK506 markedly increased the protein levels of α2δ−1 and GluN1 in PVN synaptosomes (n = 6 samples per group, each sample contained PVN tissues from 2 rats; α2δ−1: P = 1.0e-04, F(2,15) = 17.36; GluN1: P = 5.0e-04, F(2,15) = 11.97; Figure 1A and 1B). However, treatment with FK506 had no significant effect on the total protein level of α2δ−1 in the PVN lysate (Figure S2). The total protein levels of NMDARs in the PVN do not differ significantly between vehicle-treated and FK506-treated rats.5
Figure 1. Calcineurin inhibition increases α2δ−1–NMDAR interactions and synaptic levels of NMDARs in the PVN.
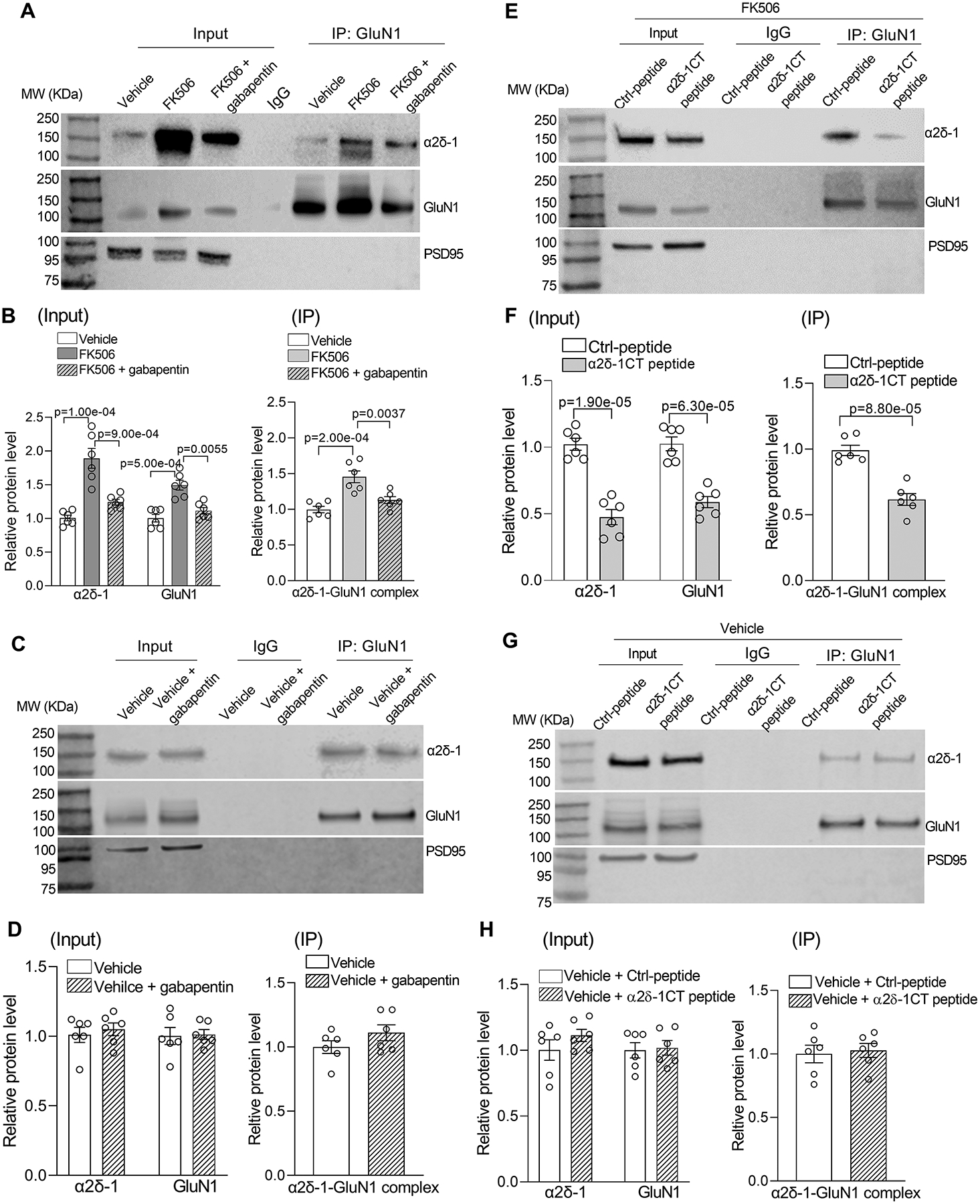
A and B, Representative gel images (A) and qualification (B) show the effects of FK506 treatment with and without 100 μM gabapentin on protein levels of α2δ−1, GluN1, and α2δ−1–GuN1 complexes in PVN synaptosomes (n = 6 samples per group; each sample included PVN tissues from 2 male rats). C and D, Original gel images (C) and qualification (D) show the lack of effect of 100 μM gabapentin on protein levels of α2δ−1, GluN1, or α2δ−1–GluN1 complexes in PVN synaptosomes from vehicle control rats (n = 6 samples per group; each sample included PVN tissues from 2 male rats). E and F, Representative gel images (E) and qualification (F) show effects of pretreatment with 1 μM control peptide (Ctrl-peptide) or 1 μM α2δ−1 C terminus peptide (α2δ−1CT peptide) on protein levels of α2δ−1, GluN1, and α2δ−1–GluN1 complexes in PVN synaptosomes from FK506-treated rats (n = 6 samples per group; each sample included PVN tissues from 2 male rats). G and H, Original gel images (G) and qualification (H) show the lack of effect of α2δ−1CT peptide on protein levels of α2δ−1, GluN1, or α2δ−1–GluN1 complexes in PVN synaptosomes from vehicle control rats (n = 6 samples per group; each sample included PVN tissues from 2 male rats). MW, molecular weight. PSD95, a synaptic protein marker, was used as a loading control. One-way ANOVA with Bonferroni’s post hoc test was used in B; two-tailed Student’s t test was used in D, F, and H.
In the hypothalamus from rats and humans, α2δ−1 physically interacts with NMDARs.34 We thus determined whether treatment with FK506 alters the α2δ−1–NMDAR interaction in the PVN. Coimmunoprecipitation analysis showed that a specific GluN1 antibody, but not the irrelevant IgG, precipitated α2δ−1 proteins in PVN synaptosomes. Treatment with FK506 substantially increased the amount of α2δ−1–GluN1 protein complexes in PVN synaptosomes (n = 6 samples per group, each sample contained PVN tissues from 2 rats; P = 2.0e-04, F(2,15) = 14.92; Figure 1A and 1B). These results suggest that calcineurin inhibition enhances the α2δ−1–NMDAR interaction and their synaptic trafficking in the PVN.
We next determined whether α2δ−1 is required for the FK506-induced increase in the level of NMDARs in PVN synaptosomes. Gabapentin is an α2δ−1 inhibitory ligand42,43 and used for treating patients with chronic neuropathic pain and epilepsy. Hypothalamic brain slices from FK506-treated rats were incubated with gabapentin (100 μM) for 30 minutes, and synaptosomes in the PVN tissue were then isolated. Immunoblotting of PVN synaptosomes showed that gabapentin treatment fully reversed the increased protein levels of α2δ−1, GluN1, and α2δ−1–GluN1 complexes by FK506 treatment (n = 6 samples per group; α2δ−1: P = 9.0e-04, F(2,15) = 17.36; GluN1: P = 0.0055, F(2,15) = 11.97; α2δ−1–GluN1 complex: P = 0.0037, F(2,15) = 14.92; Figure 1A and 1B). By contrast, gabapentin treatment had no statistically significant effect on the protein levels of α2δ−1, GluN1, or α2δ−1–GluN1 complexes in PVN synaptosomes obtained from vehicle-treated rats (n = 6 samples per group, each sample contained PVN tissues from 2 rats; Figure 1C and 1D). These findings suggest that α2δ−1 is essential for calcineurin inhibitor–induced increases in synaptic trafficking of NMDARs in the PVN.
α2δ−1 physically interacts with NMDARs via its C-terminus, which is an intrinsically disordered protein region.25,26 A cell-penetrating, Tat-fused α2δ−1 C terminus peptide (termed the α2δ−1CT peptide) that mimics the C-terminal domain of α2δ−1 effectively disrupts the α2δ−1–NMDAR interaction.25,44 We thus used α2δ−1CT peptide to determine whether calcineurin inhibition increases α2δ−1–dependent NMDAR trafficking in the PVN. Hypothalamic brain slices from FK506-treated rats were first incubated with Tat-fused α2δ−1CT peptide (1 μM) or Tat-fused scrambled control peptide (1 μM) for 30 minutes before synaptosomes in the PVN tissues were isolated. Compared with the control peptide, treatment with α2δ−1CT peptide significantly decreased protein levels of α2δ−1, GluN1, and the α2δ−1–GluN1 complex in PVN synaptosomes from FK506-treated rats (n = 6 samples per group; α2δ−1: P = 1.9e-05, t(10) = 7.57; GluN1: P = 6.3e-05, t(10) = 6.56; α2δ−1–GluN1 complex: P = 8.8e-05, t(10) = 6.30; Figure 1E and 1F). However, treatment with α2δ−1CT peptide had no significant effect on the protein levels of α2δ−1, GluN1, or α2δ−1–GluN1 complexes in PVN synaptosomes from vehicle-treated rats (n = 6 samples per group, each sample contained PVN tissues from 2 rats; Figure 1G and 1H). These data support the notion that calcineurin inhibition enhances the α2δ−1–NMDAR interaction, which promotes synaptic expression of NMDARs in the PVN.
