Abstract
The relationship between individual muscle dynamics and whole-body metabolic cost is not well established. Here we use biofeedback to modulate triceps surae (TS) activity during walking. We hypothesized: (1) increased TS activity would increase metabolic cost via shorter muscle fascicle lengths and thus reduced force capacity and (2) decreased TS activity would decrease metabolic cost via longer muscle fascicle lengths and thus increased force capacity. 23 young adults walked on an instrumented treadmill at 1.25 m/s using electromyographic (EMG) biofeedback to match targets corresponding to ±20 and ±40% TS activity during push-off (late stance). B-mode ultrasound imaged the medial gastrocnemius (MG). Participants increased net metabolic power up to 85% and 21% when targeting increased and decreased TS activity, respectively (p < 0.001). At the instant of peak gastrocnemius force, MG fascicle length was 7% shorter (p < 0.001) and gastrocnemius force was 6% larger (p < 0.001) when targeting +40% TS activity. Fascicle length was 3% shorter (p = 0.004) and force was 7% lower (p = 0.004) when targeting −40% TS activity. Participants were unable to achieve decreased activation targets. MG fascicle length and activity mediated 11.7% (p = 0.036) and 57.2% (p = 0.006) of the changes in net metabolic power, respectively. MG force did not mediate changes in net metabolic power (p = 0.948). These findings suggest that changes in the functional operating length of muscle, induced by volitional changes in TS activity, mediate the metabolic cost of walking, relatively independently of force. Thus, shifts to shorter fascicle lengths may mediate activity-induced increases in metabolic cost.
Keywords: Biomechanics, Metabolic Cost, Locomotion, Ultrasound, Plantarflexor
Introduction:
The triceps surae muscles (i.e., soleus and gastrocnemius), despite comprising less than 15% of the lower extremity muscle volume (Voronov, 2003), play a vital role in generating ankle push-off power and account for ~27% of the total metabolic cost of walking, (Umberger, 2010; Umberger and Rubenson, 2011). Forces generated by the triceps surae (TS) are governed by basic principles which prescribe an interdependent relation between neural excitation and muscle mechanics – a relation with significant metabolic implications. During the stance phase of walking, TS muscle fascicles receive some requisite level of activation to operate relatively isometrically in a narrow range of lengths near the peak of their force-length (F-L) relation (Rubenson et al., 2012). Previous work has introduced empirical relations between this requisite activation and requisite active muscle volume (Adams et al., 1992), an outcome which is in turn associated with the metabolic cost of operating the TS muscles (Beck et al., 2019; Griffin et al., 2003). Unfortunately, evidence suggests that hallmark increases in the whole-body metabolic cost of walking due to aging and/or gait pathology are accompanied by interdependent changes in TS activity and F-L operating range. For example, when older adults walk at the same speed as younger adults they use shorter TS fascicle lengths and require more muscle activity (Conway and Franz, 2020; Mian et al., 2007; Ortega and Farley, 2007; Panizzolo et al., 2013; Schmitz et al., 2009). Although basic principles, model predictions, and translational observations point to interdependence between muscle activation, F-L operating range, and metabolic cost, precisely how these relations manifest during walking remains unclear.
Theoretically, as muscles shift to lengths shorter than that at the peak of their F-L relation, their force-generating capacity (i.e., maximum force potential at that length) decreases (Beck et al., 2019; Monte et al., 2023). Accordingly, relatively shorter muscle fascicle lengths require increased activation to meet the force demands of a particular task. However, this neuromechanical interplay between a muscle’s F-L and activation dynamics is complex. Performing a particular task with increased muscle activation likely elicits shorter fascicle lengths and vice versa. For example, studies conducted using electromyographic (EMG) biofeedback have demonstrated that performing isolated contractions at higher TS activations elicits predictable shifts to shorter fascicle lengths (Clark and Franz, 2019; Hessel et al., 2021). Why would individuals walk with shorter TS operating lengths only to pay a metabolic penalty? One example is that an increase in tendon compliance, and thus an increase in tendon elongation per unit force transmitted, unavoidably provokes shorter fascicle lengths. Evidence of this structural bottleneck exists for older adults (Onambele et al., 2006) and people following stroke (Zhao et al., 2009), and the resultant effects on requisite muscle activation have been shown using model predictions (Orselli et al., 2017).
Beyond the need to activate a muscle more to produce a given force, shorter muscle fascicle lengths may also have intrinsic metabolic costs. During maximal activation, the ratio of ATP consumption to active force generation is nearly constant at optimal and longer-than-optimal fascicle lengths; however, at shorter-than-optimal lengths, this relationship uncouples, with up to an 87% increase in ATP consumption per unit force at 0.6lo (Hilber et al., 2001). Indeed, recent studies that use a dynamometer to isolate the TS while simultaneously using ultrasound to measure muscle FL dynamics and indirect calorimetry to measure whole-body metabolic cost suggest that functionally shorter muscles use a disproportionately more metabolic energy to produce the same force (Beck et al., 2022). However, the in vivo measurement of these neuromechanical relations during physiological loading in walking is challenging.
Ideally, cause-and-effect relations between muscle fascicle lengths and the metabolic cost of walking would be evaluated through an experimental protocol which directly manipulates fascicle length. For isometric contractions, muscle fascicle length can be manipulated via joint angle (Chleboun et al., 2001). In dynamic tasks like walking, this relationship is more complex. No currently available methods exist for real-time gastrocnemius fascicle length biofeedback. However, given fundamental relations between fascicle length and activation, electromyographic (EMG) biofeedback used previously during isolated contractions (Clark and Franz, 2019; Krupenevich et al., 2020) may provide a suitable alternative. TS EMG biofeedback has been used as a therapeutic strategy during walking (Colborne et al., 1994). In our experience, biofeedback also has an influential role as a means to unravel mechanistic relations underlying human neuromechanics (Clark and Franz, 2019; Munsch et al., 2020).
There are two translational implications that motivate this research. First, energetics models informed by experimentally determined relationships between muscle force, length, activation and metabolic energy use may help guide the personalized prescription of assistive devices. Second, clarifying the local muscle neuromechanical determinants of walking metabolism in young adults might provide a mechanistic framework to elucidate those in populations prone to fatigue and mobility impairment. Thus, the purpose of this study was to establish cause-and-effect relations between volitional changes in TS activation prescribed using biofeedback and walking metabolic cost, muscle mechanics, and force capacity. We hypothesized that (1) increased TS activation would increase metabolic cost via shorter muscle fascicle operating lengths and thus reduced force capacity and that (2) decreased TS activation would decrease metabolic cost via longer muscle fascicle operating lengths and thus increased force capacity. Together, these hypotheses serve to evaluate the overall premise that changes in functional muscle fascicle lengths drive requisite changes in muscle activation and metabolic cost of walking.
2. Methods:
2.1. Participants:
We recruited 23 young adults (age: 23±5 yrs., 13F/10M, mass: 67.9±20.4 kg, height: 1.73±0.1 m) to participate after providing written, informed consent according to the University of North Carolina Biomedical Sciences Institutional Review Board. Prior to participation, subjects were screened and excluded if they reported neurological disorders affecting the lower-extremity, musculoskeletal injury to the lower-extremity within the previous six months, or were currently taking medications that cause dizziness.
2.2. Experimental Protocol.
Data were collected over the course of two sessions on different days. For both sessions, subjects preconditioned their TS by walking for five minutes at 1.25 m/s on an instrumented split-belt treadmill (Bertec, Columbus, Ohio) prior to data collection. Session one consisted of a series of isometric contractions and session two consisted of a series of treadmill walking trials.
2.3. Session 1: Isolated Contractions
Participants were seated in a dynamometer (Biodex, Shirley, New York) at 85° hip flexion and 20° knee flexion. Subjects completed a series of maximal voluntary isometric plantarflexor contractions (MVIC) at six ankle joint angles (−20°, −10°, 0°, 10°, 20°, 30°). If a participants’ maximal dorsiflexion range of motion (ROM) was greater than 25°, a seventh joint angle was added that was equal to their maximum dorsiflexion ROM. We then corrected ankle joint moment for gravitational and passive moments. We calculated gastrocnemius force by scaling the TS muscle force, defined as the quotient of net ankle moment and participant-specific moment arm (described in S1–S2, Supplementary Material) by the relative physiological cross-sectional area of the gastrocnemius (24%) (Morse et al., 2005). We then calculated participant-specific force-angle relationships using a second-order polynomial fit. From these curves, we derived the maximum muscle force (Fmax) for each participant.
2.4. Session 2: Walking with Electromyographic Biofeedback.
Participants first walked for five minutes on an instrumented split-belt treadmill at 1.25 m/s while we measured TS activation using surface electromyography sensors (Delsys, Natick, Massachusetts) placed bilaterally on the lateral gastrocnemius and soleus, which were then averaged (Fig. 1). Participants then walked at 1.25 m/s while receiving near real-time biofeedback of their average TS activation. Specifically, EMG data were demeaned, full-wave rectified, and band-pass filtered (20-450 Hz) in pseudo real-time using custom MATLAB scripts (MathWorks, Natick, Massachusetts). During biofeedback trials, participants were shown a linear envelope EMG waveform that corresponded to the bilateral stance-phase average of the previous four steps (Fig. 1). The biofeedback display updated every four steps to display the average of the previous four steps. Participants were instructed to alter the magnitude of their peak TS activity during late stance to match a target line on the screen corresponding to, in four different trials, +20%, +40%, −20%, and −40% peak TS activity. The target line turned green within five percent of the target activity. Each walking trial with biofeedback lasted five minutes. Participants were given the opportunity to practice the biofeedback paradigm prior to data collection.
Figure 1.
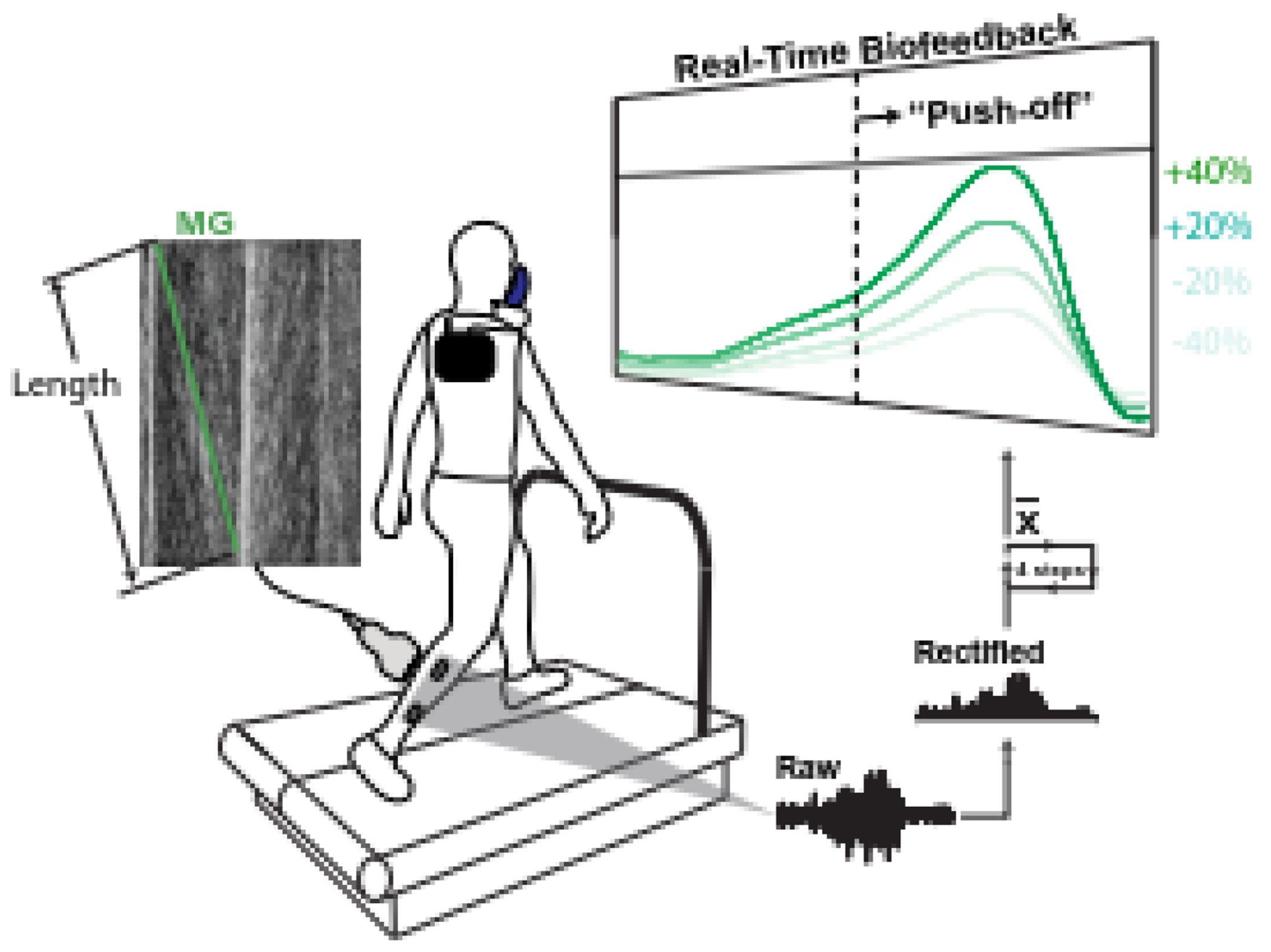
Experimental setup for walking with electromyography (EMG) biofeedback. Subjects walked on instrumented treadmill while surface EMG sensors on the right shank monitored medial gastrocnemius and soleus muscle activities – the average serving to capture triceps surae (TS) activity. TS activity was processed in real-time and the moving average of four strides was displayed on screen. Subjects were instructed to alter their TS activity to meet a prescribed target line. B-mode ultrasound of the medial gastrocnemius (MG) was recorded and metabolic energy consumption monitored.