Calcineurin inhibition potentiates synaptic NMDAR activity in PVN presympathetic neurons via α2δ−1 in rats
Tonic activation of presynaptic NMDARs increases synaptic glutamate release to PVN presympathetic neurons in CIH.5 Because calcineurin inhibition increased synaptic expression of NMDARs via α2δ−1, we next determined whether α2δ−1 mediates the increased presynaptic NMDAR activity in PVN presympathetic neurons in CIH. Hypothalamic brain slices were obtained from vehicle- and FK506-treated rats. To quantify NMDAR-mediated spontaneous quantal release of glutamate from presynaptic terminals, we recorded mEPSCs of retrogradely labeled, spinally projecting PVN neurons. The baseline frequency, but not the amplitude, of mEPSCs was significantly greater in FK506-treated than in control rats (n = 10 neurons per group; Figure 2A–2D). Bath application of AP5 (50 μM), a specific NMDAR antagonist,36,45 had no significant effect on mEPSCs in vehicle-treated rats but normalized the increased frequency of mEPSCs in FK506-treated rats, indicating increased glutamate release from presynaptic terminals via NMDARs by FK506 treatment (Figure 2A–2D). Incubation with gabapentin (100 μM) for 30 minutes significantly attenuated the higher baseline frequency of mEPSCs in labeled PVN neurons in FK506-treated rats. Subsequent bath application of AP5 had no further effect on the already decreased frequency of mEPSCs in the same neurons (n = 10 neurons per group; Figure 2C and 2D).
Figure 2. α2δ−1 inhibition with gabapentin diminishes FK506-induced increases in presynaptic and postsynaptic NMDAR activity in spinally projecting PVN neurons.
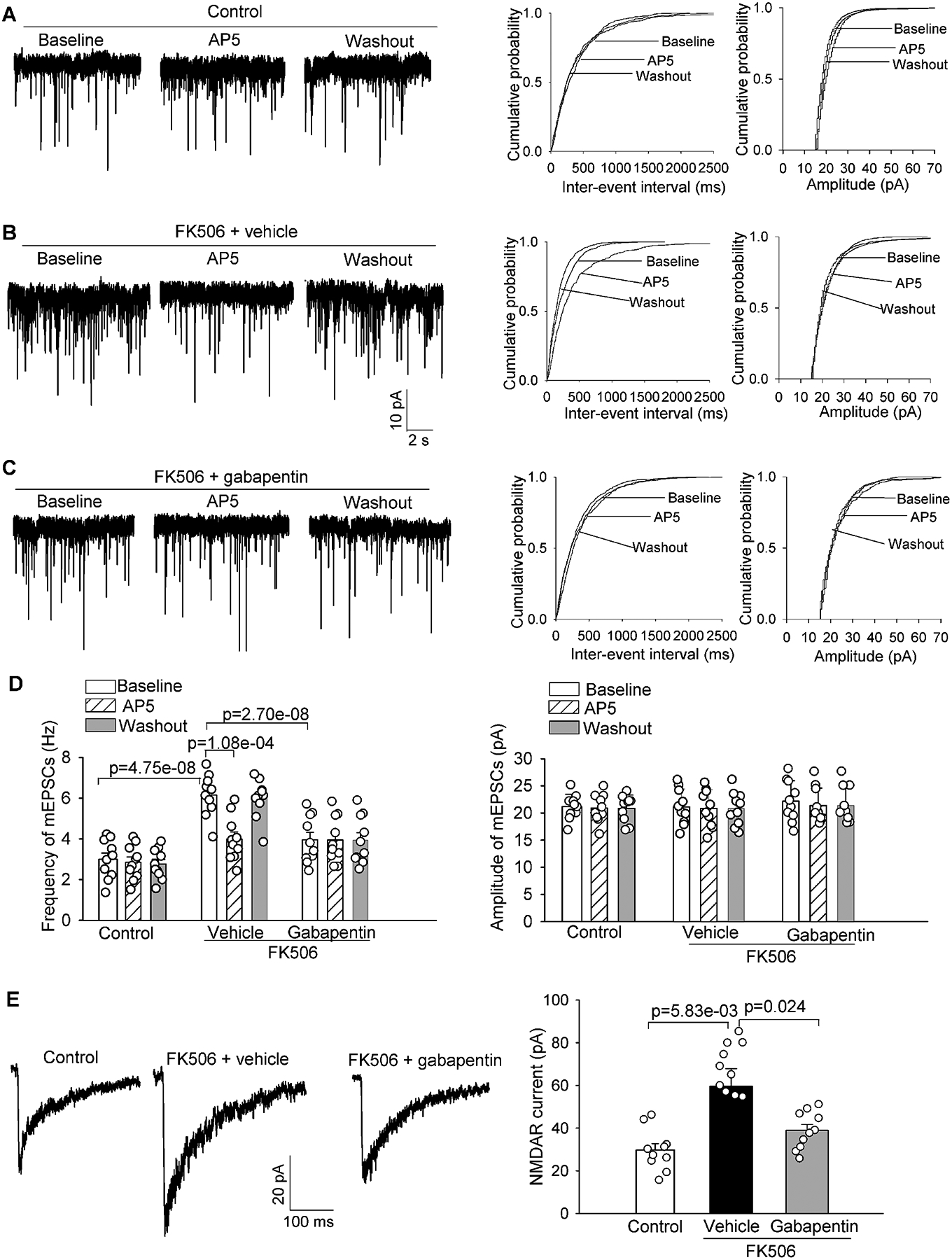
A-C, Representative recording traces and cumulative probability plots show the effects of bath application of 50 μM AP5 on the frequency and amplitude of miniature of excitatory postsynaptic currents (mEPSCs) of spinally projecting PVN neurons in brain slices pretreated with 100 μM gabapentin in FK506-treated rats. D, Summary data show the effects of gabapentin and AP5 on the frequency and amplitude of mEPSCs of labeled PVN neurons in vehicle-treated control rats and FK506-treated rats (n = 10 neurons from 4 male rats per group). E, Representative recording traces and quantification show the effect of gabapentin (100 μM) on the amplitude of puff-elicited NMDAR currents in labeled PVN neurons in brain slices from FK506-treated rats (n = 10 neurons from 4 male rats per group). The repeated measures models were fitted for statistical analysis.
Postsynaptic NMDAR activity is also increased in the PVN and plays a major role in augmenting glutamatergic excitatory input and firing activity of presympathetic neurons in CIH.5 We thus determined whether inhibiting α2δ−1 with gabapentin affects the increased postsynaptic NMDAR activity in CIH. The amplitude of puff-elicited NMDAR currents in labeled PVN neurons was much larger in FK506-treated rats than in control rats (n = 10 neurons per group; Figure 2E). Treatment with gabapentin (100 μM) for 30 minutes reversed the FK506 treatment–increased amplitude of puff NMDAR currents in labeled PVN neurons (Figure 2E). By contrast, gabapentin had no statistically significant effect on puff-elicited NMDAR currents in labeled PVN neurons in control rats (Figure S3). Together, these findings suggest an essential role of α2δ−1 in the increased presynaptic and postsynaptic NMDAR activity of PVN presympathetic neurons induced by the calcineurin inhibitor.
Genetic ablation of α2δ−1 abolishes the calcineurin inhibitor–induced potentiation of synaptic NMDAR activity in PVN presympathetic neurons in mice
Because gabapentin binds to both α2δ−1 and α2δ−2 proteins,42,43 we then attempted to use Cacna2d1 knockout (KO) mice to validate the role of α2δ−1 in synaptic NMDAR activity increased by calcineurin inhibition. We treated WT mice and Cacna2d1 KO mice with FK506 (3 mg/kg per day) for 14 days and then examined synaptic NMDAR activity in spinally projecting PVN neurons in brain slices. The frequency and amplitude of mEPSCs and the amplitude of puff-elicited NMDAR currents in labeled PVN neurons were similar in WT and Cacna2d1 KO mice (Figure 3A–3D). As expected, treatment with FK506 profoundly increased the baseline frequency of mEPSCs in labeled PVN neurons in WT mice but not in Cacna2d1 KO mice (n = 12 neurons in both vehicle-treated WT and FK506-treated Cacna2d1 KO groups, n = 13 neurons in FK506-treated WT group; Figure 3A–3D). Subsequent bath application of AP5 (50 μM) reversed the increased mEPSCs frequency in labeled PVN neurons in FK506-treated WT mice but had no such effect in FK506-treated Cacna2d1 KO mice (Figure 3A–3D).
Figure 3. α2δ−1 is essential for the increased synaptic NMDAR activity in spinally projecting PVN neurons by calcineurin inhibition.