Ground reaction forces (GRFs) were measured at 1000 Hz by the treadmill, and 3D positions of 52 retroreflective markers on the subjects’ trunk, pelvis, legs, and feet were recorded at 100 Hz using a 16-camera motion capture system (Motion Analysis, Santa Rosa, CA). GRF and marker position data were filtered using a 4th order low-pass Butterworth filter with cutoff frequencies of 100 Hz and 6 Hz respectively. VO2 and VCO2 were measured using portable indirect calorimetry (COSMED, Rome, Italy). We calculated average metabolic rate per kilogram body mass using the average VO2 and VCO2 during the last two minutes of each walking trial. We calculated net metabolic power (W/kg) by subtracting the metabolic rate of standing from the metabolic rate of walking (Brockway, 1987). Cine B-mode ultrasound images were recorded from the right MG as described in the section above. Fascicle kinematics (i.e., length and velocity) during walking were quantified from ultrasound video using an open-source deep learning software package (Cronin et al., 2020) as described in S3, Supplementary Materials. Due to the length of each ultrasound video, this state-of-the-art technique was preferred for its resistance to drift, as each frame of video is analyzed independently.
2.5. Statistical Analysis:
Our outcome measures were peak TS muscle activity, MG fascicle length and velocity at the instant of peak gastrocnemius force during the stride (tpeak), net metabolic power, and peak gastrocnemius force. Fascicle length, velocity, and gastrocnemius force were averaged during the final two 10s ultrasound recording periods (mean strides per recording = 7.86±1.03), taken during the last two minutes of the five-minute walking trials. All other outcomes were averaged over the final two minutes of each five-minute walking trial.
A one-way repeated-measures analysis of variance (rmANOVA) determined the effect of TS activity (prescribed via biofeedback) on all outcome measures. When a significant main effect was found, paired sample t-tests assessed differences between biofeedback conditions. Alpha was set at p<0.05 for un-adjusted comparisons. Effect sizes are reported as partial eta square for rmANOVA, with 0.2, 0.5, and 0.8 interpreted as small, medium, and large, respectively. Outcomes found to have significant a main effect of biofeedback target were then assessed as possible mediators of any effect of biofeedback condition on the net metabolic cost of walking using the MLMED macro (Hayes and Rockwood, 2020) in SPSS (IBM, Armonk, NY) (Fig. 3). The proportion mediated effect was calculated by dividing the indirect effect of a single mediator (i.e., mediated effect) by the sum of all indirect and direct effects (i.e., total effect) (Vuorre and Bolger, 2018). Associations between significant mediators and net metabolic cost were assessed with repeated measures correlation analysis (Bakdash and Marusich, 2017).
Figure 3.
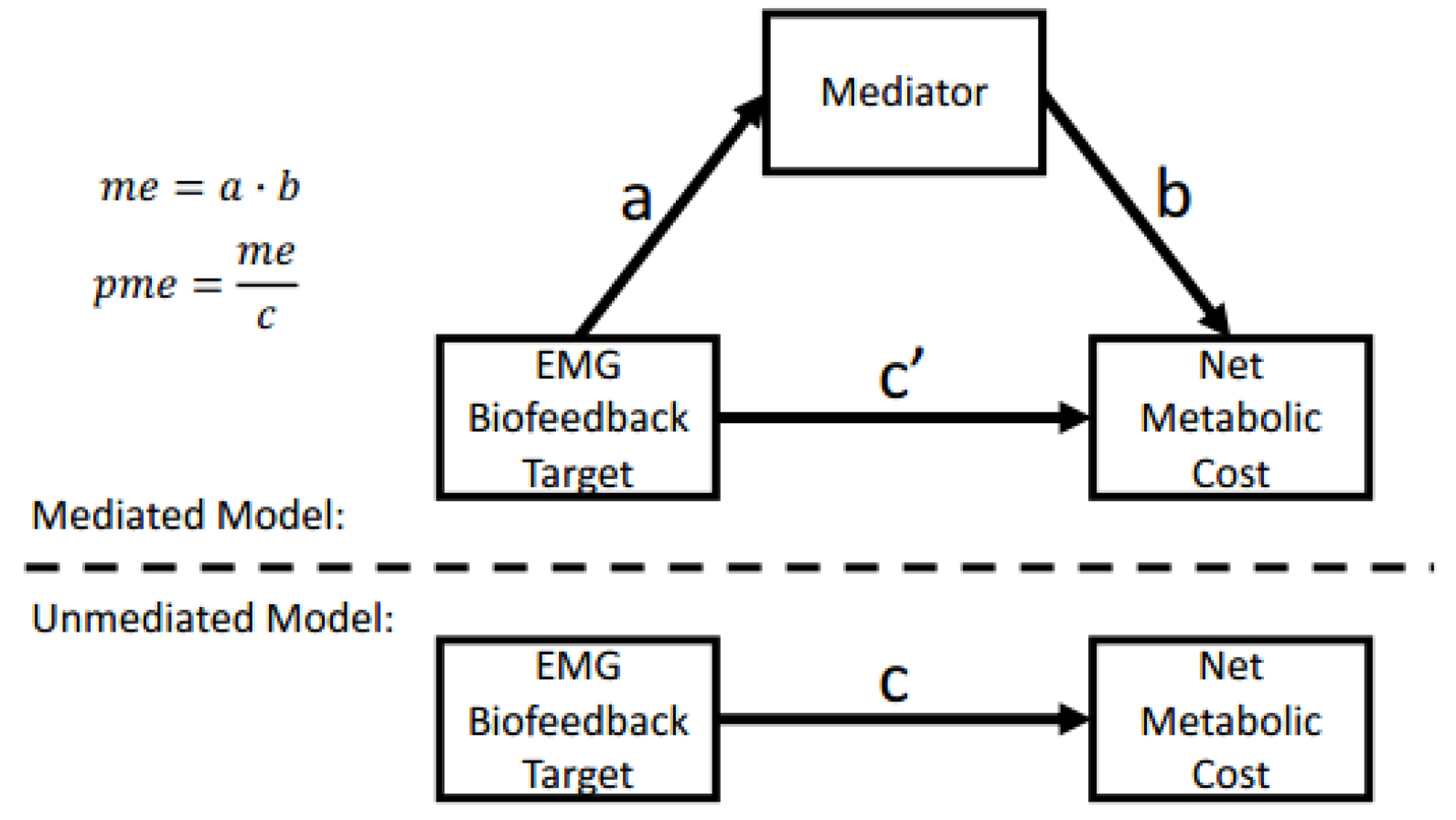
Mediation analysis scheme of identified possible mediators of the effect of EMG target biofeedback on walking metabolic energy cost. Mediation analysis model (Vuorre and Bolger, 2018). In an unmediated model, the total effect of the experimentally manipulated variable (i.e., EMG biofeedback target) on the primary outcome measure (i.e., net metabolic cost) is c. In a mediated model, the manipulated variable acts indirectly via a mediator (a and b), and directly (c’), where the mediated effect (me) is equal to a · b. The proportion of the effect mediated by the mediator (pme) is equal to me / c.