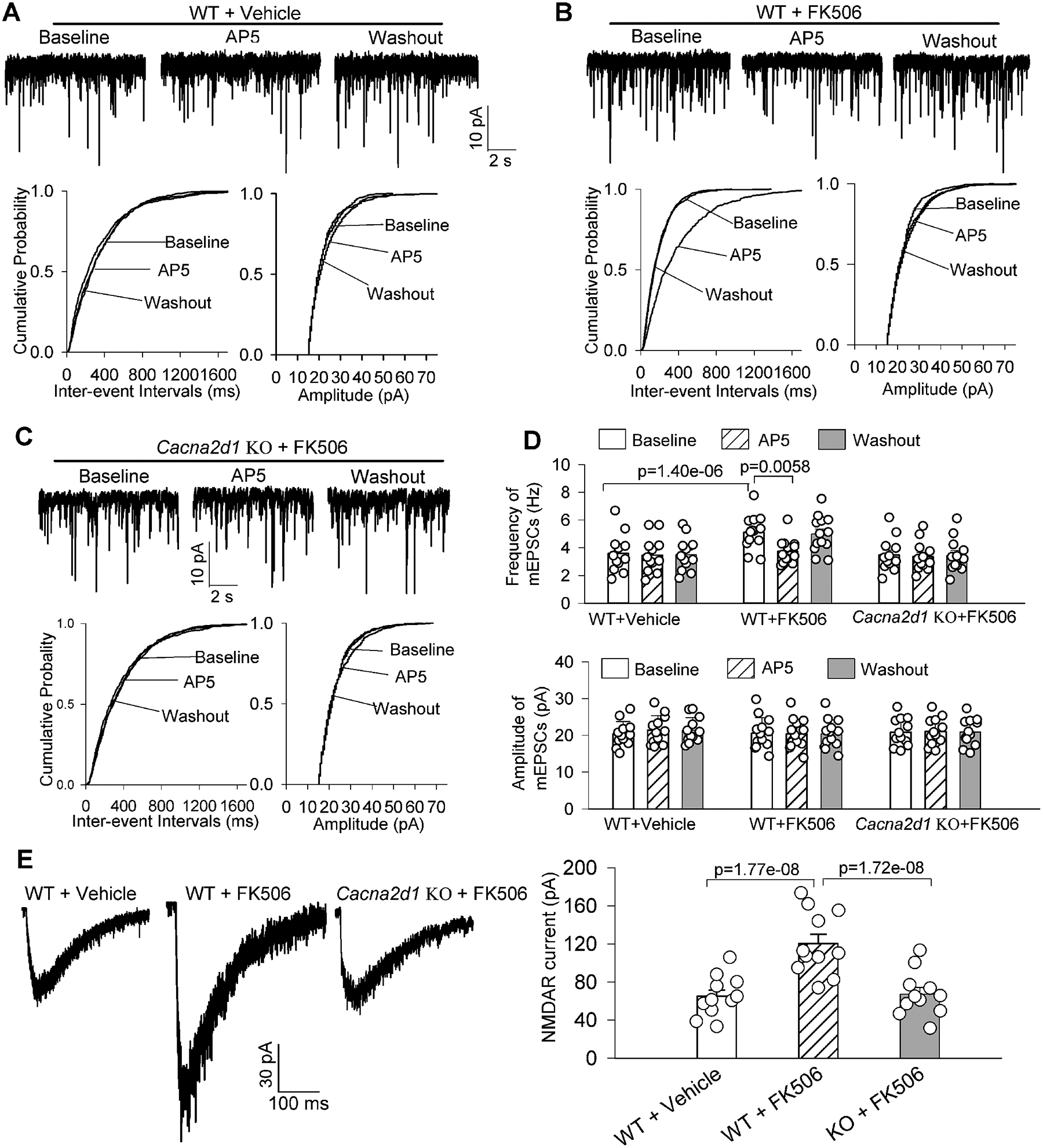
A-C, Representative recording traces and cumulative probability plots show the effects of bath application of AP5 (50 μM) on the frequency and amplitude of miniature of excitatory postsynaptic currents (mEPSCs) in labeled PVN neurons in brain slices from vehicle- treated or FK506-treated wild-type (WT) mice (A and B) and FK506-treated Cacna2d1 knockout (KO) mice (C). D, Summary data show the effects of AP5 on mEPSCs of labeled PVN neurons in brain slices from WT and Cacna2d1 KO mice treated with vehicle or FK506 (n = 12 neurons from 4 male mice in vehicle-treated WT mice and FK506-treated Cacna2d1 KO mice, n = 13 neurons from 4 male mice in FK506-treated WT group). E, Representative recording traces and quantification show the amplitude of puff-elicited NMDAR currents in labeled PVN neurons in WT and Cacna2d1 KO mice treated with vehicle or FK506 (n = 11 neurons from 4 male mice per group). The repeated measures models were fitted for statistical analysis.
In addition, FK506 treatment significantly increased the amplitude of puff-elicited NMDAR currents in labeled PVN neurons in WT mice but had no significant effect on puff-elicited NMDAR currents in labeled PVN neurons in Cacna2d1 KO mice (n = 11 neurons per group; Figure 3E). These data provide unambiguous evidence that α2δ−1 is indispensable for calcineurin inhibitor–induced potentiation in presynaptic and postsynaptic NMDAR activity in PVN presympathetic neurons.
Calcineurin inhibitor potentiates glutamatergic input to PVN presympathetic neurons via α2δ−1–bound NMDARs
To determine directly whether α2δ−1–coupled NMDARs are responsible for calcineurin inhibitor–induced glutamatergic excitatory input to PVN presympathetic neurons, we incubated hypothalamic brain slices from FK506-treated rats with 1 μM Tat-fused α2δ−1CT peptide or 1 μM Tat-fused control peptide for 30 minutes25,37 and then examined presynaptic and postsynaptic NMDAR activity in spinally projecting PVN neurons. Treatment with α2δ−1CT peptide, but not the control peptide, largely attenuated the increased baseline frequency of mEPSCs in labeled PVN neurons in FK506-treated rats (n = 10 neurons per group; Figure 4A–4C). Subsequent bath application of AP5 (50 μM) lowered the mEPSCs frequency of labeled PVN neurons treated with the control peptide (Figure 4A–4C). However, AP5 had no further effect on the mEPSCs frequency of labeled PVN neurons in brain slices treated with α2δ−1CT peptide (Figure 4A–4C).
Figure 4. α2δ−1–bound NMDARs are responsible for the increased glutamatergic input to PVN presympathetic neurons by calcineurin inhibition.
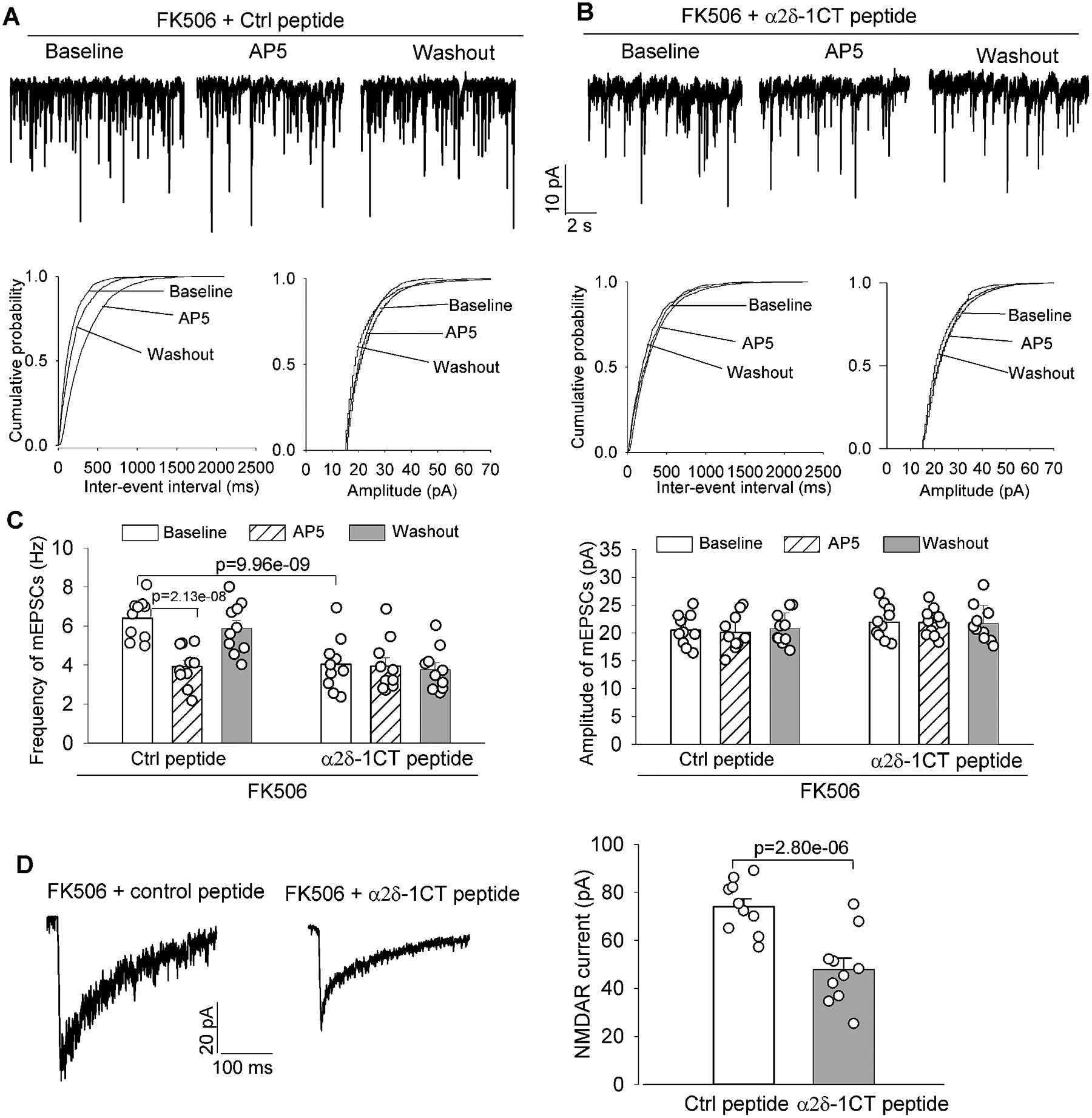
A-C, Original recording traces and cumulative probability plots (A and B) and quantification (C) show the effects of bath application of AP5 (50 μM) on miniature of excitatory postsynaptic currents (mEPSCs) in labeled PVN neurons in brain slices pretreated with 1 μM control (Ctrl) peptide or 1 μM α2δ−1 C terminus peptide (α2δ−1CT peptide) in FK506-treated rats (n = 10 neurons from 4 male rats per group). D, Representative recording traces and quantification show effects of pretreatment with 1 μM Ctrl peptide or 1 μM α2δ−1CT peptide on the amplitude of puff-elicited NMDAR currents in labeled PVN neurons in brain slices from FK506-treated rats (n = 10 neurons from 4 male rats per group). The repeated measures models were fitted for statistical analysis.