Results:
EMG Biofeedback:
EMG biofeedback significantly altered peak TS activity (main effects, p<0.001, F=39.1, ηp2=0.892; Fig. 2A). Compared to normal walking, subjects increased peak TS activity by 29% (p<0.001) and 41% (p<0.001) when targeting the +20% and +40% targets, respectively. Conversely, when targeting the −20% and −40% targets, subjects’ peak TS activity decreased by only 2% (p=0.320) and 11% (p=0.001), respectively. Participants were thus generally unable to achieve −20% and −40% changes in TS activity.
Figure 2.
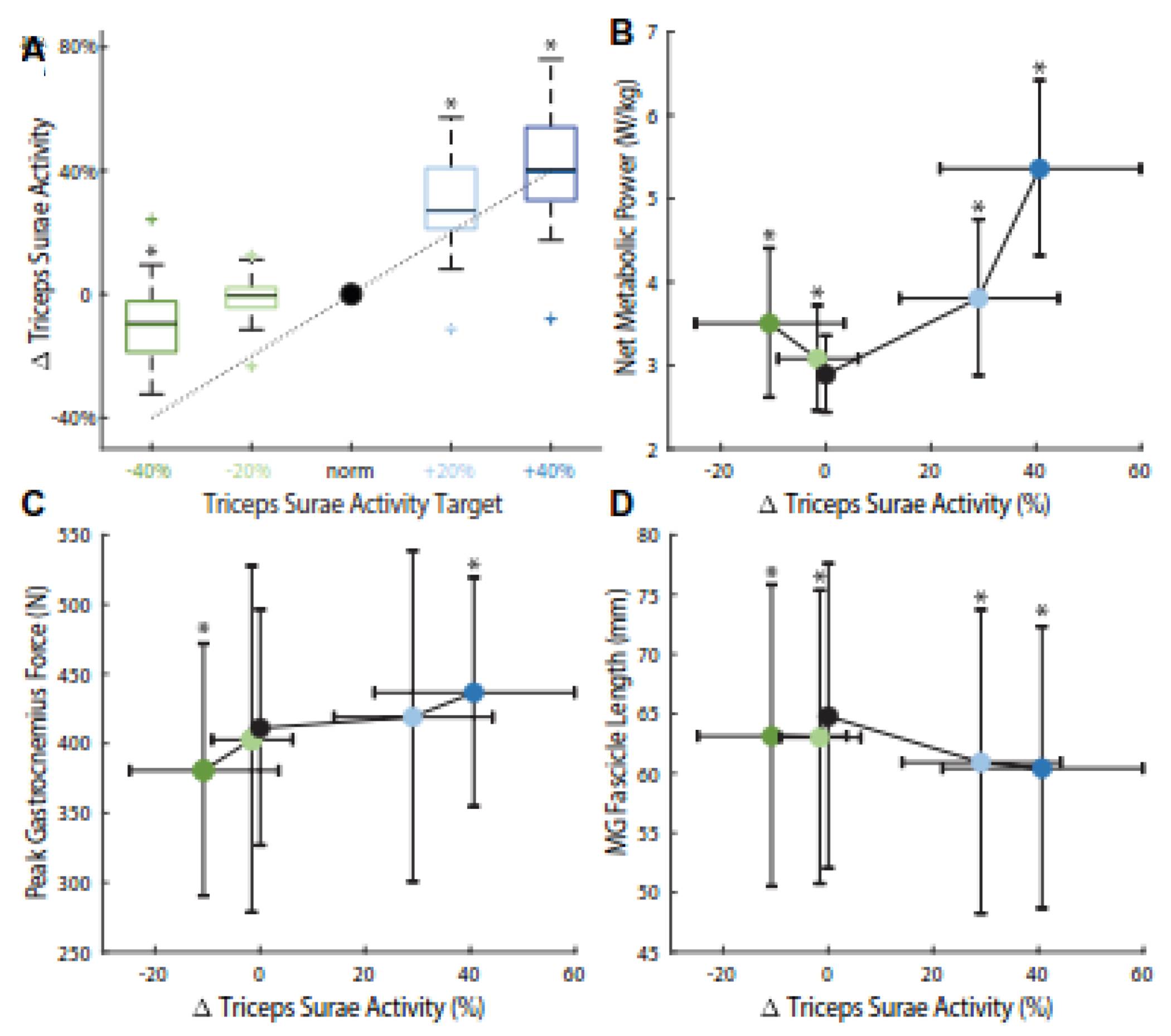
Group average ± standard deviation (A) triceps surae activity levels versus targets (dotted line and x-axis). (B) Peak gastrocnemius force, (C) net metabolic power, and (D) medial gastrocnemius (MG) fascicle length at the instant of peak gastrocnemius force (tpeak) during different triceps surae activity targets in walking versus achieved changes in activity. Asterisks (*) indicate significant (p < 0.05) pairwise difference compared to normal walking.
Spatiotemporal Characteristics:
Average stride frequency was 0.93±0.06 Hz during the Norm condition. When targeting increased activity, subjects changed their average stride frequency to 0.90±0.10 Hz (p=0.211) and 0.88±0.12 Hz (p=0.013) during the +20% and +40% conditions, respectively. When targeting decreased activity, subjects changed their average stride frequency to 0.94±0.06 Hz (p=0.271) and 1.02±0.11 Hz (p=0.001) during the −20% and −40% conditions, respectively.
Energetics and Muscle Dynamics:
TS activity biofeedback significantly altered the net metabolic cost of walking (main effect, p<0.001, F=48.8, ηp2=0.911; Fig. 2B), peak gastrocnemius force (main effect, p<0.001, F=9.41, ηp2=0.664; Fig. 2C), and MG fascicle length at tpeak (main effect, p=0.007, F=4.90, ηp2=0.508; Fig. 2D), but did not significantly affect MG fascicle velocity at tpeaK (main effect, p=0.462, F=0.941, ηp2=0.165). Increased TS activity (i.e., +20% and +40% targets) elicited up to 85% higher net metabolic power (p<0.001), 6% higher peak gastrocnemius force (p<0.001) and 7% shorter MG fascicle lengths at tpeak (p<0.001). Decreased TS activity (i.e., −40% target) elicited 21% higher net metabolic power (p<0.001), 7% lower peak gastrocnemius force (p=0.010), and 3% shorter MG fascicle lengths at the instant of peak gastrocnemius force (p=0.004).
Mediation and Correlation Analyses:
Subsequent analyses of the neuromechanical outcome measures identified two mediators of the effect of biofeedback-induced changes in TS activity on the net metabolic cost of walking (Figs. 3–4; Table 1). Biofeedback target (p=0.188) did not have a significant direct effect on the net metabolic cost of walking. Peak TS activity and MG fascicle length at tpeak mediated 57.2% (mediated effect=0.013, p=0.006) and 11.7% (mediated effect=0.003, p=0.036) of the effect of biofeedback target on net metabolic cost, respectively. Conversely, peak gastrocnemius force (p=0.948) did not significantly mediate the effect of biofeedback-induced changes in TS activity on net metabolic cost of walking. Finally, both of the identified mediators, MG fascicle length at tpeak and peak TS activity, significantly correlated with the net metabolic cost of walking. Specifically, net metabolic power was negatively associated with MG fascicle length at tpeak (r=−0.47, p<0.001) and positively associated with TS activity (r=0.70, p<0.001) (Fig. 4).
Figure 4.
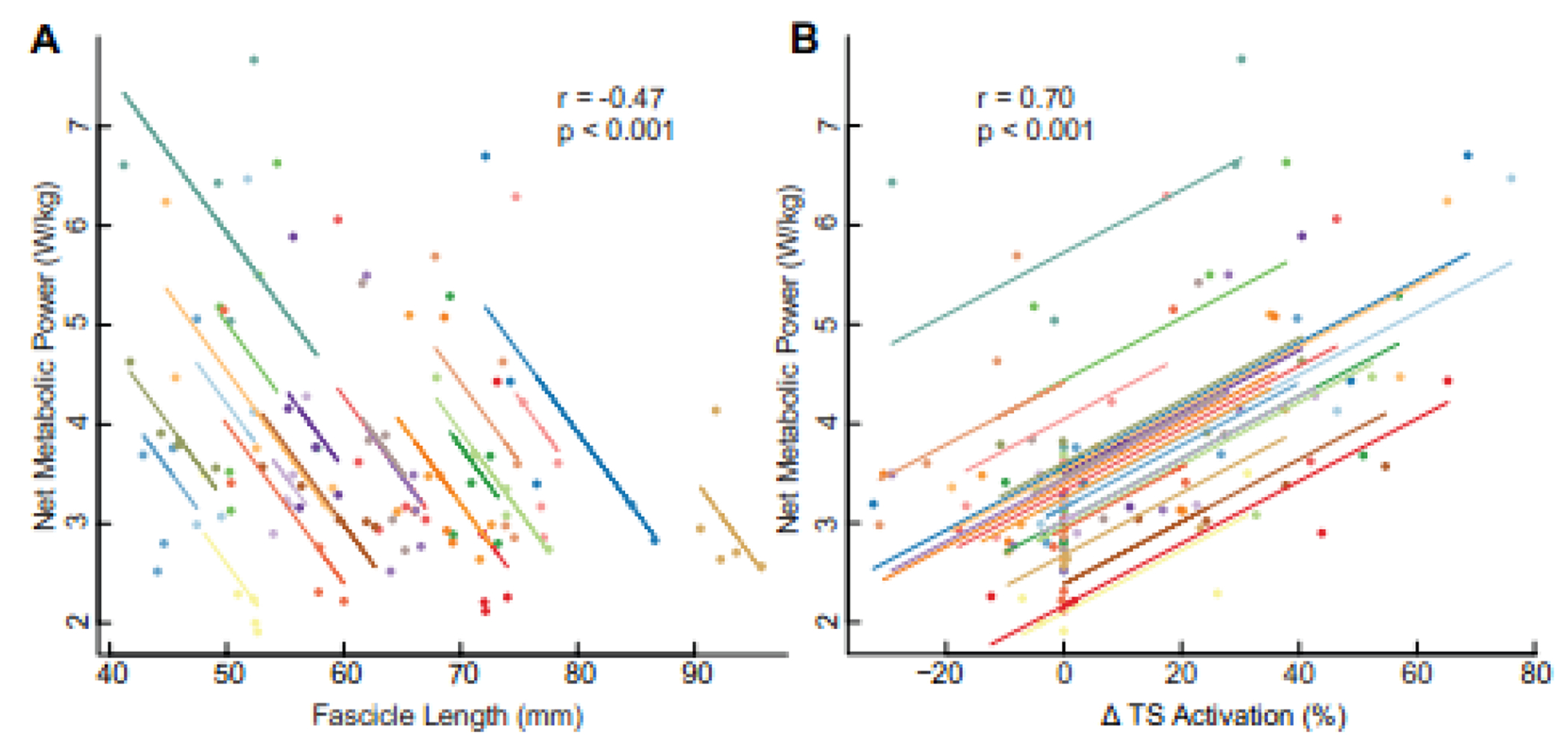
Repeated measures correlations between net metabolic power and (A) medial gastrocnemius (MG) fascicle length at the instant of peak gastrocnemius force (tpeak), and (B) peak TS activity.
Table 1.
Mediation analysis of the effect of TS activity biofeedback on the net metabolic cost of walking.
| Variable | Effect Type | Effect (p-value) | Proportion Mediated (%) |
|---|---|---|---|
| Experimental Variable | |||
|
| |||
| EMG Biofeedback Target | Direct | 0.068 (0.188) | -- |
|
| |||
| Mediators | |||
|
| |||
| Peak TS Activity | Mediated | 0.0127 (0.006) | 57.2 |
| MG Fascicle Length at tpeak | Mediated | 0.0026 (0.036) | 11.7 |
| Peak Gastrocnemius Force | Mediated | 0.0001 (0.948) | 0.45 |
TS = triceps surae, MG = medial gastrocnemius, tpeak = instant of peak gastrocnemius force
Discussion
The purpose of our study was to examine the ways in which changes in TS muscle fascicle functional lengths, steered here by prescribing requisite changes in muscle activity via biofeedback, affect the metabolic cost of walking. In full support of our first hypothesis and consistent with basic principles, we found that increasing peak TS activity to values higher than those observed during habitual walking increased metabolic cost (e.g., +85% for +40% EMG) and shortened muscle fascicles operating lengths (e.g., −7% for +40% EMG). As we describe in more detail below, participants were far less capable of walking with lesser than habitual TS activity. Perhaps accordingly, targeting lesser than habitual peak TS activity during walking also increased metabolic cost (e.g., +21% for −40% EMG) and shortened muscle fascicles (−3% for −40% EMG). However, participants’ inability to target prescribed decreases in activity precludes our ability to evaluate our second hypothesis. There are many factors that contribute to walking metabolism unaccounted for in our theoretical model and experimental paradigm. Nevertheless, our findings provide in vivo evidence that, relatively independent of muscle force output, interactions between TS fascicle operating lengths and changes in requisite TS activity can mediate changes in the metabolic cost of walking.
From basic principles largely derived from fixed-end contractions, operating muscles at shorter lengths should increase the activity required to meet the force demands of a particular task, and vice versa, thereby augmenting the metabolic cost of force generation. Indeed, as muscles shift to lengths shorter than that at the peak of their F-L relation, their force-generating capacity (i.e., maximum force potential at that length) decreases. Here, we used a novel EMG biofeedback paradigm to induce changes in TS activity as a means to interrogate the metabolic consequences of altered muscle fascicle length during walking. To help contextualize, Figure 5 illustrates the F-L operating landscape of the TS muscles on average and at the instant of peak MG force output across our experimental conditions. This reference, serving to understand functional boundaries of TS F-L behavior, also reveals normalized peak force values greater than 1. We interpret those values as arising from force enhancement due to muscle-tendon unit dynamics absent during isolated contractions. Nevertheless, Figure 5 shows how prescribed changes in TS activity shift F-L behavior in ways not constrained by a single F-L curve. Participants had little difficulty matching the prescribed EMG target values for biofeedback trials designed to increase TS activity. Moreover, when they increased peak EMG by 41%, their MG fascicles operated at 7% shorter lengths and net metabolic power increased by 85%. This large whole-body metabolic penalty aligns with in vitro fiber-level findings that reduced sarcomere length greatly increases the ATP cost of force-generation (Hilber et al., 2001) as well as experiments examining metabolic cost of isolated TS muscles during force-matched cyclic contractions on a dynamometer(Beck et al., 2022). Clinical populations with increased tendon compliance and therefore increased tendon elongation, such as older adults (Franz, 2016; Mian et al., 2007; Onambele et al., 2006) and people following stroke (Gao et al., 2009; Kramer et al., 2016; Zhao et al., 2009), similarly exhibit shorter TS muscle fascicle length, increased levels of requisite activity, and corresponding increases in the metabolic cost of walking. Thus, our findings implicate the neuromechanical consequences of those shortened fascicle lengths as a potential determinant of the higher metabolic energy costs observed in these populations.