Moreover, in labeled PVN neurons from brain slices of FK506-treated rats, the amplitude of puff-elicited NMDAR currents was much smaller in slices treated with α2δ−1CT peptide than in those treated with the control peptide (n = 10 neurons per group; Figure 4D). Additionally, neither the control peptide nor α2δ−1CT peptide had any statistically significant effect on the amplitude of puff-elicited NMDAR currents in labeled PVN neurons in control rats (Figure S3). These data suggest that α2δ−1–coupled NMDARs are responsible for calcineurin inhibitor–augmented glutamatergic synaptic input to PVN presympathetic neurons.
α2δ−1–bound NMDARs in the PVN mediate calcineurin inhibitor–potentiated sympathetic vasomotor activity
NMDAR hyperactivity in the PVN plays a critical role in the increased sympathetic output in CIH.5 Having demonstrated the essential role of α2δ−1 in synaptic NMDAR activity of PVN presympathetic neurons potentiated by the calcineurin inhibitor in brain slices, we sought to determine whether α2δ−1–bound NMDARs in the PVN mediate calcineurin inhibitor–elevated sympathetic vasomotor activity in vivo. The absolute baseline voltage level of RSNA was significantly higher in FK506-treated rats than in vehicle-treated rats (0.19 ± 0.07 μV vs. 0.09 ± 0.05 μV, P = 0.0017, t(18) = 3.68). In vehicle-treated rats, bilateral microinjection of the control peptide (50 pmol, 50 nL), α2δ−1CT peptide (50 pmol, 50 nL), or AP5 (1.0 nmol, 50 nL)13,27 into the PVN had no significant effect on RSNA, MAP, or HR (n = 9 rats, Figure S4). In FK506-treated rats, initial microinjection of the control peptide into the PVN had no significant effect on RSNA, MAP, or HR, but subsequent microinjection of AP5 significantly reduced RSNA, MAP, and HR (n = 6 rats, Figure 5A, 5C, 5E, and 5F). By contrast, microinjection of α2δ−1CT peptide alone into the PVN of FK506-treated rats markedly decreased RSNA, MAP, and HR (n = 9 rats, Figure 5B and 5D). In these rats receiving prior microinjection of α2δ−1CT peptide, subsequent microinjection of AP5 into the PVN had no further effect on RSNA, MAP, or HR (Figure 5B and 5D). These results suggest that α2δ−1–bound NMDARs in the PVN are required to support the heightened sympathetic outflow caused by the calcineurin inhibitor.
Figure 5. α2δ−1–bound NMDARs in the PVN maintain elevated sympathetic vasomotor activity in CIH.
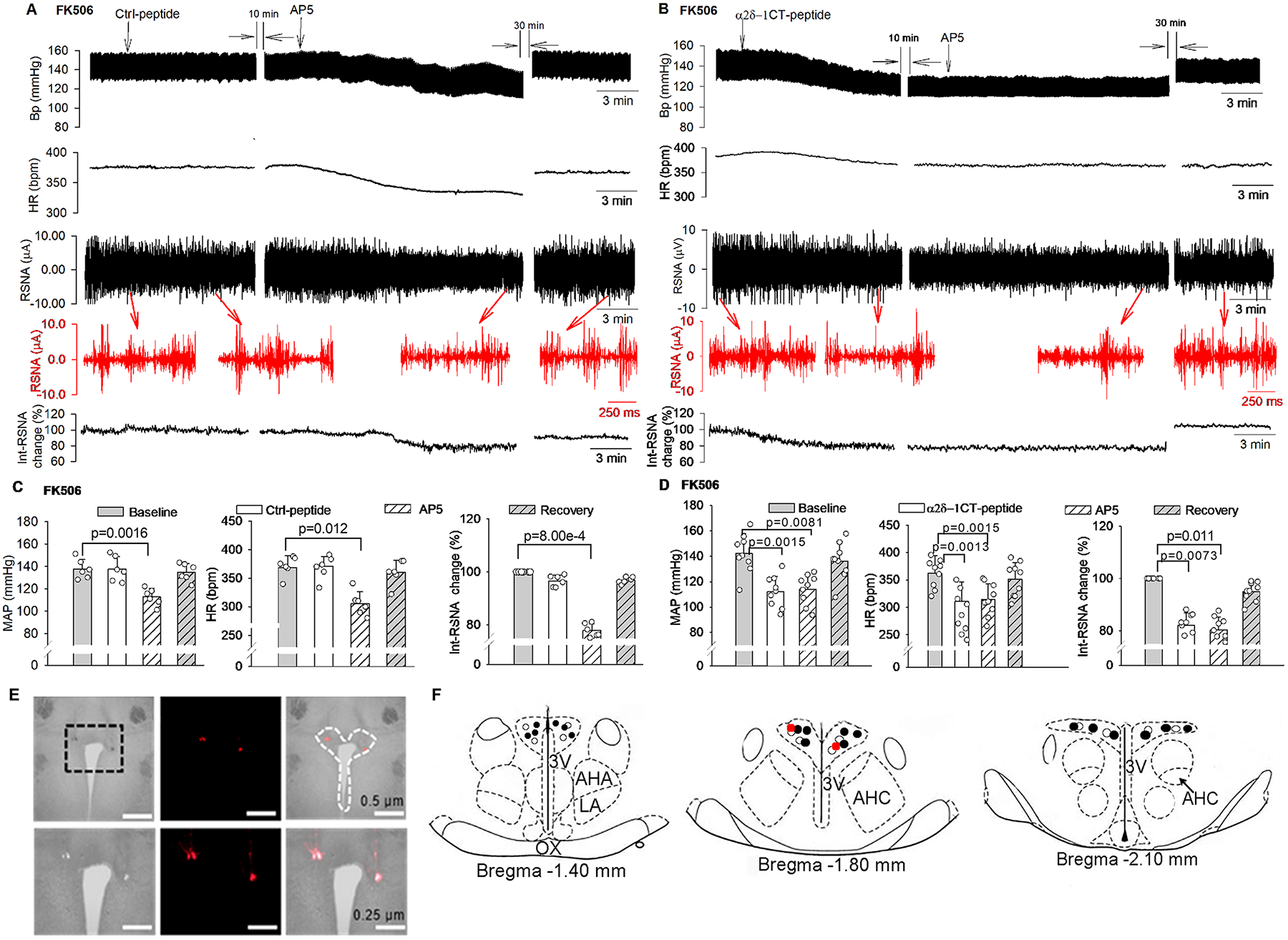
A and B, Original recording traces show the effects of bilateral microinjection of control (Ctrl) peptide (50 pmol in 50 nL, A) or α2δ−1CT peptide (50 pmol in 50 nL, B) followed by AP5 (1.0 nmol in 50 nL) into the PVN on arterial blood pressure (Bp), heart rate (HR), and integrated renal sympathetic activity (Int-RSNA) in FK506-treated rats. Insets (in red): expanded raw recording traces of RSNA. C and D, Mean data show changes in mean arterial blood pressure (MAP), HR, and Int-RSNA in FK506-treated rats after microinjection of Ctrl peptide (C, n = 6 male rats) or α2δ−1CT peptide (D, n = 9 male rats) followed by AP5 into the PVN. Repeated measures ANOVA with Dunnett’s post hoc test was used in C and D. E and F, Representative low- and high-magnification brightfield and fluorescence images (E) and schematic drawings (F, the injection sites corresponding to E are indicated in red dots) show microinjection sites in the PVN in FK506-treated rats. ○, microinjection sites in FK506-treated rats in C; ●, microinjection sites in FK506-treated rats in D. 3V, third ventricle; AHA, anterior hypothalamic area; AHC, central division of the anterior hypothalamus; LA, latero-anterior hypothalamus; OX, optic chiasm.
Systemic administration of gabapentin is effective for treating CIH in rats
To substantiate the clinical relevance of our findings, we next determined whether systemic treatment with gabapentin is effective against CIH. The gabapentin binding in the brain and other tissues is diminished in Cacna2d1 KO mice,43 and the in vivo doses of gabapentin required for effective α2δ−1 inhibition have been well documented.25,26,34,46,47 Radiotelemetry recording showed that a single intraperitoneal injection of gabapentin (60 or 100 mg/kg), which is effective in reducing pain hypersensitivity in rodent models,46,48 had no significant effect on MAP or HR in vehicle-treated male rats (n = 6 rats, Figure 6A and 6B). By contrast, a single intraperitoneal injection of gabapentin (60 mg/kg) rapidly reduced MAP and HR in FK506-treated male rats (n = 6 rats; Figure 6A and 6B). The inhibitory effect occurred within 30 minutes after gabapentin injection and lasted about 150 minutes. Intraperitoneal injection of 100 mg/kg similarly attenuated MAP and HR in FK506-treated male rats (n = 6 rats, Figure 6A and 6B). Similarly, intraperitoneal injection of 60 mg/kg or 100 mg/kg gabapentin rapidly reduced MAP and HR in FK506-treated female rats but had no such effect in vehicle-treated female rats (n = 6 rats per group, Figure S5).
Figure 6. Inhibiting α2δ−1 with gabapentin abrogates the development of CIH in rats.