Figure 5.
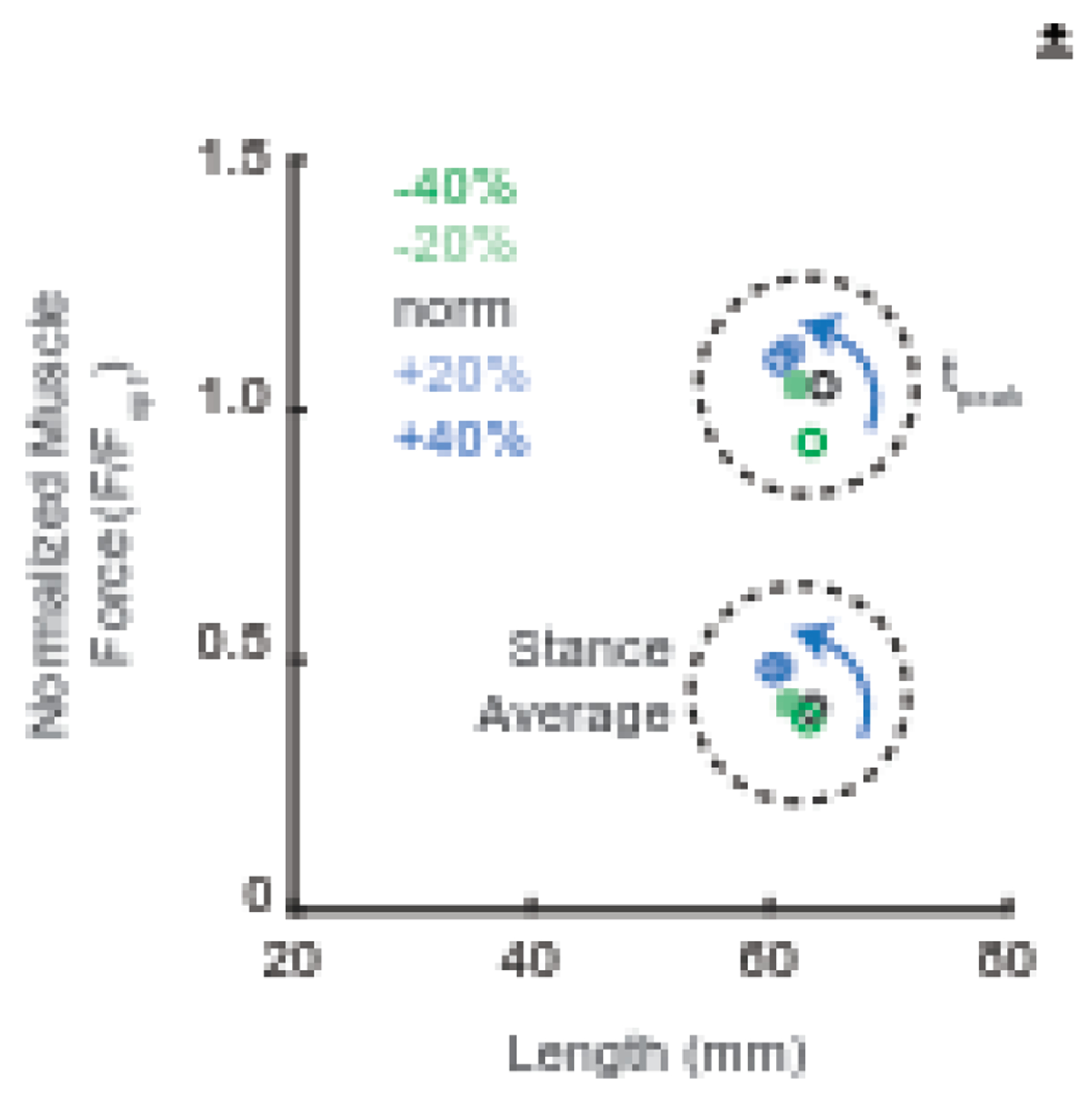
Group average normalized gastrocnemius force and fascicle length at the instant of peak gastrocnemius force (tpeak) and the stance phase average. Force values were normalized to each subject’s optimal force derived from isolated contractions.
Participants appeared to struggle to match biofeedback targets designed to decreased TS activity. For example, our subjects achieved only −11% TS activity when targeting 40% less than habitual TS activity. We suggest two possible explanations for this response. First, our younger adult participants may already operate near the minimum possible TS activity needed to generate requisite plantarflexor forces to sustain walking at a given speed. Habitual gait patterns tend to minimize the squared activity of muscles (Crowninshield and Brand, 1981), and thus minimize metabolic cost per unit distance traveled. Therefore, there may be little capacity to walk with lesser than habitual TS activity, at least for these distal leg extensor muscles. Second, the floor of possible TS activity may be governed by involuntary reflexes. Comparative evidence suggests that TS stretch reflex activity contributes approximately 25% of the overall plantarflexor force during walking (Bennett et al., 1996). This involuntary reflexive activity may limit how much participants are able to reduce TS activity during locomotion.
Contrary to our predictions, we observed shorter fascicle lengths when participants targeted decreased levels of TS activity (Fig. 2D). Despite unexpected MG fascicle length shortening, this outcome can still provide us insight into any role of fascicle length on the metabolic cost of walking. Consistent with our theoretical premise, shorter MG fascicle lengths for these conditions, which were not accompanied by changes in MTU length (Supplementary Figure 1), were similarly accompanied by large increases in metabolic cost. In addition to the direct relation between fascicle length and metabolic cost, we suspect this outcome may arise indirectly from unaccounted for coordination between the ankle and knee or a redistribution to more proximal leg muscles to power walking. Indeed, exploratory analyses allude to a proximal distribution of muscle demand when targeting decreased TS activity (Supplementary Figure 2), which may have enacted an indirect metabolic penalty.
We used mediation analyses to add objective credibility to our interpretations regarding the influence of local neuromechanical variables on the metabolic cost of walking. Biofeedback target had no significant direct effect on the net metabolic cost of walking. Instead, metabolic changes were governed via two key mediators: peak TS activity (proportion mediated: 57.2%) and MG fascicle length at the instant of peak force (proportion mediated: 11.7%). Thus, nearly 70% of the measured changes in metabolic cost appeared to be mediated by the combination of muscle activity and muscle fascicle length. The substantial mediating role of fascicle length on metabolic cost corroborates other findings that shorter muscle fascicles increase the metabolic cost of force generation (Beck et al., 2022; Nuckols et al., 2020). We also considered muscle force and fascicle velocity as other possible determinants of the metabolic energy cost of walking. Predictably, muscle force was positively associated with EMG biofeedback target (Fig. 2B); however, we did not find any evidence that muscle force independently mediated changes in metabolic cost. Finally, fascicle velocity was unaffected by our experimental manipulations. Thus, our findings about the mediating role of fascicle length can be interpreted without the confounding effect of fascicle velocity variation.
We acknowledge several potential limitations. We first interpret our results in the context of the metabolic cost to operate the TS muscles during walking. However, we acknowledge the potential for TS EMG biofeedback to elicit changes in the patterns of activity in other muscles, including more proximal and less economical leg muscles, or in the distribution of recruited fiber types. Similarly, changes in stride frequency elicited by EMG biofeedback may contribute to altered metabolic cost (Minetti et al., 1995). Future studies may consider combining EMG biofeedback with a metronome to prescribe activity and stride frequency. Nevertheless, our findings are consistent with those from isolated contractions (Beck et al., 2022), and any measured change – including those outside of the theoretical motivation for this study – was an ecologically-relevant response to an experimentally induced alteration of TS activity. We interpret our findings from a selected timepoint (i.e., the instant of peak MG force); however, more complex changes may have been elicited by biofeedback in different parts of the stride. However, exploratory time series SPM analysis (Supplementary Figure 3) indicated that changes at our selected timepoint reflected the predominant changes during stance phase across our measures of fascicle length, velocity, and force. From a practical perspective, we were unable to simultaneously record EMG and US from the MG and instead relied on lateral gastrocnemius (LG) activity. Our analysis and interpretation assumes similarity in the timing of peak force generation between the medial and lateral gastrocnemius (Sylvester et al., 2021); however, recent work suggests that MG and LG may not share the same neural drive (Levine et al., 2023) which may limit the generalizability of our results. However, extensive EMG studies suggest that conclusions drawn from the timing and magnitude of activity from one head of the gastrocnemius are very consistent if not identical with those from the other (Hamard et al., 2023). Due to a lack of direct measurements, we estimated gastrocnemius force by decomposing ankle joint moment. Thus, we could not account for antagonist co-activation. Finally, our theoretical premise that increased TS activation would increase metabolic cost via shorter muscle fascicle operating lengths and thus reduced force capacity is based on basic principles derived predominantly from studies in isolated contractions.
Consistent with prior work in isolated contractions, our results suggest that shifts to shorter fascicle lengths may mediate activity-induced increases in metabolic cost. We interpret our findings to suggest that: (1) volitional increases in TS activity can modulate the metabolic cost of walking via relatively predictable alterations in fascicle operating lengths, and (2) these shorter TS fascicle lengths contribute to increased metabolic energy consumption relatively independently of changes in muscle force. These findings may have important implications for understanding higher metabolic costs in individuals who habitually exhibit shorter TS fascicle lengths due to reduced Achilles tendon stiffness.
Supplementary Material
Acknowledgements.
This study was supported by grants from the US National Institutes of Health (R01AG058615, F32AG067675) and an Abrams Scholarship to Callum Funk from the UNC/NC State Joint Department of Biomedical Engineering. We thank the reviewers and the selection committee from the American Society of Biomechanics (ASB) for their recognition of this work, based on an abstract selected for the 2021 ASB Journal of Biomechanics award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest to disclose
References:
- Adams GR, Duvoisin MR, Dudley GA, 1992. Magnetic resonance imaging and electromyography as indexes of muscle function. Journal of Applied Physiology 73, 1578–1583. 10.n52/jappl.1992.73A1578 [DOI] [PubMed] [Google Scholar]
- Bakdash JZ, Marusich LR, 2017. Repeated Measures Correlation. Front. Psychol 8, 456. 10.3389/fpsyg.2017.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck ON, Punith LK, Nuckols RW, Sawicki GS, 2019. Exoskeletons Improve Locomotion Economy by Reducing Active Muscle Volume. Exercise and Sport Sciences Reviews 47, 237. 10.1249/JES.0000000000000204 [DOI] [PubMed] [Google Scholar]
- Beck ON, Trejo LH, Schroeder JN, Franz JR, Sawicki GS, 2022. Shorter muscle fascicle operating lengths increase the metabolic cost of cyclic force production. Journal of Applied Physiology 133, 524–533. 10.1152/japplphysiol.00720.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, De Serres SJ, Stein RB, 1996. Gain of the triceps surae stretch reflex in decerebrate and spinal cats during postural and locomotor activities. The Journal of Physiology 496, 837–850. 10.1113/jphysiol.1996.sp021731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockway JM, 1987. Derivation of formulae used to calculate energy expenditure in man. Hum Nutr Clin Nutr 41, 463–471. [PubMed] [Google Scholar]
- Chleboun GS, France AR, Crill MT, Braddock HK, Howell JN, 2001. In vivo measurement of fascicle length and pennation angle of the human biceps femoris muscle. Cells Tissues Organs 169, 401–409. 10.1159/000047908 [DOI] [PubMed] [Google Scholar]
- Clark WH, Franz JR, 2019. Activation-Dependent Changes in Soleus Length–Tension Behavior Augment Ankle Joint Quasi-Stiffness. Journal of Applied Biomechanics 35, 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborne GR, Wright FV, Naumann S, 1994. Feedback of triceps surae EMG in gait of children with cerebral palsy: a controlled study. Arch Phys Med Rehabil 75, 40–45. [PubMed] [Google Scholar]
- Conway KA, Franz JR, 2020. Shorter gastrocnemius fascicle lengths in older adults associate with worse capacity to enhance push-off intensity in walking. Gait & Posture 77, 89–94. 10.1016/j.gaitpost.2020.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin NJ, Finni T, Seynnes O, 2020. Fully automated analysis of muscle architecture from B-mode ultrasound images with deep learning. arXiv:2009.04790 [cs, eess]. [DOI] [PubMed] [Google Scholar]