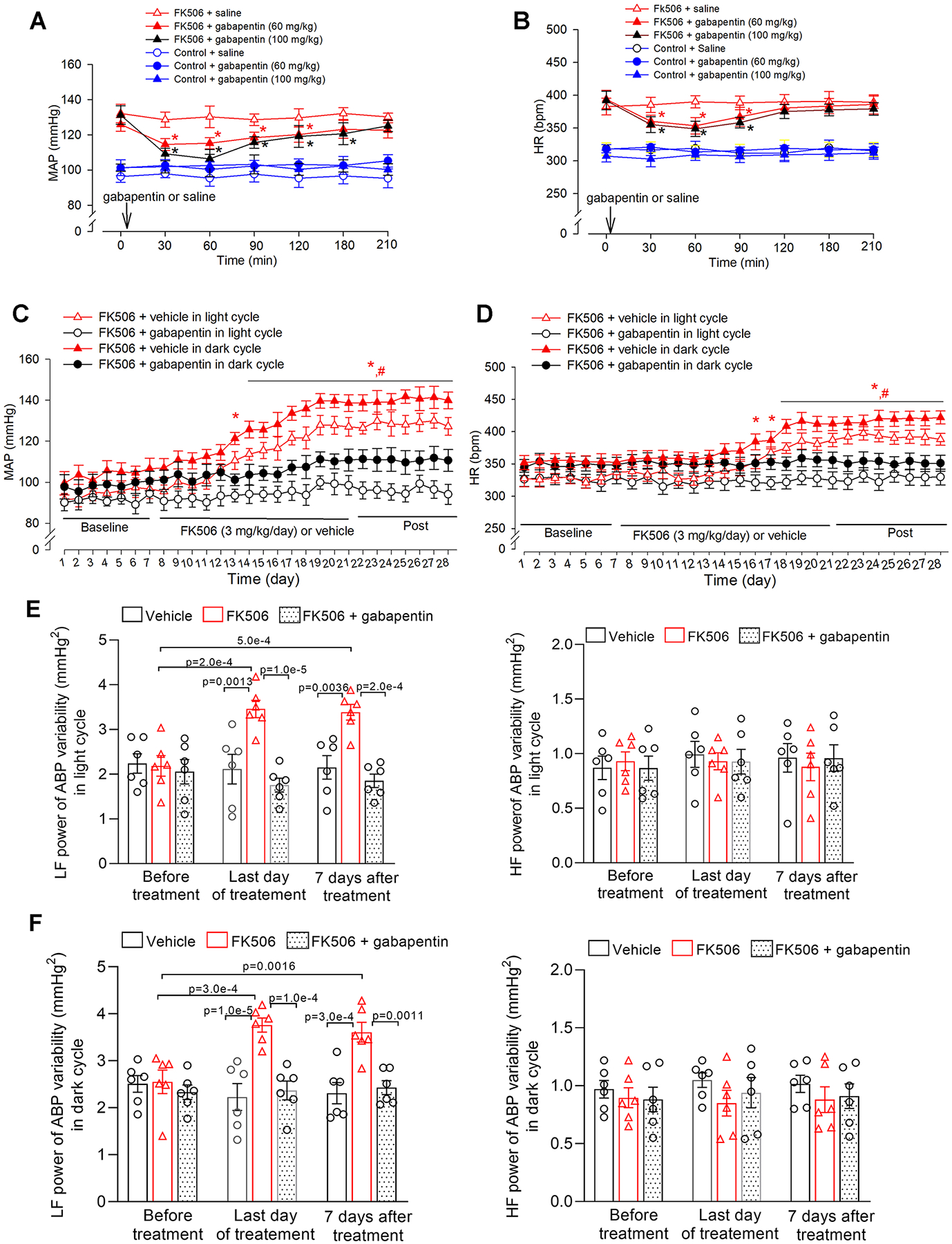
A and B, Radiotelemetry recording data show acute effects of intraperitoneal injection of gabapentin (60 mg/kg or 100 mg/kg) on mean arterial blood pressure (MAP, A) and heart rate (HR, B) in conscious rats treated with FK506 or vehicle (n = 6 male rats per group). C and D, Radiotelemetry recording shows the effect of concurrent treatment with vehicle or gabapentin (60 mg/kg/day in drinking water) on MAP (C) and HR (D) in FK506-treated male rats during light and dark cycles (n = 6 male rats per group). E and F, Power spectral analysis of systolic ABP variability shows changes in low-frequency (LF) and high-frequency (HF) power in FK506-treated male rats concurrently treated with vehicle or gabapentin during light (E) and dark (F) cycles; n = 6 rats per group). *P < 0.05, compared with respective baseline values within the same group. #P < 0.05, compared with respective values in the FK506 + gabapentin group at the same time point during light/dark cycles. Repeated measures ANOVA with Dunnett’s post hoc test was used in A and B; two-way ANOVA with Bonferroni’s post hoc test was used in C, D, E, and F. Exact P values are shown in Tables S1–S4 in Supplemental Materials.
Gabapentinoids (i.e., gabapentin and pregabalin) are orally active drugs for treating patients with epilepsy and neuropathic pain. We next determined whether concurrent treatment with gabapentin and FK506 is effective in attenuating the development of CIH. Male rats were given gabapentin, at a dose of 60 mg/kg/day49, in the drinking water during daily systemic injection of FK506 (3 mg/kg/day) for 14 days. Radiotelemetry recording showed that, compared with vehicle treatment, concurrent treatment with gabapentin diminished the increase in MAP and HR in both light and dark cycles in FK506-treated rats (n = 6 rats per group, Figure 6C and 6D).
We also performed power spectrum analysis of systolic ABP variability to determine whether it could be a useful index of sympathetic outflow in conscious animals. Prolonged treatment with FK506 significantly increased the low-frequency power in light and dark cycles when hypertension was developed (n = 6 rats per group, Figure 6E and 6F). Remarkably, concurrent treatment with gabapentin reversed the increased low-frequency power in both light and dark cycles caused by FK506 treatment (Figure 6E and 6F). Treatment with FK506 alone or with gabapentin did not significantly change the high-frequency power of systolic ABP variability. Together, these data provide strong evidence that inhibiting α2δ−1 with gabapentinoids is highly effective in treating CIH by attenuating elevated sympathetic output.
α2δ−1 is integral to the development of CIH in mice
In addition, we took the advantage of available Cacna2d1 KO mice to ascertain the role of α2δ−1 in the development of CIH. After implanting radiotelemetry, WT and Cacna2d1 KO mice were injected intraperitoneally with FK506 (3 mg/kg/day) for consecutive 14 days. The baseline MAP and HR in light and dark cycles were similar between WT and Cacna2d1 KO mice before FK506 treatment. Similar to rats, treatment with FK506 gradually and profoundly increased MAP and HR in light and dark cycles in WT mice only (Figure 7A and 7B). The increase in MAP and HR persisted at least 7 days after discontinuation of FK506 treatment in WT mice. Strikingly, prolonged treatment with FK506 failed to significantly increase MAP or HR in light and dark cycles in Cacna2d1 KO mice (Figure 7A and 7B). As expected, treatment with vehicle for 14 days had no statistically significant effect on MAP or HR in WT and Cacna2d1 KO mice (n = 6 mice per group; Figure S6).
Figure 7. Genetic ablation of α2δ−1 prevents the development of CIH in mice.
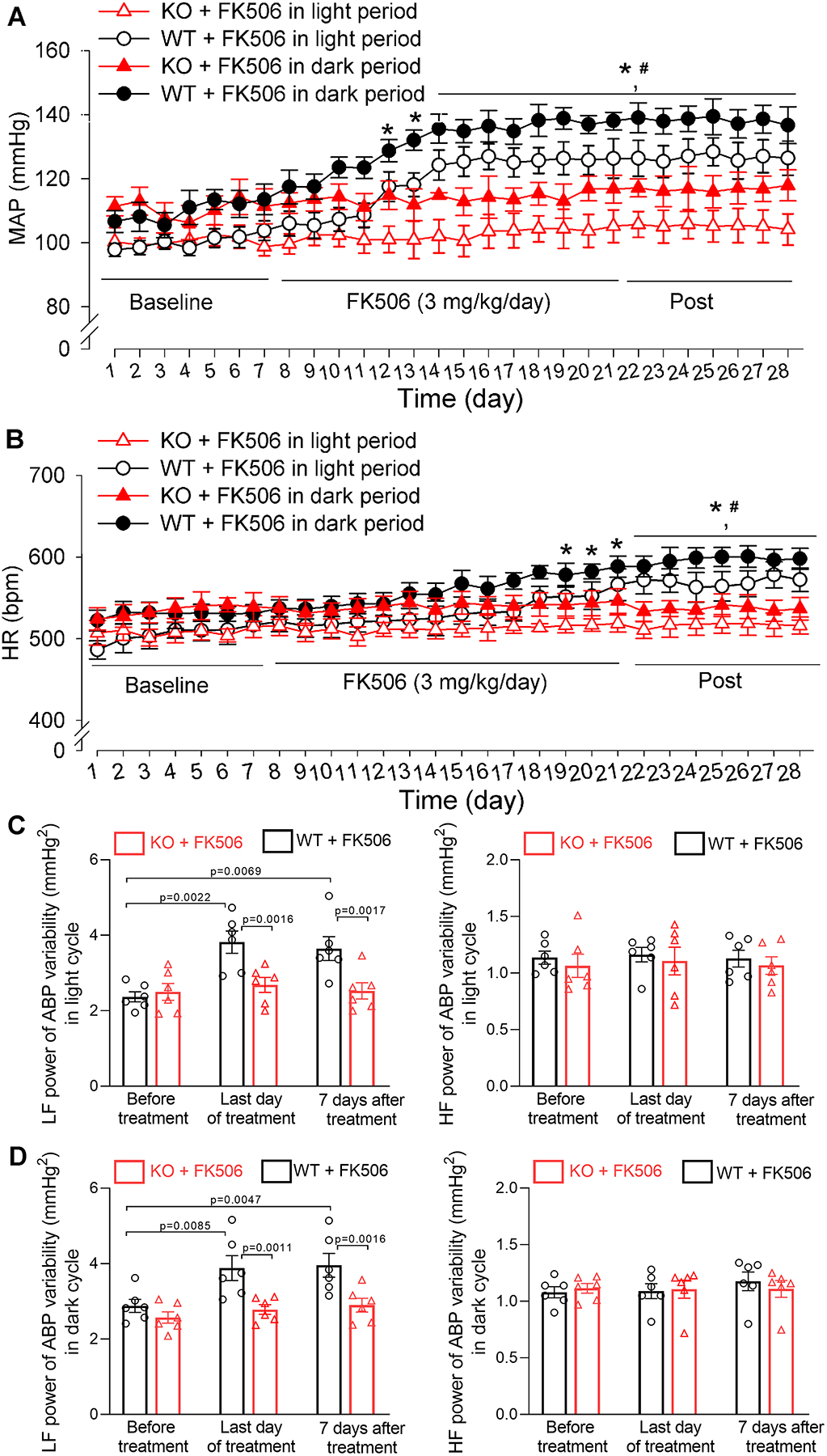
A and B, Radiotelemetry recording data show the time course of changes in mean arterial blood pressure (MAP, A) and heart rate (HR, B) in FK506-treated wild-type mice (WT) and FK506-treated Cacna2d1 knockout mice (KO) during light and dark cycles (n = 3 male and 3 female mice per group). C and D, Power spectral analysis of systolic ABP variability shows the changes in low-frequency (LF) and high-frequency (HF) power in FK506-treated WT and FK506-treated Cacna2d1 KO mice during light cycle (C) and dark cycle (D) (n = 6 mice per group). *P < 0.05, compared with respective baseline values in FK506-treated WT mice during light/dark cycles (repeated measures ANOVA with Dunnett’s post hoc test). #P < 0.05, compared with respective values in FK506-treated Cacna2d1 KO mice at the same time point during light/dark cycles (two-way ANOVA with Bonferroni’s post hoc test). Exact P values are shown in Tables S5 and S6 in Supplemental Materials.
The low- and high-frequency power of systolic ABP variability did not differ significantly between WT and Cacna2d1 KO mice at the baseline before FK506 treatment (n = 6 mice per group). Prolonged treatment with FK506 in WT mice significantly increased the low-frequency power in light and dark cycles when hypertension developed (Figure 7C and 7D). By contrast, treatment with FK506 had no such effects in Cacna2d1 KO mice. FK506 treatment did not significantly change high-frequency power of systolic ABP variability in either WT or Cacna2d1 KO mice (Figure 7C and 7D). These findings indicate that α2δ−1 is indispensable for the development of CIH and is a promising therapeutic target for CIH.
Discussion
Our study reveals that calcineurin inhibition increases NMDAR interactions with α2δ−1 and their synaptic trafficking in the PVN, extending our recent findings that the “on-target” effect of calcineurin inhibitors in the brain is the major cause of CIH. Calcineurin inhibitors have revolutionized transplant medicine by significantly prolonging graft survival and minimizing rates of acute rejection. However, persistent hypertension is a major adverse effect associated with long-term use of calcineurin inhibitors. Both cyclosporine and FK506 can readily cross the blood-brain barrier and impair normal calcineurin activity in the brain.50,51 Systemically administered FK506 profoundly inhibits calcineurin activity in the forebrain but not brainstem.5 NMDARs are highly mobile at synapses, and their synaptic levels are critically controlled by trafficking from the intracellular pool.52 NMDAR phosphorylation in the hypothalamus is balanced by relative activities of protein phosphatases and kinases, including PKC, casein kinase II, and calcineurin.5,29,45 Inhibition of the phosphatase activity by calcineurin inhibitors increases phosphorylation and synaptic expression levels of NMDARs in the PVN.5 α2δ−1 is a highly glycosylated protein that promotes synaptic and surface expression of its interacting proteins.53 α2δ−1 preferentially interacts with phosphorylated NMDARs to promote NMDAR surface trafficking in the cell line and spinal cord independently of VGCCs.26 Because α2δ−1 predominantly binds to phosphorylated NMDARs, it primarily mediates neuronal activity–dependent NMDAR hyperactivity. In this study, we demonstrated that inhibiting α2δ−1 with gabapentin or disrupting the α2δ−1–NMDAR interaction with α2δ−1CT peptide normalized synaptic levels of NMDAR in the PVN that had been increased by calcineurin inhibition, suggesting that α2δ−1 is required for NMDAR synaptic trafficking in the PVN associated with CIH. It is likely that calcineurin inhibition potentiates NMDAR phosphorylation and subsequently increases the α2δ−1–NMDAR interaction, augmenting NMDAR trafficking at the synapses in the PVN.
Another new finding of our study is that α2δ−1 is integral to the potentiation of synaptic NMDAR activity in PVN presympathetic neurons in CIH. Normal endogenous calcineurin activity in the nervous system constitutively restricts synaptic activity of NMDARs by limiting their phosphorylation levels.17,28 We showed recently that calcineurin inhibition augments the activity of NMDAR at presynaptic and postsynaptic sites in PVN presympathetic neurons.5 In this study, by recording AP5-sensitive presynaptic release of glutamate, we showed that presynaptic NMDARs in the PVN are functionally quiescent under normal conditions, which is similar to findings in other brain regions such as the nucleus accumbens37 and striatum.54 We found that treatment with gabapentin, α2δ−1CT peptide, or genetic Cacna2d1 KO fully reversed the calcineurin inhibitor–potentiated presynaptic and postsynaptic NMDAR activity in spinally projecting PVN neurons. Previous work indicates that FK506 treatment has no effect on calcineurin activity and NMDAR phosphorylation in the rostral ventrolateral medulla (RVLM)5 and that RVLM projecting and spinally projecting neurons in the PVN have the same functional properties.14,27,36,55,56 Also, RVLM projecting PVN neurons ultimately synapse with spinal cord neurons to control sympathetic outflow.14–16 Because FK506 treatment similarly increased NMDAR activity in RVLM projecting and spinally projecting neurons in the PVN,5 α2δ−1 likely has a comparable role in calcineurin inhibitor–induced NMDAR hyperactivity in both RVLM projecting and spinally projecting neurons in the PVN.
Although α2δ−1 has long been considered a VGCC subunit, blocking α2δ−1 by gabapentin or Cacna2d1 KO has little effect on overall VGCC activity25,57,58 or VGCC-mediated neurotransmitter release at presynaptic terminals.59,60 α2δ−1 predominantly interacts with NMDARs via its C-terminal domain,25 whereas α2δ−1 associates with VGCCs via the von Willebrand factor type A domain near the N terminus.61 We showed that α2δ−1CT peptide fully reversed FK506-induced synaptic NMDAR hyperactivity in presympathetic PVN neurons. Thus, it is unlikely that VGCCs are involved in glutamatergic synaptic plasticity in the PVN in CIH. Because α2δ−1 is extensively expressed in the central nervous system,62,63 calcineurin inhibitors may likewise increase the α2δ−1–NMDAR interaction to augment NMDAR activity in other forebrain regions, such as circumventricular organs,64,65 which could also contribute to augmented sympathetic outflow in CIH.
We also provide new in vivo evidence that α2δ−1–coupled NMDAR in the PVN is the key substrate required to maintain elevated sympathetic vasomotor activity caused by FK506 treatment. The PVN is the interface between the nervous and endocrine systems and plays a crucial role in coordinating sympathetic output.14 NMDAR-driven glutamatergic input in the PVN contributes predominantly to increased sympathetic outflow in the rat model of CIH.5 In this study, we revealed that disrupting the α2δ−1–NMDAR interaction with α2δ−1CT peptide in the PVN markedly reduced the elevated renal sympathetic nerve discharges and ABP by FK506 treatment. By contrast, microinjection of α2δ−1CT peptide into the PVN had no effect on the baseline RSNA or ABP in vehicle-treated rats. Together, our findings suggest that diminished phosphatase activity by calcineurin inhibitors increases NMDAR phosphorylation in the PVN, which leads to enhanced physical interaction of phosphorylated NMDARs with α2δ−1. This increased α2δ−1 and NMDAR association can subsequently augment synaptic trafficking and activity of NMDARs, thereby augmenting excitatory glutamatergic input to PVN presympathetic neurons and sympathetic vasomotor activity (Figure S7).
Our findings have clear clinical implications, because targeting α2δ−1 with gabapentinoids or α2δ−1–bound NMDARs with α2δ−1CT peptides could represent a better option for treating CIH. We showed in this study that systemic administration of gabapentin rapidly attenuated ABP and HR elevated in the animal model of CIH but had no such effect in vehicle-treated rats. Strikingly, concurrent treatment with gabapentin in rats or α2δ−1 genetic KO in mice prevented the development of hypertension caused by prolonged FK506 treatment, indicating the functional significance of α2δ−1 proteins in maintaining high sympathetic vasomotor tone in CIH. Compared with clinically used NMDAR antagonists, such as ketamine and memantine, gabapentin and pregabalin have far fewer adverse effects because they target mainly α2δ−1–bound NMDARs but do not affect basal, α2δ−1–free NMDARs.25,54
Some sex differences in ABP regulation and brain NMDAR activity have been reported.66,67 However, we found that FK506 treatment caused a similar increase in ABP and HR in male and female rats. Furthermore, gabapentin equally reduced ABP in FK506-treated male and female rats, suggesting that α2δ−1 likely has a similar role in CIH in both sexes. Previous studies on the mechanisms of CIH have focused largely on the kidney and peripheral blood vessels.6,68,69 The reported effects of calcineurin inhibitors on the kidney and blood vessels may be secondary to increased sympathetic vasomotor tone in CIH. In this regard, calcineurin inhibitor–induced excess sympathetic outflow and augmented renal sympathetic nerve activity could increase renin secretion and activation of the renin-angiotensin system, which can cause vasoconstriction and sodium retention.70,71 Remarkably, renal denervation abrogates sodium retention by calcineurin inhibition, suggesting that increased sympathetic nerve activity plays a key role in the renal effect of calcineurin inhibitors.6 A single injection of calcineurin inhibitors acutely stimulates renal afferent nerves.72 However, patients with kidney transplantation still develop CIH,2–4 suggesting that renal innervation is not essential for CIH. Nonetheless, renal afferent nerve stimulation by calcineurin inhibitors may indirectly impact NMDAR activity in the hypothalamus via activating the renin-angiotensin system.29,34
In summary, our study uncovers a new mechanism in which α2δ−1, particularly α2δ−1–bound NMDARs in the PVN, plays an essential role in calcineurin inhibitor–induced excess sympathetic outflow and hypertension. Our findings provide strong evidence linking α2δ−1 to increased synaptic NMDAR activity in the PVN and elevated sympathetic vasomotor activity in CIH. These findings not only offer new mechanistic insight into the pathogenesis of CIH but also suggest alternative, mechanism-based therapeutic targets for preventing and treating CIH. Owing to the adverse effects of the withdrawal of immunosuppressants in patients with transplants and autoimmune diseases, calcineurin inhibitors are rarely discontinued because of hypertension. Although NMDAR antagonists effectively reduce CIH,5 these drugs produce serious CNS adverse effects. Alternatively, gabapentinoids and α2δ−1CT interfering peptides have no effect on physiological, α2δ−1–free NMDARs, and thus might circumvent the adverse effects caused by general NMDAR antagonists. Because gabapentinoids are orally bioavailable, FDA-approved drugs, these agents could be readily repurposed for treating patients with CIH. Further research is warranted to validate the efficacy of gabapentinoids in patients with CIH.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Clinically used calcineurin inhibitors including immunosuppressants for transplant rejection and for treating autoimmune diseases may cause persistent hypertension.