- Crowninshield RD, Brand RA, 1981. A physiologically based criterion of muscle force prediction in locomotion. Journal of Biomechanics 14, 793–801. 10.1016/0021-9290(81)90035-X [DOI] [PubMed] [Google Scholar]
- Farris DJ, Lichtwark GA, 2016. UltraTrack: Software for semi-automated tracking of muscle fascicles in sequences of B-mode ultrasound images. Computer Methods and Programs in Biomedicine 128, 111–118. 10.1016/j.cmpb.2016.02.016 [DOI] [PubMed] [Google Scholar]
- Franz JR, 2016. The Age-Associated Reduction in Propulsive Power Generation in Walking. Exercise and Sport Sciences Reviews 44, 129–136. 10.1249/JES.0000000000000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Grant TH, Roth EJ, Zhang L-Q, 2009. Changes in Passive Mechanical Properties of the Gastrocnemius Muscle at the Muscle Fascicle and Joint Levels in Stroke Survivors. Archives of Physical Medicine and Rehabilitation 90, 819–826. 10.1016/j.apmr.2008.11.004 [DOI] [PubMed] [Google Scholar]
- Griffin TM, Roberts TJ, Kram R, 2003. Metabolic cost of generating muscular force in human walking: insights from load-carrying and speed experiments. Journal of Applied Physiology 95, 172–183. 10.1152/japplphysiol.00944.2002 [DOI] [PubMed] [Google Scholar]
- Hamard R, Aeles J, Avrillon S, Dick TJM, Hug F, 2023. A comparison of neural control of the biarticular gastrocnemius muscles between knee flexion and ankle plantar flexion. Journal of Applied Physiology. 10.1152/japplphysiol.00075.2023 [DOI] [PubMed] [Google Scholar]
- Hayes AF, Rockwood NJ, 2020. Conditional Process Analysis: Concepts, Computation, and Advances in the Modeling of the Contingencies of Mechanisms. American Behavioral Scientist 64, 19–54. 10.1177/0002764219859633 [DOI] [Google Scholar]
- Hessel AL, Raiteri BJ, Marsh MJ, Hahn D, 2021. Rightward shift of optimal fascicle length with decreasing voluntary activity level in the soleus and lateral gastrocnemius muscles. J Exp Biol 224, jeb235614. 10.1242/jeb.235614 [DOI] [PubMed] [Google Scholar]
- Hilber K, Sun Y-B, Irving M, 2001. Effects of sarcomere length and temperature on the rate of ATP utilisation by rabbit psoas muscle fibres. The Journal of Physiology 531, 771–780. 10.1111/j.1469-7793.2001.0771h.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S, Johnson L, Bernhardt J, Cumming T, 2016. Energy Expenditure and Cost During Walking After Stroke: A Systematic Review. Archives of Physical Medicine and Rehabilitation 97, 619–632.e1. 10.1016/j.apmr.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Krupenevich RL, Clark WH, Sawicki GS, Franz JR, 2020. Older Adults Overcome Reduced Triceps Surae Structural Stiffness to Preserve Ankle Joint Quasi-Stiffness During Walking. Journal of Applied Biomechanics 36, 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Avrillon S, Farina D, Hug F, Pons JL, 2023. Two motor neuron synergies, invariant across ankle joint angles, activate the triceps surae during plantarflexion. 10.1101/2022.11.11.516183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manal K, Cowder JD, Buchanan TS, 2013. Subject-specific measures of Achilles tendon moment arm using ultrasound and video-based motion capture. Physiol Rep 1, e00139. 10.1002/phy2.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigò LP, Minetti AE, Narici MV, 2007. Gastrocnemius muscle–tendon behaviour during walking in young and older adults. Acta Physiologica 189, 57–65. 10.1111/j.1748-1716.2006.01634.x [DOI] [PubMed] [Google Scholar]
- Minetti AE, Capelli C, Zamparo P, Di Prampero PE, Saibene F, 1995. Effects of stride frequency on mechanical power and energy expenditure of walking. Medicine & Science in Sports & Exercise 27, 1194–1202. [PubMed] [Google Scholar]
- Monte A, Tecchio P, Nardello F, Bachero-Mena B, Ardigò LP, Zamparo P, 2023. The interplay between gastrocnemius medialis force–length and force–velocity potentials, cumulative EMG activity and energy cost at speeds above and below the walk to run transition speed. Experimental Physiology 108, 90–102. 10.1113/EP090657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV, 2005. In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. Journal of Applied Physiology 99, 1050–1055. 10.1152/japplphysiol.01186.2004 [DOI] [PubMed] [Google Scholar]
- Munsch AE, Pietrosimone B, Franz JR, 2020. The effects of knee extensor moment biofeedback on gait biomechanics and quadriceps contractile behavior. PeerJ 8, e9509. 10.7717/peerj.9509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuckols RW, Dick TJM, Beck ON, Sawicki GS, 2020. Ultrasound imaging links soleus muscle neuromechanics and energetics during human walking with elastic ankle exoskeletons. Sci Rep 10, 3604. 10.1038/s41598-020-60360-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onambele GL, Narici MV, Maganaris CN, 2006. Calf muscle-tendon properties and postural balance in old age. Journal of Applied Physiology 100, 2048–2056. 10.1152/japplphysiol.01442.2005 [DOI] [PubMed] [Google Scholar]
- Orselli MIV, Franz JR, Thelen DG, 2017. The effects of Achilles tendon compliance on triceps surae mechanics and energetics in walking. J Biomech 60, 227–231. 10.1016/jjbiomech.2017.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega JD, Farley CT, 2007. Individual limb work does not explain the greater metabolic cost of walking in elderly adults. J Appl Physiol (1985) 102, 2266–2273. 10.1152/japplphysiol.00583.2006 [DOI] [PubMed] [Google Scholar]
- Panizzolo FA, Green DJ, Lloyd DG, Maiorana AJ, Rubenson J, 2013. Soleus fascicle length changes are conserved between young and old adults at their preferred walking speed. Gait & Posture 38, 764–769. 10.1016/j.gaitpost.2013.03.021 [DOI] [PubMed] [Google Scholar]
- Rubenson J, Pires NJ, Loi HO, Pinniger GJ, Shannon DG, 2012. On the ascent: the soleus operating length is conserved to the ascending limb of the force–length curve across gait mechanics in humans. Journal of Experimental Biology 215, 3539–3551. 10.1242/jeb.070466 [DOI] [PubMed] [Google Scholar]
- Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG, 2009. Differences in lower-extremity muscular activation during walking between healthy older and young adults. Journal of Electromyography and Kinesiology 19, 1085–1091. 10.1016/jjelekin.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester AD, Lautzenheiser SG, Kramer PA, 2021. Muscle forces and the demands of human walking. Biology Open 10, bio058595. 10.1242/bio.058595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberger BR, 2010. Stance and swing phase costs in human walking. J R Soc Interface 7, 1329–1340. 10.1098/rsif.2010.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberger BR, Rubenson J, 2011. Understanding Muscle Energetics in Locomotion: New Modeling and Experimental Approaches. Exercise and Sport Sciences Reviews 39, 59–67. 10.1097/JES.0b013e31820d7bc5 [DOI] [PubMed] [Google Scholar]
- Voronov AV, 2003. Anatomical Cross-Sectional Areas and Volumes of the Muscles of the Lower Extremities 29, 11. [PubMed] [Google Scholar]
- Vuorre M, Bolger N, 2018. Within-subject mediation analysis for experimental data in cognitive psychology and neuroscience. Behav Res 50, 2125–2143. 10.3758/s13428-017-0980-9 [DOI] [PubMed] [Google Scholar]
- Zhao H, Ren Y, Wu Y-N, Liu SQ, Zhang L-Q, 2009. Ultrasonic evaluations of Achilles tendon mechanical properties poststroke. J Appl Physiol (1985) 106, 843–849. 10.1152/japplphysiol.91212.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