Calcineurin inhibitors elevate sympathetic vasomotor activity by diminishing calcineurin activity and potentiating NMDA receptor activity in the hypothalamus.
What New Information Does This Article Contribute?
Calcineurin inhibition increases α2δ−1–NMDA receptor interactions and induces α2δ−1–dependent NMDA receptor synaptic expression and hyperactivity in the hypothalamus.
Disrupting α2δ−1–NMDA receptor interactions in the hypothalamus diminishes calcineurin inhibitor–potentiated sympathetic vasomotor activity.
Systemic treatment with gabapentin or α2δ−1 genetic knockout prevents the development of calcineurin inhibitor–induced hypertension.
Calcineurin inhibitors are immunosuppressants used clinically to treat autoimmune disorders and transplant rejection. However, their prolonged use often leads to sustained hypertension, which adversely affects allograft and patient survival. Calcineurin inhibitors augment sympathetic vasomotor activity by diminishing calcineurin activity and potentiating NMDA receptor activity in the hypothalamus. Our study demonstrates the essential role of α2δ−1 (previously known as a calcium channel subunit) in the hypothalamus in the development of elevated sympathetic outflow and hypertension caused by calcineurin inhibitors. We demonstrated that systemic treatment with tacrolimus augments α2δ−1–NMDA receptor interactions and their synaptic trafficking and activity in the hypothalamus. Inhibition of α2δ−1 with gabapentin or α2δ−1 genetic knockout abolishes tacrolimus-induced NMDA receptor hyperactivity of sympathetic-related neurons in the hypothalamus. Disrupting α2δ−1–NMDA receptor interactions in the hypothalamus reverses the tacrolimus-induced sympathetic outflow. Strikingly, concurrent treatment with gabapentin or using a α2δ−1 genetic knockout prevents the development of tacrolimus-induced hypertension. These findings uncover novel molecular mechanisms underlying the heightened sympathetic nervous system response caused by calcineurin inhibitors. Gabapentinoids (gabapentin and pregabalin), commonly used for chronic pain and epilepsy management, hold potential for repurposing in the treatment of calcineurin inhibitor-induced neurogenic hypertension.
Acknowledgments
The authors would like to thank Lei Feng in the Department of Biostatistics at MD Anderson Cancer Center for statistical consultation and support.
Sources of Funding
This study was supported by a grant (HL154512) from the National Institutes of Health and by the Pamela and Wayne Garrison Distinguished Chair Endowment.
Nonstandard Abbreviations and Acronyms:
- ABP
arterial blood pressure
- AP5
2-amino-5-phosphonopentanoic acid
- CIH
calcineurin inhibitor–induced hypertension
- HR
heart rate
- mEPSC
miniature excitatory postsynaptic current
- NMDAR
N-methyl-D-aspartate receptor
- PVN
paraventricular nuclear
- RSNA
renal sympathetic nerve activity
- RVLM
rostral ventrolateral medulla
- VGCC
voltage-gated calcium channel
Footnotes
Disclosures
None.
References
- 1.Scherrer U, Vissing SF, Morgan BJ, Rollins JA, Tindall RS, Ring S, Hanson P, Mohanty PK and Victor RG. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323:693–9. [DOI] [PubMed] [Google Scholar]
- 2.Textor SC, Canzanello VJ, Taler SJ, Wilson DJ, Schwartz LL, Augustine JE, Raymer JM, Romero JC, Wiesner RH, Krom RA and et al. Cyclosporine-induced hypertension after transplantation. Mayo Clin Proc. 1994;69:1182–93. [DOI] [PubMed] [Google Scholar]
- 3.Mange KC, Cizman B, Joffe M and Feldman HI. Arterial hypertension and renal allograft survival. JAMA. 2000;283:633–8. [DOI] [PubMed] [Google Scholar]
- 4.Koomans HA and Ligtenberg G. Mechanisms and consequences of arterial hypertension after renal transplantation. Transplantation. 2001;72:S9–12. [DOI] [PubMed] [Google Scholar]
- 5.Zhou JJ, Shao JY, Chen SR and Pan HL. Calcineurin controls hypothalamic NMDA receptor activity and sympathetic outflow. Circ Res. 2022;131:345–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moss NG, Powell SL and Falk RJ. Intravenous cyclosporine activates afferent and efferent renal nerves and causes sodium retention in innervated kidneys in rats. Proc Natl Acad Sci U S A. 1985;82:8222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu PJ, Vemulapalli S, Sabin C, Rivelli M, Bernardino V and Sybertz EJ. Sympathoadrenal stimulation, not endothelin, plays a role in acute pressor response to cyclosporine in anesthetized rats. J Pharmacol Exp Ther. 1992;261:994–9. [PubMed] [Google Scholar]
- 8.Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, O’Shaughnessy EA, Dahl DC, Silkensen JR, Sahadevan M and Snyder JJ. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43:1071–81. [DOI] [PubMed] [Google Scholar]
- 9.El-Mas MM, Omar AG, Helmy MM and Mohy El-Din MM. Interruption of central neuronal pathway of imidazoline I1 receptor mediates the hypertensive effect of cyclosporine in rats. Brain Res. 2009;1248:96–106. [DOI] [PubMed] [Google Scholar]
- 10.Morgan BJ, Lyson T, Scherrer U and Victor RG. Cyclosporine causes sympathetically mediated elevations in arterial pressure in rats. Hypertension. 1991;18:458–66. [DOI] [PubMed] [Google Scholar]
- 11.Grobecker HF, Riebel K and Wellenhofer T. Cyclosporine A-induced hypertension in SHR and WKY: role of the sympatho-adrenal system. Clin Exp Pharmacol Physiol Suppl. 1995;22:S94–5. [DOI] [PubMed] [Google Scholar]
- 12.Li DP and Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007;49:916–25. [DOI] [PubMed] [Google Scholar]
- 13.Zhou JJ, Shao JY, Chen SR, Li DP and Pan HL. α2δ−1–dependent NMDA receptor activity in the hypothalamus is an effector of genetic-environment interactions that drive persistent hypertension. J Neurosci. 2021;41:6551–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dampney RA, Michelini LC, Li DP and Pan HL. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am J Physiol Heart Circ Physiol. 2018;315:H1200–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranson RN, Motawei K, Pyner S and Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res. 1998;120:164–72. [DOI] [PubMed] [Google Scholar]
- 16.Pyner S and Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–56. [DOI] [PubMed] [Google Scholar]
- 17.Tong G, Shepherd D and Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–2. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Chen SR and Pan HL. Calcineurin regulates synaptic plasticity and nociceptive transmission at the spinal cord level. Neuroscientist. 2022;28:628–638. [DOI] [PubMed] [Google Scholar]
- 19.Chen SR, Hu YM, Chen H and Pan HL. Calcineurin inhibitor induces pain hypersensitivity by potentiating pre- and postsynaptic NMDA receptor activity in spinal cords. J Physiol. 2014;592:215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li DP, Byan HS and Pan HL. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci. 2012;32:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuno T, Mukai H, Ito A, Chang CD, Kishima K, Saito N and Tanaka C. Distinct cellular expression of calcineurin A alpha and A beta in rat brain. J Neurochem. 1992;58:1643–51. [DOI] [PubMed] [Google Scholar]
- 22.Hansen KB, Yi F, Perszyk RE, Furukawa H, Wollmuth LP, Gibb AJ and Traynelis SF. Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol. 2018;150:1081–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau A and Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525–42. [DOI] [PubMed] [Google Scholar]
- 24.Thomas SJ and Grossberg GT. Memantine: a review of studies into its safety and efficacy in treating Alzheimer’s disease and other dementias. Clin Interv Aging. 2009;4:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Li L, Chen SR, Chen H, Xie JD, Sirrieh RE, MacLean DM, Zhang Y, Zhou MH, Jayaraman V and Pan HL. The α2δ−1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep. 2018;22:2307–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou MH, Chen SR, Wang L, Huang Y, Deng M, Zhang J, Zhang J, Chen H, Yan J and Pan HL. Protein kinase C-mediated phosphorylation and α2δ−1 interdependently regulate NMDA receptor trafficking and activity. J Neurosci. 2021;41:6415–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma H, Chen SR, Chen H, Zhou JJ, Li DP and Pan HL. α2δ−1 couples to NMDA receptors in the hypothalamus to sustain sympathetic vasomotor activity in hypertension. J Physiol. 2018;596:4269–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Chen SR, Chen H, Luo Y and Pan HL. Calcineurin inhibition causes α2δ−1–mediated tonic activation of synaptic NMDA receptors and pain hypersensitivity. J Neurosci. 2020;40:3707–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma H, Chen SR, Chen H and Pan HL. Endogenous AT1 receptor-protein kinase C activity in the hypothalamus augments glutamatergic input and sympathetic outflow in hypertension. J Physiol. 2019;597:4325–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li DP, Zhou JJ, Zhang J and Pan HL. CaMKII regulates synaptic NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Neurosci. 2017;37:10690–10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye ZY, Li DP, Byun HS, Li L and Pan HL. NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J Neurosci. 2012;32:8560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva LEV, Geraldini VR, de Oliveira BP, Silva CAA, Porta A and Fazan R. Comparison between spectral analysis and symbolic dynamics for heart rate variability analysis in the rat. Sci Rep. 2017;7:8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baudrie V, Laude D and Elghozi JL. Optimal frequency ranges for extracting information on cardiovascular autonomic control from the blood pressure and pulse interval spectrograms in mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R904–12. [DOI] [PubMed] [Google Scholar]
- 34.Ma H, Chen SR, Chen H, Li L, Li DP, Zhou JJ and Pan HL. α2δ−1 is essential for sympathetic output and NMDA receptor activity potentiated by angiotensin II in the hypothalamus. J Neurosci. 2018;38:6388–6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li DP, Chen SR and Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li DP, Yang Q, Pan HM and Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008;586:1637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin D, Chen H, Chen SR and Pan HL. α2δ−1 protein drives opioid-induced conditioned reward and synaptic NMDA receptor hyperactivity in the nucleus accumbens. J Neurochem. 2022;164:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paxinos G and Watson C. The Rat Brain in Stereotaxic Coordinates: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 39.Loughran TP Jr., Deeg HJ, Dahlberg S, Kennedy MS, Storb R and Thomas ED. Incidence of hypertension after marrow transplantation among 112 patients randomized to either cyclosporine or methotrexate as graft-versus-host disease prophylaxis. Br J Haematol. 1985;59:547–53. [DOI] [PubMed] [Google Scholar]
- 40.Snanoudj R, Kriaa F, Arzouk N, Beaudreuil S, Hiesse C, Durrbach A and Charpentier B. Single-center experience with cyclosporine therapy for kidney transplantation: analysis of a twenty-year period in 1200 patients. Transplant Proc. 2004;36:83s–88s. [DOI] [PubMed] [Google Scholar]
- 41.Taler SJ, Textor SC, Canzanello VJ and Schwartz L. Cyclosporin-induced hypertension: incidence, pathogenesis and management. Drug safety. 1999;20:437–49. [DOI] [PubMed] [Google Scholar]
- 42.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R and Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J Biol Chem. 1996;271:5768–76. [DOI] [PubMed] [Google Scholar]
- 43.Fuller-Bicer GA, Varadi G, Koch SE, Ishii M, Bodi I, Kadeer N, Muth JN, Mikala G, Petrashevskaya NN, Jordan MA, Zhang SP, Qin N, Flores CM, Isaacsohn I, Varadi M, Mori Y, Jones WK and Schwartz A. Targeted disruption of the voltage-dependent calcium channel α2δ−1-subunit. Am J Physiol Heart Circ Physiol. 2009;297:H117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo Y, Ma H, Zhou JJ, Li L, Chen SR, Zhang J, Chen L and Pan HL. Focal cerebral ischemia and reperfusion induce brain injury through α2δ−1-bound NMDA receptors. Stroke. 2018;49:2464–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye ZY, Li DP, Li L and Pan HL. Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. J Neurosci. 2011;31:8271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang GF, Chen SR, Jin D, Huang Y, Chen H and Pan HL. α2δ−1 upregulation in primary sensory neurons promotes NMDA receptor-mediated glutamatergic input in resiniferatoxin-induced neuropathy. J Neurosci. 2021;41:5963–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Chen SR, Zhou MH, Jin D, Chen H, Wang L, DePinho RA and Pan HL. HDAC2 in primary sensory neurons constitutively restrains chronic pain by repressing α2δ−1 expression and associated NMDA receptor activity. J Neurosci. 2022;42:8918–8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan HL, Eisenach JC and Chen SR. Gabapentin suppresses ectopic nerve discharges and reverses allodynia in neuropathic rats. J Pharmacol Exp Ther. 1999;288:1026–30. [PubMed] [Google Scholar]
- 49.Chen SR, Samoriski G and Pan HL. Antinociceptive effects of chronic administration of uncompetitive NMDA receptor antagonists in a rat model of diabetic neuropathic pain. Neuropharmacology. 2009;57:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murakami Y, Takamatsu H, Noda A, Osoda K, Ichise R, Tatsumi M, Tabata K, Sawamoto T and Nishimura S. Pharmacokinetic animal PET study of FK506 as a potent neuroprotective agent. J Nucl Med. 2004;45:1946–9. [PubMed] [Google Scholar]
- 51.Gottschalk S, Cummins CL, Leibfritz D, Christians U, Benet LZ and Serkova NJ. Age and sex differences in the effects of the immunosuppressants cyclosporine, sirolimus and everolimus on rat brain metabolism. Neurotoxicology. 2011;32:50–7. [DOI] [PubMed] [Google Scholar]
- 52.Wenthold RJ, Prybylowski K, Standley S, Sans N and Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–58. [DOI] [PubMed] [Google Scholar]
- 53.Tétreault MP, Bourdin B, Briot J, Segura E, Lesage S, Fiset C and Parent L. Identification of glycosylation sites essential for surface expression of the CaVα2δ1 subunit and modulation of the cardiac CaV1.2 channel activity. J Biol Chem. 2016;291:4826–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou JJ, Li DP, Chen SR, Luo Y and Pan HL. The α2δ−1-NMDA receptor coupling is essential for corticostriatal long-term potentiation and is involved in learning and memory. J Biol Chem. 2018;293:19354–19364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li DP, Zhu LH, Pachuau J, Lee HA and Pan HL. mGluR5 Upregulation increases excitability of hypothalamic presympathetic neurons through NMDA receptor trafficking in spontaneously hypertensive rats. J Neurosci. 2014;34:4309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiao X, Zhou JJ, Li DP and Pan HL. Src kinases regulate glutamatergic input to hypothalamic presympathetic neurons and sympathetic outflow in hypertension. Hypertension. 2017;69:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rock DM, Kelly KM and Macdonald RL. Gabapentin actions on ligand- and voltage-gated responses in cultured rodent neurons. Epilepsy Res. 1993;16:89–98. [DOI] [PubMed] [Google Scholar]
- 58.Schumacher TB, Beck H, Steinhäuser C, Schramm J and Elger CE. Effects of phenytoin, carbamazepine, and gabapentin on calcium channels in hippocampal granule cells from patients with temporal lobe epilepsy. Epilepsia. 1998;39:355–63. [DOI] [PubMed] [Google Scholar]
- 59.Brown JT and Randall A. Gabapentin fails to alter P/Q-type Ca2+ channel-mediated synaptic transmission in the hippocampus in vitro. Synapse. 2005;55:262–9. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Chen SR, Chen H, Zhang J and Pan HL. Increased α2δ−1-NMDA receptor coupling potentiates glutamatergic input to spinal dorsal horn neurons in chemotherapy-induced neuropathic pain. J Neurochem. 2019;148:252–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor CP and Harris EW. Analgesia with gabapentin and pregabalin may involve N-Methyl-d-Aspartate receptors, neurexins, and thrombospondins. J Pharmacol Exp Ther. 2020;374:161–174. [DOI] [PubMed] [Google Scholar]
- 62.Cole RL, Lechner SM, Williams ME, Prodanovich P, Bleicher L, Varney MA and Gu G. Differential distribution of voltage-gated calcium channel α2δ subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. J Comp Neurol. 2005;491:246–69. [DOI] [PubMed] [Google Scholar]
- 63.Taylor CP and Garrido R. Immunostaining of rat brain, spinal cord, sensory neurons and skeletal muscle for calcium channel alpha2-delta (alpha2-delta) type 1 protein. Neuroscience. 2008;155:510–21. [DOI] [PubMed] [Google Scholar]
- 64.Hendel MD and Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2005;288:H680–H685. [DOI] [PubMed] [Google Scholar]
- 65.Stocker SD, Wenner MM, Farquhar WB and Browning KN. Activation of the organum vasculosum of the lamina terminalis produces a sympathetically mediated hypertension. Hypertension. 2022;79:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Briant LJ, Charkoudian N and Hart EC. Sympathetic regulation of blood pressure in normotension and hypertension: when sex matters. Exp Physiol. 2016;101:219–29. [DOI] [PubMed] [Google Scholar]
- 67.Hönack D and Löscher W. Sex differences in NMDA receptor mediated responses in rats. Brain Res. 1993;620:167–70. [DOI] [PubMed] [Google Scholar]
- 68.Meyer-Lehnert H and Schrier RW. Potential mechanism of cyclosporine A-induced vascular smooth muscle contraction. Hypertension. 1989;13:352–60. [DOI] [PubMed] [Google Scholar]
- 69.Curtis JJ, Luke RG, Jones P and Diethelm AG. Hypertension in cyclosporine-treated renal transplant recipients is sodium dependent. Am J Med. 1988;85:134–8. [DOI] [PubMed] [Google Scholar]
- 70.DiBona GF. Sympathetic nervous system influences on the kidney. Role in hypertension. Am J Hypertens. 1989;2:119s–124s. [DOI] [PubMed] [Google Scholar]
- 71.Pontes RB, Crajoinas RO, Nishi EE, Oliveira-Sales EB, Girardi AC, Campos RR and Bergamaschi CT. Renal nerve stimulation leads to the activation of the Na+/H+ exchanger isoform 3 via angiotensin II type I receptor. Am J Physiol Renal Physiol. 2015;308:F848–56. [DOI] [PubMed] [Google Scholar]
- 72.Zhang W and Victor RG. Calcineurin inhibitors cause renal afferent activation in rats: a novel mechanism of cyclosporine-induced hypertension. Am J Hypertens. 2000;13:999–1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Detailed methods can be found in the Supplemental Material.


